Note: Descriptions are shown in the official language in which they were submitted.
2198266
WO 97/03070 PCT1JP9610184I
1
DESCRIPTION
NOVEL BENZIMIDAZOLE DERIVATIVES HAVING CGMP-PHOSPHODISTERASE INHIBITING
ACTIVITY
TECHNICAL FIELD
The present invention relates to novel benz-
lmidazole derivatives and salts thereof.
BACFCGROiTND ART
Proliferation of the smooth muscle cells of
the blood vessel induces hypertrophy of the intima of
the blood vessel and, from a long-term view point, it
causes, arteriosclelerotic diseases such as myocardial
infarction, cerebral infarction and the like.
Moreover, from a short-term view paint, it induces
reobstruction of the blood vessel after treatments of
percutaneous transluminal coronary angioplasty (PTCA),
stent and/or aterectomy. In consideration of the
present situation, the therapeutic effects of conven-
tional medicines used therefor are thought of as within
certain limits, because they are classified as agents
of indirectly effective for curing the diseases induced
by the promotion factors of hypertrophy of the interim,
for example hyperlipidemia, hypertension and the like.
~ 20 Therefore, development of essentially effective
medicines therefor have been eagerly expected.
Generally, it is known that proliferation of
i
CA 02198266 2005-05-11
25717L-773
- 2 -
the smooth muscle cells is related to the influence of
cyclic guanosine 3',5'-monophosphate (cGMP). In this
connection, pharmaceutical preparations of nitro group-
containing compound known as coronary vasodilators
activate the enzymatic activity of guanylate cyclase,
and accentuate the production of cGMP, and also inhibit
the proliferation of the cells. However, the pharma-
ceutical preparations of nitro group-containing com-
pound are scarecely used for treatment of arterio-
sclerotic diseases which need to be administered for a
long period of time, because the effect of the
pharmaceutical preparations of nitro group-containing
compound can be sustained only for a quite short time,
and when they are administered repeatedly, then the
tolerance against such pharmaceutical preparations may
occur. On the other hand, in recent years, there
have been reported several pharmaceutical preparations
which can increase the concentration of cGMP by
inhibiting the enzymatic activity of the enzyme [i.e.,
cGMP-PDE (cGMP-phosphodiesterase)] for decomposition of
cGMP. However, there have not been suggested the
activity for inhibiting proliferation of the smooth
muscle cells performed by pharmaceutical preparations.
The guanine derivative which was reported recently in
an academic conference (IBC's International Conference
on RESTENOSIS, 1994, U. S. A.) is the only known com-
pound having both effects for inhibiting the enzymatic
activity of cGMP-PDE and for inhibiting proliferation
CA 02198266 2005-05-11
25711-773
- 3 -
of the smooth muscle cells.
In consideration of the present status of
such pharmacotherapy, and referring to the fact that
cGMP relates to proliferation of the smooth muscle
cells of the blood vessel, the present inventors have
made an extensive research work regarding pharmaceuti-
cal preparations having the activity for inhibiting
proliferation of the smooth muscle cells of the blood
vessel which acts directly to the cells thereof, and
can be used as the essential agent for treating
arteriosclerotic diseases.
As the results, the present inventors have
found the fact that the objective compounds, having the
activity for inhibiting proliferation of the smooth
muscle cells of the blood vessel, do exist among
benzimidazole derivatives having property for inhibit-
ing the enzymatic activity of cGMP PDE, thus the
present invention has been completed.
Related art references which disclose
compounds having chemical structural formulas similar
to those disclosed in the present invention are JP-A-2-
306916, U.S. Patent No. 4,886,803, U.S. Patent
4,551,421, JP-A-62-246546, JP-A-4-346974, JP-A-61-
167952, U.S. Patent No. 4,994,477, U.S. Patent No.
5,098,924, EP-A1-0560407, EP-A1-0407217, JP-A-64-65551,
JP-A-1-96645, and JP-A-7-133224, however, those
references neither disclose nor suggest benzimidazole
derivatives of the present invention, and do not touch
CA 02198266 2005-05-11
2571:L-773
- 4 -
on inhibition of the enzymatic activity of cGMP PDE at
all which benzimidazole derivatives of the present
invention possess.
Furthermore, references JP-A-5-222000, WO-A-
93-07124, and WO-A-94-22855 disclose structurally similar
compounds having inhibition of the enzymatic activity
of cGMP PDE, however, those references neither disclose
nor suggest benzimidazole derivatives of the present
invention.
DISCLOSURE OF THE INVENTION
Benzimidazole derivatives of the present
invention are novel compounds which have not been
reported in any literature, and are represented by the
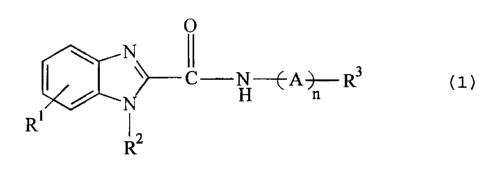
~15 general formula (1) as follows:
0
\ \
1 ~.%~N H n ( 1 )
R Rz
[wherein R1 is a hydrogen atom or a halogen atom;
RZ is a phenyl-lower alkyl group;
R3 is a heterocyclic group selected from the
group consisting of an indolyl group, indolinyl group,
1H-indazolyl group, 2(1H)-quinolinonyl group, 3,4-
dihydro-2(1H)-quinolinonyl group and 3,4-dihydro-
1,4(2H)-benzoxazinyl group, the heterocyclic group may
WO 97!03070 ~ 219 $ 2 6 6 pCT/~6/01841
have 1 to 3 substituents selected from the group
consisting of: a group of the formula -B-R', (B is a
lower alkylene group; R° is a 5- to 11-membered
saturated or-unsaturated heterocyclic group of single
5 ring or binary ring, having 1 to 4 hetero atoms
selected from the group consisting of a nitrogen atom,
oxygen atom and sulfur atom, (said heterocyclic group
may have 1 to 3 substituents selected from the group
consisting of a halogen atom, a lower alkyl group, a
lower alkoxy group and oxo group) or a group of the
formula -NRSR6 (RS and R6 are each the same or dif-
ferent, and a hydrogen atom, a lower alkyl group, a
cycloalkyl group, a pyridylcarbonyl group, an
isoxazolylcarbonyl group which may have 1 to 3 lower
alkyl groups as the substituents, a pyrrolylcarbonyl
group or an amino-substituted lower alkyl group which
may have a lower alkyl group as the substituent;
further RS and R6 may form 5- to 6-membered saturated
heterocyclic group by combining to each other, together
with the adjacent nitrogen atom being bonded thereto,
further with or without other nitrogen atom or oxygen
atom; said heterocyclic group may have 1 to 3
substituents selected from the group consisting of a
hydroxy group and a phenyl group));
a lower alkenyl group; a lower alkoxycarbonyl group;
a phenoxy-lower alkyl group which may have cyano group
as the substituents; a halogen-substituted lower alkyl
group; and a lower alkoxycarbonyl-substituted lower
;~; ~~ ; m.. 2198266
W0 97/03070 PCT/JP96/01841
6
alkyl group;
A isa lower alkylene group; and
n
~1 1S 0 Or 1 . ~ .
The benzimidazole derivatives of the present
invention possess weak activity for inhibiting the
enzymatic action of cAMP PDE, while they possess strong
activity for inhibiting the enzymatic action selec-
tively against cGMP PDE.
The benzimidazole derivatives of the present
invention possess activity for inhibiting proliferation
of the smooth muscle cells, especially they possess
strong activity for inhibiting proliferation of the
mesenchymal cells. The activity for inhibiting proli-
feration of the smooth muscle cells was determination
by measuring the activity for inhibiting proliferation
of rat A10 cell {in vitro), and confirmed. The rat
A10 cell is a cell strain derived from smooth muscle
of rat embryo thoracic aorta, and the biological
property thereof i.s described in Exptl. Cell Ress.
Vol. 98, (1976), pages 349-365, (B. W. Rimes and B. L.
Brandt). The activity for inhibiting proliferation
of the fibroblasts and mesangial cells were-determined
by measuring the activity for inhibiting proliferation
of human fibroblasts or rat mesangial cells in place of
rat A10 cell, by the procedure similar to that of
described in the same literature, and confirmed strong
activities for inhibition. The activity for inhibiting
proliferating of T-cells was determined by procedure of
i
CA 02198266 2005-05-11
25711-773
- 7 -
the experiment as described in "Current Protocol in
Immunology" Chapter 3, page 12 [compiled by Coligan,
et al., (1991), (published by Willy Interscience)],
and confirmed. As mentioned above, the benzimidazole
derivatives of the present invention also possess
immunosuppressive activity based on the activity for
inhibiting proliferation of T-cells.
The benzimidazole derivatives of the present
invention possess activity for inhibiting synthesis and
secretion of collagen. Thus, the activity was con-
firmed by culturing human fibroblast and by method
as described in Clin. Invest.,Vol. 83, (1989), pages
1160-1167 (K. Mackay, et al.) and by applying assay
method as described in Calcif. Tissue Int., Vol. 35,
(1983), pages 542-548 (M. Kumegawa, et al.).
Furthermore, the benzimidazole derivatives
of the present invention also possess antiinflammatory
activity. In case of using as a drug for external use,
the antiinflammatory activity of banzimidazole deriva-
tive was confirmed by the procedures of experiment as
described in Agents Actions, Vol. 26, (1989), page 319
(Carlson, et al.) [Test animals and administration
method were partly revised]. The inflammatory
activity of benzimidazole derivative of the present
invention was confirmed in detail as described in (6)
Determination of the activity for inhibiting TPA-
induced inflammation as described later in this
specification.
a
~''~'-:~ r~;~':.'- 2198266
WO 97/03070 PCT/JP96101841
8 _
Proliferation of the smooth muscle cells of
the blood vessel is the major cause of arterio-
sclerosis. [Nature Vol. 362, (1993), pages 801-809
(Russell Ross)]. Moreover, proliferation of fibroblast
and synthesis and secretion of collagen are also the
causes of arteriosclerosis. [Am. J. Pathol., Vol. 125,
(I986), pages 191-207 (A. M. Gown, et al.)]. In the
case of diabetes mellitus, the smooth muscle cells show
tendency of abnormal proliferation. [Eu. J. Clin.
Invest. vol. 23, (1993), pages 84-90 (M. Kawano, et
al.)]. Proliferation of fibroblast as well as
synthesis and secretion of collagen induce pulmonary
fibrosis. [Am. Rev. Respir. Dis.-, Vol 138, (1988),
pages 703-708 (G. Raghu, et al.); and CHIRYOU-GAKU
(Therapeutics), Vol. 28, (1994), pages 62-66,
(Toshihiko Sakai, et al.). Inhibition of the enzymatic
activity of cGMP PDE inhibits aggregation of platelets,
as well as effective for-prevention and treatment of
allergy, asthma, psoriasis and formation of thrombus.
[Trends Pharmacol. Sci., Vol. 12, (1991), pages 19-27
(C. D. Nicholson, et al.)]. Increase of the amount of
cGMP lowers blood-pressure. [Circ. Res., Vol. 74,
(1994), pages 416-421 (A. Koller, et al.) and DOHMYAKU
KOUKA NO BUNSHI IGAKU (Molecular Medicine of
Arteriosclerosis) (compiled by Tohru Kita, 1994,
published by YOHDO-SHA, pages 27-28 and 147-I64)].
As explained above, according to the pharma-
cological activities performed by the benzimidazole
2198266
W0 97103070 PCT/JP96Ni841
9
derivatives of the present invention, they can be
applied to-prevention and treatment of various diseases
for example, diseases related to cGMP, diseases induced
by proliferation of the smooth muscle cells and
fibroblast, diseases related to synthesis and secretion
of collagen, and they can be further applied to'
a
prevention and treatment of skin diseases relating to
immune and inflammation. As to these diseases, there
can be exemplified, reobstruction of the blood vessel
occurred after the treatments of PTCA, anginoplasy and
operation of bypass, arteriosclerotic diseases [e. g.,
angina pectoris, myocardial infarction, cerebral
infarction, cerebrovascular dementia, transient
ischemic attack (TIA), disfunction of peripheral
circulation, complication of diabetes mellitus,
atherosclerosis, arteriolosclerosis, fibrous hyper-
trophy of artery and the like], cell proliferative
diseases other than arteriosclerotic diseases (e. g.,
renal disease, asthma, bronchitis, proliferative
dermatitis, keloidosis, hyperplastic scar, glaucoma and
the like), pulmonary fibrosis, collagen disease,
psoriasis, allergic diseases (especially, atopic
dermatitis and chronic contactive dermatitis), other
dermatitises, hypertension, organopathy induced by
hypertension,- cardiac failure, hypercardia and the
Like.
The benzimidazole derivatives of the present
invention can be administered orally and non-orally by
',' ~.,,~r,' '~ s'c~ 219 8 2 6 b
WO 97/03070 PCT/JP96/01841
making them as in the form-of suitable pharmaceutical
preparations, and in case of apply to dermatic di-
seases, a pharmaceutical preparation made for external
use can be applied by coating directly onto the
5 diseased part. The effectiveness of the benzimidazole
derivatives of the present invention applied in the
form of pharmaceutical- preparations for external use
was confirmed by conducting experiments as described in
J. Dermtol. Sci., Vol. 8, (1994), page 54 (Kitagaki, et
10 al.) and Agents Actions, Vol. 26, (1989), page 319
(Carlson, et al.). (Animal test and method of admini-
stration were partly revided.] Detailed explanation
relating to activity for inhibiting TPA-induced edema
will be disclosed in Pharmacological Test (6), as
mentioned later in this specification. Models in
Dermatology, Vol. 1, pages 50-58 [(complied by H. I.
Maibach, N. J. Lowe) (published by Krager, Basel) 1985)
describes that the experiment as described in the
latter literature (Agents Actions by Carlson, et al.)
is a model of psoriasis.
As can be seen from the result of experiment
conducted by using rat carotid paratripsis model [Am.
J. Pathol., Vol. 141, (1992), pages 685-690 (U. Zeymer,
et al.) the benzimidazole derivatives of the present
invention are effective not only in vitro test but
also in vivo test.
In case of oral administration, the benz-
imidazole derivatives of the present invention can be
2198266
W097103070 PCT/JP96/0184I
11
attained to keep blood concentration sufficient to
manifestation of pharmacological affect, and the number
of oral administration per day can be set as small
number, because the duration of blood concentration and
action time thereof can be kept for quite long time.
The benzimidazole derivatives-of the present
invention are characterized by having quite weak
activity of acute vasodepression effect; systolic
potentiation effect and cardiac rate increasing effect
at the dosage of showing inhibitory effect of cGMP PDE,
and at the dosage of showing inhibitory effect of
proliferation of the smooth muscle cells.
The benzimidazole derivatives of the present
invention do not show strong toxicity even though they
are administered for short-term or administered
continuously for long-term.
As to the benzimidazole derivatives of the
present invention represented by the general formula
(1), there are various types of derivatives are
included as follows:
O1 Benzimidazol derivatives or salts thereof
represented by the general formula (1), wherein RI is a
hydrogen atom; RZ is the same as defined in the general
formula (I) as mentioned above; n is 0, and R3 is an
indolyl group-(wherein the substituents of the indolyl
group are the same as defined in the general formula
(1) as mentioned above).
O2 Benzimidazol derivatives or salts thereof
2198266
WO 97/03070 PCTIJP96/01841
12
represented by thegenera7~ formula (1), wherein R1 is a
hydrogen atom; RZ is the same as defined in the general
formula (1) as mentioned above; n is 0, and R' is an
indolinyl group (wherein the substituents of the
indolinyl group are the same as defined in the general
formula (1) as mentioned above).
Benzimidazol derivatives or salts thereof
represented by the general formula (1), wherein R' is a
hydrogen atom; RZ is the same as defined in the general
formula (1) as mentioned above; nis 0, and R3 is a
1H-indazolyl group (wherein the subsituents of the-
1H-indazolyl group are the same as defined in the
general formula (1) as mentioned above).
~ Benzimidazol derivatives or salts thereof
represented by the general formula (1), wherein RI is a
hydrogen atom; RZ is the same as defined in the general
formula (1) as mentioned above; n is 0, and R' is
2(1H)-quinolinonyl group (wherein the substituents of
the 2(1H}-quinolinonyl group are the same as defined in
the general formula (1) as mentioned above).
- Benzimidazol derivatives or salts thereof
represented by the general formula (1), wherein R1 is a
hydrogen atom; R~ is the same as defined in the general
formula (1} as mentioned above; n is 0, and R' is
3,4-dihydro-2(1H)-quinolinonyl group (wherein the
substituents of the 3,4-dihydro-2(1H)-quinolinonyl
group are the same as defined in the general formula
(1) as mentioned above).
~2~ 9a2ss
WO 97103070 PCT/JP96/OI841
13
Benzimidazol derivatives or salts thereof
represented by the general formula (1), wherein R1 is a
.
hydrogenatom; Rz is the same as defined in the general
formula (1) as mentioned above; n is 0, and R3 is
3,4-dihydro-1,4(2H)-benzoxazinyl group (wherein the
substituents of the 3,4-dihydro-1,4(2H)-benzoxazinyl
group are the same as defined in~the general formula
(1) as mentioned above).
Benzimidazol derivatives or salts thereof
represented by the general formula (1), wherein R1 is a
halogen atom; Rz is the same as defined in the general
formula (1) as mentioned above; n is 0, and R' is an
indolyl group (wherein the substituents of the indolyl
group are the same as defined in the general formula
(1) as mentioned above).
~ Benzimidazol derivatives or salts thereof
represented by the general formula (1), wherein Rt is a
halogen atom; R2 is the same as defined in the general
formula (1) as mentioned above; n is 0, and R3 is an
indolinyl group (wherein the substituents of the
indolinyl group are the same as defined in the general
formula (1) as mentioned above).
9O Benzimidazol derivatives or salts thereof
represented by the general formula (1), Wherein R' is a
t 25 halogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 0, and R3
is a 1H-indazolyl group (wherein the substituents of
the 1H-indazolyl group are the same as defined in the
v r. .,
~~~~~~~~ ~ :> 2198266
WO 97/03070 PCT/JP96/01841
14
general formula (1) as mentioned above).
Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
Ri is a halogen atom; R~ is the same as defined in the
5 general formula (1) as mentioned above; n is 0, and R3
is a Z(1H)-quinolinony]. group (wherein the substituents
of the 2{1H)-quinolinonyl group are the same as defined
in the general formula (1) as mentioned above).
11 Benzimidazol derivatives or salts
10 thereof represented by the general formula (1), wherein
R1 is a halogen atom; RZ is the same as defined in the
general formula {1) as mentioned above; n is 0, and R'
is a 3,4-dihydro-2(1H)-quinolinonyl group (wherein the
substituents of the 3,4-dihydro-2(1H)-quinolinonyl
group are the same as defined in the general formula
(1) as mentioned above).
12 -. Benzimidazol derivatives or salts ,
thereof-represented by the general formula (1), wherein
R1 is-a halogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 0, and R3
is a 3,4-dihydro-1,4(2H)-benzoxazinyl group (wherein
the substituents of the 3,4-dihydro-1,4(2H)-
benzoxazinyl group are the same as defined in the
general formula (1) as mentioned above).
~3 Benzimidazol derivatives or salts .'
thereof represented by the-general formula (1), wherein
R1 is a hydrogen atom; Rz is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
W O 97103070 ~ ~ 219 8 2 6 6 pCT/JP96/0184I
is an indolyl group (wherein the substituents of the
indolyl group are the same as defined in the general
formula (1) as mentioned above).
~4 Benzimidazol derivatives or salts
5 thereof represented by the general formula (1), wherein
R1 is a hydrogen atom; RZ is the same as defined in the
general formula (1) as mentioneri above; n is 1, and R3
is an indolinyl group (wherein the substituents of the
indolinyl group are the same as defined in the general
10 formula (1) as mentioned above).
15 Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
R1 is a hydrogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
15 is a 1H-indazolyl group (wherein the substituents of
the 1H-indazolyl group are the same as defined in the
general formula (1) as mentioned above).
1~6 Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
R1 is a hydrogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
is a 2(1H)-quinolinonyl group (wherein the substituents
of the 2(1H)-quinolinolyl group are the same as defined
in general formula (1) as mentioned above).
. 25 1~ Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
Rl.is a hydrogen atom; Rz is the same as defined in the
general formula (1) as mentioned above; n is 1, and R'
z,~i~ .-y 219266
WO 97/03070 PCT/JP96/01841
16
is a 3,4-dihydro-2(1H)-quinolinonyl group (wherein the
substituents of the 3,4-dihydro-2(1H)-quinolinonyl
group are the same as defined in the general formula
(1) as mentioned above).
18 Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
R1 is a hydrogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
is a 3,4-dihydro-1,4(2H)-benzoxazinyl group (wherein
the substituents of the 3,4-dihydro-1,4(2H)-
benzoxazinyl group are the same as defined in the
general formula (1) as mentioned above).
Q Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
RI is a halogen atom; Rz is the same as defined in the
general formula (1} as mentioned above; n is 1, and R'
is an indolyl group (wherein the substituents of the
indolyl group are the same as defined in the general
formula (1) as mentioned above).
~0 Benzimidazolderivatives or salts
thereof represented by the general formula (1), wherein
R1 is a halogen atom; Rz is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
is an indolinyl group (wherein the substituents of the
indolinyl group are the same as defined in the general ~
formula (1) as mentioned above).
Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
WO 97!03070 ~ ~ ~~ PCT/JP96/0184I
17
R1 is a halogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
is a 1H-indazolyl group (wherein the substituents of
the 1H-indazolyl group are the same as defined in the
general formula (1) as mentioned above).
22 Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
RI is a halogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
is a 2(1H)-quinolinonyl group (wherein the substituents
of the 2(1H)-quinolinonyl group are the same as defined
in the general formula (1) as mentioned above).
23 Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
Ri is a halogen atom; Rz is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
is a 3,4-dihydro-2(1H)-quinolinonyl group (wherein the
substituents of 3,4-dihydro-2(1H)-quinolinonyl group
are the same as defined in the general formula (1) as
mentioned above).
~ Benzimidazol derivatives or salts
thereof represented by the general formula (1), wherein
R1 is a halogen atom; RZ is the same as defined in the
general formula (1) as mentioned above; n is 1, and R3
is a 3,4-dihydro-1,4(2H)-benzoxazinyl group (wherein
the substituents of 3,4-dihydro-1,4(2H)-benzoxazinyl
group are the same as defined in the general formula
(1) as mentioned above).
~'~~-.,- >~' 2198266
WO 97/03070 PCT/JP96/01841
I8
The concrete examples of various substituents
as defined in R', Rz, R', A, B, R°, RS and R6 as shown
in the general formula (1) are as follows.
As to the halogen atom, such as a fluorine
atom, a chlorine atom, a bromine atom and iodine atom
can be exemplified.
As to the phenyl-lower alkyl group, a phenyl-
alkyl group in which the alkyl moiety is a straight- or
branched-chain alkyl group having 1 to 6 carbon atoms,
and said alkyl group having l to 2 phenyl groups, such
as a benzyl, 2-phenylethyl, 1-phenylethyl, 3-phenyl-
propyl, 4-phenylbutyl, 5-phenylpentyl, 6-phenylhexyl,
l,l-dimethyl-2-phenylethyl, 2-methyl-3-phenylpropyl,
diphenylmethyl and 2,2-diphenylethyl groups can be
IS exemplified.
As to the lower alkylene group, a straight-
or branched-chain alkylene-group having 1 to 6 carbon
atoms, such as a methylene, ethylene, trimethylene,
2-methyltrimethylene, 2,2-dimethyltrimethylene,
1-methyltrimethylene, methylmethylene, ethylmethylene,
tetramethylene, pentamethylene and hexamethylene groups
can be exemplified.
As to the 5- to 11-membered saturated or
unsaturated heterocyclic group of single ring or binary
ring having 1 to 4 nitrogen atoms, oxygen atoms or
sulfur atoms as the hetero atoms, such as pyrrolidinyl,
piperidinyl, piperazinyl, morpholino, thiomorpholino,
pyridyl, homopiperazinyl, 1,2,5,6-tetrahydropyridyl,
W O 97!03070 219 8 2 6 6 pCT/JP96/0184I
19
thienyl, quinolinyl, 1,4-dihydroquinolinyl, benzo-
thiazolyl, pyrazinyl, pyrimidinyl, pyridazinyl,
pyrrolyl, carbostyril, 3,4-dihydrocarbostyril, 1,2,3,4-
tetrahydroquinolinyl, indolyl, isoindolyl, indolinyl,
benzimidazolyl, benzoxazolyl, imidazolidinyl,
isoquinolinyl, quinazolidinyl, 1,2,3,4-tetrahydroiso-
quinolinyl,1,2=dihydroisoquinolinyl, quinoxalinyl,
cinnolinyl, phthalazinyl, 1,2,3,4-tetrazolyl, 1,2,4-
triazolyl, chromanyl, isoindolinyl, isochromanyl,
pyrazolyl, imidazolyl, pyrazolidinyl, imidazo[1,2-a]-
pyridyl, benzofuryl, 2,3-dihydrobenzo[bJfuryl,
benzothienyl, 1-azacycloheptyl, 4H-chromenyl,
1H-indazolyl, 2-imidazolinyl, 2-pyrrolinyl, fury!,
oxazolyl, oxazolidinyl, isoxazolyl, thiazolyl,
isothiazolyl, pyranyl, pyrazolidinyl, 2-pyrazolinyl,
quinuclidinyl, 1,4-benzoxazinyl, 3,4-dihydro-2H-1,4-
benzoxazinyl, 3,4-dihydro-2H-1,4-benzothiazinyl,
1,4-benzothiazinyl, 1,2,3,4-tetrahydroquinoxalinyl,
1,3-dithia-2,4-dihydronaphthalenyl, tetrahydro-1,3-
oxazinyl, tetrahydroxazolyl and 1,4-dithianaphthalenyl
groups can be exemplified.
As to the heterocyclic group having 1 to 3
substituents selected from the group consisting of a
lower alkyl group, a lower alkoxy group, a halogen atom
and an oxo group, a heterocyclic group having 1 to 3
substituents selected from the group consisting of a
straight- or branched-chain alkyl group having 1 to 6
carbon atoms, a straight- or branched-chain alkoxy
.~. °.: :- ~ _.~ 2198266
W0 97/03070 PCT/JP96/01841
group having 1 to 6 carbon atoms, a halogen atom and an
oxo group, such as 1-oxo-1,2,3,4-tetrahydroiso-
quinolinyl, 2-oxopiperidinyl, 2-oxo-1-azacycloheptyl,
2-oxopyrrolidinyl, 1,3-dioxoisoindolinyl, 2,4-dioxo-
5 imidazolidinyl, 2-oxooxazolidinyl, 1-methylimidazolyl,
1-propylimidazolyl, 4-methylimidazolyl, 5,6-dimethyl-
benzimidazolyl, 1,4-dimethylpyrrolyl, 2-isopropylimida-
zolyl, 4-methylpiperazinyl, 4-phenylpiperidinyl,
4-methylthiazolyl, 2-oxothiazolyl, 5-ethylthiazolyl,
10 4-phenylthiazolyl, 4-propylthiazolyl, 5-butylthiazolyl,
4-pentylthiazolyl, 2-hexylthiazolyl, 3,5-dimethyliso-
oxazolyl, 4,5-dimethylthiazolyl, 5-methoxy-4-methyl-
thiazolyl, 1-ethylimidazolyl, 4-propylimidazolyl,
5-butylimidazolyl, 1-pentylimidazolyl, 1-hexylimida-
15 zolyl, 1,4-dimethylimidazolyl, 1,4,5-trimethyl-
imidazolyl, 1-methyoxyimidazolyl, 2-ethoxyimidazolyl,
5-propoxyimidazolyl, 1-methyl-4-chloroimidazolyl,
4,5-dichloroimidazolyl, 3-methyl-1,2,4-triazolyl,
5-ethyl-1,2,4-triazolyl, 3-methyl-1,2,4-triazolyl,
20 2-oxo-1-methylimidazolyl, 2-oxoimidazolyl, 2-ethyl-
pyrrolyl, 3-propylpyrrolyl, 5-butylpyrrolyl, 4-
pentylpyrrolyl, 2-hexylpyrrolyl, 2,4,5-trimethyl-
pyrrolyl, 2-bromopyrrolyl, 2,5-dibromopyrrolyl,
2-methyl-5-methoxypyrrolyl, 2-oxopyrrolyl, 1-methyl-
1,2,3,4-tetrazolyl, 1-isopropyl-1,2,3,4-tetrazolyl,
1-ethyl-1,2,3,4-tetrazolyl, 1-propyl-I,2,3,4-
tetrazolyl, 1-butyl-1,2,3,4-tetrazolyl, 1-pentyl-
1,2,3,4-tetrazolyl, 1-hexyl-1,2,3,4-tetrazolyl,
WO 97103070 ~ - ~ ~ ~ ~ PCT/JP96J0184I
21
5-methoxyindolyl, 2-methylpyridyl, 3-ethylpyridyl,
4-propylpyridyl, 2-butylpyridyl, 3-pentylpyridyl,
4-hexylpyridyl, 2-methoxypyridyl, 3-phenylpyridyl,
4-phenylpyridyl, 2,4-dimethylpyridyl, 2,4,6-
trimethylpyridyl, 2-methyl-4-chloropyridyl, 2,4-
difluoropyridyl, 2,4,6-trichloropyridyl, 2-oxopyridyl,
4-oxopyridyl, 4-methyl-2-oxopyridyl, 2-chloro-4-
oxopyridyl, 3-methylimidazo-[1,2-a]pyridyl, 4-
ethylimidazo[1,2-a]pyridyl, 3-methoxyimidazo-
[1,2-a]pyridyl, 5-chloroimidazo[1,2-a]pyridyl,
3-methyl-1H-indazolyl, 3-iodo-1H-indazolyl,
1-methyl-1,2,3,4-tetrahydroisoquinolinyl, 5-ethyl-
1,2,3,4-tetrahydroisoquinolinyl, 6-bromo-1,2,3,4-
tetrahydroisoguinolinyl, 1-oxo-6-methyl-1,2,3,4-
tetrahydroisoquinolinyl, 1-oxo-7-methoxy-1,2,3,4-
tetrahydroisoquinolinyl, 3,4-dimethylpiprazinyl,
3-ethylpyrrolidinyl, 2-propylpyrrolidinyl, 1-methyl-
pyrrolidinyl, 3,4,5-trimethylpiperidinyl, 4-butyl-
piperidinyl, 3-pentylmorpholino, 4-hexylpiperazinyl,
3-methylthiomorpholino, 3-chloropyrrolidinyl,
2-oxo-4-methylpiperidinyl, 2-oxo-3-methylpyrrolidinyl,
2-oxo-4-fluoropiperidinyl, 4-methyl-1-azacycloheptyl,
5-methoxy-1-azacycloheptyl, 6-methyl-2-oxo-1-azacyclo-
heptyl, 1-methyl-2-oxoimidazolidinyl, 1-isobutyl-2-
oxoimidazolidinyl, 1-methyl-2-oxoimidazolidinyl,
2-oxotetrahydro-1,3-oxazinyl, 3-bromo-2-oxo-1-
azacycloheptyl, 2-oxo-tetrahydrooxazolyl, 3-chloro-
pyridyl, 4-methylpiperazinyl, 4-isobutylpiperazinyl,
WO 97103070 ~i ~ ' v'r~ ' ' 219 8 2 6 6
Pcaiar9sioasai
22
4-methylhomopiperazinyl, 3-chloropiperazinyl,
4-methoxypiperazinyl and 4-ethylhomopierazinyl groups
can be exemplified.
As to the lower alkoxy group, a straight- or
branched-chain alkoxy group having 1 to 6 carbon atoms,
such as a methoxy, ethoxy, propoxy, isopropoxy, butoxy,
art-butoxy, pentyloxy and hexyloxy groups can be
exemplified.
As to the lower alkyl group, a straight- or
branched-chain alkyl group having 1 to 6 carbon atoms,
such as a methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, tart-butyl, pentyl and hexyl groups can be
exemplified.
As to the cycloalkyl group, a cycloalkyl
group having 3 to 8 carbon atoms, such as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and
cycloactyl groups can be exemplified.
As to the isooxazolylcarbonyl group which may
have 1 to 3 lower alkyl groups as the substituents, an
isooxazolylcarbonyl group which may have 1 to 3
straight- or branched-chain alkyl groups having 1 to 6
carbon atoms as the substituents, such as isooxazolyl-
carbonyl, 3,5-dimethylisooxazolylcarbonyl, 3-methyl-
isoxazolylcarbonyl, 4-ethylisooxazolylcarbonyl,
5-propylisooxazolylcarbonyl, 3-butylisooxazolyl-
carbonyl, 4-pentylisooxazolylcarbonyl, 5-hexyl-
isooxazolylcarbonyl and 3,4,5-trimethylisooxazolyl-
carbonyl groups can be exemplified.
2198266
WO 97103070 PCT/JP96/01841
23
As to the amino=substituted lower alkyl group
which may have lower alkyl groups as the substituents,
an amino-substituted straight- or branched-chain alkyl
group having 1 to 6 carbon atoms, which may have 1 to 2
straight- or branched-chain alkyl group having 1 to 6
carbon atoms as the substituents, such as an amino-
methyl, 2-aminoethyl, 1-aminoethyl, 3-aminopropyl,
4-aminobutyl, 5-aminopentyl, 6-aminohexyl, 1,1-
dimethyl-2-aminoethyl, 2-methyl-3-aminopropyl, methyl-
aminomethyl, 1-ethylaminoethyl, 2-propylaminoethyl,
3-isopropylaminopropyl, 4-butylaminobutyl, 5-pentyl-
aminopentyl, 6-hexylaminohexyl, dimethylaminomethyl,
2-diethylaminoethyl, 2-dimethylaminoethyl, (N-ethyl-
N-propylamino)methyl and 2-(N-methyl-N-hexylamino)ethyl
groups can be exemplified
As to the 5- to 6-membered saturated hetero-
cyclic group formed by combining RS and R6 together
with the adjacent nitrogen atom being bonded thereto,
further with or without other nitrogen atom or oxygen
atom, such as pyrrolidinyl, piperidinyl, piperazinyl
and morpholino groups can be exemplified.
As to the said heterocyclic group having 1 to
3 substituents selected from the group consisting of a
hydroxyl group and a phenyl group, such as 4-phenyl-
4-hydroxypiperidinyl, 4-phenylpiperazinyl, 3-phenyl-
piperazinyl, 3-hydroxypyrrolidinyl, 4-hydroxy-
piperazinyl, 3-phenylmorpholino, 2,4-diphenyl-
piperazinyl, 3-phenylpyrrolidinyl, 2,3,4-triphenyl-
2198266
WO 97/03070 PCT/JP96/01841
24
piperazinyl, 3-hydroxymorpholino, 2-phenyl-2-
hydroxymorpholino and 3-phenyl-3-hydroxypiperazinyl
groups can be exemplified.
As to the lower alkenyl group, a straight- or
branched-chain alkenyl group having 2 to 6 carbon
atoms, such as a vinyl, allyl, 2-butenyl, 3-butenyl,
1-methylallyl, 2-pentenyl and 2-hexenyl groups can be
exemplified.
As to the lower alkoxycarbonyl group, a
straight-.or branched-chain alkoxycarbonyl group
having 1 to 6 carbon atoms, such as methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl, tert-butoxycarbonyl, pentyloxycarbonyl
and hexyloxycarbonyl groups can be exemplified.
As to the phenoxy-lower alkyl group which may
have cyano groups as the substituents on the phenyl
ring, a phenoxy group-substituted straight- or
branched-chain alkyl group having 1 to 6 carbon atoms,
which may have 1 to 3 cyano groups as the substituents
on the phenyl ring, such as a phenoxymethyl, 2-phenoxy-
ethyl, 1-phenoxyethyl, 4-phenoxybutyl, 5-phenoxypentyl,
6-phenoxyhexyl, 1,1-dimethyl-2-phenoxyethyl, 2-methyl-
3-phenoxypropyl, (2-cyanophenoxy)methyl, 2-(2-cyano-
phenoxy)ethyl, 3-phenoxypropyl, 4-(3-cyanophenoxy)-
butyl, 5-(2-cyanophenoxy)pentyl, 6-(3-cyanophenoxy)-
hexyl, (4-cyanophenoxy)methyl, 3-(2-cyanophenoxy)-
propyl, 3-(3-cyanophenoxy)propyl, 1-(3-cyanophenoxy)-
ethyl, 3-(3,4-dicyanophenoxy)propyl and 2-(3,4,5-tri-
W 0 97103070 ' ' ~ ' ~ 2 i 9 8 2 6 6
PCT/JP96/OI84i
cyanophenoxy)ethyl groups can be exemplified.
As to the halogen atom-substituted lower
s
alkyl group, a straight- or branched-chain alkyl group
having 1 to 6 carbon atoms, having 1 to 3 halogen atoms
5 as the substituents, such as trifluoromethyl, tri-
chloromethyl, chloromethyl, bromomethyl, fluoromethyl,
iodomethyl, difluoromethyl, dihromomethyl, 2-chloro
ethyl, 2,2,2-trifluoroethyl, 2,2,2-trichloroethyl,
3-bromopropyl, 3-chloropropyl, 2,3-dichloropropyl,
10 4,4,4-trichlorobutyl, 4-fluorobutyl, 5-chloropentyl,
3-chloro-2-methylpropyl, 5-bromohexyl and 5,6-dichloro-
hexyl groups can be exemplified.
As to the lower alkoxycarbonyl group-
substituted lower alkyl group, a straight- or branched-
15 chain alkoxycarbonylalkyl group in which the alkyl
group is a straight- or branched-chain alkyl group
having 1 to 6 carbon atoms, and the alkoxy-
carbonyl moiety is a straight- or branched-chain
alkoxycarbonyl group having 1 to 6 carbon atoms, such
20 as methoxycarbonylmethyl, 3-methoxycarbonylpropyl,
ethoxycarbonylmethyl, 3-ethoxycarbonylpropyl,
4-ethoxycarbonylbutyl, 5-isopropoxycarbonylpentyl,
6-propoxycarbonylhexyl, 1,1-dimethyl-2-butoxycarbonyl-
ethyl, 2-methyl-3-tert-butoxycarbonylpropyl,
25 2-pentyloxycarbonylethyl and hexyloxycarbonylmethyl
groups can be exemplified.
WO 97/03070 C 219 8 2 6 b pCT/JP96101841
26
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The benzimidazole derivatives of-.the present
l
invention can be prepared by various methods, for
example they can be prepared by the methods of Reaction
formula-1 through Reaction formula-4 as follows.
Reaction formula-1
HyY_ f A~-R 0
N n (3)
\
N~-C00H \ C_1-C a R3
N
R Rz R Rz
(2) (1.)
[wherein Ri, RZ, R3, A and n are the same as defined
above.]
The method as shown in Reaction formula-1 is
the reaction of a benzimidazole compound (a carboxylic
acid) of the formula (2) with an amine of the formula
(3) by a common amide bond formation reaction. The
acid amide bond formation reaction can easily be
carried out by the reaction conditions of amide bond
formation known in the art. For example, (a) a
mixed-acid anhydrides method: i.e., a method by
reacting a carboxylic acid (2) with an ester of
alkylhalocarboxylate to form a mixed-acid anhydride,
then by reacting it with an amine (3); (b) an activated
ester method: i.e., a method by changing a carboxylic
WO 97/03070 219 8 2 6 6 pCT/~6/OI841
27
acid (2) to an activated ester form, e.g., p-nitro-
phenyl ester, N-hydroxysuccinimide ester, 1-hydoxy-
benztriazole ester, or the like, then by reacting the
activated ester with an amine (3); (c) a carbodiimide
method: i.e., a method by reacting a carboxylic acid
(2) with an amine (3) in the presence of an activating
agent, e.g.,-dicyclohexylcarbodiimide, carbonyldiimida-
zole or the like; (d} other method; for example, a
method by changing a carboxylic acid (2) with a
dehydrating agent, e.g., acetic anhydride to form
carboxylic acid anhydride, then by reacting said acid
anhydride with an amine (3); a method by reacting an
ester of a carboxylic acid (2) and a lower alcohol,
with an amine (3) at an elevated temperature; a method
by reacting a acid halogenide of a carboxylic acid (2),
e.g., a carboxylic acid halide, with an amine (3}, and
the like can be exemplified.
The mixed acid anhydride, which is used in
the above-mentioned a mixed-acid anhydrides method, can
be prepared by a method similar to that employed in
common Schotten-Baumann reaction, said mixed-acid
anhydride is used without being isolated from the
reaction system, and reacted with an amine (3) to
obtain a ben~imidazole compound of the general formula
(1) of the present invention. The above-mentioned
Schotten-Baumann reaction is carried out in the
presence of a basic compound. As to the basic compound
to be used in the reaction, usual basic compounds used
~...3,;; ~:~ 2198266
WO 97/03070 PCTlJP96/01841
28
in Schotten-Baumann reaction, for example organic bases
such as triethylamine, trimethylamine, pyridine,
dimethylaniline, 1-methyl-2-pyrrolidinone (NMP),
N-methylmorpholine, 1,~5-diazabicyclo[4.3.0]nonene-5
(DBN), 1,8-diazabicyclo[5.4.0]undecene-7 (DBU), 1,4-
diazabicyclo[2.2.2]octane (DABCO) and the like, and
inorganic bases such as potassium carbonate, sodium
carbonate, potassium hydrogencarbonate, sodium
hydrogencarbonate and the like can be exemplified.
Said reaction is generally carried out at about -20 to
100°C, preferably at about 0 to 50°C, and the reaction
time is about 5 minutes to 10 hours, preferably about
5 minutes to 2 hours. The reaction of the thus
obtained mixed acid anhydride with an amine (3) is
carried out at about -20 to 150°C, preferably at about
10 to 50°C, and the reaction time is about 5 minutes to
10 hours, preferably about 5 minutes to 5 hours.
Generally, the mixed-acid anhydride method is carried
out in a solvent. As to the solvent to be used for the
reaction, any solvent commonly used for the mixed-acid
anhydride method can be used, specifically halogenated
hydrocarbons such as chloroform, dichloromethane and
the like; aromatic hydrocarbons such as benzene,
p-chlorobenzene, toluene, xylene and the like; ethers
such as diethyl ether, diisopropyl ether, tetrahydro-
furan, dimethoxyethane and the like; esters such as
methyl acetate, ethyl acetate and the like; aprotic
polar solvents such as N,N-dimethylformamide, dimethyl
W O 97103070 219 8 2 6 6 PCT/JP96/OI84I
29
L
sulfoxide, acetonitrile, hexamethylphosphoric triamide
and the like; and mixed solvents thereof can be
exemplified. As to the alkylhalocarbonic acid ester
used in the mixed-acid anhydride method, methyl chloro-
formate, methyl bromoformate, ethyl chloroformate,
ethyl bromoformate, isobutyl chloroformate and the like
can be exemplified. Ratio of the amounts of a
carboxylic acid (2), an alkylhalocarboxylic acid ester
and an amine (3) used in said method may be equimolar
quantities, respectively, and within the range of about
1 to 1.5 times the molar quantities of the alkylhalo-
carboxylic acid ester and the carboxylic acid (2),
respectively, can be used to 1 molar quantity of the
amine (3).
Among the methods (d), in case of using the
method by reacting carboxylic acid halide with an amine
(3), said reaction can be carried out, in the presence
of a basic compound, in a suitable solvent. As to the
basic compound to be used, known compound selected from
a wide range can be used, for example in addition to
the basic compounds used in the Schotten-Baumann
reaction, sodium hydroxide, potassium hydroxide, sodium
hydride, potassium hydride and the like can be exempli-
fied.- As to the solvent to be used in the reaction,
for example in addition to the solvents used in the
above-mentioned mixed acid anhydride method, alcohols
such as methanol, ethanol, propanol, butanol 3-methoxy-
1-butanol, ethyl cellosolve, methyl cellosolve and the
2198266
WO 97/03070 ~ ", PCT/JP96/01841
like; pyridine, acetone, water can be exemplified.
Ratio of the amount of-amine (3) and to the amount of
carboxylic acid halide is not specifically restricted
and can be suitably selected from a wide range,
5 generally, at least about an equimolar quantity,
preferably about an equimolar to 5 times the molar
quantity of the latter may be used to the former.
Generally, said reaction is carried out at about -20 to
180°C, preferably at about 0 to 150°C, and generally,
10 the reaction is completed within for about 5 minute to
30 hours.
Eurthermo~e, the amide bond formation reac-
tion shown in the above-mentioned Reaction formula-1
can also be carried out by reacting a carboxylic acid
15 (2) with an amine (3), in the presence of a phosphorus
compound as a condensing agent, such as phenylphosphin-
2,2'-dithiopyridine, diphenylphosphinyl chloride,
phenyl-N-phenylphosphoramide chloridate, diethylchloro-
phosphate, diethyl cyanophosphate, diphenylphosphoric
20 acid azide or bis(2-oxo-3-oxazolidinyl)phosphinic -
chloride, as a condensing agent.
Said reaction is carried out, in the presence
of the solvent and the basic compound used in the reac-
tion of the above-mentioned carboxylic acid halide with
25 an amine (3) generally at about -20 to 150°C, prefer-
ably at about 0 to 100°C, and the reaction is generally
completed within about 5 minute to 30 hours. The
amounts of the condensing agent and the carboxylic acid
2198266
W 0 97!03070 PCT/JP9610I841
31
(2) may be about equimolar quantity, preferably about
equimolar to 2 times the molar quantity, respectively
to the amount of the amine (3).
The reaction as shown in Reaction formula-1
can also be carried out by reacting an ester of
carboxylic acid (2) and a lower alcohol with an amine
(3) in a solvent or without~solvent, and in the
presence or absence of a basic compound. Generally,
the reaction is carried out at about room temperature
to 200C, preferably at about room temperature to 120C
and generally, the reaction is completed within 30
minutes to 5 hours. The amine (3) is used in an amount
at least 0.5 times the molar quantity, preferably 0.5
to 3 times the molar quantity to an equimolar quantity
of the ester of carboxylic acid (2) and a lower
alcohol. As to the solvent to be used in this reac-
tion,-any solvent used in the above-mentioned reaction
of a carboxylic acid halide with an amine (3) can also
be used. As to the basic compound to be used in this
reaction, in addition to the basic compounds used
in the above-mentioned method for reacting an
carboxylic acid halide with an amine (3), for example
an alkali metal alcoholate, such as sodium methylate,
sodium ethylate, potassium methylate, potassium
ethylate or the like can be exemplified.
The reaction as shown in Reaction formula-1
can also be carried out by reacting, in a suitable
solvent, an aluminum compound such as lithium aluminum
,,
., ~ Via.;,: . 2198266
W0 97103070 PCT/JP96/01841
32
hydride, trimethyl aluminum and the like as a condens-
ing agent with an amine (3), then reacting the result-
ing reaction product with an ester of carboxylic acid
(2) and a lower alcohol. As to the solvent used in
this reaction, ethers such as dioxane, diethyl ether,
diglyme, tetrahydrofuran and the like; aromatic
hydrocarbons such as benzene, toluene, xylene and the
like; aliphatic hydrocarbons such as cyclohexane,
heptane, hexane and the like; and the mixtures of .these
solvents can be exemplified. The amine (3) may be used
at least in an equimolar quantity, preferably in an
equimolar to 5 times the molar quantity of the ester of
the carboxylic-acid (2) and lower alcohol. The
condensing agent may be used at least in an equimolar
quantity, preferably in an equimolar to 1.5 times the
molar quantity of the ester of the carboxylic acid (2)
and lower alcohol. The reaction of-the condensing
agent with the amine (3) is generally carried out at
about -80 to 100°C, and the reaction is generally
completed within for about 30 minutes to 20 hours. The
subsequent ester reaction of the carboxylic acid (2)
with the lower alcohol is carried out generally at room
temperature to 200°C, preferably at about room tempera-
ture-to 150°C, and the reaction is generally completed
within 1 to 10 hours.
v ~w : : 2198266
WO 97/03070 PCT/JP96/0184I
33
Reaction formula-2
N ~I , RIX C4) ~ N II
C\~ ~C-N~A~-R3' (~ ~-C-NZA-~-R3b
N H n N H n
Rl R= Ri R2
(la) ' ~ (1b)
[wherein Rl, Rz, A and n are the same as defined
above;
Rs" is a heterocyclic group as defined
in R' which may have 1 to 2 substituents selected from
the group consisting of:
a group of the formula -B-R° (B and R° are the same as
defined above); a lower alkenyl group; a lower alkoxy-
carbonyl group; a phenoxy-lower alkyl group which may
have cyano groups as the substituents in the phenyl
ring; a halogen substituted-lower alkyl group; and a
lower alkoxycarbonyl substituted-lower alkyl group;
further R3' is a heterocyclic group as
defined in R3, having a group of the formula -NH- in
said heterocyclic group:
R36 is a heterocyclic group as defined in R;
which may have 1 to 2 substituents selected from the
group consisting of:
a group of the formula -B-R° (B and R" are the same as
defined above); a lower alkenyl group; a lower alkoxy-
'~'~ 'v =~ rr'~ 2198266
WO 97/03070 PCT/JP96/01841
34
carbonyl group; a phenoxy-lower alkyl group which may
have cyano groups as the substituents in the phenyl
ring; a halogen substituted-lower alkyl group; and a
lower alkoxycarbonyl substituted-lower alkyl group;
further Rib is a heterocyclic group as
defined in R3, having a group of the formula -N(R')- (R'
is a group of the formula -B-R4 (wherein B and R4 are
the same as defined above); a lower alkenyl group, a
lower alkoxycarbonyl group; a phenoxy-lower alkyl group
which may have cyano groups as the substituents in the
phenyl ring; a halogen substituted-lower alkyl group;
or a lower alkoxycarbonyl substituted-lower alkyl
group) in said heterocyclic group; X is a halogen atom,
a lower alkanesulfonyloxy group, an-arylsulfonyoxy
group or an aralkylsulfonyloxy group].
As to the lower alkanesulfonyloxy group,
specifically methanesulfonyloxy, ethanesulfonyloxy,
propanesulfonyloxy, isopropanesulfonyloxy, butane-
sulfonyloxy, tert-butanesulfonyloxy, pentanesulfonyloxy
and hexanesulfonyloxy groups and the like can be
exemplified. As to the arylsulfonyloxy group, specifi-
cally substituted or unsubstituted arylsulfonyloxy
groups such as phenylsulfonyloxy, 4-methylphenyl-
sulfonyloxy, 2-methylphenylsulfonyloxy, 4-nitrophenyl-
sulfonyloxy, 4-methoxyphenylsulfonyloxy, 3-chloro-
phenylsulfonyloxy and a-naphthylsulfonyloxy groups and
the like can be exemplified.
As to the aralkylsulfonyloxy group, specifi-
2198266
WO 97!03070 PCTlJP96/0184I
cally substituted or unsubstituted aralkylsulfonyloxy
groups such as benzylsulfonyloxy, 2-phenylethyl-
sulfonyloxp, 4-phenylbutylsulfonyloxy, 4-methylbenzyl-
sulfonyloicy, 2-methylbenzylsulfonyloxy, 4-nitrobenzyl-
S sulfonyloxy, 4-methoxybenzylsulfonyloxy, 3-chloro-
benzylsulfonyloxy and o:-naphthylmethylsulfonyloxy
groups can be exemplified.
The reaction of a compound (la) with a
compound (4) is carried out, generally in a suitable
10 inert solvent, in the presence or absence of a basic
substances. As to the inert solvent, for example
aromatic hydrocarbons such as benzene, toluene, xylene
and the like; ethers such as tetrahydrofuran, dioxane,
diethylene glycol dimethyl ether and the like;
15 haloqenated hydrocarbons such as dichloromethane,
chloroform, carbon tetrachloride-and the like; lower
alcohols such as methanol, ethanol, isopropanol,
butanol, tert-butanol and the like; acetic acid, ethyl
acetate, acetone, acetonitrile, pyridine, dimethyl
20 sulfoxide, dimethylformamide, hexamethylphosphoric
triamide; and mixtures of these solvents can be
exemplified_ As to the basic substances, carbonates
such as sodium carbonate, potassium carbonate, sodium
hydrogencarbonate, potassium hydrogencarbonate and the
25 like; metal hydroxide such as sodium hydroxide, potas-
sium hydroxide and the like; sodium hydride, potassium
metal, sodium metal, sodium amide; metal alcoholates
such as sodium methylate, sodium ethylate and the like;
.J :.. ;_, r, F ~-
:,' J C' V ',
WO 97103070 219 8 2 6 6 pCT/JP96/01841
36
organic bases such as pyridine,-N-ethyldiisopropyl-
amine, dimethylaminopyridine, triethylamine, 1,5-
diazabicyclo[4.3.0]nonene-5 (DBN), 1,8-diazabicyclo-
[5.4.0]undecene-7 [DBU], 1,4-diazabieyclo[2.2.2joctane
{DABCO) and the like can be exemplified. Ratio of the
amounts of compound (la) and compound (4) is not
specifically restricted and can be selected from
a wide range, generally at least about an equimolar
quantity,preferably about an equimolar to 10 times the
molar quantities of the latter may be used to the
former. The reaction is generally carried out at about
0 to 200°C, preferably at about 0 to 170°C, and
generally, the reaction is completed within 30 minutes
to 75 hours. Alkali metal halogenides such as sodium
iodide, potassium iodide; or copper metal powder-may be
added to the reaction system.
Reaction formula-3
0 RBH (5) 0
N ~I-H~A~Rg~ ~ N~~~ H~A,~Rsa
Rl N Rt i2
RZ R
(lc) (1d)
[wherein RI, RZ, A and n are the same as defined
above;
R3' is a heterocyclicgroup as defined in R3,
2198266
WO 97103070 - PCT/JP96l01841
37
which may have 1 to 2 substituents selected from the
group consisting of:
a group of the formula -B-R', (B and R' are the same as
defined above); a lower alkenyl group; a lower alkoxy-
carbonyl group; a phenoxy-lower alkyl group which may
have cyano groups as the substituents in the phenyl
ring; a halogen substituted-lower alkyl group; and a
lower alkoxycarbonyl substituted-lower alkyl group;
further R3' is a heterocyclic group as
defined in R', having a group of the formula -N(R9)-
(R9 is a halogen substituted-lower alkyl group) in said
heterocyclic group;
R'd is a heterocyclic group as defined in R3,
which may have 1 to 2 substituents selected from the
group consisting of:
a group of the formula -B-R' (B and R' are the same as
defined above); a lower alkenyl group; a lower alkoxy-
carbonyl group; a phenoxy-lower alkyl group which may
have cyano groups as the substituents in the phenyl
ring; a-halogen substituted-lower alkyl group; and a
lower alkoxycarbonyl substituted-lower alkyl group;
further, R'° is a heterocyclic group as
defined in R3, having a group of the formula -N(Rio)-
(Rlo is a group of the formula -B-R' (B and R' are the
same as defined above); or a phenoxy-lower alkyl group
which may have cyano groups as the substituents in the
phenyl ring) in said heterocyclic group;
R$ is a group of the formula -R°' (R~' is a
.:~,,~x : 2198266
WO 97/03070 PCT/JP96/01841
38
heterocyclic group as defined in R', having at least
one group of the formula -N< in said heterocyclic
f
group, or a group of the formula -NRSR6 (RS and R6 are
the same as defined above); or a phenoxy group which
may have cyano groups as the substituents in the phenyl
ring]. _
The reaction of a compound (lc) with a com-
pound (5) is carried out under the reaction condition
similar to the reaction condition of a compound (la)
with a compound (4) in the above-mentioned Reaction
formula-2.
Reaction formula-4
0 0
N~Ci H~A~R3e ~ ~ N' C[ H~A~R3r
I ~ I
R R2 R Rz
(1 e) ~ (1 f)
RIIX (6) (R14)20 RI40H (8)
RIzCORI3 (7) (9)
0 0
~~~~~.~A~.Rsa \ ~~~A~Rsn
N H n / N H n
I I I I
R Rz R R2
(1 g) (1 h)
2198266
WO 97/03070 PCT/JP96I0184i
i
39
[wherein R', Rz, A and n are the same as defined above;
1' '- Rl' is a heterocyclic group as defined in R3,
which may have 1 to 2 substituents selected from the
group consisting of:
a group of the formula -B-R° (B and R4 are the same as
defined above); a lower alkenyl group; a lower alkoxy-
carbonyl group; a phenoxy-lower alkyl group which may
have cyano groups as the substituents in the phenyl
ring; a halogen substituted-lower alkyl group; and a
lower alkoxycarbonyl substituted-lower alkyl group;
further R3' is a heterocyclic.group as
defined in R3, having a group of the formula -N(Rls)-,
(Ris is a phthalimide substituted-lower alkyl group) in
said heterocyclic group;
R3f is a heterocyclic group as defined in R3
which may have 1 to 2 substituents selected from the
group consisting of:
a group of the formula -B-R° (B and R4 are the same as
defined above); a lower alkenyl group; a lower alkoxy
carbonyl group; a phenoxy-lower alkyl group which may
have cyano groups as the substituents in the phenyl
ring; a halogen substituted-lower alkyl group; and a
lower alknxycarbonyl substituted-lower alkyl group;
further R3f is a heterocyclic group as
defined in R3, having a group of the formula -N(RIS)-
(R16 is an amino group-substituted lower alkyl group)
in the heterocyclic ring;
. 1, ; i
~~'~''~ ~ v 2198266
WO 97/03070 PCT/JP96/01841
R3g is a heterocyclic group as defined in R3,
which may have 1 to 2 substituents selected from the
group consisting of:
a group of the formula -B-R° (wherein B and R° are the
5 same as defined above); a lower alkenyl group; a lower
alkoxycarbonyl group; a phenoxy-lower alkyl group which
may have cyano groups as the substituents in the phenyl
ring; a halogen substituted-lower alkyl group; and a
lower alkoxycarbonyl substituted-lower alkyl group;
10 further R38 is a heterocyclic group as
defined in R3, having a group of the formula
-N(B-NRS'RI1)- (B is the same as defined above; RS' is
a hydrogen atom, a lower alkyl group, a cycloalkyl
group, a pyridylcarbonyl group, an isoxazolylcarbonyl
15 group which may have 1 to 3 lower alkyl groups as the
substituents; a pyrrolycarbonyl group or an amino group
substituted-lower alkyl group which may have lower
alkyl groups as the subsitituents; Ril is a lower
alkyl group, a cycloalkyl group or an amino group
2D substituted-lower alkyl group which may have lower
alkyl groups as the substituents) in said heterocyclic
group;
Rlb is a heterocyclic group as defined in R3,
which may have 1 to 2 substituents selected from the
25 group consisting of:
a group of the formula -B-R° (B and R' are the same as
defined above); a lower alkenyl group; a lower alkoxy-
carbonyl group; a phenoxy-lower alkyl group which may
.. . , 2198266
WO 97/03070 PCT/JP9610IS4I
41
have cyano groups as the substituents in the phenyl
ring; a halogen substituted-lower alkyl group; and a
lower alkoxycarbonyl substituted-lower alkyl group;
further R3h is a heterocyclic group as
defined in R3, having a group of the formula
-N(B-NR3'R'~)- (B and RS' are the same as defined
,
above; and RIe is a pyridylcarbonyl group, an
isoxazolylcarbonyl group which may have 1 to 3 lower
alkyl groups as the substituents, or a pyrrolylcarbonyl
group) in said heterocyclic group;
RlZ and R13 are each, a hydrogen atom or a
lower alkyl group, respectively].
The reaction for introducing a compound (1f)
from a compound (1e) can be carried out by reacting a
compound (1e) with hydrazine in a suitable solvent or
by hydrolysis of a compound (1e). As to the solvent
to be used in the reaction of a compound (1e) with
hydrazine, in addition to water, solvents similar to
those can be used in the reaction of a compound (la)
with a compound (4) in the above-mentioned Reaction
formula-2 can be used. This reaction is carried out
generally at about room temperature to 120°C,
preferably at about 0 to 100°C, and the reaction is
generally completed within 0.5 to 15 hours. The amount
of hydrazine is at least about an equimolar quantity,
preferably an equimolar to 5 times the molar quantities
can be used to a compound (1e).
2198266
WO 97/03070 PCT/JP96/01841
42
The above-mentioned hydrolysis reaction of
a compound (1e) can be carried out in a suitable
solvent yr without solvent, in the presence of an acid
or basic compound. As to the solvent to be used,
water, lower alcohols such as methanol, ethanol,
isopropanol and the like; ketones such as acetone,
methyl ethyl ketone and the like; ethers such as
dioxane, tetrahydrofuran, ethylene glycol dimethyl
ether and the like; fatty acids such as acetic acid,
formic acid and the like; and mixtures of these
solvents can be exemplified. As to the acid to be
used, mineral acids such as hydrochloric acid, sulfuric
acid, hydrobromic acid and the like; organic acid such
as formic acid, acetic acid, aromatic sulfonic acid
such as p-toluenesulfonic acid and the like can be
exemplified. As to the basic compound to be used,
metal carbonates such as sodium carbonate, potassium
carbonate and the like, metal hydroxides such as sodium
hydroxide, potassium hydroxide, calcium hydroxide,
lithium hydroxide and the like can be exemplified.
Generally, said reaction is suitably carried out at
about room temperature to 200°C, preferably at about
room temperature to 150°C, and generally the reaction
is completed within about 10 minutes to 25 hours.
The reaction of a compound (1f) with a com-
pound (8) is carried out under the reaction condition
similar to that of employed in the reaction of a com-
pound (2) with a compound (3) in the above-mentioned
WO 97!03070 ' 219 8 2 6 6 pCT/~6/OI841
43
Reaction formula-1.
The reaction of a compound (!f) with a
compound (6] is carried out, generally in a suitable
inert solvent, in the presence or absence of a basic
substance. As to the inert solvent to be used in the
reaction, aromatic hydrocarbons such as benzene,
toluene, xylene and the like; ethers such as tetra-
hydrofuran, dioxane, diethylene glycol dimethyl ether
and the like; halogenated hydrocarbons such as
dichloromethane, chloroform, carbon tetrachloride and
the like; lower alcohols such as methanol, ethanol,
isopropanol, butanol, tert-butanol and the like; acetic
acid, ethyl acetate, acetone, acetonitrile, pyridine,
dimethyl sulfoxide, dimethyl formamide, hexamethyl-
phosphoric triamide; or mixtures of these solvents can
be exemplified. As to the basic substances to be used
in the reaction, carbonates such as sodium carbonate,
potassium carbonate, sodium hydrogen-carbonate,
potassium hydrogencarbonate; metal hydroxides such as
sodium hydroxide, potassium hydroxide; sodium hydride,
potassium metal, sodium metal, sodium amide, metal
alcoholates such as sodium methylate, sodium ethylate
and the like; organic bases such as pyridine, N-ethyl-
diisopropylamine, dimethylaminopyridine, triethylamine,
1,5-diazabicyclo[4.3.0]nonene-5 (DBN), 1,8-diazabi-
cyclo[5.4.0]undecene-7 (DBU], 1,4-diazabicyclo-
[2.2.2]octane (DABCO] and the like can be exemplified.
Ratio of the amounts of a compound (!f] to a compound
CA 02198266 2005-05-11
25711-773
- 44 -
(6) is not specifically restricted, and can be selected
from a wide range, at least about an equimolar
quantity, preferably an equimolar to 10 times the molar
quantities of the latter may be used to the former.
This reaction is carried out generally, at about 0 to
200°C, preferably at about 0 to 170°C, and the reaction
is completed within 30 minutes to 75 hours. Into the
reaction system, an alkali metal halogenides such as
sodium iodide, potassium iodide or the like, copper
powder may be added.
The reaction of a compound (1f) with a
compound {7) is carried out without solvent or in a
suitable solvent, in the presence of a reducing agent.
As to the solvent to be used in the reaction, water;
alcohols such as methanol, ethanol, isopropanol and the
like; acetonitrile; formic acid, acetic acid; ethers
such as dioxane, diethyl ether, diglyme, tetrahydro-
furan and the like; aromatic hydrocarbons such as
benzene, toluene, xylene and the like; and mixtures of
these solvents can be exemplified. As to the reducing
agent, formic acid, ammonium formate, alkali metal
salts of fatty acid such as sodium formate; hydride
reducing agents such as sodium borohydride, sodium
cyanoborohydride,-lithium aluminum hydride-and the
like; catalytic hydrogenation reducing agents such as
palladium-black., palladium-carbon., platinum oxide,
platinum black, Raney nickel and the like can be
exemplified.
*Trade-mark
o ,v.., 2198266
PCTlJP96/OI84I
In case of using formic acid as a reducing
agent, reaction temperature is generally at about room
temperature to 200°C, preferably at about 50 to 150°C
may be suitable, and the reaction is completed within
5 about 1 to 10 hours. Formic acid may be used in a
large excess amount against a compound (1f).
In case of using hydride reducing agent,
reaction temperature is generally at about -30 to
100°C, preferably at about 0 to 70°C may be suitable,
10 and the reaction is completed for about 30 minutes to
12 hours. Reducing agent may be-used generally in
about an equimolar to 20 times the molar quantities,
preferably about 1 to 6 times the molar quantities to a
compound (1f). Particularly, in case of using lithium
15 aluminum hydride as the reducing agent, preferably
ethers such as diethyl ether, dioxane, tetrahydrofuran,
diglyme and the like; aromatic hydrocarbons such as
benzene, toluene, xylene and the like may be used.
Furthermore, in case of using a catalytic
20 hydrogenation reducing agent, the reduction is carried
out in hydrogen gas atmosphere under about normal
pressure to 20 atmospheric pressure, preferably about
normal pressure to 10 atmospheric pressure, on the
other hand in case of using reduction in the presence
25 of a hydrogen donating agent such as formic acid,
ammonium formate, cyclohexene, hydrazine hydrate or the
like, the reducing reaction may be carried out'at about
-30 to 100°C, preferably at about 0 to 60°C, and
C;:~~~~ 2198266
W0 97/03070 PCTIJP96101841
46
generally the reaction is completed within 1 to 12
hours. The catalytic hydrogenation reducing agent may
be used generally in an amount of 0.1 to 40~ by weight,
preferably 1 to 20 ~ by weight to compound (1f). The ,
hydrogen donating agent may be used in an amount of a
large excess quantity to compound (1f).
Compound (7) may be used, generally at least
in an equimolar quantity, preferably an equimolar to a
large excess quantity to compound (1f).
The reaction of compound (1f) with compound
(9) is carried out without solvent or in a suitable
solvent, in the presence or absence of a basic
compound. As to the suitable solvent, for example
aromatic hydrocarbons as previously mentioned; lower
alcohols such as methanol, ethanol, propanol and the
like; dimethylformamide, dimethyl sulfoxide and the
like; halogenated hydrocarbons such as chloroform,
methylene chloride and the like; acetone, pyridine and
the like can be used. As to the basic compound for
example, organic bases such as triethylamine, pyridine,
sodium hydroxide, potassium hydroxide, sodium hydride
and the like can be exemplified. The above-mentioned
reaction can also be carried out in a solvent, such as
acetic acid, in the presence of a mineral acid such as
sulfuric-acid. Ratio of the amount of compound (9) may
be used in an equimolar to a large excess quantity to
the starting material, and the reaction is carried out
generally at about 0 to 200°C, preferably at about 0 to
2198266
WO 97/03070 PCT/JP96/OI84I
47
150°C, and the reaction is completed within 0.5
to 20 hours.
Compound (2) and compound (3) which are used
for the starting materials are easily prepared by
methods as shown in Reaction formula-5 through Reaction
formula-9 as follows.
Reaction formula-5
I II
X2X~~Rt7
NHZ X3 (10) ~ N Xt
t ~ ~Z t / ~ 32
N X
R (9 a) R R=
(I1)
Rt90zC-CHO (10a} t8
R OH (12)
~. N
C~N?-COOH ~ ~/ N~-COORt9 -.-- ~ ~~-~ Rt Rte
N Rt$'
Rt Rz Rt RZ Rt i
R2
(2) (2 a) (1 3)
[wherein R1 and RZ are the same as defined above; R1' is
a lower alkoxy group; R18 is a lower alkoxy group; R19
is a lower alkyl group; X', XZ and X3 are each hydrogen
. ,.;, T - . .
v,~~'....: ~ 2198266
WO 97/03070 PCT/JP96/01841
4$
atom, respectively].
The reaction of a compound (9a) with a
compound (10) can be conducted in a suitable solvent in
the presence of an acid.- As to the solvent to be used .
in the reaction, for example water, lower alcohols such
as methanol, ethanol, isopropanol and the like; ketones
such as acetone, methyl ethyl ketone and the like;
ethers such as dioxane, tetrahydrofuran, ethylene
glycol dimethyl ether and the like; fatty acids such as
acetic acid, formic acid and the like; mixtures of
these solvents, can be mentioned. As to the acid to be
used in the reaction, mineral acids such as hydro-
chloric acid, sulfuric acid, hydrobromic acid and the
like; organic acids such as formic acid, acetic acid,
aromatic sulfonic acids such as p-toluenesulfonic acid
can be exemplified. A compound (10) may be used at
least in an equimolar quantity, preferably an equimolar
to 2 times the molar quantities to a compound (9a).
Said reaction is carried out preferably at about room
temperature to 200°C, desirably at about room tempera-
ture to i50°C, the reaction is generally completed
within 0.5 to 5 hours.
The reaction of a compound (11) with a com
pound (12) is carried out under the reaction condition
similar to that employed in the reaction of a compound
(la) with a compound (4) in the above-mentioned
Reaction formula-2. In the case, a compound (12) may
be used as a solvent in a large excess quantity.
W097J03070 ~ _~ _ 219 8 2 6 6 pCT1JP96/O1B4I
49
The reaction fof introducing a compound (13)
to a compound (2a), and the reaction for introducing a
compound (2a) to a compound (2) are carried out under
the reaction condition similar to that employed in the
hydrolysis for introducing a compound (1e) to a com-
pound (1f) among the reactions shown in the above-
mentioned Reaction formula-4. The reaction of a com-
pound (9a) with a compound (10a) is carried out under
the reaction condition similar to that employed in the
above-mentioned reaction of a compound (9a) with a
compound (10), or is carried out in a suitable solvent,
in the presence or absence of an acid, in the presence
of an oxidizing agent. As to the solvent to be used
therein, water; lower alcohol such as methanol,
ethanol, isopropanol and the like; ethers such as
dioxane, tetrahydrofuran, ethylene glycol dimethyl
ether and the like; fatty acids such as acetic acid,
formic acid and the like; n-hexane; aromatic hydro-
carbons such as benzene,-toluene and the like; and
mixtures of these solvents can be exemplified. As to
the oxidizing agent to be used therein, iodine, nitro
compounds such as nitrobenzene; dehydrogenating
catalysts such as palladium-carbon, can be exemplified.
A compound (10a) may be used generally at
least in an equimolar quantity, preferably in an
equimolar to 3 times the molar quantities to a compound
(9a). An oxidizing agent may be used generally in 0.1
times the molar quantity or more, preferably 0.1 to 2
P °'
WO 97/03070 2 1 9 8 2 6 6 PCT/JP96/01841
times the molar-quantities. The reaction is completed
within for about 10 minutes to 5 hours. The reaction
temperature and the acid to be used are similar to the
reaction conditions employed in the above-mentioned
5 reaction of a compound (9a) with a compound (10). In
said reaction, when an_oxidizing agent is added, then
the desired compound (2a) of high purity can be
obtained in high yield.
Reaction formula-6
R3a ~~R~a R7X (4) ~ Rzb~~~RZo
n n
(3 a) (3 b)
10 [wherein R'', R3b, A, n, R' and X are the same as defined
above; RZ° is an amino group or a group capable to
convert into an amino group].
As to a group of-RZ° capable to convert into
an amino group, groups which can be converted into an
15 amino group by conventional method, e.g., reduction,
hydrolysis or the like, such as a nitro group, a cyano
group, an azide group, a phthalimide group, can be
exemplified.
The reaction of a compound (3a) with a com-
20 pound (4) is carried out under the reaction condition
similar to that employed in the reaction of a compound
2198266
W 0 97f03070 PCT1JP96/01841
51
(la) with a compound (4) in the above-mentioned
Reaction formula-2.
Reaction formula-7
RsH (5) R~a~A~Rzo
Ra~~~,~Rza
n n
(3 c) ~ (3 d)
[wherein R3', R3d, A, n, Rz° and R8 are the same as
defined above.].
The reaction of a compound (3c) with a com-
pound (5) is carried out under the reaction condition
similar to that employed in the reaction of a compound
(lc) with a compound (5) in the above-mentioned
Reaction formula-3.
Reaction formula-8
Rs.~A~,R2a ~ R3t~A~,Rzo
a n
(3 e) ~(3 f)
R1IX (6)
(Ri~)24 R140H (8)
RIZCOR13 (7) (9)
Rsc~A.~Rzo R3h~A~Rzo
n n
(3 g) (3 h)
\ 1 , . ..y.'~. ~ !'
WO 97/03070 ' ~ 219 8 2 6 6
PCT/JP96/01841
52
[wherein Rye, A, I1, R2°, R3f, R3g R3h R11 R12, R13
i
RS' and X are the same as defined above].
The reaction for introducing a compound (3e)
to a compound (3f) is carried out under the reaction
S condition similar to that employed in the reaction of a
compound (1e) with a compound (1f) in the above-
mentioned Reaction formula-4.
The reaction of a compound (3f) with a
compound (6) or a compound (7) is carried out under the
reaction condition similar to that employed in the
reaction of a compound (1f) with a compound (6) or a
compound (7) in the above-mentioned Reaction formula-4.
The reaction of a compound (3f) with a
compound (8) or a compound (9) is carried out under the
reaction condition similar to that employed in the
reaction of a compound (1f) with a compound (8) or a
compound (9) in the above-mentioned Reaction formula-4.
Each one of compounds (3a), (3b), (3c), (3d),
(3e), (3f), (3g) and (3h) wherein RZ° is nitro group,
can be introduced to each one of the corresponding
compounds (3a), (3b), (3c), (3d), (3e), {3f), {3g) and
(3h) wherein RZ° is amino group by reducing reaction.
Said reducing reaction is carried out for example (i)
by reducing each one of the former compounds in a
suitable solvent by using a hydrogenation catalyst or
(ii) by reducing each one of the former compounds in a
suitable inert solvent, by using a chemical reducing
agent such as a mixture of a metal or metal salt with
2198266
W097I03070 PCT/JP96/01841
53
an acid; or a metal or met31 salt with an alkali metal
hydroxide, sulfide, ammonium salt; or a hydride
reducing agent such as lithium aluminum hydride.
In case of conducting the above-mentioned
method of {i) by using the hydrogenation catalyst, as
to the solvents for example, water, acetic acid,
alcohols such as methanol, ethanol, isopropanol and the
like; hydrocarbons such as hexane, cyclohexane and the
like; ethers such as dioxane, tetrahydrofuran, diethyl
ether, diethylene glycol dimethyl ether and the like;
esters such as ethyl acetate, methyl acetate and the
like; aprotic polar solvents such as N,N-dimethyl-
formamide and the like; and mixtures of these solvents
can be exemplified. As to the catalyst to be used for
catalytic hydrogenation, palladium, palladium-black,
palladium-carbon, platinum, platinum oxide, copper
chromite, Raney nickel and the like can be exemplified.
The catalyst may be used generally, in an amount of
0.02 to an equivalent quantity to the starting
material. The reaction is carried out generally at
about -20 to 150°C, preferably at about 0 to 100°C, and
under 1 to 10 atmospheric pressure of hydrogen gas, and
the reaction is completed generally within 0.5 to 10
hours. Further, an acid such as hydrochloric acid may
be added to the reaction system.
In case of conducting method of (ii) as
above, a mixture of iron, zinc, tin or stannous
chloride with a mineral acid such as hydrochloric acid
.__ .(. ; _, . 219826b
WO 97/03070 PCT/JP96/01841
54
or sulfuric acid; or iron, -ferrous sulfate, zinc or
tin with an alkali metal hydroxide such as sodium
hydroxide, a sulfide such as ammonium sulfide, ammonia
water, an ammonium salt such as ammonium chloride; or a
hydride reducing agent such as lithium aluminum hydride
may be used asa reducing agent. As to the inert
solvent to be used in the-reaction, water, acetic acid,
methanol, ethanol, dioxane or the like may be exempli-
fied. In case of using lithium aluminum hydride as the
reducing agent, ethers such as diethyl ether, dioxane,
tetrahydrofuran, diglyme and the like may preferably be
used as the solvent. The condition of the above-
mentioned reducing reaction may be suitably selected in
accordance with the reducing agent to be used, for
example, in case of using a mixture of stannous
chloride with hydrochloric acid as the reducing agent,
the reaction may be carried-out advantageously at about
0 to 80°C, and for about 0.5 to 10 hours. The reducing
agent is used at least in an equimolar quantity,
generally in an equimolar to 5 times the molar quanti-
ties to the starting compound.
Each one of compounds (3a), (3b), (3c), (3d),
(3e), (3f), (3g) and (3h), wherein RZ° is nitrile group
can be introduced to each one of the corresponding
compounds (3a), (3b), (3c), (3d), (3e), (3f), (3g) and
(3h), wherein RZ° is amino group by reducing reaction.
For this reducing reaction, a hydride reducing agent is
preferably used. As to the hydride reducing agent,
2198266
W 0 97103070 PCT/JP96101841
lithium aluminum hydride, lithium borohydride, sodium
borohydride, diborane and the like can be exemplified.
The reducing agent is used at least in an equimolar
- quantity, preferably in the range of an equimolar to 15
5 times the molar-quantities to the starting compound.
Said reducing reaction is carried out in a suitable
solvent, for example water; lower alcohols such as
methanol, ethanol, isopropanol and the like; ethers
such as tetrahydrofuran, diethyl ether, diisopropyl
10 ether, diglyme and the like; and mixtures of these
solvents, and generally at about -60 to 150°C, prefer-
ably -30 to 100°C, and for about 10 minutes to 15
hours. In case of using lithium aluminum hydride or
diborane as the reducing agent, anhydrous solvents such
15 as tetrahydrofuran, diethyl ether, diisopropyl ether,
diglyme and -the like can be used as the solvent.
Further, in case of using sodium borohydride as the
reducing agent, the reaction is advantageously
proceeded by adding a metal halide such as cobalt
20 chloride or the like to the reaction system.
Each one of compounds (3a), (3b), (3c), (3d),
(3e), (3f), (3g) and (3h), wherein RZ° is a phthalimido
group can be introduced to each one of the correspond-
ing compounds (3a), (3b), (3c), (3d), (3e), (3f), (3g)
25 and (3h), wherein RZ° is an amino group by treating
under the reaction condition similar to that of
employed in the reaction for introducing compound (1e)
to compound (1f) in the above-mentioned Reaction
,.
WO 97/03070 , ' 219 8 2 6 6 pCTIJP96/01841
56
formula-4.
Each one of compounds (3a), (3b), (3c), (3d ,
(3e), (3f), (3g) and (3h), wherein RZ° is an azido
group can be introduced to each one of the corre- -
spond.ing compounds (3a), (3b), (3c), (3d), (3e), (3f),
(3g) and (3h), wherein Rz° is an amino group by
treating under the condition similar to those employed
in the above-mentioned reduction of vitro group by
using a catalytic hydrogenation or rednction of nitrile
group by using a hydride reducing agent.
Reaction formula-9
N RZX (15) ~ N
~?-COORzi --- C~ ~~-COOHzi
Rl H R~ Nz
R
(1 4} (2 b)
jwherein Rl, RZ and X are the same as defined above; R2'
is a hydrogen atom or a lower alkyl group].
The reaction of-a compound (14) with a com-
pound (15) is carried out- under the reaction condition
similar to that employed in the reaction of a compound
(la) with a compound (4) as shown in the above-
mentioned Reaction formula-2.
A compound represented by the general formula
CA 02198266 2005-05-11
25711-773
- 57 -
(1), wherein R3 is a substituted or unsubstituted
2(1H)-quinolinonyl group can be introduced to the
corresponding compound wherein R3 is a substituted or
-- unsubstituted 3,4-dihydro-2(H)-quinolinonyl group when
the former is subjected to reducing reaction.
A compound represented by the general formula
{1), wherein R3 is a substituted or unsubstituted 3,4-
dihydro-2(1H)-quinolinonyl group can be introduced to
the corresponding compound wherein R3 is a substituted
or unsubstituted 2(1H)-quinolinonyl group when the
former is subjected to dehydrogenation reaction.
In carrying out the above-mentioned reducing
reaction, a usual catalytic hydrogenation condition can
be applied. As to the catalyst to be used in the reac-
' 15 tion, metal catalysts such as palladium, palladium-
carbon, platinum, Raney nickel and the like can be
exemplified, and such a catalyst is used in usual
catalytic quantity. Further, as to the solvent to be
used in the reaction, alcohols such as methanol,
ethanol, isopropanol and the like; ethers such as
dioxane, tetrahydrofuran and the like; aliphatic
hydrocarbons such as hexane, cyclohexane and the like;
esters such as ethyl acetate; fatty acids such as
acetic acid can be exemplified. This reducing reaction
can be carried out either under normal pressure or
under high pressure condition, and generally about
under normal pressure to 20 kg/cm2, preferably under
normal pressure to 10 kg/cm2. The reaction may be
*Trade-mark
CA 02198266 2005-05-11
25711-773
- 58 -
carried out generally at about 0 to 150°C, preferably
at about room temperature to 100°C.
The above-mentioned dehydrogenation reaction
is carried out in a suitable solvent, by using an
oxidizing agent. As to the oxidizing agent, for
example benzoquinones such as 2,3-dichloro-5,6-
dicyanobenzoquinone, chloranil(2,3,5,6-tetra-
chlorobenzoquinone) and the like; N-bromosuccinimide,
N-chlorosuccinimide, halogenating agents such as
bromine and the like; dehydrogenation catalysts such as
selenium dioxide, palladium-carbon, palladium-black,
palladium oxide, Raney nickel .and the like can be
exemplified. The amount of the halogenating agent is
not specifically restricted, and can be suitably
selected from a wide range, generally about 1 to 5
times, prefereably 1 to 2 times the molar quantities
may be used to the starting compound. The dehydrogena-
tion catalyst may be used in a usual catalytic amount.
As to the solvent, ethers such as dioxane, tetra-
hydrofuran, methoxyethanol, dimethoxyethanol and the
like; aromatic hydrocarbons such as benzene, toluene,
xylene, cumene and the like; halogenated hydrocarbons
such as dichloromethan, dichloroethan, chloroform,
carbon tetrachloride and the like; alcohols such as
butanol, amylalcohol, hexanol and the like; protic
polar solvents such as acetic acid; aprotic polar
solvents such as dimethylformamide, dimethyl sulfoxide,
hexamethylphosphoric trimamide and the like can be
*Trade-mark
W O 97!03070 ' ' - 219 8 2 6 6 PC.ly~6~01841
59
exemplified. Said reaction is carried out generally at
about room-temperature to 300°C, preferably at about
room temperature to 200°C, and is completed generally
- for about 1 to 40 hours.
Among-compounds represented by the general
formula (1)~--a-compound having acidic group can form a
salt with pharmaceutically accep~Able basic compound.
As to such basic compound for example, metal hydroxides
such as sodium hydroxide, potassium hydroxide, lithium
hydroxide, calcium hydroxide and the like; carbonates
or bicarbonates of alkali metals such as sodium
carbonate, sodium hydrogencarbonate and the like;
alkali metal-alcoholates such as sodium methylate,
potassium ethylate and the like can be exemplified.
Furthermore, among compounds represented by the general
formula (1), a compound having basic group can form a
salt with common pharmaceutically acceptable acid. As
to such acid for example, inorganic acids such as
sulfuric acid, nitric acid, hydrochloric acid, hydro-
bromic acid and the like; organic acids such as acetic
acid, p-toluenesulfonic acid, ethanesulfonic acid,
oxalic-acid, malefic acid, fumaric acid, citric acid,
succinic acid, benzoic acid and the like can be
mentioned. These salts can also be used, similar to
compounds represented by the general formula (1) in
free form, as compounds of effective ingredient in the
present invention. Moreover, compounds represented by
the general formula (1) involve inevitably their-
CA 02198266 2005-05-11
25711-773
- 60 -
sterioisomers and optical isomers, and these isomers
can also be used as compounds of effective ingredients.
The objective compounds prepared by each of
- these Reaction formulas-1 to -4 can be isolated from
5~ the reaction system by common separating methods, and
can be further purified. As to methods for separation
and purification, for example, distillation, recrystal-
lization, column chromatography, ion-exchange chromato-
graphy, gel chromatography, affinity chromatography,
preparative thin layer chromatography, solvent extrac-
tion and others can be applied.
POSSIBILITY OF INDUSTRIAL UTILIZATION
Benzimidazole derivatives represented by the
general formula (1) are used for pharmaceuticals as in
the forms of usual general pharmaceutical preparations.
The pharmaceutical preparations are formulated by
using usually used diluents such as fillers, bulking
fillers, binders, wetting agents, disintegrants,
surface active agents, lubricants; or excipients. The
pharmaceutical preparations can be selected from
various administration forms in accordance with the
therapeutic purposes. As to typical administration
forms, there can be exemplified tablets, pills,
powders, liquids, suspensions, emulsions, granules,
2~~ capsules, suppositories, injection preparations
(liquids, suspensions, etc.) and the like. For the
purpose of shaping the administration unit form into
WO 97f03070 , ' 219 8 2 6 6 pCTlJP96/01841
61
the tablets, various carriers which are well-known in
this field can be widely used. As to the examples of
carriers, excipients such as lactose, white sugar,
sodium chloride, glucose, urea, starch, calcium
carbonate, kaolin, crystal-line cellulose, silicic acid
and the like; binders such as water, ethanol, propanol,
simple syrup, glucose solution;, starch solution,
gelatin solution, carboxymethyl cellulose, shellac,
methyl cellulose, potassium phosphate, polyvinyl-
pyrrolidone and the like; disintegrants such as dry
starch, sodium alginate, agar-agar powder, Iaminaran
powder, sodium hydrogencarbonate, calcium carbonate,
polyoxyethylene sorbitan fatty acid esters, sodium
laurylsulfate, monoglyceride of stearic acid, starch,
lactose and-the like; disintegration inhibitors such as
white sugar; stearin, cacao butter, hydrogenated oils
and the like; absorption accelerators such as
quaternary ammonium salts, sodium laurylsulfate and the
like; wetting agents such as glycerin, starch and the
like; adsorbents such as starch, lactose, kaolin,
bentonite, colloidal silicic acid and the like;
lubricants such as refined talc, stearates, boric acid
powder, polyethylene glycols and the like can be
mentioned. The tablets preparations can be further
shaped into tablets coated with usual tablet coating,
for example sugar coated tablets, gelatin film coated
tablets, tablets coated with enteric coating, tablets
coated with film coating, or double layer tablets and
.-.a,- . w 2198266
WO 97/03070 PCT/JP96/01841
62
multiple layer tablets. For the purpose of shaping the
administration unit into pills, various carriers which
are .yell-known in this field can be widely used. As to
the examples of carriers, excipients such as glucose,
lactose, starch, cacao butter, hydrogenated vegetable -
oils, kaolin; talc and-the like; binders such as
powdered acacia, powdered tragacanth, gelatin, ethanol
and the like; disintegrants such as laminaran, agar-
agar and the like can be exemplified. For-the purpose
of shaping the administration unit into suppositories,
various carriers.which are well-known in this field can
be widely used. As to the- examples of carriers, poly-
ethylene glycols, cacao butter, higher alcohols, esters
of higher alcohols, gelatin, semi-synthesized
glycerides and the like can be mentioned. For the
purpose of shaping the administration unit form into
capsules, the benzimidazole derivative as the effective
ingredient is mixed with the above-mentioned various
carriers and the mixture thus obtained is placed into
rigid gelatin capsules or soft capsules. For the
purpose of shaping the administration unit into
injection preparations, liquid preparations, emulsion
preparations and suspension preparations are
sterilized, further these preparations are preferably
isotonic to the blood, and the all diluents which are
conventionally used in this field can also be used for
example, water, ethyl alcohol, macrogols, propylene
glycol, ethoxylated isostearyl alcohol, polyoxylated
21982b6
W097103070 PCT/JP96/OI84I
63
isostearyl alcohol, polyoxyethylenesorbitan fatty acid
esters-can be used. Additionally, for the purpose to
prepare isotonic injection solutions, an adequate
amount of sodium chloride, glucose or glycerin may be
added to the injection preparations, further, usual
dissolving additives, buffering agents, local
anesthetics and the like may be-added. Moreover, if
necessary, coloring agents, preservatives, spices,
flavors, sweetening agents and others may be added to
IO the pharmaceutical preparations.
The amount of the benzimidazol derivative as
effective ingredient to be contained in the pharma-
ceutical preparation of the present invention is not
specifically restricted and can be suitably selected
from a wide range, and generally about 1 to 70 ~ by
weight, preferably 5 to 50 ~ by weight of the active
ingredient may be contained in thepharmaceutical
preparations.
Methods for administering the pharmaceutical
preparation of the present invention are not
restricted, they can be administered in accordance with
various forms of preparations, age of the patient,
distinguish of sex and other conditions, the degree of
the symptom and the like, For example, tablets, pills,
liquids, suspensions, emulsions, granules and capsuled
are administered orally. While, injection preparations
are intravascularly administered, singly or by mixing,
With common transfusions such as glucose or amino acid
...: . _. ..7 . :.:. . ,v.
2198266
WO 97/03070 PCT/JP96/01841
64
solutions, and if necessary, they are singly admini-
stered intramuscularly, intracutaneously, sub-
cutaneously or intraperitonealy. Suppositories are
administered to the rectum. _
Dose of pharmaceutical preparation of the
present invention is suitably selected depend on the
usage, age of the patient, distinguish of sex and other
conditions, and degree of the symptom, and generally
the amount of effective compound may be about 0.6 to 50
mg/kg of the body weight per day. The effective com-
pound to be contained in the administration unit form
may preferably be in the range of about 10 to 1000 mg.
The amount of compound of the effective
ingredient to be formulated in the pharmaceutical
preparation for external use of-the present invention
is not specifically restricted and can be suitably
selected from a wide range, generally, 0.01-to 20 ~ by
weight, preferably 0.1 to 5 $ by weight thereof may be
formulated.
As to the basic excipients used for external
pharmaceutical preparations of the present invention,
oily bases and water-soluble bases which are well
known in this field can be selected from a wide range,
provided that they show not any phamacological activi-
ties by themselved. As to the oily bases, specifically ,
oils and fats such as peanut oil, rubber oil, soybean
oil, corn oil, rapeseed oil, cottonseed oil, castor
oil, camellia oil, coconut-oil, olive oil, cacao
2198266
WO 97/03070 PCT/JP96/0184I
s5
butter, lanolin, beef tallow, squalane and wool fat and
the like; chemically changed such as hydrogenated
reformed products of these fats and oils; mineral oils
such as vaseline, paraffin, silicone oil; higher fatty
acid esters, such as isopropyl myristate, n-butyl
myristate, isopropyl linolate, cetyl licinolate,
stearyl licinolate, diethyl sebacate, disopropyl
adipate; higher aliphatic alcohol such as cetyl
alcohol, stearyl alcohol; waxes such as bleched
beeswax, spermaceti-, Japan wax and the like; higher
aliphatic acid such as stearic acid, oleic acid,
palmitic acid and the like; mixtures of mono-, di- and
tri-glycerides of natural saturated fatty acid having
12 to 18carbon atoms and the like can be exemplified.
Among these fats and oils, the various vegetable oils
and the mixtures of mono-, di- and tri-qlycerides are
especially preferable. Furthermore, as to the water-
soluble basic excipients, specifically polyethylene
glycol, propylene glycol, glycerin, glycerogelatin,
methyl cellulose, hydroxymethyl cellulose, hydroxyethyl
cellulose, hydroxypropyl cellulose, hydroxypropylmethyl
cellulose, hydroxyvinyl polymer, polyvinyl alcohol and
the like can be exemplified. In the present invention,
these basic excipients may be used singly or may be
used by mixing with 2 or more of them.
In using the pharmaceutical preparations for
external use of the present invention, commonly used
additives such as water, surface active agents, gelling
:,
WO 97/03070 T~ 219 8 2 6 6 pCT/JP96101841
66
agents, preservatives, antioxidant, buffering agents,
pH controlling agents, wetting agents, antiseptics,
coloring agents, fragrant agents and the like can be
suitably added thereto in accordance with the
necessity.
The form of pharmaceutical preparations for
external use of the present invention is not specifi-
cally restricted, and in the forms of an ointment,
cream, lotions, emulsion and gel are preferably used,
such forms of them can be prepared in accordance with
usual methods.
EXAMPLES
In order to explain more clearly, the present
invention will be illustrated by referring to Examples
15- of pharmaceutical preparations, Reference examples,
Examples and Pharmacological tests as follow.
Example of pharmaceutical preparation-1
1-Benzyl-6-chloro-2-{1-[3-(1
pyrazolyl)propyl]indol-5
ylaminocarbonyl}benzimidazole 150 g
Avicel -
(Trademark for microcrystalline
cellulose, manufactured by Asahi
Chemical Industry CO., Ltd.) 40 g
Corn starch 30 g
Magnesium stearate 2 g
WO 97103070
pCT~JP96~01841
67
Hydroxygropylmethylcellulose IO g
Polyethylene glycol 6000 3 g
Castor oil 40 g
Ethanol 40 g
1-Benzyl-6-chloro-2-{1-[3-(1-pyrazolyl)-
propyl}indol-5-ylaminocarbonyl}benzimidazole of the
present invention, Avicel, corn starch and magnesium
stearate were mixed together and ground, the thus
obtained mixture was shaped into the form of tablets
by using a conventional pounder (R 10 mm) for sugar
coating-.- The thus obtained tablets were coated with
a film coating agent consisting of hydroxypropylmethyl
cellulose, poloxyethylene glycol 6000, castor oil and
ethanol to prepare film coated tablets.
Example of pharmaceutical preparation-2
1-Benzyl-6-chloro-2-jl-isopropyl-
tetrazol-5-yl)methyl-3,4-dihydro-
2(1H)-quinolinon-6-yl-
aminocarbonyl]benzimid_azole 150.0 g
Citric acid 1.0 g
Lactose 33.5 g
Dicalcium phosphate 70.0 g
Pluronic F-68 30.0 g
Sodium laurylsulfate 15.0 g
Polyvinylpyrrolidone 15.0 g
Polyethylene glycol (Carbowax 1500) 4.5 g
., t .,
WO 97103070 ~ ' ' - ~ ' ' ' 219 8 2 6 6 pCTIJP96101841
6$
Polyehtylene glycol (Carbowax 6000) 45.0 g
Corn starch 30.0 g
Dried sodium stearate 3.0 g
Dried magnesium stearate 3.0 g
Ethanol q.s.
1-Benzyl-6-chloro-2-[1-isopropyltetrazol-
5-yl)methyl-3,4-dihydro-2(1H)-quinolinon-6-ylamino-
carbonyl]benzimidazole, citric acid, lactose, dicalcium
phosphate, Pluronic F-68 and sodium laurylsulfate were
mixed- together.
The thus obtained mixture was sieved through
a sieve of-No. 60, the obtained sieved mixture was
granulated under wet condition with,an alcohol solution
containing polyvinylpyrrolidone, Carbowax 1500 and
6000 The granulated product was formed to paste like
lump by adding ethanol, as occasion arises. Next, corn
starch was added thereto and mixing operation of said
mixture was continued until uniform granules were
formed. The granules were sieved through a sieve-of
No. 10, then the sieved granules were placed in a tray
and dried at 100°C in an oven for 12 to 14 hours. The
dried granules were sieved-through a sieve of No. 16,
then dried sodium laurylsulfate and dried magnesium
stearysulfate were added to the dried granules. The
whole mixture of dried-granules were mixed well and
were compressed, by using a tablet machine, into the
9
desired shape of tablets to be used for the core
2198266
WO 97103070 PCT/JP96/01841
69
portions o~ coated tablets.
The above-mentioned core portions of the
tablets were treated with a varnish, and the surface
thereof were coated with talc powder for preventing
from the absorption of moisture. The surface of the
treated core portions were-further coated with a
primary coating layer, and were further coated with a
varnish to make them having a sufficient number of
layers on the surface:for preparing coated tablets for
oral administration. In order to make the coated core
portions of tablets into complete spherical form and to
make the treated surface smoothly, the coated tablets
were further coated with primary coating layers and
smoothing coating layers. The coated tablets were
color coated until the desired color of the surface
were obtained. After the coated tablets were dried,
the surface thereof-were polished to make them uniform
gloss.
Example of pharmaceutical preparation-3
1-Benzyl-6-chloro-2-~1-j3-
(imidazol-1-yl)propyl]indol-5-
ylaminocarbonyl}-
benzimidazole 5.0 g
-Polyethylene glycol (M.W. 4000) 0.3 g
Sodium chloride p,9 g
Polyoxyethylene sorbitan monooleate 0.4 g.
Sodium metabisulfite 0.1 g
2198266
,.
W0 97103070 PCT/JP96/01841
Methylparaben 0.18 g
Propylparaben 0.02 g
Distilled-water for injection 10.0 m1
The above-mentioned Methylparaben, Propyl-
5 paraben, sodium metabisulfite and sodium chloride were
dissolved in a half volume of the above-mentioned
distilled water at 80°C, under stirring. The solution
thus obtained was cooled to- 4-0°C, then 1-benzyl-6-
chloro-2-{1-[3-(imidazole-1-yl)propyl]indol-5-
10 ylaminocarbonyl~benzimidazole of the effective
ingredient of the present invention, next polyethylene
glycol and polyoxyethylene sorbitan monooleate were
dissolved in this order in the above-mentioned solu-
tion. Then to the thus obtained solution was added the
15 remaining volume of distilled water for injection to
adjust the final volume of the injection composition
into the predetermined volume, then was sterilized by
sterilizing filtration by using a suitable filter paper
to prepare injection preparation.
Reference example 1
To 100 ml of acetic acid solution containing
20 g of 2-benzylamino-4-chloroaniline was added 15 m1
of 0-methyl-trichloroacetoimidate at 0 to 25°C, and .
stirred the mixture at room temperature for 3 hours.
Then water was added to the reaction mixture, the
separated crystals were collected by filtration to
:', . 2198266
W 0 97103070 PCT/JP9610I84I
71
obtain 29.6 g of 1-benzyl-6-chloro-2-trichloromethyl-
benzimidazole in the form of pale brown powder.
1H-NMR (250 MHz, DMSO-db) 8 ppm:
5.94 (2H, s), 7.04 (2H, d, J=6.5Hz),
7.25-7.5 (5H, m), 7.88 (1H, d, J=9.OHz).
Reference example 2
Fifty (50) ml of methanol suspension
containing 5 g of 1-benzyl-6-chloro-2-trichloro-
methylbenzimidazole and 7.7 g of potassium carbonate
was heated and refluxed for 24 hours. After the
reaction mixture was filtrated, the solvent was removed
by distillation under reduced pressure, the residue
thus obtained was dissolved in chloroform, then after
removal of the insoluble matters by filtration, the
solvent was removed by distillation to obtain 4.7 g of
1-benzyl-6-chloro-2-trimethoxymethylbenzimidazole in
the form of brown oily substance. Said oily substance
was dissolved in 50 ml of acetone, and 1 g of
p-toluenesulfonic acid was added, the mixture was
refluxed for 2 hours, the solvent was removed under
reduced pressure. The residue thus obtained was
dissolved in chloroform, and the solution was washed
with water, an aqueous solution saturated with sodium
hydrogencarbonate, then was dried with anhydrous
magnesium sulfate, and the solvent was removed by
distillation. The residue was crystallized by using
diisopropyl ether-ethyl acetate to obtain 2.84 g of
r~ r
WO 97/03070 - ~ ~ - 219 8 2 6 6 PCT/JP96/01841
72
methyl 1-benzyl-6-chlorobenzimidazole-2-carboxylate in
the form of light brown powdery product.
Melting point-. 184-186°C._
Reference example 3
To 4.4 g of 5-nitro-1-(3-phthalimidopropyl)-
indole was added 200 ml of-dimethylformamide, further
was added 0.15 g of 10$ palladium-carbon and
hydrogenized at 65°C, under the pressure of 4 kg/cmz,
for 7 hours. After the reaction was finished, the
reaction mixture was filtrated, and the solvent was
removed by distillation under reduced pressure. The
thus obtained residue was treated to a silica column
chromatography (eluent: 3~ methanol/dichloromethane) to
obtain 3.4 g of 5-amino-1-(3-phthalimidopropyl)indole
in the form of brown needle crystals.
1H-NMR (250MHz, CDC13) & ppm:
2.16-2.28 (2H, m), 3.73 (2H, t, J=7Hz), 4.12
(2H, t, J=7Hz), 6.28 (1H, d, J=3Hz), 6.64-
6.69 (1H, m), 6.9 (1H, d, J=2Hz), 7.11-7.14
(2H, m), 7.7-7.73 (2H, m), 7.82-7.86 (2H, m).
By using suitable starting materials, and by
method similar to that employed in Reference example 3,
there were obtained compounds of Reference examples ,
15-22, 26-33, 47 and 49.
i
CA 02198266 2005-05-11
25711-773
- 73 -
Reference example 4
To 2.3 g of lithium aluminum hydride was
added 100 ml of tetrahydrofuran, under stirring
- condition, and 6 g of 5-cyano-1-[3-(2-isopropyl-
imidazol-1-yl)propyl)indole was gradually added
thereto. The mixture was refluxed for 4 hours, then
after confirmation of that the reaction was finished,
under cooling at 0°C, 2.3 ml of water, 2.3 ml of 10$
aqueous solution of potassium hydroxide and 7 ml of
water were gradually added thereto. The reaction
mixture was diluted with ethyl ,acetate, then filtrated
with Celite, and the solvent was removed by distil-
lation, 5.3 g of 5-aminomethyl-1-[3-(2-isopropyl-
imidazol-1-yl)propyl]indole was obtained in the form of
yellow oily product.
1H-NMR (250MHz, CDC13) 8 ppm:
1.22 (6H, d, J=7Hz), 2.3-2.4 (2H, m),
2.7-2.8 (2H, m), 3.81 (2H, t, J=7.5Hz),
3.95 (2H, s), 4.16 (2H, t, J=7Hz),
6.51 (1H, d, J=3Hz), 6.77 (1H, d, J=l.SHz),
6.98-7.04 (2H, m), 7.19 (2H, s),
7.57 (1H, s).
By using a suitable starting material and by
a method similar to that employed in Reference example
4, there was obtained a compound of Reference example 5
shown in Table 1 as follows.
*Trade-mark
2198266
WO 97/03070 PCT/JP96/01841
74
Table 1 R3-(A)n-RZ°
Reference
example No. R2° -!A)n- R' Crvstal form
NHZ -CH2- \ I I Brown solid -
H
5
Reference example 6
1.5 Grams of 5-nitroindole was dissolved in
70 ml of dimethylformamide, then 370 mg of sodium
hydride (in oil) was added thereto, the mixture was
stirred under nitrogen gas stream at 60°C for 1 hour.
Under cooling at 0°C, 1.63 g of 5-chloromethyl-1-
isopropyl-1,2,3,4-tetrazole was added, the reaction
mixture was stirred at room temperature for 4.5 hours.
After the reaction was finished, water Was added to the
reaction mixture, then the separated crystals were
collected by filtration and washed with water. The
crystals were dissolved in dichloromethane, the
solution was dried with anhydrous magnesium sulfate,
and the solvent was removed by distillation under
reduced pressure. The residue obtained was subjected
to a silica gel column chromatography (eluent:
dichloromethane ~ 3~ methanol/dichloromethane), there
was obtained 2.3 g of 1-(1-isopropyl-1,2,3,4-tetrazol-
5-ylmethyl)-5-nitroindole as in the form of yellow
powder.
_,
2198266
WO 97!03070 PCT1JP96/OI84I
1H-NMR (250MHz, CDC13) 6 ppm:
1.35 (6H, d, J=6.5Hz), 4.37-4.47 (1H, m),
5.70 (2H, s), 6.79-6.81 (!H, m),
7. Z7-7.30 (1H, m), 7.48 (1H, d, J=9Hz),
5 8.12-8.17 (1H, m), 8.59 (1H, d, J=2Hz).
By using suitable.starti.ng materials and by
method similar to that employed in Reference example 6;
there were obtained compounds of-Reference examples 7
to 49 as shown in fiables 2 to 8 as follows.
2198266
WO 97/03070 PCT/JP96/01841
76
Table 2 R3 - ;A; n-R~ °
Reference
example No. R2° -(A)n- R' Crystal form
NO - - fellow
powdery
/ I I Product
/ \
(CH~~-.-N
8 N0Z _ Yellow
OCH3 powdery
/ \ Product
(CH~y3-N /
9 NOa - Yellow
powdery
product
(CHzya
NOi - ~ Yellow
/ powdery
N product
(CH~3 / \
11 NOZ - Brown
oily
product
i N- N
--~ ~N
(~W ~,~
1' .CH3
CH
~CHS
12 NOa - Brown
powdery
product
WO 97/03070 219 8 2 6 6 pCT/~1018d1
77
Table 3 R' - (A) n-R'
Reference
example No. R2° -(A)n- R' Crystal form
13 NGa - ~ Dark ye:low
y powdery
J I I ~ product
y
(CH~~N /
14 NOi - ~ I I ~ Dark yellow
powdery
(CH~~N / product
15 NH2 _ ~ I I I
(CH~y~ /
16 NH2 - H3 Brown oily
y,,. / \ product
(~~~H /
17 NHx - Pale brown
oily product
18 NH, - Pale brown
I N oily product
° (CHp I
219266
WO 97/03070 PCTL1P96/01841
78
Table 4 R'
-
(A;
n-Rz
Reference
=-:campieNc. R2 - (A1 _ Crvstai form
n- R'
19 NHz - Biack oily
prcduct
N
-N
~
~
W1JW~N
~W.r3
~~3
2C NHz - Brown
powdery
product
21 NH, - ~ Dark violet
oily product
1' h
(CH~~N
22 NH, - Dark violet
oily product
W
(CHZ)TN
23 NHz -CHi - Yellow oily
.CH3 product
~CH3
i ' N
ccH~,N J
24 CN - Pale yellow
oily product
(CH~yCI
WO 97103070 219 8 2 6 b pC.IyJpg6/OI84I
79
Table 5 - R' - (A) n-Az °
Reference
example No. R'° -(A)n- R' Crystal form
25 NH2 -
i ~
\ O
i
CHx CH=CHI
26- NHp Brown oily
product
i N
t~H~sL~
27 NH? - Oily product
w (CH~~~
28 NHz - Brown oily
product
tCH~~N
29 NH2 - Black oily
product
(CH~y
30 NHz - Brown oily
product
(CH~~N.N
~;CH~
~i
31 NH2 - Brown oily
product
N,
CH2 -~i
~N
~CH~
2198266
WO 97/03070 PCT/JP96/01841
Table 6 R' - (A) n-R'
Reference
example No. R'° -(A)n- R' Crystal form
32 NH, - Brown needle
crystals
i i \
(CH~3
O
33 NHy - Brown oily
product
34 N08 - Ltt~ Yellow
powdery
product
OH
(CH~7
/ \
35 N02 - Yellow,
i powdery
\ product
36 NOz -- , Yellow
\ ( i O powdery
i \ product
(cH,~,
0
37 NOz - ~' i I Yellow
\ powdery
product
(CHi}~C!
38 NO= - ~ i~ i Yellow
\ powdery
(CH~3~ Product
WO 97103070 - - . ~. 219 8 2 6 6 pCT/JP96/01841
81
Table 7 R' - (A) n-R'
Reference
example No. R' ° - (A) n- R' Crystal form
39 NOi - Yellow
/ ~ ~ Powdery
product
~N
(CH~~~~
40 NOz - / Yellow oily
product
(CH~3C1
41 NOa - I I Yellow oily
N_~ product
(CH~r-~:~N ~
s
~CH~
42 NO$ - Yellow
I powdery
r' -~ product
CH2 -~ 1,1.N
~3
~3
43 NO$ - Yellow
powdery
~N product
(CHij3N 7
I
CH3
~3
44 ' CN - ~ I I ~ ~3 Pale yellow
oily product
(CH~3~N
45 NOa - / I ~ Brown
powdery
product
(CH~3Cl
WO 97/03070 ' ' 219 8 2 6 6 pCT/JP96/01841
82
Table 8 R~ - (A) n-R' °
Reference
example No. Rz° -(A)n- Rj Crystal form
46 NO= - O Yellow- solid
product
\ ~
~N
(~~3~ J
47 NH2 - ,. I ~ Black oily
\ product
i /~ N
(CH~y
/ O
48 N02 - Yellow
,~ \ powdery
(CHI ~. / product
O
49 NHz - O Dark brown
oily product
/
I/
O
2198265
WO 97/03070 - - PCT/JP96/OI84I
83
The NMR spectrum data of compounds obtained
in the above-mentioned Reference examples are shown as
follows.
Compound of Reference example 5
_'H-NMR (250MHz, CDC13) & ppm:
1.60 (2H, brs), 4.07 (2H, d, J=1Hz),
7.10-7.26 (3H, m), 7.36-7.39 (1H, m),
7.65-7.68 (1H, m) 8.12 (1H, brs).
Compound of Reference example 7
IH-NMR (250MHz, DMSO-db) & ppm:
2.2-2.4 (2H, m), 4.20 (2H, t, J=7.5Hz), 4.29
(2H, t, J=7.5Hz), 6.44 (1H, d, J=3Hz), 6.77
(1H, d, J=3Hz), 7.0-7.2 (2H, m), 7.35-7.45
(2H, m), 7.5-7.75 (3H, m), 7.97-8.02 (1H, m),
8.58 (1H, d, J=2Hz).
Compound of Reference example 8
1H-I~IR ( 250MHz , CDC13 ) 8 ppm:
2.4-2.5 (2H, m), 3.86 (3H, s),
4.07-4.13 (4H, m), 6.47 (1H, t, J=2.5Hz),
ZO 6-.69 (1H, d, J=3.SHz), 6.83-6.88 (1H, m),
7.01 (1H, d, J=3Hz), 7.07-7.18 (4H, m),
8.06 (1H, dd, J=2.5Hz, 9Hz),
8.59 (1H, d, J=2.5Hz).
WO 97/03070 2 i 9 8 2 6 b PCT/JP96/01841
84
Compound of Reference example 9
iH-NMR (250MHz, DMSO-db) b ppm:
2.2-2.3 (2H, m), 3.97 (2H, t, J=6Hz),
4.46 (2H, t, J=7Hz), 6.77 (1H, d, J=3Hz),
7.23-7.27 (1H, m), 7.37-7.51 (3H, m),
7.67-7.72 (2H, m), 7.98 (1H, dd, J=2Hz, 9Hz),
8.57 (1H, d, J=2Hz).
Compound of Reference-example 10
iH-NMR (250MHz, DMSO-db) & ppm:
2.25-2.35 (2H, m), 4.07 (2H, t, J=6Hz),
4.48 (2H, t, J=7Hz), 6.78 (1H, d, J=3Hz),
7.07-7.19 (2H, m), 7.61-7.78 (4H, m),
8.0 (1H, dd, J=2Hz, 9Hz),
8.58 (1H, d, J=2Hz).
Compound of Reference example 1I
1H-NMR (250MHz, CDC13) 8 ppm:
1.57 (6H, d, J=6.SHz), 2.2-2.3 (2H, m),
2.89 (2H, t, J=7Hz), 3.08 (2H, t, J=9Hz),
3.44 (2H, t, J=7Hz), 3.67 (2H, t, J=9Hz),
4.4-4.6 (1H, m), 6.24 (1H, d, J=9Hz),
7.85-7.9 (1H, m), 7.99-8.03 (1H, m).
Compound of Reference example 12
'H-NMR (250MHz, DMSO-db) 8 ppm:
1.9-2.0 (2H, m), 3.01 (2H, t, J=8.SHz),
3.37 (2H, t, J=9Hz), 3.66 (4H, m),
21982b6
W0 97/03070 PCTIJP9610I841
6.51 (1H, d, J=9Hz), 7.78-7.96 (6H, m).
Compound of Reference example 13
IH-NMR (250MHz, DMSO-db) 8 ppm:
2.0-2.1 (2H, m), 3.02 (2H, t, J=8.SHz),
5 3.25 (2H, t, J=7.5Hz), 3.62 (2H, t, J=8.5Hz),
3.74 (3H, s), 4.21 (2H, t, J=7Hz),
6.30-6.35 (2H, m), 6.73-6.78 (1H, m),
7.05 (1H, d, J=2.SHz), 7.32 (1H, d, J=3Hz),
7.38 (1H, d, J=9Hz), 7.80 (1H, s),
10 7.90-7.95 (1H, m).
Compound of Reference example 14
1H-NMR (250MHz, DMSO-d6) 8 ppm:
2.0-2.1 (2H, m), 3. D2 (2H, t, J=8.5Hz),
3.27 (2H, t, J=7.5Hz), 3.62 (2H, t, J=9Hz),
15 4.26 (2H, t, J=7Hz), 6.34 (1H, d, J=9Hz),
6.44-6.45 (1H, m), 6.98-7.15 (2H, m),
7.38 (1H, d, J=3Hz), 7.48-7.56 (2H, m),
7.80 (1H, d, J=2.5Hz), 7.93 (1H, dd,
J=2.5Hz, 9Hz).
20 Compound of Reference example 15
iH-NMR (250MHz, CDC13) s ppm:
2.35-2.45 (2H, m), 4.00-4.11 (4H, m),
6.33-6.34 (1H, m), 6.52 (1H, d, J=3Hz),
6.64-6.68 (1H, m), 6.94-7.22 (7H, m),
25 7.64 (1H, d, J=7.5Hz).
2198266
WO 97/03070 PCT/JP96/01841
86
Compound of Reference example 16
IH-NMR (250MHz, CDC13) & ppm:
2.35-2.45 (2H, m), 3.85 (3H, s),
3.99-4.07 (4H, m), 6.33 (1H, d, J=3Hz),
6.43 (1H, d, J=3Hz), 6.64-6.68 (1H, m),
6.83-6.88 (1H, m), 6.94-6.97 (2H, m),
7.01-7.04 (2H, m), 7.10-7.13 (2H, m).
Compound of Reference example 17
IH-NMR (250MHz, CDC13) b ppm:
2.25-2.35 (2H, m), 3.4-3.6 (2H, br),
3.85 (2H, t, J=6Hz), 4.30 (2H, t, J=6.5Hz),
6.28-6.30 (1H, m), 6.62-6.66 (1H, m),
6.92-6.97 (2H, m), 7.0-7.15 (3H, m),
7.23-7.26 (1H, m), 7.33-7.39 (1H, m)
Compound of Reference example 18
'H-NMR (250MHz, CDC13) & ppm:
2.3-2.4 (2H, m), 3.3-3.7 (2H, br),
3.89 (2H, t, J=8Hz), 4.38 (2H, t, J=8Hz),
6.26 (1H, d, J=3Hz), 6.6-6.7 (1H, m),
6.75-6.81 (1H, m), 6.91 (1H, s),
7.0-7.1 (2H, m), 7.17-7.20 (1H, m),
7.4-7.5 (1H, m), 7.58 (1H, d, J=8Hz).
Compound of Reference example 19
'H-NMR (250MHz, CDC13) 8 ppm:
1.55 (6H, d, J=6.5Hz), 2.1-2.25 (2H, m),
w 2198265
WO 97103070 PCTlJP96/01841
87
2.8-3.4 (8H, m), 4.51-4.62 (1H, m),
6.2-6.6 (3H, m)
Compound of Reference example 20
IH-NMR (250MHz, CDC13) b ppm:
1.92-2_04 (2H, m), 2.6-3.5 (8H, brm),
3.83 (2H, t, J=7Hz), 6.3-6.6 (3H, m),
7.67-7.86 (4H, m).
Compound of Reference example 21
1H-NMR (250MHz, CDC13) 5 ppm:
2.0-2.2 (2H, m), 2.8-3.0 (4H, m),
3.19 (2H, t, J=8Hz), 3.86 (3H, s),
4.25 (2H, t, J=6.5Hz), 6.2-6.3 (1H, m),
6.4-6.5 (2H, m), 6.57 (1H, s),
6.84-6.88 (1H, m), 7.08-7.11 (2H, m),
7.26 (1H, t, J=5Hz).
Compound of Reference example 22
1H-NMR (250MHz, CDC13) 8 ppm:
2.1-2.2 (2H, m), 2.8-3.0 (4H, m),
3.20 (2H, t, J=SHz), 4.29 (2H, t, J=7Hz),
6.2-6.3 (1H, m), 6.4-6.6 (3H, m),
7.1-7.3 (3H, m), 7.38 (1H, d, J=8Hz),
7.64 (1H, d, J=7.SHz).
WO 97/03070 2 1 9 8 2 6 5 pCT/JP96/O1S41
88
Compound of Reference example 23
1H-NMR (250MHz, CDC13) b ppm:
1.22 (6H, d, J=7Hz), 2.3-2.4 (2H, m), 2.7-2.8
(2H, m), 3.81 (2H, t, J=7.5Hz), 3.95 (2H, s),
4.16 (2H, t, J=7Hz), 6.51 (1H, d, J=3Hz),
6.77 (1H, d, J=l.SHz), 6.98-7.04 (2H, m),
7.19 (2H, s), 7.57 (1H, s).
Compound of Reference example 24
1H-NMR (250MHz, CDC1~) & ppm:
2.23-2.33 (2H, m), 3.45 (2H, t, J=6Hz),
4.38 (2H, t, J=6.5Hz), 6.60 (1H, d, J=3.5Hz),
7.2 (1H, s), 7.44 (2H, d, J=1Hz),
7.98 (1H, t, J=1Hz).
Compound of Reference example 25
1H-NMR (250MHa, CDC1;) 8 ppm:
2.6-2.7 (2H, m), 2.8-2.9 (2H, m),
3.55 (2H, brs), 4.4-4.6 (2H, m),
5.1-5.3 (2H, m), 5.8-6.0 (1H, m),
6.5-6.6 (2H, m), 6.8-6.9 (1H, m).
Compound of Reference example 26
IFI-NMR (250MHz, CDC13) & ppm:
2.36-2.46 (2H, m), 3.3-3.7 (2H, br), ,
4.0-4.1 (4H, m), 6.2-6.4 (2H, m),
6.6-6.7 (1H, m), 6.93 (1H, d, J=2Hz),
7.0-7.1 (2H, m), 7.30 (IH, d, J=2Hz),
WO 97/03070 ' 219 8 2 6 6 pCT/JP96/01841
89
7.56 (1H, d, J=l.SHz).
Compound of Reference example 27
1H-NMR (250MHz, CDC13) & ppm:
2.4-2.6 (2H, m), 3.1-3.8 (2H, br),
4_1-4.3 (4H, m), 6.33 (1H, d, J=3Hz),
6.68 (1H, dd, J=8.5Hz, 2Hz), 6.9-7.1 (3H, m),
7.97 (2H, d, J=l2Hz).
Compound of Reference example 28
IH-NMR (250MHz, CDC13) & ppm:
2.0-2.1 (2H, m), 2.85-2.9 (4H, m),
3.19 (2H, t, J=9Hz), 4.11 (2H, t, J=7Hz),
6.25 (1H, d, J=8Hz), 6.45-6.5 (1H, m),
6.5-6.6 (1H, m), 6.93 (1H, s), 7.08 (1H, s),
7.49 (1H, s).
Compound of Reference example 29
1H-NMR (250MHz, CDC13) & ppm:
1.7-2.0 (4H, m), 2.2-2.4 (2H, m),
2.5-2.7 (4H, m), 2.9-3.0 (4H, m),
3.01 (2H, t, J=7Hz), 3.24 (2H, t, J=8Hz),
6.37 (1H, d, J=8Hz), 6.4-6.5 (1H, m),
6.56 {1H, s), 7.3-7.4 (3H, m),
7.52 (2H, d, J=7Hz).
WO 97/03070 ~ 219 8 2 6 6 p~/,Jpg6~01841
Compound of Reference example 30
iH-NMR (250MHz, CDC1~) & ppm:
1.43 (6H, d, J=6.5Hz), 2.4-2.5 (2H, m),
2.59 (2H, t, J=8Hz), 4.1-4.2 (1H, m),
5 4.29 (2H, t, J=6.5), 6.3 (1H, d, J=2.5Hz),
6.62-6.66 (1H, m), 6.92-7.03 (3H, m).
Compound of Reference example 31
1H-NMR (250MHz, CDC13) 8 ppm:
1.25 (6H, d, J=6:SHz), 4.2-4.3 (1H, m),
10 5.57 (2H, s), 6.4 (1H, d, J=3Hz),
6.64-6.69 (1H, m), 6.9 (1H, d, J=2Hz),
6.99 (1H, d, J=3Hz), 7.1 (1H, d, J=8.5Hz).
Compound of Reference example 32
1H-NMR (250MHz, CDC13) & ppm:
15 2.16-2.28 (2H, m), 3.73 (2H, t, J=7Hz),
4.12 (2H, t, J=7Hz), 6.28 (1H, d, J=3Hz),
6.64-6.69 (1H, m), 6.9 (1H, d, J=2Hz),
7.11-7.I4 (2H, m), 7.7-7.73 (2H, m),
7.82-7.86 (2H, m).
20 Compound of Reference example 33
iH-NMR (250MHz, CDC13) 6 ppm:
2.35-2.5 (8H, m), 4.0-4.1 (4H, m),
6.35 (1H, d, J=2.5Hz), 6.67-6.70 (1H, m),
6.96-7.06 (4H, m), 7.57 (1H, s),
25 7.70 (1H, s).
2198266
W 0 97103070 PCT/JP96/0184I
91
Compound of Reference example 34
1H-NMR (250MHz, CDC13) b ppm:
1.5-1.7 (1H, br), 1.76-1.87 (4H, m),
2.1-2.2 (2H, m), 2.43-2.5 (4H, m),
2.7-2.8 (2H, m), 3.08 (2H, t, J=8.SHz),
3.33 (2H, t, J=7Hz), 3.68 (2H, t, J=8.SHz),
6.32 (1H, d, J=9Hz), 7.2-7.4 (3H, m),
7.52 (2H, d, J=7HZ), 7.88 {1H, s),
8.02-8.06 {1H, m).
Compound of Reference example 35
1H-NMR (250MHz, CDC13) & ppm:
2.1-2.18 (2H, m), 3.09 (2H, t, J=8.5Hz),
3.20 (2H, t, J=7Hz), 3.59 (2H, t, J=8.5Hz),
4.07 (2H, t, J=7Hz), 6.17 (1H, d, J=9Hz),
6.92 (1H, t, J=l.SHz), 7.12 (1H, s),
7.48 (1H, s), 7.91 (1H, s),
8.02-8.06 (1H, m).
Compound of Reference example 36
IH-NMR {250MHz, DMSO-db) E ppm:
2:1-2.2 (2H, m), 3.61 (2H, t, J=7Hz),
4.36 (2H, t, J=7Hz), 6.75 (1H, d, J=2.SHz),
7.71-7.76 (2H, m), 7.8-7.9 (4H, m),
7.99-8.04 (1H, m), 8.55 (1H, d, J=2Hz).
219266
WO 97/03070 PCTIJP96101841
92
Compound of Reference example 37
iH-NMR (250MHz, DMSO-db) & ppm:
2.18-2.29 (2H, m), 3.57 (2H, t, J=6.5Hz),
4.40 (2H, t, J=7Hz), 6.78 (1H, d, J=3Hz),
7.62-7.73 (2H, m), 8.02-8.06 (1H, m),
8.57 (1H, d, J=2Hz).
Compound of Reference example 38
1H-NMR (250MHz, CDC13) & ppm:
2.40-2.51 (2H, m), 4.11 (2H, t, J=6.5Hz),
4.19 (2H, t, J=7Hz), 6.31 (1H, t, J=2Hzj,
6.69-6.71 (1H, m), 7.24-7.34 (3H, m),
7.59 (1H, d, J=l.SHz), 8.08-8.13 (1H, m),
8.59 (1H, d, J=2Hz).
Compound of Reference example 39
1H-NMR (250MHz, DMSO-db) 6 ppm:
2.2-2.4 (2H, m), 4.19 (2H, t, J=7Hz),
4.32 (2H, t, J=7Hz), 6.77 (1H, d, J=3Hzj,
7.65-7.68 (2H, m), 7.99-8.06 (2H, m),
8.50 (1H, s), 8.58 (1H, d, J=2Hz).
Compound of Reference example 40
iH-NMR (250MHz, CDC13) 8 ppm:
2.0-2.1 (2H, m), 3.09 (2H, t, J=8Hz),
3.4-3.5 (2H, m), 3.6-3.7 (4H, m),
6.33-6.38 (1H, m), 7.89 (1H, s),
8.03-8.08 (1H, m).
2198266
WO 97!03070 PCTlJP96/0184I
93
Compound of-Reference example 41
1H-NMR (250MHz, CDC13) 6 ppm:
1.5 (6H, d, J=6.SHz), 2.4-2.6 (2H, m),
2.68 (2H, t, J=6.5Hz), 4.3-4.4 (1H, m),
4.47 (2H, t,,J=6.5Hz), 6.71 (1H, d, J=3Hz),
7.2-7.3 (2H,,m), 8.0-8.1 (1H, m),
8.59 (1H, d, J=2Hz).
Compound of Reference eXample-'42
iH-NMR (250MHz, CDC13) 8 ppm:
1.35 {6H, d, J=6.5Hz), 4.37-4.47 (IH, m),
5.70 {2H, s), 6.79-6.81 (1H, m),
7.27-7.3D (1H, m), 7.48 (1H, d, J=9Hz),
8.12-8.17 (1H, m), 8.59 (1H, d, J=2Hz).
Compound of Reference example 43
1H-NMR (250MHz, CDC13) & ppm:
2.33 (3H, s), 2.37 (3H, s),
2.45-2.56 (2H, m), 4.09-4.20 (4H, m),
6.74 (1H, d, J=3Hz), 6.94 (1H, s),
7.15-7.21 (2H, m), 7.58 (1H, s),
7.72 (IH, s), 8.05-8.09 (1H, m),
8.60 (1H, d, J=2Hz).
Compound of Reference example 44
1H-NMR {250MHz, CDC13) & ppm:
1.22 {6H, d, J=7Hz), 2.3-2.4 (2H, m),
2.6-2.8 (1H, m), 3.84 (2H, t, J=7Hz),
WO 97/03070 219 8 2 6 6 pCT/JP96/01841
94
4.19 (2H, t, J=7Hz), 6.63 (1H, d, J=3Hz),
6.78 (1H, d, J=l.SHz), 7.01 (1H, d, J=l.SHz),
7.16 (1H, d, J=3.5Hz), 7.2-7.3 (1H, m),
7.4-7.5 (1H, m), 8.0 (1H, s).
Compound of Reference example 45
1H-NMR (250MHz, CDClg) 6 ppm:
2.1-2.2 (2H, m), 3.5-3.7 (6H, m),
4.2-4.3 (2H, m), 6.63 (1H, d, J=9Hz),
7.67 (1H, d, J=2.5Hz), 7.78-7.87 (1H, m).
Compound of Reference example 46
1H-NMR (250MHz, CDC13) & ppm:
2.1-2.2 (2H, m), 3.3-3.4 (4H, m),
4.06 (2H, t, J=6.5Ha), 4.23 (2H, t, J=4.5Hz),
6.42 (1H, d, 3=9Hz), 6.95 (1H, s),
7.13 (1H, s), 7.50 (1H, s), 7.67 (1H, d,
J=2.5Hz), 7.78 (1H, d, J=2.5Hz, 9Hz).
Compound of Reference example 47
iH-NMR (250MHz, CDC13) 6 ppm:
2.0-2.1 (2H, m), 3.1-3.2 (4H, m),
4.04 (2H, t, J=7Hz), 4.22 (2H, t, J=4.5Hz),
6~.2-6.3 (2H, m),--6.35-6.45 (1H, m),
6.93 (1H, s), 7.09 (1H, s), 7,49 (1H, s). ,
21982b6
WO 97103070 PCTlJP96/01841
Compound of Reference example 48
IH-NMR (250MHz, CDC13) 8 ppm:
2.0-2.I-(2H, m), 3.4-3.5 (4H, m),
3.78 (2H, t, J=7Hz), 4.25 (2H, t, J=4.SHz),
5 6.56 (1H, d, J=9Hz), 7.64 (1H, d, J=2.SHz),
7.73-7.88 (5H, m).
Compound of Reference example 49
1H-NMR (250MHz, CDC13) S ppm:
1.95-2.04 (2H, m), 3.17-3.23 (4H, m),
10 3.77 (2H, t, J=7Hz), 4.22 (2H, t, J=4.5Hz),
6.2-6.24 (2H, m), 6.-5-6.55 (1H, m),
7.7-7.74 {2H, m), 7.83-8.02 (2FI, m).
Reference example 50
To 926 mg of 5-methoxyindole was added 30 ml
15 of dimethylformamide and 230 mg of sodium hydride (in
oil), this mixture Was stirred under nitrogen gas
stream at 60°C for 1 hour. Then 1.5 g of 1-(3-chloro-
propyl)-5-nitroindole was added to the reaction mixture
and stirred at room temperature overnight. The
20 reaction mixture was further stirred at 60°C for 5.5
hours, then water was added thereto, and the crystals
being separated were collected by filtration, and
washed with water. The washed crystals were 'subjected
to a silica gel column chromatography (eluent:
25 dichloromethane), there was obtained 1.8 g of 1-[3-(5-
methoxyindol-1-yl)propyl]-5-nitroindole as in the
2 i 9 8 2 b 6 pCT/~6/01841
WO 97/03070
96
form of yellow powdery product.
'H-NMR (250MHz, CDC13) & ppm:
2.4-2.5 (2H, m), 3.86 (3H, s),
4.07-4.13 (4H, m), 6.47 (1H, t, J=2.5Hz), .
6.69 (1H, d, J=3.5Hz), 6.83-6.88 (1H, m),
7.01 (1H, d, J=3Hz), 7.07-7.18 (4H, m),
8.06 (IH, dd, J=2.SHz, J=9Hz),
8.59 (1H, d, J=2.5Hz).
By using suitable starting materials, and by
a method similar to that employed in Reference example
50, there were obtained compounds of the above-
mentioned Reference examples 7, 9, 10, 12-18, 20-23,
26-29, 32-36, 38, 39, 43, 44 and 46-49.
Reference example 51
To 500 ml of ethanol solution containing 26 g
of 2-benzylamino-4-chloroaniline was added 45.7 g of
polymer form (45-508 toluene solution) of ethyl
glyoxylate, further 28.4 g of iodine was added and the
reaction mixture was stirred at room temperature for 20
minutes. Then 27.8 g of sodium thiosulfate aqueous
solution was added thereto; the crystals being
separated were collected by filtration, and washed with
water and ethanol, then dried. There Was obtained 26.1
g of ethyl 1-benzyl-6-chlorobenzimidazol-2-carboxylate
as in the form of pale brown-powdery product.
W O 97!03070 219 8 2 6 6 pCT/,~p96/01841
97
iH-NMR (250MHz, CDCI3) & ppm:
1.;45 (3H, t, J=7Hz), 4.49 (2H, q, J=7Hz),
5-~85 (2H, s), 7.1-7.5 (7H~ m), -
0 7.85 (1H, d, J=8.5Hz).
By using a suitable starting material, and by
a method similar to that employed in Reference example
51, compound of the above-mentioned Reference example 2
was obtained.
Example !A
A mixture of 2.2 g of methyl 1-benzyl-6-
chlorobenzimidazol-2-carboxylate and 5.3 g of 1-[3-(2-
isopropylimidazol-1-yl)propyl]-5-aminomethylindole was
stirred at 80°C for 1.5 hours, after confirmed that the
starting materials were disappeared, the reaction
mixture was dissolved in chloroform, then washed with
water and an aqueous solution saturated with sodium
chloride, and dried with anhydrous magnesium sulfate,
then the solvent was removed by distillation under
reduced pressure. The resulting residue was subjected
a silica gel column chromatography (eluent: 3~
methanol/dichloromethane), then fumaric acid was added
and recrystallized from diisopropyl ether-ethanol,
there was obtained 4 g of 1-benzyl-6-chloro-2-{1-
[3-(2-isopropylimidazol-1--yl)propyl]indol-5-
ylmethylaminocarbonyl}benzimidazole~fumarate as in the
form of pale yellow powdary product.
I i
CA 02198266 2005-05-11
2571:L-773
- 98 _
1H-NMR (250MHz, DMSO-db) 8 ppm:
1.10 (6H, d, J=7Hz), 2.1-2.3 (2H, m),
2.8-2.95 (1H, m), 3.88 (2H, t, J=7.5Hz),
4.20 (2H, t, J=7Hz), 4.55 (2H, d, J=6.5Hz),
5.98 (2H, s), 6.43 (1H, d, J=3Hz),
6.62 (2H, s), 6.66 (1H, d, J=l.SHz),
7.10 (1H, d, J=l.SHz), 7.15-7.38 (10H, m),
7.53 (1H, s), 7.77 (1H, d, J=8.5Hz),
7.84 (1H, d, J=l.5Hz), 9.57 (1H, t, J=4Hz)
Example 1B
130 Milligrams of lithium aluminum hydride
was suspended in 70 ml of tetrahydrofuran, then 2.2 g
of 6-amino-3,4-dihydro-2(1H)-quinolinone was added
gradually thereto, and the mixture was stirred at room
temperature overnight. The reaction mixture was
further stirred for 2 hours under refluxing condition,
then 1 g of methyl 1-benzyl-6-chlorobenzimidazol-2-
carboxylate was added, the reaction was continued by
refluxing for 3 hours. After the reaction was
finished, then water and 10$ aqueous solution of
potassium hydroxide were added, the reaction mixture
was diluted with ethyl acetate and filtered with
Celite, and the filtrate Was washed with chloroform,
the solvent was removed by distillation under reduced
pressure. To the residue thus obtained was added
ethanol and heated, the insoluble matters were
collected by filtration and recrystallized from
*Trade-mark
2198266
WO 97f03070 PCTlJP96/01841
99
dimethylformamide, there was obtained 0.11 g of
1-benzyl-6-chloro-2-(3,4-dihydro-2(1H)-quinolinon-
6-ylaminocarbonyl)benzimidazole as in the form of
yellow powdery product.
Melting point: Higher than 290°C
'H-NMR (250MHz, DMSO-db) s ppm:
2.44 {2H, t, J=7Hz), 2.87 (1H, t, J=7Hz),
5.99 (2H, s), 6.83 (1H, d, J=9Hz),
7.21-7.40 (6H, m), 7.57-7.6 (1H, m),
7.72 (1H, s), 7.86-7.88 (2H, m),
10.08 {1H, s).
Example 1C
To 2.2 g of 5-amino-1-[3-(1-isopropyl-5-
tetrazoiyl)propyl)indole was added 40 ml of toluene,
then this mixture was stirred under nitrogen gas
atmosphere by cooling in a methanol-ice bath. To this
reaction mixture was added 4 ml of n-hexane solution of
2M trimethylaluminum dropwise from syringe, then
reaction mixture was stirred for 20 minutes, and
further stirred at room temperature for 1 hour. 2.18
Grams of methyl 1-benzyl-6-chlorobenzimidazol-2-
carboxylate was added to the reaction mixture and was
stirred for.-5-to 6 hours under refluxing condition.
Next, 10~ hydrochloric acid was added, and the crystals
being separated were collected by filtration. Water-
chloroform was added ~o the cpstals, this solution was
made alkaline with 10~ aqueous solution of potassium
~,,
CA 02198266 2005-05-11
2571:1-773
- 100 -
hydroxide, then was filtered with Celite, the chloro-
form layer was washed with water, an aqueous solution
saturated with sodium chloride. The chloroform layer
was dried with anhydrous magnesium sulfate and the
solvent was removed under reduced pressure. The
residue thus obtained Was subjected to a silica gel
column chromatography (eluent: 3$ methanol/dichloro-
methane), and recrystallized from ethyl acetate-n-
hexane, there was obtained 2.27 g of 1-benzyl-6-chloro-
1C1 2-~1-[3-(1-isopropyltetrazol-5-yl)propyl]indol-5-
ylaminocarbonyl}benzimidazole as in the form of yellow
needle crystals.
Melting point: 190-191°C.
1-'i By using suitable starting materials, and by
methods similar to those employed in Examples 1A to 1C,
there were obtained compounds of Examples 2 to 50 as
shown in Tables 9 to 33 as follows.
*Trade-mark
0 ~ 219 8 2 6 5 pCT/JP96/01841
101
:able 9
_ . / I N~-RCV'H-(A)n-R~
iJ
R~ Rx
t
Example 2
Structure R3
I R2 , -CHZ
H
R~ ; 6-Cl - (A)n-- . -
Crystal farirt .-Grown granules
Recrystalli2ation
solvent: Ethyl acetate
Melting point . 205 - 207
Form of compound : Free form
Example 3-
Structure-R3
I / I
(CH~3~ R2 . -CHZ
R~ _ 6-CI -(A)rr- : -
Crystal form : Pale yellaw.needles
Recrystallization
solvent: Methanol
' Melting point . 187 - 188
Form of compound : Free form
WO 97/03070 219 8 2 b 6 PCT/JP96/01841
102
Table 1Q
Example 4
Structure
R3
I
(CH~3 .~ R= . -CHi /
3
Ri : 6-Cl -(A)n- . .-
Crystal form . Pale yellow needles
Recrystallization
solvent: Ethanol
Melting point . 129-130
Form of compound : Free form
Example 5
Structure
R3
\
i Rx
CHa CH = CHI
RI : 6-CI -(p)n- . -
Crystal form . Colorless needles
Recrystallization
solvent: Ethyl acetate-ethanol
Melting point . 154-155
Form of compound : Free form
WO 97!03070 219 8 2 6 5 pCT/JP96/01841
103
Table 11
- Example 6
Structure -
R~
\
(CH~y~ RZ . -CHz I
R~ : 6-C1 v' - (A)n- , -
Crystal-~orm .- Pale yellow-needles
Recrystallization-
solvent: Chloroform-ethyl acetate
Melting point . 165-166'
Form of compound : Free farm
Example 7
Structure
R3
(~~3~ ~~ R2 . -CH2
~r
R~ : 6-C1 -(A)n- _ -
Crystal form . Yellow needles-
Recrystallizatian
solvent: Methanol-ethyl acetate
Melting point . 196-197'
Form of compound : Free form
2198266
WO 97!03070 PCTIJP96/D1S41
104
Table 12
Example 8
Structure
Rs :
W
(CH~3~
r~~ 'C1 R~ -CH=
C1
R~ : 6-C( -(A)ir : -
Crystal form . Brown granules
Recrystallization
solvent: Ethyl acetate-n-hexane
Melting point . 191-192'
Form of compound : Free form
Example '9
Structure
R3
COQCH3 R2 ~ CHZ
R1 : 6-Cl -(p)~ : -
Crystal form . Pale brown powdery
Recrystallization
solvent: Ethyl acetate-diisopropyl ether
Meltinq point . 194-195
Form of compound: Free form
2198266
W097/03070 PCT/JP96/01H41
105
Table 13
Example 10
Structure
R
I
(CH~3 NHz RZ ~ -CHZ
Rt : 6-CI -(A)n- . .-
Crystal form . Yellow granules
Recrystallization
solvent: Ethyl acetate-n-hexane
Melting point . 106-108
Form of compound : Free form
Example 11
Structure
R3
~ i I
(CH~3 N~iR ~ ~ R= . -CHZ ~
H
RI : &C1 -(A)n- ; -
Crystal farm -. Yellow needles
Recrystallization
solvent: Cloroform
Melting point . 206-2-07'~
Form of compound : Free form
WO 97/03070 ' 219 8 2 6 6
PCT/JP96/01841
106
Table 14
Example 12
Structure
R~
\ ~ ~ 3
j ~~N
(~z)3 ~'HC \ O
R~ . -CHZ ~
~3
R ~ : 6-C1
Crystal form . Yellow needles
Recrystallization
soluent: Dimethylformamide-water
Melting point . 217-218'
Form of compound : Free form
Example 13
Structure
R~
~1T_ N
(~~J3-N. ' R= . -CFf= ~
N
R~ : &CI -~p)~"-: --
Crystal form . Pale yellow needles
Recrystallization
solvent: Dichloromethane-n-hexane
Melting point . 146-147
Form of compound : Free form
2198265
WO 97!03070 PCT/JP96/01841
107
Table 15
Example 14
Structure
Rs
CCH~3 ~N R . -C1?Z
Rt : b-CI -CA)m- :
Crystal form . Colorless needles
Recrystallization
solvent: Ethyl acetate-n-hexane
Melting point . -178-1791;
Form of compound : Free form
Example 15
Structure R5
W _
N
CCH~3~ ~N
CF1;CH3 R2 . -C$2
~3
RI : 6-CI - CA)n- . -
Crystal form :XeIIow Needles
Recrystalliaation
solvent: Ethyl acetate-n-hexane
Melting point . 190-1911;
Form of compound : Free form
WO 97/03070 2 1 9 ~ 2 6 6 pCTlJP96/01841
108
Table 16 -
Example 16
Structure R3:
r .
N~ .
CHi --~i N
N-
CF~~3 RZ . -CHZ I
CH3
Ri : 6-CI -(A)n- . -
Crystal form . Pale yellow needles
Recrystallization
solvent: Ethyl acetate
Melting point . 229-23:1. (decomposed)
Form of compound : Free form
Example 17 -
Structure R~
~= N
I I
CHs R~ -CHy
(CH~rN~
CH3
Ri : 6C1 -(A)rt- _ -
Crystal form . Pale yellow powdery
Recrystallization
solvent: Ethyl acetate
Melting point . 197-198'x.
Form of compound : Free form
WO 97/03070 ~- ' 219 8 2 6 6 pCT/JP96/01841
109
Table 17
Example 18
Structure
~3
,r CH3 CH3
~ CH
i
(~z)a ~N R2 . -CH
z
R i : &C1 - (A)cr- , -CHZ-
Crystal form . Pale yellow powdery
Recrystallization
sclvent: Ethanol-diisopropyl ether
Form of compound : CpzH
C02H
Example 19
Structure
R3
(CH~~~N Rz . -CHz
Rt : G-CI 1J - (A)n- .
Crystal form . Yellow powdery
Recrystallization
solvent: Methanol-diisopropyl ether
Melting point . 189-190.
Form of compound : ~pzH
1 P~
2
COyH
21982bb
WO 97/03070 PCT/JP96/01841
110
Table 18
Example 20 ,
Structure
R3
' OH
(CH~3N / \ R= . - CHi ~
RI : 6-CI - (p)~-- : -
Crystal form . Yellow needles
Recrystallization
solvent: Chlcroform
Melting point . 186-187,'",
Form of compound : Free form
Example 21
Structure
R3
R= -CHi
Rt : frCl -(A)n- . -
Crystal fnrm . Pale brown powdery
Recrystallization
solvent: Ethanol-ethyl acetate
Melting point . 277.
Form of compound : Free form
2198266
WO 97/03070 PCT/JP96/01841
111
Table 19
Example 22
Structure
R3
(CH~3~NN
CFCCH3 RI . -CHz
CH3
Rt : 6-Cl -(A)rt-
Crystal form : Yellow needles
Recrystallization
solvent: Ethyl acetate-ethanol
Melting point . 155-156
Form of compouhd : Free form
Example 23
Structure
R3
1
(CH~y3-N / R~ . -CH2
R~ . 6-Cl
- (~1)n-- : -
Crystal form . White powdery
Recrystallization
solvent: Ethyl acetate
Melting point . 16Q-161'x.
Form of compound : Free form
WO 97/03070 ~ ~ ' 219 8 2 6 5 pCT/~6/01841
112
Table 20
Example 24 .
Structure _
R5
OCH~
(CH~r-N / R= ~ -CHz
R~ : 6-CI -(Ay . -
Crystal form . Yellow powdery
Recrystallization
solvent: Ethyl acetate-dichloromethane
Melting point . 169-170
Form of compound : Free-form
Example 25
Structure
Rs
CN
(CH~~J ~ ~ Ri . . CHZ ~
R~ : 6-C1 -(A)n- , -
Crystal form . Pale yellow powdery
Recrystallization
solvent: Ethyl acetate-n-hexane
Melting point . 166-167.
Form of compound : Free form
0 219 8 2 6 6 pCT/JP96/01841
1I3
Table 27
Example Z6
Structure -
R3
(CH~3 ~ ~ R~ . -CHZ
Ri : 6-CI -(A)n- . -
Crystal form . Pale brown needles
Recrystallization
solvent: Ethyl acetate-n-hexane
Melting point . ~56~
Form of compound : Free form
Example 27
Structure
R~
OCH3
CCH~3N r Rl . -CHZ
Rt : 6C( -(A)rt- , -
Crystal form . Bright yellow Needles
Recrystalli2.ation
solvent: Ethyl acetate-n-hexane
Melting point . '157.
Form of compound : Free form
WO 97/03070 219 8 2 6 6 pCTLTP96/01841
114
Tahle 22,
Example 28 _
Structure
R3
(CH~3 ~ It
Rx . -CH.
H
Rl : 6C1 -(A)n-: -
Crystal form . Yellawgranules
Recrystallization
solvent: Dimethylformamide-water
Melting point . 213-221
Form of compound : Free form
Example 29
Structure
R3
\~
(CHi)3N / RZ . -CHz
Ri : &C1 -(A)n-
Crystal form . Bright yellow needles
Recrystallization
solvent: Ethyl acetate-n-hexane
Melting point . . 136-137°C.
Form of compound : Free form
2198266
WO 97/03070 PCT/JP96/01841
115
Table 23
Example 3D
Structure-
R3
I I / Rx
R1 : 6-CI -(A)rc- : -(CH~x'w
Crystal form . Colorless granules
Recrystallization
solvent: Ethyl acetate
Melting point . 187-T88~
Form of compound : Free form
Example 31
Structure
R3
~ ~ \
(~~3-~N R2 . -CHz
R~ : 6-Ct - (A)n- : -(CH~r-
Crystal form : White amorphous
Form of compound : Hydrochloride
WO 97/03070 '- - 219 8 2 6 6 pCT/~6/01841
116
Table 24
Example,32
Structure
R3
I l / R~ . -CHZ /
H
R~ : 6-CI -(p),:.. ; -CHZ-
Crystal form . Colorless needles
Recrystallization
solvent: Ethyl acetate-n-hexane
Melting point . 173-174'
Form of compound : Free form
Example 33
Structure R3
R~ . -CHZ
CH3
R' : 6-CI - (A)n'- : -
Crystal form -. Yellow powdery
Recrystallization
solvent: Ethanol-n-hexane
Melting point . 153-155.
Form of compound : Free form
\ ~ 3
i
(CHz)3 ~~ / N
2198265
WO 97/03070 - PCT/JP96/01841
1I7
Table 25
Example 3-4
Structure
r
i
0
(Cg~3 ~x ~ ~ R= . -CH=
R~ : 6-G -
Crystal form . Yellow needles
Recrystallizatian
solvent: Ethyl acetate
Melting point . 139-140
Form of compound : Free farm
Example 35
Structure
R3
R2 . -CHI
(CH~3 Ct
Rl : &G -(AJn- : -
Farm of compound : Free-form
i
WO 97/03070 219 8 2 6 6 pCT/JP96/01841
118
Table 26
Example 36
Structure
R3 : _
R1 . -
R~ : 6-CI - (A)n- . -
Crystal form . Yellow needles - -
Form of compound : Free form
Example 37
Structure
R3
Rz . -CH2
CHzCOOC2H5
RI : 6-CI
- tA)n- . -.
Form of compound : Free firm
2198266
WO 97103070 PCT/JP96/01841
119
Table 2Z-
Example 36-
Structure-
R1
-CHz
O
RI : 6-C1 - (~)n- _ -
Crystal form- . Brown solid
Form of compound : Free form
Example 39
Structure
R~
l
' -CHz
( tCH~~ CI R
R~ : &C1
-. (p)°- . -(CH~z
Crystal farm . Plae yellow--nily
Form of compound : Free form
I \
(~I?3
RI
y
WO 97/03070 219 8 2 6 6 pCT/JP96/01841
lao
Table 28
Example 40
Structure
R3
R= . -CH /
(CHz)s ~i
R~ : &CI -Cp.)~- : -
Crystal form . Yellow powdery
Form of compound :- Free form
Example 41
Structure
R3
\\~~'~~0 R2 . - CH2
H
RI : 6-CI -(p)I,..-.: -
Crystal form . Yellow powdery
Recrystallization
solvent: Dimethylformamide
Melting point . Higher than 290
Form of compound : Free form
WO 97/03070 219 8 2 6 6 pCT/~6101841
121
Table 29 -_.
Example 42
Structure
O Ri .
CHiCHaCH2
R1 : 6C1 - (A):r-- : -
Crystal fosm : Yellow powdery
Recrystallizatibn
solvent: Ethyl acetate-n-hexane
Melting point . 183-184
Form of compound : Free form
Example 43
Structure
RS : _
~~~~~~~0 /~ N
1~(~C1'H~~3~ Ri . -CHZ
Rt : 6-CI -(A)n- . -
Crystal form : Pale yellow powdery
Recrystallization
solvent: Ethyl acetate
Melting point . 195
Form of compound : Free form
WO 97/03070 219 8 2 6 6 pCTIJP96/01841
122
Table .3a-
Example 44
Structure
R3
O
~~3 z
(~~3ta ~ (~~32N(CH3~t R . CH
R~ : 6-Cl -(A)rt-. -
Crystal form . Yellow powdery
Recrystallization
solvent: Ethanol
Melting point . 2()Q-202'
Form of compound : ~H
COi~J'''''H
Example 45
Structure
R3
i
\\~~' ~~O
CH=--(~
~,'r ~CH~
CH NCH R~ . -CHZ I
3
R~ : 6-CI -(A)r~ : -
Crystal form . Colorless needles
Recrystallization
solvent: Ethyl acetate-ethanol
Melting point . 227-228'
Form of compound : Free form
279$266
WO 97/03070 PCT/JP96/01841
123
Table 31
Example 46
Structure
R3
R= . -CHZ
R1 : 6-G H - (A)a- : -
Crystal fnrm : Pale brown powdery
Recrystallization
solvent: Chloroform-isopropyl alcohol
Melting point : Higher than 290'
Form of compound : Free form
Example 47
Structure
R3
~. 0
\ ' N
(G~~~HC ' ~ R2 . -CH2
Ri . &G - CA)n- . -
Crystal form . Bright yellow needles
Recrystallization
solvent: Ethyl acetate-diisopropyl ether
Melting point . 146-148
Form of compound : Free form
WO 97/03070 _ ' 219 8 2 6 6 pCTIJP96101891
124
Table 32
Example 98
Structure
R3 : _
~N
(CH~3 R1 . -CHy, /
R I : 6-CI
- (A)n- . -
Crystal form . Yellow powdery
Recrystallization-
solvent: Ethyl acetate-n-hexane
Melting point . 175-176'
Form of compound : Free form
Example 49 -
Structure -
R3
\\~~~'~O Ri . - CHZ
(CI~I~~ CI
Ri : 6-Cl -(A)n- _' -
Form of compound : Free farm
WO 97103070 219 8 2 6 6 pCT/.IP96/01841
125
Table 33
Example 50
Structure
R3
0
(CHzy3 '
Rz -CHz
O
R~ : 6-CI - (AW : -
Crystal form : Yellowamorphous
Form of compound : Free form
The NMR spectrum data of compounds obtained
in the above-mentioned Examples are shown as follow.
Compound of Example 18
IH-NMR (250MHz, DMSO-d6) & ppm:
1.10 (6H, d, J=7Hz), 2.1-2.3 (2H, m),
2.8-2.95 (1H, m), 3.88 (2H, t, J=7.5Hz),
4.20 (2H, t, J=7Hz), 4.55 (2H, d, J=6.5Hz),
5.98 (2H, s), 6.43 (1H, d, J=3Hz), 6.62
(2H, s), 6.66 (1H, d, J=l.5Hz), 7.10 (1H, d,
J=l.5Hz), 7.15-7.38 (10H, m), 7.53 (1H, s),
7.77 (1H, d, J=8.5HZ), 7.84 (1H, d, J=l.5Hz),
9.57 (1H, d, J=4Hz).
WO 97/03070 219 ~ 2 b 6 pOTlJP96/01841
126
Compound of Example ZS
1H-NMR (250Mfiz, DMSO-db) 8 ppm:
1.7-1.9 (2H, m), 2.89 (2H, t, J=8Hz), 3.07 (2H, t,
J=7.5Hz), 3.2-3.4 (4H, m), 5.99 (2H, s), 6.0-6.1
(1H, m), 6.47 (1H, d, J=8.SHz), 6.7-6.8 (1H, m),
6.8-6.9 (1H, m), 7.2-7.5 (7H, m), 7.57 (1H, s),
7.s-7.9 (zH, m), s.o-a.l (1H, m)
Compound of Example 31
1H-NMR (250MHz, DMSO-db) & ppm:
2.25-2.4 (2H, m), 2.9-3.1 (2H, m), 3.5-3.65
(2H, m), 4.1-4.3 (4H, m), 5.97 (2H, s), 7.02
(1H, t, J=7.5Hz), 7.11-7.43 (9H, m), 7.60-7.64
(2H, m), 7.71-7.75 (2H, m), 7.81 (1H, d, 3=l.5Hz),
9.09 (1H, s), 9.25 (1H, t, J=8Hz).
Compound of Example 35
IH-NMR (250MHz, CDC1~) & ppm:
2.2-2.35 (2H, m), 3.4-3.5 (2H, m), 4.3-4.4
(2H, m), 6.05 (2H, s), 6.5-6.6 (1H, m), 7.1-7.2
(1H, m), 7.2-7.5 (9H, m), 7.7-7.8 (1H, m), 8.1-8.2
(1H, m), 9.62 (1H, s).
Compound of Example 36
IH-NMR (250MHz, CDC13) 8 ppm:
2.26 (2H, m), 3.75 (2H, t, J=7Hz),
4.2 (2H, t, J=7Hz), 6.06 (2H, s), 6.48
(1H, d, J=3Hz), 7.24-7.5 (10H, m),
2198266
WO 97/03070 PCT/JP96/01841
127
7.7-7.86 (5H, m), 8.07 (1H, s), 9.60 (1H, s).
Compound of Example 37
iH-NMR (250MHz, CDC13) S ppm:
1.26 (3H, t, J=7Hz), 4.15-4.3 (2H, m),
4.84 (2H, s), 6.06 (2H, s), 6.56 (1H, d, J=3Hz),
7.12 (1H, d, J=3Hz), 7.23-7.47 (7H, m), 7.75
(1H, d, J=8.5HZ), 8.12 (1H, s), 9.62 (1H, s).
Compound of Example 38
1H-NMR (250MHz, CDC13) & ppm:
1.95-2.1 {2H, m), 2.92 (2H, t, J=8.5Hz),
3.13 (2H, t, J=7Hz), 3.36 (2H, t, J=8Hz), 3.83
(2H, t, J=7.5Hz), 6.02 (2H, s), 6.44 (1H, d,
J=8.5Hz), 7.25-7.33 (7H, m), 7.39 (1H, d,
J=1.5 Hz), 7.52 (1H, s), 7.69-7.73 (3H, m),
7.82-7.85 (2H, m), 9.40 (1H, s).
Compound of Example 39
1H-NMR (250MHz, CDC13) & ppm:
2.2-2.3 (2H, m), 3.10 (2H, t, J=7Hz),
3.44 (2H, t, J=6Hz), 3.75-3.85 (2H, m),
4.30 (2H, t, J=6Hz), 5.97 (2H, s), 7.03 (1H, s),
7.12-7.37 (10H, m), 7.61-7.66 (2H, m),
7.8-7.9 (1H, m).
2198266
WO 97/03070 PCTIJP96101841
128
Compound of Example 40 -
'H-NMR (250MHz, CDC13) & ppm:
I.31 (2H, brs), 1.70-I.81 (2H, m),
2.83 (2H, t, J=7Hz), 2.97 (2H, t, J=8Hz),
3.12 (2H, t, J=7.5Hz), 3.36 (2H, t, J=8Hz),
6.02 (2H, s), 6.45 (1H, d, J=8.5Hz), 7.23-7.32
(7H, m), 7.38 {1H, d, J=2Hz), 7.53 (1H, s),
7.7I (1H, d, J=9Hz), 9.43 (1H, s).
Compound of Example 4I
1H-NMR (250MHz, DMSO-db) & ppm:
2.44 (2H, t, J=7Hz), 2.87 (2H, t, J=7Hz),
5.99 (2H, s), 6.83 (1H, d, J=9Hz), 7.21-7.40
(6H, m), 7.57-7.6 (1H, m), 7.72 (1H, s),
7.86-7.88 (2H, m), 10.08 (1H, s).
Compound of Example 46
1H-NMR (250MHz, DMSO-d6) 6 ppm
6.01 (2H, s), 6.50 (1H, d, J=9.5Hz),
7.22-7.41 (7H, m), 7.84-7.93 (4H, m),
8.25 (1H, s).
Compound of Example 49
IH-NMR (250MHz, DMSO-db) & ppm:
1.9-2.1(2H, m), 2.5-2.6 (2H, m), 2.8-3.0 (2H, m), ,
3.6-3.8 {2H, m), 3.9-4.1 (2H, m), 5.99 (2H, s),
7.1-7.5 {7H, m), 7.6-8.0 (4H, m).
219$266
WO 97/03070 PCT/JP96/01841
129
Compound of Example 50
1H-NMR {250MHz, CDC13) 8 ppm:
1.9-2.1 (2H, m), 3.28-3.34 (4H, m),
- 3.77 (2H, t, J=7Hz), 4.26 (2H, t, J=4Hz),
6.-01 {2H, s), 6.63 {1H, d, J=9Hz),
7.11-7.40 (9H, m), 7.70-7.74 (3H, m),
7.81-7.87 (2H, m), 9.34 (1H, s).
Example 51-
To 0.66 g of 1-benzyl-6-chloro-2-(indol-5-
ylaminocarbonyl)benzimidazole was-added 50 ml of
dimethylformamide, further added 170 mg of oily sodium
hydride, said mixture was stirred under nitrogen gas
atmosphere at 60°C for.l hour. Under cooling at 0°C,
0.14 ml of allyl bromide was added to the reaction
mixture, and stirred at room temperature overnight,
then water-was added thereto and extracted with ethyl
acetate, the extract was washed with water and an
aqueous solution saturated with sodium chloride. The
washed extract was dried with anhydrous magnesium
sulfate, and the solvent was removed by distillation
under reduced pressure. The residue thus obtained was
subjected to a silica, gel column chromatography
(eluent: 10~ n-hexaneJdichloromethane), recrystallized
from ethyl acetate-ethanol, there was obtained 0.35 g
of 1-benzyl-6-chloro-2-(1-allylindol-5-ylamino-
carbonyl)benzimidazo1e as in the form of colorless
needle crystals.
WO 97/03070 ~ 219 8 2 6 6 pCT/~6/01841
130
Melting point: 154-155°C.
By using suitable starting materials and by a
method similar to that of employed in Example 51, there
were obtained compounds of the above-mentioned Examples
3, 4, 6-8, 10-20, 22-29, 31, 33-40, 42-45 and 47-50.
Example 52
To 3.8 g of 1-benzyl-6-chloro-2-[1-(3-chloro-
propyl)indol-5-ylaminocarbonyl]benzimidazole was added
100 mg of dimethylformamide, further added 1.4 g of
1H-1,2,3,4-tetrazol, 2.2 g of potassium carbonate and
7.2 g of sodium iodide, the mixture was heated and
stirred at 100°C for 2 days. Water was added to the
reaction mixture, and extracted with ethyl acetate, the
extract was washed with water and an aqueous solution
saturated with sodium chloride. The washed extract was
dried with anhydrous magnesium sulfate, and the solvent
was removed by distillation under reduced pressure.
The residue thus obtained was subjected to a silica gel
column chromatography (eluent: ethyl acetate/n-hexane=
1/1), after separation of isomers, there were obtained
1.1 g of 1-benzyl.-6-chloro-2-{1-[3-(1,2,3,4-tetrazol-
1-yl)propyl]indol-5-ylaminocarbonyl}benzimidazole (A)
as in form of colorless needle crystals by recrystal-
lization from ethyl acetate-n-hexane, and 0.9 g of w
1-benzyl-6-chloro-2-{1-[3-(1,2,3,4-tetrazol-2-yl)-
propyl]indol-5-ylaminocarbonyl}benzimidazole (B) as
in the form of pale yellow needle crystals by
WO 97/03070 219 8 2 6 5 pCT/JP96/01841
13I
recrystallization from dichloromethane-~-hexane.
Melting point of (A): 178-179°C
Melting point of (B): 146-147°C
By using suitable starting materials, and by
a method similar to that of employed in Example 52,
there were_obtained compounds of the above-mentioned
Examples 3, 4, 6-8, 10-12, 17-Z0, 23-29, 31, 33, 34,
36, 38, 40, 43, 44, 47, 48 and 50.
Example 53
To 5 g of 1-benzyl-6-chloro-2-[1-(3-
phthalimidopropyl)indol-5-ylaminocarbonyl]benzimidazole
was added 100 ml of ethanol and stirred, then 0.5 ml of
hydrazine hydrate was added thereto, the mixture was
refluxed overnight. After cooled the reaction mixture
to room temperature, then white crystals were removed
by filtration. Water was added to the filtrate, and
made alkaline with 10~ aqueous solution of potassium
hydroxide. This mixture was extracted with dichloro-
methane, the extract was washed with water, an aqueous
solution saturated with sodium chloride then was dried
with anhydrous magnesium sulfate. The solvent was
removed by distillation under reduced pressure. The
residue thus obtained was crystallized from ethyl
acetate-,~-hexane, there was obtained 3.2 g of 1-benzyl-
' 25 6-chloro-2-[1-(3-aminopropyl)indol-5-ylaminocarbonyl]-
benzimidazole as in the form of yellow granular
WO 97/03070 2 l 9 8 2 6 6 Pcairn9siois4i
I32
crystals. Melting point: 106-108°C.
By using a suitable starting material, and a
T
method similar to that of employedin Example 53, there
was obtained compound of the above-mentioned Example
40.
Example 54
To 0.29 g of nicotinic acid was added 50 ml
of dimethylformamide, further 1.2 g of 1-benzyl-6-
chloro-2-[1-(3-aminopropyl)indol-5-ylaminocarbonyl]-
benzimidazole and 0.7 ml of triethylamine were added,
the mixture was stirred under cooling at 0°C. Next,
0.49 g of di~ethylcyanophosphonate was dissolved in 20
ml of dimethylformamide and added thereto and the
reaction mixture was stirred at room temperature for 1
day. After the reaction was finished, water was added
then the whole mixture was extracted with ethyl
acetate, the extract thus obtained was washed with
water and an aqueous solution saturated with sodium
chloride. The washed extract was dried with anhydrous
magnesium sulfate, then the solvent was removed by
distillation under reduced pressure. The resulting
residue was subjected to a silica gel column
chromatography (eluent: 2~ methanol/dichloromethane),
recrystallized from chloroform, there was obtained 1 g
r
of 1-benzyl-6-chloro-2-~1-[3-(pyridin-3-ylcarbonyl-
ammo)propyl]indol-5-ylaminocarbonyl}benzimidazole as
in the form of yellow needle crystals.
WO 97/03070 219 8 2 6 6 pCT/JP96/01841
133
Melting point: 206-207°C.
By using suitable starting materials and a
method similar to that of employed in example 54, there
were obtained compounds of the above-mentioned Examples
9, 12, 28, 33, 34 and 47.
Example 55
To 0.35 g of 1-benzyl-6-chloro-2-(3,4-
dihydro-2(1H)-quinolinon-6-ylaminocarbonyl)-
benzimidazole was added 30 ml of dioxane and 280 mg of
IO- . 2,3-dichloro-5,6-dicyanobenzoquinone, and the reaction
mixture was refluxed by heating. Over confirming the
proceeding of reaction by means of a thin layer
chromatography, 2,3-dichloro-5,6-dicyanobenzoquinone in
small quantity was further added and refluxed by
heating for-1 day. The crystals being separated were
collected by filtration, and recrystallized from
chloroform-isopropyl alcohol, there was obtained
1-benzyl-6-chloro-2-[2(1H)-quinolinon-6-ylamino-
caronyl]benzimidazole as in the form of pale brown
powdery product.
Melting point: higher than 290°C.
IH-NMR (250MHz, DMSO-db) 8 ppm:
6.01 (2H, s), 6.50 (1H, d, J=9.5Hz),
7.22-7.41 (7H, m), 7.84-7.93 (4H, m),
8.25 (1H, s).
i I i
CA 02198266 2005-05-11
2571:1-773
- 134 -
Example 56
A mixture of 27.9 g of ethyl 1-benzyl-6-
chlorobenzimidazol-2-carboxylate, 17.8 g of 1-[3-
(imidazol-1-yl)propyl]-5-aminoindole, 8 g of sodium
methylate and 600 ml of toluene was stirred at 100°C
for 1.5 hours. The reaction mixture was cooled to room
temperature, the crystals being separated were
collected by filtration and washed with toluene. Thus
obtained crystals were dissolved in 500 ml of chloro-
form, then 100 ml of water was added and the mixture
was filtrated with Celite The chloroform layer was
taken by separation, after washed with water, the
chloroform portion layer was dried with anhydrous
magnesium sulfate, and the solvent was removed by
distillation to obtain brown oily product. This oily
product was dissolved in methanol, further added
n-hexane and the crystals being separated were
collected by filtration, recrystallized from methanol
and dried. There was obtained 31.8 g of 1-benzyl-6-
chloro-2-~1-[3-(imidazol-1-yl)propyl]indol-5-ylamino-
carbonyl}benzimidazole.
Pale yellow needle crystals.
Melting point: 187-188°C.
By using suitable starting materials, and by a method
similar to that of employed in Example 56, there were
obtained compounds of the above-mentioned Examples
2 and 4-50.
*Trade-mark
WO 97/03070 219 8 2 6 5 pCT/JP96/01841
135
Pharmacological tests
(1) Measurement of the activity for inhibiting cGMP PDE
Separation and partial purification of PDE
~ (phosphodiesterase) from human platelets, and measure-
ment of the activity for inhibiting cGMP PDE were
conducted by the method of Hidaka, et al. [Biochimica
et Biophysica Acta, Vol. 429, (1976), pp. 485-497].
The platelets obtained from human healthy adult were
washed, and suspended in TRIS-buffer solution, then
said suspension was treated by centrifugal separation,
the supernatant was subjected to a DEAF-cellulose
treatment and fractionated into FI to FIII fractions by
a method of concentration gradient of sodium acetate.
Thus obtained FI fraction was used as the sample of
cGMP-PDE. The activity for inhibiting cGMP-PDE was
measured by using 0.4 ~M of [3H]-cGMP.
The results are shown in Table 34 as follows.
1
219266
WO 97/03070 PCT/JP96I01841
136
Table 34
Activity for inhibiting
1
Test compound of: cGMP-PDE ~ICt2 (uMl1
Example 2 - 0.02 '
Example 3 0.01
Example 4 0.06
Example 9 0.08
Example 11 0.01
Example 20 0.01
Example 21 0.02
Example 25 0.12
Example 28 < 0.01
Example 31 0.03
Example 32 0.06
Example 33 < 0.01
Example 41 0.02
Example 42 0.1
Example 44 0.014
Example 46 < 0.01
Example 47 < 0.01
(2) Measurement of the activi ty for inhibiting
proliferation of rat A10 cells
Test was conducted by the modified method of
N. Morisaki [Atherosclerosis, Vol. 71, (1988), pp.
165-171]. The rat A10 cells (purchased from Dainippon
i
Pharmaceutical Co., Ltd.) were
inoculated on a 24 wells
immunoplate at a density of 0,000 cellslwell, and were
1
2198266
W O 97!03070 PCT/JP96/01841
137
cultured in 10~ FBS (fetal bovine serum) for 2 days,
and in order to introduce the cells into the resting
stage, the cells were further cultured in a serum-free
- medium for 2 days. After that, 1~ FBS was added
thereto for stimulating the proliferation, of the cells
and at the same time, a test compound and 0.5 wCilwell
of [3H]-thymidine were added. As to the index of
synthesized amount of DNA, the quantity of [3H]-
thymidine being uptaken by the cells was measured at
the time of 24 hours after the final cultivation was
started.
The results are shown in Table 35 as follows.
Table 35 _
Activity for inhibiting pro-
- - liferation of rat A10 cells
Test cdanpound flCz" (uM)1
of:
Example 2 0.4
Example 3 0.5
Example 4 0.3
Example 6 0.66
Example 7 0.49
Example 11 0.97
Example 12 0.69
Example 18 0.48
Example 20 0.85
Example 32 0.24
Example 33 1.27
1
Example 42 0.95
- Example 47 0.67
WO 97103070 219 8 2 6 6 pCTIJP96101841
138
(3) Measurement of the activity for inhibiting
proliferation of the human fibroblast
Similar to the activity for inhibiting
proliferation-of rat A10 cells as mentioned above, the
activity for inhibiting proliferation of the human
fibroblast (purchased from Dainippon Pharmaceutical
Co., Ltd.) was determined by measuring the uptake
amount of [3H]-thymidine. Compounds of Examples 3, 16,
31, 33, 45 and 47 were used for the test, and the
measured values of ICSO were, 0.04 uM, 0.049 uM, 0.18
uM, 0.042 ~M, 0.041 ~M and 0.021 uM, respectively.
(4) Measurement of the activity for inhibiting
proliferation of the T cells
Test was conducted by the method as disclosed
in "Current Protocol in Immunology" jEdited by Coligan,
et al., (1991)] Chapter 3, page 12 (Published from
Willy Interscience, Inc.).- After sacrificed a Ba7.b/c
strain male mouse, the spleen was enucleated and a
suspension of the spleen cells in RPMI-1640 culture
medium was prepared. Said suspension was filtered
through a nylon fiber mesh, and subjected to a
centrifugal separation (1,200 rpm, for 5 minutes),
there were added on pellet 5 ml of 0.15 M ammonium
chloride, 10 mM potassium hydrogencarbonate, and 0.1 mM ,
disodium salt of EDTA (pH 7.2) per one spleen so
t
as to suspend the cells, and the suspension was
incubated at 37°C for 5 minutes. A suitable amount of
2198266
W 0 97/03070 _ PCT/JP96/01841
139
RPMI-1640 culture medium was added to the suspension,
and this mixture was subjected to a centrifugal separa-
tion (1,200 rpm, for 5 minutes),-the separated cells
were washed, after repeated further washing operations
twice, the cells were resuspended in RPMI-1640 culture
medium containing 10$ FBS (RPMI-10j. After counted the
number of cellsby using a hemacytometer, a suspension
containing cell density of 106/m1 was prepared by
diluting with RPMI-10 culture medium. A test compound
was dissolved in dimethyl sulfoxide to prepare a solu-
tion of 2 x 10'~ M, then prepared-6 stages of 10-fold
dilution sequences. Each one of these 10-fold dilution
sequences was placed on a 96-well tissue culture plate
in an amount of 10 ul/well (the final concentrations of
test compounds: 10-9 to 10-° M), and added the previ-
ously prepared cell suspension in an-amount of 190
ullwell. There was added 40 ~g/ml of Concanavaline A
in an amount of 10 ul/well, and incubated at 37° for 2
days, under 5~ carbon dioxide gas phase.
20 uCi/ml of [methyl-3H] thymidine was added
in an amount of 10 ul/well, after further incubated
overnight, by using a cell harvester the cells were
recovered on the filter of the harvester. The filter
was cut out from the cell harvester and put in a vial,
then 5 ml of a scintillation cocktail (ACS-II) was
added, and measured by using a liquid scintillation
counter. By using the compound of Example 3 as test
compound, ICso value was 2 uM.
WO 97/03070 219 8 2 b 6 rca~,rn96~ois4i
140
(5) Determination of the activity for inhibiting
chronic contactive dermatitis
f
The activity for inhibiting chronic
contactive dermatitis-[J. Dermatol. Sci., Vol. 8,
(1994), page 54] which is analogous to atopic
dermatitis being reported by Kitagaki, et al. was
discussed by using the method as follows. The
right-side ear pinna of Balb/c strain male mouse was
subjected to antigen sensitization by coating with 20
u1 of acetone solution of 1~ trinitrochlorobenzene
(TNCB). 7 Days after the antigen-sensitization, the
same right-side pinna was subjected to antigen
induction by coating with20 w1 of 1~-TNCB acetone
solution. The treatment of antigen induction was
repeated in every 2 days so as to induced chronic
contactive dermatitis. In this test, increase of
thickness of the right-side ear pinna was induced by
passing into a chronic state of the contactive
dermatitis. The test compound was dissolved in
acetone-methanol (4:1 by v/v) and 20 w1 of this
solution was administered by coating on the right-side
of ear pinna once a day from 24th day after the antigen
induction for 2 weeks. As to the control, the only
the solvent'was similarly administered. The antigen
induction by the test compound during administration
period was conducted at 30 minutes before the
1
administration. The thickness of the ear pinna of 14th
day after the administration was measured by a dial
2198266
WO 97/03070 PCTlJP96/0184i
141 '
thickness gauge.
The results are shown in Table 36 as follows.
- - Table 36
Concentration (8) Thickness of Inhibition
of compound of ear pinna rate
Example 3 (x 10 Vim)
0 (Control) 137.1 6.2 _
0.1 120.4 5.3 12
0.3 105.0 k 7.2 23
1.0 64.5 t 1.6 53
* Significant difference between the control:
p < 0.01 Dunnett's test
Number of the test animals: 8
(6) Determination of the activity for inhibiting
TPA induced inflammation
Test was conducted by the modified method of
Carlson, et al. [Agents Actions, Vol. 26, (1989),
page 3I9]. Thus, to the ear pinna of ICR strain female
mouse, 20 ~1 of acetone solution of 12-O-tetradecanoyl-
phorbol-13-acetate (TPA) in the concentration of 200
ug/ml was coated to induce inflammation. The thickness
i of ear pinna of 4 hours after the TPA coating was
measured, and difference of the last value was defined
r
as the ear pinna inflammation. Test compound was
dissolved in acetone-methanol (4:1 by v/v) to make the
WO 97/03070 ~ 219 8 2 6 5 pCT/Jpy6/01841
' 142
concentration of 1~. 20 u1 of test compound solution
was coated on the ear pinna 30 minutes before the TPA
coating. By using compound of Example 3 as test
compound, the inflammation was inhibited a~ the rate of
66~.
(7) Determination of the activity for inhibiting
proliferation of rat mesangial cells
In accordance with the method of F. Jaffer,
et al. [Am. J. Pathol., Vol. 135, (1989), pages
261-269], the mesangial cells werecollected. Under
anesthetized condition, the kidney of a rat was
aseptically enucleated and the medulla renis was cut
out, then the cortex renis-was pressed to a sieve,(120
mesh) made of stainless steel. The fraction of cortex
renis passed through the sieve was put on another sieve
of-200 mesh and washed with PBS (phosphate-buffered
saline), the fraction being remained on the sieve was
confirmed as the glomerulus. This fraction was
cultured in RPMI 1640 medium [containing 15$ of fetal
calf serum (FCS), and 5 ~g/ml of insulin] for 4 weeks.
This fraction was further subcultured twice, and the
remaining cells were thought of as the mesangial -
cells for use of this experiment.
Determination of the activity for inhibiting
proliferation of rat mesangial cells was conducted in
1
accordance with the method-of M. B. Ganz, et al. [Am.
J. Physiol., vol. 259, (1990), pages F269-F278].
WO 97/03070 Z 19 8 2 6 6 pCT/~6/01841
143
Thus 2 x 10~1m1 of mesangial cells were
inoculated on a culture plate (48 wells, each well
having the capacity of 0.5 ml), and cultured with RPMI
- medium (containing 15~ FCS) for 3 days. The RPMI
medium was then changed to another RPMI medium wherein
the concentration of FCS was decreased to 0.5$, and
further cultured for 3 days. Next, the RPMI medium was
changed to another RPMI medium containing the same
concentration of FCS, and added the test compound being
dissolved in dimethyl sulfoxide (the final concentra-
tion of dimethyl sulfoxide was lower than 0.1~) and 5
ng/ml (the final concentration) of platelet-derivered
growth factor BB (PDGF-BB). After 24 hours, 1 ~Ci/well
of ['H]-thymidine was added, and incubated for
additional 24 hours. The supernatant of the culture
medium was taken up, and the amount of [3H]-thymidine
up-taken into the cells was measured by use of
scintillation counter. ICSO value measured by using
compound of Example 3 for the test was 0.79 yiM.
i