Note: Descriptions are shown in the official language in which they were submitted.
2199656
Page 2
BACKGROUND OF THE INVENTION
This invention relates to the smelting of ferro-alloys, particularly for the
smelting of nickel-bearing ores of the lateritic type to ferro-nickel alloys.
The well known process for treating ores of this type as described in
textbooks, consists of alternating current (AC) electric arc smelting of the
ore. The AC electric arc process takes advantage of an electrical
resistance heating effect to produce molten slag and metal. A reductant
is added to the furnace so that nickel in the ore and some of the iron is
reduced to the metallic phase. it then sinks to the bottom of the furnace
where it is removed by tapping as molten ferro-nickel. The slag floats on
the surface and is also tapped from the furnace.
More recently there have been advances in electric arc furnace
technology. The direct current (DC) arc furnace was developed because
such a furnace offers certain distinct advantages over the AC furnace.
Some of these advantages are relevant to the production of ferro-nickel.
The DC arc furnace uses a single, hollow graphite electrode (generally the
cathode), positioned centrally in a circular refractory lined steel shell
above the molten bath of slag and metal. Current passes from the
electrode through the molten bath to a second electrode (generally the
P.17635/aan
2199656
Page 3
anode) in the hearth of the furnace. The top electrode is not submerged
in the slag but arcs to the slag. Current flows through the slag to the
bottom electrode via the conducting metal bath and an electrical
conducting system incorporated in the hearth. The furnace operates in
an open bath mode with no overlying charge burden.
Some advantages of DC arc furnace operation over AC operation include:
- more controllable operation;
- ability to feed fine materials continuously without problems of gas
eruptions;
- ability to feed through the hollow electrode, or peripherally by side-
feeders if required;
- energy is not transferred to the melt by electrical resistance
heating, as in AC operation, and thus slag composition, which
governs slag resistivity, is not as important a consideration; and
- the centralised energy input allows better control over slag attack
on refractories by enabling more efficient formation of a slag freeze
lining on the refractories.
However, all electric arc furnaces have the disadvantage of requiring
electrical energy, which may be costly in certain locations or countries, to
heat the charge materials and produce a melt. This is a particular
P.17635/acm
2199656
Page 4
disadvantage in smelting nickel-bearing lateritic ores because only 1 % to
3% nickel is present in the ore and thus a very large amount of slag-
forming components have to be melted relative to the amount of ferro-
nickel produced.
Despite the advantages of DC arc furnace operation, as already described,
the DC arc furnace may be at a disadvantage to the AC furnace with
respect to energy efficiency, for certain applications. its operation in an
open-bath mode results in greater energy losses by radiation compared
with the AC furnace where the layer of charge above the slag decreases
the heat energy losses.
However, both AC and DC furnaces are energy inefficient unless the ore
is preheated. This is often practised. The ore can be pre-heated in a
rotary kiln so that hot ore enters the electric arc furnace. Fiuidized bed
roasters have also been used to pre-heat the ore when it is in a finely
divided state suitable, for example, as feed to a DC furnace. Gas which
is emitted from the arc furnace can be used as fuel for the pre-heating
process.
There is another process which has been used to smelt ferro-alloys
although, to the applicant's knowledge, and at least in the case of nickel
bearing laterite ores, never on a large scale. This process is generally
P.17635/aan
CA 02199656 2001-02-08
Page 5
described as the use of oxygen or oxygen-enriched air together with a source
of fuel
which are injected into a furnace where combustion takes place. This type of
furnace is referred to herein as an oxy-air/fuel furnace. The development of
this
type of smelting furnace was carried out at the CSIRO in Australia under the
name
"SIROSMELTT""". Subsequent commercial development was carried out by two
organisations which use the trade names "ISASMELTTM" and "AUSMELTT"~". The
following references describe the development of these furnaces:
Floyd J.M. and Conochie D.S., "SIROSMELT - The First Ten Years". Extractive
Metallurgy Sypmposium. Parkville. Australasian IMM. 1984, pp 1-8 (Australasian
IMM Symposia Series, No. 36).
Brew R.B.M., "Status Report on Isasmelt"., Minerals Industry International,
March
1994, pp 15-17.
Floys J.M. and Short W.E., "Ausmelt Development of Top-submerged Lance
Technology", Minerals Industry International, March 1994, pp 18-24.
If a lance is inserted into a liquid slag bath, and air or oxygen-enriched air
and fuel,
such as coal, oil or natural gas, are injected through the lance into the
bath, then the
bath can be supplied with energy to maintain the liquid in the molten state
and to
melt the incoming ore charge with or
2199656
Page 6
without significant reduction. The lance is protected from the molten slag
by the cooling effect of the gas passing through the lance.
Reduction of the ore can be undertaken at the same time, and can be
achieved by feeding an excess of reductant in the form of coal or other
carbonaceous material. A disadvantage of this type of process is that it
generates a larger volume of gas, due to the partial combustion of the fuel
and the reduction reactions, than what is produced in an electric arc
furnace. This also leads to dust losses.
SUMMARY OF THE INVENTIQN
The invention provides a method of treating a metal bearing material for
ferro-alloy production which includes the steps of producing molten slag
by melting the material in an oxy-air/fuel furnace, and producing a ferro
alloy by reducing the molten slag in an electric arc furnace.
The operating conditions in the oxy-air/fuel furnace would include the
degree of reduction and the temperature of the slag.
The degree of reduction will be very limited and for example may be
sufficient to reduce at least 50% of the iron oxide in the ore and preferably
about 90% of the iron oxide to the Wustite form (Fe0). If a greater ratio
P.17635/$~
2199656
Page 7
of fuel to air is used such that more iron oxide is reduced then excessive
gas will be produced.
The slag temperature must be sufficiently high to ensure that the liquid
slag can be transferred.
The combination of conditions used for the two furnaces is selected to
reduce the total cost of energy consumed in the process. The total
energy cost in turn depends on the relative cost of electrical energy and
coal, and the energy input required from the electrical energy and the
coal, respectively.
The aforementioned process is particularly suited for the smelting of
nickel-bearing ores of the lateritic type, and the production of ferro-nickel.
In the oxy-air/fuel furnace, oxygen and coal or any other suitable
carbonaceous energy source may be injected into a molten slag bath
through a lance.
The oxygen may be carried in air.
The ore, or other form of metal-bearing material, may be fed continuously
or in batches into the molten slag bath. Gas and entrained dust,
P.17635Jaan
2199656
Page 8
generated in the oxy-air/fuel furnace, may be removed and separated from
each other and the dust may be fed to one, or both, of the furnaces.
Energy may be recovered from the gas, for example by cooling the gas
in a boiler to produce steam which may then be used to generate
electricity.
The electric arc furnace is preferably a DC arc furnace.
BRIEF DESCRIPTION OF THE DRAWING
The invention is further described by way of example with reference to the
accompanying drawing which schematically depicts a method according
to the invention for the smelting of a lateritic nickel-bearing ore.
DESCRIPTION OF PREFERRED EMBODIMENT
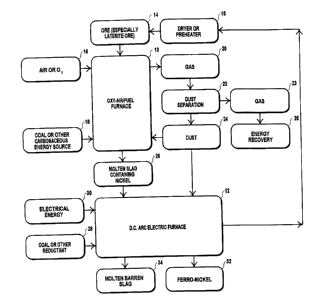
The accompanying drawing illustrates a process of heating and smelting
according to the invention, for the treating of laterite nickel ore.
The process of the invention includes two main steps namely melting the
metal bearing material or ore 14 in an oxy-air/fuei furnace 10 and a
reduction step which is carried out in a DC arc furnace 12.
P.17635/aan
2199656
Page 9
The material 14 is dried or preheated in a preheater 15 and is then fed
into the oxy-air/fuel furnace 10. The preheater 15 may be a rotary kiln, a
fluidised bed, or other suitable device which, preferably, uses as an
energy source gas, such as carbon monoxide, which is produced in the
DC arc furnace 12. After having been preheated, the material 14 is fed to
the oxy-air/fuel furnace 10 where it is melted by heat released from the
combustion of oxygen-enriched air 16 which is injected together with coal
18 or any other suitable carbonaceous energy source through a lance
which is inserted into the liquid slag bath in the oxy-air/fuei furnace 10.
The material or ore 14 is continuously fed into the molten slag bath for
melting to take place. Very little excess carbon monoxide is produced
because the gas is almost fully burnt to carbon dioxide. The extent of
reduction which takes place is not significant and is such that about 90%
of the iron oxide is reduced to Wustite (Fe0). This is beneficial for as the
degree of reduction increases, more excess gas is produced.
Gas 20 which is generated in the oxy-air/fuel furnace 10 is extracted and
fed to a separator 22 which separates entrained dust 24 from the gas.
The separated dust is fed either to the DC arc furnace 12 through its
hollow electrode, or to the oxy-air/fuel furnace, possibly after
agglomeration. The separated gas 23 may be fed to a device 25 which
contains a boiler which produces steam from the hot gas. The steam may
P.17635/aan
2199656
Page 10
then be used to generate electricity. This energy recovery step increases
the economic viability of the process of the invention.
The molten slag 26, with nickel and iron oxides still present in the slag, is
transferred to the DC arc furnace 12 continuously or on a batch basis.
Coal 28, or any other suitable reductant, is fed to the molten bath in the
DC arc furnace. No combustion takes place since no air is fed to the
furnace. Consequently no excess gas is produced and the gas, which has
a high content of carbon monoxide, as has already been described, is
suitable for subsequent post-combustion in the preheater 15 to provide
energy for drying or pre-heating of the material 14 in a rotary kiln or
fluidised-bed or similar pre-heating operation.
Electrical energy 30 to the DC arc furnace 12 is used to maintain the slag
in a molten state so that the nickel oxide in the slag and some of the iron
oxide may be reduced to the metallic phase. The metallic phase sinks to
the bottom of the furnace and is removed by tapping molten ferro-nickel
32 from the furnace. The barren slag 34 floats on the surface of the melt
and is also tapped.
The electrical energy 30 fed to the arc furnace is not used to melt the ore.
The melting takes place in the oxy-air/fuei furnace 10 and, for this step,
energy in the cheaper form of coal, or any other suitable carbonaceous
P.,~a«"
2199656
Page 11
energy source such as oil or gas is used rather than electrical energy.
The combination of the two furnaces thus optimises the cost of the
energy used in the process of the invention. The lower cost energy is
used for melting while the electrical energy is used in the reducing step.
The oxy-air/fuel furnace 10 may be of the kind developed by the CSIRO
of Australia under the name of SIROSMELT and marketed under the trade
names ISASMELT and AUSMELT. This is not limiting and any equivalent
oxy-air/fuel furnace may be used in its stead.
Exam 1e Of The Method Of The Invention
Three tons of a sample of laterite ore from Indonesia were fed to a small
oxy-air/fuel furnace built by the AUSMELT company. The furnace was
fired with natural gas. The ore was fed to the furnace at 100 kilograms
per hour. A small amount of silica sand was added as a flux. The slag
was tapped from the furnace at a temperature of 1500°C. The ore and
slag were analyzed and the results are given in the following table. The
degree of iron reduction is expressed in the form of the valency of the
iron. It will be noted that the proportion of the reduced form of iron to the
oxidized form is about 53%.
P.17635/acm
219965b
Page 12
Ni Co Fe Fe3+ Fe2+ Si02 Mg0 Ca0 AI2U3
Laterite1.95 0.10 19.4 18.3 1.1 33.4 24.0 0.08 0.78
Slag 1.73 0.12 17.2 8.0 9.2 42.2 26.2 1.63 1.93
Since the smelting furnace and the DC arc furnace were not at the same
location it was necessary to recover the smelting furnace slag by
granulation in water.
The slag was transferred to the DC arc furnace. The DC arc furnace was
operated at 200kVA. Slag was fed to the DC arc furnace together with
coke obtained from a coke oven. Four separate tests were conducted in
which the ratio of furnace slag to coke was varied. The slag and metal
recovered from the DC arc furnace were weighed and analyzed and the
results are given in the table attached.
Since the AUSMELT furnace was very small and heat losses were large
it was not possible to measure the energy consumption during the trial.
it is calculated that the energy which would be required to heat the ore
and to melt it to slag for liquid transfer to the DC arc furnace would be 0,4
Mwh per ton of slag transferred. in addition some of the iron was
reduced consuming 0,065 Mwh per ton slag. The energy measured
during the last three DC arc furnace trials was an average of 0,68Mwh per
P.1 T635/aan
2199656
Page 13
ton slag transferred. If hot transfer had taken place, the energy
consumption would have been 0,215 Mwh per ton slag. This corresponds
to 0,2 Mwh per ton ore smelted. Thus the saving in electrical energy by
using the oxy-air/fuel furnace (if hot transfer had taken place) was 0,433
Mwh per ton of ore treated. Lower cost fuel energy was used to heat the
ore in the oxy-air/fuel furnace.
15
P.17635/aan
2199656
Page 14
Recipe .' 1 ' . Z 3.
Ausmeit slag, kg 501 401 398 512
Red, kg Coke/t feed slag26.2 28.7 35.6 40.4
.
Tapping temp, C 1551 1582 1633 1618
ProdOGtynassesi : .::
k~.... :
.. .:
.;. ..
,
Spent slag 491 381 357 441
Alloy 27.5 21.7 37.7 47.2
:: ses 9'0 '~
g .....
: ,
.
.:
.
Vila
2~rralx
NIO 0.17 0.15 0.09 0.07
Co0 0.04 0.04 0.02 0.02
Fe0 16.6 14.8 11.6 9.87
Si02 42.2 44.7 47.3 49.3
Mg0 28.1 29.4 31.1 31.6
Ca0 1.93 1.94 2.05 2.18
AI203 2.87 2.53 2.79 2.69
...... ~ses~ 9~ ::
;plid~ , .: : ..
~nei ..
:.:
Co 2.5 1.7 1.2 1.0
Fe 42.3 64.8 79.6 83.i
Ni 55.1 33.5 19.2 15.9
Si 0.09 0.11 0.33 0.26
C 0.0 0.0 0.05 0.26
P 0.18 0.32 0.15 0.33
S 0.13 0.15 0.19 0.25
Element
recovery:
irt
alloy,
%.
N l 90.6 92.9 95.5 96.6
Co 54.1 65.4 78.3 84.7
Fe 6.5 18.7 39.3 46.6
_~__.
IVaieS: neu - r~euuwarm
NEC - Net Energy Consumption
P.1763,5/aan