Note: Descriptions are shown in the official language in which they were submitted.
21 8928~
E450 53-809 MIS 763 1996 10 03 D1
HIGH PURITY CHLORINE DIOXIDE PRODUCTION
The present invention relates to the production of
chlorine dioxide, a chemical used in the bleaching of
woodpulp.
Chlorine dioxide is produced commercially by the
reduction of chlorate in an aqueous acid medium. Many
such processes are effected at the boiling point of the
reaction medium while a subatmospheric pressure is
applied thereto.
The process may be depicted by the equation:
Cl03- + Cl- + 2H+ ~ ClO2 + ~Cl2 + H2O
The acid may be provided by a mineral acid,
generally sulfuric acid. Hydrochloric acid also may be
employed, which coincidentally provides the chloride
ions.
The chloride ions may be added to the reaction
medium in the form of the alkali metal chloride or may be
produced in situ when the reducing agent is methanol,
hydrogen peroxide or sulfur dioxide.
Other known chlorine dioxide generating processes
include ones involving the reduction of chloric acid,
such as by reaction with methanol or hydrogen peroxide,
by electrochemical means or by catalytic decomposition.
The present invention provides an improved process
of producing chlorine dioxide in which chlorine dioxide
of high purity is produced. In the process of the
present invention, sodium chlorate, hydrochloric acid or
hydrogen chloride and hydrogen peroxide reactants are
used to produce high purity chlorine dioxide and by-
product sodium chloride. The sodium chloride by-product
then is forwarded either to a chlor-alkali plant or a
chlorate plant to form reactants for the process.
A chlor-alkali plant produces from the forwarded
sodium chloride, aqueous sodium hydroxide by-product,
chlorine and hydrogen, which then can be burned together
to form HCl for the chlorine dioxide generating process.
21 89289
In this embodiment of the invention, chlorine dioxide and
sodium hydroxide products are formed from sodium chlorate
and hydrogen peroxide.
A sodium chlorate plant produces sodium chlorate
from the forwarded sodium chloride, which is circulated
back to the chlorine dioxide generator to provide the
sodium chlorate feed thereto. In this embodiment of the
invention, chlorine dioxide and hydrogen are produced
from feeds of HCl and hydrogen peroxide. The hydrogen
may be burned to form water.
Accordingly, in one aspect of the present invention,
there is provided a process for the preparation of an
aqueous solution of chlorine dioxide of high purity,
which comprises:
feeding sodium chlorate, hydrogen chloride and
hydrogen peroxide to a reaction zone wherein chlorine
dioxide is formed by reduction of chlorate ions in an
aqueous acid reaction medium, the chlorine dioxide is
formed into an aqueous solution thereof of high purity,
chlorine potentially contaminating said aqueous solution
of chlorine dioxide is at least partially reduced by said
hydrogen peroxide and crystalline sodium chloride is
formed,
removing said aqueous solution of chlorine dioxide
from said reaction zone,
removing said crystalline sodium chloride from said
reaction zone and forming an aqueous solution of said
removed crystalline sodium chloride,
subjecting the resulting aqueous solution of sodium
chloride to an electrolysis process operation to form
either:
(a) sodium hydroxide and hydrogen chloride, in
which event, recycling the hydrogen chloride to said
reaction zone to provide hydrogen chloride feed
thereto and recovering the sodium hydroxide, or:
21 89289
(b) sodium chlorate and hydrogen, in which event,
recycling the sodium chlorate to said reaction zone
to provide sodium chlorate feed thereto.
In one embodiment of the invention, the chlorine
dioxide may be formed and the potentially contaminating
chlorine may be reduced by the steps of feeding sodium
chlorate, hydrogen chloride and hydrogen peroxide to an
aqueous acid reaction medium to produce chlorine dioxide
therefrom substantially uncontaminated by chlorine and
dissolving the chlorine dioxide so formed in water to
provide the aqueous solution of chlorine dioxide. The
aqueous solution of chlorine dioxide may be further
contacted with hydrogen peroxide to reduce any chlorine
dissolved therein.
In this embodiment, the aqueous acid reaction medium
may be contained in a reaction vessel in the reaction
zone which reaction vessel is maintained at an
atmospheric pressure, the chlorine dioxide is removed
from the reaction vessel in admixture with an inert gas,
and the crystalline sodium chloride is formed by
evaporation of spent aqueous acid reaction zone in a
second vessel in the reaction zone. However, as noted
below, it is preferred to maintain the aqueous acid
reaction medium at its boiling point while the reaction
vessel is maintained under a subatmospheric pressure, so
that the chlorine dioxide is removed from the reaction
vessel in admixture with steam as the inert gas and the
sodium chloride is crystallized in the reaction vessel,
after saturation of the aqueous acid reaction medium
following start up.
In another embodiment of the invention, the chlorine
dioxide is formed and the potentially contaminating
chlorine is reduced by the steps of feeding the sodium
chlorate and hydrogen chloride to an aqueous acid
reaction medium to produce a gaseous admixture of
chlorine dioxide and chlorine therefrom, selectively
2 1 8928~
dissolving chlorine dioxide from the gaseous admixture to
form an aqueous solution of the chlorine dioxide
contained in the gaseous mixture contaminated by
codissolved chlorine and residual chlorine gas,
contacting the aqueous solution of chlorine dioxide
contaminated with codissolved chlorine with hydrogen
peroxide to reduce the codissolved chlorine, and reacting
the residual chlorine gas with hydrogen to form hydrogen
chloride, which is recycled to the aqueous acid reaction
medium to provide hydrogen chloride feed thereto.
In order to obtain a high efficiency of chlorine
dioxide production in the chlorine dioxide generation
step and thereby ensure a high purity of chlorine
dioxide, preferably greater than about 95% pure with the
balance being essentially chlorine, the molar ratio of
the concentration of chlorate ions to chloride ions in
the reaction medium needs to be maintained as high as
possible. This ratio, may be maximized by adding agents
depressing the solubility of NaCl, for example, highly
soluble, inert sodium salts, such as sodium nitrate or
sodium perchlorate.
In one preferred embodiment of the present
invention, there is provided a process for the production
of chlorine dioxide, which comprises:
feeding sodium chlorate, hydrogen chloride and
hydrogen peroxide to an aqueous and reaction medium in a
reaction zone to produce chlorine dioxide from the
reaction medium,
maintaining the aqueous acid reaction medium at its
boiling point while a subatmospheric pressure is applied
to the reaction zone,
removing chlorine dioxide from the reaction zone in
admixture with steam while precipitating sodium chloride
from the aqueous and reaction medium in the reaction
zone, following saturation after start-up, and
21 89289
removing the precipitated sodium chloride from the
reaction zone and forming the removed sodium chloride
into an aqueous solution thereof.
In this preferred embodiment, the resulting aqueous
solution of sodium chloride then is subjected to an
electrolysis process. Such electrolysis process may
convert such aqueous solution to sodium hydroxide,
hydrogen or chlorine so produced, in which event, the
hydrogen and chlorine so produced are reacted together to
produce hydrogen chloride, the hydrogen chloride is
recycled to the chlorine dioxide generating reaction zone
to provide hydrogen chloride feed thereto and the sodium
chloride is recovered. Alternatively, sodium chloride
may be converted directly to hydrogen chloride and sodium
hydroxide by employing an electrodialysis stack equipped
with bipolar membranes.
Alternatively, the aqueous solution of sodium
chloride may be electrolyzed to form sodium chlorate and
hydrogen, in which event, the sodium chlorate is recycled
to the chlorine dioxide generating reaction zone to
provide sodium chlorate feed thereto. The by-product
hydrogen may be burned to form water.
A catalyst can be used to further improve the purity
of the product produced in this preferred embodiment of
the invention. Such catalyst may comprise palladium
compounds, for example, palladium (II) chloride, and
complexes, particularly palladium coordinated with
ligands, including a combination of palladium (II) with
an amlno acid or an alkali metal salt thereof (as
described in USP 4,154,810), palladium (II) with ~-
diketone (as described in USP 4,051,229) or palladium
(II) with chloride ion (as described in USP 4,178,356).
Generally, the molar ratio of HCl to H202 in the feed
required to produce a given product purity is dependent
on the reaction efficiency in generating chlorine
dioxide. As illustrated graphically in Figure 1, the
21 8928~
lower the efficiency of chlorine dioxide production, the
more H2O2 relative to HCl has to be fed in order to
achieve the desired product purity. It is preferred to
operate at high levels of efficiency of chlorine dioxide
production to minimize the hydrogen peroxide requirement.
While the above-described invention is preferably
carried out at subatmospheric pressure, it is possible to
employ similar chemistry at atmospheric pressure.
In the following description, reference is made to
the accompanying drawings, in which:
Figure 1 is a graphical representation of the
relationship of chlorine dioxide generation efficiency to
the molar ratio of HCl/H2O2 in the feed to the chlorine
dioxide generating process for various chlorine dioxide
product gas purities;
Figure 2 is a schematic flow sheet of a chlorine
dioxide generating process provided in accordance with
one embodiment pf the present invention;
Figure 3 is a schematic flow sheet of a chlorine
dioxide generating process provided in accordance with
another embodiment of the invention; and
Figures 4 and 5 are schematic flow sheets of
chlorine dioxide generating processes provided in
accordance with additional embodiments of the invention,
illustrating an alternative location of introduction of
hydrogen peroxide to that illustrated in Figures 2 and 3
respectively.
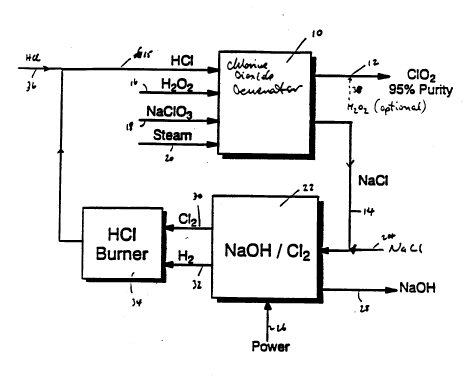
Referring first to Figure 2, there is illustrated
therein integration of chlorine dioxide generation into
a caustic-chlorine cell. As seen therein, a chlorine
dioxide generator 10 produces chlorine dioxide slightly
contaminated with chlorine and crystalline sodium
chloride, which.is removed by line 14. Chlorine dioxide
is formed in gaseous admixture with oxygen and steam and
is formed into an aqueous solution thereof. The chlorine
dioxide is produced from an aqueous acid solution of
21 89289
sodium chlorate to which is fed hydrogen chloride,
hydrogen peroxide and sodium chlorate by lines 15, 16 and
18 respectively. A premixing of hydrogen peroxide with
at least one other feedstock prior to feed to the
chlorine dioxide generator 10 is also possible. Steam is
also added by line 20 to maintain the required reaction
temperature. The aqueous acid reaction medium contained
in the chlorine dioxide generator 10 is maintained at its
boiling point under subatmospheric pressure.
Alternatively, the process may be effected at atmospheric
pressure.
The reaction medium may be maintained at a
temperature of about 40~ to about 80~C, preferably about
60~ to about 80~C, while a subatmospheric pressure of
about 80 to about 300 mm Hg, preferably about 150 to
about 240 mm Hg, is applied to the chlorine dioxide
generator.
The reactants are fed by lines 15, 16 and 18 as
required to maintain steady state conditions of
production of chlorine dioxide in the chlorine dioxide
generator 10 and a catalyst may be present in the
reaction medium in the chlorine dioxide generator 10 to
assist in achieving a high level of purity of chlorine
dioxide product, preferably about 95% or greater, in line
12. The chlorine dioxide reaction generally is carried
out to achieve a purity of chlorine dioxide in the
product gas of at least about 95%. The purity of
chlorine dioxide produced is determined by the efficiency
of the chlorine dioxide producing reaction and by the
molar ratio of HCl to H2O2 fed to the chlorine dioxide
generator 10. As may be seen from Figure 1, the lower
the efficiency of chlorine dioxide production, the more
H2O2 relative to HCl is required to achieve a desired
level of purity.
In general, the efficiency chlorine dioxide
production is at least about 90%, preferably at least
21 8928~
about 95% and molar ratios of HCl:H202 of about 1:1 to
about 3:1, preferably about 1.5:1 to about 2.5:1 may be
employed. In general, the efficiency of generation of
chlorine dioxide is higher for higher mole ratios of
chlorate ions to chloride ions in the aqueous acid
reaction medium in the chlorine dioxide generator. The
efficiency of generation of chlorine dioxide from the
reaction medium in the chlorine dioxide generator 10 may
be further improved by the addition of a suitable
catalyst, including palladium compounds and complexes.
When added, such catalyst may be present in the aqueous
acid reaction medium in an amount of about 1 mg/L to
about 40 mg/L, preferably about 5 mg/L to about 15 mg/L,
based on Pd++ ions concentration.
The aqueous acid reaction medium in the chlorine
dioxide generator 10 generally has a sodium chlorate
concentration of about 1 to about 7 M, preferably about
5 to about 7 M, maintained by the feed of aqueous sodium
chlorate in line 18, which may have a sodium chlorate
concentration of about 4 to about 8 M, preferably about
5 to about 7 M.
The aqueous acid reaction medium in the chlorine
dioxide generator 10 may have, in the absence of
buffering ions, a total acid normality of about 0.01 to
about 2 N, preferably about 0.05 to about 0.15 N,
maintained by the feed of hydrogen chloride by line 14,
which generally is in the form of hydrochloric acid
having a total acid normality of about 5 to about 12 N,
preferably about 9 to about 12 N.
As indicated above, higher molar ratios of chlorate
ions to chloride ions in the aqueous acid reaction medium
provide higher efficiencies of chlorine dioxide
production, which assists in providing high levels of
purity of chlorine dioxide produced by the process. In
general, the molar ratio of C103-:Cl- may range from about
1:5 to about 7:1, preferably about 4:1 to about 7:1.
21 8~28~
The hydrogen peroxide is fed to the chlorine dioxide
generator 10 generally as an aqueous solution thereof,
generally having concentration of about 10 to about 75
wt%, preferably about 30 to about 50 wt%. Additional
hydrogen peroxide may optionally be added to the product
chlorine dioxide stream in line 12 through line 38 to
improve the purity of the chlorine dioxide product, by
reducing contaminating chlorine contained therein.
The presence of the sodium ions and chloride ions in
the reaction medium causes the formation of sodium
chloride as a by-product of the chlorine dioxide
generating process. The sodium chloride saturates the
aqueous acid reaction medium after initial start-up and
crystallizes from the reaction medium. The crystalline
sodium chloride is removed from the generator 10 by line
14 generally by filtration from the aqueous acid reaction
medium.
The reactions occurring in the chlorine dioxide
generator 10 may be depicted by the equations:
NaCl03 + 2HCl ~ Cl02 + ~Cl2 + NaCl + H20
Cl2 + H202 ~ 2HCl + ~2
The oxygen resulting from the in-situ reduction of
chlorine is vented from the generator 10 with the
chlorine dioxide stream in line 12. The chlorine dioxide
in the product stream in line 12 generally is formed into
an aqueous solution thereof for utilization in bleaching
operations and thereby is separated from the oxygen
contained therein.
The crystalline sodium chloride in line 14 is
forwarded to chlor-alkali cell 22, generally after
formation into an aqueous solution thereof of
concentration about 2 to about 5 M, preferably about 4 to
about 5 M. Some make up sodium chloride may be fed by
line 24 to the sodium chloride feed to the chlor-alkali
cell 22. The chlor-alkali cell 22 may be of conventional
two-compartment cell configuration divided, for example,
21 ~d9289
by a cation exchange membrane or a diaphragm, in which
the aqueous sodium chloride is fed to the anode
compartment while an aqueous electrolyte, generally
sodium hydroxide, is circulated through the cathode
compartment. Water may be added to the cathode
compartment.
The solutions are electrolyzed by the application of
electrical power by line 26 to the anode and cathode of
the chlor-alkali cell 22 resulting in the formation of an
aqueous sodium hydroxide solution, which is removed by
line 28, chlorine and hydrogen, which are removed by line
30 and 32 respectively. Generally, about 2200 kWhrs of
power is used for the electrolysis to produce 1 tonne of
sodium hydroxide (100% basis). Sodium hydroxide
concentration in the product solution is generally about
5% to about 50%, preferably about 30% to about 35%. The
electrolysis may be effected at a temperature of about
80~ to about 100~C, preferably about 85~ to about 95~C.
The reactions occurring in the chlor-alkali cell 22
can be depicted by the equation:
NaCl + H2O ~ NaOH + ~C12 + ~H2
The gaseous by-product chlorine and hydrogen may be
forwarded by lines 30 and 32 to a hydrogen chloride
burner 34 in which the hydrogen and chlorine are reacted
to form hydrogen chloride, which then is recycled to the
chlorine dioxide generator 10 by line 15. As an
alternative to the addition of sodium chloride by line
24, an additional quantity of hydrogen chloride or
hydrochloric acid may be fed by line 36.
By integrating the chlorine dioxide generator 10
with the chlor-alkali cell 22 and by balancing the
various reactant feeds, high purity chlorine dioxide is
produced, substantially uncontaminated with by-product
chlorine, by reduction with a mixture of hydrogen
chloride and hydrogen peroxide while by-product sodium
chloride from the chlorine dioxide generating process is
21 892~9
used to form the hydrogen chloride utilized in the
chlorine dioxide generating process and by-product sodium
hydroxide, a chemical useful in the bleaching process of
the pulp mill.
Turning now to consideration of Figure 3, this
embodiment of the invention shows integration of the
chlorine dioxide generator 10 with a chlorate cell 40.
The chlorine dioxide generator 10 operates in the manner
and under the conditions generally described above with
respect to Figure 2 and hence this description will not
be repeated but rather is incorporated by reference
thereto.
The crystalline sodium chloride in line 14 is made
up into an aqueous solution of sodium chloride, which may
have a concentration of about 2 to about 5 M, preferably
about 4 to about 5 M. The sodium chlorate plant 40 may
be any conventional sodium chlorate plant comprising a
plurality of undivided cells in which sodium chlorate is
formed electrolytically by the application of electrical
power by line 42. Generally, 5000 kWhrs of power per
tonne of NaClO3 is used for the electrolysis to form an
aqueous solution of sodium chlorate concentration about
3 to about 6.5 M, preferably about 4.5 to about 6 M. The
electrolysis may be effected at a temperature of about
60~ to about 100~C, preferably about 75~ to about 85~C.
The reaction occurring in the sodium chlorate plant
40 can be depicted by the equation:
NaCl + 3H2O ~ NaClO3 + 3H2
The hydrogen produced according to this equation is
vented from the sodium chlorate plant 40 by line 44 and
may be burned with air or oxygen to form water for the
process.
The electrolysis in the chlorate plant 40 produces
an aqueous solution of sodium chlorate and sodium
chloride (cell liquor) which is passed by line 46 to a
sodium chlorate crystallizer 48, wherein sodium chlorate
21 89289
is crystallized from the cell liquor by any convenient
procedure. The mother liquor, from the crystallization,
which may contain sodium chlorate in an amount of about
4 to about 7 M, preferably about 5 to about 6 M, and
sodium chloride in an amount of about 1.5 to about 3 M,
is recycled by line 50 to the sodium chlorate plant 40.
The sodium chlorate crystallized in the crystallizer
48 is recycled to the chlorine dioxide generator 10,
after formation into an aqueous solution thereof of
concentration as described above with respect to Figure
3.
By integrating the chlorine dioxide generator 10
with the sodium chlorate plant 40 and by balancing the
various reactant feeds, high purity chlorine dioxide is
produced, substantially uncontaminated with by-product
chlorine, by reduction with a mixture of hydrogen
chloride and hydrogen peroxide while by-product sodium
chloride from the chlorine dioxide generating process is
used to form sodium chlorate reactant for the chlorine
dioxide generating process. Similarly, as in the case of
Figure 2, hydrogen peroxide may optionally be added
through line 52 to the product in order to improve the
chlorine dioxide purity.
Figures 4 and 5 correspond to Figures 2 and 3
respectively but contain a modification related the
addition point of H2O2. In these cases, hydrogen peroxide
is added to the chlorine dioxide product rather than to
the chlorine dioxide generator. In this modified
process, chlorine is produced in significant quantities
from the chlorine dioxide generating reaction medium and,
upon selective dissolution of chlorine dioxide with
water, an aqueous solution of chlorine dioxide with
chlorine codissolved therein is formed in line 12.
Addition of hydrogen peroxide to the chlorine dioxide
solution, following condensation of the steam and
formation of the aqueous solution of chlorine dioxide,
21 8928~
results in reduction of the codissolved chlorine to
provide high purity chlorine dioxide. This modified
process leads to minimization of hydrogen peroxide
consumption by the overall chlorine dioxide generating
process while allowing a highly pure product to be
obtained. A possible drawback of the modification shown
in Figures 4 and 5 is the loss of the part of HCl values
generated in the reaction between chlorine and hydrogen
peroxide.
In the embodiment of Figure 4, the same reference
numbers as are employed in Figure 2 are employed to
designate the same elements. In this case, hydrogen
peroxide is added by line 38 to the chlorine dioxide
product stream in line 12 to remove chlorine contained
therein by reduction. By-product chlorine, coproduced
with chlorine dioxide in the chlorine generator 10, is
forwarded by line 54 to the chlorine feed line 30 to the
HCl burner 34.
Similarly, in the embodiment of Figure 5, the same
reference numerals as are employed in Figure 3 are
employed to designate the same elements. In this case,
hydrogen peroxide is added by line 52 to the chlorine
dioxide product stream in line 12 to remove chlorine
contained therein by reduction. By-product chlorine,
2 5 coproduced with chlorine dioxide in the chlorine dioxide
generator 10, is forwarded by line 56 to an HCl burner
58, for reaction therein with hydrogen produced by the
sodium chlorate plant 40 and forwarded by line 60.
Reaction of hydrogen and chlorine in the HCl burner 58
produces HCl, which is recycled by line 62 to the HCl
feed line 15. In this embodiment of the invention, the
sodium chlorate crystallizer 48 is optional.
In summary of this disclosure, the present invention
provides a novel procedure for the production of high
purity chlorine dioxide using hydrogen peroxide and
hydrogen chloride which produces only useful by-products.
21 89289
14
Modifications are possible within the scope of the
invention.