Note: Descriptions are shown in the official language in which they were submitted.
CA 02203890 1997-04-28
wo 96/16941 PCT/EP95/04612
CARBAMATE HERBICIDES
The presen~ invention relates to novel, herbicidally active substituted N-phenyl- and
N-heteroarylalkylcarbamates, to processes for their preparation, to compositionscomprising these N-phenyl- and N-heteroarylalkylcarbamates as active ingre~ nt.c, and to
their use for controlling weeds, especially in crops of useful plants, for example cereals,
rice, maize, soya beans and cotton.
Substituted alkyl- and phenylcarbamates which are herbicidally active have already been
disclosed and are described, for exarnple, in US-A-S 078 783, US-A-5 099 0~9, US-A-5
152 827, US-A-5 194 661 and US-A-5 399 545.
There have now been found novel substituted N-phenyl- and N-heteroarylalkylcarbamates
which have herbicidal properties and are distinguished by a good activity.
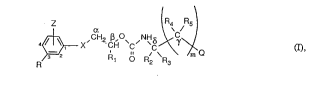
The compounds according to the invention are those of the formula I
Z a ~ /
~ R, o Rz R3 (I~,
in which
Q is a group ~ (l), ~ (2) or ~~ l (3);
R is halogen, trifluoromethyl, cyano, nitro or Cl-C3haloalkoxy;
Z is hydrogen or halogen; or
Z and R together in the 2- and 3-position of the phenyl ring form a group -OCF2O-;
Rl is Cl-Csalkyl;
R2, R3, R4 and Rs, independently of one another, are hydrogen, methyl or ethyl;
X is oxygen, sulfur, -SO- or -SO2-;
Y is hydrogen, halogen, C1-C3alkyl, Cl-C3haloalkyl, C1-C3alkoxy or cyano;
nisO, 1 or2;
n1 is 0 or 1; and
m is 0 or 1,
with the proviso that m is 1 if Q is group (1) or (2);
CA 02203890 1997-04-28
W ~96/16941 PCT~EP9',/04612
and the diastereomers and enantiomers thereof.
In the above ~lefini~ions, halogen is to be understood as meaning iodine, preferably
flllorine, chlorine and bromine.
Suitable alkyl groups are straight-chain or branched alkyl groups, for example methyl,
ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-pentyl and its isomers;
preferably methyl and ethyl.
Suitable haloaL~yl groups are mono- or polysubstituted alkyl groups, in particular alkyl
groups which are mono- to trisubstituted by halogen, halogen specifically being
understood to mean bromine or iodine and, in particular, fluorine or chlorine, for example
fluoromethyl, difluoromethyl, chloromethyl, dichloromethyl, trichloromethyl and, in
particular, trifluoromethyl.
Suitable alkoxy groups are, for example, methoxy, ethoxy, n-propyloxy and
iso-propyloxy.
Suitable haloalkoxy groups are, for example, difluoromethoxy, trifluoromethoxy,
2,2,2-trifluoroethoxy, 1,1,2,2-tetrafluoroethoxy; preferably difluoromethoxy andtrifluoromethoxy .
The N-phenyl- and N-heteroaryl-alkylcarbamates of the formula I according to theinvention have a good selectivity in crops of useful plants, such as cereals, rice, maize,
soya beans and cotton, when applied post-emergence, but in particular when applied
pre-emergence.
The result of a possible existence of at least one asymmetric carbon atom in thecompounds of the formula I in the ,~ position relative to the phenyl-X group and on the
benzyl ~- and/or ~-carbon atom if R4 and R2 differ from Rs and R3, respectively, is that the
compounds can occur both as optically active isomers and in the form of racemic
lllix.lulc;S. The optically active compounds of ~he formula I can be obtained from the
racemic mixtures by known separation methods, for example fractional cryst~ tion, or
by enantioselective synthesis. The active ingredients of the formula I in the present
invention are to be understood as meaning all pure optical antipodes, but also the
racemates. Unless specific mention is made to individual optical antipodes, the formula
given is to be understood as indicating those racemic mixtures which result from the
CA 02203890 1997-04-28
wo 96/16941 PCT/EPg5/04612
preparation process indicated.
Preferred compounds of the forrnula I are those in which the radical R is chlorine,
bromine, trifluoromethyl or trifluoromethoxy.
Other preferred compounds of the formula I are those in which Z is hydrogen or fluorine.
Other ~,efc,lcd compounds of the formula I are those in which R1 is methyl or ethyl.
Further plcrcllcd compounds of the formula I are those in which R2, R3, R4 and Rs
independently of one another are hydrogen or methyl. Particularly ~ulcrc.lcd amongst these
compounds are those in which R2, R3 and R4 are hydrogen and Rs is methyl.
Also ~lcrcllcd compounds of the formula I are those in which R2, R3 and R4 are hydrogen,
Rs is methyl or ethyl and Q is group (1), (2) or (3). Particularly preferred amongst these
compounds are those in which Rs is methyl and Q is group (1) or (2).
Further ~lcr~llcd co~npounds of the formula I are those in which X is oxygen or sulfur.
Other ~l~rcllkd compounds of the formula I are those in which Q is group (1) or (2); n is
1; and Y is bonded in the ortho- or meta-position relative to the bonding site. Particularly
~lcrellcd amongst these compounds are those in which Y is bonded in the ortho-position
relative to the bonding site.
Furthermore ~l~fcllcd compounds of the forrnula I are those in which Y is hydrogen,
fluorine, chlorine, methyl, trifluoromethyl, methoxy or cyano.
Important compounds of the formula I are those in which Q is group (1); R is chlorine,
cyano, nitro, trifluoromethyl, trifluoromethoxy or difluoromethoxy; Z is hydrogen; or Z
and R together in the 2- and 3-position of the phenyl ring form a group -OCF2O-; X is
oxygen or sulfur; Y is hydrogen, 2-fluoro, 2- or 3-chloro, 2-methyl or 2-trifluoromethyl; n
is 0 or 1; Rl is methyl or ethyl; R2 is hydrogen; and R3, R4 and Rs independently of one
another are hydrogen or methyl.
Other ""~ollant compounds of the formula I are those in which Q is the group
CA 02203890 1997-04-28
W 096/16941 PCT/~5~nscl2
~ ; R is trifluoromethyl or trifluoromethoxy, Z is hydrogen; or Z and R
together in the 2- and 3-position of the phenyl ring form a group -OCF20-; X is oxygen or
sulfur; Y is hydrogen, 3- or 5-chloro or 3-methy; Rl is ethyl; R2 is hydrogen or methyl; R3
is hydrogen; and m is 0.
Also important compounds of the formula I are those in which Q is the group
~~ ; R is trifluoromethyl or trifluoromethoxy; Z is hydrogen; or Z and R
together in the 2- and 3-position of the phenyl ring form a group -OCF20-; X is oxygen or
sulfur; Y is hydrogen, 3-chloro or 3-methyl; Rl is ethyl; R2 and R3 are hydrogen; R4 and
Rs independently of one another are hydrogen or methyl; and m is 1.
~ Y
Important compounds of the formula I are those in which Q is the group ~ ~ ; R
is trifluoromethyl or trifluoromethoxy; Z is hydrogen; or Z and R together in the 2- and
3-position of the phenyl ring form a group -OCF20-; X is oxygen; Y is hydrogen; R1 is
ethyl; R2 is hydrogen or methyl; R3 is hydrogen; and m is 0.
Other important compounds of the formula I are those in which Q is the group
~ ; R is trifluoromethyl or trifluoromethoxy; Z is hydrogen; or Z and R together
in the 2- and 3-position of the phenyl ring forrn a group -OCF20-, X is oxygen; Y is
hydrogen; R1 is ethyl; R2 and R3 are hydrogen; R4 and Rs independently of one another
are hydrogen or methyl; and m is 1.
Also important compounds of the formula I are those in which Q is the group
3 4 y
; R is trifluoromethyl or trifluoromethoxy; Z is hydrogen; or Z and R
N 6
together in the 2- and 3-position of the phenyl ring form a group -OCF20-; X is oxygen; Y
is hydrogen or 3-methyl; Rl is ethyl; R2, R3 and Rs are hydrogen; R4 is hydrogen or
methyl; and m is 1.
Particularly preferred compounds to be mentioned are those of the forrnula Ia and Ib
-
CA 02203890 1997-04-28
Wo 96/16941 pcTlEps5lQ46l2
D CH3
~ ~ o~ `CH ~C~ ~CH~ \Q (Ia)
C~3
and
ICH3
~ O' `CH C CH2 Q (Ib),
o><o
F F
in which Q is as defined for formula I. Amongst these, very particularly important are
those compounds of the formulae Ia and Ib in which Q is the group ~ (1),
~ (2) or ~ (3); Y is hydrogen, fluorine, chlorine, methyl,
trifluoromethyl, methoxy or cyano; n is 0, 1 or 2; and n1 is O or 1.
Also prerell~d compounds of the formula I are those in which the ~B-carbon atom is present
in optically pure form as the (-)-enantiomer.
The process according to the invention for the ~lel~aldlion of the compounds of the
formula I is carried out analogously to known processes and comrri~es,
a) to prepare the compounds of the formula I, reacting a compound of the formula II
,CH2~ ,OH (II),
Rl
in which R, Z, Rl and ~ are as defined for formula I with a benzyl isocyanate of the
CA 02203890 1997-04-28
Wo 96/16941 PCT/~;I 5~l0 1Cl2
formula III
( \ / \
`Ck ~Q (III),
R2 R3
in which R2, R3, R4, Rs, Q and m are as defined for formula I in an inert organic solvent in
the presence or absence of a catalyst; or
b) first chlo~ ylating a compound of the formula II
CH~ ,OH (~,
R Rl
under cll~tom~ry conditions, preferably using phosgene or diphosgene, to give a
compound of the formula IV
~ X `CH ~C~ (IV)
Rl O
where, in formulae II and IV the radicals R, Z, Rl and X are as defined for formula I, and
subsequently reacting this product with an amine of the formula V
~c~R5~
` c >mlQ (V),
R2 R3
in which R2, R3, R4, Rs, Q and m are as defined for formula I in an inert organic solvent in
the presence of a proton scavenger, for example tertiary amines or pyridine.
Process variants a) and b) follow equation l.
-
CA 02203890 1997-04-28
W 096/16941 PCT/~l53~'01Cl2
Equation 1:
z z
~ ,CH2~ HOH COC12 ~ X~ `CH C~
R II Rl R Rl o
/R4 Rs~ IV
a) ~ ~ Q R2 R3
III V
R X CH CC~Q
The ~lclition reaction in accordance with process variant a) can expediently be carried out
by reacting the alcohol of the formula II and the isocyanate of the forrnula III in an inert
aprotic organic solvent, such as an aliphatic or cyclic ether, for example diethyl ether,
1,2-dimethoxyethane, tetrahydrofuran or dioxane, or a chlonn~tt-cl aliphatic hydrocarbon,
for example methylene chloride, or an aromatic, for example toluene or xylene, or an
aliphatic ester, for example ethyl acetate, in the presence of a catalyst, for example
4-N,N-dimethylaminopyridine, triethylamine, dibutyltin dilaurate and/or dibutyltin
diacetate, preferably at temperatures of between 20C and the reflux temperature of the
reaction solution.
.
In process variant b), the chloroforrnate of the formula IV and the amine of the formula V
are expediently reacted in an inert aprotic organic solvent in the presence of an organic
base, analogously to the procedure described in process variant a), at temperatures of
between -20C and +40C, preferably between +$C and +20C. For working-up, the
resulting reaction mixture is washed, preferably using water and dilute acid, to remove
amine by-products in the form of salts.
The alcohols of the formula II (IIa: Xl = -O- or -S-; IIb; and IIc) can be prepared by
known standard methods (for example US-A-5 099 059 and WO 94/10 132), for example
CA 02203890 1997-04-28
W 096/16941 PcT/~l5~l~1cl2
as described in equation 2 below.
Equation 2
~XIH + CH2-CH2_ 1 ~X,CH.2~ ~OH
R VI VII V R IIa Rl
o Rl O
if X 1 = S ~ S CH Z
[O] e.g. H22. )=/ \C/ \OH or ~ S /CH
KMnO4, KJ04 R _ R/ o CH2 OH
IIb IIc
The intermediates of the formula IIa can also be prepared under pressure in the presence
of lithium hydroxide monohydrate and in the absence of solvents, as described inWO 94/10132.
The alcohols of the formula II can be separated to give the enantiomers for example with
the aid of liquid chromatography on chiral carriers, for example HPLC on Chiracel-OD-H
(Daicel) and 2 % isopropanol in n-hexane as the eluent.
A further possibility of obtaining enantiomerically pure alcohols of the formula II is the
enantioselective hydrogenation of a-phenoxyketones using BINAP/Ru(II) catalyst
complexes.
The (-) enantiomer of the compounds of the formula II is to be understood as the optical
antipode of the formula IIe which is first, i.e. before the (+) enantiomer, eluted by means
of HPLC on a Chiracel-OD-H column by Daicel using the mixture of n-hexane and 2 % of
isopropanol as the eluent, or which is formed predominantly (enantiomeric excess up to
> 96 %) in the enantioselective hydrogenation of a-phenoxylcetones using
Ru2Cl4[(R)-BINAP]2[N(C2Hs)3] catalyst complex (equation 2a).
The process according to the invention for the preparation of optically active compounds
of the formula IIe
CA 02203890 1997-04-28
W O 96/16941 PCT/~5Jm1~l2
Z Rl H
~ ~CH/2~0H (IIe),
in which R, Rl, X and Z are as defined in claim 1 and the ~-carbon atom is present in
optically pure forrn as the (-) enantiomer comprises subjecting the compound of the
formula VIII
Rl,
~ x \c = o (VIII),
R CH2
in which R, Rl, X and Z are as defined above to a (R)-BINAP/Ru(II)-catalysed
hydrogenation reaction in an alcoholic solvent, for example methanol or ethanol, in the
presence or absence of a catalytic amount of a protonic acid, for example hydrochloric
acid or nitric acid.
The process in which a-phenoxy ketones of the formula VIII are subjected to
çn~nti~elective hydrogenation using (R)-BINAP/Ru(II) catalysts is as shown in equation
2a.
Eguation 2a:
~X `C o H2, (R)-BINAplRu(II) Z Rl H
CH2 solvent R6-OH, H30 cat~P~ T ` ' ~ ~
VIII IIe: (-) enantiomer
+
~R~ CH~ \OR6
IX
F.n~nrioselective homogeneous hydrogenation reactions of oc-functionalized ketones with
BINAP-Ru(II) catalyst complexes are known and described, for example, in J. Am. Chem.
CA 02203890 1997-04-28
W O96/16941 PCT~EP95/04612
Soc. 110, 629 (1988).
The enantioselective hydrogenation reaction according to the invention starts from the
ketones of the formula VIII and is novel.
The ketones of the formula VIII are either known or can be prepared readily by known
processes, for example as described in J. Am. Chem. Soc. 68, 38 (1946).
The enantioselective hydrogenation reaction is expediently carried out in alcohols, for
example R6-OH, in which R6 is Cl-C4alkyl, in particular in methanol and ethanol. The
catalysts employed are BINAP/Ru(II) complexes, for example as described in
US-A-4 691 037, but in particular Ru~Cl4[(R)-~.~'-bis(diphenylphosphino)-1,1 '-
dinaphthyl]2[N(C2H5)3] = Ru2Cl4L~R)-BINAPl2lN(C2Hs)3]. The concentration of the
catalyst complex is not critical for the course of the enantioselective hydrogenation. If
desired, a protonic acid, for example hydrochloric or nitric acid, can be added to act as
co-catalyst. The hydrogenation is preferably carried out at pressures *om atmospheric
pressure to 100 bar, in particular under slightly elevated pressure up to 80 bar, and at
temperatures of from 10C to the boiling point of the solvent used, preferably at
temperatures of from 20C to 40C.
Under standard hydrogenation conditions in dilute alcoholic solutions R6-OH, in which R6
is Cl-C4alkyl, for example the 1.0 to 1.2-molar concentration of the ketone of the formula
VIII, the acetal of the formula IX is formed in variable amounts, up to 30 %, as an
undesirable, stable by-product. This acetal is also formed in hydrogenation reactions
without added acid.
The abovementioned forrnation of acetals on certain metal complexes, in particular on
Ru(II) complexes, is eX~minefl~ for example, in J. Organomet. Chem. 415, 127 (1991) and
Synlett 1993, 751.
According to J. Am. Chem. Soc. 117,4423 (1995), the acetal formation in the course of the
enantioselective hydrogenation of ,B-keto esters in the presence of i-Pr-BPE/Ru(II) as the
catalyst can be suppressed by adding water (10 % mixture of water and methanol).
Surprisingly, it is now found that the acetal formation can be suppressed largely or even
completely when the concentration of the ketone of the formula VIII in the hydrogenation
solution is increased; for example, only a maximum of 20 % of acetal of the formula IX
CA 02203890 1997-04-28
W O96/16941 PCTAEP95104612
are formed in an approximately 30 % hydrogenation solution (approximately 1.3-rnolar
concentration of ketone of the formula VIII), and no more acetal of the formula IX at all in
an approximately 65 % hydrogenation solution (approximately 2.8-molar concentration of
ketone of the formula VIII).
The yield of crude alcohol of the formula II is generally > 90 %, the enantiomeric excess
of desirable (-) enantiomer IIe being up to > 96 % under the reaction conditions in~ t~.d,
depending on the substituents R, Rl, X and Z. The absolute configuration (not known in
the present case) of the alcohol formed is determined by the configuration of the catalyst
complex used. In the en~ntioselective hydrogenation process according to the invention,
the use of Ru2Cl4[(R)-BINAP]2[N(C2Hs)3] complex mainly leads to the desirable (-)
en~ntiomeric alcohol of the formula IIe.
The optically pure alcohols of the formula IIe((-) enantiomers) are novel and therefore
also provided by the present invention.
The enantioselective synthesis of the compounds of the formula I-which are optically
active in the ~-position relative to the phenyl-X group ((-) enantiomers) from the
corresponding optically active alcohols of the forrnula IIe ((-) enantiomers) can be
effected for example by a method similar to process variants a) and b) described(equation 1).
The isocyanates of the formula III are either commercially available or can be prepared
analogously to known processes, for example as described in Houben-Weyl, "Methoden
der Org~ni~çhen Chemie" [Methods in Organic Chemi~try], Vol. VIII, page 119 et seq.,
Thieme-Verlag Stuttgart, 1952.
The amines of the formula V are either commercially available or can be preparedanalogously to known processes, for example as described in Houben-Weyl, "Methoden
der Org~nischt~n Chemie" [Methods in Organic Chemistry], Vol. XI(l), Thieme-Verlag
Stuttgart, 1957.
The chlol.,rvl"late derivatives of the formula IV are synthesized by methods known per
se, for example as described in US-A-5 099 059, US-A-5 078 783 and WO 94/10132.
The compounds of the formulae I, IIa, IIb, IIc and IV can be isolated and purified by
methods known per se. Those skilled in the art will also be aware of the sequence in which
CA 02203890 1997-04-28
W O96/16941 PCT1~ 01~12
2-
certain re~ctionc are experliently to be carried out under process variants a) and b) to avoid
potential secondary re~- tionc
Unless a targeted synthesis for isolating pure isomers is carried out, the product can be
obtained in the form of a ~ Ul~ of two or more isomers. The isomers can be separated by
methods known per se.
The intermediates of the formula IId
~ X~cH2~cH (~d),
o~><o
F F
in which Rl and X are a. defined in formula I are ~licclosecl in WO 94/10132.
The same preferences given for the compounds of the forrnula I apply to the interrnt-.fli~tPs
of the formulae II and IV.
The starting compounds of the formulae VI and VII required for the ~l~alalion processes
are either known or can be prepared by various processes known from the literature, for
example as described in equation 3 below in the case of colll~oul,ds of the formula VIa.
Equation 3: _
NH2 OH
O~ ~F l)D~ ;--" ~ ~F
F 2)Hy~olys~to ~enol ~ O F
VIa
The preparation of the starting compound required, of the forrnula ~, is described in
EP-A-0 198 797.
The form~ tio~, i.e. the compositions, preparations or products comprising the active
ingredient of the formula I in the presence or absence of one or more solid or liquid
CA 02203890 1997-04-28
W O 96/16941 PCTAEP95/04612
- 13 -
additives, are prepared in a known manner, for example by intimately mixing and/or
grinding the active ingredients with extenders, for example solvents, solid calTiers and, if
desired, surface-active compounds (surfactants).
Suitable solvents may be: aromatic hydrocarbons, in particular the fractions C8 to Cl2,
such as mixtures of alkylbenzenes, for example xylene mixtures, or aL~ylated
naphthalenes; aliphatic and cycloaliphatic hydrocarbons, such as paraffins, cyclohexane or
tetrahydronaphthalene; alcohols, such as ethanol, propanol or butanol; glycols and their
ethers and esters, such as propylene glycol or dipropylene glycol ether, ketones such as
cyclohexanone, isophorone or diacetone alcohol, strongly polar solvents, such asN-methyl-2-pyrrolidone, dimethyl sulfoxide or water, vegetable oils and their esters, such
as rapeseed oil, castor oil or soya oil: if desired also silicone oils.
Solid carriers, for example for dusts and dispersible powders, which are generally used are
ground natural minerals, such as calcite, talc, kaolin, montmorillonite or attapulgite. To
improve the physical properties, it is also possible to add highly-disperse silica or
highly-disperse absorptive polymers. Suitable particulate, adsorptive carriers for granules
are porous types, for example pumice, brick grit, sepiolite or bentonite, unsuitable
non-soll,~ive carrier materials are, for example, calcite or sand. In addition, a large number
of pregr~n~ t~rl materials of inorganic or organic nature, such as, in particular, clolomite
or comminn~ plant rç~i~nes
Suitable surface-active compounds are, depending on the nature of the active ingredient of
the formula I to be formulated, non-ionic, cationic and/or anionic surfactants which have
good emulsifying, dispersing and wetting plu~ellies. Sllrf~rt~n~ are all understood as
meaning surfactant mixtures.
Suitable anionic surfactants can be either water-soluble soaps or water-soluble synthetic
surface-active compounds.
Soaps which are suitable are the alkali metal salts, ~lk~linç earth metal salts or substituted
or unsubstituted ammonium salts of higher fa~ty acids (Cl0-C22) for exarnple the sodium
salts or potassium salts of oleic or stearic acid, or of natural fatty acid mixtures which can
be obtained from, for example, coconut oil or tallow oil. Other surfactants which may be
men~ion~ are the fatty acid methyltaurinates.
However, so-called synthetic surfactants are used more frequently, in particular fatty
CA 02203890 1997-04-28
W 096/16941 PCT/~:l5~lo~6l2
-14- .
alcohol sulfonates, fatty aIcohol sulfates, sulfonated ben7imi-1~70le derivatives or
aLkylarylsulfonates .
The fatty alcohol sulfonates or fatty alcohol sulfates are, as a rule, in the form of the alkali
metal salts, ~lk~line earth metal salts or substituted or unsubstituted ammonium salts and
have an alkyl radical having 8 to 22 carbon atoms, alkyl also including the alkyl moiety of
acyl radicals, for example the sodium or calcium salt of lignosulfonic acid, of the dodecyl
sulfuric ester or of a fatty alcohol sulfate mixture prepared from natural fatty acids. This
group also includes the salts of the sulfuric esters and sulfonic acids of fattyalcohol/ethylene oxide adducts. The sulfonated ben7imifl~7ole derivatives comprise
preferably 2 sulfonyl groups and a fatty acid radical having 8-22 carbon atoms. Examples
of alkylaryl sulfonates are the sodium, calcium or triethanolamine salts of
dodecylben7t-n~sulfonic acid, of dibutylnaphthalenesulfonic acid or of a
n~phth~lenesulfonic acid/formaldehyde condensation product.
Suitable phosphates, for example salts of the phosphoric ester of a
p-nonylphenol/(4-14)ethylene oxide adduct, or phospholipids, are also suitable.
Suitable nonionic surfactants are mainly polyglycol ether derivatives of aliphatic or
cycloaliphatic alcohols, saturated or unsaturated fatty acids and alkylphenols, which can
compri~e 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic)hydrocarbon radical and 6 to 18 carbon atoms in the alkyl radical of the alkylphenols.
Other suitable nonionic surfactants are the water-soluble polyethylene oxide adducts with
poly~ro~ylene glycol, ethylene diaminopolypropylene glycol and alkylpolypropylene
glycol which have 1 to lQ carbon atoms in the alkyl chain and comprise 20 to 250ethylene glycol ether groups and 10 to 100 propylene glycol ether groups. The
abovementioned compounds conventionally comprise 1 to 5 ethylene glycol units per
propylene glycol unit.
Exarnples of nonionic surfactants which may be mentioned are
nonylphenolpolyethoxyethanols, castor oil polyglycol ethers, polypropylene/polyethylene
oxide adducts, tributylphenoxypolyethoxyethanol, polyethylene glycol and
octylphenoxypolyethoxyethanol.
Other substances which are suitable are fatty acid esters of polyoxyethylene sorbitan, such
as polyoxyethylene sorbitan tIioleate.
CA 02203890 1997-04-28
W 09611fi941 PcTl~lJJ~o1~l2
The cationic surfactants are mainly quaternary ammonium salts which comprise, as N
subs*tllents, at least one alkyl radical having 8 to 22 carbon atoms and as further
substislle~nts lower, halogenated or unhalogenated alkyl, benzyl or lower hydroxyaL~yl
radicals. The salts are preferably in the form of halides, methylsulfates or ethylsulf~tes~ for
example stearyltrimethylammonium chloride or benzyldi(2-chloroethyl)ethyl~mmo~ mbromide.
The surfactants conventionally used in the art of formulation are described, inter alia, in
the following publications:
- "McCutcheon's Detergents and Emulsifiers Annual", Mc Publishing Corp.,
Glen Rock, New Jersey, 1988.
- M. and J. Ash, "Encyclopedia of Surfactants", Vol. I-III, Chemical Publishing Co.,
New York, 1980-1981.
- Dr. Helmut Stache "Tensid-Taschenbuch" [Surfactants Guide], Carl Hanser Verlag,
Munich/Vienna 1981.
As a rule, the herbicidal preparations comprise 0.1 to 99 %, in particular 0.1 to 95 %, of
active ingredient of the formula I, 1 to 99 % of a solid or liquid additive and 0 to 25 %, in
particular 0.1 to 25 %, of a surfactant.
While concentrated compositions are more preferred as commercially available goods, the
end consumer uses, as a rule, dilute compositions.
The compositions can also comprise other additives such as stabilizers, for example
epoxi~li7P~l or unepoxidized vegetable oils (epnxidi7~-1 coconut oil, rapeseed oil or soya
oil), antifoam, for example silicone oil, preservatives, viscosity regulators, binders,
t~rk ifi~rs and fertilizers or other active ingredients for achieving specific effects.
In particular, ~uler~,led formulations are composed as follows: (% = per cent by weight)
Emulsifiable concentrates:
Active ingredient: 1 tO 90 %, preferably 5 to 50 %
Surfactant: 5 to 30 %, preferably 10 to 20 %
Liquid carrier: 15 tO 94 %, preferably 70 tO 85 %
CA 02203890 1997-04-28
W O96/16941 PCT/~ c1Cl2
-16-
Dusts~
Active ingredient: 0.1 to 10 %, preferably 0.1 to l %
Solid carrier: 99.9 % to 90 %, preferably 99.9 to 99 %
Suspension concentrates:
Active ingredient: 5 to 75 %, preferably 10 to S0 %
Water: ~ 94 to 24 %, preferably 88 to 30 %
,Surfactant: ' 1 to 40 %, preferably 2 to 30 %
Wettable powders:
Active ingredient: 0.5 to 90 %2 preferably 1 to 80 %
Surfactant: 0.5 to 20 %, preferably 1 to lS %
Solid carrier: 5 to 95 %, preferably 15 to 90 %
Granules:
Active ingredient: 0.5 to 30 %, preferably 3 to lS %
Solid carrier: 99.5 to 70 %, preferably 97 to 85 %
A. Formulation examples for active in~redients of the formula I
(% = per cent by wei~ht)
1. Wettablepowders _ - a) b) c)
ActiveingredientofTables 1-6 20 % 50 % 0.5 %
Sodium lignosulfonate 5 % 5 % 5 %
Sodium lauryl sulfate 3 %
Sodium diisobutylnaphth~ltq,n~,-
sulfonate - 6 % 6 %
Octylphenol poiyethylene glycol
ether (7-8 mol of EO) - 2 % 2 %
Highly-disperse silica 5 % 27 % 27 %
Kaolin 67 %
Sodium chloride - - 59.5 %
The active ingredient is mixed intim~tely with the additives and the mixture is ground
thoroughly in a suitable mill. This gives wettable powders which can be diluted with water
to suspensions of any desired concentration.
CA 02203890 1997-04-28
Wo 96/16941 - PCT/~;~9~/~ 1C12
- 17 -
2. Emulsionconcentrates a) b)
ActiveingredientofTables 1-6 10 % 1 %
Czllcillm dodecylben~enesulfonate 3 % 3 %
Octylphenol polyethylene glycol ether
(4-5 mol of EO) 3 % 3 %
Castor oil polyethylene glycol ether
(36 mol of EO) 4 % 4 %
Cyclohexanone 30 % 10 %
Xylene n,i~ . 50 % 79 %
Emulsions of any desired concentration can be prepared from such concentrates bydiluting them with water.
3. Dusts = a) b)
ActiveingredientofTables 1-6 0.1% 1%
Talc 99 9 %
Kaolin 99%
Ready-to-use dusts are obtained by intim~tely mixing the carriers with the active
ingredient.
4. Extruder~ranules a) b)
Active ingredient of Tables 1-6 10 % 1 %
Sodium lignosulfonate 2 % 2 %
Carboxymethylcellulose 1% 1%
Kaolin 87 % 96 %
The active ingredient is mixed with the additives, and the mixture is ground and moistened
with water. This mixture is extruded and subsequently dried in a stream of air.
=,
5. Coated ~ranules
Active ingredient of Tables 1-6 3 %
Polyethylene glycol (MW200) 3 %
Kaolin 94 %
In a mixer, the kaolin which has been moistened with polyethylene glycol is coated
CA 02203890 1997-04-28
wo 96/16941 PCr/EPs~/04612
- 18 -
uniformly with the finely ground active ingredient. This gives dust-free coated granules.
6. Suspensionconcentrate a) b)
Active ingredient of Tables 1-6 5 % 40 %
Ethylene glycol 10 % 10 %
Nonylphenol polyethylene glycol ether
(15 mol of EO) 1 % 6 %
Sodiumlignosulfonate 5 % 10 %
Carboxymethylcellulose 1% 1%
37% aqueous formaldehyde solution 0.2 % 0.2 %
Silicone oil in the form of a 75 %
aqueous emulsion 0.8 ~7G 0.~ %
Water 77 % 32 %
The finely ground active ingredient is mixed intimately with the additives. This gives a
suspension concentrate from which suspensions of any desired concentration can be
prepared by diluting it with water.
7. Salt solution
Active ingredient of Tables I-6 5 %
Isopropylamine 1 %
Octylphenol polyethylene glycol ether
(78 mol of EO) 91 %
The compounds of the formula I are employed in unaltered form, as they can be obtained
from synthesis, or, preferably, as compositions together with the auxiliaries convention~lly
used in the art of formulation, and they are therefore processed in a known manner to give,
for example, emulsion concentrates, ready-to-spray or ready-to-dilute solutions, dilute
emulsions, wettable powders, soluble powders, dusts, granules, and also encapsnl~t;onc,
for example in polymeric substances. The methods of application, such as spraying,
atomizing, dusting, spreading or pouring, and the nature of the compositions are selected
to suit the inten(led aims and the prevailing circ--m~t~nc~es As a rule, the rates of
application are 0.00~ to 2 kg per hectare, preferably 0.01 to 1 kg per hectare.
CA 02203890 1997-04-28
wo 96/16941 = PCT/EP95/04612
- 19-
B. Preparation examples
ExampleH1: 1-(3-Trifluoromethylphenoxy)-2-butanol (intermediate)
C2HS
CF3 CH2 OH
In a bomb tube (pressurized vessel), 40.5 g of 3-hydroxybenzotrifluoride, 18.0 g of
a-butylene oxide and 1.0 g of lithium hydroxide monohydrate are heated for 16 hours at
140C. After the reaction vessel has cooled, the reaction mixture is dissolved in 200 ml of
ethyl acetate, and the organic phase is washed with water and subsequently dried over
sodium sulfate. After concentration, the desired product
1-(3-trifluoromethylphenoxy)-2-butanol is obtained in a yield of 54.0 g and in high purity.
The product can be employed in the subsequent reaction without further pllrific~tion.
Example H2: 1-(3-ChlorophenoxY)-2-butanol (intermediate) is obtained analogously to
Example H1 by using 51.4 g of 3-chlorophenol, 28.8 g of o~-butylene oxide and 1.0 g of
lithium hydroxide monohydrate in a yield of 71.4 g in the form of an oil; b.p.
83-84C/0.04 torr.
Example H3: 1-(3-Cyanophenoxy)-2-butanol (intermediate) is obtained analogously to
Example H1 by using 20.7 g of 3-cyanophenol, 13.8 g of a-butylene oxide and 0.5 g of
lithium hydroxide monohydrate in a yield of 25.9 g in the form of an oil; b.p.
116-118C/0.04 torr.
Example H4: 0-(3-TrifluoromethYlphenoxy)-2-butyl chloroformate (intermediate)
C2H5 0
~ O CH C
>=/ CH2 0 Cl
CF3
46.8 g of 1-(3-trifluoromethylphenoxy)butan-2-ol in 200 ml of toluene is added to 125 ml
of a 1.93-molar solution of phosgene in toluene and 0.5 ml of N,N-dimethylform~mi-l~
CA 02203890 1997-04-28
Wo 96/16941 PCT/EP95/04612
- 20 -
After the slightly exothermic reaction has subsided, the mixture is heated for 8 hours at
60C. After the reaction mixture has been concentrated, the desired product
0-(3-trifluoromethylphenoxy)-2-butyl chloroformate is obtained in qu~ntit~tive yield and
can be employed in subsequent reaction without further pllrifir~tion.
Example HS: O-rl-(3-Trifluoromethylphenoxy)-2-butyll-N-(2-phenylethyl) carbamate
Et O
~ OCH2 CHO- C - NH--CH2- CH2~ (Comp. No. 1.1)
CF3
A solution of 1.2 g (0.01 mol) of 2-phenylethylamine and 1.1 g (0.011 mol) of
triethylamine in 20 ml of methylene chloride is added dropwise to 2.9 g (0.01 mol) of
0-(3-trifluoromethylphenoxy)-2-butyl chloroformate in 20 ml of methylene chloride.
After 14 hours, the solution is poured into cold, dilute hydrochloric acid, and the organic
phase is separated off, washed with water, dried using sodium sulfate and evaporated on a
rotary evaporator. It is purified by means of column chromatography on silica gel using
ethyl acetate/hexane 1/3 as the eluent, resulting in 3.4 g (80.5 % of theory) of a colourless
oil of refractive index nD25 1.5015. The biologically active (-) isomer (the two (+) and
(-) isomers are separated by chromatography at the level of the alcohol of the formula II)
has the boiling point 49-50C.
Exarnple H6: (-) Enantiomer of 1-(3-trifluoromethylphenoxy)butan-2-ol (intermediate)
~,
~o /cs~ (Comp. No. 7.2)
~ CH2 OH
CF3
Using a steel capillary, 87.9 g of 1-(3-trifluoromethylphenoxy)butan-2-on, dissolved in
130 ml of methanol and 0.641 g of
Ru2Cl4[(R)-2,2'-bis(diphenylphosphino)- I, l ' -dinaphthyl]2[N(C2 Hs)3] =
Ru2C14[(R)-BINAP]2[N(C2Hs)3] and 1.2 ml of lN hydrochloric acid are transferred in
succession into a steel autoclave under an argon atmosphere. In 3 cycles (20 bar,
atmospheric pressure), the argon inert gas is displaced by hydrogen, and the mixture is
subsequently hydrogenated at ~0C undel a hydrogen pressure of 80 bar. After 69 hours,
the hydrogen uptake has ended. The reaction mixture is removed from the steel autoclave
:
CA 02203890 1997-04-28
W 096/16941 PCT/~ 01C12
- 21 -
and evaporated on a rotary evaporator. The residue obtained is dissolved in ethyl
acetate/n-hexane 1/4 and filtered through a short silica gel column to remove the catalyst.
After the collected fractions have been evapo~ated, the desired product is obtained in a
yield of 87.9 g (99 % of theory); refractive index nD 1.4611;
~ cx]365 -18 7+ 0.2 (c=1.0 in ~ anol~. .
The purity of the (-) en~ntiomer is measured by means of HPLC using a Chiracel-OD-H
column (4.6 x 250 mm) by Daicel and using the mixture of n-hexane and 2 % of
isopropanol as the eluent.
The enantiomeric excess (ee) is 94.2 % ((-) enantiomer).
Under the HPLC chromatography conditions given above, the (-) enantiomer is eluted
first, i.e. before the (+) enantiomer.
The compounds of the formula I listed in Tables 1-6 below are prepared analogously.
The (-) enantiomers listed in Tables 1 and 2 below refer to the asymmetric carbon atom in
the ,B-position relative to the phenyl-X group in the compounds of the formula I and are
prepared from the corresponding (-) ~n~ntiomeric alcohols of the formula IIe.
CA 02203890 l997-04-28
W 096/16941 PCTI~/01Cl2
- 22 -
Table 1: Compounds of the formula Ic
--CH2 CHO- C--N--C--C ~Yn (Ic)
R3 Rs
Comp. Physical data
No. R Z X n Y Rl R2 R3 R4 Rs
1.1 CF3 H O H Et H H H H n 2D5 1.5015
1.2 CF3 H O 1 2-Cl Et H H H H n D 1.5084
1.3 CF3 H O H Me H H H H
1.4 CF3 H O 1 2-Me Et H H H H n D 1.5067
1.5 CF3 H O H Et H H Me H n D1.5025
1.6 CF3 H O H Et H Me H H m.p. 48-58C
1.7 CF3 H S H Et H H H H
1.8 CF3 H O 1 2-CF3 Et H H H H
1.9 CF3 H O 1 3-CI Et H H H H
1.10 CF3 H O 1 4-F Et H H H H
1.11 CF3 H O H Et H H -Et H
1.12 CF3 H O H Et H Me Me H
1.13 CF3 H O H Et H H Me Me
1.14 OCF3 H O H Et H H H H
1.15 OCF3 H O 1 2-F Et H H H H
1.16 OCF3 H O 1 2-Me Et H H H H
1.17 OCF3 H O 1 2-Me Et H H Me H
1.18 CN H O H Et H H H H
-19 NO2 H O H Et H H H H
1.20 Cl H O H Et H H H H
1.21 -O-CF2-O- O H Et H H H H
1.22 -O-CF2-O- O 1 2-Me Et H H H H
1.23 CF3 H O 1 2-Cl Et H H Me H n D1.5113
1.24 CF3 H O 1 2-Cl Et H H Me Me n 2D 1.5135
-
CA 02203890 1997-04-28
W O96116941 PCT/~r~S/01C12
-23-
Comp. - - Physical data
No. R Z X n Y Rl R2 R3 R4 Rs
1.25 OCHP2H O H Et H H H H
1.26 CF3 H O H Et H H H H m.p. 49-50C;
(-) enantiomer
1.27 CF3 H O H Et H H Me H oil; (-) çn~n~iomer
Table 2: Compounds of the forrnula Id
Z X--Cl 12 CHO C--N--C 1~ yn1 (Id)
Comp. Physical data
No. R Z X nl Y Rl R2 R3
2.1 CF3 H O H Et H H n D 1.4989
2.2 CF3 H O H Et Me H
2.3 CF3 H S H Et H H
2.4 OCF3 H O H Et H H
2.5 CF3 H O 1 5-Cl Et H H 45-48C
2.6 CP3 H O 1 3-Me Et H H 60-62C
2.7 CF3 H O 1 3-Cl Et H H 68-70C
2.8 -O-CF2-O- O H Et H H
2.9 OCF3 H O 1 3-Me Et H H 68C; (-)enantiomer
2.10 OCF3 H O 1 3-Cl Et H H
2.11 CF3 H O H Et H H 65-66C; (-) enantiomer
2.12 CF3 H O 1 3-Me Et H H 77-78C; (-) enantiomer
CA 02203890 1997-04-28
WO 96/16941 PCT~ 95/01Cl2
- 24 -
Table 3: Compounds of the formula Ie
~ X - CH2--CH--O - C - N - C - C ~ (le)
R R3 R5
Comp. Physical data
No. R Z X nl Y Rl R2 R3 R4 Rs
3.1 CF3 H O H Et H H H H oil
3.2 CF3 H O 1 3-Me Et H H H H oil
3.3 CF3 H O 1 3-Cl Et H H H H
3.4 CF3 H O H Et H H Me H oil
3.5 CF3 H O l 3-Me Et H H Me H
3.6 CF3 H O 1 3-Cl Et H H Me H
3.7 CF3 H O H Et H H Me Me
3.8 -O-CF2-O- O H Et H H H H
3.9 CF3 H S H Et H H H H
3. lû OCF3 H O H Et H H H H
3.11 OCF3 H O H Et H H Me H
3.12 OCF3 H O 1 3-Me Et H H H H
3.13 OCF3 H O 1 3-Me Et H H Me H
CA 02203890 1997-04-28
W 096/16941 PCT/~~ o1cl2
Table 4: Compounds of the formula If
Z X - CH ~--CH--O - C - N - C ~Yn~
Comp. Physical data
No. R Z X nl Y Rl R2 R3
4.1 CF3 H O H Et H H 63-65C
4.2 CF3 H O H Et Me H
4.3 OCF3 H O H Et H H
Table S: Compounds of the formula Ig
R1 R2 R4
~ x - CH2--CH--O - C - N - C - C ~ ~ (Ig)
R R3 R5 S
Comp. Physical data
No. R Z X nl Y Rl R2 R3 R4 Rs
5.1 CF3 H O H Et H H H H 62-64C
5.2 CF3 H O H Et H H Me H 52-53C
5.3 CF3 H O H Et H H Me Me
5.4 OCF3 H O H Et H H H H
5.5 -O CF20- 0 H Et H H H H
CA 02203890 1997-04-28
Wo 96116941 PCT/EP95/04612
- 26 -
Table 6: Compounds of the formula Ih
Z R Yn
~ X - CH2--CH--O C NH ~
R 3R5
Comp. Physical data
No. R Z X n Y Rl R2 R3 R4 Rs
6.1 CF3 H O H Et H H H H oil
6.2OCF3 H O 1 3- M e Et H H H H
6.3 CF3 H O H Et H H Me H oil
6.4 OCF3 H O H Et H H Me H
Table 7: Compounds of the formula IIe ((-) enantiomers)
Z Rl H
R \ CH~ ~OH (~e)
Comp..... R Z X R1 Physical data
No.
7.1 CF3 H CH3
7.2 CF3 H C2Hs n2D2 1.4611
7.3 CF3 H O n-C3H7
7.4 CF3 H O i-C3H7
7.5 OCF3 H CH3
7.6 OCF3 H C2Hs n2D2 1.4515
7.7 O C F3 H O n-C3 H7
7.8 OCF3 H i-C3H7
7.9 -OCF2O- CH3
7.10 -OCF2O- C2Hs
CA 02203890 1997-04-28
Wo 96/lfi941 PCT/~ 01C12
~7
C. Biolo~ical Examples
Example B l: Experimental protocol for pre-emer~ence herbicidal action
Monocotyledon and dicotyledon test plants are sown in standard soil in plastic pots.
Tmm~.(1i~tely after sowing, the test substances are sprayed on in the form of an aqueous
suspension prepared with 25 % wettable powder (Formulation Example l) corresponding
to a dosage rate of 2 kg of a.i./ha (500 l of water/ha). The test plants are then grown in the
greenhouse under optimal cnn(li~ion.~ After a test period of 3 weeks, the exp~riment is
evaluated using a nine-step scale (1 = complete damage, 9 = no action). Scores of l to 4
(in particular l to 3) mean a good to very good herbicidal action. The same result is
obtained using a concentrated wettable powder (Formulation Example 3), dispersible
granules (Formulation Example 4), an emulsifiable concentrate (Formulation Example 2)
or a suspension concentrate (Formulation Example 6).
Test plants: Setaria, Sinapis, Stellaria
In this experiment, the compounds of the formula 1 of the examples in Table l exhibit a
powerful herbicidal action.
Table B l gives examples of the good herbicidal activity of the compounds of the formula
I:
CA 02203890 1997-04-28
W O96/16941 PCT~EP95/04612
-28- ---
Table B 1: Pre-emer~ence action
Comp. Dosage Setaria Sinapis Stellaria
No. [kg of
a.i./ha]
1.1 2 1 1 1
1.2 2 1 1 2
1.4 2 2
1.5 2 2
1.23 2 3 1 2
1.26 2
1.27 2
2.1 2 2 3
2.6 2 3 1 2 (r~rem~t~)
2.7 2 2 2 1 (r~cem~t~)
2.11 2
3.1 2 2 2
3.2 2 3 2
3.4 2 3 2
5.2 2 2 2 2
6.1 2
6.3 2
Exarnple B2: Experimental protocol for post-emer~ence herbicidal action (contactherbicide)
Monocotyledon and dicotyledon test plants are grown in the greenhouse in plastic pots
cnnt~ining standard soil and, in the 4- to 6-leaf stage, sprayed with an aqueous suspension
prepared with a 25 % wettable powder (Formulation Example 1) of the test substances,
corresponding to a dosage rate of 2 kg of a.i./ha (5001 of water/ha). The test plants are
then grown in the greenhouse under optimal conditions. After a test period of
approximately 18 days, the experiment is evaluated using a nine-step scale (1 = complete
damage, 9 = no action). Scores of 1 to 4 (in particular 1 to 3) mean a good to very good
herbicidal action. The same result is obtained using a concentrated wettable powder
(Formulation Example 3), dispersible granules (Formulation Example 4), an emulsifiable
concentrate (Formulation Example 2) or a suspension concentrate (Formulation Example
6).
CA 02203890 1997-04-28
Wo 96/16941 PCT/EP95/04612
- 29 -
Test plants: Setaria, Sinapis, Stellaria
In this e~Cperiment~ the compounds of the formula I of the e~ mrle in Table 1 exhibit a
powerful herbicidal action.
Table B2 gives examples of the good herbicidal activity of the compounds of the form
I:
Table B2- Post-emer~ence action
Comp. Dosage Setaria Sinapis Stellaria
No. [kg of
a.i./ha]
1.4 2 4 1 4
1.5 2 4 1 4
1.26 2 3 1 3
2.1 2 4 2 4
2.11 2 3 1 4
6.1 2 2 1 2
6.3 2 4 2 3
CA 02203890 1997-04-28
W 096116941 PCT/~5~/o1cl2
-30-
WHAT IS CLAIMED IS
1. A compound of the formula I
~R4 ~ 5
CH2 13~~ ~NH~ a),
R 1 0 R2 R3
in which
Q is a group ~ (1), ~ (2) or ~~Ynl (3);
R is halogen, trifluoromethyl, cyano, nitro or C1-C3haloalkoxy;
Z is hydrogen or halogen; or
Z and R together in the 2- and 3-position of the phenyl ring form a group -OCF20-;
Rl is Cl-C5alkyl;
R2, R3, R4 and Rs, independently of one another, are hydrogen, methyl or ethyl;
X is oxygen, sulfur, -SO- or -SO2-;
Y is hydrogen, halogen, C1-C3alkyl, Cl-C3haloalkyl, C1-C3alkoxy or cyano;
nisO, 1 or2;
nl is O or l; and
m is O or 1,
with the proviso that m is 1 if Q is group (1) or (2);
or a diastereomer or enantiomer thereof.
2. A compound according to claim 1 in which R is chlorine, bromine, trifluoromethyl or
trifluoromethoxy .
3. A compound according to claim 1 in which Z is hydrogen or fluorine.
4. A compound according to claim 1 in which Rl is methyl or ethyl.
5. A compound according to claim 1 in which R2,R3, R4 and Rs independently of one
another are hydrogen or methyl.