Note: Descriptions are shown in the official language in which they were submitted.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-1-
MICRON-SIZED NICKEL METAL POWDER AND A PROCESS FOR THE
PREPARATION THEREOF
Field of the Invention
The present invention relates to a novel, micron-sized nickel metal
powder and to a process for the production thereof. Furthermore, the invention
also
provides a method of controlling the particulate size of the produced nickel
metal
powder.
Background of the Invention
A method for the production of nickel metal powder from basic nickel
carbonate by reduction with gaseous hydrogen at elevated temperatures and
pressures
is disclosed in U. S. patent 3,399,050 to D. J. I. Evans et al. The process
utilizes a
concentrated ammoniacal solution of nickel ammonium carbonate which is
initially
diluted with water and then boiled to remove excess ammonia and carbon
dioxide.
This results in the precipitation of basic nickel carbonate (BNC), i.e. a
mixture of
nickel hydroxide and nickel carbonate, leaving essentially no nickel ions in
solution.
This slurry is then charged to the autoclave, heated to temperature and
reduced with
hydrogen. The nickel powder is effectively formed by direct reduction of the
solid
BNC.
This prior art procedure deleteriously yields a powder containing some
entrained, or encapsulated BNC, which results in a lower specific gravity and
increased
levels of oxygen and carbon which are unacceptable for certain applications.
Additionally, the prior art process is difficult to control to yield
consistent results,
since the boiling step produces variable results.
The prior art process has always used a combination of ferrous sulphate
and aluminum sulphate as the catalyst, but the iron content of up to 4000 ppm,
or the
high total metallic impurity (up to 0.8%) in the nickel metal powder precludes
its use
in certain applications.
In the paper entitled " Effect of Addition Agents on the Properties of
Nickel Powders Produced by Hydrogen Reduction" by W. Kunda, D.J.I. Evans and
V. N. Mackiw in " Modem Developments in Powder Metallurgy. Vol. I:
Fundamentals
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-2-
and Methods" Hausner, H,H, and Roll, K;. H. eds. (New York : Plenum Press,
1966),
15-49, there is detailed a discussion of a wide variety of alternative
catalysts and
additives and their effects in modifying the physical properties of the nickel
powder
produced.
During recent years, fine nickel powders have been produced
commercially for use in electronic circuitry, fuel cells and numerous other
usages.
However, in certain specialized applications, exemplary of which are
conductive pastes
used in capacitors and the like, it has been found unacceptable to utilize the
existing
available nickel powders in such pastes because of the high level of
impurities, for
example, iron, alkali metals, carbon and oxygen which deleteriously affect
conductivity. Thus, at present, the industry is using fine powders prepared
from alloys
of the platinum group metals, gold and silver in the formulation of such
pastes. As
will be readily appreciated, the smaller the particle size, the thinner the
layer of paste
which will be required for the substrate. Clearly, too, a spherical
particulate
configuration is sought after to thereby provide tighter packing concomitant
with a
layer of increased conductivity. Therefore, it is an objective of the present
invention
to provide an equally effective, but less costly replacement for the metals in
current
usage.
Additionally, it is an object of this invention to provide a process for
preparing micron-sized, spheroidal nickel metal powder having higher purity,
and a
production process exhibiting improved reproducibility.
Summary of the Invention
In accordance with a first aspect of the present invention there is
provided a novel, micron-sized nickel metal powder having a nickel content
greater
than 99% wherein the metal particles are of a generally spheroidal
configuration. The
preselected particle sizes of the nickel metal powder are in the range of 0.3
to 2.04m,
and in a preferred aspect, the particle sizes are less than 1.04m. The content
of such
undesirable trace impurities as iron, cobalt, aluminum, carbon, sulphur and
oxygen has
been greatly reduced, the nickel metal powder being characterized in having an
iron
content lower than 100 ppm.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-3-
More particularly, the chemical and physical properties of the nickel
metal powders of the invention are as follows: a chemical composition which
comprises nickel in the range of about 99 to 99.5 weight percent and contains
impurities comprising iron in the range of about 0.001 to 0.010 weight
percent;
aluminum in the range of about 0.001 to 0.005 weight percent; sulphur in the
range
of about 0.001 to 0.01 weight percent; oxygen in the range of about 0.3 to 0.8
weight
percent; carbon in the range of about 0.1 to 0.4 weight percent and silver in
the range
of about 0.01 to 0.2 weight percent. The physical properties of the nickel
metal
powder include having a surface area in the range of about 0.5 to 3.0 square
meters
per gram; an apparent density in the range of about 1.0 to 2.0 g/cc; a tap
density in
the range of about 2.0 to 4.0 g/cc; whereby said nickel metal powder possesses
micron-sized particles ranging from between about 0.3 to 1.5 m which are of a
generally spheroidal configuration.
The most preferred chemical and physical properties of the micron-sized
nickel metal powder are given below. The chemical composition comprises nickel
of
about 99.0 weight percent and includes impurities comprising oxygen less than
0.8
weight percent; and silver less than 0.3 weight percent. The physical
properties of the
nickel metal powder include having a surface area in the range of about 1.0 to
3.0
square meters per gram; an apparent density in the range of about 1.0 to 2.0
g/cc; a
tap density in the range of about 2.0 to 4.0 g/cc; whereby said nickel powder
particles
possess a micron size ranging from between about 0.3 to 0.54m and are of a
generally
spheroidal configuration.
It is also to be noted, without being bound by same, that the nickel
metal powder product of the instant invention is essentially free of entrained
or
encapsulated BNC and is believed, because of the observed high specific
gravity, to
be substantially metal powder.
As a result the thus produced spheroidal nickel metal powder particles
are particularly well adapted for the formulation of conductive pastes, and
advantageously may be utilized in the replacement of the alloys of platinum
group
metals, gold or silver previously used in certain commercial applications.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-4-
It is to be understood, however, that the utility of the powder is not to
be limited to the above-described application but will be found suitable for
any use
requiring a micron-sized nickel metal powder of this purity, composition and
morphology.
In a second broad aspect of the invention there is provided a process
for the preparation of a micron-sized nickel metal powder.
The process, in contradistinction to the prior art processes, commences
with a diluted ammoniacal nickel (II) solution, preferably a diluted
ammoniacal nickel
(II) carbonate solution, wherein neither the CO2 nor NH3 have been permitted
to boil
or partially boil out. The solution is clarified or filtered to ensure that
only soluble
nickel ions are being charged into the autoclave. A silver compound is added
to the
filtered ammoniacal nickel (II) carbonate-containing solution to obtain a
soluble silver
to nickel (II) weight ratio in the range of about 1.0 to 10.0 grams per
kilogram of
nickel (II). An organic dispersant in an amount functional to control
agglomeration of
the resultant nickel metal powder and an organic, spheroid-promoting compound
in an
amount effective to maximize the spheroidal configuration of the nickel metal
powder
are also added. The catalytic reagents, namely, silver, dispersant and
spheroid-
promoting agent, are added following the clarification/filtration step while
the solution
is charged to the autoclave. The solution is heated, with agitation,
optionally with a
hydrogen overpressure in the range of 150 to 500 kPa, to a temperature in
range of
150 C to 180 C, and then reacted with hydrogen at a pressure of 3.0 to 4.0 MPa
(i.e.,
450 to 600 psi) for a time sufficient to reduce the dissolved nickel to form a
micron-
sized nickel metal powder.
As will be described herebelow, the ratio of the soluble silver to nickel
content in the nickel metal plays a critical role in controlling the nickel
powder
particle size. The weight ratio of the added silver to nickel (II) ranges from
1.0 g to
10.0 grams per kilogram of nickel, and, most preferably, ranges from 1.0 to
2.5 grams
per kilogram of nickel.
Preferably, the anti-agglomeration agent is selected from suitable
organic compounds, such as gelatin and/or bone glue.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-5-
A suitable organic compound functional to improve spheroidal
morphology includes anthraquinone, or derivatives thereof, or alizarin alone
or in
admixture with anthraquinone.
Additionally the application of a low hydrogen overpressure during the
heating stage yields a powder having superior properties.
The preferred process for the preparation of a micron-sized nickel metal
powder from an ammoniacal nickel (II)-containing solution is as follows. The
ammoniacal nickel (II)-containing solution should contain approximately equal.
concentrations of Ni and NH31 typically about 50 g/L of each of Ni and NH31 or
in the
range of about 40 to 50 g/L each. Preferably, the ammoniacal nickel (II)-
containing
solution comprises ammoniacal nickel (II) carbonate wherein the ammonia to
nickel
mole ratio is about 3 : 1 and the COz : Ni mole ratio is about 1:1. The
solution should
contain approximately equal concentrations of Ni, NH3 and C02, typically about
50
g/L each, or in a range of about 40 to 50 g/L each. The solution is then
clarified or
filtered to ensure that it contains only nickel ions and is essentially free
of metallic
nickel. A soluble silver salt, exemplary of which would be silver sulphate or
silver
nitrate, is then added to the ammoniacal nickel carbonate solution to yield a
silver to
nickel weight ratio of about 1.0 to 10.0 grams silver per kilogram of nickel.
Gelatin
is added in an amount of 5.0 to 20.0 grams per kilogram of nickel, together
with
anthraquinone in an amount of 1.0 to 5.0 grams per kilogram of nickel. The
ammoniacal nickel (II) carbonate solution, together with the catalytic
reagents are then
heated, with agitation and with a hydrogen overpressure in the range of 150 to
500
kPa, but preferably about 350 kPa, to a temperature in the range of 150 C to
180 C,
and reacted with hydrogen at a pressure of 3.0 MPa to 4.0 MPa, preferably at
about
3.5 MPa, until the dissolved nickel (II) salt is reduced to nickel metal
powder.
Thirdly, the present inventior: -ovides a unique method for controlling
the particle size of the produced micron-sized nickel metal powder. This
method is
founded on the discovery that there exists a correlative relationship between
the
amount of silver added (i.e. grams of added soluble silver per kilogram of
nickel (II))
and the ultimate particle size obtained. Additionally, it appears that a
relationship
exists between the silver content of the produced powder and the particle size
and,
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-6-
also, that both the added silver concentration and the silver content of the
powder, in
combination, affects particle size. Moreover, increasing the amount of added
silver
decreases the particle size obtained. As will be evident to one skilled in the
art there
exists an upper limit of silver which may effectively be added, and without
being
bound by same, would appear to be of the order of 10 grams per kilogram of
nickel
(II). Clearly, therefore, this capability of producing a nickel metal powder
having a
predetermined particle size is most advantageous.
Brief Description of the Drawings
The method of the invention will now be described with reference to
the accompanying drawings, in which:
Figure 1 is a process flowsheet of the commercially operated existing process
for the production of micron-sized nickel metal powder;
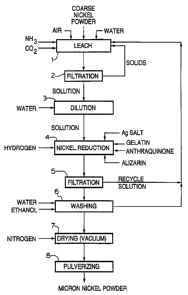
Figure 2 is a process flowsheet of the present invention;
Figure 3 is a photomicrograph of the nickel powder produced by the process
of the prior art wherein FeSO4 and A12(SO4)3 in admixture are utilized to seed
the basic nickel (II) carbonate feedstock; and
Figures 4 and 5 are photomicrographs illustrating the nickel metal powders
prepared in accordance with the process of the present invention.
Description of the Preferred Embodiment
Having reference to the flowsheet of Figure 2, a solution of nickel
ammonium carbonate may be prepared in leach step 1 by dissolving coarse nickel
powder in ammoniacal ammonium carbonate solution at 80 C at elevated air
pressure
in an autoclave. This solution is then filtered or clarified in step 2 to
ensure the
removal of solids thereby leaving a solution which is essentially free of
metallic
nickel. The solution is then diluted in step 3 and charged in an autoclave
(step 4)
wherein the catalytic reagents are added.
A soluble silver salt, preferably silver sulphate or silver nitrate, is added
in a ratio of about 1 to 10 grams of silver per kilogram of nickel (II). The
amount of
silver to be added will depend upon the desired particle size of the nickel
metal
powder.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-7-
More specifically, the amount of silver added would be dictated by the
results given in Table 1 herebelow.
TABLE I
Silver added g/kg Ni (II) Fisher No. (microns)
3.5 1.08
5.5 0.97
6.2 0.77
8.3 0.35
It has been found that the particle size of the nickel metal powder can
be controlled to produce a powder having a particle size less than, or equal
to, 1.0 m
by adding about 2.0 to 12.0 grams of silver sulphate per kilogram of nickel
(II) or
about 2.0 to 3.5 grams of silver nitrate per kilogram of nickel (II).
A dispersant such as gelatin, or bone glue, is added for agglomeration
control. The agglomeration and growth control additives are added in an amount
of
from 5.0 to 20.0 grams per kilogram of nickel (II). A spheroid-promotion
agent,
preferably anthraquinone, is added to the solution to encourage the formation
of
spherical, high density nickel metal powder particles. Alternatively,
derivatives of
anthraquinone or alizarin may be utilized as such an agent. The anthraquinone
is added
in an amount in the range of 1.0 to 5.0 grams per kilogram of the nickel (II).
A
preferred amount of anthraquinone would be about 3 grams per kilogram of
nickel
(II). An alternatively preferred agent would be a mixture of anthraquinone and
alizarin
or alizarin per se .
The slurry containing the feedstock, catalyst and additives is heated,
with agitation, to a temperature in the range of 150 to 180 C, under hydrogen
pressure
preferably about 3.5 MPa, for a time sufficient to reduce the nickel (II) to
micron-
sized nickel metal powder.
The nickel metal powder is then filtered (step 5) and subjected
in step 6 to a water/ethanol wash. Solution recovered from steps 5 and 6 is
recycled
to leach step 1. The nickel metal powder is dried under vacuum with a nitrogen
purge
in step 7. The dried nickel metal powder is then pulverized in step 8 using a
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-8-
hammermill to break up agglomerated particles. Rod milling is not desirable
because
of the minor particle distortions which result.
The product and process of the invention will now be described with
reference to the following non-limitative examples.
Experimental
EXAMPLE 1 (Prior art)
A solution of nickel ammonium carbonate containing 140 g/L Ni, 140 g/L NH31
and 130 g/L C02, was prepared by dissolving coarse nickel powder in ammoniacal
ammonium carbonate solution at 80 C at an elevated air pressure in an
autoclave. This
solution was then treated by sparging in live steam to remove excess ammonia
and
carbon dioxide and precipitate all the dissolved nickel as basic nickel
carbonate
(BNC). A solution containing ferrous sulphate, aluminum sulphate and ethylene
maleic
anhydride (EMA) was added to the slurry of BNC, which was then charged to a
600
litre autoclave. The autoclave was then heated to 180 C and pressurized with
hydrogen
to 3.5 kPa to reduce the BNC to metallic nickel powder. When the reduction was
complete the autoclave was cooled and the slurry of nickel powder in barren
liquor
was discharged and filtered. The filter cake was washed with dilute sulphuric
acid,
followed by water and methyl alcohol, and dried under vacuum with a purge of
nitrogen. The dry powder was pulverized in a hammer mill to break up
agglomerates.
The powder product was analyzed in a Fisher sub-sieve size analyzer. The
Fisher number corresponds to the approximate diameter of the powder particles
in
micrometres.
The chemical and physical analysis of the prior art nickel metal powder
are given in Table II.
CA 02204525 2006-09-01
f
WO 96/14953 PCT/CA95/00649
-9-
TABLE II
CHEMICAL ANALYSIS percent by weight
----------------------------N~ --Ai ---~e ---~o --~ ---~2 ---~ ----~~ ----
98.5 0.2 0.4 0.3 0.2 0.9 0.07 0.005
?~3-IYs1t_CAI, AN'AL~'1t9fS'---------------------------------------------------
A.D T.D F.N
1.0- . 2.0-3.5 0.7-1.2
- -------------------------------------------------------------------------
wherein A. D. is the apparent density in g/cc , T.D is the tap density in g/cc
, and F.N
is the Fisher Number.
The particle shape, at 7000 x magnification was determined as spheroidal
shaped with a minimum/m;-, ;mum diameter ratio of 0.8.
EXAMPLE II
A stock solution of nickel ammonium carbonate solution, containing 150 g/L Ni,
155 g/L NH3 and 135 g/L C02, was prepared by dissolving coarse nickel powder
in
ammoniacal ammonium carbonate solution at 80 C under 550 kPa air pressure in
an
autoclave. This solution was filtered and diluted with water to produce a
series of
solutions containing 35 to 50 g/L Ni, 35 to 50 g/L NH3 and 32 to 47 g/L CO2.
Each
diluted solution was prepared for reduction by the addition of a catalyst
solution
consisting of various combinations of silver sulphate, anthraquinone and
gelatin dissolved
in water, as specified in Table III. Each solution was charged to a 90 litre
batch autoclave
and heated to a temperature of 170 C under steam pressure only. Hydrogen was
then
introduced to the autoclave at a total pressure of 3.5 MPa, to reduce the
dissolved nickel
to nickel powder. The quantity of powder produced in each reduction test
ranged from
1.7 to 2.8 kg. When the reduction reaction was complete, the autoclave was
cooled and
discharged. The powder was filtered from the barren solution and washed with
water
followed by ethanol, and dried in a vacuum oven in an inert nitrogen
atmosphere.
The powder products were analyzed on a Fisher sub-sieve size analyzer, and all
showed Fisher numbers in the range 0.35 to 1.1 as shown in Table III. Scanning
electron
photomicrographs of these powders showed that the particle size ranged from
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-10-
0.2 to 1.0 microns, with some agglomeration. A blend of the six finer powders
analyzed 0.02% S, 0.17% C, 0.43% 02 and 0.009% Fe.
TABLE III
Head Solution Catalyst g/kg Ni Product
Composition g/L
Test Ni NH3 CO2 AO' Gelatin Ag~S,O Fisher Number
1 40 41 38 5 5 5 1.08
2 50 51 47 4 8 8 0.97
3 35 35 32 6 12 12 0.35
4 45 45 41 4.5 9 9 0.77
5 35 35 35 6 6 12 0.44
6 45 45 45 4.5 4.5 9 0.72
7 45 45 45 4.5 4.5 9 0.77
wherein AQ. is anthraquinone. The Fisher number corresponds to the approximate
diameter of the powder particles in micrometres.
A definite and reproducible particle size correlation to the amount of
silver sulphate added is evident as shown in Table IV.
TABLE IV
Silver Added, g/kg Ni 3.5 5.5 6.2 8.3
Fisher Number 1.08 0.97 0.77 0.35
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-11-
EXAMPLE III.
A stock solution of nickel ammonium carbonate solution, containing
150 g/L Ni, 155 g/L NH3 and 135 g/L C02, was prepared by dissolving coarse
nickel
powder in ammoniacal ammonium carbonate solution at 80 C under 550 kPa air
pressure in an autoclave. This solution was filtered and diluted with water to
produce
a large batch of solution containing 48 g/L Ni, 48 g/L NH3 and 43 g/L COz.
Each 60
litre charge of diluted solution was prepared for reduction by the addition of
a catalyst
solution consisting of various combinations of silver nitrate, gelatin and
either
anthraquinone, or alizarin or both, dissolved in water.
Each solution was charged into a 90 litre autoclave and heated to
175 C. Hydrogen was then introduced into the autoclave at a total pressure of
3.5
MPa, to reduce the dissolved nickel to nickel powder. The quantity of powder
produced in each reduction test ranged from 900 to 1600 grams. The powder was
filtered from the barren solution and washed with water followed by ethanol
and dried
in a vacuum oven with an inert nitrogen purge. Details of these tests and the
physical
properties of the nickel powders produced are given in Table V herebelow.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-12-
TABLE V
Test 8 9 10 11 12 13
g/charge
AgNO3 1_0 10 10 10 10 10
Gelatin 10 10 20 20 20 20
AQ 5 5 5 5 5 5
Alizarin 0 0 0 0 1 1
Fisher No. 0.88 1.00 1.34 0.75 1.23 0.75
Microtrac':
D-90, micron 8.1 6.7 2.8 2.7 2.5 2.1
D-50 2.5 2.5 1.4 1.4 1.2 1.0
D-10 0.8 0.9 0.6 0.6 0.5 0.5
A.D. g/cc 0.91 1.09 1.46 1.22 1.64 1.45
The powders produced in these tests were blended and pulverized in a hammer
mill to break up agglomerates, to simulate the commercial process. The
Microtrac ~
measurements, physical properties and chemical analyses obtained on these
blended
products are given in Tables VI and VII herebelow.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
-13-
TABLE VI
Blend A B C D E F
MICROTRAC ': micron
D- 10% 0.55 0.54 0.56 0.57 0.53 0.51
D - 50% 1.40 1.30 1.43 1.38 1.23 0.99
D- 90% 2.90 2.66 2.82 2.68 2.49 2.07
D - 7.46 3.73 7.46 3.73 3.73 3.73
100%
PHYSICAL PROPERTIES
SG 8.42 8.37 8.47 8.59 8.56 8.64
S.A. m2 2.35 3.15 1.97 1.58 3.03 2.07
/g
A.D.g/cc 1.44 1.39 1.46 1.22 1.45 1.44
T.D.g/cc 2.67 2.53 2.82 2.11 2.74 2.56
F.N. 0.94 0.93 1.34 0.75 1.23 0.94
wherein SG is the specific gravity, S.A. is the surface area, F.N. is the
Fisher
number; A.D. is the apparent density; and T.D. is the tap density.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
- 14-
TABLE VII
Blend A B C D E F
CHEMICAL ANALYSIS percent by weight
Ni+Co 98.2 98.1 98.7 98.8 99.4 99.0
Co 0.089 0.095 0.098 0.062 0.079 0.074
Cu 0.054 0.0076 0.013 0.011 0.002 0.001
Fe 0.008 0.010 0.030 0.0058 0.0075 0.0069
AI 0.0036 0.0031 0.0033 0.0036 0.0023 0.0029
Ag 0.034 0.054 0.035 0.136 0.062 0.172
Si 0.002 0.002 0.002 0.003 - -
Ca 0.0034 0.0029 0.0025 0.0015 - -
Mg 0.0010 0.0013 0.0008 0.0005 0.0008 0.0008
Na 0.0022 0.0061 0.0028 0.0027 - -
K 0.0006 0.0002 0.0005 0.0003 - -
S 0.0046 0.0014 0.004 0.008 0.0049 0.0053
C 0.184 0225 0.142 0.168 0.214 0207
0 1.1 1.2 0.72 0.59 038 0.62
EXAMPLE IV
A stock solution of nickel ammonium carbonate solution, containing 150 g/L
Ni, 155 g/L NH3 and 135 g/L C02, was prepared by dissolving coarse nickel
powder
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
- 15 -
in ammoniacal ammonium carbonate solution at 80 C under 550 kPa air pressure
in
an autoclave. This solution was filtered and diluted with water to produce a
large
batch of solution containing 52 g/L Ni, 49 g/L NH3 and 45 g/L CO2. Each 550
litre
charge of diluted solution was prepared for reduction by the addition of a
catalyst
solution consisting of various combinations of silver nitrate, gelatin and
either
anthraquinone or alizarin dissolved in water.
Each solution was charged into a 900 litre autoclave and heated to
160 C with the application of a hydrogen overpressure of 350 kPa from the
start of
heating. Hydrogen was then introduced into the autoclave at a total pressure
of 3.5
MPa, to reduce the dissolved nickel to nickel powder. The powder was filtered
from
the barren solution and washed with water followed by ethanol and dried in a
vacuum
oven with an inert nitrogen purge. Details of these tests and the physical
properties of
the nickel powders produced are given in Table VIII herebelow.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
- 16-
TABLE VIII
Test 14 15 16 17 18
g/kg Ni
AgNO31 3.3 2.2 2.2 2.2 1.7
Gelatin, 7.0 7.0 7.0 10.4 7.0
AQ, 1.7 1.7 1.7 1.7 1.7
Alizarin 0.35 0.35 0.35 0.35 0.35
Fisher No. 0.67 0.75 1.02 0.69 1.40
Microtrac* :
D-10, micron 0.74 0.77 0.95 0.76 0.98
D-50 2.90 2.64 3.15 3.37 2.79
D-90 9.66 9.32 8.19 15.42 5.78
A.D. g/cc 0.94 0.88 1.44 0.94 1.63
From the above results it will be observed that the optimum silver nitrate to
nickel (II) ratio would appear to be between 2.0-3.5 grams per kilogram.
CA 02204525 1997-05-05
WO 96/14953 PCT/CA95/00649
- 17-
It will be understood, of course, that modifications can be made in the
embodiment of the invention illustrated and described herein without departing
from
the scope and purview of the invention as defined by the appended claims.