Note: Descriptions are shown in the official language in which they were submitted.
2 1 99657
- 1 - P14525
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION:
The present invention relates to a biosensor for
quantitating a biochemical substrate (specific compound)
contained in a sample liquid such as whole blood, urine,
fruit juice and the like, with accuracy, speed and ease,
and a method for quantitating a biochemical substrate by
the biosensor. More particularly, the invention relates
to a biosensor for electrochemically quantitating a
concentration of a biochemical substrate in a sample
liquid by reacting biochemical substrates such as glucose
or cholesterol with an oxidoreductase which can react
with specificity to the biochemical substrates, and a
method for quantitating a biochemical substrate using
thereof.
2. DESCRIPTION OF THE RELATED ART:
Recently, biosensors have been proposed which can
easily quantitate a specific compound (biochemical sub-
strate) in a sample liquid such as a biological sample or
a food without diluting or stirring the sample liquid.
For example, Japanese Laid-Open Patent Publica-
tion No. 3-202764 discloses a biosensor including an
electrode system formed on an insulating substrate by
screen printing or the like, a reaction layer formed on
the electrode system and a space provided as a sample
supply path by using a cover and a spacer. The reaction
layer contains a hydrophilic polymer, an oxidoreductase,
and c~ electron acceptor. Such a biosensor can
quantitate the concentration of a biochemical substrate
in a sample liquid as follows: First, the sample liquid
21 9q657
- 2 - P14525
is dropped on the space in the biosensor so as to be sup-
plied to the reaction layer due to capillary phenomenon,
thereby dissolving the reaction layer. This causes an
enzyme reaction between the biochemical substrate in the
sample liquid and the oxidoreductase in the reaction
layer, whereby the electron acceptor in the reaction
layer is reduced. After the completion of the enzyme
reaction, the reduced electron acceptor is electrochemi-
cally oxidized, whereby the concentration of the biochem-
ical substrate in the sample liquid is quantitated by anoxidation current value.
In the above-described biosensor, potassium
ferricyanide is often used as the electron acceptor.
Biosensors using potassium ferricyanide have excellent
stability, can be produced at a low cost and thus appro-
priate in terms of mass production. However, the biosen-
sor using potassium ferricyanide as the electron acceptor
is associated with a second-order reaction velocity
between the potassium ferricyanide and the oxidoreductase
that is slower than that of a biosensor using an electron
acceptor such as quinone derivatives or another metallic
complex which is unstable or produced at a high cost.
Accordingly, in the biosensor using potassium
ferricyanide, an enzyme reaction takes substantially long
time, thereby causing a problem of not being able to
promptly quantitate the concentration of the biochemical
substrate.
Additionally, several biosensors are known which
employ ferrocene as an electron acceptor which has a
faster second-order reaction velocity than those of
potassium ferricyanide or derivatives thereof.
21 99657
P14525
Japanese Laid-Open Patent Publication No. 2-
240555 discloses a glucose sensor including a working
electrode having a photo-curing resin film containing a
ferrocene compound and a photo-curing resin film contain-
ing glucose oxidase sequentially provided on the surfaceof the working electrode. Japanese Laid-Open Patent
Publication No. 2-99851 discloses a glucose sensor
including a working electrode having a ferrocene com-
pound-containing layer and a glucose oxidase-immobilized
layer on the ferrocene compound-containing layer on the
surface of the working electrode. In the above-mentioned
biosensors, l,l'-dimethyl ferrocene, ferrocene, i.e.,
bis(cyclopentadienyl)iron(II), vinyl ferrocene or the
like is used as the ferrocene compound.
Japanese Laid-Open Patent Publication No. 5-
256812 discloses a glucose sensor including a layer
carrying glucose oxidase and a ferrocene compound on a
working electrode and a means for maintaining a prede-
termined temperature in the vicinity of the working elec-
trode. In this biosensor, ferrocene and/or derivatives
thereof is used as the ferrocene compound.
However, any ferrocene compound used in the
above-described glucose sensors normally exists as a
reduced form. Therefore, in order to achieve electron
transfer from the biochemical substrate to the working
electrode by an enzyme reaction, the ferrocene compound
needs to be converted into oxidized form on the elec-
trode.
Japanese Laid-Open Patent Publication No. 6-3316
discloses a glucose sensor (a modified electrode) modi-
21 9~657
P14525
fied by a hydrophobic redox substance (e.g., a ferrocene)which has been ionized in advance in an aqueous solution,
and a hydrophilic enzyme (e.g., glucose oxidase) on a
surface of a conductive electrode. The glucose sensor is
produced as follows: Ferrocene is electrolyzed in a
phosphate buffer to form a solution containing
ferricinium ions. Then, a glucose oxidase is added to
the solution. The resultant mixed solution is applied to
or electrodeposited on the surface of the conductive
electrode.
However, the above-described modified electrode
has a problem in that the phosphate ion and the
ferricinium ion form an ion-like complex which renders
the surface of the electrode inactive. Moreover, a step
of electrolyzing ferrocene is required in order to
produce the modified electrode. As a result, increased
production time and increased cost are required.
SUMMARY OF THE INVENTION
According to one aspect of the present invention,
a biosensor includes: an insulating substrate; an elec-
trode system formed on the insulating substrate which has
a working electrode and a counter electrode; and a
reaction layer formed on the insulating substrate which
contains an oxidoreductase and an electron acceptor. The
electron acceptor is ferricinium ion derived from
ferrocene electrolyte.
In one embodiment of the present invention, the
ferrocene electrolyte is selected from the group consist-
ing of ferrocenium hexafluorophosphate and ferrocenium
21 99~57
P14525
-- 5
tetrafluoroborate.
In one embodiment of the present invention, the
reaction layer further comprises at least one surfactant.
In one embodiment of the present invention, the
reaction layer further comprises at least one hydrophilic
polymer.
In one embodiment of the present invention, the
oxidoreductase is selected from the group consisting of
glucose oxidase; glucose dehydrogenase; lactate oxidase;
lactate dehydrogenase; uricase; cholesterol oxidase; a
combination of cholesterol oxidase and cholesterol
esterase; a combination of glucose oxidase and invertase;
a combination of glucose oxidase, invertase and mutaro-
tase; and a combination of fructose dehydrogenase and
invertase.
According to another aspect of the present inven-
tion, a method is disclosed for quantitating the concen-
tration of a biochemical substrate in a sample liquid by
using a biosensor including an insulating substrate, an
electrode system formed on the insulating substrate which
has a working electrode and a counter electrode and a
reaction layer provided on the insulating substrate which
contains an oxidoreductase and an electron acceptor. The
method includes the steps of: adding the sample liquid to
the reaction layer; and ~etecting a response current
value by applying a voltage between the working electrode
and the counter electrode. The electron acceptor is
ferricinium ion derived from ferrocene electrolyte.
21 q9657
- 6 - Pl4525
Thus, the invention described herein makes
possible the advantages of (1) providing a biosensor for
promptly quantitating a concentration of a biochemical
substrate with sufficiently short enzyme reaction time;
(2) providing a biosensor for quantitating a concentra-
tion of a biochemical substrate with accuracy without
deteriorating a detecting sensitivity; (3) providing a
biosensor produced at sufficiently low cost; and (4)
providing a method for quantitating a concentration of a
biochemical substrate using the above-mentioned biosensor
with accuracy and speed.
These and other advantages of the present inven-
tion will become apparent to those skilled in the art
upon reading and understanding the following detailed
description with reference to the accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
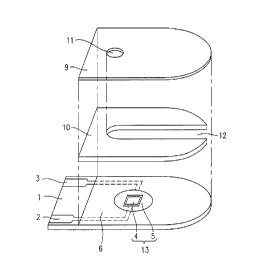
Figure 1 is an exploded isometric view showing a
biosensor according to an example of the present inven-
tion in which a reaction layer is omitted; and
Figure 2 is a schematic cross-sectional view
showing a biosensor according to an example of the
present invention in which a reaction layer is disposed
on an insulating substrate.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
A biosensor according to the present invention
includes an insulating substrate, an electrode system
formed on the insulating substrate and a reaction layer
2 1 99657
P14525
disposed on the insulating substrate. The electrode
system includes a working electrode and a counter elec-
trode.
The insulating substrate may be a synthetic resin
plate made, for example, of polyethylene terephthalate.
The electrode system including the working
electrode and the counter electrode may be provided on
the insulating substrate by a known method. For example,
leads are formed on the insulating substrate. Then, the
working electrode and the counter electrode are provided
so as to be connected to the respective leads and insu-
lated from each other. The leads and the electrodes may
be made of any of the known conductive materials.
Examples of the conductive material include carbon,
silver, platinum, gold and palladium.
The reaction layer contains at least one oxidore-
ductase and at least one electron acceptor.
The electron acceptor used in the present inven-
tion is ferricinium ion derived from ferrocene electro-
lyte. The term "ferrocene electrolyte" herein refers to
a salt which generates ferricinium ion ([(C5Hs)zFe]+) upon
preparation of a solution with an oxidoreductase which
will be described later. An anion composing the
ferrocene electrolyte is not specifically limited.
Preferred ferrocene electrolytes are, for example,
ferrocenium hexafluorophosphate and ferrocenium
tetrafluoroborate which are available from Aldrich Inc.
Although the concentration of the ferrocene elec-
21 99657
- 8 - P14525
trolyte is not specifically limited, it is preferably
about 1 to about 100 mM in the sample liquid when the
reaction layer is dissolved in a sample liquid. When the
concentration of the ferrocene electrolyte is smaller
than 1 mM in the reaction layer, a measurable range of
the concentration of a biochemical substrate may become
extremely small. When the concentration of the ferrocene
electrolyte exceeds 100 mM in the reaction layer, the
biosensor may require an increased production cost and
may cause fluctuation in a response current value and
poor stability during the storage since the reaction
layer can be broken during the formation thereof.
The oxidoreductase used in a conventional biosen-
sor such as a glucose sensor, a cholesterol sensor, a
lactic acid sensor, a uric acid sensor and a sucrose
sensor can be used in the present invention. Examples of
oxidoreductase include glucose oxidase (hereinafter,
referred to as GOD), glucose dehydrogenase, lactose
oxidase, lactose dehydrogenase, uricase, cholesterol
oxidase (hereinafter, referred to as ChOD), cholesterol
esterase (hereinafter, referred to as ChE), invertase,
mutarotase and fructose dehydrogenase, and combinations
thereof. In the case where a biosensor according to the
present invention is a glucose sensor, GOD or glucose
dehydrogenase may be used as the oxidoreductase. In the
case where a biosensor according to the present invention
is a lactic acid sensor, lactose oxidase or lactose
dehydrogenase may be used as the oxidoreductase. In the
case where a biosensor according to the present invention
is a uric acid sensor, uricase may be used as the oxido-
reductase. In the case where a biosensor according to
the present invention is a cholesterol sensor, ChOD or a
21 99657
P14525
combination of ChOD and ChE may be used as the oxidore-
ductase. In the case where a biosensor according to the
present invention is a sucrose sensor, a combination of
GOD and invertase; a combination of GOD, invertase and
mutarotase; or a combination of fructose dehydrogenase
and invertase may be used as the oxidoreductase.
The content of the oxidoreductase used in the
present invention is not specifically limited and an
appropriate content can be suitably chosen by those
skilled in the art. For example, when GOD is used, the
content of GOD is preferably about 0.1 to about 5 units
per biosensor. When a combination of ChOD and ChE is
used, the content of ChOD is preferably about 0.1 to
about 5 units per biosensor and the content of ChE is
preferably about 0.1 to about 5 units per biosensor. The
term "1 unit" herein refers to an amount of oxidoreduc-
tase required for oxidizing 1 ,umol of biochemical sub-
strate to be quantitated in one minute.
The reaction layer may further contain at least
one of hydrophilic polymers. Examples of the hydrophilic
polymer include cellulose derivatives such as carboxy
methyl cellulose (hereinafter, referred to as CMC),
hydroxyethyl cellulose, hydroxypropyl cellulose, methyl
cellulose, ethyl cellulose, ethyl hydroxy cellulose and
carboxymethyl ethyl cellulose; polyvinyl pyrrolidone;
polyvinyl alcohol; gelatin or its derivatives; acrylic
acid or its salts; methacrylic acid or its salts; starch
or its derivatives; and maleic anhydride or its salt. In
particular, CMC is preferred.
When the above-mentioned ferricinium ion is
21 9~t657
P14525
-- 10 --
employed as the electron acceptor, ferrocene is produced
during sequential enzyme reactions. Since the ferrocene
generally has low water-solubility, the produced
ferrocene molecules deposit on the reaction layer due to
water contained in a sample liquid. Thus, the reaction
layer used in the present invention preferably contains
at least one of surfactant. Examples of the surfactant
include refined lecithin derived from soybean, octyl
thioglucoside, sodium cholate, dodecyl-~-maltoside,
sodium deoxycholic acid, sodium taurodeoxycholate,
Triton-X (registered trademark), Lubrol PX (registered
trademark), DK-ester (registered trademark), BIGCHAP
(registered trademark) and DeoxyCHAP (registered trade-
mark). Specifically, the refined lecithin and the octyl
thioglucoside are preferred.
According to the present invention, a lecithin
layer containing the above-mentioned refined lecithin may
be further provided on the reaction layer. In the case
where the lecithin layer is provided on the reaction
layer, a sample liquid can be easily supplied to the
reaction layer.
Hereinafter, a preferred embodiment of a method
for producing a biosensor according to the present
invention will be described with reference to Figures 1
and 2.
First, a conductive material such as silver paste
is printed on an insulating substrate 1 by screen print-
ing to form leads 2 and 3. Then, another conductive
material containing a resin binder is printed on the
insulating substrate 1 to form a working electrode 4
21 99657
P14525
-- 11 --
which makes contact with the lead 2.
Then, insulating paste is printed on the insulat-
ing substrate 1 to form an insulating layer 6. The
insulating layer 6 covers the peripheral portion of the
working electrode 4, so as to expose a fixed area of the
working electrode 4. As is shown in Figure 1, the
insulating layer 6 also covers part of the leads 2 and 3.
Around the working electrode 4 is formed a ring-shaped
counter electrode 5 out of a conductive material contain-
ing a resin binder. The counter electrode 5 is in
contact with the lead 3. In this manner, an electrode
system 13 including the working electrode 4 and the
counter electrode 5 is formed on the insulating substrate
1.
Alternatively, the biosensor according to the
present invention may be provided with a three-electrode
system including a reference electrode (not shown) in
addition to the working electrode 4 and the counter
electrode 5 formed on the insulating substrate 1. The
three-electrode system provides stable response current,
thereby further stabilizing the measurement accuracy.
A reaction layer 7 may be formed on the insulat-
ing substrate 1 as follows:
.
An aqueous solution containing the hydrophilic
polymer is dropped and dried on the electrode system 13
to form a hydrophilic polymer layer. On the other hand,
predetermined amounts of the oxidoreductase and the
ferrocene electrolyte are dissolved in water. Prefera-
bly, a few drops of surfactant are added to this aqueous
21 9~657
Pl4525
- 12 -
solution. Then, the obtained aqueous solution containingthe oxidoreductase and the ferrocene electrolyte is added
dropwise on the hydrophilic polymer layer. As a result,
the hydrophilic polymer is dissolved in the aqueous solu-
tion. Then, the dissolved hydrophilic polymer layer isdried so that the reaction layer 7 is formed which is a
hydrophilic polymer layer incorporating an oxidoreductase
and an electron acceptor. Since the incorporation of the
oxidoreductase and the electron acceptor (i.e.,
ferricinium ion) into the hydrophilic polymer layer does
not require further steps such as stirring, only the
hydrophilic polymer exists at the interface between the
reaction layer 7 and the electrode system 13. In other
words, since the oxidoreductase and the electron acceptor
do not make contact with the surface of the electrode
system 13, inactivation of the surface of the electrode
system 13 can be avoided which is caused by adsorption of
protein on the surface.
In the case where the hydrophilic polymer layer
is not used, an aqueous solution containing oxidoreduc-
tase and ferrocene electrolyte is added dropwise and
dried directly on the electrode system 13.
For repeated application of the biosensor, the
oxidoreductase and the ferrocene electrolyte may be
immobilized on the hydrophilic polymer layer through
crosslinking with glutaraldehyde or immobilized on the
hydrophilic polymer layer together with a polymeric
material such as nitrocellulose or a conventional ion-
exchange membrane.
As shown in Figure 2, the reaction layer 7 is
2 1 99657
P14525
- 13 -
formed so as to cover the whole electrode system 13.
Then, if necessary, a predetermined amount ofsolution of refined lecithin in an organic solvent such
as toluene is spread and dried on the reaction layer 7 to
form a lecithin layer 8. Finally, as shown in Figure 1,
spacer 10 provided with a sample supply path 12 and a
cover 9 provided with a hole 11 are disposed in this
order above the insulating substrate 1 by a known method.
Thus, the biosensor according to the present invention is
produced.
A concentration of a biochemical substrate
included in a sample liquid is quantitated by using the
biosensor according to the present invention, for exam-
ple, in the following manner.
First, a sample liquid containing a biochemical
substrate is added to a reaction layer 7 directly or via
the sample supply path 12. The reaction layer 7 is
dissolved by the sample liquid. After a predetermined
period of time, a predetermined level of pulse voltage
~e.g., +0.5 V) is anodically applied to the working
electrode 4 on the basis of a voltage at the counter
electrode 5. The value of the resultant response current
is measured in a known manner. Then, the response
current value is converted into a concentration value of
the biochemical substrate by using a calibration curve
which represents the relationship between the concentra-
tion and the response current value of a biochemical sub-
strate. The calibration curve is obtained in advance by
measuring known concentrations of the biochemical sub-
strate.
21 9~657
- 14 - P14525
Hereinafter, a mechanism for obtaining response
current value using the biosensor according to the
present invention will be described.
For example, in the case where a sample liquid
contains glucose as a biochemical substrate, GOD is used
in the reaction layer 7. When the sample liquid is
contacted with the reaction layer 7, the reaction layer 7
is dissolved by the sample liquid and the glucose in the
sample liquid is oxidized by the GOD, thereby producing
gluconic lactone. At this point, electrons generated
through an oxidize reaction of the glucose reduce
ferricinium ions existing in the reaction layer 7 into
ferrocene. When the above-mentioned pulse voltage is
applied to the working electrode, an oxidation current
results which oxidizes the ferrocene. The amount of the
oxidation current is measured as a reaction current value
which is proportional to the concentration of glucose
existing in the sample liquid.
In the case where a sample liquid contains
cholesterol ester and cholesterol as biochemical sub-
strates, ChE and ChOD are used in the reaction layer 7.
When the sample liquid is contacted with the reaction
layer 7, the reaction layer 7 is dissolved by the sample
liquid and the cholesterol ester in the sample liquid is
converted into cholesterol by ChE. Then, all of the
cholesterol in the sample liquid is oxidized by ChOD,
thereby producing cholestenone. At this point, electrons
generated through an oxidizing reaction of the cholester-
ol reduce ferricinium ions existing in the reaction
layer 7 into ferrocene. When the above-mentioned pulse
voltage is applied to the working electrode, an oxidation
21 99657
P14525
- 15 -
current results which oxidizes the ferrocene. The amount
of the oxidation current is measured as a reaction
current value which is proportional to the total concen-
tration of the cholesterol ester and cholesterol existing
in the sample liquid.
Accordingly, the concentration of a biochemical
substrate included in a sample liquid can be quantitated
by the biosensor according to the present invention.
Furthermore, since ferricinium ion used as an electron
acceptor has a second-order reaction velocity faster than
that of the ferricyanide ion used conventionally, the
biosensor according to the present invention is capable
of quantitating the concentration of the biochemical sub-
strate in the sample liquid with accuracy and speed.
The biosensor according to the present invention
can be effectively used for quantitating the concentra-
tion of the biochemical substrate included in biological
samples such as whole blood, plasma, serum and urine,
materials used in food industry and product thereof
(e.g., fruit juice), or the like.
Examples
Hereinafter, the present invention will be
described by way of illustrative examples with reference
to the accompanying drawings. The present invention,
however, is not limited to the following examples. In
the accompanying drawings, same reference numerals
designate same component and the description thereof is
partially omitted for the sake of simplification.
Example l
21 99657
P14525
- 16 -
A glucose sensor was produced as follows as an
example of a biosensor according to the present inven-
tion.
As shown in Figure 1, silver paste was printed by
screen printing on an insulating substrate 1 made of
polyethylene terephthalate to form leads 2 and 3. Then,
conductive carbon paste containing a resin binder was
printed on the insulating substrate 1 to form a working
electrode 4. The working electrode 4 was formed so as to
be in contact with the lead 2.
Next, insulating paste was printed on the insu-
lating substrate 1 to form an insulating layer 6. The
insulating layer 6 covered the peripheral portion of the
working electrode 4, so as to expose a fixed area of the
working electrode 4. Moreover, conductive carbon paste
containing a resin binder was printed on the insulating
substrate 1 to form a ring-shaped counter electrode 5 so
that the ring-shaped counter electrode 5 was in contact
with the lead 3.
Then, an aqueous solution containing GOD and
ferrocenium hexafluorophosphate (produced by Aldrich
Inc.) was added and dried on the electrode system 13
(i.e., the working electrode 4 and the counter elec-
trode 5) to form a reaction layer 7. A toluene solution
containing lecithin was added dropwise on the reaction
layer 7 to spread over the entire surface of the reaction
layer 7 and dried to form a lecithin layer 8. A spac-
er 10 and a cover 9 were adhered in this order on the
lecithin layer 8, thereby producing the glucose sensor.
21 99657
- 17 - P14525
3 ,ul of 30 mg/dl aqueous glucose solution was
added to the above-described glucose sensor via a sample
supply path 12 in the spacer 10. The sample liquid
reached as high as the height of the hole 11 provided in
the cover 9 and dissolved the reaction layer 7. Then, 60
seconds after the addition of the sample liquid, a pulse
voltage of +0.5 V on the basis of a voltage at the
counter electrode 5 was anodically applied to the working
electrode 4. A response current value was measured 5
seconds after the voltage application.
Furthermore, response current values were mea-
sured with regard to aqueous glucose solutions of
45 mg/dl and 90 mg/dl, respectively in the same manner as
described above by using a fresh glucose sensor for each
measurement. The thus-obtained response current values
were proportional to the respective concentrations of the
aqueous glucose solutions.
Example 2
A glucose sensor was produced in the same manner
as described in Example 1 except for using ferrocenium
tetrafluoroborate (produced by Aldrich Inc.) instead of
the ferrocenium hexafluorophosphate. By using this
glucose sensor, response current values were measured for
aqueous glucose solutions having the same concentrations
as those described in Example 1 in the same manner as
described in Example 1. The obtained response current
values were proportional to the respective concentrations
of the aqueous glucose solutions.
Example 3
An electrode system 13 was formed on an insulat-
21 99657
P14525- 18 -
ing substrate 1 in the same manner as described in theExample 1.
Then, an aqueous solution containing 0.5 ~ by
weight of CMC was added dropwise on the electrode system
13 (i.e., working electrode 4 and the counter electrode
S) and dried to form a CMC layer. An aqueous solution
containing GOD and ferrocenium hexafluorophosphate was
added dropwise and dried on the CMC layer to form a reac-
tion layer 7. Furthermore, a toluene solution containinglecithin was added dropwise on the reaction layer 7 to
spread over the entire surface of the reaction layer 7
and dried to form a lecithin layer 8. A spacer 10 and a
cover 9 were adhered in this order on the lecithin lay-
er 8, thereby producing the glucose sensor.
3 ~l of 30 mg/dl aqueous glucose solution wasadded as a sample liquid to the above-described glucose
sensor via a sample supply path 12 in the spacer 10. The
sample liquid reached as high as a height of a hole 11
provided in the cover 9 and dissolved the reaction
layer 7. Then, 60 seconds after the addition of the
sample liquid, a pulse voltage of +0.5 V on the basis of
a voltage at the counter electrode 5 was anodically
applied to the working electrode 4. A response current
value was measured 5 seconds after the voltage applica-
tion.
Furthermore, response current values were mea-
sured with regard to glucose aqueous solutions of45 mg/dl and 90 mg/dl, respectively, in the same manner
as described above by using a fresh glucose sensor for
each measurement. The thus-obtained response current
2 1 99657
P14525
-- 19 --
values were proportional to the respective concentrations
of the aqueous glucose solutions.
Example 4
A glucose sensor was produced in the same manner
as described in Example 3 except for using ferrocenium
tetrafluoroborate instead of the ferrocenium
hexafluorophosphate. By using this glucose sensor,
response current values were measured in the same manner
as described in Example 3 for aqueous glucose solutions
having the same concentrations as those described in
Example 3. The obtained response current values were
proportional to the respective concentrations of the
aqueous glucose solutions.
Example 5
A glucose sensor was produced in the same manner
as described in Example 3 except that refined lecithin
derived from soybean (produced by SIGMA Chemical Co.) was
added as a surfactant to the aqueous solution containing
GOD and ferrocenium hexafluorophosphate. This aqueous
solution was added dropwise on the CMC layer. By using
this glucose sensor, response current values were mea-
sured in the same manner as described in Example 3 for
aqueous glucose solutions having the same concentrations
as those described in Example 3. The obtained response
current values were proportional to the respective
concentrations of the aqueous glucose solutions.
Example 6
A glucose sensor was produced in the same manner
as described in Example 3 except that ferrocenium
tetrafluoroborate was used instead of ferrocenium
21 99657
P14525
- 20 -
hexafluorophosphate and that refined lecithin derivedfrom soybean was added as a surfactant to the aqueous
solution containing GOD and ferrocenium
tetrafluoroborate. This aqueous solution was added
dropwise on the CMC layer. By using this glucose sensor,
response current values were measured in the same manner
as described in Example 3 for aqueous glucose solutions
having the same concentrations as those described in
Example 3. The obtained response current values were
proportional to the respective concentrations of the
aqueous glucose solution.
Example 7
An electrode system 13 was formed on an insulat-
ing substrate 1 in the same manner as described in
Example 1.
Then, an aqueous solution containing 0.5 ~ by
weight of CMC was added dropwise and dried on the elec-
trode system 13 (i.e., the working electrode 4 and thecounter electrode 5) to form a CMC layer. Octyl
thioglucoside was added as surfactant to an aqueous solu-
tion containing ChE, ChOD and ferrocenium
hexafluorophosphate. The obtained aqueous solution was
added dropwise and dried on the CMC layer to form a
reaction layer 7. Furthermore, the toluene solution
containing lecithin was added dropwise on the reaction
layer 7 to spread over the entire surface of the reaction
layer 7 and dried to form a lecithin layer 8. A spac-
er 10 and a cover 9 were adhered in this order on thelecithin layer 8, thereby producing the cholesterol
sensor.
21 99657
P14525
- 21 -
3 ,ul of standard solution containing 50 mg/dl ofcholesterol and 150 mg/dl of cholesterol ester was added
as a sample liquid to the above-described cholesterol
sensor via a sample supply path 12 in the spacer 10. The
sample liquid reached as high as a height of a hole 11
provided in the cover 9 and dissolved the reaction
layer 7. Then, 180 seconds after the addition of the
sample liquid, a pulse voltage of +0.5 V on the basis of
a voltage at the counter electrode 5 of the electrode
system 13 was anodically applied to the working elec-
trode 4. A response current value corresponding to the
total concentration of the cholesterol ester and choles-
terol was measured 5 seconds after the voltage applica-
tion.
Furthermore, response current values were mea-
sured with regard to a standard solution containing
300 mg/dl of cholesterol ester and 100 mg/dl of choles-
terol and a standard solution containing 450 mg/dl of
cholesterol ester and 150 mg/dl of cholesterol, respec-
tively in the same manner as described above by using a
fresh cholesterol sensor for each measurement. The thus-
obtained response current values were proportional to the
respective total concentrations of the cholesterol ester
and cholesterol existing in the sample liquids.
Example 8
A cholesterol sensor was produced in the same
manner as described in Example 7 except that ChE was not
contained in the reaction layer 7. By using this choles-
terol sensor, reaction current values were measured for
the same standard solutions containing cholesterol ester
and cholesterol, respectively. The thus-obtained re-
21 99657
P14525- 22 -
sponse current values were only proportional to therespective concentrations of cholesterol in the standard
solutions.
Example 9
A cholesterol sensor was produced in the same
manner as described in Example 7 except for using
ferrocenium tetrafluoroborate instead of the ferrocenium
hexafluorophosphate. By using this cholesterol sensor,
response current values were measured in the same manner
as described in Example 7 for standard solutions contain-
ing cholesterol ester and cholesterol of the same concen-
trations as those described in Example 7. The obtained
response current values were proportional to the respec-
tive total concentrations of cholesterol ester andcholesterol in the sample liquids.
Example 10
A cholesterol sensor was produced in the same
manner as described in Example 7 except that ChE was not
contained in the reaction layer 7 and ferrocenium
tetrafluoroborate was used instead of the ferrocenium
hexafluorophosphate. By using this cholesterol sensor,
reaction current values were measured for the same
standard solutions containing cholesterol ester and
cholesterol, respectively. The thus-obtained response
current values were only proportional to the respective
concentrations of cholesterol in the standard solutions.
Various other modifications will be apparent to
and can be readily made by those skilled in the art
without departing from the scope and spirit of this
invention. Accordingly, it is not intended that the
21 9q657
P14525
- 23 -
scope of the claims appended hereto be limited to the
description as set forth herein, but rather that the
claims be broadly construed.