Note: Descriptions are shown in the official language in which they were submitted.
~ wo 9513~983 ' - ' 2 1 9 0 8 6 2 ~ ~h~ 4
POST EXPOSURE PREVENTION OF HIV
Technical Fiel~
This inYention relates to preventing the pctAhlishmPnt of HIV in~ection
and/or ~V seroconversion in a human or simian after exposure to Hl~
Several subpopulations of society, notably health care workers, surgical
patients, medical IL~b~dlLI~ , and law ~--rul-~uL~lLL officials are subject to an
enhanced risk of coming into ArrirlPntAI contact with human
immllnnr~PfiriPnry virus (E~V). For instance, there are dozens of
0 documented b~.u.ul. . ~. ~ions in health care workers following accidental
injury from syringes or surgical instruments ~l~YiUUbly used by ~V-
infected individuals ("needle sticks"). W~ile much can be done to reduce the
risk of exposure there remains a need for an effective agent for protecting
such individuals dnd other victims of inadvertant exposure to HlV-positive
5 material from ~bLdbliblliLLg an HIV infection or HIV seroconversion.
Description of Related Art
A small number of antiviral agents, notably AZT (zidovudine) and ddI
~o (dideoxy inosine), have been developed and sho~Yn to have a clinically
si~nifir~nt action in the suppression of AIDS development or reducing viral
loads in ~V-infected individuals. There is some evidence from animal
studies that AZT may alter the patte~n of lentivirus infections if
d prophylactically. i.e. prior to exposure to ~V or SIV(simian
immllnrldPfiriPnrv virus) to delay the onset of ~iraemia and one report (Van
Rompey et al 199~ Antimicrob Agents & Chemotherapy 36 2381-Z386)
suggests that SIV infection was prevented in ~ infant mArArrlllrC with AZT
given from ~ hours before inr~rl~lAtir~n, however the low infectious dose
renders the ci~nifirAnrP of the report hard to assess. A further report
, .. _ ,, . , . , ,, ,, .. . . ,, , . , , , . .,, , _ _ _ _ _
wo ss/32ss3 2 1 9 0 8 6 2 P~_l/DI!,` ~
(Bottiger et al AIDS Res Hum R~t.uvi- UDt~D 1992; 7:1235-1238 suggests that
FLT (3'-fluorothynudine) ~ rl ~ from 8 hours prior to virus
inf~ f if ~n may provide some protection against sIv~ However the majorif~y
of literature references point to an inability to prevent infection w~th SIV
5 with prophylactically, i.e. pre-exposure ~ . l . l . l, "~ Irl ~:d AZT, ddI, ddC, d4T
and PFA (T .~lnrig~rPn et al, Antiviral Chemistry & ~ rl 11~ Ir~ Y 1990;5:299-
306, Bottiger et al, Antiviral f_hemistry & f hrll~ l.rl ~ Y 1991;6:3a7-361,
Lundgren et al, J AII~S 1991;4:498-498) The SIV and H[V-2 infections in
monkeys bear a close, r~rl ~ rP to HIV-1 infections in humars.
I~-U~l lyla~iD" as used in this technical field generally refers to theadl~ iDtla iu~l of an active agent to an at-risk individual in sufficient time to
achieve and maintain effective . -. .. r-~ in the target tissues before the
individual enters the risk ~.vi.u....... ~.~. This is the classic mearing of ulJllyla~s. The position with rev~ard to ~ " " . " ~ l ;.... of
anti-H[V agents is even less ,~ r~- 1- -, y than the above . . rl ~ 1 Ir~l
prophylactic attempts. There are currently 13 published accounts (Niu et al; J
Inf Dis 1993:168:149f~5Cl) of s~3vU~UllVfiDiU~I after accidental exposure to
HIVnolw;ll-cl~...l;..vrapidandsustained~ ,..eaLL.il~DL.c.tiv,lof
20 large doses of AZT. Despite many attempts, p. ~ treatrnent has not
prevented any HIV-1, HIV-2 or SIV infections (Niu et al, ibid). In other
words after extensive research into ~--clP~ ,, r infection ~v~lLtiUl~ and
FDA fnn~i~lPr~tif n of approval criteria (J AIDS 1991;4 513-515), not a single
agent has bePn reported which has proven to prevent the r~l~hl~l 1 l ,Pnt of
2~i ~V infection or S~:lULUllVt~lD;vll in individuals already exposed to HiV. Not
even those ~ r~ agents such as soluble CD4 and heparin sulphate
which act in vitro by preventing virus entering target cells and would thus
W09~132983 ~l q~?~,6,~ p~"~ f~ t
appear to better rAnriiri~SPc for ~lu"l~ylaxi~ than the nllrlP-ci~P analogues,
have proven to have any effect in vivo.
In the ~ il- context (as distinct from ~u~yla~is), European patent
5 application 0 391411 A2 describes a family of nIIrIrncir~P analogues,
induding the active agent defined below, whidh family is imrIir~tPr~ in the
treatment of herpes infections and to some extent exhibits in vitro activity
against H~'' in a already infected cell line. No teaching or ~ V iv,~ . as to
any prophylactic activity is apparent, whidh is in line with classical
'iO P~ C as to the efficacy of mIrIPnci~P ~n Ilnigllpc~ as discussed above.
T..~ I patent application nos. WO 88/08001 and WO 92/06102 extend
to various nlIrlPncii1P analogues of this type and rl . """~ their activity
against r-ctRhlichp~l retroviral and HIV infPrtinnc However, there is no
teadhing or ~ ;"" that any of their rnmrolmrlC can be ~
after exposure to HIV and yet prevent the PCt IhlichmPnt of Hl~,' infection or
~t iu~ullv~:~iull.
Summarv of the invention
20 The present invention provides a method for ,iJ,~v~i,Lii,g the Pct~hIichmpnt Of
~' infection and/or ~t,~ u.ullv~:liull in a human or simian ~
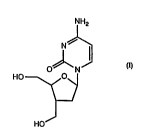
the ~11 l " " ;c~". ~ " of an effective amount of the rnmrol m rl 1-[2',3'-dideoxy-
3'C-~ y~ilu;~yll~Ll~yl)-~-D-erythro-~i-"Lo~ ,u~yl}cytosinehavingthe
formula
WO 9~132983 , ~ 9 0 8 6 2 r~ ~ - 4
,... .,~ t' '
NHz
N~
0~ N
HO ~~J
HOJ
after exposure of said human or simian to E~V. The invention further
provides ~ u~usiLio..~ for use in this method, """1" ;~ an
0 effective amount of the above defined active agent and a ~ r " l ;, f lly
acceptable diluent or carrier therefor.
AlLeL~aLivL-;y exrrPccP~ the invention relates to the use of the above defined
activeagentinthe,..~...,r~ , of a mP~lir~mPnt for~ "~
il ll l to a human or simian to prevent the pct~hlichmpnt of ~v
infection or aL~l U~ llY~l aiUll.
As explained above, not even those prior art antivirals with an Pct~hlichPLi
LlleL~L~ULiC activity against HIV-infected cells or with antiviral effects in
20 . ~V/AIDS patients can prevent irlfection when 1,1. . . ", ;~l~, ~ after exposure
of an individual to HIV, or indeed a monkey to SIV, thus the above defined
property of this particular nllrlPrci~P anomer is quite u~ e.Led.
The method and .UllL~UsiLiu.. of the invention should be contrasted with
such agents as AZT and ddI which at most appear to delay SL~lUL ullv~laiLJlL
until shortly after "treatment" is rr~nrlllrlPr7 and do not prevent the
Pct~hli chm~nt of an E~V infection and Lll LillL~Lely the resulting disease.
~ W0 951329~3 , '~ 2 1 q O 8 6 2 r~ f ~ ~ I
The method and compositions of the present in~rention u~ill be particularlv
useful for individuals at risk of ~rf~iflPnt~l orrllr~nnn~l exposure to ~-
positive material such as the staff or inmates of Pct~hlichmPntc such as
hospitals, prisons and diagnostic or research laboratories. Such
5 Pct~h7ichmPntc can keep supplies of the compositions of the invention for
rapid posL~t,u~u.~ deployment in the event o~ accidental exposure to ~V.
The accidental exposure may comprise a needle stick or other surgical injury
incurred by health care workers but in a wider context may result from any
o inadvertant exposure such as through sexual tr~ncmiccinn, assault, body
fluid splashes, erroneous blood transfusion or reuse of medical ~ ,lf~
An advantage of pn~ Yl ~ a-l .u.LisL- ~ Liu.., as distinct from prophylactic
~l~vt:llLiu~ of infection is that it avOids long term ~ inn with
potentially expensive agents and improves safety. The currently used HIV
5 agents such as AZT and ddI require life long ~ ,1 ", i . ., ~I, .1 l ;. ,,, if
s~lu.u.~v~ .. is to be avoided. ~r~rlitinn~ , p"c~,?,l" ,~", t ~.l " " ";~
can be extended to individuals not belonging to a high-risk 0l ~ 1 if ., . or
subpopulation.
~o Preferably the above defined active agent is a.L.LLLLs~ d as soon as
pr~rtir~hle following the exposure of the human or simian to HIV.
Advantageously a-L ~ isLI c Liull is ~ 1 within about one day
following exposure, especially within eight hours. Preferably a.L..i.~L.~.Liu..
of the active agent is rnmmPnrf~fl within about 3 hours following exposure
and most ~.~r~.~.bly .~ithin about I hour. This timescale allows the above
defined active agent to be ~ d as an oral rrPr~r~hnn. such as
tablets or syrups or p~ , P~ ly as a lotion, ~..,u,uosiLu. y or enema.
Preferably, however, at least the initial aLl~lLiLLiaLl~LLiu~ following discovery ûf
WO 95/32983 ` ~ r~ 2 1 9 ~ 8 6 2 P~~
the accidental exposure is parenteral, eg b~ cl-hnr--t~nr-m-c, intramuscular or
i~illlVe:llUUs routes.
While it is possible for the active agent to be admistered alone, it is5 preferable to present it as part of a ph~rm~rP~hrRI fnrTn~ tinn Such a
fnrmlllAtinn Will comprise the above defined active agent together uith one
or more acceptable carriers and optionally other L-.~. rlu~Lic ingredients. The
carrier(s) must be acceptable in the sense of being rnmp~hhlP u~ith the other
ingredients of the fnrTnlll~tinn and not rlPlr-tPrimlC to the recipient.
The fnrmlll~tir~nc include those suitable for oral, rectal, nasal, topical
(including buccal and sllhlin~l~), vaginal or parenteral (including
c~ illL~ r~ lv~lluus and inhra~prTn~l) a~
The fnrTnlll~tinnc may conveniently be presented in unit dosage form, e.g.
5 tablets and sustained release capsules, and may be prepared by any mehtods
well known in the art of pllrl Uld-y.
Such methods include the step of bringing into ,.~u~ ;~ 1 ;, ." the above defined
acitve agent with the carrier. In general, the fnrTnlll~hnnc are prepared by
~o uniformly and intimately bringing into ~ccori~hnn the active agent with
liquid carriers or finely divided solid carriers or both, and then if neoessary
shaping the product.
FnTTnTll~hnnc fororal ~,.""",~1,,. Iinn inthepresentinventionmaybe
~i presented as discrete units such as capsules, cachets or tablets each
...,.I~;,.."Fa,u.r,1PI~ l amountoftheactiveagent;asapowderor
granules; as a solution or a SllcF)Pncinn of the active agent in an aqueous
~ WO 95132983 ~ ~ ` 2 1 9 0 8 6 2 P~
liquid or a non-aqueous liquid; or as an oil-in-water liquid emuision or a
water in oil liquid emulsion and as a bolus etc.
With regard to compositions for oral ~ r 1~ e.g. tablets and
5 capsuleS), the ter~n suitable carrier indudes vehides sudh as co-m- mon
excipients e.g. binding agents, for example syrup, acacia, gelatin, sorbitol,
hA~ n~h, polyYi--y;~uy...~lidone(Povidone),methylcellulose,
ethylcellulose, sodium ~c L~u~y~ tl~ylcellulose,
~yd-u~y~uluylLLl~Lllylcellulose~ sucrûse and stardh; fillers and carriers, for
0 example corn stardh, gelatin, lactose, sucrose, micrûcrystalline cellulose,
kaolin, mannitol, dicalcium ~ , sodium chloride and alginic acid;
and lubricants sudh as m~nPCillm stearate and other metallic stearates,
stearic acid, silicone fluid, talc waxes, oils and colloidal silica. Flavouring
agents sudh as ~ LII~ L, oil of WiL~ L~LI, cherry flavouring or the like
5 can also be used. It may be desirable to add a colouring agent to make the
dosage form readily i~iPntifi~hlP Tablets may also be coated by methods
well known in the art.
A tablet may be made by ~uLL~ aaiul~ or mi~lllfiin~ optionally with one or
20 more accessory i~ La. Cu~L~ a~d tablets may be prepared by
~ULL~ iLlg in a suitable mad~ine the active agent in a free flowing form
such as a powder or granules, optionally mixed with a binder, lubricant,
inert diluent, ~e~-~ v~LLivt:, surface-active or dispersing agent. Moulded
tablets may be made by moulding in a suitable machine a mixture of the
25 powdered ri~mrol-nri . ~ i with an inert liq~ud diluent. The tablets
may be optionally be coated or scored and may be fnrm~ so as to
provide slow or controlled release of the active agent.
woss/32ss3 ~ ~ i P ~ Z ~ 9~862 .~ 7~
Fnrm~ nnn c suitable for topical adll~Lis L- ~Lion include lozenges
rnmrric;n~ the active agent in a flavoured base, usuall~ sucrose and acacia
or TrA~r~nth; pastilles ~nml~ricin~ the active agent in an inert base such as
gelatin and glycerin, or sucrose and acacia; and mouthwashes comprising
5 the active agent in a suitable liquid carrier.
Fnrm~ tinnc suitable for topical ad.",~ L aLion to the skin may be
presented as nintmPntc, creams, gels, and pastes rnmrricin~ the active agent
and a pl~ ly active carrier. An exemplary topical delivery- system
10 is a tr~ncrlPrm~l patch ~ the active agent.
Fnrm~ tinnc for rectal or vaginal ~1 1 1 1 1; 1 I;~L~; ," may be presented as a
o~iLu.y or pessary with a suitable base ~nmrricin~, for example, cocoa
buter or a salicylate. Other vaginal prPr~r~tlnnc can be presented as
tampons, cre~ms, gels, pastes, foarns or spray fnrmlllRtinnc . . ."1.,;";"~, in
addition to the active agent, such carriers as are known in the art to be
appropriate.
FnrTnlll~tinnc suitable for nasal a~L.~u~L- aLiull wherein the carrier is a solid
20 include a coarse powder having a particle size, for example, in the range 20
to 500 microns which is ad, . li.l~L~ in the manner in which snuff is taken,
i.e. by rapid inhAl~tinn from a container of the powder held up close to the
nose. Suitable fnrm~ tinnc wherein the carrier is a liquid for adlllillaLlaLi
for example, as a nasal spray or as nasal drops, include aqueous or oily
75 solutions of the active agent.
Fnrmlll~tinnc suitable for parenteral ~ , .. l ;. ." include aqueous and
non-aqueous sterile injection solutions which may contain ~nrinYifi~ntc
~ WO 95132983 2 1 9 0 8 6 2 r~l/a~
buffers, bacteriostats and solutes which render the fnrm- II Atinn isotonic ~ ith
the blood of the intended recipient; and aqueous and non-aqueous sterile
suspensions which ma~ include suspending agents and tllirkr-nin~ agents.
The fnrmlllAtirnc may be presented in unit-dose or multi-dose rnntAin-orc
5 for example sealed ampoules and vials, and may be stored in freeze-dried
(Iyophilized) condition requiring only the addition of the sterile liquid
carrier, for example water for injection, immr~liAtr-ly prior to use.
F,~ r .~r-~ ~ mjection solutions and ~IlcFPncinnc may be prepared from
sterile powders, granules, and tablets of the kind ~ ViL)L~Iy described.
In view of the l~n~r-rctAnriAhI.o shock attendant upon either the exposure to
HIV, i.e. a needle stick~, or its discovery, e.g. that a ~ rl syringe has
been been reused, the above defined active agent is a,lvA I I I ~H .1 ~ly
presented in an easily a,l.. i..aL~.~d, rapidly acting for~n. An exa~nple of such
a dosage form or package is a self-a~ d spring-loaded syringe
currently used for nerve gas antidotes.
Several aLLLL~ LlGLion routes may be All l l l l l l ;~ d ~;l l l l ll l A l lH ~ y~ for
instance a vaginal preparation, am illL. CLIIL~ L injection and a tablet may
20 be ~ rnu51y taken, e.g. in the event of a suspected sexual
.. ., to provide effective doses; ~ PI I;A IPly at the site of exposure
and in the circulatory system and for a more sustained rate via the oral
route.
2s The above defined active agent is adminstered following exposure to HIV in
an amount effective to prevent ~V infection or HlV s, ,u.u.,v~.~iv... Suitable
amounts include about 0.1 mg/kg bo,ly w~ L/day, preferably at least
about 0.; mg/kg/day and more preferably about 10 mg/kg/day. This
,
WO9!il32983 ~ ,;,; 7.1 90~ 62 r~ 4
... . --
corresponds to a preferred dosage amount of about IO mg to aDou 1~1 g De.
da~. Larger dosage regimeS will tend to be indicated L~ here a t~articuiari~
large inoculum is su5pected to have been LL~ rl~ such as througr~ a
"."1,.,l,;l,,.l"1bloodlineortrAncfllc~ heresustainedadLLLiLLi~ildhonis
impractical, such as in a military environment, u here the active agent is
A~ l, d as a single dose "morning after pill" by clinics laci~ing ~ollo~
up facilities or where there has been extended delay before the acti~e agent
is first A" " ,i. .i~l~, L d.
o The mAYimllm dosage of the active agent is of course rl~rPnri~nt on toxicit~
and the bioavailablity of the dosage route emploved but it is noted that
unlike therapeutic treatment for f~ctAhlicllPrl Hl ~,T infections uith the
currently available agents which must be aLlL-Li,LL~L~ d in r1Pfinit~ly, in order
to suppress viraemia, the present invention rnntr-mrl~trc A~
during a finite period following exposure to HIV. A~U-UiLL~ transient
toxicity which would be intnl~rAhl~ to a long term therapeutic regime may
be tolerated in the method of the present invention. Cell culture results
indicate that the above defined compound is generally well tolerated by
human cells, for instance the I~aO in MT4 cells appears to be around 4a ~1
g/ml. In vivo simian e~ indicate no detectable toxicit~ short term
at 30 mg/kg/day and bearing in mind the finite administration period,
dosages of IOO mg/kg/day or more are feasible.
A~u-ui-~ , in view of the two immr-r~iAtf~ly preceding paragraphs,
~5 preferred dosage atnounts include a range of from O.I to IOO mg/kg
bodyweight/day, particularly 0.5 mg/kg/day to aO mg/kg/day and more
particularly I.O to 2a mg/kg/day.
~ WO 95132983 ~ 2 1 9 0 8 6 2 } ~
The finite period of tiTne m~ntlnn~ri in the ponl~ltimAtP paragraph abo-~e tillbe the period of time t~rhich is sufficient to prevent the .oCtAhliChmDn~ o~ HrVinfection or Hl~T s~.u-u-, v~- ~ion when A~ d after exposure to H1~7,
but is not so long as to incur l,nn,o~.DccAry expense or risk curnulative toxicity
5 in atypically sensitive individuals. The finite period of au.l. i.,~L. d~iU~I t~
preferably be at least about one day, preferably about 3 days and may be I
or two weeks, especially where there has been si~nifirAnt delay between
exposure to I ~T and initiaT A ~ .L/ ~ l i nn of the active agent. Sustained
release dosage forms can of course be fnrml1lAt~ to release the active agnt
o for the period intended, either as a single dose, or as a twice daily, dailv or
weekly dosage unit.
In keeping with sound FhArmA~lti~Al practice in relation to antiviraT agents,
the ~ ci I i. .I l c of the invention may comprise one or more auxilliary
antiviraTs or antimicrobials as congeners etC either against other common
infective agents in HriT positive body fluids or samples, such as hepatitis B
or C, or those in use in ~v~vL-~lLiv~lal double or multiple therapy against
'f ~t,T, Examples of such Al~YilliAri~ include interferon, gamuna-globu}in, RT
and protease inhihitnrc, AZT and ddl. The . . ~ of the invention
20 may alL~ ..,dLiv~ly or A~iti nn Ally comprise an ~bu. Lir.ci~"L such as the
oestrogen derivatives employed in "morning after pills" or RU 4~6.
Detailed descriptio~L .
Embo~ LT, of the invention will now be described by way of example
25 only with reference to the following F-~mrlPc
WO 951329R3 ~ 2 ~ 9 a 8 6 2
Example 1
Preparation of 1-[2~3 -dideoxy-3~c-(hy~u~ -eLl.yl~-~-D-erythro-
p~ rlllrlln~yl}cytosine
5 The ben70yl protected sugar residue, methyl-~-O-benzoyl-3-[
L~uylu~y)methyl}2,3-dideoxy-D-erythro-p~llL~ uside is prepared
according to the method of Svensson et al; 1991, a6, 2993-2997. The base
residue is prepared by ~ 120 mg, 1.08 mmo~ cytosine in 0.2 ml
trimethylchiorosilane and 2 ml h~ul-~Ll-ylrlicil~7~n~ along with crystalline
o ,. I l l I ll .,, l , sulphate, refiuxing until clear, vacuum . . ," ~ l . r 1 I nn and
~u~vc~,uu~Liu~ with dry xylene. This preparation is dissolved in 2 ml
dichlulu~LllcLlle in a nitrogen C l Il~h~ behre 170 mg, 0.46 mmol of the
sugar residue is added followed by 0.22 ml, 0.96 mmol t-butyl-dimethyl-
triflate. Reaction proceeds for 24 hours at room ~ and is quenched
5 with saturated sodium hydrogen carbonate during agitation for half an hour.
This solution is diluted with di-l~ulveLl-c -~e~ washed in the saturated
carbonate and 111111 1-l l ~ i following drying and filtration. The resultant
~UI-~ILLl CLIt: is subjected for 24 hours at room Lt~ U~ LCLLLlle: to 20 ml of
saturated 11-~ -1 .nli~ ammonia, before being 11~ . dissolved in
20 water and extracted with riirhirlro~th~n~ A C18 column is used for reverse
phase semi ~ UCLLCLLiVt~ Illlly on the ~u~ LLcLLed aqueous phase
and eluted with 2% methanol in water. The ~ anomer, 1-[2',3'-dideoxy-3'C-
(hy~llu~yllleLllyl)-~l~D-erythro-p~ urLLLcLllu~yl}cytosine follows the c~
anomer and collected fractions yielded 27 mg with the following NMR
25 spectra in ppm (using TMS and TSP or dioxane as internal standards) [c~]
26D: +64 (c o.27, H2O); W tH2O) ~ max: 272 nm (E 9208); 1H-NMR (270
MHz, D2O): 2.2-2.46 (m, 3H, H-2); H-3'); 3.68 (d, J3~ 6' = 5.5 H_, 2H, H-6');
3.76 (dd, J4~, sla = 12.5 Hz, lH, H-5la); 3-92 (dd, J41, 5'b = 2-9 Hz, J5~a, 5'b =
WO 9SI32983 2 1 9 0 ~ 6 2 PL ~ 5
13
1~ 5 Hz, IH, H-~'b); 4.01 (m, J3, 4 = v.l Hz, J5 a, 4~ = 5.5 Hz, J~ b 4 = 2.9 Hz,
IH,H~');6.05(3,Js,6=73HZ~lH~H-5);6ll ~dd,J1',2'a'7HZ~J1,2'b=
4.0 Hz, lH,X-1'); 7.91 (d, Js, 6 = 7.3 Hz, IH, H-6); I3C-NMR (~5.05 ~Iz,
D2~: 36.1 (c-2'); 40.8 (C-3'); 62.7, 63.1 (C-5', C-6'); 84.7, 87.1 (C-l, C-4 ); 96.5
(C-5); 142.2 (C-6); 158.2 (C-2); 166.8 (C-4).
The ~luLll l laLuv~ iLrlly pure compûund is prepared into a ~
preparation by clissolving 3.00 g in 300 ml 0.9 % NaCI in pyrogen free water
followed by sterile filtration to give a 10 mg/ml solution which is
o ~ l ed sllhcllt~npnllcly at the rate ûf I mg/kg bodyweight.
Example 2a
AnimalExperiments: HIV.
nnrlll~tinn The control and test animals, 2 year old m~rr~l~RrillP monkeys
were innt~ tPri Slli~ ,y with a moderate (10 monkey infectious
doses MIDso, as r~PtPrminPd by Bottiger et al Antiviral Chemistry and
~'hPmnthDr~ry 1992;3 269-271) dose ~le~cu~lLiull of HIV-2 (SBL 6669strain)
in pl~o~l.dLe buffer
10 minutes after innr~ tir)n~ the test animals were injected sllhr1lt~nP~llcly
with a 10 mg/kg ~e~ LiL.~ of Example 3 or the cnrrpsrnnriin~ AZI
i~ . Treatment was repeated three times pêr day for 4.5 days. After
8,10,14,17, 24 and 30 days blood samples were assayed for the presence of
2s viral antigen (absorbance at 492 nm) and the presence or absence of virus in
the 30 da~ sample was assayed
WO 95/32983 ,,,;~.1 9 G 8 6 2 F~ 4
1~
AlL four l1ninn~ tPri control animals exhibited Hl~.' antigen in the blood
between days 14 and 17 and virus could be isolated after 30 days (~ months
in one animal). Both of the AZT treated animals showed minor rises in
absorbance at day 17 which later declined. However ~ irus was isolated rrom
5 both animals at day 30 and in keeping uith the usual pattern follo~ing
cessation of AZI ~ therapy, it would be expected that the antigen
(and free ~irus titre) would rise in following weeks and months.
In contrast, none of the four animals treated with the y-~ydlr~LiO~I of the
o invention showed any rise in absorbance and nor could virus be isolated
after 30 days. It is thus clear that the adl~ sL~,~Liu" of the invention
prevented the infection Pst~hii~hin~ itseLf, ~hereas AZr, the most r~ffir~imls
thPr~rP1lnr agent known to date has no such ability.
After several months, each of the protected test animals was r~innr~ tpri
with HIV and became infected in the usual u ay, thus indicating that it was
the active agent which was rPs~nnsihlP for the failure of the infection to
establish itself and not a peculiarity of the test animals.
~0 Example 2b
Animal F~ 1 i SIV
Example 2a demonstrates that the active ingredient of the mvention prevents
the r~st~hli~hmi~nt of HIV infection in the cynnnnr~ln--s monkey model. SIV,
whose native host is the monkey and which results in a more a~ ,ivl:
infection than ~V was then used to investigate ~yyl~y~ Lt dosages and
regimes. Efficacy m the more aggressive SIV model will more than reflect
efficacy in the milder HIV infection in humans.
-
WO 9~13~983 ` j~ , PCT~SE95~00524
2~ 9~62
Control and test animals were ~ year old macquaque monkeys as abo~e
which were innr~ tr-~ intraVenOUSly with 10,100 or 1 000 MID of SI~ sm as
listed in Table 1 below.
One, 3, 8, or 24 hours after irnC~ tinn with SIVsm, paired test animals were
injected su~ ,. "r~,cly with 10 mg/kg of the ~ JdldLiUII of Example 4,
repeated three times per day for 1 day or 3 days as listed in table 1. Blood
samples were extracted from the test and control animals on days 0,17, 23
0 and 30 for rl.otr-~nin~tinn of SIV antibody, SIV p26 antigen and presence of
infective ~Tirus. The methods have been described by Bottiger et al, AIDS Res
Hum R~l~ov.l us~ 1992;~:1235-1238.
With reference to table l, it is clear that the ~ . . of the present
active agent prevents the infection ~ itself in the test animals, as
monitored by the presence of viral antigen or antibody to the virus in the
blood or ability to culture virus. All of the control animals exhibited positiveSIV antibody and antigen titres within the first 23 days and ~7ithin 30 days
infectious SIV virus could be cultivated.
~o
Infection Yas prevented in all of the animals treated for three days, whether
ll l was first ~ one hour~ 3 or 8 hours after innr~ tinn
with 10 MIDso. Even waiting for as long as 24 hours before first
the ~ dldLi~ of the invention prevented infection in one of
25 the two animals.
.
With ~ dLlll~ s which were only of one day's duration, three of the four
animals showed no signs of the ~ ll l l lf-l 1 of infection if treatment
WO 95132983 2 1 9 ~ 8 6 2 ~ - ~ ? i ~ F~ 5.'C~
16
~nmmPnrP11 within I hour of innrlliAtinn u~ith 10 ~DaO. Waiting for three
hours inaeased the infection rate but some protective effect is still apparent.
Clearly a lower infectious dose, less aggressive virus, for instance HI~, o~
higher active concentration of the ~ Ja~Ali~ " ~t~ill enhance this protech~ e
5 effect.
It sho-wd be noted that an inoculum of lO in~'ectious doses is a c;~
greater amount of virus than a victim is exposed to in a normal needle stick
accident. However the ~ctAhlichmPnt of infection can be prevented even
0 following very high infectious doses (100 ~DaO) as can be seen from the
rnmhinAtinnA,ll,.. ~.l,.. li~."withinlhourplustreatmentforthreedays
(three of four animals protected~. This dosage regime also protected one of
the two animals subjected to the extremely high inoculum of 1000 ~DaO.
The~nmhinAtinnofA.ll..;,.~,AIi..llwithin3hoursplustreatmentforonlyl
day prevented infection from 100 MIDso virus in one of four monkeys
which is a ~ u~ w ~ed protective effect bearing in mind the inoc-wum, time
to first A l l l l l i l .~l l ,. l ;l l l l and duration (results not shown in Table 1).
~ wog~l32983 2 ~ 9 08 62 P~ 4
-
+ + + ~ .. ....
~o
C~
.
: ~ ++++ + $+$
t V~
-
-
E.
-
. C
O C --C O C O O O O O O O O O O O O C O
= O ~ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_
O o _ ~ X U-~
WO9S132983 ~1 9o~ ., s)~- 4 ~
.
~ ~ ' ' ' ' ' + ~ ~ + . t,
. I
$ $ $
t U~
~,
, 8.- yl
o X W X X
,~ a~ O - - _ _ _ X x Y x ~ y
O o ~ ~ ~ X ~ ~ ~ ~
~ W0 95/32983 2 ~ 9 q 8 6 2 r~
19
Example 3
Injectable ~ ,c,Liu.L
750 mg of lyophilized active agent, as prepared in Example 1, is dissolved in
100 ml of pyrogen free isotonic saline. The pH of the ,U~dL~L~iUlL is
5 adjusted to 7.5 with 0.1 M NaOH/HCl and the y~dLCLLiU~L sterile filtered
and aseptically decanted into 10 10 ml sterile vials having a rubber
diaphragm in the lid for rapid wiLl~lL~Lwdl of the contents. Each vial
le~lL-~LIL~ a dosage form ~ ;..;"~ 75 mg of active iLl~ Le~LlL suitable for
providing a dosage of I mg/kg for a normal adult male.
Exarnple 4
Capsules
12 g of active agent, as prepared in Example 1, is seived through US 60 mesh
along with 12 g of ~lPCCirRtP~l lactose and 0.1 g of " ,~ ...., stearate. The
resultant fine powder is dosed in 500 mg amounts into hard gelatin capsules
to form dosage units of 245 mg .~.,~t "Li,.g a dosage of about 32 mg/kg for
a normal adult male .
Example 5
Sustained release ~. L~dL ~L~iUll
The following materials are wet grRnlllRtP~1 in a 300 mg povidone aqueous
base:
5000 mg active agent
1000 mg E~'MC (Methocel K4M premium)
500 mg lactose (ph~nRrPll~ grade)
The granules are ulLL~l~a~Ll with a trace of mR~nPcilmn stearate into 700 mg
tablets suitable for twice daily ~.l",;,.l~L".~ ,r~n