Note: Descriptions are shown in the official language in which they were submitted.
2197178
Bicyclolactam Compounds, Use Of The Same And
Intermediate In The Production Of The Same
The present invention relates to novel bicyclolactam
compounds, use thereof, and an intermediate for preparing
the bicyclolactam compounds. The compounds have excellent
anxiolytic effect and are useful as anxiolytic agents.
With rapid diversification of the social environment
in recent years, an increasing number of people are
suffering from anxiety, and it has been expected to develop
psychosomatic therapies and excellent therapeutic agents.
Benzodiazepine compounds such as diazepam have found
wide use as anxiolytics. This group of agents, however,
generally have side effects such as hypnotic effect, muscle
relaxant effect and sedative effect. Serotonin anxiolytic
agents such as buspirone are also recently developed as
anxiolytics which are different from the benzodiazepine
compounds in activity mechanism. Reportedly these
serotonin agents are generally lesser than the
benzodiazepines in side effects such as hypnotic, muscle
relaxant and sedative effects, but they are lower in
anxiolytic effect and have the problems of diminishing
voluntary movements presumably owing to their activity as a
dopamine antagonist, and causing serotonin syndrome which
appears attributable to their properties as a full agonist
for serotonin lA receptor.
1
'~ 2~9~~7s
Compounds which are similar to the bicyclolactam
compounds of the present invention are disclosed in
International Publication No. WO 91/11434, and are known to
have a cerebral function improving effect, cerebral
metabolism activating or anoxic brain damage protecting
effect and effect against senile dementia. The present
compounds differ from those disclosed in WO 91/11434 in
that the former have a substituent directly attached to a
carbon atom on the bicyclo ring.
Further, when the compounds disclosed in International
Publication No. WO 91/11434 are orally administered, a lot
of metabolites are produced. This causes an administration
of non-effective substances and is unsuitable to develop
pharmaceuticals. The present compound produces less amount
of metabolites and is high in safety.
An object of the present invention is to provide novel
bicyclolactam compounds and an intermediate for production
of the bicyclolactam compounds. The bicyclolactam
compounds have excellent anxiolytic effect, high in safety,
and are useful as an effective component of medicinals
which are greatly diminished in side effects such as
hypnotic, muscle relaxant and sedative effects.
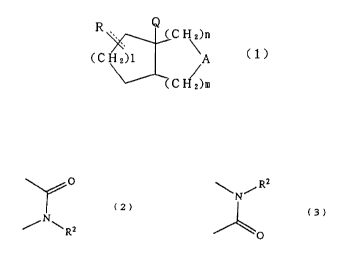
The present invention provides bicyclolactam compounds
represented by the following formula (1)
2
E
2197178
3
R. Q
( C H z)n
(CH2)1 \A (1)
C
( H z)m
wherein R is oxo or -OR', R' is a hydrogen atom or acyl group,
A is a group of (2) or (3), Q is a hydrogen atom or lower
alkyl group, 1 is 1 or 2, m is 0 or 1 and n is 0, i or 2,
provided the case where both of m and n represent 0
simultaneously is excluded
O
(2)
~N~R2
2
~N/R
(3)
O
wherein R= is benzoyl group or substituted benzoyl group.
The present invention also provides a bicyclolactam
compound represented by the following formula (4)
ORS Q
(CH~~
2 5 (CHZ)1 /A
(CHz)m
wherein A, Q, 1, m and n are as defined above, R' is benzyl
group or substituted benzyl group.
X,
2197~7s
4
The present invention further provides a process for
preparing a bicyclolactam compound represented by the
following formula (1'), comprising replacing Rg in the
bicyclolactam compound of the formula (4) by hydrogen atom in
a suitable solvent in the presence of a catalyst
HO
C H Z)n
(CHZ)1 \A (1')
C ~) m
wherein A, Q, 1, m and n are as defined above.
The present invention further provides a process for
preparing a bicyclolactam compound represented by the
following formula (1"), comprising acylating the
bicyclolactam compound of the formula (1') in a suitable
solvent
Q
C ~) n
C ~) m
wherein A, Q, 1, m and n are as defined above, R1' is acyl
group.
The present invention further provides a process for
preparing a bicyclolactam compound represented by the
following formula (i " '), comprising reacting a compound of
the formula (5) and a bicyclolactam compound of the formula
(6) in a suitable solvent in the presence of a base
219 717 8
S
R2 - X (5)
wherein R2 is as defined above, X is a halogen atom,.
O
(CHz~l ~ l C6)
N
H
wherein Q and 1 are as defined above,
(CH
RZ
wherein RZ, Q and 1 are as defined above.
The present invention further provides a
pharmaceutical composition comprising an effective amount of
the above bicyclolactam compound and a pharmaceutically
acceptable carrier.
The present invention further provides an anxiolytic
agent comprising an effective amount of the above
bicyclolactam compound and a pharmaceutically acceptable
carrier.
The present invention further includes a method of
treating anxiety comprising administering an effective amount
of the above bicyclolactam compound to mammals including man,
and also use of the above bicyclolactam compound for the
preparation of medicinals for treating anxiety.
The bicyclolactam compound of the formula (1) has an
219 7 ~ ~8
6
excellent anxiolytic effect, is high in safety, diminished in
side effects, and useful as medicinals. Further, the
bicyclolactam compound of the formula (4) is useful as an
intermediate for preparing the bicyclolactam compound of the
formula (1).
Existing as bicyclolactam derivatives of the formula
(1) or (4) are stereoisomers due to the presence of the
bicyclo ring, and also geometric isomers and optical isomers
due to the presence of the carbon atom at the bridgehead
position of the bicyclo ring and the carbon atom having R'0-
or R'0- attached thereto. The present invention includes all
of these isomers.
In view of the numbers 1, m and n, the following
fourteen (14) kinds of bicycZo ring skeletons can be present
in the compounds of the formula (1) or (4). The invention
includes all of these cases.
25
. I;y}
.~"
2197178
o (a) (d)
1N N~O
0
'N (~) ~ (d)
N
O () ~0
~..~N ~ N
/O
(g) N (h)
N' '0
O
('~ ~O G)
N
N
1 (k)
N
O O
(m) N
N
O
O
Preferable among these is the case wherein m or n is
. ,
~~r,
- 2197178
8
0, i.e. the skeleton (a) , (b) , (c) , (f) , (g) , (h) , (k) or (m) .
More preferable is the case wherein 1 is 1, m is 0, n is 2,
i . a . , (b) or (k) .
In the present invention, in the case where the
substituent R is oxo group, the bond between R and the carbon
on the bicyclo ring shows double bond.
In case of, for example, the above bicyclolactam
ring skeleton (a), the following three positions are shown
where the substituent R of the compound of the formula (1)
(or -OR' of the compound of the formula (4)] attaches to the
bicyclolactam ring. Although the invention includes all of
these cases, preferable is (p) or (r) below where R (or -OR')
attaches to the vicinal carbon atom of the brigehead atom of
the bicyclolactam ring. This is similar in the other
bicyclolactam ring skeletons (b,) to (n) .
R
N ~ R... 0
~N
CP) C4) R Cr)
According to the invention, examples of benzoyl
groups which may optionally have at least one substituent
represented by R= are benzoyl groups which may optionally
have, as a substituent, a halogen atom, lower alkyl group,
lower alkoxyl group, vitro ~qroup, cyano group, hydroxyl group
or amino group. Preferable are those which may optionally
have, as a substituent, a halogen atom, lower alkyl group or
lower alkoxyl group. More preferable is that which has, as a
2197178
9
substituent, at least one lower alkoxyl group. The number of
substituents is preferably 1 to 3. The substituent may be
present at any of the ortho-, meta- and para-positions on the
phenyl ring of the benzoyl group. Examples of benzyl groups
which m°ay optionally have at least one substituent represented
by R~ are benzyl groups which may optionally have on the
phenyl ring, as a substituent, 1 to 3 of lower alkyl group,
lower alkoxyl group, halogen atom or trifluoromethyl group.
Preferable is unsubstituted benzyl group. Examples of halogen
atoms are fluorine, chlorine, bromine and iodine atom, among
which fluorine atom is preferable. Examples of useful lower
alkyl groups are straight-chain or branched alkyl groups
having 1 to 6 ca~'bon atoms, such as methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, tert-butyl, pentyl, isopentyl
and hexyl group. Preferable among these is methyl or ethyl
group. Methyl group is more preferable. Examples of useful
lower alkoxyl groups are straight-chain or branched alkoxyl
groups having 1 to 6 carbon atoms, such as methoxy, ethoxy, n-
propoxy, isopropoxy, n-butoxy, isobutoxy, sec-butoxy, tert-
butoxy, pentyloxy, isopentyloxy and hexyloxy group, among
which methoxy or ethoxy group is preferable. Methoxy group is
more preferable.
Acyl groups represented by R' or R'' include widely
an aliphatic acyl group and aromatic acyl group. Examples of
aliphatic acyl groups are those having 2 to 6 carbon atoms,
such as formyl,-acetyl, propionyl, butyryl, isobutyryl,
pentanoyl, hexanoyl, acryloyl, propioloyl, methacryloyl and
crotonoyl. Examples of aromatic acyl groups are benzoyl, 3-
x
2197178
toluyl, 4-toluyl, 2-methoxybenzoyl, 2,4-dimethoxybenzoyl, a -
naphthylcarbonyl and ~ -naphthylcarbonyl. Preferable among
these is acetyl or benzoyl group. Acetyl group is more
preferable.
Lower alkyl group shown by Q includes the above
lower alkyl groups, among which methyl and ethyl groups are
preferable. Methyl group is more preferable.
Halogen atom shown by X includes the above halogen
atoms; among which chlorine atom is preferable.
10 Among the compounds of the formula (1) or (4),
preferable are those having a ring structure wherein m or n is
0 [provided m and n are not 0 (zero) simultaneously), and more
preferable are those wherein 1 is 1, m is 0 and n is 2. In
case of the compound (1) wherein R is -OR', preferable are
those wherein R' is a hydrogen atom or acetyl group, RZ is
benzoyl or benzoyl having lower alkoxyl group, halogen atom or
lower alkyl group, Q is a hydrogen atom, and wherein 1 is 1, m
is 0 and n is 2. Especially preferable are those wherein R' is
a hydrogen atom, RZ is a benzoyl having methoxy group, Q is a
hydrogen atom, and wherein 1 is 1, m is 0 and n is 2.
Among the compounds of the formula (1) wherein R is
oxo Qroup, preferable are those wherein R=,is a benzoyl or
benzoyl having lower alkoxyl group or lower alkyl group, ø is
a hydrogen atom or lower alkyl group, and wherein 1 is 1, m is
0 and n is 2. Especially preferable are those wherein R= is.
benzoyl having methoxy or methyl group, Q is a hydrogen atom
or methyl group, and wherein 1 is 1, m is 0 and n is 2.
Further, in case of the compound (4), preferable are
r:
11
2197~7s
those wherein R2 is benzoyl or benzoyl having lower alkoxyl
group, halogen atom or lower alkyl group, R' is benzyl group,
Q is a hydrogen atom, and wherein 1 is 1, m is 0 and n is 2.
Especially preferable are those wherein RZ is a benzoyl having
S methoxy group, Q is a hydrogen atom, and wherein 1 is 1, m is
0 and n is 2.
Examples of the compound of the above formula (1) or
(4) are 7-benzyloxy-2-(4-methoxybenzoyl)-2-azabicyclo-
[4.3.0]nonan-3-one, 7-benzyloxy-2-benzoyl-2-
azabicyclo[4.3.0]nonan-3-one, 7-benzyloxy-2-(4-fluorobenzoyl)-
2-azabicyclo[4.3.Ojnonan-3-one, 7-benzyloxy-2-(p-toluoyl)-2-
azabicyclo[4.3.0]nonan-3-one, 7-benzyloxy-2-(2,4-
dimethoxybenzoyl)-2-azabicyclo[4.3.Ojnonan-3-one,
7-benzyloxy-3.-(4-methoxybenzoyl)-3-azabicyclo[4.3.0]nonan-2-
one, 7-benzyloxy-3-benzoyl-3-azabicyclo[4.3.0]nonan-2-one, ,
7-benzyloxy-3-(4-fluorobenzoyl)-3-azabicyclo[4.3.0]nonan-2-
one, 7-benzyloxy-3-(p-toluoyl)-3-azabicyclo[4.3.Ojnonan-2-one,
7-benzyloxy-3-(2,4-dimethoxybenzoyl)-3-azabicyclo[4.3.Ojnonan-
2-one, 7-hydroxy-2-(4-methoxybenzoyl)-2-
azabicyclo[4.3.Ojnonan-3-one, 7-hydroxy-2-benzoyl-2-
azabicyclo[4.3.Ojnonan-3-one, 7-hydroxy-2-(4-fluorobenzoyl)-2-
azabicyclo[4.3.0jnonan-3-one, 7-hydroxy-2-(p-toluoylj-2-
azabicyclo[4.3.Ojnonan-3-one, 7-hydroxy-2-(2,4-
dimethoxybenzoyl)-2-azabicyclo[4.3.Ojnonan-3-one,
7-hydroxy-3-(4-methoxybenzoyl)-3-azabicyclo[4.3.Ojnonan-2-one,
7-hydroxy-3-benzoyl-3-azabicyclo[4.3.Ojnonan-2-one,
7-hydroxy-3-(4-fluorobenzoyl)-3-azabicyclo[4.3.Ojnonan-2-one,
7-hydroxy-3- (p-toluoyl) -3-azabicyclo [4 . 3. Oj nonan-2-one.,
2197178
12
7-hydroxy-3-(2,4-dimethoxybenzoyl)-3-azabicyclo[4.3.0]nonan-2-
one, 7-acetoxy-2-(4-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-
3-one, 7-acetoxy-2-benzoyl-2-azabicyclo[4.3.0]nonan-3-one,
7-acetoxy-2-(4-fluorobenzoyl)-2-azabicyclo[4.3.0]nonan-3-one,
7-acetoxy-2-(p-toluoyl)-2-azabicyclo[4.3.0]nonan-3-one,
7-acetoxy-2-(2,4-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3-
one, 7-acetoxy-3-(4-methoxybenzoyl)-3-azabicyclo[4.3.0]nonan-
2-one, 7-acetoxy-3-benzoyl-3-azabicyclo[4.3.0]nonan-2-one,
7-acetoxy-3-(4-fluorobenzoyl)-3-azabicyclo[4.3.0]nonan-2-one,
7-acetoxy-3-(p-toluoyl)-3-azabicyclo[4.3.0)nonan-2-one,
7-acetoxy-3-(2,4-dimethoxybenzoyl)-3-azabicyclo[4.3.0]nonan-2-
one, 6-benzyloxy-2-(4-methoxybenzoyl)-2-
azabicyclo[3.3.0]octan-3-one, 7-benzyloxy-3-benzoyl-3-
azabicyclo [3.3.0] octan-2-one, 8-benzyloxy-3- (4-fluorobenzoyl) -
3-azabicyclo[5.3.0]decan-2-one, 2-benzyloxy-7-(p-toluoyl)-7-
azabicyclo[4.3.0]nonan-8-one, 7-benzyloxy-2-[2,4-
dimethoxybenzoyl)-2-azabicyclo[4.4.0]decan-3-one,
2-benzyloxy-8-(4-methoxybenzoyl)-8-azabicyclo[4.3.0]nonan-7-
one, 7-benzyloxy-3-benzoyl-3-azabicyclo[4.4.0]decan-4-one,
8-benzyloxy-3-(4-fluorobenzoyl)-3-azabicyclo[5.4.0]undecan-4-
one, 9-benzyloxy-4-(p-toluoyl)-4-azabicyclo[5.4.0]undecan-3-
one, 3-(4-methoxybenzoyl)-3-azabicyclo[5.4.0]undecan-4,8-
dione, 2-(4-methoxybenzoyl)-2-azabicyclo[4.4.0]decan-3,7-
dione, 2-(4-methybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(3-ethylbenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(2-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(3-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(4-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
219 7 1 78
13
2-(2,4-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(2,6-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(3,4-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(3,5-dimethoxybenzoyl)-2-azabicyclo[4.3.0)nonan-3,7-dione,
2-(3,4,5-trimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-
dione, 6-methyl-2-(4-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-
3,7-dione and 6-ethyl-2-(4-methoxybenzoyl)-2-azabicyclo-
[4.3.0]nonan-3,7-dione.
Preferable examples are 7-benzyloxy-2-(4-
methoxybenzoyl)-2-azabicyclo[4.3.OJnonan-3-one,
7-benzyloxy-2-benzoyl-2-azabicyclo[4.3.0]nonan-3-one,
7-benzyloxy-2-(4-fluorobenzoyl)-2-azabicyclo[4.3.0]nonan-3-
one, 7-benvyloxy-2- (p-toluoyl) -2-azabicyclo [4.3~.Oj nonan-3-one,
7-benzyloxy-2-(2,4-dimethoxybenzoyl)-2-azabicyslo[4.3.0]nonan-
3-one, 7-benzyloxy-3-(4-methoxybenzoyl)-3-
azabicyclo[4.3.0]nonan-2-one, 7-benzyloxy-3-(4-fluorobenzoyl)-
3-azabicyclo[4.3.0]nonan-2-one, 7-benzyloxy-3-(p-toluoyl)-3-
azabicyclo[4.3.0]nonan-2-one, 7-benzyloxy-3-(2,4-
dimethoxybenzoyl)-3-azabicyclo[4.3.0]nonan-2-one,
7-hydroxy-2-(4-methoxybenzoyl)-2-azabicyclo[4.3.0)nonan-3-one,
7-hydroxy-2-benzoyl-2-azabicyclo[4.3.Ojnonan-3-one,
7-hydroxy-2-(4-fluorobenzoyl)-2-azabicyclo[4.3.Ojnonan-3-one,
7-hydroxy-2-(p-toluoyl)-2-azabicyclo[4.3.0]nonan-3-one, .
7-hydroxy-2-(2,4-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3-
one, 7-hydroxy-3-(4-methoxybenzoyl)-3-azabicyclo[4.3.0]nonan-
2-one, 7-hydroxy-3-(4-fluorobenzoyl)-3-azabicyclo[4.3.OJnonan-
2-one, 7-hydroxy-3-(p-toluoyl)-3-azabicyclo[4.3.Ojnonan-2-one,
7-acetoxy-2-(4-methoxybenzoyl)-Z-azabicyclo[4.3.0)nonan-3-one,
x
,.~.,
2197178
14
2-(4-methoxybenzoyl)-2-azabicyclo[4.4.Ojdecan-3,7-dione,
2-(4-methylbenzoyl)-2-azabicyclo[4.3.Ojnonan-3,7-dione,
2-(2-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(3-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(4-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(2,4-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(2,6-dimethoxybenzoyl)-2-azabicyclo[4.3.Ojnonan-3,7-dione,
2-(3,4-dimethoxybenzoyl)-2-azabicyclo(4.3.0]nonan-3,7-dione,
2-(3,5-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(3,4,5-trimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione
and 6-methyl-2-(4-methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-
3,7-dione.
More preferable examples are 7-hydroxy-2-(4-
methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3-one;
7-hydroxy-2-benzoyl-2-azablcyclo[4.3.0jnonan-3-one,
7-hydroxy-2-(4-fluorobenzoyl)-2-azabicyclo[4.3.0jnonan-3-one,
7-hydroxy-2-(p-toluoyl)-2-azabicyclo[4.3.0]nonan-3-one,
7-hydroxy-2-(2,4-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3-
one, 7-hydroxy-3-(4-methoxybenzoyl)-3-azabicyclo[4.3.0]nonan-
2-one, 7-hydroxy-3-(4-fluorobenzoyl)-3-azabicyclo[4.3.0]nonan-
2-one, 7-hydroxy-3-(p-toluoyl)-3-azabicyclo[4.3.Ojnonan-2-one,
2-(4-methylbenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2- (3-methoxybenzoyl) -2-azabicyclo [4.3.Oj nonan-3, 7-dione,
2-(2,4-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione,
2-(3,4-dimethoxybenzoyl)-2-azabicyclo[4..3.Ojnonan-3,7-dione,
2-(3,5-dimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-dione
and 2-(3,4,5-trimethoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3,7-
dione.
2197178
The bicyclolactam compound of the present invention
wherein R is -OR' can be prepared, for example, by the
following reaction process
Q (CH2)° \ Q (CNZ)n O
(CHZ)( ~0 ---~. (CH2)I
(CHZ)rn
(CH2)m ~0
Compound A Compound B
OCOPh-p-NOZ
O)-\ Q (CI-12)n O \ Q (CHZ)n O
~ a iii
(CHI b ---~- (CN2)1
(CH2)m 0 (CH2)m O
Compound traps-C Compound D
R'- X
Compound E
OH Q (CH~n O R3- x 0 \ Q {CH2)n O
iv ~ a v
-----~. (CH2)l b '-~ (CHZ)l
(CIf~rn O (CH~m O
Compound cis-C Compound F
s
OR Q (CHZ)n
vi
(CH~t ~O
(CH~m
Compound G
2197178
16
OR3 Q ((~~n O ORS Q (CH n
~'HH
C o m p o a n d G v~~ a -~ (C ~I ~ t (~ i
NH
(CH~m (CH~m 0
Compound Ha ~ Compound Hb
vii-b R2 - X
Compound 5
O \ Q (CH~n O
/ Q
(CH~)I ~ ORS z
(CHZ)t\ h / R
(CH~m N~ Rz %
+ (CHI
Compound 4a l.l,
(C ~)m C)
Compound 4b
viii
viii
O
OH Q (C!-(fin
(CH~1
(CH~mN~Rz OH Q (CH~n ~Rz
'N
Compound 1' a (CHI
H
(C ~m 0
Compound 1' b
ix
ix
R''O~ Q (CH>)~ O
(~~l
N (Z
(CH~m ~ R, s R~b~ <CHy / R s
y
Compound 1" a (~>)!
(CH~m O
Compound 1" b
wherein Q, R', R', l, m and n are as defined above, R'' is
2197178
17
acyl group, R' is a hydrogen atom, lower alkyl group, lower
alkoxyl group, halogen atom or trifluoromethyl group, X is a
halogen atom.
In the above, acyl group represented by R'' includes
same acyl groups as above, lower alkyl group, lower alkoxyl
group and halogen atom represented by R' includes same ones as
above, and halogen atom shown by X includes same ones as
above.
(Step i ) A known compound A obtained by the method
disclosed in J. Org. Chem., 42, 3764-y 3767 (1977), J. Chem.
Soc., Chem. Commun., 24, 2759 60 (1994) or Chem. Lett., _9,
1437 40 (1985) is reacted with ethylene glycol in a suitable
solvent in the presence of an acid catalyst to obtain a
compound B. The solvent to be used is not particularly limited
insofar as it does not participate in the reaction; it is, for
example, an aromatic hydrocarbon such as benzene, toluene or
xylene. Examples of useful acid catalysts are sulfuric acid,
p-toluenesulfonic acid, methanesulfonic acid,
trifluoromethanesulfonic acid and the like. The reaction is
conducted using ethylene glycol and the acid catalyst each in
an amount of about 1 to about 2 moles per mole of the compound
A. The reaction temperature is 80 °C to a temperature around
the boiling point of the solvent. For the completion of the
reaction, the reaction time is 1 to 8 hours, preferable about
4 to about 7 hours. The.compound B obtained by the invention
can be,used for the subseQuent reaction, as isolated or
without being isolated.
(Step ii) Next, the compound B is reacted with a
219 7 ~ 78
18
reducing agent in a suitable solvent to obtain a compound
traps-C Which has a hydroxyl group in the traps-position to
hydrogen atom attached to a bridgehead atom a. The solvent to
be used is not limited specifically insofar as it does not
participate in the reaction. Examples of such solvents are
methanol, ethanol, propanol, isopropanol and like alcohols,
dioxane, 1,2-dimethoxyethane, tetrahydrofuran and like ethers.
Examples of useful reducing agents are lithium aluminum
hydride, diisobutyl aluminum hydride, diborane, sodium boron
hydride and the like. The reaction is conducted using the
reducing agent in about 1 to about 1.5 moles per mole of the
compound B. The reaction temperature is -5 °C to room
temperature, preferably about 0 to about 10 'C . The reaction
time is preferably about 1 to about 3 hour-s_ The compound
traps-C resulting from the reaction can be used for the
subsequent reaction (Step m ) or (Step v ) , as isolated or
without being isolated.
(Step in ) The compound traps-C is reacted with p-
nitrobenzoic acid, triphenylphosphine and diethyl
azodicarboxylate in a suitable solvent to obtain a compound D.
The solvent to be used is not limited specifically insofar as
it does not participate in the reaction. Examples of such
solvents are dioxane, 1,2-dimethoxyethane, tetrahydrofuran and
like ethers, chloroform, dichloromethane, dichloroethane and
like hydrocarbon halides. The reaction is conducted using the
latter three reactants each in about 1. to about 3 moles per
mole of the compound traps-C. The reaction temperature is -5
to 50 'C , preferably about 0 'C to around room temperature.
X
2197178
19
The reaction times is 1 to 15 hours, preferably about 6 to
about 12 hours. The compound D resulting from the reaction can
be used for the subsequent reaction, as isolated or without
being isolated.
(Step iv ) The compound D is hydrolyzed in a suitable
solvent with use of an anion exchange resin to obtain a
compound cis-C which has a hydroxyl group in the cis-position
to hydrogen atom attached to a bridgehead atom a. The solvent
to be used is not limited specifically insofar as it will not
participate in the reaction. Examples of such solvents are
methanol, ethanol, propanol, isopropanol and like alcohols.
The reaction is conducted using the anion exchange resin in
about 1 to about 10 moles per mole of the compound D. The
reaction temperature is room temperature to 100 'C , and the
reaction time is about 10 to about 24 hours. The compound cis-
C resulting from the reaction can be used for the subsequent
reaction, as isolated or without being isolated.
(Step v ) The compound C obtained in (Step ii ) or
(Step iv) is reacted with a known compound E in a suitable
solvent in the presence of a base to obtain'a compopund F. The
solvent to be used is not limited specifically insofar as it
does not participate in the reaction. Examples of such
solvents- are N,N-dimethylformamide, N,N-dimethylacetamide,
acetonitrile and like aprotic polar solvents, dioxane, 1,2-
dimethoxyethane, tetrahydrofuran and like ethers. Examples of
useful bases are trimethylamine, triethylamine, pyridine and
like tertiary amines, potassium carbonate, sodium carbcsnate
and like alkali metal~carbonate, and potassium hydride, sodium
20 2197178
hydride and like alkali metal hydrides. For the reaction, the
base and compound E are used each in about 1 to about 2 moles
per mole of the compound C. The reaction temperature is room
temperature to 100 °~ , preferably room temperature to about 70
°C . The reaction time is 8 to 30 hours, preferably about 20 to
about 28 hours. The compound F resulting from the reaction can
be used for the subsequent reaction, as isolated or without
being isolated.
(Step vi) The compound F is subjected to a ketal
removing reaction in a suitable solvent with use of an acid to
obtain a compound G. The solvent is not limited specifically
insofar as it does not participate in the reaction. Examples
of solvents are alcohols such as methanol, ethanol, propanol
and isopropanol, and ethers such as dioxane, 1~2-
dimethoxyethane and tetrahydrofuran. Examples of useful acids
are acetic acid, trifluoroacetic acid, oxalic acid and like
organic acids, hydrochloric acid, hydrobromic acid, sulfuric
acid, nitric acid and like inorganic acids. The reaction
temperature is 0 to 60 'C , preferably about 10 to about 70 °C .
The reaction time is about 2 to about 8 hours. The compound G
resulting from the reaction can be used for the subsequent
reaction, as isolated or without being isolated.
(Step va - a) The compound G is reacted with
hydroxylamine and sodium acetate in a suitable solvent to
obtain an oxime of the compound G. The solvent is not limited
specifically insofar as it does not participarte in the
reaction. Examples of useful solvents are methanol, ethanol,
propanol, isopropanol and like alcohols, dioxane, 1,2-
219 1?8
21
dimethoxyethane, tetrahydrofuran and like ethers.
Hydroxylamine and sodium acetate are used each in about 1.5 to
2 moles per mole of the compound G. The reaction temperature
is 0 to 50 ~ , preferably room temperature. The reaction time
is preferably 5 to 8 hours.
Subsequently, the resulting oxime of the compound G
is reacted with p-toluenesulfonyl chloride in a suitable
solvent in the presence of a base to obtain a p-tosylic acid
ester of the compound G. Silica gel is added to the ester in
the same solvent, followed by a Beckmann rearrangement
reaction to obtain a mixture of compound Ha and compound Hb.
The solvent to be used is not limited specifically insofar as
it does not participate in the reaction. Examples of such
solvents are benzene, toluene, xylene and like aromatic
hydrocarbons, chloroform, dichloromethane, dichloroethane and
like hydrocarbon halides. Examples of useful bases are
trimethylamine, triethylamine, pyridine and like tertiary
amines. For the reaction, the base and p-toluenesulfonic
chloride are used each in 2 to 3 moles per mole of the oxime
of~the compound G. The reaction temperature for tosylation is
about 0 to 10 'C , and the reaction time is about 4 to about 8
hours. The 8eckmann rearrangement reaction in silica gel is
conducted at a temperature of about 10 to about 30 'C for
about 12 to about 24 hours.
(Step ~a -b) The resulting mixture of compound Ha and
compound Hb is reacted With a compound 5 in a suitable solvent
in the presence of a base to obtain a compound 4a and compound
4b. The solvent is not limited specifically insofar as it does
X
X19? 178
22
not participate in the reaction. Examples of useful solvents
are benzene, toluene, xylene and like aromatic hydrocarbons,
chloroform, dichloromethane, dichloroethane and like
hydrocarbon halides. Examples of useful bases are tertiary
amines such as trimethylamine, triethylamine and pyridine. For
the reaction, compound 5 and the base are used each in about 1
to about 2 moles per mole of the mixture. The reaction
temperature is about 0 to about 50 'C , preferably about 10 9C
to about 35 °C . The reaction time is 12 to 36 hours,
preferably about 24 to about 36 hours. The mixture of compound
4a or compound 4b resulting from the reaction can be isolated
and purified by a usual method such as chromatography and the
like. The compound 4a or compound 4b resulting from the
reaction can be used for the subsequent reaction, as isolated
or without being isolated.
(Step " ) The compound 4a or compound 4b is
hydrogenated in a suitable solvent in the presence of a
catalyst to obtain a compound 1'a or 1'b. The solvent is not
limited specifically insofar as it does not participate in the
reaction. Examples of useful solvents are methanol, ethanol,
propanol, isopropanol and like alcohols, dioxane, 1,2-
dimethoxyethane, tetrahydrofuran and like ethers, and methyl
acetate, ethyl acetate and like acetic acid esters. As a
catalyst is used for example palladium-charcoal and platinum.
For the reaction, the catalyst is used preferably in the ratio
of 0.5 to 1 by weight based on the compound 4a or 4b. The
reaction temperature is preferably around room temperature to
about 50 'C . The reaction time is about 10 to about 20 hours.
2197178
23
(Step ix) The compound 1'a or compound 1'b is
acylated in a suitable solvent by the method disclosed for
example in JP-A-106,593/1986 to obtain a compound 1"a or
1 " b. The solvent is not limited specifically insofar as it
does not participate in the reaction. Examples of useful
solvents are dichloromethane, dichloroethane, chloroform and
like hydrocarbon halides, dioxane, tetrahydrofuran and like
ethers, benzene, toluene and like aromatic hydrocarbons.
Usual acylation method is employed, and for example
acid anhydride method and acid chloride method are applicable.
In acid anhydride method, the compound 1'a or
compound 1'b is reacted with acid anhydride in a suitable
solvent in the pr-~sence or absence of dimethylaminopyridine.
As acid anhydride is used those having acyl group which should
be introduced to R''. Examples thereof are acetic anhydride,
propionic anhydride, butyric anhydride and benzoic anhydrude.
For the reaction, acid anhydride is used in about 1 to about 3
moles and dimethylaminopyridine is used in 0 to about 3 moles
each per mole of the compound 1'a or compound 1'b. The
reaction temperature is about 5 to about 50 °C , preferably
.about 10 'C to around room temperature. The reaction time is 4
to 24 hours, preferably about 6 to about 12 hours.
In acid chloride method, the compound 1'a or
compound 1'b is reacted with acyl halide (R''X) in a suitable
solvent in the presence a dehydrohalogenation agent. Examples
of dehydrohalogenation agents are sodium hydrogen carbonate,
sodium carbonate, potassium carbonate, pyridine and
triethylamine. The solvent includes those mentioned above. For
x
2197178
24
the reaction, acyl halide is used in about 1 to about 3 moles
per mole of the compound 1'a or compound 1'b. The reaction
temperature is about -30 to about 100 ~ , preferably around
room temperature to 80 °C . The reaction time is 1 to 20 hours,
preferably about 6 to about 12 hours.
Further, the bicyclolactam compound of the present
invention wherein R is oxo group can be prepared, for example,
by the following reaction process.
( C H 2 + R 2 - X ---.-j ( C H ;
R2
C ompound 6 C ompound 5 C ompound 1 ' ' '
wherein Q, RZ, 1 and X are as defined above.
According to the above reaction step (vn -b), the
compound 6 is reacted with the compound 5 in a suitable
solvent in the presence of a base to obtain a compound 1" '.
The solvent.is not limited specifically insofar as it does not
participate in the reaction. Examples of such solvents are
benzene, toluene, xylene and like aromatic hydrocarbons,
dichloromethane, dichloroethane and like.hydrocarbon halides.
Examples of useful bases are potassium carbonate, sodium
carbonate and like inorganic base's, sodium methoxide, sodium
ethoxide and like sodium alkoxides, trimethylamine,
triethylamine, pyridine and like tertiary amines. For the
reaction, the compound 5 and the base are used each in 1 to 2
moles per mole of the compound 6. The reaction temperature is
219" 178
about 0 to 50 °C , preferably 10 to 35 °C , and the reaction
time is 1 to 24 hours, preferably about 6 to about 12 hours.
The compound 6 can be prepared by the following A, B
or C process.
5 A process:
A
0 H
N 0
~o H H H
Compound I Compound 6 a
The compound I is obtained by cyclizing 2-
cyanoethyl-1,3-cyclohexanedione according to the method
di-sclosed in J.. Org. Chem., 57, 2521 (1992). The compound I is
15 reduced in a suitable solvent with hpdrogen in the presence of
palladium-charcoal to obtain the compound 6a wherein two
hydrogen atoms on both of the bridgehead carbons have cis
configuration. The solvent is not limited specifically insofar
as it does not participate in the reaction. Examples of useful
20 solvents are methanol, ethanol, isopropanol and like alcohols,
dioxane, 1,2-dimethoxyethane, tetrahydrofuran and like ethers.
For the reaction, palladium-charcoal is used in~the ratio of
0.1 to 1.2 by weight based on the compound I. The. hydrogen
pressure is about 1 to 3 atom. The reaction temperature is 0
25 to 50 'C , preferably 10 1C to around room temperature. The
reaction time is preferably about 6 to about l2 hours. The
resulting compound 6a can be used for the subsequent reaction
for obtaining the present compound 1" ', as isolated or
~19717g
26
without being isolated.
B process:
0 '~C O ZMe O C O ZMe 0 C 0 ZMe
~ 1
NH2 NHZ NHZ
C ompound J C ompound K C ompound L
O Q H
-~ ---
N 0 N O
H HH
Compound M Compound 6 b
(Step B-i ) A known compound J disclosed for example in
Synthesis-, 176 (1991) is reacted in a suitable solvent with
methyl ester of acetylenecarboxylic acid to obtain a compound
K. The solvent is not limited specifically insofar as it does
not participate in the reaction. Examples of useful solvents
are N,N-dimethylformamide, N,N-dimethylacetamide, acetonitrile
and like aprotic polar solvents, dioxane, 1,2-dimethoxyethane,
tetrahydrofuran and like ethers. Methyl ester of
acetylenecarboxylic acid is used~in an excess amount,
preferably about 4 to 7 moles per mole of the compound J. The
reaction temperature is preferably about 120 to 150 °~ , and
the reaction time is preferably about 6 to about 18 hours. The
resulting compound K can be used for the subsequent reaction,
as isolated or without being isolated.
(Step B-ii) The resulting compound K is reduced in a suitable
solvent with hydrogen in the presence of palladium-charcoal to
219 7 1~7g
27
obtain the compound L. The solvent is not limited specifically
insofar as it does not participate in the reaction. Examples
of useful solvents are methanol, ethanol, isopropanol and like
alcohols, dioxane, 1,2-dimethoxyethane, tetrahydrofuran and
like ethers. For the reaction, palladium-charcoal is used in
the ratio of 0.1 to 0.5 by weight based on the compound K. The
hydrogen pressure is about 1 to 5 atom. The reaction
temperature is 10 to 50 'C , preferably 15 to 30 °C . The
reaction time is preferably about 2 to about 5 hours. The
resulting compound L can be used for the subsequent reaction,
as isolated or without being isolated.
(Step B-m ) The resulting compound L is heated without solvent
to obtain a compound M. The heating is conducted at a
temperature of ,about 170 to about 190 °~ and for abort 1 to
about 3 hours.
Consequently, the compound.M is reduced according to
the method mentioned in the above A process to obtain a
compound 6b. The resulting compound 6b can be used for the
subsequent reaction for obtaining the present compound 1 " ',
as isolated or without being isolated.
2197 ~7g
28
C process:
O Q O
1
~C02CH3 ---~ ~ ~CONH2
0 O
C ompound N C ompound O
0 O Q
ii \\
--- j
i
<'~0
H H H
Compound P Compound 6 c
(Step C-i ) To a known compound N discolsed for example in J.
Org. Chem., 31, 1489 (1966) is added an excess amount of 25
-aqueous ammonia solution or methanol-ammonia for reaction,
~5 thereby a compound 0 is obtained. The reaction temperature is
preferably about 15 to about 30 °IC . The reaction time is
preferably about 3 to about 10 hours.
(Step C-a ) The resulting compound 0 is ring-colsed with
dehydration according to the method disclosed for example in
20 J. Org. Chem., 35, 3499 (1970) to obtain a compound P. The
reaction temperature is about 70 to about 120 'C , preferably
around a boiling temperature of solvent. The reaction time is
preferably about 2 to about 6 hours.
Consequently, the compound P is reduced according to
the method mentioned in the above A process to obtain a
compound 6c. The resulting compound 6c can be used for the
subsequent reaction for obtaining the present compound 1 " ',
as isolated or without being isolated.
x
2197178
29
The compound of the formula (1) thus obtained can be
isolated and purified by a usual method such as
recrystallization or column chromatography. The racemic
compound obtained can be divided into the desired optical
isomers, for example, by fractional recrystallization for the
separation of salts from optically active acids or by passing
a column packed with an optically active carrier. The
stereoisomers can be individually separated off and purified
by a usual method such as fractional crystallization or
chromatography.
The present bicyclolactam compound is added to a
pharmaceutical carrier to afford a pharmaceutical composition,
particularly an anxiolytic agent.
The.anxiolytic agent having incorporated the present
compound therein as an effective component can be given orally
or parenterally to mammals including man. The pharmaceutical
preparations of the present invention are not limited
specifically in the unit form of administration but can be in
various forms in conformity with preventive or therapeutic
purposes. These forms of preparations include, for example,
oral preparations, injections, suppositories, external
preparations (such as poultices and like plasters, ointments,
creams and lotions),. eye drops, nasal drops or sprays, etc.
The anxiolytic agent having incorporated the present
compound therein as an effective component is prepared and
used in the form of a composition having a desired
conventional pharmaceutical carrier or excipient incorporated
therein by a usual method.
2197178
Stated more specifically, examples of carriers for
use in formulating the agent as tablets, encapsulated
preparations, granules, powders, etc. for oral administration
are excipients such as lactose, sucrose, sodium chloride,
S glucose, urea, starch, calcium carbonate, kaolin, crystalline
cellulose and silicic acid, binders such as water, ethanol,
propanol, syrup, glucose solution, starch solution, gelatin
solution, carboxymethyl cellulose, hydroxypropyl cellulose,
hydroxypropyl starch, shellac, methyl cellulose, ethyl
10 cellulose, potassium phosphate and polyvinylpyrrolidone,
disintegrators such as dried starch, sodium alginate, agar
powder, laminaria powder, sodium hydrogencarbonate, calcium
carbonate, polyoxyethylene sarbitan fatty acid esters, sodium
laurylsulfate, stearic acid monoglyceride, starch and lactose,
15 disintegration suppressants such as sucrose, stearic acid,
cacao butter and hydrogenated oils, absorption promotors such
as quaternary ammonium bases and sodium laurylsulfate,
humectants such as glycerin and starch, absorbents such as
starch, lactose, kaolin, bentonite and colloidal silicic acid,
20 glazing agents such as purified talc, stearic acid salts,
boric acid powder and polyethylene glycol, corrigents such as
sucrose, bitter orange peel, citric acid and tartaric acid,
etc. When required, the tablets can be those having a usual
coating, such as sugar-coated tablets, gelatin-coated tablets,
25 enteric-coated tablets, film-coated tablets, double-layer
tablets and multi-layer tablets. The encapsulated preparation
is made by mixing the present compound with carriers such as
those exemplified above and filling the mixture into hard
A
2197178
gelatin capsules or soft capsules.
Liquid preparations for oral administration include
aqueous or oily suspensions, solutions, syrups and elixirs,
and are prepared in the usual manner by adding a corrigent,
buffer, stabilizer, flavoring agent, to the present compound.
In this case, examples of useful corrigents are those
exemplified above, useful buffers include sodium citrate, and
useful stabilizers include tragacanth, gum arabic and gelatin,
etc.
Injections are aqueous or oily suspensions and
solutions, or powdery fillers and freeze-dried preparations
which are dissolved when to be used. Injections are prepared
in the usual manner by adding to the present compound a pH
adjusting- agent, buffer, stabilizer, isotonic agent, di:luent,
local anesthetic, etc. Examples of pH adjusting agent. and
buffers for use in this case are sodium citrate, sodium
acetate, sodium phosphate and the like. Examples of useful
stabilizers are sodium pyrosulfite, EDTA, thioglycolic acid,
thiolactic acid, etc. Examples of useful diluents are water,
aqueous solution of lactic acid, ethyl alcohol, propylene
glycol, ethoxylated isostearyl alcohol, polyoxyisostearyl
alcohol, polyoxyethylene sorbitan fatty acid ester, etc.
Examples of useful stabilizers are sodium pyrosulfite, EDTA,
thioglycolic acid, thiolactic acid, etc. Examples of useful
local anesthetics are procaine hydrochloride, lidocaine
hydrochloride, etc.
In preparing suppositories, use can be made of
carriers such as polyethylene glycol, lanolin, cacao fat,
2197 ~ 7g
32
esters of higher alcohols, gelatin, semisynthetic glyceride,
etc., and.when required, surfactants such as Tween
(trademark) .
Ointments (pastes, creams, gels, etc.) are prepared
by admixing with the present compound a base, stabilizer,
lubricant, preservative, etc. which are usually used. Examples
of bases are fluid paraffin, white petrolatam, bleached
beeswax, octyldodecyl alcohol, paraffin and the like. Examples
of useful preservatives are methyl p-hydroxybenzoate, ethyl p-
hydroxybenzoate, propyl p-hydroxybenzoate and the like.
Plasters are prepared by applying the ointment,
cream, gel, .paste or the like to a usual support in the
conventional manner. Examples of suitabie supports are woven
or nonwoven fabrics of cot..ton, staple fiber or chemical fiber,
films of flexible polyvinyl chloride, polyethylene,
polyurethane or the like, and foamed sheets of such material.
When required, the foregoing preparations may have
further incorporated therein a coloring agent, preservative,
perfume, flavoring, sweetener and the like, and other
medicinals.
The method of administering the pharmaceutical
preparation of the invention is not limited specifically but
determined according to the form of preparation, age, sex and
other conditions of the patient and degree of symptom of the
patient. For example, tablets, pellets, powders, solutions,
suspensions, emulsions, granules and capsules are given
orally. Suppositories are introduced into the rectum.
Injections are intravenously given singly or as mixed with a
2197178
33
usual auxiliary solution such as glucose or amino acid
solution. Further when required, they are singly administered
intra-arterially, intramuscularly, intracutaneously,
subcutaneously or intraperitoneally. Ointments are applied to
the skin, mucous membrane of the oral cavity, etc. Plasters
are applied to the skin.
The dosage of the effective component of the
preparation of the invention can be suitably determined
according to the mode of administration, age, sex and other
conditions of the patient and degree, of the symptom. Generally
the effective component is administered at a daily dose
usually of 0.001 to 50 mg/kg body weight, preferably 0.01 to
10 mg/~k~ body weight. The present preparation can be given
once or in about 2 to about~four divided d~ses per day.
BEST MODE OF CARRYING OUT THE >iN'VENTION
The present invention will be described below with
reference to reference examples and examples. However, the
invention is not limited by these examples.
Reference Example 1
Preparation of 6-oxo-bicyclo[3.3.0]octan-2-
one=ethylene=acetal (Compound B-1)
A 9.62 g quantity of bicyclo[3.3.OJoctan-2,6-dione
which is known as disclosed for example in J. Org. Chem., _42,
3764 3767 (1977), 0.265 g of p-t.oluenesulfonic acid
monohydrate and 4.54 g of ethylene glycol were dissolved fn 50
ml of benzene, and the mixture was refluxed for reaction for 6
hours while removing water as an azeotropic mixture. After the
reaction, the mixture was cooled to room temperature and
xJ;
219 7 w78
34
allowed to stand for 15 minutes with addition of 3 g of sodium
hydrogencarbonate. The resulting precipitate was.filtered off
and washed with benzene. The filtrate was concentrated to
obtain a brown oily product, which was purified by column
chromatography using 180 g of silica gel and hexane-ethyl
acetate (5:1) to obtain 9.28 g of Compound mentioned above in
the form of a colorless oily substance (yield: 73 ~ ).
' H-NMR (CDCls ) a ppm . 1 . 80~- 2. 45 (m, 10H) , 3. 95 (s, 4H)
Reference Example 2
Preparation of (1RS, 2SR, 5RS)-2-hydroxybicyclo-
[3.3.Ojoctan-6-one=ethylene=acetal (Compound trans-C-1)
A 9.23 g quantity of Compound obtained in Reference
Example 1 was dissolved in 70 ml of methanol; and 1.93 g of
sodium boron hydride was added to the solution while cooling
the solution in an ice-_methanol bath. The m~.xuture was
returned to room temperature 30 minutes later,. followed by
further reaction for 1 hour. The methanol was thereafter
distilled off, 70 ml of water was added to the residue, and
the mixture was subjected to extraction with 100 ml of
dichloromethane and 50 ml of dichloromethane twice. The
dichloromethane layer obtained was washed with 50 ml of
saturated aqueous sodium chloride solution, then dried over
anhydrous sodium sulfate and distilled for the removal of
solvent, giving a colorless oily product. The product was
purified by column chromatography using 150 g of silica gel
and hexane-ethyl acetate (4:1 to 2:1) to obtain 6.63 g of
Compound mentioned above in the form of a colorless oily
substance (yield: 71
~.
2197178
' H-NMR (CDCls ) a ppm . 1 . 75~- 2. 00 (m, 8H) , 2. 10~ 2. 80 (m, 2H) ,
3. 90~ 4. 18 (m, 1H) , 3.94 (s, 4Hj
Reference Example 3
Preparation of (1RS, 2RS, SRS)-2-(4-nitrobenzoyl-
5 oxy)bicyclo-[3.3.0)octan-6-one=ethylene=acetal (Compound D-1)
A 3.68 g quantity of Compound obtained in Reference
Example 2, 6.68 g of p-nitrobenzoic acid and 10.5 g of
triphenylphosphine were dissolved in 70 ml of tetrahydrofuran,
and a solution of 7.00 g of diethyl azodicarboxylate in 10 ml
10 of tetrahydrofuran was added dropwise to the solution with ice
cooling over a period of 10 minutes. The mixture was stirred
with ice cooling for 1 hour, then returned to room temperature
and further reacted for 16 hours. The solvent was distilled
off from the reaction mixtur-e, 50 ml of ether and 30 ml of
15 hexane were added to the residue, and the resulting mixture
was aTIowed to stand in a refrigerator for. 1 day. The
resulting precipitate (triphenylphosphine oxide) was filtered
off and washed with hexane-ether (2:1). The filtrate obtained
was concentrated to obtain a yellow oily product. The product
20 was purified by column chromatography using 90 g of silica
gel, hexane and hexane-ethyl acetate (10:1), giving 5.34 g of
Compound mentioned above in the form of a light yellow oily
substance (yield: 80 94 ) .
' H-NMR (CDCls ) d ppm . 1 . 60~ 2 . 20 (m, 8H) , 2. 45~ 2 . 90 (m, 2H) ,
25 3.94 (s, 4H) , 5. 10~ 5.23 (m, 1H) , 8.24 (s, 4H)
Reference Example 4
Preparation of (1RS, 2RS, 5RS)-2-hydroxybicyclo-
[3.3.0)octan-6-one=ethylene=acetal (Compound cis-C-1)
X19 7 178
37
of 70 °C for 4 hours. The mixture was returned to room
temperature, and 80 ml of ice water was added thereto. The
resulting mixture was subjected to extraction with 60 ml of
ether three times. The ethereal layer was washed with 20 ml of
water three times and with 20 ml of saturated aqueous solution
of sodium chloride and thereafter dried over anhydrous sodium
sulfate. Removal of the solvent from the layer gave an oily
product, which was then purified by column chromatography
using 75 g of silica gel and hexane and hexane-ether (15:1),
whereby 6.65 g of Compound mentioned above was obtained as an
oily substance (yield: 70 j ).
' H-NMR (CDC1~ ) a ppm . 1 . 60~- 2 . 10 (m, 8H) , 2 . 40~ 2 . 80 (m, 2H) ,
3.. 50~- 3.80 (m, 1H) , 3.90 (s, 4H) , 4.92 (s,, 2H) , 7.31 (s, 5H)
Reference Example 6
15_ Preparation of (1RS, 2RS, 5RS)-2-benzyloxybicyclo-
[3.3.0}-octan-6-one (Compound G-1)
A 1.94 g quantity of Compound obtained in Reference
Example 5 was dissolved in 10 ml of tetrahydrofuran, and the
solution was stirred for 6 hours with 3 ml of 2N hydrochloric
acid added thereto. The tetrahydrofuran was distilled off from
the resulting reaction mixture, followed by extraction with 20
ml of ether twice. The ethereal layer was washed with 10 ml of
saturated aqueous solution of sodium hydrogencarbonate twice
and then with 5 ml of saturated aqueous solution of sodium
chloride, and dried over anhydrous sodium sulfate. Removal of
the solvent from the layer give 1.60 g of Compound mentioned
above as an oily substance (yield: 98 ~ ),
' H-NMR (CDC1~ ) a ppm . 1 . 30~ Z . 40 (m, 8N) , 2 . 60~- 3 . 00 (m, 2H) ,
x
38 '297 X78
3. 70~ 3. 90 (m, 1H) , 4. 51 (s, 2H) , 7.33 (s, 5H)
Reference Example 7
Preparation of cis-2-azabicyclo[4.4.0]decan-3,7-
dione (Compound 6a)
Methanol (100 ml) was added to 1.5 g of
2,3,4,5,6,7,8-heptahydro-1(1H)-quinoline-2,5-dione which was
known as disclosed for example in J. Org. Chem., _57, 2522
(1992). The mixture was subjected to reduction in the hydrogen
stream (1 atm.) in the presence of 0.75 g of 10 ~ palladium-
carbon. The palladium-carbon was filtered off and the solvent
was removed. The residue was purified for isolation by column
chromatography (silica gel, developer; chloroform . ethanol=
10 . 1 ) to obtain 0. 42 g of Compound mentioned above(yield:
28 96 ) . Table i shows analytical data.
Reference Examp7.e 8
Preparation of methyl 3-(3-amino-2-cyclopenten-1-
one-2-yl)-acrylate (Compound K)
To 2 ml of dimethylacetamide were added 0.2 g of 3-
amino-2-cyclopenten-1-one and 2 ml of methyl
acetylenecarbonate. The mixture was heated with stirring at
120 to 125 'C for 19 hours. After cooled, 2 ml of ether was
added and the precipitates were filtered. and washed with ether
to obtain 0. 17 g of Compound mentioned above (yield: 46 $~ ) .
m. p. 278- 279 'G
Elementary analysis; C, H~ , NOs
Calcd. C 59.67 H 6.12 N 7.73
Found C 59.34 H 6.47 N 8.06
Reference Example 9
3g
Preparation of methyl 3-(3-amino-2-cyclopenten-1-
one-2-yl) -propionate (Compound L)
To 200 ml of methanol were added 6.0 g of Compound K
obtained in Reference Example 8 and 1.5 g of 10 % palladium-
s charcoal. The mixture was reacted at room temperature in the
hydrogen stream (2 atm.) for 3 hours. After reaction, the
palladium-carbon was filtered off and the solvent was removed.
The residue was crystallized from ether to obtain 5.9 g of
Compound mentioned above (yield: 98
m. p. 223-- 224 °C
Elementary analysis; Ca H, ~ NOs ~ 0. 2 HZ 0
Calcd. C 55.61 H 6.65 N 8.11
Found C 55.58 H 6.69 N 8.31
Reference Example 10
Preparation of 2,3,4,5,6,7-hexahydro-1(1H)-pyrindin-
2 , 5-dione (Com.pound Nf)
A 2.8 g quantity of Compound L obtained in Reference
Example 9 was heated with stirring without solvent at an oil
bath temperature of 190 to 210 9C for one hour. After
reaction, when hot, isopropanol was added. After cooling, the
precipitates were filtered to obtain 1.7 g of Compound
mentioned above (yield: 74 ~ ) .
m. p. 247- 248 °C
Elementary analysis; C, H, NOZ
Calcd. C 63.56 H 6.00 N 9.27
Found C 63.25 H 6.17 N 9.30
Reference Example 11
Preparation of cis-2-azabicyclo[4.3.OJnonan-3,7-
40 2197178
dione (Compound 6b)
In 180 ml of methanol was suspended 3.8 g of
Compound N obtained in Reference Example 10 and thereto was
added 4 g of 10 % palladium-charcoal. The mixture was reacted
S in the hydrogen stream (2 to 2.5 atm.) for 12 hours. After
reaction, the palladium-charcoal was filtered off and the
filtrate was concentrated. The residue was purified by column
chromatography (silica gel, developer; ethyl acetate .
methanol= 10 . 1) to obtain 3.4 g.of Compound mentioned above
(yield: 89 % ). Table 1 shows analytical data.
Reference Example 12
Preparation of 2-methyl-2-(2-carbamoylethyl)-1,3-
cyclopentanedione (Compound 0-1)
A 1 ml quantity of 25 9S .aqueous ammonia solution
was added to 1.6 g of 2-methyl.-2.-(~ -carbomethoxyethyl)-
cyclopentan-1,3-dione which was known and disclosed for
example in J. Org. Chem., 31, 1489 (1966). The mixture was
reacted at room temperature for 5 hours. After reaction, 10 ml
of tetrahydrofuran (THF) was added. The insolubles were
filtered and recrystallized from ethanol to obtain 0.6 g of
Compound mentioned above (yield: 41 90).
m.p. 159- 162 1C
Reference Example 13
Preparation of 2,3,4,4a,5,6-hexahydro-4a-methyl-
1(1H)-pyrindin-2,5-dione (Compound P-1)
To 400 ml of toluene was added 4.42 g of Compound
0-1 obtained in Reference Example 12, and thereto was added
0.6 g of tosyl acid. The mixture was heated with stirring for
~19717g
41
3 hours with dehydration device attached. After reaction, the
solvent was removed and the residue was recrystallized from
ethanol-chloroform to obtain 3.0 g of Compound mentioned above
(yield: 75 % ) .
m.p. 228~- 230 °C
Elementary analysis; C9 H~ , NOZ
Calcd. C 65.44 H 6.71 N 8.30
Found C 65.13 H 6.67 N~8.30
Reference Example 14
Preparation of cis-6-methyl-2-azabicyclo[4.3.Oj-
nonan-3,7-dione (Compound 6c-1)
Compound mentioned above was prepared in the same
manner aj in Reference Example 11 except that Compound P-1
obtained,in Reference Example 13 was used in place of Compound
M. Table 1 shows analytical data.
25
42
Table 1
( C H ; ( C ompound 6 )
elem. anal.
CompdQ 1 yield m.p. formula calcd.(found) ~MS
No (3'6 ) ('C ) C H N +
.
(M
)
64.65 7.84 8.38
6a H 2 28 177- 178 C9 H~ s
NO
Z 167
(64.65 7.37 8.34)
CB H, 1 60. 59 7. 37 8. 83
6b H 1 ? 1 140 142 NOZ ~
153
0. 3HZ 0 (60. 41 7. 08 8.
88)
64.65 7.84 8.38
6c-1 CH9 1 77 129- 131 C9 H~ $
NO
E 167
(64.41 7.58 8.32)
Example 1
Preparation of (1RS, 6RS, 7RS)-7-benzyloxy-2-(4-
methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3-one (Compound 4a-1)
and (1RS, 6RS, 7RS)-7-benzyloxy-3-(4-methoxybenzoyl)-3-
azabicyclo [4.3.0) nonan-2-.one (Compound 4b-1)
A 1.54 g quantity of Compound obtained in Reference
Example 6 was dissolved in 15 ml of tetrah.ydrofuran, followed
by addition of 8 ml of water and thereafter by addition of
0.94 g of hydroxylamine hydrochloride and 1.84 g of sodium
acetate trihydrate. Tetrahydrofuran was subsequently added to
obtain a homogeneous mixture, which was then stirred at room
temperature for 5 hours. The tetrahydrofuran was distilled off
from the reaction mixture, followed by extraction with 80 ml
43 ~ ~.~
of ethyl acetate. The ethyl acetate layer was washed with 10
ml of water, with 10 ml of saturated sodium hydrogencarbonate
solution and subsequently with 10 ml of saturated aqueous
solution of sodium chloride, and thereafter dried over
S anhydrous sodium sulfate. Distillation of the layer for the
removal of the solvent gave 1.64 g of oxime of Compound of
Reference Example 6 as a colorless oily substance.
A 1.60 g of the oxime obtained was dissolved in 16
ml of benzene, followed by addition of 3.11 g of p-
toluenesulfonyl chloride and then by addition of 2.27 ml of
triethylamine with ice cooling. The mixture was stirred for 4
hours with ice cooling and subsequently for 2 hours at room
temperature, and thereafter diluted with 50 ml of ether. The
resulting solution was washed with 10 ml of water, with 10 ml
of 2N hydrochloric acid twice .and subsequently with 10 ml of
saturated aqueous solution of sodium chloride, and thereafter
dried over anhydrous sodium sulfate. Removal of the solvent
from the solution gave a yellow oily product. The product was
dissolved in 50 ml of anhydrous benzene, followed by addition
of 43 g of silica gel (Fuji Silysia, BW-300 as washed with 2N
HC1 and then thoroughly with water, and dried at 230 ~ for 16
hours) and further by addition of anhydrous benzene in an
amount of permitting stirring of the resulting mixture. The
mixture was shaken on a water bath having a constant
temperature of 25 °C for 18 hours. The reaction mixture was
poured into a column packed with 20 g of silica gel, and 300
ml of benzene was passed through the column to cause an excess
of p-toluenesulfonyl chloride to flow out. The solvent was
~'r
f
44
X197178
thereafter changed for benzene-methanol (6:1) to obtain an
eluate. The eluate still contained impurities and was
therefore purified by column chromatography again using 30 g
of silica gel, and chloroform and chloroform-methanol (50:1).
The product was dried in a vacuum at room temperature to
obtain 1.233 g of a light yellow oily substance. 'Ii-NMR
analysis revealed that the product was a mixture of (1RS, 6RS,
7RS)-7-benzyloxy-2-azabicyclo[4.3.0)nonan-3-one and (1RS, 6RS,
7RS)-7-benzyloxy-3-azabicyclo[4.3.0]nonan-2-one approximately
in the ratio of 2:i.
The mixture (1.18 g) obtained was dissolved in 15 ml
of dichloromethane, 1.31 g of p-methoxybenzoyl chloride and
i.34 g of triethylamine were added-to the solution, and the
mixture was stirred at room temps-rature for 36 hours. With
addition of 80 ml of ethyl acetate, the reaction mixture was
washed with 20 ml of 2N hydrochloric acid twice, with 20 ml of
saturated solution of sodium hydrogencarbonate twice and then
with 10 ml of saturated aqueous solution of sodium chloride,
and dried over anhydrous sodium sulfate. Removal of the
solvent gave a brown oily product, which was then purified by
column. chromatography using 30 g of silica gel and hexane-
ethyl acetate (4:1 to 3:1]. The component eluted first was
Compound 4b-1 mentioned above and obtained in an amount of
0.576 g as a yellow oily substance (yield: 25 ~ ). The
component eluted thereafter was Compound 4a-1 mentioned above.
The fraction was distilled for the removal of the solvent and
recrystallized from ethanol,. giving 1.05 g of the compound
(yield: 46 9S). Tables 2 to 3 show analytical data.
l
2197178
Example 2
Compounds 4a-2 to 4a-5 and Compounds 4b-2 to 4b-4
were prepared in the same manner as in Example 1 except that
various benzoic chloride derivatives were used in place of p-
5 methoxybenzoic chloride. Tables 2 to 3 show analytical data.
Example 3
Preparation of (1RS, 6RS, 7RS)-7-hydroxy-2-(4-
methoxybenzoyl)-2-azabicyclo-[4.3.0]nonan-3-one (Compound 1'a-
1)
10 A 1.05 g quantity of (1RS, 6RS, 7RS)-7-benzyloxy-2-
(4-methoxybenzoyl)-2-azabicyclo[4.3.Ojnonan-3-one (Compound
4a-1) obtained in Example 1 was dissolved in 15 ml of dioxane,
0.50 g of 10 % palladium-charcoal (product of Wako Junyaku
Co., Ltd.) was added to the solution, and the air in the
15 reactor was removed by an aspirator and replaced by hydrogen
repeatedly twice. The mixture was thereafter stirred in the
hydrogen atmosphere (1 atm.) for 16 hours. The catalyst was
filtered off and washed with dioxane. The resulting filtrate
was concentrated to obtain a colorless oily product, which was
20 then purified by column chromatography using 15 g of silica
gel and chloroform. Removal of the solvent from the product
afforded crystals, which were further recrystallized from
ether, giving 0.64 g of Compound mentioned above in the form
of a colorless powder (yield: 81 94j. Table 4 shows analytical
25 data.
Example 4
Compounds 1'a-2 to 1'a-5 were prepared in the same
manner as in Example 3 except that Compounds 4a-Z to 4a-5
x
2197 y7s
46
obtained in Example 2 were used as a starting material in
place of Compound 4a-1. Similarly, Compounds 1'b-2 and 1'b-3
were prepared in the same manner as in Example 3 except that
Compounds 4b-3 and 4b-4 obtained in Example 2 were used as a
starting material in place of Compound 4b-1. Tables 4 to 5
show yield and analytical data.
Example 5
Preparation of (1RS, 6RS, 7RS)-7-acetoxy-2-(4-
methoxybenzoyl)-2-azabicyclo-[4.3.0]nonan-3-one (Compound
1~" a-1) A 0.29 g quantity of (1RS, 6RS, 7RS)-7-hydroxy-2-(4-
methoxybenzoyl)-2-azabicyclo[4.3.0]nonan-3-one (Compound 1'a-
1) obtained in Example 3 was dissolved in 10 ml of
dichloromethane. With ice cooling, thereto were added 0.225 g
of dimethylaminopyridine and then 0.2 g of acetic anhydride.
The mixture was reacted at room temperature for 12 hours and
then thereto was added 20 ml of dichloromethane. The reaction
mixture was washed with 10 ml of 1N hydrochloric acid and then
with 10 ml of saturated aqueous solution of sodium chloride,
and dried over anhydrous sodium sulfate. Removal of the
solvent gave a light brown oily product, which was then
purified by column chromatography using 20 g of silica gel and
chloroform-methanol (20: 1) to obtain 0.23 g of Compound 1 "a-1
as an oily substance (yield: 69 9~ ) .
' H-NMR (CDC1, ) 8 ppm: 1 . 60~ 2. 70 (m; 9H) , 2.08 (s, 3H) ,
3.85 (s, 3H) , 4.70 (q, 1H) , 5.00 5. 10 (m, 1H) , 6.88 (d, 2H) ,
7. i0 (d, 2H]
,~
47 2197178
Table 2
PhCHZp H
N~0
H
~0
CR 5)n
Compd. (R5 ) n yield m. p. ' H-NMR (CDC1~ )
No . (~ ) (C ) ( 8 ppm)
4a-1 4-OCHS 46 108 . 5 1 . 30~ 2 . 70 (m, 10H) , 3 . 70~
4 . 00 (m, 1H) ,
~- 3. 83 (s, 3H) , 4. 54 (s, 2H) ,
109. 5 6. 8? (d, 2H) , 7. 34 (s, 5H) ,
7 . 58 (d, 2H)
.
.
.
4a-2 H 26 oil 1 . 50~ 2 . 31 (m, 6H) ,
2 . 40~ 2
. 57
(m, 3H).,.
3. 89 (m, 1H) , 4. 52 (m, 2H) ,
_..._._..__.__.._.__.____.____._..__...__..._._...__....__...._...._.__._.._.__
._..____.._._4_ 55_(q,.lH)__c....~_.26.".:~__.58,_(m_,._1_OH)_.:._...._....
_.
4a-3 4-F 27 of 1 1 . 10~ 2 . 25 (m, 6H) , 2 . 25~
2 . 75 (m, 3H) ,
3 . 70~ 3 . 95 (m, 1H) , 4 . 55 (dd,
2H) ,
._............_._................._.._...__..._.._....._..._...................
.._....__.__.4_ : ~ ~. ~ q .1 H ). ._...6 :.9
~ ~ ~.:. ~ ~ _(m ,. 9 H ) . ,_...._......___
4a-4 4-CHs 28 97~ 98 1 .00~- 2. 30 (m, 6H) , 2. 36 (s,
3H) ,
2 . 30~ 2 . 70 (m, 3H) , 3 . 70~
4 . 00 (m, 1H) ,
4 . 54 (dd, 2H) , 4. 69 (q,1H) ,
.......__.._..__..._....__._......_........_.__._._...____._._..__.._..._._._._
.__._._...___~. : ~ ~_".. ~ :.6 ~_ ~!~ . -9
H )...._.__._.__.___.__...__._...___.._......._.......
4a-5 2, 4- (OCHa ) = 20 oil 1 . 42~ 2 .06 (m, 5H) , 2 . 25~ 2
. 50 (m, 4H) ,
3 . 73 (s, 3H) , 3 . 79 (s, 3H) ,
3. 86 (m, 1H) , 4. 50 (d, 1H) ,
4.53 (d, 1H)-, 4.58 (q, 1H) ,
6. 50 (d, 1H) , 6. 54 (dd, 1H) ,
7. 28 (m, 1H) , 7. 32 (d, 1H) , 7.
35 (m, 4H)
48 219 7 1 7g
Table 3
PhCHzp
~N O
H 0
(R')m-IT
U
Compd (RS ) n yield m. p. ' H-NMR (CDCla )
No . (% ) (C ) ( d ppm)
4b-1 4-OCHs 25 oil 1 .303.30 (m, 10H) , 3.604. 10 (m,
1H) ,
3.83 (s,3H) , 4.52 (s,2H) ,
6. 86 (d, 2H) , _7. 33 (s, 5H) ,_
7. _54 (d, 2H)
..
.
..
..
4b-2 4-F 14 128 1 . 20~ 2 . 30 (m, 6H)
,
2 . 40~ 2 . 80 (m, 1H)
,
~ 2.903.30 (m, 1H) , 3.403.65 (m, 1H)
,
130 3.653.85 (m, 1H) , 3.954.25 (m, 1H)
,
4 . 52 (dd, 2H) ,
6 .
9
0
~ 7. 70 (m, 9H)
'
_
4b-3 4-CH$ 14 oil __
_
_
_ _____
1 . 20~ 2 . 30 (m
, 6H) , . 2.. 36'(s, 3H) , ..'
2 . 40~ 2 . 80 (m, 1H) , 2 . 90~
3 . 30 (m, 1H) ,
3.403.65 (m, 1H) , 3.653.85 (m, 1H)
,
3 . 95~ 4. 25 (m, 1H) , 4. 52 (dd,
2H) ,
...._.._..._..........._..._..__...._........__................................
......._.~ ~ ~ ~". ~:.:_'.~. (~._ 9 H ).........._.___...___........_.___
........_....._
4b-4 2, 4- (OCH' j = 17 oil 1 . 44~ 2. 52 (m, 7H) , 3 .02 (m,
1H) ,
3. 48 (m, 1H) , 3 . 69 (s, 3H) ,
3.71 (m, 1H) , 3.79 (s,3H) .
4.07 (m, 1Hj , 4.48 (d; 1Hj ,
4. 50 (d, 1H) , 6. 49 (d, 1H) , '
6. 52 (dd, 1Hj , 7. 22 (d,1Hj ,
7. 28 (m, 1Hj , 7 . 32N 7. 38 (m,
4Hj
x
2197178
Table 4
HO
~ N O
H
~0
(R 5)n
Compd (R5 ) n yield m. p. ' H-NMR (CDC19 )
No. (% ) (C ) ( 8 ppm)
1 ' 4-OCH9 81 120 1 . 20~ 2 . 70 (m, 10H) , 3 .
a-1 84 (s , 3H) ,
3. 90~ 4 . 30 (m, 1H) , 4. 20
(q, 1H) ,
121 6 . 88 (d, 2H) , 7. 59 (d, 2H)
1 ' H 37 137 . 1 . 36~ 2 . 29 (m, 7H) , 2. 45
a-2 5 (t , 2H) ,
3.96 (q, 1H) , 4.56 (q, 1H) ,
139 4. 86 (d, 1H) , 7. 41 (m, 2H)
,
7 . 48~ 7 . 56 (m, 3H)
1 ' 4-F 39 1 10 1 . 20~ 2 . 80 (m, 9H) ,
a-3
3. 90~ 4 . 25 (m, 1H) , 4. 73
(q, 1H) ,
._.._.._...._.._........_____..._._.....___.-......._......__......__ ~
~ ~m . 4 H )
~ 6
x :
2 8 5
"
7
:
. .............
. .
. .
..... ._
..
.
.
...._-..._.._..._....____...__.....__...._.
1 ' 4-CHs 27 131 1 . Z0~ 2 . 70 (m, 9H) , 2 .
a-4 37 (s , 3H) ,
~- 4.00 4. 25 (m, 1H) , 4. 71 (q,
1H) ,
.__......_..._-..___..._._.._..._..._..____.__..______________133 7 . 10~
7 . 60 (m, 4H)
1 ' a-5 2, 4- (OCH' ) = 56 1 14 1 . 28~ 2 . 27 (m, 7H) , 2. 39 (t, 3H) ,
~- 3. 73 (s, 3H) , 3. 80 (s, 3H) ,
1 15. 5 3.93 (m, 1H) , 4. 59 (q, 1H) ,
4. 84 (d, 1H) , 6. 49 (d, 1H) ,
6. 54 (dd, 1H) , 7. 31 (d, 1H)
50
219717s
Table 5
HO H
N 0
H 0
(R 5)n
Compd (R5 ) n yield m. p. ' H-NMR (CDCls )
No. (% ) (~ ) ( 8 ppm)
1 ' b-1 4-OCHg 72 108 1 . 50~ 1 . 70 (m, 2H) , 1 . 90~ 2 . 40 (m, 7H) ,
~- 3.003. 15 (m, 1H) , 3.503.65 (m, 1H) ,
109 3 .80 (s, 3H) , 3. 90~ 4. 10 (m, 1H) ,
__........_.__._.._____._..._......._______..__._._._.._......._...._.__._._...
. 6 ._ 9 ~ _~ a._~ 2 H ) , 7 . 5 5 ( d , 2 H )
1 ' b-2 4-F 30 oil 1 . 20~ 2 . 55 (m, 7H) , 2 . 9~5~ 3 . 30 (m, 1 H) ,
3 . 40~ 3 . 80 (m, 2H) , 3 . 90~ 4 . 30 (m, 2H) ,
6 . 90~ 7 . 70 (m, 4H)
1 ' b-3 4-CHs 28 104 1 . 30~ 2 . 60 (m, 7H) , 2 . 37 (s , 3H) ,
~- 2 . 90~ 3 . 30 (m, 1H) , 3 . 30~ 3 . 80 (m, 1H) ,
106 3 . 90~ 4 . 30 (m, 2H) , 7 . 10~ 7 . 65 (m, 4H)
Example 6
Preparation of cis-2-(p-toluoyl)-2-azabicyclo-
[4. 3.0] nonan-3, 7-dione (Compound 1 " '-2)
In 20 ml of dichloromethane were dissolved 0.3 g of
Compound 6b obtained in Example 11 and 0.39 g of 4-
methylbenzoyl chloride. Thereto was added dropwise 0.38 ml of
triethylamine with ice cooling. The mixture was reacted at
room temperature for 12 hours. The reaction mixture was washed
"~ X197178
51
with 0.1N hydrochloric acid, with 0.1N aqueous solution of
NaOH and then with water, and dried over anhydrous sodium
sulfate. After dried, the solvent was removed and the residue
was recrystallized to obtain 0.35 g of Compound mentioned
above (yield: 66 °/a). Table 6 shows analytical data.
Example 7
Compound 1 " ' -1 , and Compounds 1 " ' -3 to 1 " ' -1 1
were prepared in the same manner as in Example 6. Table 6
shows analytical data. Analytical data of NMR of Compounds
1 " '-3 and 1 " ' -4 were shown below.
Compound 1 " ' -3
' H-NMR (CDC13 ) 8 ppm
1 . 80--- 2. 90 {m, 9H) , 3. 75 (s, 3H) , 3 . 94~- 4. 20 (m, 1H) ,
6.70-r 7.04 (m, 2H) , 7.22 7. 48 (m, 2H)
~ 5 Compound 1 " ' -4
' H-NMR (CDC13 ) 8 ppm
1 .70~ 2.80 (m, 9H) , 3.80 (s, 3H) , 4.84- 5. 10 (m, 1H) ,
6.90-~- 7.35 (m, 4H)
25
'~ 2197~~8
52
Table 6
( C H ~ ( C ompound 1 " ' )
(R 5)n
Compound No. Q 1 (RS ) n yield (%
)
m.p. (
C
)
1"'-1 H 2 4-OCHa 83 169 171
1 ' ' ' -2 H 1 4-CHa 66 148- 149
1 " ' -3 H 1 2-OCHa 85 oil
1 "'-4 H 1 3-OCHa 89 oil
1~~~-5 H 1 4-OCHa 49 115 117
1,_6 H 1 2,4-(OCHa)z 74 153 154
1"'-7 H 1 2,6-~OCHa)z 68 144-r 145
1 " ' -8 H 1 3, 4- (OCHa ) 56 123- 125
z
1~~~-9 H 1 3,5-(OCHa)z 81 135 136
1"'-10 H 1 3,4,5-(OCHa)e 79 150 151
1 "'-1 1 CHa 1 4-OCHa 62 111 113
Preparation Example 1 Tablet
Compound 1'a-1 30 mg
Microcrystalline cellulose 50 mg
Hydroxypropyl cellulose 20 mg
Lactose 47 mg
Talc 2 mg
Magnesium stearate 1 mg
2197178
53
By the usual method, the above ingredients in the
proportions given were made into tablets each weighing
150 mg.
Preparation Example 2 Granule
Compound 1'a-5 10
mg
Lactose 400
mg
Corn starch 370 mg
Hydroxypropylmethyl cellulose 20 mg
The above ingredients in the proportions g iven were
made into a granular preparation by the usual method in an
amount of 800 mg per wrapper.
Preparation Example 3 Capsule
Compound 1'b-1 55 mg
Lactose 50 mg
Corn starch 50 mg
Microcrystalline cellulose 94 mg
Magnesium stearate 1
mg
By the usual method, the above ingredients in the
proportions given were made into a granular preparation
in an
amount of 250 mg in each capsule.
Preparation Example 4 Injection
Compound 1" '-8 10 mg
Sodium chloride 3.5 mg
Distilled water for injections, suitable amount
The above ingredients in the proportions gi ven
were made into an injection by the usual method.
Preparation Example 5 Syrup
Compound 1" '-9 50 mg
Purified sucrose 60 g
"' 2197~~s
54
Ethyl para-hydroxybenzoate Smg
Butyl para-hydroxybenzoate 5mg
Perfume suitable amount
Coloring agent suitable amount
Purified water suitable amount
The above ingredients in the proportions given
were made into a syrup by the usual method.
Preparation Example 6 Suppositories
Compound 1" '-10 50 mg
Witepsol W-35 1400 mg
(Trademark, a mixture of mono-, di- and triglyceride
of saturated fatty acids from lauric acid to stearic
acid, Dynamite Nobel Co., Ltd.)
By the usual method, the above ingredients in the
proportions given were made into suppositories.
Test examples are shown below in which 2-
azabicyclo[3.4.0]nonane-2-one disclosed in International
Public Disclosure No. WO 91/11434 was used as a comparison
compound.
Test Example 1
Anticonflict Test
1. Expermental animals
Wistar rats (males weighing 140 to 160 g) were used
for experiment in groups of 11 to 14.
2. Test agents and administration method
Compound 1'a-1, 1'a-5, i'b-1, 1"'-8, 1"'-9, the
above comparison compound, diazepam or buspirone was suspended
in a 0.5 9~ sodium carboxymethyl cellulose solution, and the
X
2197178
suspension was orally given to the animal in a volume of 5
ml/kg one hour before the start of experiment.
3. Experimental method and result
With reference to a method described in "Process in
5 Anxiolytics and Antidepressants," edited by Showa Ueki and
Tatsuo Furukawa, Ishiyaku Shuppansha, 56~ 59 (1981), the
agents were tested using experimental boxes having a grid
floor and a metal drinking tube in the floor. No water was
supplied to the rats for 48 hours before the experiment. Upon
10 lapse of first 24 hours, each group of rats were placed into
the experimental box, permitted access to water for 30 seconds
and caused to recognize the metal drinking tube. Upon lapse of
further 24 hours with access to water prevented, the rats were
placed into the box again and permitted access to water on
15 condition that an electric current was passed between the
metal drinking tube and the grid to give an electroshock for
every 20 times of water drinking behavior to measure the
frequency of water drinking behavior for 3 minutes. Anxiolytic
effect was evaluated as relieving rate of anxiety as
20 calculated by the following equation.
Relieving rate of anxiety = (C-B) / (A-B) x 100
A: Frequency of water drinking behavior of a control
group without an electroshock and no anxiety (frequency under
no punishment)
25 B: Frequency of water drinking behavior of a group
with an electroshock and anxiety (under punishment) and having
been administerd a solvent containing no test compound
(frequency under solvent-control group)
2197178
56
C: Frequency of water drinking behavior of a group
having been administered a test compound and relieved anxiety
(frequency under test compound-administered group)
Table 7 Anticonflict Test
Test compound Dose Relieving rate of
(mg/kg) anxiety (% )
Compound 1'a-1 0.01 g3
0. 1 86
1.0 76
Compound 1'a-5 0.01 g9
Compound 1'b-1 0.01 gp
Compound 1"'-8 0.01 g6
Compound 1" '-9 0.01 g6
Comparison 0.01 43
Compound
0.1 44
1.0 46
Diazepam 1.0 -3
Buspirone 1.0
From the above, the present compound decreased
anxiety almost nearly 100 $S at low doses of 0.01 to 0.1
mg/kg. Contrary, the comparison compound decreased anxiety up
to 50 ~ at the same dose. Diazepam and buspirone were found
almost ineffective even at a dose of 1.0 mg/kg. Accordingly,
the present compound exhibits extremely excellent anxiolytic
effect.
Test Example 2
X
2197 ~7g
57
Muscle Relaxant Effect (Traction Method)
1. Experimental animals and administration method
Compound 1'a-1, the comparison compound, diazepam or
buspirone was suspended in a 0.5 ~ sodium carboxymethyl
cellulose solution, and the suspension was orally administered
to 3- to 4-week-old male ddY mice (in groups of 5) in a volume
of 10 ml/kg one hour before the start of experiment.
2. Experimental method and result
With reference to a method described in Japan. J.
Pharmacol., 49. 337-349(1989), the foreleg of the mouse was
hung on a horizontal wire, having a diameter of 1.2 mm and
fixed at a level of 30 cm, three times consecutively. If the
hind leg did not touch the wire within 10 seconds each time,
the result was interpreted as positive. Thus, EDbo was
determined for evaluation. Consequently, Compound 1'a-:1 and
the comparison compound exhibited no muscle relaxant effect
even when given at a dose of 300 mg/kg. Diazepam and buspirone
were 2.2 mg/kg and 427.8 mg/kg, respectively, in EDbe-
Test Example 3
Sedative Effect (Spontaneous locomotor activity)
1. Experimental animals and administration method
Compound 1'a-1, the comparison compound, diazepam'or
buspirone was suspended in a 0.5 96 sodium carboxymethyl
cellulose solution, and the suspension was orally administered
to 3- to 4-week-old male ddY mice (in groups of 5) in a volume
of 10 ml/kg one hour before the start of experiment.
2. Experimental method and result
The test was conducted with reference to a method
i
...~
~19717g
58
described in "Evaluation of Medicinal Efficacies (1),
Pharmacological Test Method (I ), Basic Lectures on
Development of Pharmaceuticals," 50~-54(1971). More
specifically, the group of mice were given the test drug and
thereafter measured the amount of spontaneous locomotor
activity for 10 minutes per mouse using Animex MK-110TM
(Muromachi Kikai Co., Ltd.). When the amount of activity was
up to 50 % of the control group, the result was interpreted
as positive to determine EDso for evaluation. Consequently,
Compound 1'a-1 and the comparison compound exhibited no
sedative effect even at a dose of 300 mg/kg. Diazepam and
buspirone were 1.7 mg/kg and 149.7 mg/kg, respectively, in the
above value.
Test Example 4
Effect on central nervous system depressant (Ethanol
enhancing method)
1. Experimental method and administration method
Compound 1'a-1, the comparison compound, diazepam or
buspirone was suspended in a 0.5 % sodium carboxymethyl
cellulose solution, and the suspension was orally administered
to 3- to 4-week-old male ddY mice (in groups of 6) in a volume
of 10 ml/kg one hour before the start of experiment.
2. Experimental method and result
The test was conducted with reference to a method
described in Japan. J. Pharmacol., 49, 337-r 349(1989). More
specifically, the group of mice were intraperitoneally given
25 % ethanol at a dose of 20 ml/kg and checked for the time
interval between loss and recovery of the righting reflex.
2197178
59
When the time measurement was in excess of twice the
measurement of the control group, the result was interpreted
as positive to determine EDSO for evaluation. Consequently,
Compound 1'a-1 and the comparison compound produced no ethanol
enhancing effect even at a dose of 300 mg/kg. Diazepam and
buspirone were 0.48 mg/kg and 120.1 mg/kg, respectively, in
the value.
Test Example 5
Anticonvulsant Effect (Pentylenetetrazol-induced
Convulsion Method)
1. Experimental method and administration method
Compound 1'a-1, the comparison compound, diazepam or
buspirone was suspended in a 0.5 % sodium carboxyme~hyl
cellulose solution, aad the suspension was orally administered
to 3- to 4-week-old male ddY mice (in groups of 6) 1n a volume
of 10 ml/kg one hour before the start of experiment.
2. Experimental method and result
The test was conducted with reference to a method
described in "Evaluation of Medicinal Efficacies (1),
Pharmacological Test Method (I ), Basic Lectures on
Development of Pharmaceuticals," 1b7-r 172 (1971) .
More specifically, pentylenetetrazol was subcutaneously
administered to the mouse at a dose of 150 mg/kg, and when the
mouse did not die due to onset of convulsion within 60
minutes, the result was interpreted as positive to determine
ED6o for evaluation. Consequently, Compound 1'a-1 and the
comparison compound exhibited no anticonvulsant effect even at
a dose of 300 mg/kg. Diazepam and buspirone were 0.35 mg/kg
x.
2197178
and at least 300 mg/kg, respectively, in the value.
Test Example 6
Acute Toxicity Test
Five-week-old male ddY mice were used in groups of
6. The mice were orally given the test compound as suspended
in a 0.5 % sodium carboxymethyl cellulose solution and
thereafter observed for 3 days to measure the number of deaths
at each of doses. Table 8 shows the result.
Table 8 Acute Toxicity Test
Test compound Dose Number of Number
of
(mg/kg) animal death
Compound 1'a-1 2000 6 0
Compound 1'a-1 3000 6 2
Comprn. compnd 2000 6 1
Comprn. compnd 3000 6 5
From the above, although 5 mice died among 6 mice at
a dose of 3000 mg/kg in the comparison compound, only two mice
' died among 6 mice in the present compound. Accordingly, the
present compound is low in toxicity and high in safety
compared with the comparison compound.
INDUSTRIAL APPLICABILITY
The bicyclolactam derivative represented by the
formula (i) has an excellent anxiolytic effect, is reduced in
side effects such as sedative and muscle relaxant effects and
is low in toxicity. Accordingly, the agent comprising the
xJ
J
2197 178
61
present compound as an effective component is useful for
treating or preventing chronic or acute anxiety disorders (or
anxiety and fear neuroses), such as panic disorder accompanied
or not accompanied by agoraphobia, social phobia or simple
phobia, obsessive-compulsive disorder (neurosis), stress
disorder resulting from injury and systemic anxiety disorder,
and other anxiety disorders, and also for relieving healthy
persons and the aged of anxiety.
Additionally, the present invention is useful for
treating or preventing the anxiety attendant on withdrawal
symptoms due to drug dependance and/or drug addiction. Thus,
the present invention is useful for allaying withdrawal
symptoms due to alcohol dependence, nicotine dependence,
cocaine dependence and benzodiazepine dependence and
~5 withdrawal symptoms due to other drug dependence.
25
X