Note: Descriptions are shown in the official language in which they were submitted.
R'O 95135164 219 014 6 PLT~595106794
Q~EFIN POLYMERIZATION CATALYST
This invention relates to a catalyst for olefin
~ polymerization and copolymerization which comprises a
complex of a transition metal and a cocatalyst mixture
effective to activate the complex.
The catalyst of the invention exhibits very high
activities in ethylene homopolymerization and ethylene-
alpha-olefin copolymerization reactions. Ethylene
copolymers produced with this catalyst have decreased
to melting points compared to the resins produced by
commercial conventional Ziegler catalysts, which indicates
a more homogeneous compositional distribution of the
copolymers. The catalyst can be also used for
polymerization of higher alpha-olefins, in high-temperature
polymerization reactions and for the synthesis of
elastomers.
The invention resides in a catalyst for olefin
polymerization and copolymerization comprising:
(A) a complex of the empirical formula selected from the
group consisting of LMX2, LMXY, L'MX and L2M
wherein M is a transition metal: X is an alkoxy
or aryloxy group or a halogen atom; Y is an alkoxy or
aryloxy group or a halogen atom different from X, or
an oxygen atom; L is a bidentate ligand Z-R-Z or Z-R-
W-R-Z; wherein R is an alkylene, cycloalkylene or
arylene group, unsubstituted or alkyl-substituted, W
is CH2,'Q; S, N-R' or SiR'2: where R' is alkyl or aryl
group, and Z is an oxygen atom, a sulfur atom, an N-H
group or a C(=O)-O group;
L' is a tridentate ligand Z-R"-Z or Z-R"-Z
i i
Z U
where U is different from Z and is an oxygen atom, a
sulfur atom, an N-H or a C(=O)-O- group and R" is an
alkylene, cycloalkylene or arylene group: and
(B) a combination of an alkylaluminum halide and an
organomagnesium compound, wherein the combination is
VYO 95135164 21 g 014 6 pCT/US95/06794
-2
characterized by a molar [A1]:[Mg] ratio of greater
than 1.
The catalyst composition of the invention comprises a ,
transition metal complex (A) and a cocatalyst system
therefor, (B).
The cocatalyst system (B) comprises two components, an
alkylaluminum halide and an organomagnesium compound, which
are used at a molar [A1]:[Mg] ratio of-greater than 1.
The alkylaluminum halide contains alkyl groups of 1 to 8
l0 carbon atoms; and the halide is preferably chlorine.
Preferably, the alkylaluminum halide is a dialkylaluminum
halide; examples include dimethylaluminum chloride,
diethylaluminum chloride, dipropylaluminum chloride,
dibutylaluminum chloride, dipentylaluminum chloride and
dihexylaluminum chloride, in which the alkyl group is
branched or linear. The organomagnesium compound is
preferably a dialkylmagnesium, RiMgR2, in which R1 and RZ
are the same or different and are alkyl groups containing 1
to 10 carbon atoms, preferably 2 to 8 carbon atoms; thus
each of R1 and R2 may be selected from the group conisting
of methyl, ethyl, propyl, isopropyl, butyl, sec-butyl,
tert-butyl, pentyl (branched or linear), hexyl (branched or
linear), or octyl (branched or linear).
The transition metal complex (A) has an empirical
formula selected from the group consisting of
LMX2, LMXY, L'MX and L2M.
In the empirical formula of the complex, M is a
transition metal preferably selected from the group '
consisting of Ti, V, Zr, or Hf.
In the empirical formula of the complex, X is an '
alkoxy or aryloxy group or a halogen atom. The alkoxy
group may have 1 to 6 carbon atoms, and can be selected
from the group consisting of methoxy, ethoxy, propoxy,
WO 95135164 PGTIUS95106794
-3-
isopropoxy, butoxy, i-butoxy, t-butoxy, and the like. The
aryloxy may be a phenoxy, alkylphenoxy, arylphenoxy,
naphthoxy, alkyl naphthoxy or arylnaphthoxy group. The
halogen atom may be florine, chlorine, bromine or iodine,
but preferably is chlorine. Particular examples of X are
the methoxy group, the ethoxy group, the isopropoxy group
or C1; a particular example of Y is an oxygen atom.
In the empirical formula of the complex, Y is an
oxygen atom or an alkoxy or aryloxy group or a halogen atom
different from X.
In the empirical formula of the complex, L is a
bidentate ligand Z-R-Z or Z-R-W-R-Z
wherein R is alkylene group of 2 to 6 carbon
atoms; cycloalkylene group of 5 or 6 carbon atoms; or
unsubstituted or alkyl-substituted arylene group, such
as a phenyl or naphthyl group,
W is CH2, O, S, N-R' or SiR'2
where R' is an alkyl group of 1 to 10 carbon
atoms or an aryl group, and
Z is an oxygen atom, a sulfur atom, an N-H or a
C(=O)-o- group.
In the empirical formula of the complex, L' is a
tridentate ligand
Z-R"-Z or Z-R"-Z
i
Z U
where U is different from Z and is an oxygen
atom, a sulfur atom, an N-H or a C(=0)-O group, and R"
is an alkylene, cycloalkylene or arylene group.
The preferred substitution types in the multidentate
ligands L and L' are such which afford their unimpeded
coordination to the metal atom M. Particular examples of L
219014b
W O 95135164 PGTIUS95106794
-4-
and L' are alkyldioxy, alkyldiamino, alkyldicarboxy,
biaryldioxy, biaryldicarboxy or alkylaminodioxy ligands, in
which the alkyl groups contain 2 to 6 carbon atoms.
Specific ligands L or L' are derived from 1,2,6- ,
hexanetriol; 1,5-hexanediol; diglycolic acid; camphoric
acid; 2-hydroxybenzyl alcohol; 1,1'-bi-2-naphthol: bis(2-
hydroxyphenyl)methane; 2,2'-biphenol; diphenic acid; cis-
1,2-diaminocyclohexane; 1,4,7-triazacyclononane;
diethanolamine; L-glutamic acid: 1-[N,N-bis(2-
hydroxyethyl)amino]-2-propanol.
The reactions to form the transition metal complexes
are undertaken by contacting a compound of the transition
metal with a compound which is a source of L and/or L'.
The transition metal compound can be an alkoxide or halide.
Specific transition metal compounds include titanium
tetraisopropoxide: titanium tetrachloride: zirconium
tetraisopropoxide: vanadyl triisopropoxide. The contact is
undertaken in a polar solvent such as tetrahydrofuran, at
temperatures ranging from 0 to 100°C, under inert
conditions; The recovery of the transition metal complex
comprises removing solvent at temperatures of 20 to 100°C,
preferably 40 to 80°C. Removal of solvent can be by
evaporation.
The complexes containing bidentate and tridentate
liquids are crystalline solids or heavy viscous liquids.
Each complex, depending on the type of the transition
metal, the multidentate ligand and the complex composition,
has a particular color. The complexes were characterized
by their infrared spectra, as shown in the examples below.
The transition metal complexes containing multidentate
ligands can be used in a pure state or supported on inert '
carriers. The transition metal-complex portion of the
catalyst may be activated by the cocatalyst prior to its '
introduction into the polymerization reactor or it may be
activated in the polymerization reactor, whether or not the
catalyst is supported. If supported, the production of the
2190146
WO 95135164 PC17US95106794
-5-
catalyst composition may be formed by various sequences)
of steps. Preferably, the supported catalyst may contain
the transition metal complex, deposited or impregnated on
an inert porous support, which is subsequently contacted
with the cocatalyst, prior to or after introduction into
the reactor.
The carrier materials for the supported catalyst
compositions of the invention are solid, particulate,
porous, preferably inorganic materials which are inert to
the other components of the catalyst composition and to the
other active components of the reaction system. These
carrier materials include inorganic materials, such as
oxides of silicon and/or aluminum. The carrier material is
used in the form of a dry powder having an average particle
size of -1 to 250 microns, preferably from 10 to 150
microns. The carrier material is preferably porous and has
a surface area of preferably at least 50 m2/gm. The
carrier material should be free of absorbed water. Drying
of the carrier material can be effected by heating at 100C
to 1000C, preferably at about 600C. When the carrier is
silica, it is heated at at least 200C, preferably 200C to
850C and most preferably at about 600C.
In the most preferred embodiment, the carrier is
silica which, prior to the use thereof in the first
catalyst synthesis step, has been dehydrated by fluidizing
it with air or nitrogen and heating at about 600C. The
silica of the most preferred embodiment is a high surface
area, amorphous silica with a specific surface area of 300
m2/g
The carrier material is slurried in a polar solvent
and the resulting slurry is contacted with the catalyst
components of the catalyst composition of the invention.
Alpha-olefins are polymerized or copolymerized with
the catalyst according to the present invention by any
suitable process. Such processes include polymerizations
carried out in suspension, in solution or in the gas phase.
R'O 95!35164 219 014 6 PCTlU595106794~
-6
The molecular weight of the polymer may be controlled
in a known manner, e.g., by using hydrogen. The-molecular
weight distribution of the polymers prepared in the
presence of the catalysts of the present invention, as a
expressed by the MFR values, varies from 25 to 80 for LLDPE
products having a density of 0.900 to 0.940 g/cc, and an I2
(melt index) of 0.1 to 100. MFR is defined herein as the
ratio of the high load melt index (HLMI or.I21) divided by
the melt index, i-.e., MFR = I21/I2'
The linear polymers prepared in accordance with the
present invention are homopolymers of alpha-olefins [e. g.
ethylene, propylene, 4-methyl/1-pentene and the like] or
copolymers such as copolymers of ethylene with one or more
C3-C10 alpha-olefins. Thus, copolymers having two
monomeric units are possible as well as terpolymers having
three monomeric units. The copolymers can be partially
crystalline or completely amorphous, depending on
composition. Particular examples of-ethylene copolymers
include ethylene/1-butene copolymers, ethylene/1-hexene
copolymers, ethylene/1-octene copolymers and ethylene/4-
methyl-1-pentene copolymers.
The invention will now be more particularly desrcibed
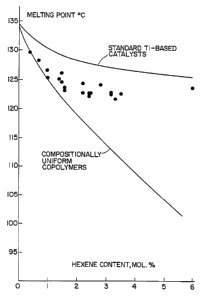
with reference to the examples and the accompanying drawing
which is a graph of melting point temperature (°C) vs. the
1-hexene content for ethylene-1-hexene copolymers prepared
with the catalyst systems of this invention (dots), with
metallocene catalysts (the lower curve) and with
conventional titanium-based catalysts (the upper curve).
3o All catalysts syntheses were carried out in dried '
solvents under nitrogen atmosphere. Polymerization
experiments were carried out in two different stainless- '
steel reactors, with volumes of 0.5-and 1.6 liters,
respectively. Each autoclave was equipped with a stirrer,
a thermocouple and several ports for adding reaction
~fi~Ofi46
W0 95/35164 PCf/US95106794
-7-
components. The reactors were purged with nitrogen flow at
100°C for 1-hour before polymerization experiments. All
solvents and monomers used in polymerization reactions were
deoxygenated and dried prior to use.
EXamolA 1
(A) ~vnthes~s of a 1~1 comblex from 1 2 6-hexanetriol and
titanium tetraisopronoxide.
1,2,6-hexanetriol (0.134 g, 1.0 mmol) was dissolved in
5 cc of dry tetrahydrofuran (THF) at 25°C. Titanium
tetraisopropoxide, Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was added
to the solution, the mixture was stirred at 55°C for 30 min
and then the solvent was removed by evaporation at 55-60°C.
The residue is a white crystalline solid (0.150 g
recovered). The OH stretching band in its IR spectrum (at
ca. 3430 cm 1) is greatly reduced compared to the spectrum
of the original 1,2,6-hexanetriol, and the C-O stretching
band is shifted from 1057 cm 1 in the spectrum of the triol
to 1126 cm 1 in the spectrum of the complex.
(B) Ethvlene-1-hexene Copolvmerization.
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 2.0 cc of
1 mol/1 solution of Al(CH3)2C1 in heptane; (b) 1.0 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C, (c) 0.0020 g (8.4'10 3 mmol)
of the complex of Example 1-A. Ethylene was admitted to
the autoclave to maintain a total pressure of 100 psig.
The polymerization reaction was carried out for 15 min to
yield 25.0 g of ethylene-hexene copolymer containing 4.2
mol.~ of hexene. The resin has an I2 value of 0.12 and the
MFR value of 44.6.
CA 02190146 2004-08-24
- $ ..
Ex~taple 2
(A) a a t'o o su o ed c c a '1
co le f om 5- ex a t t et aiso-
g~"opoxide.
Silica (Davison*955-600, calcined at 600°C in air for
16 h, 0.5 g) was mixed with 1,5-hexanediol (0.118 g, 1.0
mmol) and the mixture was slurried in 3 cc of THF at 25°C.
Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was added to the slurry and
the solvent was evaporated at 55-60'C to yield white
l0 powder.
(B) Ethylene ~-h~~,er~e Copglymerization.
0.5-liter reactor was filled with 200 ec of n-heptane
and 5o cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of Al(C2H5)2C1 in heptane: (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 100'C, (c) 0.0012 g of the catalyst
of Example 2-A (contains 2.4'10 3 mmol Ti). Reactor was
pressurized with hydrogen (10 psi) and ethylene was
admitted to maintain a total pressure of 120 psig. The
polymerization reaction was carried out for 15 min to yield
25.5 g of ethylene-hexene copolymer containing 4.4 mol.°c of
hexane (productivity ca. 22,200 g/g cat). The resin has an
I21 value of 6.4 and MFR of 59.9.
xamp~e 3
(A) S es' o ~ ex f o ac' a
titanium te,~:~a~.soprop~'de.
Diglycolic acid (0.134 g, 1.0 mmol) was dissolved in 3
cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was
added to the solution, the mixture was stirred at 55°C for
30 min and then the solvent was removed by evaporation at
55-60'C. The residue is a white crystalline solid (0.240 g
recovered).
* Trade-mark
WO 95135164 219 014 6 PCT/US95106794
_g_
(B) Ethvlene homopolvmerization. ,
A 0.5-liter reactor was filled with 200 cc of n-
heptane. The catalyst components were added to the
autoclave in the following sequence: (a) 2.0 cc of 1.5
mol/1 solution of A1(C2H5)2C1 in heptane; (b) 1.0 cc of 1.0
mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70C; (c) 0.0020 g (6.7'10 3 mmol)
of the complex of Example 3-A.- Ethylene was admitted to
the autoclave to maintain a total pressure of 100 psig.
The polymerization reaction was carried out for 15 min to
yield 30.0 g of ethylene homopolymer (productivity ca.
11,200 g/g cat).
(C) Ethylene-1-hexene Copolvmerization.-
A 0.5-liter reactor was filled with 200 cc of n-
heptane and 50 cc of 1-hexene. The catalyst components
were added to the autoclave in the following sequence: (a)
2.0 cc of 1.5-mol/1 solution of A1(C2H5)2C1 in heptane; (b)
1.0 cc of 1.0 mol/1 solution of Mg(C4H9)2 in heptane, after
which the autoclave was heated to 70C, (c) 0.0198 g of a
physical mixture prepared from 0.010gof the complex of
Example 3-A and 1.0 g of dry silica used as an inert
diluent (the mixture contains 6.6'10 4 mmol Ti). Ethylene
was admitted to the autoclave to maintain a total pressure
of 100 psig. The polymerization reaction was carried out
for 20 min to yield 12.8 g of ethylene-hexene copolymer
containing 1.0 mol.% of hexene (productivity Ca. 64,600 g/g
cat). The resin has an I21 value of 0.28. The copolymer
has a melting point of 124.7C.
(D) ~hylene-1-hexene Cobolvmerization
A 0.5-liter reactor was filled with 100 cc of 1-
hexene. The catalyst components were added to the
autoclave in the following sequence: (a) 1.0 cc of 1.5
mol/1 solution of A1(C2H5)2C1 in heptane; (b) 0.5 cc of 1.0
mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70C; (c) 0.020 g of a mixture
prepared from 0.010 g of the complex of Example 3-A and 1.0
R'O 95135164 219 014 6 PCflUS95106794
-10-
g of dry silica (6.6'10 4 mmol Ti). Ethylene was admitted
to the autoclave to maintain a total pressure of 100 psig.
The polymerization reaction wascarried outfor 60 min to
yield 13.1 g of amorphous ethylene-hexene elastomer
containing 30.0 mol.% of hexene:
Eaamole 4
(A) Preparation of a supported catalyst containing the 1:1
complex from dialvcolic acid and titanium tetraiso-
propoxide. _
Silica (Davison 955-600, calcined at 600°C in air for
16 h, 1.0 g) was mixed with diglycolic acid (0.067 g, 0.50
mmol) and the mixture was slurriedin 4 cc of THF at 25°C.
Ti(Oi-Pr)4 (0.15 cc, 0.50 mmol) was added to the slurry and
the solvent was evaporated at 55-60°C to yield white
powder.
(B) Ethylene-1-hexene Polymerization.
A 0.5-liter reactor was filled with 200 cc of n-
heptane and 5 cc of 1-hexene. The catalyst components were
added to the autoclave in the following sequence: (a) 1.0
cc of 1.5 mol/1 solution of A1(C2H5)2C1 in heptane: (b) 0.5
cc of 1,0 mol/1 solution of Mg(C4H9)2 in heptane, after
which the autoclave was heated to 100°C; (c) 0.0103 g of
the catalyst of Example 4-A (contains 5.2'10 3 mmol Ti).
Reactor was pressurized with hydrogen (25 psi) and ethylene
was admitted to maintain a total pressure of 143 psig. The
polymerization reaction was carried out for 60 min to yield
14.0 g of high density polyethylene (productivity ca. 2,700
g/mmol Ti) with I2 of 1.1 and MFR of 32.9.
(C) ~hylene-1-hexene Cobolymerization.
A 0.5-liter reactor was filled with 200 cc of n-
heptane and 50 cc of 1-hexene. The catalyst components
were added to the autoclave in the following sequence: (a) '
1.0 cc of 1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b)
0.5 cc of 1.0 mol/1 solution of Mg(C4H9)2 in heptane, after
which the autoclave was heated to 90°C; (c) 0.0023 g of the
WO 95135164 PCTlUS95/06794
-11-
catalyst of Example 4-A (contains 1.15'10 3 mmol Ti).
Reactor was pressurized with hydrogen (10 psi) and ethylene
was admitted to maintain a total pressure of 120 psig. The
polymerization reaction was carried out for 20 min to yield
28.3 g of ethylene-hexene copolymer containing 7.0 mol.% of
hexene (productivity ca. 24,600 g/g cat). The resin has an
121 value of 7.7 and MFR of 42.4.
(D) High-temperature conolymerization.
0.5-liter reactor was filled with 100 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 2.D cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b) 1.0 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 150°C: (c) 0.0198 g of the catalyst
of Example 4-A. Ethylene was admitted to the reactor to
maintain a total pressure of 15D psig. The polymerization
reaction was carried out for 60 min to yield 12.5 g of
ethylene-hexene elastomer containing 16.0 mol.% of hexene.
Example 5
(A) Synthesis of a 2:1 complex from dialycolic acid and
titanium tetraisopro~oxide.
Diglycolic acid (0.268 g, 2.0 mmol) was dissolved in 5
cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was
added to the solution, the mixture was removed by
evaporation at 55-60°C. The residue is a viscous yellow
liquid.
(B) Ethylene-1-hexene Copolymerization.
A 0.5-liter reactor was filled with 200 cc of n-
heptane and 5 cc of 1-hexene. The catalyst components were
added to the autoclave in the following sequence: (a) 1.0
cc of 1.5 mol/1 solution of A1(C2H5)2C1 in heptane: (b) 0.5
cc of 1.0 mol/1 solution of Mg(C4H9
)2 in heptane, after
which the autoclave was heated to 90°C; (c) 0.0005 g of the
complex of Example 5-A (1.6'10 3 mmol Ti). Ethylene was
2190146
W0 95/35164 PCTIUS95/06794
-12
admitted to the autoclave to maintain a total pressure of
l00 psig. The polymerization reaction was carried out for
120 min to yield 10.1 g of ethylene-hexene copolymer
containing 0.6 mol.% if--hexene (productivity ca. 6,300
g/mmol Ti) .
Example 6
(A) ~pthesis of a 2:l complex from camohoric acid and ___
~tanium tetraisobroooxide. ____ _..__ . _
Camphoric acid (0.200 g, 1.0 mmol) was dissolved in 3
cc of THF at 25'C. Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was
added to the solution, the mixture was stirred at 55°C for
30 min and then the solvent was removed by evaporation at
55-60'C. The residue is a heavy yellow liquid (0.168 g
recovered). Its IR spectrum does not contain the O-H
stretching band at ca. 2800 cm 1 and the C=O stretching
band at 1699 cm 1 which are present in the spectrum of the
acid.
(B) Ethylene-1-hexene Copolymerization. _,.,.
A 0.5-liter reactor was filled with 200 cc of n-
heptane and 50 cc of l-hexene. The catalyst components
were added to the autoclave in the following sequence: (a)
1.0 cc of 1.5 mo1/1 solution of A1(C2H5)2C1 in heptane: (b)
0.5 cc of 1.0 mol/1 solution of Mg(C4H9)2 in heptane, after
which the autoclave was heated to 70°C: (c) 0.0008 g of the
complex of Example 6-A (2.2'10 3 mmol Ti). Ethylene was
admitted to the autoclave to maintain a total pressure of
100 psig. The polymerization reaction was carried out for
18 min to yield 12.9 g of ethylene-hexene copolymer
(productivity 16,100 g/g cat; ca. 5,800 g/mmol Ti)
containing 1.4 mol.% of hexene with I21 of 0.34. The '
copolymer has a melting point of 124.7°C.
W095I35164 ~ ECTIUS95106794
-13-
Example 7 _
(A) S,,ynthesis of a 1°1 complex from 2-hvdroxvbenzvl
a~coho~ and titanium tetraisopropoxide.
2-hydroxybenzyl alcohol (0.124 g, 1.0 mmol) was
dissolved in 5 cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc, 1.0
mmol) was added to the solution, the mixture was stirred at
55-°C for 3o min and then the~solvent was removed by
evaporation at 55-60°C. The residue is a dark yellow tar,
easily soluble in toluene (0.238 g recovered).
(B) Ethvlene-1-hexene Copolylnerization.-.. _
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of-A1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 80°C: (c) 0.5 cc of toluene
solution of the complex of Example 7-A containing 1.0'10 3
mmol Ti. Ethylene was admitted to the autoclave to
maintain a total pressure of 100 psig. The polymerization
reaction was carried out for 10 min to yield 15.0 g of
ethylene-hexene copolymer (productivity 15,000 g/mmol Ti)
containing 3.5 mol.% of hexene with I2 of 0.27 and MFR of
42.3.
~RamDle 8 . ._ ...
(A) ~nthesis of a 1:1 complex from 1.1'-bi-2-naphthol and
titanium tatraigOprOpOXlde.
1,1'-bi-2-naphthol (0.290 g, 1.0 mmol) was dissolved
in 30 cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol)
was added to the solution, the mixture was stirred at 55°C
for 2 hours and then the solvent was removed by evaporation
at 55-60°C. The residue is a brown solid. Its IR spectrum
does not contain the OH stretching bands at 3485 and 3402
cm 1 which are present in the spectrum of 1,1'-bi-2-
naphthol.
VVO 95135164 219 014 6 PGTIUS95I06794
-14-
(B) Ethylene-1-hexene Copolvmerization.
0.5-liter reactor was filled with 200 cc of n-heptane ,
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 2.0 cc of
1 mol/1 solution of A1(CH3)2C1 in heptane; (b) 1.0 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C; (c) 1 cc of toluene solution
of the complex of Example 8-A containing 2.31'10 4 mmol Ti.
Ethylene was admitted to the autoclave to maintain a total
pressure of 70 psig. The polymerization reaction was
carried out for 90 min to yield 15.5 g of ethylene-hexene
copolymer containing 6.0 mol.% of hexene (productivity
44,700 g/mmol Ti'h). The resin has an I2 value of 0.99 and
the MFR value of 33.3; its melting point is 123.5°C.
(C) Proovlene polymerization.
0.5-liter reactor was filled with 100 cc of n-heptane.
The catalyst components were added to the autoclave in the
following sequence: (a) 3.5 cc of 1.5 mol/1 solution of
A1(C2H5)2C1 in heptane; (b) 1.8 cc of 1.0 moll solution of
Mg(C4H9)2 in heptane, after which the autoclave was heated
to 70'C; (c) 0.0034 g of the complex from Example 8-A
dissolved in 3 cc of toluene. Propylene was admitted to
the autoclave to maintain a total pressure of 95 psig. The
polymerization reaction was carried out for 120 min to
yield 4.3 g of semi-crystalline, partially isotactic
polypropylene with a melting point of 158.3'C.
(D) Polymerization of 4-methyl-1-pentene. _
A 50-cc glass bottle containing a magnetic stirring
bar was capped with a septum, flushed with nitrogen and
filled with 20 cc of 4-methyl-1-pentene, 3.0 cc of 1.0
mol/1 solution of A1(CH3)2C1 in heptane, and 1.5 cc of 1.0
mol/1 solution of Mg(C4H9)2 in heptane. After heating to
60'C, 0.0033 g of the complex from Example 8-A dissolved in '
1.5 cc oftoluene was added to the bottle and the
polymerization reaction was carried out for 110 min. After
that the contents of the bottle were poured into
R'O 95135164 2 1 9 0 1 4 6 PCTIUS95/06794
-15-
isopropanol and 8.8 g of amorphous poly-4-methyl-1-pentene
Were recovered.
Example 9
~(A) Synthesis of a 1:1 complex from bis(2-hvdroxvohe y,'~1
methane and titanium tetraisoprogoxide.
Bis(2-hydroxyphenyl)methane (0.200 g, 1.0 mmol) was
dissolved in 30 cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc,
1.0 mmol) was added to the solution, the mixture was
stirred at 55°C for 2 hours and then the solvent was
removed by evaporation at 55-60°C. The residue is a glossy
yellow solid.
(B) Ethylene-1-hexene Copolymerization.
0.5-liter was filled with 200 cc of n-heptane and 50
cc of 1-hexene. The catalyst components were added to the
autoclave in the following sequence: (a) 2.0 cc of 1.5
mol/1 solution of A1(C2H5)2C1 in heptane: (b) 1 cc of the
toluene solution of the complex of Example 9-A containing
2.58'10 4 mmol Ti, after which the autoclave was heated to
70°C: (c) 1.0 cc of 10 wt.% solution of Mg(C H ) in
6 13 2
heptane. Ethylene was admitted to the autoclave to
maintain a total pressure of 100 psig. The polymerization
reaction was carried out for 10 min at temperatures 80-85°C
to yield 18.2 g of ethylene-hexene copolymer containing 3.2
mol.% (productivity 423,000 g/mmol Ti'h). The resin has an
I2 value of 0.28 and the MFR value of 40.2; its melting
point is 122.4°C.
Example 10
(A) Preparation of a supported catalyst containing the 1:1
complex from bisl2-hydroxvohenvl)methane and titanium
tetraisopropoxide.
Silica (Davison 955-600, calcined at 600°C in air for
16 h, l.Og) was mixed with the solution of the complex of
Example 8-A containing 0.50 mmol (0.281 g) of the complex
WO 95135164 21 g 014 6 -is- PGT~S95/06794
in 15 cc of THF and the solvent was evaporated at 55-60°C.
(g) F+hvlane-1-hexene Copolymerization.
0.5-liter reactor was filled with 220 cc of n-heptane
and 30 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(CH3)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C; (c) 0.0040 g of the supported
catalyst from Example 10-A containing 0.00156 mmol of the
Ti complex. Ethylene was admitted to the autoclave to
maintain a total pressure of 60psig. The polymerization
reaction was carried out for 55 min to yield 23.1 g of
ethylene-hexene copolymer containing 3.3 mol.% of hexene
(productivity 16;200 g/mmol Ti'h). The melt index of the
resin is 0.21 and the MFR value is 35.6.
(C) High-temperature polymerization. , .
0.5-liter reactor was filled with 150 cc of n-heptane
and 20 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane: (b) 1.0 cc of
0.5 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 150°C: (c) 0.010 g of the complex
of Example 11-A. Ethylene was admitted to the reactor to
maintain a total pressure of 150 psig. The polymerization
reaction was carried out for 60--min to yield 10.7 g of
ethylene-hexene copolymer containing 2.6 mol.% of hexene
with I21 of 18.6 and MFR of 61.5.
Example 11
(A) S_~mthesis of 2'1 complex from 2 2'-bibhenol and
+'+anium tetraisopropoxide. ..
2,2'-biphenol (0.372 g, 2.0 mmol) was dissolved in 10
cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was '
added to the solution, the mixture was stirred at 55°C for
2 hours and then the solvent was removed by evaporation at
55-60°C. The residue is a dark brown solid. Its IA -
WO 95f35164 21 g ~ ~ 4 6 PCT/US95106794
-17-
spectrum does not contain the broad intense OH stretching
' band at 3130 cm 1 which is present in the spectrum of 2,2'-
biphenol.
(B) Ethvlene-1-hexane Cooolvmerization.
0.5-liter reactor was filled with 150 cc of n-heptane
and 10o cc of 1-hexane. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C; (c) 1.5 cc of toluene
solution of the complex of Example 12-A containing 9.60'10
4 mmol Ti. Ethylene was admitted to the autoclave to
maintain a total pressure of 80 psig. The polymerization
reaction was carried out for 60 min to yield 26.5 g of
ethylene-hexane copolymer containing 6.7 mol.% of hexane.
The melt index of the resin is 0.69 and the MFR value is
40.1. The catalyst productivity is 18,100 g/mmol Ti'h.
E~amDle 12
(A) Preparation of pre-polvmerized. supported.
preactivated catalyst containing a 1:1 complex from
2.2'-biphenol and titanium tetraisopropoxide.
Dry silica (0.50 g, Davison 955-600, calcined at 600°C
in air for 16 h and treated with AlEt3 at the Al:Si02 ratio
of 0.72 mmol/g) was slurried in 3 cc of heptane.
A1(C2H5)2C1 (2.18 mmol, 1.5 cc of heptane solution) was
added to the slurry and it was cooled to ca. 0°C.
Mg(C4H9)2 (1 mmol, 1.0 cc of heptane solution) was added
drop-wise to the slurry over a 2-min period. The slurry
was warmed to 25°C and the solution of the complex from
Example 10-A containing 0.074 g of the complex (ca. 0.21
mmol) in 3 cc of toluene was added to the slurry. After
that a stream of ethylene was passed over the stirred
slurry for 3 min to produce a pre-polymerized supported
catalyst.
2190146
O4'O 95135164 PLTIITS95106794
-18-
(B) °+hvlene-1-hexene Copolvmerizat~on._ . __ , _.... ~_.__.__
The 0.5-liter reactor was filled with 200 cc of n-
heptane and 50 cc of 1-hexene. 0.3 cc of 20 wt.% A1(C2H5)3
solution in heptane was added to the mixture, after which
the autoclave was heated to 70'C and 0.0375 g of the pre-
polymerized, supported, preactivated catalyst from Example
13-A was added to the reactor. Ethylene was admitted to
the autoclave to maintain a total pressure of 80 psig. The
polymerization reaction was carried out for 120 min to
yield 53.4 g of high molecular weight ethylene-hexene
copolymer containing 0.7 mol.% of hexene with a melting
point of 128.2°C.
E~amnle 13
(A) S~y!Dthesis of catalvst conta~n~ng,a 1~1 complex srom
~ ~' biphenol and titanium tetrachloride._,__ _
2,2'-biphenol (0.0812 g, 0.436 mmol) was dissolved in
40 cc of THF at 25'C and heated to 55°C. Titanium
tetrachloride, TiCl4 (3.0 cc of 0.145 M solution in
heptane, 0.436 mmol) was added to the solution which was
then stirred at 55°C for 40 min. 2.0 g of silica (Davison
955-600, calcined at 600°C in air for 16. h) was added to
the solution, it was stirred for 1 h and then the solvent
was removed by evaporation at 55-60°C.
(B) ~', ;hyle~~ 1-hexene Coooly~nerization. _ _" _ __,
1.6-liter reactor was filled with 750 cc of n-heptane
and 60 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 3.0 cc of
1 mol/1 solution of A1(CH3)2C1 in heptane; (b) 1.35 cc of
0.74 mol/1 solution of Mg(C4H9)2 in heptane, after which
the autoclave was heated to 80°C; (o) 0.0080 g of catalyst
from Example 14-A containing 1.64'10 3 mmol Ti. Ethylene
was admitted to the autoclave to maintain a total pressure
of 40 psig. The polymerization reaction was carried out
for 60 min to yield 67.8 g of ethylene-hexene copolymer
containing 2.4 mol.% of hexene. The resin has an I21 value
VI'O 95135164 219 014 6 P~~595106794
-19-
of 2.7; its melting point is 122.2°C. The catalyst
productivity is 41,400 g/mmol Ti'h.
$ple 14
(A) Synthesis of catalyst containing a 1:1 complex from
1.1'-bi-2-nabhthol and titanium tetrachloride.
1,1'-bi-naphthol (0.123~g, 0.430 mmol) was dissolved
in 40 cc of THF at 25~C under nitrogen and heated to 55C.
TiCl4 (2.97 cc of 0.145 M solution in heptane, 0.436 mmol)
was added to the solution which was then stirred at 55C
for 1.5 hours. 2.0 g of silica (Davison 955-600, calcined
at 600C in air for 16-h) was added to the solution, it was
stirred for 1 h and then the solvent was removed by
evaporation at 55-60C.
(B) Ethylene-1-hexene Cobolvmerization.
1.6-liter reactor was filled with 750 cc of n-heptane
and 60 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 3.0 cc of
1 mol/1 solution of A1(CH3)2C1 in heptane; (b) 1.35 cc of
0.74 mol/1 solution of Mg(C4Hg)2 in heptane, after which
the autoclave was heated to 80C; (c) 0.0109 g of catalyst
from Example 15-A containing 2.16'10 3 mmol Ti. Ethylene
was admitted to the autoclave to maintain a total pressure
of 40 psig. The polymerization reaction was carried out
for 60 min to yield 82.8 g of ethylene-hexene copolymer
containing 2.4 mol.% of hexene. The resin has an I21 value
of 2.4; its melting point is 122.4C. The catalyst
productivity is 38,300 g/mmol Ti'h.
(C) Ethylene-1-hexene Cotiolymerization.
1.6-liter reactor was filled with 750 cc of n-heptane
and 60 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 3.0 cc of
1 mol/1 solution of A1(CH3)2C1 in heptane; (b) 1.35 cc of
0.74 mol/1 solution of Mg(C4H9)2 in heptane, after which
the autoclave was heated to 80C and pressurized with 5.5
psi of hydrogen; (c) 0.0163 g of the catalyst from Example
WO 95135164 219 014 6 PCTlU595/06794
-20-
15-A containing 3.23'10 3 mmol Ti. Ethylene was admitted
to the autoclave to maintain a total pressure of 41 psig.
The polymerization reaction was carried out for 60 min to
yield 53.4 g of ethylene-hexene copolymer containing 3.2
mol.% of hexene. The resin has an I21 value of 1.25 and
MFR of 29.9: its melting point is 125.7'C. The catalyst
productivity is 16,500 g/mmol Ti'h.
Example 15 . .... _
(A) ynthesis of a 1'1 complex from 1 1'-bi-2-naohthol and
zirconium tetraisopropoxide.
1,1'-bi-naphthol (0.290 g, 1.0 mmol) was dissolved in
10 cc of THF at 25°C. Zirconium tetraisopropoxide (0.30
cc, 1.0 mmol) was added to the solution, the mixture was
stirred at 55°C for 2 hours and then the solvent was
removed by evaporation at 55-60°C. The residue is a pale-
yellow solid.
(B) F+~tlene 1-hexene Cooolymerization._,_., __ __
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 2.0 cc of
1.0 mol/1 solution of A1(CH3)2C1 in heptane: (b) 1.0 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C: (c) 2 cc of toluene solution
of complex of Example 16-A containing 0.0187 mmol Zr.
Ethylene was admitted to the autoclave to maintain a total
pressure of 100 psig. The polymerization reaction was
carried autfor 60 min to yield 13.5 g of high molecular
weight ethylene-hexene copolymer containing 1.0 mol.% of
hexene with a melting point of 126.6°C.
Example 16 y ....... . ".
(A) ynthesis of a 1'1 complex from 2.2'-biphenol and '
v_anadvl triisopropoxide._ _
2,2'-biphenol (0.186 g, 1.0 mmol) was dissolved in 5
cc of THF at 25'C. Vanadyl triisopropoxide, V(=O)(Oi-Pr)3
2190146
R'O 95135164 PGT/US95106794
-21-
(0.242 cc, 1.0 mmol) was added to the solution, the
mixture was stirred at 55C for 2 hours, and then the
solvent was removed by evaporation at 55-60C. The residue
is a black tar readily soluble in toluene.
(B) Ethylene-1-hexene Copolvmerization.
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70'C; (c) 1 cc of toluene solution
of complex of Example 16-A containing 0.0145 mmol V.
Ethylene was admitted to the autoclave to maintain a total
pressure of 100 psig. The polymerization reaction was
carried out for 60 min to yield 8.7 g of high molecular
weight ethylene-hexene copolymer containing 3.1 mol.% of
hexene with a melting point of 125.5C.
(C) Ethylene-1-hexene Copolymerization.
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70'C; (c) 1.325 mmol of 1,1,1-
trichloroethane (a catalyst modifier); (d) 1 cc of toluene
solution of complex of Example 17-A containing 0.0145 mmol
V. Ethylene was admitted to the autoclave to maintain a
total pressure of 100 psig. The polymerization reaction
was carried out for 120 min to yield 23.8 g of amorphous
ethylene-hexene elastomer containing 22.5 mol.% of hexene.
Example 17
(A) Synthesis of a 1:1 complex from cis-1.2-diamino-
cvclohexane and titanium tetraisopropoxide.
1,2-diaminocyclohexane (0.124- g, 1.09 mmol) was
dissolved in 2.5 cc of THF at 25C. Ti(Oi-Pr)4 (0.33 cc,
CA 02190146 2004-08-24
-22-
1.1 mmol) was added to the solution, the mixture was
stirred at 55'C for 30 min and then the solvent was removed
by evaporation at 55-60°C. The residue is a yellow liquid
(0.355 g recovered).
(B) ~~iene-1-hexene CopS~ uerization.
0.5-Liter reactor was filled with 200 cc of n-heptane
and 50 ca of 1-hexene. The catalyst components ware added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane: (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70'C: (c) 0.0008 g of complex of
Example 18-A in 2 cc of toluene containing 2.9'10 3 mmol
Ti. Ethylene was admitted to the autoclave to maintain a
total pressure of 100 psig. The polymerization reaction
was carried out for 25 min to yield 17.1 g of high
molecular weight ethylene-hexene copolymer (yield 21,400
g/g cat) containing 0.9 mol.~ of hexene.
Exampl a ~8
(A) Synthesis Qf a .~1 cgmg ex from 1, 4 . 7-tyiaza-
cyclon2nane and titanium tetraisobrop x~ ide.
1,4,7-triazacyciononane (0.095 g, 0.736 mmol) was
dissolved in 2.5 cc of THF at 25'C. Ti(Oi-Pr)4 (0.22 cc,
0.74 mmol) was added to the solution, the mixture was
stirred at 55'C for 30 min and then the solvent was removed
by evaporation at 55-60'C. The residue is an amorphous
brown-gray solid (0.162 g recovered) readily soluble in
toluene.
(B) F.,~~y,~_e,Be~~ hexene Co~,lo y~,g~,~zation.
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexane. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane: (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70'C: (c) 0.0025 g of complex of
Example 19-A in 2 cc of toluene containing 0.0106 mmol Ti.
~WO 95/35164 ~ ~ 9 ~ ~ 4 6 PCTIUS95106794
-23-
Ethylene was admitted to the autoclave to maintain a total
pressure of 100 psig. The polymerization reaction was
carried out for 12 min to yield 11.1 g ethylene-hexene
copolymer containing 1.6 mol.% of hexene with an I21 of
0.23. The copolymer has a melting point of 123.9°C.
~'Xdmphe 19
(A) Synthesis of a 1 ~ ~ rnmnl ev P,....... ~: _iil-nCJ l m; and
ti tan; gym- tetra i a~Dr01J07'i ria
Diethanolamine (0.234 g, 2.23 mmol) was dissolved in 5
cc of THF at 25°C. Ti(Oi-Pr)4 (0.67 cc, 2.23 mmol) was
added to the solution, the mixture was stirred at 55°C for
30 min, and then the solvent was removed by evaporation at
55-60°C. The residue is an amorphous yellow solid (0.288 g
recovered) readily soluble in toluene.
(B) Ethylene-l-hexene Cooom r;~ w
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of &1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C; (c) 1 cc of toluene solution
containing 7'10 5 g of complex of Example 20-A. Ethylene
was admitted to the autoclave to maintain a total pressure
of 100 psig. The polymerization reaction was carried out
for 120 min to yield 14.7 g ethylene-hexene copolymer
containing 0.6 mol.% of hexene with I21 of 0.12. The
copolymer has a melting point of 127.7°C.
~amele 20
' (A) Svnthe5is Of a 1°l OOmDIeX from T nliii- m;
titanium tetraiannrnnnvir7n
L-glutamic acid (0.147 g, 1.0 mmol) was dissolved in 3
cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was
added to the solution, the mixture was stirred at 55°C for
30 min, and then the,solvent was removed by evaporation at
V1'O 95!35164 219 014 6 PCT~595106794~
-24
55-60'C. The residue is a heavy yellow liquid (0.173 g
recovered).
(B) Fthy~Pne 1 hexane Copolvmerization.
0.5-liter reactor was filled with 200 cc of n-heptane ,
and 50 cc of 1-hexane. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C; (c) 0.0014 g of complex of
Example 21-A. Ethylene was admitted to the autoclave to
maintain a total pressure of 100 psig. The polymerization
reaction was carried out for 60 min to yield 8.2 g of
ethylene-hexane copolymer containing 0.9 mol.% of hexane
with I21 of 0.17.-
Example 21
(A) ~nthesis of a l 1 complex from 1-fN N-bisf2-hvdroxv-
a+-h~.iwminol-2-propanol and titanium tetraiso- ...
propoxide. _ _..__. __ _ .___
1-[N,N-bis(2-hydroxyethyl)amino]-2-propanol (0.164 g,
1.01 mmol) was dissolved in 3 cc of THF at 25'C. Ti(Oi-
Pr)4 (0.30 cc, 1.0 mmol) was added to the solution, the
mixture was stirred-at-55°C for 30 min and then the solvent
was removed by evaporation at 55-60°C. The residue is a
heavy yellow liquid (0.204 g recovered). This synthesis
was repeated using chloroform as a solvent and produced the
same product.
(B) ~'thvlene-1-hexane Cooolvmerization. __"_
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexane. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of °
1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 70°C; (c) 0.0020 g of complex of
Example 22-A. Ethylene was admitted to the autoclave to
maintain a total pressure of 1D0 psig. The polymerization
R'O 95135164 219 014 6 P~~S95106794
-25-
reaction was carried out for 32 min to yield 14.7 g of high
molecular weight ethylene-hexene copolymer containing 0.65
mol.% of hexene.
Example 22
(A) Synthesis of a 1:1 complex from diphenic acid and
titanium tetraiso_propoxide.-
Diphenic acid (0.242 g, 1.01 mmol) was dissolved in 4
cc of THF at 25°C. Ti(Oi-Pr)4 (0.30 cc, 1.0 mmol) was
added to the solution, the mixture was stirred at 55'C for
30 min and then the solvent was removed by evaporation at
55-60°C. The residue is a white crystalline solid.
(B) Ethvlene-1-hexene Copolvmerization.
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. The catalyst components were added
to the autoclave in the following sequence: (a) 1.0 cc of
1.5 mol/1 solution of A1(C2H5)2C1 in heptane; (b) 0.5 cc of
1.0 mol/1 solution of Mg(C4H9)2 in heptane, after which the
autoclave was heated to 90°C: (c) hydrogen, 20 psi: (d) 0.2
cc of toluene solution of the complex of Example 23-A
containing 7.4'10 4 mmol Ti. Ethylene was admitted to the
autoclave to maintain a total pressure of 130 psig. The
polymerization reaction was carried out for 30 min to yield
32.2 g of ethylene-hexene copolymer containing 2.2 mol.% of
hexene (productivity 85,000 g/mmol Ti'h). The resin has an
I2 value of 1.5 and the MFR value of 29.2.
Comparative Example 1.
0.5-liter reactor was filled with 200 cc of n-heptane
and 50 cc of 1-hexene. Methyl-aluminoxane (1 cc of toluene
solution, contains 15.8 wt.% A1) was used a cocatalyst.
The autoclave was heated to 70°C and the toluene solution
of the complex of Example 8-A containing 0.0069 mmol Ti was
added to the reactor. Ethylene was admitted to the
autoclave to maintain a total pressure of-180 psig. The
polymerization reaction was carried out for 50 min to yield
WO 95135164 219 014 6 -2 6- P~~S95/06794
6.0 g ethylene-hexene copolymer containing 6.0 mol.% of
hexene with the melting point of 123.9'C. The catalyst
productivity is 1040 g/mmol Ti'h, which is ca. 40 times
lower than in the case of the same Ti complex activated by
a combination of dialkyl-aluminum chloride and
dialkylmagnesium (Example 8-B).
Comparative Examples 2-7.
A series of ethylene polymerization experiments with
the complexes of Example 7, Example 8 and Example 11
(ranging in amount from 0.0005 to 0.0012 mmol) were carried
out in which various organometallic compounds were tested
as cocatalysts: A1(C2H5)3, A1(C2H5)2C1, A1(CH3)2C1,
Mg(C4H9), combinations of Mg(C4H9) with A1(C2H5)2F and with
A1(C2H5)20C2H5. None of these organometallic compounds,
when used alone, or the said combinations, could activate
the Ti complexes for ethylene polymerization.
The Figure shows a relation between the melting points
of ethylene-hexene copolymers prepared with the catalysts
of-this application as a function of their hexene content.
The figure contains two lines. The upper line gives the
melting points of the copolymers prepared with a typical
supported titanium-based polymerization catalyst using
A1(C2H5)3 as a cocatalyst. The melting points of these
copolymers decrease only slightly with an increase of their
hexene content. The lower curve gives the melting points
of ethylene copolymers prepared with metallocene catalysts
activated by methylaluminoxane, such copolymers have
uniform compositional distributions. The data for the
copolymers prepared with the catalysts of this invention
(points) show that their melting points are uniformly lower '
than those for the copolymers the same hexene content
prepared with common titanium-based polymerization
catalysts. This difference indicates that the ethylene
copolymers prepared with the catalyst systems of this
invention have quite uniform compositional distributions.