Note: Descriptions are shown in the official language in which they were submitted.
CA 02193944 1996-12-24
~ i 94 4
VO 96/02471 PCTIUS95/08799
TITLE: TURBULENT FLOW COLD-WALL REACTOR
1. Field of the Invention
This invention relates to high pressure and high temperature
reactors. More particularly, it relates to reactors used for oxidative waste
treatment under supercritical water conditions.
2. Background of the Invention
A number of different ways for disposing of waste have been used
extensively. Landfilling and incineration are the major ones, which
however, do not seem to offer the best solution,
Landfilling is becoming less and less desirable since it does not
offer elimination of waste, but just underground storage. Thus, it has
started to be used more for by-products of other types of waste
management, such as incineration for example, than for landfilling the
primary waste.
Incineration, requiring oxidation of waste at high temperatures
with high volumes of air, followed by separation of the effluent gases
from the produced ash and the entrained particulate matter, becomes
involved, complicated, and expensive, despite the fact that at first glance
1
CA 02193944 2005-02-18
it sounds to be a simple process of "just burning the waste".
In recent years, a new method, based on supercritical water oxidation, has
started
being developed. The new method achieves substantially complete oxidation of
waste by
using considerably more compact equipment, thus becoming an excellent
candidate for
elimination of waste, even on site. Supercritical water oxiciation also has
the advantage of
producing a clean water product suitable for process recycle, thereby
facilitating waste
minimization. In addition , it has the advantage of converting spent, costly
catalysts (e.g.,
noble metals in both inorganic and organically bound compounds) to forms which
may be
more easily recovered, thereby facilitating both waste minimization and cost
reduction.
to However, as with the development of any new process or equipment, there are
numerous
problems which have not been resolved so far, and which are vital for a
finally successful use
and commercial exploitation.
In a water liquid/vapor phase diagram, one may see that there is a critical
point of
temperature (about 720 F [382 C]) and a critical point of pressure (about
3,200 psia) over
which there is only one single fluid phase.
Although the single phase represents neither liquid nor vapor, it behaves and
seems
to have more of a gas character than of a liquid one at pressures near the
critical pressure.
As pressure is raised, a more
liquid like behavior is observed, including higher solubility of inorganic
matter. The single-
phase condition occurring above the critical points is called supercritical
condition.
It is worth noting that organic matter decomposes readily under
2
CA 02193944 2005-02-18
supercritical conditions; and, in the presence of oxygen, carbonaceous
compounds oxidize
completely to carbon dioxide, sulfur compounds mostly to SO3 and nitrogen
compounds
decompose mostly to molecular nitrogen. It is wori'h noting that under
supercritical water
oxidation conditions, only small amounts of nitrogen oxides are produced, if
any, in contrast with
incineration which favors the production of nitrogen oxides. Inorganic salts
are substantially
insoluble in the supercritical water single phase for pressures of the order
of 4,000 psia, while it
has been reported that they are at feast partially soluble at considerably
higher pressures, such as
10,000 psia, for example.
The use of very high pressures at elevated ternperatures presents a serious
problem in
the construction of reactors which can withstand these adverse conditions. !t
is well known that as
the temperature increases the strength of materials decreases drastically.
Supercritical pressures
(greater than about 3,200 psia) at temperatures exceeding about 1,000 F (538
C) present an
enormous challenge to any construction material, let alone higher pressures
(of the order of
10,000 psia) and temperatures, which may be desirable for a number of reasons,
including
dissolution of inorganic salts in the supercritical single phase. If in
addition to the
temperature/pressure challenge, one considers the harsh environment inside the
reactor, the
problem tends to become insurmountable.
In order to compromise with this highly undesirable situation, excessively
elongated
reactors of accordingly small diameter have been practically utilized so far.
These conventional
reactors, however, have a number of disadvantages which include, but are not
limited to
restrictions on waste feed materials to preciude corrosive feeds and products
of
3
CA 02193944 1996-12-24
'~4 4
'7VO 96/02471 PCT/US95/08799
destruction, increased plugging potential due to small diameters, waste
feed rate restrictions, increased safety hazards, increased investment
cost, and safety hazards, as it will be discussed later, in contrast to the
reactor according to this invention.
A number of patents have been dealt in general with supercritical
water oxidation of coal, organic substances, and waste, among which are
U.S. patents 4,141,829 (Thiel et al.), 4,292,953 (Dickinson), 4,338,199
(Modell), 4,377,066 (Dickinson), 4,380,960 (Dickinson), 4,543,190
io (Modell), 4,564,458 (Burleson), 4,593,202 (Dickinson), 4,594,164
(Titmas), 4,792,408 (Titmas), 4,822,394 (Zeigler et al.), 4,822,497 (Hong
et al.), 4,861,497 (Welch et al.), 4,891,139 (Zeigler et al.), 5,075,017
(Hossain et ai. ), 4,113,446 (Modell et al. ), 4,338,199 Reexamined
(Modell), 5,106,513 (Hong), 4,898,107 (Dickinson), 4,983,296 (McMahon
et al.), 5,011.614 (Gresser et al), 5,053,142 (Sorensen et al.), 5,057,231
(Mueller et al.), 5,106,513 (Hong), 5,133,877 (Rofer et al.), 5,183,577
(Lehmann), 5,192,453 (Keckler et al.), 5,221,486 (Fassbender),
5,232,604 (Swallow et al.), 5,232,605 (Baur et al.), 5,240,619 (Copa et
al.), 5,250,193 (Sawicki et al.), and 5,252,224 (Modell et al.).
U.S. Patent 3,472,632 (Hervert et al) discloses an internally lined
reactor comprising an external pressure retaining chamber, an
intermediate porous metal layer within the chamber, and a continuous
metal liner positioned along the inner wall of the porous layer. A metal
casing encompasses and is spaced from the external chamber. Partitions
divide the space between chamber and casing into a number of separate
compartments encompassing the chamber'. A number of spaced apart
leakage passageways are extended through and distributed over the
surface of the chamber, and at least one monitoring passageway for
4
CA 02193944 1996-12-24
'7 a} ~ ~
~. i 1ti.~9 4~
VO 96/02471 PCT/US95/08799
each compartment is provided through the casing.
U.S. Patent 3,515,520 (Hervert) discloses a reactor for
accommodating corrosive materials wherein a corrosion liner is
suspended within an outer reaction chamber and in a non-contacting
relationship therewith, the chamber having an inlet for non-corrosive
gases at its lower end admitting such gases to the annular space
between liner and chamber with a passageway at the top of the liner for
admission of such gases, and an adjacent corrosive fluid inlet into the
lo liner with centrally disposed discharge means from said liner for exit of
reaction products of the corrosive and non-corrosive gases.
U.S. Patent 4,199,545 (Matovich) discloses a fluid-wall reactor for
high temperature chemical reactions comprising (A) a porous reactor
tube made of fabric or fibrous refractory material and defining a reaction
zone; (B) a pressure vessel enclosing the reactor tube to define an inert
fluid plenum, the pressure vessel having at least one inlet for admitting
the inert fluid which is directed under pressure through the porous tube
wall to provide a protective blanket for the inside surface for the inside
surface of the reactor tube; (C) means for introducing at least one
reactant into the reaction zone; (D) means in the plenum for heating the
reactor tube; and (E) a heat shield disposed within the pressure vessel,
substantialiy enclosing the heating means and the reaction zone to
define a black body cavity, the heat shield reflecting radiant energy
toward the reaction zone.
U.S. Patent 4,643,890 (Schramm) discloses a reactor tube for a
high-temperature fluid wall reactor made of refractory material which
permits the tube to be heated to incandescence. The reactor tube in turn
5
CA 02193944 1996-12-24
WO 96/02471 PCT/US95/08799
radiates energy inwardly to a reaction zone to sustain the desired high-
temperature reaction. At least part of the tube is perforated to permit an
inert gas to form a protective fluid wall for preventing the reactant
products from contacting the inner surface of the tube.
U.S. Patent 4,737,348 (Levin) discloses a reactor apparatus for
continuously producing molten, solar grade purity elemental silicon by
thermal reaction of a suitable precursor gas, such as silane.
U.S. Patent 5,186,910 (Alagy et al.) discloses an oxidation reactor
having an elongated shape and including in combination, a mixing
member having a pipe for feeding oxidizing gas and a pipe for feeding
oxidizable charge; a reaction member, arranged subjacent the mixing
member; and a discharge member associated with a discharge pipe for
the products of the reaction. The reaction member includes a central
zone which has a first lining and the reactor includes at least one
peripheral zone, which has a second lining, passages in the second
lining being smaller than passages in the first lining so that the pressure
loss in the second lining is greater than that of the first lining. The
second lining forms a sleeve surround the first lining and this sleeve is
formed of at least one refractory heat insulating material. The oxidation
reactor is provided with an external sleeve steel jacket, a concrete wall
and a steel element surrounding the mixing member arranged above the
reaction member. The reaction member includes a series of single
elements which form juxtaposed channels.
U.S. Patent 5,225,169 (Elvin et al.) discloses an improved sulfider
for receiving high temperature catalysts from a hydrocarbon cracking
operation and subjecting the catalyst to a sulfur-containing gas. The
6
CA 02193944 2005-02-18
treating unit includes an outer metal housing and first refractory layer
within the metal housing for minimizing heat loss from the treatment unit.
A second refractory layer is provided within the first refractory layer and
defines an interior chamber within the treatment unit, and a plurality of
heating units are spaced circumferentially along the interface of the first
and second refractory layers. The heating units substantially minimize
the temperature differential across the second refractory layer and
thereby minimize the heat loss from the high temperature catalyst within
the chamber.
None of the above references has resolved the vital problem of
pressure/temperature/harsh-environment in a satisfactory manner, and
especially under supercritical water conditions.
U.S. Patent 5,591,415 (Dassel et al.) desc:ribes reactors of the
cold wall type. However, U.S. Patent 5,591,415 does not consider the
desirability of turbulent flow within the reactor, the role of a solid porous
thermal insulation, and other critical parameters of the present invention,
which will be discussed in detail herebelow.
3. Summary of the Invention
This invention pertains to high pressure arid high temperature
reactors. It pertains especially to reactors operating under supercritical
water conditions combined with corrosive atmosphere, wherein the
pressure/temperature/harsh environment conditions cannot be handled
by the reactors of the presently existing art. This invention relates, more
particularly, to a reactor for treating aqueous waste liquids, comprising
7
CA 02193944 1996-12-24
WO 96/02471 PCTIUS95/08799
an assembly of
a pressure vessel having a substantially cylindrical shape, a top
vessel end and a bottom vessel end opposite the top vessel end;
a reaction chamber enclosed within the pressure vessel, the
reaction chamber having a similar shape as the pressure vessel,
and also having a top chamber end, a bottom chamber end, an
inside wall and an outside wall, the pressure vessel and the
1o reaction chamber forming an annulus, the reaction chamber
defining a reaction zone, the reaction zone being isolated from the
annulus;
a feeding tube ending in a fluid exit in a reaction ignition zone in
the vicinity of the top chamber end, the fluid exit directed toward
said chamber top end in a manner to produce turbulent flow of
fluids comprising the aqueous waste liquid and oxidant exiting
said exit and impinging on said chamber top end; and
an effluent output at the bottom vessei end.
Preferably, the reactor further comprises a quencher at the bottom
vessel end, and a thermal insulator within the annulus, which preferably
is substantially filled with said thermal insulator.
The thermal insulator may be in the form of packed high-
temperature resistant material, preferably selected from a group
consisting of high temperature beads, high temperature powder, high
temperature solid porous material, and a combination thereof.
8
CA 02193944 2005-02-18
!t is important that the thermal insulator provides adequate porosity for an
inert fluid (liquid
or gas) to pass through, and preferably the thermal insulator has a strong
enough character to
provide mechanical support to the reaction chamber.
An inert fluid inlet may be used to introduce inert fluid to the annulus in
order to maintain a
pressure higher than the pressure prevailing in the reaction zone. A detector
connected to the
annulus and adaptable to detect accidental communication between the reaction
zone and the
annulus may also be used. The annular space may also be at a pressure equal to
or lower than
the pressure in the reaction chamber, providing desigri stress limitations on
the reaction chamber
wall are not exceeded. This is especially true for long reactors with very
thin walls, wherein the
to potential of buckling and/or collapsing of the reactor chamber is high. In
such a case, the pressure
in the annulus may preferably be in the range of 0-300 psia lower than the
pressure prevailing in the
reaction zone.
Although an inert gas is preferred as compared to an inert liquid, and in many
occasions in
this discussion the word inert "gas" is used for pressurizing the annulus, it
should be understood that
an inert "liquid" can also be used. Of course, the inert gas or liquid should
not decompose under the
operating conditions within the annulus.
When, for the purposes of this invention, a substance is called a gas or a
liquid, it is
substantially in the state of a gas or a liquid under the prevailing operating
conditions in each
particular case.
The inside wall of the reaction chamber may be covered with a
9
CA 02193944 2005-02-18
liner material resistant to attack by reactants, products of reaction and
effluent gases. The reaction chamber itself may be constructed of a
material resistant to attack by reactants, products of reaction, and
effluents. This material may comprise titanium, a noble metal, ceramics,
and the like. It is preferable that titanium is used in the case that the
waste feed contains halogenated compounds, whiie a noble metal,
especially in the form of a liner, may be preferably used for non-
halogenated waste streams.
In many occasions it is preferable that the reactor is removable
from the reaction chamber, so that it may be serviced, replaced, and
the like.
The reactor may further comprise a supplemental tube contained
within the feeding tube and being substantially concentric with the
feeding tube, for introducing first constituents in said feeding tube.
The reactor may also comprise an additive tube at least partially
surrounding the feeding tube and being substantially concentric with the
feeding tube, for introducing second constituents in the reaction zone.
The present invention also pertains to a method for treating
aqueous waste liquids comprising the steps of:
introducing waste into a reactor, the reactor comprising an
assembly of
a pressure vessel having a substantially cylindrical
shape, a top vessel end arnJ a bottom vessel end
CA 02193944 1996-12-24
4
'VO 96/02471 1 PCTIUS95/08799
opposite the top vessel end;
a reaction chamber enclosed within the pressure
vessel, the reaction chamber having a similar shape
as the pressure vessel, and also having a top
chamber end, a bottom chamber end, an inside wall
and an outside wall, the pressure vessel and the
reaction chamber forming an annulus, the reaction
chamber defining a reaction zone, the reaction zone
being isolated from the annulus;
a feeding tube ending in a fluid exit in a reaction
ignition zone in the vicinity of the chamber top end,
the fluid exit directed toward said chamber top end in
a manner to produce turbulent flow of fluids
comprising the aqueous waste liquid and oxidant
exiting said exit and impinging on said chamber top
end; and
an effluent output at the bottom vessel end.
maintaining within the reaction chamber a reaction zone having a
predetermined temperature and pressure in order to oxidize the
aqueous waste liquid and produce an effluent gas of substantially
complete combustion;
maintaining a pressurized inert fluid in the first annulus in a
manner to prevent any reactants, products of reaction, and
effluent gas from entering said annulus; and
11
CA 02193944 2005-02-18
disposing of the effluent gas.
It is preferable to minimize the required reaction chamber wall thickness by
maintaining
an adequately small differential pressure between the reaction chamber and the
annulus.
If the cost and nature of the construction materials allow. it, as
aforementioned, the
pressure of the inert gas in the annulus should preferably be higher than the
pressure
prevailing in the reaction zone, so that in case of a pinhole or other leakage
source, inert fluid
will flow from the annulus to the reaction zone, and no corrosive fluids will
enter the annulus
from the reaction zone. Preferably, the pressure in the annulus is 20 to 300
psi higher than the
pressure in the reaction zone, more preferably 100-:300 psi, and even more
preferably 150-250
io psi. However, a lower pressure in the annulus than in the reaction zone is
acceptable for
reasons already mentioned, if leakage consideratioris are not paramount. A
lower pressure in
the annular space is particularly desirable when the thickness of the reaction
chamber wall is
low enough as to cause serious potential of collapsing or buckling.
In either case, the differential pressure between the annular space and the
reaction
zone should be maintained within an allowable design range, preferably about
20-300 psi,
more preferably 100-300 psi, and even more preferably 150-250 psi.
The reactor of this invention may further comprise a pressure-vessel cooler,
the pressure vessel having an inside surface and ari outside surface. The
cooler may
comprise a cooling jacket at least partially surrounding the outside surface
of the pressure
vessel. The cooler may also comprise a cooling coil at least partially
surrounded the
12
CA 02193944 1996-12-24
37 944
VO 96/02471 PCTlUS95/08799
inside surface of the the pressure vessel. The cooler may also be in the
form of cooling fins on at least part of the outside surface of the pressure
vessel.
The present invention also pertains to a mett-iod as described
above, which further comprises a step of cooling the pressure vessel
externally or internally or both, with respect to the pressure vessel.
lo 4. Brief Description of the Drawing
The reader's understanding of practical implementation of
preferred embodiments of this invention will be enhanced by reference to
the following description taken in conjunction with the drawing figures,
wherein
Figure 1 shows a schematic diagram of a reactor according to a
preferred embodiment of the present invention.
Figure 2 illustrates a block diagram of a simplified supercritical
water oxidation system which includes the reactor of the present
invention.
Figure 3 shows schematically a fractional view of a preferred
configuration of a quench tube according to the present invention.
Figure 4 illustrates a different embodiment of the instant invention,
in which a supplemental concentric tube is utilized inside the feeding
tube.
13
CA 02193944 1996-12-24
~~ tl f?I
WO 96/02471 PCT/US95l08799
Figure 5 illustrates still a different embodiment of the instant
invention, in which a additive tube is utilized surrounding the feeding
tube.
Figure 6 is a schematic diagram illustrating a reactor according to
a different embodiment of the present invention, wherein the pressure
vessel is surrounded by a cooling jacket.
Figure 7 is a schematic diagram illustrating a reactor according to
io a different embodiment of the present invention, wherein the pressure
vessel is surrounded by a cooling jacket having a different cnfiguration
as compared to the jacket of Figure 6.
Figure 8 is a schematic diagram illustrating a reactor according to
1s a different embodiment of the present invention, wherein the pressure
vessel surrounds a cooling coil.
Figure 9 is a schematic diagram illustrating a reactor according to
a different embodiment of the present invention, wherein the pressure
20 vessel comprises a plurality of cooling fins on its outside surface.
5. Detailed Description of the Invention
25 As aforementioned, this invention pertains to high pressure and
high temperature reactors. More particularly, it pertains to reactors used
for oxidative waste treatment under supercritical water conditions.
As also aforementioned, the use of very high pressures at
14
CA 02193944 2005-02-18
elevated temperatures presents a serious problem in ihe construction of
reactors which can
withstand these adverse conditions. It is well known that as the temperature
increases the
strength of materials decreases drastically. Supercritical pressures (greater
than about 3,200 psia)
at temperatures exceeding about 1,000 F (538 C) present an enormous challenge
to any
construction material, !et alone higher pressures (of the order of 10,000
psia) and temperatures,
which may be desirable for a number of reasons, including dissolution of
inorganic salts in the
supercritical single phase. If in addition to the temperature/pressure
challenge, one considers the
harsh environment inside the reactor, the problem tends to become
insurmountable. The
compromised solution to combat this undesirable situsition, has been so far to
utilize tube-like
l0 excessively elongated reactors of accordingly small diameter. However, this
introduces in tum
other disadvantages, such as heat loss, easy blockage of the tubes, excessive
liner {such as
noble metal for example} casts, and the like.
Other disadvantages of the conventional reactors include, but, are not limited
to
restrictions on waste feed materials to preclude corrosive feeds and products
of destruction,
increased plugging potential due to small diameters, waste feed rate
restrictions, increased
safety hazards, increased investment cost, as discus;ced later, in contrast to
the reactor according
to this invention.
In dissimilarity, the present invention alleviates all these problems by using
a critical
combination of thermal insulation and chemical isolaticn in an annulus between
a reaction
chamber and a pressure vessel, as well as by arranging the elements of the
reactor and their
operation in a manner discussed in detail hereinafter.
CA 02193944 1996-12-24
VO 96/02471 ? 1 ~ 3 ~ ~ 4 PCTIUS95/08799
The present invention allows for the construction of relatively
large diameter reactors, which are not practical or possibie otherwise.
There are several important consequential advantages, including but not
limited to:
o A significantly lower ratio of internal reactor chamber surface area
to reaction volume
o Separation of corrosive atmosphere from containment (pressure)
vessel results in substantially improved process safety
o Reduced piugging potential
o More compact design
The lower ratio of surface area to internal volume, made possible
by the relatively large diameter enabled by the instant invention, has
several technical and economic advantages, including but not limited to:
o Reduced heat loss (this optimizes waste destruction efficiency)
and lowers operating cost
o Significantly reduced material requirement for costly liner, such
as a noble metal (e.g., may reduce requirement by more than
tenfold), due to reduced ratio of lined surface per unit of reaction
volume.
Some additional and extremely important advantages of the
present invention are:
16
CA 02193944 1996-12-24
JVO 96/02471 1 ~ -5 ~ ~ ~I' ~ PCTIUS95/08799
o Annular space pressurization allows the use of relatively thin
chamber wall, thus reducing the expense of costly materials, since
the pressure vessel itself can be constructed of high strength but
lower cost materials
o The arrangement of the feed system is such as to produce
turbulent flow within the reaction zone, and thus ensure complete
reaction of the waste material with the oxidant at a minimal reactor
length
o Very little or substantially no consumption of inert gas
o Easy and practical detection of accidental leakage between the
annulus and the reaction chamber, which presents an enormous
safety benefit, as compared to other systems
o Considerably increased integrity of the reactor, because of the
use of just one reinforced entry, the enclosure head, for all inlet
and outlet connections
o Since the only connection of the reaction chamber to the closure
head resides in a rather cool region due to quenching, there is no
need for intricate expansion joints
o Detection of leakage between the reaction zone and the annulus
is simple and reliable
o Little or no back-mixing within the reactor results in true plug
flow characteristics
17
CA 02193944 2005-02-18
Further advantages of the present invention will also be seen more clearly
later. Many
wastes naturally contain dissolved inorganic solids ;e.g., NaCI salt). It is
well known that these
come out of solution as solid particles in supercritical water conditions,
where temperatures
are in excess of 800 F (627 C). The relatively large reaction chamber
diameter, made
possible by the present invention, reduces plugging potential in the reactor.
Consequently, it
is possible to treat wastes which otherwise might result in unacceptably low
operating utility
due to plugging in the reactor. Furthermore, it is hillhly desirable to add
neutralizing agents
to waste feed prior to the waste-feed heat exchanger, so that acidic
hydrolysis products
which form in these equipment pieces can be neutralized, thereby preventing
highly
undesirable corrosion. Excess neutralizing agents and neutralization salts of
hydrolysis
products are soluble in water at temperatures norrrially experienced in the
waste-feed heat
exchanger. However, these are known to come out of solution as solid
particulates at
temperatures associated with supercritical water oxidation (e.g., temperatures
equal to or
greater than 800 F (627 C). In addition, the relatively small pressure
differential across the
reactor chamber allows for operation at pressures required to maintain salts
in solution under
supercritical conditions. This is true since the reaction chamber wall
thickness can remain
constant as system pressure is increased, providinc the annular pressure is
raised with
system pressure. The constant reaction chamber thickness allows for economical
construction at higher pressures, since the increased pressure results only in
a greater wall
thickness of the inexpensive pressure shell. Previous reactor designs required
thick walls,
often composed of expensive corrosion resistant mziterials, to resist the
higher pressures
required for salt solubilization at high temperatures.
18
CA 02193944 2005-02-18
The relatively large reaction chamber diameter, made possible by this
invention, reduces
plugging potential in the reactor due to the aforementioried source, thereby
making feasible the
highly desirable addition of neutralization agents to the heat exchanger's
waste feed. Further, thanks
to the large diameter, when there is excessive normal wear in the liner or
coating, as it will explained
in more detail later, the reaction chamber may be removed easily and
inexpensively, and
immediately replaced with a new one. In contrast, this i:: not possible with
the conventional (hot-wall)
reactors, since they play the role of both the pressure vessel and the
reaction chamber.
According to a preferred embodiment of the present invention, better
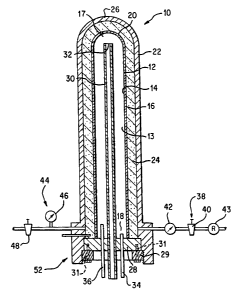
illustrated in Figure 1,
there is provided a reactor 14, which comprises a reaction chamber 12 having
an inside wail 14, an
1o outside wail 16, a bottom chamber end 18 and a top chamber end 20 opposite
the bottom
chamber end 18. The reaction chamber 12 defines or encloses a reaction zone 13
and an
ignition zone 17.
The reactor 10, also comprises a pressure vessel 22, which has preferably a
substantially
cylindrical shape, a top vessel end 26, and a bottom vessel end 28 opposite
the top vessel end 28.
The reaction chamber 12 has a similar cylindrical shape as the pressure vessel
22, and it
is enclosed within the pressure vessel 22. The pressure vessel 22 and the
reaction chamber
12 form an annulus 24, which is isolated from, and it dc-es not communicate
with the reaction zone
13 and the ignition zone 17. The reaction chamber may be designed for removal
and replacement
by means of a non permanent connection to the bottom vessel end 28. In tum,
the bottom vessel
end
19
CA 02193944 1996-12-24
WO 96/02471 PCT/US95/08799
28 may be attached to the pressure vessel 22 by means for example of a
restricting nut 29 and securing bolts 31.
The reactor 10, also comprises a feeding tube 30 passing through
the bottom vessel end 28 of the pressure vessel 22, and ending in a fluid
exit 32 within the ignition zone 17, in the vicinity of the top chamber end
20. For the purposes of this invention, the term "vicinity" represents a
region which is nominally within about two feet from the point of
reference, which in this particular case is the top chamber end 20. It is
io important that the fluid exit 32 is directed toward and impinges on the top
chamber end 20, so that it produces turbulent flow of the fluids fed, which
fluids comprise the aqueous waste liquid and oxidant.
The reactor 10, further comprises an effluent output 34 at the
bottom vessel end 28, and preferably a quench tube 36, which may
provide quench water.
An inert gas input 38, comprising a first regulating valve 40, a flow
meter 42, and a regulator 43 is provided. Also, an inert gas output 44
comprising a pressure indicator 46 and a second regulating valve 48 is
furnished.
The annulus is preferably substantially filled with high-temperature
resistant insulating material, which more preferably allows inert gas to
pass through. The insulating material in the annulus may be relatively
loosely arranged or packed tightly, and it can be selected to have a
number of different forms, well known to the art, such as beads, powder,
porous solid, fibers, and the like, very well known in the art. It is,
however, important that regardless of the form, the material may
CA 02193944 1996-12-24
{ ~ ! ~) A !
VO 96/02471 PCT/US95/08799
withstand the temperatures encountered in the annulus 24 of the reactor
10. The thermal insulator may also provide structural support to the
reaction chamber, especially if it is tightly packed or monolithic.
In order to maximize the structural integrity of the reactor, it is
preferable that all the connections are made at just one reinforced
portion of the reactor, such as for example the closure head 52. Thus,
the feeding tube 30, the effluent output 34, the querich tube 36, the inert
gas input 38, and the inert gas output 44, are all prF:ferably connected to
io the reactor 10 through the closure head 52. Connection of some of these
elements to the reactor 10 at an additional location would require
reinforcement of the additional location, and would degrade the integrity
of the reactor.
When the reactants used in the reaction chamber 12 are not
excessively corrosive, the reaction chamber may be made of any
conventional metal, such as for example stainless steel and other low
cost metals. Other construction materials include, but they are not limited
to ceramics, quartz, sintered alumina, mineral or ceramic composites,
2 o and the like. Since, as it will be discussed hereinafter, the reaction
chamber is pressurized from all sides with relatively small pressure
differentials, it only has to withstand the temperature and the atmosphere
inside the reaction chamber. Thus, the reaction chamber does not have
to be excessively strong.
A nominal wall thickness (distance between the inside wall 14 and
the outside wall 16) may be in the range of 1/8" to 3/4" . However,
thicknesses outside this range are not excluded.
21
CA 02193944 1996-12-24
4 PCT/US95/08799
WO 96f02471 94
The shape of the reaction chamber 12 is preferably tubular, with a
diameter and length depending on the particular application and desired
capacity. The reactor 10 may be installed at any angle from horizontal.
However, it is preferably installed in a vertical mode, with the ignition
zone in an upper position and the bottom vessel end at a lower position.
As aforementioned, if the atmosphere in the reaction chamber 12
is harsh and corrosive, the inside wall 14 of the reaction chamber 12
should preferably be made of or covered with a coating or liner
lo withstanding the harsh atmosphere. The exact composition of the
reaction chamber wall or wall-lining/coating is determined by the
corrosive conditions experienced with the particular waste feed. However
noble metals, such as platinum and gold for example, less expensive
metals such as titanium and zirconium for example, ceramics, ceramic
composites and other corrosion resistant materials are suitable for use.
Since the reaction chamber, for the above mentioned reasons, does not
need to be excessively strong, the use of highly expensive materials
such as platinum or goid for example, or less expensive (but still
expensive as compared to conventional construction materials) titanium
or zirconium, is viable. Since the reaction chamber has a relatively low
length to diameter ratio compared to conventional plug flow reactors, the
interior may be easily coated with a layer of corrosion resistant such as
platinum or gold for example. Prior plug flow reactors for Supercritical
Water Oxidation processing did not lend themselves to cladding or
coating, due to the high length to diameter ratios. Also, since the reaction
chamber is not a high stress bearing member, due to the annular
pressurization, a significant corrosion allowance may be designed into
the reaction chamber wall thickness. Thus, the use of materials not
normally suited for a particular corrosion service may be used since the
22
CA 02193944 1996-12-24
VO 96/02471 ,.4 3944 PCT/US95/08799
reaction chamber useful life is extended by additional corrosion
allowance. For waste containing chlorinated or in general halogenated
compounds, the preferable material of construction comprises titanium,
while for streams substantially free of halogenated compounds, the
preferred construction material, preferably in the form of a liner or
coating, is platinum.
In order to better describe the operation of the reactor of the
above embodiment, an oversimplified block diagram is shown in Figure
1.o 2, which includes a heat exchanger 62 and a heater 64. Details of the
total system and its operation, excluding the reactor of the present
invention, are well known to the art and are described in the
aforementioned patent literature.
Considering now Figures 1 and 2, the operation of a system
utilizing the reactor of the present invention may be briefly described so
that in a following detailed description, the operation of the reactor itself
may be better understood.
When the system is to start, a waste feed is pumped through the
heat exchanger 62 and heater 64 through feed path 63, and enters the
reactor 10 through feeding tube 30, preferably at or just under
supercritical conditions. An oxidant may be added to the feed prior to the
reactor, at the reactor inlet within feeding tube 30 in a supplemental
concentric tube arrangement as discussed in another embodiment of this
invention, or at the exit of the feeding tube 30 within the reaction
chamber. At this point, the heat exchanger does not offer any heating to
the waste feed, and therefore, the heater 64 has to operate at
considerably higher energy consumption. The use of heat exchanger is
23
CA 02193944 2005-02-18
optional since highly corrosive reactor effluent may prohibit its use, !n such
a case, the reactor
effluent quench is used to provide significant cooling to the effluent. The
heater 64 is
preferably a gas heater. Neutralization additives mEiy be added through the
feeding tube 30,
through additive tube arrangement as discussed in a different embodiment of
this invention,
through quench tube 36, as a part of the feed mixture, or a combination
thereof. When the
organics are oxidized, the effluent gases, being mixed with any other
constituents of the
reaction products, are subjected to a preliminary cooling by quench water
entering the reactor
through quench tube 36. The preliminarily coole-d products are in turn fed
through effluent
output 34, exit path 65 and through the other side of the heat exchanger 62,
in order to be
10 properly disposed of. As the reaction proceeds, the~ consumption of energy
in the heater 64
becomes lower, due to the heat interchange in the fieat exchanger 62, until a
steady state is
reached.
Coming now to the operation of the reactor '10 itself, as shown in Figure 1,
the initial
preparation comprises a step to ensure that the voicis left in the annulus
from the thermal
insulator are filled with inert fluid, and that the annulus 24 is isolated and
not communicating
with the reaction zone 13 of the reaction chamber 12. Inert fluid is allowed
to enter the
annulus by opening the first regulating valve 40, uniil a pressure of
preferably in the range of
to 300 psi is attained in the annulus. At this poini, the pressure in the
reaction zone is
20 about atmospheric. In sequence, if it is desired to substantially fully
remove all the oxygen
contained in the annulus 24, the first regulating va!v+: 40 is closed, the
second regulating valve
48 is opened in order to release the inert gas, the second valve 48 is closed
once more, the
first valve 40 is opened, and the annulus is pressurized with inert gas
containing no oxygen,
for
24
CA 02193944 2005-02-18
any practical purposes, preferably to the range of 20 to 300 psi.
Inert fluid far the purposes of the present invention is a fluid which does
not react with the
elements present in the annulus under operating conditions. Preferable inert
fluids are gases,
such as for example nitrogen, helium, argon, and the like.
In one method to check whether there is a leakage problem between the annulus
24 and the
reaction zone 13, provided that no other leakages are present in other
connections in the region of
the closure head, both valves 40 and 48 are closed after the pressure in the
annulus has been set
to a desired point, and said pressure is monitored on pnsssure indicator 46.
Consistent pressure
i o drop is indicative of a leakage.
A different way to check whether there is a leakage problem between the
annulus 24 and
the reaction zone 13, provided again that no other leakages are present in
other connections in the
region of the closure head, valve 48 is shut, valve 40 is attached to a source
of pressurized fluid,
and it is opened. Pressure in the annulus is set to a desired point by means
of regulator 43. The
flowmeter 42 is observed far continuous flow of gas, which is indicative of a
leakage.
Possible leakage at other points, should be checked by well known techniques
to the art, in
order to avoid false conclusions.
These methods of detecting leakage or communication between the annulus 24 and
the
reaction zone 13 may be used not only at this stage, but also during the
actual operation of the
reactor. Of course,
CA 02193944 2005-02-18
pressure and flow variations due to temperature variations and other
parameters should be
distinguished. Since pressure and flow variations due to leakage are
continuous, distinguishing the
two different types of variations is not difficult.
Still a different method to detect leakage from the reaction zone is based on
attaching a
moisture detector, very well known to the art, to monitor moisture level in
the annulus. Since the
inert gas in the annulus may be selected to be in a substantially dry state
(substantially free of
water), any leakage from the reaction chamber to the annulus will be
detectable by the moisture
detector, thus waming the operator of said leakage.
Other methods may also be used to detect leak;age. However, the three methods
described
above according to the present invention, are highly preferable as being
simple, and reliable.
After it has been established that no leakages eXist, clean pressurized water
and oxidant is
fed to the reactor through the feeding tube 30, while the pressure is raised
in the annulus and it is
maintained preferably 20 to 300 psi higher than the pressure in the reaction
zone, more preferably
100-300 psi, and even more preferably 150-250 psi. The pressure in the
reaction chamber is raised
gradually to allow for a gradual increase in the annular space pressure. When
adequate pressure
(>3,200 psi) is achieved in the reaction chamber, heat up of the fluid
entering the reactor may begin
by heater 64. When a desired temperature is reached at the reactor inlet, feed
flow may be shifted
to organic waste, and oxidant addition may be started.
26
CA 02193944 1996-12-24
VO 96/02471 PCT/US95/08799
The mixture of the waste and the oxidant ignite close to the fluid
exit 32 of the feeding tube 30, and the ignited mixture impinges on the
top chamber end 20, thereby producing high turbulence in the reaction
zone, and considerably increased reaction rates, ensuring complete
oxidation by the time the stream reaches the bottom chamber end 18.
The turbulence produces excellent mixing of the waste with the oxidant,
producing a real plug flow condition through the length of the reactor,
and removes any possibility of back-mixing (mixing of unoxidized waste
with the effluent), in contrast with other methods, such as the one
io described for example in U.S. Patent 4,822,497, wherein back-mixing is
unavoidable.
Although the temperature in the reaction zone and the chamber
walls 14 and 16 of the reaction chamber confront high temperatures, the
pressure differential between the two walls of the reaction chamber is
maintained relatively low. On the other hand, the temperature of the
pressure vessel 22 is kept low, while the pressure differential between
the inside and outside portions of the vessel is rather high. This
combination is very important, since metals lose their strength at
increased temperatures, while they may withstand high pressure
differentials at lower temperatures.
At the bottom chamber end 18, water entering through the
quench tube 36 is sprayed and cools down the stream to a certain
predetermined degree. The cooled down effluent leaves through effluent
output 34, and follows path 65, as described before, and as better shown
in Figure 2. The rest of the operation of the whole system is a shown in
Figure 2. The reactor effluent quench may be used to inject pH adjusting
additives, resolubilize salt particles, reduce the temperature of the
27
CA 02193944 1996-12-24
WO 96/02471 PCT/US95/08799
effluent so that more conventional corrosion-resistant materials may be
used, and to maintain the bottom vessel end 28 cool.
For better quenching and more efficient and uniform cooling, the
quench tube may be in a form of a coil around the feeding tube 30 with
one or more turns 52 to provide quenching water 54 in the form of fine
spray to the whole region, as better shown in Figure 3.
The oxidant may be any conventional source of oxygen, such as
lo for example oxygen-containing-gas, air, oxidizing agent, such as for
example hydrogen peroxide, ozone, persulfates, permanganates,
nitrates, and their corresponding acids, oxyacids of chlorine and their
corresponding salts, hypochlorites, chlorous acid and chlorites,
chlorates, perchlorates, and their corresponding acids. Mixtures of
oxidants may also be used.
For the purposes of this invention, oxygen, hydrogen peroxide,
and mixtures thereof are highly preferred. A free radical generating
material such as hydrogen peroxide is often a useful additive. The
2 o addition of hydrogen peroxide shortens the time required for a given
destruction level. Further, hydrogen peroxide can be used in place of
oxygen in the feeding system. This allows for a simpler system, since the
hydrogen peroxide is mixed into the waste stream before feeding, and
only one pump is needed to pressurize the stream which is fed to the
reactor through the feeding tube 30.
If there is chlorine, sulfur, or other compounds which produce
acids when oxidized under the water supercritical conditions, or in
general the conditions prevailing in the reaction zone 13, neutralization
28
CA 02193944 1996-12-24
IAVO 96/02471 1 V31944 PCTIUS95/08799
additives, such as alkali hydroxides or carbonates and the like may be
added to the reaction chamber 12 through the feeding tube 30 admixed
in the stream containing the aqueous waste liquid and the oxidant,
through additive tubes outside the feeding tube, or through concentric
tubes within the feeding tube, as described in other embodiments of this
invention. Since, however, even alkali salts may be insoluble under
water supercritical conditions and be deposited on the inside wall 14 of
the reaction chamber 12, it becomes critical in most occasions to
introduce such basic additives in the vicinity of the cooling portion, where
lo the supercritical conditions cease to exist. Therefore, it is highly
preferable that the additives are mixed with the quenching water entering
the reaction chamber 12 through the quench tube 36.
In a different embodiment of the present invention, better
illustrated in Figure 4, there is provided a supplemental tube 241, which
is substantially concentric to the feeding tube 230. It is preferable that
the concentric supplemental tube 241 ends in the vicinity of the fluid exit
232 of the feeding tube 230. For the purposes of this invention, as
already mentioned, the term "vicinity" represents a region which is
2 o nominally within about two feet from the point of reference, which at this
point is the fluid exit 232.
The supplemental concentric tube 241 is preferably used to
introduce the oxidant into the system, while the waste stream flows inside
the feeding tube 230 and outside the supplemental concentric tube 241.
The importance of this arrangement is that the reaction starts at around
or preferably after the fluid exit 232, so that the temperature of the
supplemental concentric tube remains relatively low. This in turn allows
the use of less expensive construction materials for the supplemental
29
CA 02193944 1996-12-24
WO 96l02471 PCT/US95/08799
concentric tube, and at least the inside surfaces of the feeding tube. The
high turbulence around the fluid exit 232 allows excellent mixing of the
oxidant with the waste stream.
In addition to oxidants, other first constituents, such as for
example reactants, diluents, additives, and the like may also be
introduced through the supplemental concentric tube 241.
Although the supplemental concentric tube is shown as having an
lo open end 235, the open end 235 may be actually closed and replaced by
perforations or other openings (not shown), if so desired.
More than one supplementary tubes may be used, preferably
concentric with respect to each other for feeding different first
constituents.
The operation of this embodiment is very similar to the operation
of the other embodiments, already described, with the difference that the
oxidant is mixed with the waste stream in the vicinity of the end 235 of
the supplemental concentric tube 241 instead of being premixed with
said waste stream.
In still another embodiment of the present invention, better
illustrated in Figure 5, there is provided an additive tube 343, which is
substantially concentric with feeding tube 330. The additive concentric
tube 330 has a closed end 349 with perforations 347, through which
second constituents may be introduced into the reaction zone 313.
Examples of the second constituents may comprise oxidants,
CA 02193944 1996-12-24
VO 96/02471 c PCT/US95/08799
~
other reactants, additives, recycled streams, diluents, and the like.
In most cases, it is preferable that the end 349 is disposed closer
to the bottom chamber end (18 in Figure 1), than to the fluid exit 332 of
the feeding tube 330.
Although the additive concentric tube 343 is shown as having a
closed end 349, the closed end 349 may be actually open, and the
perforations eliminated, if so desired.
More than one additive tubes may be used, preferably concentric
with respect to each other for feeding different second constituents.
The operation of this embodiment is very similar to the operation
of the other embodiments, already described, with the difference that
additives, may be added in a desired position of the reaction zone 313.
In a different embodiment of the present invention, the pressure
vessel is cooled in order to maintain an as low as possible temperature
2 o during operation. This is important, since the thickness of the pressure
vessel may be lowered and a lower-cost construction material may be
used.
Examples of ways to cool the pressure vessel are given in Figures
6 through 9.
Referring now to Figure 6, there is provided a cooling jacket 470,
surrounding the pressure vessel 422. Spacers 472 are used to hold the
jacket 470 at a distance from the outside surface 474 of the pressure
31
CA 02193944 1996-12-24
- q R
WO 96/02471 PCTIUS95/08799
vessel 422. The cooling jacket 470 has a first opening 476 and a second
opening (not shown) preferably at the other end of the jacket (not
shown), for passing cooling fluid, preferably liquid, through the space
478 between the outside surface 474 and the cooling jacket 470. The
opening 476 may be either an inlet or an outlet for the cooling fluid,
depending on the particular prevailing conditions. However, it is
preferable in most cases for the opening 476 to be an outlet of the
cooling fluid. The cooling fluid is preferably water, and more preferably
water containing rust inhibitors, well known to the art. Other liquids may
io also be used as cooling fluids.
The operation of this embodiment is similar to the operation of the
previously described embodiments, with the difference that at least part
of the pressure vessel is cooled by a cooling fluid passing through space
478 between the outside surface 474 of the pressure vessel 422, and the
jacket 470 to keep the temperature of the pressure vessel as low as
possible.
Figure 7 illustrates 510 having a cooling jacket 570 partially
surrounding the outside surface 574 of the pressure vessel 522. The
operation of this embodiment is substantially the same as the operation
of the embodiment of Figure 6, and therefore it needs no further
explanations.
In a still different embodiment of the present invention, better
illustrated in Figure 8, there is provided a cooling coil 670 inside the
annulus 624, in close proximity, and preferably in contact with the inside
surface 680 of the pressure vessel 622. The inlet and outlet (not shown)
of the coil are located preferably in the vicinity of the bottom vessel end
32
CA 02193944 1996-12-24
VO 96/02471 " 39" 4 PCT/US95/08799
(28 in Figure 1). The coil may be a separate element, or an integral part
of the pressure vessel, or even it may be embedded within the walls of
the pressure vessel.
Since the pressure inside the annulus 624 varies considerably
during different steps of the process, the use of a coil is preferable as
compared to the use of an internal jacket, since a coil may withstand high
pressures and pressure variations at considerably lower wall
thicknesses, with more effective heat transfer and considerably lower
lo cost. However, use of a coil on the outside surface of the pressure
vessel, or use of an internal cooling jacket is possibBe and within the
scope of the instant invention.
The operation of this embodiment is substantially the same as the
operation of the embodiments exemplified in Figures 6 and 7, and
therefore, it needs no further explanations.
In another preferred embodiment of the present invention, better
shown in Figure 9, there are provided cooling fins 770 attached at least
part of the outside surface 774 of the pressure vessel 722. Cooling fins
are very well known to the art. They may be in the form of thin sheets,
rods, bars, and the like, arranged in a manner to conduct heat out of the
pressure vessel, with or without the help of a cooling stream of forced air
from appropriately positioned fans (not shown).
The operation of this embodiment is substantially the same as the
operation of the embodiments exemplified in Figures 6, 7, and 8, with the
difference that air cooling is used instead of water cooling, and therefore,
it needs no further explanations.
33
CA 02193944 2005-02-18
The temperature of the pressure vessel should preferably be maintained under
704 F
(321 C), and more preferably under 500 F (260 C). Water cooling may bring the
temperature
of the pressure vessel to considerably lower levels, approaching ambient
temperature at least
in the case of the outside surface 474 of Figure 6, i'or example.
It should be pointed out that in the different embodiments of the present
invention,
oxygen could be replaced by other oxidants or mixtures of oxidants, or
mixtures of oxidants and
other gases or liquids. It should also pointed out that the reactors of the
present invention are
particularly useful in the case of supercritical water conditions, especially
in the presence of
corrosive compounds, such as for example halogeris, and the like.
The examples and the description of the above embodiments have been given for
illustrated purposes only and they should not be construed as restricting the
scope of the
present invention.
!n the different figures of the drawing, numerals differing by 100 represent
elements
which are either substantially the same or perform the same function.
Therefore, in the case
that one element has been defined once in a certairi embodiment, its re-
definition in other
embodiments illustrated in the figures by the same riumerals or numerals
differing by 100 is
not necessary, and it has been often omitted in the above description for
purposes of brevity.
34