Note: Descriptions are shown in the official language in which they were submitted.
CA 02197139 2002-02-20
A DRUG FOR THE TREATMENT' OF CANCER
FIELD OF THE INVENTION
The present invention relates to a combination of peptide analogs. The
combination may be used to block the uncontrolled multiplication of cancer
cells of the
colon, rectum, lung, breast, and kidney. The combination may be used to treat
cancers of
the colon, rectum, lung, breast, and kidney and may be used to treat leukemia
and
lymphoma. The invention also relates to a pharmaceutical composition
containing a
combination of such analogs.
BACKGROUND
Reports in the scientific literature disclose that receptors for peptides such
as VIP (vasoactive intestinal peptide j, somatostatin, and bombesin are found
on certain
tumor cells. The following Table 1 gives a list of tumor cells that secrete
and have
receptors for V1P, somatostatin, bombesin, and Substance P.
CA 02197139 2000-03-30
-2-
TABLE 1:
Peptide Secretion
And
Peptide Receptor Positivity Reference
VIP Neuroblastoma J. Molecular
Neurosciences
S (4) :231, 1994
Colorectal and N. Eng. J. Med 331
(17)
pancreatic 1116, 1994
adenocarcinoma
Somatostatin Small Cell Lung CancerEur. J. Cancer 31
A (2)
184, 1995
Thyroid carcinoma Clin. Endocrinol.
42 (1) : 31, 1995
Neuroblastoma Seminars in Oncol.
21:
38, 1994
Bombesin Small Cell Lung CancerInt. J. Cancer 60
: 82,
1995
Glioblastoma Cancer Res., 54 :
5895,
1994
Pancreatic cancer Int. J. Pancreatology,
16 : 141, 1994
Gastric cancer Cancer letters, 85
111, 1994
Prostate cancer Prostate 25 ( 1 )
: 29,
1994
Substance P Small cell lung cancerCancer Research
54 (13) : 3602-3610
(July 1, 1994)
CA 02197139 2000-03-30
-3-
SUMMARY OF THE INVENTION
The present invention provides a pharmaceutical composition useful for
killing or inhibiting multiplication of tumor cells as well as cancer cells.
The
pharmaceutical composition may also be useful in preventing, inhibiting, or
modulating
the hypersecretion of VIP, somatostatin, bombesin, Substance P, or a
combination of VIP,
somatostatin, bombesin, or Substance P. The composition may suitably comprise,
consist
of, or consist essentially of a therapeutically effective combination of
peptide analogs of
somatostatin, VIP, bombesin, and Substance P. The peptide analogs are
described in more
detail below, but constituents functionally interchangeable with those
specifically
described rnay also be employed in the claimed pharmaceutical composition.
More
particularly, the pharmaceutical composition may suitably comprise, consist
of, or consist
essentially of an analog of somatostatin and at least four peptides selected
from the group
consisting of a first analog of VIP, a second analog of VIP, a third analog of
VIP, another
analog of somatostatin, an analog of bombesin, and an analog of Substance P.
More
particularly, the composition may suitably comprise, consist of, or consist
essentially of a
therapeutically effective combination of peptide SOMz (an analog of
somatostatin) and at
least four of the following peptides: VIP, (a VIP antagonist), VIPZ (a VIP
receptor binding
inhibitor), VIP3 (a VIP receptor antagonist), SOM, (a somatostatin analog
(also
abbreviated "CTOP," which is derived from the first letters of the following
four amino
acids: Cyst, Tyr3, Orns, and PenS)), BOM, (a bombesin antagonist), and SP, (a
Substance
P antagonist). In a preferred embodiment, a pharmaceutically acceptable
carrier, diluent,
or solvent is used. The invention provides a method of treatment for humans,
mammals,
or other animals suffering from cancer or other tumors. The method may
suitably
comprise, consist of, or consist essentially of administering a
therapeutically effective
dose of the pharmaceutical composition so as to kill or inhibit the
multiplication of cancer
or tumor cells. The method of treatment of the present invention may be
particularly
useful in the treatment of cancers or tumors of the colon and rectum. The
invention also
provides a method of treatment for humans, mammals, or other animals suffering
from
hypersecretion of VIP, somatostatin, bombesin, Substance P, or a combination
of VIP,
somatostatin, bombesin, or Substance P. The method may suitably comprise,
consist of,
or consist essentially of administering a therapeutically effective dose of
the
CA 02197139 2000-03-30
-4-
pharmaceutical composition so as to prevent, inhibit, or modulate the
hypersecretion of
VIP, somatostatin, bombesin, Substance P, or a combination of VIP,
somatostatin,
bombesin, or Substance P.
DESCRIPTION OF THE DRAWINGS
Figure l, which shows the effect on tumor regression of treatment onset
time with MuJ-7, summarizes the mean tumor volume (in mm3) for all of the mice
in the
in vivo protocols described in Examples 6-13 versus the day numbers.
Figure 2 is a graph of the mean tumor volume (in mm') of the treated mice
("o") and the untreated control mice ("+") versus the day numbers for the in
vivo protocol
described in Example 6.
?0 Figure 3 is a graph of the mean tumor volume (in mm3) of the group 1
treated mice ("o"), the group 2 treated mice ("o"), and the untreated control
mice ("+")
versus the day numbers for the in vivo protocol described in Example 7.
Figure 4 is a graph of the tumor volume (in mm3) of the treated mouse
("o") and the untreated control mouse ("+") versus the day numbers for the in
vivo
protocol with SW 620 cells described in Example 8.
Figure 5 is a graph of the tumor volume (in mm3) of the treated mouse
("o") and the untreated control mouse ("+") versus the day numbers for the in
vivo
protocol with HT 29 cells described in Example 8.
Figure 6 is a graph of the tumor volume (in mm3) of the treated mouse
30 ("o") and the untreated control mouse ("+") versus the day numbers for the
in vivo
protocol with CoLo 205 cells described in Example 8.
Figure 7 is a graph of the tumor volume (in mm3) of the treated mouse
("o") and the untreated control mouse ("+") versus the day numbers for the in
vivo
protocol with L 132 cells described in Example 8.
Figure 8 is a graph of the mean tumor volume (in mm') of the treated mice
("o") and the untreated control mice ("+") versus the day numbers for the in
vivo protocol
described in Example 13.
Figure 9 is a graph of the percentage of surviving treated mice (dotted line)
and the percentage of untreated control mice (solid line) versus the day
numbers for the in
40 vivo protocol described in Example 13.
.,
a
CA 02197139 2000-03-30
-S-
DETAILED DESCRIPTION
We have observed that VIP (vasoactive intestinal peptide), somatostatin,
Substance P, and bombesin are secreted by at least some human tumor and cancer
cells
and that there are binding sites for these peptides on these cells.
Specifically, out of a
number of peptide growth regulators studied by indirect immunofluorescence,
the four
peptides (i.e., vasoactive intestinal peptide (VIP), somatostatin, Substance
P, and
bombesin) were shown to bind to tumor cells. (Herein, the terms "peptide
hormones,"
"growth factors," "peptide growth regulators," and "peptides" each refer to
VIP,
somatostatin, Substance P, and bombesin). It may be that there is an autocrine
mechanism
for cell proliferation where the peptides are secreted by tumor cells and
transduce a signal
through specific receptors on the same cell type leading to cell
proliferation.
As will be described in more detail below, the effects of the analogs of
somatostatin, VIP, bombesin, and Substance P on the tumor cell growth and
survival were
studied using different assay systems. The amino-acid sequences of the seven
analogs
(VIP,, VIP2, VIP3, SOM,, SOMZ, BOM,, and SP,) are given in Table 2. As will be
explained in more detail below, the combination of these seven analogs is
known as
MuJ-7. In the accompanying Sequence Listing section, the amino-acid sequence
for VIP,
(a VIP antagonist) is SEQ ID NO:1; and the amino-acid sequence for VIPZ (a VIP
receptor
binding inhibitor) is SEQ ID N0:2. The analogs were synthesized manually and
using a
conventional peptide synthesizer. The .purity of the peptides was established
by
performing high performance liquid chromatography and amino acid analysis,
while the
analysis was reconfirmed on a sequence analyzer.
CA 02197139 2000-03-30
- 6 -
N
p p p
z z z
0 0
a a a
x ~ ~'
z .~ '=
Q a '
''
~?
.~ a x
~ o
~ z
~
a
N z ~ N
V
O
v~ ~, ~, a
~a
H
~
a . V ~"'
N
G7
r a.
~ o
'~ ~, ~ .~
T ~
H ~ z ~, ~ x
~ ~
;, a -; z
o Qa ~ ~ '
N ~Q E
Nn. ~ ~ ~ E.~, a
~, ~
a a .~ a. o ~ o
.~ ,~ '
4,..~a a. ..
. . 'o x 7
H .a D N a C
> F; "
t~, >, ~, U E ~ C7
=~ N
Ha H ~ p
~
~ ~ is ~' o > A
v'
H a ~ o
Q w
s ~ >1 O, H N H
N ~ v
" ,-
, a E..~ H ~ 1 1
~ as '
,, U ~
~'
a U >, C7 0.
~ '~ ~ V ~
a~n
a, >, e, N . ~ . a
~ c a. ;u a,
o ~ x ca a 0 0
c~ .~
b
. .. o
.
U
o o o ~
~
,o
o
'v, o c ~a ~o 0 n
:a Q
.C c ~ C,
o ~ w
on ~. a . :
c~, ~ G a~
~
H ~ N O O .
O C y
N .-~ cd
Ar ~ ~r E E ~ .D
~
z > >.__ > v? v~ m v
N _
0, G _
,
~
U > > >
0 0
"' N
CA 02197139 2000-03-30
7-
The growth factors synthesized and secreted by tumor cells were identified
by different assay systems. For example, the peptide hormones involved in
uncontrolled
proliferation of cancer cells were identified by performing experiments on
established cell
lines. The results obtained were complemented with data obtained from
experiments
conducted on primary tumor cells of human colon adenocarcinoma as a model
tissue, for
which we have developed a novel method of establishing cell lines. The
following
article describes the novel method of establishing cell lines: Jaggi, M.,
Mukherjee, R.,
"Establishment of Tumorigenic Cell Lines from Biopsies of Human Colon
Adenocarcinomas," Journal of Basic & Applied Biomedicine, 3 (4) : 27-35
(1995).
. A sandwich ELISA for tape peptides was developed and used by the
inventors. The following article, describes the sandwich ELISA:
Jaggi, M., Mukherjee R., "New, Sensitive and Specific ELISA for
the Detection of Neuropeptides in Culture Supernatants," Journal of
Immunoassay,
15 (2) : 129-46 ( 1994). The identity of the peptides was established by
reverse phase high
performance liquid chromatography and sequence analysis. The binding sites for
VIP,
somatostatin, Substance P, and bombesin on primary human adenocarcinoma tumor
cells
of the colon were demonstrated by performing receptor-ligand assays. Two
classes of
binding sites (high affinity and moderately high affinity) were demonstrated
for VIP and
somatostatin; a single class of binding site (high affinity) was demonstrated
for bombesin;
and a single class of binding site (moderately high affinity) was demonstrated
for
Substance P.
Tables 3, 4, 5, and 6 present data on the receptor affinities for VIP,
somatostatin, bombesin, and Substance P on eight different primary tumor
cultures of
human colon adenocarcinoma. These data were obtained by performing receptor-
ligand
assays using 'z5I-VIP, 'z5I-somatostatin, 'ZSI-bombesin, and '25I-Substance P.
See the
section below entitled "Description of Protocols" for a detailed description
of the receptor-
ligand assay. In Tables 3, 4, 5, and 6, Kp(M) represents the dissociation
constant, the unit
of which is moles (M); and R (M/L.) stands for the receptor number (i.e., the
number of
receptors per tumor cell), the unit of which is moles per liter (M/L). As
described in the
"Description of Protocols" section below, Kp (M) and R (M/L) were computed
using
r,
CA 02197139 2000-03-30
-$-
LIGAND software, which did Scatchart Analysis using the raw data from the
receptor-
ligand assays.
A Kp(M) value in the range of about 10-9 to about 10''° M indicates
a
high-affinity receptor, while a Kp (M) value in the range of about 10 -6 to
about 10 '8 M
indicates a receptor with a moderately high affinity. Table 3 show two Kp (M)
values and
two R (M/L) values for each primary tumor culture because the tumor cells have
a high-
affinity receptor for VIP as well as a receptor with a moderately high
affinity for VIP.
Table 4 shows two Kp (M) values and two R (M/L) values for each primary tumor
culture
because the tumor cells have a high-affinity receptor for somatostatin as well
as a receptor
with a moderately high affinity for somatostatin. Table 5 shows only one Kp
(M) value
and one R (M/L) value for each primary tumor culture because the tumor cells
appear to
have only a high-affinity receptor for bombesin. Table 6 shows only Kp (M)
value and
one R (M!L) value for each primary tumor culture because the tumor cells
appear to have
a receptor with a moderately high affinity for Substance P.
CA 02197139 2000-03-30
-9-
Ta 1e 3 : Dissociation constant (KD (M)) in moles and receptor number
(R (M/L)) in moles per liter for VIP on eight primary tumor cultures of human
colon
adenocarcinoma.
Sample No. Kp (M) R (M/L)
PTC-1 1.04 x 10 ' 9 4.83 x 10 '"
6.33 x 10-' 1.78 x 10'8
PTC-2 1.45 x 10 -9 6.23 x 10 ' "
4.23 x 10 -' 1.03 x 10 ' 8
PTC-3 6.35 x 10 -9 2.45 x 10 -'
1.51x10 '~ 2.93x10'8
PTC-4 1.10 x 10 -8 2.75 x 10 -'
9.45x10 -' 5.03x10-8
PTC-S 1.95 x 10 '8 5.29 x 10 -'
3.51x10 -~ 8.72x10'8
PTC-6 4.41 x 10 - 9 1.05 x 10 -'
1.88x10-6 3.21x10-8
PTC-7 1.49 x 10 - 9 6.12 x 10 '"
5.55x10'' 9.37x10-9
PTC-8 1.78 x 10 ' 9 1.50 x 10 -'
8.42 x 10'6 8.49 x 10-9
CA 02197139 2000-03-30
- 10-
Table 4: Dissociation constant (Kp (M)) in moles and receptor number (R (M/L))
in
moles per liter for somatostatin on eight primary tumor cultures of human
colon
adenocarcinoma.
Sample No. Kp (M) R (~,)
PTC-1 3.23 x 10'' . 6.01 x 10 -"
9.37x10-8 4.41x10-9
PTC-2 1.70 x 10'' 8.99 x 10-"
6.35 x 10-8 2.24 x 10-9
PTC-3 1.15 x 10 -9 1.06 x 10 ''
1.34x10-' 5.09x10'9
PTC-4 8.65 x 10 ' " 4.66 x 10 -"
5.64x10-8 2.75x10-9
PTC-5 3.78 x 10 '' 5.82 x 10 '"
1.54x10-8 1.24x10'9
PTC-6 5.45 x 10 -' 6.85 x 10 ' "
4.30x 10'8 1.16x 10'9
PTC-7 1.11 x 10 ' 9 9.81 x 10 -"
1.28x 10-' 3.89x 10'9
PTC-8 9.64 x 10 -' 1.74 x 10 -'
9.92x 10-8 6.59x 10-9
CA 02197139 2000-03-30
Ta 1e Dissociation constant (KD (M)) in moles and receptor number
(R (M/L)) in moles per liter for bombesin on eight primary tumor cultures of
human colon
adenocarcinoma.
Sample No. Kp (M) R (M/L)
PTC-1 4.39 x 10 -' 2.24 x 10 -'
PTC-2 5.93 x 10 -' 3.22 x 10 -'
PTC-3 5.69 x 10 -' 2.97 x 10 -'
PTC-4 5.68 x 10 -' 2.89 x 10 -'
PTC-5 4.62 x 10-' 3.35 x 10 -'
PTC-6 4.85 x 10 -' 2.24 x 10 -'
PTC-7 6.70 x 10 -' 2.55 x 10 -'
PTC-8 8.83 x 10-' 2.85 x 10 -'
a 6: Dissociation constant (KD (M)) in moles and receptor number
(R (M/L)) in moles per liter for Substance P. on eight primary tumor cultures
of human
colon adenocarcinoma.
Sample No. Ko (M) R (M/L)
PTC-1 1.54 x 10 -' 1.85 x 10 - g
PTC-2 1.72 x 10 -' 1.71 x 10 - 8
PTC-3 1.34 x 10 -' 1.49 x 10 - 8
PTC-4 1.54 x 10 -' 1.66 x 10 - 8
PTC-5 2.10 x 10 - 8 6.2 x 10 - 9
PTC-6 2.34 x 10 -' 2.95 x 10 - 8
PTC-7 2.62 x 10 - 8 7.48 x 10 - 9
PTC-8 1.86 x 10 -' 1.32 x 10 - 8
An example of a combination within the scope of the invention comprises
SOMZ, VIP,, VIPZ, VIP3, SOM,, BOM,, and SP,. A combination, hereinafter
referred to
as MuJ-7, was prepared using the following seven peptide analogs: ( 1 ) VIP,
(the VIP
antagonist) having a molecular weight of approximately 3464.9 and a
concentration of
CA 02197139 2000-03-30
- 12-
approximately 10 -' M; (2) VIPz (the VIP receptor binding inhibitor) having a
molecular
weight of approximately 1027.55 and a concentration of approximately 10 -g M;
(3) VIP3
(the VIP receptor antagonist) having a molecular weight of approximately
3342.09 and a
concentration of approximately 10 'g M; (4) SOM, (the somatostatin analog
(CTOP))
having a molecular weight of approximately 1061.59 and a concentration of
approximately 10 -9 M; (5) SOMZ (the analog of somatostatin) having a
molecular weight
of approximately 1637.9 and a concentration of approximately 10 -g M; (6) BOM,
(the
bombesin antagonist) having a molecular weight of approximately 983.55 and a
concentration of approximately 10 -$ M; and (7) SP, (the Substance P
antagonist) having a
molecular weight of approximately 1 S 15.83 and a concentration of
approximately 10 -g M.
The preceding sentence sets forth the preferred concentrations of the seven
analogs
comprising MuJ-7. Nevertheless, it is expected that MuJ-7 would be effective
if the
concentration of each of the seven analogs ranged from approximately 10 '~ M
to
approximately 10 ''2 M.
MuJ-7 may be prepared in the following way. A stock solution of each of
the seven peptide analogs is prepared with a pH of approximately 7.0 to
approximately
7.4. Although sterile phosphate buffered saline was used to prepare the stock
solutions for
the testing described below, other diluents may be used such as RPMI 1640,
buffered
saline, isotonic NaCI, Ringer's solution, water (for injection), distilled
water, polyethylene
glycol (neat or in water), 2% TweenTM in water, dimethylsulfoxide to 50% in
water,
propylene glycol (neat or in water), balanced salt solution, glycerol, and
other
conventional fluids that are suitable for intravenous administration. To
obtain a pH in the
range of approximately 7.0 to approximately 7.4 for each stock solution, the
pH can be
adjusted by using 1 N HCI for the lowering the pH or 1 N NaOH for raising the
pH,
although other conventional agents for adjusting the pH can be used. The
concentration of
the peptide analog in each stock solution is approximately 10'' M. Aliquots of
the seven
peptides analogs are mixed together such that the MuJ-7 formulation contains
approximately equal weights of each of the seven peptide analogs. In MuJ-7,
the
concentration of VIP, is approximately 10 -' M; the concentration of VIPz is
approximately 10 '8 M; the concentration of VIP3 is approximately I 0 ~8 M;
the
concentration of SOM, is approximately 10 -9 M; the concentration of SOMz is
CA 02197139 2000-03-30
-13-
approximately 10 -g M; the concentration of BOM, is approximately 10 -8 M; and
the
concentration of SP, is approximately 10 -g M. In one exemplary embodiment,
the pH of
the MuJ-7 solution may range from approximately 7.0 to approximately 7.4. To
obtain a
pH in this range, the pH can be adjusted by using 1 N HCI for lowering the pH
or
1 N NaOH for raising the pH, although other conventional agents for adjusting
the pH can
be used.
MuJ-7 was tested against primary tumor cells of human colon
adenocarcinoma, and each of the peptide analogs comprising MuJ-7 was tested
individually against human colon adenocarcinoma tumor cells and other cancer
cell lines.
The results for primary tumor cells of human colon adenocarcinoma are
summarized in
Table 7; and the results for other tumor or cancer cell lines are summarized
in Table 8.
Tables 7 and 8 list the maximum cytotoxicity achieved for each peptide analog
and
MuJ-7.
The cytotoxicity of MuJ-7 and each of the peptide analogs listed in Tables
7 and 8 was tested by performing a one-day MTT cytotoxicity assay, which is
based on
the principle of uptake of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-Biphenyl
tetrazolium
bromide), a tetrazolium salt, by the metabolically active cells where it is
metabolized by
active mitochondria into a blue-colored formazan product, which can be read
spectrophotometrically. The following article describes the MTT assay:
Mosmann, T.,
"Rapid Colorimetric Assay for Cellular Growth and Survival: Application to
Proliferation and Cytotoxity Assays," Journal of ImmunologLcal Methods 65 : 55-
63
(1983). To prepare the MTT stock solution needed for the one-day MTT cytotoxic
assay, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-Biphenyl tetrazolium bromide)
(Sigma
catalogue number M 2128) was dissolved in phosphate buffered saline with a pH
of 7.4
to obtain an MTT concentration of S mg/ml; the resulting mixture was filtered
through a
0.22 ~ filter to sterilize and remove a small amount of insoluble residue; the
filtered
mixture was the MTT stock solution (20 p.1 per 200 ~1 of medium). Briefly, for
each
type of tumor cell, approximately 20,000-50,000 cells were seeded in a 96-well
culture
plate and incubated with each of the peptide analogs or MuJ-7 in a C02
incubator for
approximately 24 hours. The concentration of the peptide analogs and MuJ-7 are
given
in Tables 7 and 8. (In Table 8, the concentrations of the peptide
CA 02197139 2000-03-30
-14-
analogs include 10 -6 M, 10 '' M, and 10 -8 M). Controls, which were not
treated with the
peptide analogs or MuJ-7, were similarly incubated. The assay was terminated
after
approximately 24 hours by adding approximately 100 ~g (20 ~l) of MTT to each
well,
then incubating for approximately one additional hour, and finally adding
approximately
50 ~l of 10% SDS-0.01 N HCl to each well to lyse the cells and dissolve the
formazan.
After incubating for approximately one hour at 37°C., the plate
was read
spectrophotometrically at 540 nm; and the cytotoxicity percentage (i.e., the
killing
percentage or the inhibition percentage) was calculated using the following
formula:
Cytotoxicity percentage = 100 x [1 - (X/R,)],
where X = (absorbance of the treated sample at 540 nm) - (absorbance of a
blank at 540
nm), and R, _ (absorbance of the untreated control at 540 nm) - (absorbance of
a blank at
540 nm). Thus, in each of the MTT cytotoxicity assays reported herein, the
cytotoxicity
percentage was calculated according to the above formula and was based on the
proliferation of the untreated controls, the value of which was taken as 100%.
Table 7: Cytotoxic effect of individual peptide analogs and MuJ-7 on primary
tumor
cells of human colon adenocarcinoma.
Peptide Analog Concentration . % Killing
VIP, 10-' M . 61
VIPZ 10 - g M
VIP3 10 ' 8 M
SOM, 10-9 M
SOMZ 10 -8 M
BOM, 10 ' g M 64
SP, 10 -8 M 54
MuJ-7 VIP, (10'' M) + VIPZ 94
~
( 10 '8 M) + V Ipz
( 10 '8 M) +
SOM, ( 10 '9 M) + SOMZ
(10-8 M)+BOM,
( 10 -8 M) + S p ~
( 10-8 M)
CA 02197139 2000-03-30
- 15 -
~ , , , , , ,
, o
~ , , , ,
0
, 'r'
' ~ ' ' ' '
, , , , ~ N
~ , , N , ~
, , , M 00 ' , ,
0
00 "" V1 ~D C1
~. , M ,
N N
~ .-i..r C''~N , M , , 'd'
, ,
N M N '
'
, V1 , M , , , ~
o
_
~ N M M N ~ ' N ' ' ~' ,
'-'
~t O
~ ~ ' N
i r N M ~~ N ' , .-.,
.-
0
~ Ov , ""'N , , ~
,
" ~ , ~
"
, ,
o0 Ov ~ N tD ~ et
O , N ~ M ~ n
, ,..."..-. ~
a M d' O O~ p
M '~t ~ .-., y. , , p~ , ,
M ~D
M N M N ' ' M N , v7 , , d-
O w
N M ~ N ~ ' \G
N Q1 , , ,
O
O t' M N
G m M N ' ' ' ~ ,~ ~ , ,
c N
U ~ ~r
~r
~ ~ ~ ~' N t~ ' , O
'
(=r ' ~ N , N , ,
0
M ~D O
N
y. "~ ~. , N , O~ ~O
, ,
p
N l~ N t'~
~ ~
~ M N ,..,'
~
0
~ ~ ~ ~ ~ M O
, ~' M .- ' .- ' ~ ~ , , O
-~ .
N
U
M ~ ~ ~ ~
N ~. , ~ ~ ~ .-.
U ~ '~'
vo
p p ", ,
M
o w . , , ,n
c~
' ~ ~ ~ ~
U W ' ~ , ,
00
.,
N O
~ p 7 O
~ ~ ~ E-. y -
W ue O W U
W N (y ~ N p~ N ~
d
'
_ ~" ~ M M U ~ ~ ~ o
~ ~ H
~ U x x
U ~G ,~ .~ ~ v~ x v~ C7 v~ a
a
.
0 0
N
CA 02197139 2000-03-30
- 16-
In Table 8, K562 cells are human leukemia cells; MOLT-4 cells are human
lymphoma cells; L 132 cells are human lung carcinoma cells; PC3 cells are
human
pancreas tumor cells; MCF-7 cells are human breast tumor cells; HuTu80 cells
are human
duodenum tumor cells; Hu 746T cells are human stomach tumor cells; SK0.007
cells are
human myeloma cells; HT29 cells are human colon tumor cells; S W 620 cells are
human
colon tumor cells; G 401 cells are human kidney tumor cells; SK.MEL.28 cells
are human
melanoma cells; and PTC cells are human colon tumor cells.
The results in Tables 7 and 8 indicate that the peptide analogs (VIP,, VIPZ,
VIP3, SOM,, SOM2, BOM,, and SP,) are more effective when used in the MuJ-7
combination.
Five different subcombinations of the seven peptide analogs comprising
MuJ-7 were tested against human colon adenocarcinoma tumor cells. The
subcombinations are listed in Table 9. Each subcombination was tested by
performing a
one-day MTT cytotoxicity assay. Briefly, approximately 20,000-50,000 primary
tumor
cells of human colon adenocarcinoma were seeded in a 96-well culture plate and
incubated with each subcombination in a COZ incubator for approximately 24
hours. The
concentrations of the peptide analogs in each subcombination are given in
Table 9.
Controls, which were not treated with the subcombinations, were similarly
incubated. The
assay was terminated after approximately 24 hours by adding approximately 100
~cg
(20 ,u1) of MTT to each well, then incubating for approximately one additional
hour, and
finally adding approximately 50 ~1 of 10% SDS-0.01 N HCl to each well to lyse
the cells
and dissolve the formazan. After incubating for approximately one hour at 37
° C., the
plate was read spectrophotometrically at 540 nm; and the cytotoxicity
percentage (i.e., the
killing percentage) was calculated using the formula presented above. Table 9
lists the
maximum cytotoxicity achieved for each subcombination.
CA 02197139 2000-03-30
- 17-
i 0 Table 9: Cytotoxicity of subcombinations of peptide analogs on primary
tumor cells
of human colon adenocarcinoma.
Subcombination Killing percentage
Number Subcombination (approximate)
1 VIP, (10-' M) + SOM,64.7
(10-9 M) + BOM, (10-$
M)
2 VIP, (10'' M) + VIPZ75.3
(10-8 M)+SOM, (10-9
M)
+ BOM, (10 -8 M)
3 VIP, (10 '' M) + 82.9
VIPZ
(10 -g M) + SOM,
(10'9 M)
+ SOMZ (10-g M) +
SP,
( 10 -8 M)
4 VIP, (10 '' M) + 90.2
VIPZ
(10-gM)+VIPz(10-8M)+
SOM, (10 -9 M) +
SOMZ (10 -g M) +
BOM,
(10-8 M)
VIP, (10 -' M) + 94.9
VIPZ
(10-8 M)+SOM, (10-9
M)
+ SOMZ ( 10 '8 M)
+ BOM,
( 10 -8 M)
20 In another experiment, these other peptide analogs were tested:
somatostatin analog--RC-160; Substance P analogs--Substance P ,~ and
SpantideTM I;
cholecystokinin analog--CCK-33; and glucagon analog--human glucagon. Each of
these
peptide analogs was tested against primary tumor cells of human colon
adenocarcinoma.
Each peptide analog was tested at concentrations between 10 '6 M and 10
''° M by
performing a one-day MTT cytotoxicity assay. Briefly, approximately 20,000-
50,000
primary tumor cells of human colon adenocarcinoma were seeded in a 96-well
culture
plate and incubated with each peptide analog in a COZ incubator for
approximately
24 hours. Controls, which were not treated with the peptide analogs, were
similarly
incubated. The assay was terminated after approximately 24 hours by adding
30 approximately 100 ug (20 ~cl) of MTT to each well, then incubating for
approximately one
additional hour, and finally adding approximately 50 ~l of 10% SDS-0.01 N HCl
to each
CA 02197139 2000-03-30
-18-
well to lyse the cells and dissolve the formazan. After incubating for
approximately one
hour at 37°C., the plate was read spectrophotometrically at 540 nm; and
the cytotoxicity
percentage (i.e., inhibition percentage) was calculated using the formula
presented above.
The maximum cytotoxicity achieved for Substance P,_6 was approximately 35.9%;
the
maximum cytotoxicity achieved for RC-160 was approximately 58.0%; the maximum
cytotoxicity achieved for Spantide I was approximately 30.8%; the maximum
cytotoxicity
achieved for human glucagon was approximately 0%; the maximum cytotoxicity
achieved
for CCK-33 was approximately 17.8%.
Table 10 lists other VIP, analogs; Table 11 lists other somatostatin analogs;
Table 12 lists other bombesin analogs; and Table 13 lists other Substance P
analogs. The
analogs listed in Tables 10-13 may be able to replace some of the peptide
analogs
comprising MuJ-7.
In Tables 2 and 10-13 and in the Sequence Listing, "Pen" represents
penicillamine; "~Y" represents a surrogate bond; "~" represents a reduced
bond; "pGlu"
represents pyroglutamic acid (i.e., S-oxo-proline); "NicLys" represents lysine-
(Nicotinyol)
(i.e., nicotine attached to the a amino group of the lysine side chain);
"MePhe" represents
methylphenylalanine; and "Nle" represents norleucine.
The amino-acid sequences disclosed in Tables 2 and 10-13 and claimed
herein may include conservatively modified variants of the amino-acid
sequences
disclosed in Tables 2 and 10-13. It is believed that the claimed invention
would still be
etTective if the amino-acid sequences disclosed in Tables 2 and 10-13 were
shortened by
removing amino-acid residues (e.g., one, two, or perhaps more amino-acid
residues) from
the C-terminus and/or from the N-terminus. It is also believed that the
claimed invention
would still be effective if the amino-acid residues (e.g., one, two, or
perhaps more amino-
acid residues) at the C-terminus and/or at the N-terminus of the amino-acid
sequences
disclosed in Tables 2 and 10-13 were replaced with different amino-acid
residues
CA 02197139 2000-03-30
- 19-
Table 10: VIP analogs.
S. NAME SEQUENCE SEQ ID
NO. NO:
1 VIP l0-28 Tyr-Thr-Arg-Leu-Arg-Lys-Gin-MetSEQ ID
N0:3
Ala-Val-Lys-Lys-Tyr-Leu-Asn-Ser-
I le-Leu-Asn-NHZ
2 VIP Antagonist ([Ac-Tyr',D-Phe2]-Ac-Tyr-D-Phe-Asp-Ala-Ile-Phe-Thr-
Growth Hormone ReleasingAsn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-
Factor
I-29 Amide) Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu
Gin-Asp-Ile-Met-Ser-Arg-NHZ
3 VIP (6-28) Phe-Thr-Asp-Asn-Tyr-Thr-Arg-SEQ ID
N0:4
Leu-Arg-Lys-Gln-Met-Ala-Val-Lys-
Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn-
NHZ
1e 11: Somatostatin analogs.
S. NAME " SEQUENCE SEQ ID
NO. NO:
1 [D-Trpa]-SomatostatinAla-Gly-Cys-Lys-Asn-Phe-Phe-D-
Trp-Lys-Thr-Phe-Thr-Ser-Cys
2 CTAP D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-
Thr-NHZ
3 Somatostatin agonist (3-(2-Naphtyl)-D-Ala-Cys-Tyr-D-
Trp-Lys-Val-Cys-Thr-NHz
4 Somatostatin analog D-Phe-Cys-Tyr-D-Trp-Lys-Cys-Thr-
NHZ
5 [LeuB,D-Trp2z,TyrzS]-SomatostatinSer-Ala-Asn-Ser-Asn-Pro-Ala-Leu-
28 Ala-Pro-Arg-Glu-Arg-Lys-Ala-Gly-
Cys-Lys-Asn-Phe-Phe-D-Trp-Lys-
Thr-Tyr-Thr-Ser-Cys
6 [D-Trpa,Tyr"]-SomatostatinAla-Gly-Cys-Lys-Asn-Phe-Phe-D-
Trp-Lys-Thr-Tyr-Thr-Ser-Cys
7 [D-Trp"]-SomatostatinAla-Gly-Cys-Lys-Asn-Phe-Phe-Trp-
Lys-Thr-D-Trp-Thr-Ser-Cys
8 [Tyr']-Somatostatin Tyr-Gly-Cys-Lys-Asn-Phe-Phe-Trp-SEQ ID
NO:S
Lys-Thr-Phe-Thr-Ser-Cys
9 [Tyr"]-Somatostatin Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-SEQ ID
N0:6
Lys-Thr-Tyr-Thr-Ser-Cys
10 Somatostatin analog (3-(2-naphthyl)-D-Ala-Cys-Tyr-D-
Trp-Lys-Val-Cys-Thr-NHZ
11 [Des-Ala',Des-GIyZ,HisCys-His-His-Phe-Phe-D-Trp-Lys-Thr-
S,D-TrpB]-
Somatostatin Phe-Thr-Ser-Cys
CA 02197139 2000-03-30
-20-
Table 12: Bombesin analogs.
S. NAME SEQUENCE SEQ ID NO:
NO.
l [Leu "-~I'(CHZNH)Leu"]-pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-SEQ ID N0:7
Bombesin Val-Gly-His-Leu-~Y(CHzNH)Leu-NHz
2 [D-Arg',D-Pro2,D-D-Arg-D-Pro-Lys-Pro-Gln-Gln-D-Trp-Phe-
Trp'',Leu"]-SubstanceD-Trp-Leu-Leu-NHZ
P
3 (Leu "-~-Leu')-BombesinPyr-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-SEQ ID
N0:8
Gly-His-Leu-(~)-Leu-NH2 -
4 (D-Phe'~,Leu")-BombesinPyr-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-
Gly-D-Phe-Leu-Leu-NH~
5 (Tyr',D-Phe'Z)-BombesinpGlu-Gln-Arg-Tyr-Gly-Asn-Gln-Trp-Ala-
Val-Gly-D-Phe-Leu-Met-NHZ
6 [D-Phe'2]-BombesinpGlu-Gln-Arg-Leu-Gly-Asn-G(n-Trp-Ala-
(Bombesin ReceptorVal-Gly-D-Phe-Leu-Met-NHZ
Antagonist)
7 [Deamino-Phe6,His,D-Deamino-Phe-His-Trp-Ala-Val-D-Ala-His-D-
Ala",D-Pro"-~I'(CHZNH)-Pro-~I'[CHZNH]-Phe-NHZ
Phe"]-Bombesin
fragment
6-14
8 Bombesin fragmentTrp-Ala-Val-Gly-His-Leu-Met-NHZSEQ ID N0:9
8-14
9 (Tyr')-Bombesin pGlu-Gln-Arg-Tyr-Gly-Asn-Gln-Trp-Ala-SEQ ID NO:10
Val-Gly-His-Leu-Met-NH2
CA 02197139 2000-03-30
-21 -
Ta 1e 13: Substance P analogs.
S. NO. NAME SEQUENCE
1 Spantide I([D-Arg', D-Trp'9,D-Arg-Pro-Lys-Pro-Gln-Gln-D-Trp-Phe-
Leu"]-
Substance P) D-Trp-Leu-Leu-NH,
2 Spantide II D-NicLys-Pro-Thr-Pal-Pro-D-CIZPhe-
Asn-D-Trp-Phe-D-Trp-Leu-Nle-NHz
3 [D-Pro2,D-Phe',D-Trp9)-SubstanceArg-D-Pro-Lys-Pro-Gln-Gln-D-Phe-Phe-
P
D-Trp-Leu-Met-NHz
4 [D-ProZ,D-Trp'9]-SubstanceArg-D-Pro-Lys-Pro-Gln-Gln-D-Trp-Phe-
P
D-Trp-Leu-Met-NHZ
5 [D-Pro,D-Trp'']-Substance D-Pro-Gln-Gln-D-Trp-Phe-D-Trp-Leu-
P 4-11
Met-NHZ
6 [Arg6,D-Trp'-',MePheB)-SubstanceArg-D-Trp-MePhe-D-Trp-Leu-Met-NH
P 6-11
Z
7 (D-Arg',D-PheS,D-Trp'',LeuD-Arg-Pro-Lys-Pro-D-Phe-Gln-D-Trp-
")-
Substance P Phe-D-Trp-Leu-Leu-NHZ
8 [D-Pro,D-Trp''',Phe")-SubstanceD-Pro-Gln-Gln-D-Trp-Phe-D-Trp-D-Trp-
P
4-11 Phe-NHZ
9 [D-Pro,D-Trp''']Substance D-Pro-Gln-Gln-D-Trp-Phe-D-Trp-D-Trp-
P 4-I 1
Met-NH2
10 (D-Pro,D-Trp'',Nle")-SubstanceD-Pro-Gln-Gln-D-Trp-Phe-D-Trp-Trp-
P
(4-11) Nle-NHz
11 (D-Pro,D-Trp''',Val )-SubstanceD-Pro-Gln-Gln-D-Trp-Val-D-Trp-D-Trp-
P
(4-11 ) Met-NHZ
12 (Argb,D-Trp'',N-Me-Phe$)-SubstanceArg-D-Trp-N-Me-Phe-D-Trp-Leu-Met-
P
(6-11 )
NHZ
13 [D-Arg',D-Pro2,D-Phe',D-His'J-D-Arg-D-Pro-Lys-Pro-Gln-Gin-D-Phe-
Substance P Phe-D-His-Leu-Met-NHz
14 [D-Trpz'-')-Substance P Arg-D-Trp-Lys-Pro-Gln-Gln-D-Trp-Phe-
D-Trp-Leu-Met-NHz
15 [D-Arg',D-ProZ,D-Trp'9,Leu"]-D-Arg-D-Pro-Lys-Pro-Gln-Gln-D-Trp-
Substance P Phe-D-Trp-Leu-Leu-NHZ
16 [D-Arg',D-Trp'',Leu"]-SubstanceD-Arg-Pro-Lys-Pro-Gln-Gln-D-Trp-
P
Phe-D-Trp-Leu-Leu-NHZ
Another aspect of the invention provides a method for treating a mammal
(including a human being) afflicted with cancer. The types of cancer that may
be treated
include, but are not necessarily limited to, leukemia and lymphoma;
adenocarcinoma of
the stomach, pancreas, and prostate; and cancer of the colon, rectum, lung,
breast, and
CA 02197139 2002-02-20
-22-
kidney. In addition, it is expected that the invention will provide a method
for treating
other diseases and cancers characterized by hypersecretion of one or more of
the peptides
VIP, somatostatin, bombesin, and Substance P.
'the methods of this invention comprise, consist of; or consist essentially
of: administering systematically to the mammal a therapeutically effective
combination of
peptide SOMZ and at least four of the peptides: VIP,, VIP2, VIP3, SOMA, BOMB,
and SP,.
An effective dose of the combination ranges from 15 to 170 ~g (preferably 25
to 40 fig)
of the peptides per kg of the body weight of the mammal, with the dose
dependent on the
effects sought, the manner of administration, the peptides selected, and the
cancer being
treated. Systemic administration refers to oral, rectal, nasal, transdermal,
and parenteral
(i.e., intramuscular, intravenous, and subcutaneous). In accordance with good
clinical
practice, it is preferred to administer the composition at a dose that will
produce anticancer
effects without causing undue harmful side effects. The composition may be
administered
either alone or as a mixture with other therapeutic agents.
The composition may optionally and preferably contain pharmaceutically
acceptable diluents, excipients, solvents, binders, stabilizers, and the like.
Such diluents
may include: RPMI 1640 (see Table 16), buffered saline, isotonic NaCI,
Ringer's solution,
water, distilled water, polyethylene glycol (neat or in water), 2% tween in
water,
dimethylsulfoxide to 50% in water, propylene glycol (neat or in water),
phosphate
2 0 buffered saline, balanced salt solution, glycerol, and other conventional
fluids that are
suitable for intravenous administration. Pharmaceutical compositions which
provide from
about 10 to 2000 ~g of the composition per unit dose are preferred and are
conventionally
prepared as tablets, lozenges, capsules, powders, aqueous or oily suspensions,
syrups,
elixirs, and aqueous solutions. The nature of the pharmaceutical composition
employed
will, of course, depend on the desired route of administration.
The present invention is further described in detail with reference to the
following examples, which are given for the purpose of merely illustrating the
invention
without limiting it.
CA 02197139 2000-03-30
- 23 -
In Vitro Studies of MuJ-7'
Example 1:
A primary tumor cell line of human colon adenocarcinoma was established
by using fine needle aspiration cytology (FNAC) and histopathology confirmed
tumor
tissue of human colon adenocarcinoma. These cells stained positive with a
monoclonal
antibody specific for a 91 KD surface antigen present on human colon
adenocarcinoma
cells. The tumorigenicity of these cells was demonstrated by the ability of
these cells to
form tumors in nude mice on subcutaneous injection. The characteristics of the
12
primary cultures are given in Table 14. ,
Table 14: Characteristic features of human colon adenocarcinoma biopsies
cultured
in vitro. Note that cultures established from all the tumor biopsies were then
xenografted
in nude mice.
Tumor
B. Age Sex SiteFNAC Hist.Mab Pass.No.Soft Induction
No. Agar
1 52 M AC + + + 36 + +
2 47 F DC + + + 36 + +
3 74 F DC + + + 34 + +
4 68 M TC + + + 32 + +
5 60 M DC + + + 32 + +
6 58 M AC ND + + 29 + +
7 71 F DC + ND + 28 + +
8 69 M AC ND + + 18 + +
9 57 M TC + ND + 14 + +
10 73 F DC + + + 09 + +
11 50 M AC ND + + 06 + +
12 69 M AC + + + 06 + +
B. NO: Biopsy number; M.~ Male; F: Female: AC: Ascending colon; DC.'
Descending
colon; TC: Transverse colon; FNAC: Fine Needle Aspiration Cytology; Hist.:
Histopathology; Mab: Monoclonal Antibody Marker; Pass. No.: Passage number;
ND:
Not done.
CA 02197139 2000-03-30
-24-
The anti-proliferative effects of MuJ-7 were studied in a 96-well culture
plate, wherein the human colon adenocarcinoma tumor cells (approximately
50.000 cells
per well) from the each of the twelve human colon adenocarcinoma cell cultures
listed in
Table 14 were incubated in a COZ incubator at approximately 37°C. for
approximately 72
hours with MuJ-7 (approximately 20 ,u1 of MuJ-7 per well). Human colon
adenocarcinoma cells not treated with MuJ-7 served as controls. Tritiated [3H]
thymidine
(approximately l,uCi per well) was added to each well, and the plate was
incubated for
approximately 1 additional hour. The cells were harvested on filter mats, and
incorporation of [3H] thymidine into the dividing cells was counted on a Beta
plate
(Pharmacia). For each of the tritiated [3H] thymidine cytotoxicity assays
described herein,
the proliferation of cells in the untreated controls was taken as 100%. In
Example l, it
was observed that MuJ-7 inhibited proliferation of the tumor cells by
approximately 95%.
Example 2:
The cytotoxic effect of MuJ-7 was reconfirmed by a one-day MTT assay.
The method for preparing the MTT stock solution for the one-day MTT cytotoxic
assay
was described above. Briefly, the 12 human colon adenocarcinoma tumor cell
cultures,
which were described above in Table 14, were incubated in a 96-well culture
plate with
MuJ-7 (approximately 20 ,u1 of MuJ-7 were added per well at time = 0 hours)
for
approximately 24 hours at approximately 37°C. in approximately 5% COz.
The controls
were cells from the 12 human colon adenocarcinoma cell cultures that were not
treated
with MuJ-7. Then, stock MTT solution (approximately 100 ,ug of MTT per well)
was
added to each well, and incubation continued for approximately 1 additional
hour. The
formazan crystals that formed inside the cells were dissolved with a detergent
comprising
approximately 10% sodium dodecyl sulfate and approximately 0.01 N HCI; and the
optical density of each well was read spectrophotometrically at 540 nm. The
optical
density was directly proportional to the number of proliferating and
metabolically active
cells. MuJ-7 inhibited proliferation-viability in each of the 12 human colon
adenocarcinoma cell cultures as assessed by the MTT cytotoxic assay. The
inhibition
percentage ranged from approximately 80.7% to approximately 95.2%. Table 15
lists the
approximate inhibition percentages for each of the 12 human colon
adenocarcinoma cell
lines. Biopsy numbers 1 through 12 in Table 14 correspond respectively to
sample
CA 02197139 2000-03-30
-25-
numbers PTC-1 through PTC-12 in Table 15.
Table 15: Inhibition percentages for each of the I 2 human colon
adenocarcinoma cell
lines.
Sample No. Percent Inhibition
PTC-1 95.2
PTC-2 89.1
PTC-3 94.6
PTC-4 82.2
PTC-5 74.2
PTC-6 81.6
PTC-7 93.8
PTC-8 94.9
PTC-9 81.5
PTC-10 82.7
PTC-11 84.6
PTC-12 g0,7
Exam In a 3:
The cytotoxic effect of MuJ-7 was tested using a three-day MTT cytotoxic
assay on three human colon cancer cell lines: CoLo 205, HT 29, and SW 620. The
method for preparing the MTT stock solution for the three-day MTT cytotoxic
assay was
the same as the method described above for preparing the MTT stock solution
for the one-
day MTT cytotoxic assay. Briefly, CoLo 205, HT 29, and SW 620 cells were
incubated in
a 96-well culture plate (approximately 50,000 cancer cells in each well) for
approximately
72 hours at approximately 37°C. in approximately 5% CO2. MuJ-7
(approximately 20 ~cl
per well) was added to the wells of all of the treated samples at time = 0,
24, and 48 hours.
CoLo 205, HT 29, and S W 620 cells not treated with MuJ-7 served as controls.
Then,
stock MTT solution (approximately 100 ,ug of MTT per well) was added to each
well, and
incubation continued for approximately 1 additional hour. The formazan
crystals that
formed inside the cells were dissolved with a detergent comprising
approximately 10%
CA 02197139 2000-03-30
-26-
sodium dodecyl sulfate and approximately 0.01 N HCI; and the optical density
of each
well was read spectrophotometrically at 540 nm. The percentage inhibition
caused by
MuJ-7 in CoLo 205, HT 29, and SW 620 was approximately 80%, approximately 18%.
and approximately 41 %, respectively.
Example 4:
Experiments were conducted to study the cytotoxic effect of MuJ-7 on 13
human tumor cell lines using the three-day MTT cytotoxic assay. These cells
lines were:
K562 cells (human leukemia), MOLT-4 (human lymphoma), L 132 (human lung
carcinoma), MCF-7 (human breast), SW 620 (human colon), G 401 (human kidney),
CoLo 205 (human colon), HuTu80 (human duodenum), Hu 746T (human stomach), HT29
(human colon), PC3 (human pancreas), SK0.007 (human myeloma), and SK.MEL.28
(human melanoma). The method for preparing the MTT stock solution for the
three-day
MTT cytotoxic assay was the same as the method described above for preparing
the MTT
stock solution for the one-day MTT cytotoxic assay. Briefly, cells from the 13
human
tumor cell lines were incubated in a 96-well culture plate (approximately
50,000 cancer
cells in each well) for approximately 72 hours at approximately 37°C.
in approximately
S% CO2. MuJ-7 (approximately 20 ~1 per well) was added to the wells of all of
the
treated samples at time = 0, 24, and 48 hours. The controls were cells from
the 13 tumor
cell lines that were not treated with MuJ-7. Then, stock MTT solution
(approximately
100 ~cg of MTT per well) was added to each well, and incubation continued for
approximately 1 additional hour. The formazan crystals that formed inside the
cells were
dissolved with a detergent comprising approximately 10% sodium dodecyl sulfate
and
approximately 0.01 N HCI; and the optical density of each well was read
spectrophotometrically at 540 nm. The approximate percentage of cytotoxicity
(i.e., the
percent inhibition) caused by MuJ-7 in each of thirteen cell lines is listed
in Table 1 SA.
No inhibition was observed in SK0.007 (human myeloma) and SK.MEL.28 (human
melanoma).
CA 02197139 2000-03-30
-27-
Table 15A: The approximate percentage of cytotoxicity (i.e., the percent
inhibition)
caused by MuJ-7 in each of thirteen cell lines.
Cell Lines Percentage of Cytotoxicity
K562 ' 45
MOLT-4 g 1
L 132 36
MCF-7 34
S W 620 ' 41
G 401 35
CoLo 205 g0
HuTu80 g
Hu 746T 9
HT29 1 g
PC3 0
SK0.007 _
SK. MEL.28 _
am 1 5:
Primary tumor cells of human colon adenocarcinoma were seeded in five
separate flasks at a density of 104 cells/ml. Five milliliters of R.PMI 1640
containing 10%
fetal calf serum were added to each flask. MuJ-7 (approximately 200 ,u1) was
added to
four of the flasks. MuJ-7 was not added to the fifth flask, which served as
the control.
Genomic DNA from the primary tumor cells of human colon adenocarcinoma was
extracted after the cells were treated with MuJ-7 for different time intervals
ranging from
approximately 12 hours to approximately 96 hours. See the section below
entitled
"Description of Protocols" for a detailed description of the method used for
the extraction
of genomic DNA. The genomic DNA was extracted from the tumor cells in the
first flask
after approximately 12 hours; the genomic DNA was extracted from the tumor
cells in the
second flask after approximately 24 hours; the genomic DNA was extracted from
the
tumor cells in the third flask after approximately 48 hours; the genomic DNA
was
CA 02197139 2000-03-30
-28-
extracted from the tumor cells in the fourth flask after approximately 96
hours; and the
genomic DNA was extracted from the tumor cells in the control flask after
approximately
96 hours. The DNA of both untreated and treated cells was run on a 1 % agarose
gel using
ethidium bromide for staining. The DNA of tumor cells treated with MuJ-7 for
48 and
96 hours formed a smear on the gel which is indicative of programmed cell
death, while
the DNA from untreated cells formed a sharp band at 10 kb, thus demonstrating
that the
DNA from untreated cells was intact. The DNA of tumor cells treated with MuJ-7
for
24 hours did not form a smear on the gel. Therefore, the time kinetic
experiment in vitro
showed that programmed cell death occurs between approximately 24 and 48 hours
of
treatment with MuJ-7.
Formulation of a Dose of MuJ-7 for in vivo Exneriments~
A dose of the MuJ-7 formulation was prepared in the following way. A
stock solution of each of the seven peptide analogs was first prepared using
sterile
phosphate buffered saline with an approximate pH of 7.4. The concentration of
the
peptide analog in each stock solution was approximately 10 -; M. Aliquots of
the seven
peptides analogs were mixed together such that the MuJ-7 formulation contained
approximately equal weights of each of the seven peptide analogs, with the
combined
weight of the seven peptide analogs in each dose of MuJ-7 being approximately
8 to
16 ,ug, depending upon the size of the dose. In MuJ-7, the concentration of
VIP, was
approximately 10 '' M; the concentration of VIPZ was approximately 10 -8 M;
the
concentration of VIP3 was approximately 10 -8 M; the concentration of SOM, was
approximately 10 -9 M; the concentration of SOMZ was approximately 10 '8 M;
the
concentration of BOM, was approximately 10 'g M; and the concentration of SP,
was
approximately 10 -8 M. The volume of this solution was made up with sterile
RPMI 1640
to approximately 150 ~cl. RPMI 1640 is a cell culture medium that was
developed at the
Rosewell Park Memorial Institute. The components of RPMI 1640 are listed in
Table 16.
CA 02197139 2000-03-30
-29-
Table 16: Components of RPMI 1640.
COMPONENTS g/L
Calcium Nitrate - 4H20 0.1
Magnesium sulfate (anhydrous) 0.04884
Potassium chloride 0.4
Sodium chloride 6.0
Sodium Phosphate Dibasic(anhydrous) 0.8
L-Arginine (free base) 0.2
L-Asparagine (anhydrous) 0.05
L-Aspartic acid 0.02
L-Cystine 2HC1 0.0652
L-Glutamic acid 0.02
L-Glutamine 0.3
Glycine 0.01
L-Histidine (free base) 0.015
Hydroxy-L-Proline 0.02
L-Isolelucine 0.05
L-Leucine 0.05
L-Lysine HCl 0.04
L-Methionine 0.015
L-Phenylalanine 0.015
L-Proline 0.02
L-Serine 0.03
L-Threnine 0.02
L-Tryptophan 0.005
L-Tyrosine 2Na2H20 0.02883
L-Valine 0.02
D-Biotin 0.0002
Choline chloride 0.003
Folic acid 0.001
myo-Inositol 0.035
Niacinamide 0.001
p-Amino Benzoic Acid 0.001
D-Pantothenic Acid (hemicalcium) 0.00025
Pyridoxine HCl 0.001
Riboflavin 0.0002
Thiamine HCl 0.001
Vitamin B-12 0.000005
D-Glucose 2.0
Glutoathione (reduced) . 0.001
HEPES 5.958
Phenol Red (sodium) 0.0053
CA 02197139 2000-03-30
-30-
Throughout the following in vivo experiments, control mice not injected
with MuJ-7 were instead injected with RPMI 1640. The progressive growth of the
tumors
in the control mice indicates that RPMI 1640 is not critical to the
effectiveness of MuJ-7.
In the following in vivo experiments, the tumor volume was calculated
with the help of a vernier calliper. The shortest axis (a) and the longest
axis (b) of the
tumor was accurately measured, and the volume was calculated using the
following
formula:
Tumor volume = 0.4 x a2 x b
The above-mentioned formula for tumor volume is derived from H. J.
Winn, National Cancer Institute Monograph 2 (1959) 113-138.
In the following in vivo experiments, each tumor weight measurement was
taken at the end of the experiment by sacrificing the mouse and resecting out
the complete
tumor growing superficially on the posterior side immediately below the skin
over the
muscular layer. The skin and ally other tissues attached to the tumor were
removed, and
the tumor was immediately weighed on an analytical balance.
In vivo studies in tumor-bearing nude mice:
Out of 52 tumor-bearing nude mice treated with MuJ-7 according to the
various in vivo protocols described in Examples 6-13, 49 mice showed complete
or partial
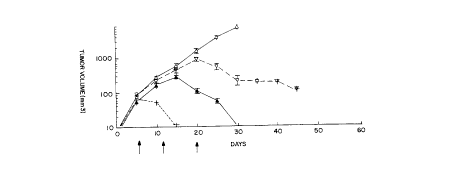
tumor regression. Figure 1, which shows the effect on tumor regression of
treatment onset
time with MuJ-7, summarizes the mean tumor volume (in mm3) for all of the mice
in the
in vivo protocols described in Examples 6-13 versus the day numbers. (Each
data point in
Figure 1 represents the mean tumor volume of different numbers of mice from
separate
experiments.) In Figure 1, the mice are grouped together into four categories:
(1)
untreated control mice ("o"); (2) treated mice (" +") that received their
first dose of MuJ-7
by day 5; treated mice (" ~") that received their first dose of MuJ-7 after
day 5 and by day
12; and treated mice ("v") that received their first dose of MuJ-7 after day
12 and by day
20. The arrows indicate days 5, 12, and 20.
Example 6:
On day 1, ten BALB/c nude nu/nu mice were implanted with primary
tumor cells of human colon adenocarcinoma (approximately 10 million tumor
cells per
mouse), and these mice received their first dose of MuJ-7 approximately one
hour after
CA 02197139 2000-03-30
-31 -
they were implanted with the tumor cells. The total daily dose of MuJ-7 for
each treated
mouse comprised approximately 1.143 ~:g of VIP,, approximately 1.143 ,ug of
VIP,,
approximately 1.143 ~cg of VIP3, approximately 1.143 ~cg of SOM,,
approximately 1.143
,ug of SOMA, approximately 1.143 ~g of BOM,, and approximately 1.143 ~g of
SP,.
Thus, the total daily dose of MuJ-7 always contained approximately equal
weights of each
of the seven peptide analogs (VIP,, VIPz, VIP3, SOM,, SOM2, BOM,, and SP,);
and the
total weight of these seven peptide analogs was approximately 8 ,ug. The total
daily dose
of MuJ-7 was divided into approximately three equal subdoses. Three times a
day at
approximately eight-hour intervals, a subdose was injected into each treated
mouse. The
first subdose of the day was injected into the tail vein; the second subdose
of the day was
injected into one gluteal muscle; and the third subdose of the day was
injected into the
other gluteal muscle. The treatment lasted for two weeks. Controls were
randomly
selected BALB/c nude nu/nu mice whose weights were similar to the weights of
the ten
treated mice. The control mice were subcutaneously injected with the same type
and
approximately the same number of human colon adenocarcinoma tumor cells as the
treated mice. The control mice did not receive MuJ-7.
The tumor volume in each treated and untreated mouse was recorded every
four days. Table 17 lists the tumor volume in mm' for each treated mouse, and
Table 18
lists the tumor volume in mm3 for each control (untreated) mouse. Figure 2 is
a graph of
the mean tumor volume (in mm') of the treated mice ("o") and the untreated
control mice
("+") versus the day numbers. In Figure 2, the arrow illustrates that the
treated mice
received their first dose of MuJ-7 approximately one hour after they were
treated with
tumor cells on day 1.
CA 02197139 2000-03-30
- 32 -
N
M
v ~ i i ~ i
N
N
' ly..~' ~ N C~ ~,
A
<t.
N
N ~ N ~ N
O A
O.
O
' O
N M ~ ~ M h
G 1 v /1
A
O
_
O O ~p N
3 ~ >, N M N M M M
' '~
0 0
H
>
~.
00 0o vo w e ~
'-' , -~ N v1 N v1 -.
c~ .-r.-,.-, .-~.-.~ .-.-..
cd
O
c,
w
O
U
b ~ .-.-N,N ~ ~ dN'~ N ~ ~ due.
O p N
...,
b
O
O O
> O
00 ~ ~O o0 ~O o0 M N ~ ~O
O a0
H
O
.-. ~ .~ N M ~t v W0 I~ oo Ov
O
cd
H
O O
N
CA 02197139 2000-03-30
- 3 "s -
a~
c~
_
M ~n d' ~O d'
M ~ a ~ a ~
O
N ~ N N N
N
N
N o0 00 I~ I~ ~D
V7 ~O M d' I~
~
_
O
N
O cd O~O~ OMOO~o~
n
,~ 01 M 00 M .--
N ~O v1 V1 I~ n
O ~
O
~_
O M M
Q ~ ~ ' 0
H cd N N N N N
A
U
N
O ~ Nv ~O
w ~
.b
a~
00
'~,O - M C'~Ice.
~' , N ~' ~IIM M
O U ~
_
b
O
.....
O
O M
~ ~t N M ~O 00
A
N
p -~ N M ~h ~n
c~
H
O
CA 02197139 2000-03-30
-34-
The treatment with MuJ-? prevented tumor growth in approximately 90%
of the treated mice. Furthermore, none of the treated mice died during the
course of the
experiment. By contrast, the control mice showed tumor growth, which
eventually
resulted in death of the mice.
Example 7:
Twenty BALB/c nude nu/nu mice, which were divided into two groups
with ten mice in each group, were implanted on day 1 with primary tumor cells
of human
colon adenocarcinoma (approximately 10 million tumor cells per mouse). The
group 1
mice received their first dose of MuJ-7 on day 12 (i.e., 11 days post
implantation on the
tumor cells on day 1). The group 2 mice received their first dose of MuJ-7 on
day 20
(i.e., 19 days post implantation of the tumor cells on day 1 ). These twenty
treated mice
were injected for 14 days with a daily dose of MuJ-7. The total daily dose of
MuJ-7 for
each treated mouse comprised approximately 1.143 ~g of VIP,, approximately
1.143 ~cg
of VIP2, approximately 1.143 ~cg of VIP3, approximately 1.143 ~cg of SOM,,
approximately 1.143 ~cg of SOM2, approximately 1.143 ug of BOM,, and
approximately
1.143 ycg of SP,. Thus, the total daily dose of MuJ-7 always contained
approximately
equal weights of each of the seven peptide analogs (VIP,, VIPZ, VIP3, SOM,,
SOM2,
BOM,, and SP,); and the total weight of these seven peptide analogs was
approximately
8 ~cg. The total daily dose of MuJ-7 was divided into approximately three
equal subdoses.
Three times a day at approximately eight-hour intervals, a subdose was
injected into each
treated mouse. The first subdose of the day was injected into the tail vein;
the second
subdose of the day was injected into one gluteal muscle; and the third subdose
of the day
was injected into the other gluteal muscle. Controls were five randomly
selected BALB/c
nude nu/nu mice whose weights were similar to the weights of the twenty
treated mice in
groups 1 and 2. (The same five mice served as controls for both the group 1
and the group
2 experiments.) The control mice were subcutaneously injected with the same
type and
approximately the same number of human colon adenocarcinoma tumor cells as the
treated mice. The control mice did not receive MuJ-7.
In the group 1 mice, the tumor volumes were recorded every five days; and
table 19 lists the mean tumor volumes for the treated mice and the five
control (untreated)
mice. Three untreated control mice died on days 31, 32, and 34; and these
deceased mice
CA 02197139 2000-03-30
- 35 -
were excluded from measurements made after their day of death.
Table 19: Mean tumor volumes in mm' for the treated group 1 mice and the five
control (untreated) mice; "R" stands for "complete regression" and
indicates that tumor volume was not measurable.
Day L_, Mean Tumor
Volume (mm')
Treated Control
17 5.1 54.9
22 9.8 122.1
27 5.6 253.3
32 5.88 1043.0
34 Not measured 1888.0
37 11.7 1499.6
42 44.8 1978.1
Eight out of ten treated group 1 mice showed complete regression of the
tumor by day 42. When complete regression occurred, measurements of tumor
weight and
tumor volume were not possible. Therefore, in Table 19 and in this paragraph,
the mean
tumor volume and the mean tumor weight for the treated group 1 mice on day 42
exclude
the eight mice showing complete regression. As shown in Table 19, the mean
tumor
volume in group 1 mice treated with MuJ-7 increased from approximately 9.8 mm3
on day
22 to approximately 44.8 mm' on day 42, while the mean tumor volume in the
control
(untreated) mice increased from approximately 122.1 mm3 on day 22 to
approximately
1978.1 mm' on day 42. The mean tumor weight on day 42 was approximately S 1 mg
in
the two treated group 1 mice that did not show complete regression of the
tumor, while the
mean tumor weight on day 42 was approximately 1196 mg in untreated control
mice.
Eight out of ten treated group 2 mice showed complete regression of the
tumor by day 34 (i.e., 33 days post implantation with cancer cells). When
complete
regression occurred, measurements of tumor weight and tumor volume were not
possible.
Therefore, in this paragraph, the mean tumor volume and the mean tumor weight
for the
treated group 2 mice on day 34 exclude the eight mice showing complete
regression. One
CA 02197139 2000-03-30
-36-
control mouse died on day 31, one died on day 32, and one died on day 34.
Therefore, in
this paragraph, the mean tumor volume and the mean tumor weight for the
control mice on
day 34 exclude the two mice that died before day 34. In the group 2 mice. MuJ-
7 caused a
reduction in the mean tumor volume in the treated mice from approximately 105
mm3 on
day 22 to approximately 3.1 mm3 on day 34, while the mean tumor volume in the
untreated control mice increased from approximately 122.1 mm3 on day 22 to
approximately 1888 mm3 on day 34. The mean tumor weight at the end of the
experiment
on day 34 was approximately 23 mg in the two treated group 2 mice that did not
show
complete regression of the tumor, while the mean tumor weight on day 34 was
approximately 746 mg in the untreated control mice.
Figure 3 is a graph of the mean tumor volume (in mm3) of the group 1
treated mice ("o"), the group 2 treated mice ("D"), and the untreated control
mice (" +")
versus the day numbers. In Figure 3, the arrow under day number 12 indicates
when the
group 1 mice were first treated with MuJ-7; and the arrow under day number 20
indicates
when the group 2 mice were first treated with MuJ-7.
Example 8:
The efficacy of MuJ-7 in the treatment of BALB/c nude nu/nu mice
bearing primary colon tumors of human origin was tested using three human
colon cancer
cell lines (namely, HT 29, SW 620 and CoLo 205). In addition, the efficacy of
MuJ-7 was
also demonstrated in the treatment of BALB/c nude nu/nu mice injected with
cells from
human lung cancer line L 132. Briefly, for each of the above-mentioned human
cancer
cell lines (HT 29, SW 620, CoLo 205, and L 132), two nude mice, one of which
served as
a control, were subcutaneously injected on day 1 with approximately 12 million
cancer
cells; and treatment of the non-control mouse with MuJ-7 started 3-11 days
after the
injection. Once the treatment had started, each treated mouse was injected
with MuJ-7 for
14 consecutive days, the daily dose of MuJ-7 being administered in
approximately three
equal subdoses. The daily dose of MuJ-7 for each treated mouse comprised
approximately 1.143 ~cg of VIP,, approximately 1.143 ~cg of VIP2,
approximately
1.143 ~g of VIP3, approximately 1.143 ~g of SOM,, approximately 1.143 ~g of
SOM2,
approximately 1.143 ~cg of BOM,, and approximately 1.143 ~g of SP,. For the
treated
mouse with HT 29 cancer cells, treatment started on day 12 (i.e., 11 days post
injection
CA 02197139 2000-03-30
-37 -
with the cancer cells); for the treated mouse with SW 620 cancer cells,
treatment started
on day 8 (i.e., seven days post injection with the cancer cells); for the
treated mouse with
CoLo 205 cancer cells, the treatment started on day 4 (i.e., three days post
injection with
the cancer cells); and for the treated mouse with L 132 cancer cells, the
treatment started
on day 5 (i.e., four days post injection with the cancer cells). The total
daily dose of
MuJ-7 was divided into approximately three equal subdoses. Three times a day
at
approximately eight-hour intervals, a subdose was injected into each treated
mouse. The
first subdose of the day was injected into the tail vein; the second subdose
of the day was
injected into one gluteal muscle; and the third subdose of the day was
injected into the
other gluteal muscle. Controls were randomly selected BALB/c nude nu/nu mice,
whose
weights were similar to the weights of the treated mice. The control mice were
subcutaneously injected with the same type and approximately the same number
of cancer
cells as the treated mice. The control mice were not treated with MuJ-7.
For the experiments involving SW 620 cells, the tumor volume in the
untreated control mouse increased from approximately 67.5 mm3 on day 6 to
approximately 508.9 mm3 on day 26 (i.e., 25 days post injection with the
cancer cells on
day 1 ), at which time the mouse died because of the tumor, while the tumor
volume in the
mouse treated with MuJ-7 decreased from approximately 47.1 mm3 on day 6 to
complete
tumor regression on day 16 (after 9 days of treatment). Table 20 lists the
tumor volume
measurements in mm3 for the untreated control mouse and the treated mouse from
day 6
until each mouse died. Figure 4 is a graph of the tumor volume (in mm3) of the
treated
mouse ("o") and the untreated control mouse (" +") versus the day numbers,
with the arrow
indicating that the treated mouse received its first dose of MuJ-7 on day 8.
Compared
with the untreated mouse, the treated mouse, which died on day 87 (i.e., 86
days post
injection with SW 620 cells on day 1), had an overall increase in survival
time of
approximately 244%. The percentage increase in survival time is calculated
herein
according to the following formula:
Percentage increase in survival time = [ (NT - N~) /N~] x 100
where NT is the day number of the day of death (or the day number
of the last known day of survival) minus the day number of
the day of injection with cancer cells for the treated mouse
CA 02197139 2000-03-30
-38-
and Nc is the day number of the day of death minus the day
number of the day of injection with cancer cells for the
untreated control mouse
Ta 1e 20: Tumor volume in mm' for the untreated control mouse and the treated
mouse, both of which were injected with S W 620 cells on day 1; "R"
stands for "regression" and indicates that the tumor volume was not
measurable.
Tumor Volume (mm')
on
Day No.
Contro 1 Treated
6 67.5 47.1
10 112.9 39.8
16 202.4 R
18 314.8 R
26 508.9 (Died) R
29 - R
43 - R
87 - R (Died)
For the experiments involving HT 29 cells, the tumor volume in the
untreated mouse increased from approximately 95.1 mm' on day 10 to
approximately
4536.3 mm3 on day 52 (i.e., 51 days post injection with the cells on day 1 ),
at which time
the mouse died because of the tumor. By contrast, the tumor volume in the
mouse treated
with MuJ-7 increased relatively gradually from approximately 159.1 mm' on day
10 to
approximately 2192.7 mm3 on day 52, after which the tumor volume decreased to
approximately 1556.4 mm3 on day 86 (i.e., 85 days post injection with the
cancer cells).
Table 21 lists the tumor volume measurements in mm3 for the untreated control
mouse
and the treated mouse from day 10 until each mouse died. Figure 5 is a graph
of the tumor
volume (in mm3) of the treated mouse ("o") and the untreated control mouse ("
+") versus
the day numbers, with the arrow indicating that the treated mouse received its
first dose of
MuJ-7 on day 12. Compared with the untreated mouse, the treated mouse, which
died on
CA 02197139 2000-03-30
-39-
day 104 (i.e., 103 days post the injection with HT 29 cells on day 1 ), had an
overall
increase in survival time of approximately 102%.
Table 21: Tumor volume in mm3 for the untreated control mouse and the treated
mouse, both of which were injected with HT 29 cells on day 1.
Day No. Tumor Volume (mm3)
on
Control Treated
10 95.1 159.1
14 486.8 282.0
19 500.2 395.5
22 1161.8 655.1
34 2576.9 511.4
48 4974.8 2129.3
52 4536.3 (Died) 2192.7
86 - 1556.4
104 - Died
For the experiments involving CoLo 205 cells, the tumor volume in the
untreated mouse increased from approximately 74.9 mm' on day 3 to
approximately
7344.9 mm3 on day 22 (i.e., 21 days post injection with the cancer cells on
day 1 ), at
which time the mouse died because of the tumor, while the tumor volume in the
mouse
treated with MuJ-7 decreased from approximately 78.2 mm3 on day 3 to complete
tumor
regression on day 8 (after 4 days of treatment). Table 22 lists the tumor
volume
measurements in mm' for the untreated control mouse and the treated mouse from
day 3
until each mouse died. Figure 6 is a graph of the tumor volume (in mm3) of the
treated
mouse ("o") and the untreated control mouse (" +") versus the day numbers,
with the arrow
indicating that the treated mouse received its first dose of MuJ-7 on day 4.
Compared
with the untreated mouse, the treated mouse, which died on day 79 (i.e., 78
days post
injection with CoLo 205 cells), had an overall increase in survival time of
approximately
271 %.
CA 02197139 2000-03-30
-40-
Table 22: Tumor volume in mm3 for the untreated control mouse and the treated
mouse, both of which were injected with CoLo 205 cells on day 1; "R"
stands for "regression" and indicates that the tumor volume was not
measurable.
Day No. Tumor Volume (mm3)
on
"'
Control Treated
3 74.9 78.2
5 497.6 29.8
8 668.9 R
11 1127.8 R
22 7344.9 (Died) R
79 R (Died)
For the experiments involving L 132 cells, the tumor volume in the
untreated mouse increased from approximately 28.5 mm3 on day 2 to
approximately
7174.3 mm3 on day 34 (i.e., 33 days post injection with the cancer cells on
day 1), at
which time the mouse died because of the tumor, while the tumor volume in the
mouse
treated with MuJ-7 decreased from approximately 38.3 mm' on day 2 to complete
regression on day 27 (after 22 days of treatment). Table 23 lists the tumor
volume
measurements in mm3 for the untreated control mouse and the treated mouse from
day 2
until each mouse died. Figure 7 is a graph of the tumor volume (in mm3) of the
treated
mouse ("o") and the untreated control mouse (" +") versus the day numbers,
with the arrow
indicating that the treated mouse received its first dose of MuJ-7 on day 5.
Compared
with the untreated mouse, the treated mouse, which died on day 70 (i.e., 69
days post
injection with the L 132 cells), had an overall increase in survival time at
approximately
3 5 109%.
CA 02197139 2000-03-30
-41 -
Table 23: Tumor volume in mm' for the untreated control mouse and the treated
mouse, both of which were injected with L 132 cells on day l; "R" stands
for "regression" and indicates that the tumor volume was not measurable.
Day No. Tumor Volume Measurements
(mm3) : L 132
Control Treated
2 28.5 38.3
4 38.5 9.1
9 147.2 5.9
12 1264.4 0.4
27 - R
34 7174.3 (Died) R
37 - R
70 - R (Died)
Example 9:
Two BALB/c nude nu/nu mice were each implanted with approximately
10 million human colon adenocarcinoma cells on day 1. On day 22 (i.e., 21 days
post
implantation on day 1 ), one mouse began to receive intraperitoneally a daily
dose of a
combination of VIP,, VIP2, VIP3, SOM,, and SOMZ. Each day for fourteen days,
this
treated mouse received approximately 8 ~g of the combination, the combination
comprising approximately 1.6 ,ug of VIP,, approximately 1.6 ,ug of VIP2,
approximately
1.6 ,ug of VIP3, approximately 1.6 ~cg of SOM,, and approximately 1.6 ~cg of
SOM2. The
' treated mouse received the last dose of the combination on day 35 (i.e., 34
days post
implantation on day 1). At the end of the experiment on day 35, the tumor
volume in the
treated mouse was approximately 720 mm3. The other mouse was untreated and
served as
a control. On day 35, the tumor volume in the untreated control mouse was
approximately
3 S 84 mm3.
CA 02197139 2000-03-30
-42-
Example 10:
Three BALB/c nude nu/nu mice were each implanted with approximately
10 million human colon adenocarcinoma cells on day I . On day 2 (i.e., one day
post
implantation on day 1 ), two mice each began to receive intraperitoneally a
daily dose of a
combination of VIP,, VIPz, VIP3, SOM,, and SOMZ. Once per day for fourteen
days, each
treated mouse received approximately 8 ug of the combination, the combination
comprising approximately 1.6 ~g of VIP,, approximately 1.6 ~g of VIP2,
approximately
1.6 ~cg of VIP3, approximately 1.6 ~cg of SOM,, and approximately 1.6 ~g of
SOM2.
(The daily dose was not divided into subdoses.) The treated mice received the
last dose of
the combination on day 15 (i.e., 14 days post implantation on day 1). At the
end of the
experiment on day 15, the tumor volume in one treated mouse was approximately
80 mm3;
and the tumor weight was approximately 0.149 g. On day 15, the tumor volume in
the
other treated mouse was approximately 0.8 mm3; and the tumor weight was
approximately
0.008 g. The third mouse was untreated and served as a control. On day 15, the
tumor
volume in the untreated control mouse was approximately 384 mm3; and the tumor
weight
in the untreated control mouse was approximately 0.406 g.
Exam 1p a 11:
Two BALB/c nude nu/nu mice were each implanted with approximately 10
million human colon adenocarcinoma cells on day 1. On day 2 (i.e., one day
post
implantation on day 1), one mouse began to receive a daily dose of a
combination of VIP,,
VIPz, VIP3, SOM,, and SOM2. Each day for fourteen days, this treated mouse
received
approximately 8 ~g of the combination, the combination comprising
approximately 1.6 ~cg
of VIP,, approximately 1.6 ~g of VIPz, approximately 1.6 ~cg of VIP3,
approximately
1.6 ~g of SOM,, and approximately 1.6 ~g of SOMz. The daily dose of the
combination
was divided into approximately three equal subdoses. Three times a day at
approximately
eight-hour intervals, a subdose was given to each treated mouse. The first
subdose each
day was given intravenously, and the second and third subdoses each day were
given via
intramuscular injections. The treated mouse received the last dose of the
combination on
day 15 (i.e., 14 days post implantation on day 1 ). At the end of the
experiment on day 15,
the tumor volume in the treated mouse was approximately 0.8 mm'; and the tumor
weight
was approximately 0.009 g. The other mouse was untreated and served as a
control. On
CA 02197139 2000-03-30
- 43 -
I 0 day 1 S, the tumor volume in the untreated control mouse was approximately
1728 mm';
and the tumor weight in the untreated control mouse was approximately 2.18 g.
Example 12:
Three randomly selected BALB/c nude nuinu mice were each implanted
with approximately 16 million primary tumor cells of human colon
adenocarcinoma on
day 1. On day 21 (i.e., twenty days post implantation on day 1 ), on day 22,
and on day 23,
two of these mice were treated with a daily dose of MuJ-7. For each of these
three days,
the total daily dose of MuJ-7 for each treated mouse comprised approximately
1.143 ,ug of
VIP,, approximately 1.143 ug of VIP2, approximately 1.143 ,ug of VIP3,
approximately
1.143 ,ug of SOM,, approximately 1.143 ,ug of SOM2, approximately 1.143 ug of
BOM,,
20 and approximately 1.143 ~cg of SP,; and the total weight of the seven
peptide analogs was
approximately 8 ~cg. On days 24 through 34, the total daily dose of MuJ-7 for
each treated
mouse comprised approximately 2.286 ,ug of VIP,, approximately 2.286 ,ug of
VIPZ,
approximately 2.286 ~g of VIP3, approximately 2.286 ~g of SOM,, approximately
2.286 ,ug of SOMz, approximately 2.286 ,ug of BOM,, and approximately 2.286
,ug of SP,;
and the total weight of the seven peptide analogs was approximately 16 ,ug.
Each day, the
total daily dose MuJ-7 was divided into approximately three equal subdoses.
Three times
a day at approximately eight-hour intervals, a subdose was given to each
treated mouse.
The first subdose each day was injected into the tail vein; the second subdose
each day
was injected into one gluteal muscle; and the third subdose each day was
injected into the
30 other gluteal muscle. The untreated third mouse, whose weight was similar
to the weights
of the two treated mice, served as a control and did not receive MuJ-7. Table
24 lists the
tumor volume measurements in mm' through day 55 for the first treated mouse
and until
the second treated mouse and the control mouse died. Compared with the
untreated
control mouse, the first treated mouse, which survived at least until day 55
(i.e., 54 days
post implantation with tumor cells on day 1 ), had an overall increase in
survival of time of
at least approximately 108%. Compared with the untreated control mouse, the
second
treated mouse, which died on day 38 (i.e., 37 days post implantation with
tumor cells on
day 1 ), has an overall increase in survival time of at least approximately
42%.
CA 02197139 2000-03-30
- 44 -
b
a~
...
c~.
~_
c~
3
a
0
0
b
o ~r ~o ~j
.r
a o~ oo ~n
M ~ M
O
O
~
N
O
4,
C
O
U
'b
cti
~
N N
O ~ N
C O
~
U O ~ N ~ M O ~t
~ N O ~ O M ~
p ~ d' G~ .w p I
C
U ~ ~
d' ~ ~ ~ n
U
z
o H
0
~'
0
H
U
w U
.,
3
b b
w O O ~ ~
N I~ c: O - I~ I~
O
O
.b M ~3'~ Ov O ~ N
N N ~ N N - w0
O O
z z
0
w
o Q,
~
E~ 3
0
N M M
N
N N N M d'
U
~D
c~
H
O O
~' N
CA 02197139 2000-03-30
- 45 -
Example 13:
On day 1, twenty-two BALB/c nude nu/nu mice were each implanted with
approximately 10 million primary tumor cells of human colon adenocarcinoma. On
day
15, twelve of these mice began to receive a daily dose of MuJ-7 for fourteen
consecutive
days. The daily dose of MuJ-7 for each treated mouse comprised approximately
1.143 ~cg
of VIP,, approximately 1.143 ~g of VIPZ, approximately 1.143 ~cg of VIP3,
approximately
1.143 ~g of SOM,, approximately 1.143 ~g of SOMz, approximately 1.143 ~cg of
BOM,,
and approximately 1.143 ,ug of SP,. Thus, the total daily dose of MuJ-7 always
contained
approximately equal weights of each of the seven peptide analogs (VIP,, VIP2,
VIP3,
SOM,, SOMz, BOM,, and SP,); and the total weight of these seven peptide
analogs was
approximately 8 ,ug. The total daily dose of MuJ-7 was divided into
approximately three
equal subdoses. Three times a day at approximately eight-hour intervals, a
subdose was
injected into each treated mouse. The first subdose of the day was injected
into the tail
vein; the second subdose of the day was injected into one gluteal muscle; and
the third
subdose of the day was injected into the other gluteal muscle. The ten
untreated mice
served as controls. The controls were ten randomly selected BALB/c nude nu/nu
mice
whose weights were similar to the weights of the twelve treated mice. The
control mice
were injected with the same type and approximately the same number of human
colon
adenocarcinoma tumor cells as the treated mice. The control mice did not
receive MuJ-7.
The tumor volume in each treated and untreated mouse was recorded
generally every five days. Table 25 lists the tumor volume in mm3 for each of
the twelve
treated mice; and Table 26 lists the tumor volume in mm3 for each of the ten
untreated
control mice.
CA 02197139 2000-03-30
- 46 -
~.
0
0
>, -o
' ~ ' . ' ' ' ' ' '
a~
U
:.d ~ 'b ~O
,O ~ ' ' N ' ' ' ' ~ '
~
.-.
O ', ~ 'ct
0 y.~ " ~ " "
.
, ~~ pp " ~ '
N
Gp
N
'"' ~ ~ b 'y p ' ~d 'b
~r N ~ ~ ~ N ~, ~ '
A
O
O
U
r., ~ ~ N ~ ~ 00 0~o O NO o0 t"~~
N
~O ~ ~ d~ 00 N ~t ~O ' ~ ~ O ,-. '
M
H
4~
~O ~ O ~ N N ~ o ~ y 0
~0
c ~ N N M _ fx M O t o
A ~ o o
~
N N N N
N
p ~ ', 00 ~O N '~t v0 v0
0o O o0 0o a\
O A ~ 0
N
'O
N M ~ M ~'' N ~ ~ '-'d
-
O
~.
O
d d' N ~ N ~ N f~ ~ 0 O
c ~ ~t o 0 ~
N 0 0
N M ~ ~ ~!1~ M M N
N.
w
N N ~ ~ ~ N ~ ~ M
cd
~ N d
'
A _ N ~ ~ N ~ ~O ~ M N
N
O ~
U O
O
C d' f~ O O w n
~
p 3 ~O ~ _ ",~~N ~ _ OOON ~ N ~ f~
O M
> ~ N N N N .--~'-'r..
O
O
H > '~ M ~ ~ ~ ~ 'o
'n
A tn M N M M
N ~ N M ~t ~ ~O I~ 00 01 ~
_N
H
O O
~' N
CA 02197139 2000-03-30
- 47 -
M Cd c~ f~ cCi ~ V~
N ~CJ~O ~n ~. M .d
?, N ~ ~ ~ ~' N_ ~ O ~ c~
t~ ~ A ~ ~n ~ o ~1
O
O N d' 01 ~ ~ ~ O
aS ,..,.,
~
O
O
O
U
4. o
cOn O N -~ ~ I~ N t~ Ov I~ d W0 l~
O ~t ~_ O I~ ~ N ~ O
I~ M M \O '~ N M OM \p
O
U
"
C.
O
U
~N ,7 ~ ~ O~ O ('~~ O~ 00 - WO 00
N N N V~ 0~lN due'N
M N N M M --~N -~ M N
O
H
U
c~
N
O
w ~ pip~ N ~ "~_, ~O ~D ~ M
00 ~O O~ ~ M M
O_
.
U
N
O N N 00 ~ ~!1
~ ' ~ '1
O
H
N
N M ~h ~ \D t~ 00 Q1
cd
H
O O
~' N
CA 02197139 2000-03-30
-48-
i 0 Figure 8 is a graph of the mean tumor volume (in mm3) of the treated mice
("o") and the untreated control mice (" +") versus the day numbers. Figure 9
is a graph of
the percentage of surviving treated mice (dotted line) and the percentage of
untreated
control mice (solid line) versus the day numbers. In Figures 8 and 9, the
arrow illustrates
that the treated mice received their first dose of MuJ-7 on day 15.
Eight of the ten control mice died from day 25 through day 35. By
contrast, five of the twelve treated mice showed complete regression of the
tumor; another
five of the twelve treated mice showed partial regression of the tumor. Thus,
ten of the
twelve treated mice showed complete or partial regression of the tumor, while
the two
remaining treated mice (mouse number 11 and mouse number 13) had different
outcomes.
20 Treated mouse number 11 responded to therapy between day 1 S and day 30,
when it was
treated with MuJ-7. After the treatment with MuJ-7 ended, the tumor in mouse
11 started
to grow; and mouse 11 eventually died on day 50. Nevertheless, the survival
time of
treated mouse 11 still exceeded the average survival time of the untreated
control mice.
Treated mouse 3 also showed a decrease in the rate of tumor growth compared to
the
average rate of tumor growth in the untreated control mice. Although treated
mouse 3
eventually died on day 50, the survival time of treated mouse 3 still exceeded
the average
survival time of the untreated control mice.
Of the ten untreated control mice, two died on day 25, two died on day 30,
four died on day 35, and two died after, day 35. The five treated mice with
complete
30 tumor regression were monitored through day S5, and no tumor recurrence was
observed.
Of the remaining seven treated mice, four died on day 40, one died on day 50,
two died on
day 55; and, thus, the average survival time of these seven treated mice
exceeded the
average survival time of the ten untreated control mice.
Description of Protocols:
Indirect Immunofluorescence: About 100 ~cl of healthy adherent tumor cell
suspension
with a density of approximately 104 cells/ml from a 3-4 day old culture were
plated on a
round, sterile cover slip in a 24-day culture plate, and incubated at
37°C. in a COz
incubator. After 24 hours, when the tumor cells started to adhere to the
culture surface,
the wells were flooded with growth medium and incubated again at 37°C.
in a COz
40 incubator. After approximately 4-5 days, the cover slips with adhering
tumor cells were
CA 02197139 2000-03-30
-49-
washed thoroughly in RPMI 1640 containing approximately S% fetal calf serum
(hereinafter referred to as "FCS") followed by washing in approximately 0.05 M
phosphate buffered saline (hereinafter referred to as "PBS"), which contained
approximately 5% FCS and which had an approximate pH of 7.4, followed by
washing in
plain PBS. The tumor cells on the cover slips were then incubated at
approximately 37°C.
for approximately one hour with a 1':50 dilution of the antipeptide polyclonal
antibody.
The cover slips were washed again as described above and the tumor cells
incubated under
the same conditions with a 1:200 dilution of anti-rabbit IgG-FITC conjugate.
After
washing, the cover slips were mounted in a medium made of carbonate-
bicarbonate buffer
and glycerol in a 1:1 ratio containing a crystal of para phenyl-diamine, and
sealed in an
inverted position on a glass slide with a clear varnish solution. The tumor
cells were
scanned under UV light on a MicrophotTM FX microscope (Nikon).
Sandwich ELISA: Wells of a round-bottomed microtitre highly activated
(Maxisorp)
plate (Nunc, catalogue number 449824) were coated with 1 ~cg of the purified
antibody in
100 u1 of approximately 0.05 M phosphate buffered saline, which contained
approximately 0.05% Tween 20 (PBS-T) and which had an approximate pH of 7.4,
and
were incubated for approximately one hour at approximately 37°C. After
incubation, the
wells were washed two times with PBS-0.2% TweenT"' in an automatic plate
washer (BDSL,
UK). To each well 100 ~1 of AmiconTM concentrated culture supernatant of
primary
tumor cultures were added, followed by incubation for approximately one hour
at
approximately 37°C. The wells were washed three times as described
previously. For
color development, 25 ~1 of substrate (1 mg/ml orthophenyldiamine + 1 ~cl
HzOz) in citrate
phosphate buffer (approximate pH of 5.~) was added to each well and incubated
in the
dark for approximately five minutes at approximately 37°C. The color
development was
terminated with the addition of 10 ~cl of SN HzSO,. The absorbance in each
well was
determined at 490 nm on a multiscan microplate spectrophotometer (Biotech,
USA).
Reverse Phase High Performance Liquid ChromatoEranhv: The supernatant of tumor
cell
cultures was run on a Waters C-18, 5 micron (46 mm X 15 cm) column. The
solvent
system comprised two solvents that were run as a gradient. Solvent A consisted
of
approximately 0.1% trifluoroacetic acid, and solvent 13 consisted of
approximately 80%
acetonitrile in solvent A. A flow rate of 1.0 ml/minute was maintained and a
gradient of
CA 02197139 2000-03-30
-$0-
approximately 40% to 100% solvent B in approximately 4$ minutes was set up. A
UV
detector at a wavelength of 230 nm was used to detect the peptide.
Receptor-Ligand AssaX: Tumor cells were grown to confluence in a 7$ cm' flask,
and the
culture medium decanted. The cells were scraped off with the help of a rubber
policeman
and suspended in a minimum volume of binding buffer comprising approximately
$%
bovine serum albumin (hereinafter referred to as "BSA") in RPMI 1640 to
achieve a
concentration of approximately 0.$ x 106 cells/$0 ~1. Increasing counts of
I-12$ peptides were added to the cells in the assay tube, and the volume was
made up to
approximately 200 ~cl with binding buffer. Radioactive counts were measured in
each
tube on a gamma counter. All the tubes were then incubated at approximately
37°C. for
approximately one hour. In order to terminate the reaction, 2 ml of ice cold
binding buffer
were added to each tube and mixed thoroughly by vortexing. The tubes were
centrifuged
at approximately 2500 rpm (revolutions per minute) for approximately 10
minutes at
approximately 4°C. The supernatant was discarded, and the tubes dried
with blotting
paper. Each tube was then counted on a gamma counter. The counts added to each
tube
were plotted against counts bound to plot the saturation curve.
The optimum cell number and tracer counts per tube were determined from
the standard curve. This corresponded to the conditions at which there was no
further
increase in the number of bound counts on addition of tracer to a fixed cell
concentration.
Cold competition experiments were performed at these saturation conditions. A
fixed cell
concentration and tracer counts, as optimized earlier, were added to the assay
tubes. This
was followed by the addition of increasing concentrations of cold VIP,
somatostatin,
bombesin, and Substance P in duplicates to the tubes, and making up the volume
to 200 ,u1
with binding buffer. The tubes were then processed in a manner identical to
the process
described for preparation of the standard curve.
To calculate Kp (M) in moles and R (M/L) in moles per liter, the average
counts of duplicate tubes were fed into LIGAND Software along with additional
data such
as molecular weight of labelled and unlabelled peptides, specific activity,
dose units, the
volume of the tubes, and the counting time. (LIGAND Software (version 3.0) is
a
radioligand binding analysis program, copyrighted by G. A. McPherson in 1986
and
published and distributed by Elsevier, BIOSOFT, 68 Hills Road, Cambridge,
United
CA 02197139 2000-03-30
-51 -
Kingdom.) The LIGAND Software performed a Scatchart Analysis by plotting on
the Y
axis the number of bound counts divided by the total number of counts added to
each tube
and plotting on the X axis the logarithm of the total number of counts added
to each tube.
The LIGAND Software used the intercept of the slope on the plot to calculate
Kp (M) and
R (M/L,).
extraction of Genomic DNA: The primary tumor cells were grown to confluence in
vitro
and the monolayer was washed twice with ice-cold Tris buffered saline
(hereinafter
referred to as "TBS"). Using a policeman, the cells were scraped into
approximately 0.5
ml of TBS. The cell suspension was transferred to a centrifuge tube and stored
on ice.
The flask was washed with an additional 1 ml (approximately) of TBS, and
washing was
combined with cell suspension in the centrifuge tube. The cells were recovered
by
centrifugation at approximately 1500 x g for approximately 10 min at
approximately 4°C.
The cells were resuspended in approximately 5-10 volumes of ice-cold TBS and
centrifugation repeated. Finally, the cells were suspended in Tris edetate
(hereinafter
referred to as "TE," the TE having an approximate pH of 8.0) at a
concentration of
approximately 5 x 10' cells/ml. Ten milliliters of extraction buffer were
added to 1 ml of
cell suspension, and the solution was incubated for approximately 1 hour at
approximately
37°C. Proteinase K to a final concentration of 100 ~g/ml was added to
this solution, and
the solution was incubated in a gently shaking water bath for approximately 12
hours at
approximately 50°C. The solution was then cooled to room temperature
and transferred
to a centrifuge tube. An equal volume of phenol-chloroform equilibrated with
0.5 M
Tris.C 1 (approximate pH of 8.0) was added, and the two phases were gently
mixed for
approximately 10 minutes. The phases were separated by centrifugation at
approximately
5000 x g for approximately 15 minutes at room temperature. The viscous aqueous
phase
was transferred to a centrifuge tube, and extraction was repeated twice with
phenol-
chloroform. Then, to the aqueous phase were added 1/2 volume of 7.5 M ammonium
acetate and two volumes of ice-cold 100% ethanol. A string of DNA formed which
was
rinsed with 70% ethanol. This was decanted and dried on a SpeedvacTM (Savant).
The
pellet was resuspended in TE.
The invention illustratively disclosed herein suitably may be practiced in
the absence of any element which is not specifically disclosed herein.
CA 02197139 2002-02-20
-52-
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT:
Rama
Mukherjee
Manu Jaggi
(ii) TITLE
OF
INVENTION:
A
Drug
for
the
Treatment
of Cancer
(iii)NUMBER
OF SEQUENCES:
(iv) CORRESPONDENCE
ADDRESS;
(A) ADDRESSEE: Marks & Clerk
(B) STREET: 55 Metcalfe Street, Suite 1380
(C) CITY: Ottawa
(D) STATE: Ontario
(E) COUNTRY: CANADA
(F) ZIP: K1P 6L5
(v) COMPUTER
READABLE
FORM:
(A) MEDIUM TYPE: Diskette, 3.5 inch,
1.44 MB storage
(B) COMPUTER: IBM PC/XT/AT or compatibles
(C) OPERATING SYSTEM: WINDOWS
(D) SOFTWARE: Word Perfect 6.1
(vi) CURRENT
APPLICATION
DATA:
(A) APPLICATION NUMBER: 2,197,139
(B) FILING DATE: February 10, 1997
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Marks Clerk, Richard J. Mitchell
(C) REFERENCE/NUMBER: 93231-1
CA 02197139 2002-02-20
-53-
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (613) 236-9561
(B) TELEFAX: (613) 230-8821
(2) INFORMATION FOR SEQ ID NO:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 28 Amino Acids
(B) TYPE: Amino Acid
(C) TOPOLOGY: Unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:1:
Lys Pro Arg Arg Pro Tyr Thr Asp Asn Tyr Thr Arg Leu Arg
10
Lys Gln Met Ala Val Lys Lys Tyr Leu Asn Ser Ile Leu Asn
20 25
(2) INFORMATION FOR SEQ ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino Acids
(B) TYPE: Amino Acid
(C) TOPOLOGY: Unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
Leu Met Tyr Pro Thr Tyr Leu Lys
5
(2) INFORMATION FOR SEQ ID N0:3:
(i) SEQUENCE CHARACTISTICS:
(A) LENGTH: 19 Amino Acids
(B) TYPE: Amino Acid
(C) TOPOLOGY: Unknown
(ii) MOLECULE TYPE: peptide
CA 02197139 2002-02-20
-54-
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:3:
Tyr Thr Arg Leu Arg Lys GIn Met AIa Val Lys Lys Tyr
10
Leu Asn Ser Ile Leu Asn
(2) INFORMATION FOR SEQ ID N0:4:
(i) SEQUENCE CHARACTISTICS:
(A) LENGTH: 23 Amino Acids
(B) TYPE: Amino Acid
(C) TOPOLOGY: Unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
Phe Thr Asp Asn Tyr Thr Arg Leu Arg Lys Gln Met Ala Val
5 10 15
Lys Lys Tyr Leu Asn Ser Ile Leu Asn
(2) INFORMATION FOR SEQ ID N0:5:
(ii) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 Amino Acids
(B) TYPE: Amino Acid
(C) TOPOLOGY: Unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:5:
Tyr Gly Cys Lys Asn Phe Phe Trp Lys Thr Phe Thr Ser Cys
5 10
(2) INFORMATION FOR SEQ ID N0:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19 Amino Acids
(B) TYPE: Amino Acid
(C) TOPOLOGY: Unknown
CA 02197139 2002-02-20
-55-
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:6:
Ala Gly Cys Lys Asn Phe Phe Trp Lys Thr Tyr Thr Ser Cys
10
(2) INFORMATION FOR SEQ ID NO: 7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(C) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:1
(D) OTHER INFORMATION:/products= "L-pyroglutamic
acid"/label= pGlu
(ix) FEATURE:
(A) NAME/KEY: Region
(B) LOCATION: (13~14)
(D) OTHER INFORMATION:/product= "OTHER"
/note= "surrogate bond followed by
(CH2NH) "
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 7:
Xaa Gln Arg Leu Gly Asn Gln Trp Ala Val Gly His Leu Leu
1 5 10
(2) INFORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(C) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
CA 02197139 2002-02-20
-56-
(ix) FEATURE
(A) NAME/KEY: Region
(B) LOCATION: 1
(D) OTHER INFORMATION:/product=" pyroglutamic
acid"/label=Pyr
(ix) FEATURE:
(A) NAME/KEY: Region
(B) LOCATION: (13~14)
(D) OTHER INFORMATION:/product= "OTHER"
/note= "reduced band"
Xaa Gln Arg Leu Gly Asn Gln Trp Ala Val Gly His Leu Leu
10
(2) INFORMATION FOR SEQ ID N0: 9:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 7 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 9:
Trp Ala Val Gly His Leu Met
5
(2) INFORMATION FOR SEQ ID NO: 10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
CA 02197139 2002-02-20
-57-
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product= "L-pyroglutamic
acid"/label== pGl.u
(xi) SEQUENCE: DESCRIPTION: SEQ ID N0: 10:
Xaa Gln Arg Tyr Gly Asn Gln Trp Ala Val Gly His Leu Met
5 10