Note: Descriptions are shown in the official language in which they were submitted.
~ Hoechst Ah~iellg~s~ drl HOE ss/F 269 ~ l 9 0 6 9 3 Dr v F
Description
5 Suhstit~ ' benzenedicarboxylic acid diguanides, process for their
,u~:pd~dliu~l,theiruseasa~ediud~e~lordiagnostic,and~ di~.d~e~l
containing them
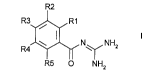
The invention relates to be~ ne~iudl~u,~ylic acid diguanides of the~0 formula I
R2
R3~1~",R1
R4~N ~ NHz
R5 ~ NH2
in which:
one of the radicals R(1), R(2), R(3) and R(4) is
-CO-N=C(NH2)2;
and the other radicals R(1), R(2), R(3) and R(4) ill each case are:
R(1) is hydrogen, alkyl having 1, 2, 3 or4 carbon atoms, F, Cl, Br, I,
-OR(32), -NR(33)R(34) or CF3;
R(32), R(33) and R(34)
independently of one another are hydrogen or alkyl having 1,
2, 3 or 4 carbon atoms;
25 R(2) and R(4)
i"d~pende, Illy of one another are hydrogen, F, Cl, Br, I, OH, -CN,
CF3, -CO-N=C(N~12)2, alkyl having 1, 2, 3, 4, 5, 6, 7 or 8 carbon
atoms, alkenyl ha~ring 2, 3, 4, 5, 6, 7 or 8 carbon atoms or
~(CH2)mR(1 4);
m is zero, 1 ol 2;
R(14) is -(C3-C8)-cycloalkyl or phenyl,
which is unsubstituted or sllhstitl~d by 1 - 3
substituents selected from the group consisting of F
Y ~ ~ 2 ~19~693
and Cl, -CF3, methyl, methoxy and -NR(15)R(16);
R(15) and R(16)
are hydrogen or-CH3;
or~ R(2) and R(4)
independently of one another are pyrrol-1-yl, pyrrol-2-yl or pyrrol-3-
yl, each of which is uns~ Ih~titl It.od or sl Ih~titllt~d by 1 - 4 substituents
selected from the group consisting of F, Cl, Br, I, -CN, (C2-C8)-
alkanoyl, (C2-C8)-alkoxycarbonyl, formyl, carboxyl, -CF3, methyl,
1 0 methoxy;
or
R(2) and R(4)
i"depellde~ y of one another are R(22)-SO2-,
R(23)R(24)N-CO-, R(28)-CO or R(29)R(30)N-S02;
R(22) and R(28)
independertly of one another are methyl or -CF3;
R(23), R(24), R(29) and R(30)
i, Idepellde, ILly of one another are hydrogen or methyl;
20 or
R(2) and R(4)
i"d~ lde~l~ly of one another are -OR(35) or -NR(35)R(36);
R(35) and R(36)
independently of one another are hydrogen or alkyl having 1,
2, 3, 4, 5 or 6 carbon atoms;
or
R(35) and R(36)
together are 4 - 7 methylene groups, of which a CH2 group
can be replaced by oxygen, -S-, -NH-, -NCH3 or-N-benzyl;
R(3) is hydrogen, -SR(Z5), -OR(25), -NR(25)R(26), -CR(25)R(26)R(27);
R(25) is hydrogen, alkyl having 1, 2, 3, 4, 5, 6, 7 or 8 carbon atoms
or phenyl,
_ . . , _ .. _ _ . . _ _ . . . . . . .
21 90693
which is unsllhstitl,tP~I or ~,lhstitlltpd by 1 - 3
substituents selected from the group consisting of F,
Cl, CF3, CH3, methoxy, hydroxyl, amino, methylamino
and dimethylamino;
5 or
R(25) is-(C~-Cg)-heteroaryl,
which is urlcl IhCtitl l'~d or sl Ihstitllt~d by 1 - 3
substituents selected from the group consisting of F,
Cl, CF3, CH3, methoxy, hydroxyl, amino, methylamino
and dimethylamino;
R(26) and R(27)
i"d~pt:~)de, ILIy of one another are defined as R(25) or are
hydrogen or alkyl having 1, 2, 3, 4, 5, 6, 7 or 8 carbon atoms;
R(5) is alkyl having 1, 2, 3 or 4 carbon atoms, F, Cl, Br, I, X-(CH2)y -CF3
or phenyl,
which is unsllhstitlltpd or sllhstitlltpd by 1 - 3 substituents
selected from the group consisting of F and Cl, -CF3, methyl,
methoxy and -NR(6)R(7);
R(6) and R~7)
indt:pelldt~ ly of one another are hydrogen or -CH3;
X is a bond or oxygen;
y is zero, 1 or 2;
and their pllal l l ,ac~utically tolerable salts.
Preferred compounds of the formula I are those in which:
one of the radicals R(1), R(2), R(3) and R(4) is
-co-N=c(NH2)2;
and the other radicals R(1), R(2), R(3) and R(4) in each case are:
R(1) is hydrogen, alkyl having 1, 2, 3 or4 carbon atoms, F, Cl, Br, -OR(32),
-NR(33)R(34) or CF3;
R(32), R(33) and R(34)
~ ~ 4 2190693
independently of one another are hydrogen or methyl;
R(2) and R(4)
independently of one another are hydrogen, F, Cl, Br, I, OH, CF3,
-CO-N=(C(NH2)2, alkyl having 1, 2, 3 or 4 carbon atoms, alkenyl
having 2, 3 or 4 carbon atoms or -(CH2)mR(14);
m is zero, 1 Ol 2;
R(14) is-(C3-C6)-cycloalkylorphenyl,
which is unsl Ihctitl ~tPd or c, IhCtitlltPd by 1 - 2
substituents selected from the group consisting of F
and Cl, -CF3, methyl and methoxy;
or
R(2) and R(4)
il,dt:~ellde, Illy of one another are pyrrol-1-yl, pynrol-2-yl or pyrrol-3-
yl, which is unsl Ihstitl 1 -' or sllhctitl ~tPd by 1 - 2 substituents
selected from the yroup consisting of F, Cl, Br, I, -CN, (C2-Cs)-
alkanoyl, (C2-Cs)-alkoxycarbonyl, formyl, carboxy, -CF3 and methyl;
or
R(2) and R(4)
independently of one another are R(22)-SO2-, R(28)-CO)- or
R(29)R(30)N-SO2;
R(22) and R(28)
i"depende,lLly of one another are methyl or -CF3;
R(29) and F~(30)
i"dt:~ellder,lly of one another are hydrogen or methyl;
or
R(2) and R(4)
independently of one another are -OR(35) or -NR(35)R(36);
R(35) and R(36)
independently of one another are hydrogen, methyl or ethyl;
or
~ ~ 5 2190693
R(35) and R(36)
together are 4 - 5 methylene groups, of which a CH2 group
can be replaced by oxygen, -S-, -NH- or -NCH3;
R(3) is hydrogen, -SR(25), -OR(25), -NR(25)R(26), -CR(25)R(26)R(27);
R(25) is hydrogen, alkyl having 1, 2, 3 or 4 carbon atoms or phenyl,
which is unsl Ihstitl ItPd or sl Ihstitl It~Pd by 1 - 2
substituents selected from the group consisting of F,
Cl, CF3, CH3, methoxy and dimethylamino;
or
R(25) -is-(C1-Cg~-heteroaryl,
which is unsl Ihstitl 1~ ' or sl Ihstitl ~tPd by 1 - 2
substituents selected from the group consisting of F,
Cl, CF3, CH3, methoxy and dimethylamino;
R(26) and R(27)
i".lepellde, Illy of one another are hydrogen or alkyl having 1,
2, 3 or 4 carhon atoms;
R(5) is alkyl having 1, 2, 3 or 4 carbon atoms, F, Cl, Br, CF3 or phenyl,
which is urlsl ~hstitl ItPd or sllh~tit~ 1' ' by 1 - 2 substituents
selected from the group consisting of F and Cl, -CF3, methyl,
methoxy and dimethylamino;
and their plla,l,,aceutically tolerable salts.
Particularly preferred col npounds of the formula I are those in which:
one of the radicals R(1), R(2), R(3) and R(4) is
-CO-N=C(NH2)2;
and the other radicals R~(1), R(2), R(3) and R(4) in each case are:
R(1) is hydrogen, alkyl having 1, 2, 3 or4 carbon atoms, F, Cl, -OR(32),
-NR(33)R(34) or CF3;
R(32), R(33) and R(34)
independently of one another are hydrogen or methyl;
R(2) and R(4)
i"d~pellder,lly of one another are hydrogen, F, Cl, OH, CF3,
~ 6 2~906~3
-CO-N=C(NH2)2, alkyl having 1, 2, 3 or 4 carbon atoms or pyrrol-1-
yl,
which is unsl Ihstitl It~d or c, Ihctitl ItPd by 1 - 2 substituents selected
from the group collsisting of F, Cl, Br, I, -CN, (C2-Cs)-alkanoyl,
(C2-Cs)-alkoxycarl~onyl, fommyl, carboxyl, -CF3 and methyl;
or
R(2) and R(4)
i"depender,~ly of one another are R(22)-SO2-;
R(22) is methyl or -CF3;
10 or
R(2) and R(4)
i"depellde"Lly of cne another are -OR(35) or -NR(35)R(36);
R(35) and R(36)
independer tly of one another are hydrogen, methyl or ethyl;
R(3) is hydrogen, -SR(25), -OR(25), -NR(25)R(26), -CR(25)R(26)R(27);
R(25) is hydrogen, alkyl having 1, 2 or 3 carbon atoms or phenyl,
which is uns~ Ihstiti ll_~ or sl Ihstitllt~ by a substituent
selected from the group consisting of F, Cl, CF3 and CH3;
or
R(25) is -(C1-Cg)heteroaryl,
which is unsl Ihstitl It~d or sl Ihstitl l'-d by a substituent
selected from the group consisting of F, Cl, CF3 and CH3;
R(26) and R(27)
i"depellde"lly of one another are hydrogen or methyl;
R(5) is alkyl having 1, 2, 3 or 4 carbon atoms, F, Cl, CF3 or phenyl,
which is unsllhstitll~ -' or Cllhctitllt~d by 1 - 2 substituents
selected from the group consisting of F and Cl, -CF3, methyl
and methoxy;
and their pha,l, ,ac~utically tolerable salts.
Very particularly preferred compounds of the fommula I are those in which:
~ 7 2190693
one of the radicals R(1), R(2), R(3) and R(4) is
-CO-N=C(NH2)2;
and the other radicals R(1), R(2), R(3) and R(4) ill each case are:
R(1 ) is hydrogen, alkyl having 1, 2, 3 or 4 carbon atoms, F, Cl, CF3 or
methoxy;
R(2) and R(4)
independently of one another are hydrogen, OH, CF3, -CO-N =
C(NH2)2, alkyl ha\ling 1, 2, 3 or 4 carbon atoms or pyrrol-1-yl,
which is urlcl Ihstitl 1'-1 or .sl IhCtitl ItPd by 1 - 2 substituents
selected from the group consisting of F, Cl, Br, I, -CN, (C2-
Cs)-alkano~l, (C2-Cs)- alkoxycarbonyl, formyl, carboxyl, -CF3
and methyl;
R(3) is hydrogen, -OR(25) or-CR(25)R(26)R(27);
R(25) is hydrogen, alkyl having 1, 2 or 3 carbon atoms or phenyl,
whicl1 is unsl Ihctitl Itpd or s~ Ihstitl Itpd by a substituent
selected from the group consisting of F, Cl, CF3 and
CH3;
or
R(25) is-(C1-C5)-heteroaryl,
which is urlc~ Ihstitlltpd or sl Ihstitl 1' ' by a substituent
selected from the group consisting of F, Cl, CF3 and CH3
R(26) and R(27)
i"d~pel1de, Illy of one another are hydrogen or methyl;
R(5) is alkyl having 1, 2, 3 or 4 carbon atoms, F, Cl, CF3 or phenyl,
which is urlsllhstitlltPd or sllhstitlltPd by 1 - 2 substituents
from the group consisting of F and Cl, -CF3 and methyl;
and their pha""aceutically tolerable salts.
The desiy"dLed alkyl radicals can be either straight-chain or branched.
(C1-Cg)-Heteroaryl is understood as meaning radicals which are derived
from phenyl or naphthyl, in which one or more CH groups are replaced by
~ ~ 8 219~693
N and/or in which at least two adjacent CH groups (with formation of a five-
"":",L,e,~d aromatic ring) are replaced by S, NH or O. In addition, one or
both atoms of the condensation site of bicyclic radicals (as in indolizinyl)
can also be N atoms.
Heteroaryl is, in particulalr, furanyl, thienyl, pyrrolyl, imidazolyl, pyrazolyl,
triazolyl, tetrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, pyridyl,
pyrazinyl, pyrimidinyl, pyridazinyl, indolyl, indazolyl, quinolyl, isoquinolyl,
pl~ dld~illyll quinoxalinyl, quinazolinyl, cinnolinyl.
1û
If one of the substituents R(1) to R(5) contains one or more centers of
asymmetry, these can be present independently of one another in either
the S or R configuration. The compounds can be present as optical
isomers, as ~id~ o",er~, as IdC~llldL~5 or as mixtures thereof.
The invention furthemmore relates to a process for the p,~,d,dlioll of the
compounds 1, which comprises reacting compounds of the formula ll
R(2~)
R(3~) ~ R(1~)
O ~ L
R(4~)
R(5~)
in which R(1') to R(5') have the meanings indicated above for R(1) to R(5),
25 but of which at least one of the substituents R(1') to R(5') is the COL group marked, and in which L is a leaving group which can be easily
nucl~ ' 'Iy substituted,
with guanidine.
30 The activated acid derivatives of the formula ll, in which L is an alkoxy
group, preferably a methoxy group or phenoxy group, a phenylthio,
methylthio or 2-pyridylthic group, or a nitrogen he~erocycle, preferably 1-
~ ~J 21~693
=~ g
imidazolyl, are advantageously obtained in a manner known per se fromthe underlying carbonyl chlorides (formula ll, L = Cl), which for their part
can in turn be prepared in a manner known per se from the underlying
carboxylic acids (fommula ll, L = OH), for example using thionyl chloride. In
5 addition to the carbonyl chlorides of the fommula ll (L = Cl) further activated
acid derivatives of the formula ll can be prepared in a manner known per
se directly from the underlying bel~el1e~i~dl ~u~ylic acid derivatives
(formula ll, L = OH), such as, for example, the methyl esters of the formula
Il where L = OCH3 by treating with gaseous HCI in methanol, the
10 i" l ' ';c'e~ of the formula ll by treating with carbon~ lidd~Ul~ [L = 1-
lmidazolyl, Staab, Angew. Chem Int. Ed. Engl. 1,351-307 (1902)], the
mixed anhydrides ll with Cl-COOC2H5 or tosyl chloride in the presence of
triethylamine in an inert solvent, and also the activation of
benzenedi~;d, ~o~ylk, acids using dicyclohexylcarl,odii"~ide (DCC) or using
0-[(cyano)ethoxycarbonyl)methylene)amino]-1,1,3,3-tetramethyluronium
tetrafluoroborate] ("TOTU") [Pl~ceedi"!Js of the 21st European Peptide
Symposium, Peptides 1990, Editors E. Giralt and D. Andreu, Escom,
Leiden, 1991].Anumberofsuitablemethodsforthep,t:pa,dli~.l,of
activated carboxylic acid derivatives of the formula ll are indicated under
20 details of source literature in J. March, Advanced Organic Chemistry, Third
Edition (John Vviley & Sons, 1985), p. 350.
The reaction of an activated carboxylic acid derivative of the formula I with
guanidine is carried out in a manner known per se in a protic or aprotic
polar but inert organic solvent. In the case of the reaction of the methyl
25 be"~t:"edica,~oxylates (Il, L = OMe) with guanidine, methanol, isop,upa~
or THF between 20~C and the boiling points of these solvents have proven
suitable here. Most reactions of compounds ll with salt-free guanidine were
advantageously carried out in inert solvents such as THF,
dimethoxyethane, dioxane or isopropanol However, water can also be
30 used as a solvent
If L = Cl, the reaction is aclvantageously carried out with addition of an acid
~ 21 90693
scavenger, e.g. in the form of excess guanidine to bind the hydrohalic acid.
The introduction of the compounds sl IhCtitl ItPIi in the phenyl moiety by
5 sulfur, oxygen or nitrogen nucleophiles is carried out by methods known
from the literature for the nucleophilic sl Ihstitl Itinl1 of derivatives of dialkyl
b~ edi~,dl ~r xylates. In this sl Ih~Ctitl Ition, suitable leaving groups on thebe,~ edi~,a,L,oxylic acid derivative have proven to be halides and
triflu~,ul"~Ll,al1esulfonates. The reaction is advantageously carried out in a
10 dipolar aprotic solvent, such as DMF or TMU, at a temperature from 0~C up
to the boiling point of the solvent, preferably from 80~C up to the boiling
point of the solvent. The acid scavenger used is advantageously an alkali
metal or alkaline earth metal salt with an anion of high basicity and low
nucleophilicity, for example K2CO3 or CsC03.
The alkyl or aryl substituents are introduced by methods known from theliterature for the palladiul-n-mediated cross-coupling of aryl halides with, forexample, u, ydl lO~il IU compounds, organu~ld, ll Idl 1~5, organoboronic acids
or organoboranes.
In general, b~ e~i~,dlL,u,~ylic acid diguanides I are weak bases and can
bind acid with formation of salts. Possible acid addition salts are salts of allpl1ar,l~olo~ ly tolerable acids, for example halides, in particular
hy~u~,lllolides, ascoiL,dl~:j, lactates, sulfates, citrates, tartrates, acetates,
25 phosphates, methylsl 1'' ldL~s and p-toluenesulfonates.
The Offenlegungsschrift WO 94/26709 describes be~ ledica,~u,~ylic acid
diguanides in which, hov/ever, none of the compounds have one of the
substituents R(5) claimed here, which corresponds to the R(3) of this
30 Offenlegungsschrift.
Surprisingly, it has been found that the introduction of certain substituents
11 21 9Q693
R(1) and/or R(5) ~iy~iriucllllly increases the activity of the compounds and
moreover positively affer~ts their pl1d,-,,dcuhi,,etics.
On account of their pl~dl " l~colùy;~l properties, the compounds I are
5 outsldl ,di"y'y suitable as antiarrhythmic pl1arl"~cel Itir,~lc having a
ca,~iùpru~ i\/e component for infarct prophylaxis and infarct treatment
and for the treatment of angina pectoris, where they also preventively
inhibit or greatly decrease the pathophysiological processes in the
formation of is~l,e",ic~'ly induced damage, in particular in the induction of
1 û is ~ " li~lly induced cardiac arrythmias. Because ûf their protective
actions against pdll loluyiudl hypoxic and ischemic situations, the
compounds of the formula I according to the invention can be used, as a
result of inhibition of the cellular Na+/H+ exchange ",eul,a"i:,"" as
pharl"~ce, Itir,~lc for the treatment of all acute or chronic damage caused
15 by ischemia or illnesses primarily or secul~dd, ily induced thereby. This
relates to their use as ",~.li.,d",el,L~. for surgicai interventions, e.g. in organ
~Idll:,pldllldlioll, where the compounds can be used both forthe protection
of the organs in the donor before and during removal, for the protection of
removed organs, for example during treatment wi~h or storage thereof in
2û physiological bath fluids, and also during transfer to the recipient's body.
The compounds are also useful pl1al",~rielltir.~1c having a protective action
when carrying out anyi~uld~lic surgical interventions, for example on the
heart and also on peripheral vessels. In accû,da"~t: with their protective
action against is~lle",i~:'y induced damage, the compounds are also
25 suitable as plla" "~ , Itir~lc for the treatment of ischemias of the nervous
system, in particular of the CNS, where they are suitable, for example, for
the treatment of stroke or of cerebral edema. Moreover, the compounds of
the formula I according to the invention are also suitable for the treatment
of forms of shock, such as, for example, of allergic, cardiogenic,
3û hypovolemic and bacterial shock.
The compounds of the formula I according to the invention are moreover
~ 21 936~3
12
distinguished by potent ir~hibitory action on the proliferation of cells, for
example fibroblast cell p" ' "rdLioll and the ~,~';' rdliull of vascular smooth
muscle cells. The compounds of the formula I are therefore suitable as
useful therapeutics for illnesses in which cell pr, ' ' dliDIl is a primary or
5 secondary cause, and can therefore be used as d"tid~l,er,,s,~le,l~i,,~,
agents against diabetic late cul~ s, Cdl~ ollld~Us disorders,
fibrotic disorders such as pulmonary fibrosis, fibrosis of the liver or fibrosisof the kidney, organ h~el 1, uuhi~s and hype, uldsids, in particular in
prostate hyperplasia or prostate hypertrophy.
10 The compounds accordin~ to the invention are effective inhibitors of the
cellular sodium-proton an~tiporter (Na+/H+ ex.,l,allger), which is raised in
numerous disorders (essential hypertension, dl~,e,uscleru~ ,, diabetes etc.)
even in those cells which are easily ~.~ce~ le to measurement, such as,
for example, in erythrocytes, platelets or leucocytes. The compounds
15 according to the invention are therefore suitable as outstanding and simple
scientific tools, for example in their use as ~iay"o~liu~ for the dt~ r" ,i, Idli
and .li,r-r-lllidliull of certain fomms of hjpe,l_"aioll, but also of
dlllt:lUscl~lusis, diabetes, proliferative disorders etc. The compounds of
the formula I are moreover suitable for preventive therapy for the
20 prevention of the genesis of high blood pressure, for example of essential
h~,~el l~l~sion.
Compared with most known compounds, the compounds according to the
invention have a siu~ ,iri~a, I~ly improved water solubility. They are therefore25 ~ ily better suited tû i.v. ddl l lil ~i~ll d~ n.
Compared with the known readily water-soluble compounds, the
compounds according to tl1e invention are distinguished by their better
bioavailability and pharmacokinetics.
PharnlAce~ti~AIc which colltain a compound I can be ad,l,i"i~t~l~d orally,
pa,i "l-r~'ly, intravenously, rectally or by inhalation, the preferred type of
_ _ _ _ .. . . . . . .
13 2~ 906~3
a l" ,i, liall dLiul, being dependent ûn the particular clinical picture ûf the
disûrder. The cûmpûunds I can be used here ûn their ûwn ûr tûgether with
~la""aceutical auxiliaries, for example in veterinary and also in human
medicine.
On the basis of his expe~t knowledge, the person skilled in the art is
familiar with which auxiliaries are suitable for the desired pha""aceutical
formulation. Beside solvents, gelling agents, suppository bases, tablet
auxiliaries and other active compound excipients, dl lliU,-i~dll~,
10 di~per~d"~, emulsifiers, antifoams, flavor corrigents, preservatives,
so'l l~ ~ or colorants, for example, can be used.
For a form for oral ddl "i, ~ dliul1, the active compounds are mixed with the
additives suitable for this purpose, such as excipients, stabilizers or inert
diluents and brought by the customary methods into the suitable
15 ad",i"i:,L,dlion forms, such as tablets, coated tablets, hard gelatin
capsules, aqueous, alcoholic or oily solutions. Inert excipients which can
be used are, for example, gum arabic, magnesia, magnesium carbonate,
potassium phosphate, lactose, glucose or starch, in particular corn starch.
P~t:pa"lliol1 can in this case take place either as dry or as moist granules.
20 Suitable oily excipients ol solvents are, for example, vegetable or animal
oils, such as sunflower oil or fish liver oil.
For subcutaneous or intravenous a l",i"i~lldlioll, lhe active compounds are
brought into solution, suspension or emulsion, if desired with the
25 substances customary fol this purpose, such as solubilizers, emulsifiers or
other auxiliaries. Possible solvents are, for example: water, physiological
saline solution or alcohols, e.g. ethanol, propanol, glycerol, a~-lilioll Iy
also sugar solutions such as glucose or mannitol solutions, or alternatively
a mixture of the various solvents "~e"li~l~ed.
Pl ,a" "ac~utical fonmulations suitable for a-ll l lil li:,~l d~ in the form of
aerosols or sprays are, for example, solutions, suspensions or emulsions
.. . ... . .. ... _ .. . ...... .. .. .. . .
~ 14 21 ~0693
of the active compound ~f the formula I in a pharmaceutically d~ JI.
solvent, such as, in particular, ethanol or water, or a mixture of such
solvents. If required, the l~ormulation can also contain still other
pharmaceutical auxiliarie~ such as surfactants, emulsifiers and stabilizers,
5 as well as a propellant. Such a preparation custolnarily contains the active
compound in a concentration from dp~luxillld~ly 0.1 to 10, in particular
from app,uxi",dl~ly 0.3 to 3~/O by weight.
The dose of the active compound of the formula I to be a~" ,i"i~ d and
1û the frequency of ad",i"i:,l,dliol1 depend on the potency and duration of
action of the compounds used; addi~iul1~lly also on the measure and
severity of the illness to be treated and on the sex, age, weight and
individual responsiveness of the mammal to be treated.
15 On average, the daily dose of a compound of the formula I in the case of a
patient weighing d,upru,~i, lldL~:ly 75 kg is at least 0.001 mg/kg of body
weight, preferably at least 0.01 mg/kg of body weight, up to at most
10 mg/kg of body weight, preferably to at most 1 mg/kg of body weight. In
acute episodes of the illness, for example i"""~ y affer suffering a
20 cardiac infarct, still higher and especially more frequent doses may also be
necessary, e.g. up to 4 individual doses per day. In particular when
ad",i, li~tt:l~d i.v., for example in the case of an infarct patient in the
intensive care unit, up to 100 mg per day may be necessary.
25 List of abbreviations:
AIBN a,~-azo-bis-isobutyronitrile
Bn benzyl
Brine saturated aqueous NaCI solution
CH2CI2 .liul ,loru, l l~ll ,ane
DCI desu",~i~l1 chemical ionization
DIP diisopropyl ether
DMA dimethyldce:ldl,,ide
2 t 90693
~ 15
DME dimethoxyethane
DMF N,N-dimeth~lru,l"d",ide
EA ethyl aceta~e (EtOAc)
El electron impact
eq equivalent
ES electrospray ionisation
Et ethyl
FAB fast atom bol"L,a,.l"le"L
HEP n-heptane
HOAc acetic acid
Me methyl
MeOH methanol
mp melting pOillt
MTB methyl tertiary-butyl ether
NBS N-bromosuccinimide
NMP N-methylpyrrolidone
RT room temperature
THF tetrahydrofuran
TMU N,N,N',N'-tetramethylurea
Tol toluene
CNS central nervous system
E~,e, i" ,t:"Ldl section
25 General procedure for the plt:pdl dLioll of ber,,ene~i.,arL,oxylic acid
diguanides (I) from dialkyl benzene.li~alL,oxylates (Il, L = O-alkyl)
5 mmol of the dialkyl ben.~ne~i.,dl LJo~ylate of the fommula (Il) and 50 mmol
of guanidine (free base) are dissolved in 5 ml of isup,upanol and boiled
30 under reflux (typical reaction time 5 minutes to 5 h) until reaction is
complete (thin-layer checking). The mixture is then diluted with 150 ml of
water and the product is lFiltered off with suction. If applup, i ' , it is
~;lllull,dLu~,dphed on silica gel using a suitable eluent, e.g. EA/MeOH 5: 1.
~i 21 9~693
16
Example 1: 4-Chloro-5-phenyl;~ophll, ' acid diguanide
O N~NH2
C~ NHz
a) 3-Bromo-2-chloro 5-methylbenzoic acid
25 9 of 2-amino-3-broma-5-methylbenzoic acid are diazotized in 500 ml of
6N aqueous HCI solutiorl at 0~C using 8.3 9 of NaNO2, then the mixture is
stirred at RT for 30 minultes and poured in portions onto a warm solution at
40~C of 22 9 of CuCI in 200 ml of a saturated aqueous HCI solution. The
mixture is stirred at 40-50~C for 20 minutes, and the precipitate is filtered
off with suction, washed with water until it is neutral and dried at 40~C
under reduced pressure. 23.3 9 of pale yellow crystals are obtained,
mp 170- 172~C.
Rf (EA/MeOH 5:1) = 0.511 MS (DCI): 249 (M+H)+
b) 5-Bromo4-cl,lo,u;sùpl,ll, ' acid
99 9 of MgSo4 x 7H2O are dissolved in 600 ml of water, then 20 9 of
3-bromo-2-chloro-5-methylbenzoic acid are added, the mixture is warmed
to 90~C, then 63 9 of KivlnO4 are added in portions at 90-100~C, and the
mixture is stirred under reflux for 2 h. It is then allowed to cool to RT,
saturated aqueous Na2SO3 solution is added dropwise until the violet color
disappears, the mixture is adjusted to pH = 12 with saturated aqueous
Na2CO3 solution and the MnO2 is filtered off with suction. It is washed with
saturated aqueous Na2SO3 solution and with hot water, the filtrate is
adjusted to pH = 1 with aqueous HCI and the precipitate is filtered off with
suction. 13.5 g of a colol less solid are obtained, mp > 275~C.
Rf (DIP/2% HOAc) = 0.18 MS (DCI). 279 (M+H)+
~, 21 90~3
17
c) Dimethyl 5-bromo-4-~;l,lo,uisopl,ll ' '
13.5 9 of 5-bromo-4-,.l,loruisopilll~ ' acid are dissolved in 200 ml of
MeOH, 20 ml of SOCI2 are added dropwise and the mixture is stirred under
reflux for 5 h. The volatile constituents are then removed in vacuo and the
residue is dried in a fine vacuum. 14 9 of colourless crystals are obtained,
mp 99~C MS (DCI): 307 (M+H)+
d) Dimethyl 4-chloro-5-phenyl;;,ophll,dldl~
3.1 9 of dimethyl 5-bromo-4-chloroisophthalate, 1.2 9 of benzeneboronic
acid, 2.1 9 of Na2CO3, 230 mg of Pd(OAc)2 and 500 mg of
triphen~ o~.l ,i"e are stirred under reflux for 6 h in 50 ml of toluene and
10 ml of water. The mixture is allowed to cool to RT, and is diluted with
300 ml of toluene and washed 3 times with 100 ml of a saturated aqueous
Na2CO3 solution each tilne. The organic phase is dried over Na2SO4, the
solvent is removed in vacuo and the residue is then ~ llldlUyld~ ed on
silica gel using EA/HEP 1:4. 1.5 9 of a colorless oil are obtained.
Rf (EA/HEP 1:4) = 0.22 MS (DCI): 305 (M+H)+
e) 4-Chloro-5-phenyli~,o~l,ll -' acid diguanide
2.6 9 of potassium teff-butoxide are dissolved in 50 ml of anhydrous DMF
and treated with 2.6 9 of guanidine hydrochloride. The mixture is stirred at
RT for 1.5 h, 700 mg of dimethyl 4-chloro-5-pherl~li ,opl l~l laldlt: are added
and it is stirred at 1 00'C for 2 h. The mixture is poured onto 1 1 of water,
and it is adjusted to pH = 8 with aqueous NaHCO3 solution and aqueous
HCI solution and then extracted 3 times with 200 ml of EA each time. The
organic phase is dried over Na2SO4 and the solvent is removed in vacuo.
The residue is suspended in 100 ml of water ancl filtered off with suction,
then the precipitate is again suspended in 50 ml of EA and filtered off with
suction. The product is dried in vacuo and 350 mg of colorless crystals are
obtained,
mp 235~C (with decomposition).
Rf (acetone/water 10:1) = 0.063 MS (ES): 359 (M+H)+
' ~ 18 2~ 90693
Fl ld~ co~ data:
Inhibition of the Na+tH+ t:X~I~dl~9el of rabbit erythrocytes
White New Zealand rabbits (Ivanovas) received a standard diet with 2%
5 .;l loltl~,ul for six weeks in order to activate Na+/H+ exchange and thus to
be able to determine the Na+ influx into the erythrocytes via Na+/H+
exchange by flame pllu~u~ , y. The blood was taken from the ear arteries
and rendered incoagulable by means of 25 IU of potassium heparin. A part
of each sample was used for the duplicate d~ r" ,i"dli~ll of the hematocrit
10 by centrifugation. Aliquots of 1 Oû ul in each case were used to measure
the Na+ starting content of the erythrocytes.
In order to detenmine the amiloride-sensitive sodium influx 1 Oû ul of each
blood sample were incubated in each case in 5 ml of a hype,us",olar salt-
sucrose medium (mmol/l: 140 NaCI 3 KCI 15û sucrose û.1 ouabain
2û tris-hydroxymethyla"",~o",t,~l,alle) at pH 7.4 and 37~C. The erythrocytes
were then washed three times with ice-cold MgCI2 ouabain solution
(mmol/l: 112 MgCI2 û.1 ouabain) and hemolyzed in 2.û ml of distilled
water. The intracellular sodium content was dt:L~II ,)i"ecl by flame
2û photometry.
The net Na+ influx was calculated from the difference between sodium
starting values and the sodium content of the erythrocytes after incubation.
The amiloride-inhibitable sodium influx resulted from the difference
25 between the sodium content of the erythrocytes after incubation with and
without amiloride 3 x 1 û4 moUI. This procedure was also used in the case
of the compounds according to the invention.
~ ~ 21 93693
Results
Inhibition of the Na+/H+ exchanger:
Example IC!jo (mmol/l)
1 0.9