Note: Descriptions are shown in the official language in which they were submitted.
CA 02199002 1997-11-24
MICROPORATION OF HUMAN SKIN FOR DRUG DELIVERY
AND MONITORING APPLICATIONS
BACKGROUND OF THE lNv~NllON
This invention relates generally to the field of
monitoring of analytes in the body and the trans~ermAl
delivery of drugs to the body. More particularly, this
invention relates to a m;n;~l ly invasive to non-invasive
method of increasing the permeability of the skin through
microporation of the stratum corneum, which can be combined
with sonic energy, chemical permeation enhAncers, pressure,
and the like for selectively ~nhAncing outward flux of
analytes from the body for monitoring thereof or the delivery
of drugs into the body. The monitoring of analytes in the
body is conducted generally for a diagnostic purpose.
The stratum corneum is chiefly responsible for the
well known barrier properties of skin. Thus, it is this
layer that presents the greatest barrier to transdermal flux
of drugs or other molecules into the body and of analytes out
of the body. The stratum corneum, the outer horny layer of
the skin, is a complex structure of compact keratinized cell
remnants separated by lipid ~nm~;nC. Compared to the oral or
gastric mucosa, the stratum corneum is much less per~Ahle to
molecules either external or internal to the body. The
stratum corneum i8 formed from keratinocytes, which comprise
the majority of epidermal cells, that lose their nuclei and
become corneocytes. These dead cells comprise the stratum
corneum, which has a thickness of only about 10-30 ~m and, as
noted above, is a very resistant waterproof membrane that
69912-294(S)
CA 02199002 1997-11-24
protects the body from invasion by exterior substances and
the outward migration of fluids and dissolved molecules. The
stratum corneum is continuously renewed by shedding of
corneum cells during des~l~m;n~tion and the formation of new
corneum cells by the keratinization process.
69912-294(S)
~ 2 1 99002
Tlle nux of a drug or analyte across the skio can be increased by ~ h~nEing either tbe
resistance ~the clirrusioll coemcient) or tlle driving force (the gradient for diffusiol~ lu:~ may
be enllallced by lhe use of so-called penetration or chemical e.lhallc. ~. Cl~e-nical enl~ancers
are well known in tlle art alld a more detailed description will follow.
Anotller me~llod of increasing the permeability of skin to dmgs is iontop11oresis.
lontopllor( sis involves the application of an external electric field and topical delivery of an
ionized fo m of dmg or an un-iollized drug carried with tlle water nux a~ociat~d with ion
llm~sllull (clectro-osmosis). While permeation enhancementwitl1 iontophoresis has been
effective, control of drug delivery and irreversible skin damage are probletns associated with
tlle techlli~lue.
Sollic energy has also bcen used to enllance pern1eability of tlle skin and syntlletic
membranes to drugs and otller molecllles. Ultrasound has been dermed as mechanical pressure
waves Witll frequencies above 20 kl-lz, Il. Lutz et al., Manual of Ultrasound 3-12 (1984)~
Sonic ene gy is generated by vil~ lg a piezoelectric crystal or otller electromechallical
element b l passing an alternating currellt througll tlle material, R. Brucks et al., 6 Pllarm. Res.
2 0 697 (1985). The use Or sonic energy to increase tlle permeability Or tlle skin to drug molecules
has been ~ermed sonoplloresis or phonopl1oresis.
~ltllollgll it llas been acknowledged tllat enllancing permeability ol the skin shollld
tlleoretically make it possible to transport molecules rrom inside the body throllgll the skin to
outside tlle body ror collection or monitoring, practicable methods have not been disclosed.
2 5 U.S. Pate lt No. 5,139,023 to Stal1ley et al. discloses an al)lJalalui~ and metllod for noninvasive
blood glucose monitoril1g. In this invel1tion, chemical permeation enl.dnce- . are used to
increase t le pelmeability of mucosal tissue or skin to glucose. Glucose thel1 passively diffilses
througl~ tlle mucosal tissue or skin and is captured in a receiving medium. The amount of
glucose in tlle receiving mediulll is measllred and correlated to determil1e the blood glucose
3 O level. }-lowever, as ~augllt in Stanley et al., tl1is me~l1od is much more efficient whel1 used 011
mucosal I issue, such as buccal tissue, whicl1 results in detectable amounts of glucose being
collected in the receiving medillm aner a lag time of about 10-20 minutes. Ilowever, the
CA 02199002 1998-08-06
method taught by Stanley et al. results in an extremely long
lag time, ranging from 2 to 24 hours dep~n~; ng on the chemical
~nhAncer composition used, before detectable amounts of
glucose can be detected diffusing through human skin
(heat-separated epidermis) in vitro. These long lag times may
be attributed to the length of time required for the chemical
permeation ~nhAncers to passively diffuse through the skin and
to enhance the permsAhility of the barrier ~tratum corneum, as
well as the length of time required for the glucose to
passively diffuse out through the skin. Thus, Stanley et al.
clearly does not teach a method for transporting blood glucose
or other analytes non-invasively through the skin in a manner
that allows for rapid monitoring, as is required for blood
glucose monitoring of diabetic patients and for many other
body analytes such blood electrolytes.
While the use of sonic energy for drug delivery is
known, results have been largely disappointing in that
enhancement of permeability has been relatively low. There is
no consensus on the efficacy of sonic energy for increasing
drug flux across the skin. While some studies report the
success of sonophoresis, J. Davick et al., 68 Phys. Ther. 1672
(1988); J. Griffin et al., 47 PhyR. Ther. 594 (1967); J.
Griffin & J. Touchstone, 42 Am. J. PhYs. Med. 77 (1963); J.
Griffin et al., 44 Am. J. Phys. Med. 20 (1965); D. Levy et
al., 83 J. Clin. Invest. 2074; D. Bo~AnnAn et al., 9 Pharm.
Res. 559 (1992), others have obtained negative results, H.
Benson et al., 69 PhYs. Ther. 113 (1988); J. McElnay et al.,
20 Br. J. Clin. Pharmacol. 4221 (1985); H. Pratzel et al., 13
75304-12(S)
CA 02199002 1998-08-06
J. Rheumatol. 1122 (1986). Systems in which rodent skin were
employed showed the most promising results, wherea~ systems in
which human skin was employed have generally shown
disappointing results. It is well known to those skilled in
the art that rodent skin is much more permeable than human
skin, and consequently the above results do not teach one
skilled in the art how to effectively utilize sonophoresis as
applied to transdermal delivery and/or monitoring through
human skin.
A significant impro~l,.allt in the use of ultrasonic
energy in the monitoring of analytes and also in the delivery
of drugs to the body is disclosed and claimed in U.S. Patent
No. 5,458,140 and U.S. Patent No. 5,445,611. In these
patents, the transdermal sampling of an analyte or the
transdermal delivery of drugs, is accomplished through the use
of sonic energy that is modulated in intensity, phase, or
frequency or a combination of these parameters coupled with
the use of chemical permeation enhAncers. Also disclosed is
the use of sonic energy, optionally with modulations of
frequency, intensity, and/or phase, to controllably push
and/or pump molecules through the stratum corneum via
perforations introduced by needle puncture, hydraulic jet,
laser, electroporation, or other methods.
The formation of micropores (i.e. microporation) in
the stratum corneum to ~nhAnce the delivery of drugs has been
the subject of various studies and has resulted in the
issuance of patents for such techniques.
Jacques et al., 88 J. Invest. Dermatol. 88-93
75304-12(S)
CA 02199002 1998-08-06
4a
(1987), teaches a method of ~m;n;stering a drug by ablating
the stratum corneum of a region of the skin using pulsed laser
light of wavelength, pulse length, pulse energy, pulse number,
and pulse repetition rate sufficient to ablate the stratum
corneum without significantly damaging the underlying
epidermis and then applying the drug to the region of
ablation. This work resulted in the issuance of U.S. Patent
4,775,361 to Jacques et al. The ablation of skin through the
use of ultraviolet-laser irradiation was earlier reported by
Lane et al., 121 Arch. Dermatol. 609-617 (1985). Jacques et
al. is restricted to use of few wavelengths of light and
expensive lasers.
Tankovich, U.S. Patent No. 5,165,418 (hereinafter,
"Tankovich '418"), discloses a method of obtaining a blood
sample by irradiating human or ~n;m~l skin with one or more
laser pulses of sufficient energy to cause the vaporization of
skin tissue so as to produce a hole in the skin exten~;ng
through the epidermis and to sever at least one blood vessel,
causing a quantity of blood to be expelled through the hole
such that it can be collected. Tankovich '418 thus is
inadequate for non-invasive or m;n;m~lly invasive
permeabilization of the stratum corneum such that a drug can
be delivered to the body or an analyte from the body can be
analyzed.
Tankovich et al., U.S. Patent No. 5,423,803 (herein-
after, "Tankovich '803") discloses a method of laser removal
of superficial epidermal skin cells in human skin for cosmetic
75304-12(S)
q ~ 3 2
applications. The method comprises applyillg a light-absorbing "cont~min~nt" to the outer
layers of t!le epidermis and rorcing some of this contaminant into the intercellular spaces in the
s~ratum carneum, aod illuminatillg the hlrlltrated skin with pulses of laser light of sufficient
intellsity t!lat tlle amoullt of energy absorbed by the col1tamillant will cause the contamhlant to
explode witll sufficient energy to tear off some of the epidermal skin cells. Tankovich '803
further teaches that there should be higll absorption of energy by the C~ nl -minsln~ at the
wavelengt~l of the laser beam, tllat tlle laser beam must be a pulsed beam of less thal~ s
duration, Ihat the contamillallt musl be rorce-l into the upper layers of the epidennis, and tllat
the contamillant must explode with sufficient energy to tear off epidermal cells upon absorption
of the laser energy. This inventioll also fails to disclose or suggest a melhod of drug delivery
or analyte collection.
ll~aven et al., WO 92/00106, describes a method of selectively removing unhealthy
tissue from a body by admhlistering to a selecled tissue a compound that is higllly absorbellt of
infiared r;ldiation of waveleng~ 50-860 nm and irradialing ~lle region with col~ lding
infrarcd rndiatioll at a power surficient to cause tllermal vaporization of ~he tissue to whicll the
compoull-l was aclmillisleled but insumciellt to cause vaporization of tissue to whicll the
compound had not been admhlistered. The absorbent compound should be soluble hl water or
serum, sucll as indocyanille green, chlolopllyll, porpllyrills, lleme-con~:lining compoullds, or
compounds containing a polyene structure, and power levels are in tlle range of 50-lO00
W/cm2 or even higller.
2 5 l~onig et al., Dl~ 259351, teaches a process for therlllal trealment of tumor tissue that
comprise~ deposiling a mediulll hl lhe tumot tissue that absorbs radiation in the rcd and/or near
red infrared spectral region, and irradiathlg tlle inilltrated tissue with an applopl;ate wavelength
of laser light. Absorbing media call inclllde melllylelle blue, reduced porpllyrin or its
aggregates, and phtllalocyallille blue. Methylelle blue, whicll strongly absorbs al 600-700 nm,
3 0 and a krypton laser emilting at 647 and 676 nm are exemplified. The power level should be at
least 200 tnW/cm2.
~. 2 t ~002
li has been showll tllat b~ stripping Ihe stratum corneum from a small area of the skin
with repented application and removal of cellopl~ane tape to the same location one can easily
collect arl)itrary quantilies of interstitial iluid, whicll can tllen be assayed for a number of
analytes cf interest. Silllilarly, tlle tape-stripped skin llas also been shown lo be permeable to
tlle transdermal delivery of compoullds into the body. Unfortunately, tape-stripping leaves a
open sore which takes weeks to heal, and for this, as well as other reasons, is not considered as
an acceptable practice for enhancing l-a..a-; .lLn~eolts transport in wide applications.
I'~S discussed above, it llas been sllowll tllat pulsed lasers, such as tlle excimer laser
operating at t93 ntn, the erl)iutn laser operating near 2.~ ~m or the CO2 laser Il ,dting at 10.2
l~m, can l~e used to e~fectively al~late small holes in the Imtnan stratum comeulll. Tllese laser
ablation teclllliques olfer tlle potential for a selectiv e and potentially non-trallmatic mell-od for
opening a delivery mldtor sampling l-ole througll tlle stratum corneum. Ilowever, due to tlle
prohibiti~ ely lligll costs associated with tllese ligllt sources, there have been no commercial
products developed bascd on tllis concept. Tlle presently disclosed invention, by clefilling a
metllod far directly conductillg thermal energy into tlle stratum corneum with very tightly
2 0 defined s~atial and temporal resolution, makes it possible to produce the desired micro-ablation
of tl~e stratum corneum using very low cost energy sources.
n view of the foregoing problems and/or deficiencies, the developmellt of a method
for safelv enhancing the permeal~ility of the skin for minhl-ally invasive or noninY.Isive
monitoring of body analytes in a more rapid time rrame would be a signirical-t advancelllent in
2 5 ll~e art. ~t would be anotller sigllificallt a Ivallcelllellt in lhe art to provide a metl-o(l of
miniltlally invasively or non-illvasively rlll.a,~ g the ll.llls lc;ln,al flux rate of a dmg into a
selected Irea of an individual s body.
BRIEF SUMMARY OF TlIE INVI::NTION
3 0 ~n object of the invention is to minimi7R the barrier properties of the stra
corneum using poration to contlollably collect analytes from withill the body througl~
p erroratiDns in the stratum corneum to enable tl~e monitoring of these analytes.
CA 02199002 1998-08-06
A first aspect of the present invention is a method
for forming a micropore in a selected area of the stratum
corneum for enhancing the permeability of the skin of an
individuals's body.
An embodiment of this aspect involves ablating the
stratum corneum by delivering sufficient energy by conduction
to the selected area of the stratum corneum with a heat source
such that the temperature of tissue-bound water and other
vaporizable substances in the selected area is elevated above
the vaporization point of the water and other vaporizable
substances thereby removing the stratum corneum and forming
micropores having a diameter of 1 to 1,000 ~m in the selected
area of the stratum corneum.
A second aspect of the present invention provides an
apparatus for forming a micropore in a selected area stratum
corneum for enhancing permeAhility of skin, which comprises:
means for delivering sufficient energy by conduction to
the selected area of the stratum corneum such that temperature
of tissue-bound water and other vaporizable substances in the
selected area is elevated above the vaporization point of the
water and other vaporizable substances, to remove the stratum
corneum and to form at least one micropore having a diameter
of 1-1,000 ~m in the selected area of the stratum corneum.
A major embodiment of the method aspect is a
diagnostic method for monitoring the concentration of an
analyte in an individual's body by ~nh~ncing the permeability
of the stratum corneum of a selected area of the individual~s
body surface to the analyte, comprising:
75304-12(S)
CA 02199002 1998-08-06
(a) porating (or ablating) the stratum corneum of the
selected area to form a micropore in the stratum corneum by
delivering sufficient energy by conduction to the selected
area with a heat source to elevate the temperature of the
selected area;
(b) collecting a selected amount of the analyte through
the micropore: and
(c) quantitating the analyte collected.
In one preferred embodiment, the method further
comprises applying sonic energy to the porated selected area
at a frequency in the range of about 5 kHz to 100 MHz, wherein
the sonic energy is modulated by means of a member selected
from the group consisting of frequency modulation, amplitude
modulation, phase modulation, and combinations thereof. In
another preferred embodiment, the method further comprises
contacting the selected area of the individual' 8 body with a
chemical enhancer with the application of the sonic energy to
further enhance analyte withdrawal.
Porating of the stratum corneum is accomplished by
means of ablating the stratum corneum by contacting a selected
area, up to about 1,000 ~m across, of the stratum corneum with
a heat source such that the temperature of tissue-bound water
and other vaporizable substances in the selected area is
elevated above the vaporization point of the water and other
vaporizable substances thereby removing the stratum corneum in
the selected area.
One preferred P~hodiment of thermally ablating the
stratum corneum comprises treating at least the selected area
75304-12(S)
CA 02199002 1998-08-06
with an effective amount of a dye that exhibits strong
absorption over the emission range of a pulsed light source
and focusing the output of a series of pulses from the pulsed
light source onto the dye such that the dye is heated
sufficiently to conductively transfer heat to the stratum
corneum to elevate the temperature of tissue-bound water and
other vaporizable substances in the selected area above the
vaporization point of the water and other vaporizable
substances. Preferably, the pulsed light source emits at a
wavelength that is not significantly absorbed by skin. For
example, the pulsed light source can be a laser diode emitting
in the range of about 630 to 1550 nm, a laser diode pumped
optical parametric oscillator emitting in the range of about
700 to 3000 nm, or a member selected from the group consisting
of arc lamps, incandescent lamps, and light emitting diodes.
A sensing system for determ;n;ng when the barrier properties
of the stratum corneum have been surmounted can also be
provided. One preferred sensing system comprises light
collection means for receiving light reflected from the
selected area and focusing the reflected light on a
photodiode, a photodiode for receiving the focused light and
g~n~; ng a gignal to a controller wherein the signal indicates
a quality of the reflected light, and a controller coupled to
the photodiode and to the pulsed light source for receiving
the signal and for shutting off the pulsed light source when a
preselected signal is received.
75304-12(S)
CA 02l99002 l998-08-06
8b
In another preferred embodiment, the method further
comprises cooling the selected area of stratum corneum and
adjacent skin tissues with cooling means such that the
selected
75304-12(S)
: ~ 2199002
area and adjacent skin tissues are hI a selected precooled, steady state, condition prior to
poration.
In still anotl~er preferred embodiment, tlle method cun~pl ises ablating tl-e stratum
COrlleUm S JCII tllat in~erslitial lluid exudcs froln llle micropores, collecting the inlerstilial fluid,
and analy~ing llle analyle in llle collected interslilial lluid. After the inle~ l fluid is
collected, Llle micropore can be sealed by apl71ying an errective amount of energy from the
laser diode or olher ligl~t source sucl- tllat intelstitial fluid remainillg in tlle micropore is caused
to coagulate rreferably, vacuum is applied to tl~e porated selected area to enhance collection
of interstilial fluid.
Il~ yet anotller preferred embodimelIt, tlle method comprises, prior to porating the
stratum ccmeulm~ illuminating at least tlIe selected area witll unfocllsed light from tlle pulsed
light source suclI lhat the selecled area illuminated will~ e ligl~t is sterili~ed.
A notller preferred mctl~od of porating tlle stralum corlIeum comprises contactillg tlle
selected area willl a metallic wire sucll tllat tlle temperatllre of tlle selected area is raised fiom
ambient sl~in temperature to greater tllan IOO~C witlIin about lO to SO ms and then returnillg
2 0 lhe tempe-ature of the selected area to approximately ambient skin temperature within about 30
to 50 IllS, whereilI tllis cycle of raishlg the temperature and returning to approximately ambient
skin temperature is repeated a number of time effective for rcducing the barrier properties of
the stratuln corneum. rreferably, tl~e slep of returning to approximately ambielIt skin
temperalure is carried out by wilhdrawilIg tlle wire from contact witlI tbe stratum cornellm. It
2 5 is also pr~ fen-cd lo plovide mealIs for molIitoring eleclrical impedalIce belween ll~e wire and
the in-3ivi,1ual's body througl~ tl~e selected area of stratum corneum and adjacent skin tissues
and means for advancing tl~e position of tlle wire sucl~ tl~at as the ablation occurs with a
colIcolllitallt reduction in resistance, tlle advancillg means advances tlIe wire such tllat tlIe wire
is in cont lct witll tlle stratum comellm dllring heatillg Or t:le wire. I~urtller, it is also preferred
3 0 to provide means for withdrawing the wire from contact witll tl~e stratum corneum, wherein Ihe
monitorhIg means is capable of detecting a cl~ange in impedance A~oe;-~ed ~Yit!I contacting an
epiderma layer ullderlying tl~e stralllm corneum and sen(ling a signal to the witlldrawing means
~ 2199002
to withdra.vll the wire from contact with the stratum corllellm. The wire can be l-eated by an
ohlllic healillg element, can have a currellt loop llaving a higll lesi~l~,lc~ point wherein the
temperature of tlle higll resistallce point is modula~ed by passing a modulated electrical currellt
tllrougll said cullent loop to efreci llle l~eating, or can be posilioned in a modulatable
alternatin~ maglletic field of an excilatioll coil such that cne.~;L;ng the excitation coil Witll
alternating current produceg eddy currents suffient to lleat the wire by internal ollmic losses.
A method for enllallcillg tlle transderlllal fl~lx rate of an active permeant into a
selected area of an individual's body compl ishlg tlle steps of e~ cillg tlle permeability of the
stratum cc-rtleum laycr of ~he seleeted area of tlle individual's body surface to the nctive
permeant ~y means of
(a) porating tlle stratum comelllll of tlle selec~cd area l~y means tllat rorm a tnicro-pore
in,the stratulll corne-lm willlout causing serious damage to the underlying tissues and tllereby
reduce thc barrier properties of the stratum corlleulll to the flux of the active pemleant; and
(I ) contacting the porated selected area witll a composition comprising an effective
amount ol the permeallt such thal the flux of lhe permeant into the body is enllanced.
2 O In a preferred embodilllellt, the method further comprises applying sonic energy to the
porated scleclcd area for a time and at an intensity and a f ~ ncy effective to create a fluid
slreamin~, crrcct and thereby enl-ance the transdelmal llux rate of the pernleallt into tlle body.
~ metllod is also provided for appyling a tatoo to a selected area of skin on anhldividua'l's l~ody,surface comprising the steps of:
2 5 (a) porating the stratum corneulll of the selected area by means tllat form a
micro-pol e in the stratum corncum without causillg serious damage to the underlying tissues
and thereby reduce the barrier propelties of the stratum corneum to the flux of a permeant; and
(i~) contactillg tl~e porated selected area with a composition comprising an effective
amount (lf a tattoing ink as a permeallt such that the flux of said ink into the body is enhanced
3 0 .~ mctllod is still furthel provided for reducing a temporal delay in diffusion of an
analyle n om blood of an hldividual to said hldividual's interstitial fluid in a selected area of
slcin coml~risillg applying means for coolillg to said selected area of skin.
~ 21 99002
A metllod is yet filrther provi~led for reducing e~,o,~.liou of interstitial fluid and the
vapor pressure thereof, wllereill said intelstitial lluid is being collected from a micropore in a
selected area Or slratum corne1lm of an individual's skin, comprising applying means for
cooling îo said selected area of skin.
I~RIEF DESCRIPTION OF l'l'lE SEVERAL VIEWS Ol; THE DI~AWINGS
Fl~. I sllows a schematic 1~ ~JIesentalion o~ a system for deliveting laser diode light
and monik~ring the progress of poration.
FIG. 2 sllows a scllellIatic represenlation of a closed-loop feedback system formonitorilI~ poration.
I~IG. 3A slIows a schelllalic lel)lc3elIt~tion of an optical poration syslem comprising a
cooling del~ice.
FlG. 3B shows a top view of a schematic l- plese..l~l;on of an illu~1lalive cooling
device acc~rding to I~IG. 3A.
FIG. 4 slIows a scl-ematic le~ se.,l<lliolt of an ohmic heatillg device with a
mecllanical actuator.
FI G. S shows a schematic It;~ se~ tlion of a high resistance current loop heating
device.
I~!G. 6 shows a schematic Icplese~ lioll of a device for modulatillg healing using
induclive llealing.
I~ G. 7 SIIOWS a schematic l~ ;.elltation of a closed loop il.lpeda,lce monitor using
changes in inpedallce to determitle tlle extent of poratioll.
F~GS. 8A-D show cross sections of l~uman skin treated witlI copper phtlIalocyanine
and tlIen subjected, respectively, to 0, 1, 5, and 50 pulses of 810 nm light with an energy
delIsily of 4000 J/Cm2 ror a pulse period of 20 IllS.
3 0 I~SGS. 9-11 show graphic l~;p~ elltations of temperature disttibution during simulated
thelmal polaLiol~ events USitlg optical poratiom
. ~ . . . . .~.. , ~ ... . .
~ 2 t 99002
12
Fl~,S. 12 and 13 sllow grapllic representatiotls of temperature as a filnction of time in
ll)e stratum cornellm and viable epidermis, ~ ectively, during simulated thermal poration
events usir~ optical poration.
FIGS. 14-16 show grapllier~pl~s~ntaliollsoFtemperatllredistributioll, temperalureasa
function ol' time in the stratulll corneum, and temperalure as a function of time in tlle viable
epidermis, respectively, during simulated lherlllal poration events using optical poralion
wllerein thD tissue was cooled pricr to poration.
F!GS. 17-19 show graphic lepl~ selltalions of telnperature distribution, temperature as a
rullction o~ time in the stratum cornellm, and temperature as a filnction of time in tlle viable
epi<lenl1is, respectively, during simlllated tllermal poration events whereill tlle tissue was lleated
with a hot wire.
liIGS. 20-22 show grapllic Ic~ ions of lc...pe,~l~ue distribution, temperature as a
fiulction af time in the stratum comeuln, and temperature as a filnction of time in tlle viable
epiderlllis respectively, during simulated tllermal poration events wllerein the tissue was heated
witll a llo~ wire and tlle tissue was cooled prior to poration.
2 0 FIGS. 23 and 24 SIIOW graplric ~ ,se~.tations of temperature distribution and
temperature as a filnctioll of time in the stratum comeum, respectively, during simulated
tllerlllal p~ralion evcnls whercill lhe tissllC is llealcd oplically according to lhe operati
parametels of Tankovicll '803.
FIG. 25 shows a graphic representation of interstitial fluid (ISF; o) and blood (*)
2 5 glucose levels as a runc~ion of time.
F IG. 26 sllows a scatter plot I~ se.l~tion of the difference ternt between the ISF
glllcose aad tlle blood glucose data of FIG. 25.
FIG. 27 shows a histogram of the relative deviation of tlle ISF to the blood glucose
levels fro~n FIG. 25.
3 0 ] IG. 28 shows a cross section oF an illustrative delivery apparatus ror deliverin~ a
drug to a selected area Oll an individual's skin.
~ .' 2~q~002
13
FIGS. 29A-C sllow grapllic representatiolls of areas of skin affected by delivery of
lidocaine to selected areas wllere tlle strat~ corneum is porated (FIGS. 29A-B) or not porated
(I~IG. 29CI.
I~IG. 30 sllows a plot comparitlg the amount of interstitial fluid harvested from
m;crol~ore!. with suctioll alone (o) and with a combination of suction and ultrasoulld (*).
I;IGS. 31, 32, and 33 sllow a perspective view of an ullrasonic ll~n~Ju-;el/vaculltn
apparatus l or llarvesling interstitial fl~lid, a cross seclion view of tlle same apparalus, and cross
sectional sellematic view of the same aplJaidtI-" respectively.
~'CS. 34~-B show a top view of a handlleld ultrasonic transducer and a side view of
the spatul~ te end tllereof, respectively.
~ DETAILED DESCRIPTION
13erore tlle presellt methocls for permeabilizing tlle stratum corneum for facilitating
transderl~ Img delivery and analyte sampling are disclosed and described, it is to be
ullderstood Illat this inventioll is not limited to the particular configurations, process steps, and
2 0 materials ~lisclosed herein as such conflglllations, process steps, and materials may vary
somewhat It is also to be understood that the terminology employed herein is used for the
pulpose oF descril~ing l~articular embodilllents only and is not intended to be limitillg since tlle
scope of tae present invention will be limited only by the appended claims and equivalents
lhereof.
2 5 ll must be noted tllat, as used in tllis specification and the appended claims, the
singIllar fi~rms "a," "an," and "the" inclllde plulal referents unless tlle context clearly dictates
otherwise Thlls, for example, reference to a metllod for delivery of "a drug" includes
reference ~o delivery of a mixture of two or more dlllgs, reference to "an analyte" incllldes
reference 'o one or more of sucll analytes, and reïercnce to "a pemleatiol1 ellllallcel" includes
3 0 reference to a mixture of two or more permeation enllancers.
I l describing and claiming tlle presellt invenlion, tlle following terminology will be
used in accotdtlllce with lhe definitiolls sct out below.
CA 02l99002 l998-08-06
14
As used herein, "poration", "microporation," or any
such similar term means the formation of a small hole or pore
in the stratum corneum in a selected area of the skin of an
individual to lessen the barrier properties of this layer of
the skin to the passage of analytes from below the skin
surface for analysis or the passage of active permeants or
drugs into the body for purposes other than treating or
preventing diseases. Preferably the hole or pore will be no
larger than about 1 mm in diameter, and more preferably no
larger than about 100 ~m in diameterj and will extend into the
stratum corneum sufficiently to break the barrier properties
of this layer without adversely affecting the underlying
tissues.
As used herein "ablation" means the controlled
removal of cells caused by kinetic energy released when the
vaporizable components of the cells have been heated to the
point that vaporization occurs and the resulting rapid
expansion of volume due to this phase change causes cells and
possibly some adjacent cells to be "blown away" from the
ablation site.
As used herein "puncture" or "micro-puncture" means
the use of mechanical, hydraulic, or electrical means to
perforate the stratum corneum.
To the extent that "ablation" and "puncture"
accomplish the same purpose of poration, i.e. the creating of
a hole or pore in the stratum corneum without significant
damage to the underlying tissues, these terms may be used
interchangeably.
75304-12(S)
CA 02199002 1998-08-06
As used herein, "penetration ~nh~ncement" or
"permeation ~nh~ncement" means an increase in the perme~ility
of skin to a drug, analyte, dye, stain, or other chemical
molecule (also called "permeant"), i.e., so as to increase the
rate at which a drug, analyte, or chemical molecule permeates
the stratum corneum and facilitates the poration of the
stratum corneum, the withdrawal of analytes out through the
stratum corneum or the delivery of drugs through the stratum
corneum and into the underlying tissues. The enhanced
permeation effected through the use of such enhancers can be
observed, for example, by observing diffusion of a dye, as a
permeant, through An;~l or human skin using a diffusion
apparatus.
As used herein, "chemical enhancer", "penetration
enhancer", "permeation ~nh~ncer", and the like includes all
enhancers that increase the flux of a permeant, analyte, or
other molecule across the skin, and is limited only by
functionality. In other words, all cell envelope disordering
compounds and solvents and any other chemical ~nh~ncement
agents are intended to be included.
As used herein, "dye", "stain", and the like shall
be used interchangeably and refer to a biologically suitable
chromophore that exhibits strong absorption at the emission
range of a pulsed light source used to ablate tissues of the
stratum corneum to form micropores therein.
As used herein, "transdermal" or "percutaneous"
means passage of a permeant into and through the skin to
achieve effective blood levels or deep tissue levels of a
75304-12(S)
CA 02199002 1998-08-06
15a
drug, or the passage of a molecule present in the body
("analyte") out through the skin 80 that the analyte molecule
may be collected on the outside of the body.
As used herein, the term "permeant", "drug", or
"pharmacologically active agent" or any other similar term
means any chemical or biological material or compound suitable
for transdermal ~m;n;stration by the methods previously known
in the art and/or by the methods taught in the present
invention, that induces a desired biological or
pharmacological effect, which may include but is not limited
to (1) having a prophylactic effect on the organism and
preventing an undesired biological effect such as preventing
an infection, (2) alleviating a condition caused by a disease,
for example, alleviating pain or inflammation caused as a
result of disease, and/or (3) either alleviating, reducing, or
completely eliminating the disease from the organism. The
effect may be local, such as providing for a local anaesthetic
effect, or it may be systemic. This invention is not drawn to
novel permeants or to new classes of active agents. Rather it
is limited to the mode of delivery of agents or permeants that
exist in the state of the art or that may later be established
as active agents and that are suitable for delivery by the
present invention. Such substances include broad classes of
compounds normally delivered into the body, including through
body surfaces and membranes, including skin. In general, this
includes but is not limited to: antiinfectives such as
antibiotics and antiviral agents; analgesics and analgesic
combinations; anorexics; antihelminthics; antiarthritics;
75304-12(S)
CA 02199002 1998-08-06
15b
antiasthmatic agents; anticon w 16ants; antidepressants;
antidiabetic agents; antidiarrheals; antihistamines;
antiinflammatory agents; antimigraine preparations;
75304-12(S)
~ 2 1 99002
16
s~n~ lSellltS; antilleoplastics; antiparkillsonislll drugs; a~ 3l~ ics; antipsychotics; antipyretics;
antispasmcdics; anticholinergics, sympatllol~ etics; xantlline derivatives; cardiovascular
preparatior s includillg ~otassium and calcium chanllel blockers, beta-blockers, all-ha-blockers,
and anliarrllylllmics, antillypertensives; diurelics and antidillretics; vasodilators includillg
general col-onaly, periplleral and celel)ral; central nervous system stimulants; vasocollstrictors;
cougll and cold preparalions, includillg decollgcOldllts; llormolles sucll as esttadiol and otller
steroids, ircluding corticosleloi(ls; llyl~notics; immull~ .l,plc,;,;ves; muscle i~Lxalllb,
~ar~sympatllolytics; psycllostfillul~nls; sedatives; and tranquilizers. By the metllod Or the
present inventioll, both ionized ansl nollionized drugs may be delivered, as can drugs of either
lligh or low molecular weigllt.
As used llerein, an "erfective" amoullt of a plla",l,lcologicallyactive agent mealls a
sufficiellt amollllt of a compoulld to provide the desired local or systemic effect and
perfomlallce at a reasonable benefi~/risk ratio :~tten(li-l~ any medical treattnellt. An "effective"
amollllt of a perlneation or cllemical enllancer as used llereill meal)s an amoullt selecled so as
lo provide llle desired inclease in skin permeability and the desired deptll of penetralion, rate
2 0 of administratioll, and amollnt of ,drug delivered.
As used llerein, "carriers" or "vehicles" refer to carrier materials without significant
pll~llmaco ogical activity at tlle quantities used tllat are suitable for administration with other
pllarlllacelltically active materials, and include any sucll materials known in tlle art, e.g., any
liquid, gel, solvent, liquid diluetlt, solubilizer, or tlle like, that is nontoxic at Ille qualltities
employed and does not interact willl tlle drug to be admillistered in a deleterious mallller.
Examples of suitable carricrs for use llerein inclllde water, mineral oil, silicone, inorgallic gels,
aqueous emlllsiolls, liquid sugars, waxes, petroleulll jelly, and a variety of other oils and
polymeric materials.
~s used llcrein, a "biological membrane" is intcllded to mean a membrane material
3 0 present w tllin a living organism tllat scpaldt~ s one area of tlle organism from anotller and, in
mally installces, tllat separatcs tlle organislll fiom its outer envirolllllellt. Skin and mucous
melllbran~ s are tl)us included.
~ 2~ 9~02
17
As used hereill, "individual" rerers to both a human and an anintal, to wllich the
present invention may be appliecl.
A!~ Ilsed llerein, "analyte" means any cllemical or biological material or compound
suitable ror passage thlougll a biological melllblane by tlle tecllnology taugllt in tllis present
invelltion, ~r by techllology previously known in the art, of which an individual might want to
know tlle concentralioll or activily inside llle body. Clucose is a specific example of an
analyte because it is a sugar suitable for passage throIlgll tlle skin, and individuals, for example
tllose llaving diabeles, migllt want to know llleir blood glucose levels. Olller examples of
analytes in-lu(3e, but are not limited to, sucll compounds as sodium, potassium, bilirubin, urea,
ammollia, ~alcium, lea~l, iron, lithium, salicylates, an(l tlle like.
A; used llereill, "ll~nsJe~lnal llux rate" ;s tlle raIc of passage of any analyle out
lhrougll th~ skin of an individual, human or animal, or tlle rate of passage of any drug,
pllarmacol ~gically active agent, dye, or pigmellt in and tllrougll the skin of an individual,
llumall or Inimal.
As used llcreill, the terms "intensity amplitude," "intensity," and "amplitude" are used
2 0 synollymo lsly and rercr to tlle amount of energy being produced by tlle sonic energy system.
As used l1erein, "rrequency modulation" or "sweep" means a contin-lous, graded or
slepped variiltioll in llle amplitude or rreqllcl1cy Or ultrasolllld in a given timc period. A
fiequency modulatioll is a graded or stepped variation in frequellcy in a given time period, for
example 5 4-5.76 Mllz in I sec., or 5-lO Mllz in 0.1 sec., or tO-5 Mllz in O.t sec., or any
2 5 otller frequellcy range or time period tllat is a~ iale to a specific applicatiom A complex
modl!latioll can incltlde varying both tl~e frequency and inteltsity simultaneously. I~or example,
I~IGS. 4A and 4B of U.S. Palent No. 5,458,140 could, respectively, ~ ,..t amplitude and
rle4nen~;r modulations being applied simultaneously to a single sonic energy transducer.
As used llerein "phase modulatioll" means tlle timillg of tlle signal has been cllanged
3 0 relative to its initial state sllown in Fig. 4C of U.S. Patent No. 5,458,140. The G~ ~lu~ncy and
amplitude of tl~e signal can remain the same. A phase modulation can be implemented witll a
~ 2 ~ 99302
18
variable delay sucll as to selecîively relard or advance the signal temporarily in reference to ils
previous state, or to ano~l~er signal.
Tl~e sonic energy, in ils various apr>lications SUCIl as with r~ .cnc.~ intensity or phase
modulatiot, or combillatiolls thereof and tlle use of chemical enllanu,~:, combilled with
modulated sonic energy, as described l-erein, can vary over a frequency range of between about
5 k~lz to 100 Mll~, wilh a range of between about 20 Icllz and 30 Mllz being preferred.
A, used hereill, "non-invasive" means not teq.li,i"g the entry of a needle, catlleter, or
olller invasive medical instrulllellt into a part of tlle body.
A s user l-erein, "minilllally invasive" refers to tlle use of mecllanical, hydraulic, or
electrical rl~ealls that invade the stlQtIllll comeIlm to create a small hole or micropore witllout
causin~ sllbs~ l dalllage to tlle undellyill~ tissues.
Means for Poration of the StratIlln Corneum
Tlle formation of a micrcpore in the stratum corneum can be accomplislled by various
state of th.~ art mealls as well as certain means disclosed herein that are improvements thereof.
2 0 The use of laser ablation as described by Jacques et al. in U.S. Patent 4,775,361 and
by Lane e~ al., supra, certainly provide one means for ablating the stratum corneum using an
excimer l~ssel. ~t 193 nln wavelenglll, and 14 ns pulsewidlll, it was found tllat about 0.24 to
2.8 /Lm of stratulll corneum could be removed by eacll laser pulse at radiant exposure of
between about 70 and 480 mJ/cm2. As the pulse energy increases, more tissue is removed
rrom tlle rtlatl-m corneum and re~ver pulses are required ror coml~lete poration of tllis layer.
The lower tllreshold of radiant exposure that must be absorbed by the stratum corneum within
the limit of tlle therlnal relaxation time to cause suitable micro-explosions tllat result in tissue
ablatiOIl i'3 about 70 mJ/cm~ witllill a 50 millisecolld (ms) time. In other words, a total of 70
mJ/cm2 nlust be dclivered within a 50 ms window. This can be done in a single pulse of 70
3 0 mJ/cm2 or in 10 pulses of 7 mJ/cm2~ or with a continuous illumination of 1.4 watts/cm~ durillg
tlle 50 mS time. The upper limit of radiant eXpOSUle is that wllich will ablate tlle stratum
corneulll witllout ~lamage to underlying tissue and can be empirically determined from the light
~ ~ 2199û02
19
source, wavelength oF light, and olher variables that are within tlle experience and knowledge
of one skil-ed in this art.
B / "deliver" is meant tl-at the stated amount of energy is abso.l,ed by the tissue to be
ablated. At the exchller laser wavelengtll of 193 mll, essentially 100% absorption occurs
witllin the first I or 2 ~n1 of stratum comellm tissue. I~cg~ming the stratum corneum is about
20 ~cm tllitk, at longer wavelengtlls, such as 670 nm, only about 5% of incidellt ligilt is
absorbed vl~itllill lhe 20 ~m layer. This mealls that about 95% of tbe hi~h po-Yer benlll pnsscs
into tlle tissues underlyillg the stlallllll corlleulll wllere it will likely cause significallt damage.
'rlle ideal is to use only as much power as is n~ y to pelr,~tt~ the stralum
corneum v ithout causing bleeding, thermal, or other damage to underlying tissues from wllicl
analytes ate to be extracted or drll~s or otller l~ermeanls delivered.
It would be beneficial to use sources of energy more economical than energy fromexcimer lasers. Excimer lasers, wllich emit light at wavelengtlls in the far UV region, are
much mor~ expensive to operate and maintaill than, for example, diode lasers that emit light at
wavelengt~1s hl visible and IR regiolls (600 to 1800 nm). tlowever, at the longer wavelengtlls,
lhe stratum corneum becomes in~"-,asillgly mole lldllsl)al~,.ll and absorption occurs primarily in
the underl ~ing tissues.
'rhe present invelltion facilitates a rapid and pahlless method of eli-nin~ing the barrier
functioll of the stratum corneum to facilitate the transc-llallc~ s transport of therapeutic
2 5 substances hlto the body whell applied topically or to access the analytes witllill the body for
analysis. rhe metllod utilizes a procedure which begins with the contact application of a small
area heat :-~ource to the targeted area of the stratum corneum.
The heat source must have several hllportant properties, as will now be described.
I;irst, the leat source must be sized SllCh that contact wilh the skin is confilled to a small area,
3 0 typically about l to 1000 llm in diameter. Second, it must have the capability to tnodulate the
temperature of the stratum corneum at the contact pOillt from ambient skin surface temperature
levels (33'C) to greater tllan 123CC and then return to apl,lu,.il"ately ambient skin temperature
-- 2 t 9qO02
~ 20
witll cycle times to minimize colldleral damage to viable tissues and sensation to tlle subject
indiYidllal. This modulation can be created electronically, mechanically, or cllelllically.
~,lditionally, an inllerellt deptll limiting feature of the microporation process can be
facililated f tbe lleat source llas botl~ a small enollgh tllerlllal mass and limited energy source
to elevate ts temperature such tllat wllen it is placed in contact witll tissues Witll more tha
30% water content, tlle tllermal dispersion in tllese tissues is sumciellt to limit the maxim
t~;llll)e~,.tule of tlle heat soulce to less tllan 100~C. Tllis feature effectively stops tlle tllermal
vapolizaliorl process once tlle heat probe llad p~lletr~led througll the stratum comelml into the
lower laye-s of tlle epidermis.
~'itll the lleat source placed in contact witll the skin, it is cycled tllrougll a series of
one or mo e modulatiolls of temperalllre from an inilial point of ambient skin temperature to a
peak temp~rature in excess of 123~C to approxmiately ambient skin lel~.pe~alule. lo millilni7e
or elimina~e tlle subject's sensory perception of the mi. Iopo.ulion process, these pulses are
limited in duratioll, and the interpulse spacing is long enough to allow cooling of tlle viable
tissue layers in tlle skin, and most particularly the enervated dermal tissues, to acheive a mean
2 0 temperatul=e of less tllan about 45~C. These palûmeters are based on the tl~ermal time consl~
of the viallle epidermal tissues (roughly 30-80 ms) located between the heat probe and tlle
enervated ~issue in lhe underlyillg dermis. Tlle result Or tllis application of pulsed thermal
energy is Illat enougll energy is conducted into tlle stratum corneum within tlle tiny target spot
that the lo-eal temperature of tllis volume of tissue is elevated sufficiently higller tban the
2 5 vaporization point of tlle tissue-boulld water contetlt in the stratum corneum. As the
temperatu e increases above 100~C, the water content of the stratum corneual (typically 5% to
15%) witllin tllis localized spot, is induced to vaporize and expand very rapidly, causing a
vapor-dri~en removal of tllose corlleocytes in the stratum corneum located in proximity to this
vapo, i~ n event. U.S. Patenl Mo. 4,775,361 teaclles lllat a slratum corneum lemperalure of
3 0 123~C represenls a lhresllold at wllicll this type of flash VdpOI i~ation occurs. As subsequellt
pulses of ~ller-nal enelgy are applied, additional layers of tlle stratum corneum are removed
unîil a mi~ropore is formed tllrougll llle stratum corneum down lo the next layer of tlle
~ 219~002
epidermis, :he stratum lucidum. By limiting the duration of the heat pulse to less than one
tllerlItal thr e constallt of tlle epiderntis and allowh-g any heat energy conducted into the
epidermis t;o dissipate ror a sufrlciently long
enough time, the elevation in temperatIlre of Ihe viable layers of
the epidemlis is minhIl;ll. Tllis allows the entire microporation process to take place without
any sensatiDn to tlle subject and no damage to the underlyilIg and surroundilIg tissues.
The present invenliolI comprises a method for pah~lessly creating microscopic holes,
i.e. micror~ores, rrom about I to 1~)00 llm across, hl the stratwtI comeum of lluman skhl. The
key to sucl essrully implementitlg lllis method is the creation of an a~ v~Jl;alc tllermal energy
source, or leat probe, whicll is held in contact with the stratum corneum. The principle
techllical cllallelIge in fabricating all al)plul ,iate heat probe is designhlg a device that has the
desired contact with the skin and that can be therllIally modulated at a sumciently higll
rrequency.
It is possible to fabricate an al"olo~";.lle heat probe by topically applying to the
stratum corlIeulll a suilable light-
2 0 al~sorbing cotnpoIlnd, sucll as a dye or stain, selecte<l because of its ability to absorb light at
the wavel~ ngtlt emitted by a selected light source. In this instance, the selected ligltt source
may be a aser diode emillhlg at a wavelength whicll would not normally be absorbed by the
skin tissues. By focusing tlle light source to a small spot on the surface of the topical layer of
the dye, tl e targeted area can be t~emperature modulated by varying the intensity of the light
flux rocused on it. It is possible to utilize the energy rrom laser sources emitting at a longer
wavelengtlt than an exchner laser by first topically applying to the stratum corneum a suitable
light-abso;bing compound, suclt as a dye or stain, selected because of its ability to absorb ligllt
at tlle wavelength emitted by the iaser source. l'he same concept cnn be applied at any
wavelenglh and one mIlst only choose an apl),u~,lidte dye or stain and optical wavelength. One
3 0 need only look to any reference manual to find which suitable dyes and wavelengtlI of the
maxhIlum absorbance of that dye. One such rererence is Green, The Si~ma-Aldrich ~landbook
Or Sla;nS~ DYeS and Ind;CatOrS, ~I(Ir;CII Chemical Company, l11C. Milwaukee, WisconsilI (1991).
.~
22 21 q9002
I;or exampl ~, copper pllthalocya~ e (rigmellt Blue 15; CrC) absorbs at about 800 nm; copper
plllllalocyal ine tetrasulfonic acid (~cid Blue 249) absorbs at about 610 nm; and Indocyanille
Green absorbs at about 775 nl1l; and Cryptocyanhle absorbs at about 703 nm. CPC is
particularly well suiled ror this eml)odiment for tlle following reasons: it is a very stable and
inert compoun~, already approved by the FDA for llse as a dye in implantal~le sulures; it
absorbs very strongly at wavelengths rrom 750 nm to 950 nm, wllicll coincide well with
numerous l ~w cost, solid state emitters sucll as laser diodes and LEDs, and in addition, this
area of optical bandwidtll is similarly not absorbed directly by the skhl tissues in any
significant amoullt; CPC llas a very higll v~p(JIi~alioll point (>550C in a vacuum) an~l goes
directly from a solid pllase to a vapor pllase with no liquid pl~ase; CPC has a relatively low
tllermal dilrusivity conslallt, allowing tl~e ligllt ener~y rocused on it to selectively lleat only
tllat area directly in the focal point with very liltle lateral spreading of tlle 'hot-spot' hlto the
SUrrOUtldill,5 CPC tllereby ctSsiStillg ill the spatial definition of the contact heat-probe.
Tl e purpose of this disclcsure is not to make an exhaustive listing oF suitable dyes or
slahls because sucll may be easily ascertailled by one skilled in the art from data readily
2 0 available.
Tlle same is true for any ~lesired particular pulsed light source. For example, this
melllod m~ y be hllplelllellled will~ a mecllallically shllUered, focused ins ~nde.5c~n~ lamp as lhe
pulse liL~ht source. Various catalogs and sales literature SIIOW numerouslasers opelaling in the
near UV, visible and near IR range. Representative lasers are H~mmntn~cu l'hotonic Systems
2 5 Model l'LI'-02 whicll opera~es a~ a power output of 2xl0 8 J, at a waveleng~h Or 415 nm;
I=lammam2tsu Photonic Systems Model PLP-05 whicll operates at a power output of 15 J, at a
wavelengtll of 685 nm; SDL, Inc., SOL-3250 Series pulsed laser whicll operates at a power
output of :!x106 J at a wavelength of al)out 800-810 nm; SDL, Inc., Model SDL-8630 wllicl
operatcs al a power output of 500 mW at a wavelengtll of about 670 nm; Unipllase Laser
3 0 Model Al~-081-15000 which operates at a power OUtpIit of 15,000 mW at a wavelength of
790-830 11!11; Toshiba America Electronic Model TOLD9150 which operates at a power output
'~ q O 0 2
23
of 30 mW at a wavelengtll of 690 nm; and LiCONlX, Model Diolite 800-50 which operates at
a power 5C mW at a wavelengtll of 780 nm.
I~or purposes of the present inventioll a pulsed laser light source can emit radiation
over a wid~ range of wavelengtl~s ran~ g fiom belween about 100 nm to 12,000 ntn. Excimer
lasers typieally will emit over a range of between about 100 to 400 nm. Commercial excimer
lasers are ~ urrently available with wavelengtlls in tlle range of about 193 nm to 350 nm.
I'referably a laser diode will have an emissioll range of between about 380 to 1550 nm. A
fre(luency ~oubled laser diode will have an emissioll range of between about 190 and 775 nln.
Longer wavelengllls rangillg from belween about 1300 and 3000 nm may be utilized USitlg a
laser diode pumped optical paramc tric oscillator. It is expectecl, given tlle amount of research
taking place Oll laser teclmology, tllat tllese rallges will expand witll time.
Delivered or absorbed energy need not be obtained from a laser as any source of light,
whetller it is from a laser, a short arc lamp such as a xenon fl~hl:~mr, an incalldescellt lamp, a
light-emitlillg diode (LED), tlle sun, or any other source may oe used. Tllus, tlle particular
instrumenl used for delivering electromaglletic radiation is less important thall the wavelengtl
2 0 and energy associated therewitll. .Any suitable instrumellt capable of delivering tlle necessary
energy at suitable wavelengtlls, i.e. in tlle range of about 100 nm to about 12,000 mn, can be
considere~ withill the scope of llle invelllioln 'I'l)e essenlilll fealure is lhat the energy must be
absorbed by tlle ligllt-absorbing compoulld to cause localized heatillg tllereof, rollowed by
conductioll of sufficiellt heat to the tissue to be ablated withill tl~e timeframe sllowed.
In one illustralive embocliment, tbe lleat probe itself is fonned from a thill layer,
preferably about S to 1000 ~ltl thick, of a solid, non-biologically active compoulld, applied
topically to a selected area of an indiviclual's skin that is large enough to cover the site where a
micropore is to be created. Tlle specific formulatioll of the chemical compound is chosen sucl
tllat it exhibits lligll absorption o~fer the spectral range of a ligllt source selected for providing
3 0 energy to ~he ligllt-absorbing compound. Tlle probe can be, for example, a sheet of a solid
COlllpOUIlC', a r~lm treated witll a higll melting point absorbing compound, or a direct
applicatio l of the ligllt-absolbillg compound to the skin as a precipitate or as a suspensioll in a
..
- 21990~2
24
carriel. R~ gardless of the confi~Illation of the lighl-absorbing heat probe, it must exhibit a low
enough laleral thermal difrIlsioll coemcient sucll that any local elevations of temperature ~Yill
remaill spalially (lerlnc~l and tlle domillallt mode of heat loss will be via direct conduction into
~he s~ratunl corlleullI througll lhe roint oF contact between the skin and the probe.
Tlle required temperature modulation of the probe can be achieved by focushlg a light
source OIIt) the light-absorbilIg compoulId and modulating the intensity of this light source. If
the energy absorbed witllill tlle illumillated area is sufficiently higlI, it will cause the light
~l~soll~ing compoIllld to ral~idly heat ul~. The amount of energy delivered, and subsequelltly
botll the rate of l-eating and lIeak temperature of tlle light-absorbing compound al the focal
I~Oillt, can be easily modulated by varying tlle plllse widtll and peak power of lhe ligllt source.
In tllis eml)odilllellt~ it is only (he small vohllIle of li~ht-absorbillg compoulld healed llp by tlle
focused, incidellt optical energy tllat forms the heat probe, additional light absorbhlg compound
wllicll ma~ llave been applied over a larger area thell the actual poration site is hIcidelltal. By
using a sclid phase light-absolbillg compound with a relatively higll melting point, sucll as
copper plrtlIalocyallille (CPC), whicll remailIs in its solid phase up to a temperature of greater
2 O than 550~~, the heat probe can be quickly brougllt up to a temperature of several hundred
degrees C, and still remain in contact with the skin, allowing this thermal energy to be
conducte~l hIto the stratUIll corne~ . In addition, this embodimellt comprises choosillg a light
source willl an emissioll s~Jel,~ nl where very little energy would norrnally be absorbed in the
skin tissu,-s.
2 5 Once tlle targeted area has the ligllt-absorbillg compoIlnd topically positioned on it, the
lleat prob~ is formed wllen the ligllt source is activated witll the focal waist of the beam
positioned to be cohlcidellt with lhe surface of the treated area. The energy density of liglIt at
the focal waist and the amount oF absorption taking place within the light-absorbing compoùnd
are set to be sumcient to bring the temperature of the light-al)so.l,il,g compound, within the
3 O area of tl e small spol defined by the focus of the light source, to greater than 123~C within a
few milliseconds. As the temperature oF the heat probe rises, conduction into the stratllm
corlleIllll dclivcrs enelgy illto lllese tissucs, elevaling tlle local tempel~.tule of tl~e slralullI
~ 2 1 ~9 002
S corneum. Whell enollgll energy has been delivered into this small area of stratutn comeum to
cause the local temperature to be elevated above tlle boiling pOillt of the water contailled in
Illese tissu~s, a llash vaporization Or tllis waler takes place, ablating tlle stratulll corneultl at this
poillt.
By tumillg the light source Oll and off, tlle temperature of tbe heat probe can be
rapidly modlllated and the selective ablation of these tissues can be achieved, allowing a very
precisely c~imellsiolled hole to be created, whicll selectively penetrates only throllgll the RlSt 10
to 30 llm ~f skim
An additional feature of tllis embodiment is tllat by choosing a light source that would
normally have very little energy absorbed by the skin or ullderlyillg tissues, and by designing
llle focllsillg and delivery optics to llave a s~lfficien~ly lligh numerical aperture, ltle small
amount ot delivered figllt that does not bappen to get absorbed in the heat probe itself, quickly
diverges as it penelrates deep illtO the body. Since there is very little absorptioll at the
delivered ~vavelengtlls, essentially no energy is delivered to the skin directly from the light
source. Tllis tllree dimensiollal dilution of coupled energy in the tissues due to beam
2 0 divergence and the low level of absorption in the u~ aled tissue results in a completely
benign intelaction between the light beam and the tissues, with no damage being done tllereby.
ln one preferred embo(lilllellt of tlle invelltion, a laser diode is used as the ligllt source
with an emission wavelength of 800 ~ 30 nm. A heat-probe can be fonned by topical
applicatio l of a transparent adhesive tape that has been treated on the adhesive side with a 0.5
2 5 cm spot fi~rmed from a deposit of finely grollnd copper r~llthalocyanine (CPC). The crc
exhibits e ctrelllely higll absorptioil coefncients in the 800 nm spectral range, typically
absorbing more thall 95% of the radiallt energy fiom a laser diode.
FIG. l sllows a system lO for delivering light from such a laser diode to a selected
area of all individual's skin and fi~r monitorillg tlle progress of the poration process. Tlle
3 0 system complises a laser diode 14 coupled to a conlroller 18, which controls tlIe intelIsity,
duration, llld spacing of the light pulses. The laser diode emits a beam 22 that is directed to a
collection lens or lenses 26, whicll focuses the beam onto a mirror 30. The beam is then
.
~ 21 99~2
2~
S retlected by tlle milror to an objective lens or lenses 34 whicll focuses tlle beam at a
preselected point 38. This preselected point corresponds with the plane of an xyz stage 42 and
the objecti~e hole 46 tllereof sucll tllat a selected area of an individual s skin can be irradiated.
The xyz stage is connected to tl~e eontroller sucll lllat tlle position of the xyz stage can be
controlled. Tlle system also comprises a monitoring system comprising a CCD camera 50
coupled to a monitor 54 Tlle CCD camera is confocally aligned Witll tlle objective lens sucl
that the progress of tlle poratioll plocess can be molIitored visually on the monitor.
In anotller illustrative eml)odimellt of tlle invention a system of sensing pllotodiodes
and colleclion optics tllat llave been confocally aligned with the ablation liglIt source is
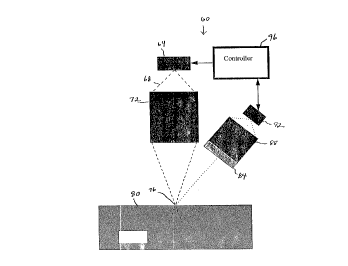
provided. FIG. Z sllows a sensor system 60 for use in tllis embodilnent. Tlle syslem
comprises I ligllt source 64 for emittill~ a beam of light 68 wllich is directed throllgll a
delivery optics system 72 that focuses the beam at a preselected point 76 such as tlle surface
of an indi~idual s skin 80. A portion of tlle light contacting the skin is reflected and otller
ligllt is en itted fioln the irradiated area. A portion of tl~is retlected and emitted ligllt passes
tlIrough a rllter 84 and then tllrougll a collection optics system 88 wlIich focuses the ligllt on a
2 0 plIototo~liode 92. A controller 96 is coupled to botll tlle laser diode and tlle pllotodiode for
respective.y controlling tlle output of the laser diode and detecting the ligllt tllat reaclles tlle
pl~otodiod~. Only selected portions of tlle spectrum emitted from tlIe skin pass throllgll the
filter. By analyzing the sllifts in tlle reflected and emitted ligllt from the targeted area the
system lIa; tlle ability to detect wllen tlle stratum comeum llas been breaclled and tllis
2 5 feedback is thell usecl to control llle ligllt source deactivating tlle pulses of ligllt whell the
microporatioll of tlle stratum corneulIl is acllieved. By employing this type of active closed
loop feedback system a self regulating ulliversally applicable device is obtained tllat produces
uniforltlly dimensioned mi l~pUI~ s in tlle strahlm corneum witlI minimal power rc~luirements
regardless of variations from one individual to tlle next.
3 0 In anothel illustrative embodiment a cooling device is incorporated into tlle system
interface ~o tlle skin. ~IG. 3A shows an illustrative schematic ~ "csel.t~tion thereof. In tllis
system lO0 a light source 104 (coupled to a controller 106) emits a beam of light 108 whicl
' ~ 219qO02
27
passes througll and is focused by a delivery optics system 112. Tlle beam is focused by tlle
delivery o ~tics system to a preselectecl point 1 16, such as a selected area of an indivi(J1lal~s
~ skin t~O. A coolhlg device 124, s1lch as a reltier device or otber means of chillillg, contacts
tlle skin to cool ll~e surrace tllereor. In a prererred embo~liment of tlle cooling device 124
(I~IG. 3B) thele is a central l1ole 128 tlnougl1 wlliclt llle l~eatn of focused light passes to
contact tlm~ skin. Referring again to ~IG. 3A, a l1eat sink 132 is also preferably placed in
contact with tl~e coolil1g device. ~y providing a cooling device with a small hole in its center
coincidenl: with the focus of tlle ligl1t, the skin tissues in the general area where the poration is
to be created may be pre-cooled to 5~C to 10~C. Tllis pre-cooling allows a greater safety
malgil1 fo- the syslem to operate in that tlle potential sensations to the user and the possibility
oF any co"lateral damage to tlle epidellllis directly below ~he poralion site are reduced
significal1~ly from non-cooled embodiment. Moreover, for monitorillg applications, pre-cooling
minimize(. evaporation of ;~ ihl fluid and can also provide adva..l~geous pllysical
properties, sucl~ as decreased surrace tensiol1 of such interstitial fluid. Still furtller, cooling Ille
~issue is known to cause a localized increase in blood nOw in such cooled tissue, tllus
promoting dimlsion of analytes fiom the blood into the interstitial fluid.
Tlle metl1od can also be applied For other micro-surgery techniques wherein the ligllt-
absorbing compo1llld/lleat-probe is applied to the area to be ablated and thell the light source is
used to selectively modulate the temperature Or the probe at tlle selected targel site, affecting
Ihe tissue~ via tlle vaporization-ablation process produced.
,~-~ fin~ller feature of tlle inYelltioll is to use the ligllt source to hclp seal the micropore
after its usefulness has passed. Specifically, in the case of monitoring for an internal analyle, a
n1icroporo is created and some amoullt of interstitial fluid is extracted through this opetlitlg.
After a sufficiellt amoullt of interslitial fluid had been collected, the light source is reactivated
at a redu~-ed power level to facilitate rapid clotling or coaglll ~tion of the interstitial fluid witllin
3 0 tlle micrcpore. By forcing the coagulalion or clolting of tlle fluid in the pore, this openillg in
tlle body is erfectively sealed, thlls reducing the risk of infection. Also, the use of the light
source itself for both the formation of the micropore and the sealing theleof is an h1l1erently
~ 2199002
28
slerile procedure, with no pllysical l~enetration into the body by any device or aplJalaIu:~.
Purtller, tlle therllIal sllock incluced by the light energy kills any microbes that may llappen to
be present at tlle ablation site.
This concept of optical stcrilization can be extended to include an additional step in
the proces~ whereilI the light source is first applied in an unfocused manner, covering tlle target
area witll ~!n illumillated area tha~ extends lO() llm or more beyond the actual size of tlle
micropore Lo be plodllced. 13y selectin~ the area over which the unfocused t7eam is to be
applied, th~ flux density can be COI~. s~Jolldillgly reduced to a level well below the ablation
tbreshold but l-igh enough to effectively sterilize the surface of the skin. After a sufficiently
long exposure of the larger area, either in one colllilIuous step or in a series of pulses, to the
sterilizin~ beam, tlle system is tlle3l conl~gItrell into tlle shalply rocused ablation mode and the
optical mieroporatioll process begins.
Anolller illustrative embodimelIt of the invention is to create the required heat probe
rrom a metallic solid, sucll as a small diameter wire. As in the previously described
embodiment, tlle contacting surface of lhe heat probe mItst be able to llave its tetnperature
2 0 modulated fiom ambient skin tempclatulcs (33~C) to temper,.(u-~,s greater than 123~C, within
the required time allowed of, preferably, between about I to 50 ms at the higll tempe~alu~e
~on-time) alld at least about lO to 50 ms al tlle low temperature (off-time). In particular, behlg
able to modul.lte the temperahlre up to greater tllall 150~C ror an "on" time of aroulld S ms
an(l an ofl thtle of 50 ms produces very effective thermal ablation with little or no sensation to
2 5 tl~e individual.
Several methods for modulating the tempc~alu-~s of the wire heat probe contact area
may be sl ccessrully implemented. For example, a short length of wire may be brought up to
the desire~ high temperature by an external heating element such as an ohmic heating element
used in the tip of a soldering iron. FIG, 4 sllows an ohmic heating device 140 with a
3 0 mechanic,ll actuator. The ohmic heating device co.. l,.ises an ohmic lleat source 144 coupled
to a wire ~leat probe 14~. The olImic lleat source is also coupled througll an insulatillg mount
152 to a mecllallical modulalioll device 156, sucll as a solenoid. In this configuratioll, a steacly
,. .~ ,
: ~ 2199002
29
state condi.ion can be reached whereill the tip of the wire probe will stabilize at some
equilibrium temperatIlre denned by tlle physical parameters of the structure, i.e., tlle
temperaturo of tlle ohmic heat source, the lengtll and diameter of tlle wire, the temperature of
tlle air surloulld.ng lhe wire, and llle material of whicll the wire is comprised. Once the
desired temperatllre is achieved, the modulalion of the temperature of the selected area of an
hldividual's skin 160 is erfected directly via the mechanical modIllation device to altenlatively
place ~lle l ot tip of llle wire in contact wilh the skin for, preferably, a S ms on-tillle and tllen
withdraw it into tlle air ror, prererably, a 50 ms o~f-time.
Another illustrative example (I;IG. 5), shows a device 170 Cv...p.;;,;llg a current source
174 coupl~ d to a controller 178. The current source is coupled to a current loop 182
conlpl-isi~ a wirc 186 rormed into a slrIlcture such lllat it presents a higll resistance point.
rreferably, the Wile i5 held on a mo~lnt 190, and an insulator 194 5Cp~ s different parts of
the curren~. Ioop. Tlle desired modlllation of temperatllre is then achieved by merely
modulatill3 the currellt througll the wire. If tlle tl~ermal mass of the wire element is
apl)lo~ lely sized and the heat sinking provided by the electrodes culllleuling it to the current
2 0 source is s urficiellt~ the warm-up and cool-down times of the wire element can be achieved in
a few mil' iseconds. Contacting the wire with a selected area of skin 198 heats the stratum
corneum to achieve the selected ablation.
Ill FIG. 6 there is shown still another illustrative example of porating the stratum
comellm with a hot wire. In this system 200, the wire 204 can be positioned withill a
2 5 modulatable alternatillg ma~netic field formed by a coil of wire 208, the excitation coil. 13y
ene,gi~ing the altemating currellt hl the excita~ion coil by means of a controller 212 coupled
thereto, eddy curlents can be induced in the wire heat probe of 5Uf~ intensity that it will
be heated up directly via the hlterllal ohltlic losses. This is essentially a miniature version of
an induct ve hea~illg system comlllollly used for heat treating the tips of tools or inducing out-
3 0 gassing fiom the electrodes in vacuum or flash tubes. The advantage of the inductive heating
method i~ that the energy delivered into ~lle wire lleat probe can be closely controlled and
modlllate~ easily via the electronic contlol of Ille excitalioll coil. If the lherlnal mass of the
~ 21 99002
wire probe itself, and tlle tllellllal mass of lhe stratllnt corneum hl contact with the tip of the
probe are known, controllillg tlle induclive energy delivered can produce very precise control
of the temperature at the contact point 216 with the skin 220. Because the skhl tissue is
essentially noll-magnetic at the lo~er freqllencies at wllich inductive heatillg can be achieved,
if appropri ltely selected frequencies are used in tlle excitation coil, tllen this alU~..a~ g
elc~l~u---agnetic field will have no efrect on tlle skhl tissues.
If a mechallically controlled contact modulation is employed, an additional feature
may be re.~ ed by h~colporating a simple closed loop control system wherein the electrical
impedance between the probe tip and the subject's skin is monitored. In this tnanner, tlle
position ol tlle probe can be brougl~t into contact with tl~e subject's skin, indicated by tl~e step-
wise reduction in resistance once conlact is made, and lhell held there for tlle desired "on-
time,~' after whicll it can be ~vitlldlawll. Several types of linear actuators are suitable for this
form of cl~sed loop control, sucll as a voice-coil merh~ni~m, a simple solenoid, a rotary
systenl wi~h a cam or bell-crank, nnd lhe like. The advantage is that as tl~e therltlal ablaîion
progresses, tlle position of the tllermal probe tip can be similarly advanced into tlle skin,
2 0 always ensurillg good a contact to facilitate the emcient transfer of tlle required thermal
energy. /~ Iso, the challge hl tlle conductivity properties of the stratum cornellm and the
epiderlllis can be used to provide an ele~ant closed loop verification that the poration process is
complete, i.e., when the res~ nce hldic;ltes that the epidermis llas been reached, it is time to
stop tlle polation process.
2 5 I~IG. 7 shows an illustrative example of sucll a closed loop impedance monitor. In
this system 230, there is an ohmic beat source 234 coupled to a wire heat probe 238. Tlle heat
source is nounted througll an inc~-la~ille tnount 242 on a mechanical modulator 246.
controller 250 is coupled to the wire and to tlle skin 254, wllerein the controller detec~s
cllanges in impedance in tlle selected area 258 Or skin, and whell a predetermined level is
3 0 obtained Ihe controller stops the poratioll process.
,~long tlle same Ihle as l~ydralllic poration means are microlallcets adapted to just
penetlate lile slratum corneum for purposes of administering a permeant, sucll as a drllg,
~ 2 1 ~93a2
31
througll the pore formed or to withdlaw an analyte througll the pore for analysis. Such a
device is callsidered to be "minimally hlvasive" as compared to devices and/or techlliques
wllicll are lon-invasive. The use of micro-lallcets that penetrate below the stratum comeum for
wilhclrawil.g blood are well knowrl. Sucll devices are commercially available from
manllractu ers such as 13ecton-Dicl~ soll and Lifescan and can be utilized in the present
invention hy controlling the depth of penetlatioll. As an example of a micro-lancet device for
collecting ~ody fluids, reference is made lo Erickson et al., Intelnationat Published rCT
Applica~ion WO 95/10223 (publislled 20 April l99~). Tl~is application shows a device for
penetratioll into tlle dermal layer of the skin, without penetration into ~ubcul~lleous tissues, to
collect body nui(ls for mollitorillg, such as for ltlood glucose levels.
roratioll of stralulll corlleulll can also be accomplislled using sonic means. Sonic-
poration is a variatiol; of tlle optical means descril!ed above except that, instead of using a light
source, a uely tightly rocused bealll of sonic energy is delivered to tlle area of the slratum
corlleulll t~ be ablated. The same levels of enelgy are required, i.e. a thresllold of 70
mJ/cm2/SC ms still must be absorbed. The same pulsed focused ultrasonic transducers as
described in parent applications Serial Nos. 08/152,442 and 08/152,174 can be utilized to
deliver tho required enelgy densit;es for ablation as are used in the delivery of sonic energy
whicll is m odulatecl in hltellsity, pllase, or fiequency or a combination of these paramelers ror
the transd~rmal samplhlg of an analyte or ll1e transdermal delivery of drugs. This has the
advantage of allowing use of the same lldl,sduGer to pusll a drug through the stratum corneum
2 5 or pull a body nuid to the surface for analysis to be used to first create a micro-pore.
~dditionally, electroporation or short bursts or pulses of electrical current can be
delivered to the stratum corneum with suff cient energy to form micropores. Eleclroporation is ~,
known h~ the art for producillg pores in biological membralles and ~Ic;~,llvpola~ion ill~tlume
are commercially available. Thu~;, a person of skill in lhis alt can select an instrument and
3 0 condilion s for use thereof witholl~ undue experimentation according to the guidelines provided
llereim
~ 2 1 99002
ï'lle micropores produced in the stratllm corneum by thc methods of the present
invention nllow higll ~lux rates of large molecular weight tl~erapeutic compounds to be
delivered transdermally. In additioll, tllese non-traumatic microscopic openings in~o the body
allow access lo various analytes withill llle body, wllich can be assayed to deterllline thcir
hltemal cancelltratiolls.
Example I
Ir tllis exaulple, skin samples were prcpared as follows. Epidermal meml)rnlle wns
separated l;om humall cadaver ~s/hole skin by the heat-separatioll n-etllod of Klingman and
Christopller, 88 Arcll. Dermatol. 702 (1963), involving the exposure of the full tllickness skin
to a temp~ rature of 60~C ror 60 seconds, after whicll time the stratum comeulll and part of tlle
epidermis (epidermal membrane) were genlly peeled from the dermis.
Example 2
I-I eat separated stralulll corlleulll saml-les prepared according to the procedure of
Example - were cut into I cm2 sections. These small samples were thall attaclled to a glass
2 0 cover slide by placin~ thettl on the slide and applying nn pressure sensiLive adllesive backed
disk wilh B G mm hole in the center over tlle skin sample. The samples were thell ready for
experimet tal testing. In some installces the skill samples were hydrated by allowing them to
soak for s veral llours in a neutral buffered phospllate solution or pure water.~s a test of these untreated skin samples, the outputs of several different infrared laser
diodes, enlittillg al rougllly 810, 905, 1480 and 1550 nanometers were applied to the sample.
The deliv~ry optics were designe(l to produce a focal waist 25 llm across witll a final objective
have a nu nerical aperture of 0.4. The total power delivered to the focal point was measured to
be between S0 and 200 tnilliwatts for the 810 and 1480 nm laser diodes, whicll were capable
of operating in a contilluous wave (CW) fashioll. The 90S and 1550 nm laser diodes were
3 0 designed r o produce high peak power pulses roughly lO to 200 nanoseconds long at repetition
rates up to S000 ~Iz. I;or the pulsed lasers tlle peak power levels were measured to be 45 watts
at 905 nn, and 3.5 watts at 1550 nm.
~ 21 99002
~;nder these operating cosIditions, there was no apparent effect on the skin samples
rrom any )f llle lasers. Tlle targeted area was illuminSlted continuollsly for 60 seconds and
then examined microscopically, revealilI~ no visible effects. In addition, tlle sample was
placed in I modified Franz cell, typically usecl to test transclermal delivery systems based on
chemical permeatioll enllatlcers, and lhe condllctivhy from one side of the membralIe to tlle
olher was measured botll before and aher the irradialion by the laser and sl~owed no cl~ange.
Based on ~hese lests whicll were mll on skin samples from four different donors, it was
concluded that at these wavelengtlls the couplillg of tlle optical energy into lhe skhl lissue was
so small t lat no erfects are detectable.
Example 3
lo evaluate the potential sensatiolI to a living subject whell ill-lmill~ted witll optical
energy ulI~ler the conditiolls of E~.ample 2, six volunteers were used and the oulput of each
laser soun~e was applied to their fillgertips, forearms, and tlle backs of their hallds. In tlle
cases of tlIe 810, 905 and 1550 tltll lasers, tlle subject was unable to sense when the laser was
2 0 turned on or orf. In tlle case of the 1480 nm laser, there was a some sensation during the
illumhIati~tl by the 1480 nm laser operating at 70 mW CW, and a short while later a thly
blisler wa3 rormed ullder lhe skhI dlle lo the absorption of the 1480 nm radiatioll by one of lhe
water absorplioll bands. Apparently tlle amount of energy absorbed was sufficient to induce
~he forma~ ion of the blister, but was not enollgll to cause the ablative removal of tlle stratum
comelll~ Iso, the absorptiolI of tlle 1480 nm light occurred pledominalltly in lhe deeper,
fully hydtated (85% to 90% water content) tissues of the epidermis and dermis, not the
relatively dry (10% to 15% water content) tissue of the stratllm corneum.
Example 4
3 0 1 3avhIg demonstrated the lack of erfect on the skin hI its natural state (Example 3), a
series of c llemical compounds was evaluated for effectiveness in absorbing the light energy and
thelI transferrilIg this absorbed energy, via conduction, into the targeted tissue of the stratum
, .. ... ..
21 99002
34
corneum. Compollnds tested included In(lia ink; "Sl-IARPIE" brantl indelible black, 171ue, and
ted marki lg l ens; me~llyletle blue; filscllian red; epoli~e #67, an absorbillg compolllld
developed for nloldil1g inlo polycarbollate lenses ror protected laser goggles; tincture of iodine;
iodine-polyvillylpyrlolidolle complex ("BETADlNle"); copper phtllalocyallille; and printers ink.
~ISillg botll of tlle CW laser diodes describcd in Example 2, positive ablation reslllts
were observed on the in vitro sanlplcs of llcal-separatedstratutn corneum prepared according to
Example I w,llcn USillg all of tllese proclucts, however some perrormed better tllan others. In
particular tlle copper plltllalocyanillc (CI~C) nn~l tlle epolite tl67 werc some of tlle most
erfective. One probal)le reason for the superior perrormance of tlle CPC is its high boiling
pOillt of ~reatcr tlle '700''C and tlle fact lllat it n~aintaitls its solid p l~ase up to tllis tetnperatltre.
F,xatnple S
~s copper pllthalocyallille llas already been approved by tlle FDA ror use in
implQntable sutures, and is listed in tlle Merck hldex as a ratller benigll and sblbile molecllle in
regard to l~umall biocompatability, tlle ncxt step taketl was to combine tlle topical applicatio
of thc CPC and tlle focused light soutce to the skin of healthy human volunteers, A
suspellsio l of finely ground CPC in isopropyl alcollol was prepared, Tlle method of
applicaticn used was to shake lllc solu(ion and tllcll apply a small drop al llle target sile. ~s
tlle alcollol evaporated, a t'inc and unifotm coating of tlle solid phase CPC was tllen len on the
surf.lce ol' tlle skin.
2 5 1'he ap paratlls sllow in ~IG I was thell applied to the site, wllerein the CPC llad been
topically eoated onto tlle skin, by placing llle selected area of the individllal's skin against a
refelellce plate. Tlle reference pl~te Collsists of a tllin ~lass window rougllly 3 Clll X 3 Clll,
Witll a 4 Inm llole ill the center. Tlle CPC covered area was tllen positioned sucll that it was
witllill tlle central llole. A conrocal video tnictoscope (lilC. I) was tllen used to bring tlle
3 0 surface of the skin into sllarp focus. Positionillg tl~c skin to acllieve tl~e sllarpest focus on the
~ideo sys~em also positiotled it sucll tllat the focal pOillt of the laser systcm was coincident
Witll tlle surface of tlle skin. 'I'lle operator tllen activated tl~e pulses of laser light wllile
. . .
~ 21 9qO02
watcilitlg lle efrects at the target ~;ite on ~he video monitor. 'I'lle amount of penetratioll was
estimated visually by the operator by gauging the amount of defocusing of the laser spot in the
micropore as the depth of the micropore increased, and this can be dynalllically corrected l~y
the operator, essentially following the ablatecl surface down into lhe tissues by tnoving the
position of tlle camera/lasersource along the "z" axis, into the skin. At the point whell tlle
stratutn cortlellm had been removed down to tlle epidermis, the al l~ea~ ,ce of the base of tlle
hole chall~ed noticeably, becoming mllcll wetter and shillier. Upon seeing tllis change, the
operalor ~'eactivalecl Ihe lascr. In mally hlstallces, depen(ling on the state of hydratioll of the
subject as well as other physiolo,~ical conditions, a dramatic outflow of interstitial fluid
occurred in response to tlle l~arrier function Or the stratutn corneutn being retnoved over Ws
small are,.. Tlle vi(leo system w~s use(l to recorcl lllis visual record of tlle accessibility of
interstitia fiuid at the poration site.
Example 6
l'he procedure of Example 5 was followed except tllat tlle CPC was applied to a
2 0 ll~.,sl,a~ent adllesive tape, wllicll was tllen caused to adbere to a selected site on the skhl of an
individua~. l'he results were sul~stalltially similal to those of Example 5.
Example 7
llistology experiments were perfonned on cadaverskin according to methods well
known in tlle art to determine ablation thresllol(l paramelers for given dye mix~ures ancl
collateral damage infomlation. Tlle top surface of the skin sample was treated with a solutio
of copper phtllalocyallille (CPC) in alcohol. After the alcohol evaporated, a topical layer of
solid phase CPC was distributed over the skin surface witll a mean thickness of lO to 20 um.
I-IG. 8A sllows a cross-section Or full tllicklless skin prior to tlle laser application, wllerein llle
3 0 CPC layer 270, stratum corneum 27~1, and underlying epidermal layers 278 are sllown. ~IG.
8B show5 the sample al~er a sin~le pulse of 810 nm ligllt was applied to an 80 um diameter
circle Wit l an cnergy density of ~lO00 J/cm2, for a pulse period of 20' ms. It is nolewortlly that
~ 2199002
36
tllere was ;till a significallt amoullt of CPC present on lhe surface of llle stratum comeum even
in tl~e mid:ile of tl~e ablated cratcr ~82. It should also be noted tl~at
laboratory measurements indicate tllat only about 10% of ~lle light energy incident on tbe CPC
is actually absorbed, with the olher 90% being reflected or b~ L~c,.l~ d. l'hlls the effective
energy llu ~ being deliverd to tlle dye layer whicll could cause the
desired helling is only about 400 J/cm2. 8C sllows the sample after 5 pulses of 810 nlll light
were applied, wllerein tlle stralum corneulll barrier was removed witl~ no damage to tlle
underlyillg tissue. Tllese results are a good ~ p~ sc.,l;ltion of tlle "ideal" optically modulate(l
thermal ablatioll performance. 1~1~. 8D sl-ows tlle sample afler 50 pulses were applied.
Damaged lissue 286 was present in the cpidermal layers due to carbonizatioll of noll ablated
lissue and thellllal delldlulillg of tlle underlyillg tissue. I~laS. 8A-8C sllow separations betwee
tlle stratum cornelllll and tlle undcrlying epidermal layers due to an artifact pf dellydratioll,
free~ing, a nd preparations for imaging.
Example 8
2 0 To examille tl~e details Or tlle tl~ermal ablation mech~nicm, a mathematical model of
tlle skin tissues was constructed upon whicl~ various different embodiments of lhe tllermal
ablaRoll melllod could be lried. 'I his motlel compules Ihe temperalure distriblllioll in a layere(l
semi-infil ite medium wilh a specified heat flux hlput locally on the surface and heat removal
rrom tlle sulrace some distance away, i.e. convection is applied between lhe two. Tlle
2 5 axisymm~tl-ic, time-dependellt dimlsion equatioll is solved in cylindrical coordinates using the
altemating-direction-implicit (ADI) mell-od. (Note: Constant Temp. B.C. is applied on lower
boundary to serve as z->inf; and zero radial heat flux is applied on max radial boundary to
serve as r->h~r). The layers are parallel to the surrace and are defined as: (I) dye; (2) stratum
corneum; (3) underlying epidermis; and (4) dermis. The depth inlo tlle semi-hlrlllite medium
3 0 and thermal properties, density (rho), specifc heat (c), and conlJu~,livily (k) must be specified
for eacll layer.
~ 2199002
37
F rst, a heat-transfer coerficient, Il, on the skin is computed based on the "steady," "I-
D," tempelature distribution delermilled by the ambient air tel"pe~idtule, skin surrace
temperatu e, and dermis temperatllle. It is assumed that there is no dye presellt and provides
"h" on the skill surface. The program then allows one to use this "h" on the dye layer surface
or input a lotller desired "h" for tl~e dye surrace. Next, the "steady" temperature distribution is
computed througllout all layers (hlclIldillg the dye layer) USillg the specified "h" at the dye
surface. 1 his te~ )elnl~llG distribution is tlle initial condition for the time-dependent lleating
problem. This con:~liIuI~3 (he "m-file" initial.lll. The program then solves for the time-
dependenl temperature distribution by marching in thlle, computing and displaying the
temperatu e fleld at each step.
Eacll eml)odimellt of the metllod described herein, ror whicll empirical data hav0 been
collected, llas been modeled for at least one set Or operational parameters, showing how
stralum corneum ablation can be achieved in a precise and controllable fashion. The output of
the simul~ tions is presented graphically hl two different formats: (I) a cross-sectional view of
the skin s!lowhlg the different ~isslle layers wi~h three isotherms plotted on top Or this view
whicll deIine lhree crilical temperature thresllolds, and (2) two different temperalure -vs- time
plots, one for the point in the mi~ldle of the stratum corneum directiy beneath tlle target site,
and the second ror lhe p oint at lhe boull-laly of lhe viable cell layers of the epidermis and the
underside of the stratum corneum. These plots show l10W the temperature at each point varies
with thne as the heat pulses are applied as if one could implant a miciuscopic thermocollple
2 5 i!!!o !he tissues. !n addition, lhe app!ica!ioll of t!:is mode! a!!ows i~:vesligP.tio:: Or lhe
parametric limits withill whicll the metllod can be employed to set the outer Ihllils for two
important aspects of the methods performance. First, general cases are presented cases that
define the envelope withill which the metllod can be employed withoIlt causing pain or
ulldesired tissue damage.
I;or any given heat source, as described in lhe several difrerent embodiments of the
invention, thele is a pOitlt at which the erfect on tlle subject's skin tissues becomes noll-optimal
in that the subject perceives a pain sensation, or that the viable cells h~ tlle undellying
~ 21~02
38
epidertnis and/or dermis sustain tempe~ntu~ s, wllicll if maintained for a long enougll duration,
will rend~ r dama8e to these tissues. Accordingly, a tesl simulation was run using tlle optically
lleated tol~ical copper pllthalocyanine (CPC) dye embodiment as a baseline method to establisl
llow ~he t lermal time constan~s of lhe dilrerent skin tissue layers essentially derme a window
Withill Wl iCIl tlle method can be employed willlout pain or damage to adjacent tissue layers.
~ IGS. 9 and 10 sl~ow scllematic cross-sectional views of the skin and tlle topical dye
Iayer. In each figure, three distinct isolllerms are displayed: (1) 123~Cl the point at whicll
vaporizat on of the water in the tassue produces an ablation of the tissue; (2) 70~C, the pOillt at
whicll vi~ble cells will be damaged if tllis temperature is ma;llt~ ed for several seconds; and
(3) 45~C, the average point at whicll a sensation of pain will be perceived l~y the subject. Tllis
l~ain tllresllold is described in several bdsic pllysiology texts, bllt experience sllows tllis
thresllold to be somewhat sul)jective. ln fact, in repeated tests on the same individual, different
poration sites within a few millimeters of each other can show significantly different amounts
of sensation, possibly due to the proximity to a nelve endillg in relationsl1ip to tlle poralion
site.
2 0 rhe dimensiol1s on the graphs sllow the different layers of the dye and skh1, as
IllCaSllred itl 11111, with flat boundaries defining them. Whereas tl1e actual skin tissues have
mllcll mcre convolllted boundaries, in a meall sense for tl~e di~ nsions involved, the mo(lel
provides a good applu~i,.-dlion cf the tllermal gradients present in tlle actual tissues. llle
dimensions used in lllis, and all "ub3c~luenl simulations, for the thicknesses of tlle CPC dye
layer and lhe various skin layers are as rOllows: dye, 10 ~Im; stratum corneum, 30 ~Im;
underlyil g epidermis, 70 ~lm; and dermis, 100 llm.
~dditiot1al conditiol1s hl1posed on the model for this particular simulation are shown
in the following tables:
' ~ 2 1 ~002
39
l'able I
Initial Conditiolls for Finile Differellce Thertnal Model
Ambiell~ Air Temperature I'a = 20~C
Skin Su~face Telllperalure Ts = 30~C
Dermis Temperatule Td = 37~C
Dye Va;~orization Temperature Tvap--550~C
S.C. Vaporization Temperatule Tc I = 123~C
Tissue l~amage Temperature Tc2 = 70~C
Pahl Temperature Tc3 = 45~C
Radius af ll-radiated Area Rh, = 30 llm
Energy Density ~pplied I~LUX = 400 Joules/cm2
l'able 2
Parameter Dye S~C. Epidermis Dermis
Thermal Con(l1lctivity0.00046.00123 0.00421 0.00421
2 01 ~ellSity 0.67 1.28 1.09 1.09
Speeific lleat 0.8 1.88 3.35 3.35
~hen these simtllations are rull the following COIl5~ e assumptions are imposed:1. While some portion of the stratum corneum may be shown as having a
2 5 temperah re already exceeded llle ablation thresllold for thermal vaporization Or the water
contellt ~ lis event is not modeled an(l tlte subsequent loss of l~eat enetgy in ~he tissues d1le 1O
lhis vapol ization is not factored into the sitnulation. This will cause a slight elevation in tlle
lemperatures sllowll in the underlyillg tissues rrom that pohlt on in the simulation run.
~ . Similarly wllen some portion of tlte copper phtllalocyallille (CPC) dye layer
3 0 is sllowtl to llave reaclled its vaporization pOitlt of 550~C tllis event is not nlodelc(l but tlle
temperature is merely hard-limited to this level. This will also cause a slight elevation of the
subsequent temperatures in the underlying layers as the simulation pl.gl- sses.
I ven with these shllplifications used in the model the correlation between the
predicted perforlllallce and the empirically observed performance based on bolh clhlical studies
3 5 and histological studies on donor tissue samples is remarkable. The key data to note in I~ICS.
9 and 10 are tlle lengtll of time whicll lhe heat pulse is applied and tlle locatioll of tlle thl-ee
different thresllold temp~ tul~ s displayed by the isotheltns.
n I~IG. 9 wilh a pulse lengtll of 21 tnilliseconds the 70~C isotherm just crosses the
boundar~ sepdldliilg the stratulll corneuln and the viable cell layers in the epidermis. In in
.. .. . .
. ~ 21~900~
vitro studies on donor skin samples under these conditions, fifty pulses of thermal energy
delivered r70 tnilliseconds apart cause delectable damage to this top layer of living cells (see
I~IG. 8D). Ilowever, it was also sl~own in tlle in vitro studies that rlve pulses of heat energy at
lllese sam, operaling parameters, did nol produce any significant damage to tllese tissues. It
seems rea30nable that even lhoIlgll llle nominal damage tl~resllold may have becn exceeded, at
least in a transient sense, tllis tempclatllre must be m:~int:lined for some cumlllative period of
thne to a( tually cause any damage to tlle cells Ncverll~eless, tlle basic inforn~ation presellte(J
by Ille simulatioll is that ir one keeps the ''on-tilI-e'~ of tlle lleat pulse to less Lhall 20
milliseconds with tlle flllx density of 400 Joules/cm2, tllen no damage to tlIe livillg cells in the
underlying epidernlis will be sustailled, even ~lIougll tlle ablation tllresllold isoll~erm llas been
moved wnll into tlIe stratum corneunn In otlIer words, by using a low flux density therlIlal
energy so lrce, modIllated such that tile "Oll time" is suitably short, al71ation of the slratullI
corneum ~an be achieved without any damage lo the adjacent cells in the ulIderlyillg epiderlnis
(see I~IC 8C) Tllis is possil)le in large palt due to llle si~nificantly different tllermal
difrusivities of tllesc two tissues layers. That is, the stratum corneum, containing only about
2 0 10% to 2;)% water content, has a much lower thermal conductivity constant, 0.00123
J/(S*cm*~), tlIan the .00421J/(SI'cm*K) of the epidemlis~ This allows the temperahlre to
build up n the stratum corneum, while mainlaining a tight spatial definitiolI, to the point at
whicll ab alion will occur
In I;IG. 10, tlle same simulation scenario started in the damage threslIold critical pOilIt
2 5 run ilhlstl ated in I~IG. 9 is carried out farlher in thne. By leaving the heat pulse on for 58
milliseco ~ds at lhe same flIlx density of 400 Joules/cm2 wilhin the 60 llm diameter circle of
dye being lleated, the pain sensoly isotllerll- al 45~C just enters the enervated layer of skin
complise I by the dermis. In ad(fition, ~he damage thleshold isolllerm moves significalllly
rartller inlo the epidenllal layer tlIan wllele it was shown to be hI ~IG. 9. Relating this
3 0 simulatioIl to tlIe numerous clinical studies conducted witll this metlIod, an excellent
verificati an of the Inodel's acculacy is obtained in tllat tlle model shows almost exactly lhe
dulation 7f 'on-time' lllat tlle lleat probe cali be applied to the skin before the hIdividllal feels
il. In cliaical tests, a controllable pulse generator was used to set tlle "on-time"and ''off-tillIe'~
of a series of ligl~t plllses applied to the topical layer of copper pllthalocyanine (CPC) dye on
3 5 the skin. While m:lintainilIg a constant "ofr-time" of 80 milliseconds, the "on-time" was
graduall~ hlcreased until the subject reporte(l a mild "pain" sensation Without exception, all
of lhe su ~jects hIvolved in tlIese studies, reported the first "pain" at an "on-time" of between 45
and 60 n illiseconds, very close to that predicted by the model. In addition, the site-to-site
variability melItiolled previously as regards llle sensation of "pnin" was noted in these clinical
4 0 studies. Accordhlgly, what is reported as "pain" is the pOillt at whicll the first unambiguous
~ 21 9qO02
41
sensation is noticeable. ~t one site this may be reported as pain, whereas at an adjacent site
tlle same ~ubject may report tllis as merely "noticeable."
Cne elemetlt of this clinical researcll is tlle realization that even at lhe same site, a
non-unifol=m p-llse-traill of heat pulses may work witll the subject's psycho~pllysiological
neuro-perceptioll to cause a genllitle rcduclioll in perceived sensation. I~or example, a series of
shorter lengtll heat pulses can be used to saturate the neurons in the area, momentarily
depleting ~he ne~lro-transmitters availal~le at this synaptic junctiotl and therefore Ih.litil.g the
ability to l;end a "pain" message. This thell allows a longer pulse following tllese sllort pulses
to be less noticeable than if it were applied at the beginnillg of the sequence. ~ccordingly, a
series of experhllellts was condllcted witll some arbitrarily created pulse trains, and tlle results
were consistellt with this llypolllesis. ~n analogy for lhis situalioll migllt be found in lhe
perceptiot. when one Rrst steps into a very hot bath that is painful at first, but quickly becomes
tolerable .-s one acclhllates to the heat sensation.
Example 9
2 O ~.n object of this invention is to acllieve a painless, micro-poration of tlle stratllm
corneum ~yilhout causing any significallt damage to the adjacent viable tissues. As described in
tlle silllulatioll ilhlstralc t in Example 8 and FIGS. 9-10, a boundary appears to exist for any
given flu~ dcnsily of lhel-lllal encl-gy willlill Illc ablaliun largcl sp(ll wilhill wllich tlle nlicro-
poratioll ~ an be acllieved in just sucll a painlcss and noll-trallmalic manner. Bolll the in vivo
and in vitro studies have sllown that this is the case, and tllis has permitted development
througll e npirical methods of solne operational parameters that appear to work very well. Tbe
following set of simulatiolls shows how the method works when tllese specific parameters are
used.
I~ the first case, a pulse train Or ten pulses, 10 milliseconds "on-time" separated by l0
3 O milliseconds "off-time" is appliecl to tlle CPC-covered skin. FIG. I l SllOWS the final
temperature distribution in tlle skin tissues immediately after this pulse train llas ended. As can
be seen, tlle isotherms .~;"~ s. .~tillg lhe three critical temperature tllresllolds show tllat strahlln
comeum 3blation has been achieved, with no sensation present in the dermal layer nerves and
very liltle cross-over of the damage thresllold into the viable cells of the underlying epidermis.
3 5 As mentiolled previously, it appears that to actually do permanent cell damage, tlle epidermal
cells must not only be heated up to a certain pohlt, but they also must be held at this
temperatl re for some period of time, generally tllought to be about five seconds. I~IGS. 12 and
13 show ~he temperature of lbe stratum corneum and the viable epidermis, respectively, as a
filnctioll of time, showillg heatillg durillg lhe "on-time" and cooling during the "off-time" for
4 O lhe entire ten cycles. Relating this simulation to the in vivo studies collducted, note tllat in
better than 90% of lhe poration altelllpts wi~h llle system parameters set to matcll the
.. .
~ ~ 2~qqo02
42
simulatiol-l, erfective poration of tlle stratum comeum was acllieved without pain to the subject,
and in sul-sequellt microscopic exan~ alioll of the poration site several days later, no noticeable
damage to tlle tissues was apparent. The in vitro studies conducted on whole thicklless dotlor
skin samples were also consistent with the model's prediction of behavior.
Example 10
l~ conducting bo~h the empirical in vivo studies, and these simulations, it appears that
prechillillg of the skin aids in OptillliZillg the micro-poration process for reducing tlle
probabilily of pain or damage to adjacellt tissues. In practice, tllis can easily be achieved llsing
a simple old-plate placed agahlst the skhl prior to the poration process. I;or example,
applyillg a I'eltier cooled plate to the I cm diameler circle surroulldillg the poration target site,
with the l~late held at roughly 5~C for a few seconds, significantly reduces lhe temperature of
tlle tissues. ~ scllematic illustration of an experimelltal device used for this purpose in the
laborator ~ is sllown in i lGS. 3A-B. By applying exac~ly the same ten-cycle pulse train as
used m the run illustrated hl Example 9, one can see, by comparing FIG. I l to Fl(3. 14, FIG.
12 to Fl~i. 15, and FIG. 13 to I~IG. 16, how mllcll improvement can be made in the control of
the temp~rature penetratioll hlto tlle skin tissues. Once again, tlle relatively low therlllal
diffilsivity and specirlc heat of the strattlm corneum as compared to the epidermis and dermis
is advallt~geous. Once cooled, the higllly hydlated tissues Or lhe epiderlllis and àcnnis require
a much 13rger lherlllal energy hlpllt to elevate lheir tempel~ s, whereas the stratum corneum,
with its lelatively dry makeup, can quickly be lleated up to the ablation thresllotd.
Example I I
3llce the basic lhellllal conduclioll mcchanislll of deliverillg llle energy hlto the skhl
tissues u lderlying the effective painless ablation and micro-poration of the stratum corneum is
3 0 understo ~d, several different specific methods to achieve the required rapid temperature
modlllatiolls of Ille contact pohlt can be conceived, such as the hot wire embodiments
illustrate~l hl FIGS. 4-7.
A basic embodiment, as described herein, uses an Ohmic heating element (FIG. 4),such as llle tip of a small cordless soldering iron, with a suitably sized, relatively non-reactive,
wire wrapped around it with a sholt amoullt of the wire left to protrude away from the body of
the heat~ r. Whell electricity is applied with a constant current source, the heater will come llp
to some lemperature and withill a few seconds, achieve a steady state with the convection
losses to the surrounding air. S;milarly, tlle wire, whicll is a part of this thermal system, will
reach a steady state such that the very tip of the wire can be raised to almost any arbitrary
tempera~ure, up to rougllly 1000~C with these types of components. The tip can be sized to
give exactly the dimension micropore desired.
.~ 2~ 99002
a,3
Il the lal)oratory, tullgstell wires witll a diameter of 80 ~Im attached to tlle replaceable
tip of a "~,rAIlL" cordless soldering iron witl~ approximately 2 mm of wire protruding from
tlle tip lla~e been utilizecl. Witll a thellllocouple, tlle temperature of tlle tip llas been measured
at its steady state, and it llas been noted tllat by varyillg the constant current settings, steady
state temp~ratllres Or greater tllan 700~C can easily l~e reached. I o acllicve tllc desired
modulatio.l, a low mass, fast response electromecllanical actuator was coupled to the tip such
tllat the positioll of the wire could be trallslated linearly more thall 2 mm at up to a 200 llz
rate. The~ y mountillg the entile al-p~ ls on a precision stage, tllis vibrating tip could very
controllably be brougllt into contact witll tlle skin surface in a manner wllere it was only in
contact rO less lllan 10 milliseconds at a limc, tlle "on-tillle," wllile an "orr-time" of arl~illatily
long pericds could be acheived by setting tlle pulse generator acc- ~.IL,gly. These in vivo
studies sl~wed that tlle poration could actually be acllieved before the sllbject being porated
even knew tbat tlle tip of tlle wire was being brougllt into contact witll tlle skim
l o compare tlle perrollllallce of tllis embodilllellt to tlle optically heated topical Cl'C
dye embodiment, the~ following simulations were run according to tlle procedure of Example 8.
2 0 EssentialPy, by only varying tlle initial conditions, tlle hot wire embodimellt can be run witll
tlle identi al simulation code. Because tlle contact with tlle wire occurs essentially instantly,
lhere is nl) time dependellt build-l3p of heat in the CPC dye layer and wllen the wire is
pl~ysically removed rrom contact witll tlle skin, tllere is a no residual lleat still lel~ on tlle
surface a~ tllere is witll tlle heated CPC dye layer. Also, as tlle wire itself defines tlle area
2 5 targeted for ablation/micro-poration, tllere shol31d be no lateral dimlsion of thermal energy
prior to ils application to the stratum corneum. The comparative performances of the "llot-
wire" embodilllellt are sllowll in I~IGS. 17-19.
Example 12
3 0 In tllis example, tlle procedure of Example 11 was followed except that tlle skin was
pre-cooled according to the procedure of Example 10. Similarly, pre-cooling tlle target site
yields similarly positive results witll tlle "llot-wire" embodimellt. 'Ihe results of the pre-cooled
simlllatio.l of tlle "llot-wire" approacll are shown in ~IGS. 20-22.
3 5 Example 13
.~s discussed in tlle background introduction of tllis disclosure, tlle Tankovicll '803
patent appears at first glance to be similar to tlle preselltly claimed invention. In tllis example,
the simulation model was set up witll the operating parameters specifled in Tankovicll '803,
i.e. a pulse widlll of I lls and a power level oF 40,000,000 W/cm2. E~IGS. 23 and 2~ show tl-at
~'L O under th~se conditions no portion of tlle stratum corneum reaches the tllreshold For Flash
vaporizalion of water, 123~C, ancl tllus no ablation/lllicroporatioll of tlle stratulll corllellm
2 t 9~00~
44
occurs. In practice, applying tllis type of higll peak power, sl~ort duration pulse to tlle topical
dye layer r lerely vaporizes tlle dye off of tl~e surrace of the skin witll no effect on tlle skin.
l l~is exam ?le, tllus, demonstrates tllat the conditio1ls specified by Tankovicll '803 are
inoperative in the presently claime~l inventiol).
Example 14
In this example, interstiti;ll nuid obtained after porating tlle skin according to tlle
procedure of Examl~le 6 was collecled and analyzed to determille tlle glucose concelll-dlion
ll)ereof~ ala were obtailled on rOul nol1-diabetic subjects and six type I diabelic subjects
ullder~oillg a 61ucose load test. Sul~ject's ages ranged rroln 27 to 43. 'I lle goal of tl)e stlldy
was to ex~ mills tl~e utility of tlle melhod ror painlessly llarvesting enougll inlerstitial tluid
(ISF) frolr the subjecls to allow the ISF samples to be assayed for glucose content, and tllen
compare t'-lese concelltratiolls to the glucose level preselltillg in the subject's ~vhole bloocl.
,411 subjects had botll llle blood and ISI; glucose assays performed willl llle "ELITE"
system frc m Miles-13ayer. ~\11 ten subjecls underwellt identical measuremellt plotocols, witl
2 0 adjustmenls being made regardillg tlle glucose load and insulill shot ror tllose subjects with
insulilt dependellt diabetes.
I he basic design of tlle study was to recruit a modest number of volunteers, some
witll diabl-tes and some without diabeles, rrom wllicll a series of sample pairs of ISI; and wllole
blood were drawn every 3 to 5 minutes throllgllout the 3 to 4 llour duration of the study
2 5 perio~l. E~otll the blood and tlle ISF samples were assayed for glucose and llle statislical
relationship bet~veen llle blood glucose levels and tlle inlerstitial fluid detemlilled. To examine
tlle hypol lesized lemr~oral lag of lhe ISP glucose levels as compared to the whole blood
glucose l~lvels, lhe sludy subjecls were induced to exhibit a signirlcant and dynalllic challge in
tlleir glucose levels. Tllis was accomplished by having each subject fast for 12 hours prior to
3 0 begimlillg tlle test and thell giving tlle subject a glucose load afler his or Iler baseline ghlcose
levels lla-Je been eslablished via a set of three fasting blood and ISF glucose levels. After the
l~aseline levcls llad bcell eslal~lislled, tlle sllbjccls were givell a glucose loa(l in ~lle rorlll of
sweet juiee based on the following guidelines:
i. For the control subjects, the glucose load was calculated based on a
3 5 75 gram glucose per pcund of body weigllt.
ii. For tlle subjecls willl insulin dependcllt diabetes tlle glucose load was
~0 grams of glucose. In addition, immediately a~ter taking tlle glucose load tlle
iiabetic subjects will self inject tlleir nomlal moming dose of fast acting insulin. In
~he case wllere tlle diabetic subject presents with fasting glucose levels above 300
4 0 mgtdL, they were asked to give themselves their insulin injectioll first, and the
~ 2 1 qqO02
glucose load was provided after lheir blood glucose levels have dropped to below 120
nlg/dL.
E'ach subject recruited was first given a complete description of the study in tlle
- "Informec' Consent" doculllellt whicll tlley were required to understand alId SiglI before they
were omeially enrolled intO tlle ~rogram. Upon acceptance, tlley completed a medical llistory
ques~ionll tire. Tlle detailed clhlical procedure implemented was:
(a) Subject rasted fiom 9:00 p.nn the nigllt berore the study visit7 COI15UI11;11g
only water. No cafreine, cigarettes, frllit juice were allowed durillg tllis period.
(b) Subject arrived at tlle tcsting facility by 9:00 a.m. the next day.
(~) Subject was seated in a reclinhlg chair provided for the subject to relax inthrouglIol.t the study procedure.
(~1) Both wllole blood and ISI; samples were taken at three to five milIute
hltervals begillllillg upon tlle subject7s arrival and contilIuing for tlle next tllree to four l10UIS.
The duralion over whicll the data were collected was based on when tlle subject7s blood
2 0 glucose Irvels had returlled to the normal range and stabili~ed aner the glucose load. The ISF
samples were harveste(l using tlle optical poration7 ISF pumping method7 described in more
detail bel~7w. Each ISI; sample was roughly S uL by volume to ensure a good fill of the
LLI'I'E test strip. l'he blood samples were obtahled via a convelltiolIal rlnger prick lancet.
Both the ISF and tlle blood samples were immediately assayed for glucose willl the ELI'I'E
2 5 home glucometer system rrom Miles-Bayer. To improve the estimate of the 7tme7 blood
glucose Icvels, two separate ELITE assays were be done on each finger stick sample.
Ie) To facilitate the continued collection of the ISF from the same site througll-
out the elItire data collection pllase ror a given hldividual, a 5 by 5 matrix of twenty five
micropor~s was created on the subject's upper forearm, eacll micropore being between 50 and
3 0 80 ~1m arross and spaced 300 llm apart. A 30 mlll diameter teilon disk witll a 6 mm hole h
the center was attaclled to tlle subject's rorearm with a pressure sensitive adhesive and
POS;t;OI1Cd SUCI1 lllat llle 6 IIIIII ccnler holc was localed over the 5 by 5 matrix Or micro-l70rcs.
Tllis atta~,hlIlellt allowed a convelliellt metllod by whicll a small suction hose could be
connecte~, applying a mild vacuulIl (10 to 12 inches of l~g) to the porated area to indllce the
3 5 ISI~ to flow out of the body througll ~he micropores. The top of the teflon disk was fitted with
a clear glass whldow allowing tlle operator to dh-ectly view the micro-porated skin beneath it.
WhelI a i uL bead of ISI; was formed ou the surface of the skin, it could easily be ascertahIed
by visually monitorillg tlle site thlough this whldow. This level of vacuum created a nominal
pressure ~radient of around 5 poullds/square inch (PSI). Witllout the micropoles, no ISI;
4 0 whatsoe~ er could be drawn from the subject's body using only the mild vacuum.
~ 2 1 q9002
46
(f~ Aner tlle first tllree sample pQirs l~ave been drawll, tlle subject was given a
glucose lold in tlle fonn of higllly sweetened orange juice. The amount of glucose given was
0.75 grams per pound of body weigllt for tlle nondiabetic subjects and 50 grams for tlle
diabelic st bjecls. Tlle diabetic subjects also self adminislered a sllot of fast acting inslllill,
(regulQI) witll the dosage appropriately calcula~ed, based on tl~is 50 gram level of ~,lucose
concurrenl witll tlle ingestion of tlle glucose load. With tlle norlllal 1.5 to 2.5 llour lag
between rcceivitlg an ins-llitl sllot and llle maximum effect of the shot, tlle diabetic subjects
were expe~ted to exhibit an upwards excursion of tlleir blood glucose levels ranging up to 300
mg/dL and tllen dropping rapidly back into the nomlal range as tlle insulin takes effect. Tlle
nondiabel'c subjects were expecled lo exllibit tlle standard glucose tolerance tesl prortles,
typically showillg a peak in blood glucose levels between 150 mg/dL and 220 mg/dI, from 45
minutes to 90 minutes after admillistering tlle glucose load, and tllen a rapid drop back to their
norlllal baseline levels over the next llour or so.
(1'.) I'ollowing llle ndlnillisll-alioll of llle glucose load or glucose load and insllli
sllot, tlle s ubjects had samples drawll, simultalleously, of ISI~ and fnger prick whole blood at
2 0 Flve millu e intervals for tlle next lllree to four hours. l'he sampling was terminated wllen lhe
blood glu-~ose levels in three successive samples indicate that llle subject's glucose llad
stabilized
llpoll examillalioll of llle daln, several fcnlures were npparellt. In parliclllal~ for any
specific bltcll of ELITE test strips, there exist a distinct sllift in the output sllown on tlle
2 5 glucometnr in mg/dL glucose as compared to tlle level indicated on the blood. /~n elevaled
reading ~ould be expected due to lhe lack Or hemalocrit in tlle ISF and to lhe tlontlal
~lifferenc~s in tlle elec~rolyle concetllraliolls belween lhe ISt and whole blood. Rcgardless of
llle under ying rensons ror tllis shiR in output, it was determitled via comparisotl to a reference
assay thal the tme ISI~ glucose levels are linearly related to the values produced by tlle ELITE
3 0 system, v, illl tl~e scaling coefficienls constant for any specific batcll of ELITE strips.
Conseqllelltly, for the compnrisoll of the ISi' glucose levels versus tlle wllole blood
mcaslllclllcllls, rnst order linear corrcc~ion was applied to tl~e ISI; data as rOllows:
'aluco~e - 0-606 ISt EL'TE} 19.5.
'''his scaling of the output of tlle ELITE glucomeler wllen used to measure ISF
3 5 glucose Icvels, allows one to examine, over the entire data set, tl~e error terms associated with
using ISF to estimate blood glucose levels. Of course, even with no linear scaling whatsoever,
llle correlations between tlle tSF glucose values and tlle blood glucose levels are tlle same as
tlle scaled version.
I?,ased on the majority ortlle publislled body of literature Oll the subject of ISF
glucose as well as preliminary data, it was originally expected that a 15 to 20 minute lag
between ~lle ISI glucose levels and tlle tllose presented in the wllole blood fiom a finger stick
.~,. ,........ , . ~:
~ 2~99~02
47
would be observed. Tllis is not wllat the data sllowed when analyzed. Specifically, wllen each
individual s data set is analyzed to determine tlle time shift required to achieve tlle msl~cimllm
correlatior between the ISI~ glucose levels and the blood glucose levels it was discovered tllat
the worst case time lag for tllis set of subjects was only 13 minutes and the average titne lag
was only 6.2 millllles, with several subjects sllowing a temporal lracking Ihat was ahllost
in~t~llt~ne )us (about I minllte).
13ased on the millilllal amollllt Or lag observed in this data set, the graph shown in
FIG. 25 pl esents all ten of tlle glucose load tests, concatellated one arter anotller on an
extended time scale. Tlle data are presented witll no time shifting whatsoever, sllowing the
l-igh level of tl-ackillg betweell the ISI~ and blood glucose levels Ihe entire clh~icnl data set
being ~lea!t willl in exaclly tlle san-e mallller. If tlle enlire dala set is sllined as a whole to find
tl1e best temporal tracking estimate, tlle correlation betweell the ISF and blood glucose levels
peaks witll a d~!ay Or two (2) mil~ cs ;It an r value of r=0.97. This is only a Irivial
illll.lovclllcll( rrOm thc ullsl~inc(J corlcl~lioll Or r=0.964. l llercrore, for lhc rcmaill(ler of tllc
analysis tlle ISI; values are treatecl witl~ no time shift imposed on thelll. That is, eacll set of
2 0 blood and ISF glucose levels is dealt with as simultaneollsly collected data pairs.
I'~fter the unsllifted Elite IS[~ readings had been scaled to reflect tlle proportional
glucose present in tlle ISP, it was possil71e to examine ttle error associated witll these data. Tlle
simplest raetllod ror Illis is to assume tllat tlle average of lhe two ELITE fingel-stick blood
glucose readings is in ract tlle absolutely correct value, and tllen to merely compare the scaled
2 5 ISli value~ to these mean blood glucose values. These data are as follows: Standard Deviation
Llood-ISI~, 13.4 mg/dL; Coemciellt of Variance of ISI:, 9.7%; Standard Deviation of the Two
Elites, 8.: mg/dL; and Coemcient of Variance of Blood (Miles), 6%.
~ ~s tllese data show, the blood based measurement already contaills an error term. Indeed,
Ihe manu racturer's published perrormance data indicates that the ELITE systeln has a nominal
3 0 Coefficie lt of Variance (CV) of Ibetween 5% and 7%, depending on the glucose levels and the
amount of hematocrit in the blood.
~n additional look at lhe dirrerence tenn belween tlle ISI; glucose and lhe blood glucose
is shown in the fonn of a scatter plot in FIG. 26. In this figure, the upper and lower bounds of
tlle gO% ,onfidence interval are also displayed for reference. It is interestillg to note that with
only two exceptions, all of the data hl the rarlge of blood glllcose levels below 100 mg/dL fall
wilhin thnse 90% confidellce interval error bars. This is important as the consequences of missing
a trend towards hypoglycelllia would be very significallt to tlle diabetic user. Tllat is, it would be
IllUCh better to under-pledict glucose levels in the 40 to 120 mg/dL tl~an to over predict them.
Esselltially, if one assumes tllat tlle basic assay error when tlle ELITE system is used on
4 0 ISF is comparable to the assay error associated witll the ELlTE's use on whole blood, thell tbe
Deviation of tlle ISI; glucose from tlle blood glucose can be described as:
~ 9 0 0 ~
48
ISl;d~ ,jon - [(ls~ u~ S~c~u,l) ]
Applying tllis equation to tl~e values shown above, one can solve for the estimated 'true'
value of tl e ISI~ error teml:
ISF,C,U,~ S~d~V~ o~)2 - (Bl~~d~c~u~l) ] -
Or, solving the equalion,
ISI~Ic,u~l = (13 .4)2 _ (~,3)21'~ = i0,5 mg/dl.
A histogram of the relative deviation of tlle ISli to the blood glucose levels is shown in
FIG 27
Dnl~ Delivery tllrou~ll Pores in lhe Strat~lm Corneulll
I I-e present invention also includes a mell-od for tl~e delivery Or drugs, including drugs
currently delivered transdermally, ~llrougll micro-pores in tlle stratum corneum. In one illustrative
en~bodim(;nt, tl~e delivery is acl~ieved by placing tl~e solution in a reservoir over ll~e poration site
In anotllel illuslralive enlbodilllcllt, a plessllre gradiellt is used to filrtller enllallce tlle delively
In still an~tller illustrative embodimellt, sonic energy is used with or withollt a pressure gradient
lo filrthet enl~ance the delivery Tlle sonic energy can be operated according to traditional
tl~usde~ al parameters or by utilizillg acoustic streaming effects, which will be described
momental ily, to pusll the delivery solution tt~rougl~ the porated stratum corneulll.
Example 15
2 5 l~l~is example sl~ows tl~e use of stratum cornellm poration for the delivery of lidocaine,
a topical analgesic. Tl~e lidocaine solution also contained a cllemical permcatioll enhancer
rormulati~n designed to enllallce Lts passive dirrusioll across the stratum cornellm. A drawillg of
an illuslr,-ltive delivery apparatus 300 is sllown in ~la. 28, whereill tlle apparatlls COlllpriSeS a
llousing304enclosingareservoir308forholdingadrug-containingsolution312. Thetopportion
of the llousillg collhprises an ultrasollic transducer 316 for providing sonic energy to aid in
ol ling tlle clrug-containing sollltion tllrougl~ micropores 320 in the stratulll corllellm 324 A
port 328 in llle ultrasollic transducer permils application of pressure thereto for rurlller aiding in
transporting the drug-containing solutioll tllrougll tl~e micropores in tl~e stratum collleum. The
delivery apparatus is applied to a selected area of an individual's skin such tllat it is positioned
3 5 over at k ast one, and preferably a plurality, of micropores. An adhesive layer 332 attached to a
lower portion of tlle llousing pcrmits tlle app~lldlu:~ to adhere to the skin sucll tllat the dmg-
containil g solution in the reservoir is in liquid conlmullicatioll willl tl~e micropores. Delivery of
tl~e drug hrollgh tlle micropores results in transport into the underlying epidermis 336 and dermis
340.
4 0 i~ive subjects were tested for the erfectiveness of drug delivery using poration togelher
witll ultrlsoulld. l he experiment used two sites Oll the subjects lert forearm al~out tllree inclles
F~
j~. 2~ ~9002
49
apart, equ~ lly spaced between the thul11b and ul-per arm. The site near lhe thul11b will be referred
to as site the site rurtllest from Llle thumb will be referred to as site 2. Site I was used as a
control where tlle lidocaine and enl1ill1cersollltion was applied using an identical delivery ~I~)paldlus
300, but ~ ithout any micro-poration of lhe stratum corneun1 or sonic energy. Sile 2 was porated
with 24 hc les spaced 0.8 millill1clcrs ap~llt in a 6rid conlained ~Yitl~in n I cm di7lllletercircle. ï'he
micropore; in Site 2 were ~eneraled according to tlle procedure of Exaînple ~. Lidocaine and low
level ultra ;ound were applied. Ultrasound applications were made with a custom mal1ufactllred
Zevex ult~asol1ic trans(lucer asseml~ly set in l~urst mode with 0.4 Volts peak to peak hlput witl1
1000 COUllt bursls occurlil1g al 10 llz will1 a 65.4 kllz rundall1ental freqllel1cy, i.e., a t~ulse
modlllalec signal witl1 the transducer enelgized ror 15 millisecond bursts, and lhell turned orf for
tlle next 8.i millisecollds. The measure(l outpllt of tlle amplifier to the t.hnsJucerwas 0.090 watts
RMS.
~ fter application of the lidocahle, sensation measuremetlts were made by rubbing a 30
gauge wire across tlle test site. ~xperimellts were executed on botll sites, Site I for 10 to 12
mil1ute dllration an(l Site 2 for two 5 mil1ute duratiol1 intervals applied serially to tl~e Salne sile.
2 0 Both sites were assessed for numblless u5il1g a scale of 10 to 0, where 10 indicated no nllmblless
and 0 indicated complete numbness as reported by tlle test subjects. lhe following sun1mary of
results is .or all 5 subjects.
llle control site, site 1, presellted little to no numbness (scale 7 to 10) at 10 to 12
minutes. At approximately 20 minutes soine numblless (scale 3) was observed at site I as the
2 5 solulion completely permeated the stratum corneul11. Site I was cleaned at the completion of the
lidocaine Ipplication. Site 2 presentedl1eallycomplelen~ 1bness (scale0 to 1) in the I cm circle
containillg the poratiol1s. Outside the I cm diameter circle the nulllbness fell of r almost lillearly
lo I at ,. 2.5 cm diameler circle with no nllmbl1ess oulside the 2.5 Clll diameler circle.
Assessmeut of site 2 a~er the second applica~ion resulted h1 a slightly larger totally nllmb circle
3 O of about ] .2 cm diameter witll numblless falling off linearly to I in an irregular oval pattern with
a diameter of 2 to 2.5 cm perpendicular to Il1e forearn1 and a diameter of 2 to 6 Clll parallel to ~he
~ro;ei~ lsido l!;e a;ca;;o ;;;;;;;b;;ess 'YVï~s ;;olc;l. A ~rap!;ic ;~ ,sc~ .tio:;of l!!t:slrativ~:es::!!s
obtained on a typical subject is shown hl FIGS. 29A-C. FIGS. 29A and 29B show tl1e results
obtained at Site 2 (porated) after 5 and 10 minutes"~ Je~liv~;ly. FIG. 29C shows the results
3 5 obtained at Site I (control with no poration).
Sonic Enl rgy and Enllancers for Enllancing l'ransdermal l~lux
'- he pl1ysics of sonic energy fields created by sonic transducers can be utilized in a
metl1od by whicl1 sonic frequellcy can be modulated to improve on flux rates acllieved by otller
n1etl1ods. As shown in I~IG. I of U.S. Patent No. 5,445,611, hereby i--c~ ol~led hereill by
reference; the energy distribu~ion of an sonic transducer can be divided into near and far fields.
2 1 99002
The near field, chatacterized by lengtll N, is lhe zone from tlle first energy minimum to lhe last
energy ma~ m. The zone distal to the last maximum is the far field. l he near (N) rleld pattern
is domitlaled by a large n~ lber of closely sllaced local pressure peaks and nulls. Tlle lengtll of
lhe near R~ld zone, N, is a runctioll of ~he rreqllellcy~ size, and shape of the transducer face, and
the speed of sound in tlle medilllll tlnollgll wllich Ille ulllasolllld travels. For a single ll~ulsducel,
intetlsity ~lariations wilhitl its norlllal operating range do not affect tlle nature of tlle sonic energy
distributioa other tllan in a Ihlear fashioll. I=lowever, for a systelll with multiple transdllcers, all
being moc.ulated in l~otll frequency and amplitude, the relative intensities of separate ll~ln~ UC. ,~
do affect t le energy distribution in the sonic medium, regardless of wllether it is skin or anolher
nledlulll.
~y challging the îrequency of the sonic energy by a modest amount, for example in the
range of about I to 20%, tlle pattern of peaks and nulls remaills relatively constant, but the lengtll
N of tlle near feld zone cllanges in dilect proportioll to the fiequency. Major challges the
fi-cquellcy say a factor of 2 or molc, will mosl likely plo(lllce a difrerellt sct Or resonfll1ces or
vibratiotla~ modes in llle transdllcer, causing a signiftcantly and unpredictably dirferent near field
2 O energy palterll. Thus, with a modest challge in the sonic rrequency, tlle complex patterll of peaks
and nlllls s compressed or expat1(led in an accordion-like manner. By selecting tlle direclion of
rrequency modlllation, llle direction of sllifl of lhese local pressure peaks can be contlolled. By
nl)plyillg '.OlliC energy al llle surface of Ihe skin, seleclive modulation of llle sonic frequellcy
controls n ovemel1t of lilese local pressllre peaks tllrougll tlle skin eilller toward Ille hlteriol of the
body or toward the surface of the body. A frequency modulation from lligll to lo~v drives the
pressure p_aks into tlle body, whereas a fiequency modulation rrom low to hi~ h pulls the pressure
peaks rrom withil1 ~lle body toward lhe surface and tllrougll lhe skin to llle outsi(le Or llle body.
I~ SSUIllillg typical parallleters ror li1is applicalioll of, for example, a 1.27 Clll dianlelel
souictlans(lllceralldallolllillalopelatillgrreqllellcyof 10 Mltzandanacollsticilllpedallcesimilar
3 0 ~o tllat of ,vater, a r~ n,cy modulation of I Ml lz prodllces a movemellt of abolll 2.5 mll1 of the
peaks and nulls of Il1e near rleld energy patlerl1 in the viCillity of llle str0tulll corneulll. Frotn llle
perspecth~e of lransdellllal an(l/ol lrallsll1llcosal will1drawal of analyles, this dcgrec of aclion
provides access to tlle area well below tlle stralum corneulll and even tlle epidermis, derlnis, and
olller tissl es bellealll it. I;or any given Irallsducer, tllere may be an optimal range of fiequetlcies
3 5 within whicl1 this freqllet1cy modulatiot1 is most effective.
1 lle flux of a drug or analyte across the skin can also be increased by cl- nging either tlle
resistallce (the diffusion coefficient) or tlle drivh1g force (the gradient for diffusion). Flux can be
enl1at1ced by tlle use of so-called penetralion or chel1lical ellllanCt;l~.
C helllical enl1ancers arc COlllpl ised Or lwo primary categories of componel1ts, i.e., cell-
4 0 envelope :lisorderhlg compounds and solvents or binary systems con~ ing both cell-envelope
disordering compoullds and solvents.
. .
2 ~ 990~2
51
Cell envelope disordering compounds are known in the art as being useful in topicQI
pharlllaceutical preparations and functioll also in analyte withdrawal througll the skin. These
compoullds are thougllt to assist in skin pene~ration by disordering the lipid structure of the stratulll
comeum cel l-envelopes. A comprellellsive list of tllese compounds is described in European Patent
~pplicaticn 43,738, pul~lisllcd Julle 13, 1982, ~vllicll is incol~.oldled herein by relé1rence. It is
believed that any cell envelôpe disorderillg compound is useful for purposes of this invention.
Suital)le solvents hlclllde water; diols, sucll as propylene glycol and glycerol; mollo-
alcohols, ~;ucll as etllanol, propanol, and higller alcohols; DMSO; dimetllylformamide; N,N-
dimethyla~etamide; 2-pyrrolidone; N-(2-llydroxyethyl) pyrrolidone, N-metllylpyrrolidone, 1-
dodecylazacyclol~eplall-2-oneand otl-er n-sul~stiluled-alkyl-a~acycloalkyl-2-olles(azol-es) and tl~e
like.
~ .S. Patent 4,537,776, Cooper, issued August 27, 1985, contains an excellentsummary
of prior alt and l~acliground inrorlllatioll detailing tlle use of certain binary systems for penneallt
enhallcelll1 llt. Because of the completeness of that disclosure, ll~e inrorl~latioll and tenninology
utilized thereill are incorporated hereill by refierence.
2 0 Similarly, European Paterlt ~pplication 43,738, referred to al)ove, teaches USillg selected
diols as sc Ivents along wilh a broad category of cell-envelope disordering compounds for delivery
of lipopllilic pllarlllacologically-activecompoullds. Because of ~he detail in disclosing tlle cell-
envelope ~isordclillg compollllds and ~he diols, this disclosure of Europeall ratcnt ~pplicalion
43,738 is also incorporated llereill by rererence.
2 5 ~ binary system for ellllancillg metoclopramide pene~ration is disclosed in UK Patellt
~pplicatioll G13 2,153,223 A, published August 21, 1985, and consists of a mollovalellt alcollol
esterofaC8-32alipllaticltlollocal1~oxylicacid(ullsaturaledand/orbrallclledifC18-32)oraC6-24
aliphatic molloalcohol (ullsatlllaled and/or l)ranclled if C14-24) and an N-cyclic compolllld sucl
as 2-pync lidone, N-methylpyrrolidone and tlle like.
3 O C'ombillaliolls of enllallcers Collsistillg Or dielllylelle glycol molloetllyl or monollletllyl
ether witll propylene glycol mollolallrate and methyl laurate are disclosed in U.S. Patent 4,973,468
as enllallcing lhe l,dnsdel,.,al delively of sleroids SIIGh as progestogens and eslrogells. A dual
enllallcer c vn~ g of glycerol monolallrate and ethallol for the transdemlal delivery of drugs is
shown in ~.S. ralent 4,820,720. U.S. Patent 5,006,342 lists null._lOU5 enhancers for transdermal
3 5 drug admulistratioll consisting of fatty acid esters or fatty alcohol ethers of C2 to C4 alkanediols,
where eac h ratty acidtalcohol portion of the ester/etller is of about 8 aO 22 carl on atoms. U.S.
I'atent 4,853,970 shows penetratian-ellllallcillgcompositiolls for topical application comprising an
active permeallt contailled in a penetratioll-ellllallcillgvellicle containing specified amoullts of one
or mole c, ll-envelopedisordering compounds such as oleic acid, oleyl alcohol, and glycerol esters
4 0 of oleic a -id; a C2 or C3 alkanol and an inert diluent such as water.
!' ~'' . ~--. .......
~ ~ 1 9q~02
52
C tller cl~emical enl)allcers, not necessarily associated witll binary systems include DMSO
or aqueou; solutions of DMSO such as taugllt h1 llerschler, U S ratent 3,551,554; llersclller,
U,S, ratert3,711,602; andllerschler,U,S, Patent3,711,606,atldtlleazones(n-sllbstituted-alkyl-
azacycloa kyl-2-ones) sucll as noted in Cooper, U.S Patent 4,557,943
Sollle chemical enllancer systems may possess negative side effects such as toxicity and
skin irritaliotl U.S. Patetlt 4,855,298 discloses compositiolls for reducing skh1 irritation caused
by cllemical enllnncercolltainillg compositiolls llaving skin irritation properties wilh an amount of
glycerin sufficient to provide an anti-irritatillg effect.
13 ccause tlle combination of microporation of the stratum corneum and the applicatiotl of
sonic enelgy acconlpallied by tlle use of cllemical enllancers can result in an improved ratc Or
analyte withdrawal or permeant delively throllglt the stratum corneum, the specific carrier vehicle
andpatticularlytltecllemicalellllallcerutilizedcallbeselectedfiolllalollglistofpliorartvellicles
sotne of ~'hiCIl are mellliolled abo~e and incol poraled heleill by rererence. To specirlcally detail
or enlll11el ate thal wllicll is readily avaihlble hl the art is not tllougllt necessary The hlvelltiol1 is
not drawn to the use pf chemical enllallcers per se and it is believed tl1at all chemical enllancers,
usefui in tlle delivery of drugs tllr~ugh the skin, will function witb dyes in optical n1icroporatioJl
and also vitl1 sonic energy in effecting measurable witlldrawal of analytes from beneatll and
throllgl1 tl e skin surrace or ~l1e delivery of permeal1ts or drugs throllgll llle skin surface
Example 16
I\ lodulated SOlliC energy and cl1enfical enl1ancers were tested for their ability to control
transdermal fluX on Imlt1at1 cadaverskin samples In these tests, the epidermal membratle llad bee
~epatdled rrom lhe Imttlan cadaver whole skhl by the heat-sel-aration method of Example 1 l he
epidermal membralle was cut and placed between two Italves of the permeation cell with tlle
3 0 stratum corneul11 facing eitller the upper (donol) compartment or lower (receiver) compartment
Modified l~ranz cells were used to hold the epidermis, as sllowll in I~IG 2 of U.S. Patent No.
5,445,61 1 Eacll l~ranz cell consists of an upper chatllber and a lower chalt1ber held togelller witll
one or more clamps. I'he lower cbatnber has a sampling port through whicll materials can be
added or lemoved. A sample of slralum comellm is held between lhe upper and lower chambers
3 5 wl1en tlley are clamped together. The upper cllamber of each Franz cell is modified to allow an
ultrasollnd transdllcer to be positioned witllin I cm of tlle stratum comeultl membrat1e. Metllylene
blue solllt on was used as an indicatol molecllle to assess tlle pcrmeatiol1 of llle stralum corneul11.
A visual r ecord Or the process and results of each experhl1ellt was oblnhled in a lime stamped
magnetic ape format with a Yideo can1era and video cassette recorder (not sllown). Additionally,
samples were witlldrawn for measuremet1t with an absorption ;,I,ccl-u-.. eter to y~lanlil~t~ the
amoullt of dye wllich had traversed tlle stratum corneum men1brane during an experiment.
~ 21 99002
53
Cllemical e 1hallcers suitable for use could vary over a wide range of solvents and/or cell envelope
disordering compoullds as nolsd above. Tlle specific ~nllancer utilized was:
etllanol/gly erol/water/glycerolmollooleate/llletllyl laurate io 50/30/15/2 512.5 volullle ratios Tlle
system for producillg and controllillg tlle SOlliC energy included a progralllmable 0-30 Mllz
arl~illary w~veforlll gellerator (Slanford Reseracll Systelns Model DS345), a 20 watt 0-30 Mllz
amplifier, ~nd two unfocused ultrasollnd imlllersioll tlallsduc~ aving peal; 1~ ~onall~es al 15 and
25 Ml-lz, respectively Six cells were prepared simultaneously for testing of stratum corneulll
samples from the same donor. Once tl~e stratum comeum samples were installed, tlley were
allowed to llydrate witll distilled water ror at least 6 llours before any tests were done
Example 17
Effects of '~OIliC Energy without Cllemical Enllancers
Asstatedal~ovein Exalnpie 16, tllel~eat-separatedepidermiswasplacedil~ tllel;ranzcclls
witll llle e3iderlllal side ~àcing up, and tlle stratulll cornellm side facing down, unless noted
otllerwise. l lle lower cllambers were filled Witll distillecl water, wllereas tlle upper cllambers were
2 0 illled will~ concentrated methylelle blue solutioll io distilled water
111 at Separated Epidermis: Imlllediately after filling tlle upper cllambers witll melhylelle
l~lue soluti ln, sonic enelgy was applie(l to one of the cells witll tlle ~Idn5d-~cdl fillly immersed.
I l\is orielllaliol~ wo~ l corlespond, ror exan~ple, lo havill~ tlle Iralls~lllcer on Ille opposile si(le Or
a rold of skin, or causing the sonic energy to be reflected off a reflector plate similarly positioned
2 5 ancl being 3sed to "pusl~" analyte Ollt of tlle otller side of the fold into a collection device. Tlle
sonic ener~y setling was initially set at tlle notnitlal operating frcquency of 25 Mllz witl~ an
intensity eqllivalellt to a 20 volt peak-to-peak (P-P) inpllt wave form. Tl~is col . . sl,ollds to rougllly
a I watt Or averagc inpllt power to tl~e tl<lnsdllCel and similarly, assullling the manufactlller's
nominal v~ lue for cl)nversioll efficiencyof 1% for tllis particulart.dnsll~ce" a sonic output power
3 0 of around 0 01 WdllS over tlle 0.78 cm2 surface of tlle active area or a sonic intensity of 0 13
watts/cm2. Tlnee oll~er control cells llad no SOlliC energy applied to tllem. After S minlltes tlle
SOI~iC ener ~y was lulned Orr No visual indicaliotl of (Iye flux across ll~e stratlltll corneum was
ol)served d ~tring tl~is interval in any of tl~e cells, indicating levels less tl~an approximately 0.0015%
(v/v) of d~ e solution in 2 tnl of receivern-edimn.
3 5 T ~sting of these same 3 control cells and I e~ . ,.t~ll cell was continued as follows.
Tlle intensi~y of sonic energy was increased to tlle maximum possible output available from tl~e
driving equipmellt of a 70 volt peak-to-peak input 12 watts average power inl)llt or (~0.13
watts/cm2) of sonic output intensity. Also, the freqllellcy was set to modulate or sweep from 30
Mllz to IC l\~llz. ï'llis 20 Ml-{z sweep was performed ten times per second, i.e, a sweep rate of
4 0 10 llz A tllese input power levels, it was nec~s~d,r to monitor the sonic energy transducer to
avoid ovetheatillg. A contact tl~erlnocollple was applied lo llle l)ody of tlle trallsducer and power
. .
2 1 990~2
54
was cycled on and off to maintain maximulll temperature of the ll~ns~l~lcel- under 42~C. ~fter
about 30 n~ ules of cycling maxil1llll1l power at about a 5~% duty cycle of l minute oll and I
minllte off, there was still no visually detectable penl1eatiol1 of the strahln1 corneum by the
melllylene l~lue dye.
A cooling water jacket was tl)en altaclled to the sonic energy ll~ulsduccl to permit
extellded e~tcitatiol1 at tlle rnaximllm enel-gy level. Using tlle same 3 controls and I experimental
cell, sonic ~nelgy was applied at maxitl1ul11 power ror 12 ilours lo tlle experimentalcell. Dming
lllis tin1e tllc temperalure of tlle fluid in tlle upper cllamber rose to only 35~C, only sliglltly above
tlle approxin1alely 31 ~C normal temperatllre of tlle stratun1 corneum in vivo. No visual evidence
of dye flux llnollgll llle stratul11 corlleul11 was apparent in any Or tlle four cells after 12 llrs. of
sonic energy applied as described above.
Example 18
Effects of ~onic Energy willlout Cllemical Enllal1cers
PerforatedSh-atlll11Corneum:SixcellswerepreparedasdescribedaboveinExample 16.
2 0 'I'lle ciamps hol(ling tlle upper and lower cllambels of tlle Franz cells were tiglltened greater lhan
tlle extent n~qlliled to nonnally seal the upper compartmel1t from tl1e lower compartl11el1t, and to
tlle extent to artiFlcially introdllce perforations and "pinl1oles" into tlle lleat-separated epiderm~l
samples. ~'llen dye solutioll was aclded lo tl~e upper cl)anlber of eacll cell, lllere were iml1lediaîe
visual indicalions of leakage of dye into the lower cllambers tluougll the perforations fontled in
2 5 the slratum ~,ornellm. Upon applica~ion of sonic energy to cells in whicl1 tlle stratum comeum was
so perforated witll slllall "pil1l1oles," a rapid illcrease ill tlle lldlls~oll of fluid tllrougll a pillllole in
~lle stlalum cornclll11 was observed. l'l1e rale of h~allsport of tlle indicator dye molecllles was
directly related to wllethel tlle sonic energy was applied or not. Tllat is, applicatioll of tlle SOlliC
energy caus d an immediate (lag tin1e approximately <0.1 second) pulse of tlle indicator molecules
3 0 tl1rougl1 tl1e pinlloles in tlle stratum corneum. This pulse of indicatol n1olecules ceased
immediately upon turning off of the sonic energy (a sllutoff lag of app~ i",ately ~0.1 second).
l'lle pulse could be repeated as desGribed.
Example 19
Eft'ects of Sonic Energy and Cl1emical Enllancers
Tv~io different chemical enl1ancer formulations were used. Chemical Enl1ancel One ot
CEI was al1 adlnixtllre of etllanol/glycerol/water/glycerol monooleale/l1letllyl laurate in a
50/30/15/2.'i/2.5 volume ratio. l'l1ese are componel1ts generally regarded as safe, i.e. GRAS, by
the FD~ for use as pl1arl11aceuticalexcipiel1ts. Chell1ical EnllancerTwo or CE2 is an experimelltal
forlnlllation sllown ~o be very effective in enllal1cillg trallsdermal dmg delivery, l)ut generally
consideled loo irritatillg for long ten1l tral1sderlllal delivery applications. CE2 contained
~ K ~ . .~ .
~ 2 t 9~032
ethanol/gl~ cerol/water/lauradone/metllyllallrate itl the volume ratios 50/30/15/2.5/2.5. Lauradone
is tlle laur~l (dodecyl) ester of 2-pyrrolidolle-5- carboxylic acid ("PCA") and is also referred to
as lauryl PCA.
Six l~ranz cells were set up as berore (Example 16) except tllat tlle heat separated
epidermis was installed witll tlle epiderltlal layer down, i.e., stratum corneum side facing up.
llydration was established by exposing each sample to distilled water overniglIt. To begin tlle
experiment, tlle distilled watcr in tl~e lower challIbers was replaced Witll metllylclle l~lue ~Iyc
solution in all six cells. The upper cllambers were filled Witll distilled water and tlIe cells were
observed ror about 30 minutes conf'il ming no passage of dye to ensure that no pinhole perforations
were prcscnt in any of the cclls. Wllcn nolle wcrc roulld, tl~e distillcd water in tlle upper challIbers
was remo~ ed frolIl four of the cells. Tlle otller two cells served as distilled water controls. The
uppel challIbers of two of the experilIlellLal cells were then filled witlI CEI and the otller two
experimental cells were filled witll C1~2.
SolIic energy was imlllediately applied to one of tlle two CE2 cells. A 25 Mllz
transdllcer was used with the ~requency sweeping every 0.1 second from I0 Mllz to 30 M~lz at
maxilllum intensity of ~0.13 watts/cm2. Afler lO-15 minutes of sonic energy applied at a 50%
duty cycle, dye flux was visually detected. No dye flux was detected in the otller five cells.
S~nic enelgy was tllen applied to one of the two cells containillg CEI at tlle same
scttings. Dye l~egan to appelll- in the upper challll~er within S milllltes. 'I'llus, sonic enelgy
together witll a chelIlical enllallcersigllificalltly incleasedtlIe t~ai~sd~.t..al flux rale of a markerdye
tlIrou~ll the strat-un corneum, as well as reduced tlle lag time.
Example 20
Effects of Sonic Energy and Chelllical EnlIallcers
l~o~ llatiolls of the two cllemical enllalIcers, CEI and CE2, were prepared minlls the
3 0 glycerin and these ncw formulati()lIs~ designated CEtMG and CE2MG, were tested as before.
Water wa; substitllted for glycernl so tllat tlle proportions of tlle otller componelIts remained
uncllallged. l'hree cells were plepalcd in modiRed l;rallz cells willl tlle epidermal side of tlle heat
separated ~pidermis samples facing toward the upper side of tlle chambers. These samples were
tllen hydnlted in distilled water for 8 hours. Afler the lIydration step, tlle distilled water in tlIe
3 5 lower cha~nbers was replaced with either CEIMG or CE2MIG and the upper chamber was filled
with the cye solutioll. Sonic energy was applied to eacll of the tllree cells sequelIti~lly.
~pon applicatiolI of pulsed, fi equelIcy-modlllated sonic energy for a total duratiolI Or less
tllan lO milIutes, a significant increase in permeability of the stratum corneum samples was
observed. The permeability of the stratum corneulIl was altered relatively uniformly across the
4 0 area expo led to l~otlI the cllemical enllancer and sonic energy. No "pinlIole" perforations tlIrougll
wlliclI the dye could traverse llle slratllm corne~ I were observed. l'lle l,~ns(lel-llal flux ratc was
2 1 99~2
56
S hlstatltly control lable by turn ing the son ic ener~y on or off. Turning the sonic energy off appeared
to installlly reduce tl~e lransderlllal flux rate SIICIl ~hat no dye was visil~ly being actively transpolted
tllrough th~ skin sample; presllltlably the rate was reduced to that of passive diffusion Tutning
the sonic ~nergy on again inslantly resumed tl~e lligll level flux rate Tl~e modulated mode
appeared to provide a regular p ulsâtile increase in the transdermal nux rate at lhe modlllated rate.
When tlle ~ onic enelgy was set to a constallt frequency, tlle maximum increase in transdermal nux
rate ror tllis confi~,lllatioll secn-c-l to occur at arollnd 27 Mllz.
I-laving obtained tlle same reslllls witll all three samples, the cells were then drahled of
all fluids al~d tluslled witll distilled waler on botll sides of the stralum corneum. 'Ille lower
cllambers w ere Ihell imlllediately filled Witll distillcd water and the upper cllambers were refilled
wilh dye solution. The cells were observed for 30 minutes. No holes in the stratultl corneu
samples w~re observed and no large amollllt of dye was detected in llle lowet challlbers. A small
amount of dye became visible in tlle lower cllambers, probably due to the dye and c.,llall~el
lrapped in llle skin samples fiom tllcir previolls exposures Arter an addilional 12 hollrs, the
amollllt of dye detected was still very small.
Example 2 1
l~rfects of Sonic Energy and Chemical Pnll~n(ers
rerroraled Slralum Cornelllll: ! lu-ee cells were prepared wilh heat-separated epidennis
sanlples w th tl~e epidermal side facing toward the llpper side of the chamber from the same donor
as in Exal lple 16 The samples were llydrated for 8 hours and then the distilled water in tlle
lower cllambers was replaced witll either CEIMG or CE2MG. The upper challlbers were tllen
filled with dye sollltioll. rinhole perforations in tlle strat~lm corneum samples permilted dye to
leak lhrou ~h lllc stratlllll corneull~ samples into tlle underlyillg enhallcer contaillillg challlbels.
Sonic energy was applied. Immediately upon application of the SOlliC energy, tlle dye molecules
3 0 were rapidly puslled throllgll the pores As sllowll al~ove, the tapid nux Or tlle dye lhrollgl~ e
pores was directly and imlllediately correlaled wilh the applicalion of the SOlliC ellCrgy.
Example 22
Effects of Sonic Energy and Chenlical Enhallcers
3 5 A low cost sonic energy transdllcer, TDK #NB-58S-OI (TDK Corp.), was tested for its
capability o enllallce transdermal flux rates The peak response of this transducer was determined
to be abo~t 5.4 Mllz wilh otller local peaks occurring at about 7 Mllz, 9 Ml-lz, 12.4 Mllz, and
16 Mllz.
Tlis TDK ll~nsd~ el was lhen tested at 5 4 Mllz for its ability to enhance ll~nlsd~ al
4 0 ~ X rate in conjunctiotl witll CE I MG. Three cells were set up with the epidermal side fachlg the
lower chalnl~er, then the skin samplcs were hydraled for 8 hrs. Ihe dye solutioll was placed iti
~ 2~9~0~
57
lhe lower ~ hnmber. Tlle transdllcei was placed in the upper chamber immersed in CEI MG. Using
swept fre~luelIcies fi om 5.3 to 5.6 Ml lz as tlle sonic energy excitation, signirlcallt quantities of dye
moved elnougll tlle stratullI comellm and were detected in the collection well of tlle cell in S
minutes. I ,ocal heatillg occurred, witlI tlle transducer reaclling a t~ pe, nLul e of 48~C. In a control
USillg CEIMG withollt sonic energy, a 24 hollr exposure yielded less dye hl the collect;on well
tllan tlle 5 minllte exposure with 5011iC ellergy.
This example demollslrates that a low cost, low frequency SOlliC ellergy Il~Jllsducc~ Call
strikillgly affect ll~usde,."al flux rate when uged in conjllnction with an ~pl,~up.;ltl~ chemical
cnllallcer. AltholJgll lligller rrequellcy sonic energy will theoretically concentrate more energy in
lhe strat~ l corllelllll, whell used wi~ll a chellIical enllal1cer, lhe lower fiequellcy mo~lulated SOIliC
energy can acceleratethe tralIsderlllal llux rate to make tlle techllology useful and practical.
Example 23
Demonslrationofmoleculemi~raliollacrosslllllllanskin:TestswitlltlleTDKlllnlsJuccl
and CElhia described al~ove were repeated at about 12.4 Mllz, olIe of the higlIest local resonant
peaks for lle transducer, witll a fiequency sweep at a ~ ~Iz rate rrom 12.5 to 12.8 Mllz and an
sonic ener 3y densily less tllan 0.1 W/cm2. The epidermal side of the heat-separated epidermis was
racilIg do~m, tlle dye solutiolI was in the lower clIaml)er, and the enllancer solution and the sonic
energy were placed in tlle ul-per chaml)er. ~Villlin 5 minutes a significant amount of dye llad
moved acl oss lhe stratultl cornellllt into tlle collection well. Ohmic heatillg in the transdllcer was
significalllly less thall willl the same transducer being driven at 5.4 Ml-lz, causillg an increase h
temperatu-e of the chellIical enhancer to only about 33~C.
Even at tllese lo-v emciency levels, tlle results obtained with CEIMC~ and sonic energy
li om tlle l DK ll ~nsdllccl were remal l~able hl the mollitorilIg direclion. I~ S. 3/~ and 3 B Or U.S.
Patent No S,445,6 11 show plots of data obtained from three separate cells witll tlle trandermal
3 0 flux rate measured in the monitorilIg direction. EvelI at the S minute time point, readily
measurabl- qualItilies of tlle dye were present in tlle chemical enhancer on the outside of the
s(ralum carnèllllI, indicatillg transpol-t rroln tlle epidermal side thlougll the stratullI conlelllll to the
"outside" area of the skin sample.
To opthllize the use of the sonic energy or lhe sonic energy/cllemical enhancer approach
3 5 ror collecling and monitorilIg analytes from the body, InealIs for assaying the amoullt of analyte
Or hIterest are required. An assay system tllat takes mllltiple readings wllile the unit is in tlle
process oE withdrawing analytes by sonic energy witll or wilhout chelllical enllalIcers makes it
un~.ecc"d y to standardize across a broad population base and nortnalize for differelIt skin
characteristics and flux rates. By plotting two or more data points in time as Ille analyte
collcet~ lion hl the collection system is increasing, a curve-ftttitlg algorithllI can be applied to
determine the parameters describillg the curve relathlg analyle witlldrawal or nux rate to the p oint
~ ~ t ~9~02
58
al wllicll e~uilibt ium is reaclled, tllereby establislling tlle measure of the interval concentratioll.
Tlle general forln of this culve is invariallt from one indiYidual to another; only the parameters
cllange. C~nce these paramelers are establislled, solvillg for the steady state solution (i.e., time
equals intinity) of tllis fullctioll, i.e., wllen full equilibrium is establislled, provides tlle
concenllatioll of tlle alnalyte witllin tlle body. Tlllls, this app.ua~,ll permits measuremellts lo be
macle to the desired level of acculacy in tlle same amount of time for all tnembels ora populatio
re~ardless of indivicl1lal variations in skin permeabilily.
S~3veralexistingdetectiollteclllliquescurrentlyexisttllatarea t~l~,t~blefortllisapplicatioll.
See, D.A. Cllristensell, in 164~ rroceedings of l~iber Ol~tic, Medical and l~ orescent Sensors and
Applicaticns 223-~6 (1992). One metllod involves tlle use of a pair of optical fibers tllat are
positiolled close together in an approxilllately parallel mallller~ One of the fibers is d source fiber,
tllrougll ~llicll ligllt energy is conducted. Tlle olher Flber is a detection fiber connected to a
pllo~osens tive diode. Whell li~ht is conducted tllrougll tlle source fiber, a portioll of tlle ligllt
encrgy,~lluevallcscelltwavc,isl~lcselltalll~esulraceof~l~efiberalldapoilionortl~isligl~teller~y
is collected by tlle detection fiber. lhe detection fiber conducts the captured evanescentwave
enelgy to the pllotosel1sitive diode wllich measllres it. Tlle fibers are treated witll a binder to
altract and bind tl~e analyte tllat is to be measured. As analyte molecules bind to tl1e surrace (sucll
as tlle ana yte glucose bindil1g to immobilized lectins sucll as concal1avalil1 A, or to immobilized
alnti-glllcose antibodies) tllc amoullt of evanescellt wave couplinL~ between tlle two ribers is
cllanged and tlle amoullt of energy captured by tlle detection filber and measurecl by tlle diode is
2 5 challged ai well. Several measurelllellts of detected evanescellt wave energy over sllort p eriods
of time SUppOlt a rapid determinaltioll of the parameters describing tlle equilibriuln curve, tllus
makil1g pessil)le calculation of the concel1tratiol1 of tlte analyte witlfin the body. Tlle experimenlal
results sllowillg meas1ll-able llux willlin 5 minutes (I IGS. 3A and 3B of U.S. ratent No.
5,4~5,GII~ Witll this system suggest sufrlcient data for an accurate final reading are collected
3 0 witllin 5 m in1ltes.
In its most basic embodiment, a device tllat can be utilized for tlle application of sonic
energy and collectioll of analyte comprises an absorbellt pad, eitller of natural or syllllletic material,
wllicll ser/es as a reservoir for the cllemical enllancer, if used, and for receiving tlle analyte from
tlle skin s lrface. Tl~e pad or reservoir is lleld in place, eitller passively or aided by apl)l("),;dt~
3 5 rastenillg aleans, sucll as a strap or adllesive tape, on tlle selected area of skin surface.
~n sonic energy transducer is positiolled sucll that the pad or reservoir is between tlle skin
surface and tlle trans(lllcer, and lleld in plalce by app,.)p, idte means. A power supply is coupled to
llle transd.lcer and activated by switcll mealls or any otller suitable mecllallisltl. Tlle transducer
is activat~ d to deliver sonic energy mod11lated in fi~ el,~;y, pbase or intensity, as desired, to
4 0 deliver tlle cllemical enllallcer, if used, fi om tlle reservoir tluougll the skin surrace followed by
collection of the analyte from tlle skin surrace into tlle reservoir. Afler tlle desired fixed or
-- 21 99002
59
variable ti~ne period, tl~e trangducel is deactivated. 'I'l-e pad or reservoir, now containillg tlle
analyte of intel est, can be removed to qualltitate tlle analyte, for example, by a laboratory utilizillg
nny numl:er Or conventiollal chemical analyses, or by a portable device. Alternalely, tlle
mecllallisl 1 for quantitatitlg tlle analyte can bc build illtO the device used for collection of tlle
analyte, eilller as an inleglal pOI(iOII of Ille devicc or as an atlaclllllellt. Deviccs ror mollilolin6
an an.llyte are described in IJ.S. Palellt No. 5,~58,140, wllicll is incorporated llerein by referel~ce.
Example Z4
A n alternate metllod for detection of an analyte, sucll as glucose, following the sample
collection tl~rougll tlle pora~ed skin surface as described above, can be acllieved througll tlle use
Or enzyl~ tic mealls. Several enzymatic melllods exist for the measurement of glucose in a
biological sample. One melllod involves oxidizing glucose in tlle sample witll glucose oxiclase to
generate ~luconolactone and hydll3~ell peroxide. Ill tlle presence of a colorless chromogen, tlle
l~ydrogen ~eroxidc is thcll convcr~cd by l~croxidase to water and a colored prodllct.
Glucose Oxidase
2 0 C lucose ~ Gluconolactone + 1~2~2
2 1~2~2 I cllrolnogell ~ 1120 + colored product
'Ille inlellsity of tl~e colored product will be proportional to the amoullt of glucose in the fluid.
Tllis COIOI can be determilled thl ough tlle use of conventional al,soll,a--ce or reflectance metllods.
~3y calibr~ltion ~villl known concentrations of glucose, tlle amount of color can be used to
determine tlle concentration of glucose in tlle collected analyte By testing to determine tlle
3 0 relationsll p, one can calculate tlle concentratioll of glucose in tlle blood of tlle subject. Tllis
informati~ n can tllen be used in tlle same way tl~at tlle informatioll obtained from a blood glucose
test from I finger r~ullctllle is use~3. Results can be available within five to ten minlltes.
Example 25
3 5 ~ ny system using a visual display or readout of glucose concentration will indicate to a
diagnosticiall or patiellt tlle need for admillislration of insulitt or olher app. up. ;ale tnedicatiom In
critical care or otller silualions wllere conslant monitorillg is desired and corre(:live action nee(ls
to be taketl almost concurrently, tlle disl~lay may be connecli-l witll a~ <,l,.iale signal means
wllicll tri~gers llle admitlistration of insulin or olller medicatioll in an al~l)lul).;nle mallller. I;or
-- 2 ~ 9qO~Z
exaltlplc~ tl~ere are insulill puml~s wllich are implanted hlto tlle peritoneum or other body cavity
wllicll can be activa~ed in respollse to external or internal stimuli. Alternatively, utilizing the
enllanced transdemlal flux rates possible with micro-poration of tlle stratum corneum and other
techniques described in this invelltion, an insulill delivery system could be implemented
transdermc:lly, witll control of the flux rates
modulated by the signal from the glucose sensing system. In this manner a complete biomedical
control sy:;tem can be available wllicll not only monitors andtor diagllO5Cs a medical need but
shllultaneously provicles corrective actioln
Biomedical control systems of a similar nature could be provided in other situatiolls sucll
as maintai ling correct electrolyte balallces or admillislerillg analgesics in response to a measured
al~alyte pa ameter sucll as prostaglalldills.
Example 26
Similar to audible sound, sonic waves can ulldergo reflection, refraction, and absorplion
whell tlley encoullter anotller mediulll witll di~gimil:lr propelties [D. Bommallllall et al., 9 rllarm.
2 0 Res. 559 ( 1992)]. Rellectors or lenses may be used to focus or o~herwise control the dislributioll
of sonic energy in a tissue of intelest. I~or many locations on the human body, a rold of flesh can
be round 1O support tllis syslem. I~or examl~le, an earlobe is a convelliellt localioll wllicll would
allow use of a rerlector or lens to assist in exerting directional conlrol (e.g., "pushillg" of analytes
or permeallts tln-ougll tlle porated strallllll corneuln) similar to wllat is realized by cl-z~nging SOlliC
freqllellcy and intensity.
Example 27
l~ultiple sonic energy b-ansducers may be used to selectively direct the direction of
transdermal flux tllrough porated stralulll corneum eitller into tlle body or fronl llle body. A fold
of skin such as an earlobe allow transducels to be located on either side of the fold. The
tl ~nsJuce s may be ene~ ;d selectively or in a pllased fasllion to enllance ll d~-sdc;- ---al flux ill tlle
desired directioll. An array of transducels or an acouslic circuit may be con~l..l~,led to use phased
~ 21 990~2
61
array concepts, similar to those developed for radar and microwavc communications systems, to
direct and focus the sonic energy into tlle area of interest.
Example 28
It tlIis example, tlle procedure of Example 19 is followed with tlle exception tllat tlle
lleat-separated epidermis samples are first treated with Qll excimer laser (e.g. model EMG/200 of
Lambda P lysik; 193 nm wavelength, 14 ns pulsewidtl~) to ablate the stratultt corneum accordin~
to tlle procedllre descril~ed in U.S. ralenl No. 4,775,361, llereby incorporated by rererence.
Example 29
In tllis exarmple, tlle procedure of Example Ig is followed with the exception tllat tlle
heat-separ~ted epidermis samples are rlrst treated witll l,l'-dietllyl-4,4'-carbocyanine iodide
(~Idrich, h m~X=703 nm) and then a total of 70 mJ/cm2/50 ms is delivered to the dye-treated sample
with a moiiel TOLD9150 diode laser (Toslliba America Electronic, 30 mW at 690 nm) to ablate
the stratulll corneum.
Example 30
In this example, llle procedure of Example 29 is followed willl the exceplion lllat llle dye
is indocya line green (Sigtlla cat. no. 1-2633; 1~ma~=775 nlIl) and the laser is a model Diolite 800-50
(LiCONi~, 50 lilW at 780 nm).
Example 3 1
In this example, tlle procedure of Example 29 is followed with the exception tbat tlle dye
is metllylene blue and the laser is a model SDL-8630 (SDL Inc.; 500 mW at 670 nm).
3 0 Exalnple 32
Il. this example, tlle procedure of Example 29 is followed witll the exception that tlle dye
is containcd in a solution comprisillg a pclllleatiolI enlIallcer~ e.g. CEI.
~ 21 99~02
62
Example 33
Ir this example, the proce~lure of Example 29 is followed with the exception that the dye
and enllan~er-containillgsolutioll are delivered to the stralum comeum witll the aid of exposure
to ~ rasomld.
Example 34
Ir tllis example, tlle procedllre of Example 31 is followed witll lhe exceptioll tllat tlle
pulsed li~llt soul-ce is a sholt arc laml7 emillill~ over tlle broad ran~e of 400 to 1100 nm but
having a b lndpass fillter placed in the system to limit the output to the ~ vt;lell~;tll region of about
650 to 70(3 Illll.
Example 35
11l this example, tlle procedure Or Example 19 is followed with tlle exception that tlle
heat-separated epidermis samples are first punctured ~vitll a microlancet (Becton l~ickhlson)
calibrated to produce a micropole ill tlle stratum comeum witllout reaclling the ullderlyillg tissue.
Example 36
In tllis example, the procedure Or rxample 19 is followed witll tlle exception tllat the
lleat-separated epidelmis samples are first treated witll focused sonic energy in the range of 70-480
mJ/cm7/50 ms to al~late tlle stratllm conleulll.
Example 37
11l tllis example, the procedure of Example 19 is followed with the exception that tlle
stratum corlleum is first punctured hydlalllically wilh a higll pressure jet of fluid to form a
miclopore of up lo about 100 ~lltl diameter.
21 99002
63
Example 38
Ir this example, the procedure of ~xample 19 is followed with the exception that the
stratum carneum is first punchlred with shol-t pulses of electricity to fonn a micropore of up to
about 100 lul1 diatmeter.
Example 39
Acoustic ~'treamillg
A new mechal1ism and applicaliol1 of sonic energy in tlle delivering of therapeulic
.cuh5~.1c~q into the body and/or harvesting fluids rrom withill the body into an external reservoir
througl1 n icro-poratiolls fonned in tlle stratum con~euln layer will now be described. An
additional aspect of (llis inventiol1 is tlle utilizaliol1 of sonic energy to create an acouslic streaming
effect on the fluids IlOWillg around and between ~he intact cells in the epidermis and dermis of the
l1uman skia. Acoustic streaming is a well documented mode by whicl1 sonic energy can interact
~vith a nui~l medium. Nyborg, Pllysical Acouslics Principles and Methods, p. 265-331, Vol ll-rart
B, Acadenlic rress, 1965. The first theoretical analysis of acoustic streaming pllenolnenoll was
given by ~ayleigh (1884, 1945). In an extensive treatnlent of the subject, Longllet-lliggills
( 1953-196 )) has given a result applicable to two dimensional flow that results hl the near vicinity
of any vib ating cylindrical surrace. A lhree dill1ellsiol1al approximation ror an arbilrary surrace
was developed by Nyborg (1958). As described by l~airbanks et al., 1975 Vltrasonics Symposium
Proceedings, IEEE Cat. #75, Cl 10 994-4SU, sonic energy, and the acoustic stream hlg phenomellon
can be of great utility in acceletatillg the fl1lx of a fiuid througll a porous mediulll, showillg
measurable increases in the flux rates by up to 50 times tllat possible passively or with only
pressure gradients being applied.
All previous transdermal delivery or extraction efforts utilizing ultrasound have focused
on metllod~ of inleracliol1 be1wecn llle sonic energy and the skin tissues designed to permeabilize
3 0 tlle stratum comeum layer. The exact mode of hlteraction involved has
been hypoll1esized to be due exclusively to the local elevation of the t.,...l,e.i.t~..e in tlle SC layer,
and the resultant melting of the lip~d domaills in the intercellular spaces between the corneocytes.
-- 21 990l~2
64
Srillivasan et al. Olller researcllers llave suggesled that miclo-cavitations and or sllearillg of llle
struclures i n the strat~ corneum opens up cllamlels tllrough which fluids may flow more readily.
In general, tlle design of the sonic systems for tlle enllancci.,n,"l of l.dnsde~ al flux rates has been
based on lle early reali~alion tllat tlle applicalioll of an existing tllerapeutic ultrasoulld Ullit
designed to produce a "deep-lleating" effect on the subject, wllen used in conjullction witll a topical
applicaliots of a gelled or liquid preparalion containillg tlle drllg to be delivered inlo llle body,
could produce a qualltifiable increase in the ~lux rate of tlle drug into tbe body. in llle conlext of
tlle metllod taugllt hereill to create micro-pores in tllis barrier layer, the use of sonic energy Inay
now be thougllt of in a totally new and different sense tllan tlle classically defined concepts of
sonopllore~is.
133sed on tlle experinlelltal discovery mentiolled in U.S. palent5 5,458,140 nnd 5,445,61 1
tllat wllen a small llole existed or was created in tlle stratum comeum (SC) in tlle Franz cells used
in tlle in vltro studies, tllat ttle application of an appropriately driven ultrasonic transducer to tlle
fluid reser.~oir on eitller side of the porated SC sample, an "acoustic streamillg" event could be
generated wllerein large ilux rates of fluid wllere capable of being pumped tllrougll tllis porated
2 0 membrane.
U'itll the metllod taugbt llerein to create the controlled micro-porations in tlle stratum
corlleutll layer in tlle living subject's skin, llle application of the fluid streamillg mode of
sollic/fluid interaclioll (o tlle inductioll of fluid into or out Or tlle body may now be practically
exploled I:or example, clinical studies llave sllown Illat by making a series of rour 80 llm
diameter r licro-poles in a ~100 ~ squale, and lllen applying a mild (10 to 12 inclles oF 11~)
suction to .llis area, an average of abouit I ~11 of interstitial fluid can be induced to leave tlle body
for extem~.l collection in an extemal cllamber. By adding a small, low power sonic transducer to
tllis systerl, configured such tllat it actively generates inwardly convergillg concentric circular
pressllre waYes in tlle 2 to 6 mm of tissue surrollllding tlle poration site, it llas been demonstrated
3 0 tllat tllis l',F flux rate can be increased by 50%.
By relieving ourselves of tlle desire to create some form of direct absprption of sonic
energy in :lle skin tissues (as requiled to generate heatillg), frequellcies of sonic energy can be
., .. _~,. .. .
' ~ 219~002
dctenllined for which the skhl tissues are virtually ll u~ l,a,c;n1 that is at the very low rrequellcy
region of I k~ Iz to 500 Kl Iz. Even at some of tlle lowest frequencies tested signiFIcant acoustic
streal11illg effects could be observed by USillg a micro-scope to watch an in vivo test wllerehl the
subject s s~;in was micro-porated and ISF was in(31lced to e:l~it tl~e body an pool on lhe SUI face oF
tlle slcin. ~1lergizing llle sonic trallsdllcer sl~owed dramatic visual indicalions of ~l~e amount of
acoustic slreamillg as small pieces of partic1llate matter were carried along witll the IS~ as it
swirled ab~ut. Typical ma~nit1lde of molion exhibited can be described as follows: for a 3 mm
diameler circular pool of ISl~ on tlle surrace of lhe skin a single visual particle could be seen to
be con~plelillg roughly 3 complete orbits per second. This equates to a linear fluid velocity of
more tban 2.5 mJll/secolld. All of lhis action was demollstrated wilh sonic power levels into tlle
tissues of ess lh;lll t00 111W/CI112.
~ ~ hile one can easily view the top surface of the skin and the fluidic activity thereon
~c~cein~ wl1at is takhlg place dynamically witllin tlle skill tissue layers in response to the coupling
into these issues of sonic energy is mucll more dimcult. One can assume that if sucl~ Iarge fluid
velocities I e.g. >2.5 mm/S) may be so easily induced on tlle sur~ace then sotne noticeable increase
in tlle flui I llOw in ~he inlercellulal chanl1els presen~ in the viable derrnal tissues could also be
realized in respollse to this sonic e nergy inp1lt. Currently an increase in harvested IS~ througll
a giYel1 set of micropolatiolls wll-m a low fiequency sonic energy was applied to llle area in a
circle surn~ulldil1g the poration sites has been quantified. In this ~ ,t;.hll ul an ISl~ hal e..l;ng
techllique based solely on a mild suction (10 to 12 inches of l-IG) was altemated witll using the
exact sam appalalus but willl Ihc sonic l~ lslucer engaged. Over a series of 10 lwo-millu1e
halvestil1g periods five with mere suctioll and five with both suction and sonic energy active it
was observed tllat by activating the sonic source roughly 50% more ISr; was collectable hl the
same time period. l hese data are sl1own in FIG. 30. This increase in ISF flux rate was realized
witll no n:ported increase in sensatioll from ~lle test subject due to the sonic energy. Tlle
3 0 at)~a.dtu;~ used for this experiment ;s illustrated in l;IGS. 31-33. The tlnnsducel assen1bly in ~IGS.
31-33 is colllprised of a tllich walled cylh1der of piezo-electricmaterial with an internal diameter
of rougl1ly 8 mlll and a wall thicklless oF 4 mm. Tlle cylinder has been polarized sucll that whell
66 2 ~ q9~02
an electri~ al field is applied across the metalized surlàces oF the outer diameter an~l inner diameter,
tlle thicklI~ss of the wall of the cyf inder expands or contracts in response to the field polarity. In
practice, t liS configIlratioll results hl a device whicll rapidly squeezes the tissue wllicll llas been
suctiolled into tlle centlal llole, causillg an hlwald radial acoustic streamillg erfect on tllose tluids
presellt in these tissues. Tllis inward acoustic streamilI~ is responsible for brillging more ISI~ to
tlle locatioll of the micro-poratiolls in the center of the hole, where it can leave the body for
external collectioll.
~ similar device showll in I~IG. 34A-B was built and tested and produced shlIilar initial
results. Ir the FIG. 34A-B version, an ultrasollic Ir~til;,duc~l built by Zevex, Inc. Salt Lake City,
Utall, was modiried by l~avhlg a spatulate extension added to tl-e SOlliC horll. A ~ mm hole was
placed in Ille 0.5 ml7I tllick spatulate end of tllis extension. wlten activated, tlle prillciple motion
is longitIulillal along the lengtll of tl~e spatula, resultillg in essentially a rapid back and forth
motioll. l'he physical perlurbatioll oF the metalic spatula casued by the placement of the ~ mlll
hole, resulls in a very active, but chaotic, large displacement behavior at this point. In use, the
skin of tlle subject was suctioned up into this hole, and tlle sonic energy was then cunductined into
tlle skin in a rashion shllilar to tllat illustrated in liIG. 33.
Tile novel aspect of this new application of ultrasowld lies in the followhIg basic areas:
1. T he fiulction Or the ultrasound is no longer needed to be focused on pern~eabilizillg
the SC balrier meltIblalle as tallgllt by Langer, Kost, l~olIlm~ln~ l and otllers.
2. A much lower frequency system can be utilized which has very little absorptioll in the
2 5 skhl tissue " yet can still create the lluidic streamillg phellomenon dcsired wilhin the intercelllllar
passageways between the epiderllIal cells whicll contahl tlle hltel~Iilidl fluid.
3. The mode of hltelactiorl with the tissues and tluids therein, is the so-called "streaming"
mode, recognized in the sonic literature as a unique and different mode than tlle classical
~ibrational interactions capable of sllearing cell membratles and accelerating the passive diffilsion
3 O process.
B ~ optimizhlg the geometric configuration, frequency, power and modulatiolls applied to
the SOlliC t-ansducer, it has been showll that significant hIcreases hl the fluid flux thlollgll the
67 2 t qq302
porated skin siles can be achieved. The optimization of lhese parameters is designed to exploit
the non-linearities governillg lh~ ftukt llow relationships in tl~is mic~uscupically scaled
environme nt. Using rrequencies under 200 kl tz, large ftuidic effects can be observed, without any
detectable l,leatillg or olhemlegative lissue hlleractiolls. T he sonic power levels required to produce
these measurable effects are very low, with average power levels typically under lO0
ntilliwatts, cm2.
T lerefore, tlle above examples are but . t,l,. ~ i,e.llative of systems whicll may be employed
in the uti ization of ultrasound or ultrasound and chemical elllldllcel:i in the collection and
quantificalion of analytes for diagnostic purposes and for ~he l-ansde-l--al delivery of permeants.
l hc invell ion is direcled to thc discovcly lhal lhe poratioll of thc stratuln cotneulll rollowed l~y
tlle proper use of ultràsound, particularly wl~en accompanied with tlle use of chemical enhancers,
enables tl~u noninvasive or minimzllly h~vasive transdermal determination of analytes or delivery
of pertnea tts. }lowever, the inventiûlt is not limited only to tlte specific illustrations. There are
nlllnerous poration techniqlles an(l enllaltcer systems, some of which may functiolt belter tltan
another, for deteclion and wilhdrawn of certain analytes or delivery of permeants throllgh the
slratulll corlleulll. Ilowever, withill the guidelines plt s~ ed herein, a certain amount of
expcrhllenlalioll lo ol)laill optimal poralioll, enllallcers, or oplhllal thne, inlellsily and rrequency
of appliec ullrasoulld, as well as modulation of r,~ .-.n.cy, amplitude and pllase of applied
ultrasound can be readily carried out by those skilled hl the art. Therefore, the inventioll is limited
hl scope only by the followhlg claims and rullctiollal equivalellts thereof.