Note: Descriptions are shown in the official language in which they were submitted.
CA 02192232 1996-12-05
ZEOLITE LAYERS WITH
CONTROLLED CRYSTAL WIDTH AND PREFERRED
ORIENTATION GROWN ON A GROWTH ENHANCING LAYER
Field of the Invention
The present invention is directed toward a new composition of matter
comprising a zeolite layer, a growth enhancing layer, and a support.
Bac ground of the Invention
Zeolite membranes have long been a goal of separation science. For a
zeolite membrane to be practical, it must have a high flex as well as
selectivity.
Obtaining such a membrane has been difficult in the past because of defects in
the
zeolite film. This has been especially true for membranes grown from low
alkaline
synthesis routes described in the literature. These membranes have a
heterogeneous
crystal structure in the membrane and require an enormous (»0 micrometer)
layer
thickness to seal pinholes and void structures. What is needed in the art is a
thin zeolite
membrane with very few defects.
A patent describing the direct synthesis of zeolite membranes has been
issued to W. Haag and J. G. Tsikoyiannis of Mobil (U.S. Patent 5,110,478,
issued May
~, 1992). A paper describing scientific results obtained with this type of
membrane was
published in an article titled "Synthesis and Characterization of a Pure
Zeolitic
Membrane" by J. G. Tsikoyiannis and W. Haag in Zeolites (Vol. 12, p. 126,
1992). The
membrane described in the above article and patent is used as a freestanding
membrane
and is not affixed or attached as a layer to a microporous support making it
mechanically fragile and leading to ready breakage during use. The physical
structure
of the membrane is such that there is a gradient of crystal sizes across the
thickness of
the membrane. This gradient of crystal sizes throughout the layer thickness
precludes
growth of a thin membrane with a minimum number of non-selective permeation
paths.
Zeolites have also been grown on supports. See, for example, "High
temperature stainless steel supported zeolite (1~IFI) membranes: preparation,
module
construction and permeation experiments," E. R. Geus, H. van Bekkum, W. Bakker
and
AMENDED SHfE1
CA 02192232 2005-04-18
J. ~.~toulij in. Vlicroporous Materials, V'ol. l , p. 13 . l ~)~) ~:
European patent publication EP 0 481 660; and U.S. patent
-1.099.69?. Other references include Deutches Patentamt No. DE X8'_'7 049 A1
relating
to a zeolitic molecular sieve for separating fluids. International PCT
Application,
Publication No. WO93: 198:10 relating to a deposition process. and
European patent publications EP 0 769 983, EP 769 980, and EP 0 769 981 all
relating
generally to compositions useful in separations processes.
f111 of the above prepared membranes are formed with several zones (larger
crystals grown on top of smaller crystals) across the membrane thickness. In
several
zones, the crystals are not grown into a dense mat that is free of
intercrystalline voids.
To obtain a permselective zeolite membrane. the above zeolite layers f
comprised of
zones) must be grown to an excessive thickness (>j0 micrometers) to seal off
voids and
defects within the membrane. This creates a great mass transfer resistance
causing
reduced flu~c. Obtaining functional zeolite membranes from high alkaline
synthesis
routes is difficult because the heterogenous crystals in the membrane require
an
enormous membrane thickness to seal pinholes and void structures which lowers
the
membrane selectivity. The presence of such pinholes and voids is the cause of
optical
scattering in as synthesized high alkaline membranes.
Brief Description of the Drawings
Figures 1, ?, and 3 show the x-ray diffraction patterns of compositions
comprising a porous support having coated thereon a growth enhancing layer,
and a
zeolite layer grown on the Growth enhancing layer, wherein the growth
enhancing layer
was inverted. as described herein, during growth of the zeolite layer. The x-
axis are
theta and the y-axis are intensity in CPS. Figures l and 2 show compositions
having C-
axis crystallographic preferred orientations of different degrees. Figure 3
shows a
composition having b-axis orientation.
Figure 4 shows a scanning electron micrograph of the morphology of a iVIFI
zeolite layer, a Growth Enhancing Layer, and a porous substrate in accordance
with the
instant invention which was fractured to reveal a cross section. The layer
labeled (A) is
the porous substrate formed of a-alumina with 800 A pores. The layer labeled
(B) is
the GEL layer which is mesoporous and clearly discernible in the micrograph.
The
CA 02192232 1996-12-05
-2a-
layer labeled (C) contains VIFI zeolite crystals which are intergrown together
in a dense
mat. The columnar nature of the zeolite crystals used is readily apparent from
the
AM'NDF.I~ ,S~aFy
CA 02192232 1996-12-05
~~. WO 96101687 PCT/IJS95108514
-3-
morphology of the fracture surface through the zeolite layer. The layer (C) is
substantially free of voids and defects.
a Figure 4(b) shows a scanning electron micrograph of the same composition
as Figure 4(a) only at higher magnification in which more detail about the
interfaces
between layers (A), (B) and (C) can be seen.
Figure 5 shows a scanning electron micrograph of the exterior surface of a
membrane of the instant invention. The surface shown is an intergrown dense
mat of
zeolite crystals which are substantially free of defects extending through the
thickness of
the layer.
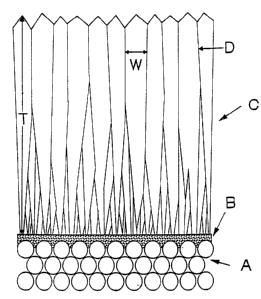
Figure 6 is a schematic view of the cross-sectional morphology, described
herein, of one of the instant compositions. (A) is the porous substrate, (B)
the growth
enhancing layer, (C) a zeolite layer, (D) a grain boundary, (T) the thickness
of one
zeolite crystal, and (W) the width of a zeolite crystal at one point on the
crystal.
Summary.of the Invention
One aspect of the present invention is directed toward a new zeolite
containing composition comprising a porous substrate (also referred to herein
as a
support) having coated thereon a mesoporous growth enhancing layer and a layer
of
zeolite crystals on said mesoporous growth enhancing layer, and wherein said
mesoporous growth enhancing layer comprises zeolites; zeolite and metal oxide;
zeolites
and metal particles; or zeolites, metal particles and metal oxides, wherein
said zeolites
are selected from the group consisting of nanocrystalline zeolites and
colloidal sized
zeolites, and wherein said mesoporous growth enhancing layer has interstices
of about
20 to about 2000 ~, and wherein said zeolite layer is a polycrystalline layer.
Preferably,
at least 99% of said zeolite crystals in the zeolite layer have at least one
point between
a adjacent crystals that is <_ 20 A. The zeolite layer will exhibit in cross-
section an
assembly of crystals of similar habit. Preferably, a zeolite layer comprised
of MFI type
zeolites, will be prepared exhibiting in cross-sectional an assembly of
crystals of
columnar habit.
The invention is further directed to a process of producing a zeolite
containing composition comprising:
CA 02192232 1996-12-05
WO 96/01687 PCTIUS95/08514
-4-
(a) coating a substrate with a growth enhancing layer wherein said growth
enhancing layer is prepared by utilizing a solution comprising zeolite;
zeolite and metal
oxide; zeoiite and colloidal metal; or zeolite, colloidal metal, and metal
oxide; and
wherein said zeolite is selected from the group consisting of nanocrystalline
and colloidal
zeolite and wherein said metal oxide is colloidal metal oxide or polymeric
metal oxide
prepared by sol-gel processing, followed by calcination at a temperature of
about 200 to
about 1000°C for at least about 2 hours;
(b) contacting said substrate having said growth enhancing layer coated
thereon with a zeolite synthesis mixture;
(c) hydrothermally treating said substrate and zeolite synthesis mixture for a
time and a temperature suffcient to form a zeolite layer on said growth
enhancing layer,
and wherein settling of particles produced from said zeolite synthesis mixture
onto said
zeolite layer is prevented;
(d) removing unreacted zeolite synthesis mixture.
Contacting as used herein includes total and partial immersion. The solution
of step (a) will contain a solvent, preferably water (distilled, deionized,
demineralized)
etc.
The process further comprises step (e) calcining said composition when said
zeolite synthesis mixture contains an organic template for a time and at a
temperature
sufficient to remove said organic template.
The compositions of the instant invention (which can be referred to as zeolite
membranes), have a large number of applications in separations. For example,
they may
be used in separation of C02 from methane, water alcohol separations, hydrogen
recovery, xylenes separation, and several other molecular separations. The
compositions are also useful in catalysizing reactions. The compositions are
often
referred to in the art as zeolite membranes and can be used as such. The
instant
compositions prepared on non-porous supports are useful as sensors.
CA 02192232 2005-04-18
-4a-
According to another aspect of the present invention, there is provided a
composition comprising a substrate having coated thereon a mesoporous growth
enhancing layer and a distinct layer of zeolite crystals on said mesoporous
growth
enhancing layer, and wherein said mesoporous growth enhancing layer comprises
zeolites; zeolite and metal oxide; zeolite and colloidal sized metals;
zeolite, colloidal
sized metal and metal oxide; or mixtures thereof, and wherein said zeolites of
said
mosoporous growth enhancing layer are selected from the group consisting of
nanocrystalline zeolites and colloidal sized zeolites, and wherein said
mesoporous growth
enhancing layer has interstices of about 20 to about 2000 1~, and wherein said
zeolite
layer is a polycrystalline layer having a thickness within the range of 0.1 to
100 um and a
dense mat of crystals closely packed together such that their exists at least
one point
between adjacent crystals of <_ 20 ~ and having substantially no voids through
the
thickness of the zeolite layer.
According to another aspect of the present invention, there is provided t~Cp
ocess
for preparing a composition comprising the steps of: (a) coating a substrate
with a growth
enhancing layer wherein said growth enhancing layer is prepared by (i)
utilizing a
solution comprising zeolite; zeolite and metal oxide; zeolite and colloidal
metal; zeolite,
colloidal metal and metal oxide; or mixtures thereof; and wherein said zeolite
of said
mesoporous growth enhancing layer is selected from the group consisting of
nanocrystalline zeolite and colloidal zeolite, and wherein said metal oxide is
colloidal
metal or metal oxide prepared from sol-gel processing, followed by (ii)
calcinations at a
temperature of <_ 1000°C; (b) contacting said substrate having said
growth enhancing
layer coated thereon with a zeolite synthesis mixture such that said growth
enhancing
layer is orientated from 90 to 2?0° in said synthesis mixture; (c)
hydrothermally treating
said substrate and zeolite synthesis mixture for a time of from 30 minutes to
300 hours
and at a temperature from 50°C to 300°C to form a distinct
zeolite layer on said growth
enhancing layer and wherein settling of particles from said zeolite synthesis
mixture
during hydrothermal treatment onto said zeolite layer is prevented; (d)
removing any
unreacted zeolite synthesis mixture, and wherein the zeolite layer is a
polycrystalline
layer having a thickness within the range of 0.1 to 100 pm and a dense mat of
crystals
closely packed together such that their exists at least one point between
adjacent crystals
of <_ 20 ~ and having substantially no voids through the thickness of the
zeolite layer.
CA 02192232 2005-04-18
-4b-
According to another aspect of the present invention, there is provided a
separation process comprising contacting a feedstock derived from petroleum,
air,
hydrocarbons, or natural gas, comprising at least two molecular species with a
composition comprising a substrate having coated thereon a mesoporous growth
enhancing layer and a distinct layer of zeolite crystals on said mesoporous
growth
enhancing layer, and wherein said mesoporous growth enhancing layer comprises
zeolites; zeolite and metal oxide; zeolite and colloidal sized metals;
zeolite, colloidal
sized metal and metal oxide; or mixtures thereof, and wherein said zeolites
are selected
from the group consisting of nanocrystalline zeolites and colloidal sized
zeolites, and
wherein said mesoporous growth enhancing layer has interstices of about 20 to
about
2000 !~, and wherein said zeolite layer is a polycrystalline layer having a
thickness within
the range of 0.1 to 100 pm and a dense mat of crystals closely packed together
such that
their exists at least one point between adjacent crystals of _< 20 A and
having substantially
no voids through the thickness of the zeolite layer.
According to another aspect of the present invention, there is provided a
process
for catalyzing a chemical reaction comprising contacting a reaction stream
with a
composition comprising a substrate having coated thereon a mesoporous growth
enhancing layer and a distinct layer of zeolite crystals on said mesoporous
growth
enhancing layer, and wherein said mesoporous growth enhancing layer comprises
zeolites; zeolite and metal oxide; zeolite and colloidal sized metals;
zeolite, colloidal
sized metal and metal oxide; and mixtures thereof, and wherein said zeolites
are selected
from the group consisting of nanocrystalline zeolites and colloidal sized
zeolites, and
wherein said mesoporous growth enhancing layer has interstices of about 20 to
about
2000 A, and wherein said zeolite layer is a polycrystalline layer having a
thickness within
the range of 0.1 to 100 p,m and a dense mat of crystals closely packed
together such that
their exists at least one point between adjacent crystals of < 20 ~ and having
substantially
no voids through the thickness of the zeolite layer.
According to another aspect of the present invention, there is provided a
process
for catalysing a chemical reaction which comprises contacting one reactant of
a
bimolecular reaction mixture with one face of a composition comprising a
substrate
having coated thereon a mesoporous growth enhancing layer and a distinct layer
of
zeolite crystals on said mesoporous growth enhancing layer, and wherein said
mesoporous growth enhancing layer comprises zeolites; zeolite and metal oxide;
zeolite
CA 02192232 2005-04-18
- 4c -
and colloidal sized metals; zeolite, colloidal sized metal and metal oxide; or
mixtures
thereof, and wherein said zeolites are selected from the group consisting of
nanocrystalline zeolites and colloidal sized zeolites, and wherein said
mesoporous growth
enhancing layer has interstices of about 20 to about 2000 ~, and wherein said
zeolite
layer is a polycrystalline layer having a thickness within the range of 0.1 to
100 ~m and a
dense mat of crystals closely packed together such that their exists at least
one point
between adjacent crystals of <_ 20 t~ and having substantially no voids
through the
thickness of the zeolite layer and is in active catalytic form, under
catalytic conversion
conditions, and controlling the addition of a second reactant by diffusion
from the
opposite face of the structure.
CA 02192232 1996-12-05
,..~.~ WO 96!01687 PCT/US95/08514
-S-
Detailed Description of the Invention
The present invention describes a new type of supported zeolite composition
' formed on the surface of a growth enhancing layer (GEL) that contains
nanocrystalline
or colloidal sized zeolites, mixtures of colloidal sized or nanocrystalline
zeolites and
metal oxides, mixtures of colloidal sized or nanocrystalline zeolite and
colloidal sized
metal and mixtures of colloidal sized or nanocrystalline zeolite, colloidal
sized metal and
metal oxide. Hence, the growth enhancing layer and the zeolite layer grown on
it
contain zeolite, but each is a distinct layer. The morphology of the zeolite
layer is such
that there are substantially no voids extending through the thickness of the
layer because
the crystals of similar habit are oriented and are grown into a
polycrystalline dense mat.
Dense mat as used herein means that at least 99%, preferably 99.9% of the
zeolite
crystals have at least one point between adjacent crystals that is <_ 20 ~. In
the instant
invention, the spacing between crystals is set by a grain boundary zone and
the
maximum grain boundary zone spacing, absent voids or defects, will be <_ 40 ~
and such
spacings are readily observable by transmission electron microscopy and may
contain
inorganic oxide material. As used herein, a grain boundary zone is defined as
the width
of the disordered zone between two adjacent ordered crystals. Preferably, the
zeolite
layer will exhibit, in cross-section, an assembly of oriented crystals of
similar habit,
preferably a columnar cross-sectional morphology (see Figure 4, views (a) and
(b))
formed by crystallographically oriented zeolite crystals. Gther habits are
possible
depending on the particular zeolite type and the composition of the zeolite
synthesis
rruxture.
The dense mat of zeolite crystals is intergrown in the composition so that
non-selective permeation paths through the membrane are blocked by the
narrowest
point of approach between crystals. Non-selective permeation pathways are
permeation
pathways which exist at room temperature that do not pass through the zeolite
crystals.
This blockage of nonpermeation pathways exists at room temperature after a
template
which occludes the pore structure is removed from the zeolite crystals.
Templates
which are used to grow the zeolite are often removed by a calcination step.
From
transmission electron microscopy (TEM) investigations, the narrowest point of
approach
between crystals of less than 20 t~ after the template is removed, can be
established.
The space between crystals at this point can contain inorganic oxide material
that
restricts non-selective permeation of molecules through the membrane. The
absence of
non-selective permeation paths can be detected by the ability to prevent the
permeation
CA 02192232 1996-12-05
WO 96101687 PCT/US95108514
-6-
at room temperature (~20°C) of dye molecules through the composition
after any
template is removed from the pore structure. Dye molecules which can be chosen
to
detect non-selective permeation pathways through the membrane should have
minimum
dimensions which are larger than the controlling aperture through the zeolite
and the
size of the dye molecule should also be less than 20 ~. Non-selective pathways
transport dye molecules which are larger than the pore size of the zeolite.
The dye
molecules should be carried in a solution made with a solvent which can be
transported
through the zeolite pore structure and the zeolite layer should not be allowed
to pick up
foreign contaminants (such as water) before being tested. It is found that the
compositions made in accordance with the present invention block the
permeation of
dye molecules at room temperature through the zeolite layer. All of the dye
molecules
chosen have at least one dimension less than approximately 20 t~. The lack of
permeation at room temperature of dye molecules with sizes less than
approximately 20
demonstrates that non-selective permeation pathways with sizes less than
approximately 20 t~ are blocked. It should be noted that this test does not
have to be
performed with a dye molecule and any molecular species that can be detected
having a
size less than 20 ~ and greater than the zeolite pore dimension can be used.
The
advantage of using a dye molecule is that it can be readily detected by
optical means.
The zeolite layer is grown on top of a mesoporous growth enhancing layer
(GEL) layer which contains colloidal sized or nanocrystalline zeolites. The
growth
enhancing layer smooths the porous support, facilitates the growth of zeolite
layer and
provides a seeding surface allowing control of the nucleation density of
zeolite crystals
formed on the support. This growth enhancing layer has to be chemically and
mechanically stable in hydrothermal synthesis conditions and the colloidal
sized or
nanocrystalline zeolites contained in it serve as nucleation sites for growth
of the zeolite
layer. Altering the density of nucleation sites alters the way the zeolite
layer grows
which determines zeolite crystal size, packing (i.e., voids and defects), and
crystal habit
or external crystal morphology.
The GEL layer contains identifiable particles with interstices between said
particles of zeolites; zeolites and metal oxides; zeolite and colloidal sized
metal and
zeolite, colloidal sized metal, and metal oxide. Said interstices are
mesoporous and have
sizes of about 20 to about 2000 t~, preferably from about 40 to about 200 t~.
Mesoporous as used herein means that there is a connected void structure
throughout
the GEL layer. Interstices in this size range provide a permeation path for
molecules
CA 02192232 1996-12-05
21~22~2 , . : ; .
_, _
through the GEL layer. Molecules can permeate through these interstices
because they
are devoid of any material which would hinder mass transport during membrane
use.
Applicants believe that the size and shape of the zeolite crystals in the
zeolite layer is
controlled by properties of the mesoporous growth enhancing layer (GEL).
Controlling
the:~morphology, orientation and shape of the zeolite crystals in the zeolite
layer reduces
the number of voids between crystals because the crystals pack together such
that only
grain boundary zones separate them (see Figures 4(a), 4 (b) and 6). The GEL
layer is
believed to nucleate the formation of the dense mat of zeolite crystals grown
on the
surface of the GEL layer. This dense mat of crystals is closely packed
together such
that there exists at least one point between adjacent crystals of _< 20 t~. As
the zeolite
layer grows from the interface at the GEL layer, crystal width may increase,
however
the individual crystals remain separated at their boundary zones by at least
one point of
spacing of _< 20 ~. This densely packed mat is the zeolite layer. Zeolite
layers grown
without the use of the growth enhancing layer do not have the degree of
perfection of
the layers described herein. The zeolite layers of the instant composition can
be formed
of crystals of a variety of habits (such as, e.g., columns or plates, as can
be determined
by SEM) or crystallographic preferred orientation (as can be determined by
XRD),
depending on the chosen zeolite, the synthesis mixture, reaction conditions
and the
composition of the GEL.
Void as used herein means spaces between adjacent zeolite crystals in the
zeolite layer larger than 40 ~. The instant compositions are virtually free of
voids in
the zeolite layer. Voids are at most about 1 volume %, preferably less than
0.1 volume
of the zeolite layer. Voids can be detected from cross-sectional images of the
zeolite
layer made in the scanning or transmission electron microscope. Defects are
connected
voids and are spaces between adjacent zeolite crystals extending through the
thickness
of the zeolite layer. In the instant composition, the total number of defects
in the zeolite
layer with sizes > 40 ~ is < 10,000 per square inch(6.4516 cmZ)), preferably <
100 per
square inch(6.4516 cm2). The number of defects larger than about 2000 ~ is
less than
per square inch(6.4516 cm2), preferably less than 1 per square inch(6.4516
cm').
Isolated defects of the type described can be detected in dye permeation
experiments. Isolated points at which dye permeates into the substrate reveal
such
defects. Defects can also be determined by examining cross-sections of the
zeolites
compositions in the scanning electron microscope. A cross-sectional view
showing a
zeolite composition which does not have any defects in the region examined is
shown
m
A~LtcNDED ,g,~EET
CA 02192232 1996-12-05
_g_
Figure ~. Gas permeation can also be used to reveal defects, in the
composition. If the
permeability of the zeolite layer to nitrogen at room temperature is less than
SxlO ~
moles/(m"-sec-Pascal) for each micrometer of thickness of the zeolite layer,
the
composition be considered to have an acceptable defect density. More
preferably, the
permeability of the zeolite layer to nitrogen at room temperature is less than
x10 ~
moles/(m2-sec-Pascal) for each micrometer of thickness of the zeolite layer.
The new architecture of the instant invention is composed of a substrate, a
growth enhancing layer containing mesoporous interstices, and a layer of
zeolite
crystals. The substrate on which the GEL layer is grown will be selected from
porous
and nonporous substrates. When a porous material is desired, it may be porous
throughout its entire thickness. Preferably an inorganic oxide will be
utilized. The
porous substrate, hence can be a ceramic, metal, zeolite, carbide, polymer or
a mixture
thereof. For example, alumina, titanic, cordierite, mullite, stainless steel,
pyrex, silica,
silicon carbide, carbon graphite and silicon nitride or mixture thereof can be
utilized.
Preferably, a porous ceramic or porous metal, mare preferably, stainless
steel, alumina,
and cordierite will be used. The porous substrate, hence may have a uniform
pore size
throughout or may be asymmetrical, having a larger pore structure throughout
the bulk
of the substrate with a smaller pore structure at the surface on which the GEL
layer is to
be grown. The substrate pore size is dictated amongst other things by mass
transfer
considerations. It is preferred that the pore structure and thickness of the
substrate be
chosen such that the mass transfer resistance does not limit the flux of
material
permeating through the zeolite membrane during use. The porous substrate will
hence
display a porosity of about 5 to about 70%, preferably about 20 to about ~0%
and an
average pore size of about 0.004 to about 2000 ~.m, preferably about 0.05 to
about 50
micrometers. It is preferred that the surface of the porous surface on which
the GEL
layer is deposited be smooth. Roughness in the substrate leads to defects in
the zeolite
layer. The substrate should have an average roughness with an amplitude of
less than
~m with an aspect ratio of the roughness less than 1:1. It is preferable that
the
average roughness of the substrate be less 0.~ ~m with an aspect ratio of the
roughness
less than 1:1. One function of the GEL is to smooth the support. If a
nonporous
substrate is utilized, it may be selected from, e.g. quartz, silicon, glass,
borosilicate
glasses, dense ceramics, for example, clay, metals, polymers, graphite and
mixtures
thereof. When nonporous supports are utilized, the finished product can be
used as a
sensor or as a catalyst.
A"~!~~IDFp ,SHEET'
CA 02192232 2005-04-18
_c
The mesoporous ~yrow2h enhancing layer is formed from a solution
containing a nanocrystalline or colloidal zeolite or a mixture of metal oxide
and
nanocrvstalline or colloidal zeolite or a mixture of nanocrvstalline or
colloidal zeolite
and colloidal metal. Preferably. nanocrvstalline or colloidal zeolite or a
mixture of
nanocnstalline or colloidal zeolite and metal oxide will be used to form the
GEL layer.
The metal oxides from which the GEL layer is prepared are colloidal metal
oxides or
polymeric metal oxides prepared from sol-gel processing. Nanocrystalline
zeolites are
crystallites having sizes from about 10 ~ to 1 pm. Nanocrystalline zeolites
can, e.g., be
prepared in accordance with the methods set forth in European patent
publication EP 0 609 304,
or other methods known to those skilled in the art. Colloidal sized particles
are between 50 and
10,000 ~ and form a stable dispersion or solution of discrete particles.
Preferably, the
colloidal particles will be 2~0 to x.000 ~, most preferably less than 1000
.=~. Colloidal
zeolites with sizes < X000 ~ are readily obtainable. The solution for
preparing the GEL
layer is coated onto the surface of the porous substrate and calcined at
temperatures <_
1000°C. preferably from about ?00 to about 1000°C, most
preferably 300-600°C.
Following calcination, a stable mesoporous 'row-th enhancing layer is formed
and is
maintained in the final composition as a distinct layer having a thickness of
about 0.1 to
?0 micrometers, preferably about 1 to ~ micrometers. This layer contains
interstices as
described above. Following calcination the zeolite will be nanocrystalline or
colloidal
sized zeolite and the metal and metal oxide will be colloidal sized metal and
metal
oxide. The GEL layer can be formed from silica. silicates. aluminosilicates,
aluminophosphates, silicoalumino-phosphates, metalloaluminophosphates,
metalloaluminophosphosilicates. and stano-silicates. Representative of
molecular
sieves (zeolites) which can be used include but are not limited to those of
structure type
AFI. AEL. BEA, EUO. FER. KFI, MAZ, MOR, MEL. MTW. OFF. TON, FAU
(includes zeolite X and zeolite Y). zeolite beta, LTA. LTL. AFS, .aFY, APC,
APD,
MTN, MTT. AEL, CHA and MFI zeolites. Preferably, an MFI zeolite with a silicon
to
aluminum ratio greater than 30 will be used including compositions with no
aluminum.
MFI zeolites with SilAl ratios greater than 300 are herein referred to as
silicalite.
Some of the above materials, while not being true zeolites are frequently
referred to in the literature as such, and the term zeolite will herein be
used broadly to
include such materials.
CA 02192232 1996-12-05
WO 96101687 PCT/US95108514 .r.,
- 10-
The metal oxides which can be used herein are selected from the group
consisting of colloidal alumina, colloidal silica, colloidal zirconia,
colloidal titanic and
polymeric metal oxides prepared from sol-gel processing and mixtures thereof.
Preferably colloidal alumina will be used. The colloidal metals which can be
used
include copper, platinum and silver.
By adjusting the ratio of colloidal zeolite and rnetal oxide, the density of
nucleation sites on the GEL can be controlled. This density <:ontrols the
morphology of
the zeolite film grown over the growth enhancing layer in .a subsequent
hydrothermal
synthesis step. The higher the nucleation density, the narrower the zeolite
crystal width
the crystals will exhibit at the GEL zeolite layer interface. Nucleation
density can be
controlled by the relative proportions of colloidal zeolites and metal oxides
(with the
density decreasing as the amount of the metal oxide utilized increases) as
well as the size
of the colloidal zeolites in the GEL. Colloidal sized zeolites in the range of
from
SO-10,000 t1 are thus used in the GEL. The larger the colloidal zeolite
crystals utilized
in the GEL, the wider the zeolite crystals in the layer will be. Applicants
believe that the
addition of metal oxide, colloidal metal or mixtures thereof to the colloidal
zeolite in the
GEL layer provides spaces between nucleation sites allowing for control of the
crystal
width in the zeolite layer.
GELs containing pure metal oxides or colloidal metal fail to produce the
necessary nucleation sites. The formulation of GEL is 100-x: wt% of colloidal
metal or
metal oxide: x wt% of colloidal zeolite, where x is at least 0.01 when the GEL
is not
formed from pure collaidal zeolite. Hence, the nucleation density is set by
the above
formula as well as the size of the particles of colloidal zeolite, colloidal
metal and metal
oxide. The smaller the particle size of the colloidal zeolite particles, the
denser the
nucleation sites which produces narrower zeolite crystals.
The preferred synthesis technique used with this invention is the growth of
zeolite crystals on the face of a GEL layer which is oriented from 90 to 270
degrees in a
low alkaline synthesis mixture. In the 180 degree orientation, the preferred
orientation,
the GEL layer is horizontal and facing downward, thus being referred to as
inverted.
Applicants believe this prevents zeolites which are homogeneously nucleated in
the
synthesis mixture from settling by gravitation and incorporating into the
growing zeolite
layer. Thus, the zeolite layer is not perturbed during the growth process. We
refer to
this synthesis technique as a Growth Enhancing Layer-Low Alkaline Inverted
Insitu
CA 02192232 1996-12-05
-. WO 96101687 PCT/US95/08514
-11-
Synthesis Crystallization (GEL-LAI-ISC) process. The herein grown MFI zeolite
compositions are optically transparent through the zeolite layer thickness in
that within
this layer they do not scatter light.
The MFI zeolite compositions also show a significant crystallographic
preferred orientation (as determined by XRD). Preferred orientation will be
different
depending on the zeolite chosen for the zeolite layer and the method of
preparation.
However, a preferred orientation will always be exhibited. The
crystallographic orienta-
tion of the MFI crystals in the preferred embodiment is such that at least 75%
of the
crystals in the zeolite layer are aligned in an orientation with the c-axis
parallel to the
growth direction (within 15° preferably 5° of the normal to the
surface of the zeolite
layer), preferably at least 90% of the crystals will display the preferred
orientation. The
crystal width in the zeolite layer can vary from 0.1 to 20 pm.
A measurement of the proportion of the crystals that have one direction
normal to the zeolite layer (such as the c-axis) may be obtained by comparison
of the
x-ray diffraction pattern of the layer with that of a randomly oriented
zeolite powder. In
the case of an MFI zeolite with a preferred c-axis orientation, for example,
the ratio of
the intensity of the 002-peak to the combined 200 and 020 peak is divided by
the same
ratio for randomly oriented powder; the quotient is termed the degree of
crystallographic preferred orientation (CPO - the orientation of which needs
to be
specified). Measured in this way, the zeolite layers of the instant invention
have a
degree of CPO along the c-axis of at least 2, and may have a degree of CPO
along the c-
axis as high as 108.
In preparing the GEL coated substrate onto which the zeolite layer is to be
gown, the substrate is first coated with the GEL followed by in-situ
crystallization. The
GEL smooths the porous substrate, facilitates the growth of the zeolite layer
and
provides a seeding surface to increase the nucleation density of the zeolite
crystals
formed on the GEL. The GEL must be chemically and mechanically stable under
the
hydrothermal conditions employed during the preparation of the final
composition and
also capable of enhancing heterogenous nucleation or surface nucleation.
Altering the
density of nucleation sites changes the zeolite crystal habit by adjusting the
width of the
crystals formed.
CA 02192232 1996-12-05
WO 96/01687 PCT/US95/08514
- 12-
The GEL layer is produced from prepared solutions by a variety of solution
coating techniques known in the art. For example, dip coating, spin coating,
and slip
casting, can be used. The coated substrate is then calcined at temperatures
ranging from
about 200°C to about 1000°C to form a stable mesoporous matrix.
The preferred
coating method is determined from the geometry of the substrate. In practical
situations, a spin coating method can be used for disks or plates. Spin
coating gives
excellent control of the coating thickness. For tubular and honeycomb type
substrates, a
dipping process can be used. The GEL will be from about 0.1 to about 20 pm
thick.
The calcination time will be sufficient to form a mechanically stable layer
thus at least
about 30 minutes, preferably 2 hours, more preferably at least about 6 hours.
Calcination of the GEL will typically be conducted with a heating rate of
about 10 to
20°C/hour from room temperature to the calcination temperature. This is
easily
determined in the art. Preferably a dilute solution of a concentration of 0.1-
10 wt%
solids, more preferably 1 wt% solids will be used to produce the GEL.
The GEL coating solution may contain small amounts of organic binders
such as PEG (polyethylene glycol), PVA (polyvinyl alcohol) or methyl
cellulose. Once
the substrate having the GEL coating is prepared, the zeolite layer is then
grown.
The hydrothermal treatment to form the crystalline zeolite upper layer is
carried out by contacting the substrate carrying the intermediate layer with a
zeolite
synthesis mixture, and hydrothermally treating for a time and at the
temperature
sufficient to effect crystallization, e.g., in an autoclave under autogenous
pressure.
Heating times may be, for example, in the range of from 30 minutes to about
300 hours.
Temperatures may be, for example, from 50°C to about 300°C and
preferably about
180°C.
Growth of the zeolite layer on the GEL coated substrate is carried out with
the GEL layer in an orientation and location in the synthesis mixture such
that the
settling of particles produced during hydrothermal treatment onto the GEL
layer, is
minimized or prevented. For example, the GEL layer is advantageously at least
5 mm,
and preferably at lest 8 mm, from a wall or, especially the base, of the
vessel to avoid
interference from particle settling. Alternatively, additional means to
inhibit settling can
be employed in the zeolite synthesis process.
CA 02192232 1996-12-05
WO 96101687 PCT/US95/08514
-13-
The zeolite layer can have either a shape preferred orientation, a
crystallographically preferred orientation, or both. Shape or
crystallographically
preferred orientations occur because of the control of the relative rates of
nucleation and
growth offered by the synthesis procedure. Specifically, daring synthesis, the
rate of
growth can be made to dominate the rate of surface nucleation of new crystals
or
incorporation of new crystals. Incorporation of new crystals is defined as
attachment
onto the surface of the growing layer of a crystal formed in the synthesis
mixture. Since
the growth rate can dominate renucleation or incorporation, crystals can
competitively
grow for long periods of time without significant addition of new crystals
into the
growing layer. Since the growing layer is composed of individual crystals and
the
synthesis method seeks to prevent renucleation or incorporation of crystals,
the resulting
composition can have shape, crystallographically preferred orientation, or
both. Shape
orientation occurs because the crystals are forced to grow with preferred
regular habits
(or morphology) at the surface of the zeolite layer. A regular habit (or
morphology) is
taken to be a regularly shaped outline of a particular crystallographic grain
in the layer.
Regularly shaped outlines are defined as those which can be ;fitted or packed
together so
that there are no interconnected spaces or voids between crystals.
Interconnected voids
will form a pore structure. A few examples of regular habits with regular
shapes are
columnar, cubic, rectangular, and prismatic. Spherical, irregular and
elliptical shapes are
not considered to be regular habits. In a shape preferred orientational,
defined layers
will have the same regular habit. This can be measured by cleaving or
fracturing the
substrate on which the layer is grown and examining the cross-sectional
morphology of
the zeolite layer with a scanning electron microscope. Exarnining the surface
of the as
grown zeolite layer can also give additional information concerning the shape
preferred
orientation in the layer. A layer with shape preferred orientation is taken to
be one
which has more than 90% of the crystals within one layer inside the zeolite
layer
exhibiting self similar regular habits. The self similar requirement means
that the same
regular habit is exhibited within a layer that can be drawn in the electron
micrograph of
the cross-section of the zeolite layer, however even though the shapes are the
same, they
do not alt have to be the same size. Because of the growth mechanism of the
zeolite
layer, it is possible to have one shape preferred orientation at the bottom
(base) of the
layer and another shape preferred orientation in a layer drawn near the
surface of the
layer. An example of this is an MFI zeolite layer which has a columnar habit
at the base
of the layer and a rectangular habit at the surface of the layer. Many MFI
zeolite layers
grown in accordance with the present invention exhibit only one habit
throughout the
thickness of the zeolite layer. Usually MFI zeolite layers with a preferred C-
axis
CA 02192232 1996-12-05
-14-
orientation exhibit a columnar habit (or morphology) throughout the entire
thickness of
the zeolite layer. Often shape preferred orientational layers have a preferred
crystallographic orientation.
In the preferred embodiment, the zeolite layer is grown by suspending a
substrate having the growth enhancing layer coated thereon in a zeolite
synthesis
mixture with the substrate oriented such that said growth enhancing layer is
oriented
from 90 to 270° in said synthesis mixture and wherein in said
180° orientation said
growth enhancing layer is horizontal and facing dou~~nward, and wherein said
growth
enhancing layer is at least about Smm from the bottom, at the lowest point, of
said
zeolite synthesis mixture, hydrothermally treating said substrate containing
zeolite
synthesis mixture for a time and at a temperature sufficient to form the
zeolite layer.
For example, at about ~0 to about 300°C, preferably about 100 to about
250°C for at
least about 30 minutes to form a zeolite layer on said growth enhancing layer.
Washing
said GEL and zeolite coated substrate with water for a time and at a
temperature
sufficient to remove any unreacted zeolite synthesis mixture, preferably at a
temperature of about 1 ~ to about 100°C for at least about 10 minutes,
more preferably
for at least about six hours. When the zeolite synthesis mixture contains an
organic
template, the composition, following washing, is calcined at a temperature of
about 400
to about 600°C for at least about one hour. Longer calcination times
will not affect
membrane performance.
The zeolite layer grown in accordance with the instant invention will have a
thickness of about 0.1-100, preferably about 0.~ to 20 micrometers. Thickness
is
herein defined as the distance from the GEL zeolite layer interface to the
uppermost
point on the zeolite crystal.
The zeolite layers of the instant invention are prepared from zeolite
synthesis mixtures. Such mixtures are any mixtures from which zeolite crystals
are
grown and are well known in the art (see e.g., Handbog~~, of Molecular Sieves,
Rosemarie Szostak, Van Nostrand Reinhold, NY 1992, Zeolite Molecular Sieves,
D.W.
Breck; R.E. Kreiger Publishing Co., Malaba, Florida. 1984 ISBN 0-89874-648-
~.). The
zeolites which can be utilized include those utilizable in the (sEL layer. A
preferred
route for MFI zeolites is from a low alkaline synthesis mixture having a pH of
about 8
to about 12 preferably about 9.5 to about 11, and from which MFI zeolite
crystals can
be grown. Such mixtures are readily prepared by those skilled in the art. For
example,
suitable mixtures
q~,~~r,,jJC~i ;,~.,~~~
:.
CA 02192232 1996-12-05
~»- WO 96/01687 PCT/US95/08514
-15-
include Na20, TPABr (tetrapropylammoniumbromide), Si02 and water. The
membranes
are grown by suspending the GEL coated porous substrate of choice in the low
alkaline
synthesis mixture. The synthesis mixture is then heated to about 50 to about
300°C,
preferably about 180°C for a period of about 30 minutes, preferably
from about 30
minutes to about 300 hours. The zeolite layer of the instant invention will
preferably be
that grown on the 90 to 270° oriented growth enhancing layer. Any
growth on the
substrate not on the GEL layer, can be easily removed by known techniques such
as
scraping or grinding, and is not part of this invention.
Once the zeolite layer has been grown, any remaining synthesis mixture is
removed, e.g., by washing with water at a temperature of about 15 to
100°C, preferably
about 80 to about 100°C for at least about 10 minutes, preferably for
at least six hours.
Excess washing for longer periods will not affect the compositions separation
capabilities.
Once the zeolite synthesis mixture is removed, if it contained an organic
template, the composition is calcined to remove the template. For example,
calcination
in air or oxygen at about 400 to 600°C can be used for at least about
one hour. Longer
calcination times will not affect the performance of the membrane. If no
organic
template is present, a drying step may optionally be conducted at temperatures
of about
100°C.
Catalytic functions can be incorporated into the compositions by methods
known in the art. When a catalytic function is incorporated, the composition
can be
used as an active element in a reactor. Several different reactor
architectures can be
constructed depending on the location of the catalystic site in the
composition. In one
case the catalytic function can be located within the zeolite layer, while in
another case
the catalytic function can be located within the support or GEL layer, and in
another
case the catalytic function can be distributed throughout the support, GEL
layer and the
zeolite layer. In addition, catalytic function can be incorporated into a
reactor by
locating conventional catalyst particles near one or more surfaces of the
composition
such that specific products or reactants are continuously and selectively
removed or
added to the reaction zone throughout the reactor. Impregnating with
catalytically
active metals such as Group VIII noble metals and particularly platinum, can
impart the
catalytic function to the composition. The catalytic activity can be
incorporated by
techniques known to those skilled in the art such as the incipient wetness
technique.
CA 02192232 1996-12-05
WO 9G/01687 PCT/US95/08514
- 16-
The amount of Group VIII noble metal to be incorporated will range from 0.1 to
about
wt%.
The compositions are useful for separation processes whereby feedstock
derived from petroleum, natural gas, hydrocarbons, or air comprising at least
two
molecular species is contacted with the composition of the invention, wherein
at least
one molecular species of said feedstock is separated from said feedstock by
said
composition and wherein said hydrocarbon feedstocks are coal, bitumen and
kerogen
derived feedstocks. Separations which may be carried out using a composition
in
accordance with the invention include, for example, separation of normal
alkanes from
co-boiling hydrocarbons, especially n-C 10 to C 16 alkanes from kerosene,
normal
alkanes and alkenes from the corresponding branched alkane and alkene isomers;
separation of aromatic compounds from one another, especially separation of C8
aromatic isomers from each other, more especially paraxylene from a mixture of
xylenes
and, optionally, ethylbenzene, and separation of aromatics of different carbon
numbers,
for example, mixtures of benzene, toluene, and mixed C8 aromatics; separation
of
aromatic compounds from aliphatic compounds, especially aromatic molecules
with
from 6 to 8 carbon atoms from CS to C 10 (naphtha range) aliphatics;
separation of
olefinic compounds from saturated compounds, especially light alkenes from
alkane/alkene mixtures, more especially ethene from ethane and propene from
propane;
removing hydrogen from hydrogen-containing streams, especially from light
refinery and
petrochemical gas streams, more especially from C2 and lighter components; and
alcohols from aqueous streams. Also alcohols from other hydrocarbons,
particularly
alkanes and alkenes that may be present in mixtures formed during the
manufacture of
alcohols.
Specifically, the following table shows some possible feedstocks derived
from petroleum, natural gas, hydrocarbons, or air and the molecular species
separated
therefrom by use of the instant compositions. The table is not meant to be
limiting.
CA 02192232 1996-12-05
WO 96!01687 PCT/US95108514
- 17-
F edst k ar t M !e u! r a i
Mixed !eves ortho, ara, meta and eth Par !eve
!benzene
Mixture of h dro en, H S, and ammonia H dro en
Mixture of normal and isobutanes Normal butane
Mixture of normal and isobutenes Normal butene
Kerosene containin C to C normal araffinsC to C normal araffins
Mixture of vitro en and o en Nitro en or o en
Mixture of h dro en and methane H dro en
Mixture of h dro en, ethane, and eth H dro en and/or eth
!eve !eve
H , ro ane and ro !eve H dro en and /or ro
!eve
Coker naphtha containing C S to C l C~ to C I 0 normal
0 normal olefins and olefins and
araffins araffins
Methane and ethane mixtures containing Helium, neon, and/or
argon, helium, argon
neon, or vitro en
Intermediate reactor catalytic reformerHydrogen, and/or light
products gases
containin h dro en and/or light ases C -C
Fluid Catalytic Cracking products containingHydrogen, and/or light
H2 and/or gases
li ht ases
Na htha containin C to C normal araffinsC to C normal araffins
Light coker gas oil containing Cg to Cg to C 1 g normal
C 1 g normal olefins olefins and
and araffins araffins
Mixture of normal and iso entanes Normal entane
Mixture of normal and iso entenes Normal entene
Mixture of ammonia, h dro en, and vitroH dro en and vitro
en en
Mixture of A10 (10 carbon) aromatics e.g. Paradiethylbenzene
DEB
Mixed butenes n-Butenes
Sulfur and/or vitro en com ounds H S and/or NH
Mixtures containing Benzene (Toluene) Benzene
~
Applicants believe that molecular diffusion is responsible for the above
separations. Additionally, the compositions can be used to effect a chemical
reaction to
yield at least one reaction product by contacting the feedstocks as described
above or
below with the compositions having a catalyst incorporated within the zeolite
layer,
support, or intermediate layer or by placing the catalyst in close enough
proximity with
the composition to form a module. A module would react the feedstock just
prior to its
entrance into the composition or just after its exit from the composition. In
this manner
one can separate at least one reaction product or reactant from the
feedstocks. The
catalysts of choice for particular process fluids are well known to those
skilled in the art
and are readily incorporated into the instant compositions ar formed into
modules by
CA 02192232 1996-12-05
WO 9G/01687 PCTlUS95108514
- 18-
one skilled in the art. The following table represents some of the possible
feedstocks/processes, in addition to those above which can be reacted and some
possible
products yielded. The table is not meant to be limiting.
Feedstock/ rocess Product Yielded
Mixed xylenes (para, ortho, Paraxylene and/or ethylbenzene
meta) and
eth (benzene
Ethane deh dro enation to eth H dro en and/or Eth lene
lene
Eth (benzene deh dro enation H dro en
to st rene
Butanes dehydrogenation butenesHydrogen
(iso's
and normals)
Pro ane deh dro enation to H dro~en and/or Pro lene
ro lene
C I p-C 1 g normal paraffin Hydrogen
deh dro enation to olefins
H dro en Sulfide decom ositionH dro en
Reforming Hydrogen, light hydrocarbons
deh dro enation/aromatization (CI-C~)
Light Petroleum Gas Hydrogen
deh dro enation/aromatization
Mixed Butenes n-butenes
Hydrocarbon streams containing
sulfur H2S and or NH3 with or without
and/or nitrogen compounds HZ
h drotreatin dro rocessin
The zeolite layer of the invention may be employed as a membrane in such
separations without the problem of being damaged by contact with the materials
to be
separated. Furthermore, many of these separations are carried out at elevated
temperatures, as high as 500°C, and it is an advantage of the supported
zeolite layer of
the present invention that it may be used at such elevated temperatures.
The present invention accordingly also provides a process for the separation
of a fluid mixture which comprises contacting the mixture with one face of a
zeolite
layer according to the invention under conditions such that at least one
component of
the mixture has a different steady state permeability through the layer from
that of
CA 02192232 1996-12-05
~" WO 96/01687 PCT/I1S95/08514
- 19-
another component and recovering a component or mixture of components from the
other face of the layer.
The invention further provides a process for catalyzing a chemical reaction
which comprises contacting a feedstock with a zeolite layer according to the
invention
which is in active catalytic form under catalytic conversion conditions and
recovering a
composition comprising at least one conversion product.
The invention further provides a process for catalyzing a chemical reaction
which comprises contacting a feedstock with one face of a zeolite layer
according to the
invention, that is in active catalytic form, under catalytic conversion
conditions, and
recovering from an opposite face of the layer at least one conversion product,
advantageously in a concentration differing from its equilibrium concentration
in the
reaction mixture. For example, a p-xylene rich mixture from the reactor or
reactor
product in a xylenes isomerization process; aromatic compounds from aliphatics
and
hydrogen in a reforming reactor; hydrogen removal from refinery and chemicals
processes such as alkane dehydrogenation in the formation of alkenes, light
alkane/alkene dehydrocyclization in the formation of aromatics (e.g., Cyclar),
ethylbenzene dehydrogenation to styrene.
The invention further provides a process for catalyzing a chemical reaction
which comprises contacting one reactant of a bimolecular reaction with one
face of a
zeolite layer according to the invention, that is in active catalytic form,
under catalytic
conversion conditions, and controlling the addition of a second reactant by
diffusion
from the opposite face of the layer in order to more precisely control
reaction
conditions. Examples include: controlling ethylene, propylene or hydrogen
addition to
benzene in the formation of ethyl benzene, cumene or cyclohexane respectively.
The invention further contemplates separation of a feedstock as described
herein wherein the separated species reacts as it leaves the composition or as
it passes
through the composition and thus forms another product. This is believed to
increase
the driving force for diiTusion through the membrane layer.
Some specific reaction systems where these compositions would be
advantageous for selective separation either in the reactor or on reactor
effluent include:
selective removal of a Para-Xylene rich mixture from the reactor, reactor
product,
CA 02192232 2005-04-18
-20_
reactor feed or other locations in a Xylenes isomerization process; selective
separation
of aromatics fractions or specific aromatics molecule rich streams from
catalytic
reforming or other aromatics generation processes such as light alkane and
alkene
dehydrocyclization processes (e.g., C3-C~ paraffns to aromatics from processes
such as
Cyclar), methanol to gasoline and catalytic cracking processes; selective
separation of
benzene rich fractions from refinery and chemical plant streams and processes;
selective
separation of olefins or specific olefin fractions from refinery and chemicals
processing
units including catalytic and thermal cracking, olefins isomerization
processes, methanol
to olefins processes, naphtha to olefins conversion processes, alkane
dehydrogenation
processes such as propane dehydrogenation to propylene; selective removal of
hydrogen
from refinery and chemicals streams and processes such as catalytic reforming,
alkane
dehydrogenation, catalytic cracking, thermal cracking, light alkanelalkene
dehydro-
cyclization, ethylbenzene dehydrogenation, parafFn dehydrogenation; selective
separation of molecular isomers in processes such as butane isomerization,
paraffin
isomerization, olefin isomerization, selective separation of alcohols from
aqueous
streams and/or other hydrocarbons.
The following examples are for illustration and are not meant to be limiting.
EXAMPLES
Materials
The following reagents were used in preparing GEL coatings: colloidal
alumina solution, colloidal titanic prepared from a sol-gel process, colloidal
silicalite
solutions, and distilled water. Several batches of colloidal silicalite
solutions prepared in
accordance with European patent publication EP 0 609 304 were used for the
preparation
of GEL coatings. More information on these solutions are shown below:
CA 02192232 1996-12-05
..,~ WO 96101687 PCT/US95/08514
-21 -
Silicalite (MFI) Silicalite
(MFI)
Batch Synthesis Silicalite Solution Solids (%) Particle
No. pH Size
Temp. C (MFI) Washed (nm)
1 68 yes 10.3 8.7 ~50
2 50 no >13 ~9 -50
3 81 yes 9.9 9.1 ~90
4 50 no >13 ~9 ~60
50 yes ~10 ~9 ~60
Remarks:
1. All suspensions were prepared from the same type of synthesis solutions
with the same raw materials.
2. Batch 4 was a duplication of 2. The solids content of batches 2 and 4
were calculated assuming 55% conversion of amorphous silica to zeolite. The
actual
solids content of these 2 unwashed samples is of course higher, e.g. for 4 the
solids
content (evaporation to dryness) was 23.3 wt%, but this includes zeolite,
amorphous
silica and residual TPAOH-NaOH.
Porous alumina and stainless steel substrates were used for the support of
GEL and zeolite coatings. The average pore size and porosity of the alumina is
about
800 A and 32%, respectively. Porous sintered stainless steel substrates from
Mott's
(0.25 pm) and Pall's (M020, 2 um) were obtained. All the substrates were
cleaned with
acetone in an ultra-sonic bath, dried at 120°C and then cooled down to
room
temperature before use.
~~L Coating
In general, a dilute solution is preferred to produce a high quality growth
enhancing layer. Dilution with distilled water to obtain a solids
concentration less than
1 wt% is generally preferred. Colloidal silicalites and metal oxides are first
diluted
separately with distilled water to the concentration of 0.5 wt%. The diluted
colloidal
silicalite solution was slowly added into the desired amount of metal oxide
solution with
CA 02192232 2005-04-18
-22-
continuous stirring. The resulting solutions with the desired wt% of colloidal
silicalite
and metal oxide were then degased for 15 minutes to remove the trapped air in
solutions.
The substrates were then spin coated with these solutions at 4000 rpm and
calcined at 400 - 500°C for 6 hours in air. The heating rate was
controlled at 20°C/hr.
H~drothermal Reaction
The hydrothermal experiments were performed using mixtures of the
following reagents: NaOH (Baker), Al(N03)3.9H20 (Baker), LudoXMAS-40 (Dupont),
NalCoag 115,2326 tetrapropylammonium bromide (98%, Aldrich), and distilled
water.
MFI membranes were prepared from two different reaction batch mixtures,
one containing silica only to make high silica MFI and the other with added
alumina to
make ZSM-5. They have the general formulation xM20:10 Si02:z AI203:p
TPABr:yH20; M can be Na, Li, K, Rb, and Cs, x eras varied from 0 to 5, and y
varied
from SO to 30,000, z varies from 0 to 0.5, and p varies from 0.2 to 1. All the
results
shown in the next section have the composition of 0.22 Na20:10 Si02:0
A1203:280
H20:0.5 TPABr with the exception of the ZSM-5 sample which contained 0.05
A1203
for ZSM-5 sample. The 1.74 g of TPABr and 0.45 g of NaOH (50 wt%) were
dissolved
in 52 ml of distilled water with stirring. To this solution, 18.8 g of Ludox
AS-40 was
then added with agitation for at least 15 minutes until a uniform solution was
formed.
TPABr may be replaced with tetrapropyl ammonium hydroxide if desired.
Substrates with GEL coating were placed inverted ( 180°
orientation) in a
Teflon lined autoclave by supporting them on the stainless steel wire frame.
The
distance between the substrate and the bottom of the autoclave was at least 5
mrrt. The
synthesis solution was then poured into the autoclave to cover the whole
substrate. The
autoclave was sealed and placed in an oven, which was preheated at the desired
temperature. The autoclaves were removed from the oven after reaction and
cooled to
room temperature. The coated substrates were washed with hot water for at
least 6
hours, then calcined at S00°C for 6 hours in air. The heating rate was
controlled at
10°C/hour.
CA 02192232 1996-12-05
~._ wo 9srolss r rc~r~s~rossia
- 23 -
Anal sis
The resulting membranes were characterized by x-ray diffraction, electron
microscopy, dye test and permeability measurements.
Results and Discussion
Pr uct
The following table shows some typical examples synthesized under different
experimental conditions, such as GEL composition, reaction time, and
substrate.
CA 02192232 1996-12-05
WO 96/01687 PCT/US95/08514
-24-
O C O
000~~CO ~~--'=-
.~.,c,. =a ~ ..CVJU~U
U U U U U U U ;:~,; .. . U
-~ . x ~Y ~ ~X
v: v; "_
.X .X ~y .Y .y .X . ,X ,X .,, cy y
U
U U U U U U U j ;j .
w.
V
H
G)
C pp ....~ N N x ~ ~ M '~ ~ ~f' ~
V x N N N N M N Q N ~!'. d' ~'
N 's
i-
c
o
,~
~
: yx~
x~xocxx
~ c
~_ ., ._. . - N
_
V
.
_
E'_' O
.~.
s~
A
L
O
d C C
L "'
O
o v
E .-- .-
~_. ... ~. .. ~ ,..~ ~ ~ tD e0
~
0
G
x O
~
O
G pC x r t~ 0
x OC O
G
tp ?
V
d
~ 3 3
3 O O
y ~n o~
d O
.. ...
c H N
...N ef
H
o
U V
o ~ y r. S V V S
.~
.~ ~- ~ V V ~ _ y
_ ~ ~, ed G
~ ,,
~. .-, X ~ a ee +~ ._a ~_ <v
in ~n Wn n ~n in ~ in ~ ~L w c ~ ~ V c
c v v
o H o c c
c o o c
.~ o c - -~ o
O 1..7iJ .rte
C L H r .N ~' O O w U
+~ r- ~ 7 S_
H ~
O O O 1-' C
r~ H H O
CS Cv ~ U C3 'fl E~ 4. ~ ~ G1 '~ H H tn
~ .~ .~ ~
L O H ~ '~
C
H H ~L ~ .~ t C7
O
:~ o 3 H s o v H
V . ag
L C tC H 3 3
~
d O ~ 3
r0 C C 3 V
19 O. 0 ~ ; > > v :5
G1
o
v, o y ~ a: s. as ~ 3
E p
r
,
c3 .- a r cs -' :~ ,~ :a O ~ 3 3 +~ +~ r.a;
:3 :3 r L
c c = c c c ~- c ; ,_ O 3 3 3 a~
y y y ' '~ '~ ' = '~ '~ '- CO O O o
'.- ' m o~ 0 0 0 o a o
_
H _ _ _ _ _ r..~~ -~ . w vn .~ ... _
O c4 c3 cs e3 cs cs :o ~ :3 H O . . L
:3 r3 cs G. a
L .. et!~ V ~ C7 t~ ..
V A
~
c X
...
a
~- ~ p
p ~ N M G. r' ~ N
:3 ~-.~ N M ef WD I~ x ~ ._. V e0 iF
._,
v
CA 02192232 1996-12-05
WO 96!01687 PCTIUS95/08514
-25-
General Observations
The x-ray diffraction pattern inverted zeolite membrane grown on a GEL
coated substrate (LAI-GEL-ISC) were observed. Reflections of MFI type zeolite
were
identified in all diagrams. No second zeolite phase was observed. The only
lines in the
patterns not associated with the zeolite identified with the porous support.
The pattern
associated with the GEL-LAI-ISC membrane was dramatically different from that
of
MFI powder. It is seen (see Figures 1 and 2) that the MFI crystal layer
prepared from
GEL-LAI-ISC exhibits pronounced 00 1 peaks with no other significant zeolite
peaks
occurring in the pattern or (o k o) peaks (see Figure 3 ). This is strong
evidence that a
preferred orientation of (0 0 1) or (o k o) directions parallel to the growth
direction
exists in the membrane. Another way of saying this is that the MFI crystal
layer in
GEL-LAI-ISC membranes shows very strong crystallographic orientation with the
c-axis normal to the GEL layer.
Figure 5 shows a plan view of a typical (C-axis orientation) GEL-LAI-ISC
membrane (sample #2). The close-packed top surface and cross-sectional
columnar
habit of the crystals was observed for the zeolite layer. Figure 4a shows the
zeolite layer
(C), the growth enhancing layer (B) and porous support (A). The major part of
Figure
4a shows the continuous growth of zeolite that completely covers the surface
of the
GEL layer. The formation of a dense packing of columnar crystals in the
zeolite layer is
apparent. The width of the columns right on the growth enhancing layer is very
narrow
and becomes larger and larger as the layer grows. As such, the cross-sectional
area of
the grains increases upwards in the layer. The columnar nature of the
microstructure is
consistent with the x-ray powder diffraction pattern. In Figure 5, it is clear
that zeolite
surface consists of a continuous array of densely packed zeolite crystals,
which are <10
pm in width.
Effect of the Densit~r of Nucleation Sites Upon the Width of MFI Crystals
The width of the zeolite crystal columns in sample #5 were smaller than
those in sample #6 which has a lower density of nucleation sites in its GEL
due to the
addition of colloidal alumina. The morphology was similar, but the grain size
is much
larger in sample #6 than in #5. Thus, by simply controlling nucleation density
the width
of the zeolite crystals in the zeolite layer can be modified and controlled.
CA 02192232 1996-12-05
2~~2Z.~2
-?6-
Effect of Substrates Used Upon the Vtorphc~logv of the MFI Crystals
Sample #9 grown on a stainless steel as substrate is very similar in
morphology to the membrane fabricated on an alumina substrate. The MFI crystal
formation seems independent of the substrate used.
Dve Permeation Test
The absence of defects in the MFI zeolite layer was measured by its inability
to pass dye molecules into the porous substrate. Any dye which wicks into the
substrate is readily visible because of a color change in the; substrate.
Rhodamine B
(0.~ w-t%) in methanol defects is added to the center of a dried membrane to
coat the
surface. Approximately 2- ~ drops were applied to a 1 inch. (2.54 cm) membrane
and
allowed to set for --30 seconds before the excess dye was blotted off.
Methanol was
then blotted on the membrane to remove any excess Rho~damine B solution on the
membrane. The membrane was then washed with methanol for 10-~0 seconds. Any
permeation of the dye into the substrate through defects in the membrane is
then readily
apparent.
Methanol permeated into the substrate, however, no Rhodamine B dye was
observed in the substrate indicating that the membrane is capable of
performing dye
separations.
When the membrane is synthesized from a zeolite synthesis mixture
containing an organic template, the dye test is performed
after calcination.
If a non-ceramic substrate is utilized, methods other than visual examination
are used to detect wicking into the membrane.
a~ ~r.,~L~C4~ ~.r,Cr~~,
= ~~ n~.