Note: Descriptions are shown in the official language in which they were submitted.
2191655
-1-
Title: Novel Antibody Compositions for Preparing Enriched Human
Hematopoietic and Tumor Cell Preparations
FIELD OF THE INVENTION
The present invention relates to novel antibody
compositions, and processes and kits for preparing cell preparations
enriched for specific cell types and the use of the cell preparations. The
invention also relates to purified cell preparations.
BACKGROUND OF THE INVENTION
Blood cells have a relatively short life span and need to be
replenished throughout life. In adults, blood cell formation or
hematopoiesis takes place in the bone marrow, but blood-forming stem cells
can also be found in peripheral blood. Hematopoietic cells represent a
hierarchy of proliferating and differentiating cells. The most abundant are
the differentiating or lineage committed cells. These cells have limited or
no proliferative capacity and represent specialized end cells that are found
in blood, and theiir immediate precursors.
T'he imniediate precursors of the differentiating cells are
the progenitor cells. Most of these cells are restricted to differentiate
along a
single lineage but they may have quite extensive proliferative capacity.
Progenitor cells appear morphologically as blast cells, and they typically do
not have specific features of the hematopoietic lineage to which they are
committed.
Progenitor cells are derived from stem cells. Stem cells
have been historically defined by their ability to self-renew as well as to
generate daughter cells of any of the hematopoietic lineages. The presence
of stem and progenitor cells may be detected by their ability to produce
colony-forming cells in culture. They may also be detected by screening for
the CD34 antigen which is a positive marker for early hematopoietic cells
including colony Eorming; cells and stem cells. At present, the long term
culture initiating cell (LTCIC) assay appears to be the best way to detect
stem
2191655
-2-
cells, or at least the most primitive progenitor cells, using tissue culture
methodologies.
There is a continued interest in developing stem cell
purification techniques. Pure populations of stem cells will facilitate
studies
of hematopoiesis. Transplantation of hematopoietic cells from peripheral
blood and/or bone marrow is also increasingly used in combination with
high-dose chemo-- and/or radiotherapy for the treatment of a variety of
disorders including malignant, nonmalignant and genetic disorders. Very
few cells in such transplants are capable of long-term hematopoietic
reconstitution, anci thus there is a strong stimulus to develop techniques for
purification of hematopoietic stem cells. Furthermore, serious
complications and indeed the success of a transplant procedure is to a large
degree dependent on the effectiveness of the procedures that are used for
the removal of cells in the transplant that pose a risk to the transplant
recipient. Such cells include T lymphocytes that are responsible for graft
versus host disease (GVHD) in allogenic grafts, and tumor cells in
autologous transplants that may cause recurrence of the malignant growth.
It is also important to debulk the graft by removing unnecessary cells and
thus reducing the volume of cyropreservant to be infused.
Hematopoietic cells have been separated on the basis of
physical characteristics such as density and on the basis of susceptibility to
certain pharmacological agents which kill cycling cells. The advent of
monoclonal antibodies against cell surface antigens has greatly expanded
the potential to distinguish and separate distinct cell types. There are two
basic approaches to separating cell populations from bone marrow and
peripheral blood using monoclonal antibodies. They differ in whether it is
the desired or urLdesired cells which are distinguished/labeled with the
antibody(s).
In positive selection techniques the desired cells are labeled
with antibodies and renloved from the remaining unlabeled/unwanted
cells. In negative selection, the unwanted cells are labeled and removed.
Antibody/complement treatment and the use of immunotoxins are
CA 02191655 2003-06-13
-3-
negative selection techniques, but FACST"' sorting and most batch wise
immunoadsorption techniques can be adapted to both positive and negative
selection. In. immunoadsorption techniques cells are selected with monoclonal
antibodies and preferen t:ial ly bound to a surface which can be remo'ved from
the remainder of the cells e,g. column of beads, flasks, magnetic particles.
Immunoadsorption techniques have won favour clinically and in research
because they maintain the high specificity of targeting cells with mc-noclonal
antibodies, but unlike FACS"11` sorting, they can be scaled up to deal
directly
with the large numbers of cells in a clinical harvest and they avoid the
dangers
of using cytotoxic reagerits such as immunotoxins, and complement.
Current positive selection techniques for the purification of
hematopoietic stem cells target and isolate cells which express CD34
(approximately 1-2% of nornlal bone marr w )(C.iviri, C.1., Trischma:nn, T.M.,
Fackler, M.J., Bernstein, 1. I)., Buhring, I-i.J., Campos, L. et al. (1989)
Report on
the CD34 cluster workshop, In: Leucocyte typing IV, White Cell Differentiation
Antigens. Kriapp, W., Dorken, B., Gilks, W.R., Reiber, E P., Schmidt, R. E.,
Stein,
H., and Kr. von den Borne, AE.G Eds., Oxford University Press. Oxford,
pp.818). Thus, the potential enrichment of hernatopoietic stem cells using
this
marker alone is approximately 50 fold. Available techniques typicall:y recover
30-70% of the CI)34+ cells in the start suspension and produce an enriched
suspension -vvhich is 50-80`%, CD34+ (Ishizawa, L. et al., In: Hematopoietic
Stem
Cells: The Mulhouse Manual eds. Wunder, E., Sovalat, H. Henon, P., and Serke,
S. AlphaMed Presa, Oh ic:) pp171-182; Shpall, E.J., et al. (1994), J. of'
Clinical
Oncology 12:28-36; Winslow, J.M., et al. (1994), Bone Marrow Transplantation
14:265-271; Thomas, T.E., (1994), Cancer Research, Therapy and Coritrol 4(2):
119-128). The positive selection procedures suffer from many disadvantages
including the presence of materials such as antibodies and/or magnetic beads
on the CD34+ cells, and damage to the cells resulting from the removal of
these
materials.
2191655
-4-
Negative selection has been used to remove minor
populations of cells frorn clinical grafts. These cells are either T-cells or
tumor cells that pose a risk to the transplant recipient. The efficiency of
these purges varies with the technique and depends on the type and
number of antibodies used. Typically, the end product is very similar to the
start suspension, inissing only the tumor cells or T-cells.
Transplants of purified stem cells without differentiated or
lineage committed cells will give short and long-term hematopoietic
support (Shpall, E.J., et al. (1994), J. of Clinical Oncology 12:28-36). Since
differentiated cells make up a vast majority of the cells in bone marrow and
blood, depletion of these cells produces a much smaller cell suspension.
The number of cells in the final product and the degree of enrichment of
progenitor/stem cells will. depend on the efficiency of the antibody targeting
and the removal of labeled cells.
T'here are several studies that enrich for hematopoietic
stem cells by depleting lineage committed cells but all require a number of
positive or nega tive selection steps to achieve the desired degree of
enrichment (50 fold). Early studies required prior density separation and
extensive incubat:ions to remove adherent cells (Linch, D.C, and Nathan,
D.G. (1984), Nature 312 20/27: 775-777; Sieff, C.A., et al. (1985), Science
230:
1171-1173; Kannourakis, G. and Bol, S. (1987) Exp. Hematol 15:1103-1108.).
More recent techniques are no less cumbersome; involving density
separation steps followed by two partial lineage depletions (Winslow, J.M.,
et al. (1994), Bone Marrow Transplantation 14:265-271) or a partial lineage
depletion using panning or FACS followed finally by positive selection
using FACS (Carlo- Stella et al. 1994, Blood 84, 10 supple.:104a; Reading, C.,
et al. (1994), Bloo(i 84, 10 supple.:399a). Most of these methods for lineage
depletion lack effective antibody combinations against myeloid cells,
erythrocytes and/or B-cells.
U.S. Patent Serial No. 5,087,570 describes a process for
preparing a hema1topoietic cell composition using a combination of positive
and negative selection. The process relies on the use of antibody to the Sca-
2191655
-5-
1 antigen which is associated with murine clonogenic bone marrow
precursors of thymocytes and progeny T-cells. The Sca-1 antibody is not
useful in isolating humail hematopoietic cells.
U.S. Patent Serial No. 5,137,809 describes a method and kit
for identifying arLd analyzing lineages and maturational stages in normal
hematopoietic cel:ls. The method uses a first monoclonal antibody labeled
with a fluorochro:me to react with all leukocytes in a sample, and a second
monoclonal antibody labeled with a second flourochrome to react with a
subpopulation of leukocytes.
SUMMARY OF THE INVENTION
T'he present inventors have developed an antibody
composition which is specifically adapted to enrich for hematopoietic stem
cells and progenitor cells and remove tumor cells. The antibodies in the
antibody composition are specific for selected markers associated with
lineage committed or differentiated cells. Some of the markers are also
expressed on some metastatic tumor cells. The composition contains
antibodies specific for glycophorin A, CD3, CD24, CD16, CD14 and optionally
CD45RA, CD38, C:D36, CD2, CD19, CD20, CD22, CD29, CD56, CD66e, and/or
CD66b, and antibodies specific for non-hematopoietic antigens expressed on
tumor cells, preferably antibodies against antigens expressed on the surface
of breast and lung carcinoma and neuroblastoma cells. The present
inventors have shown that the purging antibody composition applied in
one step to a sample of peripheral blood, bone marrow, or frozen bone
marrow containing tumor cells, results in a greater than 50% recovery of
human hematopoietic progenitor/stem cells with approximately a 3-5 log
depletion of tumoir cells.
T'he high level of enrichment obtained using the antibody
composition of the invention, does not require additional enrichment or
tumor purging steps, which would result in loss of, or damage to,
progenitor and stem cells. The recovery of CD34+ cells, CD34+CD38- cells,
colony forming cells, and LTCIC, is also much higher than with
conventional multistep techniques.
2191655
-6-
The enrichment and recovery of human hematopoietic
progenitor and stem cells using the antibody compositions of the invention
in a negative selection technique has many advantages over conventional
positive selection techniques. As mentioned above, highly enriched
progenitor/stem cell preparations can be obtained using a single step. The
human progenitor and stem cells obtained using the antibody composition
of the invention are not labeled or coated with antibodies or modified
making them highly suitable for transplantation and other therapeutic uses.
Broadly stated the present invention contemplates an
antibody composition comprising antibodies specific for glycophorin A,
CD3, CD24, CD:16, CD14, and optionally one or more of CD45RA, CD36,
CD38, CD56, CD2, CD19, C:D20, CD22, CD29, CD66e and CD66b. Preferably the
antibody composition contains antibodies specific for non-hematopoietic
antigens expressed on tumor cells, most preferably antigens expressed on
the surface of cells from breast and lung carcinoma, and neuroblastoma.
T'he present invention still further contemplates a process
for enriching and recovering normal human hematopoietic progenitor cells
and stem cells in a sample containing human hematopoietic differentiated,
progenitor, and stem cells, and tumor cells comprising
(a) reacting the sample with an antibody composition
containing antibodies capable of binding to the antigens glycophorin A,
CD3, CD24, CD16, CD14, optionally one or more of CD45RA, CD38, CD36,
CD56, CD2, CD19, CD20, CD22, CD29, CD66e, CD66b, and optionally
antibodies specific for non-hematopoietic antigens expressed on tumor cells,
under conditions so that cell conjugates are formed between the antibodies
and the cells in the sample having the antigens expressed on the tumor
cells;
(b) removing the cell conjugates; and
(c) recovering a cell preparation which is enriched in
normal human hematopoietic progenitor cells and stem cells.
T'he present invention also relates to kits useful in
performing processes of the invention comprising antibodies specific for
219165:5
-7-
glycophorin A, C'D3, CD24, CD16, CD14, optionally one or more of
CD45RA, CD36, CD38, CI)56, CD2, CD19, CD20, CD22, CD29, CD66e, CD66b,
and optionally antibodies specific for non-hematopoietic antigens expressed
on tumor cells and instructions for performing the processes of the
invention.
The invention further relates to cell preparations obtained
in accordance with the processes of the invention. The invention still
further contemplates a rnethod of using the antibody compositions of the
invention in negative selection methods to recover a cell preparation
which is enriched in human hematopoietic progenitor and stem cells.
The present invention also contemplates a tumor-
enriching antibody composition which is adapted to enrich for tumor cells,
in particular metastatic tumor cells. The composition is useful in the
detection of non-hematopoietic tumor cells from blood and bone marrow of
patients to aid in the detection of metastatic disease. The tumor-enriching
antibody composition contains antibodies specific for selected markers
associated with hematopoietic cells. In particular, the present inventors
have found usirig a negative selection technique that an antibody
composition containing antibodies specific for glycophorin A, CD3, CD19,
CD36, CD56, CD14, CD16,, CD66b, CD38, CD45, and optionally CD41, CD33,
CD20, CD22, CD29, CD2, CD45RA, and/or CD10, gives a cell preparation
highly enriched for non-hematopoietic tumor cells. The present inventors
have shown that the tumor enriching antibody composition applied in one
step to a sample of peripheral blood, frozen peripheral blood, or bone
marrow containing tumor cells results in a greater than 3 log enrichment of
the tumor cells.
T'he enrichment of non-hematopoietic tumor cells using
the tumor-enriching antibody composition of the invention has many
advantages. The composition provides for a greater than 3 log enrichment
with good recovery of metastatic tumor cells. The recovered tumor cells are
not labeled with antibody which can interfere with detection methods.
2_ 191655
-8-
7'herefore the invention contemplates a tumor-enriching
antibody composition comprising antibodies specific for glycophorin A,
CD3, CD19, CD36, CD56, CD14, CD16, CD66b, CD38, CD45, and optionally
CD41, CD33, CD20, CD22, CD29, CD66e, CD2, CD45RA and/or CD10.
The present invention also contemplates a process for
enriching for non-hematopoietic metastatic tumor cells in a sample
containing the turnor cells and hematopoietic cells comprising
(a) reacting the sample with an antibody composition
comprising antibodies specific for glycophorin A, CD3, CD19, CD36, CD56,
CD14, CD16, CD66b, CD38, CD45, and optionally CD41, CD33, CD20, CD22,
CD29, CD2, CD451RA and/or CD10, under conditions so that conjugates are
formed between the antibodies and hematopoietic cells in the sample
expressing the antiigens glycophorin A, CD3, CD19, CD36, CD56, CD14, CD16,
CD66b, CD38, CD45, and optionally CD41, CD33, CD20, CD22, CD29, CD2,
CD45RA, and/or CD10;
(b) removing the cell conjugates, and
(c) recovering a cell preparation enriched in the tumor
cells.
T'he invention still further contemplates a process for
detecting metastatic tumor cells in a sample containing the metastatic
tumor cells and hematopoietic cells comprising
(a) reacting the sample with an antibody composition
comprising antibodies specific for glycophorin A, CD3, CD19, CD36, CD56,
CD14, CD16, CD66b, CD38, CD45, and optionally CD41, CD33, CD20, CD22,
CD29, CD2, CD45RA, and/or CD10, under conditions so that conjugates are
formed between the antibodies and hematopoietic cells in the sample
expressing the antigens glycophorin A, CD3, CD19, CD36, CD56, CD14, CD16,
CD66b, CD38, CD45, and optionally CD41, CD33, CD20, CD22, CD29, CD2,
CD45RA, and/or CD10,
(b) removing the cell conjugates, and
(c) recovering a cell preparation enriched in the tumor
cells.
2191655
-9-
The present invention also relates to kits useful in
performing proceIsses of the invention comprising antibodies specific for
glycophorin A, CD3, CD19, CD36, CD56, CD14, CD16, CD66b, CD38, CD45, and
optionally CD41, CD33, CD20, CD22, CD29, CD2, CD45RA, and/or CD10, and
instructions for performing the tumor cell enriching processes of the
invention.
The invention further relates to metastatic cell
preparations obtained in accordance with the processes of the invention.
The invention still further contemplates a method of using the tumor-
enriching antibociy compositions of the invention in negative selection
methods to recover a cell preparation which is enriched in non-
hematopoietic metastatic tumor cells.
T'hese and other aspects of the present invention will
become evident upon reference to the following detailed description and
attached drawings. In addition, reference is made herein to various
publications, which are hereby incorporated by reference in their entirety.
BRIEF DESCRIPTION OF THE DRAWINGS
T'he invention will now be described with reference to the
accompanying drawings, in which:
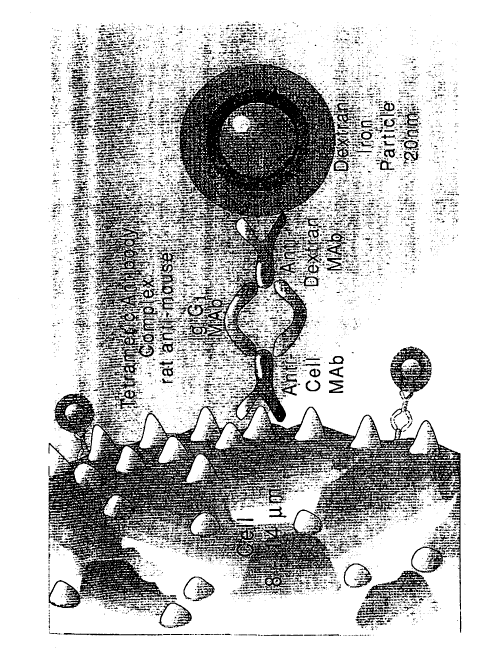
Figure 1 is a schematic representation of magnetic cell
labelling using tetrameric antibody complexes and colloidal dextran iron.
DETAILED DESCIIZIPTION OF THE INVENTION
I. HEMATOPOIETIC CELL TYPES AND TUMOR CELLS
T'he terin "differentiated cells" used herein refers to
human hematopoi.etic cells which have limited or no proliferative capacity.
Differentiated cells represent specialized end cells that are found in blood,
and their immediate precursors.
T'he term "progenitor cells" used herein refers to cells
which are the immediate precursors of the differentiating cells. Most of the
progenitor cells differentiate along a single lineage but they may have quite
extensive proliferative capacity. Progenitor cells appear morphologically as
2191655
-10-
blast cells, and they typically do not have specific features of the
hematopoietic lineage to which they are committed.
The term "stem cells" used herein refers to the cells from
which progenitor cells are derived. Stem cells are defined by their ability to
self-renew as well. as to generate daughter cells of any of the hematopoietic
lineages. Stem cells with long term hematopoietic reconstituting ability can
be distinguished by a number of physical and biological properties from
differentiated cel:ls and progenitor cells (Hodgson, G.S. & Bradley, T.R.,
Nature, Vol. 281, pp. 381-382; Visser et al., J. Exp. Med., Vol. 59, pp.
1576-1590, 1984; Spangrude et al., Science, Vol. 241, pp. 58-62, 1988;
Szilvassy
et al., Blood, Vol. 74, pp. 930-939, 1989; Ploemacher, R.E. & Brons, R.H.C.,
Exp. Hematol., Vol. 17, pp. 263-266, 1989).
T'he presence of stem cells and progenitor cells in a cell
preparation may be detected by their ability to produce colony-forming cells
in culture. They may also be detected by screening for the CD34 antigen
which is a positive marker for early hematopoietic cells including colony
forming cells and stem cells. Primitive hematopoietic stem cells with long
term hematopoietic reconstituting ability can be identified by determining
the number of clonogenic cells present after 5 to 8 weeks in long term
cultures (Sutherland et al., Blood, Vol. 74, p. 1563, 1986; Udomsakdi et al.,
Exp. Hematol., Vol. 19, p. 338, 1991; and, Sutherland et al., Proc. Natl.
Acad.
Sci., Vol. 87, p. 3584, 1990).
T'umor cells which may be removed from a sample using
the antibody compositions and processes described herein include tumor
cells which have ilon-herYlatopoietic antigens or markers expressed on their
surfaces i.e. antigens that distinguish the tumor cells from hematopoietic
progenitor cells and stem. cells. For example, specific markers have been
found to be expressed on tumor cells such as breast and lung carcinoma, and
neuroblastoma. Table 1 lists specific examples of antibodies which
recognize non-her.natopoietic antigens expressed on tumor cells.
Some metastatic tumor cells express hematopoietic lineage
markers or antigens, for example, tumor cells from B-lymphomas, multiple
2191655
-11-
myeloma, some chronic lymphocytic leukemias (CLL), and some acute
lymphocytic leukemias (ALL) express B-cell markers such as CD22, CD20,
CD29, and T cells from ALL and CLL express T-cell markers, and antibodies
to these antigens may be included in the antibody compositions of the
invention to rerriove tumor cells expressing the hematopoietic lineage
antigens.
II. ANTIBODY COMPOSITIONS
As hereinbefore mentioned, the present invention relates
to an antibody composition comprising antibodies specific for glycophorin
A, CD3, CD24, CD16, CD14, and optionally specific for CD45RA, CD36,
CD38, CD56, CD2, CD19, CD20, CD22, CD29, CD66e, CD66b, and/or non-
hematopoietic antigens expressed on tumor cells.
The antibodies in the antibody composition are selected
based on the desi:red degree of enrichment and the types of cells which are
prevalent in a particular sample. For example, in blood samples T cells are
preferably removed but it may be desirable to leave some NK cells. The T
cell load in a bone marrow sample will be much lower and including anti-T
cell antibodies in. the composition may not be absolutely necessary for
obtaining an acceptable degree of debulking. Generally an antibody
composition having at least two antibodies against T cells and two
antibodies against: B cells provides maximum enrichment.
In an embodiment of the invention the antibody
composition contains antibodies specific for glycophorin A, CD3, CD24,
CD16, CD14, CD56, CD2, CD19, and CD66b. Preferably, the compositions
contain antibodies specific for non-hematopoietic antigens, most preferably
antigens expressed on breast and lung carcinoma and neuroblastoma cells.
Pluripotent stem cells and committed progenitors express
CD34, and this CID34 compartment can be subdivided using antibodies to a
variety of cell surface markers. Stem cells co-purify in a population of
CD34+ cells which lack or have low expression of certain lineage markers
(CD38, CD33, CD45RA, CD71, CD36 and HLA-DR) (Craig et al. 1994, British
Journal of Haematology, 88:24-30; Lansdorp, P.AI. and Dragowska, W. (1992)
2191655
-12-
J. Exp. Med. 175:1501-1509; Sutherland, H,J., et al. (1989) Blood 74.1563-
1570).
Antibodies recognizing these antigens can be included in the antibody
composition to further enrich for stem cells, while losing some of the
committed mature CD34+ cells. Preferably, anti-CD45RA, anti-CD38 and
anti-CD36 are incl.uded in the antibody composition.
In an embodiment of the invention the antibody
composition contains antibodies specific for glycophorin A, CD3, CD24,
CD16, CD14, CD56, CD2, CD19, CD66b, CD36, CD45RA, and CD38, and
optionally antibodies specific for non-hematopoietic antigens, most
preferably antigens expressed on breast and lung carcinoma and
neuroblastoma cells.
7'he invention also contemplates a tumor-enriching
antibody composition comprising antibodies specific for glycophorin A,
CD3, CD19, CD36, CD56, CD14, CD16, CD66b, CD38, CD45, and optionally
CD41, CD33, CD20, CD22, CD29, CD66e, CD2, CD45RA, and/or CD10. In an
embodiment of t:he invention the tumor-enriching antibody composition
comprises glycophorin A, CD3, CD2, CD19, CD36, CD56, CD14, CD16, CD66b,
CD38, CD45RA, artd CD45.
Within the context of the present invention, antibodies are
understood to include monoclonal antibodies and polyclonal antibodies,
antibody fragments (e.g., Fab, and F(ab')2) and chimeric antibodies.
Antibodies are uriderstood to be reactive against a selected antigen on the
surface of a hematopoietic cell, differentiated cell or tumor cell if they
bind
with an appropriate affinity (association constant), e.g. greater than or
equal
to 107 M-1.
F'olyclonal antibodies against selected antigens on the
surface of hematopoietic cells, differentiated cells or tumor cells may be
readily generated by one of ordinary skill in the art from a variety of warm-
blooded animals such as horses, cows, various fowl, rabbits, mice, hamsters,
or rats. For exaniple, a inammal, (e.g., a mouse, hamster, or rabbit) can be
immunized with an immunogenic form of an antigen which elicits an
antibody respoilse in the mammal. Techniques for conferring
CA 02191655 2003-06-13
-13-
immunoger-icity on an antigen include conjugation to carriers or other
techniques well known in the art. For example, the antigen can be
administered in the presence of adjuvant. The progress of immunization can
be monitored by detectuon of antibody titers in plasma or serum. Following
immunization, antisera can be obtained and polyclonal antibodies isolated
from the sera.
Monoclonal antibodies are preferably used in the antibody
compositioris of the invention. Monoclonal antibodies specific foi- selected
antigens on the surface of hematopoietic cells, differentiated cells or tu.mor
cells
may be readily generated using conventional techniques. For example,
monoclonal antibodies may be produced by the hybridoma technique
originally developed by Kohler and Milstein (Nature 256, 495-497 (1975)). (See
also U.S. Patent Nos. RE 32,011, 4,902,614, 4,543,439, and 4,411,993; see also
Monoclonal Antibodies, E-Iybridomas: A New Dimension in Biological
Analyses, Plenum Press, Kennett, McKearn, and Bechtol (eds.), 1980, and
Antibodies: A Laboratory Manual, Harlow and Lane (eds.), Cold Spring
Harbor Laboratory Press, 1988).
Other techniques may a3so be utilized to construct
monoclonal antibodies (for example, see William D. Huse et al., "Generation of
a Large Combinational Library of the Immunoglobulin Repertoire in Phage
Lambda," Science 246:1275-1281, December 1.989; see also L. Sastry et al.,
"Cloning of the Immunological Repertoire in Escherichia coli for Generation of
Monoclonal Catalytic Antibodies: Construction of a Heavy Chain Variable
Region-Specific cDNA Li.brary," Proc. Natl. Acad. Sci USA 86:5728-5732, August
1989; Kozbor et al., Immunol. Today 4, 7 2(1983) re the human B-cell
hybridoma technique; Cole et al. Monoclonal Antibodies in Cancer Therapy
(1985) Allen R. Bliss, Inc., pages 77-96 re the EBV-:hybridoma technique to
produce human monoclonal antibodies; and see also Michelle Alting-Mees et
al., "Monoclonal Antibody Expression Libraries: A Rapid Alterriative to
Hybridomas," Strategies in Molecular Biology 3:1-9, January 1990).
Hybridoma cells can be screened
-2191655
-14-
immunochemically for production of antibodies specifically reactive with
an antigen, and rnonoclorial antibodies can be isolated.
The term "antibody" as used herein is intended to include
antibody fragments which are specifically reactive with specific antigens on
the surface of hematopoietic cells, differentiated cells or tumor cells.
Antibodies can be fragmented using conventional techniques and the
fragments screened for utility in the same manner as described above for
whole antibodies. For example, F(ab')2 fragments can be generated by
treating antibody with pepsin. The resulting F(ab')2 fragment can be treated
to reduce disulfide bridges to produce Fab' fragments.
7'he invention also contemplates chimeric antibody
derivatives, i.e., antibody molecules that combine a non-human animal
variable region and a human constant region. Chimeric antibody
molecules can include, for example, the antigen binding domain from an
antibody of a mouse, rat, or other species, with human constant regions. A
variety of approaizhes for making chimeric antibodies have been described
and can be used to make chimeric antibodies containing the
immunoglobulin variable region which recognizes selected antigens on the
surface of differeritiated cells or tumor cells. See, for example, Morrison et
al., Proc. Natl. Acad. Sci. U.S.A. 81,6851 (1985); Takeda et al., Nature 314,
452
(1985), Cabilly et al., U.S. Patent No. 4,816,567; Boss et al., U.S. Patent
No.
4,816,397; Tanaguchi et al., European Patent Publication EP171496; European
Patent Publication 0173494, United Kingdom patent GB 2177096B.
Binding partners may be constructed utilizing recombinant
DNA techniques. Within one embodiment, the genes which encode the
variable region firom a hybridoma producing a monoclonal antibody of
interest are amplified using nucleotide primers for the variable region.
These primers may be synthesized by one of ordinary skill in the art, or may
be purchased fror.n commercially available sources. The primers may be
utilized to amplify heavy or light chain variable regions, which may then be
inserted into vectors such as ImmunoZAPTM H or ImmunoZAPTM L
(Stratacyte), respectively. These vectors may then be introduced into E. coli
CA 02191655 2003-06-13
-15-
for expression. Utilizing these techniques, large amounts of a single-chain
protein containing a fusion of the VH and Vt, domains may be produced (See
Bird et al., Science 242:423-426, 1988). I.n addition, such techniques may be
utilized to change a"murin.e" antibody to a"human" antibody, without altering
the binding specificity of the antibody.
Antibodies against selected antigens on the surface of
hematopoietic cells, differentiated cells or tumor cells may also be obtained
from commercial sources.
Antib+idies may be selected for use in the antibody
compositions of the invention based on their ability to deplete targeted
hematopoietic cells, differentiated cells and/or tumor cells and recover non-
targeted cells (i.e. normal progenitor and stem cells, specific differentiated
cells,
or tumor cells), in magnetic cell separations as more particularly described
herein, and in U.S. Patent No. 5,514,340. In general, an antibody is selected
that
gives greater than 31og depletion of hematopoietic cells, differentiated cells
or
tumor cells. In an embociiment of the invention for enriching for
hematopoietic
stem cells and progenitor cells, an antibody is selected that gives greater
than 3
log depletion of differentiated cells or tumor cells, with greater than 75%
recovery of CD34+ cells (bone marrow, mobilized blood and cord blood) or
non-targeted lymphocytes (steady state blood), in test magnetic cell
separations as described herein.
The anti=-glycophorin A antibod ies contained in the antibody
compositions of the invention are used to label erythrocytes. Examples of
monoclonal antibodies specific for glycophorin A are 1OF7MN (U.S. Patent No.
4,752,582, Cell lines: ATCC accession numbers HB-8162), and D2.10
(Immunotech, Marseille, France). The concentration of antiglycophorin A
antibodies used in the antibody compositions are generally less than the
concentration that will cause agglutination (i.e. 3-10ug/ml). Preferably the
concentration of antiglycophorin A antibodies
2191655
-16-
used in the antibody compositions is between about 0.5 to 5 g/ml,
preferably 1 to 2 g/ml.
Monoclonal antibodies against CD24, CD3, CD19, CD20,
CD22, CD29, CD56, CD2 in the antibody compositions of the invention are
used to label B and T lyrnphocytes and NK cells. Examples of monoclonal
antibodies specific for CL)24, CD3, CD19, CD20, CD22, CD56, and CD2, are
32D12 (Dr. Steinar Funderud, Institute for Cancer Research, Dept. of
Immunology, Oslo, Norway,) and ALB9 (Immunotech, Marseille, France);
UCHT1 (Immunotech, Marseille, France) and Leu-4 (Becton Dickinson,
Mountain View, Calif.); J4.119 (Immunotech, Marseille, France) and Leu-12
(Becton Dickinson., Mountain View, Calif.); MEM97 (Dr. Horejsi, Institute of
Molecular Genetics Academy of Sciences of the Czech Republic, Praha,
Czech Republic, or Cedarlane Laboratories, Hornby, Ontario, Canada) and
Leu-16 (Becton Dickinson, Mountain View, Calif.); SJ10.1H11 (Immunotech,
Marseille, France'); T199 (Immunotech, Marseille, France); and 6F10.3
(Immunotech, Marseille, France), respectively. The concentration of each of
the monoclonal antibodies against CD24, CD3, CD19, CD20, CD56, CD2
contained in the antibody composition is between about 0.5 to 5 g/ml,
preferably 2 to 3 g/ml.
Monoclonal antibodies against CD14, CD16, CD66e and
CD66b in the antibody compositions of the invention are used to label
monocytes and g:ranulocytes. Examples of monoclonal antibodies specific
for CD14, CD16, CD66e and CD66b, are MEM15 and MEM18 (Dr. Vaclav
Horejsi, Institute of Molecular Genetics Academy of Sciences of the Czech
Republic, Praha, Czech Republic; Cedarlane Laboratories, Hornby, Ontario,
Canada); MEM154 (Dr. Vaclav Horejsi, Institute of Molecular Genetics
Academy of Sciences of the Czech Republic, Praha, Czech Republic;
Cedarlane Laboratories, Hornby, Ontario, Canada), Leu-lla (Becton
Dickinson, Mountain View, Calif.), and 3G8 (Immunotech, Marseille,
France); CLB/granl0 (CLB, Central Laboratory of the Netherlands, Red Cross
Blood Transfusion Service); and, B13.9 (CLB, Central Laboratory of the
Netherlands, Red Cross Blood Transfusion Service) and 80H3
2191655
-17-
(Immunotech, Marseille, France), respectively. The concentration of each of
the monoclonal aritibodies against CD14, CD16, CD66e and CD66b contained
in the antibody compositions is between about 0.5 to 5 g/ml, preferably 2-3
gg/ml.
T/Ionoclonal antibodies against CD45RA, CD38 and CD36
are used to label T-cells, B-cells plasma cells, granulocytes, platelets,
monocytes, differentiated erythroid precursors, and some committed
mature progenitors, to further enrich for stem cells. Examples of
monoclonal antibodies against CD45RA, CD38 and CD36 are 8D2.2
(StemCell Technologies, Vancouver, Canada, Craig et al., 1994, British
Journal of Haematology, 88:24-30.), Leu-18 (Becton Dickinson, Mountain
View, Calif.); T16 (Immunotech, Marseille, France); and, FA6.152
(Immunotech, Marseille, France) and IVC7 (CLB, Central Laboratory of the
Netherlands Red Cross Blood Transfusion Service), respectively. The
concentration of each of the monoclonal antibodies against CD45RA, CD38,
and CD36 contained in the antibody compositions is between about 0.5 to 5
g/ml, preferably 1 to 3 gg/ml.
Monoclonal antibodies against CD45 are used to label
leukocytes eg. lymphocytes, monocytes, granulocytes - essentially all
nucleated cells in blood and bone marrow. CD45 is not on mature
erythrocytes and plasma cells. An example of an anti-CD45 antibody is J33
(Immunotech, Niarseille, France). The concentration of monoclonal
antibodies against CD45 in the antibody composition of the invention is
between about 0.5 to 5.0 g/ml, preferably 1 to 3 gg/ml.
Monoclonal antibodies against CD41 are used to label
megakaryocytes and platelets. An example of an anti-CD41 antibody is SZ22
(Immunotech, Marseille, France). The concentration of monoclonal
antibodies against CD41 in the antibody composition of the invention is
between about 0.5 to 5.0 g/ml, preferably 1 to 3 gg/ml.
CD33 is a myeloid marker found on monocytes,
granulocytes, aind macrophage precursors. Therefore, monoclonal
2191655
-18-
antibodies against CD33 are used to label monocytes, granulocytes, and
macrophage precursors. An example of an anti-CD33 antibody is
D3HL60.251 (Irnmunotech, Marseille, France). The concentration of
monoclonal antibodies against CD33 in the antibody composition of the
invention is between about 0.5 to 5.0 g/ml, preferably 1 to 3 g/ml.
Table 2 sets out the most preferred monoclonal antibodies
specific for differentiated cells, their sources and concentrations, for use
in
the antibody conipositions of the invention. Table 1 sets out the most
preferred monoclonal antibodies specific for tumor cells, and commercial
sources/references for the antibodies.
A preferred antibody composition for removing
differentiated hentatopoietic cells and breast and lung carcinoma cells from
a sample comprises the monoclonal antibodies D2.10, UCHT1, MEM15, 3G8,
ALB9, 80H3, J4.119, 6F10.3, T199, 8D2.2, T16 and FA6.152 or the monoclonal
antibodies 10F7MN, UCHT1, 32D12, MEM154, MEM15, 80H3 or B13.29, T199,
6F10.3, J4.119, ancl optionally, 8D2.2, T16 and IVC7, and one or more of the
monoclonal antibodies specific for an antigen on the surface of a breast or
lung carcinoma as set forth in Table 1. Most preferably the monoclonal
antibodies specifiic for an antigen on the surface of cells from a breast
carcinoma used irt a composition of the invention are one or more of 5E11,
H23A, 6E7, RAR, and BRST1.
A preferred antibody composition for removing
differentiated hernatopoietic cells and neuroblastoma cells from a sample
comprises the monoclonal antibodies D2.10, UCHT1, MEM15, 3G8, ALB9,
80H3, J4.119, 6F10.3, T199, and optionally 8D2.2, T16 and FA6.152 or the
monoclonal antibodies 10F7MN, UCHT1, 32D12, MEM154, MEM15, 80H3 or
B13.29, T199, 6F10.3, J4.119, and optionally, 8D2.2, T16 and IVC7, and one or
more of the monoclonal antibodies specific for an antigen on the surface of
cells from a neuroblastoma as set forth in Table 1.
Preferred antibody compositions for enriching for non-
hematopoietic rnetastatic tumor cells from a sample containing
-- - ----------
2191655
-19-
hematopoietic cells and non-hematopoietic metastatic tumor cells comprise
the monoclonal aritibodies D2.10, UCHT1, MEM15, 3G8, 80H3, J4119, 6F10.3,
T199, 8D2.2, T16, FA6.152, and J33; or the monoclonal antibodies 10F7MN,
UCHT1, MEM15, MEM154, B13.29, J4119, 6F10.3, T199, 8D2.2 T16, IVC7, and
J33.
III. PROCESS FOR PREPARING CELL PREPARATIONS ENRICHED IN
PROGENITOR/STEM CELLS, OR TUMOR CELLS
7'he antibody compositions of the invention may be used
to enrich and recover cell preparations enriched in human hematopoietic
stem cells and progenitor cells. In accordance with a process of the
invention, a sarnple is reacted with an antibody composition of the
invention; under suitable conditions, cell conjugates form between the
antibodies contained in the antibody composition which are specific for
selected antigens on the surface of differentiated cells and/or tumor cells,
and the cells in the sample containing the antigens on their surface; and the
cell conjugates are removed.
T'he antibody composition of the invention which is a
tumor-enriching antibody composition may be used to enrich for non-
hematopoietic metastatic tumor cells in a sample. In accordance with a
process of the invention a sample containing non-hematopoietic metastatic
tumor cells and hematopoietic cells is reacted with a tumor-enriching
antibody composition; under suitable conditions conjugates are formed
between the antibodies in the composition which are specific for antigens
on the surface of hematopoietic cells in the sample expressing the antigens;
and the cell conjugates are removed to provide a cell preparation enriched
in the tumor cells.
Conditions which permit the formation of cell conjugates
may be selected having regard to factors such as the nature and amounts of
the antibodies in the antibody composition, and the estimated
concentration of targeted differentiated cells in the sample.
The antibodies in the antibody compositions may be
labelled with a marker or they may be conjugated to a matrix. Examples of
2191655
-20-
markers are biotin, which can be removed by avidin bound to a support,
and fluorochromes, e.g. fluorescein, which provide for separation using
fluorescence activated sorters. Examples of matrices are magnetic beads,
which allow for direct magnetic separation (Kernshead 1992), panning
surfaces e.g. plates, (Lebkowski, J.S, et al., (1994), J. of Cellular
Biochemistry
supple. 18b:58), dense particles for density centrifugation (Van Vlasselaer,
P., Density Adjusted Cell Sorting (DACS), A Novel Method to Remove
T u m o r Cells From Peripheral Blood and Bone Marrow StemCell
Transplants. (1995) 3rd International Symposium on Recent Advances in
Hematopoietic Stem Cell Transplantation-Clinical Progress, New
Technologies and Gene Therapy, San Diego, CA), adsorption columns
(Berenson et al. "1986, Journal of Immunological Methods 91:11-19.), and
adsorption membranes (Norton et al. 1994). The antibodies may also be
joined to a cytotoxic agent such as complement or a cytotoxin, to lyse or kill
the targeted cells.
The antibodies in the antibody compositions may be
directly or indirectly coupled to a matrix. For example, the antibodies in the
compositions of the invention may be chemically bound to the surface of
magnetic particles for example, using cyanogen bromide. When the
magnetic particles are reacted with a sample, conjugates will form between
the magnetic particles with bound antibodies specific for antigens on the
surfaces of the hematopoietic cells, differentiated cells and/or tumor cells,
and the hematopoietic cells, differentiated cells and/or tumor cells having
the antigens on their surfaces.
Alternatively, the antibodies may be indirectly conjugated
to a matrix using antibodies. For example, a matrix may be coated with a
second antibody having specificity for the antibodies in the antibody
composition. By way of example, if the antibodies in the antibody
composition are rnouse IgG antibodies, the second antibody may be rabbit
anti-mouse IgG.
CA 02191655 2003-06-13
- 21 -
The antibodies in the antibody compositions may also be
incorporated in antibcmdy reagents which indirectly conjugate to a matrix.
Examples of antibody reagents are bispecific antibodies, tetrameric antibody
complexes, and biotinylated antibodies.
Bispecific antibodies contain a variable region of an antibody
in an antibody composition of the invention, and a variable region specific
for
at least one antigen on the surface of a matrix. The bispecific antibodies may
be prepared by forming hybrid hybridomas. The hybrid hybridomas may be
prepared using the procedures known in the art such as those disclosed in
Staerz & Bevan, (1986 PNAS (USA) 83: 1453) and Staerz & Bevan, (1986,
Immunology Today, 7:241). Bispecific antibodies may a.lso be constructed by
chemical means using procedures such as those described by Staerz et al.,
(1985, Nature, 314:628) i-Ind Perez et al., (1985 Nature 37.6:354), or by
expression
of recombinant immuncyglobulin gene constructs.
A t:etnimeric immunological complex may be prepared by
mixing a first rnonoclonal antibody which is capable of binding to at least
one
antigen on the surface of a rnatrix, and a second monoclonal antibody from the
antibody composition of the .invention.. The first aiid second monoclonal
antibody are from a first animal species. T'he first and second antibody are
reacted with an about equi-no]ar amount of monoclonal antibodies of a second
animal species which are directed against the Fc-fragrnents of the antibodies
of
the first animal species. 'The first and second antibody may also be reacted
with an about equimolar amount of the F(ab')2 fragments of monoclonal
antibodies of a second animal species which are directed against the
Fc-fragments of the antibodies of the first animal species. (See U.S. Patent
No.
4,868,109 to Lansdorp for a description of tetrameric antibody complexes and
methods for preparing same).
The antibodies of the invention may be biotinylated and
indirectly conjugated to a matrix which is labelled with (strept) avidin. For
example, biotinylated antibodies contained in the antibody compositions of the
CA 02191655 2003-06-13
-22-
invention may be used in c.ombination with magnetic iron-dextran particles
that are covalently labelled with (strept) avidin (Miltenyi, S. et al.,
C:ytometry
11:231, 1990). Many alternative indirect ways to specifically cross-link the
antibodies in the antibody cornpositions artd matrices would also be apparent
to those skilled in the art.
ln an embodiment of the invention, the cell conjugates are
removed by magnetic separation using magnetic particles. Suitable magnetic
particles include particles i:n ferrofluids ancl other colloidal magnetic
solutions.
"Ferrofluid" refers to a colloidal solution containing particles consisting of
a
magnetic core, such as rnagnetite (Fe304) coated or embedded in material that
prevents the crystals froin interacting. Examples of such materials include
proteins, such as ferritin, polysaccharides, such as dextrans, or synthetic
polymers such as sulfonated polystyrene cross-linked with divinylbenzene.
The core portion is generally too small to hold a permanent magnetic field.
The ferrofluids become rnagnetized when placed in a magnetic field. Examples
of ferrofluids and methods for preparing them are described by Kemshead J.T.
(1992) in J. Hematotherapy, 1:35-44, at pages 36 to 39, and Ziolo et al.
Science
(1994) 257:219. Colloidal particles of dextran-iron complex are preferably
used
in the process of the invention. (See Molday, R.S. and McKenzie, L.L. FEBS
Lett. 170:232,, 1984; Milter-yi et al., Cytom.etry 11:231, 1990; and Molday,
R.S.
and MacKenzie, D., J. lrn.munol. Methocls 52:353, 1982; Thomas et al., J.
Hematother. 2:297 (1993); and U.S. Patent No. 4,452,733, which are each
incorporated herein. by reference).
Figure 1 is a schematic representation of magnetic cell
labeling using tetrameric antibody complexes and colloidal dextran iron.
In accordance with a tnagnetic separation method, the
sample containing the progenitor and stern cells to be recovered, is reacted
with the above described antibody reagents, preferably tetrameric antibody
complexes, so that the antibody reagents bind to the targeted differentiated
cells, tumor cells, and/or hernatopoietic cells present in the sample to form
cell
CA 02191655 2003-06-13
-23-
conjugates of the targeted differentiated cells, tumor cells, and/or
hematopoietic cells and the antibody reagents. The reaction conditions are
selected to provide the desired level of binding of the targeted
differentiated
cells, tumor cells, and/or hematopoietic cells and the antibody reagents.
Preferably the sample is, incubated with the antibody reagents for a period of
5
to 60 minutes at either s[ or antbient room temperature. The concentration of
the antibody reagents is selected depending on the estimated concentration of
the targeted differentiated cells in the sample. Generally, the concerltration
is
between about 0.1 to 50 }rg/ml of sample. The magnetic particles are then
added and the mixture is incubated for a period of about 5 minutes to 30
minutes at the selected temperature. 'The sample is then ready to be separated
over a magrietic filter device. Preferably, the magnetic separation procedure
is
carried out using the magnetic filter and methods described in U.S. F'atent
No.
5,514,340 to Lansdorp arid Thomas.
The sample containing the magnetically labelled cell
conjugates is passed through the magnetic filter in the presence of a magnetic
field. In a preferred embodiment of the invention, the magnet is a solenoid
electromagnet with a 3" diameter bore and having a magnetic field of 0.5-2
Tesla. The magnetically labelled cell conjugates are retained in the high
gradient magnetic column and the materials which are not magnetically
labelled flow through the column after washing with a buffer.
A preparation containing non-magnetically labelled cells may
be analyzed using procedures such as flow cytometry. The ability of a cell
preparation containing progenitor and stern cells to produce colony-forming
cells or long term culture initiating cells (LTCIC) in culture may also be
assessed. The efficiency of the separation procedure may also be determined
by monitoririg the recovery of CD34-+ cells, CD34+ CD38- cells and colony
forming cells.
The above described magnetic separation methods may be
adapted for enriching for tumor cells in a sample containing tumor cells and
hematopoietic cells.
IV. Uses of the Compositions and Processes of the Invention
2191655
-24-
The device and processes of the invention may be used in
the processing of biological samples including blood in particular, cord
blood and whole blood. It has also been found that the antibody
compositions of the invention can be used to prepare hematopoietic
progenitor and s1tem cell preparations and tumor cell preparations from
bone marrow samples, including previously frozen bone marrow samples.
The processes of the invention may be used to deplete or
purge erythrocytes, B and T lymphocytes, monocytes, NK cells,
granulocytes, and tumor cells from samples to prepare hematopoietic
progenitor and stem cell preparations for use in transplantation as well as
other therapeutic methods that are readily apparent to those of skill in the
art. For example, bone marrow or blood can be harvested from a donor in
the case of an allogenic transplant and enriched for progenitor and stem
cells by the processes described herein.
LJsing the process of the invention it is possible to recover
a highly purified preparation of human hematopoietic stem/progenitor
cells. In particular, a hematopoietic cell population containing greater than
50% of the hema.topoietic progenitor/stem cells present in the original
sample, and which is depleted of differentiated cells and tumor cells in the
original sample by greater than 3 logarithms may be obtained. The human
hematopoietic progenitor and stem cells in the preparation are not coated
with antibodies, or modified making them highly suitable for
transplantation and other therapeutic uses that are readily apparent to those
skilled in the art.
T'he cell preparations obtained using the processes of the
invention may be used to isolate and evaluate factors associated with the
differentiation and maturation of human hematopoietic cells. The cell
preparations may also be used to determine the effect of a substance on cell
growth and/or dii`ferentiation into a particular lineage.
T'he tumor-enriching antibody composition of the
invention is adzipted to enrich for tumor cells, in particular non-
hematopoietic metastatic tumor cells. The composition is useful in the
2191655
-25-
detection of non-hematopoietic tumor cells from blood, bone marrow, and
peritoneal and pleural effusions, of patients to aid in the diagnosis and
detection of inetastatic disease, monitoring the progression of metastatic
disease, or monitoring the efficacy of a treatment. The tumor enriching
antibody composition applied in one step to a sample of peripheral blood,
frozen peripheral blood, or bone marrow containing tumor cells results in a
greater than 3 log enrichment of the tumor cells.
The following non-limiting examples are illustrative of
the present invention:
EXAMPLES
EXAMPLE 1
PURGING BREAST CARCINOMA CELLS (BT20 or T47D CELLS)
1'etramers of anti-breast carcinoma antibodies as shown in
Table 1 were combined with a progenitor enrichment cocktail (D2.10,
UCHT1, MEM15, 3G8, ALB9, 80H3, J4.119, 6F10.3, T199, and optionally 8D2.2,
T16 and FA6.152, or 1OF7MN, UCHT1, 32D12, MEM154, MEM15, 80H3 or
B13.9, T199, 6F10.3, J4.119, and optionally, 8D2.2, T16 and IVC7) to produce a
cocktail for breast carcinoma purging and debulking. Including the lineage
depletion increases the degree of tumor purge over that seen with just anti-
tumor antibodies alone (Table 3). Breast carcinoma cell lines were added to
previously frozen marrow, peripheral blood leukapheresis or fresh bone
marrow. Tumor cell purges were performed using the anti-breast carcinoma
antibodies indicated in Table 3 with and without the standard lineage
depletion (progenitor enrichment cocktail). The recovery of hematopoietic
progenitors during lineage depletion is given in Table 4. Enrichment of
progenitors was generally 50 to 100 fold.
In summary, purging tumor cells for hematopoietic
progenitors in a one step selection using the antibody cocktail as indicated
in Table 3 achieves a niuch higher degree of tumor cell purging than
positive selection techniques while offering a similar degree of progenitor
enrichment. The recoveries of hematopoietic progenitor cells in a lineage
depletion are greater than those typically seen with positive selection.
2191655
-26-
EXAMPLE 2
Cells from the CAMA breast carcinoma cell line were
mixed with previously frozen bone marrow (BM) or peripheral blood (PB)
and processed with the enrichment antibody composition (D2.10, UCHT1,
MEM15, 3G8, 80H3, J411.9, 6F10.3, T199, 8D2.2, T16, FA6.152, and J33) in a
one step magnetic depletion. The frequency of CAMA cells in the start
suspension varied from 2% to 2/106 cells (Table 5). CAMA cells were
enriched 2-3 log.
While what is shown and described herein constitutes
various preferred embodiments of the subject invention, it will be
understood that various changes can be made to such embodiments
without departing from the subject invention, the scope of which is
defined in the appended claims.
21~1fi55
-27-
Table 1
Antibodies Recognizing Non-Hematopoietic Antigens Expressed on
Tumor Cells.
Disease Atitibody Antigen Supplier/Developer
Breast and Lung 5E11 unknown, breast carcinoma STI
Carcinoma
6E7 unknown, breast carcinoma STI
H23A unknown, breast carcinoma ATCC
RAR9941 epithelial glycoprotein Baxter, Germany
RAR9948 epithelial glycoprotein Baxter, Germany
RAR9938 crb2 Baxter, Germany
C13B5 crb2 Immunotech, Marseille, France
BR.ST 1 BCA 225 ID Labs
BR.ST 3 TAG-72 ID Labs
CA15.3 MAM-6, mucin ID Labs
CA27.29 MAM-6, mucin Cedarlane
Neuroblastoma UJ13A unknown Hurko and Walsh (1983)
Neurology 33:734
UJ181.4 unknown UJ223.8 unknown
UJ127.11 unknown
5.1.H11 unknown
390,459 unknown R.C. Seeger, L.A. Children's
Hospital, Calif.
BA,-1.2 unknown "
HSAN 1.2 unknown Reynolds and Smith (1982)
Hybridomas in Cancer p235
2~91655
-28-
Table 2
Antibodies used in Lineage Depletions
Antigen Antibody Source Concentration
ug/ml
glycophorin 10F7MN* U.S. Patent No. 4,752,582 1
D2.10 IMMUNOTECH, Marseille, France 2
CD2 6F10.3 IMMUNOTECH, Marseille, France 3
CD3 UCH7'1 IMMUNOTECH, Marseille, France 3
Leu-4 Becton Dickinson Immunocytometry,
Mountain View, Calif.
CD4 Leu-3a Becton Dickinson Immunocytometry,
Mountain View, Calif.
CD8 Leu-2a Becton Dickinson Immunocytometry,
Mountain View, Calif.
OKT3 BioDesigns
CD14 MEM 15 Dr. Vaclav Horejsi, Institute of Molecular 2
MEM "l8 Genetics Academy of Sciences of the Czech 2
Republic, Praha, Czech Republic;
Cedarlane Laboratories Hornby, Ontario,
Canada
CD16 MEM 154* Dr. Vaclav Horejsi, Institute of Molecular 2
Genetics Academy of Sciences of the Czech 3
Republic, Praha, Czech Republic;
Cedarlane Laboratories Hornby, Ontario,
Canada
3G8 IMMUNOTECH, Marseille, France
Leu-11a Becton Dickinson Immunocytometry,
Mountain View, Calif.
CD19 J4.119 IMMUNOTECH, Marseille, France 3
Leu-12 Becton Dickinson Immunocytometry,
Mountain View, Calif.
2 19 1655
- 29 -
Antigen Antibody Source Concentration
ug/ml
CD20 MEM97 Dr. Vaclav Horejsi, Institute of Molecular 3
Genetics Academy of Sciences of the Czech
Republic, Praha, Czech Republic;
Cedarlane Laboratories Hornby, Ontario,
Canada
Leu-16 Becton Dickinson Immunocytometry,
Mountain View, Calif.
CD24 32D12* Dr. Steinar Funderud, Institute for Cancer 2
Research, Dept. of Immunology, Oslo, 3
Norway
ALB9 IMMUNOTECH, Marseille, France
CD33 D3HL60.251 Immunotech 3
CD36 FA6.152 IMMUNOTECH, Marseille, France 3
IVC7 CLB, Central Laboratory of the
Netherlands, Red Cross Blood Transfusion
Service
CD38 T16 IMMUNOTECH, Marseille, France 3
CD41 P11.64 Kaplan, 5th International Workshop on 3
Human Leukocyte Differentiation Antigens
Immunotech
S222 3
CD42a Bebl Becton Dickinson Immunocytometry, 3
Mountain View, Calif.
CD45 J33 Immunotech 3
CD45RA 8D2.2 Craig et al. 1994, StemCell Technologies, 1
Vancouver, Canada
Leu-18 Becton Dickinson Immunocytometry,
Mountain View, Calif.
CD56 T199 IMMUNOTECH, Marseille, France 3
65 5
-30-
Antigen Antibody Source Concentration
ug/ml
CD66e CLB/gran10 CLB, Central Laboratory of the 3
Netherlands, Red Cross Blood Transfusion
Service
CD66b B13.29 CLB, Central Laboratory of the 3
Netherlands, Red Cross Blood Transfusion 3
Service
80H3 IMMUNOTECH, Marseille, France
~ preferred antibody based on performance in magnetic cell separations
2 19 1655
-31-
Table 3
Purging Breast Carcinoma Cells (BT20 or T47D cells).
Cell Type Lineage Depletion Anti-Breast Carcinoma Log Tumor Cell
Antibodies Depletion
Previously Purge Only 5E11 1.8
Frozen Bone 5E11, H23A 3.7, 3.7
Marrow 5E11, 6E7 3.0
Previously Lineage Depletion and 5E11 >5.8, 3.9, 4.7
Frozen Bone I'urge RAR >5.8, 4.3, 4.7
Marrow BRST1 4.9
5E11, H23A >5.2, 4.4
5E11, RAR, BRST1 >5.8
Peripheral Blood I'urge only 5E11 1.9, 1.9
Leukapheresis H23A 1.7
5E11, H23A 2.3
Peripheral Blood Lineage Depletion and 5E11, H23A 5.6
Leukapheresis Purge
Fresh Bone Lineage Depletion and 5E11, H23A 4.6, 4.4
Marrow Purge
2191655
-32-
Table 4: Recovery of Hematopoietic Colony Forming Cells During Lineage
Depletion
% Recovery
Colony Assay rangemean
CFU-Gl`/1 60-100 75
BFU-E 71-100 92
LTCIC 72->100 100
2191655
-33-
Table 5: Enrichment of CAMA Breast Carcinoma Tumor Cells From Blood
and Bone Marroiv
Exp Sample #CAMA in % CAMA in % CAMA in % Recovery Log Enrich.
# Start Start Flow CAMA CAMA
1 BM 1.1/102 1.06 91.07 72.41 1.9
2 BM 2.2/1 2.18 96.40 44.12 1.6
2.1/103 0.21 82.16 75.00 2.6
2.1/104 0.02 32.01 60.00 3.2
3 BM 2.6/103 0.26 62.54 ? 2.4
2.6/10 0.026 11.21 ? 2.6
2.6/10 0.0026 2.01 2.9
2.6/106 0.00026 0.13 ? 2.7
4 PB 1.9/1 0.19 86.21 33.33 2.7
1.9/10 0.019 37.06 18.33 3.3
1.9/105 0.0019 7.42 20.00 3.6
1.9/10 0.00019 0.77 19.33 3.6
?- Cell numbers were too low to count accurately.