Note: Descriptions are shown in the official language in which they were submitted.
W096105833 ~ ~ ~ PCT1US95110651
-1- L
METHODS OF INHIBITING ENDOMETRIAh CANCER
The uterine lining (endometrium) is composed of
tissue, blood vessels, and -glands that grow when stimulated
by the hormone estrogen. In women with normal menstrual
cycles, hormonal--fluctuations triggerthe growth and
shedding of the~endometrium each month. If conception
occurs, the endometrium nourishes the developing embryo.
Most cases of endometrial carcinoma are
associated with a precursor lesion termed endometrial
hyperplasia.~~ The classification of endometrial
hyperplasia is based on the presence or absence of
cytologic atypia, the-presence of dysplasia, and the degree-
of complexity of-the architectural pattern. Cytologic
atypia is the most predictive criterion for the likelihood
of progression-to carcinoma.
In simple or cystic hyperplasia with cytologic
atypia present there is about an 8~ chance of progression
to cancer. With complex or adenomatous hyperplasia with
cytologic atypia-present, there is 29$ chance. When no
cytologic atypia is present, the progression rate is 1$ for
simple and 3~ for complex hyperplasia.
With continuously elevated estrogen levels, the
endometrium remains in its growth phase at all time, in
some cases Leading to. an overabundance of endometrial
tissue or endometrial hyperplasia. Overgrowth of the
endometrium is often a benign condition, but it can also be
a precursor-of-endometrial cancer. Because of this risk,
doctors urge women to avoid long-term unopposed estrogen
therapy, which-can-cause endometrial overgrowth if the
lining is not continually shed, and to seek prompt
treatment for conditions that cause excessive estrogen
' production.(The-use of progesteronein-hormone
replacement therapy causes breakdown bleeding and shedding
of endometrial build up).
W096105833 . , -F, , PCTIUS95110651
2198119
_2_
Doctors base their treatment decisions on
several factors. First, they examine the cells obtained in
the biopsy or D&C. If the cells are normal but simply over ,
abundant, future development of cancer is less likely than
if the cells are atypical, displaying-enlarged nuclei-and ,
other unusual features: In some cases, a D&C will show
that cancer-has already developed.
Endometrial cancer is the most common '
gynecologic pathology and-the fourth most common malignancy
in women, after breast, colorectal, and lung caricer. --°
Approximately 30,000-40,000 new cases of endometrial cancer
are diagnosed each year. 4dhile it is the most common -
pathology, most patients present in theearly stage.
Endometrial cancer affects mainly postmenopausal
women, as the average age at diagnosis is 58 years, and
fewer than 5~ of cases occur prior to age 40. The
incidence of endometrial cancer'is higher among women with
a history of breast, endometrial, or ovarian malignancies,
and also-zn women that belong to a high socioeconomic ,
status.
The most significant risk factors for
endometrial cancer are bbesity and the presence of estrogen
unopposed by progesterone.
The inaccuracy in clinical staging of
endometrial carcinoma impedes optimal therapy and analysis
of treatment results. Unless-m~tastatic-or-systemic
disease is identified, the initial approach for all _
medically fit patients is currently a total abdominal
hysterectomy/bilateral salpingo=oophorectomy.
Adjunctive therapy, if needed; can be planned,
depending- on whether the surgical-pathologic findings ' '
indicate intrauterine only or extrauterine disease. The
patient may receive external beam radiation to the pelvis '
if pelvic nodes are positive and of external beam radiation
to the para-aortic fields if those nodes are positive.
Patients with other sites--of extrauterine disease may
VUO 96105833 ~ ~ ' ~. ~~ 1. ~ C~ pGT%fIS95110651
_3_
,
require whole abdominal irradiation. -Some patients may
need systemic therapy in addition to radiation therapy,
. depending on sites-of spread.
Patients with Stage II disease are at higher
risk for having extrauterine disease and recurrence. If
the cervix-is of normal size and grossly normal, one
appraach is an extrafasdial TAH/BSO with complete surgical
staging followed-,by postoperative irradiation. With gross
cervical involvement, two options are available. The first
is whole pelvic irradiation followed by one intracavitary
implant, which is then followed by a TAHIBSO and para-
aortic lymph-node--sampling. The second option-is a radical
hysterectomy. BSO, and pelvic and para-aortic
lymphadenectomy with irradiation tailored to the surgical
findings, if necessary.
In surgical Stage III disease,-primary surgery
with the use of a TAH/BSO with tumor debulking may be
attempted. Extrapelvic disease, depending on the site and
extent, may necessitate extended field irradiation,
systemic chemotherapy, or hormone therapy. Patients with
Stage III disease, by virtue of-vaginal or parametrial
extension, need a thorough metastatic survey and then
irradiation.
Most patients with Stage IV disease are best
treated with systemic therapy, which-includes hormones or
chemotherapy. Pelvic irradiationor hysterectomy is
reserved for palliative control purposes-:-
Patients with recurrent endometrial cancer in
the pelvis may-be treated with radiotherapy.
3.0 Unfortunately, the majority of these patients also have
" distant metastases as well. Isolated central recurrences
in the pelvis after irradiation are'rare_ However, if this
situation does occur, selected patients may be candidates
for pelvic exenterative.surgery. The majority of patients
with recurrent disease are treated with hormones or
chemotherapy.
W0961OS833 ;' ,~ PCTIUS95/10651
C.. 1
Progestins have been_used for.decades=to treat
recurrent endometrial cancer. The overall response to-:-
progestins is approximately 25$, although recent trials
demonstrate lower response gates, in the.range of 15 to
20~. Patients with endometrial carcinoma with
progesterone-positive and-estrogen-positive receptors have
a better response to endocrine therapy. Most patients-pith
positive receptors respond to progestins, whereas only-15~
with negative receptors respond. Medroxyprogesterone-:=:=_.
acetate (Provera) and megestrol-acetate (Megace) are the
agents most commonly used. Tamoxifen (NOlvadex) has also
been used to treat patients with recurring endometrial
cancer, and responses are usually seen in patients who -have
previously responded to progestins.
Several cytotoxic agents have activity for -.
endometrial cancer, but responses are short-lived, and the
treatment for advanced and recurrent-disease is considered
palliative. The two-molt active single agents are
doxorubicin and cisplatin_-- Many combinations of cytotaxic
agents have been used, but the results of multiagent
chemotherapy do not appear to be significantly better than
those of single-agent chemotherapy.
This invention provides methods of inhibiting .
25. endometrial cancer comprising administeringto a human in
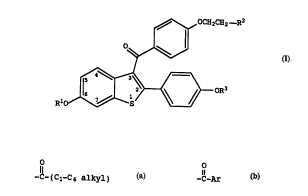
need thereof an effective amount of a compound of formula I
oCHZ-Rz
OR3
R
(I)
W 0 96105833 PCT/US95I10651
-5-
wherein R1 and R3 are independently hydrogen,
O O
~ ~~ a
-C-(C1-C6 alkyl) or -C-Ar wherein Ar is
optionally substituted phenyl; -
RZ is selected from the group consisting of -
pyrrolidino, hexamethyleneimino, and piperidino; and
pharmaceutically acceptable salts and solvates thereof.
The current invention concerns the discovery
that a select group of 2-phenyl-3-aroylbenzothiophenes
(benzothiophenes), those of-formula I, are useful for
inhibiting endometrial cancer:
The therapeutic and prophylactic treatments
provided by this invention are practiced by administering
to a human-in need thereof a dose of a compound of formula
I or a pharmaceutically acceptable salt-or-solvate thereof,
that is effQCtive to inhibit endometrial cancer.
The term "inhibit" includes its generally
accepted meaning which includes prohibiting, preventing,
resxraining, and slowing, stopping or--reversing
progression, severity or a resultant symptom. As such, the
present method includes both medical therapeutic and/or
prophylactic administration;-as appropriate.-'
Raloxifene is a-preferred compound of this
invention and it is the hydrochloride salt of a compound of
formula 1 wherein R1 and R3 are hydrogen and Rz is 1-
piperidinyl.
Generally, at least one compound of formula I
is formulated.with common excipients, diluents or carriers,
and compressed-into--tablets;-or-formulated -as-elixirs or
solutions for convenientoral administration, or
administered by the intramuscular or intravenous-routes.
The compounds can be administered transdermally, and may be
formulated as sustained release dosage forms and the like.
WO 96105833 ~ 19 8119
PCTIUS95I10651
~~ ~~.h5".~
-6-
The compounds used in the methods of the current
invention can be made according to'established procedures,
such as those detailed in U:S. Patent Nos. 4,133,814, ,
4,418,068; and 4,380;635 all of which are incorporated by
reference herein.' In general, the process starts with a ,
benzo[b7thiophene having a 6-hydroxyl group and a 2-(4-
hydroxyphenyl) group. The starting compound is protected,
acylated, and deprotected to form the formula I compounds.
Examples of the preparation of such compounds are provided
in the U.S. patents discussed above. The term "optionally
substituted phenyl" includes phenyl and phenyl substituted
once or twice with C1-C6 alkyl, C1-C4 alkoxy, hydroxy,
vitro, chloro, fluoro, or tri(chloro or-fluoro)methyl. -
The compounds used in the methods of this
invention-form pharmaceutically acceptable acid and base
addition salts with a wide variety of organic and inorganic
acids and bases and include the physiologically acceptable
salts which are often used in pharmaceutical chemistry.
Such salts are also part of this invention.-- Typical
inorganic acids used to form such salts include
hydrochloric, hydrobromic, hydroiodic, nitric, sulfuric
phosphoric, hypophosphoric and the like. Salts derived
from organic acids, such as aliphatic mono and dicarboxylic
acids, phenyl substituted alkanoic acids, hydroxyalkanoic
and hydroxyalkandioic acids, aromatic acids, aliphatic and
aromatic sulfonic acids, may also be used. Such
pharmaceutically acceptable salts thus include acetate,
phenylacetate, trifluoroacetate, acrylate, ascorbate,
benzoate, chlorobenzoate, dinitrobenzoate, hydroxybenzoate,
methoxybenzoate, methylbenzoate, o-acetoxybenzoate,
naphthalene-2-benzoate, bromide,- isobutyrate,
phenylbutyrate, i~-hydroxybutyrate, butyne-1,4-dioate,
hexyne-1,4-dioate, caprate, caprylate, chloride, cinnamate, '
citrate, formate, fumarate, glycollate; heptanoate,
hippurate, lactate, malate, maleate, hydroxymaleate,
malonate, mandelate, mesylate, nicotinate, isonicotinate;
WO96105833 '~' ~ 1 ~ g l 1 9 pCTlU895I10651
nitrate, oxalate, phthalate, teraphthalate, phosphate,
monohydrogenphosphate, dihydrogenphosphate, metaphosphate,
pyrophosphate, propiolate, propionate, phenylpropionate,
salicylate, sebacate, succinate, suberate, sulfate,
bisulfate, pyrosulfate, sulfite, bisulfite, sulfonate,
benzene-sulfonate, p-bromophenylsulfonate,
chlorobenzenesulfonate, ethanesulfonate, 2-
hydroxyethanesulfonate, methanesulfonate, naphthalene-1-
sulfonate, naphthalene-2-sulfonate, p-toluenesulfonate,
xylenesulfonate~ tartarate, and the like. A preferred salt
is the hydrochloride salt.
The pharmaceutically acceptable acid addition
salts-are typically formed by reacting a compound of
formula I-with an equimolar or excess amount of acid. The
reactants are generally combined in a mutual solvent such
as diethyl ether-Dr benzene. -The salt normally
precipitates out of.solution within about one hour to 10
days and can be isolated by filtration or the solvent can
be stripped off-by conventional-means.
Bases commonly used for formation of salts
include ammonium hydroxide and alkali and alkaline earth
metal hydroxides, carbonates, as well as aliphatic and
primary, secondary and tertiary amines,'aliphatic diamines.
Bases especially useful in the preparation of addition
salts include ammbnium hydroxide, potassium carbonate,
methylamine,-diethylamine, ethylene diamine and
cyclohexylamine.
The pharmaceutically acceptable salts generally
have enhanced solubility characteristics compared to the
compound from which they are derived, and thus are often
more-amenable to formulation as liquids or emulsions.
Pharmaceutical formulations-can be prepared by
procedures known in the art. For example, the compaunds
can be formulated with common excipients, diluents, or
carriers, and farmed into tablets, capsules, suspensions,
powders, and the like. Examples of excipients, diluents,
R'O 96/05833
PC'TIUS95110651
_g_
and carriers that are: suitable for-such formulations
include the following: fillers and extenders such as
starch, sugars, mannitol, and silicic derivatives; binding ,
agents such as carboxymethyl cellulose and other cellu3ose
derivatives, alginates~gelatin, and polyvinyl pyrrolidone; ,
moisturizing agents such as glycerol; disintegrating agents
such as calcium carbonate and sodium bicarbonate; agents
for retarding dissolutionsuch as paraffin; resorption -
accelerators such as quaternary ammonium compounds; surface
active agents such as cetyl alc9hol, glycerol monostearate;
adsorptive carriers such as kaolin and bentonite; and
lubricants such as talc, calcium and magnesium stearate,
and solid polyethyl glycols.
The compounds can also be formulated as elixirs
or solutions for convenient oral administration or as
solutions appropriate for parenteral administration, for
instance by intramuscular, subcutaneous or intravenous
routes. Additionally, the compounds are well suited to
formulation as sustained release dosage forms arid the like.
The formulations can be so constituted that they release
the active ingredient only or preferably in a particular
part of the intestinal tract, possibly over a period of
time. The coatings, envelopes, and protective matrices may
be made, for example, from polymeric substances or waxes.
The particular dosage of a compound of-formula I
required-toinhibit endometrial cancer according to this
invention will depend upon the-severity of the condition,
the route of administration, and related factors that will -
be decided by the attending physician. Generally, accepted
and effective daily doses will be from about 0.1 to about
100D-mg/day, and more typically..from about 50 to about 200
mg/day. Such dosages will be administered to a subject in
need thereof from once to about three times each day, or '
more often as needed, and for a-time to effectively treat
or prevent endometrial cancer.
F n
W 0 96105833 PCTJUS95/10651
_g _
2t is usually preferred to administer a compound
of formula 1 in the form of an acid addition salt, as is
customary in the administration of pharmaceuticals bearing
a basic group, such as the piperidino ring. For such
purposes the following oral-dosage forins are available:
Formulations
In the formulations which foI-low, "Active
ingredient" means-a compound of formula-I.
Formulation 1: Gelatin Capsules
Hard gelatin capsules are prepared using the following:
Inslredient Quantity (mc/capsule)
Active ingredient - ~ 0.1 -~10D0~~
Starch, NF D - 650
Starch flowable powder 0 - 650
Silicone fluid 350 centistokes 0 - 15
The ingredients are blended,-passed through a No. 45 mesh
U.S. sieve, and filled into hard gelatin capsules.
_Examples of specific capsule formulations of
raloxifene that have been made include those shown below:
Formulation 2: Raloxifene capsule
Ingredient ~- Quantity-(mc7Tcapsule)
Raloxifene 1
Starch, NF 112
Starch flowable powder - 225_3 ..
Silicone fluid 35D centistokes 1.7
w
219$119
j,,;;~:~ PCf/US95I10651
-10-
Formulation 3: Raloxifene capsule
Ingredient -Quantity (m /capsule)
Raloxifene
Starch, NF - 108
Starch flowable powder 225.3
Silicone fluid 350 centistokes 1.7
Formulation 4: Raloxifene capsule
Ingredient - -uantitv(ma/capsule)
Raloxifene 10 -.
Starch, NF - - 103
Starch flowable powder 225.3
Silicone fluid 350 centistokes 1 7
Formulation 5: Raloxifene capsule -
Ingredient - - uantity (mo/capsule) -
Raloxifene Sp-
Starch, NF 150 -
Starch flowable powder 397
Silicone fluid 350 centistokes 3.0
The specific formulations above may be changed
in compliance with the reasonable variations provided.
A tablet formulation is prepared using--the
ingredients below:
w
WO96105833 '~. PCT/US9511065I
-11-
Formulation 6: Tablets
Ingredient - Quantity (m /tablet)
Active ingredient 0.1 - 1000
Cellulose, microcrystalline 0 - 650
Silicon dioxide;- fumed 0 - 650
Stearate acid 0 - 15
The components are blended and compressed to form tablets.
Alternatively, tablets each containing 0.1 -
1000 mg of Active ingredient are made up as follows:
Formulation 7: Tablets
Ingredient - - Quantity-(mc/tablet)
Active ingredient 0.1 - 1000
Starch &5
Cellulose, miorocrystalline - 35~
Polyvinylpyrrolidone
(as 10$ solution in water)
Sodium carbaxymethpl cellulose~ 4_5
Magnesium stearate 0.5
Talc 1
The Active ingredient, starch, and cellulose are
passed through a No. 45 mesh U.S. sieve and mixed
thoroughly. The solution of polyvinylpyrrolidone is mixed
with the resultant powders which are then passed through a
No. 14 mesh U.S. sieve. The granules so produced are dried
at 50°-60° C and passed through a No. 18 mesh U.S. sieve.
The sodium carboxymethyl starch, magnesium stearate, and
talc, previously passed through a No. 60 U.S. sieve, are
then added to the granules which, after-mixing, are
compressed on a Lablet machine to yield tablets.
R'O 96105833 ~ ~ ~~ , , PCTIUS95110651
-12-
Suspensions each containing 0.1 - 1000-mg of
Active ingredient.per 5 mL dose are--made as follows:
Formulation 8: Suspensions
Ingredient uantitv (ma/5 ml)
Active ingredient 0.1 - 1000 mg -
Sodium carboxymethyl cellulose 50
mg
Syrup 1.25 mg
Benaoic acid solution - 0.10 mL
Flavor -
q.v.
Color
q.v.
Purified water to
The Activs ingredient is passed-through a No. 45 mesh U:S.
sieve and mixed with the sodium-c3rboxymethyl cellulose and
syrup to form a smooth paste. The benzoic acid solution,
flavor, and color are diluted with some of the water and
added, with stirring. Sufficient water is then added to
produce the required volume.
Continuous cultures of transformed endometrial
cells reflective o~ en.dometrial.carcinoma are maintained in
culture. Estrogen receptor containing cells (such as the
Ishikawa line) are assessed for proliferation in response
to estrogen and compounds of Formula I. Responsivenessis
assessed by monitoring transcription of known
estrogen/anti-estrogen-regulated genes such as progesterone
receptor, PS2 and others.
Induced Animal Models - Endometrial carcinoma'is
induced by injections of estrogenic substances such as 17-
(3-estradiol or DES during neonatal development. The
WO96l05833 - ~ j~~i~''Jt ~ p~/pS95110651
-13-
presence of carcinoma in the adult animal is confirmed by
biopsy of affected animals and/or sacrifice and biopsy of
syngeneic animals identically treated. After evaluation of
the lesions, affected animals are treated with estrogen,
compounds of formula I, or progestins for 1-8 weeks. After
treatment, lesions of surviving animals are examined for
progression, regression or stasis.
Five to fifty women are selected for the
clinical study. The women suffer from endometrial cancer.
The study has a placebo control group, i.e., the women are
divided into two groups, one of which receives a compound
of formula 1 as the active agent and the other receives a -
standard treatmeht ~or endoinetrial cancer_ Women in the
test group receive-between 50-200 mg of the drug per day by
the oral route. They continue-this therapy for 3-12
months. Accurate records are kept as to the symptoms and
status of the cancer in both groups and at the end of the
study these results are--compared. The results are compared
both between members of each group and also the results for
each patient are compared to the status reported for ea-ch
patient before the-study began.. _.
ASSAY 4
A total of 251 healthy, postmenopausal women are
recruited. Each subject has had her last menstrual period
more than 6 months but less than 6 years prior to beginning
the treatment phase of the study. Postmenopausal status of
each subject is -confirmed before beginning treatment by
- serum estradiol <120 pmol/L and by F5H >30 IU/L. Subjects
will not have been-treated with-estrogen-over at least the
° last 3 months before the study and have never been treated
with fluoride, calcitonin, or bisphosphonate. Subjects are
in good health and range in age from 46.-to 60 years.
R'O 96105833 ~ . PCT/US95I10651
-14-
The-study is a multi-center, randomized,
controlled, double-blind study. Qualified subjects who
consent are randomized to one c~f four treatment-groups:-- ,
placebo, a compound of formula I 200 mg once daily, a '-
compound offormula I 600-mg once daily, or estrogen-0.625 ,
mg once daily. All subjects also receive daily oral
calcium carbonate supplements 0520 mglday elemental
calcium). All medications and supplements are taken daily
in the morning during the 8-week treatment period_ Once
treatment is completed (Visit 5), each suject receives
Provera~ 5 mg/day for 12 days.
Using a Pipelle-catheter, a uterine biopsy is
performed at baseline and after.8 weeks of treatment. The -
biopsies are performed in a routine manner and the tissue
specimens are placed in 10~ buffered formalin. Specimens
are retrieved by pouring them into tissue paper ~iltersand
then are grossly examined and classified as to appearance
(color, texture, and consistency) and volume. Standard
histologic processing into paraffin blocks is used and the
tissues are serially sectioned onto a minimum of- two slides
which results in serial strips of 6 to 20 cross sections.
Since subjects with clinically significant endometrial -
abnormalities are to be excluded from the study or are
discontinued from the study if they develop abnormalities,
the biopsies are evaluated immediately for a descriptive
diagnosis, This is performed by one of two pathologists
and immediately reported tothe-clinical physicians. The
primary purpose ofthe biopsies is-to deteziuine the degree
of morphologic estrogenic effect of study treatment. Two .
patholor~ists are trained to-read the biopsies by reviewing
a series of-Pipelle biopsies obtained outside-the study '
that represent the full spectrum of endometrial morphology.
Using standard morphologic criteria associated with -- '
estrogen-induced proliferation, a scoring system is devised
to quantitate this estrogenic effect and-include-the more
subtle changes that may be encountered. Ten of these
WO96105833 2198 i 19 PCT/US95/10651
~t y
outside cases are then scorEd with this system by each
pathologist, and, the cases are reviewed together to assure
uniform understanding and use of the criteria
After the
.
first twenty cases from the baseline biopsies in the study
are blindly scored by each-pathologist, the scores are
reviewed to verify proper use of the system. The
.
pathologists evaluate the biopsy samples, for the following
components: 1) specimen adequacy, 2) glandular morphology,
3) stromal morphology, and 9) other changes. Additional
findings are entered as textual comments. Point scores are
generated from the glandular and stroznal morphologic
features and are totaled and graded on a 4-point
estrogenicity scale where a grade of--0- indicates typical
postmenopausal endometrium and a grade of 2 indicates a
marked estrogenic effect. Total scores for both
pathologists are averaged and then assigned a final grade
of 0 to 3_ Scoring occurs well after the initial immediate
diagnosis and usually 10 to 20 cases are scored
sequentially.
It is expected that the rate of scant tissue is
relatively high on the initial biopsy since the typical
postmenopausal endometrium is inactive and consists of a
very shallow (5 mm or less) tissue lining and the Pipelle
biopsy is a limited, blind biopsy method. Because
. endometrial glands are required to score features of
glandular and stromal morphology, the final biopsy must
have-contained glands before any conclusions can be drawn
in individual subjects. Specimen adequacy is defined as
follows:
If no tissue or debatable tissue of endometrial
' origin is present, the specimen is-deemed
inadequate and not included in the evaluation.
0
R'O 96!05833
PCTIU595I10651
21981 ~ 9
-16-
If multiple fragmenLS of endnmetrial Surface
epithelium are obtained, the -specimen-can not be
scored= However the biopsy is deemed adequate
and is assigned a grade of D nn the 4-point
scale indicating no estrogen effect. _
If disrupted endometrium with glands are
obtained, the biopsy is adequate and is scored
for the glandular and Stromal='features.-
1D
If intact endometrial tissue is obtained, the-
biopsy is adequate and is scored for the
glandular and stromal changes.- In addition, the
volume of the tissues is taken into account as
an indication of estrbgen effect.
Glandular morphology is the primary scoring
factor for adequate biopsy specimens. Stromal morphola~y
is the secondary scoring factor-for adequate biopsy
2D specimens. Tables l and 2 display the features to be used
to score=each specimen that_have glands aridlor-stroma -
present. Four features are used to classify the glands:
shape, -cellular nuclear to cytoplasmic cross sectional
areas, nuclear pseudostrati~ication, and mitotic activity.
WO 96105833 ~ ~ ~ ~ r~ ~ ~ PCTlUS95/10651
-17-
Table 1. Glandular Features: Scoring of Estrogenicity
Estrogenic Effect/POint Value
No Limited High
Glandular Estrogenicity Estrogeneicity Estrogenicity
Feature (0 Points) (1 Point) (2 Points)
Shape Small, Open, straight Open, cystic,
tubular tortuous
straight
Nucleus-to- Very High Moderate (75$ Low (<50~)
cytoplasm ,(> 75~) to 50$)
ratio
Nuclear None Limited Diffuse
pseudostrati
-fication
Mitoses None Bare Scattered to
many
Note: At least 20 gland profiles are used to grade for
mitotic activity (four serial sections of scant specimens).
In more scanty specimens a minimum of 20 gland
profiles in-serial sections are viewed before concluding no
mitoses are evident. In Table 2 the stromal and "other"
features are listed. Four features are also used to
classify the stroma: density, mitoses, metaplastic changes
in epithelia, and tissue volume.
C
W096105833 ~~ . , PCfIUS95110651
-18-
Table-2. Stromal Features: Scoring of Estrogenicity
Estrogenic Effect/POint Value . t
No Limited High
Stromal Estrogenicity Estrogeneicity Estrogenicity
Feature (0 Points) (1 Point) (2 Points)
Compact, Loosely Edematous
Density fibrous cellular
Mitoses None - Rare- Few/Many
Metaplasiag None Rare Scattered,
diffuse-
Tissue Volumeb Disrupted or Moderate, much Abundant,
few intact being intact intact._
aMetaplasia includes tubular, eo-sinophilic, and squamous
type.
bLJSed only if glands show some estrogenic effect (1 or 2
points).
Morphologic features that indicate a lack of estrogenicity
generate a score of 0 points and features indicating a
limited or significant estrogenic effect generate a score
of 1 to 2 points, respectively. Using this approach, a
biopsy can receive between 0 to 16 points.
In-addition to glandular and stromal morphology,
and other-important morphologic features including
progestational effect, inflammatory processes-, breakdown
bleeding, polypoid-growth, or other pathologic findings are
described but are not included in the scoring of
proliferative effects since the other changes are,primarily
nonproliferative.
The sum of the scores-obtained from grading the '
glandular and stromal morphology features resultin a 4-
point estrogenicity grading scale which is assigned to each
sample as follows:
W096105833 ~ 1 ~ PCT/US95110651
En v'
-19-
Grade 0 = 0 to 3 points Typical postmenopausal
endometrium with little or-no estrogenic effect
Grade 1 = 4 to 6 points Definite but limited
estrogenic effect
_._.. . _.
Grade 2 = 7 to 10 points Moderte estrogenic
ef f ect
Grade 3 _ >10 points Marked estrogenic -
effect
As noted earlier, if biopsy specimens contain
multiple fragments of endometrial surface epithelium, those
specimens are assigned a grade of 0.
For each biopsy, there are eight scores: four
assessments of the glandular morphology, two assessments of
the stromal morphology (density and mitoses scores are
combined and metaplasia and tissue volume are combined for
statistical analyses), the sum of these six scores and the
grade as defined above.
Intraclass correlation coefficients are
calculated to assess agreement between the two readers on
the sum of the scores obtained at baseline and at 8 weeks
(Fleiss, JL (1981) ,S a ~ s a~ M hod or R- c -nd
P1'ODOr ion . New York: John Wiley and Sons, p. 218.]
The baseline, week 8 and change-from-baseline to
week 8 scores for each of-the eight scores are analyzed for
treatment differences using Cochran-Mantel-Haenazel
statistical techniques adjusting for investigator [Landis,
RJ Heyman, ER and Rock, GG (1978). "Average Partial
' Association in Three-Way Contingency Tables: A Review and
Discussion of Alternative Tests". International
Statistical Review 46:237-254.].
The occurrence-of endometrial glands in the
biopsy tissue is evaluated at baseline and 8 weeks for
treatment differences using the chi-square test.
R'0, 96105833 ~ PCTIUS95I10651
-20-
Pairwise treatment comparisons between each
active treatment and placebo are performed if. the overall
treatmentdifference is statistically significant. ,
Statistical significance is judged at a two-sided 0.05 -
level of significance. A1 statistical analyses use the SAS ,
system [SAS Institute Inc. (1989),-SASISTAT Users Guide,
Version 6, Fourth Edition, Volumes 1 and 2, Gary, NC: SAS
Institute Inc_7
A positive-result in-this assay is the reduction
of the score for glandular mitoses indicating a decrease in
cell replication-relative to placebo:- _
Table 3--illustrates important results of the_
study.
Table 3. Mean (t SEM) Scores for Glandular Fatures At
Endpoint
Placebo Raloxifene Raloxifene Estrogen
(n 53) 200 mg 600 0.625
= mg mg
Variable (n 54) (n 54) (n 47)
= = =
Shape 0.44t 0.08 0.58t 0.07 0.51t 0_06.1_37t 0_06*
Pseudostrat-0.64t 0.10 0.57t 0.06 0.56Ø06 1_680.07*
- -
ification
Mitoses 0.19t 0_05 0.05t 0.02* 0.07t0_03f 0.98t D_OS*
Nucleus: 0.48t 0.09 0.58t0_D-fi 0.5810.05 1.56t D.OS*
-
Cytoplasm
* Statistically significantly different-from placebo, two-
tailed test (p<.050)
f Marginally significantly different from placebo, two-
tailed test (p = .053)
VVO 96105833 ~~ ~ PC1'IUS95110651
, , C- .
-21-
Utility of the compounds of formula I is
illustrated by ~he positive impactthey have in at least
one of the assays described above.