Note: Descriptions are shown in the official language in which they were submitted.
CA 02199344 2004-09-03 57' 9/pI(l 9
SPECIFICATION
TITLE
OPTICAL UNIT AND ELECTROLYTIC SOLUTION
BACKGROUND OF THE INVENTION
The present invention relates to optical units, for example, units for
displaying figures or characters or units for a X-Y matrix displaying or a
filter
capable of controlling transmittances in visible light (within a wavelength
range of
400,to 700nm), and also relates to electrolytic solutions used in such optical
units.
DESCRIPTION OF THE RELATED ART
Conventionally, electrochromic materials (hereinafter referred to as EC
materials) are used for displays of voltage drive type, for example, displays
for
watches which digitally display time.
Since electrochromic devices (hereinafter, referred to as ECDs) are of
non-luminescent type and utilize reflected light or transmitted light for
displaying,
Ahey have some advantages such as reduced fatigue even after viewing for long
periods, and lower electrical-power demand with a relatively low driving
voltage.
Actually, an ECD such as that disclosed in Japanese Unexamined Patent
Publication
No. 59-24879 is known as liquid-type one including a viologen molecule
derivative
used as the EC material which is an organic molecule capable of reversibly
generating a colored and a colorless state.
In response to the development of precision optical instruments, there is an
increasing demand for fine and low power type devices which control the
quantity
of light, as substitutes for conventional variable ND (Neutral Density)
filters. Under such
circumstances, it is increasingly necessary to investigate whether or not ECDs
as
described above and related techniques can be utilized for such devices.
'1
2 199 34 4
ECDs constituted with EC materials such as viologen molecule derivatives
are, however, rarely practically used since they are insufficient in response
speed and
degree of light-screening in view of practical use.
Due to this, many investigations were focused on, instead of ECDs,
reflection-type light-control devices which utilize deposition/dissolution of
metal
salts, and were conducted to develop electrochemical light-control devices
which
utilize deposition/dissolution of silver.
Although such electrochemical light-control devices satisfy the required
response speed and degree of light-screening, the transparent electrodes which
constitute the substrate readily deteriorate, and therefore, the life spans of
the devices
are short.
In particular, with indium tin oxide (ITO) electrodes, breakage readily occurs
due to an over-voltage impressed for deposition/dissolution of silver.
SUMMARY OF THE INVENTION
Accordingly, the object of the present invention is to provide an optical unit
which can be driven with a reduced over-voltage upon the electrodes therein
and has
an extended life span; and an electrolytic solution used in the optical unit.
For
achieving this object, the present invention utilizes, as an appropriate
electrolytic
solution, a solution having no absorptivity in visible light (within a
wavelength range
of 400 to 700 nm), and utilizes, as the material for an electrochemical light-
control
device, a complex silver salt which can equally screen visible light during a
colored
state of the optical unit.
The Inventors obtained a light-control device comprising an electrochemical
material by incorporating with a nonaqueous and reversible system in which
silver
from a complex silver salt can be deposited on or dissolved from the electrode
(this
event is referred to as deposition/dissolution). As a result, the Inventors
achieved a
stable optical unit, as well as an electrolytic solution to be used therein,
which can
be driven with low electrical power, can control transmittance of visible
light,
2
~3 4 4
exhibits excellent spectroscopic properties, and has a reduced possibility of
electrode
breakage, thus accomplishing the present invention.
Specifically, an aspect of the present invention is an optical unit comprising
a pair of opposing electrodes and an electrolytic solution comprising a silver
salt
solution which is disposed between the opposing electrodes such that
deposition/dissolution of silver is caused by drive-controlling these
electrodes,
wherein said electrolytic solution further contains a metal other than silver,
and silver
is co-deposited with the metal other than silver.
Additionally, another aspect of the present invention is an electrolytic
solution comprising the above-mentioned silver salt solution and the
above-mentioned metal other than silver.
Hitherto, as described above, the life spans of transparent electrodes used as
substrates were short due to deterioration of the electrodes during repeated
drive,
even though an electrolytic solution which is substantially satisfactory for
practical
use could be obtained. According to the present invention, the over-voltage
for
dissolving a deposited silver layer can be successfully reduced by co-
depositing
silver with a metal other than silver, for example, copper from a copper salt.
Due to
this, the over-voltage for the deposition/dissolution of silver can be
reduced, and the
life spans of the electrodes can be practically extended since the over-
voltage is an
important factor relating to life span.
As mentioned above, according to the present invention, the over-voltage for
dissolving a deposited silver layer can be reduced to prevent electrodes,
particularly
ITO transparent electrodes, from deteriorating. Actually, life spans of 10 to
40 times
that of a case using a conventional electrolytic solution (a Cu-free system)
have been
achieved in life tests using ITO electrodes.
To successfully obtain such an effect, the amount of the above-described
metal other than silver to be contained is suitably 0.1 to 100 mmol/liter in
the form
of a metal salt.
~
Meanwhile, as to deposition of silver from a complex silver salt, cyan
solutions used in plating baths are well known. The cyan solutions, however,
cause
some problems in relation to securing a safe working environment and disposal
of
waste fluids. Accordingly, the Inventors focused their interest on silver
salts of
non-cyan type, and conducted investigations on them.
In the investigations, various electrolytic solutions of complex silver salts
were tested with the addition of reductants, and systems having excellent
reversibility
were obtained by incorporating with solutions selected from among the
solutions
tested. The materials used in these systems, which are named as RED
(Reversible
Electro Deposition) materials, were dissolved in solvents to prepare RED
solutions.
The RED solutions (electrolytic solutions) examined up to the present were
solutions prepared using silver iodide as a halogenated silver, ascorbic acid
as a
reductant for improving reversibility, and dimethylsulfoxide (DMSO) as a
nonaqueous solvent. The solutions using silver iodide, however, have some
drawbacks. For example, in some cases, such solutions will be stained and
degraded
due to generation of iodine. during dissolution of silver. Further, image
information
in the light-screening state is frequently blurred due to deterioration in the
spectroscopic properties of the silver layer to be deposited, namely, uneven
absorptivities in visible light.
Such drawbacks may be attributed to the standard oxidation reduction
potential of iodide which is lower than those of bromine and chlorine, as
shown
below.
I, + 2e - 21- (0.536 V)
Br, + 2e - 2Br (1.065 V)
Cl, + 2e- - 2C1- (1.360 V)
(based on hydrogen)
Accordingly, in the present invention, the inventors find out that the silver
salt to be used is preferably silver bromide which has a relatively higher
standard
oxidation reduction potential. By using silver bromide, the above-mentioned
reaction gas, which has a staining effect and is generated as a by-product
during
4
dissolution of silver, can be reduced, and a system in which the silver layer
to be
deposited has even absorptivities in visible light can be obtained.
Accordingly, a
system in which the silver layer to be deposited exhibits excellent
spectroscopic
properties can be achieved, and deterioration of optical information in the
light-screening state due to irregularity of color can be effectively
prevented.
As a matter of course, RED solutions according to the present invention have
no absorptivities in visible light (within a wavelength range of 400 nm to 700
nm)
at preparation, and preferably, the solutions is prepared using a complex salt
of silver
bromide with which an even light-screening of visible light can be achieved in
the
colored state. Further, a complex salt of silver bromide is readily reversibly
deposited/dissolved by drive-controlling the electrodes.
As described above, a specific reversible system, in which silver from a
silver
salt, preferably from silver bromide is deposited and dissolved, is used in
the present
invention. As a result, the present invention can provide an optical unit such
as a
display unit or an optical filter which is of non-luminescent type, can be
driven with
a low power, and is suitably applicable for uses in relation to visible light.
In the optical unit of the present invention, the electrolytic solution to be
used
is preferably not stained due to dissolution of a metal other than silver.
As the metal other than silver, copper is preferably used, and the copper may
be contained in the solution as copper halide such as copper chloride or
copper
bromide.
If the solution is stained due to dissolving copper halide, the transparency
of
the solution should preferably be maintained with a clarifier such as a
complexing
agent or a reductant. In other words, although organic solutions will usually
have
absorptivities in visible light when copper halide is dissolved in them, which
is
undesirable for the unit of the present invention to be used in an optical
system, such
absorption by organic solutions in visible light can be sufficiently prevented
by
complexing or reducing the dissolved copper salt with a clarifier such as
triethanolamine so as to cause the following reaction as in the present
invention.
Cu-+ (colored) + e -> Cu+ (colorless)
5
,~n~ ~~ ~ ~ =A ~ 'F -1+ S
In general, the above-mentioned clarifier may be a complexing agent or a
reductant, and may comprise at least one compound selected from the group
consisting of triethanolamine, iminodiacetic acid, trans- 1,
2-cyclohexanediaminetetraacetic acid, nitrilotriacetic acid, galactitol,
ascorbic acid,
dimethylamineborane, trimethylamineborane, tetrabutylammonium borate,
triethanolamiine borate, N, N, N', N'-tetrakis (2-hydroxypropyl)
ethylenediamine,
ethylenediamine-N, N, N', N'-tetraacetic acid, salicylic acid,
2-meracaptobenzoimidazole, 1-allyl-2-thiourea, thiouracil, and
dimethylthioformamide.
Among the above-listed compounds for the above-mentioned clarifier, typical
examples of compounds acting as complexing agents are triethanolamine,
iminodiacetic acid, trans-1, 2-cyclohexanediaminetetraacetic acid,
nitrilotriacetic
acid, and galactitol. On the other hand, typical examples of compounds acting
as
reductants are ascorbic acid, dimethylamineborane, trimethylamineborane, and
tetrabutylammonium borate.
In the present invention, the optical unit may comprise a pair of opposing
electrodes, at least one of which should be in charge of
deposition/dissolution of
silver, and an electrolytic solution which comprises a solvent and a silver
salt
dissolved therein, and is disposed between the opposing electrodes in contact
with
these electrodes.
Preferably, an electrolytic solution in which silver bromide is dissolved in
water or a nonaqueous solvent, particularly a nonaqueous solvent, should be
disposed
such that a colored state and a colorless state of the optical unit can be
generated
according to deposition/dissolution of silver.
In such a case, the electrolytic solution to be used is preferably a RED
solution containing silver bromide at a concentration of 0.03 to 2.0
mol/liter, and
more preferably, 0.05 to 2.0 mol/liter.
6
4 3
Further, at least one brightener, at least one complexing agent and/or at
least
one reductant is preferably added to the solution.
The brightener may be selected from the group consisting of thiourea,
1-allyl-2-thiourea, mercaptobenzimidazole, and coumarin.
Further, the complexing agent may be selected from the group consisting of
phthalic acid, succinic acid, salicylic acid, and glycollic acid.
Moreover, the reductant may be selected from ascorbic acid,
dimethylamineborane (DMAB), trimethylamineborane (TMAB), tartaric acid, oxalic
acid, and D-glucono-1,5-lactone.
The system to be used in the present invention should preferably be
incorporated with a RED solution having high solvency for a silver salt, and
in
addition, the system should preferably be prepared to have high reversibility
by
adding at least one reductant. Concerning RED solutions, many investigations
were
focused on the use of ascorbic acid as the reductant, and the use of, as the
solvent, a
nonaqueous single solvent which consists of dimethylsulfoxide (DMSO). Such RED
solutions, however, have some problems in their characteristics at low
temperature
since the freezing point of DMSO itself is as high as 18C . As a result, for
example,
such RED solutions readily freeze during use in cold districts. For this
reason, the
solvents which could be used were limited.
Under such circumstances, the Inventors selected systems from nonaqueous
systems in which silver from a complex silver salt can reversibly be deposited
on or
dissolved from a transparent electrode, and which are incorporated with
solvents
having sufficiently low freezing points for use at low temperature without
deterioration in characteristics, and subsequently, the Inventors examined
reductants
in view of applicability to such systems.
As a result, the above-listed reductants such as DMAB and TMAB could be
recognized as reductants that were previously not examined but are applicable
as
solvents having low freezing points. The above-listed reductants such as DMAB
and
TMAB can sufficiently be used together with solvents each having a freezing
point
low enough for improving low temperature characteristics of the electrolytic
solution
7
CA 02199344 2004-09-03
to be obtained, and these reductants can be dissolved in such solvents more
readily
than ascorbic acid. By using such reductants, the freezing point of the
electrolytic
solution to be obtained is lowered, and therefore, the electrolytic solution
will not
freeze during use in cold districts. The concentration of the reductant is
preferably
within a range of 1/150 to I times that of the silver salt.
Preferable examples of solvents having low freezing points may be
nonaqueous solvents comprising at least one solvent compound selected from the
group consisting of dimethylformamide (DMF), diethylformamide (DEF),
N,N=-dimethylacetamide (DMAA), N-methylpropionic acid amide (MPA),
N-methylpyrrolidone (MP), propylene carbonate (PC), acetonitrile (AN),
2-methoxyethanol (MEOH), and 2-ethoxyethanol (EEOH).
Each of these nonaqueous solvents has a freezing point lower than that of
DMSO. Particularly, each of DMF, DEF, MEOH, and EEOH has a freezing point
70C or more lower than that of DMSO. A RED solution comprising such a solvent
and a silver salt, especially silver bromide dissolved therein has excellent
characteristics at low temperature, and sufficient applicability for use in
cold
districts.
Additionally, the RED solution may preferably further contain a supporting
salt within a range of 1/2 to 5 times the concentration of silver bromide for
the
purpose of enhancing the conductivity of the solution.
Further, silver bromide in the solution is preferably converted into a complex
silver salt by adding a supporting electrolyte which can be a resource of
halogen such
as bromine for the purpose of enhancing the conductivity Qf the RED solution
and
promoting dissolution of silver bromide. Examples of such an electrolyte are
sodium
bromide, potassium bromide, and quaternary-ammonium bromide.
Such a supporting electrolyte is added preferably within a range of 1/2 to 5
times the concentration of silver bromide.
Moreover, the deposition potential of silver onto transparent electrodes can
be reduced by a chemical or physical modification to transparent electrodes
(particularly, ITO electrodes whibh are prepared by doping indium oxide with
tin)
=8
4 4
used as working electrodes where silver is deposited/dissolved. Due to this,
silver
can readily be deposited/dissolved, and electrical damage upon the transparent
electrodes and the electrolytic solution itself can be reduced.
As such a chemical modification to ITO electrodes, a surface treatment such
as chemical plating with palladium or the like is preferably performed by
employing,
for example, a two solution method with a tin solution and a palladium
solution.
This modification treatment is, namely, surface-activating treatment with
palladium
for ITO electrodes, and ITO electrodes having a high surface activity can be
achieved
by depositing nuclei of palladium on the substrates consisting solely of ITO.
In the above treatment, the tin solution may be prepared by dissolving 0.10
to 1.0 g of tin chloride (SnC1,) in 1 liter of a HCl solution having a
concentration of
0.010 to 0.10%, and the palladium solution may be prepared by dissolving 0.10
to
1.0 g of palladium chloride (PdClz) in 1 liter of a HCl solution having a
concentration
of 0.010 to 0.10%.
On the other hand, as a physical modification, a metal less oxidizable than
silver may be vapor-deposited on ITO electrodes.
In the optical unit of the present invention; the electrolytic solution is
required
to have no absorptivities in visible light during a colorless state, and the
substrate
electrodes for generating a colored and a colorless states are preferably ITO
electrodes having no absorptivities in visible light for proper operation of
the optical
unit.
As for driving the light-control device, current modulation is preferably
employed since the RED solution to be used in the device cannot be stirred
during
the repetition of generating the colored and colorless states due to the
smallness of
the device. With current modulation, the electrochemical deposition and
dissolution
of silver can be readily quantitatively controlled.
To obtain an improved colored/colorless response (speed of silver
deposition/dissolution), the driving method for generating the colored and
colorless
states with current modulation is preferably based on the use of a current
which
varies squarely from a high current value to a low current value.
Alternatively, a
9
y{ ~'y,lp,~t t
driving method may be based on the use of a current which varies squarely from
a
low current value to a high current value, while aiming at decreasing the
damage
upon the substrate electrodes by repetition of silver deposition and
dissolution.
When a driving method based on the use of a constant current is employed, the
voltage should preferably be controlled at the critical value for generation
of
by-products to maintain the balance of electrolytes in the system.
According to the present invention, the optical units to be obtained are
useful
as units for displaying figures or characters, as units for X-Y matrix
displaying, or
as optical filters capable of controlling transmittances in visible light
(within a
wavelength range of 400 to 700 nm).
Additionally, the present invention provides an electrolytic solution to be
used for such an optical unit, the electrolytic solution comprising a solvent,
and a
silver salt and a metal other than silver dissolved in the solvent.
Desirably, the electrolytic solution of the present invention comprises water
or a nonaqueous solvent, and a silver salt such as silver bromide dissolved in
the
solvent at a concentration of 0.03 to 2.0 mol/liter. The colored and colorless
states
are generated according to the deposition/dissolution of silver. According to
the
present invention, the electrolytic solution may further contain the above-
described
additives. As occasion demands, the electrolytic solution may further contain
brighteners, complexing agents, reductants, supporting electrolytes, and other
solvents in proper amounts.
These and other features of the invention are discussed in greater detail
below in the following detailed description of the presently preferred
embodiments
with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a diagram showing the C-V curve of an optical unit based on the
present invention.
Fig. 2 is a diagram showing the C-V curve of an optical unit as a referential
example.
Fig. 3 is a spectrum diagram showing the change in transmittance of an
optical unit based on the present invention in relation to the voltage
impressed during
the colored state.
Fig. 4 is a spectrum diagram showing the change in transmittance of,an
optical unit based on the present invention in relation to the voltage
impressed during
the colorless state.
Fig. 5 is a spectrum diagram showing the change in transmittance of an
optical unit as a referential example in relation to the voltage impressed
during the
colored state.
Fig. 6 is a spectruin diagram showing the change in transmittance of an
optical unit as a referential example in relation to the voltage impressed
during the
colorless state.
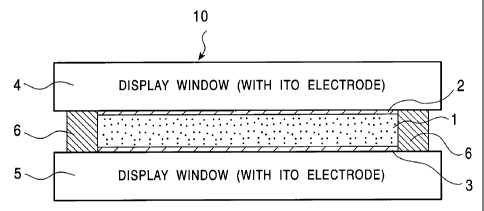
Fig. 7 is a schematic sectional view of an optical unit based on the present
invention.
Fig. 8 is a schematic perspective view of the optical unit shown in Fig. 7.
Fig. 9 is a schematic drawing showing the pattern of the ITO electrode in a
example of the optical unit based on the present invention.
Fig. 10 is a schematic sectional view of the optical unit shown in Fig. 9.
DETAILED DESCRIPTION OF THE
PRESENTLY PREFERRED EMBODIMENTS
The present invention will be further illustrated with an example below.
Initially, an example optical unit 10 used as a displaying unit or an optical
filter will be illustrated referring to Figs. 7 and R.
The optical unit 10 of this example comprises a pair of transparent substrates
4 and 5 such as glass plates which are disposed as display windows with a
pre-determined space, and which constitute a cell; and working electrodes 2
and 3
such as ITO electrodes which are disposed on the intemal surfaces of the
substrates
so as to oppositely face one another, and at least one of which is a electrode
for
11
4
generating a colored state or a colorless state. Though being shown only
schematically in the figures, these working electrodes actually have a pattern
specified depending on the purpose.
Further, a counter electrode 6 is provided so as to surround the entire
periphery of the substrates 4 and 5. The counter electrode serves as a spacer
as well,
and comprises, for example, a silver plate. Though not being shown in the
figures,
a reference electrode such as a silver wire is also provided.
Moreover, a RED solution 1 is disposed between the opposing working
electrodes 2 and 3 so as to be in contact with these electrodes, the RED
solution
comprising a nonaqueous solvent and RED materials, namely, a complex salt of
silver bromide and copper bromide or the like. One of the opposing working
electrodes 2 and 3 is set as an anode, and the other is set as a cathode. By
impressing
a driving voltage for a pre-determined time period between these electrodes to
cause
a direct current, an oxidation-reduction reaction according to the following
reaction
formula is caused in relation to the complex silver salt at the cathode.
Ag+ + e - Ag
Consequently, by the deposition of silver, the cathode turns to be colored
from being
transparent.
As a result of depositing silver on the electrode, a specific color such as a
reflected color can be observed through the display window, namely, the
obtained
unit can be a filter. This filter function according to generation of the
colored state,
namely transmittances in visible light or tones in the colored state, varies
depending
on the level of the voltage or the impressing time. By controlling these
parameters,
the optical unit can function as a transmittance-variable display unit or
filter.
In the optical unit 10, the opposing working electrodes 2 and 3 may be
disposed so as to cover the entire internal faces of the substrates.
Alternatively, the
electrodes can be composed of some portions such as shown in Figs. 9 and 10.
In detail, each of the opposing working ITO electrodes provided on the
transparent substrate 4 or 5 comprise a set of electrode portions, namely, a
central
portion 2a or 3a and ring portions 2b to 2e or 3b to 3e that are
concentrically
12
disposed around the central portion with a gap, respectively. Around the most
peripheral ring portions 2e and 3e, counter electrodes 6A and 6B are provided
to
compensate for the voltage.
These portions 2a and 3a, 2b and 3b, 2c and 3c, 2d and 3d, 2e and 3e, and
these counter electrodes 6A and 6B are respectively connected to driving
electrical
sources 8A to 8F through wires 9A to 9F each comprising a chromium thin wire
or
the like.
Further, the transparent substrates 4 and 5 are disposed so as to have a
pre-determined space between them with a spacer 7 (in Fig. 8, the counter
electrode
6 also functions as the spacer). The space is filled with a RED solution 1.
In the RED solution 1, the oxidation-reduction reaction, namely the
concentration, is controlled according to the level of the impressed voltage.
Accordingly, the quantities of silver from the RED solution deposited on the
above-described electrodes of the cathode side can be controlled by
controlling
voltages V 1 to V5 that are impressed between the portions 2a and 3a, 2b and
3b, 2c
and 3c, 2d and 3d, and 2e and 3e, respectively. Incidentally, a compensation
voltage
V6 is also impressed between the counter electrodes 6A and 6B.
When all the voltages are equally set, namely, V 1= V2 = V3 = V4 = V5, the
homogeneous colored state can be generated throughout the electrode portions
of the
cathode side, and the tone of the colored state can be homogeneously changed
by
controlling the voltages.
On the other hand, when the voltages are set differently, for example, V 1<
V2 < V3 < V4 < V5, the tone of the color generated on the electrode portions
gradually becomes deeper, namely, transmittance becomes smaller, from the
central
portion to the peripheral portions. This is useful, for example, as an optical
diaphragm used in a CCD (charge coupled device) in a television camera or the
like,
and can sufficiently cope with advance in CCD integration. When the voltage
levels
are set in reverse to the above order, the transmittance becomes larger from
the
central electrode to the peripheral electrodes.
13
3 4
Accordingly, in the optical unit provided with working electrodes each of
which comprises a set of separated electrode portions, the image pattern and
the
degree or tone of the colored state can be controlled by controlling the
voltages
impressed on the sets of separated electrode portions. As a result, the
optical unit can
also be used for various optical filters, namely, the applicability of the
optical unit
can be extended.
As illustrated above, this example is based on a concept quite different from
that of the prior art which is directed to conventional EC materials, and uses
a RED
material comprising silver bromide as a filter material for light-control in
the optical
unit. In the optical unit of this example, the tone of the colored state
generated with
the RED material can be varied by drive-controlling the opposing working
electrodes, especially by controlling the impressed voltages. By utilizing
this feature,
the display unit or optical filter to be obtained can be tone-variable.
Accordingly, by
using RED materials, there can be provided a filter which is satisfactorily
small, can
be driven with low electrical power, and has an ability as a light-control
device far
exceeding those of conventional variable ND filters which are driven in a
mechanical
manner.
Further, the RED used comprises a nonaqueous solvent such as DMF and
silver bromide as a RED material dissolved therein. Since the freezing point
of the
nonaqueous solvent is sufficiently low, the optical unit to be obtained can
possess
excellent characteristics at low temperature. This nonaqueous solvent can
satisfactorily dissolve, together with silver chloride, a reductant such as
DMAB to
be added to the RED material.
Moreover, since the RED solution further contains copper bromide, silver and
copper are co-deposited on ITO transparent electrodes. Due to such co-
deposition,
an excessive over-voltage is not impressed on the transparent electrodes, and
therefore, they incur less damage.
14
Next, the characteristics of the optical unit of the present invention will be
illustrated in detail with the following experimental examples. In the
experimental
examples, the optical units similar to those in the example shown in Figs. 7
and 8
were used.
Experimental Example 1: Evaluation of the Characteristics According to a
Cyclic Voltammetry (CV) Measurement Method
The purpose of this experimental example is to examine a system for
reversible deposition/dissolution of silver, and to evaluate
deposition/dissolution
characteristics of the system. In the system, silver bromide was used.
Dimethylformamide (DMF) was used as the solvent. The concentration of
silver bromide was set at 0.5 mol/liter. For the purpose of promoting
dissolution of
silver bromide and increasing conductivity, a quaternary anunonium salt tetra-
n-butyl
ammonium bromide (TBAB) was dissolved at 1.0 mol/liter. Additionally, thiourea
was dissolved as a brightener at 1.0 g/liter. Further, copper bromide (CuBr2)
as the
resource of copper to be co-deposited with silver was dissolved at 2.2
mmol/liter.
Although the solution had turned violet after dissolution of CuBr,, the
solution could
return transparent by dissolving a proper amount of triethanolamine. The
transparent
solution thus obtained was subjected to evaluation as an electrolytic
solution.
The CV measurements were performed on an optical unit having the
above-obtained electrolytic solution containing CuBr2, and an optical unit
having the
same electrolytic solution except for not containing CuBr,. In each optical
unit, ITO
electrodes were used as the working electrodes, a silver plate was used as the
counter
electrode, and a silver wire was used as the reference electrode. Measurement
on
each optical unit was performed with a sweep rate of 100 mV/sec. Fig. 1 shows
the
C-V curve of the electrolytic solution containing CuBr,, and Fig. 2 shows the
C-V
curve of the electrolytic solution without CuBr,, respectively.
4
According to the results of the above measurements, it was found that the
residual coloring" finally completely disappeared at around +2.0 V in the
optical unit
having the electrolytic solution without CuBr2. On the other hand, in the
optical unit
having the electrolytic solution containing CuBr2, a second dissolution
(oxidation)
peak appeared in the C-V curve at around +1.6 V versus (relative to) silver.
Although a voltage of about +1.5 to +2.0 V versus silver seems to be an
excessive
over-voltage for a system containing the copper salt, this means that the
conductivity
of the silver layer during dissolution can be successfully increased by co-
deposition
of silver and copper. Accordingly, since the second dissolution peak appeared
at
+2.0 V or below on the oxidation side, the over-voltage necessary for
completely
erasing the residual coloring" can be reduced.
As is obvious from the C-V curve shown in Fig. 1, the electrolytic solution
of the example according to the present invention has a high dissolution peak
on the
oxidation side, and is highly reversible.
Experimental Example 2: Changes in Transmittance on Deposition/Dissolution
of Silver According to a Constant Voltage Method
Using the electrolytic solution containing a copper salt obtained in
Experimental Example 1, deposition/dissolution of silver were performed
according
to a constant voltage method, and changes in transmittance on
deposition/dissolution
of silver were observed.
The driving voltage for deposition was -2.5 V versus silver, and the driving
time was 1.5 sec. On the other hand, the driving voltage for dissolution was
stepwise, namely, versus silver, +4.5 V for 20 msec., +1.6 V for 2 sec., and
+3.5 V
for 20 msec. The changes in transmittance are shown in Fig. 3 (on deposition)
and
Fig. 4 (on dissolution). Incidentally, the transmittance values are based on
those of
an ITO electrode itself.
16
CA 02199344 2004-09-03
As is obvious from the results, the case using the electrolytic solution based
on the present invention is capable of light-control (transmittance-varying).
Further,
concerning the spectroscopic properties of the deposited silver layer, the
absorptivities in visible light are homogeneous, and the transmittance changes
similarly in both deposition and dissolution, and a light-screening ability in
visible
light is exhibited.
Additionally, a unit using an electrolytic solution having the same
composition as the above except that CuBr2 was not contained was also examined
in
this experimental example. In this case, the unit was driven with a low sweep
rate.
The results on transmittance changes are shown in Figs. 5 and 6. In Fig. 5,
the
driving voltage was changed from OmV to -2000mV by 50mV/sec., then reversed
from -2000mV to OmV by 5OmV/sec. In Fig. 6, the driving voltage was changed
from
OmV to 2500mV by 50mV/sec., then reversed from 2500mV to OmV by 5OmV/sec.
In Figs. 5 and 6 (y) means the voltage in reverse cycle. As is obvious from
the
results, even in the case without CuBr2, the transmittance can be varied in
the visible
light region.
Nevertheless, life tests revealed that the ITO electrodes in the case using
the
above-described electrolytic solution containing CuBr, had life spans 10 to 40
times
those of the ITO electrodes in the case using the conventional electrolytic
solution
without CuBr2. Consequently, reducing the over-voltage on the oxidation side
by
adding CuBr2 has been found to be important in extending the life span of the
optical
unit.
17
P .9
Experimental Example 3: Low Temperature Preservation Test on Nonaqueous
Solvents
The freezing points of various nonaqueous solvents are shown in Table 1
below.
Table 1 Freezing Points of Various Solvents
(Pure Solvent)
Solvent Freezing Point ( C)
DMF -60.4
DEF -78.0
DMAA -20.0
MPA -3 0.9
N-MP -24.4
MEOH -85.1
EEOH -60.4
PC -49.0
AN -45.7
DMSO 18.0
Using above-listed solvents, low temperature preservation tests were
performed. The results are shown in Table 2 below.
18
CA 02199344 2004-09-03
Table 2 Results of Low Temperature Preservation Tests (at -40 C for 24 hours)
AgBr : 500 MM, TBAB: 1000 mM, SC(NH,)2: 1 g, and CuBr2: 2.2 mM
Solvent State
DMF Liquid
DEF Liquid
DMAA Partially Frozen
MPA Liquid
N-MP Partially Frozen
MEOH Liquid
EEOH Liquid
PC Liquid
AN Liquid
DMSO Frozen
As is obvious from the above results, the above-listed nonaqueous solvents
except for DMSO are useful since they substantially maintain liquid states
without
frozen throughout the preservation at -40 C. On the other hand, DMSO is
completely frozen to be useless by the preservation.
Experimental Example 4: Clarification Test
Using an optical unit constructed in a manner such as the example optical unit
shown in Figs. 7 and 8, a clarification test was carried out in order to
examine the
efficacy of clarifiers for an electrolytic solution.
Dimethylsulfoxide (DMSO) was used as a solvent. The concentration of
silver bromide was set at 500 mmol/liter. Further, a quaternary ammonium salt,
herein tetra-n-butylammonium bromide (TBAB), was dissolved so as to be 750
19
mmol/liter for the purpose of promoting dissolution of silver bromide and
increasing
conductivity. Moreover, as a source of metal to be co-deposited with silver,
copper
bromide (CuBr2) was dissolved so as to be 0.1 mmol/liter. The results of this
clarification test are shown in Table 3.
Incidentally, a HP8452A manufactured by Yokogawa-Hewlett-Packard, Ltd.
was used as an apparatus for measuring transparency, and samples which
exhibited
transmittances at 400nm of 80% or more were evaluated as "Satisfactory". In
Table
3, grades in the column "Result 1" show measurement results just after the
addition
of each clarifier, and grades in the column "Result 2" show measurement
results after
being left standing at 80 C for 24 hours. To leave standing at 80 C for 24
hours is a
sever condition than to leave standing in an ordinary state for 2,400 hours,
for
example, in the case where ascorbic acid is used as the clarifier.
Table 3 Results of Clarification Test in Relation to Cu to be Co-deposited
AgBr: 500 mM
TBAB: 750 mM
CuBr2: 0.1 mM
Clarifier Result 1 Result 2
Triethanolamine Satisfactory Satisfactory
Iminodiacetic Acid Satisfactory Satisfactory
Nitrilotriacetic Acid Satisfactory Pale Yellow
Trans-1, 2-cyclohexane- Satisfactory Yellow
diaminetetraacetic Acid
N, N, N', N'-Tetrakis (2- Satisfactory Yellowish Brown
hydroxypropyl) ethylene-diamine
Ethylenediamine-N,N,N',N'- Satisfactory Yellow
tetraacetic Acid
Salicylic Acid Satisfactory Satisfactory
1-Allyl-2-thiourea Satisfactory Yellow
Dimethylamineborane Satisfactory Black Cottony
Precipitation
Ascorbic Acid Satisfactory Yellow
Dimethylthioformamide Satisfactory Re-crystallized
Precipitation
As is obvious from Table 3, each sample exhibited excellent transparency in
"Result 1". As to "Result 2", although some samples were colored to some
degree,
such color appearances do not matter greatly in view of practical use.
Incidentally,
in Table 3, "Black Cottony Precipitation" means generation of a precipitate
similar
to black cotton, and "Re-crystalloid Precipitation" means generation of a
precipitate
which seems to be recrystallized dimethylthioformaide.
21
In the above, an example of the present invention was illustrated. Needless
to say, the above-described example can be further modified according to the
spirit
of the present invention.
For example, the types of the above-described RED materials, the ingredients
of the RED solution, and the metal to be co-deposited with silver and the
concentration thereof may be varied as occasion demands.
Further, the material of each structural part as well as structures such as
the
pattern of the ITO electrode, and the driving method are also not limited to
those
described above. For example, the pattern of the working electrodes, which is
concentric in the example shown in Fig. 9, may be arranged in the form of
various
stripes, grids, or the like. Moreover, a set of cells having different RED
solutions
may be separately disposed so as to be in charge of a set of separated
electrode
portions, respectively. In this case, RED solutions may be used in combination
with
conventional EC solutions.
Furthermore, the optical units according to the present invention can be
combined with other publicly-known filter members such as organic-type
electrochromic members, liquid crystals, and electroluminescent members.
Moreover, the optical units according to the present invention are broadly
applicable,
for example, to various optical systems such as optical diaphragms in CCDs,
and
light-control units in electro-photographic copying machines or optical
communication instruments.
As described above, the present invention is based on a concept quite
different from those of the prior art which are directed to conventional EC
materials,
and the optical units of the present invention have a specific reversible
system. The
specific reversible system comprises a silver-containing RED solution which
further
contains a metal other than silver to be co-deposited with silver. In the
system, silver
is reversibly deposited on or dissolved from an opposing electrode by
drive-controlling the electrodes, especially by controlling the impressed
voltage upon
the electrodes. Accordingly, based on the present invention, there can be
provided,
by using a RED material, a non-luminescent optical unit suitable for use in
relation
22
9 F? 4 41-
to visible light and drivable with low electrical power, and in addition, the
life span
of the optical unit can be extended since the over-voltage upon the electrodes
can be
reduced.
Although modifications and changes may be suggested by those skilled in
the art, it is the intention of the inventors to embody within the patent
warranted
hereon all changes and modifications as reasonably and properly come within
the
scope of their contribution to the art.
23