Note: Descriptions are shown in the official language in which they were submitted.
2-~ 96746
ULTRASOUND-ENHANCED DELIVERY OF MATERIALS THROUGH THE SKIN
This invention relates to a method of enhancing the rate of
permeation of materials through a biological membrane, for example the
5 stratum corneum of an individual, using high frequency ultrasound.
This method of enhancing permeability can be used for drug
delivery through a membrane.
Background
The delivery of drugs through the skin ("transdermal drug
10 delivery" or "TDD") provides many advantages; primarily, such a means of
delivery is a comfortable, convenient and non-invasive way of administering
drugs. The variable rates of absorption and metabolism encountered in oral
treatment are avoided, and other inherent inconveniences e.~q. gastrointestinal
irritation and the like, are eliminated as well. Transdermal drug delivery also
15 makes possible a high degree of control over blood concentrations of any
particular drug.
Skin is a structurally complex, relatively impermeable membrane.
Molecules moving from the environment into and through intact skin must
first penetrate the stratum corneum and any material on its surface. They
20 must then penetrate the viable epidermis, the papillary dermis, and the
capillary walls into the blood stream or Iymph channels. To be so absorbed,
molecules must overcome a different resistance to penetration in each type of
tissue. Transport across the skin membrane is thus a complex phenomenon.
However, it is the stratum corneum, a layer approximately 5-15 micrometers
25 thick over most of the body, which presents the primary barrier to absorptionof topical compositions or transdermally administered drugs. It is believed to
be the high degree of keratinization within its cells as well as their dense
packing and cementation by ordered, semicrystalline lipids which create in
many cases a substantially impermeable barrier to drug penetration.
, . ~
2~ 7 4 ~
Applicability of transdermal dru~ delivery is thus presently limited, because
the skin is such an excellent barrier to the in~ress of topically applied
,naterials. For example, many of the new peptides and proteins now produced
as a result of the biotechnolo~y revolution cannot be delivered across the skin
5 in sufficient quantities due to their naturally low rates of skin permeability.
Various methods have been used to increase skin permeability,
and in particular to increase the permeability of the stratum corneum (i.e., so
as to achieve enhanced penetration, through the skin, of the dru~ to be
administered transdermally). The primary focus has been on the use of
10 chemical enhancers, i.e., wherein dru~ is coadministered with a penetration
enhancin~ a~ent (or "pe""ealion enhancer~). While such compounds are
errecli-/e in increasing the rate at which drug is delivered throu~h the skin,
there are drawbacks with many per",ealion enhancers which limit their use.
For example, many permeation enhancers are associated with deleterious
15 e~rects on the skin (e.~., i..ilalion). In addition, control of dru~ delivery with
cl.e...ical enhancement can be quite difficult.
Iontophoresis has also been used to increase the permeability of
skin to dru~s, and involves ~1) the application of an external electric field, and
(2) topical delivery of an ionized form of dru~ (or of a neutral dru~ carried
with the water flux associated with ion transport, i.e., via "electroosmosis").
While permeation enhancement via iontophoresis has, as with chemical
enhancers, been ~rre.;~ e, there are problems with control of dru~ delivery
and the de~ree of irreversible skin dama~e induced by the transmembrane
passa~e of current.
The preser,lly disclosed and claimed method involves the use of
ultrasound to decrease the barrier function of the stratum corneum and thus
ir,crease the rate at which a dru~ may be delivered throu~h the skin.
"Ultrasound" is defined as mechanical pressure waves with frequencies above
3 ~ 7 ~ ~
20,000 Hz (see, e.~., H. Lutz et al., Manual of Ultrasound: 1. Basic Physical
and Technical PrinciDles ~Berlin: Springer-Verla~, 1984)).
As discussed by P. Tyle et al. in Pharmaceutical Research
6(5):355-361 ~1989), dru~ penelralion achieved via "sonophoresis" (the
5 movement of dru~s throu~h skin under the influence of an ultrasonic
perturbation; see D.M. Skauen and G.M. Zentner, Int. J. Pharmaceutics
20:235-245 (1984)), is believed to result from thermal, mechanical and
chemical alleralion of biolo~ical tissues by the applied ultrasonic waves.
Unlike ionotophoresis, the risk of skin dama~e appears to be low.
Applications of ultrasound to dru~ delivery have been discussed
in the literature. See, for example: P. Tyle et al., suDra (which provides an
overview of sonophoresis); S. Miyazaki et al., J. Pharm. Pharmacol. 40:716-
717 (1988) (controlled release of insulin from a polymer implant usin~
ultrasound); J. Kost et al., Proceed. Intern. Svm~. Control. Rel. Bioact. Mater. 16(141):294295 (1989) (overview of the effect of ultrasound on the
permeability of human skin and synthetic membranes); H. Benson et al.,
Phvsical TheraDv 69(2):113-118 (1989) (effect of ultrasound on the
percutaneous absorption of benzydamine); E. Novak, Arch. Phys. Medicine &
Rehab. 45:231-232 (1964) (enhanced penel,alion of lidocaine throu~h intact
20 skin usin~ ultrasound); J.E. Griffin et al., Amer. J. Phvs. Medicine 44(1):20-
25 (1965) (ultrasonic penel(alion of cortisol into pi~ tissue); J.E. Griffith etal., J. Amer. Phys. TheraDy Assoc. 46:18-26 (1966) ( overview of the use of
ultrasonic ener~y in dru~ therapy); J.E. Griffin et al., Phys. TheraDv
47(7):594-601 (1967) (ultrasonic penelralion of hydrocortisone); J.E. Griffin
25 et al., Phys. Therapy 48(12):1336-1344 (1968) (ultrasonic penetration of
cG.lisol into pi~ tissue); J.E. Griffin et al., Amer. J. Phys. Medicine 51(2):62-
72 (1972) (same); J.C. McElnay, Int. J. Pharmaceutics 40:105-110 (1987)
(the effect of ultrasound on the percutaneous absorption of fluocinolone
-4 ~ 96~ 6
acetonide); and C. Cscofrier et al., Bioen~. Skin 2:87-94 (1986) (Tn vitro
study of the velocity of ultrasound in skin).
In addition to the aforemenlioned art, U.S. Patents Nos.
4,767,402 and 4,780,212 to Kost et al. relate specifically to the use of
5 speciric frequencies of ultrasound to enhance the rate of permeation of a dru~ throu~h human skin or throu~h a synthetic membrane.
While the application of ultrasound in conjunction with dru~
delivery is thus known, results have for the most part been disappointin~,
i.e., enhance."enl of skin permeability has been relatively low.
, . ~
5 ~ ~67~
Summary of the In~ ion
It Is accordin~ly a pri~,.a.y object of the invcnlion to address the
aforementioned deficiencies of the prior art by providin~ a method of
enhancin~ the permeability of biolo~ical membranes and thus allowin~ for an
5 increased rate of delivery of materials therell,rou~h.
Accordin~ to a first aspect of the present invention there is
provided a "-ell-od for enhancin~ the rate of permeation of a dru~ medium
throu~h a biolo~ical membrane comprisin~:
contactin~ the membrane with the dru~ medium and
10 increasin~ the permeability by applyin~ ultrasound havin~ a frequency above
lOMHz to a selecled area of the biolo~ical membrane, at an intensity and for
a period of time errecli~e to ircrease the per,.,eability of the biolo~ical
membrane.
Brief Descri~lion of the Drawin~s
In the accompanyin~ drawin~s:
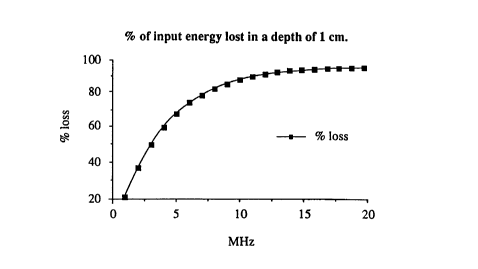
Fi~ures lA, 1B and lC are theoretical pIots of ener~y dissipation
within the skin barrier versus frequency of applied ultrasound.
Fi~ures 2, 3 and 4 are ~raphic represenlalions of the amount of
salicylic acid recovered from the stratum corneum after ultrasound lreal",ent
at different frequencies.
7 ~ ~
,
Figures 5 and 6 represent the results of
experiments similar to those summarized in Figures 2, 3
and ~, but with a shorter treatment time.
Figures 7, 8, 9 and 10 are plots of
enhancement versus ~tape-strip number,~ as described in
the Example.
Figure 11 illustrates the effect of ultrasound
on the systemic availability of salicylic acid following
topical application.
Detailed Descri~tion of Preferred Embodiments
Before the present method of enhancing the
rate of permeation of a material through a biological
membrane and enhancing the permeability of membranes
using ultrasound are disclosed and described, it is to
be understood that this invention is not limited to the
particular process steps and materials disclosed herein
as such process steps and materials may, of course,
vary. It is also to be understood that the terminology
used herein is used for purpose of describing particular
embodiments only and is not intended to be limiting
since the scope of the present invention will be limited
only by the appended claims.
It must be noted that as used in this
specification and the appended claims, the singular
forms ~a~, ~an~ and ~the~ include plural reference
unless the context clearly dictates otherwise. Thus,
for example, reference to ~a drug~ includes mixtures of
drugs and their pharmaceutically acceptable salts,
reference to ~an ultrasound device~ includes one or more
ultrasound devices of the type necessary for carrying
out the present invention, and reference to ~the method
of administration~ includes one or more different
methods of administration known to those skilled in the
~ ~ ~ fi ~ 4 6
art or which will become known to those skilled in the
art upon reading this disclosure.
In one aspect of the invention, a method is
provided for enhancing the permeation of a given
material such as a drug, pharmacologically active agent,
or diagnostic agent into and/or through a biological
membrane on an individual's body surface, which method
comprises: (a) contacting the membrane with the chosen
material in a pharmacologically acceptable carrier
medium; and (b) applying ultrasound of an intensity and
for a treatment time effective to produce delivery of
the material through the membrane. The material is
preferably a drug and it is preferable to obtain a
desired blood level of the drug in the individual. The
ultrasound is of a frequency and intensity effective to
increase the permeability of the selected area to the
applied drug over that which would be obtained without
ultrasound. The ultrasound preferably has a frequency
of more than 10 MHz, and may be applied either
continuously or pulsed, preferably continuously. The
ultrasound may be applied to the skin either before or
after application of the drug medium so long as
administration of the ultrasound and the drug medium is
relatively simultaneous, i.e., the ultrasound is applied
within about 6, more preferably within about 4, most
preferably within about 2 minutes of drug application.
The invention is useful for achie~ing
transdermal permeation of pharmacologically active
agents which otherwise would be quite difficult to
deliver through the skin or other body surface. For
example, proteinaceous drugs and other high molecular
weight pharmacologically active agents are ideal
candid~tes for transdermal, transmucosal or topical
delivery using the presently disclosed method. In an
alternative embodiment, agents useful for diagnostic
purposes may also be delivered into and/or through the
body surface using the present method.
The invention is also useful as a non-invasive
diagnostic technique, i.e., in enabling the sampling of
physiologic material from beneath the skin or other body
surface and into a collection (and/or evaluation)
chamber.
The present invention will employ, unless
otherwise indicated, conventional pharmaceutical
methodology and more specifically conventional
methodology used in connection with transdermal delivery
of pharmaceutically active compounds and enhancers.
In describing the present invention, the
following terminology will be used in accordance with
the definitions set out below.
A ~biological membrane~ is intended to mean a
membrane material present within a living organism which
separates one area of the organism from another and,
more specifically, which separates the organism from its
outer environment. Skin and mucous membranes are thus
included.
~ Penetration enhancement~ or ~permeation
e~h~ncement~ as used herein relates to an increase in
the permeability of skin to a material such as a
pharmacologically active agent, i.e., so as to increase
the rate at which the material permeates into and
through the skin. The present invention involves
enhancement of permeation through the use of ultrasound,
and, in particular, through the use of ultrasound having
a frequency of greater than 10 MHz.
~ Transdermal~ (or ~percutaneous~) shall mean
pa~s~se of a material into and through the skin to
ach~eve effective therapeutic blood levels or deep
tissuc therapeutic levels. While the invention is
--10--
described herein primarily in terms of ~transdermal~
administration, it will be appreciated by those skilled
in the art that the presently disclosed and claimed
method also encompasses the ~transmucosal~ and ~topical~
administration of drugs using ultrasound.
~Transmucosal~ is intended to mean passage of any given
material through a mucosal membrane of a living organism
and more specifically shall refer to the passage of a
material from the outside environment of the organism,
through a mucous membrane and into the organism.
~Transmucosal~ administration thus includes delivery of
drugs through either nasal or buccal tissue. 8y
~topical~ administration is meant local administration
of a topical pharmacologically active aqent to the skin
as in, for example, the treatment of various skin
disorders or the administration of a local anaesthetic.
~Topical~ delivery can involve penetration of a druq
into the skin but not through it, i.e., topical
administration does not involve actual passage of a drug
into the bloodstream.
~ Carriers~ or ~vehicles~ as used herein refer
to carrier materials without pharmacological activity
which are suitable for administration with other
pharmaceutically active materials, and include any such
materials known in the art, e.g., any liquid, gel,
solvent, liquid diluent, solubilizer, or the like, which
is nontoxic and which does not interact with the drug to
be administered in a deleterious manner. Examples of
suitable carriers for use herein include water, mineral
oil, silicone, inorganic gels, aqueous emulsions, liquid
sugars, waxes, petroleum jelly, and a variety of other
oils and polymeric materials.
By the term ~pharmacologically active agent~
or ~drug~ as used herein is meant any chemical material
.. . .. ..
4 6
or compound suitable for transdermal or transmucosal
administration which can either (1) have a prophylactic
effect on the organism and prevent an undesired
biological effect such as preventing an infection,
(2) alleviates a condition caused by a disease such as
alleviating pain caused as a result of a disease, or
(3) either alleviates or completely eliminates the
disease from the organism. The effect of the agent may
be local, such as providing for a local anaesthetic
effect or it may be systemic. Such substances include
the broad classes of compounds normally delivered
through body surfaces and membranes, including skin. In
general, this includes: anti-infectives such as
antibiotics and antiviral agents; analgesics and
analgesic combinations; anorexics: antihelminthics;
antiarthritics; antiasthmatic agents; anticonvulsants;
antidepressants; antidiabetic agents; antidiarrheals;
antihistamines; antiinflammatory agents; antimigraine
preparations antinauseants; antineoplastics;
antiparkinsonism drugs; antipruritics; antipsychotics;
antipyretics; antispasmodics; anticholinergics;
sympathomimetics; xanthine derivatives cardiovascular
preparations including potassium and calcium channel
blockers, beta-blockers, and antiarrhythmics:
antihypertensives: diuretics vasodilators including
general coronary, peripheral and cerebral; central
nervous system stimulants: cough and cold preparations,
including decongestants; hormones such as estradiol and
other steroids, including corticosteroids; hypnotics;
immunosuppressives; muscle relaxants; parasympatho-
lytics; psychostimulants; sedatives; and tranquilizers.
By the method of the present invention, both ionized and
nonionzed drugs may be delivered, as can drugs of either
high orl ow molecular weight.
7 4 b~
Proteinaceous and polypeptide drugs represent
a preferred class of drugs for use in conjunction with
the presently disclosed and claimed invention. Such
drugs cannot generally be administered orally in that
they are often destroyed in the G.I. tract or
metabolized in the liver. ~urther, due to the high
molecular weight of most polypeptide drugs, conventional
transdermal delivery systems are not generally
effective. It is also desirable to use the method of
the invention in conjunction with drugs to which the
permeability of the skin is relatively low, or which
give rise to a long lag-time (application of ultrasound
as described herein has been found to significantly
reduce the lag-time involved with the transdermal
administration of most drugs).
By a "therapeutically effective~ amount of a
pharmacologically active agent is meant a nontoxic but
sufficient amount of a compound to provide the desired
therapeutic effect. The desired therapeutic effect may
be a prophylactic effect, in preventing a disease, an
effect which allçviates a system of the disease, or a
curative effect which either eliminates or aids in the
elimination of the disease.
AS noted above, the present invention is a
method for enhancing the rate of permeation of a drug
through an intact area of an individual's body surface,
preferably the human skin. The method involves
transdermal administration of a selected drug in
conjunction with ultrasound. Ultrasound causes thermal,
mechanical and chemical alterations of biological
tissue, thereby enhancing the rate of permeation of a
given material therethrough.
While not wishing to be bound by theory,
applicants propose that the use of higher frequency
.
2~ ~67~
ultrasound as disclosed herein specifically enhances the
permeation of the drug through the outer layer of skin,
i.e., the stratum corneum, by causing momentary and
reversible perturbations within (and thus short-term,
reversible reduction in the barrier function of) the
layer of the stratum corneum. It will be appreciated by
those skilled in the art of transdermal drug delivery
that a number of factors related to the present method
will vary with the drug to be administered, the disease
or injury to be treated, the age of the selected
individual, the location of the skin to which the drug
is applied, and the like.
As noted above, ~ultrasound~ is ultrasonic
radiation of a frequency above 20,000 Hz. As may be
deduced from the literature cited above, ultrasound used
for most medical purposes typically employs frequencies
ranging from 1.6 to about 10 MHz. The present
invention, by contrast, employs ultrasound frequencies
of greater than about 10 MHz, preferably in the range of
about 15 to 50 MHz, most preferably in the range of
about 15 to 25 MHz. It should be emphasized that these
ranges are intended to be merely illustrative of the
preferred embodiment; in some cases higher or lower
frequencies may be used.
The ultrasound may be pulsed or continuous,
but is preferably continuous when lower frequencies are
used. At very high frequencies, pulsed application will
generally be preferred so as to enable dissipation of
generated heat.
The preferred intensity of the applied
ultrasound is less than about 5.0 W/cm2, more preferably
is in the range of about 0.01 to 5.0 W/cm2, and most
preferably is in the range of 0.05 to 3.0 W/cm2. The
total treatment time, i.e., the period over which drug
-
7 4 6
and ultrasound are administered, will vary depending on
the drug administered, the disease or injury treated,
etc., but will generally be on the order of about 30
seconds to 60 minutes, preferably S to 45 minutes, more
preferably S to 30 minutes, and most preferably S to 10
minutes. It should be noted that the aforementioned
ranges represent suggested, or preferred, treatment
times, but are not in any way intended to be limiting.
Longer or shorter times may be possible and in some
cases desirable. Virtually any type of device may be
used to administer the ultrasound, providing that the
device is capable of producing the higher frequency
ultrasonic waves required by the present method. A
device will typically have a power source such as a
small battery, a transducer, a reservoir in which the
drug medium is housed (and which may or may not be
refillable), and a means to attach the system to the
desired skin site.
As ultrasound does not transmit well in air, a
liquid medium is generally needed to efficiently and
rapidly transmit ultrasound between the ultrasound
applicator and the skin. As explained by P. Tyle et
al., cited above, the selected drug medium should
contain a ~coupling~ or ~contacting~ agent typically
used in conjunction with ultrasound. The coupling agent
should have an absorption coefficient similar to that of
water, and furthermore be nonstaining, nonirritating to
the skin, and slow drying. It is clearly preferred that
the coupling agent retain a paste or gel consistency
during the time period of ultrasound administration so
that contact is maintained between the ultrasound source
and the skin. Examples of preferred coupling agents are
mixtures of mineral oil and glycerine and propylene
glycol, oil/water emulsion~, and a water-based gel. A
7 ~ ~
--15--
solid-state, non-crystalline polymeric film having the
above-mentioned characteristics may also be used. The
drug medium may also contain a carrier or vehicle, as
defined alone.
A transdermal patch as well known in the art
may be used in conjunction with the present invention,
i.e., to deliver the drug medium to the skin. The
~patch~, however, must have the properties of the
coupling agent as described in the preceding paragraph
so as to enable transmission of the ultrasound from the
applicator, through the patch, to the skin.
As noted earlier in this section, virtually
any chemical material or compound suitable for
transdermal, transmucosal or topical administration may
be administered using the present method. Again, the
present invention is particularly useful to enhance
delivery of proteinaceous and other high molecular
weight drugs.
The method of the invention is preferably
carried out as follows. The drug medium, i.e.,
containing the selected drug or drugs in conjunction
with the coupling agent and optionally a carrier or
vehicle material, is applied to an area of intact body
surface. Ultrasound preferably having a frequency
greater than about 10 MHz may be applied before or after
application of the drug medium, but is preferably
applied immediately before application of the drug so as
to ~pretreat~ the skin prior to drug administration.
It should also be pointed out that the present
method may be used in conjunction with a chemical
permeation enhancer as known in the art, wherein the
ultrasound enables the use of much lower concentrations
of permeation enhancer -- thus minimizing skin
irritation and other problems frequently associated with
~ ~ ~ 6 :7 4 ~
-16-
such compounds -- than would be possible in the absence
of ultrasound. The permeation enhancer may be
incorporated into the drug medium or it may be applied
in a conventional transdermal patch after pretreatment
of the body surface with ultrasound.
The present invention may also be used in
conjunction with iontophoresis for drugs which are
particularly difficult to administer through the skin,
i.e., because of the low permeability of the stratum
corneum to such drugs. The selected area of the body
surface is pretreated with ultrasound and the drug is
then administered using conventional iontophoresis
techniques.
lS With respect to skin location, virtually any
area of the body surface may be selected so long as it
is intact, however, the thickness and permeability of
skin at the site of exposure will affect the treatment
conditions, i.e., intensity, frequency, contact time,
exposure time, and the like. The area of skin through
which the drug medium and the ultrasound will be
administered can again vary greatly, but will typically
be on the order of 1 to 100 cm2, more typically on the
order of S0 to 100 cm2, most typically on the order of
10 to S0 cm2.
Exam~le
Based on the present inventors' theoretical
analysis of the propagation of ultrasound energy in
tissue and the barrier properties of skin, it was
concluded that higher ultrasound fre~uencies might be
more effective in enhancing the flux of drug molecules
across the skin. This conclusion, based on the
assumption that enhancement is proportional to the
amount of energy dissipation within the barrier, is
supported by Figure 1, which suggests that, within the
~ ~b~74 ~
-17-
first millimeter of skin, ultrasound energy dissipation
increases exponentially with frequency.
To test the hypothesis that higher frequencies
(>10 MHz) would yield greater enhancement, in vivo
experiments were performed on hairless guinea pigs. The
setup for carrying out the experiment consisted of a
function generator, transducers tuned at different
frequeneies and a power-meter. 14C-labeled salicylic
acid was used as the ~model~ marker drug molecule. A
saturated solution of unlabeled salicylic acid in water
was prepared. Carbopol~ (~.F. Goodrich), a polymer, was
added to this solution to make a gel containing
salicylic acid at a concentration of 0.57% w/w. This
gel was then spiked with a known amount of radiolabeled
salicylic acid (approx. 2.27 ~Ci/mg gel). Approximately
30 mg gel per square centimeter of transducer
cross-sectional area was then applied to the skin
surface of the guinea pigs' flanks. This gel served as
both the drug reservoir and the coupling medium between
the transducer and skin surface. Frequencies of 1, 7.5
and 16 MHz were tested using an intensity of 0.25 W/cm2
and treatment periods (time of exposure of the skin to
both drug and ultrasound) of 10 and 20 minutes. At the
treatment site, the transducer delivered ultrasound at
the appropriate frequency for the designated period. At
the control site, in the contralateral flank, the
transducer was positioned on the skin, but not
activated. Thus, each animal served as its own control.
Enhancement was quantified in two ways: (1) by
tape-stripping the outer skin layer at the treatment and
control sites immediately after the experimental period
(radioactivity in the tape strips was then determined by
liquid scintillation counting) and (2) by measuring the
~ 7 4
-18-
cumulative amount of 14C excreted in the animals' urine
up to 14 hours after initiation of the experiment.
(1) Tape-strip procedure: It has been
established that the uppermost layer of skin, the
stratum corneum (SC), offers the most resistance to drug
penetration. Hence, it was decided to compare the
amount of radioactivity present in the SC after
ultrasound treatment with that after the control
experiment (passive diffusion, no ultrasound). Figures
2, 3 and 4 present a comparison of the total amount of
salicylic acid that had penetrated into the SC with 1,
7.S and 16 MHz exposure for 20 minutes and without
exposure to ultrasound. As can be seen, use of 16 MHz
resulted in a significantly elevated drug level in the
SC as compared to the control. Figures 5 and 6 are
comparable to Figures 3 and 4, but here the treatment
time was 10 minutes, rather than 20 minutes. Each tape
strip removed a certain amount of the SC. Hence, with
increasing tape-strip number, tissue further away from
the surface was examined. Therefore, a plot of the
amount of drug in each strip against strip number
reflects the concentration gradient of drug in the SC.
Such plots are shown in Figures 7, 8, 9 and 10. The
ordinate is the ratio of the amount of radioactivity in
the tape-strip after treatment to the amount in the
strip after control.
(2) Urinary excretion: To confirm that the
amount of drug recovered from the SC reflected the
amount of drug penetrated, radioactivity excreted in the
urine was monitored. Figure 11 graphically illustrates
a comparison of the total amount of radioactivity
excreted in urine 14 hours after a 20 minute treatment
using 16 MHz and the corresponding control. At least
4 ~
-19--
five times more drug entered the systemic circulation
with ultrasound than without.
While the present invention has been described
with reference to specific embodiments thereof, it
should be understood by those skilled in the art that
various changes may be made and equivalents may be
substituted without departing from the true spirit of
the scope of the invention. In addition, many
modifications may be made to adapt a particular
ultrasound device, drug, excipient material, process,
process step or steps, to the objective, spirit and
scope of the present invention. All such modifications
are intended to be within the scope of the claims
lS appended hereto.
.