Note: Descriptions are shown in the official language in which they were submitted.
CA 02188429 1999-11-24
-1-
CLAD COMPOSITE STENT
Backgiround of the Invention
The present invention relates.to body implantable medical devices, and more
particularly to stents and other prostheses configured for high radio-opacity
as well as
favorable mechanical characteristics.
Recently several prostheses, typically of lattice work or open frame
construction,
have been developed for a variety of medical applications, e.g. intravascular
stents for
treating stenosis, prostheses for maintaining openings in the urinary tracts,
biliary
prostheses, esophageal stents, renal stents, and vane cave filters to counter
thrombosis. One particularly well accepted device is a self-expanding mesh
stent
disclosed in U. S. IPatent No. 4,655,771 (Vllallsten). The stent is a flexible
tubular
braided structure formed of helically wound thread elements. The thread
elements can
be constructed of a biocompatible plastic or metal, e.g. certain stainless
steels,
polypropylene, polyesters and polyurethanes.
Alternatively, stents and other prostheses can be expandable by plastic
deformation, usually by expanding a dilation balloon surrounded by the
prosthesis. For
example, U. S. Patent No. 4,733,665 (Palmaz) discloses an intraluminal Qraft
constructed of stainless steel strands, either woven or welded at their
intersections with
silver. U. S. Patent No. 4,886,062 ~ktor) features a balloon expandable stent
constructed of stainless steel, a copper alloy, titanium, or gold.
Regardless of whether the prosthesis is self-expanding or plastically
expanded,
accurate placement of the prosthesis is critical to its effective performance.
Accordingly, there is a need to visually perceive the prosthesis as it is
being placed
within a blood vessel or other body cavity. Further, it is advantageous and
sometimes
necessary to visually locate and inspect a previously deployed prosthesis.
Fluoroscopy is the prevailing technique for such visualization, and it
requires
radio-opacity in the materials to be imaged. The preferred structural
materials for
prosthesis construci:ion, e.g. stainless steels and cobalt-based alloys, are
not highly
radiopaque. Consequently, prostheses constructed of these materials do not
lend
themselves well to fluoroscopic imaging.
W095/30384 PCT/1895100253
_2-
Several techniques have been proposed, in apparent recognition of this
difficulty. For example, U. S. Patent No. 4,681,110 (Wiktor) discloses a self-
expanding
blood vessel liner formed of woven plastic strands, radially compressed for
delivery
within a tube. A metal ring around the tube is radiopaque. Similarly, U.S.
Patent No.
4,830,003 (Wolff) discusses confining a radially self-expanding stent within a
delivery
tube, and providing radiopaque markers on the delivery tube. This approach
facilitates
imaging only during deployment and initial placement.
To permit fluoroscopic imaging after placement, the stent itself must be
radiopaque. The Wolff patent suggests that the stent can be formed of platinum
or a
platinum-iridium alloy for substantially greater radio-opacity. Such stent,
however, lacks
the required elasticity, and would exhibit poor resistance to fatigue. The
Wiktor'110
patent teaches the attachment of metal staples to its blood vessel liner, to
enhance
radio-opacity. However, for many applications (e.g. in blood vessels), the
stent is so
small that such staples either would be too small to provide useful
fluoroscopic
imaging, or would adversely affect the efficiency and safety of deploying the
stent or
other prosthesis. This Wiktor patent also suggests infusing its plastic
strands with a
suitable filler, e.g. gold or barium sulfate, to enhance radio-opacity. Wiktor
provides no
teaching as to how this might be done. Further, given the small size of
prostheses
intended for blood vessel placement, this technique is unlikely to materially
enhance
radio-opacity, due to an insufficient amount and density of the gold or barium
sulfate.
Therefore, it is an object of the present invention to provide a stent or
other
prosthesis with substantially enhanced radio-opacity, without any substantial
reduction
in the favorable mechanical properties of the prosthesis.
Another object is to provide a resilient body insertable composite filament
having
a high degree of radio-opacity and favorable structural characteristics, even
for stents
employing relatively small diameter filaments.
A further object is to provide a process for manufacturing a composite
filament
consisting essentially of a structural material for imparting desired
mechanical
characteristics, in combination with a radiopaque material to substantially
enhance
fluoroscopic imaging of the filament.
Yet another object is to provide a case composite prosthesis in which a highly
radiopaque material and a structural material cooperate to provide mechanical
stability
and enhanced fluoroscopic imaging, and further are selectively matched for
CA 02188429 1999-11-24
3
compatibility as 'to their crystalline structure, coefficients of
thermal expansion, and annealing temperatures.
Summary of the Invention
The present invention is concerned with a resilient body
insertable composite filament. The filament may be produced by
a process which includes the following steps:
a. providing an elongate cylindrical core
substantially uniform in lateral cross-section and having a core
diameter, and an elongate tubular case or shell substantially
uniform in lateral cross-section and having a case inside
diameter, wherein one of the core and case is formed of a
radiopaque material and the other is formed of a resilient
material having an yield strength (0.2% offset) of at least
150,000 psi, wherein the core diameter is less than the interior
diameter of the case, and the lateral cross-sectional area of
the core and case is at most ten times the lateral cross-
sectional area of the core;
b. inserting the core into the case to form an
elongate composite filament in which the case surrounds the
core;
c. cold-working the composite filament to reduce the
lateral cross-seci~ional area of the composite filament by at
least 15%, whereby the composite filament before cold-working;
d. annealing the composite filament after cold
working, to subst<~ntially remove strain hardening and other
stresses induced by the cold-working step;
e. mechanically forming the annealed composite
filament into a predetermined shape; and
f. after the cold-working and annealing steps, and
while maintaining the composite filament in the predetermined
shape, age hardening the composite filament.
In one preferred version of the process, the radiopaque
material has a linear attenuation coefficient, at 100 KeV, of at
CA 02188429 1999-11-24
3a
least 25 cm-1. The radiopaque material forms the core, and is at
least as ductile as the case. The outside diameter of the
composite filament, before cold-working, preferably is at most
about six millimeters (about 0.25 inches). The cold-working
step can include drawing the composite filament serially
W0 95130384 PCTIIB95/00253
-4- ,
through several dies, with each die plastically deforming the composite
filament to
reduce the outside diameter. Whenever a stage including one or more cold-
working
dies has reduced the cross-sectional area by at least 2596, an annealing step
should
be performed before any further cold-working.
During each annealing step, the composite filament is heated to a temperature
in the range of about 1900 - 2300° F. for a period depending on the
filament diameter,
typically in the range of several seconds to several minutes. The core
material and
cladding (case) materials preferably are selected to have overlapping
annealing
temperature ranges, and similar coefficients of thermal expansion. The core
and case
materials further can be selectively matched as to their crystalline structure
and
metallurgical compatibility.
In an alternative version of the process, the initial outside diameter of the
composite structure (billet) typically is at least fifty millimeters (about
two inches) in
diameter. Then, before cold-working, the composite filament is subjected to
temperatures in the annealing range wh'le the outside diameter is
substantially reduced,
either by swaging or by pulltrusion, in s' iccessive increments until the
outside diameter
is at most about 6 millimeters (.25 inches). The resulting filament is
processed as
before, in alternative cold-working and annealing stages.
Further according to the process, the composite filament can be severed into
a plurality of strands. Then, the strands are arranged in two oppositely
directed sets
of parallel helical windings about a cylindrical form, with the strands
intertwined in a
braided configuration to form multiple intersection >. Then, while the strands
are
maintained in a predetermined uniform tension, they are heated to a
temperature in the
range of about 700 - 1200° F., more preferably 900 - 1000° F.,
for a time sufficient to
age harden the helical windings.
The result of this process is a resilient, body implantable prosthesis. The
prosthesis has a plurality of resilient strands, helically wound in two
oppositely directed
sets of spaced apart and parallel strands, interwoven with one another in a
braided
configuration. Each of the strands includes an elongate core and an elongate
tubular
case surrounding the core. A cross-sectional area of the core is at least ten
percent
of the cross-sectional area of the strand. The core is constructed of a first
material
having a linear attenuation coefficient of at least 25 cm '' at 100 KeV. The
case is
constructed of a resilient second material, less ductile than the first
material.
CA 02188429 1999-11-24
Thus, the present invention provides a body compatible
device comprising an elongate filament substantially uniform in
lateral cross-section over its length and including an elongate
cylindrical core and an elongate tubular case surrounding the
5 core. One of the core and case is constructed of a first
material having an yield strength (0.2% offset) of at least
twice that of the second material. The other of the core and
case is constructed of a second material being radiopaque and at
least as ductile as the first material.
In a preferred embodiment, the case is constructed of a
case material having an yield strength (0.2% offset) of at least
100,000 psi and the core is constructed of a core material
comprising at least one of the following constituents: tantalum,
a tantalum-based alloy, platinum, a platinum-based alloy,
tungsten and a tungsten-based alloy.
In another preferred embodiment, the core is constructed of
a core material having a linear attenuation coefficient of at
least 25 cm-1 at 100 KeV, and the case is constructed of a case
material, the core. material being more ductile and more
radiopaque than the case material, and the case material
comprising a titanium-based alloy.
In a highly preferred version of the invention, the core is
constructed of tantalum for radio-opacity, and the case is
constructed of a cobalt-based alloy, e.g. as available under the
brand names "Elgi:Loy*", "Phynox*" and "MP35N". The "Elgiloy*"
and "Phynox*" alloys contain cobalt, chromium, nickel, and
molybdenum, along with iron. Either of these alloys is well
matched with tant<~lum, in terms of overlapping annealing
temperature range:~, coefficients of thermal expansion and
crystalline struci:ure. The tantalum core and alloy case can be
contiguous with one another, with virtually no formation
*Trade-mark
CA 02188429 1999-11-24
6
intermetallics.
When otherwise compatible core and case materials present
the risk of intermetallic formation, an intermediate layer, e.g.
of tantalum, niobium, or platinum, can be formed between the
core and the case to provide a barrier against intermetallic
formation. Further, if the case itself is not sufficiently
biocompatible, a :biocompatible coating or film can surround the
case. Tantalum, ;platinum, iridium and their alloys, or
stainless steels can be used for this purpose.
While disclosed herein in connection with a radially self-
expanding stent, 'the composite filaments can be employed in
constructing other implantable medical devices, e.g. vena cava
filters, blood filters and thrombosis coils. Thus, in
accordance with t:he present invention there is provided a
resilient, body compatible prosthesis which, despite being
sufficiently small for placement within blood vessels and
similarly sized body cavities, has sufficient radio-opacity for
fluoroscopic imaging based on the prosthesis materials
themselves.
In the Drawings
For a further understanding of the above and other features
and advantages, reference is made to the following detailed
description and to the drawings, in which:
Figure 1 is ;a side elevation of a self-expanding stent
constructed according to the present invention;
Figure 2 is an end elevational view of the stent;
Figure 3 is an enlarged partial view of one of the
composite filaments forming the stent;
Figure 4 is an enlarged sectional view taken along the line
4-4 in Figure 3;
CA 02188429 1999-11-24
6a
Figure 5-9 schematically illustrate a process for
manufacturing the stent;
Figure 10 schematically illustrates a swaging step of an
alternative process for manufacturing the stent;
Figure 11 is an end elevational view of an alternative
embodiment filament;
Figure 12 is an elevational view of several components of
an alternative composite filament constructed according to the
present invention,;
Figure 13 is an end elevational view of the composite
filament formed by the components shown in Figure 12; and
Figure 14 is an end elevational view of another alternative
embodiment composite filament .
Figures 1-6 <~ppear on sheet 1/3; Figures 7 and 11-14 appear
on sheet 2/3; and Figures 8-10 appear on sheet 3/3.
Detailed Description of the Preferred Embodiment
Turning now i~o the drawings, there is shown in Figures 1
and 2 a body implantable prosthesis 16, frequently referred to
as a stent. Stent: 16 is of open mesh or weave construction,
consisting of two sets of oppositely directed, parallel and
spaced apart helic:ally wound strands or filaments indicated at
18 and 20, respectively. The sets of strands are interwoven in
an over and under braided configuration to form multiple
intersections, onES of which is indicated at 22.
Stent 16 is :illustrated in its relaxed state, i.e. in the
configuration it assumes when subject to no external stresses.
The filaments or strands of stent 16 are resilient, permitting a
radial compression of the stent into a reduced-radius, extended-
length configuration suitable for transluminal delivery of the
stent to the intended placement site. As a typical example,
stent 16 can have a diameter of about ten millimeters in the
relaxed state, and is elastically compressed to a diameter of
about 2 millimeters (.08 inches) and an axial length of about
CA 02188429 1999-11-24
6b
twice the axial length of the relaxed stent. However, different
applications call for different diameters. Further, it is well
known to predetermine the degree of axial elongation for a given
radial compression, by selectively controlling the angle between
the oppositely directed helical strands.
Inelastic open-weave prostheses, expandable for example by
dilation balloons, provide an alternative to resilient
prostheses. Resilient or self-expanding prostheses
~WO 95130384 ~ PCTIIB95100253
-7_
often are preferred, as they can be deployed without dilation balloons or
other stent
expanding means. Self-expanding stents can be preselected according to the
diameter
of the blood vessel or other intended fixation site. While their deployment
requires skill
in stent positioning, such deployment does not require the additional skill of
carefully
dilating the balloon to plastically expand the prosthesis to the appropriate
diameter.
Further, the self-expanding stent remains at least slightly elastically
compressed after
fixation, and thus has a restoring force which facilitates acute fixation. By
contrast, a
plastically expanded stent must rely on the restoring force of deformed
tissue, or on
hooks, barbs, or other independent fixation elements.
Accordingly, materials forming the strands for filaments must be strong and
resilient, biocompatible, and resistant to fatigue and corrosion. Vascular
applications
require hemocompatibility as well. Several materials meet these needs,
including
stainless "spring" steels, and certain cobalt-based alloys: more particularly
two alloys
including cobalt, chromium, iron, nickel and molybdenum sold under the brand
names
"Elgiloy" (available from Carpenter Technology Corporation of Reading,
Pennsylvania)
and "Phynox" (available from Metal Imphy of Imphy, France), respectively.
Another
suitable cobalt-chromium alloy is available under the brand name "MP35N" from
Carpenter Technology Corporation of Reading, Pennsylvania.
Further, it is advantageous to form a prosthesis with substantial open space
to
promote embedding of the stent into tissue, and fibrotic growth through the
stent wall
to enhance long-term fixation. A more open construction also enables
substantial radial
compression of the prosthesis for deployment. In a typical construction
suitable for
transluminal implantation, the filaments can have a diameter of about 0.1
millimeter
(.004 inches), with adjacent parallel filaments spaced apart from one another
by about
1-2 millimeters (.04-.OS inches) when the stent is in the relaxed state.
Fluoroscopic imaging of a conventional open weave prosthesis is extremely
difficult. Due to their minute diameters and the materials involved, the
filaments exhibit
a relatively poor contrast to body tissue for fluoroscopic imaging purposes.
The
filaments also require a high degree of spatial resolution in the imaging
equipment
involved. Thus, a stent recognizable on X-ray film may not be distinguishable
for real
time imaging, due to the relatively poor spatial resolution of the video
monitor as
compared to X-ray film.
WO 95/30384 2 ~ 8 8 ~ 2 9 PCTIdB95100253
-8-
According to the present invention, however, prosthesis 16 is substantially
more
amenable to fluoroscopic imaging, due to the construction of strands 18 and
20. In
particular, the strands cooperate to present a sufficiently radiopaque mass at
the
tangents of device 16 (parallel to the X-rays) for satisfactory real time
imaging. As seen
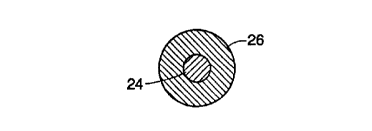
in Figures 3 and 4, a filament 18a of he prosthesis is of composite
construction, with
a radiopaque core 24 surrounded by and concentric with an annular resilient
case 26.
Core 24 is highly absorptive of X-rays, preferably having a linear attenuation
coefficient
of at least 25 (and more preferably at least 40) cm' at 100 KeV. Materials
with relatively
high atomic numbers and densities tend to have the necessary attenuation
coefficients.
More particularly, it has been found that materials with an atomic number
(elements)
or "effective" atomic number (based on a weighted average of elements in
alloys or
compounds) of at least fifty, and densities of at least 0.5 pounds per cubic
inch, exhibit
the required ability to absorb X-rays. Finally, core 24 is preferably a
ductile material so
that it readily conforms to the shape of the case.
By contrast, case 26 is formed of a highly resilient material, preferably with
a
yield strength (0.2°6 offset) of at least 150,000 psi. More preferably,
the yield strength
is at least 300,000 psi. Consequently, the mechanical behavior of composite
filament
18a in terms of elastic deformation in response to external stresses is,
essentially, the
behavior of case 26.
In addition to individual characteristics of the core and case, it is
desirable to
selectively match core and case materials based on certain common
characteristics.
The core and case materials should have the same or substantially the same
linear
coe~cients of thermal expansion. Similarity of core and case materials in
their
crystalline structure is also an advantage. Finally, the core and case
materials should
have an overlap in their annealing temperature ranges, to facilitate
manufacture of the
filaments according to the process to be explained.
In a highly preferred embodiment, core 24 is formed of tantalum, and case 26
is formed of a cobalt-based alloy, more particularly Elgiloy (brand) alloy.
Tantalum is
a ductile metal having an atomic number of 73 and a density of about 0.6
pounds per
cubic inch. Its linear attenuation coefficient, at 100 KeV, is 69.7 cm -'.
The Elgiloy alloy includes principally cobalt and chromium, and has an
effective
atomic number of less than thirty and a density substantially less than 0.5
pounds per
cubic inch. However, the alloy is body compatible, hemocompatible and highly
- WO 95130384 PCTlIB95100253
- -9-
resilient, with a yield strength (0.296 offset) of at least 350,000 psi, after
cold working
and age hardening.
Case 26 and core 24 thus cooperate to provide a prosthesis that can be viewed
in vivo, and in real time. Of course, the amount of core material in relation
to the
amount of case material must be sufficient to insure radio-opacity while
maintaining the
favorable mechanical characteristics of stent 16. It has been found that the
area of core
24, taken along a transverse or lateral plane as illustrated in Figure 4,
should be within
the range of about ten percent to forty-six percent of the filament lateral
cross-sectional
area, i.e. the area of the combined case and core.
Tantalum and the Elgiloy alloy are well matched, in that the materials have
similar linear coefficients of thermal expansion (3.6 x 10-6 per degree F. and
8.4 x 10-6
per degree F., respectively), similar crystalline structures, and annealing
temperatures
in the range of 1900 - 2300° F. Further, there is virtually no tendency
for the formation
of intermetaliic compounds along the tantalum/Elgiloy alloy interface.
Platinum and platinum alloys (e.g. platinum iridium) also are suitable as
materials for core 24. The atomic number of platinum is 78, and its density is
0.775
pounds per cubic inch. Its linear attenuation coefficient at 100 MeV is 105 cm
-1. The
linear coefficient of thermal expansion for platinum is about 4.9 x 10-6 per
degree F.
Thus, as compared to tantalum, platinum is structurally more compatible with
the Elgiloy alloy, and more effectively absorbs X-rays. Accordingly, platinum
is
particularly well suited for use in prostheses formed of small diameter
filaments. The
primary disadvantage of platinum, with respect to tantalum, is its higher
cost.
Further materials suitable for radiopaque core 24 include gold, tungsten,
iridium,
rhenium, ruthenium, and depleted uranium.
Other materials suitable for case 26 include other cobalt-based alloys, e.g,
the
Phynox and MP35N brand alloys. Cobalt-chromium and cobalt-chromium-molybdenum
orthopedic type alloys also can be employed, as well as alloys of titanium-
aluminum-
vanadium. The MP35N alloy is widely available, and has a potential for better
fatigue
strength due to improved manufacturing techniques, particularly as to the
vacuum
melting process. The titanium-aluminum-vanadium alloys are
highlybiocompatible, and
have more moderate stress/strain responses, i.e. lower elastic moduli.
Composite filaments such as filament 18a are manufactured by a drawn filled
tubing (DFT) process illustrated schematically in Figures 7-9. The DFT process
can be
WO 95130384 218 B 4 ~ ~ pCT~95100253
-10-
performed, for example, by Fort Wayne Metals Research Products corporation of
Ft.
Wayne, Indiana. The process begins with insertion of a solid cylinder or wire
28 of the
core material into a central opening 30 of a tube 32 of the case material.
Core wire 28
and tubing 32 are substantially uniform in transverse or lateral sections,
i.e. sections
taken perpendicular to the longitudinal or axis! dimension. For example, tube
32 can
have an outer diameter d. of about 0.102 inch (2.6 mm) and an inner diameter
d2
(diameter of opening 30) of about .056 inches (1.42 mm). Core or wire 28 has
an outer
diameter d3 slightly less than the tube inner diameter, e.g. .046 inches (1.17
mm). In
general, the wire outer diameter is sufficiently close to the tubing inner
diameter to
insure that core or wire 28, upon being inserted into opening 30, is
substantially radially
centered within the tubing. At the same time, the interior tubing diameter
must exceed
the core outside diameter sufficiently to facilitate insertion of the wire
into an extended
length of wire and tubing, e.g. at least twenty feet.
The values of the tubing inner diameter and the core outer diameter vary with
the materials involved. For example, platinum as compared to tantalum has a
smoother
exterior finish when formed into the elongate wire or core. As a result, the
outer
diameter of a platinum wire can more closely approximate the inner diameter of
the
tube. Thus it is to be appreciated that the optimum diameter values vary with
the
materials involved, and the expected length of the composite filament.
In any event, insertion of the core into the tube forms a composite filament
34,
which then is directed through a series of alternating cold-working and
annealing steps,
as indicated schematically in Figure 6. More particularly, composite filament
34 is
drawn through three dies, indicated at 36, 38, and 40, respectively. In each
of the dies,
composite filament 34 is cold-worked in radial compression, causing the case
tube 32
and the tantalum core wire 28 to cold flow in a mannerthat elongates the
filament while
reducing its diameter. Inftially, case tube 32 is elongated and radially
reduced to a
greater extent than core wire 28, due to the minute radial gap that allowed
the insertion
of the core into the tube. However, the radial gap is closed rapidly as the
filament is
drawn through die 36, with subsequent pressure within die 36 and the remaining
dies
cold-working both the core and case together as if they were a single, solid
filament.
In fact, upon closure of the radial gap, the cold-working within all dies
forms a pressure
weld along the entire interface of the core and case, to form a bond between
the core
and case material.
-WO 95/30384 9 PCTIIB95100253
-11-
Y
As composite filament 34 is drawn through each die, the cold-working induces
strain hardening and other stresses wftnin the filament. Accordingly,
respective heating
stages are provided, i.e. furnaces 42, 44 and 46, one heating stage to follow
each cold-
working die. At each annealing stage, composite filament 34 is heated to a
temperature in the range of from about 1900 to about 2300° F., or more
preferably 2000
- 2150° F. At each annealing stage, substantially all of the induced
stresses are
removed from the case and core, to p~:rmit further cold-working. Each
annealing step
is accomplished in a brief time, e.g. in as few as one to fifteen seconds at
annealing
temperature, depending on the size of composite filament 34.
While Figure 6 illustrates one cold-working stage and annealing stage, it is
to
be understood that the appropriate number of stages is selected in accordance
with
the final filament size, the desired degree of cross-sectional area reduction
during the
final cold-working stage, and the initial filament size prior to cold-working.
In
connection with composite filament 34, a reduction of lateral cross-sectional
area in the
range of about forty percent to eighty percent is preferred, and a range of
about fifty-
five percent to sixty-five percent is highly preferred.
The successive cold-working and annealing steps give rise to the need for
matching the core and case materials, particularly as to their coefficients of
thermal
expansion, elastic moduli in tension, annealing temperature ranges, total
elongation
capacities, and also as to their crystalline structure. A good match of
elastic moduli,
elongation, and thermal expansion coefficients minimizes the tendency for any
ruptures
or discontinuities along the core/case interface as thr composite filament is
processed.
Crystalline structures should be considered in matching core and case
materials. The
Elgifoy alloy, and other materials used to form case tube 32, commonly
experience a
transformation between the cold-working and aging steps, from a face centered
cubic
crystalline structure to a hexagonal close packed crystalline structure. The
Elgiloy alloy
experiences shrinkage as it undergoes this transformation. Accordingly, the
core
material must either experience a similar reduction, or be sufficiently
ductile to
accommodate reduction of the case.
There is no annealing after the final cold-working stage. At this point,
composite
filament 34 is formed into the shape intended for the device incorporating the
filament.
In Figure 8, several filaments or strands 34a-a are helically wound about a
cylindrical
form 48 and held in place at their opposite ends by sets of bobbins 50a-a and
52a-e.
WO 95130384 218 8 ~ 2 9 PCTIIB95100253
-t 2- .
Strands 34a-a can be individually processed, or individual segments of a
single
annealed and cold-worked composite filament, cut after the final cold-working
stage.
In either event, the filaments cooperate to form one of the two oppositely
directed sets
of spaced apart and parallel filaments that form a device such as stent 16.
While only
one set of filaments is shown, it is to be understood that a corresponding
group of
filaments, helically wound and intertwined about form 48 in the opposite
direction, are
supported by corresponding bobbins at the opposite filament ends.
A useful prosthesis depends, in part, upon correctly supporting the filaments.
The filaments are maintained in tension, and it is important to select the
appropriate
tensile force and apply the tensile force uniformly to all filaments.
Insufficient tensile
force may allow wire cast or lift effects to cause the individual filaments to
depart from
their helical configuration when released from the bobbins, and the braided
structure
of the stent may unravel.
Figure 9 illustrates two filaments 34a and 54a, one from each of the
oppositely
wound filament sets, supported by respective bobbins 50a/52a and 56a/58a in a
furnace 60 for age hardening in a vacuum or protective atmosphere. Age
hardening
is accomplished at temperatures substantially lower than annealing, e.g. in
the range
of about 700 - 1200°F., more preferably 900 - 1000°F. The
filaments overly one
another to form several intersections, one of which is indicated at 62. When
the
filaments are properly tensioned, slight impressions are formed in the
overlying filament
at each intersection. These impressions, or saddles, tend to positionally lock
the
filaments relative to one another at the intersections, maintaining the
prosthesis
configuration without the need for welding or other bonding of filaments at
their
intersections.
While only two oppositely directed filaments are illustrated as a matter of
convenience, it is to be appreciated that the age hardening stage is pertormed
after the
winding and tensioning of all filaments, i.e. both oppositely directed sets.
Accordingly,
during age hardening, the filaments are locked relative to one another at
multiple
intersections. The preferred time for age hardening is about 1-5 hours. This
age
hardening step is critical to forming a satisfactory self-expanding
prosthesis, as it
substantially enhances elasticity, yield strength, and tensile strength.
Typically, the
elastic modulus is increased by at least 1096 and the yield strength (0.296
offset) and
tensile strength are each increased by at least 2096.
_WO 95130384 ~ PCT/IB95100253
- -13-
As an alternative to the process just explained, a substantially larger and
shorter
composite filament 64 (e.g. six inches long with a diameter of approximately
ten cm)
can be subjected to a series of elongation/diameter reduction steps. Figure 10
schematically illustrates two swaging dies 66 and 68, which may be used in the
course
of a hot working billet reduction process. Of course, any appropriate number
of
swaging dies may be employed. Aftematively, the diameter reduction can be
accomplished by extrusion/pulRrusion at each stage. When a sufficient number
of
swaging steps have reduced the composite structure diameter to about 6
millimeters
(.25 inches). The composite structure or fflament can be further processed by
drawing
it through dies and annealing, as illustrated in Figure 6 for the previously
discussed
process. As before, the composite filament is ready for selective shaping and
age
hardening after the final cold-working stage.
As compared to the process depicted in Fgures 5-7, the swaging or pulltrusion
approach involves substantially increased hot and cold-working of the
composite
structure or filament, and the initial assembling of the core into the case or
shell tubing
is easier. Given the much larger inftial composite structure size, the
structure is
subjected to annealing temperatures for a substantially longer time, e.g. half
an hour
to an hour, as opposed to the one to fifteen second anneal times associated
with the
process depicted in Figure 6. Consequently, particular care must be taken to
avoid
combinations of core and case materials with tendencies for intermetailic
formation
along the core/case intertace. Further, the required hot working of the larger
billet may
not afford the same degree of metallurgical grain refinement.
In general, the preferred composite filaments have: (1) sufficient radio-
opacity
to permit in vivo viewing; (2) the preferred mechanical properties; and (3) a
sufficiently
low cost. The interrelationship of these factors requires that all three be
taken into
account in determining filament size, relationship of core 24 to case 26 as to
size, and
materials selected for the core and case.
More particularly, core 24 should be at least about 0.0015 inches in diameter,
if a stent constructed of such filament is to be visible using conventional
radiographic
imaging equipment. At the same time, structural requirements (particularly
elasticity for
a self-expanding stent) require a minimum ratio of casing material with
respect to core
material. Thus, the visibility requirement effectively imposes a minimum
diameter upon
case 26 as well as core 24. Of course, appropriate selection of core and
casing
WO 95/30384 2 ~ g g 4 ~ 9 PCT/1895100253
-14-
materials can reduce the required minimum diameters. However, potential
substitute
materials should be considered in view of their impact on cost - not only the
material
cost ep r se, but also as to the impact of such substitution on fabrication
costs.
Several composite filament structures are particularly preferred in terms of
meeting the above requirements. In the first of these structures, the core
material is
tantalum, and the casing is constructed of the Elgiloy brand cobalt-based
alloy. The
maximum outer diameter of the comocsite filament is about 0.150 mm, or about
0.006
inches. Elgiloy filaments of this diameter or larger may be sufficiently
radiopaque
without a core of tantalum or other mote radiopaque material. However, even at
such
diameters, radio-opacity is improved vu~th a tantalum core, and likewise with
a core of
a tantalum-based alloy, platinum, platinum-based alloy, tungsten, a tungsten-
based
alloy or combination of these constituents.
It has been found that the preferred core size, relative to the composite
fiber
size, varies with the filament diameter. In particular, for larger filament
(diameters of
0.10-0.15 mm or 0.004-0.006 inches), s:.ifficient radio-opacity is realized
when the cross-
sectional area of core 24 is about one-fourth of tha cross-sectional area of
the entire
fiber. For smaller filaments (e.g., 0.07-0.10 mm or 0.00276-0.0039 inches such
as the
type often used in stents for coronary applications), the core should
contribute at least
about one-third of the cross-sectional area of the composite filament.
increasing the
core percentage above about 3396 of the filament cross-sectional area
undesirably
affects wire mechanical properties and stent elasticity, reducing the ability
of a stent
constructed of the filament to fully self-axpand after is release from a
delivery device.
Composite filaments of this structure have core diameters in the range of
0.037-0.05
mm (0.0015-0.002 inches), with filament diameters up to about 0.135 mm or
about
0.0055 inches.
In a second filament structure, the core is formed of a platinum-1096 nickel
alloy,
i.e. 90~ platinum and 1096 nickel by weight. While the preferred proportion of
nickel
is 10°k, satisfactory results can be obtained with nickel ranging from
about 596 to about
1596 of the alloy. The case is constructed of the Elgiloy alloy. The platinum-
nickel
alloy, as compared to pure tantalum, has superior radiographic and structural
properties. More particularly, the alloy has a greater density, combined with
a higher
atomic number factor (z) for a 10-2096 improvement in radio-opacity. Further
as
compared to tantalum, the alloy is more resistant to fatigue and thus better
withstands
CA 02188429 1999-11-24
processes for fabricating stents and other devices. Because of
its superior mechanical properties, a core formed of a platinum-
nickel alloy can constitute up to about 40% of the total
filament cross-sectional area. Consequently the alloy is
5 particularly well suited for constructing extremely fine
filements. This structure of composite filament is suitable for
constructing stenits having diameters (unstressed) in the range
of about 3.5-6 mm.
As to all composite filament structures, purity of the
l0 elements and alloys is important. Accordingly, high purity
production techniques, e.g. custom melting (triple melting
techniques and electron beam refining) are recommended to
provide high purity Elgiloy alloy seamless tubing.
A third filament structure involves an Elgiloy case and a
15 core formed of a itantalum-10% tungsten alloy, although the
percentage of tunc3sten can range from about 5% to about 20%.
The tantalum/tungsten alloy is superior to tantalum in terms of
mechanical strength and visibility, and costs less than the
platinum-nickel a:Lloy.
According to a fourth filament structure, case 26 is formed
of the Elgiloy alloy, and core 24 is formed of a platinum-20 to
30% iridium alloy. The platinum-iridium alloy can include from
about 5 to about 50% iridium. As compared to the platinum-
nickel alloy, the platinum-iridium alloy may exhibit less
resistance to fatigue. This is due in part to segregation which
may occur during cooling of an alloy containing 30% (by weight)
or more iridium, due to the relatively high melting point of
iridium. Also, hot working may be required if the alloy
contains more than 25% iridium, thereby making the final cold
reduction of the composite difficult.
A fifth filament structure employs an Elgiloy alloy case
and a core of a platinum-tungsten alloy having tungsten in the
range of about 5-:15%, and more preferably 8%. The radio-opacity
CA 02188429 1999-11-24
15a
of this alloy is superior to the platinum-nickel alloy and it
retains the favorable mechanical characteristics.
In a sixth filament structure, casing 26 is constructed of
titanium-based a17_oy. More particularly, the alloy can be an
alloy know as "grade 10" or "Beta 3" alloy, containing titanium
along with molybdenum at 11.5% zirconium at 6%, and tin at 4.5%.
Alternatively, the' titanium-based alloy can include about from
to 15 (typicall.y about 13%) niobium, and about from 10 to 15%
(typically 13%) zirconium. Core 24 can be formed of tantalum.
10 More preferably, t:he core is formed of the platinum-10% nickel
alloy. In this event, a barrier of tantalum should be formed
between the core and case, as is discussed in connection with
Figures 12 and 13.
WO 95/30384 21 ~ ~ ~ ~ 9 PCT/IB95/00253
_1 &
The titanium-based alloy case is advantageous, particularly to patients
exhibiting
sensitivity to the nickel in the Elgiloy alloy, and may iurther be beneficial
since it
contains neither cobalt nor chrome. Also, because of the lower modulus of
elasticity
of the titanium-based alloy (as compared to Elgiloy), stents or other devices
using the
titanium-based alloy exhibit a more moderate elastic response upon release
from a
deployment catheter or other device. This may fend to reduce vascular
neointimal
hyperplasia and consequent restenosis.
Conversely, the lower elastic modulus results in a less favorable matching of
the
case and core as to elasticity. In filaments utilizing the titanium-based
alloy case, the
proportion of core material to case material must be reduced. As a result,
this
construction is suitable for filaments having diameters in the range of about
0.10-0.30
mm.
Finally, according to a seventh filament structure, core 24 is constructed of
a
tungsten-based alloy including fienium at 5-40 weight percent. More
preferably, the
alloy includes rhenium at about 25 percent by weight.
Further preferred materials for core 24 include alloys of about 85-95 weight
percent platinum and about 5-15 weight percent nickel; alloys including about
50-95
weight percent platinum and about 5-50 weight percent iridium; alloys
including at least
80 weight percent tantalum and at most 20 weight percent tungsten; and alloys
including at least 60 weight percent tungsten and at most 40 weight percent
rhenium.
Further suitable case materials are alloys including about 30-55 weight
percent cobalt,
15-25 weight percent chromium, up to 40 weight percent nickel, 5-15 weight
percent
molybdenum, up to 5 weight percent manganese, and up to 25 weight percent
iron.
Preferably the material should have a yield strength of at least 150,000 psi
(0.296 offset).
~ While less preferred, the case material can have a yield strength of at
least 100,000 psi
(0.296 offset).
Figure 1 i is an end elevation of a composite filament 74 including a central
core
76 of a structural material such as the Elgiloy alloy, surrounded by a
radiopaque case
78, thus reversing the respective functions of the core and case as compared
to
composite filament 34. Composite filament 74, as compared to filament 34,
presents
a larger and less refractive radiopaque profile for a given composite filament
diameter.
Composite filament 74, however, is more difficult to manufacture than
filaments that
employ the structural material as the case.
~O 95130384 218 8 4 ~ 9 PCT~95100253
-17-
Figures 12 and 13 show a further alternative composite filament 80, consisting
of a central radiopaque core 82, an outer annular structural case 84, and an
intermediate annular layer 86 between the core and the case. Intermediate
layer 86
provides a barrier between the core and case, and is particularly useful in
composite
filaments employing core and case materials that would be incompatible if
contiguous,
e.g. due to a tendency to form intermetallics. Materials suitable for barrier
layer 86
include tantalum, niobium and platinum. As suggested by Figure 12, the core,
barrier
layer and case can be provided as a cylinder and two tubes, inserted into one
another
for manufacture of the composite filament as explained above.
Figure 14 illustrates another alternative embodiment composite filament 88
having a central radiopaque core 90, a structural case 92, and a relatively
thin annular
outer cover layer 94. Composite filament 88 is particularly useful when the
selected
mechanical structure lacks satisfactory biocompatibility, hemocompatibility,
or both.
Suitable materials for cover layer 94 include tantalum, platinum, iridium,
niobium,
titanium and stainless steel. The composite filament can be manufactured as
explained
above, beginning with insertion of the radiopaque core into the structural
case, and in
turn, inserting the case into a tube formed of the cover material.
Alternatively, cover
layer 94 can be applied by a vacuum deposition process, as a thin layer (e.g.
from ten
to a few hundred microns) is all that is required.
The following examples illustrate formation of composite filaments according
to
the above-disclosed processes.
sample 1 -
An elongate tantalum core having a diameter of 0.46 inches (1.17 mm) was
assembled into an Elgiloy alloy case having an outer diameter of 0.102 inches
(2.6 mm)
and an inner diameter of .056 inches (1.42 mm). Accordingly, the lateral cross-
sectional
area of the tantalum core was about 2596 of the composite filament lateral
cross-
sectional area. Composite filaments so constructed were subjected to 5-6
alternating
stages of cold-working and annealing, to reduce the outer diameters of the
composite
filaments to values within the range of .004-.0067 inches. The tantalum core
diameters
were reduced to values in the range of .002-.0034 inches. The composite
filaments
were formed into a stent suitable for biliary applications, and age hardened
for up to
five hours, at temperatures in the range of 900-1000°F.
W0 95130384 PCTIiB95/00253
_1 g-
Example 2
Elongate cores of a platinum iridium alloy (2096 by weight iridium), with
initial
core outer diameters of .088 inches, were inserted into annular Elgiloy cases
with outer
diameters of .098 inches and inside diameters of .044 inches. The resulting
composite
filaments were processed through about six cold-working and annealing cycles
as in
the first example, to reduce the outer filament diameter to values within the
range of
.00276 inches-.0039 inches, and reducing the core outer diameter to values in
the
range of .0018-.0026 inches. The core thus constituted 4396 of the filament
lateral
cross-sectional area. The resulting filaments were formed into a small
vascular stent,
and age hardened for approximately three hours.
Example 3
Composite filaments were constructed and processed substantially as in
example 2, except that the core was formed of a platinum nickel alloy, with
nickel 1096
by weight.
Example 4
The composite filaments were constructed and processed as in examples 2 and
3, except that the core was formed of tantalum, and the case was formed of
MP35N
alloy, and the cold-working stages reduced the filament outer diameter to
values in the
range of .00276-.0047 inches.
In the case of all examples above, the resulting stents exhibited satisfactory
elasticity and were readily fluoroscopically imaged in real time.
In other embodiments, the device has an additional layer covering the case.
Possible materials for the additional layer include tantalum, gold, titanium,
and platinum.
The additional layer preferably has a thickness in the range of about 0.005-
5.0 microns,
~ and can be applied by methods such as thin clad overlay co-drawing,
electrochemical
deposition of the metal after fabrication of the composite filament, ion
implantation
(such as physical vapor deposition and ion beam deposition), and sputter
coating.
Preferably the additional layer is a metal having an electronegative surface
such as
tantalum.
Each of the above described composite filaments combines the desired
structural stability and resiliency, with radio-opacity that allows in vivo
imaging of the
device composed of the filaments, during deployment and after device fixation.
This
result is achieved by a drawn filled tubing process that cold works a central
core and
_WO 95130384 ~ ~ PCTlIB95100253
-19-
its surrounding case, to positively bond the core and case together such that
the
composite filament behaves as a continuous, solid structure. Pertormance of
the
filament and resulting device is further enhanced by a selective matching of
the core
and case materials, as to linear thermal expansion coefficient, annealing
temperature,
moduli of elasticity, and crystalline structure.
What is claimed is: