Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent Application: | (11) CA 2189435 |
|---|---|
| (54) English Title: | POLYETHYLENE GLYCOL DERIVATISED DIHYDRO OR TETRAHYDRO PORPHYRINS, THEIR PREPARATION AND THEIR USE AS TUMOUR LOCALISING, PHOTOSENSITISING COMPOUNDS |
| (54) French Title: | DIHYDRO OU TETRAHYDROPORPHYRINES DERIVEES DU POLYETHYLENEGLYCOL, LEUR PREPARATION ET LEUR UTILISATION EN TANT QUE COMPOSES DE PHOTOSENSIBILISATION SERVANT A LOCALISER DES TUMEURS |
| Status: | Deemed Abandoned and Beyond the Period of Reinstatement - Pending Response to Notice of Disregarded Communication |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | MARKS & CLERK |
| (74) Associate agent: | |
| (45) Issued: | |
| (86) PCT Filing Date: | 1995-05-02 |
| (87) Open to Public Inspection: | 1995-11-09 |
| Availability of licence: | N/A |
| Dedicated to the Public: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | Yes |
|---|---|
| (86) PCT Filing Number: | PCT/GB1995/000998 |
| (87) International Publication Number: | WO 1995029915 |
| (85) National Entry: | 1996-11-01 |
| (30) Application Priority Data: | ||||||
|---|---|---|---|---|---|---|
|
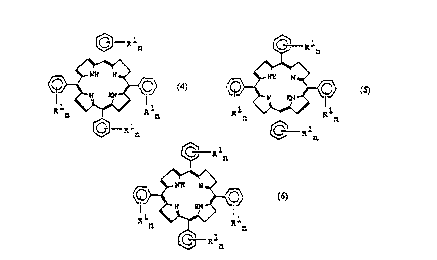
Compounds being polyethylene glycol derivatised, pharmacologically acceptable,
tumour locating dihydro or tetrahydro porphyrins of formulae (4, 5 and 6),
wherein R1n may be -OH but in at least two instances is a chain of formula (-O-
Xp-[CH2CH2O]m-Y)n wherein n = 1 to 3, m = 5 to 250 and p = 0 to 1; the group Y
may be a C1-C12 alkyl or acyl group but preferably a methyl group; the linking
group X may be present or absent, but if present may be of the following
alternative forms: R2 = -(C=O)-(CH2)q-O- where q = 1 to 5; R3 = -CH2-CH(OH)-
(CH2)s-O- where s = 1 to 4; R4 = -(C=O)-(CH2)t-(C=O)-O- where t = 1 to 4.
Improved tumour localisation of polyether-substituted photosensitising
compounds is given leading to their more efficient uptake into tumour cells.
Lower overall doses of drug give an improved side-effect profile of the
treatment especially with regard to skin photosensitisation.
Ces composés sont des dihydro ou tétrahydroporphorines dérivées du polyéthylèneglycol, acceptables sur le plan pharmacologique, servant à localiser des tumeurs et répondant aux formules (4), (5) et (6) dans lesquelles R?1¿¿n? peut représenter -OH mais, dans au moins deux cas, représente une chaîne de formule (-O-X¿p?-[CH¿2?CH¿2?O]¿m?-Y)¿n? dans laquelle n vaut 1 à 3, m vaut 5 à 250 et p vaut 0 à 1; le groupe Y peut représenter un groupe acyle ou alcoyle C¿1-12? mais représente, de préférence, un groupe méthyle; le groupe de liaison X peut être présent ou absent, mais s'il est présent, il peut représenter l'une des formes suivantes: R?2¿ = -(C=O)-(CH¿2?)¿q?-O- où q vaut 1 à 5, R?3¿ = -CH¿2?-CH(OH)-(CH¿2?)¿s?-O- où s vaut 1 à 4, R?4¿ = -(C=O)-(CH¿2?)¿t?-(C=O)-O- où t vaut 1 à 4. Ces composés de photosensibilisation à substitution polyéther permettent de mieux localiser les tumeurs grâce à leur pénétration plus efficace à l'intérieur des cellules tumorales. Des doses globalement inférieures de médicament permettent d'améliorer le profil d'effets secondaires du traitement, notamment en égard à la photosensibilisation de la peau.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
2024-08-01:As part of the Next Generation Patents (NGP) transition, the Canadian Patents Database (CPD) now contains a more detailed Event History, which replicates the Event Log of our new back-office solution.
Please note that "Inactive:" events refers to events no longer in use in our new back-office solution.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Event History , Maintenance Fee and Payment History should be consulted.
| Description | Date |
|---|---|
| Inactive: IPC expired | 2020-01-01 |
| Inactive: IPC from MCD | 2006-03-12 |
| Time Limit for Reversal Expired | 2000-05-02 |
| Application Not Reinstated by Deadline | 2000-05-02 |
| Deemed Abandoned - Failure to Respond to Maintenance Fee Notice | 1999-05-03 |
| Application Published (Open to Public Inspection) | 1995-11-09 |
| Abandonment Date | Reason | Reinstatement Date |
|---|---|---|
| 1999-05-03 |
The last payment was received on 1998-04-30
Note : If the full payment has not been received on or before the date indicated, a further fee may be required which may be one of the following
Please refer to the CIPO Patent Fees web page to see all current fee amounts.
| Fee Type | Anniversary Year | Due Date | Paid Date |
|---|---|---|---|
| Registration of a document | 1996-11-01 | ||
| MF (application, 3rd anniv.) - standard | 03 | 1998-05-04 | 1998-04-30 |
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| SCOTIA HOLDINGS PLC |
| Past Owners on Record |
|---|
| BRENDA E. REYNOLDS |
| DAVID F. HORROBIN |