Note: Descriptions are shown in the official language in which they were submitted.
~ . wogsl34373 2 1 ~ 1 438 -1- PCT/US95~06691
MONOFIL~MENT 5P~CING OF HOLLOW FIBER M~MRRZ~NF!F:
AND BLOOD OXYGENATION DEVICES INCORPORATING SAME
Present Invention
The present invention pertains generally to medical
equipment and, more particularly, to improved hollow fiber
l0 semi-permeable membranes having monofilamentous spacer
member(s) spirally disposed on the outer surface thereof,
and extracorporeal gas exchange devices incorporating such
hollow fiber semi-permeable membranes.
Backqround of the Inve~tiQn
~tracorporeal blood oxygenation systems are typically
utilized to a) oxygenate and b) remove carbon dioxide from
the blood of patients whose cardiopulmonary f unction has
been interrupted. The most common clinical situations
wherein extracorpore~Ll blood oxygenation systems are
utilized is during cardiothoracic surgical procedures
wherein cardiopulmonary bypass is employed.
The typical extracQrporeal blood oxygenation system in
use today comprises a rigid housing having a multiplicity
of tubular semi-permeable membranes known as "hollow fiber
membranes" disposed therewithin. Pure oxygen or an oxygen-
containing gas mixture is channeled through the lumens of
the hollow fiber membranes, while the patient's blood is
passed over the outer surfaces of the hollow fiber
3 0 membranes . Contact of the patient ' s blood with the outer
surfaces of the membranes permits oxygen to diffuse through
the membranes and into the blood, with concomitant back-
diffusion of carbon dioxide from the blood and into the
gas .
The partial pressures of oxygen (PO2) and carbon
dioxide (PCO2) are lnfluenced by numerous factors including;
~ ,
WO9S/34373 2 l 9143~ P~l~u~ r. 9~
'^ 2r: ~ j
a) the available surface area for gas exchange ~i.e., the
area ~herein blood actually contacts the outer surfaces of
the hollow fiber membranes; b) the relative concentrations
of oxygen and carbon dioxide in the gas mixture; c) the
pressure of the gas mixture within the lumens of the hollow
fiber membranes; d) the flow rate of blood over the outer
surfaces of the hollow fiber membranes; e) the film
thickness of blood passing over the outer surfaces of the
hollow fiber membranes; and f) the pressure and flow rate
of blood pass~g over the outer surfaces of the hollow
f iber membranes .
Although a number sf the above-listed variables may be
controlled by adjusting the pressures, flow rates and/or
relative concentrations of blood and/or gases flowing
through the device, the variable of "blood film thickness"
is largely a function of t~he design of the device and is
primarily dictated by the size of the spaces which exist
between the irdividual hollow f ibers within the device.
Inconsistencies or varia~ions in spacing between the
individual membranes may result in large variations in
oxygenator eff~Giency due to varying blood film thicknesses
(i.e., too thick or too thin) and/or the presence of "dead
spots" (i.e., constricted areas where blood does not flow)
within the f iber network .
In view of the desirability of maintaining consistent
inter-fiber spacing of the individual hollow fiber
membranes, some of the blood oxygenator devices of the
prior art have incorporated various spacer means for
maintaining the desired spacing between the individual
3 0 hollow f iber membranes . Examples of the types of spacer
means which have been utilized for spacing of hollow fiber
membranes or other similar apparatus are found in the
following: U.S. Patent No. 4,066,553 (Bardonnet); U.S.
Patent No. 4,368,124 (Brumfield); U.S. Patent No. 4,341,631
35 (Hargitay); U. 5. Patent No. 4, 622, 206 (Torgeson); U. 5.
- W0 95/34373 2 1 9 1 4 3 8 --3-- P~ S~C C9~
Patent No. 4,840,227 (Schmidt); U.S. Patent No. 4,869,059
(Austin); U.S. Patent No. 4,874,514 (Casey, Jr.); U.S.
Patent No. 4,911,846 (Akasu, et al.); U. S. Patent
No. 4,950,391 (Weickhardt); U.S. Patent No. 5,063,009
(Mizutani); U.S. Patent No. 5,141,031 (Baurmeister); U.S.
Patent No. 5,198,110 (Hanai); U.S. Patent No. 4,140,637
(Walter); U.S. Patent No. 4,031,012 (Gics); U.S. Patent
No. 4,900,314 (QuackenbUsh); U.S. Patent No. 4,293,418
(Fuji, et al.); and U.S. Patent No. 4,428,403 (Lee7 et al.)
(related to heat exchangers).
In particular, U.S. Patent No. 4,293,418 (Fuji, et
al. ) purports to describe the use of spacer yarns wrapped
about the outer surfaces of individual hollow fibers to
maintain a prescribed spacing between the individual hollow
fibers. ~he individual spacer ~yarns described in U.S.
Patent No. 4,293,418 (Fuji, et al.) are characterized as
textured yarn which is spirally wound so that substantially
equal and consistent spaces are f ormed between the hollow
fibers (Col. 2, L~ne 67-Col. 3, Line 1). Although the
textured "spacer yarns" described in U. S. Patent
No. 4,293,418 (Fuji, et al.) are purported to maintain
specific spacing between individual hollow fibers in
various types of fluid separation apparatus, the use of
such textured yarns as to space individual hollow fiber
membrane in a blood oxygenator device may be less ~ than
desirable due to the fact that the textured yarn may act as
a filtering medium such that blood will filter through the
texture of the yarn, rather than simply passing around the
individual yarns. Such filtering effect of the textured
3 0 yarn may result in retention of blood cells within the
textured yarn and on attendant potential for thrombogenic
clot formation. Additionally, the muItifilament yarns made
of many f ine f ibers present a greater than necessary
inactive surface area which is contacted by the blood, such
unnecessary surface contact presenting an increased chance
WO 95134373 2 ~ 9 1 4 3 8 ~- ~L ~, ,~ I ~,1/1)..,..,'(!~91
of inducing an undesirable bioreactive clotting response or
other bioincompata4ility reacti4n due to contact of the
blood with the constituents of the yarn surfaces with which
the blood comes in contact. Furthermore, because o~ its
compliant nature, yarn may tend to flatten out or compress
against the hollow ~iber membranes, thereby resulting in a
change of the desired spacer width resulting from the yarn
with a resultant variation or change in the ~lood f low
space (i.e., blood film thickness) between adjacent gas
exchange surfaces of the individual hollow fiber membranes.
Another problem associated with multifiliament
textured "yarns" is that such yarns tend to f latten and
spread out upon compression thereof, thereby covering more
of the surface of the adjacent hollow fiber membrane than
is necessary to effect the desired spacing function. In
view of these factors, multifilament textured "spacer
yarns" are less than optimal when employed for purposes of
maintaining specific lateral spacing between adjacent
hollow fiber membranes.
Another prior art method of spacing hollow fiber
membranes, as described in Japanese Kokai Patent
Application No. HEI3[1991]-278821 (Fuji), utilized two
spacer yarns wound in spiral fashion on the outer surface
of the hollow fiber membrane, s~ch that the "spacer yarns"
repeatedly cross one another in opposite directions. Such
oppositely wound crossing spacer yarns may be monofilament.
The oppositely wound crossing "spacer yarns" described in
Japanese Kokai Patent Application No. HEI3 (1991) -278821 can
not serve to guide a flow of blood or other liquid in a
substantially laminar flow path over the outer surfaces of
the hollow fiber membranes due tD the fact that the
opposite crossing configuration of the "spacer yarns" would
result in blocking or disruption of such lami-nar liquid
flow. Such bl4cking or disruption of blood flow may result
35 in turbulence of the blood, with resultant hemolysis or
- W09sl34373 2 ~ ~ 1 438 r ~ t~ P~
--5--
thrombogenic clot formation. Furthermore, oppositely wouna
crossing "spacer yarns" of HE13 (1991) -278821 can not
perform a consistent spacing function because they overlap
one another at cross-points, such overlapping of the
5 "spacer yarns" resulting in a doubling of the spacer yarn
width at the repetitive cross-point. Thus, if abutted
against an adjacent housing surface or an adjacent hollow
fiber membrane surfacer the cross-points of the spacer
yarns of H~13 (1991)-278821 would result in approximately
10 two times the desired spacing because of the overlapping or
crossing of the individual "spacer yarn" elements.
Yet, another prior art method of spacing hollow fiber
membranes requires the weaving or knitting of a separate
weft fiber among individual hollow fibers, as described in
U.S. Patent No. 5,141,031 (Baurmeister). The process of
weaving or knitting the weft fibers among the individual
hollow f ibers may result in damage to the hollow f iber
membranes, because such process exerts f orce upon the
surfaces of the fibers. To minimize fiber damage during
20 the weaving or knitting process, it is necessary to use
relatively strong f ibers at increased expense.
Additionally, in at least some membrane oxygenator devices,
the weft fibers are positioned perpendicular to the
direction of blood flow, thereby resulting in undesirable
25 turbulence or disruption of the f low of blood over the
outer surfaces of the hollow fiber membranes.
There remains a need in the art for development of new
spacer apparatus which may be wound about or otherwise
formed on the outer surfaces of individual hollow fiber
30 membranes to main~ain consistent prescribed spacing between
the individual membranes, while at the same time, promoting
the surf ace of the membranes .
summarY of the Invç~tion
W0 95/3~373 2 ~ 9 1 4 3 8 i ~ PCT/U595/06691
--6--
In accordance with the present invention, there are
provided elongate tubular hollow ~iber membranes having one
or more monofilament spacer memberts) disposed or formed on
the outer surfaces thereof. Preferably, the monofilament
spacer member(s) is/are disposed or formed in a spiral
configuration. Although two or more spiral monofilament
spacer members may be' disposed or formed on a single
tubular hollow f iber membrane in accordance with the
present invention, it i~ preferable that such spiral
monof ilament spacer members not overlap or cross one
another at any point so as not to block or disrupt laminar
blood flow over the outer surfaces of the hollow fiber
membranes .
In a f irst embodiment of the invention, a separate
monofilament member (e.g., a monofilamentous thread or
line) is spirally wound or wrapped about the outer surface
of a tubular hollow f iber membrane .
In accordance with a second embodiment of the
invention, the spiral monofilament spacer member may be
formed as a rl!Lised spiral rib of solid or monofilamentous
construction on the outer surface of the hollow fiber
membrane . Such raised monof ilamentous rib may be f ormed by
creating a notch or recess in an extrusion dye utilized to
extrude the tubular hollow fiber membrane. Rotational
force may then be applied to the extrusion dye, and/or to
the extruded hollow fiber emerging therefrom, so as to
cause the extruded rib to assume the desired spiral
configuration to the raised rib on the outer surface of the
tubu l ar membrane .
Further ln accordance with the invention, hollow fiber
membranes having the spiral monofilament spacer member of
the above-described f irst or $econa embodiment may be
operatively aisposed (e. g., packed) in close-spaced
parallel, side-by-side relation to one another such that
the spiral monofilamentous member of each membrane is in
.
wogs/3~373 2 1 9 1 438 _7- PcrluS95/06691
abutment with the outer surface of an adjacent membrane.
By such arrangement, the spiral monofilament spacer members
disposed or formed on the outer surfaces of adjacent side-
by-side positioned membranes will serve to define and
5 maintain consistent prescribed spacing distances between
the outer surfaces of such adjacent membranes. Similarly,
the spiral monofilament spacer members disposed or formed
on the outer surfaces of membranes which are positioned
next to a solid bulkhead or wall of a housing will serve to
lO maintain specific prescribed spacing between the outer
surfaces of such membranes and the adjacent bulkhead or
wall .
still further in accordance with the invention, there
is provided an extracorporeal membrane oxygenation device
15 which incorporates the spirally wound or spirally ribbed
hollow fiber membranes of the above-described first and/or
second embodiment therein such that the membranes are
disposed in close-spaced generally parallel side-by-side
relation to one another with the monofilamentous member or
2 o rib of each such membrane being in abutment with the outer
surface ~ of an adjacent membrane so as to define and
maintain prescribed and consistent spacing therebetween.
Still further in accordance with the invention, a
group or packet of individual hollow Iiber membranes
25 positioned in close-spaced parallel side-by-side relation
to one another may be positioned within a membrane
oxygenator or other extracorporeal blood processing device
and a flow of blood is channeled or passes over the outer
surfaces of the hollow fiber membranes such that the spiral
30 monofilament spacer members disposed on the outer surfaces
of the hollow fiber membranes will guide the path of the
blood flow over the outer surfaces of the membranes. In
embodiments wherein the f low of blood is directed parallel
to the longitudinal axis of the hollow fiber membranes, the
35 spiral monofilament spacer members will serve to cause the
WO 9S/3~373 2 1 9 1 4 3 8 ~ P~ 91 ~
blood to :Elow ln a spiral flo~ path. In embodiments where
the flow of -~ blood is ~other than parallel to the
longitudinal axis of the hollow fiber membranes, the spiral
monofilament spacer members will cause the blood to undergo
periodic def lection or of f setting each time the blood f low
comes into contact with or impinges against one of the
spiral monof ilament spacer members .
Further okjects and advantages of the invention will
become apparent to those skilled in the art upon reading
and understanding of the following detailed description and
the accompanying drawings.
Brie~ Description of the D~a.~inqs
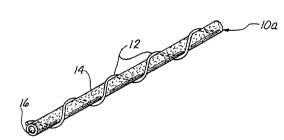
Figure l is a perspective view of an individual hollow
fiber membrane having a monofilament spacer of the present
invention spir~Llly disposed on the outer~ surface thereof.
Figure 2 is an enlarged perspective view of two side-
by-side hollow fiber membranes having monofilament spacers
of the present invention disposed thereon.
Figure 2a is a partial elevational view of a portion
2 0 of Figure 2 . --
Figure 3a is a perspective view of a heat
exchanger/membrane oxygenator device having the
monofilament-spaced hollow fiber membrane of the present
invention incorporated therein.
Figure 3b is a top plan view of the membrane
oxygenator device shown in Figure 3.
Figure 3c is an exploded view of the blood heat
exchanger/membrane oxygenator device of Figure 3a.
Figure 3d is a cross-sectional view through line 3d-3d
of Figure 3a.~
Figure ~ is a cut-away elevational view of the heat
exchanger/membrane oxygenator device of Figure 3.
Figure 4a is a cross-sectional view of a portion of
the heat exchanger/membrane oxygenator device of Figure 4.
WO gSt34373 2 ~ 9 1 ~ 3 8 , ; ~ ~ . r~l~u.. ~
_g_
Pigure 4b is an enlarged cross-sectional view of
region 4b of Figure 4a.
Figure 5a is a perspective view of an extrusion die
which may be utilized to manufacture the hollow f iber
5 membranes of the second embodiment of the present invention
having a spiral monofilamentous rib formed on the outer
surf ace thereof .
Figure 5b is a perspective view of a hollow f iber
membrane of the second embodiment of the present invention
10 emerging from the extrusion die of Figure 5a.
Figure 6 is a schematic diagram of a continuous
manufacturing method whereby a continuous hollow fiber
membrane is a) passed through a pirn apparatus which wraps
a monofilament spirally about the outer surface of the
15 hollow f iber membrane and b) subsequently wound onto a core
component of a blood processing apparatus, such as a
membrane oxygenator device.
Figure 6a is an enlarged perspective view of segment
6a of Figure 6.
Detailed DescriPtion Preferred Embodimç~ts
The following detailed description, and the
accompanying drawings, are provided for purposes of
describing and illustrating presently preferred embodiments
25 of the invention only and are not intended to limit the
scope of the claimed invention in any way.
i. Hollow Fiber Membrançs Havinq ~onQ~ilamentous
SPaCers DisPosed Thereor~
With reference to Figures 1-6, each preferred ho~low
30 fiber membrane 10 of the present invention comprises a
tubular semi-permeable membrane having an outer surface 14
and a hollow lumen 16 which extends longitudinally through
the membrane 10. One or more monofilamentous spacer
members are disposed upon or formed upon ~he outer surface
35 14 of the membrane 10. Each membrane 10 has a
WO gsl34373 2 1 9 ~ 4 3 8 PCTIUS95/0669l
--10--
semipermeable tubular wall of substantially consistent
thickness T. Each monof ilamentous spacer member 12 on the
membrane 10 has a cross-sectional dimension (e . g.,
diameter) spacer width sW. Abutment of each spacer 12
against an adjacent surface (e.g., the outer surface 14 of
an adjacent hollow fiber membrane) will serve to hold the
outer surface l~l o~ the membrane 10 upon which the spacer
12 is disposed a distance of one spacer width SW from the
outer surface 14 of an adiacent membrane and/or any
adjacent surface of the device against which the spacer 12
is abutted.
When intended for use in blood membrane oxygenator
applications, the hollow fiber membranes 10 of the present
invention wilL preferably be formed Qf semi-permeable
materials suitable for blood-gas exchange including
cellulose esters such as cellulose diacitate and cellulose
triacetate, cellulose derivatives such as cellulose ether,
polyamide, polyester, methacrylic or acrylic polymers such
as polymethyl methacrylater polyurethane, organic silicone
polymer, polyacrylonitrile and copolymer thereof,
polysulfones, ~and polyolefins such as polyethylene and
polypropylene. The hollow fiber membranes 10 are extruded
to a preferre~ wall thickness T in the range of 40-60
microns and have a preferred inner luminal diameter ID in
the range of 270-290: microns and a preferred outer diameter
OD in the range of 350-410 microns. When disposed or
formed on the outer surface of such preferred hollow fiber
membrane 10, the spiral spacer member 12 of the present
invention preferably has an effective spacer width SW
(e.g., the diameter if the spacer is of round cross-
sectional configuration) of 38-63 microns. Thus, when the
spiral spacer member 12 of one hollow fiber membrane 10
abuts against the outer surface 14 of an adjacent hollow
fiber membrane 10, such adjacent hollow fiber membranes 10
Wo 9s/3~373 2 ~ 9 ~ 4 3 8 .` -~ ~ ; r~u~ s ~G~
11
will be held apart from one another by a distance equal to
the effective spacer width SW of the spacer member(s) 12.
The longitudinal distance within which the spacer
member 12 makes one full spiral revolution about the outer
5 surface 14 of the membrane 10 is referred to herein as the
"pitch interval" P. In blood membrane oxygenator
applications wherein the wall thicknesses T of the hollow
fiber membranes 10 are in the preferred range of 40-60
microns, it will typically be preferable for the spiral
10 monofilament spacer member (s~ 12 to have pitch intervals P
in the range of 2.6-3.7 millimeters By maintaining such
pitch intervals P within the range of 2.6-3.7 millimeters,
the points at which each spaceL member 12 makes contact
with the outer surface 14 of an adjacent hollow fiber
15 membrane 10 will be sufficiently close together to result
in a consistently sized gap or space.between the adjacent
outer surfaces 14 equal to the spacer width SW or diameter
of the spacer member(s) 12.
It will be appreciated that the prescribed pitch
20 interval P may be varied depending on the relative
pliability, flexibility or wall thickness T of the hollow
f iber membrane 10 . In embodiments wherein the hollow f iber
membranes 10 are relatively flexible or pliable, it may be
desirable to utilize a short pitch interval P so as to
25 minimize the distance between the points at which each
spacer member 12 contacts the outer surface 14 of an
adjacent hollow fiber membrane 10. On the other hand, in
r~rhnl1;r~nts wherein the hoLlow fiber membranes 10 are
relatively rigid or less pliable, longer pitch intervals P
30 may be employed so as to maximize the distance between the
points at which each spacer member 12 contacts the outer
surface 14 of an adjacent hollow fiber membrane 10.
One significant advantage of the .hollow fiber
membranes 10 of the present invention is that such
35 membranes 10 may be wrapped, laid, wound, or otherwise
WO95/34373 2 ~ 9 1 438 ~ FC~ 5~6f~1
--12--
deployed into ~ny shape of channel or receiving structure,
and will conform to the shape of the particular channel or
receiving structure while maintaininq constant surface-to-
surface spacing between the outer surfaces of individual
hollow fiber membranes 10. Additionally, the hollow fiber
membranes 10 of the present invention will also maintain
constant desired spacing between the outer surfaces of the
hollow fiber membranes 10 and any ad~acent structural
members, bulkheads or walls of the particular housing,
grooYe or channel into which the hollow fiber membranes 10
have been placed. This ability of the hollow fiber
membranes 10 of the present invention to be individually
deployed within any size or shape of channel, and to
conform thereto, constltutes a significant advantage over
the prior art woven ribbons and other woven designs wherein
multiple indivldual fibers were specifically preformed or
woven into a specifically shaped group or network.
ii. Inco~Poration Of The Hollow Fiber Membranes
of The Prese~t I~vention Into A Preferre~
Blood Membrane OxYqenator Device
The monofilament-spaced hollow fiber membranes 10 of
the present invention may be incorporated into many
different types and slzes of blood oxygenators. One
particular blood oxygenator device wherein the hollow fiber
membranes 10 o~ the present invention may be incorporated
is that described in United States Patent No. 5,120,501 and
sold commercia~lly as the UnivoxD' Membrane Oxygenation
System, Baxter E~ealthcare Corporation, Bentley Labor~tories
Division, Irvine, California 92714.
The membrane oxygenator device of United States Patent
No. 5,120,501 is shown in Figures 3, 4, and 5 of this
patent application f or purposes of describing how the
hollow fiber membranes 10 of the present invention may be
incorporated into that particular membrane oxygenator
35 device. Additionally, the entire disclosure of United
Woss/34373 2 1 9 ~ 438 -13- r_~",.,,r~C ~
States Patent No. 5,120,501 is expressly incorporated
herein by ref erence .
With reference to Figures 3-3d, and 4-4b the heat
exchanger/membrane oxygenator device 40 shown is
5 connectable to an extracorporeal blood circuit for purposes
of a) controlling the blood temperature and b) maintaining
the P0z and PC02 of the blood within prescribed rangeS. _--
In general, the heat exchanger/membrane oxygenatordevice 40 comprises an outer housing or shell 42 within
10 which there is mounted a heat exchange body or bellows 44
and a heat exchange jacket 46. The bellows 44 comprises a
generally cylindrical body or core having a series of
annular ribs or pleats 48 formed circumferentially about
the outer surface thereof. The series of annular ribs 4g
15 define a corresponding series of annular, generally
parallel, blood receiving grooves or channels 50 between
adjacent ribs 48.
The heat exchange jacket 46 comprises a generally
cylindrical wall 54 having a plurality of annular flanges
20 or ribs 56 extenaing about the outer surface thereof 50 as
to form a continues fluid flow path through which heat
exchange fluid may be passed about the outer surface of the
cylindrical wall 54 and into contact with the surrounding
inner surface of bellow5 44. As such, the circulation of
25 heat exchange fluid through the flow path 58 between the
cylindrical wall 54 and the surrounding bellows 44 will
operate to either heat or cool the bellows 44 thereby
concomitantly heating or cooling the blood passing through
the blood passage channels 50 on the outer surface o
30 bellows 44.
In the particular embodiment of the heat
exchanger/membrane oxygenator device 40 shown, a single
elongate hollow fiber membrane lo is repeatedly wound about
the outer surface of the bellows 44 such that multiple
35 convolutions of the hollow fiber membrane lo become
WO 95134373 2 ~ 9 1 4 3 8 ~ r~ U ~ 91
--14 ~
disposed within each channel 50 on the outer surface of
bellows 44, with each such convolution being in generally
parallel side-by-side position to an adjacent convolution.
A vertical cut is subsequently made through the long hollow
5 fiber membrane such that each convolution becomes an
individual hollow fiber membrane 10 wound about the outer
surface of bellows 44 such that a separate individual group
or packet 84 of hollow fiber membranes 10 arranged in
substantially parallel side-by-side relation to one
10 another, resides within each channel 50.
~ ach individual group 84 of hollow fiber membranes 10
preferably consists of approximately 240-260 individual
hollow fiber membranes 10 laid or wound into each channel
50. The monofilament spacer member 12 (e.g., separate
15 monof ilament member or raised rib) of each membrane lo is
in abutment with the outer surface of an adjacent membrane
10, thereby maintaining consistent prescribed spacing
between the individual membranes lo. Also, for those
hollow ~iber membraneS 10 which lie adjacent the annular
20 ribs or pleats 48 of bellows 44, the monofilament spacer
member 12 disposed or formed thereon will abut against the
adjacent rob -or pleat 48, thereby maintaining consistent
prescribed spacing between the outer surf aces of those
hollow fiber membranes lo and the adjacent surfaces of the
25 bellows 44.
Prior to winding or laying of the individual membranes
10 into each groove 50, a space occupying member such as a
spiral plastic spring may be placed in the bottom region of
each channel 50 to prevent the hollow fiber membranes 10
30 from packing ~11 the way down into the bottom portion of
the channel 5~. Thus, the presence of the space occupying
members (e.g., plastic spring) in the l~ottom portion of
each channel 50 will form~ a blood passage gap 86 in the
innermost region of each channel 50. As such, blood may be
35 initially shunted or channeled into the blood flow gap 86
W095J3~373 21 ~143 ~ ~ r~
at the base of each channel 50 and subse~uently permitted
to percolate or pass outwardly over the outer..surfaces of
the individual hollow fiber membranes 10 grouped in each
such channel 50. Thus, the direction of the initial
5 outward blood flow is generally perpendicular to the
longitudinal axes of the hollow f iber membranes 10 disposed
in each channel 50. As the blood percolates or passes
outwardly over the outer surfaces of the individual hollow
fiber membranes 10, the blood will come into contact with
10 the monofilament spacer members 12 disposed or formed on
the outer surfaces of the individual hollow fiber membranes
10. Each such contact or impingement against a spiral
spacer member causes the f lowing blood to undergo lateral
deflection or shifting in the direction of the longitudinal
15 axis of the hollow f iber 10 . Thus, a single spiral
monof ilamentous spacer member ~2 disposed on the outer
surface of each hollow fiber membrane lQ will serve the
additional function of creating ~a desired spiral or
laterally shifted flow path of the blood as it passes over
20 the outer surfaces 14 of the hollow fiber membranes 10.
This aspect of the present invention is specifically
illustrated by the arrows on Figure 2a . This f low
directing or flow channeling function of the . single
monof ilamentous spacer members 12 could not be achieved by
25 multiple spacer members wrapped in opposite criss-crossing
directions as described in Japanese Kokai Patent
Application No. HE~I-3(1991)-278821 (Fujii) as the existence
of an oppositely wound spacer member would result in
impairment of such spirally directed blood f low and/or
30 undue turbulence of the blood due to cross stream
impingement with the second oppositely directed spacer
member .
The initial shunting or channeling of the blood into
each gap 86 allows the ~lood to initially come in contact
35 with the adjacent outer surface 48 of the bellows 44 so as
W0 95/34373 2 ~ 9 1 4 3 8 . ~ P~
--~ 6 ~
to immediately effect warming or cooling of ~he blood in
accordance with the temperature of the heat exchange medium
being circulated on the inner surface of the bellows 44.
Thus, the blood being circulated through the device 40 will
5 be at a controlled temperature before it begins to
percolate or flow outwardly from the gap 86, over the gas
exchanging outer surfaces of the individual hollow fiber
membranes 10.
The opposite ends of the hollow fiber membranes 10 of
10 each packet 84 are anchored within a solid potting material
which forms a solid block 78 on the back of a blood inlet
manifold 60. The back surface 79 of the potting material
78 is flush cut such that the open ends of the hollow fiber
lumens 16 form==open~ngS at surface 79 to permit gas to flow
15 into and out of such lumens 16 of membranes 10. Passage of
the desired gas or gas mixture through the lumens 16 of the
individual hollow fiber membranes 10 is facilitated by a
gas passage manifold 70 pos~tioned on one side of the shell
42 of the device 40, immediately outboard of the blood
20 inlet manifold 60. ~as passage manifold 70 comprises a
rigid shell or; casing having a generally hollow interior
with a solid bulkhead 72 extending vertically through the
mid-region thereof . The bulkhead 7 2 divides the inner
chamber of the gas passage manifold 70 into a gas inlet
25 chàmber 74 and a gas outlet chamber 76. The first (inlet)
ends of the hollow fiber membranes 10 are disposed in a
vertical column along the left side of the potting material
block 78 so as to be in alignment and f luidic contact with
gas inlet chamber 74 of gas manifold 70. Similarly, the
30 second ends of the hollow fiber membranes 10 are disposed
in a vertical column on the right side of the potting
material block 78 so as to be positioned in alignment and
fluidic contact with gas outlet chamber 76 of the gas
manif old 70 . - -
WO95/34373 2 1 q i 438 -17- I~ f~
By such construction , a gas or gas mixture ( i . e., pure
oxygen, oxygen/air mixture, oxygen/nitrogen mixture)
infused into the gas inlet chamber 74 of manifold 70
through a gas inlet connector 80 will subsequently pass
5 into the first open ends of the lumens 16 of hollow fiber
membranes 10 and will subsequently flow through the lumens
16 of the hollow fiber membranes 10. After passing through
the lumens of the hollow fiber membranes lo, the gas or gas
mixture will pass outwardly through the second ends of the
10 lumens 16 of the hollow fiber membranes 10 and into the gas
outlet chamber 76 of gas passage manifold 70. Thereafter,
the gas or gas mixture may be exhausted through a gas
outlet connector 82.
To facilitate circulation of blood through the blood
15 receiving channels or grooves 50, a blood inlet manifold 60
is formed or mounted on one side of the shell 42 and a
blood outlet manifold 62 is formed or mounted on the
opposite side of shell 42. A blood inlet connector 64 is
positioned on blood inlet manifold 60 to facilitate passage
20 of blood into the inlet manifold 60, while a blood outlet
connector 66 is positioned on blood outlet manifold 62 to
facilitate passage of blood out of blood outlet . manifold
62. A blood inlet flow path within blood inlet manifold 60
is configured to divide the flow of incoming blood into
25 separate streams passing out of slots 67 formed in the
inner face of the blood inlet manifold 60. Slots 67 of
blood inlet manifold 60 are positioned in correspondence
and fluidic connection with the substantially hollow blood
circulation gaps 86 formed on the innermost region of each
30 groove or channel 50 such that blood passing out of slots
67 will initially flow into the circulation gaps 86, and
will subsequently pass outwardly through the channels 50,
f lowing over the outer surf ace of the hollow f iber
membranes 10 disposed within each channel 50, thereby
35 passing a film of blood over the outer surfaces 14 of the
WO 95/34373 2 ~ 9 1 4 3 8 ~ -13~ r~ ,,S'~
membranes 10. Such contact of the blood with the outer
surfaces 14 of the hollow fiber membranes 10 allows the
blood to receive oxygen, and to give off carbon dioxide,
through the semipermeable walls of the membranes 10. The
5 resultant PC02 ~ and PO2 of the blood may be controlled by
adjusting the gaseous content (i.e., Fio2 and FiCo2) and
pressure of the gas mixture being passed through the lumens
16 of the individual hollow fiber membranes 10.
iii. OPer~tion O~ A P}eferred Membrane Oxyqenator
Device I~QrPoratinq The ~ollow Fiber Membranes
Of The Present Invention
Again, with reference to Figures 3a-3d and 4-4b, the
blood inlet connector 64 of the device 40 is connected to
a vein of the patient such that venous blood from the
15 patient will Qnter the blood inlet manifold 60 through
inlet connector 64. Similarly, the blood outlet flow
connector 66 is connected to the arterial vasculature of
the patient via tubing such that oxygenated blood f lowing
out of blood o~tflow connector 66 wlll be returned to the
20 arterial circulation of the patient. Appropriate pumping
means, such as peristaltic blood pumps or other blood
pumping apparatus are utilized to facilitate the above-
described flow of blood to and from the patient.
Source(s) of the prescribed oxygen-containing gas
25 mixture (e. g ., either pure oxygen or blends of oxygen with
other suitable gases such as nitrogen) is connected to gas
inlet connector 80 by way of tubing. By such arrangement,
the prescribed oxygen-containing gas mixture enters gas
inlet chamber 74 and passes through the lumen 16 of the
30 individual hollow fiber membranes 10 disposed within the
blood receiving grooves 50 of the device 40. After passing
through the lumens 16 of the individual hollow fiber
membranes 10, the used gas subsequently passes into gas
outlet chamber ~6 and is exhausted through gas outlet
35 connector 82.
Wo 9~/34373 2 1 9 1 4 3 8 ; 9 P~ I ~ u ~95 ~ 9 1
Apparatus for monitoring the concen~ration of oxygen
or other gases may be connected to the flow path adjacent
either the gas inlet connector 80 or the gas outlet
connector 82 so as to provide f or ongoing monitoring of the
5 relative concentrations of oxygen or other gases passing
into or out of the device 4 0 . Also, pressure sensing means
may be positioned at one or more points along the gas--
mixture pathway to permit monitoring of the pressure of the
gas-mixture within the device 40.
A device for providing a recirculating flow of
temperature-controlled heat exchange liquid (e . g ., saline
or water) is connected to a heat exchanger inlet connector
88 and a heat exchanger outlet= connector 90 so as to
provide a recirculating flow of temperature controlled heat
15 exchange medium through heat exchanger ~acket flow path 58
and into contact with the inner surface of the surrounding
bellows 44. The temperature of the blood exiting the
device 40 through blood outlet connector 66 may be
monitored and the temperature of the heat exchange medium
20 circulated through flow path 58 may be thermostatically or
otherwise adjusted so as to maintain or control the
temperature of the blood exiting the blood outf low
connector 6 6 within a prescribed temperature range .
It will be appreciated that the efficiency and
25 consistency with which the partial pressures of oz and C0;~
within the blood may be controlled is dependent upon the
maintenance of consistent prescribed spacer widths SW
between the outer surfaces 14 of the individual hollow
fiber membranes 10 such that the film thickness and surface
30 area contacted by the blood flowing through the device 40
may be optimized and maintained in a consistent fashion.
I'he disposition of the spiral monof ilamentous spacer
members 12 about the outer surfaces 14 of the individual
hollow fiber membranes lO within each membrane packet 84
35 serves to hold the individual hollow fiber membranes 10
WO 95/34373 2 1 9 1 4 3 8 ~ . PCr/US95106691
--2 0--
within each packet 84 a desired prescribed spacer width SW
from one another, thereby making certain that the film
thickness and amount of blood circulating into contact with
the outer surfaces 14 of the hollow fiber membranes 10 is
5 consistent and predictable, with minimal unit-to-unit
var iation .
Additionally, as described in detai~ herein, the
spiral or helical dispo~ition or formation of the spacer
members 12 on the outer surfaces 14 of the hollow fiber
10 membranes 10 serves to direct the blood flow in a non-
turbulent laminar f low path about the outer surfaces 14 of
the individual hollow fiber membranes 10 without
unnecessary damming, disruption or turbulence of the blood
as may result in thrombogenic consequences.
Additionally, because the individual spacer members 12
are formed of non-wetting monofilamentous material, such
spacer members 12 do not wet with blood and do not result
in hang-up or holding of blood within the device ~0, as may
occur with multif ilament, woven or yarn-type wettable 0 spacer members . ~
iv. Methods of Manufa~turinq the Monof ilament SPaced
IIollow Fiber Membranes of the Present Invention
The monofilament spaced hollow fiber membranes 10 of
the present invention may be manufactured by any suitable
25 means, including the two (2) alternative manufacturing
methods described herebelow.
In embodiments wherein the monof ilamentous spacer
member 12 consists of a separate monof ilament member which
is not formed cQntinuous with the hollow fiber wall, such
3 0 separate monof ilament member 12 may be spirally wrapped
around the outer surface 14 of an elongate tubular hollow
fiber membrane 10. Such spiral wrapping of a separate
monofilamentous spacer member 12 about the outer surface 14
of a tubular hollow fiber membrane 10 may be accomplished
35 manually or by automated machinery. One example of an
WOgS/34373 2 ~ 91438 ~ r~u
--2 i --
automated fiber-wrapping machine which may be utilized to
spirally wrap monofilamentous fibers around an elongate
hollow fiber membrane is a power driven hollow cored pirn
having a quantity of the monofilamentous fiber wrapped
5 therearound . The hollow f iber membrane is fed
longitudinally through the center of the spinning pirn and
the monofilamentous fiber is spun off of the pirn and ontb
the outer surface of the advancing hollow f iber membranes,
such that the monofilamentous fiber become spirally wrapped
10 about the outer surface of the hollow fiber membrane.
In embodiments of the invention wherein the
monof ilamentous spacer member 12 comprises a spiral rib
formed on the outer surface 14 of a tubular hollow fiber
membrane 10, such rib may be extruded as part of the body
15 of the hollow fiber membrane 10 as shown in Figures 5a and
5b. An extrusion die 100 usable to form a monofilamentous
ribbed hollow fiber membrane lOd comprises a round inner
core member 102 disposed within the hollow inner bore of a
cylindrical outer member 104. A recess 106 or recesses
20 is/are formed in the luminal surface of the cylindrical
outer core 104 such that the workpiece extruded by the die
100 will be in the form of a generally round hollow tube
lOd having a single raised rib 12d formed on the outer
surface thereof. A rotational force may be applied to the
25 outer die member 104 and/or the emerging workpiece so as to
cause the rib 12d to become spiralled about the outer
surface of the tubular hollow fiber membrane lOd. The rate
at which the outer die member 104 and/or workpiece are
rotated, relative to the rate at which the extruded
30 workpiece emerges from the die loO, will determine the
tightness or pitch interval of the spiral rib 12d.
Figure 6 shows a schematic diagram of a continuous
manufacturing process whereby a separate, hollow
monofilamentous spacer member may be initially wrapped
35 about the outer surface of an advancing hollow fiber
WO95/3~373 2 ~ ~ 1 438 -2~2 ~ P~ C 'ql --
membrane and whereby the spirally wrapped hollow f iber
membrane may be subsequently wrapped or mounted onto the
outer surface of a core member, such as the core of a
membrane oxygenator device, in a continuous fashion.
As shown in Figure 6, an elongate hollow fiber
membrane 100 is initially wound about a spool 102. The
hollow fiber membrane lO0 is continually baled or pulled
off of spool 102 and fed through the central passageway 104
of a powered pirn 106. A length of monofilamentous spacer
material 108 (i.e., monofilament line or thread) is
initially wrapped about the outer surface of the pirn 106.
As the pirn 106 spins, the monofilamentous spacer member
108 is unwound from the outer surface of the pirn 106 and
correspondingly wrapped about the outer surface of the
advancing hollow fiber membrane 100. The spiral pitch
interval P at which the monof ilamentous spacer member 108
is wrapped about the outer surface of the advancing hollow
fiber membrane 100 may be controlled and/or adjusted by
controlling or varying the feed rate of the hollow fiber
membrane 100.
After the~ powered pirn 106 has operated to spirally
wrap the monof ilamentous spacer f iber 108 about the outer
surface of the advancing hollow fiber membrane 100, the
spirally wrapp=ed hollow fiber membrane is subsequently
advanced over rollers llO, 112 and wrapped about the outer
surface of a core member 114, such as the inner core of a
blood membrane oxygenator device, an example of which is
the central bellows 44 of the heat exchanger/membrane
oxygenator device 40 shown in Figures 33-3d and 4-4c of
this patent application.
Thus, the spiral wrapping of the monofilamentous
spacer member 108 about the outer surface of the hollow
fiber membrane 100 and the subsequent deployment or
wrapping of the spirally wrapped hollow fiber membrane 10
about the outer surface of the device core member 114 is
W095/343~3 2 1 9 1 438 -2~3~- ' r- . ` PCT/US9S/06691
accomplished in a continuous process, without the need for
intervening handling or manipulation of the hollow f iber
membrane 10 0 .
Although the invention has been described herein with
5 reference to certain presently preferred embodiments, it
will be appreciated that various additions, modifications
or alterations may be madQ to the herein described
embodiments without departing from the intended spirit and
scope of the invention. For example, instead of a single
10 monof ilamentous spacer member or rib 12 being wound or
formed about the outer surface 14 of a hollow fiber
membrane 10, plural or multiple spacer members or ribs 12
may be utili~ed on each such hollow fiber membrane 10,
provided that the individual spacer members or ribs 12 do
15 not overlap one another as to cause doubling or widening of
the effective spacer width at the point of overlap or
crossing thereof. Accordingly, two or three parallel
spacer members or ribs 12 may be disposed on the outer
surface 14 of a single hollow fiber membrane 10 without
20 causing impairment of the desired flow characteristics
described herein and/or the desired consistent spacing
between the individual hollow fiber membranes 10 and their
ad j acent surf aces .