Note: Descriptions are shown in the official language in which they were submitted.
2?9349 ~-
METHOD OF CLEANING AND MAINTAINING POTABLE WATER
DISTRIBUTION PIPE SYSTEMS WITH A HEATED CLEANING
SOLUTION
It is well known that hardness,
microorganisms and suspended solids in water sources
vary widely in composition depending on the source and
will result: in scale deposition including microbial
tuberculation and sedimentation on surfaces wherever
water is a=_,ed. Scale deposition and sedimentation is
particularly troublesome in water distribution pipe
systems whi-ch service the residential and commercial
..,
SHEET
WO 95/35419 ~ , L~ ~ ~ ~ PCT/US95/07546
,, ,
~2~
customers of municipalities, private water companies
and the like along with industrial process water
distribution pipe systems as found in the mining,
petroleum,, agriculture and the like industries. In
these systems, the formation of scale, tuberculation
and sediment can reduce the water flow through the pipe
system which will limit the capacity of the pipe to
service the requirements of the customers or to provide
the required water necessary for an industrial process,
to irrigation, etc. For instance, in municipal systems
an increa:ae in the f ire risk would be obvious if the
fire hydrant did not supply sufficient water to
extinguish the fire due to scale, tuberculation and
sediment .deposits in the feed pipe line. At some
point, th~a water distribution pipe would have to be
replaced clue to these restrictions at a high cost and
with prolonged interruption of service.
Additionally, scale, tuberculation and
sedimentation will increase the nossibilitv of
2o corrosion in the water distribution pipe along with
promoting the growth of other organisms. The organisms
also can he a health hazard, promoting corrosion and
biomass which binds scale and sediment together and to
the surfaces of the system. Corrosion can eventually
lead to the leakage of the system and the necessity to
replace the leaking section.
The microbiological tuberculation found in
water distribution pipes and wells are typically due to
WO 95/35419 ~ j 9 ~ ~ 9 4 PCT/US95/07546
-3-
iron and manganese bacteria that attach themselves to
the walls of the pipe and live on the soluble iron or
manganese in the water along with other nutrients.
Their spaghetti-like features also allow them to trap
all particulate matter which is present in the water.
There are over 20 different iron bacteria
that havEa been characterized. As part of their
metabolism, they convert ferrous ion to ferric ion
which results in iron oxide (rust) accumulation in the
tuberculaition. Manganese bacteria convert manganous
ion to manganic ion which results in manganese dioxide
accumulation in the tuberculation in the water pipe.
After generations of bacteria, the iron oxide,
manganese dioxide, particulate matter and biomass
accumulation on the side of the pipe results in mounds
of tuberculated "growth" annalogous to a coral reef.
As the tuberculation grows, flow becomes
increasingly restricted and turbulant. This leads to
red water and turbidity complaints by consumers.
Restricted flow results in low pressure complaints and
poor hydrant performance. Tuberculation can also
interfere with valve and hydrant performance and
operation. There can also be corrosive sulfate
reducing bacteria that live under the tuberculation and
cause pipe. corrosion.
Strong acids have been used to clean water
wells, however, submersible pumps are removed prior to
treatment to prevent corrosion by the acids employed.
2193494
- 4 -
Also, organic acids, mixtures of mineral acids and
organic acids or inhibited acid compositions have been
found to clean water wells without the necessity of
removing the pumps or other equipment. These methods
for cleaning water wells have involved static and
surging treatment.
A proper cleaning and maintenance program for
water distribution systems will prevent decreased water
flow capacity, corrosion and the necessity to replace
the system or portions thereof. A simple and effective
method for cleaning and maintaining these systems is
needed.
French Patent 2571571 describes a mobile all-purpose
descaling apparatus with a reservoir, a pump and a moveable
chassis. The apparatus may include means for preheating a
cleaning solution to be pumped from the reservoir through a
pipe system.
French Patent 2602571 describes a method for cleaning
the pipes of a potable water distribution system in which a
pulsed air jei_ is directed into water in a pipe section.
The method replaces mechanical scraping with air/water
scraping.
U.S. F~atent 4025359 describes a pipe cleaning
composition mhich has a reduced tendency to attack
galvanised or steel pipe, the solution being an aqueous
acidic solution.
A~,?~~Y'G'tD SHEET
._. 2193494 . ,.~ , . v
., ~,
- 4a -
Intern,~tional Patent Application W092/20629 describes
a soap composition for the removal of water scale comprising
a 1:1 stoichiometric equivalent of a carboxylic acid and an
amine base.
SUMMARY OF THIS INVENTION
Thia invention is directed to a method of
cleaning and maintaining water distribution systems by
employing cleaning solutions at elevated temperatures.
Water systems having interior scale and sediment
deposits are cleaned by introducing and circulating an
effective amount of an aqueous treatment solution for
a sufficient period of time at an elevated temperature
which results in the solution, loosening and suspension
of the undesired scale and sediment. The scale is
associated with sulfate-reducing and iron bacteria
consisting primarily of iron oxide, biomass and
sediment. As developed above and hereinafter, other
bacteria such as manganese bacteria may be involved in
the microbial tuberculation found in water distribution
pipes. Thus, "scale", as the term is used herein, is
AMErde L ~ ~" ,,-.-,
L:. fu ,f
WO 95/35419 PCT/US95/07546
-5-
intended to include microbial tuberculation associated
with such bacteria or other bacteria. Thereafter, the
spent treating solution containing the dissolved or
suspended scale and sediment is flushed from the water
distribution system to provide a clean system with
improved water flow and operation. Additionally,
further f7~.ushing with high pressure water will also
remove additional scale that had been loosened by the
treating solution.
l0 It has been found that potable water
distribution systems may be cleaned and maintained by
employing cleaning or treating solutions at elevated
temperatures. In general, temperatures on the order of
about 40° to about 80°C have been employed and, more
particularly, from about 40° to about 50°C. By
employing elevated temperatures, water distribution
pipes may be cleaned of tuberculation more rapidly, for
example, tuberculated pipes cleaned at ambient
temperature over a period of about twelve hours may be
cleaned in a matter of about 1-2 hours where the
cleaning solution has been elevated in temperature to
about 75° to 80°C.
'.the cleaning solution may be acidic, neutral
or basic. :Cn the most preferred form, in potable water
pipe systems, mineral acids or organic acids, and
mixtures thereof, are employed as acidic treatment
solutions. The acidic treatment solution may contain
further additives such as inhibitors, chelating agents,
WO 95135419 , PCT/US95107546
'r:~' v ~~ 2193494
-6-
penetrating and/or dispersing agents to assist in the
removal of scale and sediment and to minimize any
adverse effects on the pipes, valves, or other system
surfaces clue to the acids employed.
This invention provides a simple, low cost
and effecaive method of removing water scale and
sediment from water distribution systems in order to
maintain proper water flow, operation and to prevent
corrosion of the system which would require the high
cost and :inconvenience of replacement.
Other advantages and objectives of this
invention will be further understood with reference to
the following detailed description and drawings.
DETAILED DESCRIPTION OF THE INVENTION
Among the acidic treatment solutions found to
be useful in practicing the method of this invention
are aqueous solutions of mineral acids such as
hydrochloric, nitric, phosphoric, polyphosphoric,
hydrofluoric, boric, sulfuric, sulfurous, and the like.
Aqueous solutions of mono-, di- and polybasic organic
acids have also been found to be useful and include
formic, acetic, propionic, citric, glycolic, lactic,
tartaric, polyacrylic, succinic, p-toluenesulfonic, and
the like. The useful treatment solutions may also be
aqueous mixtures of the above mineral and organic
acids.
Alkaline, acid, or neutral cleaning solutions
may also be employed, as indicated above, depending
2193494
_ 7 _
upon the type of scale that needs to be removed.
Sequestering or chelating agents such as EDTA
(ethylenediamine tetraacetic acid), NTA (nitrilotriacetic
acid), and derivatives, i.e., basic alkali salts, and the
like have also been found to be useful in the treatment
solution in certain cases.
The acidic treatment solution may also contain
acid inhibitors which substantially reduce the acidic
action on metal surfaces of the water distribution system,
particularly valves, fire hydrants, etc., and these various
inhibitors fo=r acids have been well documented in the
patent art. 'typical, but not necessarily all inclusive,
examples of acid inhibitors are disclosed in the following
U.S. Patents: 2,758,970; 2,807,585; 2,941,949; 3,077,454;
3,607,781; 3,668,137; 3,885,913; 4,089,795; 4,199,469;
4,310,435; 4,541,945; 4,554,090; 4,587,030; 4,614,600;
4,637,899; 4,670,186; 4,780,150 and 4,851,149.
The treatment solution may also contain
dispersing, penetrating or emulsifying agents to assist
in the removal of the scale and sediment. These
surface active agents may be anionic, cationic,
nonionic or am;photeric as defined in the art.
Compounds such as alkyl ether sulfates, alkyl or aryl
sulfates, alka:nolamines, ethoxylated alkanolamides,
amine oxides, ammonium and alkali soaps, betaines,
hydrotropes such as sodium aryl sulfonates; ethoxylated
CA 02193494 1999-OS-04
_g_
and propoxylated fatty alcohols and sugars, ethoxylated and
propoxylated alkylphenols, sulfonates, phosphate esters,
quarternaries, sulfosuccinates, and mixtures thereof, have
been found to be useful in admixture with the acid treating
solution.
DRAWINGS AND OPERATING EXAMPLES
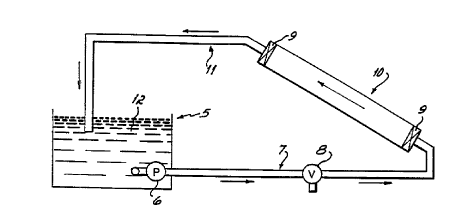
Fig. 1 is a schematic of a laboratory test system
illustrating the method of this invention.
Fig. 2 is a diagram of a field system for cleaning a
potable water distribution system.
With reference to Fig. 1, a laboratory test system is
shown to evaluate the removal of scale and sediment by
acidic treating solutions from a test pipe sample taken
from a water distribution system. This system includes a
56.8 L (15 gallon) acidic treating solution reservoir 5,
submersible ,acidic treating solution circulation pump 6
rated at 75.7 L/minute (1200 gallons per hour), 2.54 cm
(1") inlet transfer line 7, drain valve 8, heavy rubber
diaphragm seals 9 for the ends of the test pipe specimen
10, 2.54 cm (1") outlet transfer line 11 and the treating
solution 12. The test pipe specimen 10 is mounted at about
a 30 angle so that the test solution will contact
essentially the entire inner pipe surface to be treated.
A laboratory test, for example, was run on a four foot
section of 15.24 cm (6") diameter pipe which had been
removed from a potable water distribution system that had
been used for over 40 years. The scale on the inside of
lcd:km
CA 02193494 1999-OS-04
-9-
the pipe consisted of tuberculated nodules of up to 2.54 to
3 . 81 cm ( 1 to 1~ inches ) in height covering 100 0 of the
inside pipe surface which had substantially reduced the
opening inside the pipe for water to flow. Analysis of the
scale indicated it consisted of primarily iron with some
calcium, magnesium and manganese in the form oxides,
hydroxides and carbonates along with fine mineral acid
insoluble solids and some "biomass". This is typical scale
associated with manganese and iron bacteria along with the
associated corrosion.
About 37.9 L (10 gallons) of a 12.50 aqueous inhibited
hydrochloric/glycolic acid solution containing a
penetrating agent was placed in the reservoir 5 and
circulated through the test pipe 10 for a period of 24
hours at ambient temperature of about 25-30C. After 2
hours of circulation, particles of the scale were breaking
loose and could be heard in the outlet transfer line 11 and
observed entering the reservoir 5. The color of the
treating solution also become increasingly. darker with
circulation time. After 24 hours the circulation was
stopped and the system was drained of the treating
solution. The diaphragms 9 were removed and the inside of
the test pipe was observed to be about 80$ cleaned of scale
and sediment solids.
On treating the test pipe with a second identical
treating solution for a period of 21.5 hours, about 800 of
the interior surface of the test pipe was observed to still
lcd:km
CA 02193494 1999-OS-04
-10-
be covered over with a scale and/or sediment that was a
soft and paste-like semi-solid which contained some grit
and could be easily removed with a probe. The remaining
scale nodules had been substantially reduced in size since
the end of the first treatment. It was concluded that the
second treatment would probably not be necessary if a high
pressure water flush was employed to remove the insoluble
soft sediment which had coated the remaining scale nodules
after the first treatment.
With reference to Fig. 2, a field equipment and system
diagram is shown which may be employed in the cleaning of
a potable water pipe distribution system. Two 1892.7 L
(500 gallon) treating solution reservoir tanks 20 and 21
along with a 378.5 L/minute (100 gallon per minute)
circulation pump 22 and sight glass 23 are mounted on a
flat bed truck (not shown). A heating means 22A is also
shown. In this example, a 6.35 cm (2=~") inlet pipe 24 is
secured to a 198 m (650 foot) section of 15-24 cm (6")
water distribution pipe 25 after the main shut off valve
26. The fire hydrant 27 and fire hose 28 were employed for
the acidic treating solution return to tanks 20 and 21.
The section of pipe 25 to be treated was isolated by
closing off the two water main shut-off valves 26 and 29
along with all service line valves, typically 30 and 31.
With valves 32 and 33 closed, 3785 L (1000 gallons) of
acidic treating solution was prepared in tanks 20 and 21.
With the coupling 34 open, the
lcd:km
WO 95/35419 G PCT/US95/07546
-11-
treating aolution was allowed to enter the system by
opening valves 33 and 35 and turning on the circulation
pump 22. The pH of the water coming from the open
coupling was then monitored until a decrease was noted
which indicated the acid treating solution had
displaced the water in the section to be treated. The
circulation pump 22 was turned off and the coupling 34
connected. Valves 36 and 37 were then closed and valve
32 opened for circulation. The circulation pump 22 was
then started again for the treatment period. Valve 37
was closedl to allow for loosened solids to accumulate
in tank 20 while the treating solution could overflow
at 38 to tank 21 which reduces the chances of plugging
during treatment.
The treating solution was then circulated in
the system of Fig. 2 for a period of 5 hours at about
20°C. Observation of the treating solution through the
sight gl<iss 23 showed an increasingly darker
discoloration with time. At the end of the treatment
period, the circulation pump 22 was turned off, and
valves 33 .and 35 were closed. The main shut-off valve
26 was slowly opened and fresh water allowed to enter
the system until the treating solution was displaced as
noted when the tanks 20 and 21 were full. Valve 32 was
then closed. The fire hose 28 was then disconnected
from the fire hydrant 27 and the main shut-off valve 26
opened full to allow high pressure flushing of the
treated wai_er main 25. As the flush water emerged from
CA 02193494 1999-OS-04
-12-
the fire hydrant 27 it was dark in color with considerable
tuberculation or scale and sediment solids. Flushing
continued until the flush water was clean of solids for a
period of time prior to putting the treated section of the
water distribution system back into service.
The flow rate through the fire hydrant 27 prior to
treatment had been determined by a Pitot Gauge to be 2226
L/minute (588 gallons per minute). After treatment, the
flow rate was determined to be 2990 L/minute (790 gallons
per minute). This was an increase of 34.50.
Also, improved mechanical operations of the hydrants
and valves of the system were achieved. The flow of
cleaning solution may also be reversed in the system to
further improve cleaning efficiency. The above cleaning
solutions met the requirements of the National Sanitation
Foundation (NSF International, Ann Arbor, Michigan),
Standard 60 for potable water distribution systems.
Other examples of cleaning solutions may be employed as
follows:
Preblend Ingredients o by wt
31o Hydrochloric acid in water 87.14 +/- 2%
70o Glycolic acid in water 5.27 +/- 0.30
40o Sodium xylene sulfonate in water 2.06 +/- 0.20
Triethanolamine and diethanolamine 2.96 +/- 0.2$
mixture (850/15%)
Water 2.57 +/- 0.2o
In a preferred form of the invention, the above
preblended cleaning solution is used in an amount of about
12.50 by weight with water in the field for
lcd:km
WO 95/35419 ~ i g 3 ~ 9 4 P(:T/US95/07546
-13-
cleaning an underground potable water distribution pipe
system. However, more generally, the solution may be
employed :Ln amounts of from about 5 to about 50% by
weight wii=h water in the field, depending upon such
variables as the amount of tuberculation or scale,
pipe volume to be cleaned, circulation time and other
faCtOrS. The amounts of anhvrlrnttc rthctni ral ~ i n n
broader range of ingredients are about 1% to 27% HCl,
0.1% to 10% glycolic acid, 0.04% to 5% sodium xylene
sulfonate and about 0.1% to 5% of the
triethanolamine/diethanolomine mixture (hereinafter
referred to as "TEA").
It should be understood that the above
chemical ingredients may be blended for cleaning the
underground pipes, for example, hydrochloric acid may
be added to a concentrate of the glycolic acid, sodium
xylene su7lfonate and TEA. In the potable water
distribution systems, an underground section of the
pipe to be cleaned is sealed off from the rest of the
system. A:a illustrated above in Fig. 2, the cleaning
solution is then introduced from a tank into the pipe
section and, if water is in that section of pipe, it is
removed upon the introduction of the cleaning solution.
After the cleaning solution has been introduced into
the pipe section, circulation of the cleaning solution
through the underground pipe is initiated for a
sufficient period of time for solubilization, loosening
CA 02193494 1999-OS-04
-14-
and/or suspension of the scale, tuberculation and
sediments.
In the above preblends, a soap having a 1:1
stoichiometric equivalent of the acid (HC1 and glycolic
acid) and TEA base is formed with an excess of the acid.
This composition has been found to work effectively in the
field for the removal of scale and tuberculation associated
with iron bacteria consisting primarily of iron oxide,
biomass and sediment. These 1:1 soaps have also been
described in the above referred to International
Application WO 92/20629. These soaps may be more generally
categorized as soaps of mineral and/or organic acids and a
base such as an amine and ammonia. Further examples of
these soaps include 1:1 soaps of TEA and glycolic acid
(also known as hydroxyacetic acid); TEA and acetic acid;
TEA and citric acid; TEA and benzoid acid; hydrochloric
acid and ammonia; sulphuric acid and ammonia; nitric acid
and ammonia; TEA and hydrochloric acid; TEA and sulfuric
acid; TEA and nitric acid; ammonia and glycolic acid;
ammonia and benzoic acid; and ammonia and p-toluene
sulfonic acid. Accordingly, it will be understood that
other cleaning solutions of the acidic type employing 1:1
soaps may be employed to effectively solubilize, loosen
and/or suspend the scale, tuberculation and sediment from
the potable pipe in accordance with the principles of this
invention.
The comparative effects of elevated and ambient
lcd:km
CA 02193494 1999-OS-04
-15-
temperatures are illustrated by the following examples.
Example 1: CLEANING OF TUBERCULATION FROM POTABLE
WATER DISTRIBUTION PIPE AT AMBIENT AND
ELEVATED TEMPERATURES
A 10.2 cm (4") diameter potable water distribution pipe
obtained from a town in Arizona having up to 2.54 cm (1")
of tuberculation on the inside pipe wall was cut into two
0.61 m (2-foot) lengths for cleaning on the pipe testing
station. The tuberculation consisted primarily of iron
oxide, manganese oxide and biomass.
AMBIENT TEMPERATURE CLEANING
One section of the pipe was mounted in the pipe
cleaning test station. A treating solution was prepared by
mixing the "Preblend Ingredients" (by wt.) of 87o muriatic
acid, 5o glycolic acid, 2% sodium xylene sulfonate, 3%
triethanolamine/diethanolamine mixture, and water in the
test station mixing tank. The treating solution was then
circulated through the pipe section at ambient temperature
using an electric 416.4 L/minute (110 gallon/minute)
swimming pool circulating pump.
The test was run for 5 hours and the pipe section was
inspected. Considerable tuberculation was still present.
The test was continued for another 33~ hours and
inspected again. Tuberculation persisted.
The test was continued for another 4~ hours and
inspected. About 950 of the tuberculation had been removed
from the interior surface of the pipe section.
lcd:km
CA 02193494 1999-OS-04
-16
ELEVATED TEMPERATURE CLEANING
The second section of the pipe was mounted and cleaned
in the same manner except that a gasoline engine driven
pump was employed. The pump was mounted on the crank case
of the engine which caused the pump and the circulating
treating solution to be heated during the test. The pump
was rated at 586.7 L/minute (155 gallons per minute).
The test was run for 1~ hours at which time the
treating solution was hot to the touch and estimated to be
about 75-80°C. Upon inspection of the pipe section, the
interior wall of the pipe was essentially as clean as the
pipe section cleaned at ambient temperature for 13 hours.
EXAMPLE 2: CLEANING OF TUBERCULATION FROM A HEAVILY
TUBERCULATED WATER DISTRIBUTION PIPE BY
AMBIENT TEMPERATURE CLEANING FOLLOWED BY
ELEVATED TEMPERATURE CLEANING
A 15.24 cm (6") diameter 0.92 m (3') length potable
water distribution pipe obtained from a town in
Massachusetts having heavy tuberculation of about 5.08 to
6.35 cm (2" to 2~") thickness (opening was about 3.81 to
5.08 cm (l~" to 2")) was mounted on the pipe cleaning test
station. The tuberculation consisted essentially of iron
and manganese oxides, biomass and sulfate-reducing
bacteria. A treating solution was prepared as in Example
1 .
Circulation was begun using the electric 110 gallon per
minute circulation pump at ambient temperature. The test
lcd:km
CA 02193494 1999-OS-04
-17-
was run for 11 hours and the pipe section inspected. Heavy
tuberculation remained, about 1.27 cm (~") of tuberculation
had been removed.
Additional cleaning composition was added to the
treating solution thus doubling the concentration of the
treating solution. Circulation was continued at ambient
temperature for an additional 7 hours and the pipe section
inspected again. The tuberculation was still heavy and was
about 2.54 to 3.81 cm (1 to l~") thick on the interior pipe
wall .
At this point the electric pump was replaced by the
gasoline engine pump which heated the treating solution.
The temperature of the treating solution was controlled by
the circulation time. Circulation was continued for 30
minutes at which time the treating solution was 42°C.
Circulation was then discontinued and the pipe section
drained of treating solution. After the treating solution
cooled to room temperature, circulation was again started
for a period of one hour at which time the treating
solution was 50°C. Circulation was again discontinued and
the pipe section drained of treating solution. The next
day the circulation was again continued for a period of 3~
hours at which time the temperature of the treating
solution was about 50°C. The circulation was then
discontinued and the pipe section inspected. A small
amount of soft residue was in the pipe which was removed
with a water flush from a hose. The pipe was clean of
lcd:km
CA 02193494 1999-OS-04
-18_
tuberculation.
In this example, about 25~ of the cross sectional area
of tuberculation was removed after 18 hours of ambient
temperature treating solution circulation and about 750 of
the cross sectional area of tuberculation was removed by 2~
hours of periodic elevated temperature treating solution
circulation as described above.
In view of the above detailed description, other method
variations to clean domestic and industrial water
distribution systems, like houses, hotels, plants, offices,
etc., will be apparent to a person of ordinary skill in the
art. The method is especially advantageous in cleaning
underground potable water distribution systems having
tuberculation or scale associated with iron and manganese
bacteria consisting primarily of iron oxide and manganese
oxides, biomass and sediment.
lcd:km