Note: Descriptions are shown in the official language in which they were submitted.
~ - 2193146
SPECIFICATION
Title of the Invention
Fluid Fuel Reforminq Ceramic Catalysts and Their
Manufacturing Methods
Background of the Invention
This invention relates to fluid fuel reforming ceramic
catalysts that effectively increase combustion efficiency
and decrease obnoxious substances in gasoline, light oil and
other liquid fuels and natural gas and other gaseous fuels
by reforming them.
Gasoline, for example, contains approximately 30 per-
cent of benzene, acetaldehyde and other icombustible and
environment polluting substances and approximately 10 per-
cent of octane-number increasing substances, anti-freezing
agent and other additives, in addition to approximately 60
percent of combustible substances. The incombustible and
environment polluting substances are discharged as obnoxious
substances in exhaust gases as a result of incomplete com-
bustion. Reforming these incombustible and environment
polluting substances into combustible substances will
increase the ratio of combustible substances that are con-
ducive to efficient combustion. To burn the reformed sub-
stances, however, oxygen supply (or air supply) must be
increased. However, larger quantities of air inevitably
contains greater amounts of nitrogen. Then, the content of
- 21931~6
nitrogen oxides in exhaust gases inevitably increases.
Although some air is dissolved in fuels, not all of the
oxygen contained in the dissolved air contributes to combus-
tion reactions. If the dissolved oxygen not contributing to
combustion reactions is activated, then the additional
combustible substances obtained by reforming incombustible
and environment polluting substances can be efficiently
burned without increasing the amount of air supply from the
outside.
Thus, the object of this invention is to provide
catalysts that reform incombustible and environment pol-
luting substances contained in fluid fuels to combustible
substances and increase combustion efficiency and decrease
the content of obnoxious substances in exhaust gases by
activating the oxygen in the air dissolved in fuels and
methods for manufacturing such catalysts.
Summary of the Invention
To solve the above problem, this invention provides:
(1) fluid fuel reforming ceramic catalysts comprising a
core of a complex oxide ceramic of transition metals, an
intermediate layer of an alumina-based silicate ceramic
covering the core, and an outer layer of a ceramic con-
taining a noble metal alloy covering the intermediate layer;
and
(2) a method for manufacturing fluid fuel reforming
' ~ ~1931~o
ceramic catalysts comprising the steps of coating an
alumina-based silicate ceramic as an intermediate layer
covering a core of a complex oxide ceramic of transition
metals and coating a ceramic containing a noble metal alloy
as an outer layer.
Brief Description of the Drawings
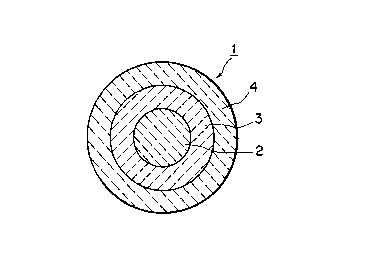
Fig. 1 is a cross-sectional view schematically il-
lustrating the structure of a ceramic catalyst according to
this invention.
Fig. 2 is a chromatogram showing the composition of a
light oil before a catalyst is immersed.
Fig. 3 is a chromatogram showing the composition of a
light oil after a catalyst is immersed.
Fig. 4 is an electron spin resonance spectrum showing
the formation of oxygen radicals in fuel.
Fig. 5 graphically shows the relationship between the
rotating speed of the automotive engine and the total al-
dehyde concentration in the exhaust gas.
Fig. 6 graphically shows the relationship between the
running speed of the automobile and the NOx concentration in
the exhaust gas.
Fig. 7 graphically shows the relationship between the
composition of the combustion gas in the engine cylinder and
the rotating speed.
Fig. 8 graphically shows the relationship between the
-
21931~
quantity of catalyst used and the octane number.
Fig. 9 graphically shows the relationship between the
air-fuel ratio and combustion efficiency.
Detailed Description of the Invention
The following paragraphs describe the fluid fuel refor-
ming ceramic catalysts according to this invention. Fig. 1
is a cross-sectional view schematically illustrating the
structure of a fluid fuel reforming ceramic catalyst accor-
ding to this invention. A fluid fuel reforming ceramic
catalyst 1 basically has a three-layer structure comprising
a core 2 of a complex oxide ceramic of transition metals, an
intermediate layer 3 of a silicate ceramic consisting essen-
tially of alumina (i.e., alumina-based) covering the core 2,
and an outer layer 4 of a ceramic containing a noble metal
alloy covering the intermediate layer 3.
The outer and intermediate layers 4 and 3 are of porous
materials having pores that allow the passage of gases and
liquids to and from the outside. The core 2 is also porous,
though the porosity is greater than in the intermediate and
outer layers 3 and 4, and allows the passage of gases and
liquids to and from the outside. The boundaries between the
individual layers are either of a slanted structure in which
composition changes gradually or of a stepped structure in
which composition changes abruptly. It should be noted that
potential energy changes more continuously in the slanted
- 21931~
structure than in the stepped structure. This permits
electrons to move smoothly at the boundaries, allows oxida-
tion-reduction reaction to proceed smoothly, and thereby
enhances the regeneration efficiency of the intermediate and
outer layers. Hence, the slanted structure is preferred for
the individual boundaries.
The ceramic catalysts according to this invention are
generally substantially spherical in shape. They may also
be shaped substantially like columns.
However, columnar catalysts cannot maintain a three-
layer structure comprising the core and the intermediate and
outer layers at both ends thereof where all of them are
exposed. By comparison, substantially spherical catalysts
maintain a three-layer structure in all directions.
Therefore, substantially spherical catalysts are preferable.
The ceramic catalysts according to this invention can
be used with liquid fuels such as gasoline, light and heavy
oils, and kerosene and gaseous fuels such as town gas and
propane. The ceramic catalysts according to this invention
are placed directly in fuels. For example, they are im-
mersed in fuel tanks.
The core consists of a complex oxide ceramic of transi-
tion metals having an oxidation-reduction catalytic action
that regenerates the outer and intermediate layers when
their catalytic activity is lost by being contaminated by
' - 21931~
trace amounts of sulphur, lead and other inorganic substan-
ces contained in fuels. Electrons in the substance making
up the core move to the intermediate and outer layers and
reduce the substances making up the intermediate and outer
layers that have been contaminated by catalyst-poisoning
impurities. With the contaminated intermediate and outer
layers thus detoxicated, their catalytic activities are
regenerated.
A complex oxide ceramic of transition metals containing
MnO2, Nio~ CoO and CuO is preferable for the core.
Preferably, the core contains 40 to 70 parts of MnO2, 10 to
20 parts each of NiO, CoO and CuO, all by weight. The four
substances described above are essential. If any of them is
absent or present in other ranges than those described
above, a perovskite-type crystal structure having pores to
store electrons contributing to the oxidation-reduction
action is not formed and, as a consequence, the regenerating
function decreases significantly. The catalysts according
to this invention may contain other substances unless they
have detrimental effects on the oxidation-reduction
catalytic action.
The intermediate layer has a function to reform the
icombustible and environment polluting substances contained
in fuels to combustible substances. For example, benzene
contained in gasoline is considered to be reformed to
- 21931~
methane, propane or other combustible substances, with the
benzene ring linkage severed and the hydrogen resulting from
the decomposition of water by the catalytic action of the
outer layer described later carrying out hydrogenation.
Acetaldehyde is considered to be decomposed to methane,
hydrogen and carbon dioxide by reacting with the hydrogen
and oxygen ions resulting from the decomposition of water by
the catalytic action of the outer layer.
The intermediate layer preferably consists of a
silicate ceramic consisting essentially of alumina (i.e.,
alumina-based) and silicate, or, preferably, kaoline and
carbon-bearing quartz. Preferably, the intermediate layer
consists of 70 to 90 parts of Al203 and 10 to 30 parts of
silicate or, preferably, 5 to 10 parts of kaoline and 5 to
20 parts of carbon-bearing quarts, all by weight. Al203
mainly functions as a carrier of silicate having a catalytic
action. While a deficiency of Al203 leads to a lowering of
mechanical strength, an excess decreases the quantity of
sillcate carried and impairs the catalytic action.
The outer layer has a function to activate the oxygen
in the air contained in fuels and evolve hydrogen and nas-
cent oxygen by decomposing the water contained in the fuel.
Therefore, the combustible substances increased by reforming
can be burnt without increasing the air supply from the
outside.
- 21931~
The outer layer preferably consists of a ceramic con-
t~;n;ng noble metal alloys containing a fired mixture of Pt-
Pd-Rh alloy and Al203, a Mo-Al203 catalyst, a LaO.5-SrO.5CoO3
catalyst, and an Al203-carried vanadium oxide catalyst and/or
a Ag-Al203 catalyst.
At least either one of the Al203-carried vanadium oxide
catalyst and Ag-Al203 catalysts is required.
Preferably, the outer layer contains 15 to 25 parts
each of the fired mixture of Pt-Pd-Rh alloy and Al203, Mo-
Al203 catalyst, LaO.5-SrO.5CoO3 catalyst, Al203-carried
vanadium oxide catalyst and/or Ag-Al203 catalyst, all by
weight. When the contents of the constituents are outside
the ranges described above, the desired ceramic is difficult
to form by firing, with a resulting decrease in the quan-
tities of oxygen activated and water decomposed.
Preferably, the Pt-Pd-Rh alloy contains approximately 5
to 7:1 to 3:1 to 3 of Pt, Pd and Rh, by weight. The Pt-Pd-
Rh alloy and Al203 are preferably mixed at a ratio of ap-
proximately 4 to 5: 5 to 6. Al203 mainly functions as a
carrier of Pt-Pd-Rh alloy having a catalytic action. While
a deficiency of Al203 leads to a lowering of mechanical
strength, an excess decreases the quantity of silicate
carried and impairs the catalytic action. The fired mixture
is prepared by firing a mixture of Pt-Pd-Rh alloy and Al203
~ 21931~
at a temperature of approximately 850~ to 930~ C. The Mo-
Al2O3 catalyst is a catalyst of Mo carried by Al2O3 at a
ratio of approximately 1:1. The LaO.s-SrO.5-CoO3 catalyst is
a fired mixture of lanthanum oxide, strontium oxide and
cobalt oxide. The Al2O3-carried vanadium oxide catalyst
consists of approximately 9:1 of Al2O3 and vanadium oxide.
The Ag-Al2O3 catalyst is a catalyst of Ag carried by Al2O3 at
a ratio of approximately 1 Ag: 9 Al2O3 .
As mentioned earlier, the method of manufacturing
ceramic catalysts according to this invention comprises the
steps of firing a core of a complex oxide ceramic of transi-
tion metal, coating an intermediate layer of an alumina-
based silicate ceramic over the core, and coating an outer
layer of a ceramic containing noble metals over the inter-
mediate layer. Methods of manufacturing the preferable
ceramic catalysts described above are given below.
Catalyst for the Core
A mixture of powders of MnO2, NiO, CoO and CuO in a
desired ratio, with the addition of a binder, is fired at a
temperature between approximately 900~ and 1000~ C and then
the fired product is pulverized. The core catalyst is
obtained by forming the pre-fired powder thus obtained into,
for example, balls of 1.5 to 2.0 mm in diameter, with the
addition of a binder, and sintered at a temperature of
- 21g3146
approximately 1150~ to 1350~ C. Sintering is performed in
the air.
Catalyst for the Intermediate Layer
A mixture of powders of alumina and silicate such as
kaoline and carbon-bearing quartz in a desired ratio, with
the addition of a binder, is fired at a temperature between
approximately 1050~ and 1200~ C and then the fired product
is pulverized. A paste of the pre-fired powder thus ob-
tained is prepared by adding a binder and a foaming agent
(which makes the sintered product porous by evolving carbon
dioxide or other gases during sintering). The paste thus
obtained is then coated over the core ball to a thickness
of, for example, approximately 1 mm. Then, a catalyst
prepared by coating the paste over the core ball is sintered
at a temperature of approximately 900~ to 1100~ C. Sin-
tering is performed in the air.
In sintering the intermediate layer catalyst at 900~ to
1100~ C, the substances making up the core and intermediate
layer catalysts melt and diffuse with each other. Hence,
the boundaries between the core and the intermediate layer
assumes a slated structure where composition changes
gradually.
Catalyst for the Outer Layer
A mixture of powders of a fired mixture of Pt-Pd-Rh
alloy and Al2O3, Mo-Al2O3 catalyst, La0.5-Sr0.5CoO3 catalyst,
-- 21~3~6
and Al2O3-carried vanadium oxide catalyst and/or Ag-Al2O3
catalyst in a desired ratio is prepared. The mixture is
made into a paste by adding a binder and a foaming agent.
The paste is then coated over the fired catalyst ball con-
sisting of the core and the intermediate layer to a thick-
ness of, for example, approximately 1 mm. The ceramic
catalyst according to this invention is obtained by firing
the coated product at a temperature of approximately 600~ to
700~ C in a reducing atmosphere. The fired mixture of Pt-
Pd-Rh alloy and Al2O3 is prepared by mixing a Pt-Pd-Rh alloy
and Al2O3 in a desired ratio and firing the mixture at a
temperature of approximately 850~ to 930~ C.
In firing the outer layer catalyst at a temperature
between 600~ and 700~ C, the substances making up the
catalysts of the outer and intermediate melt and diffuse
with each other. Hence, the boundaries between the core and
the intermediate layer have a slated structure where com-
position changes gradually.
If a stepped structure in which composition changes
abruptly is desired, the intermediate layer catalyst mixed
with wax or other viscous substance functioning as a binder
is coated over the core catalyst, with the subsequent sin-
tering process omitted. Likewise, the outer layer catalyst
mixed with the same viscous substance is coated over the
intermediate layer catalyst, with the subsequent sintering
11
2193146
process omitted.
In the manufacturing process of the ceramic catalyst
according to this invention, the core is formed into a
substantially spherical shape during sintering, with the
coatings applied subsequently to form the intermediate and
outer layers forming substantially spherical crusts. Thus,
the finished ceramic catalyst is substantially spherical in
its entirety.
When sintering is performed in a cylindrical container,
a substantially cylindrically shaped core is formed. Then,
the coatings applied subsequently to form the intermediate
and outer layers form substantially cylindrical crusts.
Thus, the finished ceramic catalyst is substantially
cylindrical in its entirety.
Embodiments
The following examples are given to illustrate specific
details of the invention. The examples are only il-
lustrative of this invention and not intended for the pur-
pose of limitation.
The embodiments described below are substantially
spherical in their entirety, with the boundaries between the
individual catalyst layers being of the slated structure in
which composition changes gradually.
Core Catalyst
To a mixture consisting of 54 g of MnO2, 15 g of NiO,
12
219314~
15 g of CoO and 16 g of CuO, all in powder form, was added
58 ml of a 7 percent by weight aqueous solution of polyvinyl
alcohol. The mixture thus obtained was fired at 950~ C and
the fired product was pulverized. A paste prepared by
adding 30 ml of a 7 percent by weight aqueous solution of
polyvinyl alcohol to the pre-fired powder was formed into
balls of approximately 2 mm in diameter. The core catalyst
was obtained by sintering the balls at 1200~ C.
Intermediate Layer Catalyst
To a mixture of 100 g consisting of 85 g of alumina, 5
g of kaoline and 10 g of carbon-bearing quartz was added 40
ml of a 7 percent by weight aqueous solution of polyvinyl
alcohol. The mixture thus obtained was fired at 1150~ C and
the fired product was pulverized. A paste was prepared by
adding 30 ml of a 7 percent by weight aqueous solution of
polyvinyl alcohol and 10 ml of a 12 percent by weight a-
queous solution of calcium carbonate to the pre-fired pow-
der. The paste thus obtained was coated over the ball-
shaped core catalyst to a thickness of approximately 1 mm.
By sintering the coated ball at 900~ C, a catalyst consis-
ting of the core coated with the intermediate layer was
obtained.
Outer Layer Catalyst
A mixture consisting of equal amounts of a Pt-Pd-Rh
alloy, which consists of Pt, Pd and Rh in a ratio of 3:1:1,
21931~16
and Al2O3 was fired at approximately 900~ C. Then, equal
amounts of the fired mixture of the Pt-Pd-Rh alloy and Al2O3,
a Mo-Al2O3 catalyst (consisting of Mo and Al2O3 in a ratio of
1:1), an Al2O3-carried vanadium oxide catalyst (consisting of
Al2O3 and vanadium oxide in a ratio of 9:1), an Ag-Al2O3
catalyst (consisting of Ag and Al2O3 in a ratio of 1:9) and a
LaO.5-SrO.5CoO3 catalyst were mixed (weighing 100 g in total).
A paste of the mixture was prepared by adding 30 ml of a 7
percent by weight aqueous solution of polyvinyl alcohol and
10 ml of a 12 percent by weight aqueous solution of calcium
carbonate. The obtained paste was coated over the fired
catalyst ball consisting of the core and intermediate layer
to a thickness of approximately 1 mm. A three-layer ceramic
catalyst was obtained by firing the coated ball at 670~ C in
a carbon monoxide atmosphere.
The following tests were made using the ceramic
catalyst thus obtained.
Reforming of Incombustible Substance to Combustible
Substance
In 1 liter of light oil was immersed 130 mg of the
catalyst balls prepared as described above. The light oil
was allowed to stand for one hour at room temperature and
gas-chromatographed. By using a Hewlett-Packard's 5290
series II chromatograph and an aluminum powder column,
14
21931~6
chromatography was carried out at 350~ C. Figs. 2 and 3
show chromatograms obtained before and after the immersion
of the catalyst balls. In Figs. 2 and 3, A1 and A2 denote
methane-based combustible substances, A3 ethane-, ethylene-
and acetylene-based combustible substances, A4 propane- and
propylene-based combustible substances, B pentane, C butane,
D methylpentene, and E benzene. As is obvious from the
chromatograms, incombustible substances, such as methylpen-
tene and benzene, decrease and combustible substances
increase after immersion of the catalyst according to this
invention.
Activation of Oxygen Dissolved in Fuel
In 1 liter of gasoline was immersed 130 mg of the
catalyst balls prepared as described above. Production of
oxygen radicals in the gasoline that was allowed to stand at
room temperature for one hour was confirmed by electron spin
resonance (ESR) spectrum (Fig. 4). a1 to a8 designate oxygen
radicals.
Decrease of AldehYde Concentration in Exhaust Gas
By immersing approximately 8 g of the catalyst prepared
as described above in the fuel tank (having a capacity of 60
liters) of automobiles equipped with a 1200 cc gasoline
engine, the relationship between the rotating speed of the
engine and the total aldehyde concentration in the exhaust
gas was determined. The concentration was determined by
2193146
measuring the absorption spectrum obtained by spectrum
analysis (by using an infrared-ray spectroscope FTIR-2
manufactured by Shimazu Corp.). Fig. 5 shows the results
obtained with and without the immersion of the catalyst
(averaged over six automobiles). AS can be seen in Fig. 5,
immersion of the catalyst significantly decreased the total
aldehyde concentration irrespective of the engine speed.
Decrease of NOx Concentration in Exhaust Gas
~ y immersing approximately 8 g of the catalyst prepared
as described above in the fuel tank (having a capacity of 60
liters) of automobiles equipped with a 1200 cc gasoline
engine, the relationship between the running speed of the
automobile and the NOx concentration in the exhaust gas was
determined. The concentration was determined by gas
chromatography. Fig. 6 shows the results obtained with and
without the immersion of the catalyst. As can be seen in
Fig. 6, immersion of the catalyst significantly (by ap-
proximately 29 to 33 percent) decreased the NOx concentra-
tion in the exhaust gas irrespective of the running speed.
The thermal decomposition temperature (ignition point)
measured by differential thermal analysis dropped by ap-
proximately 7~ C from 278~ C before the immersion of the
catalyst to 271~ C after the immersion. This temperature
drop is considered to suppress the evolution of NOx.
Composition of Combustion Gases in the Cylinders
16
21~31~6
By immersing approximately 8 g of the catalyst prepared
as described above in the fuel tank (having a capacity of 60
liters) of automobiles equipped with a 1200 cc gasoline
engine, the composition of combustion gases in the engine
cylinders was determined by gas chromatography. Five mil-
liliters of gases discharged when the pistons returned to
the original position after ignition and explosion in the
cylinders were sampled as specimens. As can be seen in Fig.
7 that shows the obtained results, the unreacted substances
significantly decreased from approximately 15 to 21 percent
before the immersion of the catalyst to approximately 1.5 to
3.5 percent after the immersion. The concentrations of
methane and ethylene also decreased greatly after the immer-
sion of the catalyst.
Relationship between Catalyst and Octane Number
Changes in octane number were determined by immersing
different quantities of the catalyst prepared as described
earlier in regular gasoline whose initial octane number was
approximately 86 before the immersion. AS shown in Fig. 8,
the immersion of the catalyst significantly increased octane
number. Octane number increased substantially linearly with
an increase in the quantity of the catalyst immersed (mg per
liter).
Relationship ~ith Combustion Efficiency
Combustion efficiencies of gasoline engines before and
' - 2193146
after immersing the catalyst in regular gasoline at a rate
of 130 mg per liter were determined. Fig. 9 shows the
combustion efficiencies before and after the immersion of
the catalyst in regular gasoline. The measurements were
taken under the conditions where constant fuel consumption
was maintained. As can be seen in Fig. 9, the catalyst
immersion increased combustion efficiency by approximately
30 percent when the air-fuel ratio was 16.7.
As discussed above, the ceramic catalysts according to
this invention enhance fuel economy and decrease noxious
substances in exhaust gases by reforming the incombustible
and environment-polluting substances in fuels to combustible
substances, activating the oxygen contained in fuels, and
evolving nascent oxygen by decomposing the water contained
in fuels.
18