Note: Descriptions are shown in the official language in which they were submitted.
21 93431
._
Polymenization Reactor and Process
FIELD OF THE INVENTION
The present invention relates to a reactor which is particularly
suitable for the solution poly",eri~dtion of olefins.
BACKGROUND OF THE INVENTION
There are a number of processes in which a relatively cooler
o stream of liquid is introd~ ~ced into a relatively warmer solution. One of the
concer.,s is the precipitation of solute from the warmer solution. One way
to minimize this problem is to provide for rapid mixing of the solutions
typically using some type of high intensity shear device such as a paddle
or agitator stirrer. Generally as the conce"l,alion of solute increases
the solution becor"es less Newtonian and the rapid mixing of the relatively
cooler solution and warm solution becomes more difficult. The problem is
acce"l,Jated if the residence time in the mixer should be relatively short.
Further difficulties arise if the solute is difficult to re-dissolve in the
solvent. This may lead to "stranding" or "spaghetti" or precipitate within
the mixer which may ultimately affect the product. This problem is
particularly acute where the process is consL,ai"ed by "heat balance"
issues.
All of the above issues are particularly relevant to bulk mass and
solution pol~ll,eri,alions (as opposed to emulsion and suspension in
which the diluent is usually water and heat of reaction is not a significant
problem) in which there is a need to remove the heat of polymerization
from a reactor. For some reactions this has led to the use of chains of
1 24can.doc 2
2 1 q343 1
reactors with the reactants being heated to successively higher
te",peralures and successively higher conversion in dirrerent reactors.
Generally where the residence time in a reactor is relatively long (e.g. in
the order of hours) and where the mixing time is relatively short (e.g. in
the order of tens of minutes) there may not be too significant a problem.
In the solution poly",eri alion of olefins there are several problems.
The residence time in the reactor is typically quite short and the lifetime of
the catalyst at higher te",,l~eralures is also relatively short. Accordingly it
is necess~ry to thoroughly mix the catalyst with the solution of alpha olefin
"~o"GI~er and polymer quickly. After the catalyst comes to the operaling
temperature of the reactor it has a short half life. The situation becomes
worse where the viscosity of the solution rises (e.g. high concent,dlion of
2 o polymer or cooler solutions - which may be used to produce higher
molecular weight polymer).
Thus it will be apparent that the design of a polymeri,alion
reaction especially one used for the solution polymerization of olefins will
involve compromises involving a desire to match mixing rates to rates of
reaction and balancing such matters as power consumption heat removal
capacity and reliability against capital and operali"g costs.
There have been several approaches to this problem. One
ap~r~ach has been to use tubular reactors. The high surface area of tube
or loop reactors assist in the removal of heat of reaction. However to
avoid problems of polymer precipitation the catalyst feed should be at
te",peralures above the precipitation temperature of the polymer from the
F i ~ 1 24can.doc 3
21q343~
solvent. The obvious answer to this problem is to increase the exit
temperature of the tube or loop reactor to increase the temperature profile
along the tube or loop at higher ter"perdlures but this i"creased
te",peralure typically leads to the formation of more low molecular weight
polymer. The problem is accenluated if the polymeri~alion catalyst
decays with te",peralure and time. Thus there is a limitation on the
temperature increase along the length of a tube or loop reactor. As a
result tube reactors tend to be run at relatively lower polymer
Col ,cenlrations to avoid problems with precipitate. In order to mitigate
these problems tubular reactors also are typically run "~di~h~tically'
(i.e. the heat of polymerization GA~ses a te",peral.lre rise between the
reactor enlrd"ce and exit with the increase being proportional to the
2 o amount of polymer prod~ ~ced).
Stirred tank reactGr~ used in the production of precipitating
polymers under solution conditions may not be limited to using feed
stream tel"peralures above the polymer precipitation tel"peralure the
same way the tubular reactors are. The mixing in such reactors however
is very demanding both on the micro and mauo level. If the micromixing
is not sufficient the catalyst efficiency usually suffers beç~ Ise it
decol"poses before the ",onoi"er diffuses to it. The ",acro",ixing is
particularly important if the polymer produced precipitates at low
temperatures. When the temperature of the feed streams into such
reactors is below the precipitation temperature of the polymer some
polymer precipitation may occur but if the macromixing is very rapid then
p~ 124can.doc 4
21 9:3431
the time during which the polymer precipitates is short and thus the
amount of precipitated polymer is small (and it will re-dissolve during the
time the solution spends in the reactor). If the macromixing is slow cold
regions and dead spots form and polymer precipitates in them (thus
impeding the mixing which son,eli",es c~uses the reactor to plug with
precipitated polymer). The amount of the precipitated polymer and the
time it takes to re-dissolve it increases very rapidly as the average reactor
temperature decreases. In many cases it is desirable to operale the
reactor at low tell,peral-lres so as, for exa",pl_, to facilitate the production
of a high molecular weight polymer. The mixing times required in such
reactors can be so short that it makes it ir"pra~;tical to design fully mixed
reactors which would operale at low temperatures with cold feed streams.
2 o This may still be manageable with low viscosity solutions but with polymersolutions which are very viscous the power requi~e",enls may become
unrealistic. Usually a cor"prol"ise must be made for example the feed
temperal"re or the operating te",peral,Jre are increased. The former
leads to drastically reduced throughput of the polymer the latter to a
polymer of comparatively poor properties. The prior art acknowledges
these difficulties but doesr, l provide many viable alternatives.
U.S. patent 4 283,339 issued Aug. 4 1981 and assigned to
National Distillers and C~,ei"i~l Corp. teaches a process for the solution
polymerization of alpha olefins in which dual autoclaves are used in
tandem. The first reactor is a relatively higher pressure reactor
(e.g. 30 000 psi). The product from the first high pressure reactor is
F ~ 1 24can.doc 5
2193431
cooled to keep the polymer in solution and avoid precipitation and then
introd~ Iced into a second reactor at relatively lower pressures
(e.g. 22 000 psi) and the poly",eri~dlion is finished. The referel,ce does
not teach or s~ ~ggest the type of mixed reactor of the present invention.
U.S. patent 4 496 698 issued Jan. 29 1985 and assigned to The
Dow Chemical Company takes a similar approach to the poly",eri alion of
ethylene in which the first reactor is operaled at pressures of greater than
50 000 kPa (about 7 500 psi) and the polyme, i~alion mass is cooled and
then fed through a cooling heat exchanger to a second reaclor which may
be a tube or loop rea~;tor.
The paper Circulation Time Prediction In The Scale-Up Of
Polymerization Reactors With Helical Ribbon Agifators by D.F. Ryan
L.P.B.M. Janssen and L.L. van Dierendonck Chemical Engineering
Science Vol. 43 No. 8 pp. 1961-1966 1988 discl ~sses a number of
devices to stir a tank reactor containing a non-Newtonian solution but
does not suggest the micromixing/l"acroi"ixing reactor of the present
invention.
Coi ,ce~lually it is highly desirable to achieve micromixing (to
ensure good mixing of catalyst and feed) and n,acro,nixing (so as to avoid
precipitation at minimal operating cost).
In the reactor of our invention we have essentially separat~d the
micromixing and the macromixing functions.
Thus the rea.;tor has a small mixing zone in which the inlet feed is
rapidly mixed with reactor coi ,lenls in a desired ratio. A very important
, ~ c ~1 24can.doc 6
2193431
element of this concept is the recognition that rapid mixing times (i.e. a
fraction of the bulk circulation time) can be achieved economically if the
blending zone is physically small particularly in highly viscous solutions.
One or more high intensi(y mixing zones with or without the inlet streams
being fed into these zones can be included in one reactor.
The mixed reactanls are then transported through the volume of the
reactor where the reaction proceeds. After a certain time the reactor
CGntel IIS return back to the high intensity mixing zone where they are
mixed with the feed stream. The product is preferably withdrawn from the
reactor at a region immedi~tely upsl,ea", from the intensive mixing zone.
If ",ecl,a"ical devices are used both in mixing and in inducing the
circulation the two devices may either be driven by separale mechanisms
2 o or be driven by the same mechanism such as a rotaling or oscillating
shaft.
As already described the reactor conlenls circulate through the
reactor volume and the high i"le, Isily mixing zone. It is advanlageous that
the distribution of the hold-up times in the re-circ~ ted reactor contents
be narrow. That is to say the reactor CGI ,lenls are re-ciru ~-ted in a loop
without much axial mixing due to short-circuiting between the forward and
return flow in the reactor. This could theoretically be achieved by
physically separating the forward and return flows by constructing the
reactor as a loop.
Conceptually the mixing device in the high intensity mixing zone is
selected so that it operales at the viscosities likely to be encountered
1 24can.doc 7
21 ~3431
during the operation of the reactor. It can be designed to provide the
required mixing only or it can also provide or aid in t,anspG, lil ,9 the mixed
sl, ear"s through the reactor volume. Desirable mixing of highly viscous
reactor co"lents with feed sl,ear"s of low viscosity can be obtained by
using a radial impeller designed so that a localized re-circulation zone for
rapid mixing and blending is produce-l Mixing can also be achieved by
o using the energy of the feed stream in the form of a jet. A simple jet may
provide sufficient mixing, or the jet may impinge on a solid surface which
may be so sculptured as to provide flow separalion, acceleration or
folding similar to those obtained by static mixing devices known in the
trade. In the addition to mixing, the jet kinetic energy may also be used to
induce the circulation in the reactor, thus a reactor with no moving parts
can be designed. The circulation of the reactor contents may also be
induced by mechanical devices such as a rolaling screw or helical ribbon
in high viscosity envirui ""ents or a combination of rotors and stators in
intermediate or low viscosity solutions. Aller"alively other principles can
conceptually be used, for example one can imagine that the circulation
could be induced by a difference in bulk density between the forward and
return flow which is produced by introducing gas to drive an upward flow.
In such a scenario, the drive gas would disengage at the top of the reactor
and the liquid would descend. The drive gas might be IIIGi ,ori,er, inert
gas, or vapors gei ,eraled by boiling the contents of the reactor.
Thus, conce,clually, it is ir,lpollal,l to achieve both micromixing and
macromixing at an ecGnol"ical cost. The prior art appears to recognize
r ~ 1 24can.doc 8
21 93431
-
this problem but doesn't provide viable answers. The Applicants have
now invented a specific reactor .:lepencle"l process in which these
concepls have been put into practical practice.
The invention is particularly suitable for the solution polymeri~ation
of olefins especi~lly when the reactor feed is "cold" (below the "critical
te""~erdlllre" i.e. below the temperature at which the polymer will
prec;pilale from the solution).
To the best of the Applicants' knowledge there is no art disclosing
the application of re-circulation to a mixing zone in the solution process
for the polymerization of olefin polymers.
The present invention seeks to provide a process in which one or
more fresh rea~;tants is mixed in a rapid mixing zone with a portion of
reactanls representative of the reactants leaving a rea,;tor and a
significant propol lion of the reactanls representative of the reactants
leaving the reaction zone are re-circl ~ted through the mixing zone. This
process provides an addiliol,al method to help control a reaction as it
increases the operator's freedom for example by permitting the operator
to control the ratio of the feed rate of fresh reactants and the re-circulation
rate of the reactants representative of the product leaving the reactor.
SUMMARY OF THE INVENTION
Accordingly the present invention provides a process for
introducing a relatively smaller volume of one or more fresh reactants into
a relatively larger volume of one or more reactal ,ls which are
representative of the desired output of a reactor then introducing said one
'Q1 24can.doc 9
2 1 ~3431
or more fresh reactants into a portion of said reactants represenldti~e of
the desired output of the reactor under conditions of rapid mixing in a
rapid mixing zone (or"micromixing zone ) and then introducing the
resultant mixture into the bulk of the reactants represenlali~e of the
reactor output in a reactor which are pumped through said reactor and a
significant portion of which is circul~ted back to the rapid mixing zone and
a small portion withdrawn as a product.
DETAILED DESCRIPTION
As noted above the ratio of the re-circulation rate of the reactants
representative of the output of the reactor to the rapid mixing zone to the
feed rate of said one or more reactanls to the rapid mixing zone may be
varied. The rate of re~irculation of reactants represenlalive of the output
of the reactor to the feed rate of the fresh reactants to the rapid mixing
zone should be greater than 1:1. Preferably the ratio of re-circulation rate
of reacta"ls represenlalive of the reactor output to fresh react&nls to the
rapid mixing zone is greater than 4:1 preferably greater than 6:1 most
pre~erably greater than 8:1. It is desirable to have the ratio high to create
as thorough a mixture as practical of reactanls representative of the
reactor output and fresh rea.;tants and which will be closer in
characteristics to the bulk propei lies of the reactants represenlali-/e of the
reactor output.
To a great extent the efficiency of mixing is controlled by the
relative viscosity of the components. Typically in accorda"ce with the
present invention the ratio of viscosity of the one or more fresh reactanls
124can.doc 10
2193431
to the viscosity or the r eacta, lls represenlali~e of the, eactor output is
from 10:1 to 1:10 000. Typically the viscosity of the one or more fresh
reactan~s will be less than the viscosity of the reaclanls representative of
the reactor output (i.e. typically the ratio will be less than 1). Preferably
the viscosity of the reactants representative of the output of the reactor is
less than 20 Pa s more prererably less than 2 Pa s.
Generally the process of the present invention may be used with an
exoll ,er",ic reaction and prererably but not necess~rily the mixture of the
one or more fresh reactants and the reactanls representative of the
reactor output are cooled before being returned to the mixing zone.
Generally the one or more fresh reaclanls have a te",perature of at least
100~C ~.rererably at least 200~C cooler than the te",perature of the
2 o reactanls representative of the output of the reactor. If the mixture of said
one or more fresh reactants with said rea~;tants representative of the
rector output is cooled before returning to the mixing zone the cooling may
be carried out by passing the mixture through a cooling device such a
jacketed tube or pipe or a cooling heat excl ,anger either before or after
passing through the quisscel ll zone (e.g. the main reador). If the reaction
is highly exothermic it is prererable to maximize the temperature
differential between the temperate of the one or more fresh, eactanls and
the re~L~, ll3 representative of the reactor output. Additionally the
resulting blend may be further cooled before being returned to the
quiescent zone and thereby subsequently to the mixing zone. However
care should be used to prevent undesired reactions. For example in the
p~ 124can.doc 1 1
2 ~ 93~3 1
-
case of mixing a stream of relatively cooler and less viscous rea~;la, lls
with a warmer more viscous stream of reactanls containing a higher
co"cenlralion of product, therel may bel precipitation of the product
(e.g. polymer) in the form of stands of "spaghetti". Elimination of
"spaghetti" may be accomplished by controlling the ratio of reactants
represei ,lali~/e of the reactor output and fresh reactanls to keep the
initially blended solution at condiliGi ,s at which precipitation will not occur.
However, care must be taken to avoid or reduce conditions so that a
significant amount of catalyst is consumed during initial mixing at too high
a temperature to produce a low molecular weight fraction (someli",es
referred to as "grease"). While excess production of such a low molecular
weight fraction is detrimental, a controlled amount of low ",olec~ ~lar weight
2 o fraction is desir~ble to modify or control certain propei lies of the resulting
polymer such as process~hility or heat sealability or even tack in
applications such as cling wrap film (or pallet wrap film). The separation
of the mixing zone from the reaction zone together with a controlled
recycle between the two zones provides the process engineer and
polymer chemist with an additional degree of r,eedoi" in producing
polymer.
While it is not essential, it is preferred that the zone of rapid mixing
and the quiescent or reaction zones be se~,ardled by reactor intervals.
The rapid mixing zone may comprise a smaller volume rea.;tor having a
means for rapid mixing such as an impeller, or an impeller in cci"b.nalion
with one or more static mixers. The mixing may be accomplished by a jet
F~ 124can.doc 12
21~3431
-
vortex mixer. In cases where the viscosi~ of the stream of reactanls
representative of the reactor output and the stream(s) of fresh feed(s) are
relatively low the mixing may be accor,l,~lished using static mixing devices.
The re-circulation of reactants through the quiescent or reaction
zone and back to the rapid mixing zone is prererably carried out under
conditions of low shear. As the fresh rea.;tants and the reactants
o representative of the reactor output have been as thoroughly mixed as
possible further mixing is not required nor in most circun)slances desired.
Rather there need only be sufficient agitation of the rea~lanls in the
reactor zone to move the reactants through the zone and provide for heat
exchange (e.g. either healing or cooling) typically by co"lacl with the walls
of the rea-;tor or by an immersion heat exchanger. The circulation through
the reaction zone may be provided by a screw or ribbon mixer.
The re-circulation of the reactants back to the zone of rapid mixing
may be under more severe conditions utilizing such equipment as a jet or
centrifugal pump or a progressive cavity pump.
In a particularly preferred embodiment the present invention is
used in association with the solution polymerization of olefins to ~.repare
linear low density polyethylene. The l"onon,ers typically comprise a
solution of at least 60% by weight of one or more C2.3 alpha olefins
optionally with up to 20 weight % of one or more C~2 ,urerera~ly C4 8.
olefin ",o.,o",ers preferably alpha olefins which may be straight chained
orbra"ched. rlefelledmG"GI"ersareethylene propylene 1-butene
1-l,exel,e and 1-octene. The ",onol"er may be dissolved in up to about
- 5 '~. ~ 'Q1 24can.doc 1 3
2~ 93431
. .
40 weight % of an aliphatic solvent typically having from about 6 to 12
preferably from about 6 to 8 carbon atoms at pressures up to about
5000psi.
The catalyst may be a Ziegler Natta type catalyst or a combination
of such a catalyst with a vanadium component together with an aluminum
co",pound as an activator optionally in the presence of a magnesium
compound soluble in the solvent. The catalyst may be a single site
catalyst such as the so-called metallocene catalysts which may be
activated with an alu",inoxane such as (methyl aluminoxane MAO") or an
ionic activator.
Typically the feed streams comprise the catalyst components which
may be prepared by in line mixing upstream of the rapid mixing zone and
the monomer stream. The feed streams may at a te",peraLure from -20~C
to 1 30~C typically from about 1 5~C to 50~C.
The feed ~l,ea",s may be fed separalely to the rapid mixing zone or
a small reactor. There they are mixed for a relatively short period of time
(typically less than about 2 preferably less than 1 minute) with a larger
propo, lioll of reactants represel ,lali~/e of the product leaving the reactor.
The ratios of mixing and the relative te""~eralures of the stream of one or
more fresh reactants and the reactants re~,resenlalive of the product
leaving the reactor have been described above.
The thoroughly mixed product is then fed to a larger reaction zone
where the reactants react at higher te",perdl,Jre again for a relatively short
time typically less than about 15 prererably less than about 8 minutes. A
.; ~. - c.'Q124can.doc 1 4
2 1 9343 1
~ . ~
significant proportion of the reactanls may be recycled back to the rapid
mixing zone, optionally through a cooler and a smaller propGrlion of the
reactanls re~resenlalive if the product of the reaction is drawn off and
separate.J from the solvent. The conversion of the feed to product in the
quiescent zone should be at least about 75%.
BRIEF DESCRIPTION OF THE FIGURE
Further details of the prefe, led embodiment will now be described
with rerere"ce to Figure 1 which is a schematic representation of a
prefer, ed reactor embodiment according to the invention.
DETAILED DESCRIPTION
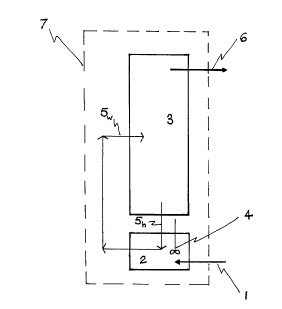
Rerer~ i"g to Figure 1, the reactor (schematically represel,led by the
dotted line 7) incl~ ~des an intensively mixed, or "micromixed", zone 2
having a volume V, and a co",parati~ely quiescent reaction zone 3 having
a volume V2.
The micromixed zone 2 is mixed by an impeller 4 having a
diameter D.
A portion of the conle"ls of the reactor is cira ~l ~ted between the
micromixed zone 2 and the reaction zone 3 as illusll aled by the arrow 5
which indicates a re-circulation pattern. The volumetric flow rate of this
re-circulation loop is QR.
Cold reactants are fed into the intensively mixed (micromixed) zone
2 through cold reactor feedline 1. The volumetric flow rate of cold
reactanls is des~ ibed by the term QF.
Hot reactor products exit through product discharge line 6.
F ~ 'Q124can.doc 1 5
21 93431
For clarity it should be noted that the micromixed zone 2 and the
comparatively quiescent reaction zone 3 may be different zones of a
single reactor as schematically suggesled in Figure 1 (or altematively
the micromixed zone 2 and the quiescent reaction zone 3 may be
physically separate reactors).
Thus in the speci~' case of the solution polymerization of olefins
o cold reactor feed (containing ",onoi"er and solvent and optionally
comonomer) is fed through the reactor feed line 1 into the micromixed
zone 2 at a volumetric flow rate of QF(m3/s). Catalyst is most pr~rerably
fed through the same reactor feed line 1 (though optionally the use of a
sepa,ale catalyst line (not shown) is also a viable option).
The impeller 4 provides intensive mixing so that the cold feed does
2 0 not cause precipitation of the warmer conte, lls of the micromixed zone 2.
A re-circulation flow (in~' ~ted by reference numerals 5) transports
reacta"ls between the micromixed zone 2 and the comparalively
quiescent reaction zone 3. As indicated by the subscript h (i.e. rt:rerence
numeral 5h), hot reactanls enter the micromixed zone 2 so as to allow the
intensive mixing with the cold feed. The resulting warm mixture of hot
and cold reactants is indiç~led by the subscript w (i.e. rerere"ce numeral
5~,) which enters the con,parali~/ely q~ _scent reaction zone 3.
The co"~pdr~li\/ely quiescent reaction zone 3 is most preferably
stirred or mixed or agitated to allow good dispersion of the heat of
poly",eri~alion through the reactor contenls. However this reaction
psc,~"~ 124can.doc 16
2193~31
.
zone 3 is "comparatively quiesce"l' (i.e. in con,parison to the "inlensively
mixed" or micromixed zone 2).
The product solution (i.e. a solution of polyethylene in solvent) exits
through product discharge line 6.
The reactor of this invention allows the solution poly",eri~alion of
olefins using cold reactor feed at a tei"perature of more than 100~C lower
than the polymerization reactor without causing problem precipitation and
without applying the large amount of energy which would be required to
micromix the entire volume of reactan~s. Thus this rea.;tor allows a very
energy efficient polymerization reaction (as a) the use of cold feed can be
used as a heat sink to remove heat of poly",eri,alion and b) this
desirable result is achieved by "micromixing" only a small volume of the
2 0 reaCtantS).
P~ arer, ad reactor conditions for the solution polymerization of
ethylene (optionally with a comG,~oi"er such as butene or octene) are
provided in Table 1.
TABLE 1
Condition rl erer, ~d Highly Frerer, ed
5<V, +V2~ 180 40~V1 +V2~70
3 ~ QF QF
2 2~QC~50 8~QC~12
QF QF
3 1 <V2<80 8'V2~10
V1 V1
4 4 c Re, ~ 100 000 100 ~ Re, c 500
where:
QF- Feed rate 1
~91 24Can dOC 1 7
~1 93431
QC - [Re-C;rC~ tion flow (QR)] plus [feed flow (QF)]
V, - Volume of micromixing zone 2
V2 - Volume of quiescent zone 3
Re, - Impeller Reynolds number (pND2/ll) in micromixing zone
N - Revolutions per second of impeller
D - Impeller dia",eter
p - Density of rea~;tor co"lents in micromixing zone
~L - Absolute viscosity of reactor co"lents in micromixing zone
Additional details are provided in the following non-limiting
examples.
Example
Ethylene was polymerized in a 600 ml reactor which had the
internal diameter of 75.5 mm and length of 150 mm. A stirrer shaft was
fitted through the centre of the reaclor top. The shaft was coupled to the
stirrer drive through a magnetic coupling. There was 1/4 inch outside
diameter OD" (about 0.635 cm OD) ll,er",owell reaching from the reactor
top down 30 mm off centre. In this thermowell there were inserted two
ll,er",ocouples measuring temperature at about 30 mm and 120 mm from
the top of the reactor cavity respectively. Ethylene cyclol ,exane solvent
and 1-butene were fed into the reactor through a 1/4 inch (0.635 cm) port
that was located in the centre of the reactor at its bottom. These three
components were mixed before entering the reactor and the temperature
of the mixed stream was controlled by heating the cyclohexane stream to
obtain the desired temperalure of the mixed stream. The reactor pressure
F ~ 'Q124can.doc 18
21 9343 1
was 20 Mpa in all cases. Catalyst was injected at the reactor bottom
through a port located 30 mm from the reactor centre. The catalyst was
pre~,ared by in-line mixing of a 6 mM solution in cyclohexane of
TiCI4/VOCI3 20/80 by mole with a 10 mM solution in cyclohexane of triethyl
aluminum. Shortly after these catalyst precursor solutions were mixed,
the resultant stream was in,ected into the reactor. The feed rate of the
transition metals was kept constant and for each run the amount of triethyl
aluminum was optimized to obtain the maximum ethylene conversion.
The changes in conversion were measured as the dirrerei ,ce between the
temperature of the reactor inlet stream and that of the reactor outlet
stream.
Comparative Example (Conventional Reactor)
The reactor stirrer shaft was fitted with two impellers. A four bladed
axial impeller was located 70 mm from the top of the reaclor cavity. The
blades of this impeller were 18 mm wide and were angled at 45 degrees
so as to pump downward. The second impeller was a four-bladed radial
impeller with blades 12.7 mm wide and it was located 100 mm from the top
of the reactor cavity. Both impellers were of 38 mm diameter. The stirrer
operated at 1300 RPM. This high rate of agitation provides "micromixing"
(i.e. intensive mixing). The Reynolds number was low (less than 10) due
to the small reactor size. (Larger, commercial size reactors typically have
a Reynolds number of from 100 to 500.) The reactor feed rate was 60
kg/hour of cyclohexane and 4 kg/hour of ethylene and 3.5 kg/hour of 1-
butene into the reactor. The feed temperature was 11 0~C, the outlet
124can.doc 19
2 1 9343 1
temperature was 180~C. The two tl,er",ocouples in the reactor indicated
temperature of 165~C at the bottom and 160~C at the top. The feed
temperalure was then lowered in small steps at a rate of about 5~C per
hour. When the feed te",peralure reached 105~C the insidé temperatures
became unstable and the temperature at the reactor bottom was lower
than that at the rea~or top. Shortly after that the conversion was lost and
the reactor stirrer shaft seized. The reactor was flashed with solvent and
opened. There were polymer strands wrapped around the propeller
blades the shaft and the thermowell. This clearly indicates that the solid
polymer formed at the reactor inlet did not re-dissolve in the warmer part
of the reactor and accumulated in the reactor.
Inventive Example 1
The 600 ml reactor described above was divided by means of
internal reactor elements into a micromixed zone and a co",parali~/ely
quiescent reaction zone.
The micromixed zone 2 had a volume V, of 60 ml and the quiescent
zone 3 had a volume V2 of 540 ml (for a VJ V, ratio of 9).
The volumetric flow ratio of [re-circulation flow QR + feed flow QF] -
[feed flow QF] was controlled at 6/1.
The residence time obtained by dividing the total volume (V, + V2)
by the feed flow QF, was between 15 and 20 seconds.
The Reynolds number was again low (less than 10) due to the
small reactor size. It will be appreci~ted by those skilled in the art that a
r i , ~ Q124can.doC 20
2 1 9343 1
-
larger reactor will require a higher Reynolds number (e.g. from 100 to 500
for the case of a commercially sized ethylene polymerization reactor).
The flow rate was 60 kg/hour of cyclohexane solvent 4 kg/hour of
ethylene and 3.5 kg/hour of butene-1. The feed tei"peralure was initially
set at 110~C and the reactor discharge te",per~ilure (or outlet
te" ,peralure) was 1 80~C. The previously described thermocouples
indicated actual te",peralures of 175~C and 160~C. The feed temperature
was then d~upped in small increments at a rate of about 5~C per hour.
Stable reactor conditions (no problematic precipitation) were obtained with
a cold feed te",peralure of 75~C (in coi"parison to the failure of the
conventional r~ador in the comparative example at a feed te",peralure of
1 05~C.
Inventive Example 2
The conditions of this example were similar to those of Example 1
except that the feed rate of ethylene was increased to 6 kg/hour. The
reactor was stable (no problematic precipitation) at a feed tei"persilure of
50~C.
~-o' l',: . ~12~can.doc 21