Note: Descriptions are shown in the official language in which they were submitted.
2197314
IMPROVED PROTEOLYTIC ENZYME CLEANER
Field of the Invention
The invention relates to enzyme containing
detergent compositions that can be used to remove food
soil from typically food or foodstuff related
manufacturing equipment or processing surfaces. The
invention relates to enzyme containing formulations in a
one and two part aqueous composition, a non-aqueous
liquids composition, a cast solid, a granular form, a
particulate form, a compressed tablet, a gel, a paste
and a slurry form. The invention also relates to
methods capable of a rapid removal of gross food soils,
films of food residue and other minor food or
proteinaceous soil compositions.
Background of the Invention
Periodic cleaning and sanitizing in the food
process industry is a regimen mandated by law and
rigorously practiced to maintain the exceptionally high
standards of food hygiene and shelf-life expected by
today's consumer. Residual food soil, left on food
contact equipment surfaces for prolonged periods, can
harbor and nourish growth of opportunistic pathogen and
food spoilage microorganisms that can contaminate
foodstuffs processed in close proximity to the residual
soil. Insuring protection of the consumer, against
potential health hazards associated with food borne
pathogens and toxins and, maintaining the flavor,
nutritional value and quality of the foodstuff, requires
diligent cleaning and soil removal from any surfaces of
which contact the food product directly or are
associatiecl with the processing environment.
The term "cleaning", in the context of the care and
maintenance of food preparation surfaces and equipment,
refers to the treatment given all food product contact
surfaces following each period of operation to
substantially remove food soil residues including any
residue that can harbor or nourish any harmful
microorganism. Freedom from such residues, however,
does not indicate perfectly clean equipment. Large
populations of microorganisms may exist on food process
surfaces even after visually successful cleaning. The
~'
2 2197314
concept of cleanliness as applied in the food process
plant is a continuum wherein absolute cleanliness is the
ideal goal always strived for; but, in practice, the
cleanliness achieved is of lesser degree.
The term "sanitizing" refers to an antimicrobicidal
treatment applied to all surfaces after the cleaning is
effected that reduces the microbial population to safe
levels. The critical objective of a cleaning and
sanitizing treatment program, in any food process
industry, is the reduction of microorganism populations
on targeted surfaces to safe levels as established by
public health ordinances or proven acceptable by
practice. This effect is termed a "sanitized surface"
or "sanitization". A sanitized surface is, by
Environmental Protection Agency (EPA) regulation, a
consequence of both an initial cleaning treatment
followed with a sanitizing treatment. A sanitizing
treatment applied to a cleaned food contact surface must
result in a reduction in population of at least 99.999%
reduction (5 log order reduction) for a given
microorganism. Sanitizing treatment is defined by
"Germicidal and Detergent Sanitizing Action of
Disinfectants", Official Methods of Analysis of the
Association of Official Analytical Chemists, paragraph
960.09 and applicable sections, 15th Edition, 1990 (EPA
Guideline 91-2). Sanitizing treatments applied to non-
food contact surfaces in a food process facility must
cause 99.9% reduction (3 log order reduction) for given
microorganisms as defined by the "Non-Food Contact
Sanitizt~r'Method, Sanitizer Test" (for inanimate, non-
food contact surfaces), created from EPA DIS/TSS-10, 07
January '82. Although it is beyond the scope of this
invention to discuss the chemistry of sanitizing
treatments, the microbiological efficacy of these
treatments is significantly reduced if the surface is
not clean prior to sanitizing. The presence of residual
food soil can inhibit sanitizing treatments by acting as
a physical barrier which shields microorganisms lying
within the soil layer from the microbicide or by
inactivating sanitizing treatments by direct chemical
interaction which deactivates the killing mechanism of
,~ .
3 2197314
the microbicide. Thus, the more perishable the food,
the more effective the cleaning treatment must be.
The technology of cleaning in the food process
industry has traditionally been empirical. The need for
cleaning treatments existed before a fundamental
understanding of soil deposition and removal mechanism
was developed. Because of food quality and public
health pressures, the food processing industry has
attained a high standard of practical cleanliness and
sanitation.
Soil removal cannot be considered a spontaneous
process because soil removal kinetics require a finite
period. The longer the cleaning solution is in contact
with the deposited soil, the more soil is removed - to a
practical limit. Final traces of soil become
increasingly difficult to remove. In the last phase of
the soil removal process, cleaning involves overcoming
the very strong adhesive force between soil and
substrate surface, rather than the weaker cohesive soil-
soil forces; and, an equilibrium state is eventually
attained when soil redeposition occurs at the same rate
as soil removal. Thus the major operational parameters
of a cleaning treatment in a food process facility are
mechanical work level, solution temperature, detergent
composition and concentration, and contact time. Of
course other variables such as equipment surface
characteristics; soil composition, concentration, and
condition; and water composition effect the cleaning
treatment. However, these factors cannot be controlled
and conSequently must be compensated for as required.
The food process industry has come to rely more on
detergent efficiency to compensate for design or
operational deficiencies in their cleaning programs.
This is not to suggest that the industry has not
addressed these factors; indeed, cleaning processes have
changed considerably during recent years because of
technological.advances in food processing equipment and
development of specialized cleaning equipment. Modern
food processing industries have revolutionized their
clean-up procedures through cleaning-in-place (CIP) and
automation.
~
4 2197314
A major challenge of detergent development for the
food process industry in the successful removal of soils
that are resistant to conventional treatment and the
elimination of chemicals that are not compatible with
food processing. One such soil is protein, and one such
chemical is chlorine or chlorine yielding compounds,
which can be incorporated into detergent compounds or
added separately to cleaning programs for protein
removal.
Protein soil residues, often called protein films,
occur in all food processing industries but the problem
is greatest for the dairy industry, milk and milk
products producers because these are among the most
perishable of major foodstuffs and any soil residues
have serious quality consequences. That protein soil
residues are common in the fluid milk and milk by-
products industry, including dairy farms, is no surprise
because protein constitutes approximately 27% of natural
milk solids, ("Milk Components and Their
Characteristics", Harper, W.J., in Diary Technology and
Engineering (editors Harper, W. J. and Hall, C. W.) p.
18-19, The AVI Publishing Company, Westport, 1976).
A growing source of protein adsorption information
is now in literature, specifically dealing with soils.
Studies have established that the same intrinsic
interactions and associations within the protein
molecule responsible for three-dimensional structure
also attract and bind proteins to surfaces. Because of
their size and complex structure, proteins contain
heterogOneous modules consisting of electrically charged
(both negative and positive) regions, hydrophobic
regions, and hydrophilic polar regions, analogous in
character to similar areas on food processing equipment
surfaces having trace soil residues. The protein can
thus interact with the hard surface in a variety of
different ways, depending on the particular orientation
exposed to the surface, the number of binding sites, and
overall binding energies.
Because biological fluids such as milk are complex
mixtures, the kinetics of the protein adsorption process
are confused by concurrent events occurring at
d'1
2197314
inte.rfacial surfaces within the bulk solution and at the
equipment surfaces. Temperature, pH, protein
populations and concentrations, and presence of other
inorganic and organic moieties have effect on rate
5 dynamics. In general, however, there is general
agreement that protein adsorption is rapid, reversible,
and randomly arranged at fractional surface coverages
less than 50%; and, the rate is mass transport
controlled, i.e. all adsorption and desorption processes
depend on transport of bulk solute to and from the
interface. As coverage exceeds 50%, surface ordering
develops, and given sufficient contact time, adsorbed
proteins undergo conformational and orientational
changes to optimize interfacial interactions and system
stability. Proteins less optimally adsorbed undergo
desorption or exchange by larger proteins having more
binding sites. The process rate becomes surface
reaction limited (mass action controlled). With
increasing residence time, protein adsorption becomes
irreversible.
Several representative articles describing food
soil deposition studies are: "Fouling of Heating
Surfaces - Chemical Reaction Fouling Due to Milk",
Sandu, C. and Lund, D., in Fouling and Cleaning in Food
Processing (editors Lund, D., Plett, E., and Sandu, C.),
pp. 122-167, University of Wisconsin-Madison Extension
Duplicating, Madison, 1985; and, "Model Studies of Food
Fouling", Gotham, S.M., Fryer, P.J., and Pritchard,
A.M., in Fouling and Cleaning in Food Processing
(editors Kessler, H. B. and Lund, D. B.), pp. 1-13,
Druckerei Walch, Augsburg, 1989; and "Fouling of Milk
Proteins and Salts - Reduction of Fouling by
Technological Measures", Kessler, H.B., Ibid., pp. 37-
45.
Researchers conducting soil removal experiments in
the 1950's with the then new concept of recirculation
cleaning (latter termed clean-in-place or CIP to
encompass different methodologies) observed the
occurrence of protein films on milk process equipment
surfaces. Subsequently, the addition of hypochlorite to
CIP alkaline detergent compounds was found to help
A
6 2197314
remove protein film; and, this technology has been
employed to-date by suppliers of cleaning compounds to
the general food process industry. (For example, see
"Effect of Added Hypochlorite on Detergent Activity of
Alkaline Solutions in Recirculation Cleaning",
MacGregor, D.R., Elliker, P.R., and Richardson, G.A.,
Jnl. of Milk & Food Technology, Vol. 17, pp. 136-138
(1954); "Further Studies on In-Place Cleaning",
Kaufmann, O.W., Andrews, R.H., and Tracy, P.H., Journal
of Dairy Science, Vol. 38, No. 4, 371-379 (1955); and,
"Formation and Removal of an Iridescent Discoloration in
Cleaned-In-Place Pipelines", Kaufmann, O.W. and Tracy,
P.H., Ibid., Vol. 42, pp. 1883-1885 (1959).
Chlorine degrades protein by oxidative cleavage and
hydrolysis of the peptide bond, which breaks apart large
protein molecules into smaller peptide chains. The
conformational structure of the protein disintegrates,
dramatically lowering the binding energies, and
effecting desorption from the surface, followed by
solubilization or suspension into the cleaning solution.
The use of chlorinated detergent solutions in the
food process industry is not without problems.
Corrosion is a constant concern, as is degradation of
polymeric gaskets, hoses, and appliances. Practice
indicates that available chlorine concentrations must
initially be at least 75, and preferably, 100 ppm for
optimum protein film removal. At concentrations of
available chlorine less than 50 ppm, protein soil build-
up is enhanced by formation of insoluble, adhesive
chloro-pr-oteins (see "Cleanability of Milk-Filmed
Stainless Steel by Chlorinated Detergent Solutions",
Jensen, J.M., Journal of Dairy Science, Vol. 53, No. 2,
pp. 248-251 (1970). Chlorine concentrations are not
easy to maintain or analytically discern in detersive
solutions. The dissipation of available chlorine by
soil residues has been well established; and, chlorine
can form unstable chloramino derivatives with proteins
which titrate as available chlorine. The effectiveness
of chlorine on protein soil removal diminishes as
solution temperature and pH decrease -- lower.
~
7 2197314
temperatures affecting reaction rate, and lower pH
favoring chlorinated additional moieties.
These problems associated with the use and
applications of chlorine release agents in the food
process industry have been known and tolerated for
decades. Chlorine has improved cleaning efficiency, and
improved sanitation resulting in improved product
quality. No safe and effective, lower cost alternative
has been advanced by the detergent manufacturers.
However, a new issue may force change upon both the
food process industry and the detergent manufacturers --
the growing public concern over the health and
environmental impacts of chlorine and organochlorines.
Whatever the merits of the scientific evidence regarding
carcinogenicity, there is little argument that
organohalogen compounds are persistent and
bioaccumulative; and that many of these compounds pose
greater non-cancer health effects -- endoctrine, immune,
and neurological problems -- principally in the
offspring of exposed humans and wildlife, at extremely
low exposure levels. It is, therefore, prudent for the
food process industry and their detergent suppliers to
refocus on finding alternatives to the use of chlorine
release agents in cleaning compositions.
A substantial need exists for a non-chlorine,
protein film stripping agent for detergent compositions
having applications in the food process industry, and
having the versatility to remedy the problems heretofore
described and presently unresolved.
Al~_Yiough enzymes were discovered in the early
1830's and their importance prompted intensive study by
biochemists, public record of research into applications
of enzymes in detergents first occurred in 1915 when
German Patent No. 283,923 issued (May 4) to 0. Rohm,
founder of Rohm & Haas for application of pancreatic
enzymes in laundry wash products. E. Jaag of the Swiss
firm Gebrueder Schnyder developed this enzyme detergent
concept further over the course of 30 years work; and,
in 1959, introduced to market a laundry product, Bio 40,
which contained a bacterial protease having considerable
advantages over pancreatic trypsin. However, this
G~.
~..
8 2197314
bacterial protease was still"not sufficiently stable at
normal use pH of 9-10 and had marginal activity upon
typical stains. It took several more years of research,
until the mid 1960's, before bacterial alkaline
proteases were commercial which had all of the necessary
pH stability and soil reactivity characteristics for
detergent applications.
Although use of enzymes in cleaning compositions
did exist prior (see for example U.S. Pat. No. 1,882,279
to Frelinghuysen issued October 11, 1932), large scale
commercial enzyme containing laundry detergents first
appeared in the United States in,test market during
1966.
The progression from dry to liquid detergent
compositions containing enzymes was a natural
consequence of inherent problems with dry powder forms.
Enzyme powders or granulates tended to segregate in
these mechanical mixtures resulting in non-uniform, and
hence undependable, product in use. Precautions had to
be taken with packaging and in storage to protect the
product from humidity which caused enzyme degradation.
Dry powdered compositions are not as conveniently suited
as liquids for rapid solubility or miscibility in cold
and tepid waters nor functional as direct application
products to soiled surfaces. For these reasons and for
expanded applications, it became desirable to have
liquid enzyme compositions.
Economic as well as processing considerations
suggest the use of water in liquid enzyme compositions.
Howeve'r,' there are also inherent problems in
formulating enzymes into aqueous compositions. Enzymes
generally denature or degrade in an aqueous medium
resulting in the serious reduction or complete loss of
enzyme activity. This instability results from at least
two mechanisms. Enzymes have three-dimensional protein
structure which can be physically or chemically changed
by other solution ingredients, such as surfactants and
builders, causing loss of catalytic effect. Alternately
when protease is present in the composition, the
protease will cause proteolytic digestion of the other
A.
9 2197314
enzymes if they are not proteases; or of itself via a
process called autolysis.
Examples in the prior art have attempted to deal
with these aqueous induced enzyme stability problems by
minimizing water content (see U.S. Pat. No. 3,697,451 to
Mausner et al. issued October 10, 1972) or altogether
eliminating water from the liquid enzyme containing
composition (see U.S. Pat. No. 4,753,748 to Lailem et
al. issued June 28, 1988). As disclosed in Mausner et
al. (Ibid.) and apparent from Lailem et al. (Ibid.),
water is advantageous to dissolve the enzyme(s) and
other water soluble ingredients, such as builders, and
effectively carry or couple them into the non-aqueous
liquid detergent vehicle to effect a homogenous,
isotropic liquid which will not otherwise phase
separate.
In order to market an aqueous enzyme composition,
the enzyme must be stabilized so that it will retain its
functional activity for prolonged periods of (shelf-life
or storage) time. If a stabilized enzyme system is not
employed, an excess of enzyme is generally required to
compensate for expected loss. Enzymes are, however,
expensive and are the most costly ingredients in a
commercial detergent even though they are present in
relatively minor amounts. Thus, it is no surprise that
methods of stabilizing enzyme-containing, aqueous,
liquid detergent compositions are extensively described
in the patent literature. (See, Guilbert, U.S. Pat. No.
4,238,345).
Whe"reas the stabilizers used in liquid aqueous
enzyme detergent compositions inhibit enzyme
deactivation by chemical intervention, the literature
also includes enzyme compositions which contain high
percentages of water, but the water or the enzyme or
both are immobilized; or otherwise physically separated
to prevent hydrolytic interaction. For example of any
aqueous enzyme encapsulate formed by extrusion, see U.S.
Pat. No. 4,087,368 to Borrello issued May 2, 1978. For
example of a gel-like aqueous based enzyme detergent,
see U.s. Patent No. 5,064,553 to Dixit et al. issued
November 12, 1991. For example of a dual component,
~
2197314
two-package composition wherein the enzyme is separated
from the alkalies, builders and sequestrants, see U.S.
Pat. No. 4,243,543 to Guilbert et al. issued January 6,
1981.
5 Enzyme containing detergent compositions presently
have very limited commercial applications within the
food process industries. A small, but significant
application for enzymes with detergents is the cleaning
of reverse osmosis and ultra filtration (RO/UF)
10 membranes -- porous molecular sieves not too dissimilar
from synthetic laundry fabrics. Hard surface cleaning
applications are almost non-existent with exception of
high foam detergents containing enzymes being used
occasionally in red meat processing plants for general
environmental cleaning.
In 1985, a paper authored by D. R. Kane and N.E.
Middlemiss entitled "Cleaning Chemicals - State of the
Knowledge in 1985" (in Fouling and Cleaning in Food
Processing; editors Lund, D. Plett, E., and Sandu, C.;
pp. 312-335, University of Wisconsin - Madison Extension
Duplicating, Madison, 1985) was delivered to the Second
International Conference of Fouling and Cleaning in Food
Processing. This paper emphasized CIP (clean-in-place)
cleaning in the dairy industry. Within the text of this
paper, the authors conclude that enzyme use in the food
cleaning industry is not widespread for several reasons
including enzyme instability at high pH and over time,
enzyme and enzyme stabilizer cost, concern about
residual enzyme and adverse effect on foodstuff quality,
enzyme Incompatibility with chlorine, slow enzyme
reactivity necessitating long cleaning cycle times, and
no commercial justification.
The present invention addresses and resolves these
issues and problems.
The patent art does contain prior disclosure of
enzyme containing detergent,compositions having
application on food process equipment. U.S. Pat. No.
4,169,817 to Weber issued October 2, 1979 discloses a
liquid cleaning composition containing detergent
builders, surfactants, enzyme and stabilizing agent.
The compositions claimed by Weber may be employed as a
~
11 2197314
laundry detergent, a laundry pre-soak, or as a general
purpose cleaner for dairy and cheese making processing
equipment. The detergent solution of Weber generally
has a pH in the range of 7.0 to 11Ø
The aforementioned prior teaching embodies high
foam surfactants and fails to provide detergents which
can be utilized in CIP cleaning systems.
U.S. Pat. No. 4,212,761 to Ciaccio issued July 15,
1980 discloses a neat or use solution composition
containing a ratio of sodium carbonate and sodium
bicarbonate, a surfactant, an alkaline protease, and
optionally sodium tripolyphosphate. The detergent
solution of Ciaccio is used for cleaning dairy equipment
including clean-in-place methods. The pH of the use
solution in Ciaccio ranges from 8.5 to 11.
In Ciaccio, no working examples of detergent
concentrate embodiments are disclosed. Ciaccio only
asserts that the desirable detergent form would be as a
premixed particulate. From the ingredient ranges
discussed, it becomes obvious to one skilled in the art
that such compositions would be too wet, sticky, and
mull-like in practice to be readily commercialized.
U.S. Pat. Nos. 4,238,345 and 4,243,543 to Guilbert
issued January 6, 1981 teach a liquid two-part cleaning
system for clean-in-place applications wherein one part
is a concentrate which consists essentially of a
proteolytic enzyme, enzyme stabilizers, surfactant and
water; with the second concentrated part comprised of
alkalies, builders, sequestrants and water. When both
parts were blended at use dilution in Guilbert, the pH
of this use solution was typically 11 or 12.
U.S. Pat. No. 5,064,561 to Rouillard issued
November 12, 1991 discloses a two-part cleaning system
for use in clean-in-place facilities. Part one is a
liquid concentrate consisting of a highly alkaline
material (NaOH), defoamer, solubilizer or emulsifier,
sequestrant and water. Part two is a liquid concentrate
containing an enzyme which is a protease generally
present as a liquid or as a slurry within a non-aqueous
carrier which is ordinarily an alcohol, surfactant,
~
CA 02197314 2005-05-24
12
polyol or mixture thereof. The use solution of
Rouillard generally has a pH of about 9.5 to about 10.5.
Rouillard teaches the use of high alkaline
materials; and, paradoxically, the optional use of
buffers to stabilize the pH of the composition.
Rouillard's invention discloses compositions wherein
unstable aqueous mixtures of inorganic salts and organic
defoamer are necessarily coupled by inclusion of a
solubilizer or emulsifier to maintain an isotropic
liquid concentrate. Rouillard further teaches that the
defoamer may not always be required if a liquid (the
assumption of term is "aqueous, stabilized") form of the
enzyme is used in the second concentrate. This
disclosure would seem to result from the use of Esperase
8.0 SLTM identified as a useful source of enzyme in the
practice of the invention and utilized in working
examples. Additional detail indicates Esperase 8.0 SLTM
is a proteolytic enzyme suspended in Tergitol 15-S-9TM, a
high foam surfactant -- hence the need for a defoamer
and for a solubilizer or emulsifier. Rouillard still
further discloses that proteolytic enzyme (Esperase 8.0
SLTM) of an by itself does not clean as effectively as a
high alkaline, chlorinated detergent unless mixed with
its cooperative alkaline concentrate.
Summary of the Invention
This invention discloses formulations, methods of
manufacture and methods of use for compositional
embodiments having application as detergents in the food
process~industry. Said compositions are used in
cleaning food soiled surfaces. The materials are made
in concentrated form. The diluted concentrate when
delivered to the targeted surfaces will provide
cleaning. The concentrate products can be a one part or
a two part product in a liquid or emulsion form; a
solid, tablet, or encapsulate form; a powder or particulate
form of size ranging from 0,05mm to 1 mm; a gel or paste; or a
slurry or mull. The concentrate products being manufactured
by any number of liquid and solid blending methods known to
the art inclusive of casting, pour-molding, compressions-
molding, extrusion-molding or similar shape - packaging
CA 02197314 2005-05-24
13
operations. Said products being enclosed in metal, plastic,
composite, laminate, paper, paperboard, disposable, or
water soluble protective packaging. Said products being
designed for clean-in-place (CIP), and clean-out-of-
place (COP) cleaning regimens in food process industries
such as dairy farm; fluid milk and processed milk by-
product; red meat, poultry, fish, and respective
processed by-products; soft drink, juice, and fermented
beverages; egg, dressings, condiments, and other fluid
food processing;and, fresh, frozen, canned or ready-to-
serve processed foodstuffs.
More specifically, the present invention describes
detergent compositions generally containing enzymes,
surfactants, low alkaline builders, water conditioning
agents; and, optionally a variety of formulary adjuvants
depending upon product form and application such as (but
not limited to) enzyme stabilizers, thickeners,
solidifiers, hydrotropes, emulsifiers, solvents,
antimicrobial agents, tracer molecules, coloring agents;
and, inert organic or inorganic fillers and carriers.
The claimed compositions eliminate the need for
high alkaline builders, axillary defoamers, corrosion
inhibitors, and chlorine release agents. Accordingly
the claimed compositions are safer to use and resulting
effluent is friendly to the environment. When used, the
claimed composition will continue to clean soiled food
process equipment surfaces equal to or better than
present, conventional chlorinated - high alkaline
detergents.
Wehave also found oxidizing sanitizing agents that
when applied to pre-cleaned and pre-rinsed surfaces as a
final sanitizing rinse, following a cleaning program
utilizing enzyme containing detersive solutions, have a
surprising profound deactivating effect upon residual
enzymes.
We have also found preferred methods of cleaning
protein containing food processing units. In the
preferred methods of the invention, the food processing
units having at least some minimal film residue derived
from the protein containing food product, is contacted
with a protease containing detergent composition of the
CA 02197314 2004-07-06
14
invention. Optionally, prior to contacting the food
processing surface with the detergent, the unit can be
prerinsed with an aqueous rinse composition to remove
gross food soil. The protein residue on the food
processing unit is contacted with a detergent of the
invention for a sufficient period of time to remove the
protein film. Any protease enzyme residue remaining on
the surfaces of the unit or otherwise within the food
processing unit, can be denatured using a variety of
techniques. The food processing unit can be heated with
a heat source comprising steam, hot water, etc. above
the denaturing temperature of the protease enzyme.
Typically, temperatures required range from about 60-
90 C, preferably about 60-80 C. Further, the residual
protease enzyme remaining in the food processing unit
can be denatured by exposing the enzyme to an extreme
pH. Typically, a pH greater than about 10, preferably
greater than about 11 (alkaline pH) or less than 5,
preferably less than about 4 (acid pH) is sufficient to
denature the enzyme.
Additionally, the protease can be denatured by
exposing any residual protease enzyme to the effects of
an oxidizing agent. A variety of known oxidizing agents
that also have the benefit of acting as a food
acceptable sanitizer include aqueous hydrogen peroxide,
aqueous ozone containing compositions, aqueous peroxy
acid compositions wherein the peroxy acid comprises a
per C1-24 monocarboxylic or dicarboxylic acid composition
or mixtures thereof.
Additionally, hypochlorite, iodophors and interhalogen
complexts- (IC1, C1Br, etc.) can be used to denature the
enzyme if used in accordance with accepted procedures.
Denatured enzyme remaining in the system after the
denaturing step can have little or no effect on any
proteinaceous food. The resulting product quality is
unchanged. Preferred foods treated in food processing
units having a denaturing step following the cleaning
step include milk and dairy products, beer and other
fermented malt beverages, puddings, soups, yogurt, or
any other liquid, thickened liquid, or semisolid protein
containing food material.
CA 02197314 2006-01-31
15.
The objectives of this product invention are thus
to:
provide the food process industry and operations
concerned about environmental hygiene with a low alkaline,
non-chlorine detergent alternative to conventional
products;
satisfy a commercial need for cost effective, user
friendly, less environmentally intrusive detergents;
facilitate utility and scope of application with a
family of said detergents having diverse physical form and
differing composition for a broad range of food soil type
and cleaning program parameter variations; and resolve
objections to the use of detersive enzymes for cleaning in
food process environments which are sensitive to enzyme
residuals by teaching cooperative cleaning and sanitizing
programs which assure complete deactivation of enzyme
prior to food contact.
In an aspect, the invention provides a low foaming
liquid stabilized enzyme-containing detergent composition
having application in the food process industry, the
composition being substantially free of an alkali metal
hydroxide and free of a source of active chlorine, the
composition comprising:
(a) about 10-90 wt % of a liquid medium;
(b) an effective proteolytic amount of an
enzyme composition;
(c) an effective enzyme stabilizing amount of
an aqueous soluble or dispersible stabilizing system
comprising an antioxidant composition and an organic water
soluble or dispersible polyol compound having 2-10
hydroxyl groups;
CA 02197314 2007-03-27
15a
(d) an effective building and conditioning
amount of a builder and a water conditioning agent; and
(e) a low foaming surfactant selected from the
group consisting of R-- (EO)e-- (PO) pH; R-- (EO)e-- (BO) bH; R--
(EO)e--Rl; [R-- (PO)p-- (EO)eH] ; R-- (PO)p-- (EO)e-- (PO)pH; R--
(PO)p--(EO)e-benzyl; (PO)p--(EO)e--(PO)p; [(PO)p--(EO)e--]2--
NCH2CH2N- - [(EO) e- - (PO) p] 2; or mixtures thereof ;
wherein R is a C6_18 alkyl group, a C6_18
alkyl or dialkyl phenol group, or a C6_18 alkyl- (PO) p-group;
R1 is a C1_8 alkyl; each e is independently about 1-20, each
p is independently about 1-20, and each b is independently
about 1-10.
The invention further provides a low foaming
liquid stabilized enzyme-containing detergent composition,
the composition being substantially free of alkali metal
hydroxides and free of sources of active chlorine, the
composition comprising:
(a) about 10-90 wt R. of a liquid medium;
(b) an effective proteolytic amount of an
enzyme composition;
(c) an effective enzyme stabilizing amount of
an aqueous soluble or dispersible stabilizing system
comprising an antioxidant composition and an organic water
soluble or dispersible polyol compound having 2-10
hydroxyl groups;
(d) an effective building and conditioning
amount of a builder and a water conditioning agent; and
(e) a low foaming surfactant comprising:
R- (EO) e- (PrO) pH; R- (EO) e- (BuO) bH; R- (EO) e-Rl;
[R- (PrO)p- (EO)eH] ; R- (PrO)p- (EO)e- (PrO)pH;
R-(PrO)p-(EO)e-benzyl; (PrO)p-(EO)e-(PrO)p;
CA 02197314 2007-03-27
15b
[(PrO) p- (EO) e] 2NCH2CH2N [(EO) e- (PrO) p] Z; or mixtures
thereof;
wherein EO is ethylene oxide; BuO is butylene
oxide; PrO is propylene oxide; R is a C6_1B alkyl group, a
C6_16 alkyl or dialkyl phenol group, or a C6_18 alkyl- (PO)p-
group; R1 is a Cl_$ alkyl; each e is independently 1-20;
each p is independently 1-20; and each b is independently
1-10.
The invention further provides a low foaming
stabilized solid block enzyme-containing detergent
composition having application in the food process
industry, the composition being substantially free of an
alkali metal hydroxide and free of a source of active
chlorine, the composition comprising:
(a) 10-90 wt t of a solidifying agent;
(b) an effective proteolytic amount of an
enzyme composition;
(c) an effective enzyme stabilizing amount of
water dispersible stabilizing system comprising an
antioxidant composition and an organic water soluble or
dispersible polyol compound having 2-10 hydroxyl groups;
(d) an effective water conditioning amount of
water conditioning agent;
(e) an effective building amount of a builder
salt; and
(f) a low foaming surfactant selected from the
group consisting of R--(EO)e--(PO)pH; R--(EO)e--(BO)bH; R--
(EO)e--Rzi [R-- (PO)p-- (EO)eH] ; R-- (PO)p-- (EO)e-- (PO)pH; R--
(PO)p--(EO)e-benzyl; (PO)p--(EO)e--(PO)p; [(PO)p--(EO)e--]2--
NCH2CH2N--[(EO)e--(PO)p]2; or mixtures thereof;
CA 02197314 2007-03-27
15c
wherein R is a C6_18 alkyl group, a C6_18
alkyl or dialkyl phenol group, or a C6_18 alkyl-(PO)P-group;
R1 is a C1_$ alkyl; each e is independently about 1-20, each
p is independently about 1-20, and each b is independently
about 1-10.
The invention further provides a low foaming
stabilized solid block enzyme-containing detergent
composition, the composition being substantially free of
alkali metal hydroxides and free of sources of active
chlorine, the composition comprising:
(a) 10-90 wt o of a solidifying agent;
(b) an effective proteolytic amount of an
enzyme composition;
(c) an effective enzyme stabilizing amount of a
water dispersible stabilizing system comprising an
antioxidant composition and an organic water soluble or
dispersible polyol compound having 2-10 hydroxyl groups;
(d) an effective water conditioning amount of a
water conditioning agent;
(e) an effective building amount of a builder
salt; and
(f) a low foaming surfactant comprising:
R- (EO) e- (PrO) PH; R- (EO) e- (BUO) bH; R- (EO) ,-Rl;
[R- (PrO)p - (EO)eH] ; R- (Pr0)P- (EO)e - (PrO)PH;
R- (PrO) P- (EO) e-benzyl; (PrO) p- (EO) ,- (PrO) P;
[(PrO)P-(EO)e]zNCH2CH2N[(EO)e-(PrO)P]z; or mixtures
thereof;
wherein EO is ethylene oxide; BuO is butylene
oxide; PrO is propylene oxide; R is a C6_18 alkyl group, a
C6_lg alkyl or dialkyl phenol group, or a C6_18 alkyl- (PO)P-
group; R1 is a C1_8 alkyl; each e is independently 1-20;
CA 02197314 2007-03-27
15d
each p is independently 1-20; and each b is independently
1-10.
The invention further provides a low foaming
stabilized particulate enzyme-containing detergent
composition adapted for use in the food process industry,
the composition being substantially free of an alkali
metal hydroxide and free of a source of active chlorine,
the composition comprising:
(a) an effective proteolytic amount of an
enzyme composition;
(b) an effective enzyme stabilizing amount of a
water dispersible stabilizing system comprising an
antioxidant composition and an organic water soluble or
dispersible polyol compound having 2-10 hydroxyl groups;
(c) an effective water conditioning amount of a
water conditioning agent;
(d) an effective building amount of a builder;
and
(e) a low foaming surfactant selected from the
group consisting of R--(EO)e--(PO)pH; R--(EO)e--(BO)bH; R--
(EO)e--Rl; [R-- (PO)p-- (EO)eH] ; R-- (PO)p-- (EO)e-- (PO)pH; R--
(PO)p--(EO)e-benzyl; (PO)p--(EO)e--(PO)p; [(PO)p--(EO)e--]2 -
-NCHzCHzN--[(EO)e--(PO)p]Z; or mixtures thereof;
wherein R is a C6_18 alkyl group, a C6_la
alkyl or dialkyl phenol group, or a C6_18 alkyl- (PO) p-group;
R1 is a C1_$ alkyl; each e is independently about 1-20, each
p is independently about 1-20, and each b is independently
about 1-10.
The invention further provides a low foaming
stabilized particulate enzyme-containing detergent
composition, the composition being substantially free of
CA 02197314 2007-03-27
15e
alkali metal hydroxides and free of sources of active
chlorine, the composition comprising:
(a) an effective proteolytic amount of an
enzyme composition;
(b) an effective enzyme stabilizing amount of a
water dispersible stabilizing system comprising an
antioxidant composition and an organic water soluble or
dispersible polyol compound having 2-10 hydroxyl groups;
(c) an effective water conditioning amount of a
water conditioning agent;
(d) an effective building amount of a builder;
and
(e) a low foaming surfactant comprising:
R- (EO) e- (PrO) pH; R- (EO) e- (BuO) bH; R- (EO) e-Rl;
[R- (PrO) P- (EO) eH] ; R- (PrO) P- (EO) e- (PrO) PH;
R-(PrO)P-(EO)e-benzyl; (PrO)p-(EO)e-(PrO)P;
[(PrO) P- (EO) e] 2NCH2CH2N [(EO) e- (PrO) P] 2; or mixtures
thereof;
wherein EO is ethylene oxide; BuO is
butylene oxide; PrO is propylene oxide; R is a C6_18 alkyl
group, a C6_18 alkyl or dialkyl phenol group, or a C6_18
alkyl- (PO)P-group; Rl is a C1_8 alkyl; each e is
independently 1-20; each p is independently 1-20; and each
b is independently 1-10.
The invention further provides a two-part, low-
foaming stabilized enzyme liquid detergent composition
having application in the food process industry, the two
part detergent being substantially free of an alkali metal
hydroxide and free of a source of active chlorine, the two
part detergent comprising a liquid enzyme part and an
aqueous builder part, each part separately packaged to
CA 02197314 2007-03-27
15f
ensure enzyme activity when blended and used, said two
part system comprising:
(a) a liquid enzyme part comprising:
(i) an active cleaning amount of a
proteolytic enzyme;
(ii) a stabilizing system comprising about
0.5 to 30 wt g of an antioxidant and about 1 to 25 wt
t of a polyol;
(iii) a liquid medium; and
(iv) an effective detersive amount of a low
foaming surfactant selected from the group consisting
of R-- (EO)e-- (PO)pH; R-- (EO)e-- (BO)bH; R-- (EO)e--Rl;
[R-- (PO)p-- (EO)eH] ; R-- (PO)p-- (EO)e-- (PO)pH; R-- (PO)p--
(EO)e-benzyl; (PO)p--(EO)e--(PO)p; [(PO)p--(EO)e--]z --
NCHzCHzN- -[( EO ) e- -( PO ) p) 2; or mixtures thereof;
wherein R is a C6_18 alkyl group, a C6_18
alkyl or dialkyl phenol group, or a C6_18 alkyl- (PO) p-
group; Rl is a C1_8 alkyl; each e is independently
about 1-20, each p is independently about 1-20, and
each b is independently about 1-10; and
(b) an aqueous builder part comprising;
(i) about 10 to 50wt o of an alkali metal
carbonate or an alkali metal silicate building salt,
or a mixture thereof; and
(ii) an effective amount of a water
conditioning agent.
The invention further provides a two-part, low-
foaming stabilized enzyme liquid detergent system, the two
part detergent being substantially free of an alkali metal
hydroxide and free of a source of active chlorine, the two
part detergent comprising a liquid enzyme part and an
CA 02197314 2007-03-27
15g
aqueous builder part, each part separately packaged to
ensure enzyme activity when blended and used, said two
part system comprising:
(a) a liquid enzyme part comprising:
(i) an active cleaning amount of a
proteolytic enzyme;
(ii) a stabilizing system comprising about
0.5 to 30 wt t of an antioxidant and about 1 to 25 wt % of
a polyol;
(iii) a liquid medium; and
(iv) an effective detersive amount of a low
foaming surfactant comprising: R-(EO)e-(PrO)pH; R-(EO)e-
(BUO)bH; R- (EO)e-Rl; [R- (PrO)p- (EO)eH] ;
R- (PrO) p- (EO) e- (PrO) pH; R- (PrO) p- (EO) e-benzyl;
(PrO)p-(EO)e-(PrO)p; [(PrO)p-(EO)e]zNCH2CH2N[(EO)e-(Pr0)p]2i
or mixtures thereof; wherein EO is ethylene oxide; BuO is
butylene oxide; PrO is propylene oxide; R is a C6_18 alkyl
group, a C6_18 alkyl or dialkyl phenol group, or a C6_18
alkyl-(PrO)p-group; R1 is a C1_8 alkyl; each e is
independently 1-20; each p is independently 1-20; and each
b is independently 1-10; and
(b) an aqueous builder part comprising;
(i) about 10 to 50wt o of an alkali metal
carbonate or an alkali metal silicate building salt,
or a mixture thereof; and
(ii) an effective amount of a water
conditioning agent.
The invention further provides a method of
cleaning a food processing unit for a protein containing
food product, which method comprises:
(a) contacting a surface of the food processing
unit having a proteinaceous film residue with a dilute
CA 02197314 2007-03-27
15h
use-solution of a low foaming protease enzyme detergent
composition for sufficient period of time to substantially
remove the proteinaceous soil from the surface of the food
processing unit, leaving residual protease activity, the
low foaming protease enzyme detergent comprising a liquid
enzyme part and an aqueous builder part, each part
separately packaged to ensure enzyme activity when blended
and used, said two part system comprising:
(A) a liquid enzyme part comprising:
(i) an active cleaning amount of a
proteolytic enzyme;
(ii) a stabilizing system comprising
about 0.5 to 30 wt o of an antioxidant and about 1 to
25 wt o of a polyol;
(iii) a liquid medium; and
(iv) a surfactant; and
(B) an aqueous builder part comprising:
(i) about 10 to 50 wt o of an alkali
metal carbonate or an alkali metal silicate builder
salt; and
(ii) an effective water conditioning
amount of a water conditioning agent; and
(b) denaturing the residual protease enzyme
activity with an oxidizing agent such that the product
made by the unit is not affected by residual enzyme
activity; whereby denatured enzymes have little or no
effect on a proteinaceous food.
The invention further provides a method of
cleaning a food processing unit for a protein containing
food product, which method comprises:
(a) contacting a surface of the food processing
unit having a proteinaceous film residue with a dilute-use
CA 02197314 2007-03-27
15i
solution of a low foaming protease enzyme detergent
composition for a sufficient period of time to
substantially remove the proteinaceous film from the
surface of the food processing unit, leaving residual
protease activity, the low foaming protease enzyme
detergent comprising a liquid enzyme part and an aqueous
builder part, each part separately packaged to ensure
enzyme activity when blended and used, said detergent
comprising:
(A) a liquid enzyme part comprising:
(i) an active cleaning amount of a
proteolytic enzyme;
(ii) a stabilizing system comprising
about 0.5 to 30 wt o of an antioxidant and about 1 to
25 wt o of a polyol;
(iii) a liquid medium; and
(iv) a surfactant; and
(B) an aqueous builder part comprising:
(i) about 10 to 50 wt o of an alkali
metal carbonate or an alkali metal silicate builder
salt; and
(ii) an effective water conditioning
amount of a water conditioning agent; and
(b) denaturing the residual protease enzyme activity
with an oxidizing agent such that the product made by the
unit is not affected by residual enzyme activity; whereby
the denatured enzymes have little or no effect on a
proteinaceous food.
The invention further provides a method of
cleaning a food processing unit for a protein containing
food product, which method comprises:
(a) contacting a surface of the food processing
CA 02197314 2007-03-27
15j
unit having a proteinaceous film residue with a dilute
use-solution of a low foaming protease enzyme detergent
composition for sufficient period of time to substantially
remove the proteinaceous soil from the surface of the food
processing unit, leaving residual protease activity, the
low foaming protease enzyme detergent comprising:
(i) about 10-90 wt % of a liquid medium;
(ii) an effective proteolytic amount of an
enzyme composition;
(iii) an effective enzyme stabilizing
amount of a aqueous soluble or dispersible
stabilizing system comprising an antioxidant
composition and an organic water soluble or
dispersible polyol compound having 2-10 hydroxyl
groups; and
(iv) a surfactant selected from the group
consisting of R-- (EO)e -- (PO) p H; R-- (EO)e -- (BO) b H;
R-- (EO)e - -Ri; R-- (PO) p -- (EO) e H; R-- (PO) p -- (EO)e --
(PO)p H; R--(PO)p --(EO)e -benzyl; (PO)p --(EO)e --
(PO)p; [(PO)p -- (EO)e - - ]z - -NCHzCHzN--[(EO)e (P)p]z;
or mixtures thereof;
wherein R is a C6_e alkyl group, a C6_18 alkyl or
dialkyl phenol group, or a C6_18 alkyl- (PO) p-group; Rl is a
C1_e alkyl; each e is independently about 1-20, each p is
independently about 1-20, and each b is independently
about 1-10; and
(b) denaturing the residual protease enzyme
activity with an oxidizing agent such that the product
made by the unit is not affected by residual enzyme
activity; whereby denatured enzymes have little or no
effect on a proteinaceous food.
The invention further provides a method of
cleaning a food processing unit for a protein containing
CA 02197314 2007-03-27
15k
food product, which method comprises:
(a) contacting a surface of the food processing
unit having a proteinaceous film residue with a dilute-use
solution of a low foaming protease enzyme detergent
composition for a sufficient period of time to
substantially remove the proteinaceous film from the
surface of the food processing unit, leaving residual
protease activity, the low foaming protease enzyme
detergent comprising:
(i) about 10-90 wt o of a liquid medium;
(ii) an effective proteolytic amount of an
enzyme composition;
(iii) an effective enzyme stabilizing
amount of an aqueous soluble or dispersible
stabilizing system comprising an antioxidant
composition and an organic water soluble or
dispersible polyol compound having 2-10 hydroxyl
groups; and
(iv) a low foaming surfactant comprising:
R- (EO) e- (PrO) pH; R- (EO) e- (Bu0) bH; R- (EO) e-R1i
[R- (Pro) p- (EO) eH] ; R- (PrO) p- (EO) e- (Pro) pH;
R- (PrO)P- (EO)e-benzyl; (PrO)P- (EO)e- (PrO)p;
[(PrO) P- (EO) e] 2NCH2CH2N [(EO) e- (PrO) p] 2; or mixtures
thereof;
wherein EO is ethylene oxide; Bu0 is butylene oxide;
Pr0 is propylene oxide; R is a C6-1.8 alkyl group, a C6-18
alkyl or dialkyl phenol group, or a C6_18 alkyl-(PO)p-group;
R1 is a C1-e alkyl; each e is independently 1-20; each p is
independently 1-20; and each b is independently 1-10; and
(b) denaturing the residual protease enzyme
activity with an oxidizing agent such that the product
made by the unit is not affected by residual enzyme
CA 02197314 2007-03-27
151
activity; whereby the denatured enzymes have little or no
effect on a proteinaceous food.
The invention further provides a method of
cleaning a food processing unit for a protein containing
food product, which method comprises:
(a) contacting a surface of the food processing
unit having a proteinaceous film residue with a dilute
use-solution of a low foaming protease enzyme detergent
composition for sufficient period of time to substantially
remove the proteinaceous soil from the surface of the food
processing unit, leaving residual protease activity, the
low foaming protease enzyme detergent comprising:
(i) 10-90 wt % of a solidifying agent;
(ii) an effective proteolytic amount of an
enzyme composition;
(iii) an effective enzyme stabilizing
amount of a water dispersible stabilizing system
comprising an antioxidant composition and an organic
water soluble or dispersible polyol compound having
2-10 hydroxyl groups;
(iv) an effective water conditioning amount
of a water conditioning agent; and
(v) a surfactant selected from the group
consisting of R--(EO)e --(PO)p H; R--(EO)e (BO)b H;
R-- (EO) e --R1; R-- (PO) p (EO) e H; R-- (PO) p (EO) e --
(PO)p H; R-- (PO)p - - (EO)e -benzyl; (PO) p -- (EO)e - -
(PO)p; [(PO)p -- (EO)e --]z --NCH2CH2N--[(EO)e (P)p]2;
or mixtures thereof;
wherein R is a C6_8 alkyl group, a C6-18 alkyl or
dialkyl phenol group, or a C6_18 alkyl-(PO)p-group; R1 is a
C1_8 alkyl; each e is independently about 1-20, each p is
independently about 1-20, and each b is independently
about 1-10; and
CA 02197314 2007-03-27
15m
(b) denaturing the residual protease enzyme
activity with an oxidizing agent such that the product
made by the unit is not affected by residual enzyme
activity; whereby denatured enzymes have little or no
effect on a proteinaceous food.
The invention further provides a method of
cleaning a food processing unit for a protein containing
food product, which method comprises:
(a) contacting a surface of the food processing
unit having a proteinaceous film residue with a dilute-use
solution of a low foaming protease enzyme detergent
composition for a sufficient period of time to
substantially remove the proteinaceous film from the
surface of the food processing unit, leaving residual
protease activity, the low foaming protease enzyme
detergent comprising:
(i) 10-90 wt %- of a solidifying agent;
(ii) an effective proteolytic amount of an
enzyme composition;
(iii) an effective enzyme stabilizing
amount of a water dispersible stabilizing system
comprising an antioxidant composition and an organic
water soluble or dispersible polyol compound having
2-10 hydroxyl groups;
(iv) an effective water conditioning amount
of a water conditioning agent; and
(v) a low foaming surfactant comprising:
R- (EO) e- (Pr0) pH; R- (EO) e- (BuO) bH; R- (EO) e-Rl;
[R- (Pr0)P- (EO)eH) ; R- (PrO)p - (EO), - (PrO) pH;
R- (Pr0)p- (EO)e-benzyl; (Pr0)p- (EO)e- (PrO)p;
[(PrO) p- (EO) e] 2NCH2CH2N [(EO) e- (Pr0) p] 2; or mixtures
thereof;
CA 02197314 2007-03-27
15n
wherein EO is ethylene oxide; BuO is butylene oxide;
Pr0 is propylene oxide; R is a C6_18 alkyl group, a C6-18
alkyl or dialkyl phenol group, or a C6_18 alkyl-(PO)p-group;
R1 is a C1_8 alkyl; each e is independently 1-20; each p is
independently 1-20; and each b is independently 1-10; and
(b) denaturing the residual protease enzyme
activity with an oxidizing agent such that the product
made by the unit is not affected by residual enzyme
activity; whereby the denatured enzymes have little or no
effect on a proteinaceous food.
"BO" and "Bu0" as used herein in reference to a
surfactant both refer to butylene oxide. "PO" and "Pr0"
as used herein in reference to a surfactant both refer to
propylene oxide. "EO" as used herein in reference to a
surfactant refers to ethylene oxide.
Brief Description of the Draaings
FIGURE 1 is Protein Film Soil Removal Test.
FIGURE 2 is Protein Film Soil Removal.
Detailed Description of the Invention
The invention comprises a use dilution, use-
solution composition having exceptional detergency
properties when applied as a cleaning treatment to food
soiled equipment surfaces and having particular cleaning
efficiency upon tenacious protein films. Preferred
embodiments of the invention provide cleaning
performance superior to conventional high alkaline,
chlorine containing detergents. The present invention
generally comprises in a low foaming formulation free of
an alkaline metal hydroxide or a source of active
chlorine.
1. an enzyme or enzyme mixture
2. an enzyme stabilizing system
3. a surfactant or surfactant mixture
4. a low alkaline builder or builder mixture
5. a water conditioning agent or mixture
6. water; and,
7. optional adjuvants
16 2197314
This invention also comprises concentrate
formulations which when dispersed, dissolved, and
properly diluted in water will provide preferred use-
solution compositions. The concentrates can be liquid
or emulsion; solid, tablet, or encapsulate; powder or
particulate; gel or paste; slurry or mull.
This invention further comprises concentrated
cleaning treatments consisting of one product; or,
consisting of a two product system wherein proportions
of each are blended.
A preferred concentrate embodiment of this
invention is a two part, two product detergent system
which comprises:
1. a concentrated liquid product comprising:
a. an enzyme or enzyme mixture
b. an enzyme stabilizing system
c. a surfactant or surfactant mixture
d. a hydrotrope or solvent or mixture
e. water; and
2. a cooperative second concentrated liquid
product comprising:
a. a low alkaline builder or builder mixture
b. a water conditioning agent or mixture; and
c. water
A detersive use solution is prepared by admixing
portions of each product concentrate with water such
that the first liquid concentrate is present in an
amount ranging from about 0.001 to 1% preferably about
0.02% (200 ppm) to about 0.10% (1000 ppm); and, the
second 1''iquid concentrate is present in an amount
ranging from about 0.02% (200 ppm) to about 0.10% (1000
ppm). Total cooperative admixture use solution
concentration ranges from about 0.01% to 2.0% preferably
about 0.04% (400 ppm) to about 0.20% (2000 ppm). The pH
range of the total cooperative admixture use solution is
from about 7.5 to about 11.5.
I. Enzymes
Enzymes are important and essential components of
biological systems, their function being to catalyze and
facilitate organic and inorganic reactions. For
~
2197314
17
example, enzymes are essential to metabolic reactions
occurring in animal and plant life.
The enzymes of this invention are simple proteins
or conjugated proteins produced by living organisms and
functioning as biochemical catalysts which, in detergent
technology, degrade or alter one or more types of soil
residues encountered on food process equipment surfaces
thus removing the soil or making the soil more removable
by the detergent-cleaning system. Both degradation and
alteration of soil residues improve detergency by
reducing the physicochemical forces which bind the soil
to the surface being cleaned, i.e. the soil becomes more
water soluble.
As defined in the art, enzymes are referred to as
simple proteins when they require only their protein
structures for catalytic activity. Enzymes are
described as conjugated proteins if they require a non-
protein component for activity, termed cofactor, which
is a metal or an organic biomolecule often referred to
as a coenzyme. Cofactors are not involved in the
catalytic events of enzyme function. Rather, their role
seems to be one of maintaining the enzyme in an active
configuration. As used herein, enzyme activity refers
to the ability of an enzyme to perform the desired
catalytic function of soil degradation or alteration;
and, enzyme stability pertains to the ability of an
enzyme to remain or to be maintained in the active
state.
Enzymes are extremely effective catalysts. In
practice, very small amounts will accelerate the rate of
soil degradation and soil alteration reactions without
themselves being consumed in the process. Enzymes also
have substrate (soil) specificity which determines the
breadth of its catalytic effect. Some enzymes interact
with only one specific substrate molecule (absolute
specificity); whereas, other enzymes have broad
specificity and catalyze reactions on a family of
structurally similar molecules (group specificity).
Enzymes exhibit catalytic activity by virtue of
three general characteristics: the formation of a
noncovalent complex with the substrate, substrate
~ rf
18 2197314
specificity, and catalytic rate. Many compounds may
bind to an enzyme, but only certain types will lead to
subsequent reaction. The later are called substrates
and satisfy the particular enzyme specificity
requirement. Materials that bind but do not thereupon
chemically react can affect the enzymatic reaction
either in a positive or negative way. For example,
unreacted species called inhibitors interrupt enzymatic
activity.
Enzymes which degrade or alter one or more types of
soil, i.e. augment or aid the removal of soils from
surfaces to be cleaned, are identified and can be
grouped into six major classes on the basis of the types
of chemical reactions which they catalyze in such
degradation and alteration processes. These classes are
(1) oxidoreductase; (2) transferase; (3) hydrolase; (4)
lyase; (5) isomerase; and (6) ligase.
Several enzymes may fit into more than one class.
A valuable reference on enzymes is "Industrial Enzymes",
Scott, D., in Kirk-Othmer Encyclopedia of Chemical
Technology, 3rd Edition, (editors Grayson, M. and
EcKroth, D.) Vol. 9, pp. 173-224, John Wiley & Sons, New
York, 1980.
In summary, the oxidoreductases, hydrolases, lyases
and ligases degrade soil residues thus removing the soil
or making the soil more removable; and, transferases and
isomerases alter soil residues with same effect. Of
these enzyme classes, the hydrolases (including
esterase, carbohydrase or protease) are particularly
preferred for the present invention.
The hydrolases catalyze the addition of water to
the soil with which they interact and generally cause a
degradation or breakdown of that soil residue. This
breakdown of soil residue is of particular and practical
importance in detergent applications because soils
adhering to surfaces are loosened and removed or
rendered more easily removed by detersive action. Thus,
hydrolases are the most preferred class of enzymes for
use in cleaning compositions. Preferred hydrolases are
esterases, carbohydrases, and proteases. The most
~
2197314
19
preferred hydrolase sub-class for the present invention
is the proteases.
The proteases catalyze the hydrolysis of the
peptide bond linkage of amino acid polymers including
peptides, polypeptides, proteins and related substances
- generally protein complexes - such as casein which
contains carbohydrate (glyco group) and phosphorus as
integral parts of the protein and exists as distinct
globular particles held together by calcium phosphate;
or such as milk globulin which can be thought of as
protein and lipid sandwiches that comprise the milk fat
globule membrane. Proteases thus cleave complex,
macromolecular protein structures present in soil
residues into simpler short chain molecules which are,
of themselves, more readily desorbed from surfaces,
solubilized or otherwise more easily removed by
detersive solutions containing said proteases.
Proteases, a sub-class of hydrolases, are further
divided into three distinct subgroups which are grouped
by the pH optima (i.e. optimum enzyme activity over a
certain pH range). These three subgroups are the
alkaline, neutral and acids proteases. These proteases
can be derived from vegetable, animal or microorganism
origin; but, preferably are of the latter origin which
includes yeasts, molds and bacteria. More preferred are
serine active, alkaline proteolytic enzymes of bacterial
origin. Particularly preferred for embodiment in this
invention are bacterial, serine active, alkaline
proteolytic enzymes obtained from alkalophilic strains
of Baciilus, especially from Bacillus subtilis and
Bacillus licheniformis. Purified or non-purified forms
of these enzymes may be used. Proteolytic enzymes
produced by chemically or genetically modified mutants
are herein included by definition as are close
structural enzyme variants. These alkaline proteases
are generally neither inhibited by metal chelating
agents (sequestrants) and thiol poisons nor activated by
metal ions or reducing agents. They all have relatively
broad substrate specificities, are inhibited by
diisopropylfluorophosphate (DFP), are all
endopeptidases, generally have molecular weights in the
~1
20 2197314
range of 20,000 to 40,000, and are active in the pH
ranges of from about 6 to about 12; and, in the
temperature range of from about 20 C to about 80 C.
Examples of suitable commercially available
alkaline proteases are AlcalaseSavinase and
Esperase -- all of Novo Industri AS, Denmark; Purafect
of Genencor International; Maxacal ,= Maxapem and
Maxatase -- all of Gist-Brocase International NV,
Netherlands; Optimase and Opticlean of Solvay Enzymes,
USA and so on.
Commercial alkaline proteases are obtainable in
liquid or dried form, are sold as raw aqueous solutions
or in assorted purified, processed and compounded forms,
and are comprised of about 2% to about 80% by weight
active enzyme generally in combination with stabilizers,
buffers, cofactors, impurities and inert vehicles. The
actual active enzyme content depends upon the method of
manufacture and is not critical, assuming the detergent
solution has the desired enzymatic activity. The
particular enzyme chosen for use in the process and
products of this invention depends upon the conditions
of final utility, including the physical product form,
use pH, use temperature, and soil types to be degraded
or altered. The enzyme can be chosen to provide optimum
activity and stability for any given set of utility
conditions. For example, Purafect is a preferred
alkaline protease for use in detergent compositions of
this invention having application in lower temperature
cleaning programs -- from about 30 C to about 65 C;
whereas, Esperase is the alkaline protease of choice
for higher temperature detersive solutions, from about
50 C to about 85 C.
In preferred embodiments of this invention, the
amount of commercial alkaline protease composite present
in the final use-dilution, use-solution ranges from
about 0.001% (10 ppm) by weight of detersive solution to
about 0.02% (200 ppm) by weight of solution.
Whereas establishing the percentage by weight of
commercial alkaline protease required is of practical
convenience for manufacturing embodiments of the present
teaching, variance in commercial protease concentrates
A
CA 02197314 2004-07-06
21
and in-situ environmental additive and negative effects
upon protease activity require a more discerning
analytical technique for protease assay to quantify
enzyme activity and establish correlations to soil
residue removal performance and to enzyme stability
within the preferred embodiment; and, if a concentrate,
to use-dilution solutions. The activity of the alkaline
proteases of the present invention are readily expressed
in terms of activity units -- more specifically, Kilo-
Novo Protease Units (KNPU) which are azocasein assay
activity units well known to the art. A more detailed
discussion of the azocasein assay procedure can be found
in the publication entitled "The Use of Azoalbumin as a
Substrate in the Colorimetric Determination of Peptic
and Tryptic Activity", Tomarelli, R.M., Charney, J., and
Harding, M.L., J. Lab. Clin. Chem. 34, 428 (1949).
In preferred embodiments of the present invention,
the activity of proteases present in the use-solution
ranges from about 1 x 10-5 KNPU/gm solution to about 4 x
10-3 KNPU/gm solution.
Naturally, mixtures of different proteolytic
enzymes may be incorporated into this invention. While
various specific enzymes have been described above, it
is to be understood that any protease which can confer
the desired proteolytic activity to the composition may
be used and this embodiment of this invention is not
limited in any way by specific choice of proteolytic
enzyme.
In addition to proteases, it is also to be
understood, and one skilled in the art will see from the
above enumeration, that other enzymes which are well
known in the art may also be used with the composition
of the invention. Included are other hydrolases such as
esterases, carboxylases and the like; and, other enzyme
classes.
Further, in order to enhance its stability, the
enzyme or enzyme admixture may be incorporated into
various non-liquid embodiments of the present invention
as a coated, encapsulated, agglomerated, prilled or
marumerized form.
CA 02197314 2004-07-06
22
II. Enzyme Stabilizing System
The enzyme stabilizing system of the present
invention is adapted from Guilbert in U.S. Pat. No.
4,238,345 issued December 9, 1980; and further disclosed
by Guilbert et al. in U.S. Pat. No. 4,243,543 issued
June 6, 1981.
A preferred stabilizing system for the present
invention consists of about 0.5 to 30wto of an antioxydant
composition and of about 1 to 25wt% of an organic water
soluble or dipersible polyol compound having 2-10 hydroxyl
groups. The antioxydant composition comprising a water
soluble metal salt of an oxidizable oxygenated-sulfur
anion such as metabisulfite, sulfite, thiosulfite,
bisulfite or mixtures thereof. The polyol comprises a
dihydric alcohol, a trihydric alcohol or mixtures thereof.
The most preferred stabilizing system for the
present invention consists of a soluble metabisulfite
salt, a glycol such as propylene glycol, and an alkanol
amine compound such as triethanolamine. The admixture
of this complete stabilizing system for maintaining
enzyme activity within the most preferred two part, two
product concentration embodiment of this invention will
typically range from about 0.5% by weight to about 30%
by weight of the total enzyme containing composition.
Within the formulary range of the total stabilizing
admixture, sodium metabisulfite will typically comprise
from about 0.1% by weight to about 5.0% by weight;
propylene glycol will typically comprise from about 1%
by weight to about 25% by weight; and, triethanolamine
will typically comprise from about 0.7% by weight to
about 15% by weight.
This stabilizing system provides stabilizing effect
to enzymes in water containing compositions consisting
of about 20% by weight to about 90% by weight of water,
per Guilbert (Ibid.). It seems obvious to conclude that
this enzyme stabilizing system would therefor provide
some degree of stabilizing effect to enzyme activity at
CA 02197314 2004-07-06
22a
all levels of free and bound waters existing in a liquid
enzyme detergent composition, typically from about 1% to
about 99% by weight of water.
We have found that incorporation of the preferred
enzyme stabilizing system has pronounced beneficial
effect upon alkaline protease cleaning performance, i.e.
enhanced protein film removal, in use-dilution
solutions. Normally, employed for shelf-life
maintenance of enzyme activity within the product
concentrate, none of the art discloses, teaches or
suggests that enzyme stabilizing systems make any
contribution to or have any expected cooperative action
with enzyme activity or manifested cleaning performance
CA 02197314 2004-07-06
23
improvement within detersive, use-dilution solution
environments.
Furthermore, none of the art discloses, teaches, or
suggests that such enzyme stabilizing systems will
profoundly demonstrate this synergistic, cooperative
effect at high temperatures otherwise destructive to
enzymes or rendering them thermolabile.
For a more detailed discussion and illustrated
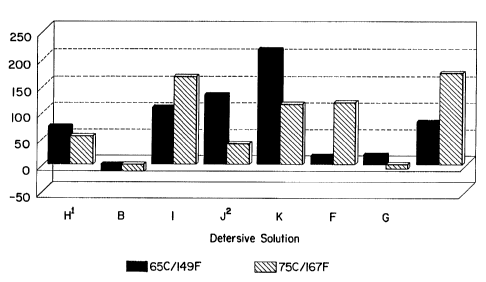
measurement of this discovery, see TABLE A and FIGURES 1
and 2.
III. Surfactant
The surfactant or surfactant admixture of the
present invention can be selected from water soluble or
water dispersible nonionic, semi-polar nonionic, anionic,
cationic, amphoteric, or zwitterionic surface-
active agents; or any combination thereof, such as R--
(EO)e--(PO)pH; R--(EO)e--(BO)bH; R--(EO)e--Rl; R--(PO)p--
(EO)eH; R--(PO)p--(EO)e--(PO)pH; R--(PO)p--(EO)e-benzyl;
(PO)p--(EO)e--(PO)p; [(PO)p--(EO)e--]2--NCH2CH2N--[(EO)e--
( PO) p] 2; or mixtures thereof, wherein R is a C6-18 alkyl
group, a C6_18 alkyl or dialkyl phenol group, or a C6-1$
alkyl-(PO)p-group; R1 is a C1-$ alkyl; each e is
independently about 1-20, each p is independently about 1-
20, and each b is independently about 1-10.
The particular surfactant or surfactant mixture
chosen for use in the process and products of this
invention depends upon the conditions of final utility,
including method of manufacture, physical product form,
use pH, use temperature, foam control, and soil type.
Surfactants incorporated into the present invention
must be enzyme compatible and free of enzymatically
reactive species. For example, when proteases and
amylases are employed, the surfactant should be free of
peptide and glycosidic bonds respectively. Care should
be taken in including cationic surfactants because some
reportedly decrease enzyme effectiveness.
The preferred surfactant system of the invention is
selected from nonionic or anionic species of surface-
active agents, or mixtures of each or both types.
Nonionic and anionic surfactants offer diverse and
CA 02197314 2004-07-06
23a
comprehensive commercial selection, low price; and, most
important,excellent detersive effect -- meaning surface
wetting, soil penetration, soil removal from the surface
being cleaned, and soil suspension in the detergent
solution. This preference does not teach exclusion of
utility for cationics, or for that sub-class of nonionic
entitled semi-polar nonionics, or for those surface-
active agents which are characterized by persistent
cationic and anionic double ion behavior, thus differing
24 2197314
from classical amphoteric, and which are classified as,
zwitterionic surfactants.
One skilled in the art will understand that
inclusion of cationic, semi-polar nonionic, or
zwitterionic surfactants; or, mixtures thereof will
impart beneficial and/or differentiating utility to
various embodiments of the present invention. As
example, foam stabilization for detersive compositions
designed to be foamed onto equipment or environmental
floor, wall and ceiling surfaces; or, gel development
for products dispensed as a clinging thin gel onto
soiled surfaces; or, for antimicrobial preservation; or,
for corrosion prevention -- and so forth.
The most preferred surfactant system of the present
invention is selected from nonionic or anionic surface-
active agents, or mixtures of each or both types which
impart low foam to the use-dilution, use solution of the
detergent composition during application. Preferably,
the surfactant or the individual surfactants
.20 participating within the surfactant mixture are of
themselves low foaming within normal use concentrations
and within expected operational application parameters
of the detergent composition and cleaning program. In
practice, however, there is advantage to blending low
foaming surfactants with higher foaming surfactants
because the latter often impart superior detersive
properties to the detergent composition. Mixtures of
low foam and high foam nonionics and mixtures of low
foam nonionics and high foam anionics can be useful in
the present invention if the foam profile of the
combination is low foaming at normal use conditions.
Thus high foaming nonionics and anionics can be
judiciously employed without departing from the spirit
of this invention.
Particularly preferred concentrate embodiments of
this invention are designed for clean-in-place (CIP)
cleaning systems within food process facilities; and,
most particularly for dairy farm and fluid milk and milk
by-product producers. Foam is a major concern in these
highly agitated, pump recirculation systems during the
cleaning program. Excessive foam reduces flow rate,
~.
2197314
cavitates recirculation pumps, inhibits detersive
solution contact with soiled surfaces, and prolongs
drainage. Such occurrences during CIP operations
adversely affect cleaning performance and sanitizing
5 efficiencies.
Low foaming is therefore a descriptive detergent
characteristic broadly defined as a quantity of foam
which does not manifest any of the problems enumerated
above when the detergent is incorporated into the
10 cleaning program of a CIP system. Because no foam is
the ideal, the issue becomes that of determining what is
the maximum level or quantity of foam which can be
tolerated within the CIP system without causing
observable mechanical or detersive disruption; and, then
15 commercializing only formulas having foam profiles at
least below this maximum; but, more practically,
significantly below this maximum for assurance of
optimum detersive performance and CIP system operation.
Acceptable foam levels in CIP systems have been
20 empirically determined in practice by trial and error.
Obviously, commercial products exist today which meet
the low foam profile needs of CIP operation. It is
therefore, a relatively straightforward task to employ
such commercial products as standards for comparison and
25 to establish laboratory foam evaluation devices and test
methods
which simulate, if not duplicate, CIP program
conditions, i.e. agitation, temperature, and
concentration parameters.
.. .
In practice, the present invention permits
incorporation of high concentrations of surfactant as
compared to conventional chlorinated, high alkaline CIP
and COP cleaners. Certain preferred surfactant or
surfactant mixtures of the invention are not generally
physically compatible nor chemically stable with the
alkalis and chlorine of convention. This major
differentiation from the art necessitates not only
careful foam profile analysis of surfactants being
included into compositions of the invention; but, also
demands critical scrutiny of their detersive properties
of soil removal and suspension. The present invention
~
CA 02197314 2004-07-06
26
relies upon the surfactant system for gross soil removal
from equipment surfaces and for soil suspension in the
detersive solution. Soil suspension is as important a
surfactant property in CIP detersive systems as soil
removal to prevent soil redeposition on cleaned surfaces
during recirculation and later re-use in CIP systems
which save and re-employ the same detersive solution
again for several cleaning cycles.
Generally, the concentration of surfactant or
surfactant mixture useful in use-dilution, use solutions
of the present invention ranges from about 0.002% (20
ppm) by weight to about 0.1% (1000 ppm) by weight,
preferably from about 0.005% (50 ppm) by weight to about
0.075% (750 ppm) by weight, and most preferably from
about 0.008% (80 ppm) by weight to about 0.05% (500 ppm)
by weight.
The concentration of surfactant or surfactant
mixture useful in the most preferred concentrated
embodiment of the present invention ranges from about 5%
by weight to about 75% by weight of the total formula
weight percent of the enzyme containing composition.
A typical listing of the classes and species of
surfactants useful herein appears in U.S. Pat. No.
3,664,961 issued May 23, 1972, to Norris.
Nonionic Surfactants, edited by
Schick, M.J., Vol. 1 of the Surfactant Science Series,
Marcel Dekker, Inc., New York, 1983 is an excellent
reference on the wide variety of nonionic compounds
generally employed in the practice of the present
invention. Nonionic surfactants useful in the invention
are generally characterized by the presence of an
organic hydrophobic group and an organic hydrophilic
group and are typically produced by the condensation of
an organic aliphatic, alkyl aromatic or polyoxyalkylene
hydrophobic compound with a hydrophilic alkaline oxide
moiety which in common practice is ethylene oxide or a
polyhydration product thereof, polyethylene glycol.
Practically any hydrophobic compound having a hydroxyl,
carboxyl, amino, or amido group with a reactive hydrogen
atom can be condensed with ethylene oxide, or its
polyhydration adducts, or its mixtures with alkoxylenes
CA 02197314 2001-10-05
27
such as propylene oxide to form a nonionic surface-
active agent. The length of the hydrophilic
polyoxyalkylene moiety which is condensed with any
particular hydrophobic compound can be readily adjusted
to yield a water dispersible or water soluble compound
having the desired degree of balance betweeri hydrophilic
and hydrophobic properties. Useful nonionic surfactants
in the present invention include:
1. Block polyoxypropylene-polyoxyethylene
polymeric compounds based upon propylene glycol,
ethylene glycol, glycerol, trimethylolpropane, and
ethylenediamine as the initiator reactive hydrogen
compound. Examples of polymeric compounds made from a
sequential propoxylation and ethoxylation of initiator
are commercially available under the trade name Pluronic
(D and Tetronicv manufactured by BASF Corp.
Pluronicc@ compolinds are difunctional (two reactive
hydrogens) compounds formed by condensinq ethylene oxide
with a hydrophobic base formed by the addition of
propylene oxide to the two hydroxyl groups of propylene
glycol. This hydrophobic portion of the molecu].e weighs
from about 1,000 to about 4,000. Ethylene oxide is then
added to sandwich this hydrophobe between hydrophilic
groups, controlled by lenqth to constitute from about
10% by weight to about 80% by weight of the firial
molecule.
Tetronic compounds are tetra-functional block
copolymers derived from the sequential addition of
propylene oxide and ethylene oxide to ethylenediamine.
The moleZ~ular weight of the propylene oxide hydrotype
ranges from about 500 to about 7,000; and, the
hydrophi_l.e, ethylene oxide, is added to constitute from
about 10% by weight to about 80% by weight of the
molecule.
2. Condensation products of one mole of alkyl
phenol wherein the alkyl chain, of straight chain or
branched chain configuration, or of single or dual alkyl
constituent, contains from about 8 to about 18 carbon
atoms with from about 3 to about 50 moles of ethylene
oxide. The alkyl group can, for example, be represented
by diisobutylene, di-amyl, polymerized propylene, iso-
CA 02197314 2001-10-05
28
octyl, nonyl, and di-nonyl. Examples of commercial
compounds of this chemistry are available on the market
under the trade name Igepal manufactured by Rhone-
Poulenc and Triton inanufactured by Union Carbide.
3. Condensation products of one mole of a
saturated or unsaturated, straight or branched chain
alcohol havinq from about 6 to about 24 carbon atoms
with from about 3 to about 50 moles of ethylene oxide.
The alcohol moiety can coris_ist of mixtures of alcohols
in the above delineated carbon r_anqe or it can consist
of an alcohol having a specific number of carbon atoms
within this range. Examples of like commercial
surfactant are available under the trade name Noedol(D
manufactured by Shell Chemical Co. and Alfonic
manufactured by Vista Chemical Co.
4. Condensation nroducts of one mole of saturated
or unsaturated, straight or branched chain carboxylic
acid having from about 8 to about 18 carbon atoms with
from about 6 to about 50 moles of ethylene oxide. The
acid moiety can consist of mixtures of acids in the
above defined carborl atoins range or it can consist of an
acid having a specific number of carbon atoms within the
range. Examples of commercial compounds of this
chemistry are available on the market under the trade
name Nopalcol(RD manutactured by Henkel Corporation and
Lipopeg manufactured by Lipo Chemicals, Inc.
In addition to ethoxylated carboxylic acids,
commonly called polyethylene glycol esters, other
alkanoic acid esters formed by reaction with glycerides,
glycerira.,-and polyhydric (saccharide or
sorbitan/sorbitol) alcohols have application in this
invention for specialized embodiments, particularly
indirect food additive applications. All of these ester
moieties have one or more reactive hydrogen sites on
their molecule which can undergo further acylation or
ethylene oxide (alkoxide) addition to control the
hydrophilicity of these substances. Care must be
exercised when adding these fatty ester or acylated
carbohydrates to compositions of the preserit irivention
containing amylase and/or lipase enzymes because of
potential incompatibility.
CA 02197314 2001-10-05
29
Low foaming alkoxylated nonionics are preferred
although other higher foaming alkoxylated nonionics can
be used without departing from the spirit of this
invention if used in conjunction with low foaming agents
so as to control the foam profile of the mixture within
the detergent composition as a whole. Examples of
nonionic low foaming surfactants include:
5. Compounds from (1) which are modified,
essentially reversed, by adding ethylene oxide to
ethylene glycol to provide a hydrophile of desiqnated
molecular weight; and, then adding propylene oxide to
obtain hydrophobic blocks on the outside (ends) of the
molecule. The hydrophobic portion of the molecule
weighs from about 1,000 to about 3,100 with the central
hydrophile comprising 10% by weight to about 80% by
weight of the final molecule. These reverse Pluronics
are manufactured by BASF Corporation under the trade
name Pluronic R surfactants.
Likewise, the Tetraonic R surfactants are produced
by BASF Corporation by the sequential addition of
ethylene oxide and propylene oxide to ethylenediamine.
The hydrophobic portiori of the molecule weiqhs from
about 2,100 to about 6,700 with the central hydrophile
comprising 10o by weight to 80% by weight of the fi.nal.
nlolecule.
6. Compounds from gr.oups (1), (2), (3) and (4)
which are modified by "capping" or "end blocking" the
terminal hydroxy group or groups (of multi-functional
moieties) to reduce foaming by reaction with a small
hydrophobic molecule such as propylene oxide, butylene
oxide, benzyl chloride; and, short chain fatty acids,
alcohols or alkyl halides containinq from 1 to about 5
carbori atoms; and mixtures thereof. Also included are
reactants such as thionyl chloride which convert
terminal hydroxy groups to a chloride group. Such
modifications to the terminal hydroxy group may lead to
all-block, block-heteric, heteric-block or all-heteric
noni.onics.
7. Additional examples of effective low foaming
nonionics include:
CA 02197314 2004-07-06
The alkylphenoxypolyethoxyalkanols of U.S. Pat No.
2,903,486 issued September 8, 1959 to Brown et al.,
represented by the formula
5
R
~ (CtH~IIOAI~ ON
in which R is an alkyl group of 8 to 9 carbon atoms, A
is an alkylene chain of 3 to 4 carbon atoms, n is an
integer of 7 to 16, and m is an integer of 1 to 10.
The polyalkylene glycol condensates of U.S. Pat.
No. 3,048,548 issued August 7, 1962 to Martin et al.,
having alternating
hydrophilic oxyethylene chains and hydrophobic
oxypropylene chains where the weight of the terminal
hydrophobic chains, the weight of the middle hydrophobic
unit and the weight of the linking hydrophilic units
each represent about one-third of the condensate.
The defoaming nonionic surfactants disclosed in
U.S. Pat. No. 3,382,178 issued May 7 1968 to Lissant et
al., having the
general formula Z[(OR)nOH]Z wherein Z is alkoxylatable
material, R is a radical derived from an alkaline oxide
which can be ethylene and propylene and n is an integer
from, for example, 10 to 2,000 or more and z is an
integer.determined by the number of reactive
oxyalkylatable groups.
The conjugated polyoxyalkylene compounds described
in U.S. Pat. No. 2,677,700, issued May 4, 1954 to
Jackson et al.,
corresponding to the formula Y(C3H60) n(C2H40) mH wherein Y
is the residue of organic compound having from about 1
to 6 carbon atoms and one reactive hydrogen atom, n has
an average value of at least about 6.4, as determined by
hydroxyl number and m has a value such that the
oxyethylene portion constitutes about 10% to about 90%
by weight of the molecule.
CA 02197314 2004-07-06
31
The conjugated polyoxyalkylene compounds described
in U.S. Pat. No. 2,674,619, issued April 6, 1954 to
Lundsted et al, having
the formula Y[(C3H60n (C2H40) mH],t wherein Y is the residue
of an organic compound having from about 2 to 6 carbon
atoms and containing x reactive hydrogen atoms in which
x has a value of at least about 2, n has a value such
that the molecular weight of the polyoxypropylene
hydrophobic base is at least about 900 and m has value
such that the oxyethylene content of the molecule is
from about 10% to about 90% by weight. Compounds
falling within the scope of the definition for Y
include, for example, propylene glycol, glycerine,
pentaerythritol, trimethylolpropane, ethylenediamine and
the like. The oxypropylene chains optionally, but
advantageously, contain small amounts of ethylene oxide
and the oxyethylene chains also optionally, but
advantageously, 'contain small amounts of propylene
oxide.
Additional conjugated polyoxyalkylene surface-
active agents which are advantageously used in the
compositions of this invention correspond to the
formula: P[(C3H6O) n(CZH40) mH] x wherein P is the residue of
an organic compound having from about 8 to 18 carbon
atoms and containing x reactive hydrogen atoms in which
x has a value of 1 or 2, n has a value such that the
molecular weight of the polyoxyethylene portion is at
least about 44 and m has a value such that the
oxypropylene content of the molecule is from about 10%
.
to about 90o by weight. In either case the oxypropylene
chains may contain optionally, but advantageously, small
amounts of ethylene oxide and the oxyethylene chains may
contain also optionally, but advantageously, small
amounts of propylene oxide.
The most preferred nonionic surfactants for use in
compositions practiced in the present invention included
compounds from groups (5), (6) and (7). Especially
preferred are the modified compounds enumerated in
groups (6) and (7).
2197314
32
Examples of especially preferred commercial
surfactants are listed in Table II.
Table II
Examples of Preferred Commercial Nonionics
General Structure Examplesa
AP-(EO)x-(PO)yH Triton CF-21
CaP (EO) 9.5 (PO) 5H
Alcohol-(EO)x-(PO)yH Sulfonic JL-80X
Cy-11 (EO) 9 (PO) 1-2H
Alcohol-(P0)x-(EO)yH Poly-Tergent SL-=42
Ca-10(PO)3(EO)5H
Alcohol-(PO)X-(EO)y-(PO)ZH Poly-Tergent SLF-18
Ca-io (PO) 16-17 (EO) 12 (PO) i-
2H
Alcohol-(PO)X-(EO)y-benzyl Triton DF-12
Cs-1o (PO) 2 (EO) 13-benzyl
Alcohol-(EO)X-(Bu0)yH Plurafac LF-221
Cio-i2 (EO) 9.5 (BuO) 1-2
Alcohol-(EO)x-alkyl Dehypon Lt-104
C16-1$ (EO) 12CHZ0C4H9
Alcohol- (EO)X-benzyl Triton DF-18
C14-16 (EO) 16-benzyl
a NMR analysis
AP = alkylphenoxy
EO = ethylene oxide
PO = propylene oxide
BuO = butylene oxide
Triton is a registered trade name of Union Carbide Chemical & Plastics
Co.
Surfonic is a registered trade name of Texaco Chemical Co.
Poly-Tergent is a registered trade name of Olin Corporation.
Plurafac is a registered trade name of BASF Corporation.
Dehypon is a registered trade name of Henkel Corporation.
A
2197314
33
Semi-Polar Nonionic Surfactants
The semi-polar type of nonionic surface active
agents are another class of nonionic surfactant useful
in compositions of the present invention. Generally,
semi-polar nonionics are high foamers and foam
stabilizers which make their application in CIP systems
limited. However, within compositional embodiments of
this invention designed for high foam cleaning
methodology, such as facility cleaning which often
employs detersive solutions dispensed onto surfaces as a
foam, semi-polar nonionics would have immediate utility.
The semi-polar nonionic surfactants include the amine
oxides, phosphine oxides, sulfoxides and their
alkoxylated derivatives.
8. Amine oxides are tertiary amine oxides
corresponding to the general formula:
Ri
R~-{0R~ N----O
1
Rs
wherein the arrow is a conventional representation of a
semi-polar' bond; and, R1, R2, and R3 may be aliphatic,
aromatic, heterocyclic, alicyclic, or combinations
thereof. Generally, for amine oxides of detergent
interest, R' is an alkyl radical of from about 8 to about
24 carbon atoms; R2 and R3 are selected from the group
consisting of alkyl or hydroxyalkyl of 1-3 carbon atoms
and mixtures thereof; R4 is an alkaline or a
hydroxyalkylene group containing 2 to 3 carbon atoms;
and n ranges from 0 to about 20.
~
34 2197314
Useful semi-polar nonionic surfactants also include
the water soluble phosphine oxides having the following
structure:
RZ
R~-P-O
RS
wherein the arrow is a conventional representation of a
semi-polar bond; and, R' is an alkyl, alkenyl or
hydroxyalkyl moiety ranging from 10 to about 24 carbon
atoms in chain length; and, R2 and R3 are each alkyl
moieties separately selected from alkyl or hydroxyalkyl
groups containing 1 to 3 carbon atoms.
Semi-polar nonionic surfactants useful herein also
include the water soluble sulfoxide compounds which have
the structure:
R~
5 ----- 0
RZ
wherein the arrow is a conventional representation of a
semi-polar bond; and, R' is an alkyl or hydroxyalkyl
moiety of about 8 to about 28 carbon atoms, from 0 to
about 5 ether linkages and from 0 to about 2 hydroxyl
substituents; and R 2 is an alkyl moiety consisting of
alkyl and hydroxyalkyl groups having 1 to 3 carbon
atoms.
Anionic Surfactants
Also useful in the present invention are surface
active substances which are categorized as anionics
because the charge on the hydrophobe is negative; or
surfactants in which the hydrophobic section of the
molecule carries no charge unless the pH is elevated to
~1
35 2197314
neutrality or above (e.g. carboxylic acids).
Carboxylate, sulfonate, sulfate and phosphate are the
polar (hydrophilic) solubilizing groups found in anionic
surfactants. Of the cations (counterions) associated
with these polar groups, sodium, lithium and potassium
impart water solubility; ammonium and substituted
ammonium ions provide both water and oil solubility;
and, calcium, barium, and magnesium promote oil
solubility.
As those skilled in the art understand, anionics
are excellent detersive surfactants and are therefore,
favored additions to heavy duty detergent compositions.
Generally, however, anionics have high foam profiles
which limit their use alone or at high concentration
levels in cleaning systems such as CIP circuits that
require strict foam control. However, anionics are very
useful additives to preferred compositions of the
present invention; at low percentages or in cooperation
with a low foaming nonionic or defoam agent for
application in CIP and like foam controlled cleaning
regimens; and, at higher concentrations in detergent
compositions designed to yield foaming detersive
solutions. Certainly, anionic surfactants are preferred
ingredients in various embodiments of the present
invention which incorporate foam for dispensing and
utility -- for example, clinging foams used for general
facility cleaning.
Further, anionic surface active.compounds are
useful to impart special chemical or physical properties
other tYfan detergency within the composition. Anionics
can be employed as gelling agents or as part of a
gelling or thickening system. Anionics are excellent
solubilizers and can be used for hydrotropic affect and
cloud point control. Anionics can also serve as the
solidifier for solid product forms of the invention, and
so forth.
The majority of large volume commercial anionic
surfactants can be subdivided into five major chemical
classes and additional sub-groups: (taken from
"Surfactant Encyclopedia", Cosmetics & Toiletries, Vol.
A
CA 02197314 2004-07-06
36
104 (2) 7 1-8 6 (1989),
A. Acylamino acids (and salts)
1. Acylgluamates
2. Acyl peptides
3. Sarcosinates
4. Taurates
B. Carboxylic acids (and salts)
1. Alkanoic acids (and alkanoates)
2. Ester carboxylic acids
3. Ether carboxylic acids
C. Phosphoric acid esters (and salts)
D. Sulfonic.acids (and salts)
1. Acyl isethionates
2. Alkylaryl sulfonates
3. Alkyl sulfonates
4. Sulfosuccin.ates
E. Sulfuric acid esters (and salts)
1. Alkyl ether sulfates
2. Alkyl sulfates
It should be noted that certain of these anionic
surfactants may be incompatible with the enzymes
incorporated into the present invention. As example,
the acyl-amino acids and salts may be incompatible with
proteolytic enzymes because of their peptide structure.
Examples of suitable synthetic, water soluble
anionic detergent compounds are the ammonium and
substituted ammonium (such as mono-, di- and
triethai;olamine) and alkali metal (such as sodium,
lithium and potassium) salts of the alkyl mononuclear
aromatic sulfonates such as the alkyl benzene sulfonates
containing from about 5 to about 18 carbon atoms in the
alkyl group in a straight or branched chain, e.g., the
salts of alkyl benzene sulfonates or of alkyl toluene,
xylene, cumene and phenol sulfonates; alkyl naphthalene
sulfonate, diamyl naphthalene sulfonate, and dinonyl
naphthalene sulfonate and alkoxylated derivatives.
Other anionic detergents are the olefin sulfonates,
including long chain alkene sulfonates, long chain
hydroxyalkane sulfonates or mixtures of alkenesulfonates
2197314
37
and hydroxyalkane-sulfonates. Also included are the
alkyl sulfates, alkyl poly(ethyleneoxy) ether sulfates
and aromatic poly(ethyleneoxy) sulfates such as the
sulfates or condensation products of ethylene oxide and
nonyl phenol (usually having 1 to 6 oxyethylene groups
per molecule. The particular salts will be suitably
selected depending upon the particular formulation and
the needs therein.
The most preferred anionic surfactants for the most
preferred embodiment of the invention are the linear or
branched alkali metal mono and/or di-(C6_14)alkyl diphenyl
oxide mono and/or disulfonates, commercially available
from Dow Chemical, for example as DOWFAX 2A-l, and
DOWFAX C6L.
Cationic Surfactants
Surface active substances are classified as
cationic if the charge on the hydrotrope portion of the
molecule is positive. Surfactants in which the
hydrotrope carries no charge unless the pH is lowered
close to neutrality or lower are also included in this
group (e.g. alkyl amines). In theory, cationic
surfactants may be synthesized from any combination of
elements containing an "onium" structure RnX+Y and could
include compounds other than nitrogen (ammonium) such as
phosphorus (phosphonium) and sulfur (sulfonium). In
practice, the cationic surfactant field is dominated by
nitrogen containing compounds, probably because
synthetic routes to nitrogenous cationics are simple and
straightfbrward and give high yields of product, e.g.
they are less expensive.
Amphoteric Surfactants
Amphoteric surfactants contain both a basic and an
acidic hydrophilic group and an organic hydrophobic
group. These ionic entities may be any of anionic or
cationic groups described in the preceding sections. A
basic nitrogen and an acidic carboxylate group are the
predominant functional groups, although in a few
structures, sulfonate, sulfate, phosphonate or phosphate
provide the negative charge. Surface active agents are
~ =
38 2197314
classified as amphoterics if the charge on the
hydrophobe changes as a function of the solutions pH -
to illustrate:
[ RNH ( CH2 ) nCO2H ] +X- 1 _ [ RN+HZ ( CH ) nC02 ] 2 RNH ( CH2 ) nC02 ] M+ 3
X represents an anion and M+ a cation.
Zwitterionic Surfactants
The presence of a positive charged quaternary
ammonium or, in some cases, of a sulfonium or
phosphonium ion; and of a negative charged carboxyl
group within a compound of aliphatic derivative
generally of betaine structure:
RRõ R_
1
R'-N{-CH=-C02 f1'-S-CHCO R1-P4-CH2-CO
R... R...
yields an amphoteric of special character termed a
zwitterion. These amphoterics contain cationic and
anionic groups which ionize to a nearly equal degree in
the isoelectric region of the molecule and develop
strong"inner-salt" attraction between positive-negative
charge ctnters. As a result, surfactant betaines do not
exhibit strong cationic or anionic characters at pH
extremes nor do they show reduced water solubility in
their isoelectric range. Unlike "external" quaternary
ammonium salts, betaines are compatible with anionics.
1 Low pH Solution: Cationic Hydrophobe
2 Intermediate pH Solution: Isoelectric Hydrophobe
3 High pH Solution: Anionic Hydrophobe
~
39 2197314
The alkyl groups contained in said detergent
surfactants can be straight or branched and saturated or
unsaturated.
The nonionic and anionic surfactants enumerated
above can be used singly or in combination in the
practice and utility of the present invention. The
semi-polar nonionic, cationic, amphoteric and
zwitterionic surfactants generally are employed in
combination with nonionics or anionics. The above
examples are merely specific illustrations of the
numerous surfactants which can find application within
the scope of this invention. The foregoing organic
surfactant compounds can be formulated into any of the
several commercially desirable composition forms of this
invention having disclosed utility. Said compositions
are cleaning treatments for food soiled surfaces in
concentrated form which, when dispensed or dissolved in
water, properly diluted by a proportionating device, and
delivered to the target surfaces as a solution, gel or
foam will provide cleaning. Said cleaning treatments
consisting of one product; or, involving a two product
system wherein proportions of each are utilized. Said
product being concentrates of liquid or emulsion; solid,
tablet, or encapsulate; powder or particulate; gel or
paste; and slurry or mull.
Builders
Builders are substances that augment the detersive
effects of detergents or surfactants and supply
alkalinity-to the cleaning solution. Builders have the
detersive properties of promoting the separation of soil
from surfaces and keeping detached soil suspended in the
detersive solution to retard redeposition. Builders may
of themselves be precipitating, sequestrating or
dispersing agents for water hardness control; however,
the builder effect is independent of its water
conditioning properties. Although there is functional
overlap, builders and water conditioning agents having
utility in this invention will be treated separately.
Builders and builder salts can be inorganic or
organic in nature and can be selected from a wide
~
40 2197314
variety of detersive, water soluble, alkaline compounds
known in the art.
A. Water soluble inorganic alkaline builder salts
which can be used alone in the present invention or in
admixture with other builders include, but are not
limited to, alkali metal or ammonia or substituted
ammonium salts of carbonates, silicates, phosphates and
polyphosphates, and borates.
Carbonates useful in the invention include all
physical forms of alkali metal, ammonium and substituted
ammonium salts of carbonate, bicarbonate and
sesquicarbonate (all with or without calcite seeds), in
anhydrous or hydrated forms and mixtures thereof.
Silicates useful in the invention include all
physical forms of alkali metal salts of crystalline
silicates such as ortho-, sesqui- and metasilicate in
anhydrous or hydrated form; and, amorphous silicates of
higher Si02 content in liquid or powder state having
Na20/Si02 ratios of from about 1.6 to about 3.75; and,
mixtures thereof.
Phosphates and polyphosphates useful in the
invention include all physical forms of alkali metal,
ammonium and substituted ammonium salts of dibasic and
tribasic ortho-phosphate, pyrophosphates, and condensed
polyphosphates such as tripolyphosphate,
trimetaphosphate and ring open derivatives; and, glassy
polymeric metaphosphates of general structure Mn+2Pn03n+i
having a degree of polymerization n of from about 6 to
about 21 in anhydrous or hydrated forms, and, mixtures
thereof. - -
Borates useful in the invention include all
physical forms of alkali metal salts of metaborate and
pyroborate (tetraborate, borax) in anhydrous or hydrated
forms; and, mixtures thereof.
B. Water soluble organic alkaline builders which
are useful in the present invention include
alkanolamines and cyclic amines.
Water soluble alkanolamines include those moieties
prepared from ammonia and ethylene oxide or propylene
oxide; i.e. mono-, di-, and triethanolamine; and, mono-,
f1
41 2197314
di-, and triisopropanolamine; and substituted
alkanolamines; and, mixtures thereof.
The preferred builder compounds for compositions of
the present invention are the water soluble, inorganic
alkaline builder salts of carbonates, silicates and
phosphates/polyphosphates.
The most preferred builder salts for the most
preference compositions of the present invention are the
salts of carbonate, bicarbonate and sesquicarbonate;
and, mixtures thereof.
Generally, the concentration of builder or builder
mixture useful in use-dilution, use solutions of the
present invention ranges from about 0% (0 ppm) by weight
to about 0.1% (1000 ppm) by weight, preferably from
about 0.0025% (25 ppm) by weight to about 0.05% (500
ppm) by weight, and most preferably from about 0.005%
(50 ppm) by weight to about 0.025% (250 ppm) by weight.
The concentration of builder or builder mixture
useful in the most preferred concentration embodiments
of the present invention ranges from about 10% by weight
to about 50% by weight of the total formula weight
percent of the builder containing composition.
Water Conditioning Agent
Water conditioning agents function to inactivate
water hardness and prevent calcium and magnesium ions
from interacting with soils, surfactants, carbonate and
hydroxide. Water conditioning agents therefore improve
detergency and prevent long term effects such as
insoluble soil redepositions, mineral scales and
., .
mixtures thereof. Water conditioning can be achieved by
different mechanisms including sequestration,
precipitation, ion-exchange and dispersion (threshold
effect).
Metal ions such as calcium and magnesium do not
exist in aqueous solution as simple positively charged
ions. Because they have a positive charge, they tend to
surround themselves with water molecules and become
solvated. Other molecules or anionic groups are also
capable of being attracted by metallic cations. When
these moieties replace water molecules, the resulting
~
2197314
42
metal complexes are called coordination compounds. An
atom, ion or molecule that combines with a central metal
ion is called a ligand or complexing agent. A type of
coordination compound in which a central metal ion is
attached by coordinate links to two or more nonmetal
atoms of the same molecule is called a chelate. A
molecule capable of forming coordination complexes
because of its structure and ionic charge is termed a
chelating agent. Since the chelating agent is attached
to the same metal ion at two or more complexing sites, a
heterocyclic ring that includes the metal ions is
formed. The binding between the metal ion and the
liquid may vary with the reactants; but, whether the
binding is ionic, covalent or hydrogen bonding, the
function of the ligands is to donate electrons to the
metal.
Ligands form both water soluble and water insoluble
chelates. When a ligand forms a stable water soluble
chelate, the ligand is said to be a sequestering agent
and the metal is sequestered. Sequestration therefore,
is the phenomenon of typing up metal ions in soluble
complexes, thereby preventing the formation of
undesirable precipitates. The builder should combine
with calcium and magnesium to form soluble, but
undissociated complexes that remain in solution in the
presence of precipitating anions. Examples of water
conditioning agents which employ this mechanism are the
condensed phosphates, glassy polyphosphates,
phosphonates, amino polyacetates, and hydroxycarboxylic
acid sal~-s and derivatives.
Like ligands which inactivate metal ions by
precipitation, similar effect is achieved by simple
supersaturation of calcium and magnesium salts having
low solubility. Typically carbonates and hydroxides
achieve water conditioning by precipitation of calcium
and magnesium as respective salts. Orthophosphate is
another example of a water conditioning agent which
precipitates water hardness ions. Once precipitated,
the metal ions are inactivated.
Water conditioning can also be affected by an in
situ exchange of hardness ions from the detersive water
~
43 2197314
solution to a solid (ion exchanger) incorporated as an
ingredient in the detergent. In detergent art, this ion
exchanger is an aluminosilicate of amorphoric or
crystalline structure and of naturally occurring or
synthetic origin commercially designated as zeolite. To
function properly, the zeolite must be of small particle
size of about 0.1 to about 10 microns in diameter for
maximum surface exposure and kinetic ion exchange.
The water conditioning mechanisms of precipitation,
sequestration and ion exchange are stoichiometric
interactions requiring specific mass action proportions
of water conditioner to calcium and magnesium ion
concentrations. Certain sequestering agents can further
control hardness ions at sub-stoichiometric
concentrations. This property is called the "threshold
effect" and is explained by an adsorption of the agent
onto the active growth sites of the submicroscopic
crystal nuclei which are initially produced in the
supersaturated hard water solution, i.e., calcium and
magnesium salts. This completely prevents crystal
growth, or at least delays growth of these crystal
nuclei for a long period of time. In addition,
threshold agents reduce the agglomeration of
crystallites already formed. Compounds which display
both sequestering and threshold phenomena with water
hardness minerals are much preferred conditioning agents
for employ in the present invention. Examples include
tripolyphosphate and the glassy polyphosphates,
phosphonates, and certain homopolymers and copolymer
salts of carboxylic acids. Often these compounds are
used in conjunction with the other types of water
conditioning agents for enhanced performance.
Combinations of water conditioners having different
mechanisms of interaction with hardness result in
binary, ternary or even more complex conditioning
systems providing improved detersive activity.
The water conditioning agents which can be employed
in the detergent compositions of the present invention
can be inorganic or organic in nature; and, water
soluble or water insoluble at use dilution
concentrations.
~
~..
44 2197314
A-1. Inorganic Water Soluble Water Conditioning Agents
Useful examples include all physical forms of
alkali metal, ammonium and substituted ammonium salts of
carbonate, bicarbonate and sesquicarbonate;
pyrophrophates, and condensed polyphosphates such as
tripolyphosphate, trimetaphosphate and ring open
derivatives; and, glassy polymeric metaphosphates of
general structure Mn+2Pn03n+1 having a degree of
polymerization n of from about 6 to about 21 in
anhydrous or hydrated forms; and, mixtures thereof.
A-2. Inorganic Water Insoluble Water Conditioning
Agents
Aluminosilicate builders are useful in the present
invention. Useful aluminosilicate ion exchange
materials are commercially available. These
aluminosilicates can be amorphous or crystalline in
structure and can be naturally-occurring
aluminosilicates or synthetically derived.
Amorphous aluminosilicate builders include those
having the empirical formula:
NZ (ZA102; ySi02 )
wherein M is a univalent cation such as sodium,
potassium, lithium, ammonium or substituted ammonium, z
is from about 0.5 to about 2; and y is 1; this material
having a magnesium ion exchange capacity of at least
about 50 milligram equivalents of CaCO3 hardness per gram
of anhydtous aluminosilicate.
Preferred crystalline aluminosilicates are zeolite
builders which have the formula:
NaZ [A102) Z (Si02) y] xH2O
wherein z and y are integers of at least 6, the molar
ratio of z to y is in the range of from 1.0 to about 0.5
and x is an integer from about 15 to about 264. Said
aluminosilicate ion-exchange material having a calcium
ion exchange capacity on an anhydrous basis of at least
45 2197314
about 200 milligrams equivalent of CaCO3 hardness per
gram.
Preferred synthetic crystalline aluminosilicate ion
exchange materials useful herein are available under the
designations zeolite crystal structure group A and X.
In an especially preferred embodiment, the crystalline
aluminosilicate ion exchange material has the formula:
Na12 [ (A102) 12 (S1.02) 121 xH2O
wherein x is from about 20 to about 30, especially about
27. This material is known as zeolite A. Preferably,
the aluminosilicate has a pore size determined by the
unit structure of the zeolite crystal of about 3 to
about 10 Angstroms; and, a finely divided mean particle
size of about 0.1 to about 10 microns in diameter.
These preferred crystalline types of zeolites are
well known in the art and are more particularly
described in the text Zeolite Molecular Sieves, Breck,
D.W., John Wiley and Sons, New York, 1974.
B. Organic Water Soluble Water Conditioning Agents
Organic water soluble water conditioning agents
useful in the compositions of the present invention
include aminpolyacetates, polyphosphonates,
aminopolyphosphonates, short chain carboxylates and a
wide variety of polycarboxylate compounds.
Organic water conditioning agents can generally be
added to the composition in acid form and neutralized in
situ; but, can also be added in the form of a pre-
neutralized salt. When utilized in salt form, alkali
metals such as sodium, potassium and lithium; or,
substituted ammonium salts such as from mono-, di- or
triethanolammonium cations are generally preferred.
~
~.
46 2197314
B-1. Aminopolyacetates
The water soluble aminopolyacetate compounds have a
moiety with the structural formula:
CH2COOM
~
R-N
CH2COOM
wherein R is selected from
CH2COOM
l
-CH2COOM; -CH2CH2OH; and -CH2CH2N
I
R'
wherein R' is
CH2COOM
-CHZCH2OH; -CH2COOM; or -CH2CH2N
CH2COOM
and each M is selected from hydrogen and a salt-forming
cation.
Aminopolyacetate water conditioning salts suitable
for use be,rein include the sodium, potassium lithium,
ammonium, and substituted ammonium salts of the
following acids:
ethylenediaminetetraacetic acid, N-(2-
hydroxyethyl)-ethylenediamine triacetic acid, N-(2-
hydroxyethyl)-nitrilodiacetic acid,
diethylenetriaminepentaacetic acid, 1,2-
diaminocyclohexanetetracetic acid and
nitrilotriacetic acid; and, mixtures thereof.
B-2. Polyphosphonates
~
47 2197314
Polyphosphonates useful herein specifically include
the sodium, lithium and potassium salts of ethylene
diphosphonic acid; sodium, lithium and potassium salts
of ethane-l-hydroxy-1,l-diphosphonic acid and sodium
lithium, potassium, ammonium and substituted ammonium
salts of ethane-2-carboxy-1,1-diphosphonic acid,
hydroxymethanediphosphonic acid, carbonyldiphosphonic
acid, ethane-l-hydroxy-1,1,2-triphosphonic acid, ethane-
2-hydroxy-1,1,2-triphosphonic acid, propane-1,1,3,3-
tetraphosphonic acid propane-1,1,2,3-tetraphophonic acid
and propane 1,2,2,3-tetraphosphonic acid; and mixtures
thereof. Examples of these polyphosphonic compounds are
disclosed in British Pat. No. 1,026,366. For more
examples see U.s. Pat. No. 3,213,030 to Diehl issued
October 19, 1965 and U.S. Pat. No. 2,599,807 to
Bersworth issued June 10, 1952.
B-3. Aminopolyphosphonates
The water soluble aminopolyphosphonate compounds
have the structural formula:
CH2P0(OM)z
R-N
{
CH2P0(OM)2
wherein R is selected from:
CH2P0 (OM) z
{
-CH2PO (OM) z; -CH2CH2OH; and -CH2CH2N
{
R
wherein R' is
CH2PO(OM)2
{
-CH2CHZOH; -CH2PO (OM) 2; or -CH2CH2N
{
CH2 PO ( OM ) Z
~
48 2197314
and each M is selected from hydrogen and a salt forming
cation.
Aminopolyphosphonate compounds are excellent water
conditioning agents and may be advantageously used in
the present invention. Suitable examples include
soluble salts, e.g. sodium, lithium or potassium salts;
of diethylene thiamine pentamethylene phosphonic acid,
ethylene diamine tetramethylene phosphonic acid,
hexamethylenediamine tetramethylene phosphonic acid, and
nitrilotrimethylene phosphonic acid; and, mixtures
thereof.
B-4.Short Chain Carboxylates
Water soluble short chain carboxylic acid salts
constitute another class of water conditioner for use
herein. Examples include citric acid, gluconic acid and
phytic acid. Preferred salts are prepared from alkali
metal ions such as sodium, potassium, lithium and from
ammonium and substituted ammonium.
B-5. Polycarboxylates
Suitable water soluble polycarboxylate water
conditioners for this invention include the various
ether polycarboxylates, polyacetal, polycarboxylates,
epoxy polycarboxylates, and aliphatic-, cycloalkane- and
aromatic polycarboxylates.
Water soluble ether polycarboxylic acids or salts
thereof useful in this invention have the formula:
R1
1
R
I
RZ
wherein R1 is selected from -CH2COOM; -CH2CH2COOM;
MOOC CODN uOOC COON
I I I I
-C-C- ; ond - CN-CH-
d1
CA 02197314 2004-07-06
49
and R2 is selected from -CH2COOM; -CH2CH2COOM;
I NOOC COOr
1 1
w00C-CM-C00M - Ch - Cn,
NOOC COOM YOOC COOM
I I I I
-CC- ; onG-CM-CM-
wherein R1 and R2 form a closed ring structure in the
event
said moieties are from:
NOOC COON u00C COON
I I I I
-C=C- ; and - CH-CH-
each M is selected from hydrogen and a salt forming
cation. The salt forming cation M can be represented,
for example, by alkali metal cations such as potassium,
lithium and sodium and also by ammonium and ammonium
derivatives.
Specific examples of this class of carboxylate builder
include the water soluble salts of oxydiacetic acid and,
for example, oxydisuccinic acid, carboxyl methyl
oxysucciiaic acid, furan tetra carboxylic acid and
tetrahydrofuran tetracarboxylic acid. Greater detail is
disclosed in U.S. Pat. No. 3,635,830 to Lamberti et al.
issued January 18, 1972.
Water soluble polyacetal carboxylic acids or salts
thereof which are useful herein as water conditioners
are generally described in U.S. Pat. No. 4,144,226 to
Crutchfield et al. issued March 13, 1979 and U.S. Pat.
No. 4,315,092 to Crutchfield et al. issued February 9,
1982.
A typical product will be of the formula:
2197314
R1(CHO)õR2
I
COOM
5
wherein M is selected from the group consisting of
alkali metal, ammonium, alkyl groups of 1 to 4 carbon
atoms, tetraalkylammonium groups and alkanolamine
groups, both of 1 to 4 carbon atoms in the alkyls
10 thereof, n averages at least 4, and R1 and R2 are any
chemically stable groups which stabilize the polymer
against rapid depolymerization in alkaline solution.
Preferably the polyacetal carboxylate will be one
wherein M is alkali metal, e.g., sodium, n is from 50 to
15 200, R1 is
CH3CH2O COOM
I {
HCO- or H3C-CO-
( 1
20 H3C COOM
or a mixture thereof, R2 is
25 OCH2CH3
I
-CH
CH3
30 = .
and n averages from 20 to 100, more preferably 30 to 80.
The calculated weight average molecular weights of the
polymers will normally be within the range of 2,000 to
35 20,000, preferably 3,500 to 10,000 and more preferably
5,000 to 9,000, e.g., about 8,000.
Water soluble polymeric aliphatic carboxylic acids
and salts preferred for application are compositions of
this invention are selected from the groups consisting
40 of:
~ ' _
51 2197314
(a) a water soluble salts of homopolymers of
aliphatic polycarboxylic acids having the following
empirical formula:
x z
I I
~ c
I I
Y 60 2H
n
wherein X, Y, and Z are each selected from the group
consisting of hydrogen methyl, carboxyl, and
carboxymethyl, at least one of X, Y, and Z being
selected from the group consisting of carboxyl and
carboxymethyl, provided that X and Y can be
carboxymethyl only when Z is selected from carboxyl and
carboxymethyl, wherein only one of X, Y, and Z can be
methyl, and wherein n is a whole integer having a value
within a range, the lower limit of which is three and
the upper limit of which is determined by the solubility
characteristics in an aqueous system;
(b) water soluble salts of copolymers of at least
two of the monomeric species having the empirical
formula described in (a), and
(c) water soluble salts of copolymers of a member
selected-from the group of alkylenes and monocarboxylic
acids with the aliphatic polycarboxylic compounds
described in (a), said copolymers having the general
formula:
R R ( C
I N J{.
COZH
CA 02197314 2004-07-06
52
wherein R is selected from the group consisting of
hydrogen, methyl, carboxyl, carboxymethyl, and
carboxyethyl; wherein only one R can be methyl; wherein
m is at least 45 mole percent of the copolymer; wherein
X, Y, and Z are each selected from the group consisting
of hydrogen, methyl, carboxyl, and carboxymethyl; at
least one of X, Y, and Z being selected from the group
of carboxyl and carboxymethyl provided that X and Y can
be carboxymethyl only when Z is selected from group of
carboxyl and carboxymethyl, wherein only one of X, Y,
and Z can be methyl and wherein n is a whole integer
within a range, the lower limit of which is three and
the upper limit of which is determined primarily by the
solubility characteristics in an aqueous system; said
polyelectrolyte builder material having a minimum
molecular weight of 350 calculated as the acid form and
an equivalent weight of about 50 to about 80, calculated
as the acid form (e.g., polymers of itaconic acid
acrylic acid maleic acid; aconitic acid; mesaconic acid;
fumaric acid; methylene malonic acid; and citraconic
acid and copolymers with themselves and other compatible
monomers containing no carboxylate radicals such as
ethylene, styrene and vinylmethyl ether). These
polycarboxylate builder salts are more specifically
described in U.S. Pat. No. 3,308,067 to Diehl issued
March 7, 1967.
The most preferred water conditioner for use in the
most preferred embodiments of this invention are water
soluble polymers of acrylic acid, acrylic acid
copolyme'l:s; and derivatives and salts thereof having the
empirical formula:
x
I
- [ -CHZ-C- ] X-
1
C=O
I
Y
where X = H, CH3Y = NH21 OH, OCH31 OC2H5, O-Na+, etc. or
copolymers with compatible monomers.
53 2197314
Such-polymers include polyacrylic acid,
polymethacrylic acid, acrylic acid-methacrylic acid
copolymers, hydrolyzed polyacrylamide, hydrolyzed
polymethacrylamide, hydrolyzed acrylamidemethacrylamide
copolymers, hydrolyzed polyacrylonitrile, hydrolyzed
polymethacrylonitrile, hydrolyzed
acrylonitrilemethacrylonitrile copolymers, or mixtures
thereof. Water soluble salts or partial salts of these
polymers such as the respective alkali metal (e.g.
sodium, lithium potassium) or ammonium and ammonium
derivative salts can also be used. The weight average
molecular weight of the polymers is from about 500 to
about 15,000 and is preferably within the range of from
750 to 10,000. Preferred polymers include polyacrylic
acid, the partial sodium salt of polyacrylic acid or
sodium polyacrylate having weight average molecular
weights within the range of 1,000 to 5,000 or 6,000.
These polymers are commercially available, and methods
for their preparation are well-known in the art.
For example, commercially available polyacrylate
solutions useful in the present cleaning compositions
include the sodium polyacrylate solution, Colloid(D 207
(Colloids, Inc., Newark, N.J.); the polyacrylic acid
solution, Aquatreat AR-602-A (Alco Chemical Corp.,
Chattanooga, Tenn.); the polyacrylic acid solutions (50-
65% solids) and the sodium polyacrylate powers (M.W.
2,100 and 6,000) and solutions (45% solids) available as
the Goodrite K-700 series from B. F. Goodrich Co.; and
the sodium or partial sodium salts of polyacrylic acid
solutions -(M.W. 1000 to 4500) available as the Acusol
series from Rohm and Haas.
Of course combinations and admixtures of any of the
above enumerated water conditioning agents may be
advantageously utilized within the embodiments of the
present invention.
Generally, the concentration of water or
conditioner mixture useful in use dilution, solutions of
the present invention ranges from about 0.0005% (5 ppm)
by active weight to about 0.04% (400 ppm) by active
weight, preferably from about .001% (10 ppm) by active
weight to about 0.03% (300 ppm) by active weight, and
6~ ;':
54 2197314
most preferably from about 0.002% (20 ppm) by weight to
about 0.02% (200 ppm) by active weight.
The concentration of water or conditioner mixture
useful in the most preferred concentrated embodiment of
the present invention ranges from about 1.0% by active
weight to about 35% by active weight of the total
formula weight percent of the builder containing
composition.
OPTIONAL ADJUVANTS
In addition, various other additives or adjuvants
may be present in compositions of the present invention
to provide additional desired properties, either of
form, functional or aesthetic nature, for example:
a) Solubilizing intermediaries called hydrotropes
can be present in the compositions of the invention of
such as xylene-, toluene-, or cumene sulfonate; or n-
octane sulfonate; or their sodium-, potassium- or
ammonium salts or as salts of organic ammonium bases.
Also commonly used are polyols containing only carbon,
hydrogen and oxygen atoms. They preferably contain from
about 2 to about 6 carbon atoms and from about 2 to
about 6 hydroxy groups. Examples include 1,2-
propanediol, 1,2-butanediol, hexylene glycol, glycerol,
sorbitol, mannitol, and glucose.
b) Nonaqueous liquid carrier or solvents can be
used for varying compositions of the present invention.
These include the higher glycols, polyglycols,
polyoxides and glycol ethers. Suitable substances are
propylene glycol, polyethylene glycol, polypropylene
glycol, diethylene glycol monoethyl ether, diethylene
glycol monopropyl ether, diethylene glycol monobutyl
ether, tripropylene glycol methyl ether, propylene
glycol methyl ether (PM), dipropylene glycol methyl
ether (DPM), propylene glycol methyl ether acetate
(PMA), dipropylene glycol methyl ether acetate (CPMA),
ethylene glycol n-butyl ether and ethylene glycol n-
propyl ether.
Other useful solvents are ethylene oxide/propylene
oxide, liquid random copolymer such as Synalox solvent
series from Dow Chemical (e.g., Synalox 50-50B). Other
suitable solvents are propylene glycol ethers such as
6~
2197314
PnB, DpnB and TpnB (propylene glycol mono n-butyl ether,
dipropylene glycol and tripropylene glycol mono n-butyl
ethers sold by Dow Chemical under the trade name Dowanol
(D
. Also tripropylene glycol mono methyl ether "TPM
5 Dowanol(D" from Dow Chemical is suitable.
c) Viscosity modifiers may be added to the
invention. These may include natural polysaccharides
such as xanthan gum, carrageenan and the like; or
cellulosic type thickeners such as carboxymethyl
10 cellulose, and hydroxymethyl-, hydroxyethyl-, and
hydroxypropyl cellulose; or, polycarboxylate thickeners
such as high molecular weight polyacrylates or
carboxyvinyl polymers and copolymers; or, naturally
occurring and synthetic clays; and finely divided fumed
15 or precipitated silica, to list a few.
d) Solidifiers are necessary to prepare solid form
compositions of the invention. These could include any
organic or inorganic solid compound having a neutral
inert character or making a functional, stabilizing or
20 detersive contribution to the intended embodiment.
Examples are polyethylene glycols or polyproylene
glycols having molecular weight of from about 1,400 to
about 30,000; and urea.
A wide variety of other ingredients useful in
25 detergent compositions can be included in the
compositions hereof, including other active ingredients,
carriers, draining promoting agents, manufacturing
processing aids, corrosion inhibitors, antimicrobial
preserving agents, buffers, tracers inert fillers, dyes,
.. .
30 etc.
The list of optional ingredients above is not
intended to be exhaustive and other optional ingredients
which may not be listed, but which are well known in the
art may also be included in the composition. The
35 examples are not intended to be limiting in any way. In
certain cases, some of the individual adjuncts may
overlap in other categories.
In general, the total proportion of adjuvants will
normally be no more than 40% by weight of the product
40 and desirably will be less than 30% by weight thereof,
more desirably less than 30% thereof. Of course, the
~
2197314
56
adjuvants employed will be selected so as not to
interfere with the detersive action of the composition
and to avoid instability of the product.
6a
57
WORKING EXAMPLE NOS. 1-10
TABLE NO. 1
ENZYME/BUILDER DUAL COMPONENT CIP (TWO PART) FORMULATIONS FOR PRODUCT LINE
PART 1
ENZYME/SURFACTANT Example Example Example Example Example Example
COMPONENT 1 2 3 4 5 6
RAW MATERIAL Percent Percent Percent Percent Percent Percent
Deionized Water 33.500 33.500 33.875 33.875 22.500 22.500
Triethanolamine, 99% 2.000 2.000 2.000 2.000 2.000 2.000
Sodium Metabisulfite 1.000 1.000 1.000 1.000 1.000 1.000
Propylene Glycol 12.250 12.250 15.000 15.000 12.000 12.000
Sodium Xylene 20.000 20.000 20.000 20.000 25.000 25.000
Sulfonate, 40%
Surfonic N95+5PO* 25.000 25.000 25.000 25.000 25.000 25.000
Purafect 4000-L, 6.250 3.125 12.500
protease**
Esperase 8.OL, 6.250 3.125 12.500
protease***
N
Ir ,
58
TABLE NO. 1 (Continued)
PART 2
:
BUILDER COMPONENT Example 7 Example 8 Example 9 Example 10
RAW MATERIAL Percent Percent Percent Percent
Deionized Water 61.24 57.30 47.80 67.30
Tetrasodium EDTA, 0.20 0.20 0.20 0,20
40%
Acusol 445N**** 26.00 26.00 26.00 26,00
Sodium Carbonate 12.56 8.25 6.50
Potassium 8.25 26.00
Carbonate
Surfonic N95+5P0 is manufactured by Texaco Chemical Company
** Purafect 4000-L, is manufactured by Genencor International, USA
*** Esperase 8.OL is manufactured by Novo Industri AS, Denmark
= **** Acusol 445N is manufactured by Rohm and Haas Company
t~.)
~
W
~
59
WORKING EXAMPLE NOS. 1-10
TABLE NO. 2
ENZYME/BUILDER DUAL COMPONENT (TWO PART) CIP PRODUCT LINE
PART 1
PRODUCT USE
EXAMPLE PRODUCT DESCRIPTION CONCENTRATION SURFACTANT
PRODUCT ENZYME/SURFACTANT (PPM) ENZYME (%) (PPM) (%) (PPM)
1 Low Temp ;"Balanced" 400 GENENCOR 12.50 50 25.00 100
Components PURAFECT 4000L
2 Low Temp; Enzyme Rich 400 GENENCOR 12.50 50 25.00 100
PURAFECT 4000L
3 Low Temp; Surfactant Rich 800 GENENCOR 3.12 25 25.00 200
PURAFECTU4000L
High Temp 1"Balanced" 400 NOVO ESPERASE 6.25 25 25.00 100
4 Components 8.OL
5 High Temp; Enzyme Rich 400 NOVO ESPERASE 12.50 50 25.00 100
8.OL
6 High Temp; Surfactant 800 NOVO ESPERASE 3.12 25 25.00 200
Rich 8.OL
N
~nt
~
60
TABLE NO. 2 (Continued)
PART 2
USE PAA
EXAMPLE PRODUCT DESCRIPTION CONCENTRATION CARBONATE (PPM) (PPM)
PRODUCT BUILDER (PPM) SOURCE (%) total ($) 100%
active
7 Standard Product 500 NaCO3/K2CO3 8.25/8.25 83 26.00 59
8 Soft Water 250 KZC03 26.00 65 26.00 29
9 Hard Water 1000 Na2CO3 6.50 65 26.00 117
Carbonate Rich; 500 K2CO3 26.00 130 26.00 59
Difficult Soil
Use temperature 30 C to 65 C.
2 Use temperature 50 C to 85 C.
N
~
61 2197314
Tables 1 and 2 contain details pertaining to a
"family" of two component enzyme/builder products for
CIP application. The CIP Product Line is described by
product design (i.e. low temp:enzyme rich) and by
product application (i.e. soft water). Basically this
"family" of products involves three products for low
temperature CIP applications (from about 30 C to about
65 C); and, three products for high temperature CIP
applications (from about 50 C to about 85 C). Within
each temperature category, products containing a
"balanced" ratio of enzyme/surfactant (25 ppm/100 ppm),
an enzyme rich ratio of enzyme/surfactant (50 ppm/100
ppm), and a surfactant rich ratio of enzyme/surfactant
(25 ppm/200 ppm) are incorporated. The low temperature
and high temperature designations reflect one major
change within the composition -- that change being
alkaline protease enzyme. All other ingredients remain
unchanged with exception of concentration.
., .
~
62
WORKING EXAMPLE NO. 11
TABLE 3
ENZYME/SURFACTANT SOLID CAST (ONE PART) CIP PRODUCTS WITH CARBONATE BUILDER
PREFERRED LIQUID PRODUCT
INGREDIENT PPM USE LEVELS
Example 11
USE CONCENTRATION: 0.10%
RAW MATERIAL (PPM)
Esperase 8.OL, protease* 25
Triton CF-21** 100
Acusol 445N*** 130
Na CO **** 63
~
-O
~
4Z-
~
63
WORKING EXAMPLE NOS. 12-19
TABLE NO. 3 (continued)
SOLID PRODUCTS
INGREDIENT PPM USE LEVELS TO EQUAL PREFERRED LIQUID
Example 12 Example 13 Example 14 Example 15
USE CONCENTRATION: CONCENTRATION FACTOR
0.10 s
(PPM) 1X 2X 3X 3.5X
RAW MATERIAL (NEEDED) (%) (%) (%) (%)
Esperase 6.OT, 19 1.9 3.8 5.7 6.7
protease*
Triton CF-21 100 10.0 20.0 30.0 35.0
Goodrite K-7058D**** 65 6.5 13.0 19.5 22.8
Sodium Carbonate 63 6.3 12.6 18.9 22.1
Polyethylene Glycol 75.3 50.6 25.9 13.4
8000
USE CONCENTRATION 0.100% 0.050% 0.033% 0.029%
PPM 1000 500 333 290
--~
64
TABLE 3 (Continued)
SOLID PRODUCT FORMULATIONS
CONCENTRATION 3X PREFERRED
Example 16 Example 17 Example Example
18 19
RAW MATERIAL PERCENT PERCENT PERCENT PERCENT
Esperase 6.OT, protease 5.60 5.60
Triton CF-21 30.00 30.00 30.00 30.00
Goodrite K-7058D 19.60 19.60 19.00 18.70
Sodium Carbonate 29.80 18.80 18.80 18.80
Polyethylene Glycol 15.00 26.00 26.00 26.00
8000
PROTECT 76-10***** 6.20
PROTECT 76-15***** 6.50
* Esperase B.OL and Esperase 6.OT are manufactured by Novo Industri AS,
Denmark.
** Triton CF-21 is manufactured by Union Carbide Chemical & Plastics Company.
*** Acusol 445N is manufactured by Rohm and Haas Company.
**** Goodrite K-7058D is manufactured by BF Goodrich Chemical Division.
***** Protect 76-10 and Protect 76-15 are encapsulates of Esperase 6.OT having
10% and 15% by weight encapsulated N
coatings comprising sodium polyacrylate, 4500 molecular weight,
65 2197314
Table 3 represents another product form of the
invention, i.e. a cast solid. Table 3 shows various
Concentration (ppm) levels of ingredients which are
delivered in detersive solutions by the preferred liquid
dual component system, then illustrates suggested
compositions which would deliver the same ppm levels at
various concentration factors, and then lists several
solid compositions actually prepared. Changes are made
in raw material selection, such as using anhydrous
polyacrylate water conditioner and prilled enzyme, to
facilitate formulation. However, the biggest formulary
change is the necessary inclusion of a solidifier,
polyethylene glycol 8000, for product form. Also
disclosed in these compositions is the concept of
encapsulated enzyme for improved stability - especially
needed during the hot melt/pour cast manufacturing
process.
., .
~
j r
66
WORKING EXAMPLE NO. 20
TABLE 4
ENZYME/SURFACTANT SOLID CAST (ONE PART) CIP PRODUCTS WITH SILICATE BUILDER
PREFERRED LIQUID PRODUCT '
INGREDIENT PPM USE LEVELS
Example 20
USE CONCENTRATION: 0.10%
RAW MATERIAL (PPM)
Esperase 8.OL, protease* 25
Triton CF-21** 100
Acusol 445N*** 130
E SILICATE**** 400
~
-10
~
67
TABLE 4 (Continued)
SOLID PRODUCT FORMULATIONS PREPARED
CONCENTRATION 3X PREFERRED LIQUID
Example 24 Example 26
2.5X 3.0X
RB-9143-9 RB-9143-9
RAW MATERIAL PERCENT PERCENT
Esperase 6.OT, protease 4.80 5.70
Triton CF-21 25.00 30.00
Acuso1n445N 16.30 16.30
SS 20 RPWD 33.90 28.00
Polyethylene Glycol 8000 20.00 20.00
* Esperase 8.OL and Esperase 6.OT are manufactured by Novo Industri AS,
Denmark.
** Triton CF-21 is manufactured by Union Carbide Chemical & Plastics Company.
*** Acusol 445N is manufactured by Rohm and Haas Company.
**** E Silicate is a liquid 36% 3.22 SiOz/NaZO silicate manufactured by PQ
Corp.
***** SS 20 Pwd is an anhydrous 98% 3.22 SiO2/Na2O silicate manufactured by PQ
Corp.
N
.d~
2197314
68
Like the enzyme/surfactant solid cast CIP products
with carbonate builder, this table illustrates that a
solid form of product can be developed having a silicate
builder. The table is laid out in similar fashion with
a comparison made to a liquid (ppms delivered) formula,
followed by prophetic solid formulas, and then concluded
with actual solid formulations prepared.
~
~ =
69
WORKING EXAMPLE NOS. 26-30
TABLE NO. 5
ALTERNATE ENZYME/BUILDER DUAL COMPONENT FORMULATION EXAMPLES
ENZYME/SURFACTANT Example 26 Example 27 Example 28 Example 29
COMPONENT
RAW MATERIAL PERCENT PERCENT PERCENT PERCENT
Experase 8.OL, 20.00 19.00 33.30 31.70
protease***
Triethanolamine, 99% 2.00 2.000
Sodium Metabisulfite 1.00 1.000
Propylene Glycol 2.00 2.00
Triton@CF-21 *** 80.000 76.00 66.70 63.30
USE CONCENTRA!'ION 0.0125d 0.0130% 0.015011 0.0155%
PPM 1225 130 150 155
IU
~
70
TABLE 5 (continued)
BUILDER COMPONENT** EXAMPLE 30
RAW MATERIAL, PERCENT
Soft Water 47.00
Acusol 445N***** 13.00
E Silicate ****** 40.00
USE CONCENTRATION 0.10%
PPM 1000
* High concentrate.
** Liquid silicate builder used in all Examples.
*** Esperase 8.OL is manufactured by Novo Industri AS, Denmark.
**** Triton CF-2l is manufactured by Union Carbide Chemical & Plastics
Company.
***** AcusolO445N is manufactured by Rohm and Haas Company.
******E Silicate is a liquid 36% 3.22 Si02/Na2O silicate manufactured by PQ
Corp.
Table 5 is included to show that the enzyme/surfactant component of the dual
products system can be formulated to a
very high active concentration, in fact excluding addition of water. Liquid
enzymes may contain water as purchased,
consequently, the formulator can either include or exclude the axillary
stabilizing system.
In addition, the builder component contains, in table 5, a silicate as the
builder rather than carbonate
~
C~!
~
~-
... ~ p
71
WORKING EXAMPLE NOS. 31-34
TABLE NO. 6
ENZYME/SURFACTANT GRANULATED CIP PRODUCTS*
Example 31 Example 32 Example 33 Example 34
RAW MATERIAL PERCENT PERCENT PERCENT PERCENT
Sodium Carbonate 56.00 51.50 56.00 51.50
Sodium Tripolyphosphate 25.00 25.00 25.00 25.00
Triethanolamine, 99% 2.00 2.00
Sodium Metabisulfite 1.00 1.00
Propylene Glycol 2.00 2.00
Surfonic N95+5P0 10.00 10.00 10.00 10.00
Purafect 4000-G, protease*** 2.50 2.50
Maxacal CST 450,000, 2.50 2.50
rotease****
Goodrite K-7058D***** 6.00 6.00 6.00 6.00
* Experimental formulas w/wo "Stabilizing Systems" for use--dilution effect.
Expected use-dilution 0.1%
(1000 ppm).
** Surfonic N95+5P0 is manufactured by Texaco Chemical Company.
*** Purafect 4000-G is manufactured by Genencor International, USA.
**** Maxacal CXT 450,000 is manufactured by Gist-Brocase International, NV. N
***** Goodrite K-7058D is manufactured by BF Goodrich Chemical Division.
(~J
-ja
72 2197314
Table 6 illustrates examples of anhydrous granulate
enzyme/builder/surfactant compositions. These are
single component formulations that show the basic
technology lends itself to this product form. STPP is
the choice of water conditioning agent in these
particular compositions. Prilled enzymes are utilized
because of product form. Because these concentrates are
anhydrous, it is the formulator's choice if a
stabilizing system is included for use-dilution effect
rather than a need for facilitating shelf-life.
., .
~
1 .
73
TABLE A
SS CLEANING CLEANING CLEANING WHOLE WI WI PERCENT
PANEL SOLUTION TEMPERATURE TIME MILK (After (After CLEANING
SOIL Soiling) Cleaning)
(2) (A) 50 C 15 min. --- 7.82 18.49 136.45
(1) (A) 50 C 15 min. 0.25% 10.42 19.40 86.19
(9) (A) 65 C 15 min. --- 8.42 9.50 12.83
(3) (B) 50 C 15 min. --- 7.80 6.67 -14.49
(11) (B) 65 C 15 min. --- 8.11 6.81 -16.03
(4) (C) 50 C 15 min. --- 8.12 23.78 192.86
(10) (C) 50 C 15 min. 0.25% 9.00 25.62 184.67
(12) (C) 65 C 15 min. --- 8.06 21.86 171.22
(21) (C) 65 C. 15 min. 0.25% 9.11 23.30 155.77
(5) (D) 50 C 15 min. --- 8.17 18.31 124.11
(13) (D) 50 C 15 min. 0.25% 9.90 22.49 127.26
(24) (D) 65 C 15 min. --- 7.96 7.96 0.00
(6) (E) 50 C 15 min. --- 7.55 28.43 276.56
(20) (E) 50 C 15 min. 0.25% 10.67 30.49 185.67
(25) (E) 65 C 15 min. --- 8.26 25.97 214.41
(22) (E) 65 C 15 min. 0.25% 8.77 29.28 233.74
(26) (F) 65 C 15 min. --- 8.33 18.22 118.73
(23) (F) 65 C 15 min. 0.25% 8.57 10.28 19.93
(41) (F) 75 C 15 min. --- 10.24 21.79 112.85
(8) (G) 50 C 15 min. --- 8.08 6.56 18.81
(30) (G) 65 C 15 min. --- 7.67 6.95 -9.39
(34) (H) 65 C 15 min. --- 11.52 19.90 72.78
(32) (H) 75 C 15 min. --- 9.61 14.87 54.68
(14) (I) 65 C 15 min. --- 12.11 25.30 108.93 Crl
(33) (I) 75 C 15 min. --- 9.71 25.99 167.75
(29) (J) 65 C 15 min. --- 10.24 23.89 133.25
(31) (K) 65 C 15 min. --- 9.07 28.58 215.23
(40) (K) 75 C 15 min. --- 10.12 21.77 115.19
2197314
74
CLEANING OF SOILED SS PANELS
Cleaning performance evaluations of the
particularly preferred concentrate embodiment of this
invention -- a two part, two product detergent system.
1) The Stainless Steel 304 panels used in this cleaning
evaluation were prepared/soiled according to Ecolab RB
No. 9419-3,4
PROCEDURE FOR PROTEIN SOILING AND CLEANING OF
STAINLESS STEEL PANELS
Purpose: To simulate the soiling and subsequent
cleaning of stainless steel equipment surfaces
in dairy plants and farms
The following reagents and test materials should be
aprepared and/or obtained prior to conducting soiling
and cleaning procedure:
1) 3" x 5" 304 stainless steel panels with #4 finish
having two 1/4" holes drilled at top and numbered.
2) 3/16" stainless steel rods approx. 15" in length.
3) 1/8" and 1/4" I.D. rubber tubing cut into 1/4"
lengths.
4) 10.5 liter tank with heating and circulation
capabilities.
5) 22.2 liter tank with drain cock.
6) A consumer type automatic dishwasher.
7) HunterLab UltraScan Spectrophotometer Model US-
8000.'
8) Lab Magnetic stir plate with heating capabilities.
9) 1000 ml. beakers.
10) Magnetic stir bars.
11) Lab thermometer.
12) Graduated cylinders and Volumetric pipettes.
13) KLENZ SOLV (a Klenzade liquid detergent-solvent
product).
14) FOAM BREAKER (a Klenzade general defoaming
product).
15) AC-300 (a Klenzade conventional acid CIP
detergent).
d7-
75 2197314
16) PRINCIPAL without chlorine (a Klenzade conventional
high alkaline CIP detergent prepared without
hyppochlorite).
17) Cleaning solutions to be evaluated.
18) Hardness solution (110.2 g/L CaC12* 2 H20 and 84.6
g/L MgC12* 6 H20) .
19) 60 gallons of Whole Milk (commercial Homogenized).
Conditioning of SS Panels Prior to Soiling and Cleaning
1) Clean SS panels with 3% by volume of Klenz Solv and
1.5% by volume of Foam Breaker in 10.5 liter tank
at 135 F for 45 min. Remove panels and rinse both
panels and tank with distilled water.
2) Passivate the SS panels with 54% by volume of AC-
300 in 10.5 liter tank at 135 F for 1 hour.
3) Remove panels, rinse well with distilled water and
allow to air dry.
4) Measure Whiteness Index (panel before soiling) of
test panels by means of the HunterLab UltraScan
Spectrophotometer, Model US-8000. The operating
procedure for the UltraScan is found in the
manufacturers manual.
Soiling of SS Panels
1) Fill the 22.2 L tank with 6 gallons of milk.
2) Place SS panels on SS rods with 1/4" rubber tube
spacers between each panel and a piece of 1/8"
rubber tube on each end to hold panels in place.
Approx. 21 panels will fit on the 15" rods.
3) Place the rack of SS panels into the tank of milk.
4) Slo4al'y drain the milk from the tank at a flow rate
of approx. 150 ml\min. Collect the milk to be used
a second time.
5) After the level of milk in the tank is below the
outlet, remove the rack of panels and place
securely in bottom of consumer dishwater.
6) Using a wash temperature of approx. 100 F, wash the
rack of panels for 2 min. in dishwasher with a
solution containing 2500 ppm PRINCIPAL without
chlorine, 60 ppm Ca and 20 ppm Mg. For a 10 liter
L~
76 2197314
machine add 25 ml PRINCIPAL and 20 ml Hardness
soln. listed above.
7) Following the wash, rinse the panels for 1.5-2 min.
using city water wihout machine drying.
8) Remove rack of panels and allow to air dry approx.
30 min. at RT prior to repeating the above seven
steps for a total of 20 cycles.
9) Fresh milk should be used every other cycle with a
total of 60 gallons of milk used.
Cleaning of Soiled SS Panels
Dipping Test
1) Prepare the cleaning solutions in City water using
1000 ml beakers.
2) Place one soiled panel in bottom of beaker filled
iwth 1000 ml of desired cleaning solution that has
been preheated to desired temperature. Agitate
solution for desired time by means of a heating,
magnetic stir place and magnetic stir bar.
3) After cleaning, rinse panels with DI water and
allow to air dry.
4) Measure Whiteness Index (panel after soiling) of
test panels.
5) Percent change (cleaning) is calculated by the
formula WI (panel after cleaning) - WI (panel after
soiling)/WI (panel after soiling). WI = Whiteness
Index.
6) Percent soil removal is calculated by the formula
WI (panel after cleaning) - WI (panel after
soi-,1ing)/WI (panel before soiling) - WI (panel
after soiling).
7) Whiteness Index (WI) measurement is per ASTM E313
(see ASTM E313-73 (Reapproved 1987)
~
77 2197314
2) The following cleaning solutions were pH before pH after
prepared in 60 ppm City water: Milk Milk
(A) 25 ppm Purafect 4000-L (0.050 gm/2000 ml) 8.67 7.69
(B) 0.05% Product A (1.00 gm/2000 ml) or 1 10.00 ---
oz./15.6 gal.
(C) 0.0496 Product B with Purafect 4000-L (0.80 8.50 7.69
gm/2000 ml) or 1 oz./19.5 gal.
(D) 25 ppm Purafect 4000-L (0.50 gm/2000 mi) & 9.95 9.54
0.05% Product A (1.00 gm/2000 ml).
(E) 0.05% Product A (1.00 gm/2000 ml) & 0.04% 9.86 9.49
Product B with Purafect 4000-L (0.80 gm/2000
ml).
(F) 0.05% Product A (1.00 gm/2000 ml) & 100 9.74 9.71
ppm Texaco NPE 9.5 P05 (0.20 gm/2000 ml) & 80
ppm Avail. Chlorine (1.60 gm 10.01% active XY-
12/2000 ml).
(G) 0.04% Product B without enzyme (0.80 8.50 ---
gm/2000 ml) or 1 oz./19.5 gal.
(H) 25 ppm Esperase 8.0 L (0.050 gm/2000 ml) 8.00 ---
(I) 0.04% Product B with Esperase 8.0 L (0.80 7.83 gm/2000 ml) or 1 oz./19.5
gal
(J) 25 ppm Esperase 8.OL (0.50 gm/2000 ml) & 9.58
0.05a Product A (1.00 gm/2000 ml).
(K) 0.05% Product A (1.00 gm/2000 ml) & 0.04% 9.49 ---
Product B with Esperase 8.0 L (0.80 gm/2000
ml).
3) 1000 ml of desired cleaning solution plus 0.25% (2.5
ml/1000 ml) milk soil when required, was placed in 1000
ml beaker. The solution was then heated to desired
temperature and one soiled panel was placed in bottom of
beaker. The solution was agitated for 15 min. while
maintaining temperature by means of a magnetic stir bar
and magnetic, heating, stir plate.
61 -
4) After cleaning, the panels were rinsed with DI water
and allowed to air dry.
5) Cleaning was measured by means of the HunterLab
UltraScan Spectrophotometer Model US-8000.
6) Settings on the instrument were RSEX\UVL ON\UVF
OUT\LAV.
7) The percent change (cleaning) was calculated by the
formula WI (panel after cleaning) - WI (panel after
~
78 2197314
soiling)/WI (panel after soiling) X 100.WI = Whiteness
Index.
This series of tables contains the majority of
laboratory evidence proving our claims that:
Table A
Alkaline protease acting of and by itself, without
cooperative effect of other detersive agents, removes
adsorbed protein (film) from food soiled surfaces. This
effect is shown on the chart of Protein Film Soil
Removal, detersive solution A, 50 C as compared to a
built, high alkaline, chlorinated commercial CIP
detergent - PRINCIPAL at 50 C utilized at recommended
use-dilutions. Also notable from Figure 1, solution A-
the enzyme, Purafect 4000L, does not perform well on
protein film by itself at.65 C; whereas, if it is used
with the stabilizing system, cleaning performance
(protein soil removal) is dramatically improved (see
Figure 1 for solution C) even at 65 C thus showing
unexpected cooperative effect at use dilution. Prior
art teaches the stabilizing effect of enzyme stabilizing
systems within the composition concentration (i.e.
shelf-life) -- nothing is discussed or disclosed
pertaining to effect at product use dilution. Also
notable from comparison of Figure 1-solution A used at
65 C (Figure 1) to PRINCIPAL (Figure 1) is that at 65 C
PRINCIPAL performs much better on protein soil than at
50 C; and, this is because of an apparent energy of
activation threshold for chlorine discovered during the
course of these experiments. In effect, this discovery
seems to indicate that low temperature CIP cleaning can
never be achieved using the standard high alkaline,
chlorinated products now utilized in the food process
industry; whereas, the present invention is ideally
suited for low temperature CIP applications. Solution
H, Figure 2 containing Esperase 8.OL (an alkaline
protease having greater high temperature tolerance)
confirms that this enzyme has higher activity in higher
temperature detersive solutions than Purafect 4000L. The
observations illustrated in Figs. 1 and 2 are again
repeated in these experiments. Noted from both Figs. 1
and 2 (one for Purafect solutions, one for Esperase
79 2197314
solutions) is that the dual product enzyme/builder
system is far superior to PRINCIPAL; that there is a
cooperative effect by combining the two solutions; and,
that the dual component performance solution K is
superior to solution F which contains the
builder/surfactant (without enzyme) and 80 ppm chlorine
(Fig.2). Disclosed in the table A is evidence that
enzyme containing systems are not affected by presence
of milk soil; whereas, chlorine containing systems are
very significantly affected (manifested by reduced
protein film removal).
~
80
TABLE B
TEST SS CLEANING CLEANING CLEANING WHOLE WI WI PERCENT
SET PANEL SOLUTION TEMPERATURE TIME MILK (After (After CLEANING
SOIL Soiling) Cleaning)
I (21) NaOH 500 50 C 60 min. --- 16.28 18.29 12.35
ppm
(22) NaOH 1000 50 C 60 min. --- 16.62 18.97 14.14
ppm
(23) NaOH 2000 50 C 60 min. --- 16.04 19.18 19.58
pm
(24) NaOH 2000 50 C 60 min. --- 15.38 22.50 46.29
ppm
(25) NaOH 50 C 60 min. --- 17.10 24.67 44.27
20000 ppm
II (21) (L) 50 C 30 min. --- 20.05 23.42 16.81
(22) (L) + 50 C 30 min. --- 20.17 24.68 22.36
NaOH 500
ppm
(23) (L) + 50 C 30 min. --- 20.36 25.22 23.87
NaOH 1000
ppm
(24) (L) + 50 C. 30 min. --- 12.90 19.90 54.26
NaOH
10000 ppm ~
II (25) (L) + 50 C 30 min. --- 18.43 38.52 109.00 ---
NaOH
20000 ppm
III (16) (M) 50 C 60 min. --- 17.17 20.89 21.67 W
IV (29) (M) + 50 C 15 min. --- 18.31 23.84 30.20 4:-
NaOC1 80
ppm
(27) (M) + 50 C 30 min. --- 18.30 32.34 76.72
NaOC1 80
81
TEST SS CLEANING CLEANING CLEANING WHOLE WI WI PERCENT
SET PANEL SOLUTION TEMPERATURE TIME MILK (After (After CLEANING
SOIL Soiling) Cleaning)
I (21) NaOH 500 50 C 60 min. --- 16.28 18.29 12.35
ppm
ppm
(28) (M) + 50 C 60 min. --- 16.57 39.73 139.77
NaOC1 80
ppm
V (31) (M) + 50 C 15 min. --- 16.97 41,20 142.78
Esperase8
.OL 100
ppm
(30) (M) + 50 C 30 min. --- 16.10 41.40 157.14
Esperase8
OL 100
ppm
V (18) (M) + 50 C 60 min. --- 11.43 41.94 266.93
Esperase
8.OL 100
PPm
VI (37) (M) + 50 C 30 min. --- 24.14 41.79 73.12
Esperase
8.OL 10
ppm
(36) (M) + 50 C 30 min. --- 23.00 41,59 80.83 ~
Esperase
8.OL
~
25 ppm
(25) (M) + 50 C 30 min. --- 18.43 38.52 109.00
Esperase
8.OL
50 ppm
VII* (38) (M) + 50 C 0-30 min. --- 22.01 41.69 89.41
lo , >
.. >
82
TEST SS CLEANING CLEANING CLEANING WHOLE WI WI PERCENT
SET PANEL SOLUTION TEMPERATURE TIME MILK (After (After CLEANING
SOIL Soiling) Cleaning)
I (21) NaOH 500 50 C 60 min. --- 16.28 18.29 12.35
pm
Esperase
8.0L
100 ppm
(39) (M) + 50 C 60-90 min. --- 21.64 42.51 96.44
Esperase
8.OL
100 ppm
VII* (40) (M) + 50 C 120-150 --- 20.71 40.70 92.29
Esperase min.
8.OL
100 ppm
(41) (M) + 50 C 180-210 --- 21.66 40.68 87.81
Esperase min.
8.OL
100 ppm
(42) (M) 50 C 240-270 --- 19.87 41.46 108.66
Esperase min.
8.OL
100 ppm
(43) (M) + 50 C 300-330 --- 17.75 39.66 123.44
Esperase min.
8 . 0 L
100 ppm
VIII (33) (M) + 50 C 30 min. 1.00% 11.59 37.20 220.97
Esperase
8. 0 L .~
100 ppm
VIII (34) (M) + 50 C 30 min. 0.10% 15.68 39.45 151.59
Esperase
83
TEST SS CLEANING CLEANING CLEANING WHOLE WI WI PERCENT
SET PANEL SOLUTION TEMPERATURE TIME MILK (After (After CLEANING
SOIL Soiling) Cleaning)
I (21) NaOH 500 50 C 60 min. --- 16.28 18.29 12.35
ppm
8.OL
100 ppm
(35) (M) + 50 C 30 min. 1.00% 16.81 18.93 12.61
NaOCl 100
m
(19) (M) + 50 C 30 min. 0.10% 21.57 30.81 42.84
NaOC1 100
p m
* (M) + Esperase 8.OL 100 ppm solutions held with agitation for 5.5 hours at
50 C.
At time 0, 1, 2, 3, 4, 5 hours, a soiled SS panel was added to agitated
solution for 30 minute increments, then
removed.
ra3
-.0
C~~I
4=--
84 21 97314
CLEANING OF SOILED SS PANELS
Comparison of high alkaline detergent solutions
without chlorine versus low alkaline detergent solutions
containing chlorine or containing proteolytic enzyme.
1) The Stainless Steel 304 panels used in this cleaning
evaluation were prepared/soiled according to Ecolab RB
No. 9419-3,4 "Procedure for Protein Soiling and Cleaning
of Stainless Steel Panels" (See page 96, line 9 through
page 99, line 5).
2) The following cleaning solutions were prepared in 60
ppm City water.
(L) PRINCIPAL without chlorine, 4000 ppm solution.
PRINCIPAL is a commercial, conventional,
chlorinated, high alkaline, CIP detergent
manufactured by Ecolab Inc.
(M) A low alkaline, non-chlorinated solution
consisting of 1000 ppm sodium
tripoly[phosphate, 500 ppm sodium bicarbonate,
and 500 ppm sodium carbonate.
3) 1000 ml of desired cleaning solution plus milk soil
when required, was placed in 1000 ml beaker. The
solution was then heated to desired temp. and one soiled
panel was placed in bottom of beaker. The solution was
agitated for 15 min. while maintaining temperature by
means of a magnetic stir bar and magnetic, heating, stir
plate.
4) After cleaning, the panels were rinsed with DI water
and allowed to air dry.
5) Cleaning was measured by means of the HunterLab
UltraScan Spectrophotometer Model US-8000.
6) Settings on the instrument were RSEX\UVL ON/UVF
OUT/LAV.
7) The percent change (cleaning) was calculated by the
formula WI (panel after cleaning) - WI (panel after
~ .;
2197314
soiling)/WI (panel after soiling) X 100. WI=Whiteness
Index.
Table B contains several experiment "sets" which add
5 additional detail to this invention:
Set I shows that solutions of caustic, even up to
2% solutions, have limited effect upon protein soil
removal (as compared to enzyme systems shown in sets V
to VIII). Set II is simply PRINCIPAL without chlorine.
10 Set III is a set of solutions combining the water
conditions agents in PRINCIPAL with the same levels of
caustic utilized in Set I. Set III is a low alkaline,
phosphate containing detergent with carbonate builder
which was utilized in early experiments with enzyme.
15 Sets IV to VIII are experiments utilizing this low
alkaline detergent (Solution M) with varying levels of
Esperase 8.OL and differing cleaning times (all
temperatures are at 50 C). Set VII is of particular
interest because these experiments would indicate that
20 Esperase 8.OL remains active for extended periods of
time -- a critical need in reuse CIP systems wherein the
cleaning solution is reused again and again for several
hours.
r.Sw/r'"'.
al
86
TABLE C
TES CLEANING CLEANING CLEANING * WI WI PERCENT
T SOLUTION TEMPERATURE TIME pH (After (After CLEANING
SET Soiling) Cleaning)
I (M) + Esperase B.OL 50 C 30 min. 8.3 22.16 42.90 93.59
50 ppm '
II (M) + Esperase 8.OL 50 C 30 min. 10.3 21.17 41.67 96.84
ppm
(M) + Esperase 8.OL 50 C 30 min. 10.3 16.50 37.41 126.73
25 ppm
III (M) + Esperase 8.OL 50 C 30 min. 8.3 16.00 40.02 150.13
50 ppm
(M) + Esperase 8.OL SO C 30 min. 9.3 17.96 39.35 119.10
50 ppm
(M) + Esperase 8.OL 50 C 30 min. 10.3 17.54 41.37 135.86
50 ppm
(M) + Esperase 8.OL 50 C 30 min. 11.3 18.68 40.33 126.61
50 ppm
IV (M) + Esperase 8.OL 50 C 5 min. 10.3 16.27 36.70 125.57
50 ppm
(M) + Esperase 8.OL 50 C 10 min. 10.3 16.44 39.02 137.35
50 ppm
(M) + Esperase 8.OL 50 C 15 min. 10.3 17.03 40.69 138.93
50 ppm
(M) + Esperase 8.OL 50 C 30 min. 10.3 19.39 41.42 113.62 NJ
10 ppm
* Normal pH of (M) solution is about 10.3. Other test pH solutions adjusted
with H3P04 or NaOH.
~
4~-
87 2197314
CLEANING OF SOILED SS PANELS
Esperase(D 8.OL cleaning performance as a function
of detersive solution pH or soil contact time.
1) The Stainless Steel 304 panels used in this cleaning
evaluation were prepared/soiled according to Ecolab RB
No. 9419-3,4 "Procedure for Protein Soiling and Cleaning
of Stainless Steel Panels" (See page 96, line 9 through
page 99, line 5).
2) The following cleaning solutions were prepared in 60
ppm City water.
(M) A low alkaline, non-chlorinated solution
consisting of 1000 ppm sodium tripolyphosphate, 500
ppm sodium bicarbonate, and 500 ppm sodium
carbonate.
3) 1000 ml of desired cleaning solution plus milk soil
when required, was placed in 1000 ml beaker. The
solution was then heated to desired temperature and one
soiled panel was placed in bottom of beaker. The
solution was agitated for 15 min. while maintaining
temperature by means of a magnetic stir bar and
magnetic, heating, stir plate.
4) After cleaning, the panels were rinsed with DI water
and allowed to air dry.
5) Cleaning was measured by means of the HunterLab
UltraScan.Spectrophotometer Model US-8000.
6) Settings on the instrument were RSEX/UVL ON/UVF
OUT/LAV.
7) The percent change (cleaning) was calculated by the
formula WI (panel after cleaning) - WI (panel after
soiling)/WI (panel after soiling) X 100. WI = Whiteness
Index.
Table C having Sets I to IV illustrates cleaning
performance of solution M with varying levels of
A
88 2197314
Esperase 8.OL at different solution pH's and with
different cleaning exposure times. This data is useful
in selection of detergent enzyme levels, CIP program
soil contact (wash) times; and, also effect of lower
pH's on detersive solutions (as might be encountered in
heavily soiled operations containing acid foodstuffs).
~
~ =
89
TABLE D
TEST SET CLEANING CLEANING CLEANING TIME WI (After WI (After PERCENT
SOLUTION TEMPERATURE Soiling) Cleaning) CLEANING
I PRINCIPAL 50 C 5 min. 7.65 10.00 30.72
PRINC PAL 50 C 10 min. 11.54 15.55 34.75
PRINCIPAL 50 C 15 min. 9.63 17.40 80.69
PRINCIPAL 65 C 5 min. 10.81 21.90 102.59
PRINCIPAL 65 C 10 min. 10.96 37.37 240.97
PRINCIPAL 65 c 15 min. 13.91 37.95 172.83
II ULTRA 4 50 C 5 min. 10.98 17.86 62.66
ULTRA 50 C 10 min. 11.63 13.35 14.79
ULTRA 50 C 15 min. 11.70 14.64 25.13
ULTRA 65 C 5 min. 11.63 12.92 11.09
ULTRA 65 C 10 min. 11.76 33.46 184.52
ULTRA 65 C 15 min. 12.08 38.29 216.97
III (M) + 50 C 10 min. 10.86 38.37 253.31
Esperase
8.OL 50 ppm
4 ULTRA is an ECOLAB commercial CIP detergent for use in industrial food
processing -generally
used at 1 oz./gal. dilution-containing potash (active K20 7.4 %) hypochiorite
(ca. 100 ppm at
dilute strength) and phosphate for controlling water hardness up to 12 grains
per gallon.
, ,.-.
90 2197314
CLEANING OF SOILED SS PANELS
Comparison of high alkaline, commercial CIP
detersive solutions containing chlorine versus low
alkaline, detersive solutions containing proteolytic
enzyme.
1) The Stainless Steel 304 panels used in this cleaning
evaluation were prepared/soiled according to Ecolab RB
No. 9419-3,4 "Procedure for Protein Soiling and Cleaning
of Stainless Steel Panels" (See page 96, line 9 through
page 99, line 5).
2) The following cleaning solutions were prepared in 60
ppm City water:
4000 ppm PRINCIPAL with about 100 ppm chlorine.
PRINCIPAL is a commercial, conventional,
chlorinated, high alkaline CIP detergent
manufactured by Ecolab Inc.
4000 ppm ULTRA with about 100 ppm chlorine.
ULTRA is a commercial, conventional, chlorinated,
high alkaline CIP detergent which contains
phosphates and silicates manufactured by Ecolab
Inc.
(M) A low alkaline, non-chlorinated solution consisting
of 1000 ppm sodium tripolyphosphate, 500 ppm sodium
bicarbonate, and 500 ppm sodium carbonate.
3) 1000 ml of desired cleaning solution plus milk soil
when required, was placed in 1000 ml beaker. The
solution,was then heated to desired temperature and one
soiled panel was placed in bottom of beaker. The
solution was agitated for 15 min. while maintaining
temperature by means of a magnetic stir bar and
magnetic, heating, stir plate.
4) After cleaning, the panels were rinsed with DI water
and allowed to air dry.
5) Cleaning was measured by means of the HunterLab
UltraScan Spectrophotometer Model US-8000.
91 2197314
6) Settin.gs on the instrument were RSEX/UVL ON/UVF
OUT/LAV.
7) The percent change (cleaning) was calculated by the
formula WI (panel after cleaning) - WI (panel after
soiling)/WI (panel after soiling) X 100. WI = Whiteness
Index.
Table D containing protein film removal performance
of PRINCIPAL5 and ULTRA and the comparison with solution
M containing Esperase 8.OL is very conclusive evidence
for the detersive effect of enzyme on protein film.
This body of evidence strongly suggests an energy
barrier for effective chlorine removal of protein film.
5 An Ecolab commercial detergent for use in food process
industries generally used at 1 oz./gal. dilution. The product
contains caustic soda (active Na20 at 12.2%) hypochlorite (ca.
100 ppm at use dilution) and a polyacrylate hardness controller
for up to 20 grains hardness component per gallon.
92
TABLE E
Non-Chlorine Exposed Low-Chlorine Exposed
Panels Panels
TEST CLEANING CLEANING CLEANING WI (After WI (After PERCENT WI WI (After
PERCENT
SET SOLUTION TEMPERATURE TIME Soiling) Cleaning) CLEANING (After Cleaning)
CLEANING
Soiling)
I NaOH 50 C 30 min. --- --- --- 12.25 10.09 -17.63
2000 ppm
NaOH 50 C 30 min. --- --- --- 4.80 4.25 -11.46
2000 ppm
NaOH 65 C 30 min. --- --- --- 7.16 7.21 0.70
2000 ppm
NaOH 50 C 60 min. 16.04 19.18 19.58 --- --- ---
2000 ppm
NaOH 50 C 60 min. 16.62 18.97 14.14 --- --- ---
1000 ppm
NaOH 50 C 30 min. --- --- --- 8.86 18.50 108.80
2000 ppm
+ NaOC1
100 ppm
NaOH 65 C 30 min. --- --- --- 5.41 41.89 674.31
2000 ppm
+ NaOC1
100 ppm
II (M) 50 C 30 min. --- --- --- 5.71 15.19 166.02
(M) 50 C 60 min. 17.17 20.89 21.67 --- --- ---
(M) + 50 C 30 min. 12.83 39.85 210.60 --- --- ---
III
Esperase
8.OL
50 ppm
(M) + 50 C 30 min. --- --- --- 4.96 18.18 266.53
Esperase
8.OL
93
Non-Chlorine Exposed Low-Chlorine Exposed
Panels Panels
TEST CLEANING CLEANING CLEANING WI (After WI (After PERCENT WI WI (After
PERCENT
SET SOLUTION TEMPERATURE TIME Soiling) Cleaning) CLEANING (After Cleaning)
CLEANING
Soiling)
50 ppm
IV (N) 50 C 30 min. 18.50 28.65 54.65 --- --- ---
(N) 50 C 30 min. --- --- --- 5.34 17.60 229.59
V (0) 500C 30 min. 15.63 40.91 161.74 --- --- ---
(0) 50 C 30 min. --- --- --- 4.18 21.96 425.36
* The "Procedure for Protein Soiling and Cleaning of Stainless Steel Panels"
described in this invention normally
employs Principal without chlorine. For these test panels only, 25 ppm NaOCI
was added with Principal to develop
chloro-protein films on the panel surfaces.
N
--+
. r~
94 2197314
CLEANING OF SOILED SS PANELS
Comparison of high alkaline detersive solutions
with and without chlorine versus low alkaline detersive
solutions containing proteolytic enzyme on chloro-
protein films.
1) The Stainless Steel 304 panels used in this cleaning
evaluation were prepared/soiled according to Ecolab RB
No. 9419-3,4 "Procedure for Protein Soiling and Cleaning
of Stainless Steel Panels" (See page 96, line 9 through
page 99, line 5).
2) The following cleaning solutions were prepared in 60
ppm City water:
(M) A low alkaline, non-chlorinated solution consisting
of 1000 ppm sodium tripolyphosphate, 500 ppm sodium
bicarbonate, and 500 ppm sodium carbonate.
(N) Soln (M) + 200 ppm Triton CF-21.
Triton CF-21 is a commercial, octyl phenol
ethoxylate propoxylate manufactured by BASF Corp.
(0) Soln (M) + 200 ppm Triton CF-21 + 100 ppm Esperase
8.OL.
3) 1000 ml of desired cleaning solution plus milk soil
when required, was placed in 1000 ml beaker. The
solution was then heated to desired temperature and one
soiled panel was placed in bottom of beaker. The
solution was agitated for 15 min. while maintaining
temperature by means of a magnetic stir bar and
magnetic, heating, stir plate.
I. -
4) After cleaning, the panels were rinsed with DI water
and allowed to air dry.
5) Cleaning was measured by means of the HunterLab
UltraScan Spectrophotometer Model US-8000.
6) Settings on the instrument were RSEX/UVL ON/UVF
OUT/LAV.
A
95 2197314
7) The percent change (cleaning) was calculated by the
formula WI (panel after cleaning) - WI (panel after
soiling)/WI (panel after soiling) X 100. WI = Whiteness
Index.
Table E makes comparisons of "non-chlorine" exposed
panels to "low-chlorine" exposed panels and establishes
another point of differentiation between enzyme
containing compositions and the high alkaline, chlorine
containing detergents now prevalent in the food
processing industry. We have found, in general, that
chloro-protein films are more difficult to remove once
formed than protein films. Chloro-protein films are
caused by the use of chlorine in detergents at low
levels (or caused by high soil conditions which
deactivate the majority of chlorine in solution). Set I
confirms that high levels of caustic have no effect on
removal of chloro-protein unless high levels of chlorine
are also present. Although enzyme containing detergents
would not contain chlorine in the formulation, hence
would not form chloro-protein, evidence contained in
Sets III and IV strongly suggest that enzyme detersive
solutions do remove chloro-protein films if present on
surfaces. This result is important from a logistics
standpoint -- when customers convert from the high
alkaline, chlorinated detergents to the enzyme
compositions of this invention, chloro-protein films may
be the first protein films encountered on surfaces until
removed completely from the CIP system.
The above specification, examples and data provide
a complete description of the manufacture and use of the
composition of the invention. Since many embodiments of
the invention can be made without departing from the
spirit and scope of the invention, the invention resides
in the claims hereinafter appended.
~ .