Note: Descriptions are shown in the official language in which they were submitted.
BASF Alctienges~ ft 930541 O.Z 0050/44978
2 1 9 3 8 8 7
U~e o~ leuco triarylmethanes for marking hydrocarbons
~he preæent invention relates to the use of leuco triarylmethanes
5 of the formula I
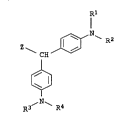
Rl
I
--CEI/¢~N~ R2
(I),
~ N~
where
20 z i8 substituted or unsubstituted phenyl, substituted or unsub-
stituted naphthyl or a radical of the formula
~ , where
y
Y is Cl--Cl~alkyl, and
30 Rl, R2, R3 and R4 are identical or different and each i8 indepen-
dently of the others hydrogen, Cl--Cl6--alkyl with or without
interrupt~on by f rom 1 to 4 oxygen atoms in ether f unction
and with or without substitution, substituted or unsubsti-
tuted phenyl or substituted or unsubstituted naphthyl,
as markers for hydrocarbons, to hydrocarbons comprising the
abovementioned leuco triarylmethanes, and to a method f or detec-
ting leuco triarylmethanes in hydrocarbons.
40 It is an object of the present invention to provide ~ovel markers
for hydrocarbons. ~he novel markers should be readily obtainable
and soluble in hydrocarbons. They should also be simple to
detect; spPr;f;--~11y~ even very small amounts of markers should
be v;c;h11i7~hl~ through a strong color reaction.
BASF Aktieng~c~llcrh~ft 930541 O.Z. 0050/44978
21 93887
a
We have found that this object is achieved by the above-defined
leuco triarylmethanes of the formula I.
Any alkyl appearing in the formulae mentioned herein may be
5 straight-chain or branched.
In any 6ubstituted phenyl or naphthyl appearing in the formulae
mentioned herein the number of ~ubstituents is generally from 1
to 3.
Substituted phenyl or naphthyl Z may have as substituents for
example C1~16--alkyl, C1--C1~alkoxy, halogen, amino or mono--or
di ( Cl--C16--alkyl ) amino .
15 Any substituted phenyl or naphthyl R1, R2, R3 or R4 may have as
substituents for example C1--C16--alkyl with or without interruption
by from 1 to 4 oxygen atoms in ether function, Cl--C16--alkoxy or
phenoxy .
20 Substituted C1--C16--alkyl R1, R2, R3 or R4 may have as substituents
for example hydroxyl, halogen or cyano. The number of sub-
stituents is generally 1 or 2.
R1, R2, R3, R4 and Y are each for example methyl, ethyl, propyl,
25 isopropyl, butyl, isobutyl, sec--butyl, pentyl, isopentyl, neo-
pentyl, tert--pentyl, hexyl, 2--methylpentyl, heptyl, octyl,
2~thylhexyl, isooctyl, nonyl, isononyl, decyl, isodecyl,
undecyl, dodecyl, tridecyl, 3, 5, 5, 7--tetramethylnonyl, i5 otri-
decyl, tetradecyl, pentadecyl or hexadecyl ~ the above designa-
30 tions isooctyl, isononyl, 1sodecyl and isotridecyl are trivialnames derived from the oxo process alcohols -- cf. Ullmann's
Encyclopedia of Industrial Chemistry, 5th Edltion, Vol. A1, pages
290 to 293, and Vol. A lO, pages 284 and 285).
35 R1, R2, R3 and R4 may each also be ~or example 2~ethoxyethyl,
2~thoxyethyl, 2--propoxyethyl, 2--is LI~LU~O~LY :thyl, 2--butoxyethyl,
2-- or 3--methc,.~y~L-,~yl, 2-- or 3~thoxypropyl, 2-- or 3--propoxy-
propyl, 2-- or 3--butoxypropyl, 2-- or 4--methoxybutyl, 2-- or
4~thoxybutyl, 2--or 4--~L~ YbU~Y1~ 3,6~ioxaheptyl, 3,6--dioxy-
40 octyl, 4, 8--dioxanonyl, 3, 7--dioxaoctyl, 3, 7~ioxanonyl, 4, 7--dioxa-
octyl, 4,7--dioxanonyl, 2--or 4--butoxybutyl, 4,8--dioxadecyl,
4~ 7~?~ n~ n~ . yl~ 3, 6, 9--trioxadecyl, 3, 6, 9--tr~ r yl,
3,6,9--tri~Y~t3n~l~cyl, 4,7,10--tr~nY~Iln~1~cyl~ 3,6,9,12--LeLLc~v~LLi--
decyl, 3, 6, 9, 12--tetraoxatetradecyl, 2--hydL~--y~Lhyl, 2--chloro-
45 ethyl, 2--cyanoethyl, 2--or 3--IIYdL~ Y~ Y1~ 2--or 3--chloropropyl,2-- or 3--cyanopropyl, 2--or 4--lly ILo~ybuLyl~ 2-- or 4--chlorobutyl,
_ _ . . . .. .. . _ . _ .. . ... . .. _ _ . _ . . . .
BASF Aktiengese~ rha~t 930541 O.Z. 0050/44978
3 2~ 93887
2-- or 4--cyanobutyl, 5--hydroxypentyl, 5--chloropentyl, 5~yano-
pentyl, 6--hydroxyhexyl, 5--chlorohexyl or 6--cyanoheYyl.
Suitable substituents for Z, as well as the aforementioned
5 C1--C16--alkyl radicals, include for example fluorine, chlorine,
bromine, mono-- or dimethylamino, mono-- or diethylamino, mono-- or
dipropylamino, mono-- or diisopropylamino, mono-- or dibutylamino,
mono--or dipentylamino, mono-- or dihexylamino, mono-- or diheptyl-
amino, mono--or dioctylamino, mono-- or bis(2--ethylhexyl~amino,
10 mono--or dinonylamino, mono--or didecylamino, mono-- or diundecyl-
amino, mono-- or didodecylamino, mono-- or ditridecylamino, mono--
or ditetradecylamino, mono- or dipentadecylamino, mono-- or
~1~ hpy~A~r-ylamino~ N--methyl-N--ethylamino, methoxy, ethoxy,
propoxy, is~ p-~Ay~ butoxy, isobutoxy, sec--butoxy, pentyloxy,
15 isopentyloxy, neopentyloxy, tert--pentyloxy, hexyloxy, 2--methyl-
pentyloxy, heptyloxy, octyloxy, 2--ethylhexyloxy, isooctyloxy,
nonyloxy, isononyloxy, decyloxy, isodecyloxy, undecyloxy,
dodecyloxy, tridecyloxy, 3,5,5,7--tetramethylnonyloxy, isotri-
decyloxy, tetradecyloxy, pentadecyloxy or hexadecyloxy.
Preference for marking hydrocarbons is given to the use of leuco
triarylmethanes of the formula I where Z i~3 substituted or unsub-
~tituted phenyl.
25 Preference for marking hydrocarbons is further given to the use
of leuco triarylmethanes of the formula I where R1, R2, R3 and R~
are each i nrl~rp~s~ntly of the others Cl--C6--alkyl .
Particular preference for the marking of hydrocarbons is given to
30 the use of leuco triarylmethanes of the formula II
Rl
~ CE~ N~ R2
[~ (II),
R3~ ~ R4
where X is hydrogen, amino or mono-- or di(C1--C16--alkyl)amino and
R1, R2, R3 and R~ are each as defined above.
45 Very particular preference for the marking of hydrocarbons is
given to the use of leuco triarylmethanes of the formula II where
X is hydrogen or di(Cl--C6--alkyl)amino, PCp~ y dimethylamino,
_ _ _ , . , ,, . ,, , , _ _ ,, _ ,,,, , ,,, _ _ _ _ _ _ _
BASF ~l~ti-~n~c~llcchaft 930541 O.Z. 0050/44978
21 93887
and Rl, R2, R3 and R4 are each ;n~lQr~n~Qrltly of the others
Cl~alkyl, especially methyl.
The leuco triarylmethanes of the formula I are known per se and
5 ~IQ~rr~hed for example in 1~. Venkataraman, The Chemistry of
Synthetic Dyes, Vol. II, Academic Press, New York, 1952, or can
be obtained for example by the methods mentioned therein.
The leuco triarylmethane of the formula II where X is hydrogen
10 and R1, R2, R3 and R4 are each methyl is leuco malachite green,
while the leuco triarylmethane of the formula II where X is
dimethylamino and Rl, R2, R3 and R4 are each again methyl is leuco
crystal violet.
15 Marking for the purposes of the present invention is the addition
to hydrocarbons of the leuco triarylmethanes of the formula I in
such a concentration that, to the human eye, the hydrocarbons
have barely any or no visible color, but the leuco triaryl-
methanes o~ the formula I are readily and distinctly visibly
20 detectable by the methods of detection more particularly
described herein.
The present invention further provides hydrocarbons containing
one or more o~ the leuco triarylmethanes of the formula I. The
25 cvl.cel,~Lation of the leuco triarylmethanes of the formula I in
the hydrocarbons is generally from 1 to 500 ppm, preferably from
5 to 50 ppm, especially about 40 ppm.
Hydrocarbons for the purposes of the present invention are
30 aliphatic or aromatic hydrocarbons which are liquid under
standard conditions, for example pentane, hexa~le, heptane,
octane, isooctane, benzene, toluene, xylene, ethylbenzene,
tetralin, decalin, dimethylnaphthalene, diisopropylnaphthalene,
chlorobenzene or dichlorobenzene. More particularly, they are
35 mineral oils, for example motor fuels, such as gasoline, kerosine
or diesel fuel, or oils, such as heating oil or engine oil.
The leuco triarylmethanes of the formula I are especially
suitable for marking mineral oils where some form of marking is
40 mandatory, for example for tax reasons. To keep the costs for
this to a minimum, it is desirable to keep the amount of marker
used to a minimum.
To mark hydrocarbons, the leuco triarylmethanes of the formula I
45 are used either without a solvent or in the form of solutions.
Suitable solvents are organic solvents. Preference is given to
using aromatic hydrocarbons, such as toluene, xylene,
, . ... _ .. _ .. ... .. , , , _ _ _ _
BASF ~l~ti ,~llschaft 930541 O.Z. 0050/44978
21 93887
dodecylbenzene, diisopropylnaphthalene or a mixture of higher
aromatlcs available from Shell as Shellsol(~) A3. To avoid the re-
sulting solution having a high viscosity, the concentration of
leuco triarylmethane I chosen for the solution generally ranges
5 from 20 to 80% by weight, based on the solution.
The solubility may be improved by using ~urther, co-solvents, for
example alcohols, such as methanol, ethanol, propanol,
isopropanol, butanol, isobutanol, pentanol, hexanol, heptanol,
10 octanol, 2--ethylhexanol or cyclohexanol, glycols, such as butyl-
ethylene glycol or methylpropylene glycol, amines, such as
triethylamine, diisooctylamine, dicyclohexylamine, aniline,
N--methylaniline, N,N--dimethylaniline, toluidine or xylidine,
AlkAn~r~in~ such as 3--(2--methoxyethoxy)propylamine, o-cresol,
15 m--cresol or p-cresol, ketones, such as diethyl ketone or cyclo-
hexanone, lactones, such as y--butyrolactone, carbonates, such as
ethylene carbonate or propylene carbonate, phenols, such as
t--butylphenol or nonylphenol, esters, such as methyl phthalate,
ethyl phthalate, 2--ethylhexyl phthalate, ethyl acetate, butyl
20 acetate or cyclohexyl acetate, amides, such as N,N--dimethyl-
formamide, N,N--diethylacetamide or N--methylpyrrolidone, or
mixtures thereof.
The leuco triarylmethanes of the formula I to be used according
25 to the present invention permit very simple detection of marked
hydrocarbons, even if, as mentioned above, the marker substances
are present only in a concentration o~ about 10 ppm or less.
In some ca3es it is also advantageous for the marker to be used
30 to comprise mixtures between leuco triarylmethanes of the formula
I.
The presence in hydrocarbons of the leuco triarylmethanes of the
formula I used as markers is advantageously detected on treating
35 the marked hydrocarbon with an oY; ~ ; n~ agent and optlonally a
protic acid in the presence of water.
This treatment gives rise to a clearly visible color rRaction and
the leuco triarylmethane I forms a triarylmethane dye and trans-
40 fers to the aqueous phase.
The resulting triarylmethane dye conforms to the formula III
BA'SF Aktieng~c~llc~-hAft 930541 O.Z. 0050/44978
1 6 21 93&87
C~N~ R2 ~n~
1 (III),
~ N~ 4
where Z, Rl, R2, R3 and R4 are each as defined above and An~ is
the equivalent of an anion (eg. sulfate, hydrogensulfate, phos-
phate, hydL~,g~llphc,~hate, di~lyd~gt~ hosphate~ nitrate, acetate,
15 lactate or citrate).
Suitable ~Y; ~; 7; nq agents include for example customary,
inorganic or organic ~Y;ri;7;ns agents known per se, such as
alkali metal r~r~~n~n~tes~ eg. potassium permanganate, ammonium
20 dichromate, alkali metal dichromates, eg. sodium or potassium
dichromate, ; peroxodisulfate, alkali metal peroxo-
disulfates such as sodium or potassium peroxodisulfate, potas3ium
pe~ sulfate, iron(III) salts, eg. iron(III) chloride or
iron(III) sulfate, hydrogen peroxide (in combination with
25 suitable catalysts ), quinones, eg . 2, 3--dichloro-5, 6--dicyanobenzo-
quinone or 2, 3, 5, 6--tetrachlorobenzoquinone, sodium perborate or
cerium(IV) salts, eg. cerium(IV) sulfate.
~oronlling on the nature of the nYi~7;n~ agents, they can be used
30 either in the form of an aqueous solution (inorganic ~Y;~;7;
agents) or in the form of a solution in an organic solvent
(organic ~Y;~;7;n~ agents~. Suitable organic solvents include for
example toluene, xylene, cy~r,hFY~n~n~, acet~honnn~, y--butyro-
lactone, 2--ethylhexyl acetate or esters of phthalic acid.
If organic ~Y;~l;7;nq agent~ are used, a dilute aqueous acid is
employed, for example from 5 to 30~ strength by weight aqueoua
acetic acid. Inorganic oY~; 7ing agents can likewise be used in
the presence of an acid, eg. sulfuric acid.
The concentration of r~Y;rl;7;nq agent in the aqueous or organic
solution is customarily from 0.001 to 596 by weight, preferably
~rom 0 . 01 to 1% by weight, in each case based on the weight of
the solution.
BASF Aktiengesellc-h-ft 930541 O.Z. 0050/44978
7 21 ~3887
If aqueous solutions of inorganic oxidizing agents are used, the
amount of acid can remain limited to small amounts, f or example a
f ew drops .
5 If organic oxidizing agents are used, the amount of the acid
used, for example from 5 to 30% strength by weight aqueous acetic
acid, is larger, since the water present in the acid serves as
aqueous phase. In this case it is advisable to choose the amount
of aqueous acid so that it c.lLL =,~,~J..ds approximately to the
10 amount of the hydrocarbon to be tested.
It is accordingly generally sufficient to treat from about 1 to
5 ml of the hydrocarbon marked according to the present invention
with from 2 to 10 ml of a solution of an organic nY;~i7;n~ agent
15 in an organic solvent and from 1 to 5 ml of aqueous acid or with
from 1 to 5 ml of an aqueous solution of an inorganic nlri~7in~
agent, optionally in the presence of an acid, at from 10 to 100 C,
preferably from 20 to 80 C, in order that the color reaction may
be obtained. This treatment advantageously takes the form of
20 extracting the hydrocarbon phase with the aqueous phase.
The leuco triarylmethanes to be used according to the present
invention are readily obtainable and readily soluble in hydro-
carbons. Moreover, they are simple to detect in that even very
25 small amounts of marker are rendered visible by a strong color
reaction .
The Examples which follow illustrate the invention.
30 Example 1
2 ml of a solution of 10 ppm of leuco crystal violet in xylene
were admixed with 3.5 ml of a solution of 50 ppm of
2,3--dichloro-5,6--dicy~nnhpn7oquinone in xylene and, after a
35 minute, the mixture was shaken up with 2 ml of 20% strength by
weight acetic acid until the dye was completely dissolved in the
aqueous phase. The aqueous phase has a deep violet color.
Example 2
2 ml of a solution of 10 ppm of leuco malachite green in xylene
were admixed with 3.5 ml of a solution of 50 ppm of
2,3--dichloro-5,6--dicy~nnhPn7oquinone in xylene and, after a
minute, the mixture was shaken up with 2 ml of 20% strength by
45 weight acetic acid until the dye was completely dissolved in the
aqueous phase. The aqueous phase has a deep green color.
BASF Aktie~ 3. F~ chaft 930541 O.Z. 0050/44978
,
1 8 21 93&87
Example 3
2 ml of a solution of 10 ppm of leuco crystal violet in commer-
cial diesel fuel were admixed with 3.5 ml of a 0.0~ strength by
5 weight solution of 2,3--dichloro-5,6--dicy~n~h~n7oquinone in xylene
and, after a minute, the mixture was shaken up with 2 ml of 20%
strength by weight acetic acid until the dye was completely dis-
solved in the aqueous phase. The aqueous phase has a deep violet
color .
Example, 4
2 ml of a ~olution of 10 ppm of leuco malachite green in commer-
cial diesel fuel were admixed with 3.5 ml of a 0.05% strength by
15 weight solution of 2,3--dichloro-5,6--dicy~n~hsn7cquinone in xylene
and, after a minute, the mixture was shaken up with 2 ml of 20~
strength by weight acetic acid until the dye was completely dis-
solved in the aqueous phase. The agueous pha~9e has a deep green
color .
Example 5
2 ml of a 0.1~ strength by weight solution of leuco crystal
violet in toluene were shaken up with 1. 5 ml of 0 .1~ strength by
25 weight aqueous iron( III ) chloride solution for 15 sec and heated
to 70 C. The aqueous phase is observed to have a distinct bluish
violet color.
Example 6
2 ml of a 0.1~ strength by weight solution of leuco crystal
violet in toluene were shaken up with 1.5 ml of 0.196 strength by
weight aS~ueous sodium peroxodisulfate solution for 15 sec and
heated to 70 C. The aqueous phase is observed to have a distinct
35 bluish violet color.
Example 7
2 ml of a 0.1~ strength by weight solution of leuco crystal
40 violet in toluene were shaken up with 1.5 ml of 0.1% strength by
weight aqueous potassium p~rr~n~snAte solution and 2 drops of 10
strength by weight sulfuric acid for 15 sec. The color of the
potassium p~srr-n~n~te disappeared and the aqueous phase took on
a distinct bluish violet color.
BASF Akti~ s^llc~~h~ft g30541 O.Z. 0050/44978
.. .
9 2 1 93887
Example 8
2 ml of a 0.1% strength by weight solution of leuco crystal
violet in toluene, 1.5 ml of 0.1% strength by weight aqueous
5 potassiu~ permanganate solution and 2 drops of 10% strength by
weight sulfuric acid were shaken up for 15 sec and heated to 70 C.
The aqueous phase is observed to have a distinct bluish violet
color .