Note: Descriptions are shown in the official language in which they were submitted.
=~ 9~48
QUANTITATIVE DETECTION OF ANALYTES
ON IMMUNOCHROMATOGRAPHIC STRIPS
Background of the Invention
Immunochromatographic strip formats are increasingly popular
for qualitative and semi-quantitative assays which use visual
detection schemes. This type of immunoassay involves the
application of a liquid test sample suspected of containing an
analyte to be detected to an application zone of an
immunochromatographic test strip. The strip is comprised of a
matrix material through which the test fluid and analyte
suspended or dissolved therein can flow by capillarity from the
application zone to a detection zone where a visible signal, or
absence of such, reveals the presence of the analyte. Typically,
the strip will include means for immunospecifically binding the
analyte to be detected with its specific binding partner which
bears a detectable label. In one such scheme; as disclosed in
U.S. Patent 4,446,232; the strip contains an enzyme labeled,
mobile binding partner for the analyte which is in a zone
downstream from the sample application zone. If analyte is
present in the test sample, it will combine with its labeled
binding partner to form a complex which will flow along the strip
to a detection zone which contains a substrate for the enzyme
label capable of providing a colored response in the presence of
the enzyme label. The strip may contain a zone in which analyte
is immobilized, so that labeled binding partner which does not
combine with analyte, due to absence of analyte in the sample,
will be captured and thereby inhibited from reaching the
detection zone. There have been published various modifications
of this technique, all of which involve some competitive specific
binding system in which the presence or absence of analyte in the
test sample is determined by the detection or lack thereof of
labeled binding partner in the detection zone. In U.S. Patent
4,868,108 there is disclosed a similar scheme with the addition
of an immobilized capture reagent for the enzyme labeled binding
partner in the detection zone to concentrate the enzyme label and
enhance its ability to react with the enzyme substrate and
thereby render the assay more sensitive.
2 ~ 98948
An alternative to the above described immunometric assay
which detects the free labeled antibody (hereafter referred to as
captured free format) is the so called sandwich format in which
the detection zone contains immobilized antibodies against an
epitope of the analyte which is different than that epitope to
which the labeled antibody is specific. In this format, there is
formed a sandwich of the analyte between the immobilized and
labeled antibodies and it is therefore an immunometric assay
which detects the bound labeled antibody species.
Not all of the schemes for immunochromatography rely on an
enzyme labeled binding partner/enzyme substrate as providing the
signal for detection of the analyte. In U.S. Patent 4,806,311
there is disclosed a multizone test device for the specific
binding assay determination of an analyte and an immobilized
binding partner therefore together with a detection zone for
receiving labeled reagent which migrates thereto from the reagent
zone. The detection zone contains an immobilized form of a
binding substance for the labeled reagent. The labeled reagent
bears a detectable chemical group having a detectable physical
property which is detectable on the basis of its own physical
properties, so that it does not require a chemical reaction with
another substance. Exemplary of such groups are colored species
fluorescers, phosphorescent molecules, radioisotopes and
electroactive moieties.
United States Patent 4,313,734 describes the use of gold
sols as labels for antibodies which are detectable without a
chemical change although this patent carries out the assay in
microtitre plates rather than on strips of absorbant material.
Immunochromatographic strip formats provide a viable system
for the determination of various analytes (whether they be
antigens or antibodies) but suffer from the limitation that they
yield results which are at best semi-quantitative when, for some
analytes, a quantitative answer is required. Accordingly, it
would be desirable and it is an object of the present invention
to provide a means for quantifying the results of analyses
carried out by the use of immunochromatographic strip formats.
3 ~2 -1 9 8 ~
iummary of the Invention
The present invention involves an improvement to a method
for determining an analyte in a test fluid which involves
applying the test fluid to an immunochromatographic matrix which
allows the test fluid; and the analyte, if present; to flow
through the matrix by capillarity and which matrix contains a
labeled binding partner for the analyte. The matrix, which is
normally in the form of a test strip, also contains at least one
detection zone in which the presence or absence of the analyte is
determined by detecting the label carried by the specific binding
partner. The improvement involves determining the concentration
of the label using an instrument having a detector capable of
determining its concentration in the detection zone(s).
In a preferred embodiment of the present invention there is
provided a test strip comprising a strip having a first region
which contains mobile specific binding partner for the analyte
which bears a detectable label and can react with the analyte to
form an analyte/labeled binding partner complex and at least one
second region which contains an immobilized analyte or analog
thereof (captured free format) as described in co-pending
application 08/380,119 or an immobilized specific binding partner
for an epitope on the analyte which is distinct from that to
which the labeled specific binding partner is bound (sandwich
format). The term analog as used herein refers to any substance
capable of being bound by the active site of the specific binding
partner.
The strip as described above is developed by applying the
test fluid sample suspected of containing the analyte thereto,
thereby allowing it to contact the mobile, labeled specific
binding partner for the analyte whereby analyte present in the
fluid test sample binds to the labeled specific binding partner
to form the complex; leaving excess, unreacted labeled binding
partner free to further react whereby the fluid test sample
carries the analyte/labeled binding partner conjugate and
unreacted labeled binding partner along the strip by capillarity
to the second region which, in the captured free format, contains
the immobilized analyte or analog thereof in which uncomplexed
7. ~
_abeled binding partner is bound to the immobilized analyte in
inverse relationship to the concentration of analyte in the fluid
test sample. In the sandwich format, there are immobilized
binding partners specific to a second epitope on the analyte for
capture of the analyte/specific binding partner complex and the
capture of this complex in the detection zone is directly
proportional to the concentration of analyte in the test fluid.
The developed strip is read on an instrument having a
detector capable of measuring the signal from the detectable
label to determine the signal from the labeled binding partner in
the second region. The concentration of the analyte in the fluid
test sample is determined by comparing the signal from the
detectable label with determinations made in a similar manner
using fluid test samples containing known concentrations of
analyte.
The sensitivity of the determination can be enhanced by
providing a strip with a third region which region contains means
for immobilizing the labeled specific binding partner species
which did not bind in the detection zone. For example, if the
labeled binding partner is a labeled mouse antibody (IgG), the
labeled mouse antibody that did not bind in the detection zone
can be captured in a zone of immobilized goat anti-mouse IgG. By
measuring the signal from the detectable label immobilized in
this third region and determining the ratio of the signal from
labeled binding partner in the second region to that in the third
region, inaccuracies caused by uneven deposition of labeled
conjugate and/or non-uniform fluid flow through the matrix can be
corrected.
Detailed Description of the Invention
The present invention is practiced by first providing a test
matrix through which the fluid test sample can flow by
capillarity. Typically, the matrix will be in the form of a
strip through which the test fluid flows horizontally although
the matrix could be set up in layers through which the test fluid
could flow vertically from top to bottom or vice-versa. The
following discussion will focus on the strip format.
~ 9 4 ~
The strip can be prepared from any matrix material through
which the test fluid and an analyte contained therein can flow by
capillarity. The matrix can be of a material which is capable of
non-bibulous lateral flow. This type of flow is described in
U.S. Patent 4,943,522 as liquid flow in which all of the
dissolved or dispersed components of the liquid are carried
through the matrix at substantially equal rates and with
relatively unimpaired flow, as opposed to preferential retention
of one or more components as would be the case if the matrix
material were capable of adsorbing or imbibing one or more of the
components. An example of such a matrix material is the high
density or ultra high molecular weight polyethylene sheet
material from Porex Technologies of Fairburn, GA. Equally
suitable for use as the matrix material from which the
chromatographic strips can be fabricated are bibulous materials
such as paper, nitrocellulose and nylon.
Various immunochromatographic strip formats are suitable for
use in conjunction with the present invention. A particularly
suitable format is that which is disclosed in U.S. Patent
4,446,232 wherein there is described a device for the
determination of the presence of antigens, which device comprises
a strip of matrix material having a first zone in which there are
provided immobilized analyte and enzyme linked antibodies
specific to the analyte to be determined. The labeled antibodies
can flow to a second zone when reacted with analyte introduced
into the first zone but will not so flow in the absence of
analyte in the test fluid due to being bound in the first zone by
interaction with the immobilized analyte. The analyte is
typically an antigen, although the format can be designed to
detect the presence of antibodies as analyte. Modifications to
this format are disclosed in U.S. Patent 4,868,108. In another
modification, the enzyme substrate is disposed in the region of a
second, immobilized antibody to thereby capture the complex
formed between the enzyme labeled antibody and the analyte. This
sort of format is particularly suitable for adaptation to the
present invention, although any physically detectable signal may
be used since the present invention need not be limited to the
interaction of an enzyme and its substrate to provide the
letectable signal. Thus, by immobilizing the conjugate in a
discrete detection zone located downstream on the strip from the
zone in which the labeled binding partner for the analyte is
bound, there are provided two zones from which the physically
detectable property of the detectable label can be measured to
determine its concentration. By measuring the signal from the
physically detectable property of the detectable label in the
second zone containing the immobilized analyte as the capture
means and the signal from the physically detectable property of
the label in the third zone, in which the immobilized antibody
against the labeled binding partner is the capture means, and
determining the ratio of these signals, the accuracy of the test
for analyte concentration can be increased. The accuracy is
increased because this technique corrects for inaccuracies in
labeled conjugate deposition and/or non-uniform fluid flow
through the matrix. More particularly, since the aforementioned
inaccuracies of labeled conjugate deposition and non-uniform
fluid flow are usually of small but significant magnitude, they
do not disturb substantially the binding equilibrium. Therefore,
the ratio of the signals in the two binding zones is a more
accurate measure of the analyte concentration than is the signal
in either zone by itself. This principle applies with equal
force when the previously described sandwich assay format is
used.
In a preferred embodiment of the present invention, there is
provided a reflectance spectrometer with means for moving the
strip or detector relative to each other such as a specimen table
on which the strip is placed which can be moved laterally under
the read head of the detector. In the case of the detectable
physical property being reflectance of light at a predetermined
wavelength, the detector is a spectrometer. This technique will
assist in providing accurate quantitation for regions of the
strip which may not have been precisely located with respect to
the detection means of the spectrometer. More specifically, the
location of the strip relative to the detector can be under
microprocessor control, so that the reflectance of any desired
region can be determined.
7 ~ ~ ~%~ ~
The method of practicing the present invention is more fully
illustrated by the following examples:
Example I
Quantitation of HSA in a Sinqle Blocking Band Format
An immunochromatographic strip containing a blocking band of
immobilized HSA and a broad area of anti-HSA:gold sol conjugate
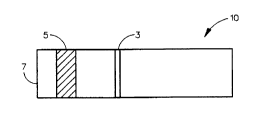
in an ImmunodyneTM nylon membrane was prepared. This strip is
illustrated by Fig. 1 wherein the strip 10 contains blocking band
3, preceded by the anti-HSA:gold sol containing region 5 and the
sample application area 7. These strips were prepared as
follows:
A 4.2 X 12.6 centimeter piece of Immunodyne~ membrane was
placed on a Comag Thin Layer Chromatography (TLC) stripping
apparatus with the long side parallel to the base and offset 1 cm
up from the O position of the Y axis. Next, a solution of human
serum albumin (HSA) with a concentration of 10 mg/mL was prepared
in phosphate buffered saline (PBS; 0.137 M sodium chloride,
0.0027 M potassium chloride, 0.010 M potassium phosphate, pH
7.4). At a Y position of 3.5 cm a 6 cm long band of the 10 mg/mL
HSA solution was stripped using the following settings of the TLC
stripper:
(a) plate = 90, (b) band = 60, (c) sec/~L + 6,
(d) volume = 6 ~L.
This gave a band 6 cm long and approximately 1 mm wide. The
stripping density was therefore 10 ~L/cm2 with a density of 100
~g of HSA/cm .
After 3 minutes the membrane was removed from the TLC
stripper and placed in a flat plastic tray containing 0.5~ casein
(Hammerstein from Schlesinger) in phosphate buffered saline (pH
7.4 from Sigma) and gently rocked on an orbital shaker for 30
minutes.
7i ~
At this point a wash buffer was prepared as 0.02% sodium
azide, 0.02% Tween 20 and 0.1% PEG 20 in PBS. The membrane was
removed from the casein blocking solution and was twice washed
with 25 mL of wash buffer for 30 minutes with gentle rocking on
an orbital buffer whereupon the membrane was removed from the
wash buffer and allowed to dry overnight at room temperature.
Gold sol was prepared by adding 2.0 mL of a 10 mg/mL
solution of acid gold chloride monohydrate (HC14Au H2O) to a
refluxing 100~C solution of tri-sodium citrate (0.00155 M). The
refluxing was continued for 30 minutes and then cooled and
filtered through a 0.2 ~M filter. Antibody-gold sol conjugate
(Ab:gold sol) was prepared by adding 240 ~g of monoclonal
antibody against human serum albumin and 50 ~L of 0.1 M potassium
carbonate to 10 mL of the gold sol solution prepared as described
above and the mixture was allowed to stir vigorously for 15
minutes, whereupon 0.5 mL of 1% (w/v) PEG-20 was added followed
by another 10 minutes of vigorous stirring. At this point, 1.0
mL of 10% bovine serum albumin (BSA) in water was added and the
mixture stirred vigorously for 10 minutes. The Ab:gold sol was
isolated by centrifugation at 14,500 X g for 30 minutes at 20~C
and then washed 10 times by suspending it in a wash buffer (1%
BSA, 0.05% PEG-20, 2 mM sodium borate, pH = 9.0) and isolated by
centrifugation as described above. After the final
centrifugation, the Ab:gold sol was suspended in 1.0 mL of wash
buffer and stored at 4~C.
The dried membrane described above was again placed on the
TLC stripper at an offset of 1 cm in the Y direction as before.
A mixture of 40 ~L of Ab:gold sol, 20 ~L of 4% casein and 20 ~L
of 1% Methocel (K4M) + 0.6% polyvinyl alcohol (PVA) was prepared
and seven adjacent bands were stripped as before between the Y
positions of 2.3 and 2.9 cm. The strip was allowed to dry at
room temperature and slit into 0.5 cm wide strips before use.
A medium specific gravity (S.G.+1.017) pool of urine was
filtered through an ultrafiltration membrane which held back
proteins larger than 30,000 daltons. The filtrate was used to
prepare HSA solutions of various known concentrations by spiking
them with an HSA solution of known concentration.
9 '~
The strips were developed by suspending them vertically in a
solution of HSA spiked urine filtrate to a depth of approximately
0.5 cm (on the end of the strip containing the Ab:gold sol
conjugate bands) and allowing 5-10 minutes for the liquid to
reach the top of the strip. These strips were allowed to air dry
at room temperature and then mounted on plastic trycite handle
material and analyzed.
The strips were developed with samples of an ultrafiltrate
of medium specific gravity urine containing 0, 1, 2, 3 and 5
mg/dL HSA. The strips from each sample concentration were read
by measuring the reflectance at 557 nm on a CT100 reflectance
photometer with scanning of the strip being simulated by cutting
one millimeter off its end between measurements.
More particularly after the strips were developed with
sample fluid and allowed to air dry at room temperature, they
were mounted on plastic handle material using double sided
adhesive. The plastic/membrane laminate was trimmed to 7 mm
(toward the sample application end) from the HSA band 3 (Fig. 1)
which was visible due to the bound Ab:gold sol. The strip was
then placed on the read table of a CT100 reflectance photometer
with the strip pushed to the end stop. In this position the read
area of the 10th pad position is 2.5 mm from the end of the
strip. The reflectance of the 10th pad position was then
measured after which 1 mm was cut from the end of the strip and
the strip pushed to the end of the read table. The 10th pad
position was again read and this process was repeated until the
end of the plastic and membrane laminate corresponded to a point
which was 3 mm past the HSA band. This technique was used to
move the read head (detector) in relation to the read zone (HSA
band) since software was not available to perform this task.
With the proper software in place, the reflectance of the strip
can be scanned by moving the read table with the plastic and
membrane laminate past the readhead.
The results of this experiment are graphically illustrated
by Fig. 2.
1 0
From Fig. 2 it can be determined that the depth of the
troughs of the reflectance scans of immunochromatographic strips
developed with urine samples containing various concentrations of
HSA are directly proportional to HSA concentration and a dose
response to HSA can be seen in reflectance. Even though the gold
sol band does not cover the entire read area, the reflectance is
lowered by between 10 and 15% when the band is in the read area.
This 10 and 15% change in reflectance is detected even though
much of the high reflectance (white) area is being measured along
with the gold sol band.
If a mask with a narrow slit were added to the readhead
area, the range of reflectance would be greatly increased because
the high reflectance white area would not be in the read area at
the same time as the gold sol band. This increased reflectance
would allow better discrimination between analyte (HSA)
concentrations. With the stepping motor, the spectrophotometer's
strip table can be moved slowly through any area on the strip
while taking consecutive readings to give good resolution for
finding trough reflectances or areas in the troughs since the
stepping motor can be moved a fraction of one revolution at a
time.
Example II
Quantitation of HSA in a Format Containing a Blocking Band and a
Capture Zone
An immunochromatographic strip was prepared according to the
methods of Example I and the format of Fig. 3. Referring to Fig.
3, the strip 10 has a blocking band of immobilized HSA 3, a mouse
anti-HSA:gold sol conjugate zone 5 and a capture band of
immobilized goat anti-mouse IgG antibody 9. In preparing this
band, a solution of goat anti-mouse IgG (sigma 8770) with a
concentration of 5 mg/mL was prepared in 0.135 M sodium chloride.
This was stripped as described above at a Y distance of 4.0 cm.
The stripping density was 50 ~g of IgG/cm2. When the sample
application zone 7 is immersed in a sample containing HSA to a
depth less than that needed to immerse the conjugate zone 5,
fluid will flow upward from the sample by capillary action. The
-HSA in the sample will complex with the gold sol:anti HSA in the
conjugate zone and will move up the strip along with conjugate
which is free since it did not find any HSA to bind as there was
a molar excess of conjugate to HSA in the sample. The free
conjugate will bind the immobilized HSA in the blocking band 3
while the gold sol-anti HSA:HSA complex will continue to flow up
the strip where it will be bound by the immobilized goat anti
mouse IgG antibody in the capture band 9.
Strips of this format were developed with samples of medium
SG urine ultrafiltrate containing concentrations of HSA of 0,
0.5, 0.8, 1.0, 1.5 and 2.0 mg/dL. Duplicate strips for each HSA
concentration were run. Reflectance data at 557 nm was collected
on the CT100 using a method for visually aligning the bands in
the center of the 10th pad position. The 10th pad position
occupies the 5 mm portion adjacent to the end of the Multistix~
10 SG urine strip product. The Multistix 10 SG product is an
approximately 10.9 cm long by 5 mm wide by 0.5 mm thick piece of
plastic to which 10 paper pads containing dried reagents each 5
mm by 5 mm are attached. The 10th pad is aligned evenly with one
end of the plastic and the spacing between the pads is 2.5 mm
leaving a 3.4 cm piece of the plastic at the other end which has
no pads and serves as a handle area. In Fig. 4a there are
plotted the results of this experiment in terms of reflectance.
In this figure, in which the reflectance is from the immobilized
HSA band 3 (Fig. 3), the dose response to HSA is linear in terms
of reflectance (R) with two outlying values. The response of the
goat anti-mouse IgG band 9 (Fig. 3) to HSA, as represented in
Fig. 4b, is more scattered and most of the reflectance drop
occurred between 0 and 0.5 mg/dL HSA. However, when the
reflectance value for the HSA band is ratioed to the reflectance
(R) value for the goat anti-mouse IgG band the variability
decreases as can be determined from Fig. 4c which plots the
concentration of HSA against the ratio of reflectance of the HSA
band to the reflectance of the goat anti-mouse IgG band. A
curved but smooth dose response to HSA concentration is observed
in Fig. 4c. This ratio can be reversed which would amount to
taking the reciprocal of each ratio which would have the same
effect as the ratio itself. Thus, the determination of the ratio
of these two reflectance values corrects for the variability of
12 ~ 1 9 ~ 9 4 8
che conjugate deposition during reagent preparation, and any
unevenness in the fluid flow during the development of the strip
with sample, i.e. if there is less gold sol:anti HSA on one strip
than on another, the ratio of the two bands will provide a result
which is corrected for the unevenness in preparation. Unevenness
in fluid flow during development of the strip can be corrected
for in a similar manner.
Example III
Quantitation of HSA and IgG in a Dual Blocking Band Format
Immunochromatography strips were constructed to measure both
human serum albumin (HSA) and human (H) IgG in order to quantify
each of these analytes independently. The strips were prepared
from ImmunodyneTM nylon according to the scheme of Fig. 5 to
comprise a sample application zone 1 followed by a zone 3
containing gold sol labeled anti-HSA and gold sol labeled anti-
(H)IgG conjugates. The strip contained two blocking bands; the
first blocking band 5, containing immobilized HSA, and the second
blocking band 7, containing immobilized (H)IgG.
These strips were developed with test samples of an
ultrafiltrate of medium SG urine containing 0, 5, 10, 15, 20, 30
and 40 mg/L HSA along with either 0 IgG or 100 mg/L (H)IgG. Two
strips for each HSA concentration were examined by measuring the
reflectance at 557 nm on a CT100 reflectance photometer by
physically cutting and aligning the strip in the 10th pad
position of the Multistix~ SG strip to obtain reflectance
readings. Fig. 6 graphically represents the reflectance for
samples containing both HSA and (H)IgG blocking bands using test
samples which did not contain IgG. Fig. 7 shows similar data for
test samples containing 100 mg/L (H)IgG along with the various
concentrations of HSA. From the data of Fig. 6 it can be
determined that the reflectance of the HSA blocking band is
directly proportional to the HSA concentration in the sample and
the reflectance of the (H)IgG blocking band is approximately 0.88
(based on a maximum total reflectance of 1.0) due to the gold
sol:anti (H)IgG which binds to it in the absence of (H)IgG in the
sample. The data in Fig. 7 show the same direct proportionality
13 ~ 1 9 8 9 4 8
-of the reflectance to HSA concentration as do the data in Fig. 6,
but the reflectance of the (H)IgG band is higher (0.91-0.92) due
to the 100 mg/L (H)IgG in the sample. The (H)IgG binds to the
gold sol-anti(H)IgG conjugate and will not allow it to bind to
the immobilized (H)IgG in the (H)IgG blocking band. This
demonstrates that the dose response to HSA is the same in the
presence or absence of (H)IgG and that there is a separate dose
response to (H)IgG. Accordingly, by using a strip with mixed
gold sol antibody conjugates against two different analytes and
in separate regions having immobilized bands of these analytes or
analogs thereof, one can obtain a separate instrumentally
detectable dose response to each analyte. This is important
because it enables one to quantitate more than one analyte using
a single immunochromatographic strip.
While the foregoing examples demonstrate the captured free
format, it can readily be understood that the ratioing concept
can be applied to the sandwich format with equal efficacy since
either format will provide a detectable signal in the second
detection zone which can be ratioed with the signal in the third
zone.