Note: Descriptions are shown in the official language in which they were submitted.
. WO 96/04948 2197758 PCT/G1395/01390
INHALER APPARATUS WITH OPTIMISATION CHAMBER
The present invention relates to a medicament dispensing
device of the type which is used for dispensing discrete
amounts of a medicament entrained in an air or propellant
stream. In particular, the invention is concerned with
devices of the metered dose inhaler type which are well known
in the art of medicine for the treatment of respiratory
complaints such as asthma.
A standard metered dose inhaler consists of three main
parts: a pressurised canister, an actuator body and a drug
delivery outlet. The pressurised canister contains a mixture
of active drug and propellant and is usually formed from a
deep drawn aluminium cup portion having a lid portion crimped
thereto which carries the metering valve assembly. The
metering valve assembly is provided with a protruding valve
stem which, in use, is inserted as a tight push fit into a so-
called "stem block" in the actuator body.
Operation of the inhaler requires the user to apply a
compressive force to the closed end of the canister. The
internal components of the metering valve assembly are spring
loaded so that, typically, a compressive force of between 15
and 30 N is required to activate the device. In response to
this compressive force, the canister moves axially with
respect to the valve stem by a distance sufficient to actuate
the metering valve and causes a metered quantity of the drug
and propellant to be expelled through the valve stem. This
is then released into the mouthpiece via a nozzle in the stem
block. A user inhaling through the drug delivery outlet of
the device at this point will thus receive a dose of the drug.
The use of metered dose inhalers has become increasingly
widespread in recent years owing to the fact that they enable
the patient to administer an accurate dose of medicament when
required. This is particularly useful for patients whose
respiratory difficulties manifest themselves suddenly.
W096l04948 2197758 PCT/GB95401390 =
2
One of the problems most frequently encountered with the
standard metered dose inhaler described as above is that the
patient has difficulty co-ordinating the action of depressing
the aerosol canister with the act of inhalation. This can be
particularly problematic when the patient is in distress due
to a sudden onset of respiratory difficulties. It is also
difficult for small children to co-ordinate these actions
properly. Such problems tend to undermine the effectiveness
of self-administration. These difficulties have now been
overcome by the introduction of breath-operated actuators such
as those described in the Applicants' International Patent
Application No. WO 92/09323.
When a pressurised inhaler is operated, the propellant
stream explosively breaks up at atmospheric pressure to
produce a range of different sizes of propellant droplets
containing the drug, either in suspension or possibly in
solution. Although the propellant in the droplets tends to
evaporate, causing the droplets to decrease in size with time,
the patient still receives a cloud of drug-containing droplets
of various sizes.
The droplets tend to separate according to their sizes
under the influence of gravitational forces and air currents.
The smaller droplets, typically less than 5 - 6 m in
diameter, pass through the patient's oro-pharynx to enter the
trachea, bronchi and lower airways where they are able to
exert a therapeutic effect.
Larger droplets, on the other hand, may be deposited in
the patient's oro-pharynx, owing to their different inertial
characteristics, having escaped impingement on the standard
actuator mouthpiece. Such oro-pharyngeal deposition is
undesirable for a number of reasons. For example, the patient
may experience an unpleasant taste, or a cooling effect as
propellant droplets are rapidly evaporated by contact with
warm mucosa. Unwanted deposition of certain classes of
compounds may even cause undesirable local side effects, such
~ WO 96104948 2197758 PCT/GB95/01390
3
as the corticosteroids which may lead to Candida infections
("thrush").
One successful approach to the elimination of problems
caused by the deposition of large droplets in unwanted areas
is the use of so-called "large volume spacers". These are
reservoirs having a typical capacity of around 750 ml which
are adapted at one end to be attached to the delivery outlet
of a pressurised inhaler, with a mouthpiece at the other end
for engagement with the user's mouth. Usually, the large
volume spacer is equipped with a one-way valve to prevent user
exhalation into the spacer cavity. In use, the inhaler is
actuated into the spacer cavity, where the emitted aerosol is
effectively trapped. Large droplets may either impinge on the
spacer walls or fall to the floor of cavity. The patient
inhales the aerosol from the spacer cavity, the inhaled cloud
consisting of predominantly fine droplets which pass with the
inhaled air stream into the lungs by virtue of their relative
aerodynamic stability.
The patient need not inhale simultaneously with inhaler
actuation; some delay is possible. Thus, the need for precise
co-ordination of actions is removed and some of the problems
associated with patient-controlled instantaneous drug
administration are alleviated. However, the means of using
large volume spacers has not been optimised and variation in
practical use is possible.
Unfortunately, the use of large volume spacers introduces
a different range of difficulties. Foremost among these is
the sheer size of a large volume spacer. Such a device cannot
be carried conveniently in the pocket or in a handbag, so the
advantage of portability which makes metered dose inhalers so
attractive is immediately cancelled out by the large volume
spacer requirement. Also, it is not always possible for
particularly frail persons or very small children to
manipulate the inhaler/spacer combination. Such patients
therefore require assistance which again undermines the
convenience of the inhaler device.
WO 96104948 2197758 PCT16B95101390
4
Attempts have been made to solve the above problems with
collapsible telescopic spacers, but these have met with only
limited success. In any case, they do not satisfactorily
overcome the co-ordination problem described earlier.
So-called "tube" spacers have been proposed as an
alternative but have enjoyed only limited success. Whilst
they are more compact, their performance as holding chambers
is ineffective because virtually all of the aerosol is
deposited on the walls of the spacer within a very short time.
Tube spacers merely trap some of the large droplets which
would otherwise be deposited in the patient's oro-pharynx and
the co-ordination problem remains unsolved. Hitherto, the
only effective way of using a tube spacer/pressurised inhaler
combination has required co-ordination of inhaler activation
with patient inhalation. As explained above, such co-
ordinated action is not easy for every category of patient.
Similar considerations apply in relation to dry powder
inhalers, where the term "particles" could be substituted for
"droplets" in the foregoing discussion of prior art devices.
It is therefore an object of the present invention to
solve the aforementioned co-ordination problem whilst
facilitating patient-administration of an effective dose of
the required drug by means of a compact inhaler system.
The invention is an inhaler device comprising an actuator
body adapted to receive a medicament dispenser, and medicament
delivery outlet means through which medicament is delivered
in an airstream in response to inhalation by a user,
characterised in that said medicament delivery outlet means
is provided with an optimisation chamber equipped with a
mouthpiece, and in that said chamber has a volume in the range
from 20 ml.to 200 ml.
Such an arrangement confers the advantage of the large
volume spacer (no requirement for co-ordination of inhaler
activation with patient inhalation) by facilitating drug
delivery in responseto patient inhalation. Moreover, the
0 WO96/04948 21Q7 75O O PCT/CB95101390
! /
advantages of the tube spacer are also present because the
inventive arrangement is compact but still allows large
= droplets/particles to be retained in the aerosol optimisation
chamber. As indicated above, the drawback of tube spacers is
5 avoided. There is no need for co-ordinated inhaler activation
and patient inhalation.
In one form of the invention, the medicament dispenser
is a pressurised container containing the medicament in
suspension or in solution in a propellant, and the actuator
body is designed to actuate the pressurised container in
response to inhalation on the part of the user.
The optimisation chamber may have internal baffle means
to promote deposition of the larger (>6 - 7 m) droplets/
particles. Conveniently, the optimisation chamber is a
separate device which can be engaged by a push-fit connection
to the mouth-piece of a conventional breath-operated inhaler
device by the patient. In an especially preferred form, the
actuator body and the optimisation chamber are pivotally
connected to each other to form a unitary device. In such an
arrangement, the optimisation chamber may be designed to serve
as a cover for the medicament delivery outlet of the device,
thereby ensuring that the air passageway of the device is
protected from dirt or other foreign body ingress. Deployment
of the optimisation chamber then opens up a passageway from
the medicament delivery outlet of the actuator body to the
patient's mouth via the chamber interior.
Surprisingly, it has been found that the apparatus
according to the present invention maintains a high respirable
fraction of the delivered dose of medicament. In relation to
breath-operated inhaler devices used without any type of
spacer, apparatus according.to the present invention delivers
a respirable fraction which is at least as great. In relation
to standard inhalers used in combination with large volume
spacers, apparatus according to the present invention delivers
a significantly higher respirable fraction.
R o 96104948 21977 C Q PCT/GB95/0 5390 ~
6 Jv
The invention will now be described by way of example
only with reference to the drawings, in which:
Figure 1 is a schematic cross-sectional view through
one embodiment of apparatus according to the
present invention;
Figure 2 is a graph comparing performance of a breath-
operated inhaler device used on its own, a
standard inhaler used with a large volume
spacer and a device in accordance with the
present invention for a 100 }eg medicament
dose, and
Figure 3 is a graph similar to Figure 2, comparing
performance for a 250 }cg medicament dose.
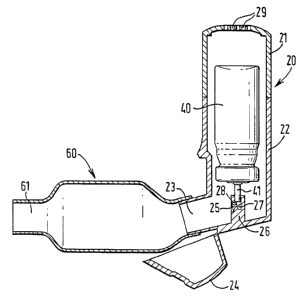
Referring now to Figure 1, an embodiment of inhaler
apparatus according to the present invention is shown which
comprises an actuator body 20, a pressurised medicament
dispenser 40 and an aerosol optimisation chamber 60. The
actuator body 20 is formed of a top section 21 and a bottom
section 22, the bottom section 22 having an integrally-formed
mouthpiece 23 and a pivotally-mounted dust cover 24 for
preventing ingress of dust through the mouthpiece when the
apparatus is not in use. The top section 21 accommodates the
mechanism for effecting breath-actuation, but in view of the
fact that the precise method by which breath-actuation is
achieved does not form part of the present invention, this
feature will not be described in detail here. As shown, top
section 21 includes a series of air inlets 29 for admitting
air to the apparatus in response to patient inhalation.
As shown, the actuator body 20 houses a pressurised
medicament dispenser 40 in the form of aerosol canister of
generally cylindrical shape. The aerosol canister has a stem
41 which contains an aerosol dispensing valve (not shown) of
conventional design. A pedestal 26 formed on the base of the
actuator body 20 "is provided with a bore 27 dimensioned to
form an airtight seal with the stem 41 of the aerosol
~ WO 96/04948 2197758 pCT/CB95/01390
7
canister. A shoulder 28 in the bore forms a seat for the tip
of the stem 41 and helps to maintain the aerosol canister in
position within the actuator body 20. Nozzle 25 communicates
between the bore 27 and the mouthpiece 23.
The apparatus is shown with the dust cover 24 pivoted to
the open position to allow attachment of aerosol optimisation
chamber 60 to the mouthpiece 23 of the actuator body 20. This
is the simplest form of the invention, in which the aerosol
optimisation chamber 60 is a separate unit adapted to be
connected to the mouthpiece 23 by an interference fit. The
optimisation chamber 60 has its own mouthpiece formation 61
at the end thereof remote from the connection to the actuator
body 20.
In use, the patient inhales through the mouthpiece 61 of
the optimisation chamber. In response to patient inhalation,
the actuation mechanism of the actuator body 20 moves the
aerosol canister so that its dispensing valve is opened,
thereby delivering a measured dose of medicament entrained in
propellant issues through nozzle 25. The measured dose
expands explosively into a cloud of droplets of various sizes
which pass into the aerosol optimisation chamber 60. Here,
large droplets (typically of diameter greater than 6 - 7 m)
impinge upon the chamber walls and are not inhaled by the
patient. Smaller droplets are relatively aerodynamically
stable and are inspired by the patient. A high proportion of
the stabilized cloud is drawn into the patient's trachea,
bronchi and lower airways where the drug is able to exert its
therapeutic effect.
Turning now to Figures 2 and 3, these show comparative
performances for various arrangements of inhaler apparatus.
The Figure 2,graph compares performance for a 100 pg dose of
beclomethasone dipropionate (BDP) dispensed through:
(A) a breath-operated inhaler device used on its own;
(B) a standard inhaler used with a large volume spacer, and
(C) an apparatus in accordance with the present invention.
WO 96104948 2191758 PCT/GB95/01390 4p
8
The so-called "Stage 1" fraction is the fraction
containing the larger droplets of diameter 6 -7 m or greater.
The "Stage 2" fraction is composed of finer droplets and is
generally regarded by persons skilled in the art as the
respirable fraction, that is the proportion of an inhaler
cloud most likely to be associated with therapeutic activity.
The Stage 1 and Stage 2 fractions were differentiated by a
two-stage impinger apparatus specified in the British
Pharmacopoeia 1994, which can be used to separate an aerosol
cloud on the basis of droplet size.
It will be noted that Figure 2 shows that, with the
breath-operated inhaler device used on its own, the Stage 1
fraction amounts to 50% of the delivered dose. The standard
inhaler combined with a large volume spacer has a Stage 1
fraction of less than 10%, whilst the apparatus according to
the present invention shows a Stage I fraction of around 15%,
both the latter showing what is likely to be clinically useful
reductions in oro-pharyngeal deposition.
However, with reference to the critical Stage 2 fraction,
it can be seen that the breath-operated inhaler device
delivers a respirable fraction of roughly 40% of the measured
dose. The apparatus according to the present invention also
shows a high respirable fraction (>45%), whereas the standard
inhaler/large volume spacer combination shows a respirable
fraction of just over 30%. For some medicaments, this would
be below the minimum threshold permitted by the British
Pharmacopoeia. It should also be borne in mind that the
respirable fraction obtained from a large volume spacer
diminishes with time, so this figure of 30% represents the
maximum respirable fraction available to the patient assuming
immediate inhalation.
The Figure 3 graph shows a corresponding analysis for a
250 g dose of beclomethasone dipropionate. This graph shows
that the trends observed for a 100 g are more sharply
accentuated at higher doses. Not surprisingly, the
unadulterated breath-operated inhaler device shows a high
WO 96/04948 219 7 7 5f3 PCT/GB95101390
9
stage 1 fraction, but delivers a respirable fraction of only
30%. The Stage 1 fraction for the standard inhaler/large
volume spacer combination is little more than 5%, but the
respirable fraction is significantly reduced to a level of
less than 20%. By contrast, the apparatus according to the
present invention not only maintains a low Stage 1 fraction
(21%), but also delivers a respirable fraction of 35%.
These figures show that the apparatus according to the
present invention gives rise to surprising synergistic effects
which are likely to result in significant patient benefits.
The fact that the apparatus uses a breath-operated inhaler
device means that it is capable of providing an accurate and
consistent injection of dose when the patient inhales. The
dose injection can be controlled by the mechanical design of
the breath-operated inhaler device, for example to occur at
an inhaled air flow rate of 25 1/min. Such actuation is
totally automatic and reproducible in response to patient
inhalation.
Moreover, the aerosol optimisation chamber which fits
onto the mouthpiece of the actuator body is of a sufficiently
small volume to ensure that air flow is immediately
transmitted to the actuating mechanism of the apparatus.
Also, the volume of inhalable air carrying the medicament dose
is within the restricted inhaled air volume of an asthmatic
patient (perhaps 500 ml).
In conclusion, an apparatus designed in accordance with
the present invention is capable of removing a significant
proportion of the large droplets of medicament-containing
propellants whilst maintaining the critical respirable
fraction of the aerosol which contains the therapeutically
,active portion of the administered medicament. Both of these
objects are achieved without requiring the patient to co-
ordinate inhaler activation with inhalation.
Wo9Gr0a948 2191 f5CJ PcTrGS95/o1390 .
Although the invention has been particularly described
with reference to only one simple embodiment, it will be
understood that various modifications are possible. In
particular, it will be understood that the inventive principle
5 herein disclosed is equally applicable to dry powder inhalers
and pressurised breath-operated devices.