Note: Descriptions are shown in the official language in which they were submitted.
WO96/12949 220 1 6 1 3 pCTlUS95111303
CHEMICAL SENSOR WITH DIFFUSION BARRIER
Field of the Invention
The present invention relates to a chemical sensor for gases which has a
rate limiting diffusion barrier.
Background of the Invention
Electrochemical sensors have at least two electrodes which are connected
by both an ionically conducting electrolyte and an electronically con~llcting
external circuit. In a sensor, one electrode is exposed to the gas to be detected
and is called the sensing electrode or working electrode. The gas may be oxidized
or reduced at the sensing electrode, depending on the nature of the gas, the
electrolyte, and the electrical potential of the electrode. The other electrode,called the counter electrode, supports a reaction to balance the oxidation or
reduction of the gas of interest, and the rate of gas reacting at the sensing
electrode is measured by the amount of current flowing in the external circuit. For
a sensor, the electrolyte is most conveniently a piece of ionically concl~lcting solid
polymer electrolyte with the two electrodes attached on opposite sides.
If the rate of reaction of the gas at the sensing electrode is controlled by
2 0 the rate of electron transfer, then the current measured in the external circuit will
depend on the electrical potential of the sensing electrode. If, on the other hand,
the rate controlling step is the rate of diffusion of the gas to the electrode, then the
measured current will be independent of potential and will be a measure of the rate
at which the gas molecules reach the electrode, which in turn is a measure of the
2 5 concentration of the gas. This is called the limiting current mode and is the
desirable condition for operating a sensor. While it is possible for a sy$em to be
operating in the limiting current mode and function as a sensor without a sep~
diffusion barrier, generally a diffusion barrier is required to ensure that the system
will perform in the limiting current mode.
3 0 Diffusion barriers can be solid nonporous films in which the gas to be
detected can dissolve and diffuse by solid state diffilsion. For e,.al.,ple, in an
pcrt~s~~/~ 3~3
~~ æs ~r 2 2 2 0 1 6 1 3
electrochemical sensor for oxygen, suitable membrane materials include
polypropylene, polyethylene, TEFLON~M and ~vfYLART~. Also disclosed for use
in oxygen sensors are silicone rubber and other rubbers.
Generally, in solid membrane diffilsion barriers, diffusion rates are low
5 which requires that the membranes must be very thin in order to achieve sufficient
sensitivity of the sensor. This can lead to handling and breakage problems. Also,
the temperature dependence of the diffusion coefficients are high which makes the
sensor response vary significantly with temperature changes.
Some of the deficiencies of solid membrane diffusion barriers may be
,- 10 overcome by using porous membranes with limited pore size or membranes with
one or more capillary openings, in which the gas diffuses through the barrier in the
gas phase rather than by solid state diffusion. Suitable materials which have been
reported include, some grades of porous unsintered polytetrafluoroethylene tape
and porous polycarbonate films. Where the porosity of the membrane is greater
than desired, the porosity can be reduced by pre-pressing membranes of
polytetrafluoroethylene or impregnating the pores with a liquid cont~ining a solid
component which partially fills the ores after removal of the liquid. L4~
tQ~ de to U~k~ 6 ~6 ~A US~ 06~ 6~s .
In porous membrane diffusion barriers, sharp changes from a cold to a
warm environment may cause water vapor to condense on the membrane which
2 0 can close the pores. Changes in temperature can also cause the sensor output to
vary. Also with a porous membrane diffilsion barrier an air pressure shock such
as the pressure wave inside a car when the door is slammed shut, or sudden
movement of the sensor can cause a burst in the sensor output and may trigger analarm.
2 5 A liquid membrane may be formed by immobilizing it between two gas-
permeable films. Such membranes have been used for separating gases from a
mixture with one of the gas-permeable films having liquid-filled pores allowing the
diffusive transfer of an active carrier species into the liquid membrane. A liquid
can also be immobilized in the pores of a porous polymer membrane. Such
3 0 membranes have been used for separating gases from a mixture. A two-step
sensing system in which an immobilized liquid membrane is used to selectively
AMEN~E~ S~U~Er
wo 96/12949 2 2 0 1 6 1 3 3 PCTIUS95/11303
separate a gas of interest from a mixture of gases, and the selected gas then passes
into a chamber where it is detected with general purpose sensors such as
semiconductor or catalytic gas sensors has been disclosed. In this two-step
process, the immobilized liquid membrane is preferably a porous polymer
5 membrane, the pores of which contain a hydrophobic, low vapor pressure,
chemically compatible liquid which wets the matrix material and has a high
solubility and diffusivity for the gas of interest.
Summary of the Invention
The present invention provides a diffusion rate limited amperometric
electrochemical sensor comprising at least two electrodes, an external circuit
connected to the electrodes, an electrolyte capable of conducting ionic charge
between electrodes, and a diffiJsion barrier coextensive with or covering one ofsaid electrodes, said diffusion barrier being a porous membrane co~ g within
15 the pores of the membrane a low vapor pressure liquid phase in which a gas to be
detected is soluble.
The present invention further provides a method of sensing the presence of
a gas comprising the steps of permitting passage of said gas through a diffusionbarrier comprising a porous membrane containing within the pores of the
2 0 membrane a low vapor pressure liquid phase in which the gas to be detected is
soluble, said gas cont~cting a sensing electrode which is proximate said diffusion
barrier and causing said gas to be oxidized or reduced, generating ions, pe~ iLLillg
ions to pass through electrolyte between electrodes, permitting ions to contact a
counter electrode, said counter electrode supporting a reaction to balance the
2 5 oxidation or reduction of the gas of interest, and quantifying the rate of gas
reacted at said sensing electrode by determining the current flow in an externalcircuit connecting said electrodes.
The present invention further provides a method of pl epa~ ing a rate limited
amperometric electrochemical sensor comprising the steps of providing a porous
3 0 membrane, imbibing said membrane with a low vapor pressure liquid in which a
WO 96/12949 PCT/US9S/11303 ~
2201 61 3 4
gas to be detected is soluble, and bringing said imbibed membrane plo~l,ldle an
electrochemical sensor.
The present invention still further provides a respirator comprising a
- facepiece defining a space covering at least the mouth and nose of a wearer, at
5 least one air inlet port, at least one air outlet port, means for filtering one or more
components from external air drawn into said space, means for detecting a gas insaid space comprising a rate limited amperometric electrochemical sensor
- comprising at least two electrodes, an external circuit connected to said
electrodes, an electrolyte capable of cond~lcting ionic charge between electrodes,
10 and a diffusion barrier coextensive with or covering one of said electrodes, and a
signal emitting means operatively connected to said external circuit, said diffusion
barrier being a microporous membrane cont~ining within the pores of the
membrane a non-evaporating liquid phase in which a gas to be detected is soluble.
The present invention also provides a supplied air respirator comprising a
15 facepiece defining a space covering at least the mouth and nose of a wearer, at
least one air inlet port, a supply of breathable air for transmission through said air
inlet port, at least one air outlet port, means for detecting a gas in said space
comprising a rate limited amperometric electrochemical sensor comprising at least
two electrodes, an external circuit connected to said electrodes, an electrolyte2 0 capable of conducting ionic charge between electrodes, and a diffusion barrier
coextensive with or covering one of said electrodes, and a signal emitfing meansoperatively connected to said external circuit, said diffusion barrier being a
microporous membrane
cont~ining within the pores of the membrane a non-evaporating liquid phase in
2 5 which a gas to be detected is soluble.
The present invention also provides a personal exposure intlic~tor or
environmental indicator comprising a diffusion rate limited ampelc.lllellic
electrochemical sensor comprising at least two electrodes, an external circuit
connectecl to the electrodes, an electrolyte capable of cond~lcting ionic charge3 0 between electrodes, and a diffusion barrier coextensive with or covering one of
said electrodes, said diffusion barrier being a porous membrane co~ g within
WO 96/12949 PCT/US95/11303
2201 61 3 5
the pores of the membrane a low vapor pressure liquid phase in which a gas to bedetected is soluble and a signal emitting means operatively connected to said
external circuit.
The rate limited amperometric electrochemical sensors of the present
invention have fast response times, excellent sensitivity, are easy to handle, and
exhibit ~ew failures due to leakage or breakage of the diffusion barrier. The
membranes of this invention are particularly easy to construct reliably and
reproducibly. The pores of the membrane material are simply imbibed with the
desired liquid and blotted to remove any excess. Also, the liquid used in the
diffusion barrier may be selected for optimum solubility for the substance beingdetected, providing a means for optimization not available with gas phase diffusion
membranes. Additionally, immobili~ed liquid membrane dif~fusion barriers are less
affected by condensation problems, air pressure shocks, or mechanical jolts thanare gas phase diffusion membranes. The diffusion barrier in this invention is rate
limiting, and the concentration dependence of the signal depends on the diffusion
properties of the membrane. Further, selective separation of the gas to be
detected is normally not required.
Brief Description of the Drawings
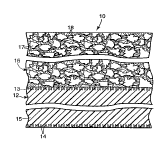
2 0 FIG. 1 is a cross-sectional view of a sensor of the present invention.
FIG. 2 shows the response curve for the hydrogen sulfide sensor of
Example 1.
FIG. 3 shows the response curve for the hydrogen sulfide sensor of
Comparative Example 1.
2 5 FIG. 4 shows the response curve for the hydrogen sulfide sensor of Example 2.
FIG. 5 shows the response curve for the hydrogen sulfide sensor of
Comparative Example 2.
FIG. 6 shows the response curve for the hydrogen sulfide sensor of
3 0 Example 3 .
WO 96/12949 PCT/US95/11303
2201 61 3 6
FIG. 7 shows the response curve for the hydrogen sulfide sensor of
Comparative Example 3.
FIG. 8 shows the response curves as a function of hydrogen sulfide
concentration for the hydrogen sulfide sensor of Example 4.
FIG. 9 shows the sensitivity as a function of hydrogen sulfide
concentration for the sensor of Example 4.
FIG. 10 shows the response curve as a fi~nction of humidity for the sensor
of Example 4.
FIG. 11 shows the response curve for 40 minute exposure to 5 ppm
1 0 hydrogen sulfide for the sensor of Example 4.
FIG. 12 shows the response curve ofthe sensor of Example 4 as a function
oftemperature at an exposure level of 10 ppm hydrogen sulfide.
FIG. 13 shows the response curve of the sensor of Example 4 as a function
of carrier flow rate.
FIG. 14 shows a respirator ofthe invention utili7ing the diffusion rate
limited amperometric electrochemical sensor.
FIG. 15 shows a supplied air respirator of the invention utili7ing the
diffusion rate limited amperometric electrochemical sensor.
FIG. 16 shows a personal exposure or environmental indicator of the
- 2 0 invention utilizing the diffusion rate limited amperometric electrochemical sensor.
FIG. 17 is a sectional view of a sensing device suitable for use with the
respirators and exposure indicators of the invention
- Detailed Description of the Invention
2 5 Amperometric electrochemical sensors useful in the present invention
include any sensors that are capable of electrochemically oxidizing or rec~ in~ an
analyte and generating a proportional current. Such sensors are described, for r- example, in U.S. Pat. No. 4,865,717 (Stetter et al.), U.S. Pat. No. 4,913,792
(Nagata et al.), U.S. Pat. No. 4,227,984 (Dempsey et al.), U.S. Pat. No.
4,025,412 (LaConti), U.S. Pat. No. 4,894,138 (Gambert et al.), U.S. Pat. No.
4,633,704 (Tantram et al.), U.S. Pat. No. 4,948,496 (Chand), and U.S. Pat. No.
WO 96/12949 PCT/US95/11303
2201613 7
4,591,414 (Zaromb et al.). Particularly preferred are sensors fabricated with the
nanostructured electrode membranes described in U.S. Pat. No. 5,338,430
(Parsonage et al.).
The porous membranes useful in the present invention are any porous
membrane capable of imbibing a liquid. The membranes have a porosity such that
simply immersing the membrane in the liquid causes the liquid to spontaneously
enter the pores by capillary action. The membranes, before imbibing p,~re-~bly
have a porosity of at least about 50%, more preferably at least about 75%. The
porous membranes preferably have a pore size of about 10 nm to 100 ~Lm, more
1 0 preferably 0.1 ~lm to I 0 ,um and a thickness of about 2.5 llm to 2500 ~m, more
preferably about 25 llm to 250 ~m. The membranes are generally prepared of
polytetrafluoroethylene or thermoplastic polymers such as polyolefins, polyamides,
polyimides, polyesters, polyether sulfones, polycarbonates, cellulosic polymers,polyvinyl chloride, polyvinylidene fluoride and the like. Examples of suitable
membranes include, for example, those disclosed in U.S. Pat. No. 4,539,256
(Shipman), U.S. Pat. No. 4,726,989 (Mrozinski), U.S. Pat. No. 4,247,498
(Castro) and U.S. Pat. No. 3,953,566 (Gore).
Suitable liquids for imbibing the membrane include, for example, mineral
oil, polypropylene glycol, silicones, and other liquid-like oligomers and polymers
2 0 The diffusion barriers may be prepared by soaking the porous membrane in
the liquid until the liquid has been imbibed and then removing excess liquid, for
example, by blotting. Alternatively, the dif~sion barriers can be prepared by
soaking the porous membrane in a solution of the liquid and then evaporating thesolvent. Further, the diffusion membrane can be prepared utili7.ing liquid-solid or
liquid-liquid phase separation techniques according to U.S. Pat. No. 4,539,256
(Shipman), U.S. Pat. No. 4,726,989 (Mrozinski) or U.S. Pat. No. 4,247,498
'` (Castro) and the blending compound may be left in the membrane if the gas to be
detected is soluble in the blending compound.
FIG. 1 shows a pl erel l ed embodiment of the diffusion rate limiting
amperometric electrochemical sensor 10 having a sensor 12 comprising a sensing
electrode 13, a counter electrode 14 and electrolyte 15 and a diffusion barrier 16
wo 96/12949 2 2 0 1 6 1 3 PCT/US95/11303 ~
comprising porous membrane 17 cont~ining a low vapor pressure liquid 18 in the
pores of the membrane. The sensor may further comprise a porous buffer layer of,for example tissue or scrim (not shown) between the sensor and the me",bl~l1e toprevent direct contact of the low vapor pressure liquid and the sensing electrode.
FIG 14 shows a respirator 20 ofthe invention. Respirator 20 contains a
pair of air purifying respirator cartridges 22, 23 disposed laterally from a face
mask 24. The diffusion rate limiting amperometric electrochemical sensor is
contained in flow through housing 26 on which is mounted a signal emitter 27.
FIG. 15 shows a supplied air or powered air purifier respirator 30 which
has a face piece 32 connected to a shroud 33 and an air supply hose 35. Air is
provided by air supply 37. a sensing device 36 can be located in the air supply line
to monitor the air supply. When the respirator is the supplied air respirator~ the air
supply would be a self-contained unit. When the respirator is a powered air
purifier respirator, ambient air would be blown through a filtering system into air
supply hose 35.
FIG. 16 shows a personal exposure indicator or an environmental indicator
40 which can be worn on a user's clothing or located in a specific area. A clip 42
may optionally be provided to attach the indicator onto a user's pocket or belt.The device has a fluid coupling membrane 44 beneath which the sensor is located
2 0 and a signal 46 may be provided as a light emitting diode.
FIG. 17 shows a sectional view of the type of sensing device 50 that could
- be used in each of the respirators and indicators. A fluid coupling membrane 51
- covers the sensing port beneath which lies the diffusion rate limited amperometric
electrochemical sensor which comprises rate limiting diffusion barrier 52, sensing
electrode 54, electrolyte 53 and counter electrode 55. The diffusion rate limited
sensor is connected to microprocessor 56 which is connected to signal means 57.
In the following examples, all parts and percentages are by weight unless
otherwise specified.
.
~ WO 96/12949 2 2 0 ~ 6 ~ 3 PCTIUS95/11303
Example 1 and Comparative Example C I
An amperometric electrochemical sensor for hydrogen sulfide was
constructed as described in U. S. Patent 5,338,430 (Parsonage et. al.). A
polynuclear aromatic hydrocarbon, N',N'-di(3,5-xylyl)perylene-3,4,9,10-
5 bis(dicarboximide), available from American Hoechst Corp. as C.I. Pigment Red149, hereinafter called "perylene red", was vacuum vapor deposited onto a
flexible, copper-coated polyimide temporary substrate, near room temperature, toa thickness of about 0.1 to 0.15 micrometers. This was annealed in a vacuum,
causing the perylene red film to convert to a layer comprising discreet, oriented
10 whiskers 1-2 micrometers in length. The whiskers
were then coated with a mass equivalent thickness of 175 nm of p~ lm by
vacuum evaporation, producing the nanostructured elements.
Next, a curable solid polymer electrolyte forrnulation was prepared
consisting of 0.06 g 90% benzene sulfonic acid (Aldrich Chemical Co.) in 1 ml
15 tetrahydrofuran, 1 ml catalyst solution consisting of 25 microliters dibutyl tin
dilaurate in 10 ml tetrahydrofuran, 2 ml 600 molecular weight poly(ethylene
glycol) and I ml DESMODURT~' N100 (available from Farbenfabriken Bayer AG)
multifunctional isocyanate. The sensor was made as follows: Approximately 0.1
ml ofthe curable solid polymer electrolyte solution was placed between two 10
2 0 mm diameter discs cut from the temporary substrate with the nanostructured
elements encapsulated in the solid polymer electrolyte supporting the
nanostructured elements. The sample was cured at approximately 40C for a
period of about 1 hour. The temporary substrate of the nanostructured elements
was then peeled away from the cured solid polymer electrolyte leaving the fresh,2 5 Pd-coated nanostructured electrodes embedded in the surface of each side of the
solid electrolyte disc. Electrical contact to both sides of the nanostructured
electrode membrane was made using 0.3 mm diameter copper wire adhered to the
electrode membrane with a trace amount of conducting silver paint ( available
from GC Electronics, Rockford, IL). One side of the membrane (counter
3 0 electrode) was then isolated by covering the entire surface with a 10 mm ~ metpr
piece of vinyl electrical tape.
WO 96/12949 PCT/US9S/11303
220~613 10
A microporous polypropylene was prepared as described in U.S. Pat. No.
4,726,989 (Mrozinski). About 0.30 weight percent of a dibenzylidine sorbitol
nucleating agent (MILLADTM 3905, available from Milliken Chemical Co.) was
dry blended with polypropylene resin (PRO-FAXTM 6823, available from Himont
5 Incorporated, Wilmin~on, Del.). This was melt-blended with 52.6 weight percentmineral oil (Amoco White Mineral Oil #31 USP Grade, available from Amoco Oil
Co.) and extruded at a melt temperature of about 205C on a BERSTORFFTM 40
mm twin screw extruder fitted with a 30.5 cm by 0.04 mm slit gap sheeting die
positioned above a water quench tank maintained at about 37.8C . The extruder
1 0 was operated at about a 227 cc/min throughput rate to produce a film collected at
- the rate of about 7.6 meters per minute. The resultant film was solvent washed in
1,1,1-trichloroethane for five minutes in a restraining device to remove the mineral
oil, and was then dried at room temperature. The film was then stretched about
1.5 to 2 times its original length and width. The properties of the stretched film
- 1 5 were: Gurley Value - 30 seconds (the time to pass 50 cc of air through the film
according to ASTM-D-726-S8 Method A); Bubble Point - 0.39 micrometers
(largest effective pore size measured according to ASTM-F-316-80); Thickness -
0.017 cm; Void Volume - 72%; and Residual Oil - 11.6%.
A diffusion barrier was formed by immersing the porous membrane
2 0 material in heavy white mineral oil (Mineral Oil, Heavy, White, catalog no.
33,076-0 available from Aldrich Chemical Co.). The mineral oil strongly wet the
membrane material resulting in a transparent film of solid con.cistçncy with no
observable void volume. The membrane was removed from the liquid and blotted
to remove excess liquid from the surface. A one centimeter diameter piece of
tissue (KIMWIPESTM No. 34133, 1-ply tissue wiper) was placed on the front of
the sensor working electrode. A one cenfimeter diameter sample of the diffusion
barrier were mounted in front of the tissue-covered sensor working electrode.
The thus-formed rate limiting amperometric electrochemical sensor was
tested in a 500 cc sample jar with exposure to 10 ppm hydrogen sulfide which
was generated by dilution from 100 ppm or 500 ppm hydrogen sulfide in an air
balance. Exposure was carried out for 10 minute~ after a 10 minute equilibration
~ WO 96112949 2 2 0 ~ 6 1 3 11 PCT~US9~ 1303
period. The sensor was connected to a Keithley 1 97A electrometer to monitor thesignal during exposure. A load resiet~nce of 100 K_ connected the working and
counter electrodes. The result is shown in FIG. 2.
l;or Co"~ ive Example 1, a sensor having no tissue or diffilsion barrier
5 on the working electrode was exposed to hydrogen sulfide in the same manner as the sensor of Example 1. The result is shown in FIG. 3 .
The efficacy of the rate limiting properties of the element is clearly
demonstrated by the approximately 80% reduction in the steady state signal
compared to when no diffusion element is present, and thus demonstrating that the
10 sensor is operating in the limiting current mode. As discussed above, to operate
the sensor in such a mode is necessary both for consistent sensitivity among
replicate sensors and also to provide signal and baseline stability.
Example 2 and Comparative Example 2
In Example 2, the rate limiting amperometric electrochemical sensor used
in Example I was exposed to relative humidity of 10% for l o minutes, 80% for 40minutes and 10% for an additional 10 minutes. FIG. 4 shows the baseline change.
In Comparative Example 2, the same humidity exposure was carried out as
in Example 2 except the sensor had no diffi~sion barrier. The results are shown in
2 0 FIG. 5.
Example 3 and Comparative Example 3
In Example 3, microporous polypropylene membrane material
(CELGARDTM 2400, available from Hoechst Celanese Corp.) having a thickness
2 5 of 2~ llm was imbibed with heavy white mineral oil (available from Aldrich
Chemical Co.) and mounted in front of the sensing electrode as in Example 1. The sensor was exposed to 5 ppm hydrogen sulfide at 22C, 10% relative humidity and
a flow rate of 10 liters per minute with a load resi~t~nce of 100 KQ. FIG. 6
shows the sensor response.
In Col"~)a,ali~/e Example 3, the sensor was subjected to the hydrogen
sulfide as in Example 3 but without the diffusion barrier. FIG. 7 shows the sensor
WO 96/12949 PCT/US95/11303 ~
2201 61 3 12
response. Again, the approximately 70% reduction in the steady state signal of
Exampole 3 relative to Co--,pa-~Li~e Example 3illustrates the efficacy ofthis
particular immobilized liquid membrane to act as a rate limiting element.
5 Example 4
- In Example 4, the rate limiting amperometric electrochemical sensor
prepared as in Example 3 and cont~ining the diffusion barrier was modified such
that the working electrode was biased +0.2 V anodically, vis-a-vis the counter
electrode, to favor oxidation. A load resistance of 200 KQ was used in this case.
10 FIGS. 8 and 9 show the response curves and calibration of the sensor as a function
of hydrogen sulfide concentration. FIG. 10 illustrates the stability of the sensor
baseline and sensitivity to changes in the ambient humidity level. FIG. 11
illustrates the signal constancy for longer-term exposure to 5 ppm hydrogen
sulfide. FIG. 12 illustrates the temperature stability ofthe baseline and sensitivity.
- 15 FIG. 13 illustrates the stability of the steady-state signal to changes in linear flow
velocity.
Example 5 and Comparative Example 4
In Example 5, a portion of the microporous membrane prepared in
2 0 Example I was imbibed with polypropylene glycol diol (625 molecular weight,
available from Aldrich Chemical Co.)and mounted on a sensing electrode as in
Example 1. The sensor was tested with 10 ppm hydrogen sulfide at 22C, 10%
relative humidity and 10 liters per minute flow rate. The response was monitoredusing a 100 KQ load resistance. The steady state response to 10 ppm hydrogen
2 5 sulfide was 3 mV.
In Comparative Example 4, a sensor having no diffusion barrier was tested
in the same manner as the sensor in Example 5. The steady state response to 10
ppm hydrogen sulfide was 14 mV. This difference in response demonstrates the
efficacy of this particular composition to act as a mass transport limiting device on
3 0 the amperometric electrochemical sensor.
~ WO96/12949 220 1 6 1 3 13 PCT/US9S/11303
Examples 6-10
In these examples, microporous me",br~nGs (CELGARDTM 2400, 25 ~m
thick, available from Hoechst Celanese Co.)were imbibed in solutions of heavy
white mineral oil (available from Aldrich Chemical Co.) in xylene (boiling range137-144C, available from EM Science) in concentrations of 5, 10, 15, 20 and 25
percent xylene by volume, respectively. The imbibed membranes were blotted to
remove excess liquid and the xylene was allowed to evaporate over 24 hours. The
samples were then mounted onto hydrogen sulfide sensors prepared as in Example
1 and tested with 10 ppm hydrogen sulfide at 30% relative humidity and 23C.
Each of the samples tested gave diffi~sion limited response of 25% or less of that
obtained without a membrane on the sensor. No correlation was observed
between the original percentage of xylene in the liquid phase and the diffusion
limited sensor response.
The various modifications and alterations of this invention will be appa~enl
to those skilled in the art without departing from the scope and spirit of this
invention and this invention should not be restricted to that set forth herein for
illustrative purposes only.