Note: Descriptions are shown in the official language in which they were submitted.
CA 02199445 1999-08-25
DESCRIPTION
Disposable Test Kit Apparatus and Method for Bacteria
Reference to Prior Application
Co-pending Canadian Patent Application No. 2,176,895, filed
on 17th May 1996 and laid-open on 19th November 1996, is directed
to a test method and test kit for the determination of bacteria,
particularly for the qualitative and quantitative determination
of total coliform bacteria and E. coli in a test sample. The
method comprises combining in a transparent, flexible, plastic
container a water sample to be tested for a total coliform
bacteria and E. coli with a powdered test composition. The
powdered test composition comprises a growth nutrient medium for
the coliform bacteria and a first agent therein which is cleaved
by an enzyme in the coliform bacteria to produce a color in the
resulting media broth, such as a blue-green color, and to
indicate by the presence of the color, the presence in the test
sample of coliform bacteria. The test composition also includes
a second, fluorescent agent, which is also cleaved by an enzyme
in the E. coli bacteria of the test sample, to indicate by the
presence of a fluorescent color the presence of E. coli bacteria
in the test sample upon exposure to ultraviolet light. The test
method includes observing the color change or absence thereof
and observing fluorometrically the fluorescent color change, or
absence thereof, to determine qualitatively the presence of
total coliform bacteria and/or E. coli bacteria in the test
sample. In one embodiment, the test method and test kit provide
a water soluble, powdered gelling agent in the test composition,
and which gelling agent reacts in situ in the presence of the
water in the test sample to form a transparent, semisolid medium
prior to incubation of the test sample, and typically, within
about two hours. The formation of the gel restricts the
mobility of the coliform
1
CA 02199445 1997-06-06
bacteria and E. coli during the subsequent incubation period
and provides for the growth of separate colonies of the
coliform bacteria in the gel and separate colonies of the E.
coli so that the total coliform bacteria, as well as the E.
coli, may be quantitatively measured.
The parent application also describes a test kit for
the determination of coliform bacteria and E. coli which
comprises a flexible, transparent, plastic bag as
container for the test sample, and which test sample
comprises water or a sample having a water content. In one
embodiment, the test container comprises a sealable,
flexible, transparent, plastic bag, for example, sealed by
a wire plastic strip, and also includes at least two
chambers separated by a dividing means, the first chamber
for the introduction of the test sample, and a separate
chamber to contain the powdered test composition, so that by
removal of the dividing means by the user, the test sample
in the first chamber and the powdered composition in the
separate chamber may be admixed by the user after sealing of
the plastic bag. In the method of operation, the plastic
bag after being kneaded for mixing of the sample with the
powdered test composition is pressed into a flat condition,
and then incubated to form a gel, and then observed to
detect qualitatively the presence of the coliform bacteria
and/or E. coli under ultraviolet light, and then also, the
total coliform bacteria or the E. coli may be quantitatively
determined in the cultured media in the flat plastic bag.
l~an_k_groLnd of the Invention
The test kit and method of the parent application
provide for a method of effective and easy determination of
total coliform bacteria and E. coli in a test sample,
particularly a large volume water sample, such as lOml to
500m1, and is subject to use in field conditions because it
is quite simple and provides for the simplified collection
and detection of the bacteria. As set forth in the parent
application, after carrying out of the test, the plastic bag
with its contents, that is, the cultured media, the test
2 ~ I
CA 02199445 1999-08-25
sample and the coliform bacteria and E. coli grown therein
during the incubation phase, may be easily disposed of as trash
after treatment, such as the employment of boiling water for ten
minutes or the employment of autoclaving the plastic bag before
disposal. The standard EPA instructions to discard a used
microbiological test media is by employing an autoclave at
121°C. However, autoclaves are not available in all
laboratories and are not available in the general retail or
consumer market. Any test kit apparatus which employs a
disposable, used microbiological media containing grown bacteria
which is used for the detection of potential pathogens, for
example, E. coli or salmonella, requires a safe disposal of the
test kit apparatus.
It is therefore desirable to provide for safe and effective
disposal of a used microbiological test media in a test
container, such as a plastic bag, particularly where the test
kit apparatus is employed by unsophisticated laboratory
personnel and in non-laboratory conditions, and which disposal
means and method provide for a safe, easy, simplified disposal
of the potentially pathologically dangerous culture media and
the grown bacteria therein.
Summary of the Invention
The present invention relates to a safe, disposable, test
kit apparatus and method for disposing of used microbiological
test media and microorganisms, including pathogen organisms,
grown thereon. In particular, the invention relates to an
effective, simple, safe, easy means of disposing of plastic bag
test kits containing used media and bacteria.
The invention relates to a safe, disposable test kit
apparatus designed for the detection of pathogens, such as
coliform bacteria and E. coli, and other pathogens in a test
3
CA 02199445 1999-08-25
sample of which the test kit apparatus after use may be easily
disposed in a bacteriological, safe manner.
According to the present invention, there is provided a
safe, disposable test kit apparatus for the detection of
pathogens in a test sample, which apparatus comprises: a) a
transparent, flexible, plastic bag packaging means of defined
internal volume, which means comprises a plurality of at least
first and second separate, adjacent, sealed compartments; b) the
packaging means having an opening at one end to introduce a test
sample to be tested in a first compartment and a means to seal
the first compartment after introduction of the test sample; c)
divider means to divide the bag into a first sample compartment
and a second media compartment and to permit a user to remove
the divider means between the first and second compartments for
the admixing of the ingredients in the said compartments; d) the
first sample compartment adapted to receive therein a defined
volume of a test sample to be tested for pathogens; e) a second
media compartment containing a growth media and a pathogen
indicator in the compartment to provide for the growth on
incubation of the pathogen and the detection of the pathogen to
be determined in the test sample; and f) sanitizing means
containing a sanitizing composition to provide for the
destruction of the pathogens grown on the cultured growth media
after incubation and determination of the pathogens in the test
sample, the sanitizing agent to be introduced into the packaging
means after incubation and reading of the test results by a
user, thereby permitting for the destruction of the grown
pathogens in the incubated media to provide for the safe
disposal of the packaging means.
According to the present invention, there is also provided
a safe, disposable test apparatus for the detection of pathogens
in a test sample, which test apparatus comprises: a) a
3a
CA 02199445 1999-08-25
transparent, flexible, multicompartment, plastic bag packaging
means of defined internal volume which comprises a plurality of
at least first, second and third separate, adjacent, sealed
compartments; b) the said packaging means having a first sample
compartment which is open to receive a test sample therein to be
tested for pathogens and which includes means to seal the open
end of the test compartment after receipt of the test sample; c)
seal means to seal each of the compartments from each other and
to permit a user to remove the seal means for the sequential
mixing of the ingredients in the respective separate, sealed
compartments as desired; d) a second media compartment
containing a powdered growth nutrient medium therein and an
indicator for the detection of the pathogens to be detected; and
e) a third sanitizer compartment containing a sanitizing
composition therein to provide for the destruction of the
pathogens grown on the media after incubation and detection of
the test results, thereby permitting the safe disposal of the
packaging means after such destruction of the pathogens.
According to the present invention there is also provided a
test method for determination of pathogens in a test sample,
which method comprises: a) introducing a test sample into a
sample compartment in a plastic bag packaging means and sealing
the test sample within the sample compartment; b) providing a
media in a separate, sealed media compartment in the plastic bag
packaging means, the media compartment containing a growth
medium for the growth of the pathogens to be detected in the
test sample and having an indicator agent therein to provide for
detection of selected pathogens in the test sample; c) providing
a separate sanitizing compound; d) removing the sealing means
between the test sample compartment and the media compartment
and admixing the media with the test sample;
3b
CA 02199445 1999-08-25
e) incubating the test sample in the media in the sample and
media compartments; f) observing and detecting the pathogens in
the test sample in the plastic bag packaging means; g)
introducing the sanitizing agent into the media compartment to
provide for the total destruction of the pathogens grown in the
media company during incubation; and h) disposing of the plastic
bag packaging means after destruction of the total pathogens.
In one embodiment, the test kit apparatus comprises a
transparent, flexible, plastic, multicompartment packaging
means, such as a bag of defined internal volume to receive a
test sample, such as a water
3c
CA 02199445 1997-06-06
sample of lOml to 500m1, and which comprises a plurality of
at least first, second and third separate, adjacent, sealed
compartments in the packaging means.
The packaging means includes a first compartment having
an open end to receive a test sample and means to seal the
open end of the first compartment after receipt of the teat
sample. The package may also include seal or divider means
to seal or divide each of the compartments from each other
and to permit a user to remove the seal or divider means for
the sequential mixing of ingredients in the respective
compartments. The packaging means includes a first sample
compartment adapted to receive therein a defined volume of
a liquid test sample, such as a water sample to be tested
for E. coli and total coliform bacteria, as an illustrative
example. The packaging means includes a second media-type
compartment containing a culture growth medium and a
selected bacteria indicator, and particularly comprises a
powdered test medium which includes a test medium for the
growth of the bacteria and agents which change color to
detect for example coliform bacteria and a fluorescent agent
to detect E. coli bacteria under ultraviolet light.
The packaging means includes a third adjacent or
separate sanitizes compartment which contains a sanitizes,
which sanitizes is selected to destroy and render inactive
any of the grown microorganisms in the cultured growth media
within the packaging means, such as within the plastic bag,
after incubation and detection, either qualitatively or
quantitatively, of the microorganisms.
In the sanitizes test kit apparatus, the test sample is
introduced into the first compartment and then the
compartment sealed. The test sample is then admixed with
the culture medium in the second compartment after the
removal of the seal means between the first and second
compartments, and this admixture of the test sample and
medium is then incubated, typically with the plastic bag
pressed in a flat condition, where the culture medium
contains a gelling agent. The incubated admixture is then
-4-
CA 02199445 1997-06-06
read to detect qualitatively and/or quantitatively the
pathogens in the test sample. Prior to disposal, and after
incubation and reading of the test sample, the used,
incubated culture media with the test sample and the grown
pathogens thereon is subsequently admixed with the sanitizes
of the third compartment by the removal of the means between
the second and third compartments or the separate
introduction of the sanitizes by any means wherein the
presence and admixture of the sanitizes destroys or renders
inactive the growth pathogens and thereby permits the safe
disposal of the entire packaging means or sealed plastic bag
into the trash. The test kit and method are particularly
adapted for test kits and apparatus used in the field and
used by unsophisticated laboratory personnel or by
consumers, and thus avoids the necessity of heat treating
the sealed plastic bag or autoclaving the bag prior to
removal of the dangerous pathogen components therein.
The sanitizing compounds employed may comprise a wide
variety of chemical antibacterial or antipathogenic agents,
either alone or in admixtures, such as sanitizers or
oxidizers which are well known and routinely used in
hospitals, the food industry and consumer markets for the
control of microorganisms. The user of the test kit can
provide for the destruction of the known pathogens within
the plastic bag at the end of the test, and thus permit the
safe disposal of the test kit apparatus. The sanitizing
agent should be selected to kill all live pathogens, such as
the total coliform bacteria and E. coli, in the particular
test method employed, so that the resulting test container
can then be safely discarded.
Sanitizing compounds employed may vary and include
oxidizing-type chemicals, such as the hypohalides, or more
particularly sodium hypochloride, and may include organic
acids, such as peracetic acid; peroxides, including hydrogen
peroxide and perborate oxides or halogens like iodine and
iodide compounds; and also aldehydes, such as formaldehyde
and glutaraldehyde. .Other sanitizers which are detergent-
-5-
CA 02199445 1997-06-06
type sanitizers would include, but are not limited to,
quaternary ammonium compounds or other sanitizers which have
detergent or surface action effects, such as more
particularly benzalkonium halides, such as benzalkonium
chlorides, as well alkyl benzyl quaternary ammonium bromides
or chromides, more particularly as dimethylalkyl quaternary
ammonium chlorides, or fatty acid pyridinium halides like
cetyl pyridinium chloride or bromide, as well as alkali
metal lauryl sulfates, such as sodium lauryl sulfate. The
sanitizing agents may be used alone or in combination. For
example, an effective sanitizing agent may include an
oxidizing-type chemical together with a quaternary ammonium-
type chemical. Typically, the sanitizing agent is employed
in liquid form in the third compartment or as a separate
capsule alone or in the plastic bag compartment. The
concentration of the sanitizes compound should be sufficient
to provide for the total destruction of the pathogenic
organisms in the cultured growth media. The concentration
of the sanitizing agent may vary; however, generally the
concentration should be at least as great as, or typically
more than sufficient to provide for the destruction of all
the suspected pathogenic organisms.
Additionally, but preferably, it is also desirable to
employ an indicating agent in combination with the
sanitizes, such as an indicating dye, for example, black or
other brightly visible color, water soluble dye, such as a
food dye, in combination with the sanitizes, so that when
the sanitizes is released for example from the third
compartment or separately introduced into the cultured media
and pathogens, the dye may act as an indicator to establish
mixing of the cultured media and pathogens with the
sanitizes to ensure total mixture and destruction of the
grown pathogens. A black food dye is one preferred dye
indicator selected for use with the sanitizes, since a black
color would prevent children from contacting or drinking the
contents of the bag or vial. Further, other colors like
light yellow, red or blue which are indicators of the test
-6-
CA 02199445 1997-06-06
results preferably should not be used, since these colors
may encourage children to drink the contents if they
mistaken it as to a juice-type product. Thus, the dye
indicator selected should be child safe, and particularly
where consumer use is intended of the test kit apparatus, to
indicate that the sanitizer has been totally mixed with the
grown culture media and pathogens.
The sanitizing agent may be incorporated into the test
kit apparatus in a variety of ways. In one embodiment
wherein a compartment is employed in the flexible plastic
bag and the compartment is then sequentially opened to first
provide for a mixing of the test sample with the culture
medium, and then after incubation, mixing of the culture
medium with the grown pathogen with the sanitizing agent.
There are also other techniques employed to introduce the
sanitizing agent into the cultured medium and grown
pathogens. For example, a separate, burstable capsule
containing the sanitizing agent may be employed. The
buratable capsule may, for example, be a pressure-sensitive
capsule, such as one burst by hand pressure, and placed
within the bag either before or after incubation, and
sufficient so that the capsule may not be activated or burst
during the incubation period, or in fact, the pressure-
sensitive capsule may be introduced into the bag aftez~
incubation, though this is not a preferred method, and then
the pressure-sensitive capsule burst after reading of the
test results, thus to provide for the destruction of the
. pathogens within the bag.
In another embodiment, a pressure-sensitive pocket or
plug may be built into the flexible plastic bag of the test
kit apparatus as an integral part thereof, and after
incubation and reading of the test results, pressure applied
by the user to force the sanitizing agent in the pressure
sensitive pocket, alone or together with the color
indicator, into the incubated media and pathogens. In a
further embodiment of the invention, a time released means
may be employed that is activated by the addition of water.
CA 02199445 1999-08-25
For example, the plastic bag may have an elongated neck or plug
or other device in the plastic bag plugged with a mixture of a
water-soluble material, such as starch or amylase or a solid
lipid and lipase, so that on contact with the water test sample,
the enzyme is then activated, which slowly digests the plug over
a selected time period greater than the incubation time period.
The length of the plug and the rate of digestion is
precalculated in use, so that at the end of the incubation
period, for example, 24 hours at 35°C or 36, 48 or 72 hours as
desired, the plug would be burst exposing the sanitizer in the
compartment to the cultured media and the grown pathogens.
Thus, the sanitizing agent may be employed in a precalculated,
time release manner activated by the test sample or by
incubation temperature, so as to prevent for example the
inadvertent nonuse of the sanitizer by a user, such as a
consumer user, so that the flexible bag with the cultured media
and the grown pathogens would be automatically decontaminated
and safe to dispose regardless of the failure to act of the
user.
It is often desirable, particularly with water test
samples, that the water sample be neutralized prior to admixture
with a medium, so as to reduce the effect of halogens, such as
chloride, in the water test samples, for example by the use of a
thiosulfate prior to admixture of the powdered test medium, such
as the powdered, gellable test medium as described in the parent
application. Thus, the packaging means may include an
intermediate, separate, sealed compartment containing a
neutralizing agent, such as a water solution of a thiosulfate,
to neutralize chlorine as recommended by the EPA in the water
test sample prior to admixing the neutralized water sample with
the powdered test medium in the second compartment.
8
CA 02199445 1999-08-25
The packaging means employed as set forth more particularly
in Canadian Application No. 2,176,895, would include and
comprise a flexible plastic bag
8a
CA 02199445 1997-06-06
having an open one end and means to seal the open one end of
the bag. The plastic bag is typically transparent to permi
the reading of the test results in the bag and is flexible
so that the bag may be pressed into a flattened condition
for incubation, where gelling agents are used. The bag
would also contain printed on a portion thereof containing
the medium and the test sample a plurality of grids or
squares, so that the grown pathogens therein, for example,
the total coliform bacteria and/or E. coli may be easily
quantitatively read by.a user. Generally, the plastic bag
would have a means to seal the open mouth of the bag after
the introduction of the test sample therein. For example,
it may contain a plastic wire strip extending around and
outwardly from the mouth, so that the user may merely twirl
the open mouth of the bag around and use the wire strip to
seal completely the open mouth of the bag after the
introduction of the test sample.
i
The flexible plastic bag typically would have at least
two compartments which may be separated by a dividing means.
For example, the dividing means may include a clip means,
that is, a plastic, tension-biased clip divider which is
merely clipped to the outside of the bag to divide the bag
into separate compartments, and wherein the clip is removed
by a user in use to permit the sequential mixing of the
ingredients in the compartments as required in the
particular test. Thus, for example, in the parent
application, a flexible plastic bag is employed with a
disposable plastic divider clip to divide the bag into a
medium compartment for a powdered, gellable test composition
containing the growth medium, the agents and the gelling
agents, and a separate compartment for the introduction of
the water sample, with which the powdered test composition
is to be admixed after sealing of the plastic bag.
Further, the plastic bag or the packaging means may be
subdivided employing a seal line which extends across the
plastic bag, so as to form and provide for separate, sealed
compartments, which sealed compartments would have a seal
_g_
CA 02199445 1997-06-06
line, such as a heat seal line, or an adhesive line
extending across the length of the plastic fag to provide
sealed compartments, which seal line to the sealed
compartments may be easily ruptured by hand pressure of the
user, so as to permit the sequential mixing of the
ingredients in the compartments. The seal line may be
formed by separate adhesives used or by merely the heat
sealing together of the thermoplastic component film of the
bag. Thus, the bag for example may comprise a bag which is
peripherally heat sealed, having an open mouth with a wire
sealing means to seal the open mouth, and a plurality of
compartments, for example, at least two, but preferably
three or four, divided by plastic clip means, or preferably
by sealing lines extending across the plastic bag, which
I
seal lines are burstable by a user.
The packaging may comprise a plastic disposable bag of
a thermoplastic material which is readily heat sealed about
its periphery to form heat sealing lines or which may be
readily sealed by employing adhesives. However, it is
recognized that the flexible plastic bag employed may
comprise a multiple layer film bag, for example, employing
a moisture impermeable layer so as to protect the medium and
sanitizing agents to be stored in the bag to permit long
time storage and employing inner and outer films over the
other film which may be heat or adhesive sealable. Thus,
the transparent, flexible plastic bag may be formed of a
single thermoplastic film material or may be composed of a
plurality of layers of different film materials as desired
with typically a central oxygen barrier layer and an oute~
layer which is printable to permit the imprinting of the
grid on the outside of at least a part of the plastic bag
and also to permit instructions to be imprinted on the
remaining part of the bag for the user.
The disposable, safe test kit apparatus comprises a
packaging means, such as a flexible, transparent, plastic
bag containing the media and containing means to receive the
test sample, a separate compartment for the medium to mix
-10-
CA 02199445 1997-06-06
with the test sample and containing means, such as a
pressure burstable capsule, a time release plug or a
separate compartment for the sanitizing agent, so as to
provide for, the subsequent sanitization of the grown
pathogens after incubation and reading of the test results.
The test apparatus may be easily employed in the field and
by consumers and would merely comprise the flexible plastic
bag containing the medium therein and the sanitizer and
include incubation means, such as a simple oven, for
incubation of the sealed plastic bag and an ultraviolet
light as the fluorescent agent to determine E. coli or other
pathogens where required.
Also, the test kit apparatus may comprise a plastic bag
of two compartments and a separate, pressure releasable
capsule containing the sanitizer agent separately applied or
added to one of the compartments or may contain a plug
within the bag. It is desirable to provide for a plastic
bag composed of plastic materials which would withstand the
incubation conditions and preferably having a plastic
material which would withstand pasteurization conditions of,
for example, 75aC or more. Otherwise, incubation conditions
would have to be adjusted to the tolerance of the plastic
film that composes the plastic bag, such as for example,
incubating at a lower temperature for a longer period of
i
time. Preferably, however, the plastic bag would be made up
of plastic polymeric components which will be pasteurized
before use of the plastic bag with the components therein,
such as for example, at 75~C for 30 minutes or longer, so
that the entire contents may be pasteurized after packaging.
Otherwise, the plastic bag and its components, such as the
powdered media, the sanitizing agent and the thiosulfate and
other components used therein must be assembled and packaged
under sterile conditions, which add to the cost and expense
of the packaging means.
The invention will be described for purposes of
illustration only in connection with certain preferred
embodiments; however, it is recognized that various
-11-
CA 02199445 1997-06-06
modifications, changes, additions and improvements may be
made to the illustrated embodiments without departing from
the spirit and the scope thereof.
Erief Description of the Drawinag
Fig. 1 is a perspective, illustrative view of one
embodiment of the test kit apparatus of the invention;
Fig. 2 is a schematic illustration of a test kit
apparatus of the invention which includes the flexible
plastic bag of Fig. 1;
to Fig. 3 is a perspective, illustrative view of the
invention of Fig. 1 in a post-use, ready-to-dispose
condition;
Fig. 4 is a sequence of method steps in the use of the
test kit apparatus of the invention;
Fig. 5 is a perspective, illustrative view of another
embodiment of the test kit apparatus of the invention;
Fig. 6 is a perspective, illustrative view of the test
kit apparatus of Fig. 4 in position for diagnosis; and
Fig. 7 is a perspective, illustrative view of another
embodiment of the test kit apparatus of the invention.
Descr ration of the Embodiments i
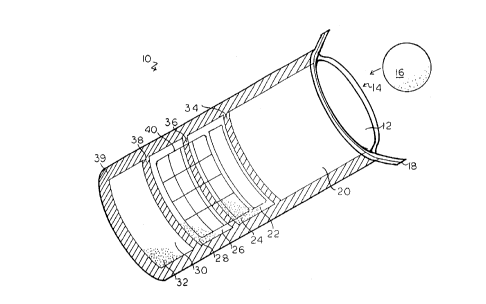
In the drawings, Fig. 1 shows the test kit apparatus of
the invention 10 in perspective, illustrative view. The
media bag 12 has an open top 14 to permit the entry of a
water sample 16. A closing wire 18 extends across the top
of the open top 14 of the water sample collecting
compartment 20. A rupturable heat seal 34 seals the water
sample collecting compartment 20 from the sample neutralizer
compartment 22, which has a neutralizer 24 sealed therein.
A second rupturable heat seal 36 separates the sample
neutralizer compartment 22 from the media compartment 26,
which has the media and indicator 28 sealed therein. A
final rupturable heat seal 38 separates the media
compartment 26 from the sanitizes compartment 30, which has
a sanitizes in powder form 32 sealed therein. An outer heat
seal 39 extends around the three edges of the media bag 12i
to seal all compartments of the media bag 12. An indicator
-12-
CA 02199445 1999-08-25
grid 40 is printed on the top surface of the media and indicator
compartment 26 to provide for reading of the mixed media sample
52. It is recognized that other printing or informative indicia
may be printed on the media bag 12 as required and desired.
Fig. 2 shows the test kit method of the invention 50, with
the test kit of the invention 10 in a perspective illustrative
position. The media bag 12 is shown with the open top 14 of
Fig. 1 folded over and secured with the closing wire 18 of Fig.
1 to form a tie seal 44. The punctures of the puncturable heat
seals 34 and 36 are shown as openings 46 and 48 respectively.
Further, the mixed media sample 52, comprised of the water
sample 16, neutralizer 24, and media/indicator 28 (as shown in
Fig. 1) is shown in position under the indicia grid 40. The
flattened media bag 12 is shown ready for placement in the
incubator 54, with the ultraviolet light indicator 56 shown in a
ready-to-use position after the test sample is incubated.
Fig. 3 shows the test kit of the invention 10 after use,
with the rupturable heat seal 38 separating the media/indicator
compartment 26 shown punctured and open 58 to allow for the
sanitizer 32 to enter the media/indicator compartment 26 and
sanitize the sample. The media bag 12, with the sanitized
mixture 59 therein, is shown being disposed of in an appropriate
waste receptacle 60.
Fig. 4 shows a schematic diagram of the method of the
invention, with the process steps shown and described.
Fig. 5 shows a perspective, illustrative view of another
embodiment of the invention 70. The media bag 72 has an open
top 74 to permit entry of a water sample directly into a
media/indicator compartment 80. A closing wire 78 extends
across the top of the open top 74 of the compartment 80. A
removable plastic clip 94 separates the compartment 80 from the
sanitizer compartment 90, which has a sanitizer in capsule form
13
CA 02199445 1999-08-25
92 sealed therein. An outer heat seal 99 extends around the
three sides of the media bag 72 to seal all compartments of the
media bag 72. An indicator grid 100 is printed on the top
surface of the media and indicator compartment 80 to provide for
reading of the mixed media sample. It is recognized that other
printing or informative indicia may be printed on the media bag
as required and desired.
Fig. 6 shows the test kit 70 in use, in a folded over,
flattened position to enable incubation and subsequent reading
of the incubated sample. The sanitizes capsule 92 is shown
separated from the test sample by plastic clip 94.
Fig. 7 shows a further embodiment of the invention 100,
with a time-release plug 120 containing sanitizes 122 in the
single compartment 128. The sample to be tested 116 is placed
directly into the main compartment 128 which compartment
contains the media/indicator 114. The compartment is shown
sealed by a tie seal means 118 and with print indicia 124
thereon.
In use, the water sample 16 to be tested is introduced into
the open top 14 of the first sample compartment 20. The
compartment is sealed by the user holding the two ends of the
closing wire 18 and twirling the bag two or three times to
create a tight foldover 42, with the user then forming a tie
seal 44 with the closing wire 18. The test sample is then
admixed with the neutralizer in the second compartment 24 after
the removal of the rupturable heat seal 34 between the first 20
and second 22 compartments. Next, this admixture of the sample
16 and neutralizer 24 is admixed with the medium and indicator
28 in the third media/indicator compartment 26, after the
removal of the rupturable heat seal 36 between the second 22 and
third 26 compartments. This admixture of the neutralized
14
CA 02199445 1999-08-25
test sample 16, 24 and medium 28 is then incubated, typically
with the bag pressed in a flat condition.
After incubation, the admixture is evaluated by means of
the indicia grid 40 to detect qualitatively and/or
quantitatively the pathogens in the test sample. Prior to
disposal, and typically after incubation and reading of the
14a
CA 02199445 1997-06-06
teat sample, the used, incubated culture media with the teat
sample and the grown pathogens thereon is subsequently
admixed with the sanitizer 32 of the fourth compartment 30
by the removal of the rupturable heat seal means 38 between
the third 26 and fourth 30 compartments wherein the
admixture of the sanitizer 32 destroys or renders inactive
the growth pathogens and thereby permits the safe disposal
of the entire packaging means or sealed plastic bag into the
trash. The test kit and method are particularly adapted
for test kits and apparatus used in the field and used by
unsophisticated laboratory personnel or by consumers, an~
thus avoids the necessity of heat treating the sealed
plastic bag or autoclaving the bag prior to removal of the
dangerous components therein.
-15-