Note: Descriptions are shown in the official language in which they were submitted.
21 88784
I:lLE, ~H~S ~ N~E~
T~Al~JS~ATI~
64133.587
O-Acyl-4-phenyl-CYclalkanolsr their salts,
medicaments containing such compounds, and their
use, as well as a method of preparing them
The present invention relates to O-acyl-4-phenyl-
cycloalkanOlS, the salts thereof with physiologically
acceptable organic and inorganic acids, processes for
preparing these compounds and pharmaceutical compositions
containing them and the use thereof.
The compounds according to the invention are inhibitors of
cholesterol biosynthesis, more particularly inhibitors of the
enzyme 2,3-epoxysqualene-lanosterol-cyclase, a key enzyme in
cholesterol biosynthesis. The compounds according to the
invention are suitable for the treatment and prophylaxis of
hyperlipidaemia, hypercholesterolaemia and atherosclerosis.
Other possible fields of application consist of the treatment
of hyperproliferative skin and vascular disorders, tumours,
gallstone problems and mycoses.
Compounds which intervene in cholesterol biosynthesis are of
importance in the treatment of a number of syndromes.
~ Particular examples are hypercholesterolaemias and
-hyperlipidaemias which are risk factors for the development of
atherosclerotic vascular changes and their sequelae such as,
for example, coronary heart disease, cerebral ischaemia,
claudicatio intermittens and gangrene.
The significance of elevated serum cholesterol levels as a
main risk factor in the occurrence of atherosclerotic vascular
changes is generally recosnised. Extensive clinical trials
have led to the finding that the risk of coronary heart
disease can be reduced by lowering serum cholesterol (Current
Opinion in Lipidology 2(4), 234 [1991~). Since the majority
of cholesterol is synthesised in the body and only a small
proportion is taken in with food, the inhibition of
21 88784
biosynthesis constitutes a particularly attractive method of
lowering elevated cholesterol levels.
Other possible fields of application for cholesterol
biosynthesis inhibitors consist of the treatment of
hyperproliferative skin and vascular disorders as well as
tumoral diseases, the treatment and prophylaxis of gallstone
problems and use in mycoses. This latter case involves
intervention in the ergosterol biosynthesis in fungal
organisms, which proceeds to a considerable extent in the same
way as cholesterol biosynthesis in mammalian cells.
Cholesterol or ergosterol biosynthesis proceeds, starting from
acetic acid, via a large number of reaction steps. This
multi-step process presents a series of possible
interventions, of which the following may be mentioned by way
of example:
For inhibiting the enzyme 3-hydroxy-3-methylglutaryl-coenzyme
A (HMG-CoA) synthase, ~-lactones and ~-lactams with a
potential antihypercholesterolaemic activity may be mentioned
(see J. Antibiotics 40, 1356 [1987], US-A-4,751,237,
EP-A-0 462 667, US-A-4,983,597~.
Inhibitors of the enzyme HMG-CoA-reductase are 3,5-
dihydroxycarboxylic acids of the statin type and the ~-
lactones, of which lovastatin, simvastatin and pravastatin are
used in the treatment of hypercholesterolaemia.
Other possible uses for these compounds are fungal infections
(US-A-4,375,475, EP-A-0 113 881, US-A-5,106,992~, skin
diseases (EP-A-0 369 263) and gallstone problems and tumoral
diseases (US-A-5,106,992; Lancet 339, 1154-1156 [1992]).
Another possible therapy is the inhibition of the
proliferation of smooth muscle cells using lovastatin
` - 21 88784
-- 3
(Cardiovasc. Drugs. Ther. 5, Suppl. 3, 354 [1991]).
Inhibitors of the enzyme squalene-synthetase include, for
example, isoprenoid-(phosphinylmethyl)phosphonates which have
been described as suitable for the treatment of
hypercholesterolaemia, gallstone problems and tumoral diseases
in EP-A-0 409 181 and in J. Med. Chemistry 34, 1912 [1991], as
well as the squalestatins with a cholesterol-lowering and
antimycotic effect (J. Antibiotics 45, 639-647 [1992] and J.
Biol. Chemistry 267, 11705-11708 [1992].
Known inhibitors of the enzyme s~ualene-epoxidase are
allylamines such as naftifin and terbinafin, which have been
used in therapy as dru~s to combat fungal diseases, as well as
the allylamine NB-598 which has an antihypercholesterolaemic
effect (J. Biol. Chemistry 265, 18075-18078, [1990]) and
fluorosqualene derivatives having a hypocholesterolaemic
effect (US-A-5,011,859). In addition, piperidines and
azadecalins having a potential hypocholesterolaemic and/or
antifungal activity have been described, the mechanism of
activity of which has not been adequately explained and which
constitute squalene epoxidase and/or 2,3-epoxysqualene-
lanosterol-cyclase inhibitors (EP-A-0 420 116, EP-A-0 468 434,
US-A-5,084,461 and EP-A-0 468 457).
Examples of inhibitors of the enzyme 2,3-epoxysqualene-
lanosterol-cyclase include diphenyl derivatives
(EP-A-0 464 465), aminoalkoxybenzyl derivatives
(EP-A-0 410 359) and piperidine derivatives (J. Org. Chem. 57,
2794-2803, [1992]) which have an antifungal activity.
Moreover, this enzyme is inhibited in mammalian cells by
decalins, azadecalins and indane derivatives (WO 89/08450, J.
Biol. Chemistry 254, 11258-11263 [1981], Biochem. Pharmacology
37, 1955-1964 [1988] and J 64 003 144) and also by 2-aza-2,3-
dihydrosqualene and 2,3-epiminosqualene (Biochem. Pharmacology
` - 21 887~4
34, 2765-2777 [1985]), by squalene-oxide-epoxide-enol ethers
(J. Chem. Soc. Perkin Trans. I, 1988, 461) and 29-methylidene-
2,3-oxidosqualene (J. Amer. Chem. Soc. 113, 9673-9674 [1991]).
Finally, steroid derivatives having a potential
antihyperlipaemic activity may also be mentioned as inhibitors
of the enzyme lanosterol-14~-demethylase and at the same time
they have an effect on the enzyme HMG-CoA-reductase
(US-A-5,041,432, J. Biol. Chemistry 266, 20070-20078 [1991],
US-A-5,034,548). Furthermore, this enzyme is inhibited by
antimycotics of the azole type which constitute N-substituted
imidazoles and triazoles. This category includes, for
example, the antimycotics ketoconazole and fluconazole which
are on the market.
The compounds of general formula I which follows are new. It
has been found, surprisingly, that they are highly effective
inhibitors of the enzyme 2,3-epoxysqualene-lanosterol-cyclase
(International Classification: EC5.4.99.7).
The enzyme 2,3-epoxysqualene-lanosterol-cyclase catalyses a
key stage of cholesterol or ergosterol biosynthesis, namely
the conversion of 2,3-epoxysqualene into lanosterol, the first
compound with a steroid structure in the biosynthesis cascade.
Inhibitors of this enzyme lead one to expect the advantage of
higher selectivity over inhibitors of earlier biosynthesis
stages, such as, for example, HMG-CoA-synthesis and HMG-CoA-
reduction, since the inhibition of these early biosynthesis
stages leads to a reduction in biosynthetically formed
mevalonic acid and, as a result, may have a negative effect on
the biosynthesis of the mevalonic acid-dependent substances
dolichol, ubiquinone and isopentenyl-t-RNA (cf. J. Biol.
Chemistry ~, 18075-18078 [1990]).
In the inhibition of biosynthesis stages after the conversion
2 1 88784
of 2,3-epoxysqualene into lanosterol there is the risk of the
accumulation of intermediate products having a steroidal
structure in the body and the triggering of toxic effects
connected therewith. This has been described, for example,
for triparanol, a desmosterol reductase inhibitor. This
substance has had to be taken off the market owing to the
formation of cataracts, ichthyosis and alopecia (mentioned in
J. Biol. Chemistry 265, 18075-18078 [1990]).
As has already been explained, inhibitors of 2,3-
epoxysqualene-lanosterol-cyclase have been individually
described in the literature. However, the structures of these
compounds are completely different from the structure of the
compounds according to the invention which conform to general
formula I hereinafter.
The invention relates to the preparation of
antihypercholesterolaemic substances suitable for the
treatment and prophylaxis of atherosclerosis and, compared
with known active substances, characterised by an improved
antihypercholesterolaemic activity with greater selectivity
and hence increased safety. Since the compounds according to
the invention are also able to inhibit ergosterol biosynthe~is
in fungal organisms, thanks to their considerable
effectiveness as inhibitors of the enzyme 2,3-epoxysqualene-
lanosterol-cyclase, they are also suitable for the treatment
of mycoses.
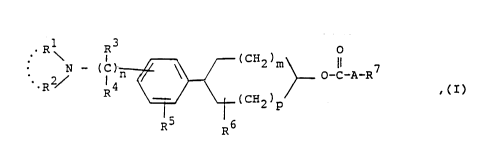
The present invention relates to new O-acyl-4-phenyl-
cycloalkanols of general formula I
` ~18~784
Rl 3 ~ (C~2~p
wherein
n denotes the number O or 1,
m denotes the number 1 or 2,
p denotes the number O or 1,
R1 and R2, which may be identical or different, denote a
hydrogen atom, a straight-chained or branched C16-alkyl group,
a straight-chained or branched C3 -6 - alkenyl or alkynyl group,
the double and triple bonds thereof being isolated from the
nitrogen-carbon bond, whilst the above-mentioned alkyl,
alkenyl and alkynyl groups may also be substituted by an
amino, hydroxy, alkoxy, alkylcarbonyloxy, alkylcarbonylamino,
carboxyl, alkoxycarbonyl, aminocarbonyl or cyano group, and
the above-mentioned amino, hydroxy, alkoxy, alkylcarbonyloxy
and alkylcarbonylamino group may not be bound to an
unsaturated carbon atom and may not be bound to the carbon
atom in position 1, or
Rl and R2 together with the nitrogen atom between them denote a
5- to 7-membered saturated monocyclic heterocyclic ring,
whilst in a 6-membered saturated monocyclic heterocyclic ring
thus formed a methylene group in the 4-position may be
replaced by an oxygen or sulphur atom or by an optionally
alkyl-substituted imino group,
R3 and R4, which may be identical or different, denote a
hydrogen atom or a straight-chained or branched C14-alkyl
21 8~784
group,
R5 denotes a hydrogen atom, a straight-chained or branched
Cl4-alkyl group or a C14-alkoxy group,
R5 denotes a hydrogen atom or a straight-chained or branched
Cl4-alkyl group,
R7 denotes a hydrogen atom, a C37-cycloalkyl group, a phenyl
group optionally mono- or disubstituted by a fluorine,
chlorine or bromine atom or by a hydroxy, alkyl, alkoxy,
phenylalkoxy, phenyl, nitro, amino, alkylamino, dialkylamino,
alkylcarbonylamino, cyano, carboxy, alkoxycarbonyl,
aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl,
trifluoromethyl, alkylcarbonyloxy, aminosulphonyl,
alkylaminosulphonyl or dialkylaminosulphonyl group, wherein
the substituents may be identical or different and two
adjacent hydrogen atoms in a phenyl group may be replaced by a
methylenedioxy or 1,2-ethylenedioxy group, a phenyl group
substituted by two chlorine or bromine atoms and an amino
group, a naphthyl or tetrahydronaphthyl group, a thienyl,
furyl or pyridyl group substituted by a halogen atom or by one
or two alkyl groups, and
A denotes a bond, a straight-chained or branched Cll7-alkylene
group or a C2l7-alkenylene or alkynylene group,
whilst all the above-mentioned alkyl and alkoxy moieties,
unless otherwise specified, may contain 1 to 3 carbon atoms,
and any halogen atom mentioned hereinbefore may be a fluorine,
chlorine or bromine atom, the enantiomers, diastereomers and
geometric isomers thereof and the salts thereof, more
especially for pharmaceutical use the physiologically
acceptable salts with organic or inorganic acids.
`- 21 88784
The preferred compounds are those of general formula Ia
,. Rl~ R3 r(CH2)m\ 11 7
~,R2 / l4 ~ ~ O-C-A-R (Ia)
wherein
n, m and p each denote the number 1,
R1 and R2, which may be identical or different, denote a
hydrogen atom, a straight-chained or branched C16-alkyl group,
a straight-chained or branched C36-alkenyl or alkynyl group,
the double and triple bonds thereof being isolated from the
nitrogen-carbon bond, whilst the above-mentioned alkyl,
alkenyl and alkynyl groups may be substituted by an amino,
hydroxy, alkoxy, alkylcarbonyloxy, alkylcarbonylamino,
carboxyl, alkoxycarbonyl, aminocarbonyl or cyano group, and
the above-mentioned amino, hydroxy, alkoxy, alkylcarbonyloxy
and alkylcarbonylamino group may not be bound to an
unsaturated carbon atom and may not be bound to the carbon
atom in position 1, or
R1 and R2 together with the nitrogen atom between them denote a
5- to 7-membered saturated monocyclic heterocyclic ring,
whilst in a 6-membered saturated monocyclic heterocyclic ring
thus formed a methylene group in the 4-position may be
replaced by an oxygen or sulphur atom or by an optionally
alkyl-substituted imino group,
R3 to R6, which may be identical or different, each denote a
hydrogen atom or a methyl group,
R7 denotes a hydrogen atom, a C37-cycloalkyl group, a phenyl
`- 21 887~4
group optionally mono- or disubstituted by a fluorine,
chlorine or bromine atom or by a hydroxy, alkyl, alkoxy,
phenylalkoxy, phenyl, nitro, amino, alkylamino, dialkylamino,
alkylcarbonylamino, cyano, carboxy, alkoxycarbonyl,
aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl,
trifluoromethyl, alkylcarbonyloxy, aminosulphonyl,
alkylaminosulphonyl or dialkylaminosulphonyl group, whilst the
substituents may be identical or different and two adjacent
- ~ hydrogen atoms in a phenyl group may be replaced by a
methylenedioxy or 1,2-ethylenedioxy group, a phenyl group
substituted by two chlorine or bromine atoms and an amino
group, a naphthyl or tetrahydronaphthyl group, a thienyl,
furyl or pyridyl group substituted by a chlorine or bromine
atom or by one or two alkyl groups, and
A denotes a bond, a straight-chained or branched C11O-alkylene
group or a C2l0-alkenylene or alkynylene group,
wherein all the above-mentioned alkyl and alkoxy moieties,
unless otherwise specified, may contain 1 to 3 carbon atoms,
the enantiomers, diastereomers and geometric isomers thereof
and the salts thereof, particularly for pharmaceutical use the
physiologically acceptable salts thereof with inorganic or
organic acids.
Particularly preferred compounds are those of general formula
Ia
wherein
n, m and p each denote the number 1,
R1 denotes a hydrogen atom, a straight-chained or branched
Cl4-alkyl group which may be substituted by an aminocarbonyl
group or in the 2-, 3- or 4-position by a hydroxy or alkoxy
group, or a 2-propenylene group, and
21 887~4
- 10 -
R2 denotes a hydrogen atom, a Cl4-alkyl group or a 2-
propenylene group, or
R1 and R2 together with the nitrogen atom between them denote a
5- or 6-membered saturated monocyclic heterocyclic ring,
whilst in a 6-membered saturated monocyclic heterocyclic ring
thus formed, a methylene group in the 4-position may be
replaced by an oxygen atom or by an optionally alkyl-
~ ~I substituted imino group,
R3 to R6 each denote a hydrogen atom,
R7 denotes a hydrogen atom, a C36-cycloalkyl group, a phenyl
group optionally monosubstituted in the 4-position by a
fluorine, chlorine or bromine atom or by an alkyl, alkoxy,
phenyl, nitro or trifluoromethyl group, a phenyl group
disubstituted by two chlorine atoms, one chlorine atom and an
alkyl or amino group or two alkoxy groups, a phenyl group
trisubstituted by two chlorine atoms and an amino group, a
3,4-methylenedioxyphenyl group, a naphthyl or
tetrahydronaphthyl group, a 2-furyl group or a 2-thienyl group
optionally substituted by a chlorine atom in the 5-position or
a 3-pyridyl group,
A denotes a bond, a straight-chained or branched C16-alkylene
group or a C25-alkenylene group,
whilst all the above-mentioned alkyl and alkoxy moieties,
unless otherwise specified, may contain 1 to 3 carbon atoms,
the enantiomers, diastereomers and geometric isomers thereof
and the salts thereof, more particularly for pharmaceutical
use the physiologically acceptable salts thereof with
inorganic or organic acids.
Most particularly preferred compounds are those of general
21 ~784
-- 11
formula Ia
whereln
n, m and p each denote the number 1,
R1 denotes a hydrogen atom, a straight-chained or branched
Cl4-alkyl group which may be substituted by an aminocarbonyl
group or, in the 2-, 3- or 4-position, by a hydroxy or alkoxy
group, or a 2-propenylene group,
R2 denotes a hydrogen atom or a C14-alkyl group,
R3 to R6 each denote a hydrogen atom,
R7 denotes a phenyl group optionally substituted in the 4-
position by a fluorine, chlorine or bromine atom or by a
methyl, trifluoromethyl, methoxy, phenyl or nitro group, a
3,4-dichlorophenyl, 2,4-dichlorophenyl, 4-chloro-3-
methylphenyl, 4-amino-3-chlorophenyl, 3,4-dimethoxyphenyl,
3,4-methylenedioxyphenyl, 4-amino-3,5-dichlorophenyl or 2-
naphthyl group, and
A denotes a bond, a straight-chained or branched C1s-alkylene
group or a C23-alkenylene group,
wherein all the above-mentioned alkyl and alkoxy moieties,
unless otherwise specified, may contain 1 to 3 carbon atoms,
the enantiomers, diastereomers and geometric isomers thereof
and the salts thereof, particularly for pharmaceutical use the
physiologically acceptable salts thereof with inorganic or
organic acids,
and in particular the compounds
(1) cis-0-(4-chlorobenozyl)-4-(4-dimethylaminomethylphenyl)-
- 21 88784
cyclohexanol
(2) cis-0-(4-phenyl-3-butenoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
(3) trans-0-(4-chlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
(4) cis-0-(5-methylhexanoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
(5) trans-0-(2-phenylpropionyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
(6) trans-0-(4-fluorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
(7) trans-0-(3,4-dichlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
(8) cis-0-(4-fluorocinnamoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
(9) trans-0-(p-tolylacetyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
(10) trans-0-(4-[trifluoromethyl]-phenylacetyl)-4-(4-
dimethylaminomethylphenyl)-cyclohexanol
(11) trans-0-(2-naphthylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
(12) trans-0-(4-nitrophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
21 88784
(13) trans-0-(4-bromophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
(14) trans-0-(2,4-dichlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
(15) trans-0-([4-amino-3-chlorophenyl]acetyl)-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
(16) trans-0-(4-methoxyphenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol,
(17) trans-0-(4-chlorophenylacetyl)-4-(4-methylaminomethyl-
phenyl)-cyclohexanol,
and the salts thereof.
Methods of preparation:
The compounds of formula I may be prepared, for example, by
the following methods:
a) By reacting a 4-phenylcycloalkanol of general formula II
R3
.-R \
N ~ (C)n ~ ~ ~ 2)m\
-R2 / R4 ~ (S~2)
R5 R6
wherein
n, m, p and R1 to R6 are as hereinbefore defined, with a
carboxylic acid or the reactive derivatives thereof of general
formula III
21 ~87~4
- 14 -
R7 - A - COX (III)
wherein
R7 and A are as hereinbefore defined and X denotes a hydroxy
group or a reactive leaving group, e.g. a halogen atom, such
as a chlorine or bromine atom, a trimethylsilyloxy group, a
sulphonyloxy group, e.g. the p-toluenesulphonyloxy group, an
N-heteroaryl group, e.g. the 1-imidazolyl or 1-benzotriazolyl
~ i group, or an O-isourea group, e.g. the O-(N,N'-dicyclohexyl)-
lsourea group.
The reaction is conveniently carried out in a solvent such as
benzene, toluene, xylene, diisopropylether, dioxan,
tetrahydrofuran, dimethylformamide, dichloromethane or
chloroform and optionally in the presence of a base such as
triethylamine, pyridine or 4-dimethylaminopyridine, or in the
presence of an acid, particularly if X in the general formula
III denotes a hydroxy group, e.g. in the presence of boron
trifluoride etherate or an acid cation exchanger at a
temperature between -10 and 150C, but preferably at a
temperature between -10 and 80C.
If the groups R1 and/or R2 contain free hydroxy, amino or
carboxy groups, it is advisable to protect them in a suitable
manner before the reaction, e.g. by converting the hydroxy
into an ether group, e.g. a 2-methoxyethoxymethyl, tert.-butyl
or benzylether group, the amino into a carbamate group, e.g. a
trichloroethyl, 9-fluorenylmethyl or 2,4-dichlorobenzyl-
carbamate group and the carboxyl into an ester group, e.g. a
2,2,2-trichloroethyl, tert.-butyl or benzylester group, and
cleaving the protective groups by known methods once the
reaction has ended.
b) By reacting an O-acyl-4-phenylcycloalkanol of general
formula IV
21 ~8784
Y ~ (C)n ~ (C~2 m ~ o-C-A-R7 (IV)
R5 R6
whereln
n, m, p, R3 to R7 and A are as hereinbefore defined and
Y denotes a reactive leaving group such as a halogen atom,
e.g. a chlorine or bromine atom, or a sulphonyloxy group, e.g.
a methylsulphonyloxy group, with an amine of general formula V
Rl ~ (V)
: NH
' R2/
wherein
R1 and R2 are as hereinbefore defined.
The reaction is conveniently carried out in a suitable solvent
such as ethanol, tert.-butanol, dimethylformamide or
tetrahydrofuran, optionally in the presence of a base such as
potassium carbonate, sodium ethoxide, potassium tert.butoxide
or sodium hydride, and optionally under phase transfer
conditions, at a temperature between 0 and 100C.
c) In order to prepare compounds of general formula I wherein
Rl is as hereinbefore defined and R2 denotes a straight-chained
or branched Cl6-alkyl group which may be substituted by a
hydroxy, alkoxy, alkylcarbonyloxy, alkylcarbonylamino,
carboxyl, alkoxycarbonyl, aminocarbonyl or cyano group,
wherein the hydroxy, alkoxy, alkylcarbonyloxy or
- 21 ~8784
- 16 -
alkylcarbonylamino group is not bound to the carbon atom in
position 1:
reacting an O-acyl-4-phenylcycloalkanol of general formula VI
Rl\ R3
R ~ ~CH )
R5 R6
wherein
n, m, p, R3 to R7 and A are as hereinbefore defined and R1 has
the meanings given above, with a compound of general formula
VII
R2l z1 (VII)
wherein
R2' denotes a straight-chained or branched C16-alkyl group
which may be substituted by a hydroxy, alkoxy,
alkylcarbonyloxy, alkylcarbonylamino, carboxyl,
alkoxycarbonyl, aminocarbonyl or cyano group, wherein the
hydroxy, alkoxy, alkylcarbonyloxy or alkylcarbonylamino group
is not bound to the carbon atom in position 1, and Z1 denotes
a reactive leaving group such as a halogen atom, e.g. a
chlorine or bromine atom, or a sulphonyloxy group, e.g. a
methylsulphonyloxy group.
The reaction is conveniently carried out in a solvent or
mixture of solvents such as ethanol, tert.-butanol,
tetrahydrofuran, dimethylsulphoxide or dimethylformamide,
optionally in the presence of an acid binding agent, such as
sodium carbonate, potassium carbonate, sodium hydroxide,
sodium hydride, sodium methoxide, potassium tert.-butoxide,
triethylamine or pyridine, wherein the latter two may
`- 21 88784
simultaneously be used as solvent, optionally under phase
transfer conditions, preferably at temperatures between 0 and
100C, e.g. at temperatures between 20 and 50C.
In the reactions described above, any reactive groups present
such as hydroxy, amino, alkylamino, imino or carboxyl groups
may be protected during the reaction by conventional
protecting groups which are cleaved again once the reaction
has ended.
For example, a protective group for a hydroxy group might be a
trimethylsilyl, acetyl, benzoyl, methyl, ethyl, tert.-butyl,
2-methoxyethoxymethyl, benzyl or tetrahydropyranyl group, a
protecting group for an amino, alkylamino or imino group might
be an acetyl, benzoyl, ethoxycarbonyl or benzyl group and a
protecting group for a carboxyl group might be a 2,2,2-
trichloroethyl, tert.-butyl or benzylester group.
The optional subsequent cleaving of any protecting group used
is preferably carried out by hydrolysis in an aqueous solvent,
e.g. in water, isopropanol/water, tetrahydrofuran/water or
dioxan/water, in the presence of an acid such as hydrochloric
or sulphuric acid or in the presence of an alkali metal base
such as sodium hydroxide or potassium hydroxide at
temperatures between 0 and 100C, preferably at the boiling
temperature of the reaction mixture. However, a benzyl group
is preferably cleaved by hydrogenolysis, e.g. with hydrogen in
the presence of a catalyst such as palladium/charcoal in a
solvent such as methanol, ethanol, ethyl acetate or glacial
acetic acid, optionally with the addition of an acid such as
hydrochloric acid at temperatures between 0 and 50C, but
preferably at ambient temperature, under a hydrogen pressure
of 1 to 7 bar, but preferably 3 to S bar.
The compounds of general formula I prepared by the above
- 21 B8784
- 18 -
methods may be purified and isolated using known methods, e.g.
crystallisation or chromatography.
In addition, the compounds of general formula I obtained may
if desired be converted into the acid addition salts thereof,
particularly for pharmaceutical use into the physiologically
acceptable salts with inorganic or organic acids. Suitable
acids include, for example, hydrochloric acid, hydrobromic
acid, sulphuric acid, phosphoric acid, fumaric acid, succinic
acid, lactic acid, citric acid, tartaric acid or maleic acid.
In the compounds of formula I according to the invention,
stereoisomers such as diastereomers, geometric isomers or
optical isomers may occur, depending on the position of the
substituents on the cycloalkane ring or the form of the
substituents Rl to R7. The invention includes both the pure
stereoisomers and the mixtures thereof.
Start; ng compol~nds:
The starting compounds of general formula II may be prepared
by the following method, for example:
1. By reduction of 4-phenylcycloalkanones of general formula
VIII
.,Rl\ l3
; - (C) ~ (C 2)m
R5 R6
wherein
n, m, p and Rl to R6 are as hereinbefore defined.
- ~ 21 88784
-- 19 --
By a suitable choice of reducing agents, e.g. sodium
borohydride or lithium tri-sec.-butyl-borohydride (L-
selectrides) the reaction may be steered so as to produce
mainly the e,e-isomer or the e,a-isomer of a compound of
general formula II.
The ketones of general formula VIII may be prepared by known
methods, e.g. by reacting monoethylene ketals of general
formula IX
~ (C 2)m X ~ (IX)
with an organometallic compound of general formula X
R \ R
~R2 / 14 ~ Me (X)
- R5
wherein
n and R1 to R5 are as hereinbefore defined and Me denotes a
lithium atom or a group -MgHal, wherein Hal is a halogen atom,
preferably a chlorine atom, with subsequent cleavage
using water, hydrogenation of the resulting double bond and
hydrolysis of the ketal grouping.
The process may be modified so that a ketone of general
formula VIII wherein R6 denotes a hydrogen atom is converted,
after the above reaction æequence has been carried out, into a
- 21 ~8784
- 20 -
ketone of general formula VIII wherein R denotes a C14-alkyl
group, e.g. by alkylation of the ketone-enolate ion.
Another method of preparing compounds of general formula VIII
consists of Dieckmann cyclisation of dicarboxylic acid esters
of general formula XI
.R2 R3
R3/ IG~ ~ m COOR (XI)
R5 R6
wherein
n, m, p and R1 to R6 are as hereinbefore defined and
R8 and R9, which may be identical or different, denote an
alkyl, aralkyl or aryl group, and subsequent saponification
and decarboxylation by known methods.
2. The starting compounds of general formula IV may be
prepared, for example, by halomethylation of O-acyl-4-
phenylcycloalkanols of general formula XII
~ ~ - o-C-A-R7 (XII)
- ~ ~t(C~2)P
R5 R6
wherein
m, p, Rs to R7 and A are as hereinbefore defined, with a
corresponding aldehyde and hydrogen halide, e.g. hydrogen
chloride or hydrogen bromide, in the presence of a Friedel
` - 21 88784
Crafts catalyst e.g. zinc chloride, and optional subsequent
replacement of the halogen atom by another suitable reactive
leaving group.
3. The starting compounds of general formula VI may be
prepared, for example, from O-acyl-4-phenyl-cycloalkanols of
general formula XIII
R \ 13
N ~ (C)n ~ ~ ( 2)m\ 11 7
z2 / R4 ~ ~ O-C-A-R (XIII)
R5 R
wherein
n, m, p, R1, R3 to R7 and A are as hereinbefore defined and Z2
denotes a suitable protective group, by cleaving this
protective group. An example of a protecting group may be a
tert.-butoxycarbonyl, 1-(3,5-di-tert.-butylphenyl)-1-
methylethoxycarbonyl or 2-(4-pyridyl)ethoxycarbonyl group.
The compounds of general formula XIII may be synthesised, for
example, using the method described in Process 1.
The starting compounds of formulae III and V are known from
the literature or may be obtained by methods known from the
literature.
The compounds of general formula I have useful biological
properties. They are inhibitors of cholesterol biosynthesis,
particularly inhibitors of the enzyme 2,3-epoxysqualene-
lanosterol-cyclase. In view of their biological properties
they are particularly suitable for the treatment and
prophylaxis of hyperlipidaemias, particularly
hypercholesterolaemia, hyperlipoproteinaemia and
21 88784
hypertriglyceridaemia and the resulting atherosclerotic
vascular changes with their sequelae such as coronary heart
disease, cerebral ischaemia, Claudicatio intermittens,
gangrene and the like.
In order to treat these diseases the compounds of general
formula I may be used either on their own in monotherapy or in
conjunction with other cholesterol- or lipid-lowering
substances, the compounds preferably being given orally but
optionally in rectal form. Drugs which may be used in
conjunction with them include, for example:
- gallic acid binding resins such as cholestramine,
cholestipol and the like,
- compounds which inhibit cholesterol resorption such as
sitosterol and neomycin,
- compounds which are involved in cholesterol biosynthesis,
e.g. HMG-CoA-reductase inhibitors such as lovastatin,
simvastatin, pravastatin and the like,
- squalene-epoxidase inhibitors such as NB 598 and analogous
compounds and
- squalene-synthetase inhibitors such as, for example,
compounds of the category of the isoprenoid-
(phosphinylmethyl)phosphonates and squalestatin.
Other possible combinations may include the fibrates such as
clofibrate, bezafibrate, gemfibrozil and the like, nicotinic
acid, the derivatives and analogues thereof such as acipimox
and also probucol.
The compounds of general formula I are also suitable for
21 88784
- 23 -
treating diseases connected with excessive cell proliferation.
Cholesterol is an essential cell component and has to be
present in sufficient quantities for cell proliferation, i.e.
cell division. The inhibition of cell proliferation by
inhibiting cholesterol biosynthesis is described with
reference to the example of the smooth muscle cells with the
HMG-CoA-reductase inhibitor of the statin type, lovastatin, as
mentioned hereinbefore.
Examples of diseases connected with excessive cell
proliferation include in particular tumoral diseases. In cell
culture and in vivo experiments it has been shown that a
reduction of the serum cholesterol or intervention in
cholesterol biosynthesis by HMG-CoA-reductase inhibitors
reduces tumour growth (Lancet 339, 1154-1156 [1992]). The
compounds of formula I according to the invention are
therefore potentially suitable for treating tumoral diseases
on the basis of their inhibitory effect on cholesterol
biosynthesis. They may be used on their own or to support
known types of therapy.
Other examples include hyperproliferative skin diseases such
as psoriasis, basal cell carcinoma, plate epithelial
carcinoma, keratosis and keratinisation disorders. The term
"psoriasis" used here refers to a hyperproliferative skin
disease which changes the regulating mechanism of the skin.
In particular, lesions are formed which constitute primary and
secondary changes in proliferation in the epidermis,
inflammatory skin reactions and the expression of regulatory
molecules such as lymphokines and inflammatory factors.
Psoriatic skin is characterised morphologically by an
increased turnover of epidermis cells, thickened epidermis,
abnormal keratinisation of inflammatory skin infiltrates in
the dermis and polymorphonuclear leukocyte infiltration into
the epidermis, leading to an increase in the basal cell cycle.
21 ~8784
- 24 -
In addition, hyperkeratotic and parakeratotic cells are
present. The terms "keratosis", "basal cell carcinoma",
"plate epithelium carcinoma" and "keratinisation disorders'~
refer to hyperproliferative skin diseases in which the
regulating mechanism for the proliferation and differentiation
of the skin cells has been disrupted.
The compounds of formula I are effective as antagonists of
skin hyperproliferation, i.e. as agents which inhibit the
hyperproliferation of human keratinocytes. Consequently, they
are suitable as agents for treating hyperproliferative skin
diseases such as psoriasis, basal cell carcinoma,
keratinisation disorders and keratosis. In order to treat
these diseases the compounds of formula I may be administered
either orally or topically, and may be used either on their
own in form of monotherapy or in conjunction with known active
substances.
Hyperproliferative vascular diseases such as stenosis and
vascular occlusions based on the proliferation of smooth
muscle cells, which are triggered by surgical procedures such
as PT Q (percutaneous transll]m- n~ 1 coronary angioplasty) or
bypass operations may also be mentioned. As stated
hereinbefore, this cell proliferation can be suppressed, as is
well known, by HMG-CoA-reductase inhibitors of the statin type
such as lovastatin. On the basis of their inhibitory effect
on cholesterol biosynthesis, the compounds of general formula
I are suitable for treatment and prophylaxis of these
diseases, and may be used either on their own or in
conjunction with known active substances such as intravenously
adminiRtered heparin, preferably in oral forms.
Another possible use of the compounds of general formula I
according to the invention is in the prevention and treatment
of gallstone problems. The formation of gallstones is
21 88784
triggered by an unfavourable ratio of cholesterol to bile acid
in the bile liquid, as a result of which the solubility of
cholesterol is exceeded and cholesterol is precipitated in the
form of gallstones. The effectiveness of the HMG-CoA-
reductase inhibitor lovastatin in dissolving gallstones,
particularly in conjunction with ursodeoxycholic acid, is
described in Gastroenterology 102, No. 4, Pt.2, A319 [1992].
In view of their mode of activity the compounds of general
formula I are therefore also important in the prevention and
treatment of gallstone problems. They may be used either on
their own or in conjunction with known therapies such as, for
example, treatment with ursodeoxycholic acid or shockwave
lithotripsy, and preferably administered orally.
Finally, the compounds of formula I are suitable for treating
infections caused by pathogenic fungi such as Candida
albicans, Aspergillus niger, Trichophyton mentagrophytes,
Penicillium sp., Cladosporium sp. and others. As already
mentioned above, the end product of sterol biosynthesis in the
fungal organism is not cholesterol but ergosterol which is
essential to the integrity and functioning of the fungal cell
membranes. Inhibiting the biosynthesis of ergosterol
therefore leads to disruption in growth and may possibly kill
off the fungal organisms.
In order to treat mycoses the compounds of general formula I
may be administered either orally or topically. They may be
used on their own or in conjunction with known antimycotic
substances, particularly those which intervene in other stages
of sterol biosynthesis, such as, for example, the squalene
epoxidase inhibitors terbinafin and naftifin or the
lanosterol-14a-demethylase inhibitors of the azole type such
as ketoconazole and fluconazole.
Another possible use of the compounds of general formula I
21 887a4
- 26 -
concerns their use in poultry rearing. Lowering the
cholesterol content of eggs by administering the HMG-CoA-
reductase inhibitor lovastatin to laying hens has been
described (FASEB Journal 4, A 533, Abstracts 1543 [1990]).
The production of low-cholesterol eggs is of importance since
the cholesterol load in the body can be reduced by the use of
eggs with a reduced cholesterol content without changing
eating habits. In view of their inhibitory effect on
cholesterol biosynthesis, the compounds of general formula I
may also be used in poultry rearing to produce low cholesterol
eggs, the substances preferably being given as a feed
additive.
The biological effect of compounds of general formula I was
determined using the following methods:
I. Measuring the inhibition of l4C-acetate incorporation into
the steroids which can be precipitated with digitonin:
Method:
Human hepatoma cells (HEP-G2) were grown for 3 days and then
stimulated for 16 hours in cholesterol-free medium. The
substances to be tested (dissolved in dimethylsulphoxide,
final concentration 0.1~) were added during this stimulation
phase. Then, after the addition of 200 ~Mol/l 2-l4C-acetate,
incubation is continued for a further two hours at 37C in an
incubator.
After the cells have been removed and the sterol esters have
been saponified, digitonin is added after extraction and the
sterols precipitated are isolated. The l4C-acetate
incorporated in the sterols capable of being precipitated by
digitonin is measured by scintillation counting.
The inhibitory effect was investigated at test concentrations
- 2 1 88784
of 10-7 mol/l and 10-8 mol/l. It was found that the following
compounds A to Q of general formula I, for example, exhibited
a good inhibitory effect at these test concentrations, e.g.
they showed an inhibitory effect of at least 50~ at a test
concentration of 10-~ mol/l:
A = cis-0-(4-chlorobenzoyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
B = cis-0-(4-phenyl-3-butenoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
C = trans-0-(4-chlorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
D = cis-0-(5-methylhexanoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
E = trans-0-(2-phenylpropionyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
F = trans-0-(4-fluorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
G = trans-0-(3,4-dichlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
H = cis-0-(4-fluoroclnn~moyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
I = trans-O-(p-tolylacetyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
J = trans-0-(4-[trifluoromethyl]-phenylacetyl)-4-(4-
dimethylaminomethylphenyl)-cyclohexanol
21 ~8784
- 28 -
K = trans-0-(2-naphthylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
L = trans-0-(4-nitrophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
M = trans-0-(4-bromophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
N = trans-0-(2,4-dichlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
O = trans-0-([4-amino-3-chlorophenyl]acetyl)-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
P = trans-0-(4-methoxyphenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
Q = trans-0-(4-chlorophenylacetyl)-4-(4-methylaminomethyl-
phenyl)-cyclohexanol.
The percentages by which the above compounds inhibit 14C-
acetate incorporation are given in the following Table:
21 88~84
- 29 -
mol/l 10-7 lo-8
A -85 -51
B -87 -58
C -83 -66
D -88 -53
E -89 -72
F -86 -66
G -89 -74
H -86 -51
I -90 -72
J -89 -87
K -86 -54
L -83 -67
M -84 -64
N -85 -67
O -79 -51
P -73 -52
Q -79 -50
As already mentioned, individual inhibitors of the enzyme 2,3-
epoxysqualene-lanosterol-cyclase have already been described
in the literature but they are structurally very different
from the compounds of formula I according to the invention.
The compounds which are most closely related in structure to
the compounds of general formula I are described in
EP 0 468 457. By way of a comparison, therefore, Example 1 of
this publication was tested by the method described above in
test concentrations of 10-5 mol/l and 10-6 mol/l. The
inhibitory values of 41~ and 13~ obtained show that these
compounds are significantly inferior to the compounds of
general formula I according to the invention.
2 1 88784
- 30 -
II. Measurement of the in vivo activity in the rat after oral
administration
Inhibition of the enzyme 2,3-epoxysqualene-lanosterol-cyclase
brings about an increase in the 2,3-epoxysqualene levels in
the liver and plasma. The quantity of 2,3-epoxysqualene
formed therefore serves as a direct measurement of the potency
on the animal as a whole. The measurement is carried out as
follows:
Male Wistar rats (weighing 160-190 g) are given the substance,
suspended in 1.5~ aqueous methyl cellulose, by oesphageal
tube. 5 hours after administration, blood is taken
retroorbitally from the venus plexus. Plasma is worked up
using the method of Bligh and Dyer (Canad. J. Biochem.
Physiol. 37, 912, [1959]), purified using a preliminary column
and then analysed with HPLC. The peaks obtained are
identified and quantified using calibrating substances. An
internal standard is used to test the reproducibility of the
results.
The tests were carried out with concentrations of 0.1 and
1.0 mg/kg. In the Table which follows, the test data of the
above-mentioned substances B, C, J, M, N and P are shown, by
way of example, for the 2,3-epoxysqualene levels obtained in
rat plasma. No measurable 2,3-epoxysqualene levels occur
under the test conditions in the control animals.
21 88784
2 3-F~oxysqualene levels in plasma (rat)
2,3-Epoxysqualene [~g/ml]
Substance 0.1 mg/kg 1.0 mg/kg
B 0.4 1.1
C 0.6 4.2
J 0.5 3.6
M 0.6 3.5
N 0.1 2.2
P 0.3 0.9
None of the inhibitors of the enzyme 2,3-epoxysqualene-
lanosterol-cyclase described in the literature has hitherto
been found to inhibit cholesterol biosynthesis in the whole
animal.
The compounds prove totally non-toxic at the curative dose.
For example, compound C shows no side effects in the rat, and
compounds J and M show no side effects in the mouse, after
oral administration of 100 mg/kg once a day for 5 days.
For pharmaceutical use the compounds of general formula I may
be incorporated in the usual pharmaceutical preparations for
oral and topical administration in a manner known per se.
Formulations for oral use include, for example, plain or
coated tablets and capsules whilst suppositories are
preferably used for rectal administration.
Topical formulations include gels, creams, lotions, ointments,
powders, aerosols and other conventional preparations for
using therapeutic agents on the skin. The quantity of active
substance for topical use is 1 to 50 mg per gram of
- 21 88784
- 32 -
preparation but preferably 5 to 20 mg per gram of preparation.
As well as being used on the skin the topical formulations
according to the invention may also be used in the treatment
of mucosa which are accessible for topical treatment. For
example, the topical formulations may be applied to the mucosa
of the mouth, lower colon and elsewhere.
The oral or rectal daily dose is between 1 and 1200 mg for a
person weighing 60 kg, but preferably the daily dose is from 5
to 100 mg for a person weighing 60 kg. The daily dose is
preferably divided into 1 to 3 individual doses.
For topical use the compounds are administered in preparations
containing about 1 to 1000 mg, more particularly 10 to 300 mg
of active substance per day. The daily dose is preferably
divided into 1 to 3 individual doses.
For use in poultry rearing in order to produce low cholesterol
eggs, the active substances of general formula I are given to
the animals in the form of an additive to their feed by normal
methods. The concentration of active substances in the
complete feed is normally 0.01 to 1~, but preferably 0.05 to
0.5%.
The active substances may be added to the feed as such. Thus,
the feedstuffs according to the invention for laying hens will
contain, apart from the active substance and possibly a
conventional vitamin/mineral mixture, maize, soya flour,
meatmeal, edible fat and soya oil, for example. One of the
above-mentioned compounds of formula I is added to this
feedstuff as an active substance in a concentration of 0.01 to
1~, but preferably 0.05 to 0.5~.
21 887~34
The Examples which follow are intended to illustrate the
lnventlon:
In the following Examples the thin layer chromatography was
carried out using ready-made TLC plates produced by Messrs.
E. Merck of Darmstadt, the plates being specifically:
a) silica gel 60 F254
b) aluminium oxide F2s4 (Type E)
Preparation of the starting compounds
Exam~le I
4-(4-Dimethylaminomethylphenyl)-cyclohexanone
a) 4-(4-Dimethylaminomethylphenyl)-4-hydroxycyclohexanone-
ethylene ketal
To a solution of 36.4 g (0.17 mol) of 4-bromo-N,N-
dimethylbenzylamine in 250 ml of dry tetrahydrofuran, cooled
to -70C, are added dropwise, under a nitrogen atmosphere and
with stirring, 112 ml (0.179 mol) of a 1.6 molar solution of
n-butyllithium in hexane in such a way that the temperature
does not exceed -65C. The orange solution is stirred for a
further 15 minutes at -70C and then within 10 minutes a
solution of 27.6 g (0.172 mol) of 1,4-cyclohexanedione-
monoethylene ketal in 110 ml of tetrahydrofuran is added,
whilst the temperature must not exceed -65C.
The reaction mixture is stirred first for 30 minutes at -70C
and then without external cooling until a temperature of +20C
is reached, then poured into 600 ml of ice water and extracted
with 200 ml of ethyl acetate. The organic phase is separated
off and the aqueous phase is extracted several times with
21 88784
- 34 -
ethyl acetate. The combined organic extracts are dried with
sodium sulphate, evaporated down in vacuo and the residue
rem~;n;ng is recrystallised from diisopropylether. 41.9 g
(85% of theory) of 4-(4-dimethylaminomethylphenyl)-4-
hydroxycyclohexanone-ethylene ketal are obtained, m.p.
84-86C.
b) 1-(4-Dimethylaminomethyl)phenyl-4-ethylenedioxy-1-
cyclohexene
A mixture of 22.4 g (0.077 mol) of 4-(4-dimethylaminomethyl-
phenyl)-4-hydroxycyclohexanone-ethylene ketal, 15.0 g
(0.079 mol) of p-toluenesulphonic acid monohydrate, 39 ml of
ethylene glycol and 240 ml of toluene is refluxed for 3~ hours
with stirring and the reaction water produced is continuously
removed. The cooled reaction mixture is poured into 200 ml of
water and adjusted to pH 12-13 with 2N NaOH. The organic
phase is separated off and the aqueous phase is extracted
several times with toluene. The combined organic extracts are
dried with sodium sulphate and evaporated down in vacuo. 21 g
(about 100~) of the title compound are obtained in the form of
a yellow oil.
c) 1-(4-Dimethylaminomethyl)phenyl-4-ethylenedioxy-1-
cyclohexane
A solution of 21 g (0.077 mol) of crude 1-(4-dimethylamino-
methyl)phenyl-4-ethylenedioxy-1-cyclohexene in 200 ml of ethyl
acetate and 100 ml of methanol is combined with 5 g of
palladium/barium sulphate catalyst and hydrogenated for 1.5
hours under a hydrogen pressure of 5 bar. After the catalyst
has been removed the residue is evaporated down in vacuo.
20 g (about 100~) of the title compound are obtained as a
yellowish-brown oil.
` - 2 1 88784
- 35 -
d) 4-(4-Dimethylaminomethyl)phenyl-cyclohexanone
A mixture of 20 g (0.077 mol) of crude 1-(4-dimethylamino-
methyl)phenyl-4-ethylenedioxy-1-cyclohexane and 110 ml of 2N
hydrochloric acid is stirred for 3.5 hours at ambient
temperature. The aqueous solution formed is extracted several
times with ethyl acetate; the organic extracts are discarded.
The aqueous phase is adjusted to pH 13-14 with 50~ sodium
hydroxide solution whilst being cooled and is extracted
several times with ethyl acetate. The combined organic
extracts are washed with saturated sodium chloride solution,
dried with sodium sulphate and evaporated down in vacuo. 14 g
t79~ of theory) of 4-(4-dimethylaminomethyl)phenyl-
cyclohexanone are obtained, melting point 64-67C, as a light
yellow product. An analytical sample is recrystallised from
petroleum ether 60/90.
Melting point: 65-67C
C1sH21NO (231.34)
Calculated: C 77.88 H 9.15 N 6.05
Found: 77.69 9.32 5.98
Ex~le II
trans-4-(4-Dimethylaminomethylphenyl)-cyclohexanol
To a solution of 11.1 g (0.048 mol) of 4-(4-
dimethylaminomethylphenyl)-cyclohexanone in 100 ml of absolute
methanol, which has been cooled to -10C, is added 1.82 g
(0.048 mol) of sodium borohydride in batches with stirring.
The reaction mixture is allowed to react for 1.5 hours at
ambient temperature and then evaporated down in vacuo. The
residue remaining is mixed with water, acidified with
concentrated hydrochloric acid, stirred for 30 minutes at
ambient temperature, made alkaline with 50~ sodium hydroxide
solution and extracted several times with chloroform. The
` - 21 887~4
- 36 -
combined extracts are dried with sodium sulphate and
evaporated down in vacuo. The residue r~m~;n;~g, which
consists of a mixture of trans/cis-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol (cis fraction ~ 10~) is purified by
column chromatography (aluminium oxide neutral, activity stage
III, ICN; petroleum ether/methylethyl ketone = 5:1).
White crystals are obtained, melting point 63-65C.
Yield: 8.8 g (79~ of theory),
l C1sH23NO (233.36)
Calculated: C 77.21 H 9.93 N 6.00
Found: 77.34 10.02 5.89
Example III
cis-4-(4-Dimethylaminomethylphenyl)-cyclohexanol
50 ml (0.05 mol) of a 1 molar solution of lithium tri-sec.-
butyl-borohydride in absolute tetrahydrofuran are diluted with
100 ml of absolute tetrahydrofuran under a nitrogen atmosphere
and then, at -65C to -70C, with stirring and within 10
minutes, a solution of 5.8 g (0.025 mol) of 4-(4-
dimethylaminomethylphenyl)-cyclohexanone in 50 ml of absolute
tetrahydrofuran is added thereto. The reaction mixture is
then left to react for 3 hours at -70C and then heated to
ambient temperature within 1 hour. It is then hydrolysed with
20 ml of 75% aqueous ethanol and the organoborane is oxidised
with alkaline hydrogen peroxide (10 ml of 6 M NaOH/15 ml 30~
H2O2). The organic phase is separated off, the aqueous phase
is saturated with potassium carbonate and extracted with 50 ml
of ethyl acetate. The combined organic extracts are dried
with sodium sulphate and evaporated down in vacuo. The greasy
residue r~;n;ng, which consists of a mixture of cis/trans-4-
(4-dimethylaminomethylphenyl)cycloh~x~nol (trans fraction
< 5~) is purified by column chromatography (aluminium oxide
neutral, acti~ity stage III, ICN; petroleum ether/methylethyl
21 887~4
- 37 -
ketone = 5:1).
The product is obtained as a colourless oil.
Yield: 4.1 g (71~ of theory).
1H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.5-2.0 (2m,8H); 2.25 (s,6H); 2.4-2.65 (m,lH); 3.4 (s,2H);
4.1-4.18 (m,lH); 7.15-7.3 (m,4H).
Example IV
trans-O-Acetyl-4-(4-chloromethylphenyl)-cyclohexanol
a) 4-Phenylcyclohexanol
To a solution of 31.4 g (0.18 mol) of 4-phenylcyclohexanone in
500 ml of absolute methanol, which is cooled to -10C, are
added 6.8 g (0.18 mol) of sodium borohydride in batches with
stirring. The reaction mixture is allowed to react for 0.5
hours at -10C and for 3 hours at ambient temperature and then
evaporated down in vacuo. The residue remaining is mixed with
water and acidified with 2N hydrochloric acid. The suspension
formed is stirred for 1 hour and the crystalline product is
suction filtered, dried and recrystallised from
diisopropylether. 21 g (66~ of theory) of 4-
phenylcyclohexanol are obtained, melting point: 112-114C.
b) O-Acetyl-4-phenylcyclohexanol
To a mixture of 20.3 g (0.115 mol) of 4-phenylcyclohexanol,
14.2 ml (0.15 mol) of acetanhydride and 29 ml of triethylamine
are added, with stirring and at ambient temperature, 2.3 g
(0.02 mol) of 4-dimethylaminopyridine, a clear solution being
produced in an exothermic reaction. This is heated to 80C
for 3 hours and the reaction mixture is then poured into ice
water. The crystalline product precipitated is suction
filtered, dissolved in ether, washed with sodium bicarbonate
` - 21 88784
- 38 -
solution, dried and evaporated down in vacuo. 23 g (92% of
theory) of O-acetyl-4-phenylcyclohexanol are obtained. The
product is obtained initially as an oil but crystallises when
left to stand.
Melting point: 43-45C.
c) trans-O-Acetyl-4-(4-chloromethylphenyl)-cyclohexanol
7 A solution of 24.3 g (0.11 mol) of O-acetyl-4-
phenylcyclohexanol in 1300 ml of methylene chloride is
combined with 26.0 g (0.86 mol) of paraformaldehyde and 26.0 g
(o.l9 mol) of zinc chloride. Hydrogen chloride is introduced
into this suspension, with stirring, for 2.5 hours, whilst the
temperature rises to about 30C and a substantially
homogeneous solution is formed. The mixture is then allowed
to react for 15 hours at ambient temperature and the reaction
mixture is then hydrolysed with stirring in about 1.5 litres
of ice water. The organic phase is separated off, the aqueous
phase is extracted with methylene chloride again and the two
organic phases are combined. They are washed until neutral,
dried and evaporated in vacuo. The yellow oil rem~;n;ng is
crystallised by trituration with diisopropylether and the
solid product is recrystallised from diisopropylether. White
crystals are obtained, melting point 87-89C.
Yield: 12.7 g (43% of theory).
Cl5H1gClO2 (266.77)
Calculated: C 67.53 H 7.18 Cl 13.29
Found: 67.68 7.29 13.11
2l ~8184
- 39 -
Exam~le V
cis/trans-O-(4-Chlorophenylacetyl)-4-(4-N-[tert.-
butoxycarbonyl]-methylaminomethyl)phenyl-cyclohexanol
a) 4-(4-Methylaminomethyl)phenyl-4-hydroxycyclohexanone-
ethylene ketal
A solution of 94 g (0.47 mol) of 4-bromo-(N-methyl)-
benzylamine in 460 ml of dry tetrahydrofuran is combined first
with 300 ml (0.48 mol) of a 1.6 molar solution of n-butyl
lithium in hexane and then with 52.5 g (0.48 mol) of
trimethylchlorosilane, under a nitrogen atmosphere and at -30
to -25C. The reaction mixture is stirred for a further 15
minutes at this temperature and then cooled to -75~C. Then
another 320 ml (0.51 mol) of a 1.6 molar solution of n-
butyllithium in hexane are added so that the temperature does
not exceed -70C. The mixture is stirred for a further 20
minutes at -75C and then, within 20 minutes, mixed with a
solution of 76 g (0.47 mol) of 1,4-cyclohexanedione-
monoethylene ketal in 200 ml of tetrahydrofuran, whilst the
temperature should not exceed -65C. The reaction mixture is
then stirred first for 30 minutes at -70C and then without
external cooling until a temperature of +20C is reached. It
is then decomposed in ice cold aqueous ammonium chloride
solution and extracted several times with methylene chloride.
The combined organic extracts are dried with sodium sulphate,
the solvent is eliminated and the residue rem~1n1ng is
recrystallised from diisopropylether. 77 g (59~ of theory) of
4-(4-methylaminomethyl)phenyl-4-hydroxycyclohexanone-ethylene
ketal are obtained, melting point 95-97C.
b) 1-(4-Methylaminomethyl)phenyl-4-ethylenedioxy-1-cylohexene
A mixture of 68 g (0.24 mol) of 4-(4-methylaminomethyl)-
` ~188784
- 40 -
phenyl-4-hydroxycyclohexanone-ethylene ketal, 51 g (0.27 mol)
of p-toluenesulphonic acid monohydrate, 150 ml of ethylene
glycol and 900 ml of toluene is refluxed for 2.5 hours with
stirring and the reaction water formed is continuously
removed. The cooled reaction mixture is made alkaline with lN
sodium hydroxide solution (pH 12-13), the organic phase is
separated off and the aqueous phase is extracted several times
with ethyl acetate. The combined organic phases are dried
with sodium sulphate and evaporated down in vacuo. 63 g
(about 100~ of theory) of 1-(4-methylaminomethyl)phenyl-4-
ethylenedioxy-1-cyclohexene are obtained as a yellowish oil.
c) 1-(4-N-[tert.-Butoxycarbonyl]-methylaminomethyl)phenyl-4-
ethylenedioxy-1-cyclohexene
A solution of 63 g (0.24 mol) of the crude 1-(4-methylamino-
methyl)phenyl-4-ethylenedioxy-1-cyclohexene in 350 ml of
absolute tetrahydrofuran is combined, with stirring, with a
solution of 58 g (0.26 mol) of di-tert.-butyldicarbonate in
100 ml of absolute tetrahydrofuran, the temperature being
maintained at between 15 and 20C by cooling. After the
development of CO2 has died away, the mixture is left for a
further 10 hours at ambient temperature, the solvent is
distilled off in vacuo, the residue is mixed with water and
extracted several times with ether. After drying with sodium
sulphate and evaporation 84 g (about 100~ of theory) of 1-(4-
N-[tert.-butoxycarbonyl]-methylaminomethyl)phenyl-4-
ethylenedioxy-1-cyclohexene are obtained as a yellowish oil.
d) 4-(4-N-[tert.-Butoxycarbonyl]-methylaminomethyl)phenyl-
cyclohexanone
A solution of 84 g (0.24 mol) of the crude 1-(4-N-[tert.-
butoxycarbonyl]-methylaminomethyl)phenyl-4-ethylenedioxy-1-
cyclohexene in methanol/ethyl acetate (250+250 ml) is combined
21 88784
with 10 g of palladium/barium sulphate catalyst and
hydrogenated for 4 hours at ambient temperature under a
hydrogen pressure of 3 bar. The catalyst is separated off,
the solvent is distilled off in vacuo, the oily residue is
dissolved in acetone/water (1400+140 ml), and after the
addition of 8.5 g (0.034 mol) of pyridinium tosylate, it is
refluxed for 15 hours. The solvent is then distilled off in
vacuo, the residue is combined with water and extracted
several times with methylene chloride. After the organic
phase has been dried with sodium sulphate and evaporated down,
61 g (77~ of theory) of 4-(4-N-[tert.-butoxycarbonyl]-
methylaminomethyl)phenyl-cyclohexanone are obtained as a pale
yellow oil which solidifies when left to stand for a length of
time.
Melting point: 55-57C.
e) 4-(4-N-[tert.-Butoxycarbonyl]-methylaminomethyl)phenyl-
cyclohexanol (cis/trans mixture)
1.31 g (0.035 mol) of sodium borohydride is added in batches,
with stirring, to a solution of 11 g (0.035 mol) of 4-(4-N-
[tert.-butoxycarbonyl]-methylaminomethyl)phenyl-cyclohexanone
in 70 ml of absolute methanol, cooled to -10C. The reaction
mixture is allowed to react for 0.5 hours at -10C and for 2
hours at ambient temperature and then evaporated down in
vacuo. The residue rem~;n;ng is mixed with water and stirred
for 1 hour at ambient temperature. The solid product produced
is suction filtered, dissolved in ethyl acetate and this
solution is dried over sodium sulphate. After evaporation in
vacuo, 8.6 g (77~ of theory) of a mixture of the cis- and
trans-forms of 4-(4-N-[tert.-butoxycarbonyl]-
methylaminomethyl)phenyl-cyclohexanol are left in the form of
a colourless oil. This can be separated into the pure isomers
by column chromatography (aluminium oxide neutral, activity
stage III, ICN; petroleum ether/ethyl acetate = 3:1).
21 88784
- 42 -
Rf value (aluminium oxide; petroleum ether/ethyl acetate =
3:1): 0.21 (trans) and 0.31 (cis).
f) 0-(4-Chlorophenylacetyl)-4-(4-N-[tert.-butoxycarbonyl]-
methylaminomethyl)phenyl-cyclohexanol (cis/trans mixture)
A mixture of 0.54 g (0.0032 mol) of 4-chlorophenylacetic acid,
0.52 g (0.0032 mol) of N,N'-carbonyldiimidazole and 20 ml of
xylene is heated to 60C for 1 hour with stirring. Then a
solution of 0.85 g (0.0027 mol) of 4-(4-N-[tert.-
butoxycarbonyl]-methylaminomethyl)phenyl-cyclohexanol
(cis/trans mixture) in 10 ml of xylene is added and the
reaction mixture is heated to 160C for a further 8 hours.
After cooling, it is evaporated down in vacuo, the residue is
mixed with water and extracted with ethyl acetate. The
organic phase is dried over sodium sulphate and evaporated
down in vacuo. 1. 3 g (about 100% of theory) of 0-(4-
chlorophenylacetyl)-4-(4-N-[tert.-butoxycarbonyl]-
methylaminomethyl)phenyl-cyclohexanol (cis/trans mixture) are
left as a reddish-brown oil.
Rf value (aluminium oxide; petroleum ether/ethyl acetate =
3:1): 0.78 (trans) and 0.85 (cis).
The following substance was synthesised analogously:
(1) trans-O-(4-chlorophenylacetyl)-4-(4-N-[tert.-
butoxycarbonyl]-methylaminomethyl)phenyl-cyclohex~nol
from trans-4-(4-N-[tert.-butoxycarbonyl]-methylaminomethyl)-
phenyl-cyclohexanol and 4-chlorophenylacetic acid/N,N'-
carbonyldiimidazole.
White crystals.
Melting point: 94-96C.
2 1 887~4
- 43 -
Preparat;on of the end products:
~xample 1
trans-O-(4-Chlorobenzoyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
A solution of 1.0 g (0.0043 mol) of trans-4-(4-
dimethylaminomethylphenyl)-cyclohexanol and 0.6 ml of
triethylamine in 50 ml of methylene chloride is combined,
dropwise, with 0.75 g (0.0043 mol) of 4-chlorobenzoylchloride,
with stirring, and refluxed for 3 hours. After cooling, 50 ml
of water are added, the mixture is adjusted to pH 12-13 with
sodium hydroxide solution, the methylene chloride phase is
separated off and the aqueous phase is extracted once more
with methylene chloride. The combined organic phases are
dried over sodium sulphate and evaporated down in vacuo. The
solid residue is purified by column chromatography (aluminium
oxide neutral, activity stage III, ICN; petroleum ether/ethyl
acetate = 40:1). White crystals are obtained with a melting
point of 94-95C.
Yield: 1.1 g (69~ of theory)
lH-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.55-1.8 (m,4H); 1.9-2.1 (m,2H); 2.15-2.3 (s+m,6+2H); 2.5-2.7
(m,lH); 3.4 (s,2H); 4.9-5.1 (m,lH); 7.15-7.3 (m,4H); 7.4
(d,2H); 8.0 (d,2H).
The following substances were synthesised analogously:
(1) trans-O-acetyl-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
acetylchloride/triethylamine.
21 ~8784
- 44 -
Colourless syrup.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.45-1.7 (m,4H); 1.9-2.05 (m,2H); 2.05-2.15 (s+m,3+2H); 2.23
(s,6H); 2.4-2.65 (m,lH); 3.4 (s,2H); 4.7-4.9 (m,lH); 7.1-7.3
(m,4H).
(2) trans-O-butyryl-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
butyric acid chloride/triethylamine.
Colourless oil.
lH-NMR spectrum (200 MHz, CDCl3); signals at ppm:
0.9-1.02 (t,3H); 1.45-1.75 (m,6H); 1.89-2.05 (m,2H); 2.05-2.18
(m,2H); 2.18-2.38 (s+t,6+2H); 2.4-2.6 (m,lH); 3.4 (s,2H);
4.7-4.9 (m,lH); 7.1-7.3 (m,4H).
~3) trans-O-cyclopropanoyl-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
cyclopropane carboxylic acid chloride/triethylamine.
Colourless wax.
lH-NMR spectrum (200 MHz, CDCl3); signals at ppm:
0.81-0.87 (m,2H); 0.95-1.02 (m,2H); 1.45-1.7 (m,4H); 1.9-2.0
(m,2H); 2.05-2.15 (m,2H); 2.24 (s,6H); 2.4-2.63 (2m,2H); 3.4
(s,2H); 4.73-4.83 (m,lH); 7.12-7.25 (m,4H).
(4) trans-O-cyclohexanoyl-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from tran~-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
cyclohexane carboxylic acid chloride/triethylamine.
White crystals.
Melting point: 66-68C.
21 88784
- 45 -
(5) cis-O-(4-chlorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
chlorophenylacetylchloride/triethylamine.
Colourless oil.
lH-NMR spectrum (200 MHz, CDC13); signals at ppm:
1.5-1.75 (m,6H); 1.88-2.05 (m,2H); 2.25 (s,6H); 2.4-2.65
(m,lH); 3.4 (s,2H); 3.65 (s,2H); 5.05-5.15 (m,lH); 7.08
(d,2H), 7.2-7.4 (m,6H).
(6) trans-O-(4-phenyl-3-butenoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
phenyl-3-butenoic acid chloride/triethylamine.
White crystals.
Melting point: 90-91C.
(7) cis-O-(4-phenyl-3-butenoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
phenyl-3-butenoic acid chloride/triethylamine.
White crystals.
Melting point: 71-73C.
Ex~m~le 2
trans-O-(4-Chlorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
To a mixture of 0.43 g (0.0025 Mol) of 4-chlorophenylacetic
acid and 30 ml of xylene are added 0.41 g (0.0025 Mol) of
21 88784
- 46 -
N,N'-carbonyldiimidazole, a white product being produced with
the release of CO2. The reaction mixture is heated to 60C for
1 hour with stirring and then 0.5 g (0.0021 mol) of trans-4-
(4-dimethylaminomethylphenyl)-cyclohexanol are added. The
mixture is heated to 160C for 12 hours with stirring, cooled
to ambient temperature, mixed with water and adjusted to pH
12-13 using 2N sodium hydroxide solution. The xylene phase is
separated off, the aqueous phase is extracted several times
with ethyl acetate, the organic phases are combined, dried and
evaporated down in vacuo. The solid residue is purified by
column chromatography (aluminium oxide basic, activity stage
III, ICN; petroleum ether/ethyl acetate = 10:1). White
crystals are obtained with a melting point of 75-77C.
Yield: 0.7 g (86~ of theory).
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.4-1.7 (m,4H); 1.8-2.15 (m,4H); 2.25 (s,6H); 2.4-2.6 (m,lH);
3.38 (s,2H); 3.6 (s,2H); 4.7-4.9 (m,lH); 7.1-7.35 (m,8H).
The following substances were synthesised analogously:
(1) trans-O-(5-methylhexanoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 5-
methylhexanoic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 35-36C.
(2) cis-O-(5-methylhexanoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohex~nol
from cis-4-(4-dimethylaminomethylphenyl)-cycloh~nol and 5-
methylhexanoic acid/N,N'-carbonyldiimidazole.
Colourless oil.
lH-NMR spectrum (200 MHz, CDCl3); signals at ppm:
21 88784
- 47 -
0.9 (d,6H); 1.15-1.32 (m,2H); 1.5-1.88 (m,9H); 1.95-2.1
(m,2H); 2.25 (s,6H); 2.3 (d,2H); 2.48-2.69 (m,lH); 3.4 (s,2H);
5.08-5.18 (m,lH); 7.12-7.3 (m,4H).
(3) trans-O-cyclohexylacetyl-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
cyclohexylacetic acid/N,N'-carbonyldiimidazole
White crystals.
Melting point: 37-39C.
(4) trans-O-(2-butenoyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
crotonic acid/N,N'-carbonyldiimidazole
White crystals.
Melting point: 69-71C.
(5) trans-O-(2-hexenoyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 2-
hexenoic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 40-42C.
(6) trans-O-(3-cyclohexylpropenoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 3-
cyclohexylpropenoic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 46-47C.
21 88784
- 48 -
(7) trans-O-benzoyl-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
benzoic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 68-70C.
(8) trans-O-(4-chloro-3-methylbenzoyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
chloro-3-methylbenzoic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 100-102C.
(9) trans-O-(2-naphthoyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
naphthoic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 110-112C.
(10) trans-O-phenylacetyl-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
phenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 38-40C.
(11) trans-O-(4-fluorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
2 1 ~878~
- 49 -
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
fluorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 68-70C.
(12) cis-O-(4-fluorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
fluorophenylacetic acid/N,N'-carbonyldiimidazole.
Colourless oil.
1H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.5-1.75 (m,6H); 1.85-2.05 (m,2H); 2.3 (s,6H); 2.4-2.65
(m,lH); 3.43 (s,2H); 3.65 (s,2H); 5.05-5.15 (m,lH); 7.0-7.15
(m,4H); 7.2-7.38 (m,4H).
(13) trans-O-(4-bromophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
bromophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 72-74C.
(14) trans-O-(3,4-dichlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
3,4-dichlorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 95-97C.
(15) cis-O-(3,4-dichlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
21 88784
- 50 -
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 3,4-
dichlorophenylacetic acid/N,N'-carbonyldiimidazole.
Colourless oil.
lH-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.5-1.75 (m,6H); 1.9-2.05 (m,2H); 2.28 (s,6H); 2.4-2.65
(m,lH); 3.4 (s,2H); 3.62 (s,2H); 5.8-5.17 (m,lH); 7.05-7.3
(m,5H); 7.35-7.49 (m,2H).
(16J trans-O-(2,4-dichlorophenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
2,4-dichlorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 78-80C.
(17) trans-O-(p-tolylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and p-
tolylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 40-42C.
(18) trans-O-(4-[trifluoromethyl]-phenylacetyl)-4-(4-
dimethylaminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
(trifluoromethyl)-phenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 73-75C.
(19) trans-O-(4-methoxyphenylacetyl)-4-(4-dimethylamino-
methylphenyl)-cyclohexanol
21 88784
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
methoxyphenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 47-49C.
(20) trans-O-(4-nitrophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
nitrophenylacetic acid/N,N'-carbonyldiimidazole.
Yellowish crystals.
Melting point: 136-137C.
(21) trans-O-[3-(4-fluorophenyl)-propionyl]-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 3-
(4-fluorophenyl)-propionic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 58-59C.
(22) trans-O-[3-(4-chlorophenyl)-propionyl]-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 3-
(4-chlorophenyl)-propionic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 85-87C.
(23) trans-O-(4-biphenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
biphenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
- 2 1 88784
- 52 -
Melting point: 88-89C.
(24) trans-0-(2-naphthylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 2-
naphthylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 85-87~.
(25) trans-O-[2-(1,2,3,4-tetrahydro)naphthoyl]-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 2-
(1,2,3,4-tetrahydro)naphthoic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 95-96~.
(26) cis-0-[2-(1,2,3,4-tetrahydro)naphthoyl]-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 2-
(1,2,3,4-tetrahydro)naphthoic acid/N,N'-carbonyldiimidazole.
Colourless oil.
1H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.55-1.85 (m,6H); 1.9-2.1 (m,3H); 2.15-2.32 (s+m,6+1H)i
2.4-2.65 (m,lH); 2.72-2.95 (m,3H); 3.05 (d,2H); 3.4 (s,2H);
5.1-5.2 (m,lH); 7.05-7.3 (2m,8H).
(27) trans-O-(2-phenylpropionyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 2-
phenylpropionic acid/N,N'-carbonyldiimidazole.
Colourless wax.
~1 887~
1H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.25-1.7 (d+m,3+3H); 1.8-2.15 (m,5H); 2.3 (s,6H); 2.38-2.6
(m,lH); 3.4 (s,2H); 3.7 (q,lH); 4.68-4.9 (m,lH); 7.15 (d,2H);
7.18-7.38 (d+m,2+5H).
(28) cis-O-(2-phenylpropionyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 2-
phenylpropionic acid/N,N'-carbonyldiimidazole.
Colourless oil.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.3-1.7 (d+m,3+6H); 1.8-2.05 (m,2H); 2.25 (s,6H); 2.35-2.58
(m,lH); 3.4 (s,2H); 3.78 (q,lH); 5.0-5.1 (m,lH); 7.0 (d,2H);
7.2 (d,2H); 7.25-7.4 (m,5H).
(29) trans-O-(4-fluorocinnamoyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
fluoroc'nn~mic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 118-120C.
(30) cis-O-(4-fluoroc;nn~moyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
fluorocinnamic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 66-68C.
(31) trans-0-(4-chloroclnn~moyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
~1 8~784
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
chlorocinnamic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 131-133C.
(32) cis-0-(4-chloroc;nn~mQyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
chloroc;nn~m;c acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 88-90C.
(33) trans-0-(4-[trifluoromethyl]-cinnamoyl)-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
(trifluoromethyl)-c; nn~m; C acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 134-136C.
(34) cis-0-(4-[trifluoromethyl]-c;nn~moyl)-4-(4-
dimethylaminomethylphenyl)-cyclohexanol
from cis-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
(trifluoromethyl)-c;nn~m;c acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 61-63C.
(35) trans-0-(5-chloro-2-thenoyl]-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 5-
chloro-2-thiophenecarboxylic acid/N,N'-carbonyldiimidazole.
White crystals.
21 88784
Melting point: 95-97C.
(36) trans-O-nicotinoyl-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
nicotinic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 86-88C.
(37) trans-O-(2-furoyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 2-
furancarboxylic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 58-60C.
(38) trans-O-(3,4-dimethoxyphenylacetyl)-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
3,4-dimethoxyphenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 32-34C.
(39) trans-O-(4-amino-3-chlorophenylacetyl)-4-(4-dimethyl-
aminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
amino-3-chlorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 83-85C.
(40) trans-0-(4-amino-3,5-dichlorophenylacetyl)-4-(4-
21 88784
dimethylaminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and 4-
amino-3,5-dichlorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 78-80C.
(41) trans-O-(4-chlorophenylacetyl)-4-(4-diethylaminomethyl
phenyl)-cyclohexanol
;
from trans-4-(4-diethylaminomethylphenyl)-cyclohexanol and 4-
chlorophenylacetic acid/N,N'-carbonyldiimidazole.
Colourless oil.
lH-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.05 (t,6H); 1.4-1.72 (m,4H); 1.9-2.2 (m,4H); 2.4-2.6
(q+m,5H); 3.5 (s,2H); 3.6 (s,2H); 4.7-4.9 (m,lH); 7.12 (d,2H);
7.18-7.35 (m,6H).
(42) trans-O-(4-chlorophenylacetyl)-4-(4-dipropylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-dipropylaminomethylphenyl)-cyclohexanol and 4-
chlorophenylacetic acid/N,N'-carbonyldiimidazole.
Colourless oil.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
0.88 (t,6H); 1.38-1.7 (m,8H); 1.9-2.19 (m,4H); 2.35 (q,4H);
2.4-2.6 (m,lH); 3.5 (s,2H); 3.6 (s,2H); 4.7-4.9 (m,lH); 7.1
(d,2H); 7.15-7.38 (m,6H).
(43) trans-O-(4-chlorophenylacetyl)-4-(4-[N-
methylbutylamino]methylphenyl)-cyclohexanol
from trans-4-(4-[N-methylbutylamino]-methylphenyl)-
cyclohe~nol and 4-chlorophenylacetic acid/N,N'-
carbonyldiimidazole.
~1 ~878~
Colourless oil.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
o.9 (t,3H); 1.2-1.7 (m,8H); 1.8-2.15 (m,4H); 2.18 (s,3H); 2.35
(t,2H); 2.4-2.6 (m,lH); 3.41 (s,2H); 3.6 (s,2H); 4.7-4.9
(m,lH); 7.1 (d,2H); 7.2-7.35 (m,6H).
(44) trans-O-(4-chlorophenylacetyl)-4-(4-diallylaminomethyl-
phenyl)-cyclohexanol
from trans-4-(4-diallylaminomethylphenyl)-cyclohexanol and 4-
chlorophenylacetic acid/N,N'-carbonyldiimidazole.
Colourless oil.
1H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.4-1.75 (m,4H); 1.9-2.2 (m,4H); 2.4-2.6 (m,lH); 3.0-3.18
(dd,4H); 3.5 (s,2H); 3.6 (s,2H); 4.7-4.9 (m,lH); 5.1-5.3
(m,4H); 5.75-6.0 (m,2H); 7.12 (d,2H); 7.15-7.38 (m,6H).
(45) trans-O-(4-chlorophenylacetyl)-4-(4-[N-pyrrolidino]-
methylphenyl)-cyclohexanol
from trans-4-(4-[N-pyrrolidino]methylphenyl)-cyclohexanol and
4-chlorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 57-59C.
(46) trans-O-(4-chlorophenylacetyl)-4-(4-[N-piperidino]-
methylphenyl)-cyclohexanol
from trans-4-(4-[N-piperidino]methylphenyl)-cyclohexanol and
4-chlorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 87-89C.
(47) trans-O-(4-chlorophenylacetyl)-4-(4-[N-morpholino]-
methylphenyl)-cyclohexanol
21 8~784
from trans-4-(4-[N-morpholino]methylphenyl)-cyclohexanol and
4-chlorophenylacetic acid/N,N'-carbonyldiimidazole.
White crystals.
Melting point: 114-116C.
(48) trans-O-(4-chlorophenylacetyl)-4-(4-[N-methyl-N'-
piperazino]methylphenyl)-cyclohexanol
~1 from trans-4-(4-[N-methyl-N'-piperazino]methylphenyl)-
cyclohexanol and 4-chlorophenylacetic acid/N,N'-
carbonyldiimidazole.
White crystals.
Melting point: 97-99C.
(49) trans-O-(3,4-[methylenedioxy]-phenylacetyl)-4-(4-
dimethylaminomethylphenyl)-cyclohexanol
from trans-4-(4-dimethylaminomethylphenyl)-cyclohexanol and
3,4-(methylenedioxy)-phenylacetic acid/N,N'-
carbonyldiimidazole.
Colourless oil.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.4-1.7 (m,4H), 1.8-2.0 (m,2H), 2.0-2.15 (m,2H), 2.25 (s,6H),
2.4-2.6 (m,lH), 3.38 (s,2H), 3.5 (s,2H), 4.7-4.9 (m,lH), 5.94
(s,2H), 6.7-6.85 (m,3H), 7.1-7.3 (m,4H).
21 88784
- 59 -
Ex~m~le 3
cis-O-(4-Chlorobenzoyl)-4-(4-dimethylaminomethylphenyl~-
cyclohexanol
0.24 g (0.001 mol) of cis-4-(4-dimethylaminomethylphenyl)-
cyclohexanol, 0.34 ml (0.0025 mol) of triethylamine and 0.12 g
(0.001 mol) of dimethylaminopyridine are dissolved in 20 ml of
methylene chloride, mixed with 0.175 g (0.001 mol) of 4-
chlorobenzoylchloride and stirred for 12 hours at ambient
temperature. The reaction mixture is combined with water and
adjusted to pH 12-13 using sodium hydroxide solution. The
methylene chloride phase is separated off, the aqueous phase
is extracted several times with methylene chloride and the
combined organic phases are washed with saturated saline
solution, dried and evaporated down in vacuo. The residue is
purified by column chromatography (aluminium oxide neutral,
activity stage III, ICN; petroleum ether/ethyl acetate = 45:1)
White crystals.
Melting point: 96-97C.
Yield: 0.27 g (73~ of theory)
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.7-2.0 (m,6H); 2.1-2.25 (m,2H); 2.28 (s,6H); 2.55-2.75
(m,lH); 3.4 (s,2H); 5.33-5.4 (m,lH); 7.15-7.3 tm,4H)i 7-45
(d,2H); 8.2 (d,2H).
Ex~m~le 4
trans-O-Acetyl-4-(4-diethylaminomethylphenyl)-cyclohexanol
A solution of 1 g (3.75 mmol) of trans-O-acetyl-4-(4-
chloromethylphenyl)-cyclohexanol in 10 ml of dimethylformamide
is mixed with 0.52 g (3.75 mmol) of potassium carbonate and
0.27 g (3.75 mmol) of diethylamine. This mixture is heated to
21 8~784
- 60 -
50C for 6 hours with stirring, then water is added and the
mixture is extracted with methylene chloride. The organic
phase is dried, evaporated down in vacuo and the residue
rem~;ni~g is purified by column chromatography (aluminium
oxide basic, activity stage III, ICN; petroleum ether/ethyl
acetate = 15:1).
Colourless oil.
Yield: 0.79 g (69~ of theory)
1H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.05 (t,6H); 1.45-1.75 (m,4H); 1.9-2.2 (s+m,7H); 2.4-2.6
(q+m,5H); 3.55 (s,2H); 4.68-4.9 (m,lH); 7.12 (d,2H); 7.28
(d,2H).
The following substances were synthesised analogously:
(1) trans-O-acetyl-4-(4-dipropylaminomethylphenyl)-
cyclohexanol
from trans-O-acetyl-4-(4-chloromethylphenyl)-cyclohexanol and
dipropylamine.
Colourless oil.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
0.9 (t,6H); 1.35-1.75 (m,8H); 1.9-2.2 (s+m,7H); 2.3-2.6
(q+m,5H); 3.5 (s,2H); 4.65-4.9 (m,lH); 7.1 (d,2H); 7.25
(d,2H).
(2) trans-O-acetyl-4-(4-[N-methylbutylamino]-methylphenyl)-
cyclohexanol
from trans-O-acetyl-4-(4-chloromethylphenyl)-cyclohexanol and
N-methylbutylamine.
Colourless oil.
1H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
0.9 (t,3H); 1.2-1.75 (m,lOH); 1.9-2.2 (2s+m,8H); 2.38 (t,2H);
2.4-2.6 (m,lH); 3.45 (s,2H); 4.7-4.9 (m,lH); 7.15 (d,2H); 7.25
~1 88~4
(d,2H).
(3) trans-O-acetyl-4-(4-diallylaminomethylphenyl)-
cyclohexanol
from trans-O-acetyl-4-(4-chloromethylphenyl)-cyclohexanol and
diallylamine.
Colourless oil.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.4-1.7 (m,4H); 1.9-2.18 (s+m,7H); 2.4-2.6 (m,lH); 3.09
(dd,4H); 3.52 (s,2H); 4.7-4.9 (m,lH); 5.01-5.3 (m,4H);
5.75-6.0 (m,2H); 7.12 (d,2H); 7.35 (d,2H).
(4) trans-O-acetyl-4-(4-[N-pyrrolidino]methylphenyl)-
cyclohexanol
from trans-O-acetyl-4-(4-chloromethylphenyl)-cyclohexanol and
pyrrolidine.
Colourless crystals.
Melting point: 43-45C.
(5) trans-O-acetyl-4-(4-[N-morpholino]methylphenyl)-
cyclohexanol
from trans-O-acetyl-4-(4-chloromethylphenyl)-cyclohexanol and
morpholine.
Colourless crystals.
Melting point: 53-55C.
(6) trans-O-acetyl-4-(4-[N-piperidino]methylphenyl)-
cyclohexanol
from trans-O-acetyl-4-(4-chloromethylphenyl)-cyclohexanol and
.
plperldlne.
Colourless crystals.
21 8~78~
Melting point: 62-64C.
(7) trans-O-acetyl-4-[N-methyl-N'-piperazino]methylphenyl)-
cyclohexanol
from trans-O-acetyl-4-(4-chloromethylphenyl)-cyclohexanol and
N-methylpiperazine.
Colourless crystals.
Melting point: 50-52C.
~xam~le 5
trans-0-(4-Chlorophenylacetyl)-4-(4-methylaminomethylphenyl)-
cyclohexanol
A solution of 8.9 g (0.019 Mol) of trans-O-(4-chlorophenyl-
acetyl)-4-(4-N-[tert.-butoxycarbonyl]-methylaminomethyl)-
phenyl-cyclohexanol in 200 ml of methylene chloride is
combined with 35 ml of trifluoroacetic acid and stirred for 2
hours at ambient temperature. Then the volatile components
are distilled off in vacuo, the residue is taken up in
methylene chloride and washed to neutral with saturated sodium
hydrogen carbonate solution. The organic phase is dried over
sodium sulphate and evaporated down in vacuo. A yellowish oil
rem~; ns which is purified by column chromatography (aluminium
oxide basic, activity stage III, ICN; petroleum ether/ethyl
acetate/methanol = 10:10:1). Yellowish white crystals are
obtained, melting point 65-67C.
Yield: 6.4 g (91~ of theory).
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.4-1.7 (m,4H); 1.8-2.0 (m,2H), 2.0-2.2 (m,2H), 2.4-2.6
(s+m,3+1H), 3.58 (s,2H), 3.7 (s,2H); 4.7-4.9 (m,lH), 7.1-7.35
(m,8H).
21 88784
- 63 -
The following substance was synthesised analogously:
(1) 0-(4-chlorophenylacetyl)-4-(4-methylaminomethylphenyl)-
cyclohexanol (cis/trans mixture)
from 0-(4-chlorophenylacetyl)-4-(4-N-[tert.-butoxycarbonyl]-
methylaminomethyl)phenyl-cyclohexanol (cis/trans mixture) and
trifluoroacetic acid.
Yellow oil.
Rf value (aluminium oxide; petroleum ether/ethyl
acetate/methanol = 10:10:1): 0.28-0.53.
Example 6
O-(4-Chlorophenylacetyl)-4-(4-N-[carboxamidomethyl]-
methylaminomethyl)phenyl-cyclohexanol (cis/trans mixture)
A mixture of 1.0 g (0.0027 mol) of 0-(4-chlorophenylacetyl)-4-
(4-methylaminomethylphenyl)-cyclohexanol (cis/trans mixture),
0.5 g (0.0027 mol) of iodoacetamide, 0.37 g (0.0027 mol) of
potassium carbonate and 5 ml of dimethylformamide is heated to
50C for 2 hours with stirring. After it has cooled to
ambient temperature, water is added and the mixture is
extracted with ethyl acetate. The organic phase is dried over
sodium sulphate, the volatile components are distilled off in
vacuo and the residue is purified by column chromatography
(aluminium oxide basic, activity stage III, ICN; petroleum
ether/ethyl acetate/methanol = 60:40:2.5).
A white crystalline product is obtained which sinters from
110C and melts between 128-132~C.
H-NMR spectrum (200 MHz, CDCl3); signals at ppm:
1.4-1.75 (m,5H); 1.8-2.2 (m,3H), 2.32 (dd,3H), 2.4-2.63
(m,lH), 3.0 (dd,2H), 3.5-3.7 (dd+dd,2+2H), 4.7-4.9 (m,0.5H),
5.08-5.15 (m,0.5H), 7.0-7.4 (m,8H).
21 887&4
- 64 -
The following substances were synthesised analogously:
(1) trans-O-(4-chlorophenylacetyl)-4-(4-N-[carbethoxymethyl]-
methylaminomethyl)phenyl-cyclohexanol
from trans-O-(4-chlorophenylacetyl)-4-(4-methylaminomethyl-
phenyl)-cyclohexanol, ethylbromoacetate and potassium
carbonate/dimethylformamide.
White solid product.
Melting point: 40-42C.
(2) trans-O-(4-chlorophenylacetyl)-4-(4-N-[3-hydroxypropyl]-
methylaminomethyl)phenyl-cyclohexanol
from trans-O-(4-(chlorophenylacetyl)-4-(4-methylaminomethyl-
phenyl)-cyclohexanol, 3-bromopropanol and potassium
carbonate/dimethylformamide.
White crystals.
Melting point: 75-77C.
Ex~m~le 7
trans-O-(4-Chlorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol-hydrochloride
A solution of 0.39 g (0.001 mol) of trans-O-(4-chlorophenyl-
acetyl)-4-(4-dimethylaminomethylphenyl)-cyclohexanol in 10 ml
of diethyl ether is combined at ambient temperature, with
stirring, with a 1.5 times equimolar amount of hydrogen
chloride in isopropanol added dropwise. The precipitate
formed is left for 1 hour at ambient temperature, suction
filtered, washed repeatedly with diethyl ether and dried.
White crystals are obtained, melting point 231-233C.
Yield: 0.32 g (76% of theory).
21~78~
C23H29Cl2NO2 (422.40)
Calculated: C 65.40 H 6.92 N 3.32 Cl 16.79
Found: 65.33 7.06 3.45 16.92
Exam~1e 8
trans-O-(4-Chlorophenylacetyl)-4-(4-dimethylaminomethyl-
phenyl)-cyclohexanol-tartrate
First 0.15 g (0.001 mol) of anhydrous tartaric acid and then
0.39 g (0.001 mol) of trans-O-(4-chlorophenylacetyl)-4-(4-
dimethylaminomethylphenyl)-cyclohexanol are dissolved in 7 ml
of absolute ethanol. Diethyl ether is then added to the clear
solution until it becomes slightly cloudy and it is then left
to stand for 8 hours at +4C. The crystalline product
precipitated is suction filtered, washed with diethyl ether
and dried.
Melting point: 169-171C.
Yield: 0.46 g (86~ of theory).
C27H34ClNO8 (536.02)
Calculated: C 60.50 H 6.39 N 2.61 Cl 6.61
Found: 60.37 6.38 2.65 6.73
2 1 887~34
- 66 -
The following Examples illustrate the preparation of some
pharmaceutical administration forms:
F.~m~le I
Tablets containing 5 mg of trans-O-(4-chlorophenylacetyl)-4-
(4-dimethylaminomethyl-phenyl)-cyclohexanol
Composition:
1 tablet contains:
Active substance 5.0 mg
Lactose 148.0 mg
Potato starch 65.0 mg
Magnesium stearate 2.0 mg
220.0 mg
Method of preparat;on
A 10~ mucilage is prepared from potato starch by heating. The
active substance, lactose and the rem~;n;ng potato starch are
mixed together and granulated with the above mucilage through
a 1.5 mm mesh screen. The granules are dried at 45C, rubbed
through the same screen again, mixed with magnesium stearate
and compressed to form tablets.
Weight of tablet: 220 mg
Punch: 9 mm
2 1 887~4
- 67 -
Example II
Coated tablets containing 5 mg of trans-O-(4-chlorophenyl-
acetyl)-4-(4-dimethylaminomethyl-phenyl)-cyclohexanol
The tablets prepared according to Example I are coated, by a
known method, with a coating consisting essentially of sugar
and talc. The finished coated tablets are polished with
beeswax.
Weight of coated tablet: 300 mg
Example III
Suppositories containing 5 mg of trans-O-(4-chlorophenyl-
acetyl)-4-(4-dimethylaminomethyl-phenyl)-cyclohexanol
Composition:
1 suppository contains:
Active substance 5.0 mg
Suppository mass (e.g. Witepsol W 45~)1 695.0 m~
1 700.0 mg
Method of prep~rat~on:
The finely powdered active substance is suspended in the
molten suppository mass which has been cooled to 40C. At 37C
the mass is poured into slightly chilled suppository moulds.
Weight of suppository 1.7 g
` 21 88784
- 68 -
~m~le IV
Capsules containing 5 mg of trans-O-(4-[trifluoromethyl]-
phenylacetyl)-4-(4-dimethylaminomethyl-phenyl)-cyclohexanol
Composition:
1 capsule contains:
Active substance 5.0 mg
Lactose 82.0 mg
Starch 82.0 mg
Magnesium stearate 1.0 mg
170.0 mg
Method of preparation:
The powder mixture is mixed thoroughly and packed into size 3
hard gelatine capsules in a capsule filling machine, the end
weight being continuously monitored.
~x~m~le V
Tablets containing 5 mg of trans-O-(4-bromophenylacetyl)-4-(4-
dimethylaminomethyl-phenyl)-cyclohexanol
Composition:
1 tablet contains:
Active substance 5.0 mg
Lactose 148.0 mg
Potato starch 65.0 mg
Magnesium stearate 2.0 mg
220.0 mg
`- 21 88784
- 69 -
Method of preparation:
A 10~ mucilage is prepared from potato starch by heating. The
active substance, lactose and the remaining potato starch are
mixed together and granulated with the above mucilage through
a 1.5 mm mesh screen. The granules are dried at 45C, rubbed
through the same screen again, mixed with magnesium stearate
and compressed to form tablets.
Weight of tablet: 220 mg
Punch: 9 mm
Example VI
Cream for topical administration containing 1 g of trans-O-(4-
chlorophenylacetyl)-4-(4-dimethylaminomethyl-phenyl)-
cyclohexanol
A formulation for topical administration of the compounds of
formula I may have the following composition:
1. Active substance 1.0 g
2. Stearyl alcohol 4.0 g
3. Cetyl alcohol 4.0 g
4. Mineral oil 3.0 g
5. Polysorbate 60 4.5 g
6. Sorbitan stearate 4.5 g
7. Propyleneglycol 10.0 g
8. Methylparaben 0.18 g
9. Propylparaben 0.02 g
10. Water q.s. ad 100.00 g
Ingredients 2-6 are heated to 80C until they have all melted.
Then ingredient 1 is dissolved in the oily phase. Ingredients
7 and 10 are heated to 90C and ingredients 8 and 9 are
8~:8~
- 70 -
dissolved in the aqueous phase thus obtained. The aqueous
phase is then added to the oil phase and quickly stirred to
obtain an emulsion. The mixture is then slowly cooled to 50C
in order to solidify the emulsion. The preparation is cooled
to ambient temperature whilst stirring is continued.
The following Example describes the preparation of a feed for
laying hens:
Ex~m~le VII
Feed for laying hens containing as active substance trans-O-
(4-chlorophenylacetyl)-4-(4-dimethylaminomethylphenyl)-
cyclohexanol
Maize 633 g/kg
Soya bean flour 260 g/kg
Meatmeal 40 g/kg
Edible fat 25 g/kg
Soya oil 17 g/kg
Bicalcium phosphate 12 g/kg
Calcium carbonate 6 g/kg
Vitamin/mineral mixture 5 g/kg
Active substance 2 g/kg
These components in the quantities specified, when mixed
thoroughly, yield 1 kg of feed.