Note: Descriptions are shown in the official language in which they were submitted.
CA 02205122 1997-05-12
HYDROGEN PEROXIDE PICKLING OF STAINLESS STEEL
BACKGROUND OF THE INVENTION
This invention relates to a process for acid descaling ferrous alloys
containing
chromium. More specifically, oxide on hot rolled or annealed ferrous alloys
containing
chromium is removed by sequentially immersing the alloy in an inorganic acid
and then
applying an aqueous solution containing hydrogen peroxide to the pickled
alloy.
One of the most environmentally intensive problems relating to steel
manufacturing
is pickling of steel to remove oxide or scale formed during hot processing
such as rolling
on a hot strip mill or annealing. Most low carbon steels may be descaled in
hydrochloric
acid at high speeds. The scale on stainless steel, however, has a very fine
structure and is
tightly adherent usually requiring mechanical scale cracking such as shot
blasting, roll
bending or roll leveling of a steel strip to loosen the scale prior to acid
pickling.
Additionally, stainless steel pickling acids such as hydrofluoric, sulfuric,
nitric or mixtures
thereof generally must be more aggressive than those required for low carbon
steel. The
immersion time required for stainless steel is much longer than that required
for low carbon
steel and may require electrical assistance to help remove the scale as well.
A major
motivation for improving the scale removing process is the capital and
environmental
disposal costs associated with pickling acids. A major disadvantage of
chemical descaling
using hydrofluoric and nitric acids is the environmental problems related to
their disposal.
It is known to use acid mixtures containing hydrogen peroxide for pickling and
cleaning stainless steel. For example, US patent 5,154,774 teaches adding an
oxygenated
agent, e.g., hydrogen peroxide, potassium permanganate or air, to a
hydrofluoric acid for
2 5 pickling stainless steel to convert ferrous ions to ferric ions. US patent
5,164,016 teaches
adding hydrogen peroxide to an organic acid such as formic, acetic, propionic,
lactic,
benzoic, phthalic and naphthoic for pickling stainless steel. Hydrogen
peroxide is added to
the acid to control the ratio of ferrous/ferric ions within the range of 10/90
to 40/60.
Japanese patent application 63-20494 discloses a method for chemically
removing scale
3 0 from stainless steel by adding an adhesive to a solution containing
hydrogen peroxide,
phosphoric acid and hydrogen fluoride. The adhesive is not decomposed by the
hydrogen
peroxide and gives viscosity to the cleaning solution and forms a pasty
liquid. Japanese
patent application 60-243289 discloses reducing smut on steel using an acid
bath containing
hydrofluoric acid, hydrogen peroxide and hydrochloric or sulfuric acid.
Japanese patent
3 5 application 54-64022 discloses providing a viscous pickling agent for
removing stain and
scale from stainless steel. Abrasive particles such as alumina, Cr oxide, Si
carbide or silica
are added to an acidic solution containing hydrogen peroxide, sulfuric acid,
hydrochloric
CA 02205122 1997-05-12
acid and a surfactant agent. Japanese patent application 58-110682 discloses
pickling hot
rolled stainless steel with a solution containing sulphamic acid, nitric acid,
hydrofluoric
acid and hydrogen peroxide.
Although these acids are effective for removing scale from stainless steel,
their use
creates certain undesirable problems and have their limitations. For example,
using sulfuric
acid alone for removing scale from stainless steel is undesirable because this
acid leaves a
black smut on the pickled steel. Using hydrochloric acid alone results in a
bright stainless
steel surface but is undesirable because of slow reaction with the tightly
adhering scale.
More aggressive acids such as nitric and hydrofluoric to remove scale from
stainless steel
are especially undesirable because their use creates environmental problems
requiring fume
abatement equipment to handle fumes from the pickling tanks, special equipment
for
storing the acids, and their pickling by-products require special handling and
costly
disposal. Other disadvantages include safety and health risks associated from
chronic
exposure to these acids and limits on allowable nitrate and fluoride discharge
in effluents
from treated wastes. An organic acid is undesirable because it would not be
useful for
descaling of a stainless steel.
Accordingly, there remains a need for a process for pickling ferrous alloys
containing chromium that does not include nitric acid, hydrofluoric acid or a
fluoride
compound. There remains a further need for a process for pickling ferrous
alloys
containing chromium that does not create costly environmental disposal
problems of
pickling waste by-products. Another need includes being able to obviate the
need for
expensive pollution control and waste treatment facilities associated with
using nitric acid,
hydrofluoric acid or a fluoride compound.
2 5 BRIEF SUMMARY OF THE INVENTION
A principal object of the invention is to provide a ferrous alloy containing
chromium
having a bright, oxide free surface, using a hydrochloric or sulfuric pickling
solution
whose by-products do not cause an environmental disposal problem.
3 0 Another object of the invention is to provide a ferrous alloy containing
chromium
having a bright, oxide free surface without using nitric acid, hydrofluoric
acid or a fluoride
compound.
Another object of the invention includes providing a hydrochloric or sulfuric
pickling process wherein the chemical cost is no greater than that otherwise
required for
3 5 nitric, hydrofluoric acid or a fluoride compound.
Another object of the invention is to pickle a ferrous alloy strip containing
chromium at a speed of at least 30 nVmin.
2
CA 02205122 2005-10-14
The invention relates to a hot rolled or annealed ferrous alloy strip
containing
chromium being descaled with an acid. The hot rolled or annealed ferrous alloy
strip is
pretreated to crack the scale and then immersed into at least one pickling
tank containing an
inorganic acid from the group consisting of hydrochloric or sulfuric acid to
remove the
cracked scale. Thereafter, an aqueous solution containing hydrogen peroxide is
applied to
the pickled alloy strip wherein any residual scale becomes activated by the
peroxide so that
the residual scale can be removed by the inorganic acid thereby providing a
clean chromium
ferrous alloy strip.
Another feature of the invention is for the aforesaid aqueous solution to
contain at
least about 10 g/1 hydrogen peroxide.
Another feature of the invention is for the aforesaid aqueous solution to
contain the
inorganic acid for removing the residual scale.
Another feature of the invention is for the aforesaid aqueous solution to
contain at
least about 5 g/1 of the inorganic acid.
Another feature of the invention is for the aforesaid aqueous solution being
disposed
of in the pickling tank.
Another feature of the invention is for the aforesaid pickling tank to contain
at least
about 50 g/1 of the inorganic acid.
Another feature of the invention is for the aforesaid pickling tank acid to
have a
temperature of at least about 60 C.
An advantage of the invention includes using hydrochloric or sulfuric acid for
removing hot roll mill scale or annealing scale from a ferrous alloy strip
containing
chromium rather than using nitric acid, hydrofluoric acid or fluoride
compounds. Another
advantage of the invention includes increased pickling speeds without using
nitric acid,
hydrofluoric acid or fluoride compounds. Other advantages include fewer
environmental
concerns, a hydrogen peroxide containing waste solution being compatible with
hydrochloric or sulfuric acid waste by-products, a smut free chromium alloyed
strip,
obviating the need for electrical assistance for removing scale and a more
passive corrosion
resistant pickled ferrous chromium alloyed surface.
In one aspect, the present invention resides in a process for removing scale
from a
ferrous alloy containing chromium, comprising: providing a ferrous alloy strip
containing
3
CA 02205122 2007-01-30
chromium covered by scale, pretreating the strip to crack the scale, immersing
the pretreated
strip into at least one pickling tank containing an inorganic acid selected
from the group
consisting of hydrochloric and sulfuric acid to remove the cracked scale, and
applying an
aqueous solution containing hydrogen peroxide to the pickled strip wherein any
residual
scale on the strip becomes activated by the hydrogen peroxide and removed by
contacting
the pickled strip with the inorganic acid at a point in the process selected
from the group
consisting of during the application of the solution containing hydrogen
peroxide and after
the application of the solution containing hydrogen peroxide to thereby form a
clean strip.
In another aspect, the present invention resides in a process for removing
scale from
a ferrous alloy containing chromium, comprising: providing a ferrous alloy
strip containing
chromium covered by scale, pretreating the strip to crack the scale, immersing
the pretreated
strip into at least one pickling tank containing an inorganic acid selected
from the group
consisting of hydrochloric and sulfuric acid to remove the cracked scale, and
applying an
aqueous solution containing at least 20 g/l hydrogen peroxide and at least 5
g/1 of thc
inorganic acid to the pickled strip wherein any residual scale on the strip
becomes activated
by the peroxide and simultaneously removed by the inorganic acid contained in
the aqueous
solution thereby fonming a clean strip.
In a further aspect, the present invention resides in a process for removing
scale from
a ferrous alloy containing chromium, comprising: providing a ferrous alloy
strip containing
chromium covered by scale, pretreating the strip to crack the scale, immersing
the pretreated
strip into at least one pickling tank containing at least 50 g/1 of inorganic
acid selected from
the group consisting of hydrochloric acid and sulphuric acid at a temperature
of at least 77 C
to remove the cracked scale, applying an aqueous solution containing at least
20 g/l of
hydrogen peroxide and at least 20 g/1 of the inorganic acid to activate any
residual scale on
the strip, immersing the activated strip into another pickling tank containing
at least 50 g/1
of the inorganic acid at a temperature of at least 77 C to remove any
activated residual scale,
and applying the aqueous solution containing at least 20 g/1 of the hydrogen
peroxide and at
least 20 g/1 inorganic acid to the pickled strip wherein any residual scale on
the strip
becomes activated by the hydrogen peroxide and simultaneously removed by the
inorganic
acid contained in the aqueous solution thereby forming a clean strip.
3a
CA 02205122 2007-01-30
In another aspect, the present invention resides in a process for removing
scale from
a ferrous alloy containing chromium, comprising: providing a ferrous alloy
strip containing
chromium covered by scale, pretreating the strip to crack the scale, immersing
the pretreated
strip into at least one pickling tank containing an inorganic acid from the
group consisting of
hydrochloric and sulfuric acid to renlove the cracked scale, and applying an
aqueous
solution containing hydrogen peroxide to the pickled strip wherein any
residual scale on the
strip becomes activated by the hydrogen peroxide and removed by the inorganic
acid
thereby forming a clean strip.
The above and other objects, features and advantages of the invention will
become
apparent upon consideration of the detailed description and appended drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 schematically illustrates a pickling line incorporating the process of
the
invention,
3b
CA 02205122 1997-05-12
FIG. 2 schematically illustrates another embodiment of a pickling line
incorporating
the process of the invention, and
FIG. 3 schematically illustrates another embodiment of means for applying an
inorganic acidic solution containing hydrogen peroxide of the invention to a
pickled ferrous
alloy strip containing chromium.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
This invention relates to a process using inorganic acid for descaling a
ferrous alloy
containing chromium, such as ferritic stainless steel strip. More
specifically, oxide or scale,
hereafter referred to as scale, on a hot rolled or an annealed ferrous alloy
containing
chromium is removed by immersing the alloy into an inorganic acid of
hydrochloric (HC1)
or sulfuric acid (H2SO4), and then rinsing the pickled strip with an aqueous
solution
containing hydrogen peroxide (H202). Any residual scale remaining on the strip
is
activated by the hydrogen peroxide contained in the aqueous rinsing solution
and then
removed by inorganic hydrochloric or sulfuric acid. The inorganic acid for
removing
residual scale can be sprayed onto the strip after activation by the hydrogen
peroxide, the
activated strip can be immersed into a tank containing the inorganic acid or
preferably the
inorganic acid is contained in the aqueous solution containing the hydrogen
peroxide
rinsing.
By activating any residual scale with a solution containing hydrogen peroxide,
it
has been determined the use of nitric or hydrofluoric acids and/or fluoride
containing
compounds is not required to adequately remove scale from ferrous alloys
containing
chromium during high speed pickling. Not being bound by theory, what is meant
by
2 5 activating the scale is that hydrogen peroxide reacts with the base metal
of the steel alloy to
loosen and/or decompose the scale tightly adhering thereto thereby aiding in
the removal of
the scale from the substrate by the inorganic acid.
By a ferrous alloy containing chromium is meant an alloy of iron and chromium,
e.g., chromium alloyed steel, stainless steel, in which the chromium content
is at least
3 0 about 5% Cr, preferably at least 10% Cr and up to about 30% Cr. The alloy
preferably is a
ferritic stainless steel including up to about 0.5% Al, up to about 0.3% of C,
up to about
1% of one or more of Si, Ti, Nb, Zr; up to about 5% of Ni and/or Mo and up to
about
1.5% Mn. All percentages are by wt.%. These alloys also may include purposeful
additions of one or more of Ta, Ca, Cu, B and N as well.
3 5 After a scale is formed during hot processing such as by rolling on a hot
strip mill
or in a continuous annealing furnace, continuous stainless steel strip or foil
or cut to length
sheets, referred to hereafter as strip, is given a mechanical scale cracking
treatment such as
4
CA 02205122 1997-05-12
shot blasting or roll bending to loosen the scale. Thereafter, the strip is
immersed into a
pickling tank containing an inorganic acid to remove the cracked scale. For
this invention,
the inorganic acid is defined to include either of sulfuric acid or
hydrochloric acid. An
important feature of the invention is to thereafter apply an aqueous solution
containing
hydrogen peroxide onto the pickled strip wherein any remaining residual scale,
smut, dirt,
and the like becomes activated by the hydrogen peroxide. Preferably, the
aqueous solution
contains inorganic acid and any activated residual scale is simultaneously
removed by the
inorganic acid in the aqueous solution containing the hydrogen peroxide.
Preferably, the
pickling tank contains the same inorganic acid as that used to remove the
residual scale.
This remaining scale then becomes removed when the strip is rinsed with the
solution
containing the inorganic acid and hydrogen peroxide and when the strip then is
brushed and
rinsed with water.
The pickling tank preferably contains the same inorganic acid as that used to
remove
residual scale so that the spent aqueous solution containing hydrogen peroxide
and
inorganic acid can be disposed of in the pickling tank after being used to
activate and aid in
the removal of any residual scale on the strip. Considerable make up solution
is required in
the pickling tank because of evaporation when the acid is hot. The aqueous
solution
advantageously can be disposed of by being sent to the pickling tank as part
of this make
up requirement.
Hydrogen peroxide rapidly reacts with ferrous iron (Fe+2) resulting in ferric
iron
(Fe+3) when iron is removed from steel strip and becomes dissolved into a
solution
containing the hydrogen peroxide. For this reason, the hydrogen peroxide of
the invention
preferably is dissolved into a hydrochloric or sulfuric acid and applied
directly to the
surface of the strip rather than being stored within an immersion tank. If the
peroxide were
2 5 dissolved within the inorganic acid stored within an immersion tank, the
peroxide would
break down and become ineffective after a relatively short period of time no
longer
activating any residual scale remaining on the pickled strip. Hydrogen
peroxide is
consumed when ferrous ions (Fe+2) dissolved in the inorganic acid are oxidized
to ferric
ions (Fe+3).
3 0 It was surprisingly discovered any residual scale advantageously need only
remain
in contact with an aqueous solution containing hydrogen peroxide a very short
period of
time to become sufficiently activated by the hydrogen peroxide thereby easily
being
removed by the inorganic acid. If the inorganic acid is contained in the
aqueous solution,
any residual scale is simultaneously removed by the acid. By simultaneously
removing any
3 5 residual scale from the strip is meant having an activation time as short
as about 1 second,
preferably at least 3 seconds and more preferably less than 10 seconds. A
major advantage
5
CA 02205122 1997-05-12
of this invention is that it is not necessary to apply the inorganic acid to
the steel strip for
removing residual scale apart from the hydrogen peroxide.
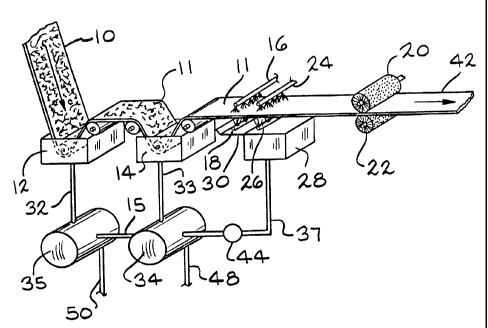
FIG. 1 illustrates one embodiment of a pickling line incorporating the process
of the
invention. More specifically, reference numeral 10 schematically illustrates a
ferrous alloy
strip containing chromium such as stainless steel covered with a scale such as
from rolling
on a hot strip mill. The scale on strip 10 would have been cracked such as
being passed
through a shot blasting machine or roll leveler (not shown). The scale of a
stainless steel
should be loosened whenever nitric, hydrofluoric acid and/or fluoride
compounds are not
used to enhance the descaling effect. Thereafter, the strip is immersed into
sulfuric or
hydrochloric acid contained within one or more pickling tanks such as tanks 12
and 14. If
the pickling line of the invention includes a plurality of pickling tanks, the
acid in the
pickling tanks preferably is counter current flowed such as through a pipe 15
through the
tanks in a direction opposite the direction of travel of the strip.
Thereafter, the strip
normally may have residual a.mounts of tightly adherent scale 11. This tightly
adherent
residual scale is activated by being contacted with an aqueous solution
containing hydrogen
peroxide and then removed by hydrochloric or sulfuric acid. This aqueous
solution may be
sprayed onto the strip such as by a spray header 16 extending completely
transversely
across and positioned above the strip and another spray header 18 extending
completely
transversely across and positioned below the strip. Preferably, another pair
of spray
2 0 headers 24 and 26 extending transversely completely across the strip is
provided. Using
multiple spray headers above and below the strip increases the activation time
of the tightly
adherent residual scale by the hydrogen peroxide. Ferrous alloy strip
containing chromium
having a very clean surface was pickled at a speed of at least 30 m/min. Any
peroxide spray
dripping from the strip may be collected onto a catch pan 30 and flowed into a
tank 28. If it
2 5 is desired to apply the hydrochloric or sulfuric acid to the strip
separately from the aqueous
solution, another pair of spray headers can be positioned a short distance
down stream
from spray headers 24 and 26 for this purpose. Any hydrochloric or sulfuric
acid spray
dripping from the strip may then also be collected onto catch pan 30 and
flowed into tank
28. After the hydrogen peroxide rinse, it is desirable to abrade the pickled
steel strip by one
3 0 or more pairs of brushes 20 and 22. These brushes are of a grit
impregnated polymer
construction. The strip also will be rinsed with water. If the acid in tanks
12 and 14 is
sulfuric, a black smut may remain on the strip surface exiting from tank 14.
This smut is
easily removed from the strip by the inorganic acid contained in the hydrogen
peroxide
solution to improve the cleanliness of a cleaned strip 42.
3 5 When tanks 12 and 14 contain hydrochloric acid, a collected sprayed
hydrogen
peroxide solution containing hydrochloric acid can be disposed of in either of
tanks 34 and
6
CA 02205122 1997-05-12
35 as makeup for liquid lost to evaporation through pipes 32 and 33
respectively. The used
acidic hydrogen peroxide solution can flow by gravity to tanks 34 and 35
through a line 37
by opening a valve 44. After the acid becomes saturated with iron, this acid
is replaced with
fresh acid. Spent acid may be periodically withdrawn from tanks 34 and 35
through a line
50 and sent to an acid recovery plant (not shown). Fresh acid would be
returned to tanks
34 and 35 through a return line 48. The fresh acid, along with the used
aqueous solution
originally containing the hydrogen peroxide, would be pumped from tank 34 to
pickling
tank 14. -
FIG. 2 illustrates another embodiment of a pickling line incorporating the
process
of the invention. In this embodiment, components that are the same as in the
embodiment
illustrated in FIG. 1 have like numerals. If scale 11 on strip 10 is extremely
adherent and/or
it is desired to operate the pickling line at very high speeds, it may be
necessary to repickle
the strip by passing the pickled strip through another tank 38 containing
inorganic acid.
Thereafter, if the strip still has any residual scale 11, this remaining scale
may be
reactivated by the aqueous solution containing the inorganic acid and hydrogen
peroxide
sprayed onto the strip by a second set of multiple spray headers 52, 54
extending
completely transversely across and positioned above the strip and another set
of multiple
spray headers 56, 58 extending completely transversely across and positioned
below the
strip. By using a second set of multiple spray headers above and below the
strip, the
2 0 activation time of the residual scale by the hydrogen peroxide may be
twice as long as that
illustrated in FIG. 1 and insures a very bright strip 42 so that a ferrous
alloy strip
containing chromium can be pickled to a very clean surface at speeds in excess
of 60
m/min. Any peroxide spray dripping from the strip at this second set of spray
headers may
be collected onto a catch pan 60 and flowed into a tank 62. Thereafter, the
pickled steel
2 5 strip is abraded by brushes 20 and 22 and rinsed with water. Used peroxide
solution can
flow by gravity from tank 62 to a tank 36 by opening a valve 68.
Alternatively, the spent
peroxide solution can be pumped to a waste water holding tank 66 though a line
64 by
opening a valve 70. The waste water in holding tank 66 may then be sent to a
waste water
treatment plant (not shown) though a line 46. The acid solution in tank 36 may
be pumped
3 0 to the acid recovery plant though a line 40.
In the embodiment of FIG. 2, the aqueous solution containing spent hydrogen
peroxide collected in tank 28 is flowed into and disposed of in acid tank 14
through a line
17. Since the inorganic acid in pickling tank 14 contains dissolved iron, any
hydrogen
peroxide remaining in the aqueous solution will break down into water and
oxygen.
3 5 Other means for applying the aqueous solution containing hydrogen peroxide
to the
pickled steel may include using laminar flow or an absorbent contact roller
for contacting
7
CA 02205122 1997-05-12
each side of a steel strip. FIG. 3 illustrates another embodiment for applying
the aqueous
solution containing peroxide to the pickled steel using laminar flow. The
pickled strip is
passed through means 72 for laminar flowing the aqueous solution. Laminar flow
means
72 includes a pair of juxtaposed panels 74 and 76 sealably joined to a strip
entry end 78 and
a strip exit end 80. Ends 78 and 80 include squeegee type wipers for sealing
the ends of the
applicator. The aqueous solution containing hydrogen peroxide is pumped into
laminar
flow means 72 through a line 82. The steel strip would be immersed into the
aqueous
solution. Spent aqueous solution would be continuously withdrawn from laminar
flow
means 72 through a line 84 for disposal to one of the acid tanks to prevent
accumulation of
dissolved iron.
An important feature of this invention is that the aqueous solution containing
the
hydrogen peroxide must be metered onto the pickled strip such as by a spray
header,
laminar flow or using a contact roller rather than being contained within an
immersion tank.
Hydrogen peroxide readily oxidizes ferrous iron to ferric iron. If a pickled
steel strip were
to be continuously immersed into a tank containing the aqueous peroxide
solution, the
solution would continuously dissolve iron from the steel strip thereby
continuously
consuming the hydrogen peroxide. This would result in a very inefficient use
and wasting
of hydrogen peroxide. It is important not to contaminate the aqueous solution
containing
hydrogen peroxide with iron, e.g., ferrous ions, prior to the aqueous solution
being
2 0 applied to the pickled steel strip. Accordingly, it is important that the
aqueous solution
containing hydrogen peroxide remain free of ferrous ions prior to the aqueous
solution
being applied to the pickled steel strip.
Another important feature of the invention is that any waste waters containing
the
spent aqueous solution containing the hydrogen peroxide and inorganic acid not
contain
2 5 free hydrogen peroxide. When hydrogen peroxide is present and the pH of
the solution is
at least equal to about 7, trivalent chromium, i.e., Cr+3, is readily oxidized
to hazardous
hexavalent chromium, i.e., Cr+6. Trivalent chromium can be readily
precipitated as an
environmentally safe insoluble chromium hydroxide whereas hazardous hexavalent
chromium tends to remain soluble and can not be safely disposed such as in an
unsecured
3 0 landfill. Accordingly, the spent aqueous solution containing the inorganic
acid and
hydrogen peroxide will be mixed with waste water containing dissolved ferrous
iron or
spent inorganic acid containing dissolved ferrous iron to break down the spent
hydrogen
peroxide into water and oxygen.
The strip is initially pickled in tanks 12, 14 and 38 in a hot inorganic acid
such as
3 5 hydrochloric or sulfuric maintained at a temperature of at least 60 C.
Preferably, the strip is
pickled at a temperature of at least 77 C, more preferably at least 82 C and
most preferably
8
CA 02205122 2007-09-12
at least 88 C in hydrochloric acid in pickling tanks 12, 14 and 38.
Preferably, the
hydrochloric acid is maintained at 50 g/1, more preferably at least 75 g/1 and
most preferably
at least 100-200 g/1.
The concentration of the hydrogen peroxide in the aqueous solution should be
at least
10 g/l. If it is not at least 10 g/l, the peroxide will not effectively
activate stainless steel scale.
Preferably, the hydrogen peroxide concentration in the aqueous solution will
be at least 25 g/1,
more preferably, at least 30 g/1 and most preferably at least 40 g/1. The
hydrogen peroxide
preferably is dissolved in an aqueous solution containing at least 5 g/1 of
inorganic acid.
Preferably, the aqueous solution will contain at least 20 g/1 inorganic acid,
more preferably, at
least 40 g/1 inorganic acid and most preferably at least 50 g/1 inorganic
acid.
Example I
In an example, a 409 grade stainless steel was hot rolled on a continuous
strip mill and
then shot blasted. Thereafter, the steel strip was cut into coupons which were
pickled
in a solution containing 280 g/l sulfuric acid at 99 C and then pickled in 150
g/1 hydrochloric
acid at 88 C. The coupons then were removed from the acid, rinsed with water,
brushed and
dried. The coupons contained small amounts of scale and a large amount of
smut. The dirty
appearance of the coupons would result in the steel being unacceptable for
many exposed
applications.
Example 2
In another example, the hot rolled stainless steel of Example 1 was processed
in
accordance with the invention. The samples were processed in a manner similar
to that
described in Example 1 except as noted herein. After being pickled in the hot
sulfuric acid,
the coupons were immersed for 5 seconds into an aqueous solution at 88 C
containing 20 g/l
of sulfuric acid and 40 g/1 H202. The coupons were removed from the aqueous
solution,
rinsed with water, brushed and then pickled again in hydrochloric acid at 88
C. The coupons
then were removed from the second acid, rinsed with water, brushed and dried.
Unlike the
coupons of Example 1, this time the coupons contained no scale and no smut.
These samples
processed according to the invention had a very bright appearance and resulted
in a steel
acceptable for all exposed applications. This demonstrated the importance of
adding the
hydrogen peroxide to the aqueous solution to obtain a clean surface free of
smut and scale.
9
CA 02205122 1997-05-12
Example
In another example, a 409 type stainless steel was hot rolled on a continuous
strip
mill and then pretreated in a shot blasting machine. Thereafter, the steel
strip was processed
in accordance with the invention by being pickled in a solution containing 150
g/1
hydrochloric acid heated to 82 C and processed at a speed of 20 m/min. After
being pickled
in the hot hydrochloric acid, the strip was sprayed for about 2 seconds with
an aqueous
solution containing 50 g/l of hydrochloric acid and 50 g/1 H202. This
activated strip then
was brushed and rinsed with water. The strip contained no visible scale or
smut. The strip
processed according to the invention had a very bright appearance and resulted
in a steel
acceptable for all exposed applications. This example demonstrates the
importance of
adding the hydrogen peroxide to the aqueous hydrochloric acid solution to
obtain a clean
surface free of smut and scale. This trial also demonstrates that had the
strip been pickled
using three acid tanks instead of just one, the line speed could have been
increased to 60
m/min.
Example 4
In another example, a 409 type stainless steel was hot rolled on a continuous
strip
2 0 mill and then pretreated in a shot blasting machine. Thereafter, the steel
strip was processed
in accordance with the invention by being pickled in two acid tanks each
containing a
solution containing 250 g/l sulfuric acid heated to 112 C and processed at a
speed of 40
m/min. After being pickled in the hot sulfuric acid, the strip was sprayed for
about 2
seconds with an aqueous solution containing 40 g/l of sulfuric acid and 40 g/1
H202. This
2 5 activated strip then was brushed and rinsed with water. The strip
contained no visible scale
or smut. The strip processed according to the invention had a very bright
appearance and
resulted in a steel acceptable for all exposed applications.
Example 5
In another example, a 409 type stainless steel was hot rolled on a continuous
strip
mill and then pretreated in a shot blasting machine. Thereafter, the steel
strip was processed
in accordance with the invention by being pickled in two acid tanks each
containing a
solution containing 250 g/l sulfuric acid heated to 112 C and processed at a
speed of 60
3 5 m/min. After being pickled in the hot sulfuric acid, the strip was sprayed
for about 2
seconds with an aqueous solution containing 40 g/l of sulfuric acid and 40 g/l
H202. After
being brushed and rinsed with water, this activated strip then was immersed
again in an
CA 02205122 1997-05-12
acid tank containing a solution containing 150 g/l hydrochloric acid heated to
82 C. After
being pickled in the hot hydrochloric acid, the strip was sprayed again for
about 2 seconds
with an aqueous solution containing 40 g/l of sulfuric acid and 40 g/l H202.
This activated
strip then was brushed and rinsed with water. The strip contained no scale or
smut. The
strip processed according to the invention was fully descaled, totally free of
smut, with a
bright appearance and resulted in a steel acceptable for all exposed
applications.
It will be understood various modifications may be made to the invention
without
departing from the spirit and scope of it. Therefore, the limits of the
invention should be
determined from the appended claims.
11