Note: Descriptions are shown in the official language in which they were submitted.
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95114319
1
IMPLANTABLE REFILLABLE CONTROLLED RELEASE DEVICE TO
DELIVER DRUGS DIRECTLY TO AN INTERNAL
PORTION OF THE BODY
Technical Field
The present invention relates to an implantable,
refillable, rate-controlled drug delivery device, with a
hollow reservoir, and a drug delivery tube communicating
with the hollow reservoir. The drug delivery tube
includes at least one rate-limiting permeable membrane
which regulates drug delivery. A method of controlling
the delivery of a drug to an internal portion of a body
is also disclosed.
Backcrround
Implantable devices for drug delivery are known.
U.S. Patent No. 3,310,051 to Schulte discloses a
ventricular catheter with a reservoir, a backing member
and a tube.
U.S. Patent No. 5,511,355 to Franetzki et al.
discloses an infusion device intended for implantation
into the human body. The device comprises a flexible
walled reservoir in a housing to contain the infusion
liquid; a dosing unit for conveying the infusion liquid
from the housing to a catheter, means permitting the
refilling of the reservoir. The device is useful for
delivering insulin.
U.S. Patent No. 4,969,871 to Theeuwes et al.
. discloses a drug delivery device comprising a reservoir
containing a beneficial agent to be delivered. The
delivery device includes a drip chamber attached to
tubing which is further attached to a transdermal
delivery device. The tubing may have attached a
CA 02204789 1997-OS-07
WO 96114834 PCT/US95/14319
2
formulator which includes a flow distributor. The
delivery device is a transdermal drug delivery device
comprising a reservoir attached to the skin by an
adhesive.
U.S. Patent No. 4,568,331 to Fischer et. al.
discloses a disposable medicine dispensing device. The
device comprises a dispenser with walls which define a
chamber which holds a medicament; a removable cap for
refilling the reservoir; a delivery tube and a valve
means for controlling delivery of the solution through
the tube.
Heyer Shulte Medical Catalog discloses an Ommaya
reservoir with either an on-off flushing device, or with
a pressure pump to regulate brain pressure. Codman
Medical Catalog discloses an Ommaya reservoir with a
pressure flow control. P.8. Medical Catalog discloses an
Ommaya reservoir with a pressure flow control.
"Osmet" by Alza is a pump which works by osmotic
pressure, and polyanhydride matrices by Nova. Polyanhy
drides are bioerodible polymers and this system uses
matrices of drug (BCNU) in a polyanhydride base. The
processes involved in release are dissolution of BCNU,
diffusion through the matrix and breakdown of the
polyanhydride. These systems work by a different
mechanism to that reported here.
Drugs for the treatment of tumors are known. For
example, U.S. Patent No. 5,087,616 to Meyers et al.
discloses cytotoxic drug conjugates and their delivery to
tumor cells. The cytotoxic substances include
daunomycin, bleomycin, melphalan, chlorambucil,
cysplatin, vinblastine, vincristine, or anti-metabolites
such as methotrexate.
U.8. Patent No. 5,051,257 to Pietronigro discloses
anti-neoplastic solutions for direct intra-tumoral
delivery. The anti-neoplastic agents include
nitrosourea, preferably BCNU. (1,3-bis(2-chloroethyl)-1-
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95/14319
3
nitrosourea). The compound may be needle injected into
brain tumors.
U.S. Patent No. 4,273,761 to Matsuda et al.
discloses an anti-tumor composition containing bleomycin
and an absorption enhancer for the treatment of a gastro
. intestinal tumor site. The drug is administered orally
or through subcutaneous injection.
U.S. Patent No. 5,229,500 to Barde et al. discloses
a brain derived neurotrophic factor (or BDNF) which can
be delivered via an Ommaya reservoir (see column 35,
lines 33-35).
U.S. Patent No. 5,180,820 to Barde et al. discloses
antibodies directed toward brain derived neurotrophic
factor which can be delivered via an Ommaya reservoir.
R.A. Morantz et al., "Bleomycin and brain tumors.
A review." J. Neurooncol. (United States), 1983, pp.
249-55. This publication discloses the delivery of
bleomycin to brain tumors using an Ommaya reservoir.
S. Nakazawa et al., "New management of brain
neoplasms. 2. Local injection of adriamycin." No Shinkei
Geka (Japan), August 1983, 11 (8?, pp. 821-7. This
publication discloses the delivery of bleomycin to brain
tumors using an Ommaya reservoir.
S. Nakazawa et al., "A new treatment of malignant
brain tumor -1: local injection of bleomycin." No
Shinkei Geka (Japan) , December 1981, 9 (13) , pp. 1487-93.
This publication discloses the delivery of bleomycin to
brain tumors using an Ommaya reservoir which is implanted
subcutaneously under the scalp.
F. Nakajima et al., "A case of alpha-fetoprotein
producing primary intracranial embryonal carcinoma
treated with combination chemotherapy with cis-platinum,
vinblastine and bleomycin." No Shinkei Geka (Japan),
1981, 9 (3), pp. 371-5. This publication discloses the
delivery of bleomycin to brain tumors using an Ommaya
reservoir.
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95/14319
4
T. Shimura et al., "Disseminated necrotizing
leukoencephalopathy accompanied with multiple calcium
deposits following antineoplastic and radiation, therapy
in a case of intracranial germ cell tumor: Computerized
tomographical study." No Shinkei Geka (Japan), June
1989, 17 (6), pp. 573-7. This publication discloses the
intraventricular delivery of bleomycin to brain tumors
using an Ommaya reservoir.
RØ McKeran et al., "A potential application for
the intracerebral injection of drugs entrapped within
liposomes in the treatment of human cerebral gliomas."
J. Neurol. Neurosurg. Psychiatry (England), December
1985, 48 (12), pp. 1213-9. The publication discloses the
delivery of bleomycin to brain tumors using an Ommaya
reservoir.
Known devices may include pressure releasing~valves
to release or regulate pressure in the brain or drain
fluid from the brain. No rate-limiting permeable
membrane designed to release the drug to be delivered at
a controlled rate or means for controlling drug delivery
is present in existing implantable drug delivery devices .
,The present device overcomes the deficiencies of the
prior art by providing an implantable, refillable, drug
delivery device with a means for controlling drug
delivery with at least one rate-limiting permeable
membrane.
Disclosure of the Inven ion
It is an object of this invention to provide a
device which, once implanted, gives continuous access to
internal regions of the body without requiring additional
needle penetrations into these regions. Instead, a
tubular portion of the device remains in the body and
extends to the affected area where it serves ~as a
continuously-available conduit placed there but once.
Thereafter, a syringe or other device need only be placed
CA 02204789 1998-06-15
in fluid communication with this conduit to inject, withdraw or
mix fluids in the interior region. The delivery tube is
equipped with at least one rate-limiting permeable membrane
designed to release the drug to be delivered at a controlled
rate.
In a preferred embodiment the device is adapted to
deliver a drug to a brain tumor at a controlled rate.
According to one aspect of the present invention,
there is provided an implantable, refillable, rate-controlled
drug delivery device, comprising a hollow reservoir, and a drug
delivery tube communicating with the hollow reservoir, wherein
said device comprises at least one rate-limiting permeable
membrane in said drug delivery tube which regulates drug
delivery.
According to another aspect of the present invention,
there is also provided a method of controlling the delivery of
a anti-tumor drug to an internal portion of a body comprising
administering an anti-tumor drug to an internal portion of the
body through a device as above described.
According to another aspect of the present invention,
there is also provided a method of treating a brain tumor
comprising administering an anti-tumor drug to a brain tumor
through a device as above described.
According to another aspect of the present invention,
there is also provided an implantable, refillable, rate-
controlled drug delivery device, comprising a hollow reservoir,
a drug delivery tube communicating with the hollow reservoir,
and one or more rate-limiting elements, said one or more
elements consisting essentially of a permeable membrane in said
drug delivery tube, which membrane passively regulates drug
delivery.
According to another aspect of the present invention
there is also provided a method of controlling the delivery of
a anti-tumor drug to an internal portion of a body comprising
administering an anti-tumor drug to an internal portion of the
body through a device as above described to control the
CA 02204789 2001-06-15
Sa
delivery of the anti-tumor drug.
According to another aspect of the present invention,
there is also provided a method of treating a brain tumor
comprising administering an anti-tumor drug to a brain tumor
through a device as above described to regulate drug delivery
to the brain tumor.
According to a further aspect of the present
invention, there is also provide an implantable, refillable,
rate-controlled drug delivery device, comprising a hollow
reservoir, and a drug delivery tube communicating with the
hollow reservoir, wherein the device comprises at least one
rate-limiting permeable membrane which regulates drug delivery
disposed in an opening between the reservoir and the drug
delivery tube.
The above and other objects of the invention will
become readily apparent to those of skill in the relevant art
from the following detailed description and figures, wherein
only the preferred embodiments of the invention are shown and
described, simply by way of illustration of the best mode of
carrying out the invention. As is readily recognized the
invention is capable of modifications within the skill of the
relevant art without departing from the spirit and scope of the
invention.
FIG. 1 shows a schematic of the control delivery
device of the present invention.
FIG. 2 shows release of bleomycin from the reservoirs
was pseudo zero order for the first 7 days. This corresponds to
approximately 25% bleomycin release. After this time release
began to slow reflecting depletion of the reservoir. Refilling
the tubes repeatedly did not appear to affect the percentage
CA 02204789 2001-06-15
Sb
release per unit time.
FIG. 3 shows the release rate over the first 7 days
after refilling was found to be directly proportional to the
concentration of bleomycin in the reservoir.
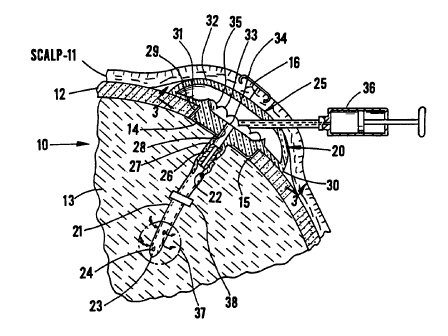
FIG. 4 is a cross-section of a portion of a skull,
showing the device of the invention, partly in cutaway cross-
section and in its normal, unstressed condition.
FIG. 5 is a top view of the invention, partly in
cutaway cross-section.
CA 02204789 1997-OS-07
WO 96/14834 PCTIUS95/14319
6
FIG. 6 is a bottom view of the device taken at line
3-3 of FIG. 4; and
FIG. 7 is~a fragmentary cross-section of a portion
of FIG. 4, showing the device in another of its
conditions.
Description of the Invention
This invention provides a device which, once
. implanted, gives continuous access to internal regions of
the body without requiring additional needle penetrations
into these regions. Instead, a tubular portion of the
device remains 'in the body and extends to the affected
area where it serves as a continuously-available conduit
placed there but once. Thereafter, a syringe or other
device need only be placed in fluid communication with
this conduit to inject, withdraw or mix fluids in the
interior reservoir. The device has a rate-limiting
permeable membrane designed to release the drug to be
delivered at a controlled rate. In a preferred
embodiment the device further comprises a backing member,
such as a membrane, circumscribing the hollow reservoir.
,In a more preferred embodiment, the device is
designed to deliver a drug to a brain tumor at a
. controlled rate. The apparatus of the invention is
composed of a refill-able reservoir containing a drug
solution connected to a delivery tube. The tube is
inserted into the target region of the brain (tumor)
through a hole drilled in the skull. The reservoir
remains outside the skull under the scalp so it can be
easily refilled. Diffusion of drug from the reservoir
into the tube and hence the brain is controlled by a
rate-limiting permeable membrane. The rate of delivery
into the brain is controlled by the membrane and by the
drug concentration in the reservoir.
This device enables a large variety of drugs and
other agents to be delivered into any internal region of
CA 02204789 1997-OS-07
WO 96114834 PCT/US95/14319
7
the body, preferably the brain. Bleomycin is a preferred
drug used in the reservoir delivery device for the
treatment of brain tumors.
Brain tumors are the second leading cause of death
from cancer in pediatrics and the fourth leading cause
among middle aged men. Each year in the United States
approximately 40,000 people are diagnosed with a primary
brain tumor or secondary brain malignancy (1) [see
References below] and 10,000 die (2). Glioblastoma is
particularly devastating. Despite aggressive multimodal
therapy the 2 year survival rate is approximately 25% (3)
and new treatment modalities are urgently needed.
. In the brain cellular proliferation is normally
extremely limited so it could be expected that brain
tumors would be susceptible to cell cycle specific
agents. There are however, two major problems which
limit the usefulness of chemotherapy. Firstly, although
high levels of drugs can be achieved in the blood many
drugs penetrate the blood brain barrier poorly and so
fail to achieve therapeutic levels in the target area.
This is especially true for hydrophilic drugs (4, 5) . The
second problem is that in glioblastomas is the cells
involved with grow slowly (although much faster than
healthy brain tissue). Usually less than 10% of the
cells in a brain tumor are actively cycling at any one
t ime ( 2 ) .
The most direct route of administration to brain
tumors is direct intratunioral (IT) injection. An
advantage of this route is that most primary brain tumors
and metastases are localized in a single region so IT
injection can localize, at least initially, delivery to
the target area. Human and animal studies with IT
delivery of antibiotics such as adriamycin (6) and
lomustine, (7) have demonstrated high concentrations of
' 35 drug in the brain and, in some cases, increased survival
times (6) .
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95/14319
8
In human studies, IT administration is normally
performed with an indwelling catheter implanted into the
brain and linked to a refillable reservoir under the
scalp (8). Despite the use of a variety of cytotoxic
agents clinical results have been disappointing (8-10).
The slow growth rate of brain tumors suggests that= better
results would be obtained if therapeutic concentrations
of cytotoxic agents, especially cell cycle specific ones,
could be maintained for a prolonged period.
Bleomycins are a group of large (molecular weight
1400) hydrophilic compounds isolated from streptomyces
verticillus. Bleomycins are believed to interact with
DNA and cause strand scission, a review of the mode of
action is given by Byrnes et al., 1990 (11). Bleomycin
causes an accumulation of cells in the G2 phase in vitro
(12) and are used in a variety of tumors including
squamous carcinomas of the skin, neck and lungs in
addition to lymphomas and testicular tumors (12).
Bleomycin localizes on and within tumor cells (13) and
has a special affinity for ectodermal tissues (14).
Bleomycin has been shown to inhibit the growth of human
glioma cells in culture at a concentration of over 0.47
~,M (15). As gliomas are ectodermal in origin, bleomycin
could be expected to be effective against these tumors.
Bleomycin is preferentially taken up by malignant brain
tissue and 5'Co labelled bleomycin scintigraphy can be
used to detect brain metastases (16).
EXAMPLE 1
Materials and methods
Polyvinyl alcohol, 98% hydrolyzed, was purchased
from Aldrich Chemical Co. The molecular weight range was
70 - 80k. Bleomycin sulphate (Blenoxane) was obtained
from Nippon Kayaku Co. Ltd. of Japan. Sodium phosphate
(mono and di basic was purchased from Fisher Scientific) .
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95/14319
9
Release of Hleomycin from Implantable. Rate Controlled
Drug Delivery Reservoir
Solutions of polyvinyl alcohol (PVA) were prepared
by dissolving 2 g of PVA in 100 ml of deionized water at
80°C. After cooling to room temperature the solution was
. poured onto a glass plate and allowed to dry forming a
thin film. The film was then removed and heated to 120°C
for 2 hours. 1 cm2 pieces of the film were cut and fixed
(figure 1). The hollow reservoirs were filled with
phosphate buffer (pH 7.4) containing 3 U/mL bleomycin and
the delivery tubes immersed in 5 mL buffer. Care was
taken to ensure that no air bubbles were trapped in the
reservoir, tube or connector. Samples (1 mL) were
periodically removed and assayed by uv detection at 290
nm.
After 10 days the bleomycin solution was removed
from the reservoir with a 5 ml syringe and a 20 gauge
needle. The reservoir was then refilled with a 10 U/mL
bleomycin solution and the release again measured for 10
days. The same process was repeated once more with a
22.5 U/ml bleomycin solution.
Implantation into the Rat Hraia
Animals were anesthetized by subcutaneous injection
of 0.4 ml of a ketamine/rompun mixture (55%:45%). The
skull was then exposed with a #11 scalpel and a 3 mm hole
drilled through the skull to expose the dura. The tip of
the tube was then inserted through the dura to a depth of
3 mm. The tube was then fixed in place with dental
acrylic and the wound closed with silk suture. The
reservoir was then filled with a 3 U/ml bleomycin
solution. An incision was then made in the rat's back
and the reservoir implanted under the skin.
Release of Bleomycin from Reservoir
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95l14319
Release of bleomycin from the reservoirs was pseudo
zero order for the first 7 days (figure 2). This
corresponds to approximately 25% bleomycin release.
After this time release began to slow reflecting
5 depletion of the reservoir. Refilling the tubes
repeatedly did not appear to affect the percentage
release per unit time and the release rate over the first
7 days after refilling was found to be directly
proportional to the concentration of bleomycin in the
10 reservoir (figure 3).
Implantation into the Rat Hrain
Animals appeared to fully recover after implantation
with no observable loss of mobility or motility. The in
vitro data shows that release from the devices is pseudo-
.zero order and is directly proportional to the
concentration in the reservoir. The data also shows that
the devices can be refilled without changing the release
properties. Previous work has shown that direct
intracranial infusions of bleomycin can be tolerated in
rats and humans ( 18 ) . Similarly devices of PVA have been
implanted into a variety of sites in the body with no ill
effects (19,20) and implantable reservoirs are well
tolerated. The work presented here shows that
implantable reservoirs containing bleomycin and a
membrane of PVA are well tolerated.
A device according to this invention comprises a
tube having a central passage, the tube being adapted to
be inserted in to an internal portion of the body so that
its passage communicates with a selected region therein.
A hollow reservoir is adapted to be connected to this
tube and to be placed beneath the skin of the body. This
hollow reservoir comprises an enclosure which has an
internal periphery and a first and second wall, these
walls facing each other inside the reservoir. The wall
next to the skin is tolerant to, and self-sealing after,
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95/14319
11
needle puncture. One of the walls has a passage through
the tube. The tube is adapted to include a rate-limiting
permeable membrane to control the rate of drug delivery
to the target area. In a preferred embodiment the
delivery tube comprises two rate-limiting permeable
membranes.
Thus, the invention relates to an implantable,
refillable reservoir containing a drug solution connected
to a delivery tube. The tube is inserted into an
internal target region of the body. Diffusion of the
drug from the reservoir into the tube and into the target
area of the body is controlled by a rate-limiting
permeable membrane. The rate of delivery into the target
area of the body is controlled by the membrane and by the
drug concentration in the reservoir.
According to a preferred but optional feature ~of the
invention, one of the walls is flexible and palpable
through the skin by force exerted by the hand. The
material of the flexible wall is flexible enough to be
moved toward the other wall and thereby reduce the volume
of the reservoir, and also sufficiently springy to return
to its normal unstressed condition, thereby providing for
pumping means to create bi-directional flow in the
passages for mixing purposes.
The above and other features of this invention will
be fully understood from the following detailed
description and the accompanying drawings in which:
FIG. 4 illustrates a skull 10, showing the scalp 11
and the bone 12 of the skull surrounding the brain 13.
The drawing of the anatomical portions is simplified
because these form no portion of the present invention.
It will be noted that the device is installed within a
burr hole 14 having an internal wall 15 in the bone of
the skull, and is held beneath the skin of the scalp by
' 35 means of a sutured slit 16.
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95l14319
12
The reservoir, elements 20 comprising the invention
includes a tube 21 having a central passage 22 and a
closed end 23. Perforations 24 through the wall provide
for fluid communication between the passage and the
outside of the tube. The tube is connected to a
reservoir 25 by means of a connector 26, which connector
is attached to a passage extension 27 which has a passage
28 that enters the reservoir itself through one wall
thereof. The tube is equipped with at least one rate-
' 10 limiting permeable membrane 38 for controlling the rate
of drug delivery to the target area of drug delivery. In
an alternative embodiment the rate-limiting permeable
membrane may be place in any location in the tube or
between the opening of the reservoir and the tube. The
reservoir has a boss 29 which fits within burr hole 14
and provides for lateral restraint of the device. The
reservoir outward of the boss rests on the skull so as to
give longitudinal restraint.
The reservoir is a continuous enclosure and includes
a circumferential periphery defined by circumferential
edge 30. First and second interior walls 31, 32 are
provided on opposite sides of the periphery. TYie first
wall lies closer to the skull, and includes an interior
crown 33 which surrounds the opening 34 of passage 28
into the interior of the reservoir. The crown has a
plurality of notches 35 formed therein.
Second wall 32 is generally domed and has a central
section which is thicker than the edges. This wall is
flexible and, as best shown in FIG. 4, is adapted to be
palpated by finger pressure through the scalp to move it
toward the first wall, thereby reducing the volume of the
reservoir. In order to prevent the first and second
walls from adhering to each other and closing the
passages, the crown is provided to hold them apart.
However, the notches provide for fluid flow past the
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95/14319
13
crown to the passage even when the second wall is pressed
against the crown.
The entire structure is made of material which is
compatible with the human tissue with which it comes in
contact. A convenient substance is silicone rubber
manufactured by Dow Corning Company, of Midland,
Michigan. In a preferred embodiment the material of the
device is polyvinyl alcohol. If a backing member is
present in a preferred embodiment, the backing member may
be composed of any material tolerated by the human body,
preferably ethylene vinyl acetate, Teflon, silicone,
silastic and nylon. The rate-limiting permeable membrane
may be composed of a composition such as polyvinyl
alcohol,. ethylene vinyl acetate, silicone, nylon,
polypropylene, polcarbonate, cellulose, cellulose
acetate, cellulose esters or polyether sulfone.
The thickness of the second wall is selected such
that it is springy enough to restore itself to its
normal, unstressed condition shown in FIG. 4, and yet
sufficiently flexible that it can be deflected to the
configuration shown in FIG. 7. It is also tolerant to
repetitive needle punctures (with a reasonable number),
because, as best shown in FIG. 4, material may be
injected from a hypodermic syringe 36 by puncturing the
scalp and the second wall with the needle. When the
needle is withdrawn, the material of the second wall will
seal the opening made by the needle.
The use of the device should be evident from the
foregoing. The device may be used to control the rate of
. 30 drug delivery to any internal region of the body.
In a most preferred embodiment the device is adapted
for only drug delivery. In an alternative embodiment, if
it is desired either to withdraw fluid from region 37
within the brain or to introduce medication thereto, the
tube will be forced into this region by means well known
in the surgical arts. Once in place, this tube is
CA 02204789 2001-06-15
14
connected to the reservoir, and the reservoir is placed
in position as shown, and the scalp sutured over the
reservoir. Medication may be introduced to the region by
a syringe as shown, mixing being attained either by
depressing the second wall or by pumping with the
syringe.
It will thereby be seen that this device provides a
reservoir of fluid which in effect is an extension of a
region under treatment or study. Only one puncture of
1o the brain or other region is necessary because Che tube
remains in place for sensible periods of time to provide
a continuously-available conduit to the region, and the
dome is available for introduction and withdrawal, of
material even were the second wall to be too stiff to
flex. On the other hand, should the device be made
flexible enough for this purpose, then even better
results may be attained as a result of the more complete
bi-directional flow attainable thereby.
A phase I trial of IT bleomycin for recurrent malignant
gliomas was completed using the implantable reservoir according
to the present invention. See University of Kentucky IRB # 93-
30026, in its entirety. The device was extremely well
tolerated. Nine patients were enrolled in a dose escalation
study and received weekly doses of bleomycin beginning at 4.9
units per week and escalating to 24.5 units per week. The study
determined that the maximum tolerable weekly doses of
intratumoral bleomycin is 16 units per week. Toxicity, when
present, consisted of drowsiness, lethargy and increased
seizure frequency in those patients with existing seizure
disorders. Two patients who received cumulative does of 300
units developed skin ulcers around the injection sites. No .-
CA 02204789 1997-OS-07
WO 96/14834 PCT/US95/14319
other neurological or systemic toxicity from bleomycin
occurred. The median survival after start of IT
bleomycin therapy was 39 weeks, and this compares
favorably with the 8-12 week expected survival with
5 patients with recurrent high grade gliomas. The phase I
study showed that the implantable reservoir of the
invention worked well without adverse effects and that IT
dose of bleomycin was relatively non-toxic and well
tolerated.
10 In sum, the invention provides an implantable,
refillable reservoir containing a drug solution connected
to a delivery tube. The tube is inserted into a specific
target region of the body or may be placed in a region
such as a blood vessel, to provide systemic distribution
15 of the drug.
Diffusion of the drug from the reservoir into the
tube and into the target area of the body is controlled
by at least one rate-limiting permeable membrane. The
rate of delivery into the target area of the body is
controlled by the membrane and by the drug concentration
in the reservoir.
REFERENCES
1. Rutka, J.T., Trent, J.M. and Rosenblum, M.L.
Cancer Invest. 8:425-438 (1990).
2. Saleman, M. Neurologic Clinics 3,2:229-257 (1985).
3. Saleman, M., Kaplan, R.S., Ducker, T.B. et al.
Neurosurgery 10:44-463 (1982).
4. Chen, H.-S. G. and Gross, J.F. Cancer Treat. Rep.,
64:31-40 (1980).
5. Stewart, D.J., Prog. Exp. Tumor Res. 28:32-50
(1984) .
6. Shimura, T. and Nakazawa, S. No Shinkei Geka.
8:35-42 (1980).
7. Tator, C.H. Surg. Neurol. 7:73-77 (1977).
CA 02204789 2001-06-15
16
8. Ringkjob, R. Acta. Neurol. Scand. 44:318-322
(1968) .
9. Weiss, S.R. and Radkind, R. Internat. Surg.
31:149-155 (1969).
10. Newton, W.A., Sayers, M.P. and Samuels, L. Cancer
Chemother. Rep. 52:52:257-261 (1968).
11. Byrnes, R.W., Templin, J., Sem D., Lyman, S. and
Petering, D.H. Cancer Res. 50:5275-5286 (1990).
12. Goodman and Gilman's The Pharmacological Basis of
Therapeutics Eds. A.G. Gilman, T.W. Rall, A.S. Nies
and P. Taylor. Pergamon Press, 1990, 8th Ed.
13. Fujimoto, J. Cancer Res. 34:2969 (1974).
14. Blum, R.H., Carter, S.K. and Agre, K. Cancer.
31:903 (1973).
15. Kanno, T., Kudo, T. and Nakazawa, T. Clin. Neurol.
10:409-414 (1970).
16. Nieweg, O., Piers, D.A. and Beekhuis, H. Clin.
Neurol. Neurosurg. 90-92, 109-111 (1988).
17. Adamson, I.Y.R., and Bowden, D.H. Am. J. Path.
77:185 (1975).
18. Morantz, R.A., Kimler, B.F., Vats, T.S. and
Henderson, S.D. J. Neuro. Oncol. 1:249-255
( 1983 ) .
19. Sanborn, G.E., Anand, R., Tori, R.E., Nightingale,
S.D., Cal, S.X., Yates, B., Ashton, P. and Smith,
T.J. Arch. Ophthalmol. 110:188-195 (1992).
20. Blandford, D.L., Smith, T.J., Brown, J.D., Pearson,
P.A. and Ashton, P. Invest. Ophthalmol. Vis. Sci.
In press.
30. The purpose of the above description and examples is
to illustrate some embodiments of the present invention
without implying any limitation. It will be apparent to
those of skill in the art that various modifications and
variations may be made to the apparatus and method of the
present invention without departing from the spirit or
scope of the invention.