Note: Descriptions are shown in the official language in which they were submitted.
WO95133031 ~ ~ PCT1~1S95f06S60
DETERGENT COMPOSITION CONTAINING OLEOYL SARCOSINATE AND ANIONIC SURFACTANTS
IN OPTIMUM RATIOS
i
io FIELD OF TIIE INVENTION
The present invention generally relates to a detergent composition having
improved cleaning performance. More particularly, the invention is directed to
a
detergent composition containing oleoyl sarcosinate and one or more anionic
surfactants selected from the group consisting of alkyl benzene sulfonates,
alkyl ester
sulfonates, alkyl sulfates, alkyl ethoxy sulfate, secondary alkyl sulfates and
mixtures
thereof. The anionic surfactants described herein are combined with oleoyl
sarcosinate in optimum ratios.
BACKGROUND OF INVENTION . _ .
Typically, conventional detergent compositions contain mixtures of various
zo surfactants in order to remove a wide variety of soils and stains from
surfaces. For
example, various anionic surfactants, especially the alkyl benzene sulfonates,
are
useful for removing particulate soils, and various nonionic surfactants, such
as the
alkyl ethoxylates and alkylphenol ethoxylates, are useful for removing greasy
soils.
While the art is replete with a wide variety of surfactants for those skilVed
in
z5 the art of detergent formulation, most of the available surfactants are
specialty
chemicals which are not suitable for routine use in low cost items such as
home
laundering compositions. The fact remains that many home-use laundry
detergents
still comprise one or more of the conventional alkyl benzene sulfonates or
primary
alkyl sulfate surfactants.
3o The limited solubility of alkyl sulfate surfactants including both primary
and
secondary alkyl sulfates is especially prevalent in modern granular laundry
detergents
which are typically used in cold temperature (e.g. 5°C to 30°C)
washing solutions
and are formulated in "condensed" or "compact" form for low dosage usage. For
the
consumer, the smaller package size attendant with compact detergent products
s5 provides for easy storage and handling. For the manufacturer, unit storage
costs,
shipping costs and packaging costs are lowered.
W095133031 2191314 PCT~S95/06560
_2_
The manufacture of acceptable compact or condensed granular detergents has
its difficulties. In a typical compact detergent formulation, the so-called
"inert"
ingredients such as sodium sulfate are substantially eliminated. However, such
ingredients do play a role in enhancing solubility of conventional detergents.
As a
consequence, compact detergents often suffer from solubility problems,
especially in
cold temperature laundering solutions. Moreover, conventional compact or low '
density detergent granules are usually prepared by spray drying processes
which
result in extremely porous detergent particles that are quite amenable to
being
dissolved in aqueous washing solutions. By contrast, compact detergents are
to typically comprised of less porous, high density detergent particles which
are less
soluble. Thus, since the compact form of granular detergents typically
comprise
particles or granules which contain high levels of detersive ingredients with
little or
no room for solubilizing agents, and since such particles are intentionally
manufactured at high bulk densities, the net result can be a substantial
problem with
regard to in-use solubility.
It would also be desirable to incorporate effectively certain alkyl sulfate
surfactants into heavy duty liquid detergents, in order to benefit from their
good
overall cleaning ability. Whereas the lower alkyl chain sulfate surfactants
can be
easily incorporated into light duty liquid detergents, formulators in the art
have
2o experienced difficulty in incorporating alkyl sulfates with 14 or more
carbon atoms
into liquid laundry detergents. It is these longer chain surfactants that are
generally
preferred for laundry and other textile cleaning applications.
Accordingly, there remains a need for a detergent composition which has
improved solubility, especially in cold temperature washing solutions, and
improved
clearting performance. This need is especially prevalent in the art of compact
or high
density granular detergents currently being used by consumers. There is also a
need
for such a detergent composition which also can be in liquid form and
effectively
include longer chain alkyl sulfate surfactants for improved cleaning
performance.
EACKGROUND ART
3o Oleoyl sarcosinate is described in the following patents and publications:
U. S.
2,542,385; U.S. 3,402,990; U.S. 3,639,568; U.S. 4,772,424; U.S. 5,186,855;
European Patent Publication 505,129; British Patent Publication 1,211,545;
Japanese
Patent Publication 59/232194; Japanese Patent Publication 62/295997; Japanese
Patent Publication 02/180811; and Chemical Abstracts Service abstracts Nos.
61:3244q, 70:58865x, and 83:181020p.
CA 02191314 1999-09-02
SLINiNIARY OF THE INVENTION
The present inventions meets the needs identified above by providing a
detergent composition which provides improved cleaning performance as well as
improved solubility in aqueous laundering solutions. The invention provides a
detergent composition which can be in granular or agglomerated form, or in
liquid
form which can include higher chain length alkyl sulfates. It is the oleoyl
sarcosinate
component which largely contributes to the unexpectedly superior cleaning and
solubility results achieved by the invention. Fortunately, a relatively
inexpensive
process by which the oleoyl sarcosinate can be produced has been found and is
set
1o forth herein. In light of the foregoing, the detergent composition of the
invention is
practical and affordable for modern day consumers. The compositions also
provide
excellent color care for dyed fabrics and excellent skin mildness for handwash
operations.
As used herein, the phrase "improved solubility" means that the solubility of
the anionic surfactants of the detergent composition is enhanced by at least
5% in the
laundering solution when employed in the manner of this invention, as compared
to
the solubility of the same anionic surfactants per se, under the same test
conditions
(i.e. water temperature and pH, stirring speed and time, particle size, water
hardness,
and the like). As used herein, the term "agglomerates" refers to particles
formed by
2o agglomerating particles which typically have a smaller mean particle sue
than the
formed agglomerates. All percentages, ratios and proportions used herein are
by
weight, unless otherwise specified.
In accordance with one aspect of the invention, a detergent composition
surprisingly having improved solubility in aqueous laundering solutions and
cleaning
performance is provided. Specifically, the detest composition comprises from
1% to 55% of a surfactant system containing at least one anionic surfactant
selected
from the group consisting of alkyl benzene sulfonates, alkyl ester sulfonates,
alkyl
sulfates, alkyl ethoxy sulfates, secondary alkyl sulfates and mixtures
thereof. Also
included is from 0.1% to 90% of oleoyl sarcosinate, wherein the ratio of
oleoyl
sarcosinate to the surfactant system is from about 1:20 to about 10:1. The
overall
cleaning performance of the detergent composition and solubility in cold
temperature
laundering solutions is surprisingly improved.
In other embodiments of the invention, the detergent composition further
comprises at least about I% by weight of a detergency builder, wherein the
detergency builder is selected from the group consisting of sodium carbonate,
zeolites and mixtures thereof. Yet another embodiment entails including
adjunct
R'095/3303t 219 I 314 PCTIUS95/06560
ingredients selected from the group consisting of bleaches, bleach activators,
suds
suppressors, enzyme stabilizers, polymeric dispersing agents, dye transfer
inhibitors
and soil release agents into the detergent composition.
The detergent composition may be in the form of agglomerates as opposed to
spray dried granules and has an overall density of at least about 600 g/l for
purposes
of commercialization as a compact detergent product. Other more preferred
embodiments are directed to the inclusion of from about 0.1% to about 45% by
weight of a mixture of alkyl sulfate and alkyl ester sulfonate surfactants in
the
composition; and/or the inclusion of from about O.I% to about 20% of
polyhydroxy
to fatty acid amide surfactant.
In another embodiment, the detergent composition also contains from about
0.1% to about 20% of at least one nonionic surfactant selected from the group
consisting of alkyl ethoxylates, alkyl phenol alkoxylates,
alkylpolyglucosides, and
mixtures thereof; wherein the ratio of the nonionic surfactant to the
surfactant system
is from about 1:1 to about 1:30.
The detergent composition may be in the form of a laundry bar or liquid
detergent composition. The invention also provides a method of laundering
soiled
fabrics comprising the step of contacting the fabrics with an aqueous medium
containing an effective amount of a detergent composition according to the
invention
2o described herein.
Accordingly, it is an object of the invention to provide a detergent
composition which has improved cleaning performance and improved solubility,
especially in cold temperature washing solutions. It is also an object of the
invention
to provide such a detergent composition which also can be in liquid form and
effectively include longer chain alkyl sulfate surfactants for improved
cleaning
performance. These and other objects, features and attendant advantages of the
present invention will become apparent to those skilled in the art from a
reading of
the following detailed description of the preferred embodiment and the
appended
claims.
3o DETAILED DESCRIPTION OF THE PREFERRED EMBODWENTS
The present invention is directed to a detergent composition preferably
comprising at least 1%, more preferably from about IO% to about 55%, and most
preferably from about 15% to about 40%, by weight of a surfactant system
containing at least one anionic surfactant as described herein. Also included
is oleoyl
sarcosinate in amounts ofat least about 0.1%, preferably from about 0.1% to
about
90%, more preferably from about 1% to about 50%, and most preferably from
about
3% to about 30%, by weight of the detergent composition.
WO 95133031 2191314 PCT/US95/065i60
-5-
Additionally, the ratio of oleoyl sarcosinate to the surfactant system is
preferably from about 1:20 to about 10:1, more preferably from about 1:20 to
about
5:1, and most preferably from about 1:20 to about 2:1. In this way, the
solubility of
the surfactant system is improved as well as the overall cleaning performance
of the
composition. The "improved solubility" achieved by the detergent composition
is
concerned with enhanced solubility of the surfactants contained in the
surfactant
system. Preferably, the improvement represents at least a 5% increase in
solubility of
the anionic surfactants in the wash solution over the solubility of the same
surfactants
if they were dissolved alone or without being contained in a detergent
composition as
1o defined herein. More preferably, the solubility improvement is from about
10% to
about 50%. As those skilled in the art will appreciate, any comparison of
anionic
surfactant solubility should be completed under the same laundering
conditions, e.g.
water temperature, hardness and pH, stirring speed and time, and particle
size.
Those skilled in the art should also appreciate the numerous ways in which
the amount of the surfactant system in the washing solution can be determined.
For
example, in the so-called "catS03" titration technique, samples ofthe aqueous
laundering solution containing the detergent composition can be taken after
one
minute and filtered with 0.45 um nylon filter paper, after which the filtered
solution
can be titrated with a cationic titrant, which can be cotrnnercially
purchased, e.g.
2o from Sigma Chemical Company under the trade name Hyamine, in the presence
of
anionic dyes. From the foregoing, the amount of anionic surfactant which was
dissolved in the washing solution can be determined.
As an option, the invention also contemplates the addition of one or moue
nonionic surfactants to the detergent composition. In that regard, the amount
of
nonionic is preferably from about 0.1% to about 20%, more preferably from
about
0.2% to about 10%, and most preferably from about 0.4% to about 4%, by weight
of
a nonionic surfactant. The ratio of nonionic surfactant to anionic surfactant
in the
composition described herein is preferably from about 3:1 to about i :30, more
preferably from about 1:2.5 to about 1:20, and most preferably from about 1:5
to
3o about 1:20.
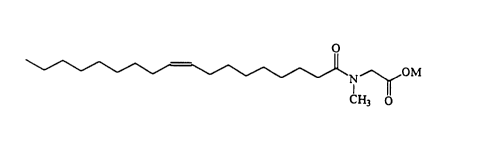
Oleoyl_Sarcosinate
The present invention compositions comprise oleoyl sarcosinate, in its acid
and/or salt form selected as desired for the compositions and uses herein,
haviqg the
following formula:
CA 02191314 1999-09-02
-6-
N~ 'OM
CH3 ~0
wherein M is hydrogen or a cationic moiety. Preferred M are hydrogen and
alkali
metal salts, especially sodium and potassium. Oleoyl sarcosinate is
commercially
s available, for example as Hamposyl O~ supplied by W.R. Grace & Co.
In addition to the commercially-available oleoyl sarcosinate, oleoyl
sarcosinate useful herein can also preferably be prepared from the ester
(preferably
the methyl ester) of oleic acid and a sarcosine salt (preferably the sodium
salt) under
anhydrous reaction conditions in the presence of a base catalyst with a
basicity equal
~to or greater than alkoxide catalyst (preferably sodium methoxide). For
example, the
reaction may be illustrated by the scheme:
CH3 O
~N~ONa
NaOCH3 (cat)
This salt may optionally be neutralized to form the oleoyl sarcosinate in its
acid
15 form.
The preferred method for preparing oleoyl sarcosinate is conducted at a
temperature from about 80°C to about 200°C, especially from
about 120°C to about
200°C. It is preferred to conduct the reaction without solvent although
alcohol
solvents which have a boiling point of at least 100°C and are stable to
the reaction
2o conditions (ie. glycerol is not acceptable) can be used. The reaction may
proceed in
CA 02191314 1999-09-02
-7-
about 85% yield with a molar ratio of methyl ester reactant to sarcosine salt
reactant
to basic catalyst of about 1:1:0.05-0.2.
Methyl ester mixtures derived from high oleic content natural oils (preferably
having at least about 60%, more preferably at least about 75%, and most
preferably
s at least about 90% oleic content) are especially preferred as starting
materials.
Examples include high-oleic sunflower and rapeseed/canola oil. In addition, a
high-
oleic methyl ester fraction derived from either palm kernel oil or tallow is
acceptable.
It is to be understood that such oils typically will contain some levels of
impurities,
including some fatty acid impurities that may be converted to sarcosinate
compounds
1o by this synthesis method. For example, commodity canola/rapeseed oil may
comprise
a majority of oleic acid, and a mixture of fatty acid impurities such as
palmitic,
stearic, linoleic, linolenic and/or eicosenoic acid, some or all of which are
converted
to the sarcosinate by this reaction method. If desired for formulation
purposes, some
or all of such impurity materials may be excluded from the starting oil before
15 preparing the oleoyl sarcosinate to be used in the present compositions.
Finally, sarcosine remaining in the reaction mixture can be converted to an
amide by addition of malefic or acetic anhydride to the mixture, thereby
minimiz;ng
the sarcosine content and any potential for formation of undesired nitrogen-
containing impurities.
2o The synthesis of oleoyl sarcosinate may be carried out as follows to
prepare
the sodium oleoyl sarcosinate.
Synthesis of Oleoy~l Amide of Sarcosine Sodium Salt - A 2 L, 3-neck, round
bottom flask is fitted with thermometer, Dean-Stark trap with condenser,
mechanical
stirring, and a gas inlet adapter through which nitrogen is passed over the
reaction
25 mixture. The reaction vessel is charged with sarcosine (43.3 g, 0.476 mol),
sodium
methoxide 25% in methanol (97.7 g, 0.452 mol), and methanol (400 mL). The
reaction is refluxed 15 min to neutralize the sarcosine and then methyl ester
derived
from Cargill~"'~ regular high-oleyl sunflower oil (148.2s g, 0.5 mol) is
added. After the
methanol is removed with the Dean-Stark trap, reaction mixture is heated to
170°C
3o for 1 hr to drive off any water. The reaction is initiated by the addition
of sodium
methoxide 25% in methanol (15.4 g, 0.0714 mol). Reaction is kept at
170°C for 2.5
hr during which methanol is collected in the Dean-Stark trap. The reaction is
allowed to cool slightly and then methanol (200 g) is added. Malefic anhydride
(9.43
g, 0.095 mol) is added to the methanol solution and the reaction is stirred at
60°C for
35 0.5 hr. Then most of the methanol is removed by rotary evaporation and
acetone (2
L) is added to precipitate the product. The product is collected by suction
filtration
and allowed to air dry to give an off white solid. Analysis of the reaction
mixture by
W095133031 ~ 19 I 314 PCTIUS95106560
_g_
GC indicates the majority of the product is oleoyl sarcosinate, with minor
amounts of
the following impurities: sarcosine, oleic acid, and the sarcosinates derived
from
palmitic acid, stearic acid, and linoleic acid.
Surfactant Svstem
Nonlimiting examples of the preferred anionic surfactants useful in the
surfactant system include the conventional C11-Clg alkyl benzene sulfonates
("LAS"), primary, branched-chain and random Clp-C2p alkyl sulfates ("AS"), the
Cl0-Clg secondary {2,3} alkyl sulfates ofthe formula CHg(CH~x(CHOS03-M+)
CH3 and CH3 (CH~y(CHOS03-M+) CH2CH3 where x and (y + 1 ) are integers of
io at least about 7, preferably at least about 9, and M is a water-
solubilizing cation,
especially sodium, unsaturated alkyl sulfates such as oleyl sulfate, and the
C10-C18
alkyl alkoxy sulfates ("AEXS"; especially x up to about 7 EO ethoxy sulfates).
Optionally, other exemplary surfactants useful in the composition of the
invention include and C 10-C 1 g alkyl alkoxy carboxylates (especially the EO
1-5
i5 ethoxycarboxylates), the C 10-18 glycerol ethers, the C 10-C 1 g alkyl
polyglycosides
and their corresponding sulfated polyglycosides, and C12-Clg alpha-sulfonated
fatty
acid esters. If desired, the conventional nonionic and amphoteric surfactants
such as
the C12-Clg alkyl ethoxylates ("AE") including the so-called narrow peaked
alkyl
ethoxylates and C6-C 12 alkyl phenol alkoxylates (especially ethoxylates and
mixed
2o ethoxylpropoxy), C12-Clg betaines and suifobetaines ("sultaines"), C10-Clg
amine
oxides, and the like, can also be included in the overall compositions. The
C10-C18
N-alkyl polyhydroxy fatty acid amides can also be used. Typical examples
include
the C12-Clg N-methylglucamides. See WO 9,206,154. Other sugar-derived
surfactants include the N-alkoxy polyhydroxy fatty acid amides, such as Cl0-C
1 g N-
25 (3-methoxypropyl) glucamide. The N-propyl through N-hexyl C 12-C 1 g
glucamides
can be used for low sudsing. C 10-C20 conventional soaps may also be used. If
high
sudsing is desired, the branched-chain C 10-C 16 soaps may be used. Mixtures
of
anionic and nonionic surfactants are especially useful. Other conventional
useful
surfactants are listed in standard texts.
30 Adtunct In~redient~
The compositions herein can optionally include one or more other detergent
adjunct materials or other materials for assisting or enhancing cleaning
performance,
treatment of the substrate to be cleaned, or to modify the aesthetics of the
detergent
composition (e.g., colorants, dyes, etc.). The following are illustrative
examples of
35 such adjunct materials.
Detersive Builder - Detergent builders can optionally be included in the
compositions herein to assist in controlling mineral hardness. Inorganic as
well as
. W095133031 ~ 191314 PCTlUS951065~60
-9-
organic builders can be used. Builders are typically used in fabric laundering
compositions to assist in the removal of particulate soils.
The level of builder can vary widely depending upon the end use of the
composition and its desired physical form. When present, the compositions wilO
typically comprise at least about 1% builder. Granular formulations typically
comprise from about 10% to about 80%, more typically from about 15% to about
50% by weight, of the detergent builder. Lower or higher levels of builder,
however,
are not meant to be excluded.
Inorganic or P-containing detergent builders include, but are not limited to,
to the alkali metal, ammonium and alkanolammonium salts of polyphosphates
(exemplified by the tripolyphosphates, pyrophosphates, and glassy polymeric
meta-
phosphates), phosphonates, phytic acid, silicates, carbonates (including
bicarboinates
and sesquicarbonates), sulphates, and aluminosilicates. However, non-phosphate
builders are required in some locales. Importantly, the compositions herein
function
15 surprisingly well even in the presence of the so-called "weak" builders (as
compared
with phosphates) such as citrate, or in the so-called "underbuilt" situation
that may
occur with zeolite or layered silicate builders.
Examples of silicate builders are the alkali metal silicates, particularly
those
having a Si02:Na20 ratio in the range 1:I to 3.2:1 and layered silicates, such
as the
20 layered sodium silicates described in U.S. Patent 4,664,839, issued May 12,
19;87 to
H. P. Rieck. NaSKS-6 is the trademark for a crystalline layered silicate
marketed by
Hoechst (commonly abbreviated herein as "SKS-6"). Unlike zeolite builders, the
Na
SKS-6 silicate builder does not contain aluminum. NaSKS-6 has the delta-
Na2Si05
morphology form of layered silicate. It can be prepared by methods such as
those
25 described in German DE-A-3,417,649 and DE-A-3,742,043. SKS-6 is a highly
preferred layered silicate for use herein, but other such layered silicates,
such as those
having the general formula NaMSix02x+1'YH20 "'herein M is sodium or hydrogen,
x is a number from 1.9 to 4, preferably 2, and y is a number from 0 to 20,
preferably
0 can be used herein. Various other layered silicates from Hoechst include
NaSKS-5,
3o NaSKS-7 and NaSKS-11, as the alpha, beta and gamma forms. As noted above,
the
delta-Na2Si05 (NaSKS-6 form) is most preferred for use herein. Other silicates
may
also be useful such as for example magnesium silicate, which can serve as a
cri sping
agent in granular formulations, as a stabilizing agent for oxygen bleaches,
and as a
component of suds control systems.
35 Examples of carbonate builders are the alkaline earth and alkali metal
carbonates as disclosed in German Patent Application No. 2,321,001 publisheef
on
November 15, 1973.
WO 95133031 , J~ PCT/US95J06560
-10-
Aluminosilicate builders are useful in the present invention. Aluminosilicate
builders are of great importance in most currently marketed heavy duty
granular
detergent compositions, and can also be a significant builder ingredient in
liquid
detergent formulations. Aluminosilicate builders include those having the
empirical
formula:
MzlnL(~02)z(Si02)yJ~xH20
wherein z and y are integers usually of at least 6, the molar ratio of z to y
is in the
range from 1.0 to 0, and x is an integer from 0 to about 264, and M is a Group
IA or
IIA element, e.g., Na, K, Mg, Ca with valence n.
io Useful aluminosilicate ion exchange materials are commercially available.
These aluminosilicates can be crystalline or amorphous in structure and can be
naturally-occurring aiuminosilicates or synthetically derived. A method for
producing aluminosilicate ion exchange materials is disclosed in U.S. Patent
3,985,669, Kiummel, et aI, issued October 12, 1976. Preferred synthetic
crystalline
15 aluminosilicate ion exchange materials useful herein are available under
the
designations Zeolite A, Zeolite P {B), Zeolite MAP and ZeoIite X. In an
especially
preferred embodiment, the crystalline aluminosilicate ion exchange material
has the
formula:
Nal2~(~02)12(Si02)12)'~20
2o wherein x is from about 20 to about 30, especially about 27. This material
is known
as Zeolite A. Dehydrated zeolites (x = 0 - 10) may also be used herein.
Preferably,
the aluminosilicate has a particle size of about 0.1-10 microns in diameter.
Organic detergent builders suitable for the purposes of the present invention
include, but are not restricted to, a wide variety of polycarboxyiate
compounds. As
25 used herein, "polycarboxylate" refers to compounds having a plurality of
carboxylate
groups, preferably at least 3 carboxylates. Polycarboxytate builder can
generally be
added to the composition in acid form, but can also be added in the form of a
neutralized salt. When utilized in salt form, alkali metals, such as sodium,
potassium,
and lithium, or alkanolammonium salts are preferred.
3o Included among the poiycarboxylate builders are a variety of categories of
useful materials. One important category of polycarboxylate builders
encompasses
the ether polycarboxylates,including oxydisuccinate, as disclosed in Berg,
U.S.
Patent 3,I28,287, issued April 7, 1964, and Lamberti et ai, U.S. Patent
3,635,830,
issued January 18, 1972. See also "TMSITDS" builders of U.S. Patent 4,663,071,
35 issued to Bush et al, on May 5, 1987. Suitable ether polycarboxylates also
include
cyclic compounds, particularly alicyclic compounds, such as those described in
U.S.
Patents 3,923,679; 3,835,163; 4,158,635; 4,120,874 and 4,102,903.
CA 02191314 1999-09-02
-E1-
Other useful detergency builders include the ether hydroxypolycarboxylates,
copolymers of malefic anhydride with ethylene or vinyl methyl ether, 1, 3, 5-
trihydroxy benzene-2, 4, 6-trisulphonic acid, and carboxymethyloxysuccinic
acid, the
various alkali metal, ammonium and substituted ammonium salts of polyacetic
acids
such as ethylenediamine tetraacetic acid and nitrilotriacetic acid, as well as
polycarboxylates such as mellitic acid, succinic acid, oxydisuccinic acid,
polymaleic
acid, benzene 1,3,5-tricarboxylic acid, carboxymethyloxysuccinic acid, and
soluble
salts thereof.
Citrate builders, e.g., citric acid and soluble salts thereof (particularly
sodium
to salt), are polycarboxylate builders of particular importance for heavy duty
liquid
detergent formulations due to their availability from renewable resources and
their
biodegradability. Citrates can also be used in granular compositions,
especially in
combination with zeolite and/or layered silicate builders. Oxydisuccinates are
also
especially useful in such compositions and combinations.
15 Also suitable in the detergent compositions of the present invention are
the
3,3-dicarboxy-4-oxa-1,6-hexanedioates and the related compounds disclosed in
U.S.
Patent 4,566,984, Bush, issued January 28, 1986. Useful succinic acid builders
include the CS-C20 alkyl and alkenyl succinic acids and salts thereof. A
particularly
preferred compound of this type is dodecenylsuccinic acid. Specific examples
of
2o succinate builders include: laurylsuccinate, myristylsuccinate,
palmitylsuccinate, 2-
dodecenylsuccinate (preferred), 2-pentadecenylsuccinate, and the like.
Laurylsuccinates are the preferred builders of this group, and are described
in
European Patent Application 0,200,263, published November 5, 1986.
Other suitable polycarboxylates are disclosed in U.S. Patent 4,144,226,
25 Crutchfield et al, issued March 13, 1979 and in U.S. Patent 3,308,067,
Diehl, issued
March 7, 1967. See also Diehl U.S. Patent 3,?23,322.
Fatty acids, e.g., C 1 Z-C 18 monocarboxylic acids such as oleic acid and/or
its
salts, can also be incorporated into the compositions alone, or in combination
with
the aforesaid builders, especially citrate and/or the succinate builders, to
provide
3o additional builder activity. Such use of fatty acids will generally result
in a
diminution of sudsing, which should be taken into account by the formulator.
In situations where phosphorus-based builders can be used, and especially in
the formulation of bars used for hand-laundering operations, the various
alkali metal
phosphates such as the well-known sodium tripolyphosphates, sodium
pyrophosphate
35 and sodium orthophosphate can be used. Phosphonate builders such as ethane-
1-
hydroxy-1,1-diphosphonate and other known phosphonates (see, for example, U.S.
CA 02191314 1999-09-02
-12-
Patents 3,159,581; 3,213,030; 3,422,021; 3,400,148 and 3,422,137) can also be
used.
Enzymes - Enrymes can be included in the formulations herein for a wide
variety of fabric laundering purposes, including removal of protein-based,
5 carbohydrate-based, or triglyceride-based stains, for example, and for the
prevention
of refirgee dye transfer, and for fabric restoration. The enrymes to be
incorporated
include professes, amylases, lipases, cellulases, and peroxidases, as well as
mixtures
thereof. Other types of enzymes may also be included. They may be of any
suitable
origin, such as vegetable, animal, bacterial, fungal and yeast origin.
However, their
1o choice is governed by several factors such as pH-activity and/or stability
optima,
thermostability, stability versus active detergents, builders and so on. In
this respect
bacterial or fugal enzymes are preferred, such as bacterial amylases and
proteases,
and fungal cellulases.
Enzymes are normally incorporated at levels sufficient to provide up to about
15 5 mg by weight, more typically about 0.001 mg to about 3 mg, of active
enzyme per
gram of the composition. Stated otherwise, the compositions herein will
typically
comprise from about 0.001% to about 5%, preferably 0.01%-2% by weight of a
commercial enzyme preparation. Protease enzymes are usually present in such
commercial preparations at levels sufficient to provide from 0.005 to 0.1
Arson units
20 (AU) of activity per gram of composition.
Suitable examples of professes are the subtilisins which are obtained from
particular strains of B. subtilis and B. licheniforms. Another suitable
protease is
obtained from a strain of Bacillus, having maximum activity throughout the pH
range
of 8-12, developed and sold by Novo Industries A/S under the registered trade
mark
25 ESPERASE. The preparation of this enzyme and analogous enzymes is described
in
British Patent Specification No. 1,243,784 of Novo. Proteolytic enzymes
suitable for
removing protein-based stains that are commercially available include those
sold
under the trade marks. ALCALASE and SAVIT1ASE by Novo Industries A/S
(Denmark) and hZAXATASE by International Bio-Synthetics, Inc. (The
3o Netherlands). Other professes include Protease A (see European Patent
Application
130,756, published January 9, 1985); Protease B (see European Patent
Application
251,446, published January 7, 1988, and European Patent Application
130,756, Bott et al, published January 9, 1985); and professes made by
Crenencor
International, Inc., according to one or more of the following patents:
Caldwell et al,
3s U.S. Patent Nos. 5,185,285, 5,204,015 and 5,244,791. Most preferred is what
is
called herein "Protease C", which is a variant of an alkaline serine protease
from
Bacillus. particularly Bacillus lentus. in which arginine replaced lysine at
position 27,
CA 02191314 1999-09-02
-13-
tyrosine replaced valine at position 104, serine replaced asparagine at
position 123,
and alanine replaced threonine at position 274. Protease C is described in
WO 91/06637; U.S. Patent No. 5,185,250; and U.S. Patent No. 5,204,015. Also
preferred are protease which are described in copending application U.S.
Patent No.
5,679,630, entitled Protease-containing Cleaning Compositions and U.S. Patent
No.
5,677,272, entitled Bleaching Compositions Comprising Protease Enzymes.
Genetically
modified variants, particularly of Protease C, are also included herein.
Amylases include, for example, a-amylases described in British Patent
Specification No. 1,296,839 (Novo), RAPIDASE'~'''~, International Bio-
Synthetics, Inc.
and TERMAMYL~'~'', Novo Industries.
The cellulase usable in the present invention include both bacterial or fungal
cellulase. Preferably, they will have a pH optimum of between 5 and 9.5.
Suitable
cellulases are disclosed in U.S. Patent 4,435,307, Barbesgoard et al, issued
March 6,
1984, which discloses fungal cellulase produced from Humicola insolens and
Humicola strain DSM1800 or a cellulase 212-producing fungus belonging to the
genus Aeromonas, and cellulase extracted from the hepatopancreas of a marine
mollusk (Dolabella Auricula Solander). suitable cellulases are also disclosed
in GB-
A-2.075.028; GB-A-2.095.275 and DE-OS-2.247.832. CAREZYME"~ (Novo) is
2o especially useful.
Suitable lipase enzymes for detergent usage include those produced by
microorganisms of the Pseudomonas group, such as Pseudomonas stutzeri ATCC
19.154, as disclosed in British Patent 1,372,034. See also Gpases in Japanese
Patent
Application 53,20487, laid open to public inspection on February 24, 1978.
This
Zs lipase is available from Amano Pharmaceutical Co. Ltd., Nagoya, Japan,
under the
trade mark Lipase P "Amano," hereinafter referred to as "Amano-P." Other
commercial lipases include Amano-CES, Gpases ex Chmmobacter viscosum, e.g.
Chromobacter viscosum var. Gpolyticum NRRLB 3673, commercially available from
Toyo Jozo Co., Tagata, Japan; and further Chromobacter viscosum Gpases from
U.S.
3o Biochemical Corp., U.S.A. and Disoynth Co., The Netherlands, and lipases ex
Pseudomonas gladioli. The LIPOLASET"' enzyme derived from Humicola lanuginosa
and commercially available from Novo (see also EPO 341,947) is a preferred
lipase
for use herein.
Peroxidase enzymes are used in combination with oxygen sources,
35 e.g., percarbonate, perborate, persulfate, hydrogen peroxide, etc. They are
used for
"solution bleaching," i.e. to prevent transfer of dyes or pigments removed
from
substrates during wash operations to other substrates in the wash solution.
CA 02191314 1999-09-02
-14-
Peroxidase enzymes are known in the art, and include, for example, horseradish
peroxidase, ligninase, and haloperoxidase such as chloro- and bromo-
peroxidase.
Peroxidase-containing detergent compositions are disclosed, for example, in
PCT
International Application WO 89/099813, published October 19, 1989, by O.
Kirk,
assigned to Novo Industries A/S. It may be desired to use, in combination with
these
peroxidases, materials viewed as being peroxidase accelerators such as
phenolsulfonate and/or phenothiazine.
A wide range of enzyme materials and means for their incorporation
into synthetic detergent compositions are also disclosed in U.S. Patent
3,553,139,
to issued January 5, 1971 to McCarty et al. Enzymes are further disclosed in
U.S.
Patent 4,101,457, Place et al, issued July 18, 1978, and in U.S. Patent
4,507,219,
Hughes, issued March 26, 1985, both. Enzyme materials useful for liquid
detergent
w formulations, and their incorporation into such formulations, are disclosed
in U.S.
Patent 4,261,868, Hora et al, issued April 14, 1981. Enzymes for use in
detergents
can be stabilized by various techniques. Enzyme stabilization techniques are
disclosed and exemplified in U.S. Patent 3,600,319, issued August 17, 1971 to
Gedge, et al, and European Patent Application Publication No. 0 199 405,
' published October 29, 1986, Venegas. Enzyme stabilization systems are also
described, for example, in U.S. Patent 3,519,570.
2o E~me Stabilizers - The enzymes employed herein are stabilized by the
presence of water-soluble sources of calcium and/or magnesium ions in the
finished
compositions which provide such ions to the enzymes. (Calcium ions are
generally
somewhat more effective than magnesium ions and are preferred herein if only
one
type of cation is being used.) Additional stability can be provided by the
presence of
various other art-disclosed stabilizers, especially borate species: see
Severson, U.S.
4,537,706. Typical detergents, especially liquids, will comprise from about 1
to
about 30, preferably from about 2 to about 20, more preferably from about 5 to
about 15, and most preferably from about 8 to about 12, millimoles of calcium
ion
per liter of 5nished composition. This can vary somewhat, depending on the
amount
of enzyme present and its response to the calcium or magnesium ions. The level
of
calciutrt or magnesium ions should be selected so that there is always some
minimum
level available for the enzyme, aRer allowing for complexation with builders,
fatty
acids, ete., in the composition. Any water-soluble calcium or magnesium salt
can be
used as the source of calcium or magnesium ions, including, but not limited
to,
calcium chloride, calcium sulfate, calcium malate, calcium maleate, calcium
hydroxide, calcium formate, and calcium acetate, and the corresponding
magnesium
salts. A small amount of calcium ion, generally from about 0.05 to about 0.4
2191314
WO 95133031 PC1'IUS9510fi560
-15-
millimoles per liter, is often also present in the composition due to calcium
in the
enzyme slurry and formula water. In solid detergent compositions the
formulation
may include a sufficient quantity of a water-soluble calcium ion source to
provide
such amounts in the laundry liquor. In the alternative, natural water hardness
may
suffice.
It is to be understood that the foregoing levels of calcium and/or magnesium
ions are sufficient to provide enzyme stability. More calcium and/or magnesium
ions
can be added to the compositions to provide an additional measure of grease
removal
performance. Accordingly, as a general proposition the compositions herein
will
to typically comprise from about 0.05% to about 2% by weight of a water-
soluble
source of calcium or magnesium ions, or both. The amount can vary, of course,
with
the amount and type of enzyme employed in the composition.
The compositions herein may also optionally, but preferably, contain various
additional stabilizers, especially borate-type stabilizers. Typically, such
stabilizers will
be used at levels in the compositions from about 0.25% to about 10%,
preferably
from about 0.5% to about 5%, more preferably from about 0.75% to about 3%, by
weight of boric acid or other borate compound capable of forming boric acid in
the
composition (calculated on the basis of boric acid). Boric acid is preferred,
ailthough
other compounds such as boric oxide, borax and other alkali metal borates
(e.g.,
2o sodium ortho-, meta- and pyroborate, and sodium pentaborate) are suitable.
Substituted boric acids (e.g., phenylboronic acid, butane boronic acid, and p-
bromo
phenylboronic acid) can also be used in place ofboric acid. It is to be
recogni.?ed that
such materials may also be used in formulations as the sole stabilizer as well
ass being
used in combination with added calcium and/or magnesium ions.
Finally, it may be desired to add chlorine scavengers, especially to protease-
containing compositions, to protect the enzymes from chlorine typically
present in
municipal water supplies. Such materials are described, for example, in U.S.
Patent
4,810,413 to Pancheri et al.
Bleaching Comr?ounds - Bleaching Agents and Bleach Activators - The.
3o detergent compositions herein may optionally contain bleaching agents or
bleaching
compositions containing a bleaching agent and one or more bleach activators.
When
present, bleaching agents will typically be at levels of from about I% to
about 30%,
more typically from about 5% to about 20%, of the detergent composition,
especially
for fabric laundering. If present, the amount of bleach activators will
typically be
from about 0.1% to about 60%, more typically from about 0.5% to about 40% of
the
bleaching composition comprising the bleaching agent-plus-bleach activator.
2191314
wU 95133031 PCTIUS95106560
-16-
The bleaching agents used herein can be any of the bleaching agents useful for
detergent compositions in textile cleaning or other cleaning purposes that are
now
known or become known. These include oxygen bleaches as well as other
bleaching
agents. Perborate bleaches, e.g., sodiurtl perborate (e.g., mono- or tetra-
hydrate) can
be used herein.
Another category of bleaching agent that can be used without restriction
encompasses percarboxylic acid bleaching agents and salts thereof. Suitable
examples of this class of agents include magnesium monoperoxyphthalate
hexahydrate, the magnesium salt of metachloro perbenzoic acid, 4-nonylamino-4-
io oxoperoxybutyric acid and diperoxydodecanedioic acid. Such bleaching agents
are
disclosed in U.S. Patent 4,483,781, Hartman, issued November 20, 1984, U.S.
Patent Application 740,446, Burns et al, filed June 3, 1985, European Patent
Application 0,133,354, Banks et al, published February 20, 1985, and U.S.
Patent
4,412,934, Chung et al, issued November 1, 1983. Highly preferred bleaching
agents
i5 also include 6-nonylamino-6-oxoperoxycaproic acid as described in U.S.
Patent
4,634,551, issued January 6, 1987 to Burns et al.
Peroxygen bleaching agents can also be used. Suitable peroxygen bleaching
compounds include sodium carbonate peroxyhydrate and equivalent "percarbonate"
bleaches, sodium pyrophosphate peroxyhydrate, urea peroxyhydrate, and sodium
20 peroxide. Persulfate bleach (e.g., OXONE, manufactured commercially by
DuPont)
can also be used.
A preferred percarbonate bleach comprises dry particles having an average
particle size in the range from about 500 micrometers to about 1,000
micrometers,
not more than about 10% by weight of said particles being smaller than about
200
25 micrometers and not more than about 10% by weight of said particles being
larger
than about 1,250 micrometers. Optionally, the percarbonate can be coated with
silicate, borate or water-soluble surfactants. Percarbonate is available from
various
commercial sources such as FMC, Solvay and Tokai Denka.
Mixtures of bleaching agents can also be used.
30 Peroxygen bleaching agents, the perborates, the percarbonates, etc., are
preferably combined with bleach activators, which lead to the in situ
production in
aqueous solution C.e., doting the washing process) ofthe peroxy acid
corresponding
to the bleach activator. Various nonlimiting examples of activators are
disclosed in
U.S. Patent 4,915,854, issued April 10, 1990 to Mao et al, and U.S. Patent
35 4,412,934. The nonanoyloxybenzene sulfonate (HOBS) and tetrascetyl ethylene
diarnine (TAED) activators are typical, and mixtures thereof can also be used.
See
also U.S. 4,634,551 for other typical bleaches and activators useful herein.
CA 02191314 1999-09-02
-17-
Highly preferred amido-derived bleach activators are those of the formulae:
R1N(RS)C(0)R2C(0)L or R1C(O)N(RS)R2C(O)L
wherein R1 is an alkyl group containing from about 6 to about 12 carbon atoms,
R2
is an alkylene containing from 1 to about 6 carbon atoms, RS is H or alkyl,
aryl, or
alkaryl containing from about 1 to about 10 carbon atoms, and L is any
suitable
leaving group. A leaving group is any group that is displaced from the bleach
activator as a consequence of the nucleophilic attack on the bleach activator
by the
perhydrolysis anion. A preferred leaving group is phenyl sulfonate.
Preferred examples of bleach activators of the above formulae include (6-
to octanamido-caproyl)oxybenzenesulfonate, (6-nonanamidocaproyl)oxybenzenesul
fonate, (6-decanamido-caproyl)oxybenzenesulfonate, and mixtures thereof as
described in U.S. Patent 4,634,551.
Another class of bleach activators comprises the benzoxazin-type activators
disclosed by Hodge et al in U.S. Patent 4,966,723, issued October 30, 1990.
15 A highly preferred activator of the benzoxazin-type is:
O
II
CEO
~,C
'N
Still another class of preferred bleach activators includes the aryl lactam
activators, especially aryl caprolactams and aryl valerolactams of the
formulae:
O C-C H2-C HZ 0 C-C H2-C H2
Re'-C-~ iC H2 RB-C-~
CH -C
20 C~-C~ , 2 H2
wherein R6 is H or an alkyl, aryl, alkoxyaryl, or alkaryl group containing
from 1 to
about 12 carbon atoms. I~ghly preferred lactam activators include benzoyl
caprolactam, octanoyl caprolactam, 3,5,5-trimethylhexanoyl caprolactam,
nonanoyl
caprolactam, decanoyl caprolactam, undecenoyl caprolactam, benzoyl
valerolactam,
25 octanoyl valerolactam, decanoyl valerolactam, undecenoyl valerolactam,
nonanoyl
valerolactam, 3,5,5-trimethylhexanoyl valerolactam and, mixtures thereof. See
also
U.S. Patent 4,545,784, issued to Sanderson, October 8, 1985, which discloses
aryl
caprolactams, including benzoyl caprolactam, adsorbed into sodium perborate.
W095133031 ~ 1 ~ ~ ~ ~ ~ PCTIUS95/06560
-18-
Bleaching agents other than oxygen bleaching agents arealso known in the
art and can be utilized herein. One type of non-oxygen bleaching agent of
particular
interest includes photo activated bleaching agents such as the sulfonated zinc
andlor
aluminum phthalocyanines. See U.S. Patent 4,033,718, issued July 5, I977 to
Holcombe et al. If used, detergent compositions will typically contain from
about
0.025% to about 1.25%, by weight, of such bleaches, especially sulfonate zinc
phthalocyanine.
If desired, the bleaching compounds can be catalyzed by means of a
manganese compound. Such compounds are well known in the art and include, for
to example, the manganese-based catalysts disclosed in U.S. Pat. 5,246,621,
U.S. Pat.
5,244,594; U.S. Pat. 5,194,416; U.S. Pat. 5,114,606; and European Pat. App.
Pub.
Nos. 549,271A1, 549,272A1, 544,440A2, and 544,490A1; Preferred examples of
these catalysts include MnN2(u-O)3(1,4,7-trimethyl-1,4,7-triazacyclo-
nonane)2(PF6)2, Mn~2(u-O)i(u-OAc)2(1,4,7-trimethyl-1,4,7-triazacyclononane)2-
(C104)2, Mn~4(u-O)6(1,4,7-triazacyclononane)4(CI04)4, MnEIMnIV4(u-O)1(u-
OAc)2_(1,4,7-trimethyl-1,4,7-triazacyclononane)2(C104)g, Mn~(1,4,7-trimethyl-
1,4,7-triazacyclononane)- (OCH3)3(PF6), and mixtures thereof. Other metal-
based
bleach catalysts include those disclosed in U.S. Pat. 4,430,243 and U.S. Pat.
5,114,611. The use of manganese with various complex ligands to enhance
bleaching
2o is also reported in the following United States Patents: 4,728,455;
5,284,944;
5,246,612; 5,256,779; 5,280,117; 5,274,147; 5,153,161; and 5,227,084.
As a practical matter, and not by way of limitation, the compositions and
processes herein can be adjusted to provide on the order of at least one part
per ten
million of the active bleach catalyst species in the aqueous washing liquor,
and will
z5 preferably provide from about 0.1 ppm to about 700 ppm, more preferably
from
about 1 ppm to about 500 ppm, of the catalyst species in the laundry liquor.
Polymeric Soil Release,Agent - Any polymeric soil release agent known to
those skilled in the art can optionally be employed in the compositions and
processes
of this invention. Polymeric soil release agents are characterized by having
both
3o hydrophilic segments, to hydrophilize the surface of hydrophobic fibers,
such as
polyester and nylon, and hydrophobic segments, to deposit upon hydrophobic
fibers
and remain adhered thereto through completion of washing and rinsing cycles
and,
thus, serve as an anchor for the hydrophilic segments. This can enable stains
occurring subsequent to treatment with the soil release agent to be more
easily
3s cleaned in later washing procedures.
The polymeric soil release agents useful herein especially include those soil
release agents having: (a) one or more nonionic hydrophile components
consisting
CA 02191314 1999-09-02
-19-
essentially of (i) polyoxyethylene segments with a degree of polymerization of
at least
2, or (ii) oxypropylene or polyoxypropylene segments with a degree of
polymerization of from 2 to 10, wherein said hydrophile segment does not
encompass
any oxypropylene unit unless it is bonded to adjacent moieties at each end by
ether
5 linkages, or (iii) a mixture of oxyalkylene units comprising oxyethylene and
from 1 to
about 30 oxypropylene units wherein said mixture contains a sufficient amount
of
oxyethylene units such that the hydrophile component has hydrophilicity great
enough to increase the hydrophilicity of conventional polyester synthetic
fiber
surfaces upon deposit of the soil release agent on such surface, said
hydrophile
1o segments preferably comprising at least about 25% oxyethylene units and
more
preferably, especially for such components having about 20 to 30 oxypropylene
units,
at least about 50% oxyethylene units; or (b) one or more hydrophobe components
comprising (i) C3 oxyalkylene terephthalate segments, wherein, if said
hydrophobe
components also comprise oxyethylene terephthalate, the ratio of oxyethylene
15 terephthalate:C3 oxyalkylene terephthalate units is about 2:1 or lower,
(ii) C4-C6
alkylene or oxy C4-C6 alkylene segments, or mixtures therein, (iii) poly
(vinyl ester)
segments, preferably polyvinyl acetate), having a degree of polymerization of
at least
2, or (iv) Cl-C4 alkyl ether or C4 hydroxyalkyl ether substituents, or
mixtures
therein, wherein said substituents are present in the form of C1-C4 alkyl
ether or C4
2o hydroxyalkyl ether cellulose derivatives, or mixtures therein, and such
cellulose
derivatives are amphiphilic, whereby they have a sufficient level of C1-C4
alkyl ether
and/or C4 hydroxyalkyl ether units to deposit upon conventional polyester
synthetic
fiber surfaces and retain a sufficient level of hydroxyls, once adhered to
such
conventional synthetic fiber surface, to increase fiber surface
hydrophilicity, or a
2s combination of (a) and (b).
Typically, the polyoxyethylene segments of (axi) wiU have a degree of
polymerization of from about 200, although higher levels can be used,
preferably
from 3 to about 150, more preferably from 6 to about 100. Suitable oxy C4-C6
alkylene hydrophobe segments include, but are not limited to, end-caps of
polymeric
3o soil release agents such as M03S(CH2)nOCH2CH20-, where M is sodium arid n
is
an integer from 4-6, as disclosed in U.S. Patent 4,721,580, issued January 26,
1988
to Gosselink.
Polymeric soil release agents useful in the present invention also include
cellulosic derivatives such as hydroxyether cellulosic polymers, copolymeric
blocks
3s of ethylene terephthalate or propylene terephthalate with polyethylene
oxide or
polypropylene oxide terephthalate, and the like. Such agents are commercially
available and include hydroxyethers of cellulose such as METHOCELT"' (Dow).
CA 02191314 1999-09-02
-20-
Cellulosic soil release agents for use herein also include those selected from
the
group consisting of C 1-C4 alkyl and C4 hydroxyalkyl cellulose; see U. S.
Patent
4,000,09;, issued December 28, 1976 to Nicol, et al.
Soil release agents characterized by polyvinyl ester) hydrophobe segments
include graft copolymers of polyvinyl ester), e.g., C1-C6 vinyl esters,
preferably
polyvinyl acetate) grafted onto polyalkylene oxide backbones, such as
polyethylene
oxide backbones. See European Patent Application 0 219 048, published April
22,
1987 by Kud, et al. Commercially available soil release agents of this kind
include
the SOKALAN'~M type of material, e.g., SOKALAN HP-22, available from BASF
(West Germany).
One type of preferred soil release agent is a copolymer having random blocks
of ethylene terephthalate and polyethylene oxide (PEO) terephthalate. The
molecular
weight of this polymeric soil release agent is in the range of from about
25,000 to
about 55,000. See U.S. Patent 3,959,230 to Hays, issued May 25, 1976 and U.S.
Patent 3,893,929 to Basadur issued July 8, 1975.
Another preferred polymeric soil release agent is a polyester with repeat
units
of ethylene terephthalate units contains 10-15% by weight of ethylene
terephthalate
units together with 90-80% by weight of polyoxyethylene terephthalate units,
derived
from a polyoxyethylene glycol of average molecular weight 300-5,000. Examples
of
2o this polymer include the commercially available material ZELCONTM 5126
(from
DuPont) and MILEASE'~M T (from ICI). See also U.S. Patent 4,702,857, issued
October 27, 1987 to Gosselink.
Another preferred polymeric soil release agent is a sulfonated product of a
substantially linear ester oligomer comprised of an oligomeric ester backbone
of
terephthaloyl and oxyalkyleneoxy repeat units and terminal moieties covalently
attached to the backbone. These soil release agents arc described fully in
U.S. Patent
4,968,451, issued November 6, 1990 to J. J. Scheibel and E. P. Gosselink.
Other
suitable polymeric soil release agents include the terephthalate polyesters of
U. S.
Patent 4,711,730, issued December 8, 1987 to Gosselink et al, the anionic end-
3o capped oligomeric esters of U.S. Patent 4,721,580, issued January 26, 1988
to
Gosselink, and the block polyester oligomeric compounds ofU.S. Patent
4,702,857,
issued October 27, 1987 to GosseGnk.
Preferred polymeric soil release agents also include the soil release agents
of
U.S. Patent 4,877,896, issued October 31, 1989 to Maldonado et al, which
discloses
35 anionic, especially sulfoarolyl, end-capped terephthalate esters. Still
another
preferred soil release agent is an oGgomer with repeat units of terephthaloyl
units,
sulfoisoterephthaloyl units, o~iyethyleneoxy and oxy-1,2-propylene units. The
repeat
CA 02191314 1999-09-02
-21-
units form the backbone of the oligomer and are preferably terminated with
modified
isethionate end-caps. A particularly preferred soil release agent of this type
comprises about one sulfoisophthaloyl unit, S terephthaloyl units,
oxyethyleneoxy
and oxy-1,2-propyleneoxy units in a ratio of from about 1.7 to about 1.8, and
two
end-cap units of sodium 2-(2-hydroxyethoxy)-ethanesulfonate. Said soil release
agent also comprises from about 0.5% to about 20%, by weight of the oligomer,
of a
crystalline-reducing stabilizer, preferably selected from the group consisting
of xylene
sulfonate, cumene sulfonate, toluene sulfonate, and mixtures thereof.
If utilized, soil release agents will generally comprise from about 0.01% to
to about 10.0%, by weight, of the detergent compositions herein, typically
from about
0.1% to about 5%, preferably from about 0.2% to about 3.0%.
Chelating Agents - The detergent compositions herein may also optionally
contain one or more iron and/or manganese chelating agents. Such chelating
agents
can be selected from the group consisting of amino carboxylates, amino
1s phosphonates, polyfunctionally-substituted aromatic chelating agents and
mixtures
therein, all as hereinafter defined. Without intending to be bound by theory,
it is
believed that the benefit of these materials is due in part to their
acceptional ability to
remove iron and manganese ions from washing solutions by formation of soluble
chelates.
2o Amino carboxylates useful as optional chelating agents include
ethylenediaminetetracetates, N-hydroxyethylethylenediaminetriacetates, nitrilo-
triacetates, ethylenediamine tetraproprionates,
triethylenetetraaminehexacetates,
diethylenetriaminepentaacetates, and ethanoldiglycines, alkali metal,
ammonium, and
substituted ammonium salts therein and mixtures therein.
2s Amino phosphonates are also suitable for use as chelating agents in the
compositions of the invention when at lease low levels of total phosphorus are
permitted in detergent compositions, and include ethylenediaminetetrakis
(methylenephosphonates) as DEQUESTz''''. Preferred, these amino phosphonates
to
not contain alkyl or alkenyl groups with more than about 6 carbon atoms.
3o Polyfunctionally-substituted aromatic chelating agents are also useful in
the
compositions herein. See U.S. Patent 3,812,044, issued May 21, 1974, to Connor
et
al. Preferred compounds of this type in acid form are dihydroxydisulfobenzenes
such
as 1,2-dihydroxy-3,5-disulfobenzate.
A preferred biodegradable chelator for use herein is ethylenediamine
35 disuccinate ("EDDS"), especially the [S,S] isomer as described in U.S.
Patent
4,704,233, November 3, 1987, to Hartman and Perkins.
W095/33031 ~ ~ ~ ~ ~ ~ ~ PCT/IJS95/06560
-22-
If utilized, these chelating agents will generally comprise from about 0.1% to
about 10% by weight of the detergent compositions herein. More preferably, if
utilized, the chelating agents will comprise from about 0.1% to about 3.0% by
weight
of such compositions.
Clay Soil Removal/Anti-redeR_o_sition Agents - The compositions of the
present invention can also optionally contain water-soluble ethoxylated amines
having clay soil removal and antiredeposition properties. Granular detergent
compositions which contain these compounds typically contain from about 0.01%
to
about 10.0% by weight of the water-soluble ethoxylates amines; liquid
detergent
to compositions typically contain about 0.01% to about 5%.
The most preferred soil release and anti-redeposition agent is ethoxylated
tetraethylenepentamine. Exemplary ethoxylated amines are further described in
U.S.
Patent 4,597,898, VanderMeer, issued July 1, 1986. Another group of preferred
clay
soil removal-antiredeposition agents are the cationic compounds disclosed in
European Patent Application 111,965, Oh and Gosselink, published June 27,
1984.
Other clay soil removaVantiredeposition agents which can be used include the
ethoxylated amine polymers disclosed in European Patent Application 111,984,
Gosselink, published June 27, 1984; the zwitterionic polymers disclosed in
European
Patent Application 112,592, Gosselink, published July 4, 1984; and the amine
oxides
2o disclosed in U.S. Patent 4,548,744, Connor, issued October 22, 1985. Other
clay
soil removal and/or anti redeposition agents known in the art can also be
utilized in
the compositions herein. Another type of preferred antiredeposition agent
includes
the carboxy methyl cellulose (CMC) materials. These materials are well known
in
the art.
Polymeric Dispersing_A_gents - Polymeric dispersing agents can
advantageously be utilized at levels from about 0.1% to about 7%, by weight,
in the
compositions herein. Suitable polymeric dispersing agents include polymeric
polycarboxylates and polyethylene glycols, although others known in the art
can also
be used.
3o Polymeric polycarboxylate materials can be prepared by polymerizing or
copoiymerizing suitable unsaturated monomers, preferably in their acid form.
Unsaturated monomeric acids that can be polymerized to form suitable polymeric
polycarboxylates include acrylic acid, malefic acid (or malefic anhydride),
fumaric acid,
itaconic acid, aconitic acid, mesacottic acid, citraconic acid and
methylenemalonic
acid. The presence in the polymeric polycarboxylates herein or monomeric
segments,
containing no carboxylate radicals such as vinylmethyl ether, styrene,
ethylene, etc. is
2191314
W 0 95133031 PC1'IUS95106560
-23-
suitable provided that such segments do not constitute more than about 40% by
weight.
Particularly suitable polymeric polycarboxylates can be derived from acrylic
acid. Such acrylic acid-based polymers which are useful herein are the water-
soluble
salts of polymerized acrylic acid. The average molecular weight of such
polymers in
the acid form preferably ranges from about 2,000 to 10,000, more preferably
from
about 4,000 to 7,000 and most preferably from about 4,000 to 5,000. Water-
soluble
salts of such acrylic acid polymers can include, for example, the alkali
metal,
ammonium and substituted ammonium salts. Soluble polymers of this type are
known materials. Use of polyacrylates of this type in detergent compositions
has
been disclosed, for example, in Diehl, U.S. Patent 3,308,067, issued March 7,
1967.
Acrylic/maleic-based copolymers may also be used as a preferred component
of the dispersing/anti-redeposition agent. Such materials include the water-
soluble
salts of copolymers of acrylic acid and malefic acid. The average molecular
weight of
such copolymers in the acid form preferably ranges from about 2,000 to
100,000. A
preferred copolymer has an average molecular weight of about 2,000 to 15,000,
more preferably about 6,000 to about 13,000, and most preferably about 7,000
to
about 12,000. Other preferred copolymers have an average molecular weight from
about 5,000 to 75,000, most preferably from about 7,000 to 65,000. The ratio
of
zo acrylate to maleate segments in such copolymers will generally range from
about
30:1 to about 1:2, more preferably from about 10:1 to 1:1, and most preferably
about
2.5:1 to 1:1. Water-soluble salts of such acrylic acid/maleic acid copolymers
can
include, for example, the alkali metal, ammonium and substituted ammonium
salts.
Soluble acrylatelmaleate copolymers of this type are known materials which are
described in European Patent Application No. 66915, published December 15,
1982,
as well as in EP 193,360, published September 3, 1986, which also describes
such
polymers comprising hydroxypropylacrylate. Still other useful dispersing
agents
include the maleic/acrylic/vinyl alcohol terpolymers. Such materials are also
disclosed in EP 193,360, including, for example, the 45/45/10 terpolymler of
acrylic/maleic/vinyl alcohol.
Particularly preferred dispersant polymers are low molecular weight
modified polyacrylate copolymers. Such copolymers contain as monomer units: a)
from about 90% to about 10%, preferably from about 80% to about 20% by weight
acrylic acid or its salts and b) from about 10% to about90%, preferably from
about
20% to about 80% by weight of a substituted acrylic monomer or its salt and
have
the general formula: -[(C(R2)C(RI)(C(O)OR3)]- wherein the incomplete valencies
inside the square braces are hydrogen and at least one of the substituents Rl,
R2 or
CA 02191314 1999-09-02
-24-
R3, preferably R1 or R2, is a 1 to 4 carbon alkyl or hydroxyalkyl group, R1 or
R'
can be a hydrogen and R3 can be a hydrogen or alkali metal salt. Most
preferred is a
substituted acrylic monomer wherein R1 is methyl, R2 is hydrogen and R3 is
sodium.
The low molecular weight polyacrylate dispersant polymer preferably has a
molecular weight of less than about 15,000, preferably from about 500 to about
10,000, most preferably from about 1,000 to about 5,000. The most preferred
polyacrylate copolymer for use herein has a molecular weight of 3500 and is
the fully
neutralized form of the polymer comprising about 70% by weight acrylic acid
and
about 30% by weight methacrylic acid.
to Other suitable modified polyacrylate copolymers include the low molecular
weight copolymers of unsaturated aliphatic carboxylic acids disclosed in U.S.
Patents
4,530,766, and 5,084,535.
Agglomerated forms of the present invention may employ aqueous solutions of
polymer dispersants as liquid binders for making the agglomerate (particularly
when
the composition consists of a mixture of sodium citrate and sodium carbonate).
Especially preferred are polyacrylates with an average molecular weight of
from
about 1,000 to about 10,000, and acrylate/maleate or acrylateJ fumarate
copolymers
with an average molecular weight of from about 2,000 to about 80,000 and a
ratio of
acrylate to maleate or fumarate segments of from about 30:1 to about 1:2.
Examples
of such copolymers based on a mixture of unsaturated mono- and dicarboxylate
monomers are disclosed in European Patent Application No. 66,915, published
December 15, 1982.
Other dispersant polymers useful herein include the polyethylene glycols and
polypropylene glycols having a molecular weight of from about 950 to about
30,000
2s which can be obtained from the Dow Chemical Company of Iv~dland, l~chigan.
Such compounds for example, having a melting point within the range of from
about
30° to about 100°C can be obtained at molecular weights of 1450,
3400, 4500, 6000,
7400, 9500, and 20,000. Such compounds are formed by the polymerization of
ethylene glycol or propylene glycol with the requisite number of moles of
ethylene or
3o propylene oxide to provide the desired molecular weight and melting point
of the
respective polyethylene glycol and polypropylene glycol. The polyethylene,
polypropylene and mixed glycols are referred to using the formula HO(CH2CH20)m
(CH2CH(CH3)O)n (CH(CH3)CH20)oH wherein m, n, and o are integers satisfying
the molecular weight and temperature requirements given above.
3s Yet other dispersant polymers useful herein include the cellulose sulfate
esters
such as cellulose acetate sulfate, cellulose sulfate, hydroxyethyl cellulose
sulfate,
CA 02191314 1999-09-02
-25-
methylcellulose sulfate, and hydroxypropylcellulose sulfate. Sodium cellulose
sulfate
is the most preferred polymer of this group.
Other suitable dispersant polymers are the carboxylated polysaccharides,
particularly starches, celluloses and alginates, described in U.S. Pat. No.
3,723,322,
5 Diehl, issued Mar. 27, 1973; the dextrin esters of polycarboxylic acids
disclosed in
U.S. Pat. No. 3,929,107, Thompson, issued Nov. 11, 1975; the hydroxyalkyl
starch
ethers, starch esters, oxidized starches, dextrins and starch hydrolysates
described in
U.S. Pat No. 3,803,285, Jensen, issued Apr. 9, 1974; the carboxylated starches
described in U.S. Pat. No. 3,629,121, Eldib, issued Dec. 21, 1971; and the
dextrin
1o starches described in U.S. Pat. No. 4,141,841, McDanald, issued Feb. 27,
1979.
Preferred cellulose-derived dispersant polymers are the carboxymethyl
celluloses.
Yet another group of acceptable dispersants are the organic dispersant
polymers, such as polyaspartate.
15 Another polymeric material which can be included is polyethylene glycol
(PEG). PEG can exhibit dispersing agent performance as well as act as a clay
soil
removal-antiredeposition agent. Typical molecular weight ranges for these
purposes
range from about 500 to about 100,000, preferably from about 1,000 to about
50,000, more preferably from about 1,500 to about 10,000.
2o Polyaspartate and polyglutamate dispersing agents may also be used,
especially in conjunction with zeolite builders: In compositions containing
detergent
builders, it is believed, though it is not intended to be limited by theory,
that
polymeric dispersing agents enhance overall detergent builder performance,
especially zeoGte and/or silicate builders, when used in combination with
other
2s builders (including lower molecular weight polycatboxylates) by crystal
growth
inlu'bition, particulate soil release peptization, and anti-redeposidon.
Dispersing
agents such as polyaspartate preferably have a molecular weight (avg.) of
about
10,000.
Bri toner - Any optical brighteners or other brightening or whitening agents
3o known in the art can be incorporated at levels typically from about 0.05%
to about
1.2%,,by weight, into the detergent compositions herein. Commercial optical
brighteners which may be useful in the present invention can be classified
into
subgroups, which include, but are not necessarily limited to, derivatives of
stilbene,
pyrazoline, coumarin, carboxylic acid, methinecyanines, dibenzothiphene-5,5-
dioxide,
3s azoles, 5- and 6-membered-ring heterocycles, and other miscellaneous
agents.
Examples of such bcighteners are disclosed in "The Production and Application
of
CA 02191314 1999-09-02
-26-
Fluorescent Brightening Agents", M. Zahradnik, Published by John Wiley & Sons,
New York ( 1982).
Specific examples of optical brighteners which are useful in the present
compositions are those identified in U.S. Patent 4,790,856, issued to Wixon on
December 13, 1988. These brighteners include the PHORWHITE'~M series of
brighteners from Verona. Other brighteners disclosed in this reference
include:
Tinopal''M LJNPA, Tinopal CBS and Tinopal SBM; available from Ciba-Geigy;
Antic
White''' CC and Antic White CWD, available from Hilton-Davis, located in
Italy; the 2-
(4-stryl-phenyl)-2H-napthol[1,2-d]triazoles; 4,4'-bis- (1,2,3-triazol-2-yl)-
stil- benes;
l0 4,4'-bis(stryl)bisphenyls; and the aminocoumarins. Specific examples of
these
brighteners include 4-methyl-7-diethyl- amino coumarin; 1,2-bis(-venzimidazol-
2-
yl)ethylene; 1,3-Biphenyl-phrazolines; 2,5-bis(benzoxazol-2-yl)thiophene; 2-
stryl-
napth-[1,2-d]oxazole; and 2-(stilbene-4-yl)-2H-naphtho- [1,2-d]triazole. See
also
U.S. Patent 3,646,015, issued February 29, 1972 to Hamilton. Anionic
brighteners
is are preferred herein.
Suds Suno_reccnrc - Compounds for reducing or suppressing the formation of
suds can be incorporated into the compositions of the present invention. Suds
suppression can be of particular importance in the so-called "high
concentration
cleaning process" as described in U.S. 4,489,45s and 4,489,574 and in front-
loading
2o European-style washing machines.
A wide variety of materials may be used as suds suppressers, and suds
suppressers are well known to those skilled in the art. See, for example, Kirk
Othmer Encyclopedia of Chemical Technology, Third Edition, Volume 7, pages 430-
447 (John Wiley & Sons, Inc., 1979). One category of suds suppresser of
particular
2s interest encompasses monocarboxylic fatty acid and soluble salts therein.
Sec U.S.
Patent 2,9s4,347, issued September 27, 1960 to Wayne St. John. The
monocarboxylic fatty acids and salts thereof used as suds suppresser typically
have
hydrocarbyl chains of 10 to about 24 carbon atoms, preferably 12 to 18 carbon
atoms. Suitable salts include the alkali metal salts such as sodium,
potassium, and
3o lithium salts, and ammonium and alkanolammonium salts.
. The detergent compositions herein may also contain non-surfactant suds
suppressers. These include, for example: high molecular weight hydrocarbons
such
as paraffin, fatty acid esters (e.g., fatty acid triglycerides), fatty acid
esters of
monovalent alcohols, aliphatic Clg-C40 ketones (e.g., stearone), etc. Other
suds
3s inhibitors include N-alkylated amino triazines such as tri- to hexa-
alkyimelamines or
di- to tetra-alkyldiamine chlortriazines formed as products of cyanuric
chloride with
two or three moles of a primary or secondary amine containing 1 to 24 carbon
atoms,
CA 02191314 1999-09-02
-27-
propylene oxide, and monostearyl phosphates such as monostearyl alcohol
phosphate
ester and monostearyl di-alkali metal (e.g., K, Na, and Li) phosphates and
phosphate
esters. The hydrocarbons such as paraffin and haloparaffin can be utilized in
liquid
form. The liquid hydrocarbons will be liquid at room temperature and
atmospheric
5 pressure, and will have a pour point in the range of about -40°C and
about 50°C, and
a minimum boiling point not less than about 110°C (atmospheric
pressure). It is also
known to utilize waxy hydrocarbons, preferably having a melting point below
about
100°C. The hydrocarbons constitute a preferred category of suds
suppresser for
detergent compositions. Hydrocarbon suds suppressers are described, for
example,
1o in U.S. Patent 4,265,779, issued May 5, 1981 to Gandolfo et al. The
hydrocarbons,
thus, include aliphatic, alicyclic, aromatic, and heterocyclic saturated or
unsaturated
hydrocarbons having from about 12 to about 70 carbon atoms. The term
"paraffin,"
as used in this suds suppresser discussion, is intended to include mixtures of
true
paraffins and cyclic hydrocarbons.
15 Another preferred category of non-surfactant suds suppressers comprises
silicone suds suppressers. This category includes the use of
polyorganosiloxane oils,
such as polydimethylsiloxane, dispersions or emulsions of polyorganosiloxane
oils or
resins, and combinations of polyorganosiloxane with silica particles wherein
the
polyorganosiloxane is chemisorbed or fused onto the silica. Silicone suds
Zo suppressers are well known in the art and are, for example, disclosed in
U.S. Patent
4;265,779, issued May 5, 1981 to Gandolfo et al aad European Patent
Application
No. 354,016, published February 7, 1990, by Starch, M.S.
Other silicone suds suppressers are disclosed in U.S. Patent 3,455,839 which
relates to compositions and processes for defoaming aqueous solutions by
25 incorporating therein small amounts of polydimethylsiloxane fluids.
Mixtures of silicone and silanated silica are described, for instance, in
German
Patent Application DOS 2,124,526. Silicone defoamers and suds controlling
agents
in granular detergent compositions are disclosed in U.S. Patent 3,933,672,
Bartolotta
et al, and in U.S. Patent 4,652,392, Baginski et aI, issued March 24, 1987.
3o An exemplary silicone based suds suppresser for use herein is a suds
suppressing amount of a suds controlling agent consisting essentially of
(i) polydimethylsiloxane fluid having a viscosity of from about 20 cs. to
about 1,500 cs. at 25°C;
(ii) from about 5 to about 50 parts per 100 parts by weight of (i) of siloxane
35 resin composed of (CH3)3Si01~ units of Si02 units in a ratio of from
(CH3)3 SiOl~ units and to Si02 units of from about 0.6:1 to about
1.2:1; and
3 I 4 pCTIUS95106560
WO 95!33031
_28_
(iii) from about I to about 20 parts per 100 parts by weight of (i) of a solid
silica gel.
In the preferred silicone suds suppressor used herein, the solvent for a
continuous phase is made up of certain polyethylene glycols or polyethylene-
polypropylene glycol copolymers or mixtures thereof (preferred), or
polypropylene
glycol. The primary silicone suds suppressor is branchedlcrosslinked and
preferably
not linear.
To illustrate this point further, typical liquid laundry detergent
compositions
with controlled suds will optionally comprise from about 0.001 to about 1,
preferably
l0 from about 0.01 to about 0.7, most preferably from about 0.05 to about 0.5,
weight
of said silicone suds suppressor, which comprises (1) a nonaqueous emulsion of
a
primary antifoam agent which is a mixture of (a) a polyorganosiloxane, (b) a
resinous
siloxane or a silicone resin-producing silicone compound, (c) a finely divided
filler
material, and (d) a catalyst to promote the reaction of mixture components
(a), (b)
and (c), to form silanolates; (2) at least one nonionic silicone surfactant;
and (3)
polyethylene glycol or a copolymer of polyethylene-polypropylene glycol having
a
solubility in water at room temperature of more than about 2 weight %; and
without
polypropylene glycol. Similar amounts can be used in granular compositions,
gels,
etc. See also U.S. Patents 4,978,471, Starch, issued December 18, 1990, and
4,983,316, Starch, issued January 8, 1991, 5,288,431, Huber et al., issued
February
22, 1994, and U.S. Patents 4,639,489 and 4,749,740, Aizawa et al at column 1,
line
46 through column 4, line 35.
The silicone suds suppressor herein preferably comprises polyethylene glycol
and a copolymer of polyethylene glycol/polypropylene glycol, all having an
average
molecular weight of less than about 1,000, preferably between about 100 and
800.
The polyethylene glycol and polyethylene/polypropylene copolymers herein have
a
solubility in water at room temperature of more than about 2 weight %,
preferably
more than about 5 weight %.
The preferred solvent herein is polyethylene glycol having an average
3o molecular weight of less than about 1,000, more preferably between about
100 and
800, most preferably between 200 and 400, and a copolymer of polyethylene
glycoVpolypropylene glycol, preferably PPG 200/PEG 300. Preferred is a weight
ratio ofbetween about 1:1 and 1:10, most preferably between 1:3 and 1:6, of
polyethylene glycol:copolymer of polyethylene-polypropylene glycol.
The preferred silicone suds suppressors used herein do not contain
polypropylene glycol, particularly of4,000 molecular weight. They also
preferably
CA 02191314 1999-09-02
-29-
do not contain block copolymers of ethylene oxide and propylene oxide, like
PLURONIC'~'' L101.
Other suds suppressorswseful herein comprise the secondary alcohols (e.g.,
2-alkyl alkanols) and mixtures of such alcohols with silicone oils, such as
the silicones
disclosed in U.S. 4,798,679, 4,075,118 and EP 150,872. The secondary alcohols
include the C6-C 16 alkyl alcohols having a C 1-C 16 chain. A preferred
alcohol is 2-
butyl octanol, which is available from Condea under the trademark ISOFOL'M 12.
Mixtures of secondary alcohols are available under the trademark ISALCHEM~ 123
from Enichem. Mixed suds suppressors typically comprise mixtures of alcohol +
1o silicone at a weight ratio of 1:5 to 5:1.
For any detergent compositions to be used in automatic laundry washing
machines, suds should not form to the extent that they overflow the washing
machine. Suds suppressors, when utilized, are preferably present in a "suds
suppressing amount. By "suds suppressing amount" is meant that the formulator
of
~5 the composition can select an amount of this suds controlling agent that
will
sufFtciently control the suds to result in a low-sudsing laundry detergent for
use in
automatic laundry washing machines.
The compositions herein will generally comprise from 0% to about 5% of
suds suppressor. When utilized as suds suppressors, monocarboxylic fatty
acids, and
Zo salts therein, will be present typically in amounts up to about 5%, by
weight, of the
detergent composition. Preferably, from about 0.5% to about 3% of fatty
monocarboxylate suds suppressor is utilized. Silicone suds suppressors are
typically
utilized in amounts up to about 2.0%, by weight, of the detergent composition,
although higher amounts may be used. This upper limit is practical in nature,
due
Z5 primarily to concern with keeping costs minimized and effectiveness of
lower
amounts for effectively controlling sudsing. Preferably from about 0.01% to
about
1% of silicone suds suppressor is used, more preferably from about 0.25% to
about
0.5%. As used herein, these weight percentage values include any silica that
may be
utilized in combination with polyorganosiloxane, as well as any adjunct
materials that
3o may be utilized. Monostearyl phosphate suds suppressors are generally
utilized in
amounts ranging from about 0.1% to about 2%, by weight, of the composition.
Hydrocarbon suds suppressors are typically utilized in amounts ranging from
about
0.01% to about 5.0%, although higher levels can be used. The alcohol suds
suppressors are typically used at 0.2%-3% by weight of the finished
compositions.
35 Fabric Softeners ~ Various through-the-wash fabric softeners, especially
the
impalpable smectite clays of U.S. Patent 4,062,647, Storm and N~rschl, issued
December 13, 1977, as well as other softener clays known in the art, can
optionally
2191314
W0 95133031 PCTlUS95106560
-3 0-
be used typically at levels of from about 0.5% to about 10% by weight in the
present
compositions to provide fabric softener benefits concurrently with fabric
cleaning.
Clay softeners can be used in combination with amine and cationic softeners as
disclosed, for example, in U.S. Patent 4,375,416, Crisp et al, March I, 1983
and U.S.
Patent 4,291,071, Harris et al, issued September 22, 1981.
I?ve Transfer Inhibiting Aeents -The granular compositions of the present
invention may also include one or more materials effective for inhibiting the
transfer
of dyes from one fabric to another during the cleaning process. Generally,
such dye
transfer inhibiting agents include polyvinyl pyrrolidone polymers, polyamine N-
oxide
io polymers, copolymers ofN-vinylpyrrolidone and N-vinylimidazole, manganese
phthalocyanine, peroxidases, and mixtures thereof. If used, these agents
typically
comprise from about 0.01% to about 10% by weight ofthe composition, preferably
from about 0.01% to about 5%, and more preferably from about 0.05% to about
2%.
More specifically, the polyamine N-oxide polymers preferred for use herein
contain units having the following structural formula: R-Ax-P; wherein P is a
polymerizable unit to which an N-O group can be attached or the N-O group can
form part of the polymerizable unit or the N-O group can be attached to both
units; A
is one of the following structures: -NC(O)-, -C(O)O-, -S-, -O-, -N=; x is 0 or
1; and
R is aliphatic, ethoxylated aliphatics, aromatics, heterocyclic or alicyclic
groups or
any combination thereof to which the nitrogen of the N-O group can be attached
or
the N-O group is part of these groups. Preferred polyamine N-oxides are those
wherein R is a heterocyclic group such as pyridine, pyrrole, imidazole,
pyrrolidine,
piperidine and derivatives thereof.
The N-O group can be represented by the following general structures:
O
(Rtht ~ WRz~: =N-(Ruc
(Rs)z
as
wherein Rl, R2, R3 are aliphatic, aromatic, heterocyclic or alicyclic groups
or
combinations thereof; x, y and z are 0 or 1; and the nitrogen of the N-O group
can be
attached or form part of any of the aforementioned groups. The amine oxide
utat of
the polyamine N-oxides has a pKa <10, preferably pKa <7, more preferred pKa
<6.
Any polymer backbone can be used as long as the amine oxide polymer
formed is water-soluble and has dye transfer inhibiting properties. Examples
of
suitable polymeric backbones are poiyvinyls, polyalkylenes, polyesters,
polyethers,
polyamide, polyimides, polyacrylates and mixtures thereof. These polymers
include
random or block copolymers where one monomer type is an amine N-oxide and the
CA 02191314 1999-09-02
-31-
other monomer type is an N-oxide. The amine N-oxide polymers typically have a
ratio of amine to the amine N-oxide of 10:1 to 1:1,000,000. However, the
number of
amine oxide groups present in the polyamine oxide polymer can be varied by
appropriate copolymerization or by an appropriate degree of N-oxidation. The
polyamine oxides can be obtained in almost any degree of polymerization.
Typically,
the average molecular weight is within the range of 500 to 1,000,000; more
preferred
1,000 to 500,000; most preferred 5,000 to 100,000. This preferred class of
materials
can be referred to as "PVNO".
The most preferred polyamine N-oxide useful in the detergent compositions
to herein is poly(4-vinylpyridine-N-oxide) which as an average molecular
weight of
about 50,000 and an amine to amine N-oxide ratio of about 1:4.
Copolymers of N-vinylpyrrolidone and N-vinylimidazole polymers (referred to
as a class as "PVPVI") are also preferred for use herein. Preferably the PVPVI
has
an average molecular weight range from 5,000 to 1,000,000, more preferably
from
1s 5,000 to 200,000, and most preferably from 10,000 to 20,000. (The average
molecular weight range is determined by light scattering as described in
Barth, et al.,
Chemical Analysis_ Vol 113. "Modern Methods of Polymer Characterization".)
The PVPVI copolymers typically have a molar ratio of N-vinylimidazole to N-
vinylpyrrolidone from 1:1 to 0.2:1, more preferably from 0.8:1 to 0.3:1, most
20 preferably from 0.6:1 to 0.4:1. These copolymers can be either linear or
branched.
The present invention compositions also may employ a polyvinylpyrrolidone
("PVP") having an average molecular weight of from about 5,000 to about
400,000,
preferably from about 5,000 to about 200,000, and more preferably from about
5,000
2s to about 50,000. PYP's are known to persons skilled in the detergent field;
see, for
example, EP-A 262,897 and EP-A-256,696, incorporated herein by reference.
Compositions containing PVP can also contain polyethylene glycol ("PEG")
having
an average molecular weight from about 500 to about 100,000, preferably from
about
1,000 to about 10,000. Preferably, the ratio of PEG to PVP on a ppm basis
delivered
3o in wash solutions is from about 2:1 to about 50:1, and more preferably from
about
3:1 to about 10:1.
The detergent compositions herein may also optionally contain from about
0.005% to 5% by weight of certain types of hydrophilic optical brighteners
which
also provide a dye transfer inhibition action. If used, the compositions
herein will
35 preferably comprise from about 0.01% to 1% by weight of such optical
brighteners.
The hydrophilic optical brightenen useful in the present invention are those
having the structural formula:
CA 02191314 1999-09-02
-3 2-
R~ R
Z
~--N H H N
N N C=C
~- N ~~ N
/ N H H N
RZ S03M S03M
Ri
wherein RI is selected from anilino, N-2-bis-hydroxyethyl and NH-2-
hydroxyethyl;
R2 is selected from N-2-bis-hydroxyethyl, N-2-hydroxyethyl-N-methylamino,
morphilino, chloro and amino; and M is a salt-forming cation such as sodium or
s potassium.
When in the above formula, R1 is anilino, R2 is N-2-bis-hydroxyethyl and M
is a canon such as sodium, the brightener is 4,4',-bis[(4-anilino-b-(N-2-bis-
hydroxyethyl)-s-triazine-2-yl)amino]-2,2'-stilbenedisulfonic acid and disodium
salt.
This particular brightener species is commercially marketed under the trade
mark
to Tinopal-LJNPA-GX by Ciba-Geigy Corporation. Tinopal-UNPA-GX is the
preferred
hydrophilic optical brightener useful in the detergent compositions herein.
When in the above formula, R1 is anilino, R2 is N-2-hydroxyethyl-N-2-
methylamino and M is a cation such as sodium, the brightener is 4,4'-bis[(4-
anilino-6-
(N-2-hydroxyethyl-N~methylamino~s-triazine-2-yl)amino]2,2'-stilbenedisulfonic
acid
1s disodium salt. This particular brightener species is commercially marketed
under the
trade mark Tinopal SBM-GX by Ciba-Geigy Corporation.
When in the above formula, Rl is anilino, R2 is morphilino and M is a cation
such as sodium, the brightener is 4,4'-bis[(4-anilino-6-morphilino-s-triazine-
2-
yl)amino]2,2'-stilbenedisulfonic acid, sodium salt. This particular brightener
species is
2o commercially marketed under the trademark Tinopal AMS-GX by Ciba Geigy
Corporation.
The specific optical brightener species xleeted for use in the present
invention
provide especially effective dye transfer inhibition performance benefits when
uxd in
combination with the xlected polymeric dye transfer inhibiting agents
hereinbefore
25 described. The combination of such xlected polymeric materials (e.g., PVNO
and/or
PVPVn with such selected optical brighteners (e.g., Tinopal UNPA-GX, Tinopal
sBM-GX, Tinopal-PLC, and/or Tinopal AMS-GX) provides significantly better dye
transfer inhibition in aqueous wash solutions than does either of these two
detergent
composition components when used alone. Without being bound by theory, it is
3o believed that such brighteners work this way because they have high
aflznity for
fabrics in the wash solution and therefore deposit relatively quick on thex
fabrics.
The extent to which brighteners deposit on fabrics in the wash solution can be
defined
by a parameter called the "exhaustion coefficient". The exhaustion coef5cient
is in
X191314
W0 95/33031 PCTIUS95/06560
-33-
general as the ratio of a) the brightener material deposited on fabric to b)
the initial
brightener concentration in the wash liquor. Brighteners with relatively high
exhaustion coefficients are the most suitable for inhibiting dye transfer in
the context
of the present invention.
Of course, it will be appreciated that other, conventional optical brigh~tener
types of compounds can optionally be used in the present compositions to
pravide
conventional fabric "brightness" benefits, rather than a true dye transfer
inhibiting
effect. Such usage is conventional and well-known to detergent formulations.
Other Ingr di n ~ - A wide variety of other ingredients useful in detergent
1o compositions can be included in the compositions herein, including other
active
ingredients, carriers, hydrotropes, processing aids, dyes or pigments,
solvents for
liquid formulations, solid fillers for bar compositions, etc. If high sudsing
is desired,
suds boosters such as the CIO-C16 alkanolamides can be incorporated into the
compositions, typically at 1%-10% levels. The CIO-C14 monoethanol and
diE;thanol
amides illustrate a typical class of such suds boosters. Use of such suds
boosters
with high sudsing adjunct surfactants such as the amine oxides, betaines and
sultaines
noted above is also advantageous. If desired, soluble magnesium salts such as
MgCl2, MgS04, and the like, can be added at levels of, typically, 0.1 %-2%, to
provide additional suds and to enhance grease removal performance.
Various detersive ingredients employed in the present compositions
optionally can be further stabilized by absorbing said ingredients onto a
porous
hydrophobic substrate, then coating said substrate with a hydrophobic coating.
Preferably, the detersive ingredientis admixed with a surfactant before being
absorbed into the porous substrate. In use, the detersive ingredient is
released from
the substrate into the aqueous washing liquor, where it performs its intended
detersive function.
To illustrate this technique in more detail, a porous hydrophobic silica
(trademark SIPERNAT DLO, DeGussa) is admixed with a proteolytic enzyme
solution containing 3%-5% of C13-15 ethoxylated alcohol (EO 7) nonionic
3o surfactant. Typically, the enzyme/surfactant solution is 2.5 X the weight
of silica.
The resulting powder is dispersed with stirring in silicone oil (various
silicone oil
viscosities in the range of 500-12,500 can be used). The resulting silicone
oil
dispersion is emulsified or otherwise added to the final detergent matrix. By
this
means, ingredients such as the aforementioned enzymes, bleaches, bleach
activators,
bleach catalysts, photo activators, dyes, fluorescers, fabric conditioners and
hydrolyzable surfactants can be "protected" for use in detergents, including
liquid
Laundry detergent compositions.
CA 02191314 1999-09-02
-34-
Liquid detergent compositions can contain water and other solvents as
carriers. Low molecular weight primary or secondary alcohols exemplified by
methanol, ethanol, propanol, and isopropanol are suitable. Monohydric alcohols
are
preferred for solubilizing surfactant, but polyols such as those containing
from 2 to
5 about 6 carbon atoms and from 2 to about 6 hydroxy groups (e.g., 1,3-
propanediol,
ethylene glycol, glycerine, and 1,2-propanediol) can also be used. The
compositions
may contain from 5% to 90%, typically 10% to 50% of such carriers.
Granular detergents can be prepared, for example, by spray-drying (final
product density about 520 g/!) or agglomerating (final product density above
about
1o b00 g/I) the Base Granule. The remaining dry ingredients can then be
admixed in
granular or powder form with the Base Granule, for example in a rotary mixing
drum,
and the liquid ingredients (e.g., nonionic surfactant and perfume) can be
sprayed on.
The detergent compositions herein wiD preferably be formulated such that,
during use in aqueous cleaning operations, the wash water will have a pH of
between
15 about 6.5 and about 11, preferably between about 7.5 and 10.5. Liquid
dishwashing
product formulations preferably have a pH between about 6.8 and about 9Ø
Laundry products are typically at pH 9-11. Techniques for controlling pH at
recommended usage levels include the use of buffers, alkalis, acids, etc., and
are well
known to those skilled in the art.
2o In order to make the present invention more readily understood, reference
is
made to the following examples, which are intended to be illustrative only and
not
intended to be limiting in scope.
EXAMPLE I
This Example illustrates heavy duty granular detergents containing a
2s surfactant system and oleoyl sarcosinate in accordance with the invention.
The
ingredients in the typical granular detergents exemplified herein are set
forth in Table
I below.
AB
('/. W~,- _
30 Comuoneot
C 12-I8 ~yl ethoxy (0.6) sulfate10.1 8.9 4.9
C 11-13 X3'1 ~~ne sulfonate - - 9.
3
Neodof~"'' 23-9' 0.5 0.4 2.2
Neodol 23-32 0.5 0.4 -
C 12-14 N-methyl glucamide 0.9 0.8 -
Oleoyl sarcosinate 5.7 5.0 5.6
Polyacrylate (MW=4500) 3.0 2.6 2.9
X191314
WO 95133031 PCT/US95/06560
-35-
Polyethylene glycol (MW=4000) 1.2 1.7 1.5
Sodium silicate _ - 0.7
Sodium Sulfate 16.6 14.5 14.6
Aluminosilicate 24.3 21.3 ,-25.7
Sodium carbonate 27.2 23.8 14.5
Protease enzyme 0.4 03 0.3
Amylase enzyme 0.1 _ _
Lipase enzyme 0.2 _ _
Cellulase enzyme 0.1 - 2.1
NOBS3 - 4.7 4.9
Sodium perborate - 4.3 3.3
Minors (water, perfume, etc.) 9~
100.0 100.0 100.0
1 C12-13 alkyl ethoxylate (EO=9) commercially available from Shell Oil
Company.
2 C12-13 alkyl ethoxylate (EO=3) commercially available from Shell Oil
Company.
3 Nonanoyloxybenzene sulfate
Initially a base formula containing the starting liquids and powders is made
via one of a variety of known processes including conventional spray drying
techniques or agglomeration in apparatus such as powder mixers and fluid beds
commercially available from Liidige and Aeromatic, respectively. Agglomeration
is
especially suitable for preparing modern compact granular detergents and
entails
initially forming a surfactant paste using standard mixers, after which the
paste is
agglomerated into agglomerates and dried. Such processing techniques are well
known in the art. Detergent ingredients such as enzymes are dry mixed into the
base
formula and/or ingredients such as perfumes are subsequently sprayed onto the
base
formula so as to form the final granular detergent compositions exemplified
herein.
EXAMPLE II
is This Example illustrates liquid laundry detergent compositions according to
the invention. Table II illustrates the various ingredients of the liquid
laundry
detergent.
TABLE iI
(% Weiahtl
Component
Oleoyl sarcosinate 9.0 7.0
C 12-18 alkyl ethoxy (0.6)12.0 14.0
sulfate
C12-14 N-methyl glucamide 6.0 6.0
C9-11 alkyl ethoxylate 3.0 3.0
(EO=8)
C12-20 fatty acid 4.0 4.0
291314
w0 95133031 PCT/US95106560
-36-
Citric acid 0.5 0.5
Protease enzyme 0.5 0.5
Lipase enzyme 0.2 0.2
Amylase enzyme 0.1 0.1
Cellulase enzyme 0.1 0.1
Misc. (water, solvent, perfume, etc.) _58.1 _58.1
100.00 100.00
EXAMPLE III
This Example illustrates laundry bars in accordance with the invention. The
laundry bars exemplified herein are prepared by standard extrusion processes
so as to
be suitable for handwashing soiled fabrics. Table III sets forth the various
ingredients
in the laundry bars.
TABLE III
L/o Weight)
Component r
C 12-18 alkyl ethoxy (0.6) 10.1 8.9
sulfate
Neodo123-91 0.5 0.4
Neodol23-32 0.5 0.4
C12-14 N-methyl giucamide 0.9 0.8
Oleoyl sarcosinate 5.7 5.0
Sodium pyrophosphate 7.0 7.0
Sodium tripolyphosphate 7.0 7.0
Sodium carbonate 25.0 25.0
Aluminosilicate 5.0 5.0
Carboxymethyl cellulose 0.2 0.2
Polyacrylate (MW=1400) 0.2 0.2
Protease enzyme 0.4 0.4
Iv&nors (water, filler, 37 ~. 7
perfume, etc.)
.""..,
I C12-13 alkyl ethoxylate (EO=9) commercially available from Shell Oil
Company.
2 C12-13 alkyl ethoxylate (EO=3) commercially available from Shell Oil
Company.
Having thus described the invention in detail, it will be clear to those
skilled in
the art that various changes may be made without departing from the scope of
the
invention and the invention is not to be considered limited to what is
described in the ,
specification.