Note: Descriptions are shown in the official language in which they were submitted.
CA 0220393~ 1997-04-28
WO96/1~01 PCT/GB9S/02S86
TRANSGENIC ORGANISMS AND THEIR USES
The present invention relates to transgenic organisms
having cells with one or more selectable phenotypes and
their uses.
Transgenic organisms, including transgenic animals, have
been known for a number of years. Although the term has
occasionally been applied to any organism which contains
foreign DNA, the term "transgenic organism" is used
herein in its more usual sense to denote eukaryotic
organisms (and in particular, animals or plants, and
especially vertebrates e.g. mammals) and their progeny
which contain heterologous chromosomal DNA in the germ
line. The heterologous chromosomal DNA comprises a coding
sequence which is hereinafter referred to as a
"transgene~. Thus, every (or at least most) of the cells
of a transgenic organism - both somatic and germ - may
contain one or more copies of the transgene(s).
Transgenic organisms can be produced by many different
methods. The methods are well documented in the prior art
and their practice forms part of the technical repertoire
of those skilled in the art. Methodological approaches
CA 0220393~ 1997-04-28
W O96/14401 P~ll~b5S~X6
commonly used are described for example in First and
Haseltine (Eds.~, Transgenic Animals (1991) Butterworth-
Heineman MA USA.
According to one known method, the transgene is inserted
into embryonic stem cells which are then injected into
fertilized zygotes at a stage when only a small number of
cells are present. The engineered embryonic stem cells
become incorporated into the zygote, and cells derived
therefrom go on to differentiate into many or all of the
different cell types of the animal's body. Such cells may
also include those contributing to the germline, and the
progeny of such (chimaeric) animals may therefore be
fully transgenic.
Other methods involve the introduction of the transgene
into the pronucleus or into the fertilized or
unfertilized ovum.
It is also known in the art that cells can be routinely
engineered or induced to express gene(s) which confer any
of a wide variety of selectable phenotypes thereon. Such
genes are known as selectable markers. They are normally
introduced into cells as part of a recombinant expression
vector. The selectable phenotype conferred by a
CA 0220393~ 1997-04-28
W O96/14401 PCT/~b5~2r~
--3--
selectable marker may be classed as either po~itive or
negative.
A positive selectable phenotype is one which permits
survival under particular conditions which would kill tor
at least prevent or impair the growth of) cells which do
not exhibit the positive selectable phenotype.
A negative selectable phenotype is one which results in
the destruction (or the prevention or impairment of
growth) of the cell under particular conditions which are
relatively innocuous to cells which do not exhibit the
negative selectable phenotype.
A wide variety of selectable markers are available.
Genes that are widely applied as positive selectable
markers include the bacterial neomycin phosphotransferase
(neo; Colbere-Garapin et al. (1981) 150:1), hygromycin
phosphotransferase (hph; Santerre et al. (1984), Gene
30:147) and xanthineguanine phosphoribosyl transferase
(gpt; Mulligan and ~erg (1981), P.N.A.S., USA 78:2072).
Also used as positive selectable markers are the Herpes
simplex virus type 1 thymidine kinase (HSV-l TR; Wigler
et al. (1977), Cell 11:223), adenine phosphoribosyl-
CA 0220393~ 1997-04-28
WO96/1~01 PCTIGB95/02~6
--4--
transferase (APRT; Wigler et al. (1979), P.N.A.S. USA
76:1373) and hypoxanthine phosphoribosyltransferase
(HPRT; Jolly et al. (1983), P.N.A.S. USA 80:477). These
latter markers must be used in cells having a particular
mutant genotype (viz. one which leads to a deficiency in
the gene product on which the selection is based).
Some of the aforementioned genes also confer negative as
well as positive selectable phenotypes. They include the
HSV-1 TR, APRT, HPRT and gpt genes. These genes encode
enzymes which can catalyse the conversion of certain
nucleoside or purine analogues to cytotoxic
intermediates. For example, the nucleoside analog
ganciclovir (GCV), i5 a good substrate for the HSV-1
thymidine kinase but a poor substrate for the natural
thymidine kinase found in mammalian cells. Consequently,
GCV can be used for efficient negative selection against
cells expressing the HSV-l TR gene (St. Clair et al.
(1987), Antimicrob. Agents Chemother. 31:844).
Xanthineguanine phosphoribosyl transferase can be used
for both positive and negative selection when expressed
in wild type cells (Besnard et al. (1987), Mol. Cell.
Biol. 7:4139).
Selectable markers are usually used in both prokaryotic
CA 0220393~ 1997-04-28
W 096/14401 P~l/~S,'~2
and eukaryotic genetic engineering to permit the
recovery, from a mixed population, of cells which have
undergone a relatively rare genetic change. For example,
they can be used in physical association with another
gene which encodes a product of interest (for example, a
therapeutic protein) to select cells which have taken up
that other gene along with the selectable marker. For
example, the neo gene has been used to monitor
genetically modified cells taken from patient samples
after gene therapy has taken place.
It has also been proposed to use negative selectable
markers as a safety device in gene therapy. Many gene
therapies involve the removal of somatic cells from the
patient, the introduction therein of a therapeutic gene
(the expression of which repairs a biochemical lesion),
followed by reintroduction of the cells back into the
patient. Since the reintroduced genetically modified
cells may ultimately prove deleterious to the health of
the patient (for example, if they prove to be
immunologically incompatible or become malignant), a
negative selectable marker may be introduced along with
the therapeutic gene to permit (if necessary) subsequent
selective elimination of the genetically modified cells.
CA 0220393~ 1997-04-28
W O96tl4401 PCTt~b9~1~2S'
--6--
A number of vectors bearing positive or negative
selectable markers have been made and are readily
available to those skilled in the art (for review see
Miller (1992), Nature 357:455-460). Others may be
readily assembled using standard gene cloning techniques.
Transgenic organisms bearing a selectable marker as a
transgene are known in the art. Generally, the selectable
marker is introduced to permit the recovery of cells
which have also taken up a gene of interest to which the
selectable marker was linked. For example, many such
transgenic organisms have been constructed in the course
of work involving the introduction into the germline of
genetic information which disrupts normal development
(see WO/91/13150).
Transgenic organisms bearing a selectable marker have
also been constructed in the course of the construction
of animals bearing a specific genetic lesion. Here, the
selectable marker is inserted (usually by homologous
recombination) into a particular gene which is thereby
insertionally inactivated. The selectable phenotype
conferred by the selectable marker is then used as the
basis for the identification and recovery of cells
bearing an insertionally inactivated copy of the gene.
CA 0220393~ 1997-04-28
W 096/14401 1~-ll~b55/02586
Examples of transgenic Ani~-ls produced by such methods
are described in Piedrahita et al. (1992), PNAS 89, pages
4471-4475.
In vitro tissue/cell culture methods are fundamental to
pharmaceutical, clinical, agricultural and industrial
research. For example, tissue/cell culture methods are
used in the pharmaceutical industry for the preparation
of medicaments (for example, therapeutically active
protein products) and in assays or screens for potential
drugs.
However, these in vitro cell/tissue culture techniques
are slow, laborious and expensive. One important problem
is culture infection (usually arising from microbial
contamination by bacteria, yeasts and/or fungi). This is
usually countered by the use of various antibiotics which
are added to the culture medium to eliminate or reduce
the growth of contaminants.
However, the use of antibiotics does not completely
eliminate the risk of infection, especially that arising
from yeast and/or fungal contaminants (many of which are
resistant to the commonly used antibiotics).
CA 0220393~ 1997-04-28
W O96114401 PCTIGB9S/02S86
Another important problem arises from the need for
cultures of a single tissue or cell type. Growth in vitro
from single cells may be difficult (often requiring the
use of feeder cells and/or mixtures of growth factors and
other supplements) and homogenous in vitro populations
cannot therefore be easily obtained.
There is therefore a need for a convenient source of
cells/tissue of all types for primary culture or other
purposes.
It has now been found that transgenic organisms bearing
positive and/or negative selectable markers have
previously unrecognized utility in cell culture
techniques.
The present invention provides transgenic organisms which
inter alia constitute a very convenient source of
material for the isolation, identification, culture and
analysis of cells from any tissue of the organism' 5 body.
Tissue dissected from the transgenic organisms of the
invention can be particularly easily grown (even as
homogenous populations of a particular cell/tissue type)
in vitro and used in a wide variety of applications,
including pharmaceutical assays, tissue transplant
CA 0220393~ 1997-04-28
W~96114401 PCT/GB95/02586
synthesis, drug delivery and protein production.
According to one aspect of the present invention there is
provided a transgenic eukaryotic organism having cells
cont~ining heterologous DNA comprising a transgene
encoding a positive selectable marker and a transgene
encoding a negative selectable marker. ~ut for the
selectable phenotypes arising from the transgenes, the
organism may be essentially normal, the transgenes for
example not being located such that they insertionally
inactivate a gene.
The term "essentially normal" as used herein may indicate
that the organism is not mutant for any significant
character or trait with respect to the wild type and/or
exhibits normal tissue differentiation and development.
For example, the organism may be essentially normal in
the sense that the transgenes are resident in a silent
(i.e. non-expressed) region of the genome and/or in a
region of the genome where the transgenes do not
significantly perturb the replication, segregation,
organisation or packing of the chromosome or its
interaction with cellular components such as DNA hi n~ling
proteins (including histones and regulatory elements).
CA 0220393~ 1997-04-28
WO96/14401 PCT/~b~5i~2'~
--1 0--
The provision of transgenes encoding both positive and
negative selectable markers provides great flexibility
during subsequent manipulation of cells derived from the
transgenic organism in vitro. Moreover, where the
invention is used to generate tissue transplants, cells
of a particular type may be isolated from the transgenic
animal of the invention by positive selection. The cells
so isolated may then be transplanted into a non-
transgenic animal to determine whether the transplant has
any therapeutic effect. The transplant may then be
ablated by negative selection to provide a control to
determine whether the transplant was having a direct
therapeutic effect.
In another aspect, the invention provides a transgenic
eukaryotic organism having cells cont~ining heterologous
DNA comprising a transgene encoding a positive selectable
marker and/or a transgene encoding a negative selectable
marker, the organism being essentially normal but for the
selectable phenotypes arising from the transgene(s). A
positive and negative selectable marker may be provided
by a single transgene, since (as explained above) some
markers can be used as both positive and negative
selectable markers (depending upon the selection
conditions used).
CA 0220393~ 1997-04-28
WO96/14401 PCT/~b5S~2S~6
The transgenic eukaryotic organism of the invention is
preferably an animal or a plant, for example a vertebrate
(e.g. a mammal, for example a rat, rabbit, pig or mouse).
The transgenic organism preferably has a genotype which
is essentially wild type but for the presence of the
heterologous DNA.
In addition, that portion of the heterologous DNA which
is expressed in the cells may consist of a transgene
encoding a positive selectable marker and/or a transgene
encoding a negative selectable marker, each transgene
being operably linked to an expression element or
elements. The absence of expression of any other
transgenically derived genetic sequences makes this
preferred transgenic organism suitable for a wide range
of experimental research requiring an effectively wild
type genetic background.
At least one of the selectable markers may be operably
linked to a regulatable expression element or elements,
for example a tissue- or cell-specific expression element
or elements. In such circumstances, each selectable
marker is advantageously differentially regulated, each
marker for example being linked to a different tissue- or
CA 0220393~ l997-04-28
W 096/14401 ~ ~b9S/02S86
-12-
cell-specific expression element or elements. This
permits the expression of the selectable marker to be
limited to a selected class of cells or tissue, so
providing e.g. for the selective culture in vitro of the
selected class of cells or tissue from a mixed primary
cell culture.
The present invention does not rely on the use of
transgenic organisms produced by any one method: any
transgenic procedure may be used in the practice of the
invention. Moreover, in most circumstances, the precise
nature of the selectable markers for use in the present
invention is unimportant: in general, any selectable
marker gene may be used so long as it confers a positive
or negative selectable phenotype on the cell.
For example, the positive selectable marker may be
selected from neomycin phosphotransferase, hygromycin
phosphotransferase, xanthineguanine phosphoribosyl
transferase, the Herpes simplex virus type 1 thymidine
kinase, adenine phosphoribosyltransferase and
hypoxanthine phosphoribosyltransferase.
The negative selectable marker may for example be
selected from Herpes simplex virus type 1 thymidine
CA 0220393~ 1997-04-28
W 096/14401 PCT/~b5~'02S86
-13-
kinase, ~Pnine phosphoribosyltransferase, hygromycin
- phosphotransferase and hypoxanthine
phosphoribosyltransferase.
The selectable markers are conveniently derived (e.g. by
subcloning using restriction endonucleases) from any of a
large number of known vectors, examples of which are
described in e.g. Molecular Cloning: A laboratory Manual
Second Edition Edited by Sambrook J, Fritsch and Maniatis
T 1989 Cold Spring Harbour Laboratory Press).
The expression elements for use in the invention may take
any form so long as they can (under at least some
circumstances) be made to direct and/or control the
expression of the genes with which they are operably
coupled. Expression elements for use in the invention may
comprise transcriptional and/or translational elements,
and include promoters, ribosome binding sites, enhancers
and regulatory sites including activator and repressor
(operator) sites. Preferred expression elements comprise
promoters selected from a wide range available for use,
examples of which are shown in Table 1. This Table, which
is non-exhaustive, also indicates the use to which each
promoter may be put in the methods of the invention
described infra.
- - -
CA 0220393~ 1997-04-28
W O96/14401 ~ ~b5~ o6
By way of example only, the expression elements for use
in the invention may be selected from: promoters and/or
enhancers which are specifically active in: (i)
dopaminergic, serotoninergic, GABAergic, cholinergic or
peptidergic neurones and sub-populations thereof; (ii)
oligodendrocytes, astrocytes and sub-populations thereof;
(iii) the endocrine glands, lungs, muscles, gonads,
intestines, skeletal tissue or part or parts thereof;
(iv) epithelial, fibroblast, fat, mast, mesenchymal or
parenchymal cells; (v) particular stages of
embryogenesis, and (vi) components of the blood system
(e.g. T-lymphocytes, B-lymphocytes and macrophages).
Alternatively they may be selected from promoters and/or
enhancers which direct the transcription of genes for:
(i) neurotransmitter-specific receptors; (ii) ion
channels; (iii) receptors involved in ion channel gating
and (iv) cytokines, growth factors and hormones.
At least one of the selectable markers may advantageously
be constitutively expressed. This ensures uniform
expression of the selectable marker in every transgenic
cell of the transgenic organism under all conditions,
which is particularly useful where the transgenic
organism is for general use as a source organism for
cell/tissue culture.
CA 0220393~ 1997-04-28
W 096/14401 ~ ~b55~ 6
Table 1
Promoter Tissue/cell-type Application Reference
_
5 Tyrosine Catecholamin- Alzheimer's
hydroxylase -ergic neurones Parkinson's
_________________________________________________________
TSH Thyroid cells Hypothyroidism 2
receptor
-- ----------------__________________________________
BSFl GABAergic Epilepsy 3
neurones
_________________________________________________________
Human dopamine Noradr~Al in Alzheimer's 4
3-hydroxylase neurones
_________________________________________________________
Thyroglo~ulin Thyroid cells Hypothyroidism 5
_________________________________________________________
Serotonin 2 Glial cells in Neuro- 20 receptor serotoninergic degenerative
projection areas diseases
_________________________________________________________
CA 0220393~ l997-04-28
W O96/14401 PCTtGB95/02586
-16-
Table 1 (cont.)
________________________________________________________
Mouse inter- bone cells and inflammatory 7
leukin 4 heaematopoietic processes
system
_________________________________________________________
CD4 receptor CD4 expressing AIDS 8
T-lymphocytes
_________________________________________________________
human choline Acetylcholine Alzheimer's 9
acetyltrans- neurones Motor-neurone
ferase disease
15 References
1: Stachowick et al. ( 1994), Molecular Brain Research
22(1-4): 309-319.
2: Ikuyama and Nawata (1994), Japanese Journal of
Clinical Medecine 52(4): 962-968
20 3: Motejlek et al. ( 1994), Journal of Biological
Chemistry 269(21): 15265-15273
4: Hoyle et al. ( 1994), Journal of Neuroscience 14( 5 Pt
1): 2455-2463
5: Pichon et al. (1994), Biochemical Journal, 298 (pt
3): 537-541
CA 0220393~ 1997-04-28
WO96/1~01 ~1/~b5SI'~2S~
-17-
Table 1 (cont.)
6: Ding et al. (1993), Molecular Brain ~esearch, 20(3):
181-191
7: Bruhn et al. (1993), PNAS USA, 90(20): 90(20): 9707-
9711
8: Nakayama et al. (1993), International Immunology,
5(9): 817-924
9: Li et al. (1993), Neurochemical Research, 18(3):
271-275
10: Sohanberg et al. (1991), PNAS, 99(2): 603-607
Constitutive expression may be achieved for example vla
the use of a promoter which directs the expression of a
"house-keeping~ gene. A "house-keeping" gene is one
which is expressed in all cell types. Their translated
products are required as part of general cell metabolism
or cell structure and, consequently, they are not
specifically expressed in a particular cell or tissue
type. House-keeping gene promoters, therefore, need to
be active in a broad range of (and sometimes in all) cell
types in order to ensure constitutive gene expression.
An example of a constitutively-expressed promoter useful
in the present invention is that for the
histocompatability complex H-2Kb class 1 promoter (Weiss
CA 0220393~ 1997-04-28
WO96/1~01 PCT/GB9S/0~86
-18-
et al. (1983) Nature, 301, 671-674; Baldwin and Sharp
(1987), Mol. Cell. Biol. 7, 305-313; Kimura et al.
(1986), Cell 44, 261-272) which has been shown to express
downstream coding sequences in cells generally when used
as a promoter in a transgene (Jat et al (1991), PNAS USA
88, 5096-5100). Another example is the viral SV40 early
promoter.
The promoters for use in the present invention are not
restricted to those derived from mammalian cells but may
also include avian- and fish-derived promoters.
Additionally, virally derived promoters, some of which
have biological activity in a broad range of mammalian,
fish and avian cells as well as other eukaryotes, could
also be used in performing the invention. Examples are
the simian virus-40 derived early or late promoters, or
the Long Terminal Repeats (LTR'S) of retroviruses which
comprise promoter as well as enhancer elements and have
the ability to promote expression of sequences under
their influence in a broad range of eukaryote cells.
These promoters along with supporting sequences such as
~nhAncer elements and other regulatory elements are well
known to the man skilled in the art (see e.g. Molecular
Cloning: A laboratory Manual Second Edition Edited by
Sambrook J, Fritsch and Maniatis T 1989 Cold Spring
CA 0220393~ 1997-04-28
WO96/14401 PCT/~b5S~ 6
--19--
Harbour Laboratory Press).
The transgenic organism of the invention may also contain
heterologous DNA which further comprises a reporter
transgene, for example 3-galactosidase or luciferase. The
reporter transgene may be itself operably linked to an
expression element or elements which are subject to cell-
or tissue-specific regulation.
Such reporter transgenes facilitate subsequent analysis
of cells/tissue cultured from the transgenic organism and
in particular permit the response (to for example an
induced deficit in a particular class of cells/tissue) of
a particular expression element or class of expression
elements to be monitored in vivo or in vitro.
In another aspect, the invention provides a method of
culturing cells and/or tissues in vitro, comprising the
steps of: ta) providing a transgenic animal or plant
having cells contAining genetic material which confers a
selectable phenotype thereon; (b) generating a primary
culture from cells and/or tissue of the transgenic
organism of step (a); and (c) selectively growing the
primary culture on the basis of the selectable phenotype
~ 25 conferred by the genetic material contained in the cells
CA 0220393~ 1997-04-28
WO96/1~01 PCT/GB9S/0~6
-20-
of the transgenic organism.
Preferably, the cell/tissue culture method of the
invention is based on the use of a transgenic organism
having a selectable marker operably linked to a tissue-
or cell-specific expression element or elements, whereby
in step (c) a particular cell/tissue type is selectively
grown on the basis of the tissue- or cell-specific
expression therein of said at least one selectable
marker.
This preferred method of the invention finds application
for example in the selection of thyroid follicular cells
from a primary (mixed cell) culture. This method may
provide a primary stromal cell population of the thyroid
gland in the absence of the thyroid follicular cells and
constitutes a unique cell culture system useful for the
study of thyroid biology and in the development of new
therapeutic drugs for the treatment of thyroid diseases.
The cell/tissue culture method of the invention may also
be practised such that step (c) reduces or eliminates
microbial contamination of the tissue culture, there~y
alleviating or eliminating a common problem in cell
culture systems, viz. culture contamination (particularly
CA 0220393~ 1997-04-28
W 096/14401 PCT/~b9
-21-
by fungi and yeasts).
The transgenic organisms of the invention can also be
used as a source of lymphocytes in methods for the
production of monoclonal antibodies.
Monoclonal antibodies are of fundamental importance in
biotechnology. Their preparation involves a sequence of
steps including: (a) immunizing an animal by injecting
the antigen of interest, (b) removing the spleen from the
animal and preparing lymphocytes therefrom, (c) fusing
the lymphocytes with immortal (usually myeloma) cells to
produce a hybridoma, (d) selectively growing hybridomas
and (e) cloning the hybridomas to produce a clone
secreting the monoclonal antibody of interest.
The step of selectively growing the hybridoma (step (d),
above) is usually achieved on the basis of a HPRT-
genotype in a myeloma fusion partner which prevents
unfused myeloma cells from growing in selective media
cont~in;ng hypoxanthine, aminopeterin and thymidine (HAT
medium). This restricts the choice of fusion partners.
Thus, according to a further aspect of the present
invention, there is provided a method of making a
CA 0220393~ 1997-04-28
W Og6/14401 ~1/~b5~ 6
-22-
monoclonal antibody specific for an antigen, comprising
the steps of: (a) providing a transgenic AnimAl (for
example a rat, rabbit, pig or mouse) having lymphocytes
cont~;n;ng genetic material which confers a selectable
phenotype thereon; (b) immunizing the transgenic organism
with the antigen; (c) removing the lymphocytes from the
transgenic animal; (d) fusing the lymphocytes of step (c)
with immortal cells (for example tumour cells, e.g.
myeloma cells) to produce hybridomas; and (e) selectively
culturing the hybridomas on the basis of the selectable
phenotype conferred by the genetic material contained in
the lymphocytes.
The presence of the selectable marker in the lymphocyte
preparations from the transgenic animal obviates the
requirement for eg. a HPRT selection process and expands
the repertoire of fusion partner cells that can be used
in hybridoma formation.
The transgenic organisms of the present invention also
find application in relation to diseases or disorders
involving cell loss.
Many diseases and disorders are known to be associated
with specific cell and/or tissue loss. For example, in
CA 0220393~ 1997-04-28
W O96/14401 P~1/~D~5~6
neurodegenerative disorders such as Par~inson's disease,
Huntington's chorea and Alzheimer's disease one or more
sub-populations of neurotransmitter-identified cells are
lost during the course of the disease.
In Parkinson's disease, this loss is principally of the
dopaminergic neurones of the substantia nigra region of
the brain, although other cell types also decline.
In Huntington's chorea, there is a more general loss of
neurones, but in this case the deficits are restricted
largely to the Rtriatum.
In Alzheimer's disease, there is a decrement in
acetylcholine-, serotonin- and noradrenAl;ne- contA;ning
neurones projecting to the neo- and palaeocortex.
Other neurological diseases and disorders also stem from
neural cell degeneration; the demyelination occurring in
multiple sclerosis, for instance, is due to the
destruction of oligodendrocytes in the brain.
The Human Immunodeficiency Virus (HIV) is known to enter
cells that express the CD4 receptor and cell infection
appears to lead ultimately to cell death. The loss of CD4
CA 0220393~ 1997-04-28
WO96/1~01 PCT/~9~'C25
-24-
cells causes a catastrophic block of the entire immune
system and death of the infected person.
The molecular/cellular basis of HIV induced-disease is
poorly understood. This is due, at least in part, to the
lack of model systems to study the pathogenesis of the
disease, particularly in-vivo.
The use of SIV (simian immunodeficiency virus) infected
primates has been considered as a paradigm, but SIV
monkeys do not acquire full-blown AIDS. In many
instances, they show no symptoms at all. Alternative
models that have been proposed include HIV-infected
chimpanzees. Apart from the potential ethical
considerations, the manisfestation of AIDS-like symptoms
in such a model may take several years, substantially
hindering research and the development of effective
therapies.
Thus, animal models of the various diseases and disorders
discussed a~ove are essential as test subjects for
potential pharmaceuticals and in basic clinical research.
The choice of these anLmal models is presently very
limited because of the difficulties associated with
qelectively destroying specific cell and/or tissue types.
CA 0220393~ 1997-04-28
WO96/1~01 PCTIGB95/02~6
-25-
Thus, according to a further aspect of the present
invention there is provided a method of selectively
el;~inAting or depleting a particular tis~ue or cell type
in an organism, comprising the steps of: (a) providing a
transgenic organism having a negative selectable marker
operably linked to an expression element (e.g. a
promoter) specific for the tissue or cell type to be
eliminated or depleted, and (b) administering a selective
agent to the organism to eliminate or deplete that tissue
or cell type on the basis of the expression therein of
the negative selectable marker.
The selective agent may be ~i ni stered by any route.
Where systemic administration is required, oral,
parenteral or intravenous routes may be used. Where
localized administration is required (for example where
the tissue or cell-type to be eli~inAted is restricted to
a particular organ or to a particular region of the body)
targeted injection, implantation (e.g. slow release
capsules) or catheterization may be used. For example,
tissue in particular regions of the brain may be
specifically targeted by intracerebral injection.
The method of selectively eliminating or depleting a
- 25 particular tissue or cell type of the invention may be
CA 0220393~ 1997-04-28
WO96/1~01 PCT/~b5Sl~G
-26-
employed to provide in vivo models of disea~es/disorders
involving disease-related cell loss, for example
immunodegenerative or neurodegenerative
diseases/disorders (such as AIDS, Parkinson's and
Alzheimer's disease).
Accordingly, in a further aspect the present invention
provides a method of modelling disease/disorder-related
cell/tissue loss or atrophy comprising the steps of: (a)
providing a transgenic organism having a negative
selectable marker operably linked to an expression
element (e.g. a promoter) specific for the tissue or cell
type which is subject to disease-related elimination or
atrophy; and (b) administering a selective agent to the
organism to eliminate or deplete the tissue or cell type
on the basis of the expression therein of the negative
selectable marker.
The invention also provides a method (e.g. an in vitro
method) of determining the effect of a deficit in a first
class of cells on the characteristics of a second class
of cells in an organism, the method comprising the steps
of; ( a) providing a transgenic organism having a first
negative selectable marker operably linked to an
expression element specific for the first class of cells
CA 0220393~ 1997-04-28
W 096/14401 P~ll~b5S~!~2
-27-
and either; (i) a positive selectable marker operably
linked to an expression element specific for the second
class of cells, or (ii) a second negative selectable
marker linked to an expression element which directs the
expression of the negative selectable marker in all cells
of the organism except the second class of cells; (b)
administering a selective agent to the organism to induce
a deficit in the first class of cells on the basis of the
expression therein of the negative selectable marker; (c)
removing cells from the organism; and (d) selectively
culturing cells of the second class from those cells
removed in step (c) on the basis of; (i) the expression
therein of the positive selectable marker, or (ii) the
lack of expression therein of the negative selectable
marker.
In another aspect the invention provides a method of
screening compounds for pharmacological activity against
a disease or disorder involving cell/tissue loss or
atrophy, comprising the steps of: (a) providing a test
model of the disease via the steps of; (i) providing a
transgenic organism having a negative selectable marker
operably linked to an expression element (e.g. a
promoter) specific for the tissue or cell type which is
subject to disease/disorder-related elimination or
CA 0220393~ 1997-04-28
W 096/14401 ~1/~b~S~ 6
-28-
atrophy, and then (ii) a~;nistering a selective agent to
the organism to eliminate or deplete the tissue or cell
type on the basis of the expression therein of the
negative selectable marker to produce a test model; (b)
administering the compound to be tested to the test
model; (c) screening the compound to be tested on the
basis of its effect on the test model of step (a).
The methods of the invention may be usefully applied to
any disease or disorder which is associated with
cell/tissue loss or atrophy. In particular, the methods
of the invention find particular utility in respect of
neurodegenrative or immunodegenerative diseases and
disorders, for example: (a) Parkinson's disease (the
tissue or cell-type to be eliminated or depleted
comprising dopaminergic neurones in the substantia
nigra); (b) Huntington's chorea (the tissue or cell-type
to be eliminated or depleted comprising neural cells of
the striatum; (c) Alzheimer's disease (the tissue or
cell-type to be eliminated or depleted comprising
acetylcholine-, serotonin- and/or noradrenaline- neurones
associated with the neo- and palaeocortex; (d) multiple
sclerosis (the tissue or cell-type to ~e eliminated or
depleted comprising brain oligodendrocytes), (e) immune
disease and the cell-type to be eliminated or depleted
CA 0220393~ 1997-04-28
W O96/14401 PCT/GB9S/02S86
-29-
comprises CD3, CD4 and/or CD8 cells and (f) AIDS and the
cell-type to be eliminated or depleted comprises CD4
cells.
In the case of AIDS models, the method of the invention
could be used to specifically deplete or eliminate CD4
cells by linking a negative selectable marker to a CD4
cell-specific promoter (e.g. the CD4 receptor promoter).
This would permit the generation of an in vivo model of
AIDS by regulating the proportion of cells expressing CD4
by negative selection in vivo.
Furthermore, in the case where the transgenic animal
model carries both a positive and negative selectable
marker, any residual CD4 expressing cells could later be
isolated from the transgenic tissue of the animal model
by positive selection in vitro for further study.
Examples of various promoters suitable for use in the
methods of the invention described above are listed in
Table 1, along with the disease(s) in which each promoter
may find application.
The invention also contemplates cell/tissue cultures
derived from the transgenic organisms of the invention
CA 0220393~ 1997-04-28
WO96/1~01 PCTtGB95/02~6
-30-
(or produced by the cell culturing methods of the
invention), and also to various therapeutic uses of the
inventlon .
The invention will now be described in more detail by way
of specific examples. These examples are not intended to
be taken as limiting in any way.
The methods and technologies required to construct
plasmid vectors, for example, in order to generate the
invention are well known to the man skilled in the art.
The constructed sequences given below represent examples
of numerous constructs that could be used to perform the
invention. The invention should not be construed as
~eing limited to their use only.
A. Materials
i. Vectors
pBabeneo plasmid vector Morgenstern, J.P. & Land, H.
Nucl. Acids Res. 18(1990) 3587-
3596 (plasmid is freely
available).
pCI plasmid vector Promega, 2800 Woods Hollow Rd,-
Madison, USA
25 CD2 plasmid vector Blaese, M.R., NIH, Bethesda, USA
CA 0220393~ 1997-04-28
WO96/1~01 P~-l/~b~S1~6
(plasmid freely available).
Mullen, C.A., Kilstrup, M.,
Blaese, R.M., Proc. Natl. Acad.
Sci. USA., 89(1992) 33-37.
Austin, E.A. & Huber, B.E. Mol.
Pharmacol., 43(1993) 380-387.
Wallace, P.M., ~C~Acter~ J.F.,
Smith, V.F., Rerr, D.E., Senter,
P.D. & Cosand, W.L. Cancer Res.
54(1994) 2719-2723.
TG-TKa plasmid vector Wallace, H., Rings Buildings,
University of Edin~urgh, UR
(plasmid freely availa~le).
Wallace, H., Ledent, C.,
Vassart, G., Bishop, J.O. &
AlShawi, R. Endocrinology,
129(1991) 3217-3226.
pPBS plasmid Morgan, Nucleic Acids Research
(1992), 20, pages 1293-1299
ii. Molecular BioloqY Reaqents
Restriction endo- Promega, 28000 Woods
nucleases Hollow Rd, Madison, USA
- 25 DNA modifying enzymes, Promega, 28000 Woods Hollow -
CA 02203935 l997-04-28
W O96/14401 P~ll~b5SJ~ 6
-32-
ligase, CIP, T4 Rd, Madison, USA
polymerase etc.
Agarose for electo- Sigma Chemical Co., St.
phoresis Louis, USA
Polynucelotide kinase New England 13iolabs Ltd.,
and buffers 3397 American Drive, Unit 12,
Mississauga, Ontario, Canada
B. Construction of qenes
ti) Thyroqlobulin-thymidine kinase-internal ribosomal
entry site-neomvcin resistance (TG-TK-a-IRES-neor~
The neomycin resistance gene (neor) was obtained from the
pBabe Neo plasmid (Morgenstern & Land, Nucl. Acids Res.
15 18(1990)3587-3596) by digestion with Hind III/Cla I and
retrieval of the 1165 b.p. fragment containing the neor
gene by gel electrophoresis and the Promega Wizard PCR
kit.
The pPBS plasmid (Morgan, Nucl. Acids Res. (1992) 20,
pages 1293-1299) comprising the poliovirus derived
internal ribosomal entry site sequence was digested with
Hind III~Cla I. However, this could not be done
sLmultaneously, or, in sequence, since the restriction
sites were too close together. In order to overcome-this
CA 0220393~ 1997-04-28
W 096/14401 P~l/~b5
problem, the plasmid was initially digested with Hind III
and a 200 b.p. fragment of DNA containing Hind III
restriction sites at both the 5' and 3' ends was inserted
in order to separate the sites. The pPBS plasmid could
then be digested first with Cla I and then with Hind III.
TerminA1 phosphate groups were removed from the Hind
III/Cla I cut pPBS vector using calf intestinal
phosphatase (CIP). The vector was gel-purified using a
1% agarose gel and a band contAining the DNA was excised
and electroeluted.
The neomycin gene was then ligated into the pP~S plasmid
overnight~at 15~C and the ligation reaction transformed
into freshly-made MC1061 competent cells.
Positive colonies were identified by digestion of
prepared plasmids with Hind III/Cla I. The neor gene and
plasmid being detected electrophoretically in plasmid
preparations from positive colonies. Plasmids from the
positive colonies were then digested with Hinc II and Sac
I (both restriction enzymes leaving digested DNA with
blunt ends). The resulting Sac I/Hinc II digestion
contA i n ing the IRES-neoR fragment was run on a 1%
electrophoresis gel and the appropriate size band was-
CA 0220393~ 1997-04-28
WO96/1~01 PCT/GB95/02~6
-34-
excised and the DNA electroeluted and ethanol-
precipitated.
The TG-TRa plasmid (freely available from Ge~h~nk, NIH,
USA accession No. JO2224, Santelli et al 1993) DNA was
preparet using Promega Wizard mini preps and digested
with Nar I. The ends of the plasmid were blunted using
T4 Polymerase at 37~C for 1 h followed by removal of the
terminal phosphate groups using CIP. The CIP was
inactivated by treatment of the DNA with
phenol/chloroform followed by ethanol precipitation. The
resulting plasmid was electrophoresed on a 1% agarose gel
and the DNA was recovered and ligated with the insert in
a 1:3 molar ratio of plasmid to insert.
The ligation was incubated at 15~C overnight, and was
then used to transform competent MC1061 cells. Positive
colonies were selected by digestion of prepared plasmids
with BamH I (the correct construct provided restriction
fragments of size 3980, 1663, 3102 and 1039 b.p.).
Linearization of the plasmid was achieved by digestion of
prepared plasmids with Sal I restriction enzyme. The
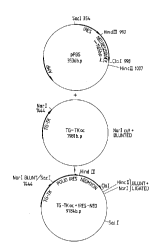
construction is shown in Figure 1.
CA 0220393~ 1997-04-28
W O96/14401 PCT/~b55~2S
(ii) Cvtomeqalovirus-cytosine deAm;nAse-sv40 Promoter-
neomycin resistance (CMV-CD-SV40-neor, or CD2-neor)
pCD2 plasmid (Mullen et al., PNAS 89(1992)33-37) was
digested with EcoR I and EcoR V, and the digest was
electrophoresed on a 1% agarose gel where the 2.5 kb.
fragment containing the cytosine deA~;nAse gene, the SV40
promoter and the neomycin resistance gene was retrieved
by electroelution followed by ethanol preciptation.
To ensure terminal phosphate groups were present in the
fragment it was treated with polynucleotide kinase.
The pCI vector was digested with EcoR 1 and Sma I (a
restriction enzyme leaving the DNA with blunt ends), and
the term;~Al phosphate groups were removed using CIP and
the enzyme was inactivated with phenol/chloroform
followed by ethanol precipitation. The band was then
gel-purified and recovered by electroelution.
The ligation was set up contA;ning a 3:1 molar ratio of
insert to vector and was carried out at 15~C overnight.
The ligation mixture was used to transform freshly-
prepared MC1061 competent cells and positive colonies
were selected by digestion of prepared plasmids with EcoR
CA 0220393~ 1997-04-28
WO96/14401 PCT/GB95/02586
-36-
l and Hind III to provide restriction fragments of length
1868 b.p. and 5062 b.p., respectively. Linearization of
the plasmid was achieved by digestion with Bgl I.
The construction is shown in Figure 2.
C. Production of transqenic animals
Transgenic rats were produced by established methods
(Hogan, B., Constantini, F. & Lacy, E. (1986)
Manipulating The Mouse Embryo - A Laboratory Manual, Cold
Spring Harbor Lab., Cold spring Harbor, NY). In brief,
approximately 2 pl of the plasmid were microinjected at a
concentration of 5~g/ml into the pronucleus of outbred
Sprague-Dawley embryos. Embryos were then implanted into
pseudopregnant recipients, and after identification of
transgenic animals, lines were isolated and established.
Lines were maintained as transgenic hemizygotes by mating
hemizygous females with non-transgenic males.
D. Positive/neqative selection of cells from transqenic
animals in vitro
i. Fibroblast cells.
Fibroblast cultures derived from lung of adult CD2/neor,
TG/TK/neor and control animals were produced and expAn~PA
CA 0220393~ 1997-04-28
WO96/1~01 PCT/~b5S~2S~
-37-
by routine methods (Freshney (1987), Alan R. Liss, New
York). Twenty-four hours after plating, geneticin
(400~g/ml) was added to cultures originating from both
types of transgenic rats and from control rats, and
S replaced every three days with fresh medium. When
required, cells were subcultured (1:3) to prevent them
becoming confluent, again by basic culture methods
(Freshney 1987). Cell counts were made manually in 20
fields chosen randomly and the values at each time point,
after allowing for changes due to subculturing, were
aggregated. As can be seen from Table 2, no fibroblast
cells derived from control animals or the TG/TK/neor
transgenic survived more than 10 days treatment with
geneticin.~ In the absence of added geneticin, no change
in cell survival from either of the transgenic An;~ls
was observed.
The effects of 5-fluorocytosine (5FC) were also
determined. 5-fluorocytosine at a concentration of
lOOyg/ml had no effect on fibroblast cells derived from
control animals or from the TG/TK/neor transgenic. In the
cells derived from the CD2/neor transgenic animal,
however, 94% of the originally-plated cells died, or were
non-functional (as determined by their failure to exclude
trypan blue) after 10 days culture in the presence of 5FC
CA 0220393~ 1997-04-28
WO96/1~01 PCTIGB9S/02S86
-38-
(Table 2). By contrast, no significant difference in
cell counts was found between cultures from control rats
in the absence and presence of SFC, or between controls
and cultures taken from CD2/neor rats in the absence of
added 5FC (Table 2).
Table 2: Survival of lung fibroblast cells derived from
control and transgenic rats, and effects of
various drugs.
Days in culture
GenotvPe/druq 1 3 5 7 9 11
Control 100 100 100 100 100 100
TG/TK/neor 98 97 98 95 95 96
CD2/neor 92 93 92 98 105 98
Control + geneticin 99 101 85 23 5 2
TG/TK/neor + geneticin97 105 108 101 105 111
CD2/neor + geneticin 91 94 91 93 107 105
Control + 5FC 101 105 9B 97 93 96
TG/TK/neor + 5FC 98 96 95 92 92 93
CD2/neor + 5FC 94 5 3 3 4 4
Drugs were added at day 2 in culture. Values are related
to the number of cells found in control cultures without
drug additions at various times after plating, and
allowing for dilutions resulting from passaging. Figures
are the means of three separate deterrinAtions~ the
stAn~Ard errors all being less than 15% of the mean.
CA 0220393~ 1997-04-28
WO96/14401 PCT/GB95/02
-39-
ii. Thvroid cells
Thyroid cultures derived from the thyroid gland of adult
CD2/neor, TG/TK/neor and control ~nir-l s were produced by
routine methods (Freshney, 1987). Twenty-four hours
after plating, geneticin (400~g/ml) was added to cultures
originating from both types of transgenic and the control
rats, and replaced every three days with fresh medium.
When required, cells were subcultured (1:2) to prevent
their becoming confluent. Cell counts were made manually
in 20 fields chosen randomly, and the values at each time
point, after allowing for changes due to subculturing,
were aggregated (Table 3). Ten days after the initial
application of geneticin, lO~g/ml acycloguanosine (ACG,
Sigma) was added to thyroid cells originating in the
TG/TK/neor transgenic. Ten days later, cell counts were
again made of 20 fields chosen at random. Results are
given in Table 3. To summaraize, cells derived from both
types of transgenic animal survived the geneticin
treatment, whereas the control cells did not. Cells
derived from the TG/TK/neor transgenic did not survive ACG
treatment, whereas the cells derived from the control
animals did. The results were as expected in view of the
specific and non-specific expression of the positive and
negative selection markers, in the TG/TK/neor and CD2/neor
CA 0220393~ 1997-04-28
WO96/1~01 PCT/~b5S~2~6
-40-
transgenics, respectively. TG/TK/neor transgenic rat
thyroid cells cultured in the absence of any added drug
did not exhibit any differences in their growth or
survival compared to control thyroid cell cultures (Table
3).
Table 3: Survival of thyroid cells derived from control
and transgenic rats, and effects of various
drugs.
Days in culture
Genotype/druq 1 3 5 7 9 11
Control 100 100 100 100 100 100
TG/TK/neor 91 95 93 92 101 99
CD2/neor 99 103 102 97 89 91
Control + geneticin 95 91 85 56 9 4
TG/TK/neor + geneticin104105 98 88 93 98
CD2/neor + geneticin 91 94 91 93 107 105
Control + ACG 94 97 98 91 92 102
TG/TK/neor + ACG 98 38 12 10 8 7
CD2/neor + ACG 98 90 93 93 97 88
Drugs were added at day 2 in culture. Values are related
to the number of cells found in control cultures without
drug additions at various times after plating, and allow
for dilutions resulting from passaging. Figures are the
means of three separate det~r~;nAtions, the stAn~Ard
errors all being less than 15% of the mean.
CA 0220393~ 1997-04-28
W 096/14401 P~ll~b5SI'~2S''
-41-
E. Enhanced sterility in tissue culture usinq cells from
CD2-neor transqenic ~ni~l S
Cultures of spinal cord cells derived from the CD2-neor
transgenic rat were grown in small flasks, using
previously established methods (Foster et al., (l990~
Eur. J. Neurosci. 3, 32-39). At the beginning of the
experimental period, in the region of 100 yeast spores,
and unknown amounts of other common laboratory
microbiological contaminants, were introduced into the
flask. At the same time geneticin (lmg/ml) was added.
Thereafter, all manipulations of the medium and additions
to the flask were conducted outside the sterile
environment of the laminar flow cabinet. Sixty days
after the initial plating, no evidence of any form of
contamination was apparent. Indeed, the survival and
development of the neural cells appeared unimpaired
compared to uninfected control spinal cord cells grown in
the absence of geneticin (see Fig. 3, which shows phase-
contrast photomicrographs of 60 days in vitro spinal cordcultures derived from control rat embryos (A) and from
CD2/neor transgenic rat embryos (~) at day 14 of
gestation. The latter cultures were deliberately
infected with yeast and other microorganisms, and
simultaneously treated with geneticin. No infection could
CA 0220393~ 1997-04-2X
WO96/1~01 PCT/GB9S/02~6
-42-
be discovered after geneticin application, whereas
infected cultures without geneticin were overrun with
microorganism growth within 4 or 5 days).
F. Ablation of thvroid follicle cells in vivo
Adult female rats (250g) were injected intra-peritoneally
with 50 mg of ACG per day for a period of 5 days. Seven
days after the final injection, serum levels of T3 and T4
were measured (Amersham, UK), and found to have fallen in
transgenic animals from 0.76 @ 0.05 nM to less than 0.06
nM (T3) and from 58.2 @ 3.2 nM to less than 2.5 nM
(T4)(N=6). ~ n;stration of saline to transgenic animals
resulted in a small but non-significant fall in T4 to 0.68
@ 0.07 nM (N=6). The thyroid glands of transgenic rats
treated with ACG for 12 days had shrunk to 7% of the
original weights. Histochemical analysis of these
thyroid glands revealed an almost complete loss of
follicular cells, with only non-follicular, perhaps
calcitonin-producing cells, remaining. Administration of
lower amounts of ACG per day resulted in a partial loss
of T3 and T4. In most other tissues from transgenic
Ani~-~s, HSV-thymidine kinase activity (Brinster et al.
(1981) Cell 27, 223-231; Jamieson et al. (1974) J. Gen.
Virol. 24, 481-492) was not expressed in detectable
CA 0220393~ 1997-04-28
W 096/14401 ~CTIGBgS/02S86
-43-
amounts. No histochemical evidence of cell 1Os8 was
demonstrable in parathyroid, submaxillary or adrenal
glands, nor in heart, kidney or brain.
In summary, both types of transgenic ~ni~-l, or the cells
therefrom, were apparently normal until application of
either ACG or 5FC, as appropriate. After such
application, either in vivo or in vitro, the cells upon
which sensitivity had been conferred were rapidly
destroyed. In addition, cells from both transgenic
animals were resistant to the cytotoxic effects of
geneticin, whereas cells from non-transgenic controls
were completely eradicated.
lS Example 2: ProPosed protocol for the Production of a
transqenic mouse bearinq both Positive and neqative
selectable markers
The herpes simplex virus (HSV) thymidine kinase gene (tk)
(operably linke~ to the tk promoter) and the bacterial
neomycin phosphotransferase (neo) gene (operably linked
to the SV40 early promoter) are cloned into the
appropriate cloning sites of a plasmid vector.
The plasmid vector is digested with restriction
CA 0220393~ 1997-04-28
WO96/14401 PCT/~b9S,~
endonucleases and a fragment cont~;ning both the tk and
neo selectable markers (along with the expression
elements operably linked thereto) is isolated on an
agarose gel.
s
The fragment isolated on the gel is then purified and
injected into male pronuclei of fertilized one-cell mouse
eggs at a concentration of 1-2 ug/ml DNA in TE buffer
(lOmM Tris, Ph 7.5, 0.2 Mm EDTA~. The eggs are those
derived from a CBA x C57BL/10 mating.
The eggs which survive micro-injection are then
transferred to pseudopregnant females as described e.g.
in Wagner et al. (1981) PNAS 78, 5016, and allowed to
develop to term.
At 7-14 days of age, each pup is analysed to determine
whether the transgenes are present. DNA is prepared from
a section of the tail by the method described in Sambrook
et al. (1989) "Molecular Cloning", Cold Spring Harbor.
The presence of the neo and tk genes is determined by
probing with labelled tk and neo-specific probes.
The transgenic pups so identified are mated and their
offspring also analysed to check for Mendelian transfer
CA 0220393~ 1997-04-28
PCT/GB9S/02~6
WO96/14401
of the transgenes.
Example 3: Proposed Protocol for the selective culture of
mouse thyroid follicular cells
Transgenic mice are prepared as described in Example 1,
except that the neo gene is placed under the control of a
thyroglobulin promoter (e.g. described by Christophe et
al. (1989) Molecular and cellular endocrinology 64(1) 5-
18; Christophe et al (1987), 19, Suppl. 17, pp 70-73; and
Ledent et al. (1990), PNAS, 87 (16), pp 6176-6180).
The transgenic mice are sacrificed and the thyroid tissue
removed and a primary culture prepared in the presence of
lS antibiotic G418. This antibiotic kills cells not
expressing the neo gene, and results in the selective
culturing within the primary (mixed cell) culture of
thyroid follicular cells.
ExamPle 4: ProPosed protocol for the Preparation of
monoclonal antibodY
The bacterial neomycin phosphotransferase (neo) gene
(operably linked to the SV40 early promoter) is cloned
into the appropriate cloning site of a plasmid vector.
CA 0220393~ 1997-04-28
WO96/l~01 PCT/GB95/02S86
-46-
The plasmid is then digested with restriction
endonucleases and a fragment containing the neo
selectable marker is isolated on an agarose gel, and
transgenic mice bearing the neo transgene are then
prepared essentially as described in Example 1.
The antigen against which a monoclonal antibody is
required is purified and injected into the transgenic
mouse prepared as described above along with Freund's
adjuvant. The mouse is then sacrificed and the spleen
removed and placed in tissue culture fluid. The spleen is
teased apart to release the lymphocytes and these are
isolated by centrifugation. The lymphocytes are then
mixed with a myeloma fusion partner in the presence of
polyethylene glycol to induce fusion and produce
hybridomas.
Hybridomas are selected by supplementing the culture
medium with the antibiotic G418 on the basis of the
presence of the neo selectable marker in the mouse
lymphocytes. The hybridomas are then cloned by limiting
dilution and the relevant clone identified by screening
via the appropriate binding assay.
The myeloma cell line does not need to have a negative
CA 0220393~ 1997-04-28
W O96/14401 PCT/~b5SI'~86
selectable marker (e.g. ~PRT-). Moreover, the presence of
G418 in the culture medium reduces or eliminates the risk
of culture infection.
ExamPle 5: ProPosed Protocol for the preParation of a
rattine model of Parkinson's disease
The herpes simplex virus (HSV) thymidine kinase gene (tk)
is operably linked to a promoter which is active only in
dopaminergic neurones in the substantia nigra and cloned
into the appropriate cloning site of a plasmid vector.
The plasmid is digested with a restriction endonuclease
and a fragment contAi n; ng the tk selectable marker is
isolated on an agarose gel, and transgenic rats bearing
the tk transgene are then prepared essentially as
described in Example l.
Ganciclovir is then administered by injection into the
substantia nigra regions of the brain of the transgenic
rats to specifically eliminate or deplete the
dopaminergic neurones expressing the negative selectable
tk marker, thus providing a rattine model of Parkinson's
disease.
- 25
CA 0220393~ 1997-04-28
PCT/~,;bgS,'~,2'~
WO96/1~01
-4~-
ExamPle 6: ProPosed Protocol for the preParation of a
rattine model of Alzheimer's disease
The herpes simplex virus (HSV) thymidine kinase gene (tk)
is operably linked to a promoter which is active only in
acetylcholine-, serotonin- and/or noradren~line- neurones
associated with the neo- and palaeocortex is cloned into
the appropriate cloning site of a plasmid vector.
The plasmid is digested with a restriction endonuclease
and a fragment containing the tk selectable marker is
isolated on an agarose gel, and transgenic rats bearing
the tk transgene are then prepared essentially as
described in Example 1.
Ganciclovir is then administered by injection into the
aapropriate region of the brains of the transgenic rats
to specifically eliminate or deplete the acetylcholine-,
serotonin- and/or noradrenaline- neurones associated with
the neo- and palaeocortex expressing the negative
selectable tk marker, thus providing a rattine model of
Alzheimer's disease.