Note: Descriptions are shown in the official language in which they were submitted.
WO 96/08466 21/ 3398 PGT/dP95/01796
- 1 -
DESCRIPTION
' BENZOCYCLOALKENE COMPOUNDS, THEIR PRODUCTION AND USE
= TECHNICAL FIELD
This invention relates to a novel benzbcycloalkene
derivative having an excellent binding affinity for
melatonin receptor, a process for producing it and use.
BACKGROUND ART
Melatonin (N-acetyl-5-methoxytryptamine), which is
a hormone synthesized and secreted principally in the
pineal gland, increases in dark circumstances and
decreases in light circumstances. Further, melatonin
exerts suppressively on pigment cells or female gonad
and acts as a synchronous factor of biological clock
while taking part in transmittance of photoperiodic
code. Therefore, melatonin is considered to be
possibly used for the therapy of diseases related with
melatonin activity, such as reproduction and endocrinic
disorders, sleep-awake rythm disorders, jet lag
syndrome and various disorders related to ageing.
Recently it was reported in Ann. N.Y. Acad. Sci., Vol.
719, PP.456-460 (1994) that the production of melatonin
decline steadily into old age and the supplementing
melatonin could reset the body's aging clock. However,
in "Bioorganic & Medicinal Chemistry Letters, Vol.4,
p.1485 (1994)", there is described that, when the
action on central nervous system is expected, melatonin
is shown to be inactive by a peripheral administration.
As a compound having a melatonin receptor
affinity,
1) a compound having acylamino group at the 2-position
of tetrahydronaphthalene and of the formula
WO 96108466 219 3 3 9 8 PCU'TP95l01796
2
R ga ~
N C-R,
ga (
QR 5
wherein R1 and R2 are independently, H, alkoxy etc., R3
and R5 are H etc., R4 is aryl, C1_4 alkyl etc., is
described in EP-A-420064,
2) a compound having acylaminoethyl group at the 1-
position of naphthalene and of the formula
CHZCHZNHCOR
He0 O
wherein R is propyl, butyl, cyclopropyl etc.,
is described in "Journal of Medicinal Chemistry, No.35,
pp.1484-1486, (1992)" and
3) (naphthylethyl)ureas are described in EP-A-530087.
And, as compounds having a benzocycloalkene
structure
1) a compound of the formula
Ra
H1
(CHa)nK~g~
wherein R, R1 and R2 are independently H, lower alkoxy
etc., R3 and R4 are independently H, lower acyl (-CO-R5)
etc., R5 is lower alkyl, n is 1 to 4, and having
adrenaline activity and being useful as therapeutic =
agents in the treatment of hypertension is described in
GB 2093837,
WO 96/08466 2193398 PCT/JP95101796
- 3 -
2) a compound of the formula
.
OR2
gi /
~
/g3
N\g+
wherein Rl, R2, R3 and R4 are independently H, lower
alkyl, and having aZ-adrenergic receptor agonist
activity and being useful for hypertension is described
in CA 1221639,
3) a compound of the formula
HsC CH2CH2N(CH3)C0CH3
CH'~
which is used as an intermediate of eptazocin
hydrobromide being useful as a narcotic-antagonizing
analgesic for relieving post-operative pains is
described in EP-A-384917,
4) a compound of the formula
CH2NHCOCF3
CH3
- CH3
which is used as a starting material of tetralin having
dopamine activity is described in "Journal of Medicinal
chemistry, No. 26, p. 813, (1983)".
None of them refers to the melatonin receptors
affinities.
A melatonin agonist, which is different from
melatonin in the structure, has stronger activities
than those of melatonin, is metabolicaly stable and is
excellent in the transferability into brain, can be
expected to show superior therapeutic effects to those
of melatonin. And, when the antagonistic activities of
WO 96/08466 21 p Z Z Q O' PCT/JP95/01796
-14lJ-JlU =
melatonin are desired, creation of a new melatonin
antagonist is necessary. =
At the present circumstances, no compounds which
are fully satisfactory in the activities of inelatonin =
receptors, in metabolical stability and in
transferability into brain have been found. So,
development of compounds, which are different from the
above-mentioned known compounds in chemical structure,
have excellent melatonin receptor affinities and are
fully satisfactory as medicines, is ardently desired.
DISCLOSURE OF INVENTION
The present inventors diligently conducted
extensive study and succeeded in creation of the novel
compound which is characterized in having a R3 CO-amino-
C2_3 alkyl group or a R'CO-amino-C2_5 alkylidene group (R'
is of the same meanings as defined hereinafter) at the
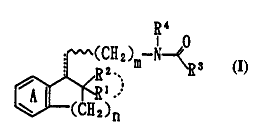
1-position of benzocycloalkenes of the formula:
1
Of,(CH2)n
wherein all symbols are of the same meaning as defined
hereinafter and represented by the formula:
- g4
(CHZ) -No
R2.'. ID 3 (I~
~A i?
~ (CHa)n
wherein R1 and R 2 independently represent a hydrogen
atom, an optionally substituted hydrocarbon group or an
optionally substituted heterocyclic group, or Ri and
RZ, taken together with the adjacent carbon atom, may
form an optionally substituted spiro ring;
R3 represents an optionally substituted hydrocarbon
= WO 96/08466 2193398 PCT/JP95101796
-
group, an optionally substituted amino group, a
= substituted hydroxyl group or an optionally substituted
heterocyclic group;
= R4 represents a hydrogen atom or an optionally
5 substituted lower alkyl group;
ring A represents a substituted benzene ring;
m and n independently represent an integer of l to 4;
and ........ represents a single bond or a double bond, or
a salt thereof, and found that the compound (I) or a
salt thereof and compound (Ia) containing the compound
(I) and represented by the formula:
R'
1 0
RHzID Ng (Ia)
(CHa)n
wherein ring A1 represents an optionally substituted
benzene ring and other symbols are of the same meaning
as defined above, or salt thereof are excellent in
affinity for melatonin receptors as melatonin agonists
or antagonists and are satisfactory as medicines.
Based on these findings, the present inventors have
completed the invention.
More specifically, this invention relates to
(1) the compound (I) or salts thereof,
(2) the compound described in the above (1), in which
R1 and R 2 are independently a hydrogen atom or an
optionally substituted hydrocarbon group, or R1 and R2,
taken together with the adjacent carbon atom, may form
a spiro ring, R' represents an optionally substituted
hydrocarbon group, an optionally substituted amino
group or a substituted hydroxyl group,
= (3) the compound described in the above (1), in which
the hydrocarbon group is a C1_6 aliphatic hydrocarbon
= group, a C3_6 monocyclic saturated hydrocarbon group or
WO 96/08466 21p Z ZJQ 8 PGT/JP95/01796 =
V-lJ6-J
a C6_10 aromatic hydrocarbon group,
(4) the compound described in the above (1), in which
the heterocyclic group is a 5- to 7-membered
heterocyclic group having 1 to 3 hetero-atoms selected
from nitrogen, oxygen and sulfur,
(5) the compound described in the above (1), in which
the spiro ring is a 3 to 8-membered ring,
(6) the compound described in the above (1), in which
the substituent of the amino group is an optionally
substituted lower alkyl group or an optionally
substituted aryl group,
(7) the compound described in the above (1), in which
the substituted hydroxyl group is an optionally
substituted lower alkoxy group,
(8) the compound described in the above (1), in which
Rt and RZ are independently a hydrogen atom, a lower
alkyl group or an aryl group,
(9) the compound described in the above (1), in which
R1 and RZ are independently a hydrogen atom or a lower
alkyl group,
(10) the compound described in the above (1), in which
R3 is (i) an optionally substituted lower-alkyl group,
(ii) an optionally substituted lower cycloalkyl group,
(iii) an optionally substituted lower alkenyl group,
(iv) an optionally substituted aryl group, (v) an
optionally substituted lower alkylainino group, (vi) an
optionally substituted arylamino group, (vii) an
optionally substituted 5- or 6-membered nitrogen-
containing heterocyclic group or (viii) an optionally
substituted lower alkoxy group,
(11) the compound described in the above (1), in which
R3 is an optionally halogenated C1_3 alkyl group,
(12) the compound described in the above (1), in which
R4 is a hydrogen atom,
(13) the compound described in the above (1), in which ......... is a single
bond,
WO 96/08466 2193398 PCTIJP95/01796
7
(14) the compound described in the above (1), in which
' n is an integer of 1 to 3,
(15) the compound described in the above (1), in which
n is 1,
(16) the compound described in the above (1), in which
m is 1 or 2,
(17) the compound described in the above (1), in which
the ring A is a benzene ring having 1 to 3 substituents
selected from the group consisting of (i) a halogen
atom, (ii) a lower alkyl group, (iii) a lower alkoxy
group optionally substituted with an aryl group, (iv)
hydroxyl group and (v) a mono-lower alkylamino group,
(18) the compound described in the above (1), in which
the ring A is represented by the formula
gs'
wherein R5 is an optionally substituted lower alkoxy
group,
(19) the compound described in the above (1), in which
m is 1, n is 2 and ......... is a single bond,
(20) the compound described in the above (1), in which
m is 1, n is 2 and ........ is a double bond,
(21) the compound described in the above (1), in which
m is 1 or 2, n is 1 and ........ is a single bond,
(22) the compound described in the above (1), in which
m is 1, n is 3 and ........ is a double bond,
(23) the compound described in the above (1), in which
RI, R 2 and R 4 all are a hydrogen atoms,
(24) the compound described in the above (23), in which
R3 is an optionally halogenated lower alkyl group,
(25) the compound described in the above (23), in which
R3 is a lower cycloalkyl group,
(26) the compound described in the above (18), in which
R1, RZ and R 4 all are a hydrogen atoms, R3 is an
WO 96108466 2193398 PCf/JP95101796
- 8 -
optionally halogenated lower alkyl group and both of m
and n are 1,
(27) the compound described in the above (1), which is
the compound represented by the formula:
g4
_el~(CHz)m Ks
gz==
flfl
wherein symbols are of the same meaning as defined
above,
(28) the compound described in the above (1), which is
(S)-1-[2-(acetylamino)ethyl]-6-methoxyindan, (S)-6-
methoxy-l-[2-(trifluoroacetylamino)ethyl]indan, (S)-1-
[2-(cyclopropylcarbonylamino)ethyl]-6-methoxyindan,
(S)-1-[2-(propionylamino)ethyl]-6-methoxyindan, (S)-1-
[2-(isobutyrylamino)ethyl]-6-methoxyindan, (S)-1-[2-
(acetylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene, (S)-7-methoxy-l-[2-
(trifluoroacetylamino)ethyl]-1,2,3,4-
tetrahydronaphthalene or (S)-1-[2-
(cyclopropylcarbonylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene,
(29) the compound described in the above (1), which is
(S)-1-[2-(trifluoroacetylamino)ethyl]-6-methoxyindan,
(30) the compound described in the above (1), which is
(S)-1-[2-(propionylamino)ethyl]-6-methoxyindan,
(31) (S)-1-(2-aminoethyl)-6-methoxyindan or a salt
thereof,
(32) a procsss for producing a compound described in
the above (1), which comprises reacting a compound
represented by the formula: =
WO 96/08466 2193398 PCTlJP95/01796
9 -
(CHz)m NH-E"
g
. ~ (CHZ)n
wherein all symbols are of the same meaning as defined
above, or a salt thereof, with a carboxylic acid of the
formula: R'COOH (R3 is as defined above), a salt or
reactive derivative thereof, or an isocyanate
derivative of the formula: R3' N=C=O (R' has the same
meaning as R3 defined above except -NH),
(33) a pharmaceutical composition which comprises a
compound described in the above (1), if necessary
together with a pharmaceutically acceptable carrier,
(34) the composition described in the above (33), which
has a binding affinity for melatonin receptor,
(35) the composition described in the above (34), which
is a regulating agent of circadian rhythm,
(36) the composition described in the above (35), which
is a regulating agent of sleep-awake rhythm,
(37) the composition described in the above (36), which
is a regulating agent of time zone change syndrome,
(38) the composition described in the above (35), which
is a therapeutic agent of sleep disorders,
(39) a composition having a binding affinity for
melatonin receptor which comprises a compound (Ia) or a
salt and a pharmaceutically acceptable carrier, and
(40) the composition described in the above (39), which
is a melatonin receptor agonistic composition.
"Hydrocarbon group" of the term "optionally
substituted hydrocarbon group" used in this
specification include, among others, aliphatic
hydrocarbon groups, monocyclic saturated hydrocarbon
groups and aromatic hydrocarbon groups. The carbon
number of the hydrocarbon group is preferable 1 to 16.
An alkyl group, an alkenyl group, an alkynyl group, a
WO 96/08466 219339$ PCT/.iP95101796
- 10 - ~
cycloalkyl group and an aryl group are exemplified.
"Alkyl group" is preferably a lower alkyl group,
for example, C1_6 alkyl groups such as methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-
=
butyl, pentyl and hexyl are used.
"Alkenyl group" is preferably a lower alkenyl
group, for example, C2_6 alkenyl groups such as vinyl,
1-propenyl, allyl, isopropenyl, butenyl and isobutenyl
are used.
"Alkynyl group" is preferably a lower alkynyl
group, for example, C2_6 alkynyl groups such as ethynyl
and 1-propynyl are used.
"Cycloalkyl group" is preferably a lower
cycloalkyl group, for example, C3_6 cycloalkyl groups
such as cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl are used.
"Aryl group" is preferably C6_14 aryl groups such
as phenyl, xylyl, 1-naphthyl, 2-naphthyl, biphenylyl,
2-indenyl and 2-anthlyl. Among others, a phenyl group,
for example, is used.
Examples of the substituents, which "hydrocarbon
group" of "optionally substituted hydrocarbon group"
may optionally have, include halogen atoms (e.g.
fluorine, chlorine, bromine and iodine), nitro group,
cyano group, hydroxyl group, optionally halogenated Ci_6
alkyl groups (e.g. methyl, chloromethyl,
difluoromethyl, trichloromethyl, trifluoromethyl,
ethyl, 2-bromoethyl, 2,2,2-trifluoroethyl,
pentafluoroethyl, propyl, 3,3,3-trifluoropropyl,
isopropyl, butyl, 4,4,4-trifluorobutyl, isobutyl, sec-
butyl, tert-butyl, pentyl, isopentyl, neopentyl, 5,5,5-
trifluoropentyl, hexyl and 6,6,6-trifluorohexyl, a
lower alkoxy group (e.g. Ci_6 alkoxy groups such as
methoxy, ethoxy, propoxy, isopropoxy, butoxy, =
isobutoxy, pentyloxy and hexyloxy), amino group, mono-
lower alkyl amino group (e.g. mono-C1_6 alkylamino group =
2193398
WO 96/08466 PCT7JP95/01796
- 11 -
such as methylamino and ethylamino), di-lower
alkylamino group (e.g. di-C1_6 alkylamino group such as
dimethyamino and diethylamino), carboxyl group, lower
alkylcarbonyl group (C1_6 alkyl-carbonyl group such as
acetyl and propionyl), lower alkoxycarbonyl group (e.g.
C3_6 alkoxy-carbonyl group such as methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl and butoxycarbonyl),
carbamoyl group, mono-lower alkylcarbamoyl group (e.g.
mono-C1_6 alkylcarbamoyl group such as methylcarbamoyl
and ethylcarbamoyl), di-lower alkylcarbamoyl group
(e.g. di-Ci_6 alkylcarbamoyl group such as
dimethylcarbamoyl and diethylcarbamoyl), arylcarbamoyl
group (e.g. C6_10 arylcarbamoyl group such as
phenylcarbamoyl and naphthylcarbamoyl), aryl group
(e.g. C6_10 aryl group such as phenyl and naphthyl) and
aryloxy group (e.g. C6_10 aryloxy group such as
phenyloxy and naphthyloxy), optionally halogenated
lower alkylcarbonylamino group (e.g. optionally
halogenated C1_6 alkyl-carbonylamino group such as
acetylamino, trifluoroacetylamino). "Hydrocarbon
group" of "optionally substituted hydrocarbon group"
may optionally have 1 to 5, preferably 1 to 3, of these
substituents. When the number of the substituents is
two or more, they may be the same one or different from
one another.
"Heterocyclic group" of the term "optionally
substituted heterocyclic group" used in this
specification includes, for example, 5- to 14-membered
(preferably 5- to 10-membered) (mono-, di- or
tricyclic, preferably monocyclic or dicyclic)
heterocyclic group containing, besides carbon atom, 1
to 4 hetero atoms, preferably 1 to 3, selected from
nitrogen atom, oxygen atom and sulfur atom. As the
"heterocyclic group", use is made of 5-membered cyclic
, 35 group containing, besides.carbon atom, 1 to 4 hetero
atoms selected from oxygen atom, sulfur atom and
WO 96/08466 21933G'' 8 PCTlJP95101796
- 12-7
nitrogen atom, as exemplified by 2- or 3-thienyl, 2- or
3-furyl, 1-, 2- or 3-pyrrolyl, 1-, 2- or 3-
pyrrolidinyl, 2-, 4- or 5-oxazolyl, 3-, 4- or 5-
isooxazolyl, 2-, 4- or 5-thiazolyl, 3-, 4- or 5-
isothiazolyl, 3-, 4- or 5-pyrazolyl, 2-, 3- or 4-
pyrazolidinyl, 2-, 4- or 5-imidazolyl, 1,2,3-triazolyl,
1,2,4-triazolyl, and 1H- or 2H-tetrazolyl; 6-membered
cyclic group containing, besides carbon atom, 1 to 4
hetero atoms selected from oxygen atom, sulfur atom and
nitrogen atom, as exemplified by 2-, 3- or 4-pyridyl,
N-oxido-2-, 3- or 4-pyridyl, 2-, 4- or 5-pyrimidinyl,
N-oxido-2-, 4- or 5-pyrimidinyl, thiomorpholinyl,
morpholinyl, piperidino, 2-, 3- or 4-piperidyl,
pyranyl, thiopyranyl, 1,4-oxazinyl, 1,4-thiazinyl, 1,3-
thiazinyl, piperazinyl, triazinyl, 3- or 4-pyridazinyl,
pyrazinyl and N-oxido-3- or 4-pyridazinyl; dicyclic or
tricyclic condensed cyclic group (preferably a group
formed by condensation of the above-mentioned 5- to 6-
membered cyclic group with one or two of the 5- to 6-
membered cyclic groups optionally containing, besides
carbon atom, 1 to 4 hetero atoms selected from oxygen
atom, sulfur atom and nitrogen atom), as exemplified by
indolyl, benzofuryl, benzothiazolyl, benzoxazolyl,
benzimidazolyl, quinolyl, isoquinolyl, phthalazinyl,
quinazolinyl, quinoxalinyl, indolizinyl, quinolizinyl,
1,8-naphthyridinyl, dibenzofuranyl, carbazolyl,
acridinyl, phenanthridinyl, chromanyl, phenothiazinyl
and phenoxazinyl. Among others, a 5- to 7-membered
heterocyclic group having 1 to 3 hetero-atoms selected
from nitrogen atom, oxgen atom and sulfur atom is
preferable.
Examples of the.substituent, which the
"heterocyclic group" of this "optionally substituted
heterocyclic group" may optionally have, include
halogen atoms (e.g. fluorine, chlorine, bromine and
iodine), lower alkyl groups (e.g. C1_6 alkyl groups such
= WO 96/08466 2193398 PCI /JP95/01796
- 13 -
as methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
sec-butyl, tert-butyl, pentyl and hexyl), cycloalkyl
groups (e.g. C3_6 cycloalkyl groups such as cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl), lower alkynyl
groups (e.g. C2_6 alkynyl groups such as ethynyl, 1-
propynyl and propar(jyl), lower alkenyl groups (e.g. C2_6
alkenyl groups such as vinyl, allyl, isopropenyl,
butenyl and isobutenyl), aralkyl groups (e.g. C7_11
aralkyl groups such as benzyl, a-methylbenzyl and
phenethyl), aryl groups (e.g. C6_10 aryl groups such as
phenyl and naphthyl, preferably phenyl group), lower
alkoxy groups (e.g. C1_6 alkoxy groups such as methoxy,
ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-
butoxy and tert-butoxy), aryloxy groups (e.g. C6-10
aryloxy groups such as phenoxy), lower alkanoyl groups
(e.g. C1_6 alkanoyl groups such as formyl, acetyl,
propionyl, butyryl and isobutyryl), benzoyl group,
naphthoyl group, lower alkanoyloxy groups (e.g. C1_6
alkanoyloxy groups such as formyloxy, acetyloxy,
propionyloxy, butyryloxy and isobutyryloxy),
arylcarbonyloxy groups (e.g. C6_10 aryl-carbonyloxy
groups such as benzoyloxy and naphthoyloxy), carboxyl
group, lower alkoxycarbonyl groups (e.g. C1_6 alkoxy-
carbonyl groups such as methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl, isobutoxycarbonyl and tert-
butoxycarbonyl), aralkyloxycarbonyl groups (e.g. C7_11
aralkyloxycarbonyl groups such as benzyloxycarbonyl),
carbamoyl group, mono-, di- or tri-halogeno-lower alkyl
groups (e.g. mono-, di- or tri-halogeno-C1_4 alkyl
groups such as chloromethyl, dichloromethyl,
trifluoromethyl and 2,2,2-trifluoroethyl), oxo group,
amidino group, imino group, amino group, mono-lower
alkylamino groups (e.g. mono-C1_4 alkylamino groups such
as methylamino, ethylamino, propylamino, isopropylamino
WO 96108466 2193398 PCT13P95101796
- i147- v
and butylamino), di-lower alkylamino groups (e.g. di-
C1_4 alkylamino groups such as dimethylamino,
diethylamino, dipropylamino, diisopropylamino and
dibutylamino), 3- to 6-membered cyclic amino groups
optionally containing, besides carbon atom and one
nitrogen atom, one to three hetero atoms selected from
oxygen atom, sulfur atom and nitrogen atom (e.g. 3- to
6-memberedcyclic amino groups such as aziridinyl,
azetidinyl, pyrrolidinyl, pyrrolinyl, pyrrolyl,
imidazolyl, pyrazolyl, imidazolidinyl, piperidyl,
morpholinyl, dihydropyridyl, pyridyl, N-
methylpiperazinyl and N-ethylpiperazinyl),
alkylenedioxy group (e.g. C1_3 alkylenedioxy groups such
as methylenedioxy and ethylenedioxy), hydroxyl group,
nitro group, cyano group, mercapto group, sulfo group,
sulfino group, phosphono group, sulfamoyl group,
monoalkylsulfamoyl group (e.g. C1_6 monoalkylsulfamoyl
groups such as N-methylsulfamoyl, N-ethylsulfamoyl, N-
propylsulfamoyl, N-isopropylsulfamoyl and N-
butylsulfamoyl), dialkylsulfamoyl group (e.g. di-C1_6
alkylsulfamoyl groups such as N,N-dimethylsulfamoyl,
N,N-diethylsulfamoyl, N,N-dipropylsulfamoyl and N,N-
dibutylsulfamoyl), alkylthio group (e.g. Ci_6 alkylthio
groups such as methylthio, ethylthio, propylthio,
isopropylthio, butylthio, sec-butylthio and tert-
butylthio), arylthio group (e.g. C6_10 arylthio groups
such as phenylthio, naphthylthio), lower alkylsulfinyl
groups (e.g. C1_6 alkylsulfinyl groups such as
methylsulfinyl, ethylsulfinyl, propylsulfinyl and
butylsulfinyl), arylsulfinyl group (e.g. C6_1o
arylsulfinyl groups such as phenylsulfinyl and
naphthylsulfinyl), lower alkylsulfonyl groups (e.g. C1_6
alkylsulfonyl groups such as methylsulfonyl,
ethylsulfornyl, propylsulfonyl and butylsulfonyl) and
arylsulfonyl group (e.g. C6_10 arysulfonyl groups such
as phenylsulfonyl and naphthylsulfonyl).
WO 96/08466 219 3 3 9 8 PGT/JP95/01796
- 15 -
The "heterocyclic group" of the "optionally
substituted heterocyclic group" may have 1 to 5,
preferably 1 to 3, of the above-described substituents
at any possible position. When the number of the
substituents is two or more, they may be the same one
or different from one another.
The term "optionally substituted amino group" used
in this specification means amino group which may
optionally have, as the substituents, one or two of the
above-mentioned "optionally substituted hydrocarbon
group" for example. Preferable examples of the
substituents, which this "amino group" may optionally
have, include an optionally substituted CI_6 alkyl group
and an optionally substituted C6_10 aryl group. The
substituents of the alkyl or aryl group are, for
example, the same substituents which above-mentioned
"hydrocarbon group" may optionally have.
The term "substituted hydroxyl group" used in this
specification means the hydroxyl group which have, in
place of the hydrogen atom of the hydroxyl group, one
~optionally substituted hydrocarbon group" mentioned
above. Preferable examples of "substituted hydroxyl
group" include hydroxyl group substituted with one
lower alkyl group. Examples of the "lower alkyl group"
includes C1_6 alkyl groups such as methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl and tert-
butyl. The substituents which "lower alkyl group" may
optionally have is, for example, the same ones as the
above-mentioned "hydrocarbon group" may optionally
have.
"Lower alkyl group" of the term "optionally
substituted lower alkyl group" used in this
specification includes, for example, C1_6 alkyl groups
such as methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl and tert-butyl, which may
optionally have 1 to 3 substituents which, for example,
WO 96108466 2193 3 98 PCf/JP95l01796 =
- 16 -
the above-mentioned "hydrocarbon group" may optionally
have.
"Spiro ring" of the term "optionally substituted
spiro ring" used in this specification includes, for
example, 3- to 8-membered ring formed by R' and Ra
combinedly taking the adjacent carbon atom as the spiro
atom, as exemplified by a lower cycloalkane (e.g. C3_8
cycloalkane such as cyclopropane, cyclobutane,
cyclopentane and cyclohexane) and a lower cycloalkene
(e.g. C3_8 cycloalkene such as cyclopropene,
cyclobutene, cyclohexene). C3_8 cycloalkane is
preferable.
The substituents and its number which "spiro ring"
may optionally have are, for example, the same ones as
the above-mentioned "heterocyclic group" may optionally
have. The "spiro ring" may condense with aromatic
rings, for example, 6-membered aromatic rings such as
benzene and pyridine.
The term "substituted benzene ring" used in this
specification means benzene rings which have, at any
possible position, one to three substituents selected
from halogen atoms (e.g. fluorine, chlorine, bromine
and iodine), optionally substituted hydrocarbon groups,
substituted hydroxyl groups (preferably, optionally
substituted lower (C1_6) alkoxy groups such as methoxy,
ethoxy, propoxy and isopropoxy), hydroxyl group,
optionally substituted amino group, amido groups (e.g.
C1_6 acylamino groups such as acetamido), and lower
alkylenedioxy groups (e.g. C1_b alkylenedioxy groups
such as methylenedioxy, ethylenedioxy).
As these "optionally substituted hydrocarbon group",
"substituted hydroxyl group" and "optionally
substituted amino group", the same ones as the above-
mentioned are used. When the number of the
substituents is two or more, they may be the same one
or different from one another. Preferable examples of
CA 02193398 2005-07-12
26456-138
- 17 -
the above-mentioned "substituted benzene ring" include
benzene rings having 1 to 3 substituents selected from
halogen atoms (e.g. fluorine and chlorine), C1_6 alkyl
groups (e.g. methyl and ethyl), C1_6 alkoxy groups (e.g.
methoxy and ethoxy) which may have C6_10 aryl group,
hydroxyl group and mono-C1_6 alkylamino group,
especially preferable one being, for example, benzene
ring substituted with one, for example, C1_6 alkoxy
group (e.g. methoxy).
The term "optionally substituted benzene ring"
used in this specification includes, for example, the
benzene ring which may optionally have 1 to 3
substituents which the above-mentioned "substituted
benzene ring" has.
In the above formulae, R1 and R 2 are each
a hydrogen atom, an optionally substituted hydrocarbon
group or an optionally substituted heterocyclic group.
R1 and R2 may form an optionally substituted spiro ring
together with the adjacent carbon atom. Each of R1 and
R 2 is preferably a hydrogen atom, a'lower alkyl group
(e.g. a C1_6 alkyl group such as methyl, ethyl, propyl
and isopropyl) or an aryl group (e.g. a C6_10 aryl group
such as phenyl, 1-naphthyl and 2-naphthyl), especially
a hydrogen atom and a lower alkyl group. And, the case
where R' and R 2 combinedly form, taken together with
the adjacent carbon atom, a group which may be
condensed with an aromatic ring, for example, 6-
membered aromatic ring such as benzene, of, for
example, the following is preferable.
>1 >0 >0
Among others, R1 and R 2 are preferably a hydrogen atom.
7 Z{~ Q PC17JP95101796
WO 96/08466 219J/U
- 18 J-
In the above formulae, R3 is an optionally
substituted hydrocarbon group, an optionally
substituted amino group, a substituted hydroxyl group
or an optionally substituted heterocyclic group.
Preferable examples of "hydrocarbon group" of "an
optionallysubstituted hydrocarbon group" shown by R3
include alkyl groups (e.g. C1_6 alkyl groups such as
methyl, ethyl, propyl and isopropyl), alkenyl groups
(e.g. C2_6 alkenyl groups such as vinyl), alkynyl groups
(e.g. CT_6 alkynyl groups such as ethynyl), cycloalkyl
groups (e.g. C3_6 cycloalkyl groups such as cyclopropyl,
cyclobutyl,cyclopentyl and cyclohexyl) and aryl groups
(e.g. C6_14 aryl groups such as phenyl). Among others,
alkyl groups (e.g. Ci_b alkyl groups such as methyl) and
cycloalkyl groups (e.g. C3_6 cycloalkyl groups such as
cyclopropyl) are preferably used. The "alkyl group",
"alkenyl group", "alkynyl group", "cycloalkyl group"
and "aryl group" may optionally have, for example, 1 to
5, preferably 1 to 3 substituents, which the above-
mentioned "hydrocarbon group" may optionally have,
(preferably halogen atoms such as fluorine).
Preferable examples of the substituents of
"optionally substituted amino group" shown by R3
include one or two of optionally substituted lower
alkyl groups and optionally substituted aryl groups.
Especially, one lower alkyl group which may optionally
have substituent is used. Examples of the "lower alkyl
group" include C1_6 alkyl groups such as methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl and tert-
butyl. The "lower alkyl group" may optionally have,
for example, 1 to 3 substituents which the above-
mentioned "hydrocarbon group" may optionally have.
Examples of the "aryl group" include C6_10 aryl groups
such as phenyl group. The "aryl group" may have, for
example, 1 to 5, preferably 1 to 3 substituents, which
the above-mentioned "hydrocarbon group" may optionally
WO 96108466 21/33Y8 PGT13P95101796
- 19 -
have, (preferably halogen atoms such as fluorine and
chlorine, and CI_6 alkoxy groups such as methoxy and
ethoxy). Examples of the "optionally substituted amino
group" include phenylamino groups substituted with 1 to
3 lower alkoxy groups (e.g. methoxy) and amino groups
mono-substituted with a lower alkyl group (e.g. methyl,
ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl
and tert-butyl). Among others, a mono-Ci_6 alkyl amino
group is preferable.
Examples of the substituents which the
"substituted hydroxyl group" shown by R3 may have
include an optionally substituted lower alkyl group.
Examples of the "lower alkyl group" include C1_6 alkyl
groups such as methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl and tert-butyl. The "lower alkyl
group" may optionally have, for example, 1 to 3
substituents which the above-mentioned "hydrocarbon
group" may optionally have. Preferable examples of
"substituted hydroxyl group" include optionally
substituted lower alkoxy groups (e.g. C1_6 alkoxy groups
such as methoxy, ethoxy, propoxy, isopropoxy and
butoxy). Examples of the substituents of the "lower
alkoxy groups" include the substituents which the
above-mentioned "hydrocarbon group" may optionally
have.
Preferable examples of "heterocyclic group" of
"optionally substituted heterocyclic group" shown by R3
include 5- or 6-membered heterocyclic group containing,
besides carbon atom, 1 to 3 hetero atoms selected from
nitrogen atom, oxygen atom and sulfur atom, as
exemplified by 1-, 2- or 3-pyrrolidinyl, 2- or 4-
imidazolinyl, 2-, 3- or 4-pyrazolidinyl, piperidinyl,
2-, 3- or 4-piperidyl, 1- or 2-piperazinyl, morpholino,
2- or 3-thienyl, 2-, 3- or 4-pyridyl, 2- or 3-furyl,
pyrazinyl, 2-pyrimidinyl, 3-pyrrolyl, 3-pyridazinyl, 3-
isothiazolyl or 3-isooxazolyl. Especially, 6-membered
WO 96108466 2193 3 g g PG7/JP95101796
- 20 -
nitrogen-containing heterocyclic groups such as pyridyl
are preferably used.
Preferable examples of substituents of "optionally
substituted heterocyclic group" shown by R3 include,
for example, halogen atoms, C1-6 alkyl groups, C1_6
alkoxy groups and aralkyloxycarbonyl groups.
Preferable examples of R3 include (i) optionally
substituted lower alkyl groups, (ii) optionally
substituted lower cycloalkyl groups, (iii) optionally
substituted lower alkenyl groups, (iv) optionally
substituted aryl groups (v) optionally substituted
lower alkylamino groups, (vi) optionally substituted
arylamino groups, (vii) optionally substituted 5- or 6-
membered nitrogen-containing heterocyclic groups, or
(viii) optionally substituted lower alkoxy groups.
Preferable examples of the "lower alkyl group" include
C1_6 alkyl groups such as methyl, ethyl, propyl,
isopropyl, butyl, pentyl and hexyl. Preferable
examples of the "lower cycloalkyl group" include C3-6
cycloalkyl groups such as cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl. Preferable examples of the
"lower alkenyl group" include C2_6 alkenyl groups such
as vinyl, 1-propenyl and butenyl. Preferable examples
of the "aryl group" include C6-10 aryl groups such as
phenyl, 1-naphthyl and 2-naphthyl. Preferable examples
of the "lower alkylamino group" include mono- or di-C1_6
alkylamino groups such as methylamino, ethylamino,
propylamino, butylamino, tert-butylamino,
dimethylamino, diethylamino and methylethylamino.
Preferable examples of the "arylamino group" include
C6-1o arylamino groups such as phenylamino. Preferable
examples of the "5- or 6-membered nitrogen-containing.
heterocyclic group" include 5- or 6-membered nitrogen-
containing heterocyclic groups such as 2-, 3- or 4-
pyridyl. Preferable examples of the "lower alkoxy
group" include C1-6 alkoxy groups such as methoxy,
WO 96108466 219JZ 398 PCT/JP95101796
21-
ethoxy, propoxy and isopropoxy. The substituents which
these groups may optionally have is, for example, 1-5
of those which the above-mentioned "hydrocarbon group"
may optionally have. Among others, i) C1_6 alkyl groups
optionally substituted by halogen atoms or C1_6 alkoxy
groups, ii) C3_6 cycloalkyl groups, iii) C2_6 alkenyl
groups, iv) C6_10 aryl groups optionally substituted by
a) C1_6 alkoxy, b) nitro, c) halogeno-Ci_6 alkyl-
carbonylamino or d) halogen atoms, v) mono- or di-Ci_6
alkylamino groups, vi) C6_lo arylamino groups optionally
substituted by 1 to 3 C1_6 alkoxy, vii) 6-membered
nitrogen-containing heterocyclic groups optionally
substituted by C7_11 aralkyloxy-carbonyl and viii) Ci_6
alkoxy groups are preferably used. Especially, for
example, optionally halogenated C1_6 alkyl groups (e.g.
methyl, chloromethyl, difluoromethyl, trichloromethyl,
trifluoromethyl, ethyl, 2-bromoethyl, 2,2,2-
trifluoroethyl, pentafluoroethyl, propyl, 3,3,3-
trifluoropropyl, isopropyl, butyl, 4,4,4-
trifluorobutyl, isobutyl, sec-butyl, tert-butyl,
pentyl, isopentyl, neopentyl, 5,5,5-trifluoropentyl,
hexyl and 6,6,6-trifluorohexyl), C3_6 cycloalkyl groups
(e.g. cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl) and mono- CL_6 alkylamino groups (e.g.
methylamino, ethylamino, propylamino, isopropylamino,
butylamino and tert-butylamino) are used. Typically,
optionally halogenated C1_6 alkyl groups and mono- Ci_6
alkylamino groups, especially, halogenated Ci_3 alkyl
groups are preferred.
In the above formulae, R 4 is a hydrogen atom or an
optionally substituted lower alkyl group. R 4 is
preferably a hydrogen atom or a Ct_4 alkyl group (e.g.
methyl), especially a hydrogen atom.
In the above-mentioned formulae, ring A is a
substituted benzene ring, and ring A1 is an optionally
WO 96/08466 2193398 PCT/JP95101796
- 22 _
substituted benzene ring. Preferable examples of ring
A and ring A' include
ES A gs
wherein RS is an optionally substituted lower alkoxy
group (e.g. CI_6 alkoxy group such as methoxy and
ethoxy). Preferable examples of RS include C1_6 alkoxy
groups optionally substituted by C6_10 aryl groups.
Especially, C1_6 alkoxy groups (e.g. methoxy) are
preferable.
Preferable examples of
A ga ~
(CHn
include
g$
(CHZ)n
wherein symbols are of the same meaning as defined
above.
m and n independently denote an integer of 1 to 4,
preferably 1 to 3. Preferably, m is 1 or 2, especially
1. Preferably, n is 1 or 2, especially 1.
In the above formulae, ........ means a single bond
or double bond, preferably, single bond, and AMM
means, when ........ is double bond, E-isomer, Z-isomer or
a mixture of them.
Examples of the compound (I) of the present
invention include those having the following structural
formulae.
= WO 96/08466 211 3398 PCT/JP95/01796
- 23 -
0 0 0
'~N~Es 4N~&s 4N~
/ $2.'=. ~ BZ'=. / g2...
~A L.=' , ~A L: , ~A L,.' ,
0 0
g4~Nxgs g4Nxg g'\NAIgs
gz.= gz., gz.
A L=' A L.% A L. %
0 0 0
g'\NJ~gs E ~Nxgs g Nxgs
g2 . B~ =. Rz'=
\ L.% \A L.~' \ gL.%
wherein symbols are of the same meaning as defined in
the foregoing.
Preferable example of the compound (I) include the
compound of the formula:
g
N 0
~CCH2)m - ~ s
2==
A g l :
(CHZ)n
wherein all symbols are of the same meaning as defined
above. R' and RZ are preferably hydrogen.
More preferably; in the above formulae, (i) the
CA 02193398 2005-07-12
26456-138
- 24 -
compound in which m is 1, n is 2 and ..., is a single
bond, (ii) the compound in which m is 1, n is 2 and
......... is a double bond, ( iii ) the compound in which m
is 1 or 2, n is 1 and ........ is a single bond, and (iv)
the compound in which m is 1, n is 3 and ........ is a
double bond.
In the compound (I) and (Ia), preferable is the
compound wherein R' and R 2 are a hydrogen
atom, a lower alkyl group or C6_10 aryl group or Rl and
R 2 may combinedly form, taken together with the
adjacent carbon atom, a C3_8 spiro ring which may be
condensed with a 6-membered aromatic ring;
R3 is (i) optionally substituted lower alkyl groups,
(ii) optionally substituted lower. cycloalkyl groups,
(iii) optionally substituted lower alkenyl groups, (iv)
optionally substituted aryl groups (v) optionally
substituted lower alkylamino groups, (vi) optionally
substituted arylamino groups, (vii) optionally
substituted 5- or 6-membered nitrogen-containing
heterocyclic groups, or (viii) optionally substituted
lower alkoxy groups, the substituents which these
groups may optionally have is 1 to 5 of those which the
above-mentioned "hydrocarbon group" may optionally
have;
R' is a hydrogen atom or a lower alkyl;
ring A or ring A' is a substituted benzene ring;
m is 1 or 2; and
n is integer of 1 to 3.
More preferable is the compound.wherein R' and R 2
are a hydrogen atom, a C1_3 alkyl group or
C6_10 aryl group;
R3 is i) C1_6 alkyl groups optionally substituted
by halogen atoms or C1_6 alkoxy, (ii) C3_6 cycloalkyl
groups, (iii) C2_6 alkenyl groups, iv) C6_10 aryl groups
optionally substituted by a) C1_6 alkoxy, b) nitro, c)
CA 02193398 2005-07-12
26456-138
- 25 -
halogeno-C1_6 alkyl-carbonylamino or d) halogen, v)
mono- or di-C1_6 alkylamino groups, vi) C6_10 arylamino
groups optionally substituted by 1 to 3 C1_6 alkoxy,
vii) 6-membered nitrogen-containing heterocyclic groups
optionally substituted by C7_11 aralkyloxy-carbonyl or
viii) C1_6 alkoxy groups;
R4 is a hydrogen atom or a C1_4 alkyl group;
ring A or ring A' is a benzene ring substituted by a
lower alkoxy group which may have a C6_10 aryl group;
and
m and n are 1.
Especially, the compound wherein Ri, R 2 and R' are
a hydrogen atom; R' is a C1_6 alkyl group
optionally substituted by halogen atoms or mono-C1_6
alkylamino group; ring A or ring A1 is a benzene ring
substituted by a C1_3 alkoxy group; and m and n are
1, is preferable.
Preferable examples of the object compound (I) of
the present invention and compound (Ia) include (E)-1-
[2-(acetylamino)ethylidene]-7-methoxy-1,2,3,4-
tetrahydronaphthalene, (E)-7-methoxy-l-[2-
(trifluoroacetylamino)ethylidene]-1,2,3,4-
tetrahydronaphthalene, (E)-1-[2-
(cyclopropylcarbonylamino)ethylidene]-7-methoxy-
1,2,3,4-tetrahydronaphthalene, 1-[2-
(acetylamino)ethyl]-6-methoxyindan, 1-[2-
(trifluoroacetylamino)ethyl]-6-methoxyindan, 1-[2-
(cyclopropylcarbonylamino)ethyl]-6-methoxyindan, 1-[2-
(propionylamino)ethyl]-6-methoxyindan, 1-[2-
(isobutylylamino)ethyl)-6-methoxyindan, (E)-1-[2-
(acetylamino)ethylidene]-6-methoxyindan, (E)-1-[2-
(trifluoroacetylamino)ethylidene]-6-methoxyindan, (E)-
1-[2-(cyclopropylcarbonylamino)ethylidene]-6-methoxyi-
ndan, 1-[2-(acetylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene, 7-methoxy-l-[2-
WO 96/08466 2193398 PCT/3P95/01796 =
26 -
(trifluoroacetylamino)ethylJ-1,2,3,4-
tetrahydronaphthalene and 1-[2-
(cyclopropylcarbonylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene.
More preferable is (S)-1-[2-(acetylamino)ethyl]-6-
methoxyindan, (S)-1-[2-(trifluoroacetylamino)ethyl]-6-
methoxyindan, (S)-1-[2-
(cyclopropylcarbonylamino)ethyl]-6-methoxyindan, (S)-1-
[2-(propionylamino)ethyl]-6-methoxyindan, (S)-1-[2-
(isobutyrylamino)ethyl]-6-methoxyindan, (S)-1-[2-
(acetylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene, (S)-7-methoxy-l-[2-
(trifluoroacetylamino)ethyl]-1,2,3,4-
tetrahydronaphthalene and (S)-1-[2-
(cyclopropylcarbonylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene.
Examples of the salts of the compound (I) and (Ia)
include pharmaceutically acceptable salts, which are
exemplified by salts with inorganic bases, salts with
organic bases, salts with inorganic acids, salts with
organic acids and salts with basic or acidic amino
acids. Preferable salts with inorganic bases include
alkali metal salts such as sodium salt and potassium
salt, alkaline earth metal salts such as calcium salt
and magnesium salt as well as aluminum salt and
ammonium salt. Preferable salts with organic bases
include salts with, for example, trimethylamine,
triethylamine, pyridine, picoline, 2,6-lutidine,
ethanolamine, diethanolamine, triethanolamine,
cyclohexylamine, dicyclohexylamine and N,N'-
dibenzylethylenediamine. Preferable salts with
inorganic acids include salts with, for example,
hydrochloric acid, hydrobromic acid, nitric acid,
sulfuric acid and phosphoric acid. Preferable salts
with organic acids include salts with, for example,
formic acid, acetic acid, trifluoroacetic acid,
= WO 96/08466 2193398 PGT/JP95/01796
- 27 -
phthalic acid fumaric acid, oxalic acid, tartaric acid,
maleic acid, citric acid, succinic acid, malic acid,
methanesulfonic acid, benzenesulfonic acid and p-
toluenesulfonic acid. Preferable salts with basic
amino acids include salts with, for example, arginine,
lysine and ornithine. Preferable salts with acidic
amino acids include salts with, for example, aspartic
acid and glutamic acid.
Among them, pharmaceutically acceptable salts are
preferable, which are exemplified by, when the compound
(I) or (Ia) has a basic functional group, salts with
inorganic acids such as hydrochloric acid, hydrobromic
acid, nitric acid, sulfuric acid or phosphoric acid,
and, salts with organic acids such as acetic acid,
phthalic acid, fumaric acid, tartaric acid, maleic
acid, citric acid, succinic acid, methanesulfonic acid
and p-toluenesulfonic acid, and, when the compound (I)
or (Ia) has an acidic functional group, alkali metal
salts such as sodium salt and potassium salt, alkaline
earth metal salts such as calcium salt and magnesium
salt, and ammonium salt.
On the production of the compound (I) or salts
thereof of this invention (hereinafter simply referred
to as compound (I)) and the compound (Ia) or salts
thereof (hereinafter simply referred to as compound
(Ia)), the following description is given.
The compound (I) and compound (Ia) can be produced
in accordance with, for example, the reaction scheme-1
or 2. These reaction schema are shown below.
All symbols of the compounds in the reaction
schema are of the same meaning as defined above.
WO 96/08466 21933 9$ PCT/AP95101796 =
- 28 -
Reaction scheme - 1
CN
HO R~
condensation RZ
' ~ (CHp)n
O O Rt
alkylation ~ ~, RZ ; (Xi)
-
(oH,)n ~ A(cHr,~~ dehydration
CN
(II) (III) condensation cc
(IV)
0
NH2 R4 11 N R3
~C~m (CH2)m
R
R2
reduction R
a)n (CH2)n
(V) (i)
WO 96/08466 2193398 PCf/3P95101796
_ 29 _
Reaction scheme-2
c,ooz
- '=
condensation HO R~
~ A ccHZ~
0 eFR
(VI)'~
OH
fAOZ
(III) I R' '. ; R+ = .
Rz- reduction Rz='
condensation ~ A tCH'I I A tca
(VII) (Vlll)
X oORo
N
R~ R'
halogenation Rz substitution Rz='
~ A ccHa~~ ---~ ~ A (IX) (X)
0
4
NH2
R ~N R3
R~ R~
deprotection ' Rz R
M (I)
In the present invention, the compound (II) can be
produced by a paZ Sg known method or a method analogous
thereto, for example, by the method described in
Journal of the Chemical Society, (C), P.990 (1966).
The compound (III) can be produced by a per ~cg
WO 96/08466 91398 PCT/JP95/01796
~t
known method of a method analogous thereto, for
example, by methods described in Journal of Organic
Chemistry, 21, P.27 (1961) and 51, P.1874 (1990),
Journal of-the American Chemical Society, IDI, 3992
(1983), Journal of the Chemical Society Perkin Trans.
I, 3399 (1988), Journal of the Chemical Society (c),
P.217 (1969) and Liebigs Annalen Chemie, P.263 (1987),
or methods analogous thereto.
The compound (V) can be produced by a g= ,e known
method of an analogous method thereto, for example, the
method described in Canadian Journal of Chemistry
P.3681 (1975).
The compound (IV) can be produced by allowing
phosphonate carboanion, produced by processing
alkylphosphonic acid diester with a base, to react with
the compound (III) as a configuration isomer singly or
a mixture of E- and Z-isomers. Relative to 1 mol. of
the compound (III), about 1 to 3 mol., preferably about
1 to 1.5 mol., of alkyl phosphonic acid diester is
used. As the base, is used sodium hydride, sodium
amide or metal alcoholate, for example, in an amount of
about 1 to 3 mol., preferably about 1 to 1.5 mol.
relative to 1 mol. of alkylphosphonic acid diester.
This reaction is conducted advantageously in the
presence of an inert solvent. As the solvent, any one
can be used unless it hampers the proceeding of the
reaction. Preferable examples of the solvent include
alcohols such as methanol, ethanol and propanol,
aromatic hydrocarbons such as benzene, toluene and
xylene, ethers such as tetrahydrofuran, dioxane, 1,2-
dimethoxyethane, diethyl ether and diisopropyl ether,
and amides such as N, N-dimethylformamide, N, N-
dimethylacetamide and hexamethylphosphoramide, and
sulfoxides such as dimethyl sulfoxide. The reaction
time ranges usually from 1 to 24 hours, preferably 1 to
6 hours. The reaction temperatures ranges usually from
= WO 96/08466 219 3 3 98 PCTIJP95l01796
- 31 -
0 to 150 C, preferably from 0 to 100 C. And, the
compound (IV) can also be produced by allowing
carbanion, produced by processing acetonitrile with a
base, to react with the compound (III) and by
subjecting the reaction mixture to dehydration.
Acetonitrile is used in an amount of about 1 to 1.5
mol., preferably equimol. relative to 1 mol. of the
compound (III). As the base, lithium amide (e.g.
lithium diisopropylamide and lithium 1,1,1,3,3,3-
hexamethyldisilazide) is used in an amount of about 1
to 1.5 mol., preferably about 1 to 1.1 mol. relative to
1 mol. of the compound (III). This reaction is
advantageously conducted in the presence of an inert
solvent. As the solvent, any one can be used if only
the reaction proceeds, and ethers such as
tetrahydrofuran, dioxane, 1,2-dimethoxyethane, diethyl
ether and diisopropyl ether for example, are
preferable. The reaction time ranges usually from 30
minutes to 2 hours. The reaction temperature ranges
usually from -78 to 50 C, preferably from -78 to 0 C.
As the catalyst for dehydration, for example, an acid
(e.g. hydrochloric acid, sulfuric acid, phosphoric
acid, potassium hydrogen sulfate, oxalic acid, p-
toluenesulfonic acid, boron trifluoride ether complex),
and a base (e.g. sodium hydroxide and potassium
hydroxide) are used. The dehydration also proceeds by
using, for example, a dehydrating agent such as N,N-
dicyclohexylcarbodiimide, or, alumina, sodium dioxide,
phosphorus oxychloride, thionyl chloride, iodine,
anhydrous copper sulfate and methanesulfonyl chloride.
It is advantageous that this reaction is conducted in
the absence of solvent or in the presence of a solvent
inert to the reaction. As the solvent, any one can be
used so long as the reaction proceeds. Preferable
examples of the solvent include alcohols such as
methanol, ethanol and propanol, ethers such as
CA 02193398 2005-07-12
26456-138
- 32 -
tetrahydrofuran, dioxane, 1,2-dimethoxyethane, diethyl
ether and diisopropyl ether, and aromatic amines such
as pyridine. The reaction time ranges usually from 30
minutes to 6 hours, preferably from 30 minutes to 2
hours. The reaction temperature ranges usually from 0
to 300 C, preferably from 10 to 100 C. The mixture of
isomers of the compound (IV) can be isolated and
purified by conventional separating methods such as
recrystallization, distillation and chromatography to
give a mixture of E- and Z-isomers or a simple
substance of E-isomer or Z-isomer, respectively.
The compound (V) can be produced by subjecting the
compound (IV) or a salt thereof to reduction. This
reduction is conducted by hydrogenation using a metal
hydride (e.g. aluminum hydride or diisobutylaluminum
hydride), a metal hydride complex (e.g. lithium
aluminum hydride or sodium borohydride) or a Raney*
nickel catalyst or a Raney cobalt catalyst. A metal
hydride is used in an amount of about 0.5 to 10 mol.,
preferably about 1.0 to 3.0 mol., relative to 1 mol. of
the compound (IV). A metal hydride complex is used in
an amount of about 0.5 to 10 mol., preferably 1.0 to
3.0 mol., relative to 1 mol of compound (IV). Raney*
catalyst is used in an amound of 10 to 1000 w/w%,
preferably 50 to 300w/w% of the compound (IV). It is
advantageous that this reaction is conducted by using
an inert solvent. As the solvent, any one can be
employed so long as it does not hamper the proceeding
of reaction. Preferable examples of the solvent
include alcohols such as methanol, ethanol and
propanol, aliphatic hydrocarbons such as cyclohexane
and hexane, carboxylic acids such as formic acid and
acetic acid, ethers such as tetrahydrofuran, dioxane,
1,2-dimethoxyethane, diethyl ether and diisopropyl
ether, and amides such as N,N-dimethylformamide, N,N-
dimethylacetamide and hexamethylphosphoramide. When a
*Trade-mark
CA 02193398 2005-07-12
26456-138
- 33 -
Raney nickel catalyst or a Raney cobalt catalyst is
used, it is preferable, in some instances, to
supplement, for example, ammonia or hydrazine to
suppress undesirable side reactions. The reaction time
ranges usually from 1 to 24 hours, preferably from 1 to
6 hours, while varying with the activity and amount of
the reducing agent then employed. The reaction
temperature ranges usually from 0 to 100 C, preferably
from 20 to 50 C. The pressure ranges usually from 1 to
100 kgf/cmZ. The compound (V) can be isolated and
refined by conventional separating methods, for
example, distillation and chromatography to give a
mixture of E- and Z-isomers or a simple substance of E-
isomer or Z-isomer, respectively. And, by employing
adequate conditions, the double bond can be reduced to
single bond simultaneously with the reduction of
nitrile. Further, it is possible that merely the
nitrile of either one of E-isomer or Z-isomer in their
mixture is reduced selectively to give the amine
compound of either one of E-isomer or Z-isomer
selectively.
The compound (I) is produced by allowing the
compound (V) to react with carboxylic acid represented
by R3COOH (R3 is of the same meaning as defined above)
or salt or a reactive derivative thereof. Examples of
the salts include alkali metal salts such as sodium
salt and potassium salt, alkaline earth metal salts
such as calcium salt and magnesium salt, ammonium salt
and organic bases such as trimethylamine,
triethylamine, pyridine and picoline. Examples of the
reactive derivatives of carboxylic acid include acid
halogenides (e.g. acid chlorides and acid bromides),
acid amides (e.g. imidazolides), acid anhydrides, acid
azides, active esters (e.g. N-phthalimidoester and N-
succinimidoester. And, instead of using the reactive
derivative, the carboxylic acid may be allowed to react
*Trade-mark
R'O 96/08466 219339 8 PCI'/JP95/01796
- 34 -
directly with the compound (V). In this case, it is
preferable to allow the reaction to proceed in the
presence of a coupling reagent such as N,N'-
dicyclohexylcarbodiimide or 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (WSC).
The carboxylic acid or a reactive derivative thereof is
used in an amount ranging usually from about 1 to 3
mol., preferably from about 1 to 1.2 mol. relative to 1
mol. of the compound (V). It is advantageous that this
reaction is conducted in the presence of an inert
solvent. As the solvent, any one can be used so long
as it does not hamper the proceeding of the reaction.
Preferable examples of the solvent include ethers such
as tetrahydrofuran, dioxane, 1,2-dimethoxyethane,
diethyl ether and diisopropyl ether, amides such as
N,N-dimethylformamide, N,N-dimethylacetamide and
hexamethylphosphoramide, and halogenohydrocarbons such
as dichloromethane, chloroform, tetrachloromethane and
1,2-dichloroethane. When an acid halogenide is used as
the reactive derivative of carboxylic acid, it is
preferable that an amine, for example, triethylamine,
pyridine or 4-dimethylaminopyridine is previously added
to the reaction system. While the reaction time varies
with the reagent or solvent then employed, it ranges
usually from 30 minute to 24 hours, preferably from 30
minutes to 4 hours. The reaction temperature ranges
usually from 0 to 100 C, preferably from 0 to 70 C.
When the compound (I) is a urea compound, it is also
produced by subjecting the compound (V) to condensation
with an isocyanate compound represented by R3'N=C=O (R3'
has the same meaning as R3 defined above except-NH.).
Usually, 1 to 1.5 mol., preferably equimol. of an
isocyanate-compound is used relative to 1 mol. of the
compound (V). As the solvent, any one can be used so
long as it does not hamper the proceeding of reaction.
Preferable examples of the solvent include aromatic
WO 96108466 2193398 PCT/JP95101796
- '35 -
hydrocarbons such as benzene, toluene and xylene,
ethers such as tetrahydrofuran, dioxane, 1,2-
dimethoxyethane, diethyl ether and diisopropyl ether,
amides such as N,N-dimethylformamide, N,N-
dimethylacetamide and hexamethylphosphoramide,
halogenohydrocarbons such as dichloromethane,
chloroform, carbon tetrachloride and 1,2-
dichloroethane, and ketones such as acetone and methyl
ethyl ketone. While the reaction time varies with the
reagent and solvent then employed, it ranges usually
from 30 minutes to 24 hours, preferably from 30 minutes
to 4 hours. The reaction temperature ranges usually
from 0 to 100 C, preferably from 0 to 70 C. A mixture
of isomers of the compound (I) can be isolated and
refined by conventional methods, for example,
recrystallization, distillation or chromatography to
give a mixture of E- and Z-isomers or a simple
substance of E-isomer or Z-isomer, respectively.
The compound (VI) shown in the reaction scheme - 2
can be produced by allowing carbanion, which is
produced by subjecting a compound represented by
CH3COOZ (Z represents an optionally substituted
hydrocarbon group) to processing with a base, to react
with the compound (III). The "optionally substituted
hydrocarbon group" by shown Z includes, for example,
the above-mentioned "optionally substituted hydrocarbon
group". Relative to 1 mol. of the compound (III), the
compound of CH3COOZ is used in an amount of about 1 to
1.5 mol., preferably equimol. As the base, lithium
amide (e.g. lithium diisopropylamide and lithium
1,1,1,3,3,3-hexamethyldisilazide) is used in an amount
of about 1 to 1.5 mol., preferably about 1 to 1.1 mol.
relative to 1 mol. of the compound (III). This
reaction is advantageously conducted using a solvent
inert to the reaction. As the solvent, while any one
can be used, so long as it does not hamper the
WO 96108466 2 193 3 9 g PCTlJP95/01796
- 36 -
proceeding of reaction, ethers such as tetrahydrofuran,
dioxan, diethyl ether anddiisopropylether, for
example, are preferably used. The reaction time ranges
usually from 30 minutes to 6 hours, preferably from 30
minutes to 2 hours. The reaction temperature ranges
usually from -78 to 50 C, preferably from -78 to 0 C.
The compound (VII) can be produced by processing
the compound (VI) to cause dehydration to proceed. As
the catalyst for dehydration, for example an acid (e.g.
hydrochloric acid sulfuric acid, phosphoric acid,
potassium hydrogen sulfate, oxalic acid, p-
toluenesulfonic acid, 10-camphorsulfonic acid and boron
trifluoride ether complex) and a base (e.g. sodium
hydroxide and potassium hydroxide) are used. And,
depending on cases, dehydration also proceeds by the
use of a dehydrating agent such as N,N-
dicyclohexylcarbodiimide, or by the use of alumina,
sodium dioxide, phosphorus oxychloride, thionyl
chloride, iodine, anhydrous copper sulfate and
methanesulfonyl chloride. It is advantageous that this
reaction is conducted in the absence of solvent or
using a solvent inert to the reaction. As the solvent,
while any one can be employed, so long as it does not
hamper the proceeding of reaction. Preferable examples
of the solvent include alcohols such as methanol,
ethanol and propanol, ethers such as tetrahydrofuran,
dioxane, 1,2-dimethoxyethane, diethyl ether and
diisopropyl ether, and aromatic amines such as
pyridine. The reaction time ranges usually from 30
minutes to 6 hours, preferably from 30 minutes to 2
hours. The reaction temperature ranges usually from
room temperature (10 35 C) to 300 C, preferably from
room temperature to 100 C.
And, the compound (VII) can be produced by
allowing phosphonate carbanion, produced by processing
alkylphosphonic diester.with a base, to react with the
21 9339,8
WO 96/08466 PGTlJP95/01796
~ - 37 -
compound (III), as the respective isomers singly or a
= mixture of E- and Z-isomers. Relative to 1 mol. of the
compound (III), alkylphosphonic diester is used in an
amount of about 1 to 3 mol., preferably about 1 to 1.5
mol. As the base, for example, sodium hydride, sodium
amide and metal alcoholate is used in an amount of
about 1 to 3 mol., preferably about 1 to 1.5 mol.
relative to 1 mol. of alkylphosphonic diester. This
reaction is advantageously conducted by the use of an
inert solvent. As the solvent, any one can be used, so
long as it does not hamper the proceeding of reaction.
Preferable examples of the solvent include alcohols
such as methanol, ethanol and propanol, aromatic
hydrocarbons such as benzene, toluene and xylene,
ethers such as tetrahydrofuran, dioxane, 1,2-
dimethoxyethane, diethyl ether and diisopropyl ether,
and sulfoxides such as dimethylsulfoxide. The reaction
time ranges usually from 1 to 24 hours, preferably from
1 to 6 hours. The reaction temperature ranges usually
from 0 to 150 C, preferably from 0 to 100 C. A mixture
of isomers of the compound (VII) can be isolated and
purified by conventional separating methods such as
recrystallization, distillation and chromatography to
give a mixture of E- and Z-isomers or a simple
substance of E-isomer or Z-isomer, respectively.
The compound (VIII) is produced by subjecting the
compound (VII) to reduction.
The compound (VIII), in which the ester group is
reduced, can be produced by processing the compound
(VII) with a metal hydride (e.g. aluminum hydride and
diisobutylaluminum hydride) or a metal hydride complex
compound (e.g. lithium aluminum hydride and sodium
borohydride) in an amount of about 1 to 3 mol.,
preferably about 1 to 1.2 mol. relative to 1 mol. of
the compound (VII). This reaction is advantageously
conducted by the use of an inert solvent. As the
WO 96/08466 2193398 PCTJJP95/01796
- 38 -
solvent, any one can be used, so long as it does not
hamper the proceeding of reaction. Preferable examples
of the solvent include alcohols such as methanol,
ethanol and propanol, aromatic hydrocarbons such as
benzene, toluene and xylene, aliphatic hydrocarbons
such as cyclohexane and hexane, ethers such as
tetrahydrofuran, dioxane, 1,2-dimethoxyethane, diethyl
ether and diisopropyl ether, and halogenohydrocarbons
such as dichloromethane, chloroform, carbon
tetrachloride and 1,2-dichloroethane. The reaction
time ranges usually from 30 minutes to 12 hours,
preferably from 30 minutes to 4 hours. The reaction
temperature ranges usually from -20 to 150 C,
preferably from -20 to 80 C A mixture of isomers of
the compound (VIII) can be isolated and purified by a
conventional separating methods such as
recrystallization, distillation and chromatography to
give a mixture of E- and Z-isomers or a simple
substance of E-isomer or Z-isomer, respectively.
The compound (IX) can be produced by allowing a
halogenide to react with the compound (VIII).
Preferable examples of the halogenide include hydrogen
halogenide (e.g. hydrogen bromide and hydrochloric
acid), phosphorus halogenide (e.g. phosphorus
pentachloride, phosphorus trichloride and phosphorus
tribromide), thionyl halogenide (e.g. thionyl chloride
and thionyl bromide) and halogenide with phosphine
(e.g. carbon tetrabromide or carbon tetrachloride with
triphenylphosphine). The halogenide is used in an
amount of 0.2 to 5.0 mol., preferably 0.5 to 2.0 mol.
relative to 1 mol. of the compound (VIII). Preferable
examples of the solvent include aromatic hydrocarbons
such as benzene, toluene and xylene, ethers such as
tetrahydrofuran, dioxane, 1,2-dimethoxyethane, diethyl
ether and diisopropyl ether and halogenohydrocarbons
such as dichloromethane, chloroform,
WO 96/08466 21O33(~ Q PGT/dP95/01796
- 379 - /v
carbontetrachloride and 1,2-dichloroethane. The
reaction time ranges usually from 30 minutes to 12
hours, preferably from 30 minutes to 3 hours. The
reaction temperature ranges usually from room
temperature to 150 C, preferably from room temperature
to 100 C. A mixture of isomers of the compound (IX)
can be isolated and purified by conventional separating
methods such as recrystallization, distillation and
chromatography to give a mixture of E- and Z-isomers or
a simple substance of E-isomer or Z-isomer,
respectively.
The compound (X) can be produced by subjecting 1
mol of the compound (IX) to condensation with about 1
to 5 mol., preferably about 1 to 1.2 mol., of potassium
phthalimide. The condensation is advantageously
conducted, when desired, in the presence of a base, in
the absence of a solvent or using an inert solvent.
Preferable examples of the base include triethylamine,
sodium amide, sodium hydride, sodium alkoxide and
lithium diisopropylamide. As the solvent, any one can
be used, so long as it does not hamper the proceeding
of reaction. Preferable examples of the solvent
include alcohols such as methanol, ethanol and
propanol, aromatic hydrocarbons such as benzene,
toluene and xylene, ethers such as tetrahydrofuran,
dioxane, 1,2-dimethoxyethane, diethyl ether and
diisopropyl ether, and halogenohydrocarbons such as
dichloromethane, chloroform, carbon tetrachloride and
1,2-dichloroethane. The reaction time ranges usually
from 30 minutes to 12 hours, preferably from 30 minutes
to 4 hours. The reaction temperature ranges usually
from -5 to 150 C, preferably from 5 to 80 C. A mixture
of isomers of the compound (IX) can be isolated and
purified by conventional separating methods such as
recrystallization, distillation and chromatography to
give a mixture of E- and Z-isomers or a simple
WO 96108466 L ! 93398 PCr/3P95l01796 =
- 40 -
substance of E-isomer or Z-isomer, respectively.
The compound (V) can be produced by using,
relative to 1 mol. of the compound (X), amines (e.g.
methylamine and ethylamine), hydrazines (e.g.
hydrazine, phenylhydrazine), alkali sultides (e.g.
sodium and potassium), and a mineral acids (e.g.
hydrochloric acid and sulfuric acid) in an amount of
usually from about 1 to 20 mol., preferably from about
1 to 5 mol. It is advantageous that this reaction is
conducted using a solvent inert to the reaction. As
the solvent, any one can be used, so long as it does
not hamper the proceeding of reaction. Preferable
examples of the solvent include alcohols such as
methanol, ethanol and propanol, and ethers such as
tetrahydrofuran, dioxane, 1,2-dimethoxyethane, diethyl
ether and diisopropyl ether. The reaction time ranges
usually from 30 minutes to 12 hours, preferably from 30
minutes to 4 hours. The reaction temperature ranges
usually from room temperature to 150 C, preferably from
room temperature to 100 C. A mixture of isomers of the
compound (V) can be isolated and purified by
conventional methods such as distillation and
chromatography to give a mixture of E- and Z-isomers or
a simple substance of E-isomer or Z-isomer,
respectively.
The compound (V) can be led to the compound (I) by
substantially the same procedure as described in the
above-mentioned reaction scheme - 1. .
And, in each of the reactions described above,
when the starting compound has amino group, carboxyl
group or hydroxyl group as a substituent, these groups
may be protected with a protective group generally used
in the field of peptide chemistry. After completion of
the reaction, these protective groups may be removed
depending on necessity to give the object compound.
Examples of amino-protective groups include formyl
~ WO 96/08466 2193398 P~/jP9SI01796
- 41 -
group or an optionally substituted C1_6 alkyl carbonyl
(e.g. acetyl and propionyl), phenylcarbonyl, Cj_6 alkyl-
oxycarbonyl (e.g. methoxycarbonyl and ethoxycarbonyl),
phenyloxycarbonyl, C7_10 aralkyl-oxycarbonyl (e.g.
benzylcarbonyl), trityl and phthaloyl groups. Examples
of these substituents include halogen atoms (e.g.
fluorine, chlorine, bromine and iodine), C1_6 alkyl-
carbonyl (e.g. acetyl, propionyl and valeryl) and
nitro, and the number of these substituents ranges from
about 1 to 3.
Examples of carboxyl-protective groups include
optionally substituted Ci_6 alkyl (e.g. methyl, ethyl,
propyl, isopropyl, butyl and tert-butyl), phenyl,
trityl and silyl groups. As these substituents, for
example, halogen atoms (e.g. fluorine, chlorine,
bromine and iodine), formyl, Ci_6 alkyl-carbonyl (e.g.
acetyl, propionyl and butyl carbonyl), Ci_6 alkyl (e.g.
methyl, ethyl and tert-butyl), C6_10 aryl (e.g. phenyl
and naphthyl) and nitro group, and the number of these
substituents ranges from about 1 to 3.
Examples of hydroxyl-protective groups include
optionally substituted C1_6 alkyl (e.g. methyl, ethyl,
propyl, isopropyl, butyl and tert-butyl), phenyl, C7_10
aralkyl (e.g. benzyl), formyl, C1_6 alkyl-carbonyl (e.g.
acetyl and propionyl), phenyloxycarbonyl, C7_11 aralkyl-
oxycarbonyl (e.g. benzyloxycarbonyl),
tetrahydropyranyl, tetrahydrofuranyl and silyl groups.
As these substituents, for example, halogen atoms (e.g.
fluorine, chlorine, bromine and iodine), C1_6 alkyl,
(e.g. methyl, ethyl and tert-butyl) C7_11 aralkyl (e.g.
benzyl) C6-10 aryl (e.g. phenyl and naphthyl) and nitro
group are used, and the number of these substituents
' ranges from about 1 to 4.
For removing these protective groups, a per ~g
known method or analogous methods thereto are employed.
WO 96/08466 2 193 3 g g PCT/JP95/01796
- 42 -
For example, methods using acid, base, ultra-violet
ray, hydrazine, phenylhydrazine, sodium N-methyl =
dithiocarbamate, tetrabutylammonium fluoride and
palladium acetate or reduction.
Starting compounds of the above-mentioned compound
(I) of the present invention may be in the form of a
salt. While there is no specific restriction on kinds
of the salt, so long as the reaction proceeds, are
used, for example, substantially the same salts as
those which the above-mentioned compound (I) may form.
Referring to the configurational isomers (E- and
Z-isomers) of the compounds (I), (IV), (V), (VII),
(VIII), (IX) and (X), when such isomerization takes
place, the isomers can be isolated and purified by a
conventional separating methods such as extraction,
recrystallization, distillation and chromatography to
give pure compounds. And, in accordance with the
methods described in "Shin Jikken Kagaku Koza" 14
(compiled by The Chemical Society of Japan), pp.251-
253, "Fourth Edition Jikken Kagaku Koza" 19 (compiled
by The Chemical Society of Japan), pp.273-274 and
analogous methods thereto, isomerization of the double
bond is allowed to proceed by heating, using an acid
catalyst, a metal catalyst, a radical catalyst, light
irradiation or using a strongly basic catalyst to give
the corresponding pure isomer.
Incidentally stating, the compound (I) gives rise
to stereoisomers depending of the kinds of
substituents, and these isomers, singly or as a mixture
of them, are included in the present invention.
In any of such cases, when further desired, the
compound (I) can be synthesized by deprotection,
acylation, alkylation, hydrogenation, oxidation,
reduction, carbon chain elongation, substituent-
exchange reaction singly or by combination of two or
more of them.
WO 96/08466 219 3 3 9 8 pCr/,Tp95/01796
=
- 43 -
In the case where the object compound is obtained
in the free form by the above reaction, it may
optionally be converted into a corresponding salt by
conventional methods, and, in the case where the object
compound is obtained as a salt, it can be converted
into the free form or any other salt. The obtained
compound (I) can be isolated from the reaction mixture
and purified by conventional methods such as phasic
transfer, concentration, solvent-extraction, fractional
distillation, crystallization, recrystallization and
chromatography.
Additionally stating, in the case where the
compound (I) is present as, for example,
configurational isomer, diastereomer or conformer, they
can be isolated, when desired, respectively by the
above-mentioned isolation and purification means. And,
when the compound (I) is a racemic compound, it can be
resolved into d-isomer and 1-isomer by a conventional
means for optical resolution.
Effects
The compound (I) and (Ia) shows a high affinity
for the melatonin receptor, and is less in toxicity and
undesirable side effects, which is thus useful as a
medicine.
The compound (I) and (Ia) acts, as a melatonin
agonist or antagonist, in mammals (e.g. mouse, rat,
hamster, rabbit, cat, dog, cow, sheep, monkey and man),
and, therefore, it can be used as a pharmaceutical
composition having a binding affinity to the melation
receptor, especially, melatoninagonist or-antagonist,
for the therapy of diseases possibly affected by
melatonin, for example, sleep-awake rhythm disorders,
jet lag, abnormal physical conditions caused by a
three-shift labor system, seasonal melancholia,
disorders in reproduction and neurosecretion, senile
dementia including Alzheimer's disease, various
WO 96/08466 2193398 PCT11P95/01796
- 44 -
disorders accompanied with aging, cerebral circulation
disorders, stress, epilepsy, convulsion, anxiety,
parkinsonism, hypertension, glaucoma, cancer and
insomnia, or it can be used as an agent of controlling
ovulation. Especially the compound (I) and (ia) can be
used as a therapeutic agent for insomnia and circadian
rhyzm disturbance including sleep-awake rhyzm
disturbance (e.g. jet lag and insomnia of shift
workers).
The compound (I) and (Ia) can be safely
administered orally or non-orally as it is or as
medicinal preparations mixed with a pharmaceutically
acceptable carriers in accordance with a per -g known
method, for example, tablets (including sugar-coated
tablets and film-coated tablets), powdery preparations,
granular preparations, capsules (containing soft-
capsules), liquid preparations, injectable
preparations, suppository preparations, sustained
release preparations, plasters and chewing gum.
The amount of the compound (I) or (Ia) in the
composition of the present invention is about 0.01 to
100 w/w %. The daily dose varies with, for example,
subjects to be administered, administration routes and
diseases to be treated, and, it is preferable, when
administered to, for example, an adult patient
suffering from sleep disorders, to administer once
daily or severally divided dosages in an amount ranging
from about 0.1 mg/kg to 20 mg/kg body weight,
preferably from 0.2 mg/kg to 10 mg/kg body weight, more
preferably from 0.5 mg/kg to 10 mg/kg body weight, in
terms of the effective component (the compound (I) or
(Ia)).
The compound (I) or (Ia) may be used with another
active component, for example, benzodiazepine such as
triazolam, regulating agents of sleep rhythm such as
butoctamide and salts thereof, sleep inducing
WO 96/08466 219 3 3 9 8 PCT/,TP95101796
- 45 -
substances such as cis-9,10-octadecenamide. The
compound (I) or (Ia) can be used as a pharmaceutical
composition, for example, tablet, powdery preparation,
granular preparation, capsule (containing soft
capsule).
As pharmaceutically acceptable carriers, various
organic or inorganic carriers, which are conventionally
employed in the field of formulation of pharmaceutical
preparations, can be used, and they are incorporated as
excipients, lubricants, binders and disintegrants in
solid compositions; and as solvents, solubilizers,
suspending agents, isotonizing agent, buffering agent
and pain-easing agents in liquid compositions. And,
depending on necessity, further additives such as
preservatives, anti-oxidants, coloring agent and
sweeteners can also be supplemented. Preferable
examples of excipients include lactose, sugar, D-
mannitol, starch, crystalline cellulose and more
volatile silicon dioxide. Preferable examples of
lubricants include magnesium stearate, talc and colloid
silica. Preferable examples of binders include
crystalline cellulose, sugar, D-mannitol, dextrin,
hydroxypropyl cellulose, hydroxypropylmethyl cellulose
and polyvinyl pyrrolidone. Preferable examples of
disintegrants include starch, carboxymethyl cellulose,
carboxymethyl cellulose calcium, cross carmelose sodium
and carboxymethyl starch sodium. Preferable examples
of solvents include water for injection, alcohol,
propylene glycol, macrogol, sesame oil and corn oil.
Preferable examples of solubilizers include
polyethylene glycol, propylene glycol, D-mannitol,
benzyl benzoate, ethanol, tris-aminomethane,
cholesterol, triethanolamine, sodium carbonate and
sodium citrate. Preferable examples of suspending
agents include surfactants such as stearyl
triethanolamine, sodium lauryl sulfate, lauryl
WO 96108466 2193398 PCTIJP95/01796 =
-4fi-
aminopropionic acid, lecithin, benzalkonium chloride,
benzetonium chloride and monostearic glyceryl ester;
and hydrophilic polymers such as polyvinyl alcohol,
polyvinyl pyrrolidone, sodium carboxymethyl cellulose,
methyl cellulose, hdyroxymethyl cellulose, hydroxyethyl
cellulose and hydroxypropyl cellulose. Preferable
examples of isotonizing agents include sodium chloride,
glycerin and D-mannitol. Preferable examples of
buffering agents include buffering solutions such as
phosphate, acetate, carbonate and citrate. Preferable
examples of pain-easing agents include benzyl alcohol.
Preferable examples of preservatives include para-
hydroxybenzoic acid esters, chlorobutanol, benzyl
alcohol, phenethyl alcohol, dehydroacetic acid and
sorbic acid. Preferable examples of anti-oxidants
include sulfite, ascorbic acid and a-tocopherol.
BEST MODE FOR CARRYING OUT THE INVENTION
Examples
The present invention will be described in further
detail by the following Reference Examples, Working
Examples and Experimental Examples, but they are mere
examples and are not intended by way of limitation upon
the scope of this invention, and they may be modified
within the range which does not deviate the scope of
this invention.
In the following Working Examples, Reference
Examples and Experimental Examples, "room temperatures"
means 0 to 30 C, and other definitions have the
following meanings.
s: singlet
d: doublet
t: triplet
q: quartet
m: multiplet
br: broad
= WO 96108466 21+ 33/ 8 PCT/JP95/01796
- 47 -
J: coupling constant
Hz: Hertz
CDC13: deuterochloroform
D20: deuterium oxide
THF: tetrahydrofuran
DMF: N,N-dimethylformamide
d6-DMSO: (dimethylsulfoxide)-d6
NMR: proton-nuclear magnetic resonance
Reference Example 1
(1,2,3,4-tetrahydro-7-methoxy-l-
naphthylidene)acetonitrile
To a solution of 60% sodium hydride (6.24 g, 156
mmol) in tetrahydrofuran (100 ml) was gradually added
dropwise, under ice-cooling, diethyl cyanomethyl
phosphonate (30.4 g, 172 mmol). The mixture was
stirred for 15 minutes. To the reaction mixture was
then added dropwise a solution of 7-methoxy-l-tetralone
(25.2 g, 143 mmol) in THF (50 ml). The reaction
mixture was heated for 3 hours under reflux. The
reaction mixture was poured into water, and the organic
layer was subjected to extraction with chloroform. The
extract solution was washed with brine and water, which
was dried over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was purified by means of a silica gel column
chromatography (hexane:ethyl acetate=9:1) to give the
above-titled compound (a mixture of isomers) (28 g,
yield 98%, oil). The above-titled compound (a mixture
of isomers) was further purified by a silica gel column
chromatography (hexane:ethyl acetate=98:2) to
fractionally refine the respective isomers.
E-isomer
m.p. 59-61 C
NMR(CDC13)S: 1.85-2.00(2H,m), 2.76-2.91(4H,m),
3.81(3H,s), 5.70(1H,s), 6.91(1H,dd,J=2.6Hz,8.4Hz),
7.03(1H,d,J=2.6Hz), 7.10(1H,d,J=8.4Hz).
WO 96108466 219 3398 PCf/JP95l01796
- 48 -
Z-isomer
oil
NMR(CDC13)6: 1.87-2.02(2H,m), 2.54-2.64(2H,m),
2.83(2H,t,J=6.2Hz), 3.85(3H,s), 5.28(1H,s),
6.93(1H,dd,J=2.6Hz,8.4Hz), 7.09(1H,d,J=2.6Hz),
7.86(1H,d,J=8.4Hz).
Reference Example 2
(Z)-(1,2,3,4-Tetrahydro-7-methoxy-l-
naphthylidene)acetonitrile
To a solution of (1,2,3,4-tetrahydro-7-methoxy-l-
naphthylidene)acetonitrile (4.0 g, 20 mmol) in ethanol
(10 ml) were added a saturated ammonia/ethanol solution
(5 ml) and Raney cobalt (ODHT-60, 1 g). The reaction
mixture was stirred for 3.5 hours at room temperature
under hydrogen atmosphere (about 4 kgf/cmZ). The Raney
cobalt was filtered off, then the solvent was distilled
off under reduced pressure. To the residue was added
dilute hydrochloric acid, then the organic layer was
subjected to extraction with ethyl acetate. The
extract solution was washed with a saturated aqueous
solution of sodium hydrogencarbonate and water, which
was dried over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was purified by means of a silica gel column
chromatography to give the above-titled compound (yield
2.14 g, oil).
NMR(CDC13)S: 1.87-2.02(2H,m), 2.54-2.64(2H,m),
2.83(2H,t,J=6.2Hz), 3.85(3H,s), 5.28(1H,s),
6.93(1H,dd,J=2.6Hz,8.4Hz), 7.09(1H,d,J=2.6Hz),
7.86(1H,d,J=8.4Hz).
Reference Example 3-A
1-(2-Aminoethylidene)-7-methoxy-1,2,3,4-
tetrahydronaphthalene
To a solution of (1,2,3,4-tetrahydro-7-methoxy-i-
naphthylidene)acetonitrile (15.0 g, 25 mmol) in ethanol
(100 ml) were added a saturated ammonia/ethanol
WO 96108466 2~ 93Z 98 PCT/JP95101796
- 49 -/
solution (30 ml) and Raney nickel (W-2, 3 g). The
' reaction mixture was stirred for 8 hours at 50 C under
hydrogen atmosphere (3-4 kgf/cmZ). The Raney nickel
was filtered off, then the solvent was distilled off
under reduced pressure to give the above-titled
compound (a mixture of isomers (20.4 g, yield 80%,
oil). This compound was used in the subsequent
reaction without further purification.
Reference Example 3-B
1-(2-Aminoethylidene)-7-methoxy-1,2,3,4-
tetrahydronaphthalene
To a solution of (Z)-(1,2,3,4-tetrahydro-7-
methoxy-l-naphthylidene)acetonitrile produced in
Reference Example 2 (1.84 g, 9.23 mmol) in ethanol (10
ml) were added a saturated ammonia/ethanol solution (5
ml) and Raney cobalt (ODHT-60, 1.8 g). The mixture was
stirred for 3 hours at room temperature under hydrogen
atmosphere (about 4 kgf/cmZ). The Raney cobalt was
filtered off, then the solvent was distilled off to
give the above-titled compound (a mixture of isomers)
(yield 1.46 g, 78%, oil). This compound was used in
the subsequent reaction without further purification.
Reference Example 4
(6-Methoxyindan-1-ylidene)acetonitrile
To a solution of 60% sodium hydride (2.71 g, 67.9
mmol) in THF (150 ml) was added dropwise, under ice-
cooling, diethyl cyanomethylphosphonate (11.5 g, 64.8
mmol). The mixture was stirred for 15 hours, to which
was then added dropwise a solution of 6-methoxy-l-
indanone (10.0 g, 61.7 mmol) in THF (30 ml). The
reaction mixture-was stirred for 30 minutes at room
temperature. The reaction mixture was poured into
water, and the organic layer was subjected to
extraction with ethyl acetate. The extract solution
was washed with brine and water, followed by drying
over anhydrous magnesium sulfate. The solvent was then
WO 96108466 21C) 33C~ 8 PCP7JP95/01796
-I /50 -
distilled o_ff under reduced pressure.- The residue was
purified by means of a silica gel column chromatography
(hexane:ethyl acetate=8:2). Recrystallization of the
product from ethyl acetate/hexane afforded the above-
titled compound (6.00 g, yield 53%).
m.p.95-96 C (recrystallized from ethyl acetate/hexane)
NMR(CDC13)S: 3.01-3.18(4H,m), 3.83(3H,s),
5.61(1H,t,J=2.4Hz), 6.96-7.03(2H,m),
7.27(1H,d,J=8.8Hz).
Reference Example 5
1-(2-Aminoethyl)-6-methoxyindan
To a solution of (6-methoxyindan-1-ylidene)
acetonitrile (4.00 g, 21.6 mmol) in ethanol (80 ml)
were added a saturated ammonia/ethanol solution (40 ml)
and Raney nickel (W-2, 4 g). The reaction mixture was
stirred, under hydrogen atmosphere (3 kg f/cm2), for 5
hours at room temperature, then for 2 hours at 50 C.
The Raney nickel was filtered off, then the solvent was
distilled o_f.f under reduced pressure. The residue was
purified by means of a silica-gel column chromatography
(chloroform:methano1=97.3 to
chloroform:methanol:triethylamine=90:7:3) to give the
above-titled compound (3.30 g, yield 80%, oil).
NMR(CDC13)S: 1.50-1.76(2H,m), 1.90-2.08(1H,m), 1.22-
1.34(1H,m), 2.65-3.20(5H,m), 3.79(3H,s),
6.71(1H,dd,J=2.6Hz,8.2Hz), 6.76(1H,br s),
7.12(1H,d,J=8.2Hz).
Reference Example 6
1-Cyanomethyl-l-hydroxy-7-methoxy-2,2-dimethyl-
1,2,3,4-tetrahydronaphthalene
To a solution of 1,1,1,3,3,3-hexamethyldisilazane
(4.74 g, 29.4 mmol) in THF (30 ml) was gradually added
dropwise, at -78 C, a butyllithium hexane solution
(1.56 M hexane solution, 18.8 ml, 29.4 mmol). The
mixture was stirred for 10 minutes, to which was then
added acetonitrile (1.41 ml, 26.9 mmol). The reaction
WO 96/08466 2193J! 4J PCIYJP95l01796
- 51 -
inixture was stirred for further 20 minutes, to which
was then added dropwise a solution of 7-methoxy-2,2-
dimethyl-l-tetralone (5.0 g, 24.5 mmol) in THF (10 ml),
followed by stirring for 2 hours. The reaction mixture
was poured into water, and the organic layer was
subjected to extraction with chloroform. The extract
solution was washed with brine and water, which was
dried over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was purified by means of a silica gel column
chromatography (ethyl acetate:hexane=2:8) to give the
above-titled compound (5.3 g, yield 88%).
m.p.107-108 C
NMR(CDC13)S: 0.97(3H,s), 1.15(3H,s), 1.60-1.95(2H,m),
1.97(1H,s), 2.69-2.98(4H,m), 3.83(3H,s),
6.81(1H,dd,J=2.7Hz,8.4Hz), 7.00(1H,d,J=8.4Hz),
7.34(1H,d,J=2.7Hz).
Elemental Analysis for C15H19NOz:
Calcd.: C, 73.44; H, 7.81; N, 5.71.
Found : C, 73.64; H, 7.74; N, 5.83.
Reference Example 7
(1,2,3,4-Tetrahydro-7-methoxy-2,2-dimethyl-l-
naphthylidene)acetonitrile
To a solution of 1-cyanomethyl-l-hydroxy-7-
methoxy-2,2-dimethyl-1,2,3,4-tetrahydronaphthalene
(5.27 g, 21.5 mmol) in toluene (50 ml) was added
camphor sulfonic acid (0.5 g, 2.15 mmol). The mixture
was heated for one hour under reflux. The reaction
mixture was poured into a saturated aqueous solution of
sodium hydrogencarbonate, and the organic layer was
subjected to extraction with chloroform. The extract
solution was washed with brine and water, which was
dried over magnesium sulfate, followed by distilling
off the solvent under reduced pressure. The residue
was purified by means of a silica gel column
chromatography (ethyl acetate:hexane=2:8) to give the
WO 96/08466 219 3 98 PCT/JP95/01796
- 52 -
Above-titled compound (a mixture of isomers) (4.88 g,
quantitative).
NMR(CDC13)S: 1.16(4H,s,Z-isomer), 1.52(2H,s,E-isomer),
1.66(0.67H,t,J=6.6Hz,E-isomer), 1.75(1.33H,t,J=6.6Hz,Z-
isomer), 2.70(0.67H,t,J=6.6Hz,E-isomer),
2.83(1.33H,t,J=6.6Hz,Z-isomer), 3.81(1H,s,E-isomer),
3.86(2H,s,Z-isomer), 5.37(0.67H,s,Z-isomer),
5.70(0.33H,s,E-isomer), 6.87-7.12(2.33H,m,E-isomer+Z-
isomer), 7.64(0.67H,d,J=2.6Hz,Z-isomer).
Reference Example 8
1-(2-Aminoethylidene)-7-methoxy-2,2-dimethyl-
1,2,3,4-tetrahydronaphthalene
By substantially the same manner as in Reference
Example 3-B, a mixture of isomers of the above-titled
compound was produced from (1,2,3,4-tetrahydro-
7-methoxy-2,2-dimethyl-l-naphthylidene)acetonitrile
(yield 99%).
NMR(CDC13)S: 1.11(4H,s,Z-isomer), 1.30(2H,s,E-isomer),
1.56(0.67H,t,J=6.9Hz,E-isomer), 1.66(1.33H,t,J=6.9Hz,Z-
isomer), 2.61(0.67H,t,J=6.9Hz,E-isomer),
2.75(1.33H,t,J=6.9Hz,Z-isomer), 3.63(2H,d,J=6.8Hz,Z-
isomer), 3.70(1H,d,J=6.8Hz,E-isomer), 3.80 and
3.81(3H,sX2,E-isomer+Z-isomer), 5.54(0.67H,t,J=6.4Hz,Z-
isomer), 5.89(0.33H,d,J=7.1Hz,E-isomer), 6.68-
7.10(3H,m,E-isomer+Z-isomer).
Reference Example 9
(2-Methoxy-6,7,8,9-tetrahydro-SH-benzocyclohepten-
9-ylidene)acetonitrile
By substantially the same manner as in Reference
Example 1, the above-titled compound was produced from
2-methoxy-6,7,8,9-tetrahydro-SH-benzocyclohepten-9-one.
This compound was used in the subsequent reaction
without purification.
Reference Example 10
(E)-9-(2-Aminoethylidene)-2-methoxy-6,7,8,9-
tetrahydro-5H-benzocycloheptene
WO 96/08466 2193398 PCT7JP95101796
- 53 -
To a solution of (2-methoxy-6,7,8,9-tetrahydro-5H-
benzocyclohepten-9-ylidene)acetonitrile (2.00 g, 9.38
mmol) in ethanol (10 ml) were added a saturated
solutioin of ammonia/ethanol (5 ml) and Raney nickel (W-
2, 2 g). The mixture was stirred for 4 hours at 50 C
under hydrogen atmosphere (4 kgf/cm2). The Raney
nickel was filtered off, then the solvent was distilled
off under reduced pressure. The residue was purified
by means of a silica gel column chromatography
(chloroform:methanol=9:1 to
chloroform:methanol:triethylamine=90:8:2) to give the
above-titled compound (1.50 g, yield 74%, oil).
NMR(CDC13)S: 1.60-1.75(4H,m), 2.33-2.41(2H,m), 2.61-
2.72(2H,m), 3.47(2H,d,J=7.OHz), 3.79(3H,s),
5.53(1H,t,J=6.6Hz), 6.66-6.75(1H,m), 6.96-7.02(1H,m),
7.14-7.17(1H,m).
Reference Example 11
(E)-1-(2-Aminoethylidene)-6-methoxyindan
To a solution of (6-methoxyindan-1-ylidene)
acetonitrile (1.60 g, 8.64 mmol) in ethanol (80 ml)
were added a 2M ammonia/ethanol solution (40 ml) and
Raney cobalt (1.6 g). The mixture was stirred, under
hydrogen atmosphere (4 kgf/cmZ), for 32 hours at 40 C,
and for further 8 hours at 70 C. The Raney cobalt was
filtered off, then the solvent was distilled off under
reduced pressure. The residue was purified by means of
a silica gel column chromatography
(chloroform:methano1=9:1 to
chloroform:methanol:triethylamine=90:8:2) to give the
above-titled compound (yield 1.40 g, 86%, oily).
NMR(CDC13)6: 2.70-2.80(2H,m), 2.89-2.97(2H,m),
3.48(2H,d,J=6.6Hz), 3.81(3H,s), 5.91-6.01(lH,m),
6.77(1H,dd,J=2.4Hz,8.2Hz), 6.96(1H,d,J=2.4Hz),
7.13(1H,d,J=8.2Hz).
Reference Example 12
(5,6-Dimethoxyindan-1-ylidene)acetonitrile
WO 96/08466 2193398 PCTIJP95101796
- 54 -
In substantially the same manner as in Reference
Example 1, the above-titled compound was produced from
5,6-dimethoxy-indanone. The product was used in the
subsequent reaction without purification.
Reference Example 13
1-(2-Aminoethyl)-5,6-dimethoxyindan hydrochloride
To a solution of (5,6-dimethoxyindan-1-ylidene)
acetonitrile (1.70 g, 7.90 mmol) and Raney nickel (1.7
g) in ethanol (80 ml) was added 2M ammonia/ethanol
solution (40 ml). The mixture was stirred for 12 hours
at 50 C under hydrogen atmosphere (3.2 kgf/cm2). The
Raney cobalt was filtered off, and then the solvent was
distilled off. The residue was dissolved in ethanol
(25 mL), to which was added 5% palladium-carbon (1 g,
content 50%). The mixture was stirred for 1.5 hours at
room temperature under hydrogen atmosphere. The
palladium-carbon was filtered off. To the filtrate was
added 10% HC1/ethanol, and the mixture was
concentrated. The concentrate was recrystallized from
ethyl acetate-isopropyl ether to give the above-titled
compound (0.90 g, yield 44%).
m.p.175-179 C (decomp.)
NMR(CDC13)6: 1.59-1.97(2H,m), 2.18-2.40(2H,m), 2.77-
2.86(2H,m), 3.05(2H,t,J=8.OHz), 3.18(1H,br s),
3.84(3H,s), 3.85(3H,s), 6.73(1H,s), 6.75(1H,s).
Reference Example 14
1-(2-Aminoethyl)-7-methoxy-2,2-dimethyl-1,2,3,4-
tetrahydronaphthalene hydrochloride
To a solution of 1-(2-aminoethylidene)-7-methoxy-
2,2-dimethyl-1,2,3,4-tetrahydronaphthalene (10.2 g,
44.1 mmol) in ethanol (50 ml) was added 5% Pd-C (50%
hydrous, 1 g). The mixture was subjected to catalytic
reduction at room temperature under hydrogen atmosphere
(latm). After completion of hydrogenation in a
theoretical volume, the Pd-C catalyst was filtered off.
From the filtrate, the solvent was distilled off. The
WO 96/08466 21 p Z Z,~ 8 PGT/JP95101796
- l557 -I J
residue was neutralized with hydrogen chloride to give
the above-titled compound (yield 10.7 g, 90%), m.p.141-
143 C (recrystallized from ethanol).
NMR(d6-DMSO,D20)8: 0.83(3H,s), 1.01(3H,s), 1.30-
1.80(5H,m), 1.90-2.10(1H,m), 2.25-2.40(1H,m), 2.60-
2.96(4H,m), 3.72(3H,s), 6.63(1H,d,J=2.2Hz),
6.73(1H,dd,J=2.2Hz, 8.4Hz), 7.03(1H,d,J=8.4Hz).
Elemental Analysis for C15H23NO=HC1:
Calcd.: C, 66.77; H, 8.97; N, 5.19; Cl, 13.14
Found : C, 66.61; H, 9.02; N, 5.20; Cl, 13.19
Reference Example 15
(1,2,3,4-Tetrahydro-5,7-dimethyl-l-
naphthylidene)acetonitrile
By substantially the same procedure as in
Reference Example 1, a mixture of isomers of the above-
titled compound was produced from 5,7-dimethyl-l-
tetralone and diethyl cyanomethylphosphonate (yield
93%), m.p.71-73'C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)S: 1.96(2H,m), 2.23(3H,s), 2.30(3H,s),
2.72(2H,t,J=6.2Hz), 2.79-2.88(2H,m), 5.69(1H,s),
7.06(1H,s), 7.22(1H,s).
Elemental Analysis Calcd for C14HisN:
Calcd.: C, 85.24; H, 7.66; N, 7.10.
Found : C, 85.19; H, 7.59; N, 7.13.
Reference Example 16
1-(2-Aminoethylidene)-5,7-dimethyl-1,2,3,4-
tetrahydronaphthalene
By substantially the same procedure as in
Reference Example 3-B, a mixture of isomers of the
above-titled compound was produced from (1,2,3,4-
tetrahydro-5,7-dimethyl-l-naphthylidene)acetonitrile
(yield 88%, oil).
NMR(CDC13)S: 1.75-1.93(2H,m), 2.15-2.30(6H,m), 2.40-
2.90(4H,m), 3.44-3.65(2H,m), 5.85-6.08(1H,m), 6.85-
7.30(2H,m).
--- -- -
WO 96/08466 2193398 PCIYJP95101796
- 56 -
Reference Example 17
(1,2,3,4-tetrahydro-6,7-dimethoxy-l-
naphthylidene)acetonitrile
By substantially the same procedure as in
Reference Example 1, the above-titled compound was
produced from 6,7-dimethoxy-l-tetralone and diethyl
cyanomethylphosphonate (yield 95%, oil).
NMR(CDC13)S: 1.85-2.00(2H,m), 2.80-3.00(4H,m),
3.89(3H,s), 3.90(3H,s), 5.57(1H,s), 6.63(1H,s),
6.99(1H,s).
Reference Example 18
1-(2-Aminoethylidene)-6,7-dimethoxy-1,2,3,4-
tetrahydronaphthalene
By substantially the same procedure as in
Reference Example 3-B, a mixture of isomers of the
above-titled compound was produced from (1,2,3,4-
tetrahydro-6,7-dimethoxy-l-naphthylidene)acetonitrile
(yield 65%, oil).
NMR(CDC13)Ss 1.75-1.95(2H,m), 2.20-2.90(4H,m), 3.44-
3.60(2H,m), 3.80-4.00(6H,m), 5.77-6.00(1H,m), 6.55-
7.20(2H,m).
Reference Example 19
2-Isopropoxy-6,7,B,9-Tetrahydro-5H-
benzocyclohepten-9-one
To a suspension of 2-hydroxy-6,7,8,9-Tetrahydro-
5H-benzocyclohepten-9-one (9.69 g, 56.3 mmol.) and
potassium carbonate (23.3 g, 0.17 mol) in DMF (60 ml)
was added dropwise, under ice-cooling, isopropyl
bromide (34.6 g, 0.28 mol). The reaction mixture was
stirred for one hour at 100 C, which was poured into
water, followed by extraction of the organic substance
with ethyl acetate. The extract solution was washed
with brine and water, which was dried over anhydrous
magnesium sulfate, followed by distilling off the
solvent. The residue was purified by means of a silica
gel column chromatography (hexane:ethyl acetate=9:1) to
WO 96/08466 2193398 PC.'T/3P95/01796
57 -
give the above-titled compound (11.4 g, yield 93%,
oil).
NMR(CDC13)S: 1.33(6H,d,J=5.7Hz), 1.70-1.92(4H,m),
2.72(2H,t,J=5.9Hz), 2.87(2H,d,J=5.9Hz), 4.58(1H,m),
6.95(1H,dd,J=2.7Hz,8.2Hz), 7.10(1H,d,J=8.2Hz),
7.26(1H,d,J=2.7Hz).
Reference Example 20
(2-Isopropoxy-6,7,8,9-tetrahydro-5H-
benzocyclohepten-9-ylidene)acetonitrile
By substantially the same procedure as in
Reference Example 1, a mixture of isomers of the above
titled compound was produced from 2-isopropoxy6,7,8,9-
tetrahydro-5H-benzocyclohepten-5-one and diethyl
cyanomethylphosphonate (yield 94%, oil).
NMR(CDC13)S: 1.20-1.43(6H,m), 1.60-2.20(4H,m), 2.40-
2.80(4H,m), 4.40-4.60(1H,m), 5.30-5.43(1H,m), 6.60-
7.20(3H,m).
Reference Example 21
9-(2-Aminoethylidene)-2-isopropoxy-6,7,8,9-
tetrahydro-5H-benzocycloheptene
By substantially the same procedure as in
Reference Example 3-B, a mixture of isomers of the
above-titled compound was produced from (2-isopropoxy
6,7,8,9-tetrahydro-SH-benzocyclohepten-9-
ylidene)acetonitrile (yield 91%, oil).
NMR(CDC13)S: 1.20-1.43(6H,m), 1.60-1.85(4H,m), 2.20-
2.80(4H,m), 3.18-3.50(2H,m), 4.40-4.60(1H,m), 5.47-
5.65(1H,m), 6.55-7.10(3H,m).
Reference Example 22
6-Methoxy-2,2-dimethyl-l-indanone
To a suspension of 60% sodium hydride (2.22 g,
55.5 mmol) in 1,2-dimethoxyethane (20 ml) was added,
under ice-cooling, a suspension of 6-methoxy-l-indanone
(3.00 g, 18.5 mmol) in 1,2-dimethoxyethane (10 ml).
The mixture was stirred for 5 minutes, to which was
added dropwise methyl iodide (4.61 mL, 74.0 mmol), and
WO 96108466 211('7ZJ3{fl8 PC'1'1JP95/01796 =
-758 -I
the mixture was stirred for 20 minutes. To the
reaction mixture was added water, and the mixture was
subjected to extraction with ethyl acetate. The
extract solution was washed with brine, which was dried
over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was purified by means of a silica gel column
chromatography (hexane - hexane/ethyl acetate, 9:1) to
give 3.15 g (yield 90%) of the above-titled compound as
an oily product.
NMR(CDC13)S: 1.23(6H,s), 2.92(2H,s), 3.84(3H,s), 7.16-
7.34(3H,m).
Reference Example 23
(Z)-(6-methoxy-2,2-dimethylindan-l-
ylidene)acetonitrile and (E)-(6-methoxy-2,2-
dimethylindan-1-ylidene)acetonitrile
To a solution of 1,1,1,3,3,3-hexamethyldisilazane
(4.13 ml, 19.6 mmol) in THF (80 ml) was added dropwise,
under argon atmosphere at -78 C, a 1.56M solution of n-
butyllithium hexane solution (12.5 ml, 19.6 mmol). The
mixture was stirred for 10 minutes, to which was added
acetonitrile (0.94 ml, 17.9 mmol), and the mixture was
stirred for further 15 minutes. To the mixture was
added a solution of 6-methoxy-2,2-dimethyl-l-indanone
(3.00 g, 16.3 mmol) in THF (10 ml), which was stirred
for 15 minutes. To the reaction mixture was added
water so that the temperature was gradually reverted to
room temperature. The reaction mixture was subjected
to extraction with ethyl acetate. The extract solution
was washed with brine, which was dried over anhydrous
magnesium sulfate, followed by distilling off the
solvent under reduced pressure. The residue was
dissolved in toluene (100 ml). To the solution was
added 10-camphor sulfonic acid (0.5 g), and the mixture
was heated for 1.5 hours under reflux. The reaction
mixture was cooled, to which was added water. The
WO 96/08466 219 3 3 9 8 PCr/JP95/01796
- 59 -
pixture was subjected to extraction with ethyl acetate.
The extract solution was washed with brine, which was
dried over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was subjected to a silica gel column
chromatography (hexane/ethyl acetate, 97:3 - 9:1) to
give 1.03 g (yield 30%) of (Z)-(6-methoxy-2,2-
dimethylindan-1-ylidene)acetonitrile as an oily
product.
NMR(CDC13)S: 1.26(6H,s), 2.83(2H,s), 3.86(3H,s),
5.17(1H,s), 7.01(1H,dd,J=2.4Hz,8.2Hz),
7.20(1H,d,J=8.2Hz), 7.88(1H,d,J=2.4Hz).
The elution on the silica gel column
chromatography was further continued to give 1.78 g
(yield 52%) of (E)-(6-methoxy-2,2-dimethylindan-l-
ylidene)acetonitrile as an oily product.
NMR(CDC13)S: 1.51(6H,s), 2.91(2H,s), 3.83(3H,s),
5.65(1H,s), 6.92(1H,d,J=2.4Hz),
7.01(1H,dd,J=2.4Hz,8.4Hz), 7.19(1H,d,J=8.4Hz).
Reference Example 24
1-(2-Aminoethyl)-6-methoxy-2,2-dimethylindan
hydrochloride
By substantially the same procedure as in
Reference Example 3-A, a free base of the above titled
compound was produced from (E)-(6-methoxy-2,2-
dimethylindan=1-ylidene)acetonitrile. The base was
converted to hydrochloride by using an ethanol solution
of hydrogen chloride to give the above-titled compound
(yield 74%), m.p.194-195 C (recrystallized from
ethanol/isopropyl ether).
NMR(d6-DMSO,)S: 0.93(3H,s), 1.07(3H,s), 1.59-
1.94(2H,m), 2.55-2.69(3H,m), 2.91(2H,t,J=8.OHz),
3.72(3H,s), 6.69(1H,dd,J=2.4Hz,8.2Hz),
6.78(1H,d,J=2.4Hz), 7.08(1H,d,J=8.2Hz), 7.94(2H,br s).
Reference Example 25
(E)-1-(2-Aminoethylidene)-6-methoxy-2,2-
WO 96,08466 219 3 398 PGTIJP95101796 =
- 60 -
Siimethylindan
By substantially the same procedure as in
Reference Example 3-B, the above-titled compound was
produced from (E)-(6-methoxy-2,2-dimethylindan-l-
ylidene)acetonitrile (yield 96%) as an oily product.
NMR(CDC13)6: 1.34(6H,s), 2.79(2H,s),
3.64(2H,d,J=7.4Hz), 3.80(3H,s), 5.95(1H,t,J=7.4Hz),
6.78(1H,dd,J=2.4Hz,8.2Hz), 6.92(1H,d,J=2.4Hz),
7.04(1H,d,J=8.2Hz).
Reference Example 26
(Z)-1-(2-Aminoethylidene)-6-methoxy-2,2-
dimethylindan
By substantially the same manner as in Reference
Example 3-B, the above-titled compound was produced
from (Z)-(6-methoxy-2,2-dimethylindan-l-
ylidene)acetonitrile (yield 98%) as an oily product.
NMR(CDC13)6: 1.18(6H,s), 2.73(2H,s), 3.76-3.84(2H,m),
3.81(3H,s), 5.46(1H,t,J=6.2Hz),
6.79(1H,dd,J=2.4Hz,8.2Hz), 7.00(1H,d,J=2.4Hz),
7.14(1H,d,J=8.2Hz).
Reference Example 27
Ethyl (6-methoxyindan-1-yl)acetate
To a suspension of 60% sodium hydride (1.84 g,
46.0 mmol) in THF (200 ml) was added dropwise, under
ice-cooling, triethyl phosphonoacetate (10.3 g, 46.0
mmol). The mixture was stirred until the reaction
mixture became a homogeneous solution. To the solution
was added a suspension of 6-methoxy-l-indanone (7.10 g,
43.8 mmol) in THF (30 ml). The mixture was stirred for
2 hours at room temperature and for-further 12 hours at
70 C. To the reaction mixture was added water, which
was subjected to extraction with ethyl acetate. The
extract solution was washed with brine, which was dried
over anhydrous magnesium sulfate, followed by
concentration under reduced pressure. The concentrate
was dissolved in ethanol (200 ml), to which was added
WO 96/08466 2 1 9 3 3 5' 8 PCT1JP95101796
- 61 -
5% Pd-C (50% hydrous, 2.5 g). The mixture was stirred
for 1.5 hours at 50 C under hydrogen atmosphere. The
reaction mixture was subjected to filtration, and the
filtrate was concentrated under reduced pressure. The
concentrate was purified by means of a silica gel
column chromatography (hexane/ethyl acetate, 97:3 -
4:1) to give 6.55 g (yield 64%) of the above-titled
compound as an oily product.
NMR(CDC13)S: 1.28(3H,t,J=7.2Hz), 1.67-1.83(1H,m), 2.30-
2.47(2H,m), 2.69-2.95(3H,m), 3.47-3.62(1H,m),
3.78(3H,s), 4.18(2H,q,J=7.2Hz), 6.69-6.75(2H,m),
7.11(1H,d,J=8.6Hz).
Reference Example 28
1-(2-Hydroxyethyl)-6-methoxyindan
To a suspension of lithium aluminum hydride (1.06
g, 27.9 mmol) in THF (150 ml) was added dropwise, under
ice-cooling, a solution of ethyl (6-methoxyindan-l-
yl)acetate (6.53 g, 27.9 mmol) in THF (20 ml), and the
mixture was stirred for 15 minutes. To the reaction
mixture was added water (lml), to which were further
added ethyl acetate, anhydrous magnesium sulfate and
celite. The mixture was subjected to filtration. The
filtrate was concentrated under reduced pressure to
give 4.96 g (yield 93%) of the above-titled compound as
an oily product.
NMR(CDC13)6: 1.35(1H,br s), 1.60-1.82(2H,m), 2.06-
2.41(2H,m), 2.69-2.96(2H,m), 3.15-3.28(1H,m), 3.75-
3.88(2H,m), 3.79(3H,s), 6.68-6.79(2H,m),
7.12(1H,d,J=8.OHz).
Reference Example 29
1-(2-Bromoethyl)-6-methoxyindan
To a solution of 1-(2-hydroxyethyl)-6-methoxyindan
(4.95 g, 25.7 mmol) in dichloromethane (100 ml) was
added dropwise at -5 C phosphorus tribromide (0.86 ml,
27.0 mmol). The mixture was stirred fos 30 minutes.
To the reaction mixture was added water, which was
WO 96/08466 219339U PCT/JP95/01796
- 62 -
pubjected to extraction with chloroform. The extract
solution was washed with brine and dried over anhydrous
magnesium sulfate, followed by distilling off the
solvent under reduced pressure. The residue was
purified by means of a silica gel column chromatography
(hexane/ethyl acetate, 7:3 - 1:1) to give 1.80 g (yield
27%) of the above-titled compound as an oily product.
NMR(CDC11)5: 1.60-1.78(1H,m), 1.88-2.06(lH,m), 2.24-
2.41(2H,m), 2.70-2.96(2H,m), 3.21-3.38(1H,m), 3.41-
3.60(2H,m), 3.79(3H,s), 6.68-6.78(2H,m),
7.12(1H,d,J=7.6Hz).
Reference Example 30
1-(2-Cyanoethyl)-6-methoxyindan
To a solution of 1-(2-bromoethyl)-6-methoxyindan
(1.75 g, 6.86 mmol) in dimethyl sulfoxide (80 ml) was
added sodium cyanide (0.35 g, 7.20 mmol). This mixture
was stirred-for 40 minutes at 60 C. To the reaction
mixture was added water, which was subjected to
extraction with ethyl acetate. The extract solution
was washed with brine and dried over anhydrous
magnesium sulfate, followed by distilling off the
solvent under reduced pressure. The residue was
purified by means of a silica gel column chromatography
(hexane/ethyl acetate, 85:15) to give 1.28 g (yield
93%) of the above-titled compound as an oily product.
NMR(CDC13)S: 1.62-1.89(2H,m), 2.03-2.48(4H,m), 2.71-
2.96(2H,m), 3.18-3.33(1H,m), 3.80(3H,s), 6.72-
6.78(2H,m), 7.13(1H,d,J=9.OHz).
Reference Example 31
1-(3-Aminopropyl)-6-methoxyindan
By substantially the same procedure as in
Reference Example 3-A, the above-titled compound was
obtained (yield 96%) from 1-(2-cyanoethyl)-6-
methoxyindan as an oily product.
NMR(CDC13)S: 1.20-1.95(8H,m), 2.20-2.38(lH,m), 2.68-
2.94(3H,m), 3.01-3.15(1H,m), 3.79(3H,s), 6.67-
___
= WO 96108466 2 1g 3 3 g g PCf13P95101796
- 63 -
6.78(2H,m), 7.04-7.14(1H,m).
Reference Example 32
(E)-1-(2-Aminoethyliden)indan
In substantially the same manner as in Reference
Example 1, (indan-1-ylidene)acetonitrile was produced
from 1-indanone as a mixture of its isomers (yield
76%). By reducing the cyano group in substantially the
same procedure as in Reference Example 3-B, the above-
titled compound was obtained (yield 47%) as an oily
product.
NMR(CDC13)S: 2.70-2.82(2H,m), 2.96-3.05(2H,m),
3.48(2H,d,J=7.OHz), 5.96-6.07(1H,m), 7.16-7.30(3H,m),
7.42-7.51(lH,m).
Reference Example 33
5,6,7-Trimethoxy-l-indanone
A mixture of 3-(3,4,5-trimethoxyphenyl)propionic
acid (9.40 g, 39.1 mmol) and polyphosphoric acid (50 g)
was stirred for 2 hours at 80 C. To the reaction
mixture was added water, which was subjected to
extraction with ethyl acetate. The extract solution
was washed with brine, which was dried over anhydrous
magnesium sulfate, followed by distilling off the
solvent under reduced pressure. The residue was
recrystallized f.rom ethyl acetate/isopropyl ether to
give 7.71 g (yield 89%) of the above-titled compound,
m.p.114-115 C'.
NMR(CDC'13)S: 2.61-2.70(2H,m), 2.99-3.08(2H,m),
3.86(3H,s), 3.94(3h,s), 4.05(3H,s), 6.68(1H,s).
Reference Example 34
(E)-(5,6,7-Trimethoxyindan-1-ylidene)acetonitrile
In substantially the same manner as in Reference
Example 1, the above-titled compound was produced from
5,6,7-trimethoxy-l-indanone (yield 57%). This compound
was used for the subsequent reaction without
purification.
WO 96105466 2 19 3 3 9 8 PCT1JP95/01796
- 64 -
Reference Example 35
(E)-1-(2-Aminoethylidene)-5,6,7-trimethoxyindan
In substantially the same manner as in Reference
Example 3-B, the above-titled compound was produced
from (E)-(5,6,7-trimethoxyindan-1-ylidene)acetonitrile
(yield 97%) as an oily product.
NMR(CDC13)6: 1.46(2H,br s), 2.68-2.80(2H,m), 2.87-
2.98(2H,m), 3.44(2H,d,J=6.8Hz), 3.85(6H,s), 3.92(3H,s),
6.25-6.38(1H,m), 6.56(1H,s).
Reference Example 36
(E)-Ethyl 3-(4-methoxy-3-methylphenyl)acrylate
In substantially the same manner as in Reference
Example 27, the above-titled compound was produced from
4-methoxy-3-methylbenzaldehyde. This compound was used
for the subsequent reaction without purification.
Reference Example 37
Ethyl 3-(4-methoxy-3-methylphenyl)propionate
In substantially the same manner as in Reference
Example 14, the above-titled compound was produced from
(E)-ethyl 3-(4-methoxy-3-methylphenyl)acrylate. This
compound was used for the subsequent reaction without
purification.
Reference Example 38
Ethyl 3-(3-Bromo-4-methoxy-5-
methylphenyl)propionate
To a solution of ethyl 3-(4-methoxy-3-
methylphenyl)propionate (derived from 10.0 g (66.6
mmol) of 4-methoxy-3-methylbenzaldehyde) in chloroform
(200 ml) was added dropwise bromine (10.6 g, 66.6
mmol). The mixture was stirred for 12 hours at room
temperature. The reaction mixture was washed with
water, an aqueous solution of sodium thiosulfate, which
was dried over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was purified by means of a silica gel column
chromatography (hexane - hexane/ethyl acetate, 9:1) to
WO 96108466 219 3 3 9 8 PCf/JP95/01796
- 65 -
give 17.0 g (yield 85%; 3 steps) of the above-titled
compound as an oily product.
NMR(CDC13)S: 1.24(3H,t,J=7.2Hz), 2.29(3H,s),
2.57(2H,t,J=7.OHz), 2.84(2H,t,J=7.OHz), 3.78(3H,s),
4.12(2H,q,J=7.2Hz), 6.95(1H,d,J=1.6Hz),
7.21(1H,d,J=1.6Hz).
Reference Example 39
3-(3-Bromo-4-methoxy-5-methylphenyl)propionic acid
To a solution of 17.0 g (56.4 mmol) of ethyl 3-(3-
bromo-4-methoxy-5-methylphenyl)propionate in methanol
(30 ml) was added a 8N aqueous solution of sodium
hydroxide (200 ml). The mixture was stirred for 18
hours at room temperature. The reaction mixture was
acidified with 5N HC1, which was subjected to
extraction with chloroform. The extract solution was
washed with brine, which was dried over anhydrous
magnesium sulfate. The solvent was distilled off under
reduced pressure to give 14.8 g (yield 96%) of the
above-titled compound as an oily product.
NMR(CDC13)S: 2.30(3H,s), 2.60-2.68(2H,m), 2.81-
2.89(2H,m), 3.78(3H,s), 6.95(1H,d,J=1.8Hz),
7.22(1H,d,J=1.8Hz).
Reference Example 40
5-Bromo-6-methoxy-7-methyl-l-indanone and 7-bromo-
6-methoxy-5-methyl-l-indanone
A mixture of 3-(3-bromo-4-methoxy-5-methylphenyl)
propionic acid (14.8 g, 54.2 mmol) and polyphosphoric
acid (75 g) was stirred for one hour at 80 C. The
reaction mixture was cooled, to which was added water.
The mixture was subjected to extraction with ethyl
acetate. The extract solution was washed with brine,
which was dried over anhydrous magnesium sulfate,
followed by distilling off the solvent under reduced
pressure. The residue was purified by means of a
silica gel column chromatography (hexane/ethyl acetate,
9:1 - 4:1). to give 6.06 g (yield 44%) of 5-bromo-6-
WO 96/08466 L ') 1933 C 8 PG7/3P95/01796
-66 -
}nethoxy-7-methyl-l-indanone, m.p.76-77 C
(recrystallized from ethyl acetate/isopropyl ether).
NMR(CDC13)6: 2.62(3H,s), 2.64-2.71(2H,m), 2.99-
3.06(2H,m), 3.80(3H,s), 7.51(1H,s).
The elution of the silica gel column
chromatography was continued to give 4.00 g (yield 29%)
of 7-bromo-6-methoxy-5-methyl-l-indanone, m.p.108-109 C
(recrystallized from ethyl acetate/isopropyl ether).
NMR(CDC13)6: 2.41(3H,s), 2.69-2.76(2H,m), 2.96-
3.03(2H,m), 3.83(3H,s), 7.22(1H,s).
Reference Example 41
(E)-(5-Bromo-6-methoxy-7-methylindan-l-
ylidene)acetonitrile
By substantially the same procedure as in
Reference Example 1, the above-titled compound was
produced from 5-bromo-6-methoxy-7-methyl-l-indanone
(yield 34%), m.p.126-128 C (recrystallized from ethyl
acetate/isopropyl ether).
NMR(CDC13)6: 2.45(3H,s), 2.99-3.07(2H,m), 3.10-
3.20(2H,m), 3.78(3H,s), 5.69-5.72(1H,m), 7.43(1H,s).
Reference Example 42
(E)-1-(2-Aminoethylidene)-5-bromo-6-methoxy-7-
methylindan
By substantially the same procedure as in
Reference Example 3-B, the above-titled compound was
produced from (E)-(5-bromo-6-methoxy-7-methylindan-l-
ylidene)acetonitrile (yield 97%) as an oily product.
NMR(CDC13)6: 2.47(3H,s), 2.70-2.80(2H,m), 2.85-
2.93(2H,m), 3.50(2H,d,J=7.OHz), 3.76(3H,s), 6.00-
6.08(1H,m), 7.29(1H,s).
Reference Example 43
(E)-(7-Bromo-6-methoxy-5-methylindan-l-
ylidene)acetonitrile
In substantially the same manner as in Reference
Example 1, the above-titled compound was produced from
7-bromo-6-methoxy-5-methyl-l-indanone (yield 73%),
WO 96108466 2 l 9 3 3 9 8 PCT/JP95/01796
- 67 -
m.p.124-125 C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)6: 2.37(3H,s), 2.98-3.05(2H,m), 3.10-
3.20(2H,m), 3.80(3H,s), 6.70(1H,t,J=2.4Hz), 7.13(1H,s).
Reference Example 44
(E)-1-(2-Aminoethylidene)-7-bromo-6-methoxy-5-
methylindan
In substantially the same manner as in Reference
Example 3-B, the above-titled compound was produced
from (E)-(7-bromo-6-methoxy-5-methylindan-l-
ylidene)acetonitrile (yield 96%) as an oily product.
NMR(CDC13)6: 2.31(3H,s), 2.73-2.82(2H,m), 2.85-
2.96(2H,m), 3.50(2H,d,J=6.8Hz), 3.78(3H,s), 6.90-
7.00(2H,m).
Reference Example 45
1-(2-Aminoethyl)-6-ethoxyindan
To a solution of l-[2-(acetylamino)ethyl]-6-
hydroxyindan (1.00 g, 4.56 mmol), ethanol (0.32 ml,
5.47 mmol) and triphenylphosphine (1.32 g, 5.02 mmol)
in THF (20 ml) was added dropwise, under ice-cooling,
diethyl azodicarboxylate (0.87 g, 5.02 mmol). The
mixture was stirred for 15 hours at room temperature.
The reaction mixture was concentrated under reduced
pressure. To the concentrate was added water, and the
mixture was subjected to extraction with ethyl acetate.
The extract solution was washed with brine, which was
dried over anhydrous magnesium sulfate. The solvent
was distilled off under reduced pressure. The residue
was subjected to a silica gel column chromatography
(ethyl acetate - ethyl acetate/methanol, 95:5) to give
1-[2-(acetylamino)ethyl]-6-ethoxyindan. To this
compound was added hydrazine hydrate (20 ml). The
mixture was heated under reflux for 15 hours under
argon atmosphere. The reaction mixture was cooled, to
which was added water. The mixture was subjected to
extraction with ethyl acetate. The extract solution
WO 96108466 2 193398 PCT/JP95/01796
- 68 -
was.washed with brine and dried over anhydrous
magnesium sulfate. The solvent was distilled off under
reduced pressure to give 0.34 g (yield 36%) of the
above-titled compound as an oily product.
NMR(CDC13)8: 1.40(3H,t,J=7.OHz), 1.52-1.78(2H,m), 1.95-
2.22(1H,m), 2.23-2.39(1H,m), 2.50-2.60(3H,m), 2.72-
2.96(3H,m), 3.06-3.20(1H,m), 4.01(2H,q,J=7.OHz), 6.66-
6.76(2H,m), 7.10(1H,d,J=8.OHz).
Reference Example 46
1-(2-Aminoethyl)-6-(2-phenylethoxy)indan
In substantially the same manner as in Reference
Example 45, 1-[2-(acetylamino)ethyl)-6-(2-
phenylethoxy)indan was produced from 1-(2-
(acetylamino)ethyl)-6-
hydroxyindan and )3-phenethyl alcohl. This product was
subjected to substantially the same procedure as in
Reference Example 45 to give the above-titled compound
(yield 36%) as an oily product.
NMR(CDC13)S: 1.55-1.82(2H,m), 2.20-2.37(2H,m), 2.65-
2.85(2H,m), 2.90-3.20(5H,m), 4.13(2H,t,J=7.2Hz),
4.87(2H,br s), 6.68-6.76(2H,m), 7.07(lH,d,J=8.2Hz),
7.16-7.32(5H,m).
Reference Example 47
7-Amino-l-tetralone
Under ice-cooling, 1-tetralone (15.0 g, 0.103 mol)
was gradually added dropwise to fuming nitric acid (100
ml). The reaction mixture was stirred for 30 minutes,
which was poured into water. Resulting crystalline
precipitate was collected by filtration and washed with
water, which was dissolved in ethyl acetate. The
solution was washed with brine, dried over anhydrous
magnesium sulfate and concentrated under reduced
pressure. The residue was crystallized from ethyl
acetate/isopropyl ether to give 7-nitro-l-tetralone.
This compound was dissolved in ethanol (100 ml), to
which 5% Pd.-C (50% hydrous, 1 g) was added. The
WO 96/08466 2193398 PCT/JP95101796
- 59 -
lnixture was stirred for 2 hours at room temperature
under hydrogen atmosphere. The reaction mixture was
subjected to filtration, and the filtrate was
concentrated. The concentrate was purified by means of
a silica gel column chromatography (hexane/ethyl
acetate, 3:2), followed by recrystallization from ethyl
acetate/isopropyl ether to give 1.70 g (yield from 1-
tetralone, 10%) of the above-titled compound, m.p.141-
143 C.
NMR(CDC13)6: 2.09(2H,quintet,J=6.OHz),
2.61(2H,t,J=6.OHz), 2.85(2H,t,J=3.OHz), 3.70(2H,br s),
6.83(1H,dd,J=2.6Hz,8.2Hz), 7.06(1H,d,J=8.2Hz),
7.32(1H,d,J=2.6Hz).
Reference Example 48
7-Formylamino-l-tetralone
To a solution of 7-amino-l-tetralone (1.70 g, 10.5
mmol) in formic acid (3 ml) was added a mixture of
formic acid (8 ml) and acetic anhydride (3 ml),
followed by stirring for 10 minutes at room
temperature. The reaction mixture was concentrated
under reduced pressure. The concentrate was
neutralized with a saturated aqueous solution of sodium
hydrogencarbonate, which was subjected to extraction
with ethyl acetate. The extract solution was washed
with brine and dried over anhydrous magnesium sulfate,
followed by distilling off the solvent under reduced
pressure. The residue was recrystallized from ethyl
acetate/isopropyl ether to give 1.80 g (yield 91%) of
the above-titled compound, m.p.137-138 C.
NMR(CDC13)S: 2.08-2.20(2H,m), 2.62-2.71(2H,m), 2.91-
2.99(2H,m), 7.21-7.30(1H,m), 7.75-7.88(1.5H,m), 8.00-
8.17(1.5H,m), .8.42(0.5H,d,J=1.4Hz),
8.73(0.5H,d,J=11.4Hz).
Reference Example 49
1-(2-Aminoethyl)-7-formylamino-1,2,3,4-
tetrahydronaphthalene
WO 96108466 2193398 PCT/JP95/01796
- 70 -
By substantially the same procedure as in
Reference Example 1, from 7-formylamino-l-tetralone, a
cyano compound was produced as a mixture of isomers at
the double bond. The cyano group of this cyano
compound was reduced in substantially the same manner
as in Reference Example 3-B to give the corresponding
amino compound. The double bond of this amino compound
was hydrogenated in substantially the same manner as in
Reference Example 14 to give the above-titled compound.
Yield was 99%. The compound thus obtained was used for
the subsequent reaction without purification.
Reference Example 50
(E)-1-[(2-Trifluoroacetylamino)ethylidene]-
1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-
1,2,3,4-tetrahydronaphthalene and trifluoroacetic
anhydride (yield 62%). The mixture was subjected to a
silica gel column chromatography and recrystallization
to separate the above titled compound in a pure state
(yield 24%), m.p.99-102 C (recrystallized from hexane).
NMR(CDC13)S: 1.86(2H,m), 2.57(2H,t,J=6.3Hz),
2.80(2H,t,J=6.1Hz), 4.18(2H,t,J=6.3Hz), 5.96(1H,br
t,J=7.lHz), 6.37(1H,br s), 7.05-7.30(3H,m), 7.50-
7.60(1H,m).
Elemental Analysis for C34Ht4F3N0:
Calcd.: C, 62.45; H, 5.24; N, 5.20; F, 21.17.
Found : C, 62.34; H, 5.24; N, 5.22; F, 21.29.
Reference Example 51
1-[(2-Acetylamino)ethylidene]-1,2,3,4-
tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-
1,2,3,4-tetrahydronaphthalene and acetyl chloride
WO 96/05466 219339" PCT/JP95/01796
71 -
.(yield 68%), m.p.62-65 C (recrystallized from ethyl
acetate/hexane)
NMR(CDC13)&: 1.75-2.05(5H,m), 2.50-2.80(4H,m), 3.30-
4.10(2H,m), 5.65(1H,br s), 5.85-6.05(1H,m), 7.00-
7.60(4H,m).
Elemental Analysis for C14H17NO:
Calcd.: C, 78.10; H, 7.96; N, 6.51.
Found : C, 78.22; H, 7.91; N, 6.66.
Reference Example 52
1-[2-(4-Nitrobenzoylamino)ethylidene]-1,2,3,4-
tetrahydronaphtalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-
1,2,3,4-tetrahydronaphthalene and p-nitrobenzoyl
chloride (yield 78%), m.p.138-139 C (recrystallized
from ethyl acetate/hexane).
NMR(CDC13)6: 1.80-2.00(2H,m), 2.50-2.90(4H,m), 3.60-
4.40(2H,m), 5.90-6.20(1H,m), 6.25-6.45(1H,br s), 7.10-
8.40(BH,m).
Elemental Analysis for CteHieN20s:
Calcd.: C, 70.79; H, 5.63; N, 8.69.
Found : C, 70.76; H, 5.59; N, 8.70.
Reference Example 53
1-[2-(4-Trifluoroacetylaminobenzoylamino)ethyl]-
1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in
Reference Example 11 and Working Example 1, the above-
titled compound was produced from 1-(2-
aminoethylidene)-1,2,3,4-tetrahydronaphthalene and
trifluoroacetic anhydride (yield 68%), m.p.165-167 C
(recrystallized from ethyl acetate).
NMR(CDC13)S: 1.60-2.10(6H,m), 2.70-2.80(2H,m), 2.82-
3.00(1H,1tn), 3.50-3.70(2H,m), 6.15-6.23(1H,br s), 7.02-
7.20(4H,m), 7.60-7.78(4H,m), 8.52(1H,br s).
Elemental Analysis for CZ1HZ1F3Na0Z:
WO 96108466 21() ZJ3QQ PCT7JP95/01796 =
- 17J2- / v
Calcd.: C, 64.61; H, 5.42; N, 7.18; F, 14.60.
Found C, 64.62; H, 5.39; N, 7.23; F, 14.58.
Reference Example 54
(E)-1-j2-(Trifluoroacetylamino)ethylideneJindan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
(E)-1-(2-aminoethylidene)indan and trifluoroacetic
anhydride (yield 22%), m.p.101-103 C (recrystallized
from isopropyl ether/hexane).
NMR(CDC13)S: 2.76-2.85(2H,m), 3.01-3.09(2H,m),
4.15(2H,t,J=6.4Hz), 5.84-5.96(lH,m), 6.39(1H,br s),
7.18-7.24(3H,m), 7.41-7.50(1H,m).
Elemental Analysis for C13H12F3NO:
Calcd.: C, 61.18; H, 4.74; N, 5.49.
Found C, 61.21; H, 4.74; N, 5.54
Reference Example 55
1-[2-(Trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
(E)-1-[2-(trifluoroacetylamino)ethylidene]indan (yield
76%), m.p.67-68 C (recrystallized from isopropyl
ether/hexane).
NMR(CDC13)S: 1.64-1.82(2H,m), 2.07-2.42(2H,m), 2.79-
3.06(2H,m), 3.12-3.23(1H,m), 3.51(2H,q,J=7.OHz),
6.32(1H,br s), 7.20(4H,s).
Elemental Analysis for C13H14F3NO:
Calcd.: C, 60.70; H, 5.49; N, 5.44.
Found C, 60.60; H, 5.24; N, 5.49
Reference Example 56
4-(3-Bromopropyl)-6-methoxy-1,2-dihydronaphthalene
To a suspension of magnesium (2.9g) in THF (100m1)
was added bromocyclopropane (14.4g, 11.9mmol) dropwise
at 0 C under argon atmosphere. The mixture was stirred
for 30 minutes at room temperature and then a solution
of 7-methoxy-l-tetralone (15g, 85.1mmo1) in THF (50m1)
was added..The mixture was refluxed for 2 hours and
WO 96/08466 2193398 PCT/JP95/01796
- 73 -
then cooled. Saturated aqueous ammonium chloride was
introduced and the product was extracted with ethyl
acetate. The extract was washed with brine and water,
dried over anhydrous magnesium sulfate, and the solvent
was removed in vacuo. The residue was dissolved in
acetic acid (100m1) and 20% hydrobromic acid (75m1) was
added. The mixture was stirred for 15 hours at room
temperature and then concentrated. To the residue was
added saturated aqueous sodium hydrogen bicarbonate and
the product was extracted with ethyl acetate. The
extract was washed with brine and water, dried over
anhydrous magnesium sulfate, and the solventremoved in
vacuo. The residue was purified by a silica gel column
chromatography (ethyl acetate:hexane=l:9) to afford the
titled compound (20g, yield 84%, oil).
NMR(CDC13)S: 2.00-2.15(2H,m), 2.17-2.30(2H,m),
2.55-2.70(4H,m), 3.45(2H,t,J=6.6Hz), 3.80(3H,s),
5.94(1H,t,J=4.4Hz), 6.69(1H,dd,J=2.6Hz,8.1Hz),
6.83(1H,d,J=2.6Hz), 7.06(1H,d,J=8.lHz).
Elemental Analysis For C14H17BrO
Calcd.: C,59.80;H,6.09.
Found : C,59.77;H,6.32.
Reference Example 57
2-[3-(7-Methoxy-3,4-dihydronaphthalen-l-
yl)propyl]isoindole-l,3-dione
A mixture of 4-(3-bromopropyl)-6-methoxy-1,2-
dihydronaphthalene (lOg, 35.6mmol), potassium
phthalimide (7.9g, 42.7mmol) and DMF (50m1) was heated
at 100 C for 1 hour and then cooled. The mixture was
diluted with water and the product was extracted with
ethyl acetate. The extract was washed with brine and
water, dried over anhydrous magnesium sulfate, and the
solvent was removed in vacuo. The residue was purified
by a silica gel column chromatography (ethyl
acetate:hexane=2:8) to afford the titled compound
(11.8g, yield 95%, oil).
WO 96108466 2193398 PGT/JP95/01796 =
- 74 -
NMR(CDC13)6: 1.95(2H,m), 2.12-2.27(2H,m),
2.48(2H,t,J=7.7Hz), 2.63(2H,t,J=7.7Hz),
3.70-3.93(5H,m), 5.92(1H,t,J=4.6Hz),
6.67(1H,dd,J=2.6Hz,8.1Hz), 6.78(1H,d,J=2.6Hz),
7.03(1H,d,J=8.1Hz), 7.65-7.90(4H,m).
Elemental Analysis for CaiH221NO3:
Calcd.: C,76.06;H,6.09;N,4.03.
Found : C,76.23;H,6.23;N,3.99.
Reference Example 58
3-(7-Methoxy-3,4-dihydronaphthalen-l-
yl)propylamine
A solution of 2-[3-(7-methoxy-3,4-
dihydronaphthalen-l-yl)propyl]isoindole-1,3-dione
(11.8g, 34.0mmo1) and hydrazine monohydrate (5.1g,
O.lmol) in ethanol (150m1) was refluxed for 1 hour and
then cooled in an ice bath. The resulting insoluble
material was removed by filtration and the solvent was
removed in vacuo to afford the titled compound (5.7g,
yield 77%, oil). This compound was provided for the
next reaction without further purification.
NMR(CDC13)S: 1.68(2H,m), 2.15-2.30(2H,m),
2.46(2H,t,J=7.5Hz), 2.60-2.80(4H,m), 3.80(3H,s),
5.89(1H,t,J=4.4Hz), 6.68(1H,dd,J=2.4Hz,8.2Hz),
6.83(1H,d,J=2.4Hz), 7.06(1H,d,J=8.2Hz).
Reference Example 59
4-[3-(Trifluoroacetylamino)propyl]-6-methoxy-l,2-
dihydronaphthalene
By substantially the same procedure as in Working
Example 1, the above-titled compound was prepared from
3-(7-methoxy-3,4-dihydronaphthalen-1-yl)propylamine and
trifluoroacetic anhydride (yield 87%, oil).
NMR(CDC13)S: 1.84(2H,m), 2.16-2.30(2H,m),
2.50(2H,t,J=6.8Hz), 2.67(2H,t,J=7.9Hz),
3.40(2H,q,J=6.6Hz), 3.80(3H,s), 5.91(1H,t,J=4.6Hz),
6.35(1H,br s), 6.70(1H,dd,J=2.8Hz,8.2Hz),
6.77(1H,d,J=2.8Hz), 7.07(1H,d,J=8.2Hz).
wo 96/09466 2 19 3 39$ PCTlJP95101796
- 75 -
, Elemental Analysis for C16H18F3NOZ:
Calcd.: C,61.34;H,5.79;N,4.47;F,18.19.
Found : C,61.22;H,5.77;N,4.63;F,18.22.
Reference Example 60
4-[3-(Acetylamino)propyl]-6-methoxy-1,2-
dihydronaphthalene
By substantially the same procedure as in Working
Example 1, the above-titled compound was prepared from
3-(7-methoxy-3,4-dihydronaphthalen-1-yl)propylamine and
acetyl chloride (yield 90%, oil).
NMR(CDC13)S: 1.75(2H,m), 1.95(3H,s), 2.13-2.30(2H,m),
2.46(2H,t,J=7.4Hz), 2.66(2H,t,J=7.9Hz),
3.29(2H,q,J=6.5Hz), 3.80(3H,s), 5.50(1H,br s),
5.89(1H,t,J=4.4Hz), 6.69(1H,dd,J=2.2Hz,8.1Hz),
6.79(1H,d,J=2.2Hz), 7.07(1H,d,J=8.1Hz).
Elemental Analysis for C16H21NOZ:
Calcd.: C,74.10;H,8.16;N,5.40.
Found : C,74.23;H,8.21;N,5.33.
Reference Example 61
4-(3-Cyanopropyl)-6-methoxy-l,2-dihydronaphthalene
A mixture of 4-(3-bromopropyl)-6-methoxy-l,2-
dihydronaphthalene (lOg, 35.6mmo1), sodium cyanide
(1.92g, 39.1mmol) and dimethyl sulfoxide (20m1) was
stirred for 1 hour at room temperature. The mixture was
diluted with water and the product was extracted with
ethyl acetate. The extract was washed with brine and
water, dried over anhydrous magnesium sulfate, and the
solvent was removed in vacuo. The residue was purified
by silica gel column chromatography (ethyl
acetate:hexane=l:9) to afford the titled compound
(7.5g, yield 93%, oil).
NMR(CDC13)6: 1.80-1.98(2H,m), 2.18-2.30(2H,m),
2.35(2H,t,J=7.0Hz), 2.50-2.75(4H,m), 3.80(3H,s),
5.95(lH,t,J=4.6Hz), 6.70(1H,dd,J=2.6Hz,8.1Hz),
6.78(1H,d,J=2.6Hz), 7.07(1H,d,J=8.1Hz).
Elemental Analysis for C15H17N0:
WO 96/08466 2193398 PCT/JP95/01796
- 76 -
_Calcd.: C,79.26;H,7.54;N,6.16.
Found : C,79.23;H,7.66;N,6.36.
Reference Example 62
4-(4-Aminobutyl)-6-methoxy-1,2-dihydronaphthalene
By substantially the same procedure as in
Reference Example 2, the above-titled compound was
prepared from 4-(3-cyanopropyl)-6-methoxy-l,2-
dihydronaphthalene (yield 90%, oil).
NMR(d6-DMSO)5:1.30-1.60(4H,m), 2.05-2.67(lOH,m),
3.73(3H,s), 5.87(1H,t,J=4.OHz),
6.70(1H,dd,J=2.OHz,8.1Hz), 6.76(1H,d,J=2.OHz),
7.05(1H,d,J=8.1Hz).
Referece Example 63
4-[4-(Trifluoroacetylamino)butyl]-6-methoxy-1,2-
dihydronaphthalene
By substantially the same procedure as in Working
Example 1, the above-titled compound was prepared from
4-(4-aminobutyl)-6-methoxy-1,2-dihydronaphthalene and
trifluoroacetic anhydride (yield 97%, oil).
NMR(CDC11)S: 1.40-1.70(4H,m), 2.15-2.30(2H,m),
2.38-2.55(2H,m), 2.67(2H,t,J=7.9Hz), 3.30-3.42(2H,m),
3.80(3H,s), 5.87(1H,t,J=4.6Hz), 6.27(1H,br s),
6.69(1H,dd,J=2.6Hz,8.1Hz), 6.78(1H,d,J=2.6Hz),
7.07(1H,d,J=8.1Hz).
Elemental Analysis for C17HZOF3NO2:
Calcd.: C,62.38;H,6.16;N,4.28;F,17.41.
Found : C,61.94;H,6.14;N,4.14;F,17.45.
Reference Example 64
4-[4-(Acetylamino)butyl]-6-methoxy-1,2-
dihydronaphthalene
By substantially the same procedure as in Working
Example 1, the. above-titled compound was prepared from
4-(7-methoxy-3,4-dihydronaphthalen-1-y1)butylamine and
acetyl chloride (yield 95%), m.p. 79-81 C
(recrystallized from ethyl acetate/hexane).
NMR(CDC13)S: 1.49-1.62(4H,m), 1.95(3H,s),
~ WO 96/08466 2193398 PCTlJP95/01796
- 77 -
2.14-2.30(2H,m), 2.36-2.50(2H,m), 2.66(2H,t,J=8.1Hz),
3.20-3.33(2H,m), 3.80(3H,s), 5.44(1H,br s),
5.87(1H,t,J=4.4Hz), 6.68(1H,dd,J=2.4Hz,8.2Hz),
6.80(1H,d,J=2.4Hz), 7.06(1H,d,J=8.2Hz).
Elemental Analysis for C17H23NO2:
Calcd.: C,74.69;H,8.48;N,5.12.
Found : C,74.66;H,8.30;N,5.01.
Reference Example 65
(E)-(6-Methoxy-2-phenylindan-l-
ylidene)acetonitrile
By substantially the same procedure as in
Reference Example 23, the above-titled compound was
prepared from 6-methoxy-2-phenyl-l-indanone(yield 16%),
m.p. 112-114 C (recrystallized from ethyl
acetate/isopropyl ether).
NMR(CDC13)S: 3.03(1H,d,J=17.OHz),
3.59(1H,dd,J=8.2Hz,17.OHz), 3.86(3H,s),
4.49(1H,d,J=8.2Hz), 5.69(1H,d,J=2.6Hz), 6.95-
7.32(8H,m).
Reference Example 66
3-(2-Aminoethyl)-5-methoxy-2-phenyl-lH-indene
hydrochloride
By substantially the same procedure as in
Reference Example 3-B, the free base of the titled
compound was prepared from (E)-(6-Methoxy-2-
phenylindan-1-ylidene)acetonitrile. The above-titled
compound was prepared from the free base and
HC1/ethanol (yield 58%), amorphous. The compound thus
obtained was used for the subsequent reaction without
purification.
Reference Example 67
5-Methoxy-2-phenyl-3-12-
(trifluoroacethylamino)ethyl]-1H-indene
By substantially the same procedure as in Working
Example 1, the above-titled compound was prepared from
3-(2-Aminoethyl)-5-methoxy-2-phenyl-lH-indene
WO 96/08466 2; lJ{) Z Z(~ p PCT/JP95/01796
- 78-J JU
hydrochloride and trifluoroacetic anhydride (yield
92%), m.p. 138-139 C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)6: 3.03(2H,t,J=7.2Hz), 3.61(2H,q,J=7.2Hz),
3.71(2H,s), 3.88(3H,s), 6.29(1H,broad s),
6.81(1H,dd,J=2.2Hz,8.4Hz), 7.03(1H,d,J=2.2Hz),
7.39(1H,d,J=8.4Hz), 7.40(5H,s).
Elemental Analysis for C20H18F3NO2:
Calcd.: C,66.48;H,5.02;N,3.88.
Found : C,66.23;H,4.90;N,3.65.
Working Example 1
(E)-1-[2-(Acetylamino)ethylidene]-7-methoxy-
1,2,3,4-tetrahydronaphthalene
To a solution of 1-(2-aminoethylidene)-7-methoxy-
1,2,3,4-tetrahydronaphthalene (2.0 g, 9.74 mmol) and
triethylamine (1.5 g, 14,6 mmol) in THF (20 ml) was
gradually added dropwise, under ice-cooling, acetyl
chloride (0.76 g, 9.74 mmol). The mixture was stirred
for 30 minutes at room temperature. The reaction
mixture was poured into water, and the organic layer
was subjected to extraction with chloroform. The
extract solution was washed with brine and water, which
was dried over anhydrous magnesium sulfate. The
solvent was then distilled off under reduced pressure.
The residue was purified by means of a silica gel
column chromatography (ethyl acetate), followed by
recrystallization from ethyl acetate/hexane to give the
above-titled compound (yield 0.96 g, 40%).
m.p.92-94 C.(recrystallized from ethyl acetate/hexane)
NMR(CDC13)5: 1.73-1.90(2H,m), 2.01(3H,s),
2.51(2H,t,J=5.8Hz), 2.71(2H,t,J=6.2Hz), 3.80(3H,s),
4.06(2H,t,J=6.2Hz), 5.62(1H,br s), 5.94(1H,m),
6.75(1H,dd,J=2.6Hz,8.4Hz), 7.01(1H,d,J=8.4Hz),
7.06(1H,d,J=2.6Hz).
Elemental Analysis for C15HtyNO2a
Calcd.: C, 73.44; H, 7.81; N, 5.71.
WO 96/08466 219 3 3 98 PC'IYJP95/01796
- 79 -
Found : C, 73.52; H, 7.86; N, 5.73.
Working Example 2
(Z)-1-[2-(Acetylamino)ethylidene]-7-methoxy-
1,2,3,4-tetrahydronaphthalene
In substantially the same manner as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-7-
methoxy-1,2,3,4-tetrahydronaphthalene and acetyl
chloride. This mixture of isomers was purified by
means of a silica gel column chromatography (ethyl
acetate:hexane=6:4) to give the above-titled compound
(yield 30%).
m.p.71-73 C
NMR(CDC13)6: 1.81-1.97(2H,m), 2.42(2H,d,J=6.6Hz),
2.75(2H,t,J=6.6Hz), 3.79(3H,s), 4.19(2H,t,J=6.OHz),
5.41(1H,t,J=6.8Hz), 5.60(1H,br s), 6.72-6.82(2H,m),
7.05(1H,d,J=8.4Hz).
Working Example 3
(E)-1-[2-(Cyclopropylcarbonylamino)ethylidene]-7-
methoxy-1,2,3,4-tetrahydronaphthalene
In substantially the same manner as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethylidene)-7-methoxy-1,2,3,4-
tetrahydronaphthalene and cyclopropanecarbonyl chloride
(yield 59%).
m.p.130-1320C (recrystallized from ethyl
acetate/hexane)
NMR(CDC13)S: 0.70-0.82(2H,m), 0.90-1.08(2H,m), 1.25-
1.43(1H,m), 1.81(2H,m), 2.52(2H,t,J=5.5Hz),
2.71(2H,t,J=6.2Hz), 3.80(3H,s), 4.09(2H,t,J=6.2Hz),
5.71,1H,br s), 5.96(1H,m), 6.75(1H,dd,J=2.6Hz,8.4Hz),
7.01(1H,d,J=8.4Hz), 7.07(1H,d,J=2.6Hz).
Elemental Analysis for C37H21NDZ:
Calcd.: C, 75.25; H, 7.80; N, 5.16.
Found : C, 75.02; H, 7.85; N, 5.05.
Working Example 4
WO 96/08466 21'~ 33Q8 PCl'lJP95/01796
- 810 -
(E)-1-[2-(Valerylamino)ethylidene]-7-methoxy-
1,2,3,4-tetrahydronaphthalene
In substantially the same manner as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethylidene)-7-methoxy-1,2,3,4-
tetrahydronaphthalene and valeryl chloride (yield 54%).
m.p.64-66 C
NMR(CDC13)S: 0.92(3H,t,J=7.2Hz), 1.25-1.45(2H,m), 1.50-
1.90(4H,m), 2.20(2H,t,J=7.6Hz), 2.52(2H,t,J=5.9Hz),
2.71(2H,t,J=6.2Hz), 3.80(3H,s), 4.07(2H,t,J=6.2Hz),
5.50(1H,br s), 5.94(1H,t,J=7.OHz),
6.75(1H,dd,J=2.6Hz,8.4Hz), 7.01(1H,d,J=8.4Hz),
7.06(1H,d,J=2.6Hz).
Elemental Analysis for C18HZ5N0Z:
Calcd.: C, 75.22; H, 8.77; N, 4.87.
Found : C, 74.92; H, 8.79; N, 4.79.
Working Example 5
(E)-1-[2-[3-(4-Methoxyphenyl)ureido]ethylidene]-7-
methoxy-1,2,3,4-tetrahydronaphthalene
To a solution o-f 1-(2-aminoethylidene)-7-methoxy-
1,2,3,4-tetrahydronaphthalene (3.0 g, 14.8 mmol) in THF
(20 ml) was gradually added dropwise, under ice-
cooling, 4-methoxyphenyl isocyanate (2.2 g, 14.8 mmol).
The mixture was stirred for 30 minutes at room
temperature. The reaction mixture was then poured into
water. The organic layer was subjected to extraction
with 10% methanol/chloroform. The extract solution was
washed with brine and water, which was dried over
anhydrous magnesium sulfate, followed by distilling off
the solvent under reduced pressure. The residue was
purified by means of a silica gel column chromatography
(ethyl acetate:hexane=6:4), followed by
recrystallization from ethyl acetate/methanol to give
the above-titled compound (2.8 g, yield 54%).
m.p.168-170 C (recrystallized from ethyl
acetate/methanol)
WO 96108466 21 / J 3/ 8 PCT1JP95101796
- 81 -
NMR,(CDC13)S: 1.77(2H,m), 2.47(2H,t,J=5.9Hz),
2.68(2H,t,J=6.OHz), 3.78(6H,s), 4.03(2H,t,J=5.9Hz),
4.85(1H,br s), 5.93(1H,t,J=6.5Hz), 6.38(1H,br s),
6.73(1H,dd,J=2.6Hz,8.4Hz), 6.84(2H,d,J=9.OHz),
6.99(1H,d,J=8.4Hz), 7.04(1H,d,J=2.6Hz),
7.17(2H,d,J=9.OHz).
Elemental Analysis for C2.iH24NZ03:
Calcd.: C, 71.57; H, 6.86; N, 7.95.
Found : C, 71.55; H, 6.82; N, 7.93.
Working Example 6
(E)-1-[2-[3-(2,4-Dimethoxyphenyl)ureido]-
ethylidene]-7-methoxy-1,2,3,4-tetrahydronaphthalene
In substantially the same manner as in Working
Example 5, the above-titled compound was produced from
1-(2-aminoethylidene)-7-methoxy-1,2,3,4-
tetrahydronaphthalene and 2,4-dimethoxyphenyl
isocyanate (yield 34%).
m.p.140-143 C (recrystallized from ethyl acetate)
NMR(CDC13)S: 1.70-1.88(2H,m), 2.51(2H,t,J=6.2Hz),
2.70(2H,t,J=6.2Hz), 3.77(3H,s), 3.78(3H,s), 3.79(3H,s),
4.08(2H,br s), 4.80(1H,br s), 5.98(1H,t,J=6.6Hz), 6.40-
6.55(3H,m), 6.74(1H,dd,J=2.6Hz,8.1Hz),
7.00(1H,d,J=8.1Hz), 7.07(1H,d,J=2.6Hz),
7.69(1H,d,J=9.5Hz).
Elemental Analysis for Cz2H26N204:
Calcd.: C, 69.09; H, 6.85; N, 7.32.
Found : C, 69.17; H, 6.89; N, 7.42.
Working Example 7
1-[2-(Acetylamino)ethyl]-6-methoxyindan
To a solution of 1-(2-aminoethyl)-6-methoxyindan
(0.80 g, 4.18 mmol) and triethylamine (0.44 g, 4.39
mmol) in dichloromethane (15 ml) was gradually added
. dropwise, under ice cooling, acetyl chloride (0.33 g,
4.18 mmol). The mixture was stirred for 10 minutes at
room temperature. The reaction mixture was poured into
water. The organic layer was subjected to extraction
WO 96/08466 2193398 PCT1JP95/01796
82 -
with chloroform. The extract solution was washed with
1N hydrochloric acid, a saturated aqueous solution of
sodium hydrogencarbonate and brine, which was dried
over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was recrystallized from ethyl acetate/isopropyl
ether to give the above-titled compound (yield 0.68 g,
70%).
m.p.73-74 C (recrystallized from ethyl
acetate/isopropyl ether)
NMR(CDC13)S: 1.50-1.80(2H,m), 1.93-2.15(1H,m),
1.97(3H,s), 2.22-2.40(1H,m), 2.68-2.86(2H,m), 3.05-
3.18(1H,m), 3.33(2H,q,J=5.8Hz), 3.78(3H,s), 5.49(1H,br
s), 6.67-6.76(2H,m), 7.1l(1H,d,J=8.OHz).
Elemental Analysis for C14H19NO2:
Ca1cd.: C, 72.07; H, 8.21; N, 6.00.
Found : C, 72.16; H, 7.94; N, 6.17.
Working Example 8
1-(2-(Trifluoroacetylamino)ethyl]-6-methoxyindan
In substantially the same manner as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-methoxyindan and trifluoroacetic
anhydride (yield 68%).
m.p.66-67 C (recrystallized from isopropyl
ether/hexane)
NMR(CDC13)6: 1.60-1.80(2H,m), 2.02-2.20(lH,m), 2.24-
2.41(1H,m), 2.77-2.96(2H,m), 3.05-3.21(1H,m),
3.50(2H,q,J=7.2Hz), 3.79(3H,s), 6.32(lH,br s), 6.70-
6.77(2H,m), 7.12(1H,d,J=8.4Hz).
Elemental Analysis for C14H16F3NO2:
Calcd.: C, 58.53; H, 5.61; N, 4.88.
Found : C, 58.30; H, 5.41; N, 5.08.
Working Example 9
1-[2-(Cyclopropylcarbonylamino)ethyl]-6-
methoxyindan
In substantially the same manner as in Working
WO 96/08466 L 1 9 J3/ U PCT/3P95/01796
- 83 -
,Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-methoxyindan and
cyclopropanecarbonyl chloride (yield 78%).
m.p.105-106 C (recrystallized from ethyl
acetate/isopropyl ether)
NMR(CDC13)S: 0.68-0.77(2H,m), 0.93-1.02(2H,m), 1.24-
1.38(1H,m), 1.55-1.80(2H,m), 1.99-2.15(1H,m), 2.25-
2.41(lH,m), 2.70-2.92(2H,m), 3.06-3.20(1H,m), 3.38-
3.46(2H,m), 3.79(3H,s), 5.63(1H,br s), 6.69-6.77(2H,m),
7.11(1H,d,J=8.OHz).
Elemental Analysis for Cl6HZ1NO2:
Calcd.: C, 74.10; H, 8.16; N, 5.40.
Found : C, 73.90; H, 7.89; N, 5.44.
Working Example 10
1-[2-(Valerylamino)ethyl]-6-methoxyindan
In substantially the same manner as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-methoxyindan and valeryl chloride
(yield 56%).
m.p.66-67 C (recrystallized from isopropyl
ether/hexane)
NMR(CDC13)S: 0.91(3H,t,J=7.OHz), 1.23-1.42(2H,m), 1.51-
1.80(4H,m), 1.97-2.20(3H,m), 2.23-2.40(iH,m), 2.69-
2.95(2H,m), 3.06-3.19(1H,m), 3.35-3.44(2H,m),
3.79(3H,s), 5.45(1H,br s), 6.70-6.79(2H,m),
7.1l(1H,d,J=8.4Hz).
Elemental Analysis for C17H25NOZ:
Calcd.: C, 74.14; H, 9.15; N, 5.09.
Found : C, 73.93; H, 9.00; N, 5.16.
Working Example 11
1-[2-(Acetylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene
To a solution of (E)-1-[2-
(acetylamino)ethylidene]-7-methoxy-1,2,3,4-
tetrahydronaphthalene (2.55 g, 10 mmol) in ethanol (20
ml) was added 5% palladium/carbon (50% hydrous, 400
2193398 PC77JP95/01796
WO 96/08466
- 84 -
mg). The mixture was subjected to catalytic reduction
at normal pressure under hydrogen atmosphere. After
the completion of hydrogenation, the palladium/carbon
was then filtered off, and the solvent was distilled
off under reduced pressure. The residue was purified
by means of a silica gel column chromatography
(chloroform:methanol=98:2) to give the above-titled
compound (2.2 g, yield 86%, oil).
NMR(CDC13)'8: 1.60-2.10(6H,m), 1.96(3H,s),
2.68(2H,t,J=5.1Hz), 2.80(1H,m), 3.36(2H,m), 3.78(3H,s),
5.50(1H,br s), 6.64-6.72(2H,m), 6.98(1H,d,J=9.2Hz).
Working Example 12
1-[2-(Cyclopropylcarbonylamino)ethyl]-7-methoxy-
1,2,3,4-tetrahydronaphthalene
To a solution of (1,2,3,4-tetrahydro-7-methoxy-l-
naphthylidene)acetonitrile (1.0 g, 5.02 mmol) in
ethanol (10 ml) were added a saturated ammonia/ethanol
solution (5 ml) and Raney nickel (W-2, 1 g). The
mixture was stirred for 4 hours at 50 C under hydrogen
atmosphere (3-4 kgf/cmZ). The Raney nickel was
filtered off and, then, the solvent was distilled off
under reduced pressure to give 1-(2-aminoethyl)-7- ,
methoxy-1,2,3,4-tetrahydronaphthalene. To a solution
of this 1-(2-aminoethyl)-7-methoxy-1,2,3,4-
tetrahydronaphthalene and triethylamine (0.76 g, 7.53
mmol) in THF (20 ml) was gradually added dropwise,
under ice-cooling, cyclopropanecarbonyl chloride (0.63
g, 6.02 mmol). The mixture was stirred for 30 minutes
at room temperature. The reaction mixture was poured
into water. The organic layer.was subjected to
extraction with chloroform. The extract solution was
washed with brine and water, which was then dried over
anhydrous magnesium sulfate, followed by distilling off
the solvent. The residue was purified.by means of a =
silica gel column chromatography (ethyl
acetate:hexane=2:8) to give the above-titled compound
WO 96/08466 21 /339C3 PCT/3P95/01796
- 85 -
.(1.04 g, yield 76%, oil).
NMR(CDC13)S: 0.70-0.80(2H,m), 0.92-1.06(2H,m), 1.22-
1.41(1H,m), 1.60-2.10(6H,m), 2.62-2.90(3H,m), 3.32-
3.50(2H,m), 3.78(3H,s), 5.66(1H,br s), 6.64-6.74(2H,m),
S 6.98(1H,d,J=8.7Hz).
Working Example 13
1-[2-(Isobutyrylamino)ethyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene
In substantially the same manner as in Working
Example 12, the above-titled compound was produced
from (1,2,3,4-tetrahydro-7-methoxy-l-
naphthylidene)acetonitrile and isobutyryl chloride.
(yield 72%).
m.p.47-49 C
NMR(CDC13)S: 1.13(6H,d,J=6.OHz), 1.60-2.00(6H,m), 2.28-
2.39(1H,m), 2.62-2.90(3H,m), 3.30-3.46(2H,m),
3.78(3H,s), 5.45(1H,br s), 6.64-6.73(2H,m),
6.98(1H,d,J=9.5Hz).
Elemental Analysis for C17H25N02:
Calcd.: C, 74.14; H, 9.15; N, 5.09.
Found : C, 73.98; H, 9.09; N, 5.22.
Working Example 14
1-[2-[3-(4-Methoxyphenyl)ureido]ethyl]-7-methoxy-
1,2,3,4-tetrahydronaphthalene
In substantially the same manner as in Working
Example 11, the above-titled compound was produced from
(E)-1-[2-[3-(4-methoxyphenyl)ureido]ethylidene]-7-
methoxy-1,2,3,4-tetrahydronaphthalene (80%).
m.p.123-125 C (recrystallized from ethyl
acetate/hexane)
NMR(CDC13)S: 1.60-1.90(6H,m), 2.66(2H,t,J=4.6Hz),
2.78(1H,m), 3.34(2H,m), 3.75(3H,s), 3.78(3H,s),
4.76(1H,br s), 6.36(1H,br s), 6.62-6.70(2H,m),
6.85(2H,d,J=8.8Hz), 6.92-7.00(1H,m),
7.16(2H,d,J=8.8Hz).
Elemental Analysis for C21H2,6NZO3:
WO 96/08466 219 330j 8 PCT/JP95l01796
- 86 -
Calcd.: C, 71.16; H, 7.39; N, 7.90.
Found : C, 70.96; H, 7.37; N, 7.91.
Working Example 15
1-[2-(Acetylamino)ethylidene]-7-methoxy-2,2-
dimethyl-1,2,3,4-tetrahydronaphthalene
In substantially the same manner as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-7-
methoxy-2,2-dimethyl-1,2,3,4-tetrahydronaphthalene and
acetyl chloride (yield 78%, oil).
NMR(CDC13)6: 1.09(4H,s,Z-isomer), 1.32(2H,s,E-isomer),
1.57(0.67H,t,J=6.OHz,E-isomer), 1.63(1.33H,t,J=6.8Hz,Z-
isomer), 2.00(2H,s,Z-isomer), 2.02(2H,s,E-isomer),
2.61(0.67H,t,J=6.OHz,E-isomer), 2.75(1.33H,t,J=6.8Hz,Z-
isomer), 3.79(2H,s,Z-isomer), 3.80(1H,s,E-isomer),
4.18(1.33H,t,J=7.OHz,Z-isomer), 4.28(0.67H,d,J=7.3Hz,E-
isomer), 5.43(0.67H,t,J=7.OHz,Z-isomer), 5.55(1H,br s),
5.76(0.33H,t,J=7.3Hz,E-isomer), 6.62-7.07(3H,m,E-
isomer+Z-isomer).
Working Example 16
1-[2-(Acetylamino)ethyl]-7-methoxy-2,2-dimethyl-
1,2,3,4-tetrahydronaphthalene
In substantially the same manner as in Working
Example 11, the above-titled compound was produced
from 1-[2-(acetylamino)ethylidene]-7-methoxy-2,2-
dimethyl-1,2,3,4-tetrahydronaphthalene (yield 91%,
oil).
NMR(d6-DMSO)S: 0.81(3H,s), 0.97(3H,s), 1.18-1.43(2H,m),
1.58-1.90(2H,m), 1.80(3H,s), 2.21(1H,dd,J=2.9Hz,8.8Hz),
2.60-2.73(2H,m), 3.06(2H,q,J=7.0Hz), 3.71(3H,s), 6.63-
7.02(3H,m), 7.87(1H,t,J=5.2Hz).
Working Example 17
(E)-9-[2-(Trifluoroacetylamino)ethylidene]-2-
methoxy-6,7,8,9-tetrahydro-5H-benzocycloheptene
In substantially the same manner as in Working
Example 1, the above-titled compound was produced from
WO 96/08466 2193398 PCT1JP95/01796
- 87 -
.(E)-9-(2-aminoethylidene)-2-methoxy-6,7,8,9-tetrahyro-
5H-benzocycloheptene and trifluoroacetic anhydride
(1.97 g, yield 91%). A portion of this compound was
recrystallized from isopropyl ether-hexane to give a
crystalline product, m.p.101-103 C.
NMR(CDC13)S: 1.69-1.79(4H,m), 2.39-2.47(2H,m), 2.65-
2.71(2H,m), 3.80(3H,s), 5.45(2H,t,J=7.OHz), 6.36(1H,br
s), 6.68-6.75(2H,m), 7.00(1H,d,J=8.OHz).
Elemental Analysis for C16Ht8F3N02:
Calcd.: C, 61.34; H, 5.79; N, 4.47.
Found : C, 61.29; H, 5.69; N, 4.55.
Working Example 18
9-[2-(Trifluoroacetylamino)ethyl)-2-methoxy-
6,7,8,9-tetrahydro-SH-benzocycloheptene
In substantially the same manner as in Working
Example 11, the above-titled compound was produced from
9-[2-(trifluoroacetylamino)ethylidene]-2-methoxy-
6,7,8,9-tetrahydro-SH-benzocycloheptene (yield 97%,
oil).
NMR(CDC13)S: 1.65-1.96(7H,m), 2.08-2.25(1H,m), 2.72-
2.89(3H,m), 3.22-3.38(1H,m), 3.40-3.60(1H,m),
3.78(3H,s), 6.18(1H,br s), 6.61-6.68(2H,m),
7.02(1H,d,J=8.OHz).
Working Example 19
(E)-6-Methoxy-1-[2-
(trifluoroacetylamino)ethylidene]indan
In substantially the same manner as in Working
Example 1, the above-titled compound was produced by
(E)-1-(2-aminoethylidene)-6-methoxyindan and
trifluoroacetic anhydride (yield 49%).
m.p.95-96 C (recrystallized from isopropylether-hexane)
NMR(CDC13)6: 2.78-2.87(2H,m), 2.93-3.01(2H,m),
3.82(3H,s), 4.15(2H,t,J=6.4Hz), 5.83-5.92(1H,m),
6.34(1H,br s), 6.83(1H,dd,J=2.2Hz,8.4Hz),
6.94(1H,d,J=2.2Hz), 7.17(1H,d,J=8.4Hz).
Elemental Analysis for C14H14F3NOZ:
WO 96105466 21r] Z Z98 PCP/JP95l01796
- 88 J- 1J
Calcd.: C, 58.95; H, 4.95; N, 4.91.
Found C, 58.73; H, 5.08; N, 5.02.
Working Example 20
(E)-1-[2-(Acetylamino)ethylidene]-6-methoxyindan
In substantially the same manner as in Working
Example 1, the above-titled compound was produced from
(E)-1-(2-aminoethylidene)-6-methoxyindan and acetyl
chloride (yield 47%).
m.p.100-102 C (recrystallized from ethyl acetate -
isopropyl ether)
NMR(CDC13)S: 2.01(3H,s), 2.76-2.87(2H,m), 2.90-
3.00(2H,m), 3.81(3H,s), 4.03(2H,t,J=6.2Hz), 5.53(1H,br
s), 5.82-5.93(1H,m), 6.80(1H,dd,J=2.2Hz,8.2Hz),
6.93(1H,d,J=2.2Hz), 7.15(1H,d,J=8.2Hz).
Elemental Analysis for C14Ht7NOz:
Calcd.: C, 72.70; H, 7.41; N, 6.06.
Found C, 72.65; H, 7.31; N, 6.20.
Working Example 21
5,6-Dimethoxy-l-[2-
(trifluoroacetylamino)ethyl]indan
In substantially the same manner as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-5,6-dimethoxyindan hydrochloride and
trifluoroacetic anhydride (yield 73%).
m.p.114-115 C (recrystallized from isopropyl ether -
hexane)
NMR(CDC13)S: 1.60-1.82(2H,m), 2.03-2.19(1H,m), 2.25-
2.43(1H,m), 2.72-2.98(2H,m), 3.07-3.22(1H,m),
3.49(2H,dd,J=7.2Hz,13.6Hz), 3.86(3H,s), 3.87(3H,s),
6.30(1H,br s), 6.73(1H,s), 6.77(lH,s).
Elemental Analysis for CuH18F3NO3:
Calcd.: C, 56.78; H, 5.72; N, 4.41.
Found C, 56.73; H, 5.79; N, 4.55.
Working Example 22
1-[2-(Acetylamino)ethyl]-5,6-dimethoxyindan
In substantially the same manner as in Working
= WO 96108466 2193398 PCT/JP95101796
- 89 -
Bxample 1, the above titled compound was produced from
1-(2-aminoethyl)-5,6-dimethoxyindan hydrochloride and
acetyl chloride (yield 94%, oil).
NMR(CDC13)6: 1.48-1.80(2H,m), a.96-2.12(1H,m),
1.98(3H,s), 2.24-2.40(1H,m), 2.70-2.95(2H,m), 3.03-
3.18(1H,m), 3.37(2H,dd,J=7.4Hz,13.6Hz), 3.85(3H,s),
3.86(3H,s), 5.60(1H,br s), 6.74(1H,s), 6.76(1H,s).
Working Example 23
1-[2-(Trifluoroacetylamino)ethyl)-7-
methoxy-2,2-dimethyl-1,2,3,4-tetrahydronaphthalene
To a solution of 1-(2-aminoethyl)-7-methoxy-
1,2,3,4-tetrahydro-2,2-dimethylnaphthalene
hydrochloride (1.5 g, 5.56 mmol) in pyridine (10 ml)
was added gradually dropwise, under ice-cooling,
trifluoroacetic anhydride (2.34 g, 11.1 mmol). The
mixture was stirred for 4 hours at room temperature,
which was poured into water, followed by subjecting the
organic layer to extraction with chloroform. The
extract solution was washed with brine and water, which
was then dried over anhydrous magnesium sulfate, then
the solvent was distilled off under reduced pressure.
The residue was purified by means of a silica gel
column chromatography (hexane:ethyl acetate=9:1) to
give the above-titled compound (1.55 g, yield 85%,
oil).
NMR(CDC13)6: 0.87(3H,s), 1.04(3H,s), 1.36-2.10(4H,m),
2.26(1H,dd,J=3.6Hz,9.1Hz), 2.70-2.80(2H,m), 3.20-
3.61(2H,m), 3.78(3H,s), 6.13(1H,br s),
6.57(1H,d,J=2.6Hz), 6.72(1H,dd,J=2.6Hz,8.4Hz),
7.02(1H,d,J=8.4Hz).
Elemental Analysis for C17H22F3N02:
Calcd.: C, 61.99; H, 6.73; N, 4.25; F, 17.30.
Found : C, 61.64; H, 6.74; N, 4.29; F, 17.58.
Working Example 24
7-Methoxy-l-[2-(methoxycarbonylamino)ethyl]-2,2-
dimethyl-1,2,3,4-tetrahydronaphthalene
WO 96108466 2193 3'98 PCT/JP95/01796 =
- 90 -
By substantially the same procedure as in Working
Example 23, the above-titled compound was produced from
1-(2-aminoethyl)-7-methoxy-2,2-dimethyl-1,2,3,4-
tetrahydronaphthalene hydrochloride and methyl
chlorocarbonate (yield 81%, oil)
NMR(CDC1j)S: 0.86(3H,s), 1.02(3H,s), 1.35-1.55(2H,m),
1.65-2.05(2H,m), 2.23(1H,dd,J=3.OHz,8.8Hz), 2.68-
2.80(2H,m), 3.10-3.32(2H,m), 3.66(3H,s), 3.78(3H,s),
4.60(1H,br s), 6.59(1H,d,J=2.7Hz),
6.70(1H,dd,J=2.7Hz,8.3Hz), 6.99(1H,d,J=8.3Hz).
Elemental Analysis for C13H25NO3:
Calcd.: C, 70.07; H, 8.65; N, 4.81.
Found : C, 69.87; H, 8.46; N, 4.93.
Working Example 25
7-Methoxy-2,2-dimethyl-l-[2-(3,3-
dimethylureido)ethylidene]-1,2,3,4-
tetrahydronaphthalene
In substantially the same manner as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced (yield 81%, oil) from 1-(2-
aminoethylidene)-7-methoxy-2,2-dimethyl-1,2,3,4-
tetrahydronaphthalene and dimethylcarbamyl chloride.
The product was subjected to a silica gel column
chromatography and recrystallization to separate and
purify the respective isomers.
E-isomer
m.p.146-148 C (recrystallized from petroleum ether
/diethyl ether)
NMR(CDC13)8: 1.34(6H,s), 1.57(2H,t,J=6.OHz),
2.61(2H,t,J=6.OHz), 2.92(6H,s), 3.80(3H,s),
4.26(2H,dd,J=5.3Hz,7.1Hz), 4.40(1H,br s),
5.82(1H,t,J=7.1Hz), 6.72(1H,dd,J=2.6Hz,8.4Hz), 6.96-
7.04(2H,m).
Elemental Analysis for C18HZ6NZ0Z:
Calcd.: C, 71.49; H, 8.67; N, 9.26.
Found : C, 71.32; H, 8.62; N, 9.36.
= WO 96/08466 2193398 PCT/JP95/01796
- 91 -
Z-isomer
Oil
NMR(CDC13)S: 1.09(6H,s), 1.66(2H,t,J=6.8Hz),
2.75(2H,t,J=6.8Hz), 2.90(6H,s), 3.78(3H,s),
4.17(2H,t,J=6.OHz), 4.21(1H,br s), 5.50(1H,t,J=6.4Hz),
6.69(1H,d,J=2.6Hz), 6.76(1H,dd,J=2.6Hz,8.2Hz),
7.03(1H,d,J=8.2Hz).
Elemental Analysis for C1aH26N202:
Calcd.: C, 71.49; H, 8.67; N, 9.26.
Found : C, 71.51; H, 8.72; N, 9.18.
Working Example 26
(Z)-1-[2-(3-tert-Butylureido)ethylidene]-7-
methoxy-2,2-dimethyl-1,2,3,4-tetrahydronaphthalene
To a solution of 1-(2-aminoethylidene)-7-methoxy-
2,2-dimethyl-1,2,3,4-tetrahydronaphthalene (1.5 g, 6.48
mmol) in THF (30 ml) was gradually added dropwise,
under ice-cooling, Tert-butyl isocyanate (0.84 g, 8.43
mmol). The mixture was stirred for 2 hours at room
temperature. The reaction mixture was then poured into
water. The organic layer was subjected to ethyl
acetate. The extract solution was washed with brine
and water, which was dried over anhydrous magnesium
sulfate, followed by distilling off the solvent under
reduced pressure. The residue was purified by means of
a silica gel column chromatography (hexane:ethyl
acetate=8:2), followed by recrystallization to give the
above-titled compound (yield 780 mg, 36%), m.p.175-
176 C (recrystallized from ethyl acetate/diethyl
ether).
NMR(d6-DMSO)S: 1.05(6H,s), 1.21(9H,s),
1.60(2H,t,J=6.8Hz), 2.70(2H,t,J=6.8Hz), 3.73(3H,s),
3.81(2H,t,J=5.9Hz), 5.42(1H,t,J=6.8Hz), 5.60(1H,br s),
5.78(1H,t,J=6.8Hz), 6.68(1H,d,J=2.6Hz),
6.79(1H,dd,J=2.6Hz,8.6Hz), 7.06(1H,d,J=8.6Hz).
Elemental Analysis for C2OH3oN202a
Calcd.: C, 72.69; H, 9.15; N, 8.48.
WO 96108466 2193JZ 98 PCTlJP95l01796 =
- 92 -
Found : C, 72.81; H, 9.07; N, 8.65.
Working Example 27
7-Methoxy-l-[2-(methoxyacetylamino)
ethylidene]-2,2-dimethyl-1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-7-
methoxy-2,2-dimethyl-1,2,3,4-tetrahydronaphthalene and
methoxyacetyl chloride (yield 75%, oil).
NMR(CDC13)6: 1.00-1.15(6H,m), 1.50-1.70(2H,m), 2.70-
2.80(2H,m), 3.38-3.50(3H,m), 3.80-4.00(5H,m), 4.20-
4.40(2H,m), 5.40-5.80(1H,m), 6.60-7.20(3H,m).
Elemental Analysis for C18H2.,NO3:
Calcd.: C, 71.26; H, 8.31; N, 4.62.
Found : C, 71.33; H, 8.38; N, 4.51.
Working Example 28
7-Methoxy-l-[2-(4-methoxybenzoylamino)ethyl]-2,2-
dimethyl-1,2,3,4-tetrahydronaphthalene
By substantially the same procedures as in Working
Example 1 and 11, The above-titled compound was
produced from 1-(2-aminoethylidene)-7-methoxy-2,2-di-
niethyl-1,2,3,4-tetrahydronaphthalene and p-
methoxybenzoyl chloride (yield 71%, oil).
NMR(CDC13)S: 0.86(3H,s), 1.04(3H,s), 1.32-2.16(4H,m),
2.30(1H,m), 2.68-2.80(2H,m), 3.25-3.72(2H,m),
3.76(3H,s), 3:82(3H,s), 6.10(1H,br s), 6.65-6..74(2H,m),
6.87(2H,d,J=8.8Hz), 7.00(1H,d,J=8.4Hz,
7.62(2H,d,J=8.8Hz).
Elemental Analysis for C23HT9N03=0.5HZ0:
Calcd.: C, 73.37; H, 8.03; N, 3.72.
Found : C, 73.30; H, 7.78; N, 3.55.
Working Example 29
(E)-1-[2-(Trifluoroacetylamino)ethylidene]-5,7-
dimethyl-1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
= WO 96108466 2193398 PCT/JP95101796
-93-
I.-(2-aminoethylidene)-5,7-dimethyl-1,2,3,4-
tetrahydronaphthalene and trifluoroacetic anhydride
(yield 89%), m.p.143-146 C (recrystallized from ethyl
acetate).
NMR(CDC13)S: 1.89(2H,m), 2.21(3H,s), 2.47(3H,s),
2.50(2H,t,J=6.OHz), 2.66(2H,t,J=6.2Hz),
4.17(2H,t,J=6.4Hz), 5.92(1H,t,J=7.3Hz), 6.35(1H,br s),
6.93(1H,s), 7.23(iH,s).
Elemental Analysis for C16H18F3N0:
Calcd.: C, 64.64; H, 6.10; N, 4.71; F, 19.17.
Found : C, 64.47; H, 6.17; N, 4.81; F, 19.32.
Working Example 30
(E)-5,7-Dimethyl-1-[2-(3,3-
dimethylureido)ethylidene]-1,2,3,4-
tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-5,7-
dimethyl-1,2,3,4-tetrahydronaphthalene and
dimethylcarbamyl chloride (yield 89%). The mixture was
subjected to a silica gel column chromatography and
recrystallization to separate the above-titled compound
in a pure state (yield 25%), m.p.141-143 C
(recrystallized from ethyl acetate).
NMR(CDC13)6: 1.86(2H,m), 2.20(3H,s), 2.28(3H,s),
2.50(2H,t,J=5:9Hz), 2.64(2H,t,J=6.2Hz), 2.92(6H,s),
4.04(2H,t,J=6.2Hz), 4.37(1H,br s), 5.98(1H,t,J=7.OHz),
6.89(1H,s), 7.26(1H,s).
Elemental Analysis for C17H24N20:
Calcd.: C, 74.96; H, 8.88; N, 10.28.
Found : C, 75.16; H, 8.92; N, 10.32.
Working Example 31
5,7-Dimethyl-l-[2-(3,3-dimethylureido)ethyl]-
1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
WO 96108466 - 21p'y Z O Q PCP1JP95/01796 =
- 94 J-J 1 v
5,7-dimethyl-l-[2-(3,3-dimethylureido)ethylidene]-
1,2,3,4-tetrahydronaphthalene (yield 80%), m.p.121-
123 C (recrystallized from ethyl acetate/hexane).
NMR(CDC13)S: 1.65-2.00(6H,m), 2.17(3H,s), 2.26(3H,s),
2.50-2.63(2H,m), 2.75-2.87(1H,m), 2.86(6H,s), 3.28-
3.44(2H,m), 4.30(1H,br s), 6.82(1H,s), 6.84(1H,s).
Elemental Analysis for C17H26N20:
Calcd.: C, 74.41; H, 9.55; N, 10.21.
Found : C, 74.45; H, 9.56; N, 10.13.
Working Example 32
1-[2-(Trifluoroacetylamino)ethyl]-5,7-dimethyl-
1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound.was produced from
1-[2-(trifluoroacetylamino)ethylidene]-5,7-dimethyl-
1,2,3,4-tetrahydronaphthalene (yield 83%), m.p.103-
105 C (recrystallized from hexane).
NMR(CDC13)S: 1.60-2.00(6H,m), 2.18(3H,s), 2.26(3H,s),
2.50-2.63(2H,m), 2.75-2.90(1H,m), 3.40-3.57(2H,m),
6.23(1H,br s), 6.80(1H,s), 6.84(1H,s).
Elemental Analysis for C16HaoF3NO:
Calcd.: C, 64.20; H, 6.73; N, 4.68; F, 19.04.
Found : C, 64.22; H, 6.73; N, 4.67; F, 19.18.
_Working Example 33
(E)-1-[2-(Trifluoroacetylamino)ethylidene]-7-
methoxy-1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-7-
methoxy-1,2,3,4-tetrahydronaphthalene and
trifluoroacetic anhydride (yield 85%). The mixture was
subjected to a silica gel column chromatography and
recrystallization to separate the above-titled compound
in a pure state (yield 37%), m.p.98-100 C
(recrystallized from diethyl ether).
NMR(CDC13)S: 1.84(2H,m), 2.53(2H,t,J=5.5Hz),
WO 96108466 2193 3 9 g PGT/JP95/01796
- 95 -
2.73(2H,t,J=6.2Hz), 3.81(3H,s), 4.18(2H,t,J=6.2Hz),
5.93(1H,t,J=7.1Hz), 6.40(1H,br s),
6.78(1H,dd,J=2.6Hz,8.4Hz), 7.00-7.10(2H,m).
Elemental Analysis for C15H16F3N0Z:
Calcd.: C, 60.20; H, 5.39; N, 4.68.
Found : C, 60.23; H, 5.39; N, 4.76.
Working Example 34
1-[2-(Trifluoroacetylamino)ethyl]-7-methoxy-
1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
1-[2-(trifluoroacetylamino)ethylidene]-7-methoxy-
1,2,_~,4-tetrahydronaphthalene (yield 82%, oil).
NMR(CDC13)&: 1.60-2.10(6H,m), 2.69(2H,t,J=5.4Hz),
2.84(1H,m), 3.47(2H,q,J=7.OHz), 3.78(3H,s), 6.30(1H,m),
6.65-6.76(2H,m), 6.99(1H,d,J=7.7Hz).
Elemental Analysis for Cl5H18F3NOZ:
Calcd.: C, 59.79; H, 6.02; N, 4.65.
Found : C, 60.09; H, 6.06; N, 4.40.
working Example 35
1-[2-(Acetylamino)ethyl]-7-hydroxy-1,2,3,4-
tetrahydronaphthalene
To a solution of 1-[2-(acetylamino)ethyl]-7-
methoxy-1,2,3,4-tetrahydronaphthalene (3.14 g, 12.7
mmol) in dichloromethane (70 ml) was added dropwise,
under ice-cooling, boron tribromide (6.4 g, 25.4 mmol).
The reaction mixture was stirred for 50 minutes at room
temperature, which was poured into water. The organic
layer was subjected to extraction with chloroform. The
extract solution was washed with brine and water, which
was dried over anhydrous magnesium sulfate, followed by
distilling off the solvent under reduced pressure. The
residue was purified by means of a silica gel column
chromatography (chloroform:methanol =9:1) to give the
above-titled compound (1.54 g, yield 52%), m.p.153-
155 C (recrystallized from ethyl acetate).
WO 96/08466 211933 98 PC17JP95101796 =
- 96 -
NMR(CDC13)8: 1.55-2.10(6H,m), 1.98(3H,s),
2.66(2H,t,J=5.8Hz), 2.68-2.82(1H,m), 3.28-3.42(2H,m),
5.72(1H,br s), 6.61-6.70(2H,m), 6.85(1H,br s),
6.92(1H,d,J=7.7Hz).
Elemental Analysis for C14H19NOZ:
Calcd.: C, 72.07; H, 8.21; N, 6.00.
Found : C, 72.01; H, 8.34; N, 6.01.
Working Example 36
(E)-1-[2-(Trifluoroacetylamino)ethylidene]-6,7-
dimethoxy-1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-6,7-
dimethoxy-1,2,3,4-tetrahydronaphthalene and
trifluoroacetic anhydride (yield 75%). The mixture was
subjected to a silica gel column chromatography and
recrystallization to separate the above-titled compound
in a pure state (yield 29%), m.p.117-119 C
(recrystallized from ethyl acetate/hexane).
NMR(CDC13)8: 1.85(2H,m), 2.53(2H,t,J=6.4Hz),
2.74(2H,t,J=6.2Hz), 3.88(3H,s), 3.90(3H,s),
4.18(2H,t,J=6.2Hz), 5.83(1H,t,J=7.1Hz), 6.36(1H,br s),
6.59(1H,s), 7.03(1H,s).
Elemental Analysis for C16Hl8F3NO3:
Caicd.: C, 58.36; H, 5.51; N, 4.25.
Found : C, 58.16; H, 5.60; N, 4.22.
Working Example 37
(E)-1-[2-(Acetylamino)ethylidene]-6,7-dimethoxy-
1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 1-(2-aminoethylidene)-6,7-
dimethoxy-1,2,3,4-tetrahydronaphthalene and acetyl
chloride (yield 78%). The mixture was subjected to a
silica gel chromatography and recrystallization to
separate the above-titled compound in a pure state
WO 96/08466 2193398 PCT/JP95101796
- 97 -
,(yield 39%), m.p.131-133 C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)S: 1.83(2H,m), 2.02(3H,s),
2.51(2H,t,J=5.8Hz), 2.73(2H,t,J=6.2Hz), 3.87(3H,s),
3.89(3H,s), 4.07(2H,t,J=6.1Hz), 5.58(1H,br s),
5.84(1H,t,J=7.1Hz), 6.58(1H,s), 7.05(1H,s).
Elemental Analysis for_C16H21N03:
Calcd.: C, 69.79; H, 7.69; N, 5.09.
Found : C, 69.63; H, 7.46; N, 4.99.
Working Example 38
1-[2-(Acetylamino)ethyl]-6,7-dimethoxy-1,2,3,4-
tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, , the above-titled compound was produced
from 1-[2-(acetylamino)ethylidene]-6,7-dimethoxy-
1,2,3,4-tetrahydronaphthalene (yield 78%, oil).
NMR(CDC13)6: 1.60-1.93(6H,m), 1.98(3H,s),
2.68(2H,t,J=4.9Hz), 2.76(1H,m), 3.22-3.49(2H,m),
3.84(3H,s), 3.86(3H,s), 5.57(1H,br s), 6.56(1H,s),
6.66(lH,s).
Elemental Analysis Calcd for C16HZ3N03:
Calcd.: C, 69.29; H, 8.36; N, 5.05.
Found : C, 69.86; H, 8.33; N, 4.89.
Working Example 39
1-[2-(Trifluoroacetylamino)ethyl]-6,7-dimethoxy-
1,2,3,4-tetraliydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
1-[2-(trifluoroacetylamino)ethylidene]-6,7-dimethoxy-
1,2,3,4-tetrahydronaphthalene (yield 53%), m.p.76-78 C
(recrystallized from ethyl acetate/hexane).
NMR(CDC13)S: 1.60-2.10(6H,m), 2.69(2H,t,J=5.5Hz), 2.75-
2.87(lH,m), 3.40-3.57(2H,m), 3.84(3H,s), 3.86(3H,s),
6.33(1H,br s), 6.57(1H,s), 6.63(1H,s).
Elemental Analysis for C16H2pF3N03:
Calcd.: C, 58.00; H, 6.08; N, 4.23.
WO 96108466 2jQ3Z~C~ 8 PGT/JP95/01796
- 9181-
Found : C, 57.94; H, 5.94; N, 4.37.
Working Example 40
1'-[2-(Trifluoroacetylamino)ethyl]-1,3,3',4'-
tetrahydro-7'-methoxyspiro[2H-indene-2,2'(1'H)-
naphthalene]
By substantially the same procedure as in
Reference Example 6, 7, Working Example 11, Reference
Example 3-B and Working Example 1, the above-titled
compound was produced from 1,3,3',4'-
tetrahydrospiro[2H-indene-2,2'(1'H)-naphthalene]-1'-one
(yield 13%), m.p.149-152 C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)5: 1.60-2.12(4H,m), 2.44-3.13(7H,m), 3.20-
3.72(2H,m), 3.80(3H,s), 6.10(1H,br s),
6.58(1H,d,J=2.6Hz), 6.77(1H,dd,J=2.6Hz,8.2Hz), 7.10-
7.22(5H,m).
Elemental Analysis for C23HZ,,F3NOZ:
Calcd.: C, 68.47; H, 6.00; N, 3.47.
Found : C, 68.69; H, 5.98; N, 3.55.
Working Example 41
l'-[2-(Acetylamino)ethyl]-1,3,3',4'-tetrahydro-7'-
methoxyspiro[2H-indene-2,2'(1'H)-naphthalene]
By substantially the same procedure as in
Reference Example 6, 7, Working Example 11, Reference
Example 3-B and Working Example 1, the above-titled
compound was produced from 1,3,3',4'-
tetrahydrospiro[2H-indene-2,2'(1'H)-naphthalen]-1'-one
(yield 16%), m.p.139-141 C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)S: 1.41-2.06(4H,m), 1.92(3H,s), 2.45-
3.10(7H,m), 3.10-3.60(2H,m), 3.80(3H,s), 5.38(1H,br s),
6.63(1H,d,J=2.6Hz), 6.74(1H,dd,J=2.6Hz,8.2Hz), 7.00-
7.21(5H,m).
Elemental Analysis for C2.3HZ7NOZ:
Calcd.: C, 79.05; H, 7.79; N, 4.01.
Found : C, 79.23; H, 7.82; N, 4.00.
2193398 PCT/3P95/01796
WO 96(08466
- 99 -
Working Example 42
(E)-9-[2-(Acetylamino)ethylidene]-2-
isopropoxy-6,7,8,9-tetrahydro-5H-benzocycloheptene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled
compound was produced from 9-(2-aminoethylidene)-2-
isopropoxy-6,7,8,9-tetrahydro-SH-benzocycloheptene and
acetyl chloride (yield 80%). The mixture was subjected
to a silica gel chromatography to separate the above-
titled compound in a pure state (yield 33%, oil).
NMR(CDC13)6: 1.32(6H,d,J=6.OHz), 1.71(4H,br s),
2.01(3H,s), 2.40(2H,br s), 2.65(2H,t,J=5.4Hz),
4.02(2H,t,J=5.5Hz), 4.52(1H,m), 5.43(1H,t,J=6.9Hz),
5.68(lH,br s), 6.60-6.72(2H,m), 6.97(1H,d,J=8.4Hz).
Elemental Analysis for C18HuNO2.:
Calcd.: C, 75.22; H, 8.77; N, 4.87.
Found : C, 75.41; H, 8.58; N, 4.81.
Working Example 43
9-[2-(Acetylamino)ethyl]-2-isopropoxy-6,7,8,9-
tetrahydro-5H-benzocycloheptene
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
9-[2-(acetylamino)ethylidene]-2-isopropoxy-6,7,8,9-
tetrahydro-5H-benzocycloheptene (yield 92%, oil).
NMR(CDC13)S: 1.32(6H,d,J=6.2Hz), 1.50-2.20(8H,m),
1.91(3H,s), 2.70-2.95(3H,m), 3.07-3.50(2H,m),
4.51(1H,m), 5.45(lH,br s), 6.60(1H,dd,J=2.6Hz,8.1Hz),
6.66(1H,d,J=2.6Hz), 6.98(1H,d,J=8.1Hz).
Elemental Analysis for C18HZ7NO2:
Calcd.: C, 74.70; H, 9.40; N, 4.84.
Found : C, 74.58; H, 9.37; N, 4.76.
Working Example 44
(E)-9-[2-(Trifluoroacetylamino)ethylidene]-2-
isopropoxy-6,7,8,9-tetrahydro-5H-benzocycloheptene
By substantially the same procedure as in Working
Example 1, a mixture of isomers of the above-titled -
R'O 96/08466 217// 78 PCT/JP95/01796
- 100 -
compound from 9-(2-aminoethylidene)-2-isopropoxy-
6,7,8,9-tetrahydro-5H-benzocycloheptene and
trifluoroacetic anhydride (yield 89%). The mixture was
subjected to a silica gel chromatography to separate
the above-titled compound in a pure state (yield 38%,
oil).
NMR(CDC13)S: 1.33(6H,d,J=6.2Hz), 1.73(4H,br s),
2.42(2H,br s), 2.65(2H,br s), 4.14(2H,t,J=6.4Hz),
4.52(1H,m), 5.44(1H,t,J=7.OHz), 6.40(1H,br s), 6.63-
6.74(2H,m), 6.98(1H,d,J=8.4Hz).
Elemental Analysis for C18H22F3NOZ:
Calcd.: C, 63.33; H, 6.50; N, 4.10.
Found : C, 63.51; H, 6.62; N, 4.26.
Working Example 45
9-[2-(Trifluoroacetylamino)ethyl]-2-isopropoxy-
6,7,8,9-tetrahydro-5H-benzocycloheptene
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
9-[2-(tri~luoroacetylamino)ethylidene]-2-isopropoxy-
6,7,8,9-tetrahydro-5H-benzocycloheptene (yield 91%,
oil).
NMR(CDC13)S: 1.32(6H,d,J=6.2Fiz), 1.45-2.25(8H,m), 2.70-
2.90(3H,m), 3.17-3.60(2H,m), 4.51(1H,m), 6.20(1H,br s),
6.58-6.68(2H,m), 6.99(1H,d,J=8.4Hz).
Elemental Analysis for C18H24F3N02:
Calcd.: C, 62:96; H, 7.04; N, 4.08.
Found : C, 62.81; H, 7.00; N, 3.97.
Working Example 46
6-Methoxy-2,2-dimethyl-l-[2-
(trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-methoxy-2,2-dimethylindan
hydrochloride and trifluoroacetic anhydride (yield 93%,
oil).
NMR(CDC13)S: 1.01(3H,s), 1.12(3H,s), 1.61-1.97(2H,m),
WO 96108466 219 3 3 9 8 PCT1JP95101796
- 101 -
2.51-2.71(3H,m), 3.53(2H,dd,J=7.2Hz, 13.8Hz),
3.80(3H,s), 6.32(1H,br s), 6.70(1H,dd,J=2.4Hz, 8.0Hz),
6.76(1H,d,J=2.4Hz), 7.07(1H,d,J=8.OHz).
Working Example 47
(E)-6-Methoxy-2,2-dimethyl-l-[2-
(trifluoroacetylamino)ethylidene]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
(E)-1-(2-aminoethylidene)-6-methoxy-2,2-dimethylindan
and trifluoroacetic anhydride (yield 96%, oil).
NMR(CDC11)5: 1.37(6H,s), 2.83(2H,s), 3.81(3H,s),
4.32(2H,dd,J=6.OHz, 7.4Hz), 5.82(1H,t,J=7.4Hz),
6.36(lH,br s), 6.83(1H,dd,J=2.4Hz, 8.0Hz),
6.89(1H,d,J=2.4Hz), 7.10(1H,d,J=8.OHz).
Working Example 48
(Z)-6-Methoxy-2,2-dimethyl-l-[2-
(trifluoroacetylamino)ethylidene]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
(Z)-1-(2-aminoethylidene)-6-methoxy-2,2-dimethylindan
and trifluoroacetic anhydride (yield 43%), m.p.107-
108 C (recrystallized from isopropyl ether/hexane).
NMR(CDC13)S: 1.20(6H,s), 2.75(2H,s), 3.81(3H,s),
4.41(2H,t,J=6.4Hz), 5.35(1H,t,J=6.4Hz), 6.39(1H,br s),
6.85(1H,dd,J=2.2Hz, 8.4Hz), 6.95(1H,d,J=2.2Hz),
7.17(1H,d,J=8:4Hz).
Elemental Analysis for CL6H18F3NOZ:
Calcd.: C, 61.34; H, 5.79; N, 4.47.
Found : C, 61.25; H, 5.92; N, 4.52.
Working Example 49
6-Methoxy-l-[3-(trifluoroacetylamino)propyl]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(3-aminopropyl)-6-methoxyindan and trifluoroacetic
anhydride (yield 97%, oil).
NMR(CDC13)S: 1.40-1.94(5H,m), 2.01-2.38(1H,m), 2.69-
W0 96108466 2193398 PCT/JP95/01796
- 102 -
2.90(2H,m), 3.02-3.18(1H,m), 3.42(2H,q,J=6.6Hz),
3.80(3H,s), 6.30(1H,br s), 6.69-6.75(2H,m), 7.08-
7.15(1H,m).
Working Example 50
1-(3-(Acetylamino)propyl]-6-methoxyindan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(3-aminopropyl)-6-methoxyindan and acetyl chloride
(yield 69%), m.p.74-75 C (recrystallized from isopropyl
ether).
NMR(CDC13)S: 1.35-1.92(5H,m), 1.98(3H,s), 2.20-
2.38(1H,m), 2.71-2.90(2H,m), 3.02-3.16(1H,m),
3.29(2H,dd,J=7.0Hz, 13.0Hz), 3.80(3H,s), 5.49(1H,br s),
6.67-6.74(2H,m), 7.08-7.14(1H,m).
Elemental Analysis for CuH21NOZ:
Calcd.: C, 72.84; H, 8.56; N, 5.66.
Found : C, 72.81; H, 8.49; N, 5.97
Working Example 51
6-Methoxy-l-[2-(propionylamino)ethyl]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-methoxyindan and propionyl chloride
(yield 60%), m.p.76-77 C (recrystallized from isopropyl
ether/hexane).
NMR(CDC13)S: 1.15(3H,t,J=7.8Hz), 1.50-1.80(2H,m), 1.97-
2.40(2H,m), 2:19(2H,q,J=7.8Hz), 2.68-2.95(2H,m), 3.04-
3.18(1H,m), 3.33-3.45(2H,m), 3.79(3H,s), 5.46(1H,br s),
6.68-6.76(2H,m), 7.11(1H,d,J=8.OHz).
Elemental Analysis for C15H21NO2,:
Calcd.: C, 72.84; H, 8.56; N, 5.66.
Found : C, 72.82; H, 8.42; N, 5.62
Working Example 52
(E)-5,6,7-Trimethoxy-l-(2-(trifluoroacetylamino)
ethylidene]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
= WO 96/08466 21 733! U PCT/JP95101796
- 103 -
(E)-1-(2-aminoethylidene)-5,6,7-trimethoxyindan and
trifluoroacetic anhydride (yield 83%), m.p.97-98 C
(recrystallized from isopropyl ether).
NMR(CDC13)S: 2.75-2.83(2H,m), 2.90-3.02(2H,m),
3.84(3H,s), 3.86(3H,s), 3.93(3H,s), 4.12(2H,t,J=6.4Hz),
6.20-6.40(2H,m), 6.57(1H,s).
Elemental Analysis for C16H18F3NO4:
Calcd.: C, 55.65; H, 5.25; N, 4.06.
Found : C, 55.64; H, 5.23; N, 4.10
Working Example 53
5,6,7-Trimethoxy-l-[2-
(trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
(E)-5,6,7-trimethoxy-l-[2-(trifluoroacetylamino)
ethylidene]indan as an oily product. The yield was
97%.
NMR(CDC13)S: 1.62-1.95(2H,m), 2.18-2.38(1H,m), 2.69- -
2.84(1H,m), 2.90-3.12(2H,m), 3.21-3.34(1H,m), 3.55-
3.77(2H,m), 3.84(3H,s), 3.85(3H,s), 3.93(3H,s),
6.60(2H,br s).
Working Example 54
(E)-5-Bromo-6-methoxy-7-methyl-l-[2-
(trifluoroacetylamino)ethylidene]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
(E)-l-(2-aminoethylidene)-5-bromo-6-methoxy-7-
methylindan and trifluoroacetic anhydride (yield 89%),
m.p.138-139 C (recrystallized from isopropyl ether).
NMR(CDC13)6: 2.44(3H,s), 2.76-2.86(2H,m), 2.88-
2.99(2H,m), 3.76(3H,s), 4.15(2H,t,J=6.4Hz), 5.87-
5.96(1H,m), 6.41(1H,br s), 7.32(1H,s).
Elemental Analysis for Ci5H15BrF3NO2:
Calcd.: C, 47.64; H, 4.00; N, 3.70.
Found : C, 47.59; H, 3.96; N, 3.60.
Working Example 55
WO 96/08466 21J J39U PCT/JP95/01796
- 104 -
6-Methoxy-7-methyl-l-[2-
(trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
(E)-5-bromo-6-methoxy-7-methyl-l-[2-
(trifluoroacetylamino)ethylidene]indan (yield 68%),
m.p.126-127 C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)S: 1.62-2.32(4H,m), 2.16(3H,s), 2.74-
3.05(2H,m), 3.22-3.58(3H,m), 3.81(3H,s), 6.17(1H,br s),
6.69(1H,d,J=8.2Hz), 7.02(1H,d,J=8.2Hz).
Elemental Analysis for C15HL8F3N0Z:
Calcd.: C, 59.79; H, 6.02; N, 4.65.
Found : C, 59.96; H, 5.95; N, 4.62.
Working Example 56
1-[2-(Crotonoylamino)ethyl]-6-methoxyindan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-methoxyindan and crotonoyl chloride
(yield 37%), m.p.107-1080C (recrystallized from ethyl
acetate/isopropyl ether).
NMR(CDC13)S: 1.52-1.78(2H,m), 1.85(3H,dd,J=1.6Hz,
6.8Hz), 2.01-2.18(1H,m), 2.25-2.41(1H,m), 2.69-
2.95(2H,m), 3.05-3.20(lH,m), 3.40-3.51(2H,m),
3.79(3H,s), 5.47(1H,br s), 5.77(lH,dd,J=1.6Hz, 15.2Hz),
6.70-6.93(3H,m), 7.11(1H,d,J=8.2Hz).
Elemental Analysis for C16HZ1NO2:
Calcd.: C, 74.10; H, 8.16; N, 5.40.
Found : C, 73.80; H, 8.22; N, 5.28.
Working Example 57
6-Methoxy-l-[2-(methoxycarbonylamino)ethyl]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-methoxyindan and methyl
chloroformate (yield 67%) as an oily product.
NMR(CDC13)S: 1.50-1.80(2H,m), 1.97-2.14(1H,m), 2.23-
WO 96/08466 2193393 PCT/JP95101796
- 105 -
2.40(1H,m), 2.69-2.96(2H,m), 3.02-3.20(1H,m), 3.25-
3.38(2H,m), 3.68(3H,s), 3.80(3H,s), 4.72(1H,br s),
6.72(1H,dd,J=2.6Hz, 8.4Hz), 6.74(1H,br s),
7.11(1H,d,J=8.4Hz).
Working Example 58
(E)-7-Bromo-6-methoxy-5-methyl-l-(2-
(trifluoroacetylamino)ethylidene]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
(E)-1-(2-aminoethylidene)-7-bromo-6-methoxy-5-
methylindan and trifluoroacetic anhydride (yield 88%),
m.p.117-118 C (recrystallized from ethyl
acetate/isopropyl ether).
NMR(CDC13)S: 2.33(3H,s), 2.78-2.88(2H,m), 2.90-
2.98(2H,m), 3.79(3H,s), 4.17(2H,t,J=6.2Hz), 6.42(1H,br
s), 6.81-6.91(lH,m), 7.03(1H,s).
Elemental Analysis for C15HLSBrF3NOZ:
Calcd.: C, 47.64; H, 4.00; N, 3.70.
Found : C, 47.85; H, 3.90; N, 3.75
Working Example 59
6-Methoxy-5-methyl-l-[2-
(trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 11, the above-titled compound was produced from
(E)-7-bromo-6-methoxy-5-methyl-l-[2-
(trifluoroacetylamino)ethylidene]indan (yield 49%),
m.p.105-1-06 C (recrystallized from ethyl
acetate/hexane).
NMR(CDC13)S: 1.61-1.80(2H,m), 2.04-2.41(2H,m),
2.19(3H,s), 2.70-2.96(2H,m), 3.08-3.21(1H,m),
3.50(2H,q,J=7.OHz), 3.82(3H,s), 6.30(1H,br s),
6.68(1H,s), 7.00(1H,s).
Elemental Analysis for C15Hi8F3NO2:
Calcd.: C, 59.79; H, 6.02; N, 4.65.
Found : C, 59.44; H, 6.04; N, 4.71
Working Example 60
WO 96/08466 2 193 3 g8 PCT7JP95/01796
- 106 -
1-[2-(Acetylamino)ethyl]-6-hydroxyindan
To a solution of 1-[2-(acetylamino)ethyl]-6-
methoxyindan (6.40 g, 27.4 mmol) in dichloromethane
(150 ml) was added dropwise, under ice-cooling, boron
tribromide (5.19 ml, 54.8 mmol). The mixture was then
stirred for one hour. The reaction mixture was poured
into water. The mixture was stirred for 8 hours at
room temperature, which was subjected to extraction
with chloroform. The extract solution was washed with
brine, which was dried over anhydrous magnesium
sulfate, followed by distilling off the solvent under
reduced pressure. The residue was recrystallized from
ethy7 acetate/isopropyl ether to give 4.70 g (yield
78%) of the above-titled compound, m.p.107-108 C.
NMR(CDC13)S: 1.50-1.75(2H,m), 1.90-2.08(1H,m),
1.98(3H,s), 2.20-2.35(1H,m), 2.66-2.87(2H,m), 2.98-
3.13(lH,m), 3.35(2H,dd,J=7.OHz, 13.2Hz), 5.80(1H,br s),
6.64-6.74(2H,m), 7.04(1H,d,J=7.BHz).
Elemental Analysis for C13H17NO2:
Calcd.: C, 71.21; H, 7.81; N, 6.39.
Found : C, 70.93; H, 7.90; N, 6.21.
Working Example 61
1-Benzyloxycarbonyl-N-[2-(6-methoxyindan-l-
yl)ethyl]-4-piperidinecarboxamide
To a solution of 1-(2-aminoethyl)-6-methoxyindan
(1.00 g, 5.23 mmo1), 1-benzyloxycarbonyl-4-
piperidinecarboxylic acid (1.38 g, 5.23 mmol) and 1-
hydroxy-lH-benzotriazole (0.78 g, 5.75 mmol) in DMF (20
ml) was added 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (WSC;
1.10 g, 5.75 mmol). The mixture was stirred for 6
hours at room temperature. To the reaction mixture was
added water, and the mixture was subjected to
extraction with ethyl acetate. The extract solution
was washed with brine, which was dried over anhydrous
magnesium sulfate, followed by distilling off the
WO 96108466 21p3JZ9~9 PCT1JP95101796
- 107 - U
solvent under reduced pressure. The residue was
purified by means of a silica gel column chromatography
(chloroform/methanol, 95:5) to give 2.10 g (yield 92%)
of the above-titled compound, m.p.132-133 C
(recrystallized from ethyl acetate/isopropyl ether).
NMR(CDC13)6: 1.50-1.88(6H,m), 1.95-2.40(3H,m), 2.70-
2.95(4H,m), 3.02-3.20(1H,m), 3.33-3.45(2H,m),
3.78(3H,s), 4.13-4.26(2H,m), 5.12(2H,s), 5.42(1H,br s),
6.67-6.74(2H,m), 7.1l(1H,d,J=7.6Hz), 7.35(5H,s).
Elemental Analysis for Ca6H3zNz0o=
Calcd.: C, 71.53; H, 7.39; N, 6.42.
Found : C, 72.00; H, 7.53; N, 7.09.
Working Example 62
6-Ethoxy-l-[2-(trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-ethoxyindan and trifluoroacetic
anhydride (yield 36%), m.p.85-86 C (recrystallized from
isopropyl ether/hexane).
NMR(CDCl3)8:'1.41(3H,t,J=7.0Hz), 1.62-1.81(2H,m), 2.04-
2.20(1H,m), 2.28-2.41(lH,m), 2.71-2.98(2H,m), 3.08-
3.21(1H,m), 3.50(2H,q,J=7.OHz), 4.02(2H,q,J=7.OHz),
6.32(1H,br s), 6.71-6.76(2H,m), 7.12(1H,d,J=8.4Hz).
Elemental Analysis for CuH18F3NOZ:
Calcd.: C, 59.79; H, 6.02; N, 4.65.
Found : C, 59.31; H, 6.00; N, 4.85
Working Example 63
6-(2-Phenylethoxy)-1-[2-
trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 1, the above-titled compound was produced from
1-(2-aminoethyl)-6-(2-phenylethoxy)indan and
trifluoroacetic anhydride (yield 63%), m.p.114-115 C
(recrystallized from isopropyl ether/hexane).
NMR(CDC13)5: 1.60-1.80(2H,m), 2.02-2.18(1H,m), 2.22-
2.40(lH,m), 2.70-2.95(2H,m), 3.04-3.19(3H,m),
WO 96/08466 21(a33 C~ g PCI'/JP95/01796
- 108 -
3.48(2H,q,J=7.2Hz), 4.15(2H,t,J=7.OHz), 6.23(1H,br s),
6.69-6.74(2H,m), 7.10(1H,d,J=8.6Hz), 7.20-7.38(5H,m).
Elemental Analysis for CZ1HZZF~N0Z: _
Calcd.: C, 66.83; H, 5.88; N, 3.71.
Found : C, 66.86; H, 5.77; N, 4.04.
Working Example 64
1-[2-(Acetylamino)ethyl]-1,2,3,4-tetrahydro-7-(N-
methylamino)naphthalene
To a solution of 1-(2-aminoethyl)-7-formylamino-
1,2,3,4-tetrahydronaphthalene (1.80 g, 8.25 mmol) in
THF (20 ml) was added added, under ice-cooling, lithium
aluminum hydride (0.63 g, 16.5 mmol). The mixture was
heated for 4 hours under reflux under argon atmosphere.
The reaction mixture was cooled with ice, to which was
added water (0.9 ml), followed by further addition of
ethyl acetate, anhydrous magnesium sulfate and celite,
successively. The mixture was subjected to filtration,
and the filtrate was concentrated under reduced
pressure. The concentrate was dissolved in DMF (20
ml). To the solution was added 4-nitrophenyl acetate
(1.33 g, 7.34 mmol), and the mixture was stirred for 20
minutes at room temperature. To the reaction mixture
was added water, which was subjected to extraction with
ethyl acetate. The extract solution was washed with
brine, which was dried over anhydrous magnesium
sulfate, followed by distilling off the solvent under
reduced pressure. The residue was purified by means of
a silica gel column chromatography (methanol/ethyl
acetate, 97:3 - 96:5) to give 1.10 g (yield 54%) of the
above-titled compound as an oily product.
NMR(CDC13)S: 1.60-2.00(6H,m), 1.95(3H,s), 2.60-
2.85(3H,m), 2.81(3H,s), 3.20-3.50(3H,m), 5.63(1H,br s),
6.40-6.47(2H,m), 6.89(1H,d,J=7.8Hz).
Working Example 65
(R)-1-[2-(Acetylamino)ethyl)]-6-methoxyindan
1-[2-(Acetylamino)ethyl]-6-methoxyindan was
WO 96108466 2193398 PCTIJP95101796
- 109 -
subjected to optical resolution by means of a high
performance liquid chromatography (pump, L-6000;
detector, L-4000; date processing device, D-2500;
autosampler, AS-2000; column, Ceramospher RU-1: mobile
phase, methanol; flow rate, 0.6ml/min; column
temperature, 50 C; detective wave-length, 290 nm;
sample concentration, 6%; volume to be fed, 0.1 ml;
feeding frequence, 100 times) to give the above-titled
compound (99 mg), m.p.95-96 C. [a]H8365 -61.3 (c 0.3,
CHC13 ) .
Working Example 66
(S)-1-[2-(Acetylamino)ethyl]-6-methoxyindan
By substantially the same procedure as in Working
Example 65, 1-[2-(acetylamino)ethyl]-6-methoxyindan was
subjected to optical resolution by means of a high
performance liquid chromatography to give the above-
titled compound (119 mg), m.p.93-940C. [a] Hg365 +80.7
(c 0.3, CHC13) .
Working Example 67
(R)-1-[2-(Trifluoroacetylamino)ethyl]-6-
methoxyindan
A mixture of (R)-1-[2-(acetylamino)ethyl]-6-
methoxyindan (50 mg, 0.21 mmol) and hydrazine hydrate
(1 ml) was heated under reflux for 26 hours under argon
atmosphere. The reaction mixture was cooled, to which
was added brine, followed by extraction with
chloroform. The extract solution was washed with
brine, followed by drying over anhydrous magnesium
sulfate. The solvent was distilled off under reduced
pressure to give 39 mg (yield 95%) of (R)-1-(2-
aminoethyl)-6-methoxyindan as an oily product. This
product was dissolved in chloroform (0.8 ml), to which
was added triethylamine (57 L, 0.41 mmol), and the
mixture was cooled with ice. To the mixture was added
dropwise trifluoroacetic anhydride (43 L õ 0.31 mmol),
which was stirred for 20 minutes. To the reaction
WO 96/08466 2193398 PCT7JP95/01796
- 110 -
mixture was added water, which was subjected to
extraction with ethyl acetate. The extract solution
was washed with brine, which was dried over anhydrous
magnesium sulfate, followed by distillation of the
solvent under reduced pressure. The residue was
purified by means of a silica gel column chromatography
(hexane/ethyl acetate, 7:3) to give 41 mg (yield 70%)
of the above-titled compound, m.p.65-66 C
(recrystallized from isopropyl ether/hexane).
[a]Hg365 -51.8 (c 0.3, CHC13)
Working Example 68
(S)-1-[2-Trifluroacetylamino)ethyl]-6-methoxyindan
By substantially the same procedure as in Working
Example 67, 38 mg (yield 93%) of (S)-1-(2-aminoethyl)-
6-methoxyindan was produced from (S)-1-[2-
(acetylamino)ethyl]-6-methoxyindan (50 mg, 0.21 mmol).
A small portion of this product was converted to
hydrochloride using HC1/ethanol. The product was
precipitated by the addition of diethyl ether.
[a]p -30.0 (c 0.15, HZ0) m.p.180-181 C. The free base
was subjected to trifluoroacetylation in substantially
the same manner as in Working Example 67 to give the
above titled compound (yield 63%), m.p.65-66 C
(recrystallized from isopropyl ether/hexane). [a]H8aes
+54.9 (c 0.3, CHC13).
Working Example 69
1-[2-(Isobutyrylamino)ethyl]-6-methoxyindan
By substantially the same procedure as in Working
Example 1, the above-titled compound was prepared from
1-(2-aminoethyl)-6-methoxyindan and isobutyryl chloride
(yield 94%), m.p. 104-105 C (recrystallized from ethyl
acetate/isopropyl ether).
NMR(CDC13)S: 1.14(6H,d,J=6.6Hz), 1.50-1.81(2H,m),
1.96-2.14(1H,m), 2.25-2.40(2H,m), 2.68-2.95(2H,m),
3.02-3.18(1H,m), 3.32-3.44(2H,m), 3.78(3H,s),
5.49(lH,br s), 6.67-6.75(2H,m), 7.11(1H,d,J=8.OHz).
WO 96/08466 217 PCTlJP95/01796
p~~~p tJ
- 111 -
Elemental Analysis for C16HZ3N0Z:
Calcd.: C, 73.53; H, 8.87; N, 5.36.
Found : C, 73.64; H, 9.02; N, 5.35.
Working Example 70
(S)-1-[2-(3-Ethylureido)ethyl]-6-methoxyindan
To a suspension of (S)-1-(2-aminoethyl)-6-
methoxyindan hydrochloride (0.lOg, 0.44mmol) and
triethylamine (61 1, 0.44mmo1) in acetonitrile (3m1)
was added ethyl isocyanate (35 1, 0.44mmol). The
mixture was stirred for 30 min at room temperature,
then diluted with water and the product was extracted
with ethyl acetate. The extract was washed with 1N
hydrochloric acid and brine, dried-over anhydrous
magnesium sulfate, and the solvent was removed in
vacuo. The residue was recrystallized from ethyl
acetate and isopropyl ether to afford the titled
compound (79mg, yield 69%), m.p. 104-105 C. [a.]x8365
+48.8 (c 0.5, CHC13)
NMR(CDC13)S: 1.14(3H,t,J=7.2Hz), 1.52-1.80(2H,m),
1.97-2.15(1H,m), 2.26-2.40(1H,m), 2.68-2.95(2H,m),
3.07-3.37(5H,m), 3.79(3H,s), 4.20-4.35(2H,m),
6.68-6.75(2H,m), 7.11(1H,d,J=8.2Hz).
Working Example 71
1-[2-(4-Bromobenzoylamino)ethyl]-6-methoxyindan
By substantially the same procedure as in Working
Example 1, the above-titled compound was prepared from
1-(2-aminoethyl)-6-methoxyindan and 4-bromobenzoyl
chloride (yield 94%), m.p. 142-143 C (recrystallized
from ethyl acetate).
NMR(CDC13)6: 1.62-1.95(2H,m), 2.03-2.24(1H,m),
2.26-2.43(1H,m), 2.70-2.96(2H,m), 3.12-3.30(1H,m),
3.56-3.63(2H,m), 3.77(3H,s), 6.09(1H,br s),
6.68-6.77(2H,m), 7.12(1H,d,J=8.OHz), 7.55(4H,s).
Elemental Analysis for C19HZoBrNO2:
Calcd.: C, 73.53; H, 8.87; N, 5.36.
Found : C, 73.64; H, 9.02; N, 5.35.
R'O 96/08466 21193398 PCI'/JP95101796
~
- 112 -
Working Example 72
6-Methoxy-2-phenyl-l-[2-
(trifluoroacetylamino)ethyl]indan
By substantially the same procedure as in Working
Example 11, the above-titled compound was prepared from
5-methoxy-2-phenyl-3-[2-
(trifluoroacetylamirio)ethyl]-1H-indene (yield 68%),
m.p. 109-111 C (recrystallized from ethyl acetate and
hexane).
NMR(d6-DMSO)S: 1.20-1.45(2H,m), 2.95-3.38(5H,m),
3.70-3.82(1H,m), 3.75(3H,s), 6.76(1H,dd,J=2.4Hz,8.2Hz),
6.90(1H,d,J=2.4Hz), 7.16-7.36(6H,m), 9.31(1H,br s).
Elemental Analysis for C20H20F3N02:
Calcd.: C, 66.11; H, 5.55; N, 3.85; F, 15.68.
Found : C, 66.04; H, 5.58; N, 3.79; F, 15.73.
Working Example 73
1-[3-(Trifluoroacetylamino)propyl]-7-
methoxy-1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound was prepared from
4-[3-(trifluoroacetylamino)propyl]-6-methoxy-l,2-
dihydronaphthalene (yield 91%, oil).
NMR(CDC13)8: 1.53-1.90(8H,m), 2.69(2H,t,J=5.7Hz),
2.77(1H,m), 3.30-3.46(2H,m), 3.78(3H,s), 6.33(1H,br s),
6.65-6.73(2H,m), 6.99(1H,d,J=9.2Hz).
Elemental Analysis for C16H2OF3NOZ: _
Calcd.: C, 60.94; H, 6.39; N, 4.44.
Found : C, 61.01; H, 6.30; N, 4.39.
Working Example 74
1-[3-(Acetylamino)propyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound was prepared from
4-[3-(acetylamino)propyl]-6-methoxy-l,2-
dihydronaphthalene (yield 84%), m.p. 71-73 C
(recrystallized from ethyl acetate and hexane).
= WO 96108466 21{~ 33~ 8 PCl'lJP95101796
- 1137 -
NMR(CDC13)S: 1.50-1.95(SH,m), 1.97(3H,s),
2.61-2.81(3H,m), 3.20-3.35(2H,m), 3.78(3H,s),
5.47(1H,br s), 6.63-6.71(2H,m), 6.98(1H,d,J=9.2Hz).
Elemental Analysis Calcd for Cl6HZ3NOZ:
Calcd.: C, 73.53; H, 8.87; N, 5.36.
Found : C, 73.26; H, 8.66; N, 5.38.
Working Example 75
1-[4-(Trifluoroacetylamino)butyl]-7-
methoxy-1,2,3,4-tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound was prepared from
4-[4-(trifluoroacetylamino)butyl]-6-methoxy-1,2-
dihydronaphthalene (yield 58%, oil).
NMR(CDC13)S: 1.30-1.90(10H,m), 2.61-2.80(3H,m),
3.38(2H,q,J=6.7Hz), 3.78(3H,s), 6.33(1H,br s),
6.64-6.72(2H,m), 6.98(1H,d,J=9.1Hz).
Elemental Analysis for C17HZZF3N02:
Calcd.: C, 61.99; H, 6.79; N, 4.25; F, 17.30.
Found : C, 61.79; H, 6.72; N, 4.11; F, 17.25.
Working Example 76
1-[4-(Acetylamino)butyl]-7-methoxy-1,2,3,4-
tetrahydronaphthalene
By substantially the same procedure as in Working
Example 11, the above-titled compound was prepared from
4-[4-(acetylamino)butyl]-6-methoxy-1,2-
dihydronaphthalene (yield 86%, oil).
NMR(CDC13)S: 1.30-1.90(10H,m), 1.97(3H,s),
2.62-2.80(3H,m), 3.25(2H,q,J=6.4Hz), 3.78(3H,s),
5.52(1H,br s), 6.63-6.72(2H,m), 6.98(1H,d,J=8.8Hz).
Elemental.Analysis for C17H25N0Z:
Calcd.: C, 74.14; H, 9.15; N, 5.09.
Found : C, 73.92; H, 9.10; N, 5.04.
The chemical structures of the compounds obtained
by the working Examples are as follows.
WO 96108466 219339 8 PCTl3P95/01796
- 114 -
CN NC NH CN
H3COI\ I H3CO I\ I HgCO '\ I 2 H3CO I\
Reference Example 1 Reference Example 2 Reference Example 3 Reference Example
4
1 NH2 CN CN N
'
H
HyCO \ H3CO I\ 0 CH3 H3CO \ CHa H3CO C a
CH3 CH3
Reference Example 5 Reference Example 6 Reference Example 7 Reference Example
8
CN CN
NH2 NHz I
H3CO I\ I H3CO I\ I H3CO I H3CO
/ I H3CO
~
Reference Example 9 Reference Example 10 Reference Example 11 Reference
Example 12
NH2 , CN NHt
H3CO I\ H3CO CH HyC \ I H,C \ S
H,CO / HCI CH3 I/ ~/
=HCI CH3 CHa
Reference Example 13 Reference Example 14 Reference Example 15 Reference
Example 16
CN NHz OII CN
H3CO ~ I H3CO \ I H3C~O H,C 0
~ / CH, ,.
H,CO ~ / HjCO ' / CH3
Reference Example 17 Reference Example 18 Reference Example 19 Reference
Example 20
~ NH2 0 CN NH2
HOCYO HaCO I\ CH HOCO I\ I CH HjCO \ CH
a a a
CH3 CH, CH3 / CHg
=HCI
Reference Example 21 Reference Example 22 Reterence Example 23 Reference
Example 24
~ WO 96/08466 219 3 3 9 8 PCP/JP95/01796
- 115 -
0
H3CO NH2 H3CO HzN I 0~CH3 OH
CH3 \ CH3 H3CO I\ H3CO I\
= I ! CH3 I ! CH3
Reference Example 25 Reference Example 26 Reference Example 27 Reference
Example 28
Br CN NH2 NH2
H3CO \ HAO H3CO
I I
\ ' \
~
Reference Example 29 Reference Example 30 Reference Example 31 Reference
Example 32
OCH3O OCH3 OCH3 NH2 H3CO \
H~CO H3C0 ~ CN H3CO \ ~
I I H~C / / OvCH3
H~CO H3CO H~CO ! 0
Reference Example 33 Reference Example 34 Reference Example 35 Reference
Example 36
Br Br
H3CO I\ H3CO bll-~ HzCO;
H3C ! OEt H3C O~CH3 HaC I! OH
0 O 0
Reference Example 37 Reference Example 38 Reference Example 39
CHs 0 Br O CH3 CN CHa NHx
H~CO - l ~ H3CO HsCO l~ ~ H3CO \ ~
I''
Br H3C Br Br
Reference Example 40 Reference Example 41 Reference Example 42
CN Br
H3CO Br ~ H3CO' NH2 NH2 NHz
\ % l \ C2H50 \ \ O \
!
H3C I! HaC
Reference Example 43 Reference Example 44 Reference Example 45 Reference
Example 46
4
0 OI' NHp
HzN~ OHCNH OHCNH \
I !
Reference Example 47 Reference Example 48 Reference Example 49
WO 96/08466 2193 398 PCT/JP95/01796
- 116 -
0 0 0
I H~CF3 H~CH3 H ~\
()I I \~ NOz
/ /
Reference Example 50 Reference Example 51 Reference Example 52
0 0 N H ~ \ 0 ~ H~CF3
~ \ / H~CF3 / ~
\
Reference Example 53 Reference Example 54
0
0
N x CF3 Br N
H H3CO 0\ H3CO I\\ 0
Reference Example 55 Reference Example 56 Reference Example 57
H H
NH2
N uCF3 N uCH3
H3CO I~ \ H3CO I\ \ IoI H3CO I\ \ I0I
.. /
Reference Example 58 Reference Example 59 Reference Example 60
CN
NHz
H3CO I \ \ H3CO I \ \
,i
Reference Example 61 Reference Example 62
0 0
NAICFg Nlul CH3
H3CO I\ \ H H3CO
~\ \ H
Reference Example 63 Reference Example 64
~ WO 96/08466 2193398 PCT7JP95101796
- 117 -
NH2 0
CN H3
CO
H3CO _ H3CO H CF3
~ / =HCI \ I \ ~ /
Reference Example 65 Reference Example 66 Reference Example 67
WO 96/08466 21933J U PGT/JP95101796 ~
- 118 -
0
NI, CH3 H3 C~N N~
H3CO H HaCO H3CO H
Working Example 1 Working Example 2 Working Example 3
0 0Ir
N~~~CH3 !~
I H ~ H H ~~ OCH3
H3CO H3CO
/ I /
Working Example 4 Working Example 5
x x ~
H CO H N H N i~ OCH3 H CO H CH3 N CF3
a OCH 3 H3CO H
3
Working Example 6 Working Example 7 Working Example 8
O 0 0
N"IV N" v vCH3 SN)~ CH3
H3C0 \ H H3CO \ H H3C0 H
~ / ~ / 1 /
Working Example 9 Working Example 10 Working Example 11
0 0 0
N-kV NKyCH, NxN ~ ~ OCH3
H3CO H H3CO \ H CH3 H3CO \ H H
i/ I/
Working Example 12 Working Example 13 Working Example 14
N" CH3 N" CH3 I H~CF3
H3CO \ S CH3 H3CO \ CH3 H3CO
I/ CH3 ~/ CH3
Working Example 15 Working Example 16 Working Example 17
~ WO 96/08466 2" 93 3 9 '" PGT/JP95101796
- 119 -
0
N 'A,CF3 x x
H N CH3
HaCO I~ H H3CO I~ H CF3 H3CO I~ 3
/ /
Working Example 18 Working Example 19 Working Example 20
O
NCFy Nk CH3
" CF3
~ N
H3CO H H3CO H H CO H CH3
H3CO H3CO ~/ CH3
Working Example 21 Working Example 22 Working Example 23
OH3C CH3
0 0 HN)~ N" 'CH3
N~OCH3 ~/ N" N'CHy H
H3CO H3 H3CO ~ S H3 'CHa H3CO CHa
~/ CH3 CH3 CH3
Working Example 24 Working Example 25 Working Example 26
0
O ~ H ~CF3
N~OCH3 N H3C \
H3CO I H H3CO ~ CH3 I/ OCH3 I/
CH3 ~/ (CH3 CH3
Working Example 27 Working Example 28 Working Example 29
0 0f' 0
ll
~ CH3 N N, CH3 N'k CF3
N N' H CH3 H3C H
H3C H
CH3 H3C ) ~
/
/
CH3 CH3
CH3
Working Example 30 Working Example 31 Working Example 32
WO 96/08466 21p3.JZ O Q PCT/JP95/01796
- 120 -Ju
O 0 0
N )~ CF3 N IkCF3 N )~ CH3
H3CO I\ I H H3CO I\ H HO H
/
Working Example 33 Working Example 34 Working Example 35
0
0
N )~ CF3 N lk CH3 N )~ CH3
H3CO \~ H H3CO \ ~ H H3CO H
I \
H3C0- I/ H3Co I/ H3CO /
Working Example 36 Working Example 37 Working Example 38
lk CF3 HN~CF3 HN~CH3
N
H3CO H
D H3CO CH30 H3CO
Working Example 39 Working Example 40 Working Example 41
0 0
H3C N)~ CH3 H3C N~CH3
~O H ~O H
H3C I \6 H3C \
/ ~/
Working Example 42 Working Example 43
0 0 0
x N ~CF3 N{ ~CF3
H3C I H CF3 H3C H ~
~O \ ~-O \ H3C0 N. CH3
H3C ~ H3C ~ I/ /
CH3
Working Example 44 Working Example 45 Working Example 46
~ WO 96108466 2 1 933 9 a PCT/1P95/01796
-121-
+
0
O HN CF3 H
H3CO N CF3
H3Co / ~ CH~CF3 H3CO CH3
~ H
\ CHs \ CH3 H3
Working Example 47 Working Example 48 Working Example 49
H O 0
N CH3 x
y ~CH3 OCH3 N CF3 N H3CO 0 H3CO / H H3CO H
\ ~ H3C0 \ ~
Working Example 50 Working Example 51 Working Example 52
0 0
OCH3 NA
CFa CH3 N~CF
H3CO / H H3CO
~ I H ~
H3CO \ I B. Working Example 53 Working Example 54
0 0
~ lk~CH3
H CO CH3 HCF3 H3CO H
3 ~
\ I \ I
Working Example 55 Working Example 56
o ~ 0
NAOCH B~ N CF3 N CF3
H~CO i H 3 H3C0 , ~ H H3CO / H
~ \~ \~
\ Me Me
Working Example 57 Working Example 58 Working Example 59
WO 96108466 2193398 PCTIJP95/01796 -122-
.
O 0
N HO H ~CH3 N /
/ HsCO HNUp ~ ~
~ 'I
~ O
Working Example 60 Working Example 61
0 0
H3CV0 / H
N
CF3 o H
~CF3
~I o
Working Example 62 Working Example 63
0
H ~CH3
H3CNH /
\ I
Working Example 64
0 0
N )JI CHa 'A, CH3
H3C0 / H H3Cp / H
ztl (R)-form rn~
(S)-form
Working Example 65 Working Example 66
0 0
N' 'CF3 _"--N t'
" 'CF5
H3CO H H3CO H
(R)-form (S)-form
Working Example 67 Working Example 68
~ WO 96108466 2193398 PCT/JP95101796
- 123 -
O 0
'
NAT/CH3 x I' 11%,
/
H3CO H CH3 H3CO H N CH3
H H
Working Example 69 Working Example 70
0 0
N ~ N~C
H3CO H H3CO / H 3
Br F
~ -
/
Working Example 71 Working Example 72
H H
N CF3 NuCH3 y HaCO / 0 H3CO IoI
~~
Working Example 73 Working Example 74
0 0
H ~CF3 N ~CH3
H3CO H
H3CO /' ~ ~
/
\
Working Example 75 Working Example 76
WO 96/08466 2193398 PCT/JP95/01796
- 124 -
Formulation Example 1 -
(1) Compound of Working Example 68 10.0 g
(2) Lactose 60.0 g
(3) Corn starch 35.0 g
(4) Gelatin 3.0 g
(5) Magnesium stearate 2,0"g
Using 30 ml of a 10 weight % aqueous solution of
gelatin (3.0 g in terms of gelatin), a mixture of 10.0
g of the compound produced in Working Example 68, 60.0
g of lactose and 35.0 g of corn starch was granulated
through a sieve of 1 mm mesh. The granular product was
dried at 40 C, which was sieved again. The granules
thus-obtained were blended with 2.0 g of magnesium
stearate, and the mixture was subjected to compression.
The core tablet thus obtained was sugar-coated with an
aqueous suspension containing sucrose, titanium
dioxide, talc and gum arabic. The coated tablets were
polished with bee-wax to prepare 1000 tablets.
Formulation Example 2
(1) Compound of Working Example 68 10.0 g
(2) Lactose 70.0 g
(3) Corn starch 50.0 g
(4) Soluble starch 7.0 g
(5) Magnesium stearate 3.0 g
-With 70 ml of an aqueous solution of soluble
starch (7.0 g in terms of soluble starch), 10.0 g of
the compound produced in Working Example 68 and 3.0 g
of magnesium stearate were granulated and dried,
followed by blending with 70.0 g of lactose and 50.0 g
of corn starch. The mixture was subjected to
compression to prepare 1000 tablets.
Experimental Example 1
The farebrains of 7-day-old chicken (white
leghorn) were homogenized with ice-cold assay buffer
(50 mM Tris-HC1, pH 7.7 at 25 C) and centrifuged at
44,000 x g for 10 minutes at 4 C. The pellet was
WO 96108466 2 19 3 3 9 8 pC1YJP95101796
- 125 -
washed once with the same buffer and stored at -30 C
until use. For the assay, the frozen tissue pellet was
thawed and homogenized with the assay buffer to make a
protein concentration of 0.3 - 0.4 mg/ml. An 0.4 ml
aliquot of this homogenate was incubated with a test
compound and 80 pM 2-[125I]iodomelatonin in a total
volume of 0.5 ml for 90 minutes at 25 C. The reaction
was terminated by adding 3 ml of ice-cold assay buffer
immediately followed by vacuum filtration on Whatman
GF/B which was further washed twice with 3 ml of ice-
cold assay buffer. The radioactivity on the filter was
determined by means of y-counter. Specific binding was
calculated by subtracting non-specific binding which
was determined in the presence of 10-5M melatonin. The
50% inhibiting concentration (IC50) was determined by
the log-probit analysis.
Table 1
Action of inhibiting 2-[LUI]iodomelatonin binding
Compounds of IC50 (nX)
Working Example
8 0.64
33 0.72
35 0.95
51 0.45
66 0.70
68 0.16
melatonin 1.1
From the results shown in Table 1, it is
considered that the compound (I) of the present
invention has an excellent melatonin receptor agonistic
activity.
WO 96/08466 219 3 3 9 8 pCr/Jp95101796
- 126 -
Industrial Applicability
The compounds (I) of the present.invention and the
compound (Ia) show excellent affinity for a melatonin
receptor, they can provide clinically useful
prophylactic and therapeutic agents of diseases related
with the action of inelatonin in living bodies.