Note: Descriptions are shown in the official language in which they were submitted.
~ 2195759
WO 96/05200 PCT/GB95/01820
-1-
TETItAHYDROPYRIDINYLMETHYL DERNATIVES OF
PYRROLO12,3-b1 PYRIDINE
This invention relates to a particular class of heteroaromatic
compounds. More particularly, the invention is concerned with certain
substituted tetrahydropyridinylmethyl derivatives of pyrrolo[2,3-b]-
pyridine. These compounds are ligands for dopamine receptor subtypes
within the body, in particular the dopamine D4 receptor subtype. They
are therefore of use in the treatment and/or prevention of disorders of the
dopamine system, including schizophrenia, depression, anxiety, nausea,
Parkinson's disease, tardive dyskinesias and extrapyramidal side-effects
associated with treatment by conventional neuroleptic agents, neuroleptic
malignant syndrome, disorders of hypothalamic-pituitary function such as
hyperprolactinaemia and amenorrhoea, and delusional disorders (cf.
1s Catalano et al., Biol. Psychiatry, 1993, 34, 459).
Upper gastrointestinal tract motility is believed to be under
the control of the dopamine system. The compounds according to the
present invention may thus be of use in the prevention and/or treatment
of gastrointestinal disorders, and the facilitation of gastric emptying.
Dependence-inducing agents such as cocaine and
amphetamine have been shown to interact with the dopamine system.
Compounds capable of counteracting this effect, including the compounds
in accordance with the present invention, may accordingly be of value in
the prevention or reduction of dependence on a dependence-inducing
agent.
Dopamine is known to be a peripheral vasodilator; for
example, it has been shown to exert a dilatory effect on the renal vascular
bed. This implies that the compounds of the present invention may be
beneficial in controlling vascular blood flow.
The localisation of dopamine receptor mRNA in rat heart and
large vessels has been noted. This suggests a role for dopamine receptor
2155759 ~
WO 96/05200 PGT1GB95101820
-2-
ligands in controlling cardiovascular function, either by affecting cardiac
and smooth muscle contractility or by modulating the secretion of
vasoactive substances. The compounds according to the present invention
may therefore be of assistance in the prevention andlor treatment of such
conditions as Ilypertension and congestive heart failure.
By virtue of their activity as ligands for dopamine receptor
subtypes within the body, the compounds in accordance with the present
invention may also be of benefit in enhancing cognitive function, and in
treating andlor preventing cognitive disorders induding presenile and
senile dementia (also known as Alzheimer's disease and senile dementia
of the Alzheimer type respectively).
Molecular biological techniques have revealed the existence
of several subtypes of the dopamine receptor. The dopamine D, receptor
subtype has been shown to occur in at least two discrete forms. Two forms
of the D2 receptor subtype, and at least one form of the D3 receptor
subtype, have also been discovered. More recently, the D4 (Van Tol et al.,
Nature (London), 1991, 350, 6 10) and Ds (Sunahara et al., Nature
(Londora,), 1991, 350, 614) receptor subtypes have been described.
The compounds in accordance with the present invention,
being ligands for dopanmine receptor subtypes within the body, in
particular the D4 receptor subtype, are accordingly of use in the treatment
andlor prevention of disorders of the dopamine system, induding
schizophrenia.
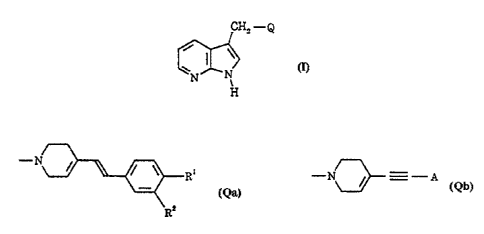
The present invention provides a compound of formula I, or a
salt or prodrug thereof:
Cfiz -Q
N x
k
H
ll)
2195759
WO 96/05200 PCTIcB95101820
_g.
wherein Q represents a moiety of formula Qa or Qb:
-N
R' -N_ ir---A
2
(Qa) (Qb)
wherein
Ri represents hydrogen, fluoro, cyano, trifluoromethyl,. Ci.s
alkyl, Ci.s alkoxy or di(Ci.c)alkylamino; and
R2 represents hydrogen, halogen, cyano, trifluoromethyl, Ci.6
alkyl or di(Ci-s)alkylamino; or
Rl and R2 together represent methylenedioxy;
provided that Ri and R2 are not both simultaneously hydrogen; and
A represents a group of formula (i), (ii) or (iii):
R R"
Ra
V
0) (h) (W)
in which
V represents oxygen, sulphur or N-R5;
R3 represents halogen, cyano, trifluoromethyl, Ci-s alkyl or
Ci.s alkoxy;
R¾ represents hydrogen, halogen, cyano, trifluoromethyl, C,.s
alkyl or Ci.s alkoxy; and
R$ represents hydrogen or Ci.s alkyl.
2195?5 ~
WO 96/05200 PCT/t'>B95l0]820
-4-
The compounds in accordance with the present invention are
encompassed within the generic scope of co-pending International Patent
Applfcation No. PCT1GB94f00384 (published on 15 September 1994 as WO
94/20459). There is, however, no specific disclosure therein of compounds
corresponding to those of formula I above wherein Q is as defined above.
For use in medicine, the salts of the compounds of formula I
will be pharmaceutically acceptable salts. Other salts may, however, be
useful in the preparation of the compounds according to the invention or
of their pharmaceutically acceptable salts. Suitable pharmaceutically
acceptable salts of the compounds of this invention include acid addition
salts which may, for example, be formed by mixing a solution of the
compound according to the invention with a solution of a pharmaceutically
acceptable acid such as hydrochloric acid sulphuric acid, fumaric acid,
maleic acid, succinic acid, acetic acid, benzoic acid, oxalic acid, ci.tric
acid,
tartaric acid, carbonic acid or phosphoric acid. Furthermore, where the
compounds of the invention carry an acidic moiety, suitable
pharmaceutically acceptable salts thereof may include alkali metal salts,
e.g. sodium or potassicun salts; alkaline earth metal salts, e.g. calrium or
magnesium salts; and salts formed with suitable organic ligands, e.g.
quaternary ammonium salts.
As used herein, the expression Ci.6 alkyl" relates to methyl
and ethyl groups, and straight-chained or branched propyl, butyl, pentyl
and hexyl groups. Particular alkyl groups include methyl, ethyl, n-propyl,
isopropyl and t-butyl. Derived expressions such as "C1.6 alkoxy" and
"di(Ci.s)alkylamino" are to be construed accordingly.
The term "halogen" as used herein includes fluorine,
chlorine, bromine and iodine, especially fluorine and chlorine.
The present invention includes within its scope prodrugs of
the compounds of formula I above. In general, such prodrugs will be
functional derivatives of the compounds of formula I which are readily
convertible in vivo into the required compound of formula I. Conventional
2'95759
=
WO 96/05200 PCT/GB95/01820
-5-
procedures for the selection and preparation of suitable prodrug
derivatives are described, for example, in Design of Prodrugs, ed. H.
Bundgaard, Elsevier, 1985.
Where the compounds according to the invention have at
least one asymmetric centre, they may accordingly exist as enantiomers.
It is to be understood that all such isomers and mixtures thereof in any
proportion are encompassed within the scope of the present invention.
Where the compounds of formula I above contain a moiety of
formula Qa, the group Rl suitably represents fluoro, cyano,
trifluoromethyl, methyl, methoxy or dimethylamino. Particular values of
R' include fluoro, cyano, trifluoromethyl, methyl and methoxy.
Suitably, R2 represents fluoro, chloro, cyano, trifluoromethyl,
methyl or dimethylamino, in particular fluoro or chloro, and especially
chloro.
Moreover, one of R' and R2 may represent hydrogen when the
other of Rl and R2 is other than hydrogen. Preferably, one of RI and R2
represents hydrogen and the other of R1 and R2 is other than hydrogen.
Where the compounds of formula I above contain a moiety of
formula Qb, the integer V suitably represents sulphur.
Suitably, R3 represents fluoro, chloro, cyano, trifluoromethyl,
methyl or methoxy. Particular values of R3 include fluoro, chloro,
trifluoromethyl, methyl and niethoxy.
Suitably, R4 represents hydrogen, fluoro, chloro, cyano,
trifluoromethyl, methyl or niethoxy, especially hydrogen.
Suitably, R5 represents hydrogen or methyl, especially
methyl.
Particular values for the substituent A include fluorophenyl,
chlorophenyl, cyanophenyl, trifluoromethylphenyl, methylphenyl,
methoxyphenyl, pyridinyl and thienyl.
2 '9J159
WO 96/05200 PC TdGB95i01820
-6-
A particular sub-class of compounds according to the present
invention is represented by the compounds of formula IIA, and salts and
prodrugs thereof:
ii
~ R
Nr N
H
(IL4)
wherein Rll represents fluoro, cyano, trifluoromethyl, Cr.s alkyl, Ci.s
alkoxy or di(Ca.e)alkylamino.
Suitably, R" represents fluoro, cyano, trifluoromethyl,
methyl, methoxy or dimethylamino.
Particular values of R" include fluoro, cyano,
trifluoromethyl, methyl and methoxy.
Another sub-class of compounds according to the present
invention is represented by the compounds of formula IIB, and salts and
-5 prodrugs thereof:
N
N N R3a
H
(IIB)
wherein R12 represents halogen, cyano, trifluoromethyl, CI.e alkyl or
di(Ci.s)alk,ylamino.
Suitably, R12 represents fluoro, chloro, cyano, trifluoromethyl,
methyl or dimethylamino.
= 2?95759
WO 96/05200 PCT/GB95101820
-7-
Particular values of R12 include fluoro and chloro, especially
chloro.
A further sub-class of compounds accordin.g to the present
invention is represented by the compounds of formula IIC, and salts and
s prodrugs thereof:
R
/
~
~ \ Rss
N
H
(TIC)
wherein
R" is as defined with reference to formula IIA above; and
R22 represents fluoro, chloro, trifluoromethyl or Cis alkyl.
Suitably, R22 represents fluoro, chloro, trifluoromethyl or
methyl.
Suitably, Rli and R22 both represent fluoro.
A still further sub-class of compounds according to the
present invention is represented by the compounds of formula IID, and
salts and prodrugs thereof:
N_ ~}--_
~
N \
H
' (IID)
wherein R3 is as defined with reference to forniula I above.
2'9~:i759
WO 96105200 PCT1GB95101820
-8-
A yet further sub-dass of compounds according to the present
invention is represented by the compounds of formula I as defined above,
and salts and prodrugs thereof, wherein Q represents a moiety of formula
Qb in which A represents a pyridin-3-yl, thien-2-yl or thien-3-yl group.
Specific compounds within the scope of the present invention
include:
3-[4-(4-trifluoromethylphenyl)ethynyl-1,2,3,6-tetrahydropyridin-l-
yl]methyl-lH-pyrro.lo [2 , 3-b]pyridine;
3-[4-(pyridin-3-yl)ethynyl-1,2,3,6-tetrahydropyridin-1-yl] methyl-lH-
1o pyrrolo[2,3-b]pyridine;
3-[4-(4-methoxyphenyl)ethynyl-1,2,3,6-tetrahydropyridin-1-yl] methyl-1H-
pyrrolo[2,3-b]pyridine;
3-[4-(4-methylphenyl)ethynyl-1,2,3,6-tetrahydrop yridin-l-yl]meth.yl-1H-
pyrrolo [2, 3-b]pyrid.ine;
3-[4-(4-fluorophenyl)ethynyl-1,2,3,6-tetrahydropyridin-1-yl]methyl-lH-
pyrrolo[2,3-b}pyridine;
3-[4-(4-chlorophenyl)ethynyl-1,2,3,6-tetrahydropyridin-1-yl}methyl-lH-
pyrrolo[2,3-b]pyricline;
3-[4-(t,hien-3-yl)ethynyl-1,2,3,6-tetrahydropyridin-1-yl]methyl-lH-
pyrrolo[2,3-b]pyridine;
3-[4-(thien-2-yl)etihynyl-1,2,3,6-tetrahydropyridin-l-yl]methyl-lH-
py'rrolo[2,3-b]pyridine;
(E)-3-[4-(2-(4-trifluoromethylphenyl)ethenyI)-1,2, 3,6-tetrahydropyridin-l-
yl] methyl-lH-pyrrolo[2,3-b]pyridine;
(E)-3-[4-(2-(4-methoxyphenyl)ethenyl)-1,2,3,6-tetrahydropyridin-l-
yl]methyl-lH-pyrrolo[2,3-b]pyridine;
(E)-3-[4-(2-(4-methylphenyl)ethenyl)- 1,2,3,6-tetrahydropyridin-1-
yl] methyl-lH-pyrrolo[2, 3-b]pyridine;
(E)-3-[4-(2-(4-cyanophenyl)ethenyl)-1,2,3,6-tetrahydropyridin-l-yl]methyl-
1H-pyrrol0[2,3-bjpyridine;
= 2 195759
WO 96105200 PCT/GB95P01820
-9-
(E)-3-[4-(2-(4-fluorophenyl)ethenyl)-1,2,3,6-tetrahydropyridin-l-yl]methyl-
1H-pyrrolo[2 ,3-b]pyridine;
(E')-3-[4-(2-(3-chlorophenyl)ethenyl)-1,2,3,6-tetrahydropyridin-l-
yl] methyl-lH-pyrrolo[2,3 -b]pyridine;
and salts and prodrugs thereof.
Additional specific compounds within the scope of the present
invention also include:
(E)-3-[4-(2 -(3-fluorop henyl)ethenyl)-1,2,3,6-tetrahydropyridin-l-yl]methyl-
1H-pyrrolo [2,3-b]pyridine;
(E)-3-[4-(2-(3,4-methylenedioxyphenyl)ethenyl)-1,2,3,6-tetrahydropyrid.in-
1-yl]methyl-lH-pyrrolo[2,3-b]pyridine;
()T)-3-[4-(2-(3-methylphenyl)ethenyl)-1,2,3,6-tetrahydropyridin-l-
yl]methyl-lH-pyrrolo [2,3-b]pyridine;
(E)-3 -[4-(2-(3-cyanophenyl)ethenyl) -1,2,3,6-tetrahydropyridin-l-yl]methyl-
1H-pyrrolo [2,3-bjpyridine;
(E)-3-[4-(2-(3,4-difluorophenyl)ethenyl)- 1,2,3,6-tetrahydropyridin- 1-
yl]methyl -1H-pyrrolo [2, 3-b]pyridine;
and salts and prodrugs thereof.
Further specific compounds within the scope of the present
invention include:
(E)-3-[4-(2-(3-fluoro-4-methoxyphenyl)ethenyl)-1,2,3,6-tetrahydropyridin-
1-yl]methyl-1H-pyrrolo[2,3-b]pyridine;
and salts and prodrugs thereof.
The invention also provides pharmaceutical compositions
comprising one or more compounds of this invention in association with a
pharmaceutically acceptable carrier. Preferably these compositions are in
unit dosage forms such as tablets, pills, capsules, powders, granules,
= sterile parenteral solutions or suspensions, metered aerosol or liquid
sprays, drops, ampoules, auto-injector devices or suppositories; for oral,
parenteral, intranasal, sublingual or rectal administration, or for
administration by inhalation or insufflation. Alternatively, the
2'957a9
=
WO 96/05200 PCTlGB9.5101820
-i0-
compositions may be presented in a form suitable for once-weekly or once-
monthly administration; for example, an insoluble salt of the active
compound, such as the decanoate salt, may be adapted to provide a depot
preparation for intramuscular injection. An erodible polymer containing
the active ingredient may be envisaged. For preparing so.lid compositions
such as tablets, the principal active ingredient is mixed with a
pharmaceutical carrier, e.g. conventional tableting ingredients such as
corn starch, lactose, sucrose, sorbitol, talc, stearic acid, magnesium
stearate, dicalcium phosphate or gums, and other pharmaceutical
diluents, e.g. water, to form a soiid preformulation composition containing
a homogeneous mixture of a compound of the present invention, or a
pharmaceutically acceptable salt thereof. When referring to these
preformulation compositions as homogeneous, it is meant that the active
ingredient is dispersed evenly throughout the composition so that the
1s composition may be readily subdivided into equally effective unit dosage
forms such as tablets, pills and capsules. This solid preformulation
composition is then subdivided into unit dosage forms of the type
described above containing from 0.1 to about 500 mg of the active
ingredient of the present invention. Favoured unit dosage forms contain
from 1 to 100 mg, for example 1, 2, 5, 10, 25, 50 or 100 mg, of the active
ingredient. The tablets or pills of the novel composition can be coated or
otherwise compounded to provide a dosage form affording the advantage
of prolonged action. For example, the tablet or pill can comprise an inner
dosage and an outer dosage component, the latter being in the form of an
envelope over the fornier. The two components can be separated by an
enteric layer which serves to resist disintegration in the stomach and
permits the inner component to pass intact into the duodenum or to be
delayed in release. A variety of materials can be used for such enteric
layers or coatings, sucll materials including a number of polymeric acids
and mixtures of polymeric acids with such materials as shellac, cetyl
alcohol and cellulose acetate.
~
WO 96/05200 2195759 PCT/GB95/01820
-11-
The liquid forms in which the novel compositions of the
present invention may be incorporated for administration orally or by
injection include aqueous solutions, suitably flavoured syrups, aqueous or
oil suspensions, and flavoured emulsions with edible oils such as
s cottonseed oil, sesame oil, coconut oil or peanut oil, as well as elixirs
and
similar pharmaceutical vehicles. Suitable dispersing or suspending
agents for aqueous suspensions include synthetic and natural gums such
as tragacanth, acacia, alginate, dextran, sodium carboxymethylcellulose,
methylcellulose, polyvinyl-pyrrolidone or gelatin.
In the treatment of schizophrenia, a suitable dosage level is
about 0.01 to 250 mg/kg per day, preferably about 0.05 to 100 mg/kg per
day, and especially about 0.05 to 5 mg/kg per day. The compounds may be
administered on a regimen of I to 4 times per day.
In order to alleviate the symptoms of schizophrenia without
causing sedation or extrapyramidal side-effects, it is believed that the
dosage level of the active ingredient should be selected such that the dose
administered is effective in substantially completely blocking the
dopamine D., receptor subtype in human brain whilst displaying no or
negligible dopamine D2 receptor subtype occupancy. A suitable dosage
level in this regard is about 0.001 to 5.0 mg/kg per day, more particularly
about 0.005 to 1.0 mg/kg per day, and especially about 0.01 to 0.5 mg/kg
per day.
If desired, the compounds in accordance with this invention
may be co-administered with another medicament, for example a known
anti-schizophrenic agent which produces its effects via dopamine D2
and/or 5-HT2 receptor blockade. Such co-administration may be desirable
where a patient is already on an established treatment regime, for
example one involving conventional anti-schizophrenic medicaments such
as haloperidol or chlorpromazine.
2195759 WO 96/05200 PCTlG$95/01820
-12-
The compounds in accordance with the present invention
may be prepared by a process which comprises reacting a compound of
formula III with a compound of formula IV:
i
~ i H-N'
R
(TIl) (IV)
wherein Q1 represents the residue of a moiety of formula Qa or Qb as
defined above, and RF represents a hydrogen atom or a suitable protecting
group; in the presence of a substantially equimolar amount of
formaldehyde; followed, where required, by removal of the prote.ctinng
group R.P.
The reaction is conveniently carried out by stirrin.g the
reactants in aqueous acetic acid, ideally in the presence of a buffer such as
sodium acetate tri.hydrate, suitably at room temperature.
The formaldehyde may be utilised in the form of
parafornlaldehyde; or as a solution of formaldehyde in an inert solvent,
e.g. 37% aqueous formaldehyde.
The protecting group Rr, when present, is suitably an acyl
moiety such as acetyl, which can conveniently be removed as necessary by
treatment under strongly basic conditions, e.g. sodium methoxide in
methanol. Alternatively, the protecting group RP may be a carbamoyl
moiety such as t-butoxycarbonyl (BOC), which can conveniently be
removed as necessary by treatment under mildly acidic conditions.
In an alternative procedure, the compounds according to the
present invention may be prepared by a process which comprises reacting
a compound of formula IV as defined above with a compound of formula V:
= 2195759
WO 96/05200 PCT/GB95/01820
-13-
CH~
I 6~ N
N
R
(V)
wherein Rp is as defined above, and L represents a suitable leaving group;
followed, where required, by removal of the protecting group W.
The leaving group L is suitably a halogen atom, e.g. chlorine
or bromine; or a dialkylami.no group, e.g. dimethylamino.
When L represents a halogen atom, the reaction between
compounds IV and V is conveniently carried out by stirring the reactants
under basic conditions in a suitable solvent, for example potassium
carbonate in N,N-dimethylformamide, or triethylamine in
tetrahydrofuran or acetonitrile. Where L represents a dialkylamino
group, the reaction is conveniently effected by heating the reactants in an
inert solvent such as toluene, typically at the reflux temperature of the
solvent.
Where they are not commercially available, the starting
materials of formula III, IV and V may be prepared by procedures
analogous to those described in the accompanying Examples, or by
standard methods well known from the art.
Where the above-described processes for the preparation of
the compounds according to the invention give rise to mixtures of
stereoisomers, these isomers may be separated by conventional techniques
such as preparative chromatography. The compounds may be prepared in
racemic form, or individual enantiomers may be prepared either by
enantiospecific synthesis or by resolution. The compounds may, for
example, be resolved into their coniponent enantiomers by standard
techniques such as preparative HPLC, or the formation of diastereomeric
pairs by salt formation with an optically active acid, such as (-)-di-p-
2195759
. =
WO 96/05200 PC77GB95/01820
-14-
toluoyl-d-tartaric acid and/or (+)-di-p-toluoyl-l-tartaric acid, followed by
fractional crystallization and regeneration of the free base. The
compounds may also be resolved by formation of diastereomeric esters or
amides, followed by chromatographic separation and removal of the chiral
auxiliary.
During any of the above synthetic sequences it may be
necessary and/or desirable to protect sensitive or reactive groups on any of
the molecules concerned. This may be achieved by means of conventional
protecting groups, such as those described in Protective Groups in Organic
Chemistry, ed. J.F.W. McOmie, Plenum Press, 1973; and T.W. Greene &
P.G.M. Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons,
199 1. The protecting groups may be removed at a convenient subsequent
stage using methods known from the art.
The following Examples illustrate the preparation of
t5 compounds according to the invention.
The compounds useful in this invention potently inhibit [gH]-
spiperone binding to human dopamine D4 receptor subtypes expressed in
clonal ce.ll lines.
2o [3H]-Spiperone Binding Studies
Clonal cell lines expressing the human dopamine D4 receptor
subtype were harvested in PBS and then lysed in 10 mM Tris-HCl pH 7.4
buffer containing 5 mlY1 MgSO4 for 20 min on ice. Membranes were
25 centrifuged at 50,000g for 15 min at 4 C and the resulting pellets
resuspended in assay buffer (50 mM Tris-HCl pH 7.4 containing 5 mM
EDTA, 1.5 mM CaClz, 5 mM MgCl2, 5 mM KCI, 120 mM NaCl, and 0.1%
ascorbic acid) at 20 mglml wet weight. Incubations were carried out for 60
min at room temperature (22 C) in the presence of 0.05-2 nM [9H]-
3e spiperone or 0.2 p.M for displacement studies and were initiated by
addition of 20-100 g protein in a final assay volume of 0.5 ml. The
~ 2?95759
WO 96/05200 PCTIGB95/01820
15-
incubation was terminated by rapid filtration over GFB filters presoaked
in 0.3% PEI and washed with 10 ml ice-cold 50 mM Tris-HC1, pH 7.4.
Specific binding was determined by 10 gM apomorphine and radioactivity
determined by counting in a LKB beta counter. Binding parameters were
determined by non-linear least squares regression analysis, from which
the inhibition constant K could be calculated for each test compound.
The compounds of the accompanying Examples were tested
in the above assay, and all were found to possess a K; value for
displacement of [3H]-spiperone from the human dopamine D4 receptor
subtype of below 1.5 M.
EXANTPLE 1
$-[4-(4-Tri.fluoromethvl)phenvlethvnyl-1 2 3 6-tetrahvdronvridin-l-
y1]methvl-1H-12.3-binyridine
Step 1: 1-tert-Butoxycarbonv1-4-oineridone
Triethylamine (92ml, 0.66mo1) was added dropwise to a cold
(0 C) suspension of 4-piperidone monohydrate hydrochloride (100g,
0.65mo1) and di-tert-butyl dicarbonate (142g, 0,65mo1) in dichloromethane
(400m1) under a nitrogen atmosphere. The cooling bath was removed
when about a quarter of the triethylamine had been added.
After 2 hours stirring at room temperature the vigorous
bubbling had ceased, the mixture was diluted with water (500m1), the
phases were separated and the aqueous was extracted with
dichloromethane (3 x 250ni1). The combined organics were washed with
brine (250m1), dried (MgSOa) and evaporated irt vacuo to give a light
brown solid. The solid was dissolved in ethyl acetate (11) and treated with
silica gel (150g). Filtration and evaporation gave the title compound as a
white waxy solid (111.7g, 86%); SH (CDCIs) 1.49 (9H, s, OC(CH3)3), 2.44
2195759 WO 96/05200 PC1'1GB95/01820
-16-
(4FI, t, J 6.1Hz, 2 x piperidone CH2CO) and 3.72 (4H, t, J 6.1Hz, 2 x
piperidone CH2-N).
Step 2: 1-tert-Butoxvcarbonvl-4-trimethvlsilylethvnvl-1.2.3.6-
tetrahvdronyridine
n-Butyl lithium (2.5M in hexanes, I lOml, 276mmol) was
cannulated into a solution of trimethylsilylacetylene (40m1, 276mmo1) in
tetrahydrofuran (500m1) at -70 C, under a nitrogen atmosphere, at such a
rate that the temperature did not exceed -60 C. Once the addition was
complete the solution was stirred at -70 C for one hour. The cooling bath
was removed and the solution was cannulated into a solution of 1-tert-
butoxycarbonyl-4-piperidone in THF (500m1) at -70 C under a nitrogen
atmosphere at such a rate that the temperature did not exceed -65 C.
Once the addition was complete the mixture was stirred at -70 C for 10
minutes before warming to room temperature. Following a one hour stir
at room temperature the mixture was cooled to 0 C and saturated
ammonium chloride (500m1) was added. The solvent was removed itt
vacuo and the residue extracted with dichloromethane (3 x 500m1). The
organics were dried (MgSO4) and evaporated in vucuo to give an orange
residue (89g). The residue was dissolved in diehloromethane (11) under a
nitrogen atmosphere, triethylamine (52.5m1, 375mmo1) was added and the
mixture was cooled to -10 C. Mesyl chloride was then added at such a
rate that the temperature did not exceed 0 C. Once the addition was
complete the mixture was stirred at 0'C for 10 minutes, allowed to warm
to room temperature and stirred for 20 hours.
The solution was treated with sodium bicarbonate (sat.,
400ml), the phases were separated and the aqueous extracted with
dichloromethane (2 x 250m1). The combined organics were washed with
brine (250ml), dried (MgSO4) and evaporated in vacuo to give a pale oil
which was purified by column chromatography on silica, eluting with
ethyl acetate/petroleum ether 60/80 (1:1), to give the title compound (51g,
2195759
WO 96/05200 PCT7GB95101820
-17-
72%) as a pale straw coloured oil; Sx (CDCla) 1.46 (9H, s, OC(CHs)a), 2.24
(2H, br s, tetrahydropyridinyl CH2), 3.47 (2H, t, J 5.6Hz,
tetrahydropyridinyl CH2), 4.12 (2H, d, J 7.1Hz, tetrahydropyridinyl CH2)
and 6.06 (1H, br s, tetrahydropyridinyl CH).
Step 3: 1-tert-Butoxvcarbonvl-4-ethynvl-1.2.3.6-
tetrahvdronyridine
Potassium carbonate (1.0g, 7.2mmol) was added to a solution
of 1-tert-butoxycarbonyl-4-trimethylsilylethynyl-1,2,3,6-
tetrahydropyridine (44.9g, 161mmo1) in methanol (250m1) under a
nitrogen atmosphere and the mixture was stirred at room temperature for
3 hours. The solution was evaporated in vacuo without heating. The
residue was dissolved in ether (250m1), washed with sodium carbonate
(sat., 100m1), water (50m1) and brine (50m1) before drying (MgSO4) and
evaporation in vacuo to give the title compound as a white waxy solid
(30.0g, 90%); 5x (CDC13) 1.46 (9H, s, OC(CHa)s), 2.24-2.26 (2H, m,
tetrahydropyridinyl CH2), 2.89 (1H, s, ethynyl CH), 3.49 (2H, t, J 5.7Hz,
tetrahydropyridinyl CH2), 3.95-3.97 (2H, m, tetrahydropyridinyl CH2) and
6.10 (1H, s, tetrahydropyridinyl CH).
Step 4: 1-tert-Butoxycarbony1-4-1(4-trifluoromethyl)-
phenvl ethvnvll-1.2.3.6-tetrahvdropvridine
Bis(triphenylphosphine)palladium(II) chloride (61mg,
0.09mmo1) and copper(I) iodide (17mg, 0.09mmo1) were added to a solution
of 1-tert-butoxycarbonyl-4-ethynyl-1,2,3,6-tetrahydropyridine (2.0g,
9.6mmol) and 4-iodobenzotrifluoride (3.15g, 11.6mmol) in diethylamine
under a nitrogem atmosphere. The mixture was stirred at room
temperature for 18 hours before evaporation in vacuo. The residue was
diluted with dichloromethane (50m1) and washed with water (50m1). The
aqueous was extracted with dichloromethane (2 x 50m1) and the combined
organics were washed with brine (50m1), dried (NIgSO4) and evaporated in
2195759
WO 96105200 PQT/GB95/01820
-18-
uacuo to give a red brown oil which solidified on standing. Purification by
column chromatography on silica eluting with ethyl acetatelpetrnl (60-80 )
(1:9) gave the title compound as a beige solid (2.58g, 76%); Sa (CDCIs)
2.24-2.30 (2H, m, tetrahydropyridinyl CHa), 3.01 (2H, t, J 5.7Hz,
tetraliydropyridinyl CH2), 6.23-6.26 (1H, m, tetrahydropyridinyl CH) and
7.49-7.58 (4H, m, ArH).
Step 5: 4-(4-Trifluoromethvl)nhen ~ Ilethynvl-1.2.3.6-
tetrahydropvridine
Trifluoroacetic acid (lOml) was added to a solution of 1-terG-
butoxycarbonyl-4-[(4-trifluoromethyl)phenylethynyl] -1,2,3,6-
tetrahydropyridine (2.40g, 6.83mmol) in dichloromethane (15m1) under a
nitrogen atmosphere and the solution was stirred at room temperature for
30 minutes. The mixture was evaporated in vacuo and the residue diluted
with dichloromethane (40m1) and washed with sodium hydroxide solution
(1N, 40m1). The aqueous was extracted wth dichloromethane (2 x 40m1)
and the combined organics were washed with brine (40m1), dried (KaCOs)
and evaporated ira vacuo to give the title compouind as a yellow brown
solid (1.69g, 99%); SFi (CDCIa) 2.24-2.30 (2H, m, tetrahydropyridinyl CHz),
3.01 (2H, t, J 5.7Hi, tetrahydropyridinyl CH2), 3.46-3.50 (2H, m,
tetrahydropyridinyl CH2), 6.24-6.26 (1H, m, tetrahydropyri(iinyI CH) and
7.49-7.58 (411, m, ArI-1).
Step 6: 3-f(4-Trifluoromethyl)phenyletbvnvI-1.2.3.6-
tetrahvcixonyri.din-I-vllmethyl-IH-nvrrolo12.3-binvizdine.
A mixture of 4-(4-trifluoromethyl)phenylethynyl-1,2,3,6-
tetrahydropyridine (800mg, 3.2mmol) and 3-di.methylaminomethyl-IH-
pyrrolo[2,3-b]pyridine (613mg, 3.5mmol) in dry toluene (30m1) under a
nitrogen atmosphere was heated at reflux for 20 hours. The mixture was
cooled to room temperature and the solid precipitate collected by filtration,
washed krith toluene and dried in vacuo. The solid was recrystallised
~ 2'9575g
WO 96/05200 PCT/GB95101820
19-
from toluene to give the title compound as a white solid (615mg, 51%),
m.p. 191-193 C (toluene); (found: C, 69.54; H, 4.65; N, 10.98. CzzHiaFaNs
requires C, 69.28; H, 4.76; N, 11.02%); 611 (DMSO-d6) 2.27-2.30 (2H, m,
tetrahydropyridinyl CHz), 2.58 (2H, t, J 5.6Hz, tetrahydropyridinyl CH2),
3.05 (211, m, tetrahydropyridinyl CH2), 3.73 (211, s, NCHzAr), 6.24 (1H, m,
tetrahydropyridinyl CH), 7.04 (1H, dd, J 7.8 & 4.6Hz, 5-H), 7.38 (1H, d, J
2.3Hz, 2-H), 7.62 (2H, d, J 811z, ArH), 7.72 (2H, d, J 8Hz, ArH), 8.02 (1H,
dd, J 7.8 & 1.4Hz, 4-H), 8.20 (1H, dd, J 4.6 & 1.5Hz, 7-H) and 11.48 (111,
br s, NH); m/z (ES') 382 (M+1)4.
EXAMPLE 2
3-[4-(4-Methoxy)phenvlethynyl-12 3 6-tetrahvdronvridin 1 vl]methvl-IH
p ynolo(2.3-b2nvridine
M.p. 207-209 C (toluene); (found: C, 76.65; H, 6.08; N, 12.08.
C22H2iN30 requires C, 76.94; H, 6.16; N, 12.24%); Sx (DMSO-ds) 2.23 (2H,
br s, tetrahydropyridinyl CHz), 2.56 (2H, t, J 5.5Hz, tetrahydropyridinyl
CHz), 3.02 (2H, d, J 2.9Hz, tetxahydropyridinyl CH2), 3.72 (2H, s,
NCH2Ar), 3.76 (3H, s, OCHa), 6.08 (1H, m, tetrahydropyridinyl CH), 6.90-
6.93 (2H, m, ArH), 7.03 (111, dd, J 7.8 & 4.6Hz, 5-H), 7.33-7.38 (3H, m,
ArH), 8.02 (1H, dd, J 7.8 & 1.3Hz, 4-H), 8.19 (IH, dd, J 4.6 & 1.5Hz, 6-H)
and 11.47 (1H, br s, NFi); m/z (ES*) 344 (M+1)'.
EXAMPLE 3
3-(4'-(3-Pvridinvl)ethynyl-1.2.3.6-tetrahvdropvridin-1-y llmethvl-lH-
pyrrolo [2.3-blpyridin e
The title compound was synthesised from 3-iodopyridine
(prepared using the procedure of L.O. Shnaidmann et al., Tr. Inst.
ti
2195759 PCT/GB99/01820
WO 95l05200
-20-
Eksperim. Klinisch. Med. Akad. Nauk Latu. SSR, 1962, 27, 1-14) using
chemistry analogous to Example 1.
M.p. 170-172 C (toluene); (found: C, 76.28; H, 5.69; N, 17.34.
CaoHrsN4Ø025 (C7Hs) requires C, 76.52; H, 5.79; N, 17.69%); 5x (DMSO-
de) 2.26-2.29 (2H, m, tetrahydropyridinyl CHz), 2.58 (2H, t, J 5.6Hz,
tetra:hydropyridinyl CHa), 3.05 (2H, d, J 3.2Hz, tetrahydropyridinyl CHa),
3.73 (2H, s, NCHaAr), 6.21 (1H, br s, tetrahydropyridinyl CH), 7.04 (1H,
dd, J 7.8 & 4.7Hz, 5-H), 7.38-7.41 (2H, m, ArH), 7.81-7.84 (1H, m, ArH),
8.02 (111, dd, J 7.8 & 1.3Hz, 4-H), 8.19 (1H, dd, J 4.6 & 1.5Hz, 6-H), 8.52
(1H, dd, J 4.8 & 1.6Hz, ArH), 8.61 (iH, dd, J 2.1 & 0.6Hz, ArH) and 11.48
(1H, br s, NIi); m/z (ES+) 315 (M+1)'.
EXAMPLE 4
8-f 4-(3-Thienvl)ethynvl-1.2.3.6-tetrah ydrov vridin-1-vl]methyl-lH-
pvrrolo (2.3-b1 pvri dine
M.p. 203-205`C (methanol); (found: C, 70.45; H, 5.23; N,
12.83. Ci9Hi7N3SØ25 (HaO) requires C, 70.45; H, 5.45; N, 12.97%); 611
(I)MSO-dc) 2.28 (2H, br s, tetrahydropyridinyl CHa), 2.56 (2H, t, J 5.6Hz,
tetrahydropyridinyl CHa), 3.02-3.03 (2H, m, tetrahydropyridinyl CH2),
3.72 (2H, s, NCHaAr), 6.10 (1H, br s, tetrahydropyridinyl CH), 7.03 (1H,
dd, J 7.8 & 4.6Hz, 5-H), 7.12 (1H, dd, J 5.0 & 1.1Hz, ArH), 7.37 (1H, s, 2-
H), 7.58 (1H, dd, J 5.0 & 2.9Hz, ArH), 7.72 (1H, dd, J 2.9 & 1.0Hz, ArH),
8.02 (1H, dd, J 7.8 & 1.4Hz, 4-H), 8.19 (1H, dd, J 4.6 & 1.5Hz, 6-H) and
11.47 (111, br s, NH); m/z (ES+) 320 (N,1+1)`.
~ 2195759
WO 96/05200 PCT/cs95/01820
-21-
EXAMPLE 5
3-f4-(4-Chloro)nhenyle hvnvl-1.2.3.6-tetrahydropyridin-l-yllmethyl-lH-
nyrro1o f 2, 3-b1 oyridine
M.p. 114-116 C (methanol); (found: C, 72.19; H, 5.29; N,
11.85. CaiHiaNsC1 requires C, 72.51; H, 5.22; N, 12.08%); Sx (DMSO-ds)
2.24 (2H, br s, tetrahydropyridinyl CHz), 2.57 (2H, t, J 5.5Hz,
tetrahydropyridinyl CHz), 3.03 (2H, d, J 3.2Hz, tetrahydropyridinyl CH2),
3.72 (2H, s, NCH2Ar), 6.16 (1H, m, tetrahydropyridinyl CH), 7.03 (1H, dd,
J 7.8 & 4.6Hz, 5-H), 7.37 (1H, d, J 2.3Hz, 2-H), 7.42 (4H, s, ArH), 8.02
(1H, dd, J 7.9 & 1.4Hz, 4-H), 8.19 (1H, dd, J 4.7 & 1.6Hz, 6-H) and 11.47
(1H, br s, NH); m/z (ES*) 348 (M+1)*.
1s EXAMPLE 6
3-f4-(4-Methvl)aheanvlethynyl-1,2,3,6-tetrahydronyridin-l-vllmethyl-lH-
pvrrolof 2.3-blpvridine
M.p. 219-221 C (methanol); (found: C, 79.80; H, 6.39; N,
12.64. C22H2N3Ø2 (H20) requires C, 79.82; H, 6.52; N, 12.69%); Sx
(DMSO-ds) 2.23 (2H, br s, tetrahydropyridinyl CHa), 2.30 (3H, s, ArCHs),
2.56 (2H, t, J 5.6Hz, tetrahydropyridinyl CH2), 3.02 (2H, d, J 3.2Hz,
tetrahydropyridinyl CHs), 3.72 (2H, s, NCHzAr), 6.10 (1H, m,
tetrahydropyridinyl CH), 7.03 (1H, dd, J 7.8 & 4.7Hz, 5-H), 7.17 (2H, m,
ArH), 7.29 (2H, m, ArH), 7.37 (111, d, J 2.3Hz, 2-H), 8.02 (1H, dd, J 7.8 &
1.4Hz, 4-H), 8.18 (1H, dd, J 4.7 & 1.5Hz, 6-H) and 11.47 (1H, br s, NH);
m/z (ES*) 328 (M+1)*.
2195759 WO 96/05200 PCT1GB95101820
-22-
EkAMPLE 7
344-(4-F1 uoro)oh envlethvnvl-1.2.3.6-tetrahvdron vridin -1-vll methyl-1H-
Dvrrolof2.3-blovridine
M.p. 191-192 C (methanol); (found: C, 75.98; H, 5.37; N,
12.54. CztHisFNa requires C, 76.11; H, 5.48; N, 12.68%); Sfi (DMSO-ds)
2.24 (2H, m, tetrahydropyridinyl CH2), 2.57 (2H, t, J 5.5Hz,
tetrahydropyridinyl CH2), 3.03 (2H, d, J 3.0Hz, tetrahydropyridi:nyl CH2),
3.72 (2H, s, NCH2Ar), 6.14 (1H, br s, tetrahydropyridinyl CH), 7.04 (1H,
dd, J 7.8 & 4.6Hz, 5-.H), 7.18-7.23 (2H, m, ArH), 7.38 (1H, s, 2-H), 7.44-
7.48 (2H, m, ArH), 8.02 (IH, dd, J 7.8 & 1.5Hz, 4-H), 8.19 (1H, dd, J 4.7 &
1.5Hz, 6-H) and 11.47 (111, br s, NH); m/z (ES`) 332 (M+1)*.
EXAMPLE 8
344-(2-Thienvl)ethynvl 12 3 G tetrahydropvridin-l-yllmethyl-iH-
Pyrrolof2.3-blpvridine
Step 1: 4-(2-Thieny)ethynvl-1.2,3,6-tetrahydropyridine
A solution of 1-tert-butoxycarbonyl-4-(2-thienyl)ethynyl-
1,2,3,6-tetrahydropyridine (530mg, 1.8mmol) (prepared in a manner
analogous to Example 1) in diethyl ether (10m1) at room temperature
under a nitrogen atmosphere was treated with excess ethereal hydrogen
chloride. Following a 3 hour stir at room temperature the dark mixture
was diluted with water and extracted with diethyl ether (2 x 30ml). The
aqueous phase was made basic with potassium carbonate solution (sat.)
and extracted with diethyl ether (3 x 50ml). The extracts were dried
(IisCQs) and evaporated in vacuo to give the title compound as a brown oil
(267mg, 77%); 5a (CDCls) 2.24-2.29 (211, m, tetrahydropyridinyl CH2),
3.00 (2H, J 5.7Hz, tetrahydropyridinyl CH2), 3.45-3.47 (2H, m,
2195759
WO 96/05200 PCTPGB9S/01$20
-23-
tetrahydropyridinyl CH2), 6.17-6.19 (1H, m, tetrahydropyridinyl CH),
6.94-6.97 (1H, m, ArH), 7.16-7.17 (1H, m, ArH) and 7.22-7.26 (1H, m,
ArH).
Step 2: 3-f4-(2-Thieyl)ethynyl-1 2 3 6-tetrahvdropyridin-l-
yllmethvl-lHQyrrolol2,3-binvridine
The title compound was prepared from 4-(2-thienyl)ethynyl-
1,2,3,6-tetrahydropyridine using chemistry analogous to Example 1.
M.p. 191-193 C (methanol); (found: C, 71.38; H, 5.24; N,
13.01. Ci9HI7N3S requires C, 71.44; H, 5.36; N, 13.16%); Sx (DMSO-ds)
2.36 (2H, br s, tetrahydropyridinyl CHz), 2.56 (2H, t, J 5-6Hz,
tetrahydropyridinyl CH2), 3.03 (2H, d, J 3.1Hz, tetrahydropyriclinyl CHs),
3.72 (2H, s, NCHaAr), 6.15 (1H, br s, tetrahydropyridinyl CH), 7.02-7.07
(2H, m, ArH), 7.26 (1H, dd, J 3.6 & 1.2Hz, ArH), 7.37 (1H, d, J 2.3Hz, 2-
H), 7.58 (IH, dd, J 5.1 & 1.0Hz, ArH), 8.02 (1H, dd, J 7.8Hz, 4-H), 8.19
(1H, dd, J 4.6 & 1.5Hz, 6-H) and 11.47 (1H, br s, NH); m/z (ES*) 320
(M+1)+-
2o EXAMPLE 9
(E)-3-f4-f2-(4-Fluoron henvl)ethenyll-1 2 3 6-tetrahvdropyridin-1
yllmethvlnvrrolo f 2, 3-bi n vri dine
Step 1: (E)-4-f2-(4-Fluorophenvl)ethenvllnyridine
A mixture of 4-picoline (15.6m1, 161mmo1) and 4-
fluorobenzaldehyde (20g, 161mmo1) in acetic anhydride (125m1) was
= heated at reflux overnight. The reaction was cooled and the solvent
evaporated. Water (100m1) was added to the residue, and the mixture
stirred at room temperature for 30 minutes. Saturated sodium carbonate
solution (100m1) and ethyl acetate (150m1) were then added, and stirring
- - -- -
2195759
WO 96/05200 PCTlGB95101820
-24-
continued for a further 30 minutes. The two phases were separated, and
the aqueous phase extracted with ethyl acetate (3 x 200m1). The combined
organic phases were dried (MgSO4) and evaporated. The residue was
purified by flash chromatography, eluting with 50% ethyl acetate/petrol
(60-80 ). The appropriate fractions were combined and concentrated to
yield (E)-4-[2-(4-fluorophenyl)ethenyl]pyridine (19g, 60%) as a beige solid.
Sx (CDCb) 6.93 (1H, d, J 16.3Hz, ArCHCH), 7.05-7.13 (2H, m, ArH), 7.26
(1H, d, J 16.3Hz, ArCHCID, 7.34-7.36 (2H, m, ArH), 7.48-7.54 (2H, m,
ArH) and 8.56-8.60 (2H, m, ArH).
Step 2: (E)-1-Benzvl-4-f2-(4-fluorophenvl)ethenvll-I ?.3.6-
tetrahvdropyridine
To a solution of (E)-4-[2-(4-fluorophenyl)ethenyljpyridine
(18.75g, 94.2mmo1) in dimethylformamide (30m1) at 900C was added
is benzyl bromide (11.9m1, 100mmo1) and the reaction stirred at 90 C for 1
hour. The reaction was cooled to room temperature, diluted with ethanol
(400m1) and sodium borohydride (4g, 105mmol) added in portions. The
reaction was stirred at room temperature for 2 hours, after which time the
solvent was evaporated to a slurry. Methanol was added, and the
resultant solid filtered and dried to yield (E)-1-benzyl-4-[2-(4-
fluorophenyl)ethenyi]-1,2,3,6-tetrahydropyridine (12g, 44%) as a white
solid. Sx (CDCIa) 2.41 (2H, br s, tetrahydropyridinyl CH2), 2.68 (2H, t, J
5.8Hz, tetrahydropyridinyl CH2), 3.14 (2H, br s, tetrahydropyridinyl CHa),
3.63 (2H, s, NCHzEIr), 5.80 (1H, br s, tetrahydropyridinyl 5-CH), 6.40 (1H,
d, J 16.2Hz, ArCHCHC), 6.69 (2H, d, J 16.2Hz, ArCHCHC), 6.96-7.02 (211,
m, ArH) and 7.25-7.39 (711, m, ArH).
Step 3: (E)-4-[2-(4-Fluorophenyl)ethenyl]-1,2.3.6-
tetrahvdrouyridine hvdrochloride
To a.solution of (E)-1-benzyl-4-[2-(4-fluorophenyl)ethenyl]-
1,2,3,6-tetrahydropyridine (I1.6g, 37.5mmol) in dichloromethane (500m1)
?195759
WO 96105200 PCT/GB95/01820
-25-
at 0 C was added 1-chloroethyl chloroformate (4.85m1, 45mmol). The
reaction was stirred initially at 0 C, and allowing it to warm to room
temperature over 2 hours. The solvent was evaporated and methanol
(500m1) added. The mixture was heated at reflux for 1 hour, cooled to
room temperature, and the solvent evaporated to a slurry. Diethyl ether
was added and the resultant precipitate filtered and dried to yield (E)-4-
[2-(4-fluorophenyl)ethenyl]-1,2,3,6-tetrahydrapyridine hydrochloride
(6.2g, 69%) as a white solid. ba (DMSO-ds) 2.49-2.53 (2H, m,
tetrahydropyridinyl CHz), 3.23 (2H, t, J 6.1Hz, tetrahydropyridinyl CH2),
to 3.69 (2H, br s, tetrahydropyridinyl CH2), 5.90 (1H, br s,
tetrahydropyridinyl5-CH), 6.61 (1H, d, J 16.3Hz, ArCHCHC), 6.92 (1H, d,
J 16.3Hz, ArCHCHC), 7.15-7.20 (2H, m, ArH), 7.55-7.59 (7H, m, ArH) and
9.45 (1H, br s, NH).
Step 4: -3- 4- 2-(4-Fluorophenvl)ethenvll-1.2.3.6-
tetrahyclronyridin-l-vl]methylnyrrolof 2.3-blpyridine
A mixture of 3-dimethylaminomethyl-lH-pyrrolo[2,3-
b]pyridine (1.57g, 9mmol) and (E)-4-[2-(4-fluorophenyl)ethenyl]-1,2,3,6-
tetrahydropyridine (2g, 9mmo1) in toluene (50m1) was refluxed overnight.
The hot solution was filtered, and the product crystallised from toluene to
give (E)-3-[4-[2-(4-fluorophenyl)ethenyl]-1,2,3,6-tetrahydropyridin-i-
yl]methylpyrrolo[2,3-b]pyridine (1.2g, 40%). M.p. 211-213 C
(isopropanol). (Found: C, 75.05; H, 5.99; N,12.27; CziHzoFNa, 0.15H20
requires C, 75.04; H, 6.09; N, 12.50%); Sa (DMSO-dr) 2.29 (2H, br s,
tetrahydropyridinyl CHz), 2.61 (211, t, J 5.GHz tetrahydropryridinyl CH2),
3.06 (2H, br s, tetrahydropyridinyl CH2), 3.73 (2H, s, NCHzAr), 5.87 (1H,
br s, tetrahydropyridinvl5-CH), 6.45 (1H, d, J 16.3Hz, ArCHCHC), 6.83
(1H, d, J 1G.3Hz, ArCHCHC), 7.01-7.04 (1H, m, ArH), 7.14 (2H, t, J 8.9Hz,
ArH), 7.38 (1H, s, ArH), 7.47-7.51 (2H, m, ArH), 8.02 (1H, d, J 7.9Hz,
ArH), 8.18-8.20 (111, m, ArH) and 11.45 (1H, br s, NH); m/z (CI*, NHs),
334 (M+1)'.
2195759 WO 96/05200 PCT/GB95101820
-26-
EXAMPLE 10
(E)-3-f4-(2-(3-Chloronhenyl)ethenyll-12 3 6-tetrahvd.ronvridin-l-
yllmethyln.yrroloL2.3-binyridine
M.P. 208-210 C (isopropanol). (Found: C, 71.86; H, 5.74; N,
11.66; Ca,HsoCINa requires C, 72.09; H, 5.76; N, 12.01%). 511(DMSO-ds)
2.28 (2H, br s, tetrahydropyridinyl CHz), 2.61 (211, t, J 5.5Hz
tetrahydropyridinyl CH2), 3.07 (2H, br s, tetrahydropyridinyl CHa), 3.73
(2H, s, NCHzAr), 5.93 (1H, br s, tetrahydropyridinyl 5-CH), 6.44 (1H, d, J
16.3Hz, ArCHCHC), 6.95-7.05 (2H, m, ArCHCHC, ArH), 7.24 (1H, d, J
8.1Hz, ArH), 7.30-7.43 (3H, m, ArH), 7.54 (1H, s, ArH), 8.02 (1H, d, J
7.8Hz, t1rH), 8.19 (1H, d, J 4.6Hz, ArH) and 11.45 (1H, br s, NH); m/z
(CI,, NH3) 350 (M+1)*.
EXAMPLE 1 i
(E}-3-f4-[2-(4-Cvanophenyl)ethenvll-1.2.3.6-tetrahydropyridin-i-
2o y-ljmethvlp MoloL.3-blpvridine
M.P. 213-215 C (toluene). (Found: C, 76.62;1I, 5.76; N,
16.05; C22H2oN4, 0.25H20 requires C, 76.61; H, 5.99; N, 16.24%). SF3
(DMSO-de) 2.31 (2H., br s, tetrahydropyridinyl C112), 2.62 (211, t, J 5.6Hz,
tetrahydropryridinyl CH2), 3.09 (2H, br s, tetrahydropyridinyl CHz), 3.74
(2H, s, NCHzAr), 6.01(1H, br s, tetrahydropyridinyl5-CH), 6.52 (1H, d, J
16.2Hz, ArCHCHC), 7.01-7.06 (2H, m, ArCHCHC, ArH), 7.38 (1H, d, J
2.3Hz, ArH), 7.64 (2I1, d, J 8.4Hz, ArH), 7.75 (2H, d, J 8.4Hz, ArH), 8.03
(1H, d, J 7.8Hz, ArH), 8.18-8.20 (1H, m, ArH) and 11:46 (1H, br s, NH);
m/z ((;I', NHs) 341 (M+1)*.
2195759
=_ `'
WO 96/05200 PC"T1GB95/01820
-27-
EXEIMPLE 12
(E)-3-14-[2-(4-Methvlnhenvl)ethenvll-1 2 3 6-tetrahvdronyri ~n-1-
r~l methvlnyrrolof2.3-binvridine
M.p. 209-212 C (xylene). (Found: C, 80.18; H, 7.13; N, 12.57;
C23H23Ns requires C, 80.21; H, 7.04; N, 12.76%). Sa (DNISO-ds) 2.27-2.29
(5H, m, tetrahydropyridinyl CH2, ArCHs), 2.61 (2H, t, J 5.7Hz,
tetrahydropyridinyl CH2), 3.06 (2H, br s, tetrahydropyridinyl CH2), 3.73
(2H, s, NCH2Ar), 5.84 (1H, br s, tetrahydropyridinyl5-CH), 6.41 (IH, d, J
16.3Hz, ArCHCHC), 6.82 (1H, d, J 16.2Hz, ArCHCHC), 7.01-7.04 (1H, m,
ArH), 7.12 (2H, d, J 8.0Hz, ArH), 7.32-7.38 (3H, m, ArH), 8.03 (1H, d, J
8.0Hz, ArH), 8.18-8.20 (1H, m, ArH) and 11.46 (1H, br s, NH); mlz (CIt,
NHa) 330 (M+1)*
EXAMPLE 13
(E)-3-f4-f2-(4-Methoxvnhenvl)ethenyll-1 2.3 6-tetrahydropyridin-l-
y-IlmethYlnvrrolof2 3-bjpvridine
M.p. 201-204 C (xylene). (Found: C, 76.20; H, 6.62; N, 12.12;
C22H23NsO requires C, 76.49; 11, 6.71; N, 12.16%). Slx (DMSO-ds) 2.25 (2H,
br s, tetrahydropyridinyl CH2), 2.60 (2H, t, J 5.7Hz, tetrahydropyridinyl
CH2), 3.05 (2H, br s, tetrahydropyridinyl CH2), 3.72 (2H, s, NCH2Ar), 3.74
(3H, s, OCH3), 5.81. (1H, br s, tetrahydropyridinyl 5-CH), 6.40 (1H, d, J
16.3Hz, ArCHCHC), 6.73 (1H, d, J 16.3Hz, ArCHCHC), 6.88 (2H, d, J
8.8Hz, ArH), 7.01-7.05 (1H, m, ArH), 7.37 (1H, s, ArH), 7.38 (2H, d, J
8.8Hz, ArH), 8.02 (1H, dd, J 7.8, 1.4Hz, ArH), 8.19 (1H, dd, J 4.6, 1.5Hz,
ArH) and 11.45 (1H, br s, NH); m/z (ES*) 346 (M+1)'.
3Q
;.; 2195759
WO 96/05200 PCT/GB95/01821)
-28-
E%AMPLE 14
(E)-3-I4-f2-(4-TrifluoromethvlnhenyI)ethenyll-1.2.3.6-tetrahvdropvidin-1-
xl] methylp vrrolof 2.3-bl u vridine
s M.p. 203-205 C (xyiene). (Found: C, 68.70; H, 5.28; N, 10.98;
Cz3H2aFsNs requires C, 68.92; H, 5.25; N, 10.96%). Sa (DMSO-ds) 2.32 (2H,
br s, tetrahydropyridinyl CH2), 2.62 (2H, t, J 7.9Hz, tetrahydropyridinyl
CH2), 3.09 (2H, br s, tetrahydropyridinyl CH2), 3.74 (2H, s, NCHsAr), 5.99
(1H, br s, tetrahydropyridinyl 5-CH), 6.54 (1H, d, J 16.2Hz, ArCHCHC),
7.01-7.09 (2H, in, ArCHCHC, ArH), 7.39 (1H, d, J 3.4Hz, ArH), 7.66 (4H,
s, 2'-H, 3'-H, 5'-H, 6'-H), 8.03 (1H, dd, J 10.4, 2Hz, ArH), 8.19 (1H, dd, J
6.7, 2.3Hz, ArH) and 11.49 (1H, br s, NH); mCz (ES*) 384 (M+1)*.
EXAMPLE 15
M-3-14-f2-(3-FluoronhenvInyl_1-1,2,3,G-tetrLhydrgRyridin-l-
YIlrr1&4h.YlpXTr1f2,3 b1PYridine
Ivl.p. 199-201 C (propan-2-ol); (Found:, C, 75.42; H, 5.95; N,
12.86. C21ffioFN3 requires C, 75.65; H, 6.05; N, 12.60%); 5H '(Db'ISO-de)
2.29 (2H, m, tetrahydropyridinyl CH2), 2.61(2H, t, J 5.5Hz,
tetrahydropyridinyl CH2), 3.07 (2H, br s, tetrahydropyridinyl CHs), 3.72
(2H, s, ArCH2N), 5.93 (1H, br s, tetrahydropyridinyl 5-CH), 6.45 (1H, d, J
16.2Hz, ArCHCHC), 6.93-7.05 (3H, m, ArCHCHC, ArH), 7.26-7.39 (4H, m,
ArH), 8.02 (1H, d, J 7.8Hz, ArH), 8.19 (1H, dd, J 4.6, 1.5Hz, ArH) and
11.48 (1H, br s, NH); mlz (ES+) 334 (M+1)*.
2195759
= -
WO 96105200 PCT/GB95/01820
-29-
EXAMPLE 16
M-3 f4-f2-(3.4-Methylenediox shenvllethen~l 1.2.3.6-tetrahydropyridin-
1-vllmethylnyrrolo f 2.3-bjpyridine
M.p. 206-208 C (propan-2-ol); (Found: C, 72.39; H, 5.80; N,
11.22. C22H2iN302 requires C, 72.61; H, 5.95; N, 11.54%); Sa (DMSO-d6)
2.65 (2H, br s, tetrahydropyridinyl CH2), 2.60 (2H, t, J 5.7Hz,
tetrahydropyridinyl CH2), 3.05 (2H, br s, tetrahydropyridinyl CH2), 3.72
(2H, s, ArCH2N), 5.81 (1H, br s, tetrahydropvridinyl5-CH), 5.99 (2H, s,
OCH2O), 6.37 (1H, d, J 16.2Hz, ArCHCHC), 6.74 (113, d, J 16.2Hz,
ArCHCHC), 6.82-6.89 (2H, m, ArH), 7.01-7.04 (1H, m, ArH), 7.11 (1H, s,
ArH), 7.37 (1H, s, ArH), 8.02 (1H, d, J 8.7Hz, ArH), 8.19 (1H, d, J 4.7Hz,
ArH) and 11.45 (1H, br s, NH); m/z (ES') 360 (M+1)*.
EXAMPLE 17
(E)-3-f4-f2-(3-Methylphenvl)ethenyll-1 2 3 6-tetrahvdrqpvridin-l-
yllmethylnvrrolo f 2,3-1ipvridine
M.p. 210-212 C (propan-2-ol); (Found: C, 80.37; H, 6.89; N,
12.64. C22H23N3 requires C, 80.21; H, 7.04; N, 12.76%); Sa (DMSO-ds) 2.28
(2H, br s, tetrahydropyridinyl CH2), 2.61 (2H, t, J 5.6Hz,
tetrahydropyridinyl CH2), 3.07 (2H, br s, tetrahydropyridinyl CH2), 3.73
(2H, s, ArCH2N), 5.87 (1H, br s, tetrahydropyridinyl 5-CH), 6.41 (1.H, d, J
16.3Hz, ArCHCHC), 6.86 (1H, d, J 16.4Hz, ArCHCHC), 7.01-7.04 (2H, m,
ArH), 7.16-7.27 (3H, m, ArH), 7.38 (1H, s, ArH), 8.03 (1H, d, J 7.7Hz,
ArH), 8.19 (1H, d, J 4.7Hz, ArH) and 11.46 (1H, br s, NH); m/z (ES') 330
(1\'1+1)`=
2195759 WO 96/05200 PCTIGB95/01820
-30-
EX"PLE 18
(F')-3-f4-f2-(3-Cyanonhenvl)ethenyll-12 3 6-tetrahvdropyridin-i-
vllmethylnyrrolo[2.3-binvridine
M.p. 213-215 C (propan-2-ol); (Found: C, 77.39; H, 5.77; N,
16.25. C22H2oNa requires C, 77.62; H, 5.92; N, 16.46%); Sa (DMSO-de) 2.29
(2H, br s, tetrahydropyridinyl CH2), 2.62 (2H, t, J 5.7Hz,
tetrahydropyridinyl CH2), 3.08 (2H, br s, tetrahydropyridinyl CH2), 3.73
(2H, s, ArCH2N), 5.96 (1H, br s, tetrahydropyridinyl 5-CH), 6.49 (1H, d, J
16.3Hz, ArCHCHC), 7.02-7.08 (2H, m, ArCHCHC, ArH), 7.38 (1H, d, J
2.4Hz, ArH), 7.51 (1H, t, J 7.8Hz, ArH), 7.64 (1H, d, J 7_711z, ArH), 7.79
(1H, (1, J 8.OHz, ArH), 7.94 (1H, br s, ArH), 8.03 (1H, d, J 29Hz, ArH),
8.19 (1H, d, J 4.6Hz, ArH) and 11.46 (1H, br s, NH); m/z (HS') 341 (M+1)+.
F.XAMPLE 19
(L+1-3-f 4-f 2 -(3.4-DifTuoronhenvl)ethenyll-1.2.3.6-tetrahvdrop,yridin-l-
vllmethvlnvrroiof 2.3-blrrvridine
M.p. 208-210 C (propan-2-ol); (Found: C, 71.60; H, 5.36; N,
12.17. C21Hi9F2N3 requires C, 71.78; H, 5.45; N, 11.96 .6); Sa (D14i$O-d6)
2.28 (2H, br s, tetrahydropyridinyl CH2), 2.61 (2H, t, J 5.7Hz,
tetrahydropyridinyl. CH2), 3.07 (2H, br s, tetrahydropyridinyl CH2), 3.73
(2H, s, ArCHzM, 5.91 (1H, br s, tetrahydropyridinyl5-CIl), 6.43 (1H, d, J
16.2Hz, ArCHCHC), 6.90 (1H, d, J 16.2Hz, ArCHCHC), 7.01-7.04 (1H, m,
ArH), 7.28-7.39 (3H, m, ArH), 7.54-7.60 (1H, m, ArH), 8.02 (1H, d, J
8.2Hz, ArH), 8.19 (1H, dd, J 4.3, 1.6Hz, ArH) and 11.46 (1H, br s, NH);
m/z (ES') 352 (M+1)+.
2195759
WO 96/05200 PCT/GB95101820
31-
EXAMPLE 20
(E)-3-f4-f2-(3-Fluoro-4-methoxpphenvl)ethenyl1 -1 2 3 6-tetrahydropylidin-
1-vll m ethvhayrrol o f 2. 3-bl a yri di n e
M.p. 199.5-201.5 C (MeOH); (found: C, 73.12; H, 6.03; N,
11.53. C22I-i22FN30 requires C, 72.71; H, 6.10; N, 11.56%); Sx (DMSO-ds)
2.28 (2H, br s, tetrahy(iropyridinyl CH2), 2.61 (2H, t, J 5.7Hz,
tetrahydropyridinyl CH2), 3.07 (2H, br s, tetrahydropyridinyl CHz), 3.73
(2H, s, NCH2Ar), 3.83 (3H, s, ArOCHs), 5.85 (1H, m, tetrahy(iropyridinyl
5-CH), 6.39 (1H, d, J 16.2Hz, CH=CHAr), 6.80 (1H, d, J 16.2Hz,
CH=CHAr), 7.04 (1H, dd, J 7.8, 4.6Hz, 5-H), 7.10 (1H, t, J 8.7Hz, 5'-H),
7.21 (1H, m, 6'-H), 7.35-7.39 (211, m, 2-H, 2'-H), 8.03 (IH, dd, J 7.8, 1.4Hz,
4-H), 8.20 (1H, dd, J 4.6, 1.4Hz, 6-H), and 11.46 (1H, br s, NH); m/z (ES')
364 (M+1)*.