Note: Descriptions are shown in the official language in which they were submitted.
__
WO 96/12171 PGT/US95/13308
HIGH BPEED FLOW CYTOMETER DROPLET FORMATION SYSTEM
I. TECHNICAL FIELD
Generally this invention relates to droplet flow cytometers
such as are used for the analysis and sorting of substances
contained within separate droplets. Specifically, the invention
relates to aspects of such systems which act to form regular
droplets after exit from a nozzle orifice.
II. BACRGROOND ART
Droplet flow cytometers have been in clinical and research
use for many years. Basically, the systems act to position small
amounts of a substance within individual droplets of a sheath
fluid. These droplets can be made uniform by utilizing an
oscillator which emits a predominant frequency. These
oscillations are usually applied to the nozzle container. Since
droplet flow cytometry is heavily utilized in both research and
clinical environments, such systems have been the subject of much
refinement. One of the facets of these systems which has been
particularly challenging, however, is the aspect of controlling
the drop formation. As to this aspect it has not only been
difficult to practically achieve processing rates of much more
than 40 kilohertz, it has also been difficult to deal with the
incidents of using relatively high power to drive the oscillators
involved.
It should be noted that each of the challenges faced in the
field of droplet formation for flow cytometers is largely unique
to that field. Even seemingly similar fields such as those
involving channel-type flow cytometers are not very analogous as
they do not face such problems. Their operation as continuous
flow devices rather than droplet formation devices makes much of
the understandings available in that field inapplicable to the
challenges and problems faced in flow cytometry droplet formation
systems.
To some degree the challenges for droplet formation may be
the result of the fact that although drop formation has been
modeled with significant theoretical detail, in practice it still
WO 96112171 PCT/US95/13308
remains a somewhat empirical subject. While on one level
exhaustive mathematical predictions are possible, in practice
these predictions can be greatly tempered - and are often
revised - by the fact that materials limitations, inherent
substance variations, and the like contribute heavily to the end
result. A number of "advances" in this field have even proved
to be either unnecessary or unworkable in practice.
The level of oscillation energy required in order to achieve
uniform droplet formation has, prior to the present invention,
been very subject to empirical constraints. This power (often
expressed as a voltage amplitude applied to a piezoelectric
crystal oscillator) has previously been in the ten volt range.
Unfortunately, this relatively high voltage not only results in
a need for more robust circuitry, but it also has the undesirable
practical consequence of resulting in undesirable electromagnetic
emissions. These emissions can impact the sensitivity of the
flow cytometer or other nearby equipment. Further, as the desire
for higher processing frequencies is pursued, this problem is
compounded. Although these problems have been know for years,
prior to the present invention it has apparently been an accepted
attitude that in order to achieve higher frequencies, still
higher oscillation energies are a physical requirement. This
invention proves this expectation to be untrue. An example of
the extremes to which this rational had been applied is shown in
U.S. Patent No. 4361400 to Gray where droplet formation
frequencies in the range of 300 to 800 kilohertz had been
achieved. This design had required an oscillator powered by
approximately 80 volts. The apparent physical requirement of
higher powers in order to achieve higher droplet frequencies may
have been one reason that most practical droplet flow cytometers
operated only in the range of 10 to 50 kHz. The present
invention shows that such a relationship is not a physical
requirement and, in fact, shows that droplet formation speeds in
the 100-200 kHz range are actually possible with only millivolts
s
of power applied to an oscillator.
-2-
WU 96/121?i ~ ~ ~ ~ PCT/US95/13308
Yet another problem practically encountered in this field
was the challenge of resonances existing within the nozzle
assembly. Again, this appears to have simply been accepted as
a necessary incident of workable systems and may have resulted
in an attitude among those having ordinary skill in the art that
it was not practical to vary frequency without unacceptable
changes in the performance of the entire system. There also
seems to have been some confusion as to the appropriate way to
apply the droplet forming oscillations. U.S. Patent No. 4302166
shows that the oscillations are applied to the nozzle container
perpendicular to the fluid flow, whereas, U.S. Patent No. 4361400
suggests applying the oscillations to the nozzle container
parallel to the lines of flow. In fact, the present invention
discloses that each of these systems are suboptimal in that they
may even act to generate the resonances and variations in
frequency response of the nozzle system.
An even more paradoxical situation exists with respect to
the problem of maintaining laminar flow within the nozzle system
of a droplet flow cytometer. Although those having ordinary
skill in this field have known for years that maintaining laminar
flow was desirable, until the present invention, practical
systems utilizing replacement tips have not been optimally
designed so as to achieve the goal of truly laminar flow. For
instance, U.S. Patent No. 4361400 as well as the 1992 publication
by Springer Laboratory entitled "Flow Cytometry And Cell
Sorting", each show replaceable nozzle tip designs in which
laminar flow is disrupted at the junction between the nozzle body
and the nozzle tip. Again, such designs seem to present almost
a paradox in that they obviously are not optimum from perspective
of a goal which has long been known as those having ordinary
skill in the art. The present invention not
only recognizes this goal but also demonstrates that a solution
has been readily available.
Yet another problem encountered in this field is the need
to vary parameters to optimize actual conditions encountered in
-3-
~~~~32~
WO 96/12171 PGT/US95113308
processing. Again theory and practice did not mix well. While
systems were usually designed for optimum conditions, in actual
usage such conditions rarely existed. Thus, as U.S. Patent No.
4070617 recognized, designs which allow variation of the
substance output velocity within the sheath fluid were desirable.
Although such systems permitted some variation, it was recognized
that such variations necessarily made conditions within the flow
cytometer suboptimal for the simple reason that there is a very
definite physical relationship between the sheath substance and
drop parameters which must be maintained. Since these parameters
are well known to those having ordinary skill in the art (as also
indicated in U.S. Patent No. 4302166), the variations required
in practice appear to have been accepted as a necessary evil.
To some extent, the resulting reduced resolution appears to have
been accepted without question. Again, the present invention
realizes that approaches which moved conditions away from optimal
were not a necessary incident of adapting to conditions
practically encountered; it shows that solutions which allow for
variation and yet maintain optimal flow conditions are possible.
As explained, most of the foregoing problems had long been
recognized by those having ordinary skill in the art. Solutions,
however, had either been perceived as unlikely or not been
recognized even though the implementing elements had long been
available. This may also have been due to the fact that those
having ordinary skill in the art may not have fully appreciated
the nature of the problem or may have been due to an actual
misunderstanding of the physical mechanisms involved. These
appear to have included the misunderstanding that actually moving
the nozzle was the proper way to induce the droplet forming
oscillations and the simple failure to realize that it was
possible to coordinate the desire for replaceable nozzle tips
with the desire for laminar flow within the flow cytometer nozzle
assembly. Similarly, those skilled in the art had long attempted
to achieve higher frequency systems which were practically
implementable and had attempted to achieve variations which would
to the largest extent possible maintain optimal conditions.
-4-
CA 02201324 2005-03-02
Their attempts often led them away from the technical
directions taken by the present invention and may even have
resulted in the achievements cf the present invention being
considered an unexpected result of the approach taken.
III. DISCLOSURE 1~F THE INVENTION
The present invention involves a number of
improvements which are applicable to a flow cytometer
droplet system. These improvements each offer independent
to advantages and may be combinec. synergistically to produce a
great increase in the performance of droplet flow
cytometers. The preferred embodiment involves a
piezoelectric oscillator contained within the sheath fluid
above a continuously conver~~ing nozzle container. This
nozzle container acts to amplify the oscillations which are
directly and directionally coupled to the sheath fluid.
Further, the location of the substance introduction tube
may be adjusted within a convergence zone so as to vary the
rate at which the substance is introduced relative to the
2o rate at which the sheath fluid is introduced to maintain
optimal conditions. In addition, a replaceable nozzle tip
is fit within an edge insert and sealed on its outer
surface so as to maintain laminar flow and enhance the
amplification of the oscillation throughout the converging
nozzle body. As a result of the combination of these
various features, the present invention not only achieves
practical processing at frequencies of many multiples of
typical prior art devices, but it also achieves these
processing rates at oscillat=_on powers which are several
orders of magnitude less than chose typically utilized.
Accordingly, one of the objects of aspects of the
CA 02201324 2005-03-02
invention is to provide for a low power system which allows
high processing rates. In keeping with this object, one
goal is to achieve direct coupling of the oscillations to
the sheath fluid and thus minimize any losses associated
with material interfaces. Ir.. keeping with this object,
another goal is to provide for a system which actually
amplifies the oscillations ~o as to produce acceptable
fluid variations at the nozzle tip.
to Yet another object of an aspect of the invention is to
minimize the impacts of resonance frequencies within the
nozzle system. In keeping with this object, a goal is to
directionally couple the osci=~lations to the sheath fluid.
It is also a goal to isolate the oscillations from
i5 imparting upon the sheath fluid in more than the desired
direction.
A further goal of the i:zvention is to provide for a
system which allows for the maintenance of laminar flow
2o within the entire nozzle assembly while allowing for both
replaceable nozzle tips and for internal variations. The
present invention achieves the, first obj ect by providing a
design which avoids the unnecessary impacts of a seal on
the flow condition within the nozzle container. The second
25 object is achieved by providing a system which varies the
location at which a substance is introduced while still
maintaining optimal, laminar conditions.
Still another object of .gin aspect of the invention is
3o to provide for a practically implementable system. In
keeping with this object, one goal is to provide a system
which can be easily cleaned and for which components can be
f.
CA 02201324 2005-03-02
easily replaced. A goal is a:Lso providing a design which
can be relatively easily and inexpensively manufactured.
Naturally, further objects of aspects of the invention
are disclosed throughout other areas of the specification
and claims.
In accordance with another aspect of the present
inventi on, there is provided a method of creating a droplet
to from jet of a flow cytometer comprising the steps of:
a
a. establishing a :ZOZZle volume within a
continuously converging nozzle;
b. introducing a flow of sheath fluid into said
nozzle volume;
i5 c. introducing a flow of a substance within said
sheath fluid in said nozzle volume;
d. establishing an oscillator coupled to said nozzle
volume and located predominantly within said
nozzle volume such i~hat oscillations are coupled
2o from the oscillator to the sheath fluid without
passing through the nozzle;
e. applying an alternative voltage with an amplitude
of less than one hundred millivolts to said
oscillator;
25 f. allowing said sheath fluid to exit from said
nozzle volume; and
g. forming at least one droplet from said sheath
fluid after allowing said sheath fluid to exit
from said nozzle volume.
In accordance with another aspect of the present
invention, there is provide3 a system fflr creating a
6a
CA 02201324 2005-03-02
droplet from a jet of a flow c~rtometer comprising:
a. a nozzle container establishing a nozzle volume
and having a nozzle that continuously converges
to a nozzle exit;
b. a sheath fluid port. located within said nozzle
volume wherein said sheath fluid port introduces
a sheath fluid;
c. a substance introduction port located within said
nozzle volume;
to d. an oscillator to which said sheath fluid is
responsive and located predominantly within skid
l
ed
nozzle volume such that oscillations are coupl
from the oscillator to the sheath fluid without
passing through the nozzle container;
e. an alternating voltage source having an
alternating voltage amplitude of less than one
hundred millivolts connected to said oscillator;
and
f. a free fall area below said nozzle exit and
2o within which said droplet forms.
IV. BRIEF DESCRIPTION OF THE DRAWINGS
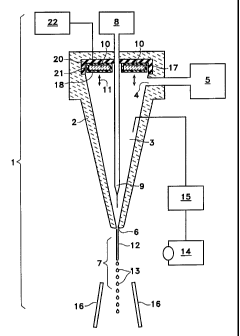
Figure 1 is a schematic cross sectional view of an
embodiment of the invention showing the various features
combined.
Figure 2 is a plot of the droplet onset energy of
prior art designs compared to that of the present
invention.
6:~
CA 02201324 2005-03-02
Figure 3 is a schematic cross sectional view of an
alternative design showing the automatic substance
adjustment
6~
2~~'~324
WO 96/12171 PGT/US95/13308
feature and a directionally coupled, external oscillator.
Figure 4 is a cross sectional view of a replaceable tip
design according to one embodiment of the invention.
Figure 5 is a cross sectional view of a prior art
replaceable nozzle tip design.
0. BEST MODE FOR CARRYING OUT THE INVENTION
As mentioned, the present invention involves an improved
flow cytometer droplet nozzle system which incorporates a variety
of features. As shown in figure 1, the flow cytometer system (1)
involves nozzle container (2) which establishes nozzle volume
(3). Nozzle volume (3) is supplied a liquid by sheath fluid port
(4) which acts to introduce a sheath fluid from some sheath
reservoir (5). During operation, the sheath fluid flows through
nozzle container (2) and out nozzle exit (6) into free fall area
(7) .
Since the sheath fluid is typically an unreactive substance
such as a saline fluid and is an analytically transparent, it has
introduced within it some desirable substance such as cells or
parts of cells or other items. This substance is maintained in
substance reservoir (8) and is introduced to nozzle volume (3)
through substance introduction port (9). Through hydrodynamic
focusing, the substance flows and is separated into single cell
units within the sheath fluid and exits at nozzle exit (6).
In order to form regular droplets, the preferred embodiment
utilizes a piezoelectric crystal (10) to cause oscillations (11)
within the sheath fluid. These oscillations are transmitted as
pressure variations through to nozzle exit (6) and act to allow
jet (12) to form regular droplets (13) through the action of
surface tension. These processes are well understood and are
further explained in a number of references including the 1992
reference entitled "Flow Cytometry and Cell Sorting" by A.
Radbruch (~ Springer-Verlag Berlin Heidelberg) and the 1985
reference entitled "Flow Cytometry: Instrumentation and Data
CA 02201324 2005-03-02
Analysis" edited by Marvin A. Van Dil:la, et al. (m Academic Press
Inc. (London) Ltd.);
As shown in figure 1, one of thn features. of the preferred
embodiment is the location of piezoelectric crystal (10) within
nozzle volume 3. BY his feature the oscillator acts to initiate
oscillations (11) within the nozzle v~~lume. The oscillator thus
may be directly coupled to the sheath fluid.. These oscillations
are transmitted through the sheath fluid as it flows out nozzle
exit (6) and fogs droplets (13) below nozzle (6) in freefall
area (7). Naturally, although shown to be directly below it is
possible that the nozzle assembly co~ild be oriented on its side
or in some other relationships and s:o droplets (13) might form
at some other location and yet still :be characterized as "below"
nozzle tip (6) since they will form in the direction that jet
(12) is emitted from nozzle exit (6).
As is well understood, by allowing sheath fluid and the
substance to exit from nozzle container (2), cells or cell
fragments may be isolated in singular fashion within separate
droplets (13) for analysis by ser,~sor (14) which feeds its
information to analysis equipment (15). Analysis equipment (15)
may provide the necessary data or nay act to further process
droplets (13) through some equipment such as an electrode in
nozzle volume (3) in combination with sorting electrostatic field
equipment (16) as is well known in t:ne art. When electrostatic
potentials are applied, they may be. applied differentially to
each droplet based upon the delay in droplet formation. This
analysis equipment (15) may also include a separate laser which
induces fluorescence and the like :gin specific cells to allow
further sensing and facilitate conducting analysis as well.
As may be easily understood from figure 1, this type of flow
cytometer, a droplet flow cytometer, operates quite differently
from a channel forming flow cytome~~er. In channel-type flow .
cytometers, oscillators and the theories involved are not
relevant as no freefall or dropl~at formation is required.
_g_
zz~~~z~
WO 96/12171 - PGTlUS95/13308
Further, while the nozzle exit orifice is approximately 50 to 150
microns in diameter in droplet forming flow cytometers, in
channel-type flow cytometers, the orifice can be much larger -
on the order of 1000 microns. This causes extremely different
conditions and has resulted in the two fields being treated
somewhat differently by those involved.
Another feature of the invention is how the oscillator
couples to actually cause the formation of droplets (13). As
shown in figure 1, the oscillator is in this embodiment
piezoelectric crystal (10). While, naturally, a variety of
different devices could be used in order to achieve oscillation
(11), by using piezoelectric crystal (10) a host of different
frequencies and powers are possible. It should be understood,
however, that while the use of some piezoelectric crystal is
usually the preferred technique, the invention should not be
considered as limited to that type of oscillator as its teachings
can be broadly applied.
As shown in figure 1, piezoelectric crystal (10) is
configured as a ring-shaped crystal which occupies most of the
top end of nozzle container (2). This ring is mounted directly
to nozzle container (2) in a manner so as to be situated within
nozzle volume (3). It need not vibrate the nozzle container and,
indeed is designed to avoid it. Its oscillations (il) may also
be made to occur generally in a direction parallel to the central
axis of nozzle container (2) as shown. Further, these
oscillations (11) are essentially coupled to the. sheath fluid,
not to the nozzle container. Thus, rather than taking the
directions suggested by some of the prior art involving moving
the actual nozzle container, the present invention acts directly
upon the sheath fluid to cause pressure variations within the
sheath fluid. These pressure variations move down nozzle volume
(3) and may actually be amplified by the shape of nozzle
container (2) so as to cause surface tensions variations in jet
(12) as it emerges from nozzle exit (6). These variations act
to pinch off jet (12) and thus form droplets (13). Since the
-9-
WO 96/12171 PCT/US95I13308
sheath fluid is not substantially compressible, these pressure
variations may pass relatively unattenuated and in fact may be
amplified through nozzle volume (3) to achieve the desired
droplet formation effect. While others may have considered the
desire to coupling directly to the sheath fluid, they failed to
recognize ways to do this and did not recognize that they could
have positioned the oscillator within the sheath fluid for most
efficient coupling.
Both the direct coupling of oscillations (11) to the sheath
fluid and the directional nature of the oscillations (11)
contribute to the invention's ability to achieve droplet
formation at power levels which are several orders of magnitude
less than those of the prior art. As may be understood from
figure 1, piezoelectric crystal (l0) may directly transfer the
vast majority of its energy to the sheath fluid. To further
enhance the transfer of the majority of the energy into the
sheath fluid (rather than the nozzle as often suggested by the
prior art), the invention may also incorporate the designing of
massive nozzle container elements so as to minimize the transfer
of energy through these elements. As may be easily understood,
by positioning the oscillator within the sheath fluid, frequency
dependencies and resonances which are caused by the vibration of
the entire nozzle container can be greatly reduced. Thus,
contrary to the teachings of the prior art which suggested
vibrating the entire nozzle container, the present invention can
specifically avoid such vibrations. This acts to avoid resonance
frequencies as might occur through vibrations perpendicular to
the lines of flow which may be inevitable whenever the entire
nozzle assembly is vibrated. Contrary to those teachings which
have suggested mounting the entire nozzle assembly on a flexible
membrane so as to allow the entire nozzle assembly to move, the
present invention relies not on movement of nozzle container (2)
but rather on pressure waves within the sheath fluid in nozzle
volume (3). This aspect greatly reduces the amount of power
necessary to cause droplet formation and greatly reduces the
appearance of resonance frequencies which occur as a result of
-10-
220132
WO 96/12171 PGT/US95/13308
the entire vibration of nozzle container (2) among other aspects.
Referring to figure 2, the dramatic impact of these
reductions can be understood. Figure 2 shows a conceptual plot
of the rough energy of droplet formation onset versus frequency
anticipated for the present invention. As shown in figure 2, the
energy (expressed in terms of volts applied to a given
piezoelectric crystal) is reduced by orders of magnitude. This
reduction has been demonstrated for a number of frequencies. As
shown in figure 2, the prior art which typically operated in the
10 volt range now only requires ten millivolts or so.
In addition, as shown in figure 2, it can be seen that the
prior art was also subject to a great number of resonance
frequency variations (shown by the peaks and valleys in the plot
of the prior art). These peaks and valleys were to a large
extent caused not only by the amount of power required but also
by designs which were based upon movement of the entire nozzle
assembly rather than merely pressure waves within the nozzle
assembly. In sharp contrast to the prior art characteristic
conceptually shown in Figure 2, the present invention not only
achieves droplet formation with dramatically lower voltages but
it also achieves these levels over a relatively large frequency
range with very small resonance variations compared to those of
the prior art. These relative plots are believed to represent
significant differences in result between prior art designs and
those of the present invention. While naturally variations will
occur due to the particular nozzle designs ultimately chosen, it
is believed that through the teachings of the present invention
these dramatic variations should be practically achievable in
many cases.
Referring again to figure 1, it can be seen that besides
merely positioning the oscillator within nozzle volume (3), the
embodiment also is designed to minimize the number of material
interfaces through which the oscillations must pass before being
imparted upon the sheath fluid. While, naturally, it would be
-11-
~~0~3~4
WO 96/12171 PCT/US95/13308
possible to position piezoelectric crystal (10) directly exposed
to the sheath fluid, for contamination and other reasons, the
preferred embodiment allows for the inclusion of protective
coating (17) over piezoelectric crystal (10). This protective
coating (17) may actually be some type of epoxy or other coating
which has no tendency to interfere either with the sheath
material or the oscillations (il) of piezoelectric crystal (10).
Again, contrary to the teachings of the prior art which involve
numerous material interfaces between the oscillator and the
sheath fluid, the present invention minimizes the number of
material interfaces through which oscillations (11) must pass.
Since any change in material can cause reflection and energy
losses, the preferred design allows for only one interface
material such as protective coating (17). Thus, only one
interface material exists between oscillator surface (18) and the
sheath fluid. By positioning piezoelectric crystal (10) within
nozzle volume (3) not only can the interface material be limited
to the simple epoxy coating mentioned, but also, the oscillator
surface (18) can be positioned so as to face directly to the
sheath fluid.
As mentioned, another aspect which helps the invention
achieve its extraordinary reduction in oscillation drive power
is the fact that the oscillator is directionally coupled to the
sheath fluid. In order to avoid resonances and energy
transmissions in other than the desired direction, the present
invention recognizes that unidirectional coupling is desirable.
In order to achieve this, as shown in figure 1 the embodiment
provides for positioning piezoelectric crystal (10) so that it
is detached from the sides of nozzle container (2). Since all
piezoelectric crystals act in a manner so as to conserve volume
during oscillations, this avoids coupling the inherent
perpendicular oscillations to nozzle container (2). Again, _
through this recognition, the invention can achieve a uniform
pressure wave within the sheath fluid. Since oscillator surface
(18) is oriented perpendicular to the primary flow direction, the
oscillations (11) are coupled substantially only as a flow
-12-
CA 02201324 2005-03-02
direction deemed to be prima~.-y, whether the average flow
direction, a specific location's flow direction, or even the
direction at the nozzle exit (6). This allows for the
oscillations to be unidirectionally applied to the sheath fluid
and also aids in the reduction of resonance frequencies. As
shown in figures 1 and 3, this unidirectional coupling can be
achieved through the inclusion c~f a directional isolator (19).
As shown in figure 3, directional isolator (19) may be a separate
element such as a rubber or ether material which does not
transmit frequencies of the predominant oscillation frequency.
As shown in figure l, the directional isolator (19) may actually
be spacer ( 2 0 ) . Spacer ( 2 0 ) may be a separate element or, as
shown in figure 1, may be an integral portion of the top or cap
of nozzle container (2) so as to simply act to space oscillator
side (21) away from nozzle container (2). The unidirectional
coupling of oscillations (11) t~ the sheath fluid may be enhanced
by making oscillator surface (18) planar as shown in figure 1 and
by making it cover most of the top surface area. The oscillator
is thus established substantially throughout a perpendicular
cross sectional area (perpendicular to the primary flow
direction) and will~cause osci11,3tions throughout it. Thus the
ring shaped crystal design coordinates the desire to maximize the
surface area of oscillator surfa~~e (18) with the unidirectional
desire by making it match the t~~pically circular cross section
of nozzle container (2). NatuZ-ally other shapes can also be
used. Further, the coupling, .sh~~wn in figure 1 and in figure 3
as the portion of the top section of the nozzle container (2) may
also be planar and may also be coupled along only one plane.
These each contribute to making 'the main oscillation area cause
only one direction of oscillation as can be easily understood.
To further enhance the reduction in oscillation power
achievable through the present invention, nozzle container 2 is
also designed as a continuousl~~ converging nozzle container.
This acts to not only maintain laminar flow throughout nozzle
volume (3), but also to effectively amplify oscillations (il) as
they travel in pressure waves 1-~rough the sheath fluids ,from
-1_~-
~2~ ~~~~~
WO 96/12171 PCT/US95/13308
piezoelectric crystal (l0) to nozzle exit (6). As may be
understood from figure 1, by continuously converging it is not
meant that nozzle volume (3) must constantly or uniformly
converge throughout its length, rather, it need only converge at
all locations. Thus, nozzle volume (3) has a largest cross-
sectional area located at or near its top and has continuously
diminishing cross-sectional areas along its length through to
nozzle exit (6).
Having a continuously converging nozzle container also helps
in maintaining laminar flow up to nozzle exit (6). In this
regard nozzle exit (6) should be understood to exist not only at
the actual end location of the orifice but more accurately at the
point at which there is a significant increase in the pressure
gradient so as to make changes in the angle of convergence less
important. Unlike the teachings of the prior art which
frequently involve straight cylindrical sections within nozzle
container (2), this aspect of the invention specifically avoids
such possibilities. This is somewhat surprising and may be
treated with skepticism by those of ordinary skill in the art
because traditional theories provide that once laminar flow is
established such flow should continue in most applications when
the nozzle container does not expand sharply. In contrast, this
aspect of the invention suggests otherwise. While these,
traditional laminar flow theories may be appropriate in some
instances, the continuous convergence of the sheath fluid appears
desirable in most droplet flow cytometers. To some extent this
may be due to the fact that the required acceleration of the
sheath fluid and pressure, and the resulting increase in the
friction of the sheath fluid against nozzle container (2), each
make a constant convergence desirable to avoid nonlaminar flow
results. Basically it has been empirically found that through
a continuously converging nozzle container optimal conditions for
maintaining laminar flow can be created.
In addition to the aspect of maintaining laminar flow, the
continuously converging nozzle container can provide
amplification of the oscillations (11). Similar to horn and
-14-
~~Q X324
WO 96/12171 PCT/US95/13308
other designs, the continuous convergence combines with the
principals of conservation of energy so that the amplitude of the
oscillations actually increases as it passes from piezoelectric
crystal (10) to nozzle exit (6). This amplification may be
maximized not only by positioning the oscillator at or near the
largest cross-sectional area but also by making oscillator
surface (18) to have an area substantially as large as the
largest cross-sectional area. In this regard by "substantially"
it is meant that the oscillator should be as large as practically
possible after consideration of the typical desire to introduce
substance through the center axis of nozzle volume (3) as well
as this invention's unique desire to maintain oscillator side
(21) spaced apart from nozzle container (2). The amplification
may also be enhanced by providing for continuous convergence from
sheath fluid port (4) through to nozzle exit (6). As mentioned
earlier, each of the foregoing aspects also contribute to the
present invention's extraordinary reduction in input power
requirements.
To create oscillations (il), piezoelectric crystal (10) is
powered through an alternating voltage source (22) as those
skilled in the art can easily understand. Through the teachings
of the present invention, alternating voltage source (22) may be
configured to stimulate the oscillator with the voltage amplitude
of less than 100 millivolts and thus represents orders of
magnitude of reduction in the typical voltage applied to
piezoelectric crystals in such systems. This voltage may be
greater than 10 millivolts or so as that was a representative
level at which droplet formation seems to occur. It should be
understood, however, that this limitation should not be taken as
a lower limit since the teachings of this invention may become
refined and alternative designs may be developed which result in
further reduction in power.
Yet another independent feature of the present invention is
its design to allow the nozzle section to be easily replaced or
cleaned while permitting laminar flow. Referring to figure 4,
-15-
WO 96/12171 PCT/US95/13308
it can be seen that the entire nozzle container (2) may be made
of several components. Nozzle container (2) may consist of cap
section (23) to which piezoelectric crystal (10) may be attached.
Cap section (23) may be attached in some sealing fashion or may
even be integral to nozzle body (24) as shown in figure 1.
Similarly, nozzle body (24) may be sealingly attached to nozzle
tip (25). Each of these seals may consist of O-rings as but one
example of the types of seals shown in figure 4. Nozzle tip (25)
may be a ceramic fabricated item which includes an exit situated
at its tip. This exit may actually be an orifice made through
techniques known by those skilled in the art (such as the use of
tungsten wire and the like) so as to create a small orifice of
about 50 to 150 microns in diameter.
Unlike the designs shown in the prior art such as those
shown in figure 5, nozzle tip (25) need not be sealed to nozzle
body (24) on its inner surface. Instead, the nozzle body inner
surface (26) joins smoothly with the nozzle tip inner surface
(27) at tip joint (28). This smooth transition is to the degree
necessary to maintain laminar flow in the particular application.
It can be achieved through the inclusion of edge insert (29)
within nozzle body (24) so as to allow nozzle tip (25) to be
inserted into nozzle body (24). In this fashion seal (30) can
be positioned so as to contact the outer surface (31) of nozzle
tip (25) and thus avoid any adverse impacts on laminar flow
within nozzle volume (3). By locating seal (30) off of inner
surface (27) of nozzle tip (25), the seal can be kept away from
areas which are important to laminar flow. As may be understood,
a great variety of designs may be accomplished to achieve this
goal. Importantly, it should be understood that inner surface
3 0 ( 2 7 ) of noz z le tip ( 2 5 ) is def fined ~ merely with respect to its
function, namely, the surface which contacts and directs the flow
of sheath fluid of nozzle volume (3). Further, the definition
of "smooth" is also relatively defined as those transitions which
do not significantly interrupt laminar flow and thus do not
degrade the performance of the flow cytometer. It should also
be understood that the seal between any two components such as
-16-
zzo~~z~
WO 96/12171
PCT/US95/13308
the seal between nozzle body (24) and nozzle tip (25) may be
direct or indirect through the use of intervening materials or
components.
Yet another independent aspect of the invention is the
aspect of being able to adjust the location at which the
substance is introduced. As mentioned earlier, those skilled in
the art have long recognized the need to achieve variations in
the entire process to accommodate variations in conditions
practically experienced. As shown in figure 3, the present
invention affords the ability to vary the rate at which substance
is introduced without disrupting laminar flow and the like. This
is achieved through positioning substance introduction port (9)
within convergence zone (32) as may be easily understood and by
varying the location of substance introduction port (9) within
convergence zone (32). As shown, substance introduction port (9)
may move along the primary flow direction to maintain an optimal
relationship to the flow of the sheath fluid. Through this
technique, the relative concentrations of the substance
introduced and the sheath ,fluid can be varied. This can act to
avoid the resolution drop and the like which the prior art
appeared to consider unavoidable as they adapted to changing
conditions.
Further, since it may be desirable to maintain equal
velocities at substance introduction port (9), and since
substance tube (33) may be moved, it is possible to include a
controller (34) which receives signals from some type of sensor
(14) and which may act to control a movement mechanism (35) and
thus automatically adjust the location of substance introduction
port (9) within nozzle container (2). Further, controller (34)
may act to additionally control the pressure of substance
reservoir (8) and sheath reservoir (5) for automatic correlation
of the various factors based upon location or other parameters
sensed. Since the theoretical relationship between these factors
is well known for optimal conditions and since the programming
or wiring of such a design could be easily achieved by those
-17-
~2t~1~24r
WO 96/12171 PCT/US95/13308
skilled in the art, a variety of designs may be implemented to
achieve this goal. Given the great variety of flow cytometer
systems possible, it should be understood that a great variety
of sensed values may be used ranging from concentration of the
substance contained within substance reservoir (8), to the actual
location of substance introduction port (9), to the pressure of
the various sheath fluid or substance fluids, to some other
property of the substance sensed by sensor (14). Each of these
- or any combination of them and other factors - may be
adjusted automatically to achieve desired relationships or to
simply optimize results without regard to the actual predicted
values. Naturally, in keeping with this broad concept it should
be understood that sensor (14) may not be just one sensor but may
in fact be a host of different sensors positioned at various
locations depending upon the particular condition existing within
the flow cytometer desired to be sensed. While, of course, the
sensor (14) will only ascertain specific values, these values can
indicate results which may be used to more appropriately adjust
the location of the substance introduction port.
Similarly, a host of different designs for the location
adjuster (shown in figure 3 as movement mechanism (35)) are
possible. The location adjuster may also include some type of
screw means (36), that is, some type of device which allows
relatively continuous movement with fine adjustment. It may also
include telescoping substance tube (37) (shown in figure 3 as
potentially a redundant location adjuster for illustrative
purposes only) or perhaps some type of slide design through the
cap section. In applications in which the conditions remain
relatively stable, a replacement substance tube of fixed length
may also be provided. Thus, various substance tubes may be
selected based upon the conditions encountered in that particular
type of application. In this fashion, the limitation experienced
by the prior art whereby variations in pressure were used but
undesirably resulted in unequal fluid velocities at the location
of substance introduction port (9) can be avoided. This affords
an increase in the resolution.
-18-
WO 96/12171 PCT/US95I13308
The foregoing discussion and the claims which follow
describe the preferred embodiment of the present invention.
Particularly with respect to the claims and the broad concept
discussed, it should be understood that changes may be made
without departing from the essence of this patented invention.
It is intended that changes are permissible to accommodate
varying applications and will still fall within the scope of this
patent. It is simply not practical to describe and claim all
possible revisions nor is it practical to claim all combinations
of the varying features. To the extent revisions utilize the
essence of the present invention, each would naturally fall
within the breath or protection encompassed by this path. This
is particularly true for the present invention since its basic
concepts and understandings are fundamental in nature and can be
broadly applied. It is also particularly true since the present
invention involves a number of potentially independent features
which may be combined in synergistic ways for particular
applications.
-19-