Une partie des informations de ce site Web a été fournie par des sources externes. Le gouvernement du Canada n'assume aucune responsabilité concernant la précision, l'actualité ou la fiabilité des informations fournies par les sources externes. Les utilisateurs qui désirent employer cette information devraient consulter directement la source des informations. Le contenu fourni par les sources externes n'est pas assujetti aux exigences sur les langues officielles, la protection des renseignements personnels et l'accessibilité.
L'apparition de différences dans le texte et l'image des Revendications et de l'Abrégé dépend du moment auquel le document est publié. Les textes des Revendications et de l'Abrégé sont affichés :
| (12) Demande de brevet: | (11) CA 2189435 |
|---|---|
| (54) Titre français: | DIHYDRO OU TETRAHYDROPORPHYRINES DERIVEES DU POLYETHYLENEGLYCOL, LEUR PREPARATION ET LEUR UTILISATION EN TANT QUE COMPOSES DE PHOTOSENSIBILISATION SERVANT A LOCALISER DES TUMEURS |
| (54) Titre anglais: | POLYETHYLENE GLYCOL DERIVATISED DIHYDRO OR TETRAHYDRO PORPHYRINS, THEIR PREPARATION AND THEIR USE AS TUMOUR LOCALISING, PHOTOSENSITISING COMPOUNDS |
| Statut: | Réputée abandonnée et au-delà du délai pour le rétablissement - en attente de la réponse à l’avis de communication rejetée |
| (51) Classification internationale des brevets (CIB): |
|
|---|---|
| (72) Inventeurs : |
|
| (73) Titulaires : |
|
| (71) Demandeurs : |
|
| (74) Agent: | MARKS & CLERK |
| (74) Co-agent: | |
| (45) Délivré: | |
| (86) Date de dépôt PCT: | 1995-05-02 |
| (87) Mise à la disponibilité du public: | 1995-11-09 |
| Licence disponible: | S.O. |
| Cédé au domaine public: | S.O. |
| (25) Langue des documents déposés: | Anglais |
| Traité de coopération en matière de brevets (PCT): | Oui |
|---|---|
| (86) Numéro de la demande PCT: | PCT/GB1995/000998 |
| (87) Numéro de publication internationale PCT: | WO 1995029915 |
| (85) Entrée nationale: | 1996-11-01 |
| (30) Données de priorité de la demande: | ||||||
|---|---|---|---|---|---|---|
|
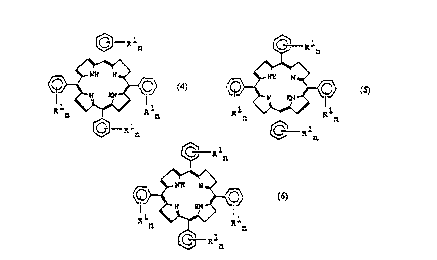
Ces composés sont des dihydro ou tétrahydroporphorines dérivées du polyéthylèneglycol, acceptables sur le plan pharmacologique, servant à localiser des tumeurs et répondant aux formules (4), (5) et (6) dans lesquelles R?1¿¿n? peut représenter -OH mais, dans au moins deux cas, représente une chaîne de formule (-O-X¿p?-[CH¿2?CH¿2?O]¿m?-Y)¿n? dans laquelle n vaut 1 à 3, m vaut 5 à 250 et p vaut 0 à 1; le groupe Y peut représenter un groupe acyle ou alcoyle C¿1-12? mais représente, de préférence, un groupe méthyle; le groupe de liaison X peut être présent ou absent, mais s'il est présent, il peut représenter l'une des formes suivantes: R?2¿ = -(C=O)-(CH¿2?)¿q?-O- où q vaut 1 à 5, R?3¿ = -CH¿2?-CH(OH)-(CH¿2?)¿s?-O- où s vaut 1 à 4, R?4¿ = -(C=O)-(CH¿2?)¿t?-(C=O)-O- où t vaut 1 à 4. Ces composés de photosensibilisation à substitution polyéther permettent de mieux localiser les tumeurs grâce à leur pénétration plus efficace à l'intérieur des cellules tumorales. Des doses globalement inférieures de médicament permettent d'améliorer le profil d'effets secondaires du traitement, notamment en égard à la photosensibilisation de la peau.
Compounds being polyethylene glycol derivatised, pharmacologically acceptable,
tumour locating dihydro or tetrahydro porphyrins of formulae (4, 5 and 6),
wherein R1n may be -OH but in at least two instances is a chain of formula (-O-
Xp-[CH2CH2O]m-Y)n wherein n = 1 to 3, m = 5 to 250 and p = 0 to 1; the group Y
may be a C1-C12 alkyl or acyl group but preferably a methyl group; the linking
group X may be present or absent, but if present may be of the following
alternative forms: R2 = -(C=O)-(CH2)q-O- where q = 1 to 5; R3 = -CH2-CH(OH)-
(CH2)s-O- where s = 1 to 4; R4 = -(C=O)-(CH2)t-(C=O)-O- where t = 1 to 4.
Improved tumour localisation of polyether-substituted photosensitising
compounds is given leading to their more efficient uptake into tumour cells.
Lower overall doses of drug give an improved side-effect profile of the
treatment especially with regard to skin photosensitisation.
Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2024-08-01 : Dans le cadre de la transition vers les Brevets de nouvelle génération (BNG), la base de données sur les brevets canadiens (BDBC) contient désormais un Historique d'événement plus détaillé, qui reproduit le Journal des événements de notre nouvelle solution interne.
Veuillez noter que les événements débutant par « Inactive : » se réfèrent à des événements qui ne sont plus utilisés dans notre nouvelle solution interne.
Pour une meilleure compréhension de l'état de la demande ou brevet qui figure sur cette page, la rubrique Mise en garde , et les descriptions de Brevet , Historique d'événement , Taxes périodiques et Historique des paiements devraient être consultées.
| Description | Date |
|---|---|
| Inactive : CIB expirée | 2020-01-01 |
| Inactive : CIB de MCD | 2006-03-12 |
| Le délai pour l'annulation est expiré | 2000-05-02 |
| Demande non rétablie avant l'échéance | 2000-05-02 |
| Réputée abandonnée - omission de répondre à un avis sur les taxes pour le maintien en état | 1999-05-03 |
| Demande publiée (accessible au public) | 1995-11-09 |
| Date d'abandonnement | Raison | Date de rétablissement |
|---|---|---|
| 1999-05-03 |
Le dernier paiement a été reçu le 1998-04-30
Avis : Si le paiement en totalité n'a pas été reçu au plus tard à la date indiquée, une taxe supplémentaire peut être imposée, soit une des taxes suivantes :
Veuillez vous référer à la page web des taxes sur les brevets de l'OPIC pour voir tous les montants actuels des taxes.
| Type de taxes | Anniversaire | Échéance | Date payée |
|---|---|---|---|
| Enregistrement d'un document | 1996-11-01 | ||
| TM (demande, 3e anniv.) - générale | 03 | 1998-05-04 | 1998-04-30 |
Les titulaires actuels et antérieures au dossier sont affichés en ordre alphabétique.
| Titulaires actuels au dossier |
|---|
| SCOTIA HOLDINGS PLC |
| Titulaires antérieures au dossier |
|---|
| BRENDA E. REYNOLDS |
| DAVID F. HORROBIN |