Note: Descriptions are shown in the official language in which they were submitted.
~ WO96110213 2 1 9 7 ~ 6 q A ~ 1~
LASER ~nn~T~ RTP~ ~P~IC ELEMENT8
~ Field of the Invention
The present invention relates to novel
lO th~L - ~hic imaging elements and more particularly,
it relates to th~L JL~hic imaging elements that can
be directly imaged using an infrared laser diode. The
present invention further relates to processes for
imaging the inventive thermographic imaging elements
15 using an infrared laser diode.
Back~rouna of the Invention
In the imaging arts, imaging elements that can be
imagewise exposed by means of light or heat are well
20 known. Silver halide conventional photographic and
photothermographic elements are the most representative
elements of the class of light-sensitive materials. In
both conventional photographic ("wet silver") and
photothermographic ("dry silver") elements, exposure of
25 the silver halide in the photosensitive emulsion to
light ~Lvduces small clusters of silver atoms tAg~).
The imagewise distribution of these clusters is known
in the art as a latent image. Generally, the latent
image formed is not visible by ordinary means and the
30 photosensitive emulsion must be further processed in
order to produce a visible image. In both dry and wet
silver systems the visible image is produced by the
reduction of silver ions, which are in catalytic
proximity to silver halide grains bearing the clusters
35 of silver atoms, i.e. the latent image. This produces
a blacX-and-white image.
~: .
- 21 9746q
WO96110~13 l~1~ S .
Conventional photographic silver halide elements
require a wet devel-~ L process to render the latent
image visible. The wet chemistry used in this process
requires special hAn~l ing and disposal of the spent
5 chemistry. The process equipment is large and requires
special plumbing.
In photothermographic elements the photographic
silver halide is in catalytic proximity to a non-
photosensitive, reducible silver source (e.g., silver
lO behenate) so that when silver nuclei are generated by
light exyo-ure of the silver halide, those nuclei are
able to catalyze the reduction of the reducible silver
source. The latent image is rendered visible by
application of uniform heat across the element.
15 Thermal devices used for developing photothermographic
elements address the problems in conventional
photographic elements by using a dry process. However,
photothermographic elements developed using these
devices may have uneven or non-uniform image density,
20 image distortions, and/or surface abrasion defects.
Non-uniform image density defects may occur during the
development process due to, for instance, surface
variations on the heated member, the presence of
foreign matter on the photothermographic element or the
25 heated member, and insufficient allowance for
outgassing of volatile materials generated during
development. Image distortions can occur due to
uncontrolled dimensional changes in the base of the
phototh~ hic element during heating and/or
30 cooling of the photothermographic element. Surface
abrasions or marring may occur by dragging the
photothermographic element across a stationary
component in the heating devlce. In many applications,
such as text and line drawings, these defects may be
35 acceptable. However, users of medical diagnostic,
industrial, graphic arts, printed circuit boards, and
~ WO961~0213 2 ~ 9 7 4 6 9
other imaging applications desire uniform and high
quality images.
U.S. Patent No. 5,041,369 describes a process
which capitalizes on the advantages of a dry processed
5 photo~he~ -phic element without the need for surface
contact with a heating device. The photothermographic
element is imagewise exposed with a laser which splits
the beam using a second harmonic generation device. In
this process, the element is simultaneously exposed
lO with one wavelength of light and thermally activated by
the absorption of a light-to-heat near-infrared (NIR)
dye at the second wavelength of light. Even though
this process has the advantage of simultaneous ~X~Oau~ e
and heat development of the image, the equipment
15 required is complex and limited by laser outputs
capable of generating two useful separate wavelengths.
In addition, the photosensitive emulsion still requires
both light and heat activation to generate an image.
Photosensitive emulsions which contain silver
20 halide are well known in the art to be capable of
causing high minimum density (Dmin)~ both in the
visible and ultraviolet (W) portions of the spectrum.
The high W Dmin is due to the inherent absorption in
the near W of silver halides, particularly silver
25 bromide and silver iodide, and to high haze when silver
halide and organic silver salts are present together.
High W Dmin is undesirable for graphic arts scanner
and imagesetting films since it increases the exposure
time required during contact exposure with other media
30 such as W sensitive printing plates, proofing films,
and papers. High haze can also lead to loss of image
resolution when imaged photothermographic elements are
used as contact films. It is also well known that
silver halides in photothermographic elements can lead
35 to poor light stability of the background image density
leading to fogging.
--3--
2~ 97469
WO96110tl3 I~ .c
A class of imaging elements that do not rely on
silver halide-based chemistry is theL - aphic
elements. These materials are widely used in facsimile
r-rh;nPC, labels, tickets, charts for recording the
5 output of medical or scientific monitoring apparatus,
and the like. In the most common form, the
thP -,L~phic element comprises a support carrying a
coating of a thermally-sensitive composition comprising
a color former, usually a substantially colorless
lO electron-donating dye precursor, and a color developer,
usually an electron-accepting compound. Heat is
imagewise applied to the element by means of a thermal
head, a thermal pen or a laser beam, and upon imagewise
applied heating, the color former instantaneously
15 reacts with the color developer to form an image. U.S.
Patent No. 4,904,572 describes a thermographic element
which uses leuco dyes to enhance the developed image.
A leuco dye is the reduced form of a color-bearing dye.
It is generally colorless or very lightly colored. In
20 this application, silver behenate acts as a Lewis acid
which reacts with the leuco dye upon imagewise
application of heat to form a colored image. A black
image is achieved by the combination of subtractive
colors (cyan, yellow, and magenta~. It is well known
25 in the art that it is very difficult to achieve a high
density neutral tone black using subtractive colors.
Since the image is generated by colored dye formation,
the absorption of the image in the ultraviolet is weak
and therefore, provides little utility as a W masking
30 film.
Conventional thermographic films typically require
imaging dwell times of from l to 5 seconds . Processing
times of this extent are not practical in a laser
imaging application. In order to provide an
35 appropriate imaging dwell time for a laser addressable
system, there is a need for a thermographic film
~ WO96110213 2 1 9 7 4 6 9 r~ r7
UUna~L ~u~ion which is capable of forming an in-situ
image in micros~con~c.
Each of the abu~ ioned classes of imaging
elements has some disadvantage. For example,
~ 5 conventional silver halide photographic materials have
a high environmental impact due to the wet procpccing
~ chemistry; photothermographic materials have lower
image fastness, limited optical density, and poor
dimensional stability; silver halide-based emulsions
lO typically use visible sensitizers which need to be
bleached or removed and need to be handled in the dark
or subdued light; both conventional photographic and
phototh~L ,L aphic elements require a two-step process
('_X~Oa~L~ and dev~1 ~ t); and conventional
15 thermographic elements require high imaging energy,
relatively long thermal imaging dwell times, and have
lower image fastness and limited W optical density.
What is needed in the industry are imaging
elements and processes which help to overcome the
20 above-disclosed problems. It was against this
background that the present inventions were developed.
BummarY of the Invention
In one ~ho~i r -nt, the present invention provides
25 a thermographic imaging element comprising a substrate
having coated on at least one surface thereof a
th~L ~phic imaging system comprising at least one
layer comprising light-insensitive organic silver salt;
reducing agent for silver ion; a binder; toner; and a
30 dye which absorbs radiation in the wavelength range of
about 750-llO0 nm, wherein the at least one layer
comprising the light-insensitive organic silver salt
forms an image density of greater than about l.0 when
exposed to 0.10-2.0 joules/cm2 of radiation (having a
35 wavelength in the range of about 750-llO0 nm)in 0.2-200
microseconds.
--5--
2 1 9 7 4 6 ~
WO96110213 -
In another Pmho~ir--t~ the present invention
provides a th~ , _phiC imaging element comprising a
substrate coated with a thermographic imaging system,
the th, ~ aphic imaging system comprising at least
5 two adjacent layers, one of the adjacent layers
comprising light-insensitive organic silver salt;
reducing agent for silver ion; a binder; toner; and
optionally, a dye which absorbs radiation having a
wavelength in the range of about 750-llO0 nm and the
lO other adjacent layer consisting essentially of dye
which absorbs radiation in the wavelength in the range
of about 750-llO0 nm and binder such that the layer
comprising the light-insensitive organic silver salt
forms an image density of greater than about l.0 when
lS exposed to O.lO -2.0 joules/cm2 of radiation (having a
wavelength in the range of about 750-llO0 nm)in 0.2 -
200 microseconds.
In a further Pmho~ L, the present invention
provides a prccess for forming an image comprising the
20 step of exposing a thermographic imaging element
comprising a substrate coated ~ith a thermographic
imaging system, comprising at least one layer
comprising light-insensitive organic silver salt;
reducing agent for silver ion; a binder; a dye which
25 absorbs radiation in the wavelength range of about 750-
llO0 nm; and toner, to radiation in the range of about
750-llO0 nm such that the at least one layer comprising
the light-insensitive organic silver salt forms an
image density of greater than about l.0 when exposed to
30 O.lO - 2.0 joules/cm2 of radiation in (having a
wavelength in the range of about 750-llO0 nm) in 0.2 -
200 microsecon~q.
In still another embodiment, the present invention
provides a process for forming an image comprising the
35 step of exposing a thermographic imaginq element
comprising a substrate coated with a thermographic
~ WO96110213 2 1 974 69 J~
imaging system, the ~h~ ,L~phic imaging system
comprising at least two~adjacent layers, one of the
adjacent layers comprising light-insensitive organic
silver salt; reducing agent for silver ioni a binder;
5 toner; and optionally, a dye which absorbs radiation
having a wavelength in the range of about 750-1100 nm
and the other adjacent layer consisting essentially of
binder and dye which absorbs radiation having a
wavelength in the range of about 750-1100 nm, to
10 radiation having a wavelength range of about 750-1100
nm which is directed to the thermographic imaging
element through the layer comprising the light-
insensitive organic silver salt before striking the
adjacent layer consisting essentially of binder and dye
15 such that the layer comprising the light-insensitive
organic silver salt forms an image density of greater
than about 1.0 when exposed to 0.10 - 2.0 joules/cm2 of
radiation ~having a wavelength in the range of about
750-1100 nm) in 0.2 - 200 microseconds.
In a preferred Dmho~; - ~ nt to the above inventions,
an image density of greater than about 2.00 and, more
preferably, greater than about 2.50, and most
preferably, greater than about 2.75 comprising metallic
silver is formed in the layer comprising light-
25 insensitive organic silver salt, reducing agent, etc.,
upon exposure to 0.10 - 2.0 joules/cm2 of radiation
(having a wavelength in the range of about 750-1100
nm)in 0.2 - 200 microsecon~c.
The layers ("theL ,L~phic silver emulsion
30 layers") comprising light-insensitive organic silver
salt, reducing agent for silver ion, etc., in all of
the above-disclosed Pmho~ir~nts of the present
invention can incorporate up to about 1.0 wt% silver
halide, based upon the total weight of the layer.
The silver-based thermographic imaging elements
and methods for using the thermographic imaging
WO96/10213 21 9746q l~lIL~__.'O~
elements as laser addressable direct-write fllm
provided by the present invention ~v~ many of the
problems seen in current systems. Since the
thl , a~hic imaging element is thorr~lly-sensitive~
5 rather than photosensitive, it is white light-
handleable and does not require removal of a visible
sensitizer. Unlike wet silver and photothermographic
elements, no post-processing steps are required for
development of the image. When a high power laser
lO diode is scanned across the thermographic imaging
element, an in-situ black image is printed out in the
th -_ ~phic silver emulsion, thus enabling many
useful applications such as an on-line inspection
system for printed circuit board phototool mask
15 production. In addition, heat shrinkage of the film is
minimized since only the imaged portion of the emulsion
is heated and the temperature of the substrate is
relatively unchanged. This is ocperi~lly important for
applications where registration is critical, such as
20 image-setting films for color reproduction and printed
circuit board phototools. In addition, the
~h~ ~ ~phic imaging element is capable of producing
high resolution halftone images which are useful in
color reproduction processes.
Other aspects, advantages, and benefits of the
present invention are apparent from the detailed
description, the examples, the drawings, and the
claims.
Brief DescriDtion of the D~awin~s
FIG. l shows a schematic representation of a laser
sensitometer.
FIG. 2 shows a graph of distance versus the
relative intensity of a laser diode comparing the plots
35 for theoretical versus actual profile data for a flat-
topped cone shaped laser spot on the film plane.
--8--
~ WO 96110~13 2 1 9 7 4 6 q
FIG. 3a shows a graph of the total incident
e~o-uLe energy plotted against the distance across the
laser beam in the cross/scan direction.
FIG. 3b shows a microdensitometer profile of a
S line imaged with the energy profile depicted in FIG.
3a onto a thermographic element. (Example 16, Sample N
not shown.)
FIG. 4 shows a graph of density versus the log of
the e~o~uLe using the data shown in FIG. 3a.
FIG. 5 shows a graph of absorption versus
wavelength comparing the imaged and non-imaged areas of
a fh~ ~,LaphiC imaging element. (Example 16, Sample N
not shown.)
Det~iled DescriPtion of the Invention
As used herein, "thermographic imaging element"
refers to a substrate coated on at least one surface
thereof with a ~l~hP r,Laphic imaging system". The
thermographic imaging system comprises at least one
theL-~,Laphic silver emulsion layer containing light-
20 insensitive organic silver salt; reducing agent for
silver ion; binder; toner; and a dye which absorbs
radiation having a wavelength in the range of about 750
- 1100 nm. Additionally, the thermographic imaging
system may comprise a layer adjacent to the
25 thermographic silver emulsion layer which contains
further radiation-absorbing dye and binder.
In the present invention, the thermographic silver
emulsions which are utilized comprise a light-
insensitive silver salt; a reducing agent for silver
30 ion; a dye which absorbs radiation having a wavelength
in the range of about 750-1100 nm; a toner, binder; and
an optional development accelerator.
The light-insensitive silver salts are materials,
which in the presence of a reducing agent, undergo
35 reduction at elevated temperatures, e.g., 60~-225~C, to
form silver metal. Preferably, these materials are
_g_
wo g6~l0~l3 2 ~1 9 7 4 6 9 ~ 5 ~
silver salts of long chain alkanoic acids (also knoun
as long chain aliphatic carboxylic acids or fatty
acids) containing 4 to 30 carbon atoms; more
preferably, 8 to 28 carbon atoms; and most preferably,
5 10 to 22 carbon atoms. The latter are also known in
the art as "silver soaps".
Non-limiting examples of silver salts of aliphatic
carboxylic acids include silver behenate, silver
stearate, silver oleate, silver erucate, silver
lO laurate, silver caproate, silver myristate, silver
pa~mitate, silver maleate, silver ~umarate, silver
tartarate, silver linoleate, silver camphorate, and
mixtures thereof. Complexes of organic or inorganic
sllver salts wherein the ligand has a gross stability
15 constant between 4.0 to 10.0 can also be used. Silver
salts of aromatic carboxylic acids and other carboxyl
L~LUU~ ~OIlLailling ~ C include silver benzoate, a
substituted silver benzoate such as silver
3,5-dihydroxybenzoate, silver o-methylbenzoate, silver
20 m-methylbenzoate, sllver p-methylbenzoate, silver
2,4-dichlorobenzoate, silver acetamidobenzoate, silver
p-phenyl benzoate, etc., silver gallate, silver
tannate, silver phthalate, silver terephthalate, silver
salicylate, silver phenylacetate, silver pyromellitate,
- 25 silver salts of 3-carboxymethyl-4-methyl-4-
thiazoline-2-thiones or the like as described in U.S.
Pat. No. 3,785,830; and silver salts of aliphatic
carboxylic acids containing a thioether qroup as
disclosed in U.S. Pat. No. 3,330,663. Silver salts of
30 _ olln~ containing mercapto or thione groups and
derivatives thereof can also be used. Preferred
examples of these compounds include silver
3-mercapto-4-phenyl-1,2,4-triazolate, silver
2-mercaptobenzimidazolate, silver 2-mercapto-5-
35 amino~h~ olate, silver 2-(S-
ethylglycolamido)benzothiazolate; silver salts of
--10--
~ Wos6/10213 2~ 97469 r~.,. ~ . .
t_ioglycolic acids such as silver salts of S-alkyl
thioglycolic acids (wherein the alkyl group has from 12
to 22 carbon atoms~; silver salts of dithiocarboxylic
acids such as silver dithioacetate, silver
5 thio~mi~ate, silver 1-methyl-2-phenyl-4-thiopyridine-
5-carboxylate, silver triazinethiolate, silver 2-
sulfidoh~n7~Yazole; and silver salts as disclosed in
U.S. Pat. No. 4,123,274. Furthermore, silver salts of
a compound containing an amino group can be used.
10 Preferred examples of these compounds include silver
salts of benzotriazoles, such as silver
benzotriazolate; silver salts of alkyl-substituted
benzotriazoles such as silver methylbenzotriazolate,
etc.; silver salts of halogen-substituted
15 benzotriazoles such as silver 5-chlorobenzotriazolate,
etc.; silver salts of carboimi~h~n7Otriazoles, etc.;
silver salts of 1,2,4-triazoles and l-H-tetrazoles as
described in U.S. Pat. No. 4,220,709; silver salts of
imidazoles; and the like. Preferably, the light-
20 insensitive silver salt material should constitute from
about 5 to 60% by weight and more preferably, from
about 30 to 50% by weight, based upon the total weight
of the thermographic silver emulsion layer.
Any reducing agent for silver ion can be used in
25 the present invention. Such reducing agents are well-
known to those skilled in the art. Examples of such
reducing agents include, but are not limited to, methyl
gallate; hindered phenols; catechol; pyrogallol;
hydroquinones; substituted-hydroquinones; ascorbic
30 acid; ascorbic acid derivatives; leuco dyes; and the
like. The most preferred reducing agents are methyl
gallate, butyl gallate, and propyl gallate. Whatever
reducing agent is employed in the present invention is
preferably used in an amount of about 5.0 to 25.0% by
r 35 weight and more preferably, from about 10.0 to 20.0% by
--11--
WO96/10213 2 1 9 7 4 6 9 r ~ 5 ~
weight, based upon the total weight of the
th~ phic silver emulsion layer.
Toners are also used in the thermographic silver
~ lcion layer(s~. Examples of toners include
5 phthalazinone, phthalazine, barbituric acid,
sllccin;m;~e~ and phth~lim;de. Combination of toners
have been found to be especially useful, the preferred
combinations being phthalazinone with barbituric acid
and phthalimide with barbituric acid and most preferred
10 being succinimide with barbituric acid. The toner(s)
should preferably be present in an amount in the range
of about 0.2 to 10.0~ by weight; more preferably, about
1.0 to 8.0% by weight; and most preferably, about 2.0
to 6.0% by weight, based upon the total weight of the
15 thermographic silver emulsion layer.
AnY11 i ~ry reducing agents or development
accelerators may be optionally included in the
thermographic silver emulsion layer ~p~n~ing upon the
silver source used. Preferably, the auxiliary reducing
20 agent comprises a 3-indazolinone or urea compound as a
development accelerator.
3-indazolinone - ~n~c used in the present
invention preferably have the following structure:
0
~""
wherein R is selected from the group consisting of:
hydrogen; an alkyl group of 1 to 4 carbon atoms;
halogen; -COOH and -RlCOOH wherein Rl is an alkyl group
having from 1 to 4 carbon atoms. Preferably, R is
35 hydrogen or an alkyl group having from 1 to 4 carbon
atoms and most preferably, R is hydrogen.
~ WO9~10213 2 1 9 7 4 69 ~I/L~
Such 3-indazolinone _ '- can be synthP~; 70~
according to procedures well known to those skilled in
the art of synthetic organic chemistry. Alternatively,
such materials are cially available, such as from
5 Aldrich Chemical Company of Milwaukee, Wisconsin;
Lancaster ~hP~irAl Company of Windham, New ~ -~ire;
~ and K&K Laboratories of Cleveland, Ohio.
As is well understood in this area, a large degree
of substitution is not only tolerated, but is often
10 advLsable. Thus, as used herein the phrase "group" is
intended to include not only pure hydrocarbon
substituents such as methyl, ethyl, and the like, but
also such hydrocarbon subctituents bearing conventional
substituents in the art such as hydroxy, alkoxy,
15 phenyl, halo (F, Cl, Br, I), cyano, nitro, amino, etc.
Urea compounds used in the present invention
preferably have the following formula:
o
R2-NH-C-NH-R3
wherein R2 and R3 each independently represent
hydrogen; a Cl-Cl0 alkyl or cycloalkyl group; or
phenyl; or R2 and R3 together form a heterocyclic group
25 containing up to 6 ring atoms. Preferably, R2 and R3
represent ll~dLUY~I; a C1 to C~ alkyl or cycloalkyl
group; or phenyl; or R2 and R3 together form a
heterocyclic group containing up to 5 ring atoms. Such
urea _ -ullds can be readily synthesized and are
30 commercially available. Non-limiting examples of such
urea compounds include urea; 1,3-diphenyl urea; 1,3-
diethyl urea; butyl urea; and 2-imidazolidone. The
most preferred development accelerator is 2-
imidazolidone.
The thermographic imaging elements of the present
invention are not light-sensitive in the traditional
sense and therefore, do not need to contain
photosensitive agents such as silver halides;
-13-
W096tlO213 2 1 9 7 4 6 9 1~I/L~
photoinitiator; or photogenerated bleaching agents.
The ~h- -, ~phic silver emulsion layers can have less
than 1%, less than 0.75%, less than 0.5%, or 0% by
weight, based upon the total weight of the
5 thermographic silver emulsion, and perform well. The
silver halide is deemed to be ineffective if it does
not catalyze formation of a latent image.
Light stabilizers such as benzotriazole,
phenylmercaptotetrazole, and other light stabilizers
10 known in the art may be added to the thermographic
silver emulsion. The preferred light stabilizer is
benzotriazole. The light stabilizer should preferably
be present in an amount in the range of about 0.1% to
3.0% by weight of the thermographic silver emulsion
15 layer and more preferably, from 0.3 to 2.0 wt%.
The thermographic silver emulsion layer utilized
ln the present invention also employs a binder. Any
conventional polymeric binder known to those skilled in
the art can be utilized. For example, the binder may
20 be selected from any of the well-known natural and
synthetic resins such as gelatin, polyvinyl acetals,
polyvinyl chloride, cellulose acetate, polyolefins,
polyesters, polystyrene, polyacrylonitrile,
polycarbonates, and the like. Copolymers and
25 terpolymers are, of course, included in these
definitions, examples of which, include, but are not
limited to, the polyvinyl aldehydes, such as polyvinyl
acetals, polyvinyl butyrals, polyvinyl formals, and
vinyl copolymers. Preferably, the binder should be
30 present in an amount in the range of 10 to 50~ by
weight and more preferably, 15 to 50% by weight, based
upon the total weight of the thermographic silver
emulsion layer.
The thermographic element of the present invention
35 employs a dye which absorbs electromagnetic radiation
having a wavelength in the range of between about 750-
-14-
~ WO96~10213 2 1 9 7 4 6 9 , ~
llO0 nm, preferably in the range of about 750-900 nm,
and most preferably, in the range of about 750-870 nm.
The dye should be soluble in the coating solvent,
preferably ketones or aromatic solvents, such as methyl
- 5 ethyl ketone or toluene. The dye should also be
miscible with the binder and compatible with the silver
salts, activators, and developers used in the emulsion.
For use in W (ultraviolet) contact film or mask
applications the optical density of the dye is
lO preferably greater than l.0 optical density units with
a concomitant weak absorption of less than 0.2 optical
density units in the W region corresponding to the
wavelength of ~OSUL e devices for which the material
will be used as a mask (250-450 nm). The optical
15 density is measured using a ~acBeth Model TDS23
densitometer eguipped with a status 18A filter. It is
also desirable, but not necessary, for the dye to have
a low visible background absorption.
The radiation-absorbing dye can be employed in the
20 same layer as the light-insensitive organic silver
salt; reducing agent for silver ion; toner; and binder.
Alternatively, the dye can be employed in the foregoing
layer as well as in an adjacent layer or primarily in
the adjacent layer. The radiation-absorbing dye may be
25 added directly to the thermographic silver emulsion
layer or indirectly by allowing the dye to migrate from
the adjacent layer, containing the dye, into the
theL -, ~p1.ic silver emulsion layer during the
manufacturing process of the thermographic imaging
30 element.
Suitable dyes include, but are not limited to,
oxonol, squarylium, chalcogenopyrylarylidene,
bis(chalcogenopyrylo)polymethine,
bis(aminoaryl)polymethine, merocyanine, trinuclear
35 cyanine, indene-bridged polymethine, oxyindolizine,
ferrous complex, quinoid, nickel dithiolene complex,
.
WO96110213 2 1 9 7 4 6 9
and cyanine dyes such as carbocyanine, aza~a~bo~anine,
hemicyanine, styryl, diazacarbocyanine,
triazacarbocyanine, diazahemicyanine,
polymethinecyanine, azapolymethinecyanine, holopolar,
5 indocyanine, and ~i~ 7 ~ h Pm i cyanine dyes.
The amount of dye present in the th~L ,L aphic
imaging element will be ~PpP~dP~t upon whether the dye
is incuL~o,ated solely into the thermographic silver
emulsion layer or in an adjacent layer as well. When
lO the dye is present solely in the thermographic silver
emulsion layer, the dye will be present in an amount of
from 0.10-5.0 wt~ and preferably from 0.2 - 3.0 wt%,
based upon the total weight of the thermographic silver
emulsion layer.
When present in an adjacent layer, the dye will be
present in the thermographic silver emulsion layer in
an amount of from 0 - 5.0 wt% and preferably, from
about 0 - l.0 wt~, based on the total weight of the
thermographic silver emulsion layer. In the adjacent
20 layer containing dye and binder, the dye will be
present in an amount of from 1-25 wt~ and preferably, 5
- 20 wt%, based upon the total weight of the adjacent
layer.
Any suitable base or substrate material known to
25 those skilled in the art can be used in the present
invention. Such materials can be opaque, translucent,
or transparent. Commonly employed base or substrate
materials utilized in the thermographic arts include,
but are not limited to, paper; opaque or transparent
30 polyester and polycarbonate films; and sppc~ rly light
reflective metallic substrates such as silver, gold,
and aluminum. As used herein, the phrase 'lsppr~llarly
light reflecting metallic substrates" refers to
metallic substrates, which when struck with light,
35 reflect the light at a particular angle as opposed to
reflecting the light across a range of angles.
-16-
~ w09~02~3 - 21 9 74 69 r~
Optionally, a protective or anti-stick layer,
positioned on top of the theLIr, ~phic imaging element,
may be used. Any conventional anti-stick material may
be employed in the present invention. Examples of such
5 anti-stick materials, include, but are not limited to
waxes, silica particles, styrene-containing elastomeric
block copolymers such as styrene-butadiene ~LyLane,
styrene-isoprenc ~LyL~ne, and blends thereof with such
materials as cellulose acetate, cellulose acetate
10 butyrate, cellulose acetate propionate, and poly(vinyl
butyral).
Additional layers may be in~GL~oL~ted into the
thermographic elements of the present invention such as
a primer layer or anti-static layer. Furthermore, an
15 anti-static or anti-stick layer may optionally be
applied to the back of the support. Materials for such
purposes are well known to those skilled in the art.
The th~. .,L~phic imaging system, anti-stick,
infrared or near-infrared dye absorbing, and anti-
20 static layers employed in the present invention can beapplied by any conventional method such as knife
coating, roll coating, dip coating, curtain coating,
hopper coating, etc. If desired, two or more layers
may be coated simultaneously by the procedures
25 described in U.S. Patent No. 2,761,791 and British
Patent No. 837,095.
The th~ phic imaging elements of the present
invention are imaged by ~o~u~e to infrared or near-
infrared laser radiation, typically from a infrared or
30 near-infrared laser diode. As is well known in the
thermal imaging arts, infrared or near-infrared laser
diodes may be advantageously arranged in an array to
increase imaging speed. Lasers that can be used to
provide infrared or near-infrared radiation include
~ 35 substantially any laser capable of generating light in
the infrared and near-infrared region of the
- -17-
W096/10213 2 1 9 7 4 6 9
ele~LL~ gnetic ~e~L~- from about 750 to 1100 nm,
including dye lasers; solid state diode lasers such as
~ m1mlm gallium arsenide diode lasers that emit in the
region of 780 to 870 nm; and diodc p, -~ solid state
5 lasers such as Nd:YAG, Nd:YLF, or Nd:glass.
The following non-limiting examples further
illustrate the present invention.
UPr.l:!R
Materials used in the following examples are
available from standard commercial sources such as
Aldrich Chemical Co. (~ilwaukee, WI) unless otherwise
specified.
Silver behenate and silver laurate homogenates
15 may be prepared as ~;CClns~d in U.S. Pat. No. 4,210,717
(column 2, lines 55-57) or U.S. Pat. No. 3,457,075
(column 4, lines 23-45 and column 6, lines 37-44).
The following dyes were used in various examples
which follow:
SO~NH2
H2NO2S~< Cl
N ~ N
Dyel
jO3 ~03 ~
Pr~ration of Dve 1: 5-Sulfamoyl-2,3,3-
trimethylindolenine was prepared according to the
-18-
~ WO96/102U 2 1 9 7 4 6 9
method described in U.S. Patent No. 4,062,682. A
mixture of 37.0g of 5-sulfamoyl-2,3,3-
trimethylindolenine, 16.7mL of 2-chloroeth~n~ fonyl
chloride, and 200mL of acetonitrile were refluxed for 6
- 5 hours. After the addition of 18.5mL of water, the
mixture was stirred overnight. The separated solid was
~ filtered, washed with acetonitrile, and dried to give
ll.Og of a 1-sulfoalkylated quaternary salt
int~ te.
A mixture of 6.5g of chlorocyclopentene
dialdehyde, 26g of the quaternary salt int~ ~iate
prepared above, 108mL of acetic anhydride, and 72mL of
acetic acid was stirred for 10 minutes at room
temperature. After addition of 12.8mL of
15 diisopropylethylamine, the mixture was stirred
overnight. The separated solid was filtered, washed
with the solvent mixture, and dried to give 20.0g of
dye 1.
SO2NH2
H2NO2S~X Cl ~
~ ~ N r nye2
Pre~aration of Dve 2: In a 3L flask, 385g of trimethyl
sulphonamido indolenine was added with 250mL of
butyronitrile. To this mixture was added, with no
25 exotherm, 225mL (364g~ of butyliodide followed ~y 750mL
of additional butyronitrile. The mixture was heated to
reflux with efficient overhead stirring for 22.5 hours.
The heat was removed and the mixture allowed to cool to
--19--
2 1 974 6~
WO96/10213 r~". ~o
about 40~C. Stirring was continued for 1 hour, after
the addition of lL of ethylacetate. The solid was
filtered, washed with ethylacetate, and dried to give
595.6g of N-butyl-2,3,3-trimethyl-5-sulphonamido
5 indoleninium iodide.
To a solution of 370mL of methylene chloride and
558mL of dimethylformamide, cooled to below 5~C, was
added dropwise over 1 hour 277mL of rhncrhnrus
oxychloride at a rate such that the temperature did not
10 exceed 5~C. After the completion of the addition, the
external cooling was removed and the mixture was
stirred for 1 hour. Over a 30 minute period, 75mL of
cyclopentanone was added in two portions. After the
first addition, a slow increase in temperature and
15 color was observed to about 35~C, at which time the
second portion was added resulting in a large exotherm.
After the exotherm subsided, the mixture was heated to
reflux for 4 hours. The mixture was distilled under a
slight vacuum after the addition of lL of ethylacetate.
20 Approximately 250mL of liquid was collected to which
700mL of ethylacetate was added when a precipitate
started to form. The mixture was stirred overnight. The
solid was filtered, washed with lL of ethylacetate,
followed by heptane, and dried under vacuum at 35~C for
25 4 hours giving rise to 115.8g of crude
chlorocyclopentene dialdehyde.
The crude chlorocyclopentene dialdehyde was
dissolved in 1250 mL of water. crystals started to
appear after about 1 hour. The mixture was allowed to
30 stand over the weekend. The brownish solid was
filtered, washed with water, and dried under vacuum at
35~C for 7 hours giving rise to 61.0g of
chlorocyclopentene dialdehyde.
To a solution of 450mL of acetic acid and 450mL of
35 acetic anhydride was added 278.7g of N-butyl-2,3,3-
trimethyl-5-slllrhon~mi~ indoleninium iodide and 47.6g
-20-
~ WO96~10213 2197469 r .,. . ~
of cyclopentene dialdehyde. To the stirred mixture was
added dropwise 90mL of triethylamine over 5 minutes at
60-65~C. No large exotherm was observed. The mixture
was heated for an additional 30 minutes, after which
- 5 the heat was removed and the reaction mixture cooled to
15~C. The resultant golden-brown solid was filtered and
washed with a 1:1 mixture of acetic acid:acetic
~nhydride until the washings were greenish rather than
purple. Residual acetic acid and acetic anhydride were
10 removed by suspending the solid in lL of ethyl acetate,
followed by stirring for so minutes. The solid was
filtered and washed with ethyl acetate. The filtrate
had a pink hue. The solid was dried at 45~C under
vacuum overnight giving rise to 250.0g of Dye 2.
SO~
~o2S ~ a ~
~N/~N ~ Dye 3
Pre~aration of Dve 3: A mixture of 0.1 moles of Dye 2
20 prepared above, 0.1 moles of sodium tetraphenylborate,
and 500mL of methanol was refluxed with stirring for 10
minutes. The solid was filtered, washed with methanol,
followed by water, and then dried to give 0.97 moles of
Dye 3.
- 25
-21-
WO96/10213 . ?l9 74 69 rcl,u~c~
o- Dye 4
H ~ ~ " H
Hgc>(~ ><CHg
Pr~n~ration of Dve 4: A stirred mixture of 26.05g of
1,8-di~min~n~rhthalene, 32.66g of 2-tride~nnn~, 55mg
of p-toluenesulfonic acid monohydrate, and 250mL of
toluene was heated to reflux under a nitrogen
15 at~ ph~re using a Dean-Stark trap to remove the water
evolved from the reaction for 5 hours. The mixture was
then washed with saturated sodium bicarbonate solution,
dried over anhydrous potassium carbonate, filtered, and
the solvent removed under reduced pressure. The
20 product was distilled to yield 48.86g of
dihydroperimidine intermediate; b.p. 192-213~C at 0.3
to 0.4 torr.
A stirred mixture of 8.0g of the dihydroperimidine
intermediate prepared above, 1.48g of squaric acid,
25 64mL of n-butanol, and 64mL of toluene was heated to
reflux under a nitrogen atmosphere using a Dean-Stark
trap to remove the water evolved from the reaction for
3 hours. The mixture was filtered, poured into 600mL
of petroleum ether (b.p. 35-60~C), and kept at 5~C for
30 18 hours. The product was f iltered of f, washed with
petroleum ether, and air dried to give 6.45g of Dye 4.
-22-
' 2 1 97469 ~ 5~-~
WO96110213
OCH~
CHJo~mX a ~o; Nl~
- 5 ~ ~ ~ ~ ~ 9~N ~
N~O9S '~' ~ ~ ~ SO;N~ Dye 5
SOJ
15 PreParation of DYe 5: With stirring, 28g of 2,3,3-
trimethyl-5-methoxyindolenine was added to 80mL of
fuming sulfuric acid ~10% of 5O3). The mixture was
stirred overnight at room temperature and then poured
onto 500g of crushed ice. The aqueous solution was
20 neutralized with a 30% sodium hydroxide solution and
evaporated to dryness under vacuum. The residue was
extracted with methanol and the solution evaporated.
The solid was picked up in ethanol, filtered, washed
with ethanol and dried under vacuum to give 20g of an
25 indolenine sulfate salt intermediate as yellowish
prisms.
A mixture of 20g of the indolenine sulfate salt
int~ te prepared above, 20g of 2,4-butanesulfone,
and 80mL of benzonitrile was refluxed with stirring for
30 5 hours. The solid formed was filtered, washed with
ethylacetate, and dried under vacuum to give 25g of a
sulfonalkylated quaternary salt intermediate as light-
brown prisms.
A mixture of 14.2g of the sulfonalkylated
35 quaternary salt intermediate prepared above, 7.5g of N-
(2-chloro-3-(dimethylamino)methylene)-1-cyclohexen-1-
-23-
~096110213 2 ~ 9 7 4 6 9 ~ 7 ~
yl~_ethylene)-N-methylme~h~n~min1um chloride (prepared
as described in EPO Application No. 0288261~, 5.4mL of
dicyclohexylethylamine, and 75mL of benzonitrile was
stirred at room temperature overnight. The mixture was
5 filtered and the ~iltrate added to ethylacetate. The
solid was filtered and dried. The solid was dissolved
in 50mL of ethanol and 3g of sodium iodide added. The
precipitate was filtered, washed with acetonitrile, and
dried to give 3g of Dye 5 as green prisms.
~ ~3 ~3
~ 3 H3C ~
-CH ~ I ~ Dye 6
( ~ 4so3Na ( ~ ~SO3
Dve 6 is commercially available from Eastman Kodak Co.,
Rochester, NY.
r les 1-3
The following coating solutions were used in the
preparation of Examples 1-3:
Silver E~llcion:
Silver behenate homogenate 160g
(10% by weight in methyl ethyl ketone)
Butvar B76 poly(vinyl butyral~, available from 20g
~onsanto Co.
~hermogramhic Coatin~ Solutions:
~ WO96110213 2 1 9 74 69 .~-I/U~ 5,~
The ~he , GphiC coating solutions for Examples
1-3 were y~.pa~ed by mixing the following ingredients
with 20g of the silver emulsion described above:
5 ~aterial Example 1 Ex~mple 2 Example 3
Methyl Gallate 0.6g 0.6g 0.6g
Pyrogallol 0.2g 0.2g 0.2g
Phthalazinone 0.2g 0.2g 0.2g
Succinimide O.lg O.lg O.lg
10 2-imidazolidone O.lg O.lg O.lg
Barbituric acid 0.05g 0.05g
Benzotriazole 0.02g
Each of the solutions were coated onto a 0.08mm t3
15 mils) polyester substrate at a O.lmm (4 mils) wet
thickness and air dried at 60~C for 3 minutes.
An infrared-absorbing topcoat solution was
prepared by mixing 0.08g of Dye 1, l.Og of CA 398-6
cellulose acetate resin, and 20.0g MEK. The topcoat
20 solution was coated onto the thermographic layer at a
0.05mm (2 mils) wet thi~kn~c and air dried for 3
minutes at 60~C.
E~ples 4-6
The following coating solutions were used in the
preparation of Example~ 4-6:
Silver E~yl~iQn:
Silver laurate full soap hr , n~te 160g
(10% by weight in methyl ethyl ketone)
BX-l poly~vinyl butyral), available from lOg
Sekisui ~h~m; r~ 1 CO.
35 Thermo~ra~hic Coating Solutions:
2 1 9 7 4 6 q
WO96110213
The th~L _ ~phic coating solutions for r l~c
4-6 were prepared by mixing the following ingredients
with 20g of the silver emulsion described above:
N~terial Ex~mplo ~ Examplo 5 Example 6
Methyl Gallate 0.6g 0.6g 0.6g
Pyrogallol 0.2g 0.2g 0.2g
Phthalazinone 0.2g 0.2g 0.2g
10 Succinimide O.lg O.lg O.lg
2-imidazolidone O.lg O.lg O.lg
Barbituric acid 0.05g 0.05g
Benzotriazole 0.02g
Each of the solutions were coated onto a 0.08 D (3
mils) polyester substrate at a O.lmm (4 mils~ wet
thickness and air dried at 60~C for 3 minutes.
An infrared-absorbing topcoat solution was
prepared by mixing 0.08g of Dye 1, l.Og of CA 398-6
20 cellulose acetate resin, and 20.0g MEK. The topcoat
solution was coated onto the thermographic layer at a
0.05mm (2 mils) wet thickness and air dried for 3
minutes at 60~C.
Table 1 summarizes the results of exposing the
25 materials of Examples 1-6 with a 810 nanometer laser
diode (available from Spectra Diode Labs of San Jose,
CA) at 1.75 J/cm2 focused on the film plane at (a 7
micron spot size). The visible optical densities were
measured using a Perkin Elmer microdensitometer PDS
30 lOlOM and the W optical densities were measured using
a MacBeth TD523 densitometer equipped with a status 18A
filter. The W light stability was de~ermined by
-26-
~ WO961~0~13 2 i 9 7469 .~ s~i- .
allowing the sample to stand in a fluu~3cent (1,000
foot candles, so~Fj light box for 24 hours.
~able 1
Example l 2 3 ~ 5 6
Visible Dmax3.06 3.23 3.25 3.87 3.84 3.83
Visible Dmin0.05 0.05 0.05 0.07 0.07 0.07
UV Dmin 0.15 0.14 0.13 0.19 0.15 0.16
~V Dmin 0.55 0.61 0.23 0.53 0.59 0.25
24hr stability
Examples 3 and 6 clearly show a significant
~ uv~ -~t in UV Dmin light stability when
benzotriazole is added to the thermographic silver
emulsion.
Exam~le 7
The f ollowing coating solutions were used in the
preparation of Example 7:
Silver r lsion:
Silver behenate homogenate
(10~ by weight in methyl ethyl ketone) 160g
BX-1 poly(vinyl butyral), available from Sekisui
Chemical Co. lOg
Thermoqra~hic Coatinq Solution.
The th~ , aphic coating solution was prepared by
adding 20g of the silver emulsion to 0.6g of methyl
gallate, O.lg of succinimid~ O.lg phth~limide~ O.lg
35 tetrachlorophthalic anhydride, 0.02g of benzotriazole,
0.05g of barbituric acid with 4mL of methanol, and lmL
-27-
WO96/10213 2~97469 .~ J~-S ~
of MEK. The solution was coated onto a 0.08mm (3 mils)
polyester substrate at a 0.08mm (3 mils) wet thickness
and air dried at 60~C for 3 minutes.
An infrared-absorbing topcoat solution was
5 prepared by mixing 0.08g of Dye 1, 0.5g of Sekisui BX-l
poly(vinyl butyral), and 20.0g MEK. The topcoat
solution was coated onto the thermographic layer at a
0.05mm (2 mils) wet thickness and air dried for 3
minutes at 60~C.
Example 7 was exposed with a 810 nanometer laser
diode (available from Spectra Diode Labs of San Jose,
CA) at 1.75 J/cm2 focused on the film plane at (a 7
micron spot size). The imaged film gave rise to a
visible Dmax of 3.4, visible Dmin of 0.08, W Dmax of
15 3.6 and W Dmin of 0.17. The visible optical densities
were measured using a Perkin Elmer microdensitometer
PDS lOlOM. The W optical densities were measured using
a MacBeth Model TD523 densitometer equipped with a
status 18A filter.
~x~mPle 8
The following coating solutions were used in the
preparation of Example 8:
25 Silver Emulsion:
Silver behenate homogenate 160g
(10% by weight in methyl ethyl ketone)
BX-l poly(vinyl butyral), available from 15g
Sekisui Chemical Co.
30 Acryloid A-21 acrylic resin, available from 6g
Rohm and Haas
Methyl ethyl ketone (MEK) 50g
35 Th~LI..4~rauhic Coatin~ Solution:
The thp ,LaphiC coating solution was prepared by
adding 15g of the silver emulsion to 0.6g of methyl
-28-
~ W096/10213 21 9 74 69 r .,
gallate, O.lg phth~lA7inon~ O.lg 2-imidazolidone, O.lg
tetrachlorgFh~h~l i r anhydride, 0.05g of barbituric acid
with 4mL of methanol, lmL of MEK, and lmL of
tetralLydLuruL~n. Before coating, 0.13g of Dye 1 was
~ 5 added to the solution. The solution was coated onto a
0.08mm (3 mils) polyester substrate at a 0.08mm t3
- mils) wet thir~nPcc and air dried at 50~C for 3
minutes.
A topcoat solution containing a 2.4~ by weight
10 solution of BX-l poly(vinyl butyral) was overcoated
onto the thermographic layer at a 0.05mm (2 mil) wet
thic~n~cc and air dried at 50~C for 3 minutes.
Examples 9-10
The following coating solutions were used in the
preparation of Examples 9-10:
Silver Em~lcion:
Silver behenate homogenate 160g
(10% by weight in methyl ethyl ketone)
BX-1 Poly~vinyl butyral), available from 5g
Sekisui ~h~mic~l Co.
25 Thermoqra~hic Coat~inq Solution:
The thermographic coating solutions were prepared
by adding 15g of the silver emulsion to 0.6g of methyl
gallate, O.lg of succinimide, O.lg of 2-imidazolidone,
O.lg of tetrachlorophthalic anhydride, 0.05g of
30 barbituric acid with 4mL of methanol, and lmL of MEK.
Before coating, 0.08g of Dye 2 was added to the
solution in Example 2 and 0.08g of Dye 3 was added to
the solution in Example 3. The solution was coated
onto a 0.08mm (3 mils) polyester substrate at a 0.08mm
(3 mils) wet thickness and air dried at 50~C for 3
minutes.
A topcoat solution containing a 2.4% by weight
solution of BX-1 poly(vinyl butyral) was overcoated
-29-
2 1 9 7 4 6 9 ~.,~
W096/10213
onto the th~L - ~phic layer at a 0.05mm (2 mils) wet
thickness and air dried at 50OC for 3 minutes.
r les 11-12
5 The following coating solutions were used in
preparation of Examples 11-12:
silver Emulsion:
Silver behenate homogenate 160g
(10% by weight in methyl ethyl ketone)
Butvar B-76 poly(vinyl butyral) 20g
TheL ._La~hic Coatina Solution:
The thermographic coating solution was yL~a~ed by
adding 15g of the silver emulsion to 0.8g of methyl
gallate, 0.2g of succinimide, O.lg of phthalaz,inone,
O.lg of 2-imidazolidone in 4mL of methanol, and lmL of
methyl ethyl ketone. Before coating, 0.05g of Dye 4
20 was added to the solution in Example 11 and 0.08g of
Dye 5 was added to the solution in Example 12. The
solutions were coated onto a .08mm (3 mils) polyester
substrate at a O.lmm (4 mils) wet thickness and air
dried at 21~C for 10 minutes.
A topcoat solution containing a 2.4~ by weight
solution of CA398-6 cellulose acetate, available from
Eastman Kodak Co., was overcoated onto the
thermographic layer at a O.O5mm (2 mils~ wet thickness
and air dried at 21~C for 20 minutes.
Table 2 summarizes the results of exposing the
materials of Examples 8-12 with a 810 nanometer laser
diode (available from Spectra Diode Labs of San Jose,
CA) at 1.75 J/cm2 focused on the film plane at (a 7
micron spot size). Maximum (DmaX) and minimum (Dm~n)
35 optical densities were measured using a MacBeth Model
TD523 densitometer equipped with a status 18A filter.
-30-
WO96110213 I~
~ 2~9746~
T~ble 2
Example # 8 9 10 11 12
EX~U~ULe time 72 45 45 45 72
(microseron~c)
D~.y 3.2 3.01.55 2.5 3.1
W Dmjn 0.19 0.160.13 0.180.18
Visible D~ n O . 07 0.070.07 0.070.13
~Yam~le 13
To show the effect of having halide ion present in
the thermographic silver emulsion, 0.2g of calcium
bromide was added to the thermographic coating solution
of Example 9. The thermographic layer turned totally
15 blacX when air dried at 21~C for 3 minutes.
~Y~mnle 14
The following coating solutions were used in the
preparation of Example 14:
20 Silver E~lcion:
Silver behenate homogenate 160g
(10~ by weight in methyl ethyl ketone)
Butvar B-76 poly(vinyl butyral) 20g
Th~L ~ hic Coatinq Solution:
The thermographic coating solution was prepared by
adding 0.6g of methyl gallate, 0.2g of phthalazinone,
O.lg of succin;mide~ O.lg of 2-imidazolidone, and 0.2g
30 of pyrogallol to 20g of the silver emulsion. The
solution was coated onto a .08mm (3 mils) polyester
substrate at a O.lmm (4 mils) wet ~hir~nPsc and air
dried at 21~C for 10 minutes.
An infrared-absorbing topcoat solution was
35 prepared by mixing 0.03g of Dye 6, l.Og of CA 398-6
cellulose acetate resin, and 20.0g MEK. The topcoat
solution was coated onto the th- D r aphic layer at a
. .
WO96/10213 2 l 9 7 4 6 ~
O.O5mm (2 mils) wet ~hirkn~cc and air dried for 3
minutes at 60~C.
~y~m~le ~5
The following coating solutions were used in the
preparation of Example 15:
Silver F~llcion:
Silver behenate homogenate 160g
tlOi by weight in methyl ethyl ketone1
Butvar~ B-76 poly(vinyl butyral) 20g
The~ phic Coatinq Solution:
The thermographic coating solution was prepared by
adding 0.6g of methyl gallate, O.lg of succinimide,
O.lg of 2-imidazolidone and 0.2g of L-ascorbic acid
palmitate to 20g of the silver emulsion. The solution
was coated onto a .08mm (3 mils) polyester substrate at
20 a O.lmm (4 mils) wet thickness and air dried at 60~C
for 3 minutes.
An infrared-absorbing topcoat solution was
prepared by mixing 0.03g of Dye 6, l.Og of CA 398-6
cellulose acetate resin, and 21.0g MEK. The topcoat
25 solution was coated onto the thermographic layer at a
0.05mm (2 mils) wet thickness and air dried for 3
minutes at 60~C.
Table 3 summaries the laser exposure results for
each example. Maximum and minimum optical densities
30 were measured using a MacBeth Model TD523 densitometer
equipped with a status 18A filter.
Table 3
Example 14 15
Dmax 3.73 2.73
Dmin 0.10
-32-
~ ~os6l~02~3 21 97469 1~., J;-f~
The radiation-absorbing dye may be incorporated
primarily into the thermographic silver emulsion layer.
It is believed that the th~ -, aphic silver emulsion
layer is heated above its glass transition temperature,
-5 thereby allowing the reducing agent for silver ion to
migrate to the light-insensitive organic silver salt
~(e.g., silver behenate) within the layer. The silver
behenate is reduced by the reducing agent to elemental
silver, forming a brown/black image. Toners are
10 incorporated into the formulation to obtain a more
neutral black color. The formation of the elemental
silver in the imaged area not only provides W opacity
of the image in the final element, but also is an
infrared-absorber which accelerates the image-forming
15 process. The intensity of the infrared laser beam
decreases exponentially as it penetrates into the
thermographic silver emulsion layer. The thickness of
the thermographic silver emulsion layer and the
concentration of infrared dye will effect the sharpness
20 of the image due to the decreasing intensity of the
laser beam as a function of distance through the layer.
The thickness of the thermographic silver emulsion
layer is preferably between about 1 and 10 microns and
more preferably, between about 2 and 6 microns. The
25 concentration of the infrared dye and the thickness of
the layer is adjusted such that the IR absorption of
the layer is generally between 20% to 99~; preferably,
50 - 90%; and more preferably, 60 - 85%.
In high resolution imaging conditions, where the
30 pixel dwell time is short and the laser peak intensity
is high, ablation may occur if the infrared dye is
incorporated solely in the thermographic emulsion layer
~of the construction. The heating rate is higher at the
surface where the laser beam enters into the
35 thermographic silver emulsion layer. As the elemental
silver forms, the absorption of the laser beam
-33-
21 97469
WO96110213 .~
lncreases. This can cause the ~hP ~hic silver
~ 1 ~inn layer to overheat, thereby causing smoke,
damage, or ablation.
By eliminating or decreasing the concentration of
5 infrared dye in the th- -, ~phic silver emulsion layer
and adding infrared dye in a layer adjacent to the
~h- -~aphic silver emulsion layer, the penetration of
the laser beam into the fhl aphic silver emulsion
layer can be increased. The thermographic imaging
10 element is exposed by directing the laser beam through
the thermographic silver emulsion layer before striking
the adjacent layer containing infrared dye. The
infrared-absorbing layer can be positioned either above
or below the thermographic silver emulsion layer
15 relative to the substrate upon which the adjacent
layers are deposited. The concentration of infrared
dye in the infrared-absorbing layer is chosen such that
the highest heating rate occurs at the interface
between the infrared-absorbing layer and the
20 th~L -, ~phic silver emulsion layer. The concentration
of infrared dye will depend upon the thickness of the
thermographic layer and the physical properties of the
dye. For example, the concentration of infrared dye in
a 1 micron thick th. ,L~phiC layer is adjusted to
25 achieve an absorption of preferably about 90% or more.
During the course of an imaging laser pulse,
elemental silver forms at this interface. The
elemental silver formed increases the infrared
absorption in this region of the thermographic silver
30 emulsion layer and acts as a heat source for the image
area within the th~ aphic silver emulsion layer.
As the elemental silver density i5 built up adjacent to
the infrared-absorbing layer, the intensity near the
opposite surface of the thermographic silver emulsion
15 layer is attenuated, thus reducing overheating in this
region. The profile of the pixel image would resemble
-34-
~ wog6no~l3 2 1 9 7 4 6 9 r~"~
an hour-glass shape, thus giving rise to a sharper
image.
r,~m~le 16
This example demonstrates the effect of the
5 ~hirknPe5 of the th~ , aphic silver emulsion layer,
the resin/silver ratio, the concentration of infrared
A dye, and the type of topcoat on the imaging
characteristics of the inventive thermographic imaging
element.
The following coating solutions are used in
preparation of Samples A-P. X are variables specified
in Table 4.
Silver Emulsion:
15 Silver behenate homogenate 160g
tlO% by weight in methyl ethyl ketone)
Butvar~ B-76 poly(vinyl butyral) Xg
20 Thermoqra~hic Coatlnq Solution:
The th~ phic coating solution was prepared by
adding 15g of the silver emulsion to 0.8g of methyl
gallate, 0.2g of sl~rcinimide~ O.lg of phthalazinone,
O.lg of 2-imidazolidone in 4mL of methanol and lmL of
25 methyl ethyl ketone. Before coating, Xg of Dye 2 was
added to the solution. The solutions were coated onto
a .08mm (3 mil) polyester substrate at X wet thirknPc~
and air dried at 70~C for 3 minutes.
A topcoat solution comprising a 2.4% by weight
30 solution of CA398-6 cellulose acetate; Scripset 540
~yL~ne -~leic anhydride copolymer available from
Monsanto Company;, Tyril 880B styrene-acrylonitrile
resin available from Dow Chemical Company, or
poly(vinyl alcohol) (PVA), Airvol 523 available from
35 Air Products and Chemicals, Inc., Allentown, PA and X%
Dye 2 was overcoated onto the theL -,L~hic layer at X
wet thirknP~s and air dried at 50~C for 3 minutes.
-35-
WO 96110213 2 1 q 7 4 6 9 T~
Samples A-P were scanned with a laser sensitometer
at several different 6can 5peed6 ranging from 20 to 60
cm/s. Density profiles of these lines at 415
nanometers were measured using a Perkin-Elmer
5 microdensitometer PDS l0l0M. Optical density
measurements were taken at 826 nA- ors (laser diode
wavelength) and at 415 nanometers for the unimaged
Pl~ ~ u8ing a ~:hir~~7u S~e.;~ul,l.otometer MPC-
3 l00/W3 l0 lPC .
Tnbl~
Sarnple # A B C D E F G H
" .'' 0.10 0.05 0.10 0.05 0.10 0.05 0.10 0.10
Layer thicicness
(wet, rn n)
Top Coat Layor 0 0 0 0 0 0 0.05 005
thicicness (wet,
mm)
Top Coat Resin none none none none none none PVA PVA
Type
Butvar content in20g 208 lOg lOg Sg Sg 0 0
Silver L yer
infrareci Dye in O.OSg O.OSg O.OSg O.OSg O.OSg O.OSg 0 0.01gTl _
L yer
infrre~iDyein 0 0 0 0 0 0 O.lOg O.lSg
Top Coat
D min (415mn) 0.95 0.14 1.1 0.3 1.24 0.75 0.34 0.53
Opticai Density 0.65 0.34 0.7 0.36 0.79 0.27 1.80 3.20
oD 825 nm
S~nnple~Y I J K L M
Tl _ .- l~yer 0.10 0.05 0.10 0.05 010
thicicness (wet, rmn)
Top Coat Layer 0.05 005 005 005 o o5
thicicness (wet, r~n)
Top Coat Resin Type Celluiose CelluioseCeliuiose Ceiiulose TyriiTU
Acetate Aceb~te Acetate Acetate
Butvar content in10g 10g 10g lOg o
Silver I~yer
--36--
SUBSTITUTE SHEET (RULE 26)
WO 96110213 2 1 9 7 4 b 9 P~
t
Infiur~ Dyo in 0.07g 0.07g O.lOg O.lOg O.OSg
Tl _ , ' ' l~yer
Inf~ed Dyo in 0 0 0 0 0
Top Co~t
Dmin (415tun) 0.23 0.07 0.42 0.12 0.55
Opticd Density e~ 825 nm 0.75 0.30 1.40 0.84 0.76
Sllmple~ N O P
Tl _ ,' Iayer 0.10 0.10 0.10
tbic~noss (wet, mm)
Top Co~lt L~yer 0,05 0.05 0.05
thiclolocs (vvet, rmn)
Top Collt ResinTypoScripset~MScripset~ Scnpset~U
Butv~r conterlt in 0 0 0
Silver IJyer
Infr~red Dye in O.Olg O.Olg 0
Tl " ,-' I~yer
~nf~red Dyo m 0. IOg 0 158 O.lOg
2 0 Top Coct
Dm~ (415~) 0.35 0.29 0.42
Opticd Density all 825 nm 0.84 1.45 1.11
A laser sen6itometer (l), shown in FIG. 1, was
25 used to evaluate the t h~ phic imaging elements in
Example 16. A 700 milliwatt beam (2) emitted from a
2361-P2 fiber coupled laser diode (3) (available from
Spectra Diode ~abs) was focused onto a rotating drum
(4). The core diameter of the fiber (5) was 100
30 micrometers and the wavelength of the laser diode (3)
was 826 nanometers. The power on the rotating drum (4)
was 210 milliwatts and the spot shape was a flat-topped
cone with a spot size of 45 microns at full width half
maximum (FWHH). The flat-topped cone profile is
35 characterized by rO, the radius of the peak intensity
of the cone, and rl, the outer radius of the cone where
the intensity is nearly zero. A sc~nning slit beam
-37-
SUSSTITUTE SHEET (RULE 26)
W096110213 2 1 9 7 4 6 9
profiler was used to measure the intensity profile of
the laser spot. Since the profiler integrates the
intensity in the direction perpendicular to the slit
- ~ ~, the actual spot profile was inferred from the
5 profiler data. FIG. 2 shows a comparison of the
profiler data (6) and the calculated profile data t7)
expected for a flat-topped cone intensity profile with
rO equal to 10 micrometers and rl equal to 36
micrometer6. The curve was computed by integrating the
10 model flat-topped cone intensity profile in one
direction and rpcral; nq.
As the intensity profile is scanned across the
film, points lying under the spot profile will receive
a finite exposure energy. This exposure energy is
15 ~f~pan~Pnt upon the location of the point with respect
to the scanned spot as well as the spot scan speed.
FIG. 3a shows the total incident exposure energy
plotted versus the distance across the beam in the
cross/scan direction. The curve was calculated for the
20 fiber-coupled sensitometer model beam shape, aR-nm;nq a
scan speed of 40 centimeters/seconds. In FIG. 3b, a
microdensitometer profile of a line imaged with the
energy profile shown in FIG. 3a, onto the thermographic
element is shown. (Example 16, Sample N not shown.)
25 The density data was collected using a narrowband
filter at 415 nanometers. The density edges in FIG. 3b
exhibit gradients that are larger than that of the
incident exposure profile shown in FIG. 3a, indicating
that the thf ~l~phic element (Example 16, Sample N
30 not shown) has a high contrast.
The contrast of the fhr~ phic element can be
PY~minPd more quantitatively using a D-logE curve. A
D-logE curve is a plot of the imaged film density
versus the logarithm of the incident ~X~U~UL a energy.
35 The theoretical form for this curve is given by D =
ylog (EEF/Eo); where y is equal to the slope of the D-
-38-
~ ~096110213 2 1 9 7 4 6 9 F~./ ~ : .
logE curve, E is equal to the incident exposure energy,
EF i8 equal to the fog or backyr~.,d level effective
energy, Eo is equal to the minimum energy required to
begin development of the image, and D is equal to the
5 optical density of the element when exposed to ex~o
energy E. The backyround density i5 equal to ~log EF
Using the data shown in FIG. 3, a D-logE curve was
computed and plotted in FIG. 4. From the model curve
described by the optical density equation, the gamma or
lO contrast of the element corresponds to the slope of the
D-logE curve. The gamma value for the D-logE curve in
FIG. 4 is 34. For a relative comparison, a typical
rapid access wet-processed silver halide film has a
gamma of approximately lO. The higher contrast is an
15 advantage for graphic arts applications since high
contrast hal~tone dots are desired for consistent tone
curve control and also for new stochastic screening
processes. Similar advantages apply to printed circuit
board phototool applications.
The D-logE curve in FIG. 4 shows that the density
development begins at approximately 0.9 J/cm2 and that
density saturation at maximum density (DmaX) occurs at
l.2 J/cm2. It is to be understood that the optimum
imaging speed and scanner exposure conditions will be
25 unique for the particular scanner used to image the
thermographic element.
Each of the samples described in Table 4 were
scanned with the laser sensitometer (l) of FIG. l at
Qeveral different scan speeds ranging from 20 to 60
30 centimeters/second. D-logE curves were calculated with
a Perkin-Elmer microdensitometer using the data from
the density profiles of these lines at 415 nanometers,
The model parameters in the optical density equation
described above were determined from the D-logE curves
35 and are summarized below in Table 5.
-39-
WO 96/10213 ~ 2 1 9 7 4 6 ~
Tztbl~ i
S mpledl A B C D E
Dma ~ 4 5
(415 ~m)
mD Eo, 50 0.77 0.85 0.74 0.93 0.60
mD Esat, 501.30 1.40 1.40 1.80 1.23
mD Dma~, 503.50 3.50 3.65 3.50 3.60
mD Dmin, 500.85 0.40 0.55 0.65 0.80
gamma, 50 11.65 14.30 11.19 9-93 8.98
mD Eo, 100 0.966 0.94 0.96 1.00 1.03
mD Dsat, 1001.27 1.30 1.26 10.00 1.32
mD Dmal~, 100 3.5 3.0 3.4 0.0 3.6
mD Dmin, 1000.80 0.35 0.80 0.00 0.80
gamn~a, 10022.7~ 18.82 22.02 0.00 25.99
Gamma 100 1.95 1.32 1.97 0.00 2.89
/Gamlna 50
Sample~ E 1 ~ K L
Dma~ (415 nm)
mD Eo, 50 0.65 0.80 1.13 0.56 0.35
mD Esat, 501.60 1.26 2.00 1.30 1.00
mD Dnw, 50 3.70 3.60 3.50 3.70 3.60
mD Dmin, 500.70 0.40 0.30 0.60 0.35
gamma, 50 7.80 16.22 12.90 8.47 7.12
mD Eo, 100 1.00 0.90 1.00 0.66 0.661
mD Dsat, 10010.001.16 10.00 1.00 1.042
mD Dma~, 1000.0 3.5 0.0 3.6 3.5
mD Dmin, 1000.00 0.30 0.00 0.70 0.40
gamma, 100 0.00 29.03 0.00 16.07 15.88
Gamma 100 0.00 1.79 0.00 1.90 2.20
IGamma 50
--40--
WO 96/10213 2 1 9 7 4 6 9 ~ c
S~lmplelt N O
DrGa~ (415 nm) 5.00 4.4
mD Eo, 50 0.95 0.88
mD Es t, 50 1.65 1.26
mD Dmax, 50 3.70 3.80
mD Dmin, 50 0.70 0.50
gamma, 50 12.51 21.16
mD Eo, 100 0.96 0.97
mD Dsat, 1001.20 i.76
mD Dmax, 100 3.7 3.8
mD Dmin, 1000.40 0.50
ganuna, 100 34.05 29.05
Gamma 100 2.72 1.37
/Gamma 50
mD Eo,S0 = Eo value from D-lo~E curve, scan spoed at 20 . '. ' (cmts)
mD Esat,S0 e Esst valuc from D-logE curve, scam speed ~t 20 cmts
mD Dmax,50 = Maximum Density at 20 cmts
mD Dmmn,50 = Minimum Density at 20 cmts
20 gamm~,S0 = ~mma value when scanning at 20 cmts
mD Eo,100 = Eo value from D-loxE curvo, scan speed at 40 cmts
mD Esat,100--Esat value from D-lo~E curve, sc n speed at 40 cmls
mD Dmax,100 = Maximum Density at 40 cmts
mD Dmin,100 = Minimum Density at 40 cmls
25 ~amma,100 ~ gamma value when scannin~ 40 cmts
gnmma 100/gamma 50 = (gamma st 40 cmls) divi~le~l by (~amma at 20 cmts)
The average value Eo for samples A through L is
0. 8 + 0 . 2 Joules/cm2 at a scan speed of 20 cm/s and 0.9
30 + 0.2 Joules/cm2 at a scan speed of 40 cm/s. The
minimuD e~o~uLe energy re~uired to begin density
development is relatively independent of the scan
speed. The Esat values are ;n~r~pPn~Pnt of speed as
well. The average value for Esat is 1.3 + 0.2
35 Joules/cm2 at a scan speed of 20 cm/s and 1.2 + 0.1
Joules/cm2 at a scan speed of 40 cm/s. The gamma
values show evidence of imaging performance differences
at the two speeds. The average gamma value for Samples
A through ~ at a scan speed of 20 cm/s is 12 + 4 while
-41-
21 ~746q
WO96110213 I~
the average gamma value at 40 cm/s is 24 i 6. Thus.
the gamma values appear to increase significantly as
the scan speed is increased. It is possible that at
the lower scan speeds the heat diffusion i5 more
5 significant giving rise to loss of sharpness of the
edges and thus, reducing the gamma of the image.
Unlike photothermographic silver media, thermographic
silver elements show a more pronounced effect of
~O~UL e conditions.
Samples C, I, and K were coated with different
infrared dye concentrations. Samples C and I have an
80% absorption at the laser diode wavelength of 826
nanometers. Sample K was coated to the same thickness,
but was loaded with more infrared dye so that it
15 absorbs 96% at 826 nanometers. The average Eo and Esat
values for C and I are 0.93 Joules1cm2 and l.21
Joules/cm2, respectively, at a scan rate of 40 cm/s.
The Eo and Esat values for Sample K are 0.66 Joules/cm2
and l.0 Joules/cm2, respectively. The sensitivity of
20 the film appears to be slightly improved by the 16%
increase in the layer absorption.
The effect is more pronounced for thinner
coatings. Samples D, J, and L were coated at half the
thi~kn~ss of C, I, and K. Samples D and J absorb only
25 about 50% of the incident laser radiation, and do not
image at the 40 cm/s scan rate. Sample L absorbed 85%
of the incident laser radiation. The average Eo and
Esat values for D and J are l.0 Joules/cm2 and l.9
Joules/cm2, respectively at 20 cm/s, whereas the Eo and
30 Esat values for Sample L are 0.35 Joules/cm2 and l.0
Joules/cm2, respectively. The ~O~ULe energy values
~or L are lower than that of D and J. Sensitivity is
~nh In~d with increasing laser absorption in the
thermographic silver emulsion layer, or with increasing
35 infrared dye conc~ L~tion. The edges of the imaged
lines scanned at 40 cm/s in Samples K and L were
21 ~7469
~096~02~3
smoother than the other single infrared layer samples.
The line edge sharpness can be improved by increasing
the infrared dye concentration in the layer.
A comparison of samples with different
~ 5 thi~nP~epe~ but similar absorption percentages,
indicates that a thinner coating with a higher infrared
dye ~oncell~Lation is more sensitive than one with a
thicker coating. The Eo and Esat values for R are 0.56
and 1.3 Joules/cm2, respectively, at 20 cm/s, whereas
10 the Eo and Esat values for L are 0.35 and 1.0
Joules/cm2, respectively. The thickness of Sample L is
half that of K, but it absorbs 85~ of the laser
radiation, which is roughly comparable to that of K.
Increasing the infrared dye concentration may cause
15 ablation due to increased peak temperatures in the
th~. ~ aphic layer. The sensitivity of a single
thermographic silver emulsion layer containing infrared
dye can be r-Yimi7ed by coating the thermographic
silver emulsion layer as thin as possible with the
20 highest achievable infrared dye concentration, while
maintaining the desired maximum density.
The quality of the imaged line is affected by the
resin to silver ratio. The exposure energy values and
the gamma values are not significantly affected by
Z5 changes in the resin to silver ratio, as shown in Table
5 for Samples A, C, and E. However, the micrographs of
these samples indicate that the resin to silver ratio
does affect the image quality of thc lines. As the
resin to silver ratio is decreased, the edges of the
30 lines become rough and jagged, and the density
uniformity across and along the imaged line decreases.
Decreasing the resin concentration should enhance the
sensitivity of the material due to less bulk material
to heat. However, the jagged edges appear to have
35 offset this advantage. The resin to silver ratio is
preferably between about 25 - 50 wt~.
-43-
Wo96/10213 2 1 9 7 4 6 9 r~l,. 5 96~1
Another ~mhoA i r ~ t of the present invention
comprises the addition of infrared dye in a layer
adjacent to the ~h~ ,LGphiC silver emulsion layer.
Samples N, 0, G, H, and P in Example 16 evaluate the
5 addition of an infrared dye in the topcoat of a
thermographic element. For some unknown reason,
Samples M, G, and H imaged poorly and therefore, are
not included in Table 4. Samples N, 0, and P exhibited
improved line quality compared to the samples
lO containing infrared dye in the th~ ,LGphic layer
only. The thermographic silver layers in Samples N, 0,
and P were overcoated with a .05 millimeter coating of
Scripset resin that contains a high concentration of
infrared dye. A piece of pressure-sensitive adhesive
15 tape was used to separate the topcoat from the
thermographic silver emulsion layer to verify that the
two layers had not intermixed. Samples N and 0 have
gamma values greater than 34 and 30, respectively, at a
scan speed of 40 cm/s. These gamma values are
20 comparable to a conventional silver halide duplication
film. For comparison, a typical rapid access silver
halide film has a gamma of approximately lO. The
quality of Sample P is similar to N and 0, although a
D-log E curve was not computed for this sample. Both N
25 and 0 exhibit sharp smooth line edges with an
approximate l micron edge roughn~CC. The samples
containing infrared dye in the thermographic silver
layer only had rougher edges than Samples N and 0. The
density uniformity of Samples N, 0, and P were within
30 +5%. The sensitivity of Samples N and 0 are comparable
to the samples having no topcoat containing infrared
dye. No ablation was observed in Samples N, 0 and P.
Improved edge contrast, edge sharpness, and density
uniformity can be achieved by the addition of infrared
35 dye in a layer adjacent to the thermographic silver
layer. In addition, the susceptibility to ablation is
-44-
- 21 974 69 r~ J -
WO96110213
also reduced in this cvn~LLu~Lion. The concer.LL~tion
of the infrared dye in the th~ -,L~pllic silver layer
i5 in an amount such that the absorption Or the laser
radiation in the th. , ~phiC layer is preferably less
~ 5 than or equal to 40~ and more preferably, less than or
equal to 35%.
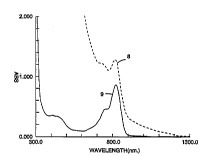
FIG. 5 shows the imaged t8) and non-imaged
(background) (9) transmission spectra for Example 16,
Sample N. The ~nhAncPd infrared dye absorption peak at
10 820 nanometers is clearly evident. The density at the
laser diode wavelength of 826 nanometers increases from
0.84 (14.5~ transmission) to 1.26 (5.5% transmission),
while the density at 415 nanometers increases from
0.355 t44.2~ transmission) to 5.0 (nearly 0%
15 trAn~ sion). The elemental silver formed in the
thermographic layer during ~O~UL a provides an
~nhAnre~ absorption difference in the ultraviolet (W),
which is an advantage in UV mask applications. In
Table 5, the D~aX measured by the microdensitometer was
20 3.7 which is lower than the value obtained from the
spectrophotometer. Apparently, the maximum optical
density that can be measured by the microdensitometer
is limited to about 3.7. This implies that many of the
gamma values computed in Table 5 are lower than the
25 true values and therefore, should be treated as
~rv~tive estimates.
In order to compare the imaging characteristics of
the thermographic elements with little or no effects
due to heat dissipation, Examples 1, 2, 3, 4, 5, 6 and
30 16 (Sample N) were imaged using a 150 milliWatt (110
milliWatts at the image plane) laser diode (SDL-5422,
available from Spectra Diode Labs) emitting at 811
nanometers. The beam was focused to an 8 micrometer
spot size (full width at 1/e2 level) and scanned at 213
35 centimeters/second with a 4.5 micrometer cross scan
-45-
WO96110213 2 1 9 7 4 6 9
llne spacing. Table 6 summarizes the results from this
evaluation.
. Table 6
Exzunpl~ Dmax, Dmin, Dmax- Dmax, Dmin, Dmax-
365nm365nmDmin, 415nm 415nmDmin,
365nm 415nm
1 2.170.31 1.85 2.48 0.2s 2.23
2 2.580.39 2.19 2.94 0.29 2.65
3 1.780.39 1.39 1.85 0.29 1.56
4 3.050.63 2.42 3.13 0.57 2.56
10 S 4.500.57 3.93 S.00 0.42 4.58
6 4.04o.90 3.14 S.00 O.S9 4.01
16, 4.400.47 3.92 S.00 0.53 4.46
8ampln U
The data shows that the higher contrast(Dmax-Dmin)
films are achieved when silver laurate is used in
combination with barbituric acid in the thermographic
~ silver emulsion layer (Examples 5 and 6) or a higher
concentration of methyl gallate is used in the silver
20 behenate formulation (Example 16, Sample N~. In order
to provide workable W contract applications, the
contrast is preferably greater than about 2.50. The
data also shows that the addition of benzotriazole
inhibits the speed Or the film; however, the decrease
25 in speed is minimized when silver laurate is used for
the silver soap. Even though a slight decrease in speed
may be observed, the improved light stability, as shown
in Table l, provides an advantage for jnm~ ng
benzotriazole in the th~ ~~ mphic silver emulsion.
pP~c~n~hle variations and modifications are
possible from the foregoing disclosure without
departing from either the spirit or scope of the
present invention as defined in the claims.
-46-