Note: Descriptions are shown in the official language in which they were submitted.
CA 02205586 1997-05-16
WO 96/16052 PCr/US95/14987
CERTAIN SUBSTITUTED l-ARYL-3-PIPERAZIN-l'-YL PROPANONES
BAC~GROUND OF THE INVENTION
l. Field of the Invention
This invention relates to substituted l-aryl-3-piperazin- l'-yl prop~nones and 1-arvl-3-
piperidin-1'-yl propanones. These coll,pou.lds are useful in the l,~ t of Al7hr.imL~r's
Disease, neopl~stic disease, and ba~t~ or fungal i~ cli
2. Description of the R~l~t~ Art
0 Al7hrimer's Disease is a progressive neurodegenerative disorder affecting 7% of the
population over 65 years of age and characterized clinically by progressive loss of intel1~ctu~l
function. This in,pai"l.ent of function is caused by the presence of neuri~ic plaques in the
ne~co l~.~ and the loss of ~l~sy~la~Llc m~l~cl~~cholinergic neurons. Neuritic plaques are
composed of degcn~ lg axons and nerve terrnin~lc~ often surrounding an amyloid core and
I5 usually containing reactive glial elements. Another characteristic pathologic feature of
~l7heimer's Disease is the neurofibrillary tangle, which is an intraneuronal mass which
C~ ,o,lds to an accum~ tiQn of abnormally phosphorylated tau protein polymerized into
fibrillar structures termed paired helical hl~m~onrs In addition, the neurofibrillary ~angle also
contains highly phosphorylated neurofii~mrnt proteins. Even the earliest papers on .A17~ 'S
20 Disease were clear that both "senile" plaques and neurofibrillary tangles had to be present in
ablln-i~nr,e to allow a post-mortem diagnosis of the disease. Before any un~iersr:~n~i;ng of the
molecular nature of these ~LIuClul~;S was obtained, efforts were being made to understand the
relationship ~el~n the ~lulllb~ of these lesions and the progression or severity of the ~lemrrln~
expr ienre~ by the patient. The limit~ion.c of silver staining were such that early workers in this
25 field were unable to appreciate the complexity of these abnormal ~illU~;IulGs (Ball, MJ, Neurobiol.
of Aging, 8: 564-565, I987). Although early at~G,llpls to correlate pathologic lesion counts with
clinical S~ (JIlls generally yielded poor results, it was clear that the inri~lenre of lar_e llulll~ls
of both type lesions were invariably associated with ~iemen~ia (Perry et al. J. ~eural
Transmission, 24: 131-136, 1987; Alafuzoff, I., Acta Neuropath, 74: 209-225, 1987;
SUBSTlTUtE SHEET (RULE 26)
CA 0220~86 1997-0~-16
WO 96/16052 PCI/US95114987
Wll;~ ho"se et al, Prog. Clin. Biol. Res., 317: 131-142, 1989; Tomlincon~ NeuloyaLllol. Appl.
Neurobiol, 15: 491-512, 1989).
More recent work has allowed a clearer clefinition of the nature of the lesions stained by
silver salts. "Senile" plaques are actually two distinct types of ~Llu-;LulcS. the cl~ccic~l plaques
S described by Al7heimçr being composed of degenerating neuronal elements (neurites)
~ulluundillg a central core of amyloid, while the other so-called "diffuse" or "pli~lliLivc" plaque is
usually a deposit of amyloid without a halo of de~ ;,.g neurites (Tagliavini,, et al, Neurosci.
Lett., 93, 191-196, 1988; Dickson, DW, et al, Am. J. Path., 132, 86-101, 1988). These
~Llu~_lul~,s are liffie-llt to ~ictin~llich from each other with silver stains or with the widely used
10 nuor~,scc.,L dye, thioflavin S. Early attempts, using silver staining, at correlations bCL..~,.,ll
plaque number and extent of ~1~ .. l ;~ as well as some more recent studies using thioflavin S,
were flawed by this inability to define what type of plaque was being counted (Hansen, LA, et al,
Neurology, 38, 48-54, 1988; ~t7.m~n, R, et al, Ann. Neurol., 23, 138-144, 1988). This
bcco.l..,s very obvious in recent studies, which have corlcictently l~polL~d that amyloid plaques
15 can be found in large nu~ in the brains of the lllaJol;Ly of the very elderly (over 80 years),
and are only rarely ~ccoci~ted with clem~nti~ when present without evidence of de~ul .a~il,g
n~lritç5 (Davies, L, et al, Neurology, 38, 1688-1693, 1988; Delaere, P, et al, Nc~lusci. Lett.,
116, 87-93, 1990; Dickson, DW, et al, Neurobiol. Aging, 13, 179-189, 1991).
The counting of neurofibrillary tangles also fails to give an ~cur~t~ picture of the extent
20 of neurofibrillary degeneration. Again, the presence of large numbers of tangles is invariably
associated with a profound dçmtonti~, and some index of disease severity can be gained from
tangle counts (Tomlinson, BE, Neul~palllol. Appl. Neurobiol., 15, 491-512, 1989; Delaere, P,
et al, Acta Ne~l,u~atll., 77, 645-653, 1989). Electron mi~luscuy~ first revealed that tangles were
cc.lll~osed of masses of paired helical fil~m~-,nt~ (PHF) filling the cytoplasm of neurons (Terry,
25 RD, et al, pages 145-155, In CIBA Fol-n~tion Symposium on ~ c;ll~'s Disease and Related
Con-1ition~ Ed. by Wolstenholme, GEW, O'Conner, M, J and A Churchill, London, 1970).
These studies also clearly showed that degenerating neurites also co-,lai-,cd PHF, a fact only
widely appreciated much later, when the use of antibodies to PHF became common (Wolozin,
BL, et al, Sciçnre, 232, 648-650, 1986). Thus, both the plaques and the tangles of Al~ c 's
SUBSTITUTE SHE-tT (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95114987
Disease contain PHF. The use of antibodies to PHF also revealed the widespread presence of
these paired helical fil~mentc in neuronal processes (Brun, A, Prog. Clin. Biol. Res., 317, 285-
293, 1989~. Thus, the presence of PHF is much more widespread than was first s~lcpecte,l and
is today concidered to be an infliratinn of a widespread neuronal disease, of which the neuritic
5 plaque and the neurr~fibrill~ry tangle are only the most obvious signs (McKee, AC, et al, Ann.
Neurol., 30, 156-165, 1991). It has been ectim~ted that as much ~s 90% of the PHF in the
cortex of the average ~l,l.e~ . case is present in neuronal ~ ce,sses r~ther than in the plaque or
tangle (Wolozin, BL, Ann. Neurol., 22, 521-526, 1987). Much of the early and indeed the
present confusion with regard to ~ gnosic and severity of ~17~ f ~ 'S Disease comes from the
10 failure to a~l, ciale the complexity of the pathology in this disease. ~Ithough the oc~ tnce of
si~ni~r~nt ~ 0~ of PHF in the brain is always ~cso~ tr~ with severe Citl,.f -,l;~ (Hyman, BT,
Current Opinions in Neurology and Neurosurgery, S, 88-93, 1992). These results suggest that
good coll~laLions can be obtailled bcL~ I the conce ~ JII of PHF proteins (COIllf.l;ll~f S referred
to as ,A~ e;--~"'S Disease ~ccoci~d Proteins or ADAP) in the cerebr31 cortex and the degree of
5 tl~ n~;~ measured prior to the death of the patient (Gh~nb~ i, HA~ et al, JAMA, 263, 2907-
2910, 1990).
It is noted that the terminology used to describe the abnormal protein complexescGIll~)osing the fibrillar pathology in Alzheimer's Disease can be confusing. PHF is a
morphological Aecign~ti~n, describing abnormal fil~ment.c found in neurofihrill~ry tangles. An
'~0 abnormal protein species migr~tinP at 68 kDa, elc~,llopho.cucally, and observed on immlmt blots
of tissue from AD brains was termed A68 (Wolozin, B, et al, Science,232, 648-650, 1986). A
protein complex shown to be specific for AD by immnnochemical analyses was design~t~7
~l~h~im~r~s Disease ~coci~le~l Protein or by the acronym, ADAP (Ghanbari, HA, et al, JAMA,
263, 2907-2910, 1990). It is now known that A68 and ADAP, when viewed by the electron
r 2~ microscope, are PHFs. These PHFs have been further shown to contain, primarily, the
microtubule-~ccoci~rt~(l protein, tau. Hence, the terms A68, ADAP, PHF and PHF-tau are often
used int~l~h~l-ge~hly. Thus, they are meant to be interch~n~l~ herein.
A series of important studies have suggested that the progression of ~l7h~im~r's Disease
can be defined pathologically by careful n~ulu~n~lo~ studies of large nU~11~1S of brains from
SUBSTITUTE SHEET (RlJLE 26)
CA 02205586 1997-05-16
WO 96/160~2 PCT/US95114987
elderly hl1m~ns, (Braak, H, et al, Neurosci. Lett., 103, 24-28, 1989; Braak, H, et al,
Ntulu~alh. and Applied Neurobiol., lS, 13-26, 1989; Braak, H, et al, Acta Nc.llupdlll., 82,
239-259, 1991; Braak, H, et al, European Neurology, 33, 403-408, 1993). The Braak's focus
is almost entirely on the dc~, elu~ ,l of neurofihnll~ry pathology, which begins in the ~ ,. h; . .~l
S cortex perhaps years before the clinical signs of the disease are app~cnt. The progression of
A~ F ;~ 'S Disease is thought to be soll~ l,at unllc~ , in the sense that the disease ~ ,a3eS
by the involvement of more and more neurons in the formation of PHF, in an ~n~tomic sequence
which is to some extent pre~iirt~hl~. In the initial stages, only those neurons of the ~ \lo,~
cortex contain PHF, in latçr stages, neurons of the CA1 and CA2 pyramidal layer of the
10 hippocampus contain these StluCLul~,S, and still later, neurons of the ~ccoci~tinn cortex begin to
show evidence of PHF forrnation. Clearly, pl~,.lLion of PHF formation even after the process
is underway in the hippocampus could limit the disease to this brain region, at which point
clinical problems are co, r; r~ to some loss of short terrn Ill~.llul y. Patients first seek help at a
stage when PHF pathology is largely limited to the l~i~oc~ll~us (Berg, L, et al, pages 9-25, in
15 Al,l.~ 's Disease, Eds., Terry, RD; K~r7rn~n, R; Bick, Kl. Raven Press, New York, 1994).
To prevent the pr~ ion of ~l7l.. ~ 's Disease at this point would clearly be a major benefit
to the patient (Davies, P, pages 327-334, in Al,l.- ;...- 's Disease, Eds., Terry, RD; ~t7m~n, R;
Bick, KL. Raven Press, New York, 1994; Kh~rh~tllri~n ZS, et al, pages 445-454, in
,,'s Disease, Eds., Terry, RD: K~t7m~n, R; Bick, KL. Raven Press, New York, 1994).
Attempts to cl~lr-.,.. ;n~ the mol~ocul~r nature of the PHF were h~~ lc;d at first by an
approach which attempted to purify PHF from isolated neurofibrillary tangles. Once
h~cc,l~ulaL~d into the llla ;lulllolecular CO~1CA that is the tangle, PHFs become eAI-~.llely difficult
to soll-bili7.o for protein analysis. The microtubule-acsoci~t~ protein tau appears to be a major
~nri~eniC c~ n~llt of PHF. The repeat region of microtubule-~c~oci~ 1 protein tau forms part
25 of the core of the paired helical fil~m~nt of ~I,l.. ;...-, 's Disease (Goedert, M, et al, Proc. Nat'l.
Acad. Sci., 8S, 4051-4055, 1988; Wischik, CM, et al, Proc. Nat'l. Acad. Sci., 85, 4506-4510,
1988).
The involvement of prolein phosphorylation in the fr)rm~tion of PHF has been su~esteA
by a variety of studies which have shown that tau in PHF is hy~ hf ~l~hs~ ylated (Kosik, KS, et
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96116052 PCT/US95tl4987
al, Ann. Med., 21, 109-112, 1989; Goedert, M, Trends in Neuroscienres~ 16, 460-465, 1993;
Iqbal, K, et al, Act Neurobiologiae E~ t,.li!c~ 53, 325-335, 1993). There is su~ h,~,
evidence that several other ~rolcins, especi~lly neurofil~mrntc and other ll.icluL-~L ule ~soci~trd
proteins (MAPs) are also hy~ ho ,~hr~ylat~d in the brains of patients with ~1,1.~ IIIF~- 's Disease
.~ S (Lovestone, S, et al, Current Opinions in Neurology and ~ u:,ul~,.,,y, S, 883-888, 1992).
In 1985, a monoclonal antibody ALZ50 was raised (produced by hybridoma cell lineATCC No. HB9205) that recognized a 68 kDa polypeptide (termed A68) in imm--nnb1Ots of the
vulnerable brain regions in AD, but not in control brains, (Wolozin, B, et al, Science,232, 648-
650, 1986). T.. nohi~loc~ .. ;r~l analysis with this anti'oody in~'ir~.rçd an intense reaction with
10 neurofibrill~ry tangles and. in later cA~ illJ~nls, with the PHF's, as well. The discovery of A68
was the first report of a bioçhrmically ~ ...i.,e~ abnormal protein species ~ccoci~t~fl with the
fibrillar pathology in AD.
In o~her iaboratories in 198~5, monoc~onal and polyclonal antibodies recognizing the
microtubule-associated protein, tau, were shown to react with neurofibrillary tangles, and
15 subsequently, with PHFs (Grundke-Iqbal, I, et al, Proc. Nat'l,. Acad. Sci., 83, 4913-4917,
1986; Kosik, KS, et al, Proc. Nat'l. Acad. Sci., 83, 4044-4048, 1986). Furthermore,
biocl....,;~l analyses of the ~lutGase-resistant "core" of the PHF d~ oll~L,attd the ~lesencG of
peptides cont~ining the tau selluence, (Goedert, M, et al, Proc. Nat'l. Acad. Sci., 85, 4051-
4055, 1988; Wischik, CM, et al. Proc. Nat'l. Acad. Sci., 85, 4506-4510, 1988). ~cldition~lly~
20 monoclonal antibodies, whose epiLol,eS sp~nnr-l the tau molecule, all were shown to react with
neurofibrillary tangles in AD brain (Kosik, KS, et al, Neuron, 1, 817-825, 1988). Much later, it
was ~letermin~l that the ALZ50 antibody reacts with the e~ cllle amino terrninus of the tau
mrJlecllle (Goedert, M, et al, Neurosciçnr,e Letters, 126, 149-154, 1991).
In 1990, in an attempt to purify A68, a more readily soluble ~ Ou~lion of PHFs was
25 isolated (Greenberg, S and Davies, P, Proc. Nat'l. Acad. Sci., 87, 5827-5831, I990).
Tmml~nnblots of these ~,~uclulcs in~ir~tçd that three closely migrating cleclluyhulclic species, the
slowest migrating of which was ca. 68 kDa in size, all reacted with ALZ50 and tau antibodies
(Lee, V, et al, Science, 251, 675-678, 1991). This in~ic~tç~l that the A68 complex of proteins
SUBSTITUTE Sl IEET (RULE 26)
CA 022055X6 1997-05-16
WO 96~16052 PCT/US95/14987
is, to a large extent, coll.yo3ed of the tau protein. This polymerized form of tau was shown over
the years to be in a hyy~hn~l~horylated state.
Other monoçlon~l antibodies raised against human PHF l,l~aldlions are the antibodies
PHF-1 and TG3. While these antibodies display robust affinity for paired helical fil~m~-
5 ~ alalions~ they only have trace l-,aclivily towards normal adult tau. Further studies revealed
that the mo~oclon~l antibody PHF-1 has a reduced affinity for PHF which has been treated with
alkaline phosphatase or hy~LunLIulic acid, thereby suggesting that this antibodv recognized a
ph~spl.o.ylat.,d epitope(s) in paired helical fil~m~n-c These pho~yllolylated ~,piluyCS (recogniæd
by PHF-1) reside within the C-tçrTnin~l fragment of the tau protein, at serine 396 and 404
10 (Trojanowski, J.Q., et al, Clin. Neurosci., 1, 184-191, 1993). The epitope(s) l~cûg-li~cd by
TG3 has not been mapped yet.
To date, it has not been possible to c ?ncictently induce the fo~nation of ~l71,f ;."f -like
e~iluyes either in vivo or in vitro. Hence~ there is a need for an assay for the ric~r , l l;l~ on of tau
protein, paired helical fil~mf ntc and ..,fcl-~-;cmc involved with ~17hçimf r's Disease. There is
15 further a need for an assay suitable for S~ ling for drugs which may prevent or decrease
Al,l.~ . 's Disease activity.
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCTJUS95/14987
SUMMARY OF THE INVENTION
The co~ -ds of this invention and an al,Lil yscl~otic agent, chlo~ r are active in
an assay indicative of the potential of certain drugs to inhibit a h:~llm~rk trait of ~ 's
Disease. Further, chlol~ e is shown herein to be capable of treating and/or preventing
5 ~l7hçim~r~s Disease. Const.lu~ iy~ the compounds of this invention are useful for treating
and/or ~.c~".Lillg the disease.
The human neurobl~ctom~ MSNla cell line G~ 5CS the plvt~;ns known to be involvedin PHF formation (inclllfling tau, other MAPs and neurofil~mf nt plc,Le;~,s, and thus is a cell line
in which ~l7llf ;...l 's Disease e~iLo~e,s can be G~ ,ssGd. (Arias et al, J. Ne.,-~he,ll., 61: 673-
10 682, 1993; Vincent et al, J. Nc.~ ., 62: 715-723, 1994).
Immunohistochemi~ ~l st~inin~ of brains from normal and Alzheimer's Disease (AD)patients using the monoclonal antibodies PHF-1 and TG3 inrii~pr~s that these monoclonal
antibodies specificallv stain c~ of AD brain, but not normal brain (hgure 1). C`onrlitions
were est~klich~od which led to hyperphosphorylation of the proteins known to be involved in
15 PHF formation. Using monoclonal antibodies PHF-1 and TG3 generated to human PHF
aldtions, the effects of a variety of manipulations of the pho5phorylation state of these
proteins were tested. These tests c~luilcd that most of the aLncl ,lldl c~ilo~es l~,co~ cd by these
monoclonal antibodies in the human ~l7h~imer brain should be gcll~ tcd in the MSNla cells.
The llc~ nr of MSNla cells with okadaic acid and other selecte~ inhibitors of protein
20 phosphatases has been found to generate the epitopes associated with these ~l~h~im~:r brain
specifi. monoslona! antibodies, thereby meeti;.g, the re4ui~cil.c.its described above. These
e~i~s are either absent or present at a very low level in ~Illllc~Lt~d MSNla cells.
The AD specifi~ity of the PHF-l and TG3 ~ntiho~ s was also demonstrated using anELISA assay. Shown in Figure 2A are bar graphs (percent of AD) of the ELISA
25 immllnoreactivity of brain plc~dlions (AD or normal) to either PHF-1 or TG3. Shown in
Figure 2B are bar graphs (percent of MSN+) of the ELISA immnnoreactivity of MSNla cell
Jaldlions (minus or plus okadaic acid) to either PHF- 1 or TG3. The MSNla cell ~ ~dlions
plus vs minus okadaic acid closely parallel the AD vs normal brain l,lep~ ;o~c This clearly
shows the relationship belv~ecn disease pathology (i.e. AD brain) and what is recognized by the
SUBSTITUTE SHEET (RULE 26)
CA 0220~x6 1997-0~-16
WO 96116052 PcrluS95/14987
antibodies in the MSNla in vilro cell culture assay. The cell culture system according to the
LiOIl in which ~.p;~f,S C~ af`~ tiC of PHF can be readily ge~ tf -i and sul: s~ y
l~eeo~ i by the specific ~1~1.. ;,.... brain specifie antibodies provides an assay suitable for
screening cu~ oullds for their ability to block the ~luJI~ l;ol. of these pi~osL.hfj ylated c~itupcs.
PHF tau protein has lower affinity for microtubules as colllpdlcid to normal tau protein,
which is likely the result of the abnf)rm~l pho~ .ulylatiûn (Lindwall et al, J. Biol. Chem., 259:
5301-5305, 1984). As a co..se~l~le..ce, microtubule ~lest~kili7~tion occurs which i~telr~..,s with
illll,c,lia.ll neuronal ~lucesses such as rapid axonal Llansl,uli and, subsequently, this process
leads to the follllaLiol. of PHF and the neurofibrillary tangles (Bancher, et al, Brain Res., 477,
10 90-99, 1989). Thus, inhibition of the ~ iul,l;on of the abnorrnal phosphorylated PHF f~)iL~)e S
will i~ .r~.e with and/or prevent the fO..l-atioll of PHF. Since PHF formation is a major feature
of 1~ f l pathology, the development of co~ ol"~ds that intu.rcl~ with all or part of this
process inhibit or prevent the ~lu~ ion of this disease.
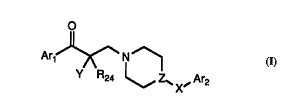
The invention provides co-l-yuul-ds of formula I:
O
Ar~ N ~~
Y R24 ~ Z~ X~ Ar2
I
or the l,h~ e~ ;c~lly ~cceptA hle salts thereof wherein
X is a c~ L~nyl, sulfonyl, methylene, or methylene ~ ,sl ;l l l~. cl with optionally snh~l ;n ~l-,d
phenyl; and
Z is nitrogen or C~I; and
Arl and Ar2 in~rp~.,Ae.~tly ~ylcis~n~ aryl groups; and
R24 is hydrogen; or when Y is other than hydrogen, R24 is hydrogen, loweralkyl, or
optionally ~ l;nllrA phenyl; and
Y is hydrogen, or Y and a substit~Pnt on the Arl group togethem~l~sellt a CH2.
CH2CH2, CH20, or CH2S group to form a five or six l-~-.-~.~d ring where the ring is
optionally slll~ llrA with loweralkyl orphenyl.
-8-
SUBSTITUTE SHEET (RULE 26)
,
CA 02205586 1997-05-16
WO 96/160S2 PCT/US95/14987
These co~ )(,ullds are useful for treating ~l7heimçrs Disease neoplastic disease and
bat~teri~1 or fungal infections. Thus these coll,~ou,lds are antilleoplastic. ~nrihacterial. and
5 ~ntifim~l agents.
The coll",~)u"ds of this invention by preventing or decreasing the production ofah.l.~. ",~11y phnspht. ~ldl~d paired helical fil~mt m e~ u~es ~csoçi~rt t1 with the prot1l~ctin~l of the
neurofibri11~ry tangles, a cl,a. .r~ ;r pathological feature of ~l,l.- h..~ s Disease. are useful in
the Ll~ of this n~ cleg~ .- alive t1i~nrt1er.
The colll~ullds of forrnula I inhibit and/or prevent the formation of abnornallyphosphorylated epi~opes on PHF tau. Through inhibitory il~L~ ~acLions of the phosphorvlation
events associated with the forrnation of PHF tau and other proteins involved in PHF ~",~I;Ol"
one can rno~ te the ~çmhly of tnicrotubules, thereby affecting neuronal processes such as
rapid axonal transport, thus preventing the forrnation of PHF and the production of the
15 neurofih ill~ry tangles. Thus, the co",~ou,~ds of the present invention are lhc. .~ 11y useful
in various neurodeg.--.~ aLive disorders such as ~ ;".t~ s Disease. In ~t1tlition the com~ou"ds
of the present invention are useful for the ll~allll nl of certain neoplastic ~ e~es. and can be
used as ~nti~rt~ori~l and antifun~l agents.
Thus the invention provides m~oth xls of ~ e.i~ii,g and/or treating ~ s Disease
20 in a patient comprising ~ mini~tering to the patient a co",pou,.d of formula I in an amount
effective to inhibit forrnation of ah,-- ",~11y ~ho~l,o,ylated paired helical fil~m~nt epitopes.
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
Wo 96/16052 PCT/US95/14987
BRIEF DESCRIPTION OF THE DRAWrNGS
Figures lA-lD are images produced by light miclùsco~,y showing immnnoh~ e...~
staining of brain tissue from normal ~l~heim~ s Disease patients with monoclon~l ~ntibo~lies
PHF-l and TG3.
SFigure 2A is a graphic CO~ O.I of normal (Norm) and Al,l.P:..... 's Disease (AD) brain
hu...ngf ~t.o. ;...~ ,acLiv-ity toward PHF-1 and TG3 antibodies ~I,~sscd as a percent of total
AD ;~ a~;livity.
Figure 2B is a graphic cu---~ ;con of MSNla cell lysate (minus or plus okadaic acid)
immllnoreactivity toward PHF-1 and TG3 antibodies expressed as a percent of total
10 ;~ U~ a~,livi~y in lysates of MSN+ okadaic acid.
Figure 3 is a western blot showing PHF-1 ;....... ~ ,.eactivity in Iysates from control and
chloll,lo...~ trea~ed MSN cells treated with okadaic acid.
Figure 4A is a chart showing relative ADAP conc~.~udLions in Brodman Area 10 in
's Disease p~tient~, normal controls and patients treated with chlul~lo---~ (Rx).
Figure 4B is a chart showing relative ADAP col-~e.~dlions in Brodman Area 38 in
.Al,l.~;...~ ~ 's Disease pati~ont~l normal controls and patients treated with chl~ 7;~
-10-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
Wo 96/16052 PCT/US95/14987
DETAILED DESCRIPrION OF THE INVENTION
The invention provides colllyuu,lds of formula I or the pharm~eut~ y acceptable salts
thereof wherein
X ~ carbonyl, sulfonyl, methylene, or methylene ~ulJ~1 with optionally ~ul
. 5 phenyl;
Z is ni~u~c.l or CH with the proviso that when Ar1 is phenyl or ~ phenyl Z is ni~UO_.l;
Arl is a group of the forrnula:
R2R~
wherein
Rl, R2 and R3 in~epen~l~ntly ~y.~sent hydrogen, fluoro, iodo, phenyl, nitro,
trifluoroaL~cyl, thioloweralkoxy, cyclohexyl, amino, acetyl, morpholino, cyano,
piperidinyl, trifluoroalkoxy, alkylsulfonylamino, optionally substitllted
phenylethynyl, 4~s-~ih~loimi~l~7ole~ optionally suhstitl-te(l phenoxy, optionally
~ d phenyl amino, optionally sul~ 1 phenyl~lfi~1e, provided that Rl,
R2 and R3 are not all hydrogen; or
two of R1, R2 and R3 taken together can form an ethvlenedioxy, ethyleneoxy, or
a lower straight alkylene chain alkyl bridge of 3-5 atoms op~ionally ~lb~ cl by
loweralkyl; or
Rl, R2 and R3 are the same or dirr~.cl~- and lGyl~s~n~ a group of the fo~
l~N J~; or
Ar1 is a group of the forrnula:
SUBSTITUTE SHEET (RULE 26
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
r\ S~ ~R2s R7~$
R23 R23 ~
R26_N~N~R R25 N)~N~ 50~--R 54--R
R23 /~ R23~ ~R23
R23~_R23 R23--~N3--
R27 R27
~R23 ~f~ R28~ R2-~R23
R6~o R~
or ~0
wherein
X2 is oxygen or sulfur;
Rs is hydrogen, halo, loweraL~cyl, loweraLIcoxy or thioloweraL~coxy;
R6 is hydrogen, halo, or lowera~cyl;
R7 is hydrogen, lowerakyl, halo, nitro, cyano, optionally substitl1t~ri thienyl, 5
optionally substitllteci phenyl, morpholino, piperidinyl, piperazinyl, optionally
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
substituted phenylmethylenylpiperazinyl, optionally substituted
phenylsulÇonyl~ h~yl, or optionally sllhstitl~t~o~ phenylca~ lyl~ ,.~illyl,
R8 is loweralkyl or phenyl;
Rg and Rlo are independently selecte~l from hydrogen, halo, loweralkoxy,
f 5 IIYC1~VAY;
R14 is s~lect~ from hydrogen, loweralkyl, nitro or halo;
Rlg and R20 are intle~e fie..lly select~l from hydrogen, loweralkyl, halo, nitro,
oroptionally ~.b~ d phenyl;
R 21 is hydrogen, loweralkyl, nitro, halo, cyano, thienyl, phenyl, or
phenylethynyl;
R22 is hydrogen, C~U ;~I1~ hUAY~ optionally ~.lbsl ;l ~.lrd phenyl or loweralkyl;
R23 is hydrogen or loweralkyl;
R24 is hydrogen; or when Y is other than hy~ug~ll R24 is hydrogen, loweralkyl,
or optionally ~ d phenyl;
R25 is hydrogen, loweralkyl or thio~lko,lcy;
R26 is loweralkyl, optionally substitutecl phenyl or optionally sllb~
phenylmethylenyl; and
R27 is loweralkyl, optionally subsl;~ e~ phenyl or optionally sub~itulcd
ph~.lylln~ ylenyl; and
R28 is hydrogen, optionally substitute~ phenyl, loweralkyl, or optionally
A .t.hieny!; ~nd
R2g is hydrogen, nitro, or loweralkyl; and
provided that R7, R14 and R2g are not all hydlugell; and
providcd that R23 and R28 are not both hydrogen; and
Ar2 is a group of the formula:
R
r ~Rl Z
~UBSTITUTE SHEET (~ULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95tl4987
where Rl 1 and R12 independently lc~ulcsent hydrogen, halo, trifluo~u,l~ yl,
amino, nitro, cyano, acetyl, thioloweraLIcoxy, or loweralkyl;
or Rl 1 and R12 together form a methyl~ncdioxy or ethylen~~ yy bridge; or
Ar2 is naphthyl, optionally su~stitntP~l thienyl, furyl, 1~2~3-thi~Ai~7olyl~ or a group of the
S fn~m-lR
CF~ R4
wherein
R4 illd~p.,.ldently lc~ scnts lly~gen, halo, or loweralkyl;
R13 is selected from hydrogen or halo; and
Y is hydrogen, or Y and Rl, R2, R3, R1g, R20, R22, R23, or R2g together lc~l~se.lt
CH2, C(R24)2cH27 C(R24)2o~ or C(R24)2S forming a five or six membered ring.
f~.l~ coll~.uul,ds of formula I are those where Arl is a group of the formula:
R1~,~
R2~J
R3
wherein
Rl, R2 and R3 in~epen~ently represent hydrogen, phenyl, nitro, trifluoroalkyl,
thioloweraL~coxy, cyclohexyl, amino, acetyl, morpholino, cyano, piperidinyl,
trifluoroaL~oxy, arylsulfonylamine, alkylsulfonylamino, optionally substituted
phenylethynyl, 4,5-dihak>imi~701e, optionally sl1bstitllt~rl phenoxy, optionallysubstit~ted phenylclllfi~le, provided that Rl, R2 and R3 are not all hydrogen; or
-14-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
two of Rl, R2 and R3 taken together can form an ethylenedioxy, ethyleneoxy, or
a lower straight alkylene chain aLkyl bridge of 3-5 atoms optionally ~u~ t~A by
lowe~aLkyl;
Z is N; X is CH2; and Ar2 is sn~l ;lul~ phenyl.
Most pl~,r~ ,d culll~ounds of formula I are those where Arl is nilluphe.lyl. Other
~ul~ d colll~ûunds of formula I are those where Arl is iodo- or fluc~luphellyl. ~ef,.l~d iodo-
or nulu~h~llyl colllpûullds are those where the halogen is in the para position with respect to the
plu~ ol~e group. More ~ ,ll~ iodo- or fluluphellyl co~ uunds are those where the halogen
is in the meta position. Particularly ~ d iodo- or Ilulu~hc.lyl colll~oullds are those where
10 the halogen is in the ortho position.
~ ef~ d c~.lllpou.lds of formula I having a het-,.u~uyl group as the Arl sut-stitllent are
those where Arl is N-arylsulf~,nylpyllulyl, more preferably optionally s~ d isoxazolyl,
and still more plci~,ably, optionally ~"~ "I A thiazolyl. Most ~IGr~ d colll~oullds of formula
I having a hot~,-v~yl group as the Arl substinle~t are those where-Arl is optionally ~ .Jt~
15 thienyl. E~Cr.,ll~d colll~ùullds of formula I where Arl is optionally sllhstit~lto-d thienyl are those
where X is CO. Particularly pler.,l,~d col"~oullds of formula I where Arl is optionally
s,.l,~l;l.,t~A thienyl are those where X is methylene.
The invention further provides cc,l,lpounds of foîmula II:
Ar1J~ N ~
1 Z~ X~ Ar2
wherein
X l~ ,StnL~ Cdll~llyl, sulfonyl, or methylene;
Z is ni~ug~ll or CH with the proviso that when Arl is phenyl or s~ ;lul~l phenyl Z is nitrogen;
25 Arl is a group of the formula:
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
WO 96/16052 PCr/US95/14987
R,~
R2
R3
wherein
Rl, R2 and R3 il~d~ n~ y l~,~cse.l~ hydlug~n, fluorine, iodine, phenyl, nitro,
I-inuul~alkyl, thioloweraL~coxy, cyclohexyl, amino, acetyl, morpholino, cyano,
piperidinyl, trifluoroalkoxy, alkylsulfonylamino, optionally substituted
phenylethynyl, 4,5-dihaloimin'~7ole, optionally substitut~q' phenyl amino,
optionally snb~ d phenoxy, optionally substituted pheny1~lllfin7e~ provided
that Rl, R2 and R3 are not all nyd-ùg~,.l, or
Arl is a group of the formula:
or R1~;
wherein
R6 is hydrogen, halo, or loweralkyl;
Rg and Rlo are independently selected from hydrogen, halo, loweralkoxy,
hydroxy; and
Ar2 is a group of the formula:
R11
~R12
where Rl 1 and R12 independently l~plesent hydrogen, halo, trifluolulllelllyl,
amino, nitro, cyano, acetyl, thioloweralkoxy, loweralkyl, or taken together forrn
a methyleneaioxy bridge.
-16-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
Wo 96/16052 PCr/US95/14987
The invention also provides co~ uullds of formula m
Ar
s m
wherein
Rl 1 and R12 ind~ ie~nly ,c~,~,se.~l hydlugcn, halo, l~ifluun"llcllly-l, nitro, cyano, acetyl,
amino, thioloweralkoxy, loweralkyl, or taken together form a methylenedioxy bridge;
Arl is a group of the formula:
R~
R2~J
R3
wherein
Rl, R2 and R3 in~epçndlontly lG~ ;S'eU~ hy..l-ugc~, fluorine, iodine, phenyl, nitro,
trifluoroalkyl, thioloweralkoxy, cyclohexyl, acetyl, amino, morpholino, cyano,
piperidinyl, loweralkyl, trifluoroalkoxy, arylsulfonylamine, alkylsulfonylamino,optionally su~s~ çd phenylethynyl, 4,5- iih~loimir~7t~le, optionallv ~I,h~
~}~_-,o~y, optionally ~ !;llllr(~ phenyi.culfi~e, provided that Rl, R~ and R3 are
not all hydrogen; or
Rl, R2 and R3 are the same or dirrc~cnL and lc~lcS~ L a group of the forrnula:
~N~
SUBSTITUTE SHcET (~ULE 26)
~=
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
The invention further e.~ro, ~ cces cull.~unds of Formula IV:
~~ ~N~Ar2
02N
IV
wL~ Ar2 ~c~lescnl~ phenyl or phenyl mono or riicub~stitlltpc~ with halogen, cyano, nitro, or
optionally s~ thienyl groups.
The invention further en~o~ cses culn~u,,ds of Formula V:
R3 O
R2~N~
~S ~N~x,Ar2
Rl
V
wherein
X f~ sellla c~ul~llyl, sulfonyl, or methylene; and
Rl lcpl~,sellts hydrogen, loweralkyl, halogen, cyano, nitro, optionally substituted
thienyl, optionally substinlt~i phenyl, morpholino, piperidinyl, piy~,~a~ yl7 optionally
substituted phenylmethylenylpiperazinyl, optionally substituted
phenyl~ulr~"lyll.;pr.i.7;..yl, or optionally substih-tetl phenylc~u-AJ"yl~ip~ lyl; and
R2 is hydrogen, nitro, loweralkyl or halogen; and
R3 is hydrogen, nitro, or loweralkyl; and
provided that not all of Rl, R2 and R3 are c;mlllt~ntoollcly hydrogen; ar d
Ar2 represents thienyl, phenyl or phenyl mono or riicllbstituted with halogen, cyano,
nitro, loweralkyl, or loweraLlcoxy groups; or Ar2 is a group of the fnrrnlli~
~Rs
-18- = = =
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCTIUS95/14987
wl~ ,;n R4 and RS are indepenrl~ntly selected from hydrogen, h~lo, or loweralkylgroups.
Re~.~,stl,ld~i~e ~.ef~..~ cc,.l~ou.lds of formula V are those where Arl is a thienyl group
s~ t. A as follows:
02N
Cl_~~5sS Brl~ 55s 02N~5SS
~ H3C~ j~,
s 02N--~ NC ~ SCH3
The inven~ion further ~ o,..l~c.cbs comyvullds of Forrnula VI:
R~ ~ X'
Vl
wherein
X ~ c:sel.ls carbonyl, sulfonyl, or methylene; and
Rl ,~ sel.t~ halogen, loweralkyl, nitro, or cyano; and
Ar2 le~r~se-,Ls phenyl or phenyl mono or disubstituted with halogen, cyano, or nitro
groups.
The invention further ellco.~ cces cum~ou.1ds of Forrnula VII:
-19-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCI~/US95/1~987
R2 ~N~ ,.
~S ~N~Ar2
R,
VII
wherein
Rl and R2 are independently selected from hydrogen, halogen, loweraLkyl, nitro, or
S optionally ~.u~ lr~ phenyl; and
Ar2 ~ scnts phenyl or phenyl mono or riicuhstitute~ with halogen, cyano, nitro,
loweraL~cyl or loweralkoxy groups.
Re~ senlative ~lcîtll~,d compoullds of formula VII are those where Rl and R2 arehydrogen
The invention further encomp~cses cOlllpC ull~S of ~o~nula VIII:
R1
N~N~
~N~x,Ar2
vm
wherein
X lc,vl~ ,ents carbonyl, or methylene; and
R1 l~,~lcs7cllL~. hydrogen, optionally sllhstinl~qd phenyl, C~I~I11~LhCSAY7 or loweralkyl; and
R2 Lyd~ ,n, or loweralkyl; and
Ar2 represents phenyl or phenyl su'Qstit-lted with halogen, cyano, nitro, loweralkyl, or
loweralkoxy groups.
Re~l~,se.l~ti~e ~lcf~,.l.,d cc.lllp.Jul.ds of formula VIII are those where Rl is phenyl and
R2 is methyl.
The invention further encomp~cces compounds of Formula IX:
-20-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCr/US95114987
R1--<~N~
c S ~ N~, Ar2
IX
wherein
Rl lcpl~,scnts hydl.,gen, phenyl, or phenyl sub~litul~d with halogen, cyano, or nitro
groups; and
Ar2 l~,~,csent~ phenyl or phenyl s~lbs-itnt~i with halogen, cyano, nitro, loweralkyl, or
loweralkoxy groups.
The invention further encc,~ ac.ces co,lll,ou,lds of Forrnula X:
R1~N~
N--O ~ N~, Ar2
X
wherein
Rl lcpn,sents hydrogen, optionally substituted phenyl, c3~ llcLiloxy, or loweralykyl
groups; and
Ar2 represents phenyl or phenyl sllbstitnte~ with halogen, cyano, nitro. loweralkyl, or
loweralkoxy groups.
The invention further er~comracces colllyoullds of Formula XI:
--N~
S2 ~N~X~Ar2
-21-
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 l997-0~-l6
Wo 96/160S2 PCItUS95/14987
wherein
X r~ .,Ls carbonyl, sulfonyl, or methylene; and
Ar2 re~,Sel~l~ phenyl or phenyl subs*tll~e~i with halogen, cyano, nitro, lowerallcyl, or
loweralkoxy groups.
The inven*on further e~ro".l~c~es co,.,l~ull~c of Forrnula XII:
~N~
)~ O ~,N~, Ar2
R,
XII
wherein
R~ ,S~illL:~ optionally s~lhsl;L~ phenyl, op*onally ~ub~ d thienyl orloweraLcyl
groups; and
Ar2 r~ sel,L~ phenyl or phenyl sllb~ A with halogen, cyano, nitro, loweraLI~yl, or
loweralkoxy groups.
The invention further ~.nr~,l"l ~cces cc~ ounds of Forrnula XIII:
~N~N~ Ar2
XIII
wherein
R~ csel,ts hvdrogen, halo, thioloweraLkoxy, loweralykyl, or loweralkoxy groups;
and
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
Ar2 lc~lcse.ll~ phenyl or phenyl subs~ with halogen, cyano, nitro, loweraL~yl, or
loweraL~coxy groups.
The invention further e ~oll ll.hc.cf s ~olllyounds of Forrnula XIV:
S
NO ~N~f 3 R1
~3 R2
XIV
wherein
Rl and R2 in~f penrlf nrly lc~l~,sent hydrogen or h~log~n
The invention further e'n~ CCeS COlll~)vullllS of Forrnula XV:
R1~` N ~ ~N~, Ar2
XV
15 wherein
R1 and R2 infi~ pf...rl~ y lc~c;~cnt hydrogen, nitro or halogen; and
Ar2 lc~lcsenl~ phenyl or phenyl ~LIb~Lulcd with halogen, cyano, or nitro groups.
The invention further encomp~scec col~lyoul1ds of Forrnula XVI:
-23-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCI`/US95/14987
R2~=~R3 1--N~ ~Ar2
R1--N`N~ `J
XVI
wherein
R 1 is loweralkyl, optionally substituted phenyl, or optionally substituted
~lle.. yll~ ylenyl;and
R2 and R3 are hydlu~,~,n or lower alkyl; and
X lel,.esen~ carbonyl, sulfonyl, or methylene; and
Ar2 l~V~SclllS phenyl or phenyl sub~ lerl wi~h halogen, cyano. nitro, loweraLlcyl, or
loweraL~oxy groups.
The invention further e-~o~ cs culll~uullds of Formula XVII:
R ~ N ~
R _N~ R3 ~ N~ X~ Ar2
XVII
wherein
R 1 is loweralkyl, optionally substituted phenyl, or optionally substituted
phenylmethylenyl; and
R2 and R3 are llydlu~ or lower alkyl; and
X ~ s~.lLs carbonyl, sulfonyl, or methylene; and
Ar2 IG~lcsenl~ phenyl or phenyl substitllt~-l with halogen, cyano, nitro. IoweraL~cyl, or t
loweralkoxy groups.
The invention further e..~-....l.~c~es co-l-~ou,-ds of Formula XVIII:
-24-
SUBSTITUTE SHEET (RULE 26
CA 02205586 1997-05-16
WO 96/16052 PCr/US95/14987
(~ ~Z~ , Ar2
xvm
wherein
Z is CH or nillug_.n,
S X is carbonyl, sulfonyl, or methylene;
M is CH2, C(Rl)2CH2, C(RI)2O, or C(RI)2S;
Rl is hy~llu~e,l, loweraL~cyl, optionally ~ d phenyl;
Ar2 is optionally sllb ~ d phenyl, optionally Sl11~ r~ thienyl, or furyl; and
("the A ring") lc~l~se.ll~. an aryl or ncter~,~yl ring having from zero to three
he~ero atoms selecteA from the group cQncicting of oxygen, sulfur and nitrogen.
Aryl and heteroaryl groups l~le~.e.,~d by the A ring are aromatic or hetcr~3uulna~ic
syste ns rl, ..~rt~ d by 4n+2 7~ electrons.
The invention further e.,( l~lllr~7cses cc,~ uunds of Formula XIX:
R_~ ~ X'
R1
XIX
wherein
Z is CH or lli~lugC~l; and
X is carbonyl, sulfonyl, or methylene; and
M is CH2, O, S, or a direct bond; and
Rl is hydrogen, loweralkyl, optionally substin~trA phenyl; and
-25-
SUBSTITUTE SHEET (RULE 2~)
_
CA 02205586 1997-05-16
WO ~6/16052 PCT/US95114987
R2 and R3 are i..~- y,~ nt1y sel~cl~d from hydrogen, halogen, loweralkyl, nitro, cyano,
alkylsulfon~mi~lo, arylsulfon~mido, optionally s~ phenyl, or R2 and R3 taken
t~,~ e. ~I,ylcsel~L a methylhlc~io~ ring; and
Ar2 is optionally sl1b~ ;n ~lr~ phenyl, optionally ~ thienyl, or furyl.
.
The invention further en~rJ~p~cses coll~uullds of Formula XX:
R3 ~ N~z~ X' Ar2
XX
wherein
Z is CH or nitrogen; and
X is c~ul,onyl, sulfonyl, or methylene; and
M is CH2, O, S, or a direct bond; and
Rl is hydrogen, loweralkyl, option~11y Sl1b~ 1 phenyl; and
R2 and R3 are il,dey."ldently selected from hydrogen, halogen, loweralkyl, nitro, cyano,
or optionally s1~bstit-1t~A phenyl; and
R4 is hydrogen, loweralkyl, optionally substituted phenyl, or optionally substituted
ph~l,yLllcL}l~lenyl; and
Ar2 is optionally ~.u~iLuL~d phenyl, optionally substirl-t.od thienyl, or furyl.0
The invention further e.~co, .p~cceS colll~oul,ds of Forrnula XXI:
R 1,
~ N
R3--N
N ~ M ~ R 1 ~ Z~ X~ Ar2
XXI
-26-
SUBSTITUTE SHEET (RULE 26
,
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
wherein
Z is CH or ~ ug~ , and
X is carbonyl, sulfonyl, or methylene; and
M is CH2, O, S, or a direct bond; and
S Rl is lly~L~Jg~"~, loweralkyl, optionally s~lkal;lu~-A phenyl; and
R2 is hydrogen, halogen, loweralkyl, nitro, or cyano; and
R3 is hydrogen, loweralkyl, optionally subsl;l.,l.,d phenyl. or optionally sub~
phenyL~ l,yle,.yl, and
Ar2 is optionally ~..h~ phenyl, optionally sub~l ;l it~.l thienyl, or furyl.
The invention further .oncomr~cces coll,~x>ullds of Fonnula XXII:
o R1
R2~ X~
R1
XXII
wherein
Z is CH or uillugf;n; and
X is carbonyl, sulfonyl, or methylene: and
M is CH2, O, S, ûr a direct bond; and
Rl is hydrogen, loweralkyl, optionally Su'c~ ;llllf ~ phenyl; and
R2 is hydrogen, halogen, loweralkyl, nitro, cyano, optionally substituted phenyl,
alkylclllfon~miAo, arylsulfon~miAc)7 or thioloweralkyl; and
Ar2 is optionally sub~ ut.,d phenyl, optionally subs~ led thienyl, or furyl.
The invention further encomracses colll~oullds of Fo~nula xxm
SUBSTITUTE SHEET (RULE 2B)
CA 0220~86 1997-0~-16
WO 96116052 PCr/US95/14987
~Sx~ N~
XXIII
wherein
Z is CH or nitrogen; and
S X is ca~ lyl, sulfonyl, or methylene; and
M is CH2, O, S, or a direct bond; and
Rl is hydrogen, loweraLcyl, optionally ~k~ rl phenyl; and
R2 is hvdrogen, halogen, loweralkyl, nitro, cyano, optionally substit1lte~i phenyl,
alkylsulfonamido, arylsulfon~mi~o, or thioloweraL~cyl; and
Ar2 is optionally sul~ A phenyl, optionally sul~ lr~ thienyl, or furyl.
The invention further e ~ , . .p~cses cc,lllyOullds of Formula XXIV:
R2~ ll R1
R3~ ~Z~ X~ Ar2
XXIV
wherein
Z is CH or nitrogen; and
X is carbonyl, sulfonyl, or methylene; and
M is CH2, O, S, or a chçmic~l bond; and
Rl is hydrogen, lowe~L~cyl, optionally sub~LiLuLed phenyl; and
Y is O or S; and
R2 and R3 are independently selected from hydrogen, halogen, loweraLlcyl, nitro, cyano,
or optionally i~b~ lr~ phenyl; and
-28 -
SUBSTITUTE SHEET (RULE 26
CA 02205586 1997-05-16
WO 96/16052 PCr/US95/14987
Ar2 is optionally suk~ e~ phenyl, optionally sl1hstitut~rl thienyl, or furyl.
The instant invention in~ os optically active cc .lll,oul,ds as well as racemic I~ ,s of
such co~ oullds. The invention further in~ es the pk,.. ~r~ ~,I;e~lly acceptable salts of such
compounds or racemic ll.i~lu.es. The invention still further includes pharm~eutiral
COlllyOC l l l~ c,
As used herein, the term "loweralkyl" means straight or branched chain saLuldud
hyd~ uL~n radicals having 1 to 4 carbon atoms, such as methyl, ethyl, n-propyl, ~-butyl and the
10 like.
As used herein, the term "thioloweralkoxy" refers to -SRIs, wherein R1s is loweralkyl.
As used herein, the term "halo or halogen" refers to fluorine, chlorine, bromine, or
iodine.
As used herein, the term "aryl" refers to systems ch~dc~ ed by 4n+2 ~ electrons, i.e.,
15 aromatic carbocyclic groups having a single ring (e.g., phenyl), mnltirle rings (e.g., IJil~h~nyl)
or m~lltirlP conflence~i rings in which at least one is aromatic, (e.g., 1,2,3,4-tetrahy~u-laphthyl,
naphthyl, anthryl, or phe-n~nth yl), which can optionally be u~ ~ or Sub~ 'lled with
e.g., halogen, lower alkyl, lower alkylthio, t.inuulull.~,.l-yl, lower acyloxy, aryl, and hetelua.yl.
As used herein, the term "heteroaryl" means ~, 6, or 7 membered aromatic ring systems
20 having at least one hetero atom selected from the group consisting of nitrogen, oxygen and
sulfur. Examples of heteroaryl groups are pyridyl, pyrimidinyl, pyrrolo. pyrazolo, pyrazinyl,
pyridazinyl, oxazolo, furanyl, quinoline, isoquinoline, thiazole, thi~ 7nle, isoxazole, and
thienyl, which can optionally be ~ncukstitntPA or ~b~,l;Llll~ with, e.g., halogen, hydroxy, lower
alkyl, lower alkoxy, lower alkylthio, trifluoromethyl, lower acyloxy, cyano, nitro, aryl, and
25 heteroaryl.
As used herein, the term "alkylsulfonylamino" means R16S(0)2NR17 - wherein R16 is
loweralkyl, phenyl, or suhstit~ phenyl, and R17 is hydrogen, loweralkyl or -S02R16.
-29-
SUBSTITUTE SHEET (RULE 26)
-
CA 0220~86 1997-0~-16
WO 96/16052 PCr/US95/14987
As used herein, the term "tnfluoroallcoxy!' refers to -R1gCF3, wherein R1g denotes a
loweralkoxy group as defined above, atrAehed to the parent molecular moiety through the oxygen
atom. Rel"~ sen~ es of such groups include mr thlr~nr oxy, ethyleneoxy and the like.
As used herein, the term "optionally substinlte~ phenyl" refers to a phenyl ring having
5 from zero to three substiturntc independently selected from loweralkyl, halogen, hydroxy,
loweralkoxy, am~no, thioloweralkoxy, nitro and cyano.
As used herein, the term "optionally substituted thienyl" refers to a thiophene ring with
from zero to three sllbs!;~ r~ inriepen(irntly selected from loweralkyl, halo, phenyl, cyano and
nitro.
As used herein, the term "optionally substituted phenoxy" refers to the phenoxy ring
;I- I~A as defined foroptionally s~ ~ phenyl.
As used herein, the term "optionally s,.b:,lin.led phenylsulfide" refers to a thioph~nyl ring
..I.~I;n.t~A as defined from optionally su~ t~ ~ phenyl.
The terrn "~hA..n~ Ally a~ceptAhle salts~' refers to the phz1rm~ceuticAIIy acceptable,
15 leld.i~,~ly non-toxic, inorganic, or organic acid addition salts of the co.ll~uuu,-ds of this invention.
These salts can be pl~?d,Cd in situ during the final icolAtir n and pnnfirAtion of the com~uullds or
by sepz~r~t~ly reacting the free base with a suitable organic or inorganic acid. Rc~,~se.,ldlive salts
include the hydrochloridr7 hydlub~ ide, sulfate, phosphzltç, nitrate. bicn1fzltç~ acetate, oxalate,
valerate, oleate, palmitrate, mr~th~Anesulfonate~ stearate, laurate. borate, ben70At~, lactate,
20 phosphate, tosylate, citrate, mztlrAt~ ru",a.a~e, ~uc~.-inAIr tar~rate. napsylate. lactobionate, and the
like. It will be app&cl~L to those skilled in the art that, depending upon the number of available
amino groups for salt formation, the salt of this invention can be per N-salts.
The present invention also provides pharmzl~euti~AI cc,lllposiLions which comprise one or
more of the culllpoul,ds of fonnnlzle I-XXIV above formlllAt~od together with one or more non-
25 toxic phAnmAreutirAlly acceptable carriers. The pharmzl~ ellticAl co~ x~iLions may be specifirz~llyfi~nmlllzAt~ for oral a~minictration in solid or li~uid fiorm, for ~al~.ne~dl injection, or
for rectal
~rlmini5tration.
The phArmAreutirZ~l compositions of this invention can be A-lministered to humans and
other zlnimAIc orally, rectaly, parenrerally (i.e. intravenously, intramuscularly, or sub-
-30-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/160!;2 PCT/US95/14987
cutaneously), intracicterll~lly, intravaginally, intraperitoneally, topically (as by powders,
Oi.. l.. lc or drops), t,,i,-cA~.. ~lly, bucally, or as an oral or nasal spray.
Pl,~...A~eutil~l coll,~ositions of this invention for parenteral injection comprise
pl~ e~ lly acceptable sterile aqueous or nonagueous solutionc~ dispersions, sucpçn~ionc
S or emulsions as well as sterile powders for l~,con~ n into sterile injectable solutions or
;o~c just prior to use. F~r~mplec of suit~ble aqueous and nona~ueous carriers, rlilu~ntc,
solvents or vehicles include water, eth~nol, polyols (such as glycerol, propylene glycol,
polyethylene glycol, and the like), and sni~h~ Lul~S thereof, veget~hle oils (such as olive
oil), and injectable organic esters such as ethyl oleate. Proper fluidity can be m~int~ined, for
10 er~mrle, by the use of coating m~t~ri,~l.c such as lecithin, by the n~int ..~n-e of the required
particle size in the case of ~ ;onc~ and by the use of s~ ri1-.,;...,c
These CU~ Jo~iLions may also contain adjuvants such as preservative, wetting agents,
emulsifying agents, and dispersing agents. E~ ,ntion of the action of microorg;lni~mc may be
ensured by the inclusion of various ~ntiba~tlorial and antifungal agents, for ex~mrle, p~r~hen,
15 chlorobutanol, phenol sorbic acid, and the like. It may also be desirable to include isotonic
agents such as sugars, sodium chlotide~ and the like. Prolonged absorption of the injectable
ph~ en~ l form may be brought about by the in~lll$inn of agents which delay absorption
such as ~1.. ;.~.. " 111(1115~ t~ and gelatin.
If desired, and for more effective distribution, the colll~unds can be incul~-,laL~d into
20 slow release or targeted delivery systems such as polymer matrices, liposomes, and
The injectable formulations can be sterilized, for example, by filtration through a
bacterial-retaining filter. or by incorporating sterilizing agents in the form of sterile solid
compositions which can he dissolved or dispersed in sterile water or other sterile injectable
25me~ m just prior to use.
Solid dosage forms for oral ~Aminic~ration include capsules, tablets, pills, powders, and
"granules. In such solid dosage forms, the active compound is mixed with at least one inert,
ph~rm~re-ltic~lly arcept~hle eYcirient or carrier such as sodium citrate or rlic~ -m phosphate
and/or a) fillers or e~rt~n~l~rs such as starches, lactose, sucrose, glucose, m~nnitQl, and silicic
-31-
SUBSTITUTE SHEET (RULE 26
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
acid, b) binders such as, for example, carboxymethylcellulose, alingnates. gelatin,
polyvinyl~yllolidone~ sucrose, and acacia, c) hl.n,~ nl~ such as glycerol, d) disintegrating
agents such as agar-agar, c~lri-lm carbonate, potato or tapioca starch, alginic acid, certain
Cilir~tes~ and sodium carbonate, e) solution retarding agents such as paraffin. f) absorption
5 accelc,a~ such as 4l~ . . .;., y ~" ", .on ;~.. ., co.ll~ou,lds, g) wetting agents such as, for e~ lc,
cetyl alcohol and glycerol IlllJno~ P~ h) absc,~ such as kaolin and benl-J~;Ie clay, and i)
Inl..;r,~ such as talc, calcium ste~rat~, m~nP~ st~oA-ate, solid polyethylene glycols, sodium
lauryl sulfate, and llli~cLul~.~ thereof. In the case of capsules, tablets and pills, the dosage form
may also co...~ b..r~ .;"g agents.
Solid c.,~ u~;l;ons of a similar type may also be employed as fillers in soft and hard-
filled gelatin c~ps--lPs using such txci~ s as lactose or milk sugar as well as high molec~ r
weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsulPs, pills, and granules can be ~
with co~tingc and shells such as enteric coatings and other coatings well known in the
15 ph~.,,-A~ l;r~l formlll~ting art. They may optionally contain opacifying agents and can also be
of a cc,lll~osiLion that they release the active il,~l~diel-L(s) only, or ~l~r~.c.lLially, in a certain part
of the in~pstin~l tract, optionally, in a delayed manner. FY~"l,l~ S of emhedriing colllpo~iLions
which can be used include polymeric subst~nrec and waxes.
If desired, and for more effective distribution, the co...~oL,nds can be incolluulalcd into
20 slow release or targeted delivery systems such as polymer matrices, liposomes. and
microspheres.
The active colll~ullds can also be in micrc~nr~rslli~teri form, if ayl,lUluliate. with one or
more of the above-mPntion~l PYripiet~tc
Liquid dosage forrns for oral ~riminictration include pharrn~reutic~lly acceptable
25 emlllcinnc, solutions, sncpencionc, syrups and elixirs. In addition to the active co~ o~dds, the
liquid dosage forms may contain inert diluents cc,llllllollly used in the art such as, for example,
water or other solvents, solubilizing agents and emlllcifiers such as ethyl alcohol, isopropyl
alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl bPn7nate propylene glycol, 1,3-
butylene glycol, dimethyl fol",~ oils (in particular, coSI~ ec~l gr~!un~lnl-t corn, germ,
-32-
SUBSTITUTE Sl ~'EET (RULE 26)
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
olive, castor, and sesame oils), glycerol, tetrallydlorullulyl alcohol, polyethylene glycols and
fatty acid esters of sorbitan, and ~ ul~,s thereof.
Besides inert rlihl~nt~ the oral co,llpo~ilions can also include adjuvants such as wetting
agents, emulsifying and snspen~ing agents, s-.eet~ g, flavoring, and p~,.ru,llil,g agents.
S S.. ~ ;ollc, in ~drlitil~n to the active colllp~llllds, may contain ~ e ~rlhlg agents as, for
example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters,
,l~ly~L~lline celllllnse, ~II....in~.... metahydroxide, bentonire, agar-agar, and ~g~nth, and
ix lw- s thereof.
Collll,osilions for rectal or vaginal ~lminictration are preferably ~u~pO~ ;F-S which can
10 be ~l~p~ed by mixing the cc,llll)uu,~ds of this invention with suitable non-irritating excirient~ or
carriers such as cocoa butter, polyethylene glycol or a ~ul,l)osilo~y wax which are solid at room
e~ a~ but liquid at body ~ e and therefore melt in the rectum or vaginal cavity and
release the active co,l,~uul,d.
Dosage forms for topical ~rlmini~tration of a compound of this invention include15 powders, sprays, oimmlontc and inh~ nsc. The active co~l~pound is mixed under sterile
con~itic n~ with a ph~. ,n~r~ ;c~lly acceptable carrier and any needed preservatives, buffers, or
propellants which may be re~uired. Ophth~lmir form-ll~tions, eye ointmrnt~ powdc.~ and
sol~tion~ are also conr~ tecl as being within the scope of this invention.
Actual dosage levels of active ingredients in ~he ph~rm~reutical co"l~uo~ilions of this
20 invention may be varied so as to obtain an amount of the ac~ive compound(s) tha~ is effective to
achieve the desired therapeutic response for a particular patient, co"lpositions, and mode of
a~lmini~tration. The selected dosage level will depend upon the activity of the particular
compound, the route of a~lmini~tration, the severity of the condition being treated, and the
condition and prior mtoAir~l history of the patient being treated. However, it is within the skill of
25 the art to start doses of the co"l~ound at levels lower than required for to achieve the desired
therapeutic effect and to gradually increase the dosage until the desired effect is achieved.
Generally dosage levels of about 0.1 to about 200, more preferably of about 05 to about 150,
and most preferably about 1 to about 125 mg of active collJpo.lnd per kilogram of body weight
per day are ~rlmini~tered orally to a .. ~.. ~li~n patient suffering from ~ hP.. r.'s Disease,
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 l997-0~-l6
Wo 96tl60S2 PCr/US95/14987
neoplastic disease, or bacterial or fungal infections. If desired. the effective daily dose may be
divided into m1llrirl~ doses for ~u~l,oses of ~flmini~tration, e.g. two to four Sc~dlc doses per
day.
All rloc~ e.g., patents and journal articles, cited above or below are hereby
5 il~Cul~ul.. t~l by lcr~ c"cc in their entirety. - =
One skilled in the art will recognize that m~iifir~tion~ may be made in the present
invention without deviating from the spirit or scope of the invention. The invention is illnst-~t~
further by the following eY~mpleA which are not to be con:,llued as limiting the invention or
scope of the specific IJloc~luu~;s d~osor~ herein.
GENERAL PROCEDURE
As is more fully e~pl~in~d in the following scheme and ~ ...ples, the com~oul.ds of the
invention may be ylcp~u~c1 by adding an ~lv~liate seconrl~ry amine (l mole) to a solution of
c~ e~ni.tcd hy~llucllloric acid (2-5 moles) in suffisient iso~luyallol to achieve an amine
col-~e..l. a~ion of about 0.1 M-0.4 M. To the solution of amine hydrochl~ri~e salt which has been
15 forrned in situ is added p~arollllaldehyde (1-2.2 moles) followed by the desired aryl or
hetc.û~L~cetone (1.1-2 moles), and the resulting reaction mixture is then refluxed for about 12-
72 hours. The reaction is cooled and the res1llting solid is filtered and recryst~lli7~d from
l or lliLul~l~ with ...~ l or ethanol to afford the desired product.
The co..lyuul~ds of the invention may be ~rcl,a.cd according to the reactions set forth in
20 the following reaction scheme.
ArlJlCH2
+ ~PrOH ~ Ar~ N ~
HN ~ HCI Y ~ Z~ X~ Ar2
~Z~x,Ar2
where Arl, X, Y, Z and Ar2 are as defined above for forrnula I.
Using the above-described procedure, the cûllllJu~ ds described in the followingeY~mples were yl~
-34-
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
Wo 96/16052 PCr/US95/14987
FY~mrle 1
G
~ ~ ~N v~3
l-~D-Iodoph~ 1)-3-(4'-benzyl-1'~ "a7il~yl)-l-~o~allolledihydrochloride: mpæ4 C (dec.);
lHNMR(300MHz,DMSO-d6) ol2.0(brm,2H),8.0(d,2H),7.65(d,2H),7.6(m,2H),
5 7.45 (m, 3 H), 4.4 (brs, 2 H), 4.0-3.2 (m, 12 H).
FY~rnrl~ 2
~ uph~nyl)-3-(4'-benzyl-l'-pi~illyl)-1-~ panone dihydrochloride: mp > 240 C; lH
NMR of the free base (300 MHz, CDC13) o 8.0 fm, 2 H), 7.3 (m, 5 H), 7.13 (t, 2 H), 3.5 (s, 2
H), 3.15 (t, 2 H), 2.85 (t, 2 H), 2.55 (brs, 8 H).
FY~mrle 3
1-(3",4"-Dichk~lu~hfnyl)-3-(4'-benzyl-1'-pi~"a~i~lyl)-1-pl~dn(~lle dihydrochloriA~-: The solid
was converted to its free base using NH4OH, e.~ a~ ;i with dichlolu~ tl ~l-f and purified via
flash ch~,nlal.,graphy (Silica, EtOAc). The resulting oil was converted to its dihydrochl~ride salt
by ~AAition of drv ether-HCI to give the desired ~I~Klu~; mp 220 C (dec.); lH NMR of the free
15 base (300 MHz. CDC13) ~ 8.03 (d, 1 H), 7.77 (dd, 1 H), 7.53 (d, 1 H), 7.3 (m, 5 H), 3.5 (s, 2
H), 3.12 (t, 2 H), 2.82 (t, 2 H), 2.5 (brs, 8 H).
Exarnple 4
1 -~D-Chlorophenyl)-3-(4'-benzyl- 1 '-piperazinyl)- 1 -plo~anone dihydrochloride: mp > 230-C; lH
NMR of the free base (300 MHz, CDC13) ~ 7.9 (d, 2 H), 7.43 (d, 2 H), 7.28 (m, 5 H), 3.5 (s,
20 2 H), 3.15 (t, 2 H), 2.83 (t, 2 H), 2.5 (brs, 8 H).
Fx~mrlt~- 5
l-~D-Nitrophenyl)-3-(4'-benzyl-1'-pi~dzillyl)-1-~1ul,dllunc dihydrochloride: A mixture of p-
nitroacetophenone (6.09 g, 0.037 mol), I-benzylpiperazine (5.00 g, 0.023 mol),
-35-
SUBSTITUTE SHEET (RULE 26)
- - =
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
p~ar~ ehyde (1.11 g, 0.037 mol), and concc~.t.d~A hydrochloric acid (7.00 mL.
0.084 mol) in 100 mL isopropanol were refluxed for 16 h. The reacton was cooled. filtered,
and the flter calce was !- ;~ A with m~ nol (4x) to give 4.6 g (51%) of the desired p.~duct:
mp 250-C; IH NMR (300 MHz, DMSO-d6) ~ 11.5 (brm, 2 H), 8.4 (d, 2 H), 8.2 (d, 2 H),
5 7.66-7.36 (m, 5 H), 4.3 (brm, 2 H), 3.8-3.2 (brm, 12 H).
FY~mrle 6
1-(3"-Thienyl)-3-(4'-benzyl-1'-pi~ yl)-1-pl~.p~ edihydT~ch~ Ae mp223 C; lHNMR
(300 MHz, DMSO-d6) o 11.5 (brs, 2 H), 8.56 (s, 1 H), 7.8-7.3 (m, 7 H), 4.3 (brs, 2 H), 3.9-
3.0 (brm, 12 H).
F.y~mrlto 7
1-(2"-Thienyl)-3-(4'-benzyl-1'-pi~.,~inyl)-1-~1ul)ano"cdihydrochln~iA~o: mp240-241 C; lH
NMR (300 MHz, D2O) ~ 7.82 (m, 2 H), 7.4 (brs, 5 H), 7.1 (m, 1 H), 4.3 (s, 2 H), 3.5 (brs,
12 H).
FY~mri~ 8
~3~ H~c~
1-[3"-~-Chlol~,~hc"yl)snlfon~miAophenyl]-3-t4'-benzyl- 1 '-piperazinyl)- l-p~ one
dihydrochln~ mp 151-153 C; lH NMR (300 MHz, D20) ~ 7.7-7.14 (m, 13 H), 4.2 (s, 2
H), 3.55-3.2 (brs, 12 H).
FY~mrle 9
o
F3C ~ N~3
CF3
-3~
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
1-[3",5"-Bis-(~inllol"~ yl)phenyl~-3-(4'-benzyl- 1'-~ yl)- l-~lul)al~ûne
~lihy~ nn~ The solid was basified with NH40H, e~ ed with dichlo~ e and
purificd via flash clll.~ u~rhy (Silica, EtOAc). The free amine was converted to its
dihydl~ nTide salt by the ~ ition of dry ether-HCI: mp 255 C; IH NMR of the free base (300
~, S MHz, CDC13) o 8.38 (s, 2 H), 8.07 (s, 1 H), 7.29 (m, 5 H), 3.5 (s, 2 H), 3.23 (t, 2 H), 2.87
(t, 2 H), 2.5 (brm, 8 H).
F.Y~nrle 10
O
Il
~~~ ~N~
l-(Bc,,zurL,1-2''-yl)-3-(4'-benzyl-l'-~ i,,yl)-l-plu~ànone dihydro~hlnritle mp 255-257 C;
10 IH NMR (300 MHz, D2O) o 7.7 (m, 2 H), 7.55-7.18 (m, 8 H), 4.3 (s, 2 H), 3.5 (brs, 12 H).
FY~mrl-o. 1 1
1-(m-Nilluph~.,yl)-3-(4'-benzyl-1'-~ .a~ yl)-1-p.~allone di}~y~ llloride: mp 222 C(dec.);
lH NMR (300 MHz, DMSO-d6) ~ 12.40-11.70 (brs, 2 H), 8.70 (s, 1 H), 8.50 (d, 1 H), 8.35
(d, 1 H), 7.90 (dd, 1 H), 7.85-7.35 (m, 5 H), 4.35 (brs, 2 H), 4.00-2.10 ~m, 12 H).
FY~rnrlto 12
l-~D-M~ yl~h~,,lyl)-3-(4'-benzyl-1'-~i~cla~illyl)-1-propanonedihydrochlon~s mp 196-198-C;
lH NMR (300 MHz, CDC13) of the free base: o 7.85 (d, 2 H), 7.40-7.10 (m, 7 H), 3.5 (s, 2
H), 3.15 (t, 2 H), 2.82 (t, 2 H), 2.65-2.40 (m, 8 H), 3.50 (s, 3 H).
FY~mrle 13
20 l-(o-Flu~ vhcllyl)-3-(4~-benzy~ -pipclazillyl)-l-propanonedihydroehlt~nrle mp 185-190C;
lH NMR (300 MHz, DMSO-d6) ~ 12.00-11.20 (brs, 2 H), 7.90-7.30 (m, 9 H), 4.35 (s, 2 H),
4.10-3.00 (m, 12 H).
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCTtUS95/lq987
FY~mpl~ 14
l-~-McLl,ylLlliophenyl)-3-(4'-benzyl-1'-pi~ lyl)-1-plupahollc dihydrochlonrl~: mp 216-
220 C; lH NMR (300 MHz, DMSO-d6) o 12.10-11.70 (brs, 2 H), 7.95 (d, 2 H), 7.75-7.35
(m, 7 H), 4.35 (s, 2 H), 3.85-3.20 (m, 12 H), 2.75 (s, 3 H).
FY~mrle 15
l-(m-chlul"l,he~lyl)-3-(4~-benzy~ ip~ yl)-l-plo~nolle dihydroehlond~ mp 196-l99 C;
1H NMR (300 MHz, DMSO-d6) o 12.30-11.55 (brs, 2 H), 8.15-7.30 (m, g H), 4.35 (brs, 2
H), 4.00-3.00 (m, 12 H).
Example 16
10 1-~-BIullluph~.nyl)-3-(4'-benzyl-l'-pi~ ~i,,yl)-1-propanone dihydrochlnnrie mp 205-C
(dec.); IH NMR (300 MHz, DMSO-d6) ~ 12.00- 11.20 (brs, 2 H), 7.90 (d, 2 H), 7.80 (d, 2
H), 7.70-7.35 (m, 5 H), 4.35 (brs, 2 H), 4.00-3.10 (m, 12 H).
FY~mrl~ 17
l-(m-Ni~ophenyl)-3-~4'-GD-nuc,lu~",yl)- 1 '-piperazinyl]- i-plu~ c dihydrochloride: mp
15 210 C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.50-11.60 (brs, 2 H), 8.70 (s, 1 H), 8.5
(d, 1 H), 8.40 (d, 1 H), 7.90 (dd, 1 H), 7.80-7.60 (m, 2 H), 7.30 (m, 2 H), 4.35 (s, 2 H),
4.00-3.00 (m, 12 H).
Example 18
l-~D-Tlinu~ o~Ly~ yl)-3-(4~-benzy~ yl)-l-propanonedihydrochlnntl~: mp
20 l95 C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.00-11.70 (brs, 2 H), 8.12 (dd, 2 H), 7.80-
7.30 (m, 7 H), 4.40 (brs, 2 H), 3.85-3.10 (m, 12 H).
Example 19
SUBSTITUTE SHEET (~ULE 26
CA 02205586 1997-05-16
WO 96/16052 PC~/US95/14987
~O r ~N~ J~
1 -(2",3"-Dihydro- I ",4"-benzodioxan-6"-yl)-3-(4'-benzyl- 1 '-pi~ azinyl)- I -propanone
dil~ydl~l.loride: mp 215-C (dec.); 1H NMR (300 MHz, DMSO-d6) ~ 11.85-11.60 (brs, 2 H),
7.65-7.30 (m, 7 H), 7.00 (d, 1 H), 4.70-4.20 (m, 4 H), 4.00-3.30 (m, 14 H).
Fy~mrle 20
o
~ N~ ~
1-(1",2",3",4"-Tetrahydronaphth-6"-yl)-3-(4'-benzyl-1'-pi~ u~ olle
dil-~ocl-lnn~ mp 200 C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.35-11.70 (brs, 2 H),
7.80-7.15 (m, 8 H), 4.35 (brs, 2 H), 4.10-3.10 (m, 12 H), 2.90-2.65 (brs, 4 H), 1.70-1.90
10 (brs, 4 H).
Example 21
1 -(4"-Fluoronaphth- I "-yl)-3-(4'-benzyl- 1 '-I~ip~.dzinyl)- I -propanone dihydrochloride: mp
220 C (dec.); 1H NMR (300 MHz, DMSO-d6) ~ 12.50-11.80 (brs, 2 H), 8.75 (d,l H), 8.40-
8.10 (m, 2 H), 7.90-7.30 (m, 8 H), 4.45 (brs, 2 H), 4.10-3.10 (m, 12 H).
Example 22
o
N~ J~3
-39-
SUBSTITUTE SHEEt (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCTtUS95/14987
1-p-Biphenyl-3-(4'-benzyl-1'-~il,~,.,.,;.~yl)-l-pl"yanone dihydro~hlori~ mp 225-C (dec.); lH
NMR (300 MHz, DMSO-d6) ~ 12.55-11.50 (brs,2 H), 8.10 (d, 2 H), 7.90 (d, 2 H), 7.80-7.30
(m, 10 H), 4.35 (brs, 2 H), 4.00-3.10 (m, 12 H).
FY~mri~ 23
S 1-(m-TIinuulul.lGlhyll,h~",yl)-3-(4'-benzyl-l '-pi~.~lyl)- l-~l~anone dihydrochloride: mp
215 C (dec.); 1H NMR (300 MHz, DMSO-d6) ~ 12.20-11.00 (brs, 2 H), 8.55-8.00 (m, 2 H),
8.10 (d, 1 H), 7.85 (dd, 1 H), 7.70-7.40 (m, 5 H), 7.60-4.00 (brs, 2 H), 4.00-3.00 (m, 12).
FY~mrl~ 24
1l
N ~----N~
H3C
10 1-(1"-Methyl-l"-H-pyrazol-4"-yl)-3-(4'-benzyl-1'-~i~"~,lyl)-1-~ one dihydrochlt~nrle
mp 221 C (dec.); lH NMR (300 MHz, DMSO-d6) o 12.45-11.60 (brs, 2 H), 8.45 (s, 1 H),
7.95 (s, 1 H), 7.75-7.30 (m, 5 H), 4.35 (brs, 2 H), 3.90 (s, 3 H), 3.80-3.10 (m, 12 H).
FY~mrl~ 25
I-(p-Fluoro-m-b;omophenyl)-3-(4'-benzyl-1'-pi~ zi,lyl)-1-~1.,pano"e dihydrochlonn'~: mp
15 220 C (dec.); 1H NMR (300 MHz, DMSO-d6) o 12.40-11.40 (brs, 2 H), 8.32 (d. 1 H), 8.05
(m, 1 H), 7.80-7.30 (m, 6 H), 4.35 (brs, 2 H), 4.15-3.10 (m, 12 H).
FY~mrle 26
o
CH3SO2NH ~ ~N
40-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
1 -[p-(h~ nf ~ fon~m~ )phenyl]-3-(4~-benzyl- l '-pi~ ~il.yl)- 1-plù~ onc dihydrochloride:
mp 202-205 C; lH NMR (300 MHz, DMSO-d6) o 12.30-11.20 (brs, 2 H), 8.60 (d, 2 H), 7.75
(d, 2 H), 7.65-7.35 (m, 5 H), 4.33 (brs, 2 H), 4.06-310 (m, 12 H), 3.55 (s, 6 H).
FY~mrl.o 27
5 1-~D-Cycloh~Ayl~h~.lyl)-3-(4'-benzyl-1'-pi~.~u~yl)-l-plu~ o~edihydr~l~lnnde: mp231-C
(dec.); lH NMR (300 MHz, DMSO-d6) o 12.40-12.10 (brs 1 H), 12.1-11.70 (brs 1 H), 7.90
(d, 2 H), 7.80-7.20 (m, 7 H), 4.70-4.20 (brs, 2 H), 4.20-3.10 (m 12 H), 2.70-2.40 (m, 2 H),
2.00-1.15 (m, 9 H).
FY~mple 28
o
~ ~N~
1-(Fluoren-2"-yl)-3-(4'-benzyl-1'-p;~ ,;..yl)-1-propanonedihydro~hlnrir1e: mp225C(dec.);
lH NMR (300 MHz, DMSO-d6) ~ 12.40-11.30 (brs, 2 H), 8.40-7.95 (m, 4 H), 7.75-7.30 (m,
8 H), 4.60-4.20 (brs, 2 H), 4.05 (s, 2 H), 3.95-3.05 (m, 12 H).
FY~mr1~ 29
15 1-~D-Iodophenyl)-3-[4'-(o-fluolubo,l~yl)-1'-P;l~ yl~-1-plul)anoncdihydrochloride: mp215-
217 C; lH NMR (300 MHz, DMSO-d6) ~ 11.6 (br, 2 H), 8.0 (d, 2 H), 7.7 (m, 3 H), 7.5 (m,
1 H), 7.3 (m, 2 H), 4.3 (brs, 2 H), 3.8-3.0 (brm, 12 H).
Example 30
1 -(o-Fluorophenyl)-3-[4'-(o-fluorobenzyl)- 1 '-~i~c. d~hlyl]- 1 -~lupanol~c dihydrochloricle The
20 solid formed was converted to its free base using NH40H, ~ d with dichlululllr~ n~ and
purified via flash chromato~l~hy (Silica, EtOAc). The resulting colll~ùul-d was converted to its
dihydrochlnride salt by the addition of dry ether-HCI to give the desired product: mp 213-216-C;
-41-
SUBSTITUTE SHEET (RULE 2G)
CA 02205586 1997-05-16
Wo 96/16052 PCT/US95/14987
lH NMR of the free base (300 MHz, CDC13) ~ 7.83 (t, I H), 7.5 (m, 1 H), 7.4-6.95 ~m, 6 H),
3.6(s,2H),3.2(m,2H),2.8(t,2H),2.5(brs,8H).
FY~mrie 31
l-(m-NiLlù~hc.,yl)-3-[4'-(o-lluulùb~""yl)~ .r ~ yl]-l-~ dlloncdihydrochloride: lH
5 NMR of the free base (300 MHz, CDC13) o 8.78 (s, 1 H), 8.42 (d, 1 H), 8.28 (d, 1 H), 7.7 (t,
lH),7.37(t, lH),7.24(m, lH),7.15-6.95(m,2H),3.6(s,2H),3.22(t,2H),2.88(t,2
H), 2.56 (brs, 8 H).
FY~rnrlP 32
l-~D-Nil,u~hellyl)-3-r4'-(o-flllolu~,~yl)-1'-~ "~h~yl]-1-~ )anone dihydrochlnrid~ The
10 solid formed was converted to its free base using NH40H, cAIla.;l~d with dichlcl~ hAl~e and
p~ified via flash cl~ull,a~ography (Silica, 9:1 EtOAc~eOH). The resulting colll~uu"d was
C~ Lt;d back to its ~ yd~u~ hlotirl~ salt by the A~drlition of dry ether-HCl and recrystAlli7~d from
nol (2x) to give the desired ~ludu~ H NMR of the free base (300 MHz, CDC13) o 8.3
(d,2H),8.1(d,2H),7.9-6.95(m,4H),3.6(s,2H),3.2(t,2H),2.85(t,2H),2.5(brs,8
15 H).
FYAmrl~ 33
l-~D-NiL~o}~ht~yl)-3-r4~-(2"~5"-difluulu~l~y~ à~illyl]-~ ol~e dihydrochloride:
The solid was basified with NH40H, c,.uacLcd with dichlc,lu..~ Ih~ purified via flash
cll~u~ y (Silica. EtOAc), and converted to the dihydrochlc-n~e salt by the addition of dry
20 ether-HCI. This was recryst~lli7~oA in meth~nol (2x) to give the desired product: mp 212-215-C;
lH NMR of the free base (300 MHz, CDC13) o 8.3 (d, 2 H), 8.1 (d, 2 H), 7.2-6.8 (m, 3 H),
3.55 (s, 2 H), 3.2 (t, 2 H), 2.85 (t, 2 H), 2.55 (brs~ 8 H).
Example 34
-42-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO g6116052 PCTIUS9~/14987
3~ / ~--N~
l-~D-NiL~ yl)-3-(4'-~ ,.u~ yl)-1-~ 0l~e d~hy~u~ Ot~ The solid was
basified with NH40H, ~xl.n~ ;I with dichlu~ I h ~ and purified via flash ch-~ ug.,.lll.y
(Silica, EtOAc). The l~,..ulling co~ ,ou,ld was converted to the dihydroehlntid~ salt by the
5 ~riition of dry etner-HCl and recryst~lli7~ in ~ nol to give the desired ~ lu~;l. mp 211
217 C; lH NMR of the free base (300 MHz, CDC13) o 8.3 (d, 2 H), 8.1 (d, 2 H), 6.9-6.65 (m,
3H),5.95(s,2H),3.4(s,2H),3.21 (t,2H),2.83(t,2H),2.5(brm.8H).
FY~mp3e 35
o
N2~ ~N~
~3
10 1-(p-Nitrophenyl)-3-~4'-(1"-p-chlorophenyl-1"-phenylmethyl)-1'-pi~ yl]-1-propanone
dihydrochloride: The reaction was cO.I~f .~ ted in vacuo, basified with NH40H, ~ d with
dichlorometh~n~ and purified via flash chlv . .~ graphy (Silica, EtOAc). The resulting
co~ ,ou~ld was converted to its dihydrochloride salt by the arl~isinn of dry ether-HCl to give the
desired product: mp 148-153 C; IH NMR of the free base (300 MHz. CDC13) ~ 8.3 (d, 2 H),
15 8.1 (d, 2 H), 7.45-7.1 (m, 9 H), 4.2 (s, 1 H), 3.2 (t, 2 H), 2.85 (t, 2 H), 2.7-2.3 (brm, 8 H).
Example 36
l-(p-l~oth~n~slllfon~mi~iophenyl)-3-(2l~7s~-difluo~benzyl-l~ yl)-l-~ulu~ one
dihydrochlnri~le: mp 230-232 C; lH NMR (300 MHz, D20) â 7.88 (d, 2 H), 7.3-7.05 (m, 5
H), 4.28 (s, 2 H), 3.8-3.3 (brm, 12 H), 3.06 (s, 3 H).
-43-
SUBSTITUTE SHEET (RULE 26
=
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
Exarnple 37
1 -(3''-Thienyl)-3-[4'-(m-fluc,.uboll ,yl)- 1 '-pip. . ~,; . ,yl]- 1 -propanone dihydrochlnnr~ The solid
was basified with NH40H, e~.LIaCh;l with dichlc lu, . ~ nç and purified via flash
clll~ ".pl,y (Silica, EtOAc). The free amine was converted to its dihydro~lnntle salt by the
5 addition of dry ether-HCl to give the desired ~10 IU~;L~ lH NMR of the free base (300 MHz,
CDC13) ~ 8.06 (d, 1 H), 7.53 (d, 1 H), 7.29 (m, 2 H), 7.08 (m, 2 H), 6.93 (m, 1 H), 3.5 (s, 2
H),3.12(t,2H),2.85(t,2H),2.54(brm,8H).
FY~mpl~ 38
1 -(3 "-Thienyl)-3-[4'-(2"',5"'-difluol ù~"z.yl)- 1 '-pip~,. ~inyl] - 1 -1,l upa~one dihydrochloride: 1 H
10 NMR of the free base (300 MHz, CDC13) o 8.05 (d, 1 H), 7.54 (d, 1 H), 7.32 (m, 1 H), 7.12
(m, 1 H), 6.94 (m, 2 H), 3.57 (s, 2 H), 3.1 (t, 2 H), 2.83 (t, 2 H), 2.56 (brs, 8 H).
FY~rnrl~ 39
l-~D-NiLlu~llC~yl)-3-([4'-(o-llinuululll~ lyl)benzoyl3-l~ yl)-l-~ p~ nt The solid
was basified with NH40H, ~ 1t-lrrl with dichlc/l.J~ ,ç and purified via flash
15 clllulllatugraphy (Silica, EtOAc) to give the desired plodu.;l. mp 179-181 C; lH NMR (300
MHz CDC13) ~ 8.32 (d, 2 H), 8.1 (d, 2 H), 7.72 (d, 1 H), 7.56 (m, 2 H), 7.33 (d, 1 H), 3.82
(m, 2 H), 3.2 (m, 4 H), 2.88 (t, 2 H), 2.6 (m, 2 H), 2.62, (m, 2 H).
FY~rnpl~ 40
o
NO2~ N~N~
O F
20 l-~-Ni~ophenyl)-3-[4'-(2'',6''-diflllolu~ll2oyl)-ll-pil~e~ yl]-l-l,lupallol~e hydrochlnri~
mp 225-227 C; lH NMR (300 MHz, D2O/CD3OD) ~ 8.21 (d, 2 H), 8.03 (d, 2 H), 7.42 (m, 1
H), 6.98 (t, 2 H), 3.78-3.15 (brm, 12 H).
SUBSTITUTE ~HEET (RULE 26
-
CA 0220~86 1997-0~-16
WO 96tl6052 PCTIUS95/14987
FY~mpl~ 41
1 -(o-Fluu.~h~nyl)-3-r4'-(2'',6''-difluorobell~.,yl) 1 '-~ip~ yl]- I -p,opanon~ hydrochlnn-le:
mp 185-187 C; IH NMR (300 MHz, CDC13) ~ 13.55-13.73 (brs, 1 H), 7.87-7.65 (m, 1 H),
7.65-7.35 (m, 2 H), 7.35-6.90 (m, 4 H), 2.92 (d, I H), 4.15 (t, 1 H), 3.90-3.40 (m, 8 H),
5 3.05-2.80 (m, 2 H).
Example 42
1-~7-NiLluph~ yl)-3-[4l-(m-flllo~ yl)-1'-~ a~ yl]- 1-~l.p~lolledihy~ll~hlûride: mp
210-C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.10-11.30 (brs, 2 H), 8.40 (d, 2 H), 8.25
(d, 2 H), 7.60-7.20 (m, 4 H), 4.45-3.00 (m, 14 H).
Fs~mr1-~ 43
1-(o-Flllolu~he,lyl)-3-[4'-(o-llinuolullleLl.ylbenzoyl)-1'-~ yl]-1-~ nol~e hydrochloride:
mp 175-176 C; lH NMR (300 MHz, CDC13) ~ 13.55-13.45 (brs, 1 H), 7.98-7.50 (m, 5 H),
7.45-7.10 (m, 3 H), 4.92 (d, 1 H), 4.12 (t, 1 H), 3.95-3.35 (m, 8 H), 3.10-2.70 (m, 2 H).
FY~mrk~ 44
15 1-(o-Fluolul)h~"~yl)-3-[4'-~D-fluorobenzyl)-1'-~ 7;nyl]- l-propanone dihydl~ 1nn~l~o: mp
209 C (dec.); IH NMR (300 MHz, DMSO-d6) ~ 12.00-11.40 (brs, 2 H), 7.95-7.20 (m, 8 H),
4.30 (s, 2 H), 3.85-3.20 (m, 12 H).
Example 45
1-GD-Ni~Jphe.lyl)-3-r4'-~D-fluorobenzyl)-ll-~ illyl]-1-~1u~ Onf dihydrochlc-n~is: mp
20 200 C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.50-11.60 (brs, 2 H), 8.50 (d, 2 H), 8.25
(d, 2 H), 7.85-7.20 (m, 4 H), 4.39 (s, 2 H), 4.00-3.00 (m, 12 H).
Example 46
1-GD-Fluc~ h~nyl)-3-[4'-(p-fluorobenzyl)-1'-pi~ zinyl]-1-propanone dihydrochlori(le mp
224 C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.40-11.70 (brs, 2 H), 8.05-7.20 (m, 8 H),
25 4.35 (brs, 2 H), 3.95-3.10 (m, 12 H).
-45-
SUBSTITUTE SHEET IRI ILE 2~)
-
CA 0220~86 l997-0~-l6
WO 96/16052 PCr/US95/14987
FY~mrl~ 47
1-(m-Fluolu~hc.,yl)-3-[4'-~-fluu,u~llL~ yl]-1-~v~dihydrochloride: mp
215 C (dec.); 1H NMR (300 MHz, DMSO-d6) o 12.05-11.75 (brs, 2 H), 7.95-7.15 (m, 8 H),
4.35 (brs, 2 H), 3.9S-3.15 (m, 12 H).
Example 48
1-~-Iodc~hc.~yl)-3-[4'-~D-fluolu~,,~y-l)-l'-pil,~,~i,,yl]-l-~ru~ Jl,cdihydrochlt-rirl~: mp
215 C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.25-11.70 (brs, 2 H), 7.95 (d, 2 H), 7.80-
7.50 (m, 4 H), 7.40-7.20 (m, 2 H), 4.30 (s, 2 H), 3.90-3.10 (m, 12 H).
Example 49
(a) 4-Fluo~u~nLuyl~ zine: p-Fluolub.~n~ùylcllloride (6.00g) in 30mL
CH3CN was added in one portion to a stirred so1u~inn of pi~,e.~ine (16.25 g) dissolved in
lNHCl (285 mL). The reaction was stirred at room t~mrçr.~h~re for 3.5 hrs. then an ~ itinn~l
50 mL of lN HCl was added. The sollltion was c,~L~ ed with 2 x 100 mL EtOAc. Thea~ucous acidic layer was made basic with lN KOH then e~.LI~L~d with 2 x 150 rnL CH2C12.
15 The organic layer was 5~ ",~ , dried (Na2S04), filtered and e~pUldt~, affording a yellow oil
which soii-iifi~d upon st~nriing (5.60 g; 71 % yield). M+ 208. lH NMR (300 MHz, CDC13) o
7.50-7.35 (m, 2 H), 7.18-7.00 (m, 2 H), 3.90-3.30 (brd, 4 H), 3.00-2.70 (brs, 4 H), 1.85 (s, 1
H).
(b~ 3-Flu~lubcn~oyl~i~.~ne: Utilizing the ~,l~lulc of FY~mple 49a but repl~ing
20 p-fluorobenzoylchlc ri~ with m-fluolu~n,~ylchloride (3.00 g) afforded the desired product
(3.20 g; 82% yield). M+ 208. lH NMR (300 MHz, CDC13) ~ 8.50-7.32 (m, 1 H), 7.25-7.05 (m, 3 H), 3.75 (brs, 2 H), 3.40 (brs, 2 H), 3.05-2.65 (m, 4 H), 1.90 (s, 1 H).
(c) 2-Fluoro~n~uyl~ 7inç: Using the l"ucedu.~ of Example 49a and
o-fluorobenzoylchloride (2.00 g) gave the desired product (2 40 g; 90% yield). M+ 208. lH
25 NMR (300 MHz, CDC13) ~ 7.50-7.00 (m, 4 H), 2.90-3.70 (m, 2 H), 3.45 (brs, 2 H), 3.10-
2.65 (m, 4 H), 1.85 (s, 1 H).
~6-
SU3STITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
WO 96tl6052 PCI/US95/14987
(d) 2,5-Dinu~u~,lzoyl~ à~ e Using the plOCcdulC of Example 49a with
2,5-difluoro~nz~ylchloride (5.00 g) gave the desired product (3.00 g; 51% yield). M+ 226.
lH NMR (300 MHz, CDC13) ~ 7.20-7.00 (m, 3 H), 3.86-3.70 (m, 2 H), 3.40-3.25 (m, 2 H),
3.05-2.75 (m, 4 H), 1.97 (s, 1 H).
(e) 4-Tlinuulul~ ylbenzoyl~ a~ e: Usingthe ~/rlX`~lUlc ofExample49awith
p-llinuurol~lell~ylbenzoyl~hlorirls (5.00 g) gave the desired product (1.85 g; 30% yield).
M+ 258. IH NMR (300 MHz, CDC13) o 7.67 (d, 2 H), 7.50 (d, 2 H), 3.75 (brs, 2 H), 3.35
(brs, 2 H), 3.05-2.75 (m, 2 H), 1.85 (s, 1 H).
(f~ 2,3-Dinuulu~.-~uylp;l~ ;--f - Using the l,.uc,~ulc of FY~mrlt~ 49a with
2.3-difluorobenzovlchlori(1e (5.00 g) gave the desired product (3.00 g; 47% yield). M+ 226.
lH NMR (300 MHz, CDC13) ~ 7.35-7.05 (m, 3 H), 3.75 (brs, 2 H), 3.30 (brs, 2 H), 3.06-
2.75 (m, 4 H), 1.75 (s, 1 H).
(g) 2-Trifluolulllclllylbenzoyl~;lh,.~l, ;"e Using the ~locedulc of Example 49a with
o-~ifluululllc~ lbenzoylchloride (5.00 g) gave the desired product (3.4 g; 55% yield).
M+ 258. lH NMR (300 MHz, CDC13) ~ 795-7.45 (m, 3 H), 7.40-7.25 (m, 1 H), 3.90-3.70
(m, 2 H), 3.25-3.05 (m, 2 H), 3.00-2.85 (m, 2 H), 2.85-2.65 (m, 2 H), 1.70 (s, 1 H).
(h) 4-Nillu~.l,uylpi~-,.~ine: Using the l"uc~lulc of Fy~mrle 49a with
p-nitrobenzoylchloride (7.00 g) gave the desired product (4.50 g; 50% yield). M+ 235. lH
NMR (300 MHz, CDC13) ~ 8.27 (d, 2 H), 7.07 (d, 2 H), 3.90-3.60 (brs, 2 H), 3.45-3.25 (brs,
2 H), 3.10-2.70 (m, 4 H), 1.75 (s, 1 H).
(i) 2,~DifluOlu~ll~oyl~i~x,a7;-.c: Using the plvcedulc of Fy~mrle 49a with
2,6-difluorobenzovlchlcn-1e (5.00 g) gave the desired product (3.25 g; 51% yield). M+ 226.
lH NMR (300 MHz, CDC13) ~ 7.45-7.25 (m, 1 H), 7.05-6.85 (m, 2 H), 3.70-3.40 (brs, 2 H),
3.40-3.25 (brs, 2 H), 3.05-2.75 (m, 4 H), 1.70 (s, 1 H).
(1) 4-Iodobenzoyl~,ipe,d~u,c: Using the procedure of Fy~mrle 49a with
4-iodobenzoylchloride (4.24 g) gave the desired product (0.70g; 14% yield). M+-316. 1H
47-
SUE~STlTUTc S~Y~ET (RUL~ 26)
CA 0220~86 1997-0~-16
Wo 96/16052 P~USg5114987
NMR (300 MHz, CDC13) o 7.85-7.15 (d,2 H),7.20-7:05 (d,2 H),3.85-3.05 (brs,2 H),
3.55-3.25 (brs, 2 H), 3.10-2.65 (m,4 H),1.75 (s, 1 H).
(k) ~ .An~, ,..Il.hf~..~...;~in a~ernph.onnnlo A CH2C12 (130 mL) solution co,~
4-,....;~.o~e~ f ..on~ (5.00 g) and triethylamine (5.6 mL) was cooled to 0 C and placed under
S niL.~g~ll. Then, ..l Ill~npslllfonyichlnririe (3.1 mL) in CH2C12 (5 mL) was added ~ wise
over 10 min. Upon co...l-k ~ ad~iition the reaction was sti~rcd at 0 C for an ~ririinnn~l 10 min.
then allowed to warm to room ~ ,,,.l...c with stirring c~ .;Q~ for 2 hrs. Then lN KOH
was added and the layers s~ A The basic layer was made acidic, then c~LIa~ ;i with
CH2C12 (2 x 75 mL). The organic extracts were comhinçci, dried (Na2S04), filtered and
10 c~ia~ulaled affording a pale yellow solid (4.10 g; 52% yield). M+ 213. lH NMR (300 MHz,
CDC13) o 7.97 (d, 2 H), 7.27 (d, 2 H), 3.10 (s, 3 H),2.07 (s,3 H).
(1) 3 ~ ". ~ .hollA...;~io a~ .- .o.~e- Using the ~luc~ule of FY~mrl~ 49k but
starting with 3-~mino~cet~h~ .-one (5.00 g) gave the desired product (6.00 g; 76% yield).
M+ 213. lH NMR (300 MHz, CDC13) o 7.92-7.45 (m, 5 H),3.52 (s, 3 H),2.65 (s,3 H).
(m) 2-Fluorobtl"ylpi~,~,.~i,.e; The product from Fy~mrl~ 49c (3.10 g) was
dissolved in dry T~F (75 mL) then a lM B2H6THF solution (45 mL) was added dropwise
over ca. 15 min. under N2. Upon cOl. .~ rlition~ the sol-lhnn was refluxed under N2 for 3
hrs. The reaction was cooled to 0 C in an ice bath and then lN HCl (ca. 70 mL) was carefully
added d,~pwise. Upon comrlete ~iditiQn, the reaction was allowed to warm to room20 le~ ... c followed by extraction with EtOAc (2 x 100 mL). The organic layer was discarded
and the acidic layer made basic with KOH. This was C.~ dcl~ with CH2C12 (2 x 150 mL).
The organic extracts were cnmhineA, dried (Na2S04), filtered and e~a~uldL~ giving an oil that
began to solidify upon st~n~1ing This was dissolved in IN HCl (75 mL) then refluxed 1.5 hrs.
The reaction was cooled to 0C then made basic with KOH followed by CH2C12 e~ ion
25 (2 x 100 mL). The organic extracts were co...l~ fA, dried (Na2S04), filtered and e~d~c~ldled
~fforn'ing a pale yellow oil (1.2 g; 41% yield). M+ 194. lH NMR of the dihydrochlori~le salt
(300 MHz, CDC13) o 10.00-9.30 (brs, 2 H), 7.15-6.85 (m, 4 H), 3.62 (s, 2 H), 3.35-3.15 (m,
2 H), 2.90-2.70 (m, 2 H).
-48-
SUBSTITUTE SHEET ~RULE 26)
~ ==
CA 0220~86 1997-0~-16
WO 96tl6052 PCT/US95/14987
(n) 2,3-Dinuolù~l~yl~i~G~zine: Using the product from Example 49f (1.14 g) and
the ~lucGdu~c of Fy~mrle 49m gave the desired product (0.45 g; 43% yield). M+ 212. lH
NMR (300 MHz, CDC13) ~ 7.20-6.97 (m, 2 H), 3.60 (s, 2 H), 2.90 (t, 4 H), 2.55-2.35 (brs, 4
H), 1.67 (s, 1 H).
(o) 4-Ni~ù~yl~ e: Using the product from FY~mrl~ 49h (0.50 g) and the
procedure of Fy~mr!e 49m gave the desired product (0.28 g; 59% yield). M+ 221. 1H NMR
(300 MHz, CDC13) o 8.27 (d, 2 H), 7.15 (d, 2 H), 3.75 (s, 2 H), 3.30-3.15 (m, 4 H), 2.85-
2.70 (m, 4 H).
(p) 2,5-Dinuclubell~yl~ cl~le: Using the co.nl)ounA of FY~mrle 49d (2.90 g)
and the ~lue~lwci of FY~mrle 49m gave the desired product (2.3û g; 83% yield). M+ 212. lH
NMR (300 MHz, CDC13) o 7.36-7.22 (m, 1 H), 7.10-6.90 (m, 3 H), 3.55 (s, 2 H), 3.30-3.10
9m, 4 H), 2.85-2.65 (m, 4 H).
(q) 4-FlllOlu~l~.yl~i~a~ille; Using the co~llpuund of Exarnple 49a (5.25 g) and the
~ruc~luuG of Fy~mrle 49m gave the desired product (4.35 g; 75% yield). M+ 194. lH NMR
(300 MHz, CDC13) ~ 8.95-8.70 (brs, 1 H), 7.40-7.25 (m, 2 H), 7.25-7.05 (m, 2 H), 3.50 (s,
2 H), 3.15-2.95 (brs, 2 H), 2.65-2.35 (m, 2 H).
(r) I-Be.~ lfonylpiperazine: Be~ .lfonyl chloriA~. (2.00 g, 0.011 mol) in
25 mL CH3CN was added in one portion to a stirred solution of ~i~Gl~ine (4.90 g, 0.057 mol)
dissolved in lN HCl (100 mL). The reaction was stirred at room IC.ll~.dtu~ for 30 minntes
The solution was e~ L~ with EtOAc and the a4u20us acid layc-r was made basic with
lN KOH and then extracted with 2 x 10 mL CH2CI2. The organic layer was s~ated, dried
(MgS04), filtered, and e~u~ted affording 1.40 g (50%) of a pale yellow solid: mp 109-
111 C; IH NMR (300 MHz, CDC13) o 7.75 (m, 2 H), 7.65-7.40 (m, 3 H), 2.96 (m, 8 H).
,~
(s) 1-(2'-Chloro-6'-methylpyrido-4'-yl)l,ip~.dzille: 2-Chloro-6-methylpyridine-4-
c~l~nyl chloriA~o~ (5.00 g, 0.026 mol) in 50 mL CH3CN was added in one portion to a stirred
solution of pil)~.azille (11.31 g, 0.13 mol) dissolved in lN HCl (180 mL). The reaction was
stirred at room le~ .alu~ for 3 hours, then an ~AAitinn~l 50 mL of lN HCl was added. The
-49-
SUBSTITUTE SHEET ~RULE 26)
CA 0220c,c,86 1997-0ct-16
Wo 96tl6052 PCT/US95/14987
solm nn was tAI~tlcl,~i with EtOAc (2 x 150 mL), the a~ueous acid layer was made basic with
IN KOH and then e,.ll..cled with 2 x 10 mL CH2C12 (2 x 200 mL). The organic layer was
se~ AtcA, dried (MgS04), filtered and cQnçe..~ eA, affording 3.30 g (53%) of a light yellow
solid: mp 164-165C; IH NMR (300 MHz, CDC13) ~ 7.12 (s, 1 H), 7.06 (s, 1 H), 3.74 (m, 2
5 H),3.33(m,2H~,2.95(m,2H),2.83(m,2H),2.57(s,3H), 1.72(s, lH).
(t) p-Toll~- IP ~u1rullr~ e Using the ~luee~lul~ of FY~mple 49r withp-
~tu~l~le l~slllfonyl chloride gave the desired ~ ~lu~;l. mp 98-101C; IH NMR (300 MHz, CDCl3)
o 7.64 (d, 2 H), 7.34 (d, 2 H), 2.95 (m, 8 H), 2.44 (s, 3 H), 1.60 (brs, 1 H).
(u) p-B~u~l~ù~ ..t~lllrul Iyl~ t~;1le- Usingthe~,ucedu~ofExarnple49rwithp-
0 L tlUII10~U~ 1fonyl chloride gave the desired plu~lu~l; mp 117-121C; IH NMR (300 MHz,
CDC13) ~ 7.66 (dd, 4 H), ~.97 (m, 8 H), 2.44 (s, 3 H), 1.60 (brs, 1 H).
FYS~m~ S 50 and 51
o
~N ~ ~N~
o
H3C~ ¦ ~N ,
51
1,4-Bis-t3'-(4"-benzyl-1"-~il)el~i"yl)-propanoyl] benz, "c (50) and 1-(p-Acetylphenyl)-3-(4'-
benzyl-l'-~ .,,,;,,yl)-1-~,,u~,~,one dihydr~chloride (51): Using the general method gave bodl
20 collll~ullrl~ afterpnrifir~ti( n via flash Chl~JIIIQ~ hY (Silica, EtOAc). Each was converted to
-50-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/160S2 PCT/US95/14987
its dihydrochlo~ salts by the addition of dry ether-HCl. Higher Rfl = .43 (8:2 EtOAc/MeOH)
gave 8 mg (50): mp ~ 250-C; lH NMR of the free base (300 MHz, CDCl3) ~ 8.0 (s, 4 H), 7.3
(m, 5 H), 3.5 (s, 2 H), 3.2 (t, 2 H), 2.85 (t, 2 H), 2.65 (s, 3 H), 2.5 (brm, 8 H). Lower Rf2 =
.14 (8:2 EtOAc/MeOH) gave 0.21 g (51): mp > 245 C; lH NMR of the free base (300 MHz,
5 CDC13) o 8.0 (s, 4 H), 7.3 (m, 10 H), 3.5 (s, 4 H), 3.2 (t, 4 H), 2.85 (t, 4 H), 2.5 (brm, 16
H).
FY~mrl~c 52 and 53
O O
CH3J~ ~ N~N~3
O O
10 ~ N~ ~50~--N~N
1-(8"-Acetylph~nnY~thiin-2"-yl)-3-(4'-benzyl-1'-pi~ a~ yl)-1-pl(,pa.~olle dihydrochloride (52)
and 2,8-Bis-[3'(4"-benzyl-1"-~ui~.d~,~yl)propanoyl] ph~.,o~ h;;~ (53): According to
Examples 50 and 51, 1,4-diacetyl~..~e"c was replaced with 2,8-diace.vlyhcnox~thiin (0.25 g,
15 0.88,nrnol). After pnrifif~tir)n by flash chlul . .~tography (Silica. EtOAc) the two L ludu- l~. were
icol~t~A Rfl = .47 (8:2 EtOAc/MeOH) was converted to its collc~ onding salt by the ~ln'itinn
of dry ether-HCl to give 95 mg (52): mp > 250'C; 1H NMR of the free base (300 MHz,
CDCl3) ~ 7.8-7.5 (m, 4 H), 7.3 (m, 5 H), 7.15-6.95 (m, 2 H), 3.5 (s, 2 H), 3.1 (m, 2 H), 2.8
(m, 2 H), 2.65-2.3 (brm, 11 H). Rf2 = .24 (8:2 EtOAc/MeOH) was converted to its
20 co~l~ollding hydrochlo~i~e salt to give 35 mg (53): mp ~ 250 C; lH NMR of the free base
(300 MHz, CDCl3) â 7.8-7.5 (m, 4 H), 7.3 (m, 10 H), 7.15 (d, 1 H), 7.05 (d, 1 H), 3.55 (s, 4
H), 3.1 (m, 4 H), 2.85 (m, 4 H), 2.5 (brm, 16 H).
F~mrl~ 54
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
Wo 96/16052 PCT/US95tl4987
02N~ ~N~
O F
l-~o-Ni~ h~ rl)-3-(4'-o-fl, o.o~n~oyl-l'-pi~.e.azi-lyl)-l-propanone dihydrochlonri~ The
solid was basified with NH40H, e ~ l . j.rt~ A with dichlo~ L~ ç and purified via flash
cLI..."~lo~".l.l-y (Silica, EtOAc). The free amine was converted to its hydrochl(~ salt by the
5 addition of dry ether-HCI; filtered and dried: mp 217-218-C; 1H NMR of the free base (300
MHz, CDC13) o 8.32 (d, 2 H), 8.11 (d, 2 H), 7.4 (m, 2 H), 7.27-6.99 (m, 2 H), 3.8 (m, 2 H),
3.48-3.1 (m, 4 H), 2.89 (t, 2 H), 2.7-2.3S (m, 4 H).
FY~mpl~ S5
1-(~7-NiLIuphe~lyl)-3-[4'-(3'',4''-difluolu~,lzyl)-l'-pip~ inyl]-l-plop~ulone dihydrochloride:
10 mp 210-212 C; lH NMR (300 MHz, D20? ~ 8.22 (d, 2 H), 8.04 (d, 2 H), 7.37-7.09 (m, 3 H),
4.35 (s, 2 H), 3.7-3.35 (brm, 12 H).
FY~mrie 56
o
~~ ~N J~3
1 -(S"-Phenyl-2"-thienyl)-3-(4'-benzyl- 1 '-~ ~llyl)- 1 -p-upallone dihydrochloride: mp 224-
15 247 C; IH NMR (300 MHz, D20) ~ 7.76 (m, 1 H), 7.67 (m, 2 H), 7.37 (m, 9 H), 4.33 (s, 2
H), 3.5 (brm, 12 H).
Example 57
1-(~"-Bromo-2"-thienyl)-3-(4'-benzyl-1'-,,,;l.e.~.7;.~yl)-1-propanone dihydroçhlon~ The solid
was basified with NH40H, G~ ,L~d with dichlc lur . ~ ~ h~ le and purified via flash
20 cll~.,.",.~ rhy (Silica, EtOAc). The free amine was converted to its hydrochl~n~l~ salt by the
-52-
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
WO 96/16052 PCI/US95/14987
a~dition of dry ether-HCl; filtered and dried: mp 242-244-C; IH NMR (300 MHz, D20) ~ 7.58
(d, 1 H), 7.37 (brs, 5 H), 7.15 (d, 1 H), 4.32 (s, 2 H), 3.5 (brm, 12 H).
Example 58
~~ N~
S l-(Biphenylen-2"-yl)-3-(4'-benzyl-1'-pi~ ,inyl)-1-p~,~none: mp 133-134-C; lH NMR (300
MHz, CDC13) ~ 7.46 (d, 1 H), 7.29 (m, 5 H), 7.17 (s, 1 H), 6.9-6.65 (brm, 5 H), 3.52 (s, 2
H),3.04(t,2H),2.81 (t,2H),2.52(brs,8H).
FY~mpl~ 59
1 -(5 "-Chloro-2"-thienyl)-3-(4'-benzyl- 1 '-I)i~, d~ in~l)- 1 -prop~none dihydrochlnri~ mp 248-
10 249-C; lH NMR (300 MHz, D2O) ~ 7.63 (d, 1 H), 7.36 (brs, 5 H), 7.0 (d, 1 H), 4.28 (s, 2
H), 3.44 (brm, 12 H).
FY~mpl~ 60
1-(m-Cyanophenyl)-3-(4'-benzyl-1'-~ yl)-1-~ulu~,,..,one dihydrochloride: The solid was
basified with NH40H. t;~ acL~,d with dichlulu..~ and purified via flash chlulllalû~;lapl~y
15 (Silica, EtOAc). The free amine was converted to its hydr~chlr-rirl~ salt by the ~n~n~inon ûf dry
ether-HCl; filtered and dried: mp 247-248 C; lH NMR (300 MHz, CDCl3) o 8.28-8.1 (m, 2
H), 7.84 (d, 1 H), 7.61 (t, 1 H), 7.3 (brm, 5 H), 3.S1 (s, 2 H), 3.17 (t, 2 H), 2.84 (t, 2 H), 2.5
(brm, 8 H).
~Y~mrl~ 61
20 1 -(6"-Fluoro-2"-methylphenyl)-3-(4'-benzyl- 1 '-pi~,~;l~inyl)- 1 -propanone dihydrochloride: mp
210-C (dec.); lH NMR (300 MHz, DMSO-d6) o 12.15-11.40 (brs, 2 H), 8.05-7.95 (m, 1 H),
7.70-7.40 (m, 5 H), 7.30-7.20 (m, 2 H), 4.55-4.20 (brs, 2 H), 3.90-3.20 (m, 12 H).
Example 62
-53-
SUBSTITUTE SHEET (RULE 26)
=
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
N~ ~ r
O F
l-(S'''-Bromo-2'''-thienyl)-3-[4'-(2'',6''-dinuo,ubenzoyl)-l' ~ ,.;nyl]-l-~lu~allolle
hy~Lo~ l~ln.,i~1e mp 225-226-C; lH NMR (300 MHz, DMSO-d6/D20) o 7.9 (d, 1 H), 7.63 (m,
1 H), 7.49 (d, 1 H), 7.28 (t, 2 H), 4.65 (brs, 2 H), 3.95-3.03 (m, 1~ H).
FY~mrl~ 63
l-~D-Nillu~hc~lyl)-3-r4'-(2'',3''-difluo~u~l~2url)-ll-~ dzillyl]~ lu~ olle dihydrochloril1e
The solid was basified with NH~LOH, extracted with dichlo,u,. ,I.~ne and purified via flash
C1~ t~ lllY (Silica, EtOAc). The free amine was converted to its hydr~chlnrirl~ salt by the
~rlrlitinn of dry ether-HCI; filtered and dried: mp 213-214 C; IH NMR (300 MHz, DMSO-d6)
10 10.7 (brs, 1 H), 8.39 (d, 2 H), 8.23 (d, 2 H), 7.58 (m, 1 H), 7.34 (m, 2 H), 3.85-3.0 (m, 12
H).
FY~mrle 64
l-(o-Chlo,ul,henyl)-3-(4'-benzyl-1'-pil,e~dzinyl)-1-p~panone dihydrochloride: The solid was
basified with NH40H. rx~ d with dichlo~ ne and purified via flash cl~lul~laLography
l5 (Silica, EtOAc). The free arnine was converted to its dihydror~hlnrirl~o salt by the addition of dry
ether-HCI; filtered and dried: mp ~ 250-C; lH NMR (300 MHz, D20) ~ 7.8-7.18 (m, 9 H),
4.55 (s, 2 H), 3.64 (brs, 12 H).
Exarnple 65
I-(o-Nitrophenyl)-3-(4'-benzyl-1'-pip~"~nyl)-1-propanone dihydrochloride: The solid was
20 basified with NH40H, extracted with dichlolu.~ ne and purified via flash cl.l~"latugraphy
(Silica, EtOAc). The free amine was converted to its dihydrochlon~l~ salt by the addition of dry
ether-HCI; filtered and dried: mp 226-228-C; lH NMR (300 MHz, D20) ~ 8.25 (d, 1 H),7.89
(t, 1 H), 7.79 (t, 1 H), 7.56 (m, 6 H), 4.4 (s, 2 H), 3.7-3.28 (brm, 12 H).
-54-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
FY~mrle 66
1-(o-Dlu~ .he.~yl)-3-(4'-benzyl-1'-pi~ yl)-1-~ one dihydrochlo7~ mp > 250 C; lH
NMR (300 MHz, DMSO-d6, H20) o 7.73 (d, 2 H), 7.64-7.3 (m, 7 H), 4.25 (brm, 2 H), 3.52
(brs, 12 H).
FY~mrl~ 67
o
~ ~N~
Me
1-(5"-Methyl-2"-furanyl)-3-(4'-benzyl-1'-pipe.~illyl)-1-propanone hydrochlori~ie mp 213-C
(dec.); lH NMR (300 MHz, DMSO-d6) o 12.40-11.60 (brs, 2 H), 7.75-7.55 (brs, 2 H), 7.55-
7.35 (brs, 4 H), 6.4 (d, 1 H), 4.55-4.20 (brs, 2 H), 3.80-3.20 (m, 12 H), 2.35 (s, 3 H), 3.90-
10 3.30(m,20H).
FY~mpie 68
o
N ~--N~N `~1
~ O
l-(p-N-MoIph~ n~1~h~ yl)-3-r4~-p-iodobenzoy~ illyl]-~ lol~e ~ chlf..~
mp 240 C; lH NMR (300 MHz, CDC13) o 7.91 (d, 2 H), 7.80 (d, 2 H), 7.17 (d, 2 H), 6.85
15 (d, 2 H).
- E,Yample 69
~S~ N~N
-55-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 Pcr/uS95/14987
l-p-p~ y~ yl-3-(4~-benzy~ yl)-3-~ dnon~ dihydro~hlor~ mp 210 C
(dec.); lH NMR (300 MHz, DMSO-d6) ~ 12.30-11.10 (brs, 2 H), 7 90 (d, 2 H), 7.70-7.40
(m, 10 H), 7.30 (d, 2 H), 4.60-3.90 (brs, 2 H), 3.90-3.10 (m, 12 H).
FY~mrl~ 70
O
~ ~N~
O
1-(2"-Thienyl)-3-[4'-(o-fluo,~J'oel,2oyl)-l'-pi~ yl~ u~anonelly~lluehloride: mpl83-
184 C: IH NMR (300 MHz, CDC13) o 10.30-10.07 (brs, 1 H), 7.90 (d, 1 H), 7.76 (d, 1 H),
7.55-7.35 (m, 2 H), 7.30-7.05 (m, 3 H), 4.97-4.80 (brd, 1 H), 4.15-3.95 (m, 1 H), 3.90-3.40
(m, 8 H), 3.05-2.8 (m, 2 H).
FY~mrl~ 71
1-[4~-(p-IIyLu~y-m~ upll~nyl)~-3-(4~-'oenzyl-1'-~i~e ~,~ u~anone dihydrochloride:
mp 209-212 C; lH NMR (300 MHz, D20) o 8.75 (s, 1 H), 8.24 (d, 1 H), 7.65-7.50 (brs, 5
H), 7.31 (d, 1 H), 4.51 (s, 2 H), 3.86-3.60 (m, 12 H).
FY~mrl~ 72
15 1-~D-N-Morpholinophenyl)-3-(4'-benzyl-1 '-pire~7inyl)- 1 -~lu~al~olle dihydrochloride: mp
205 C (dec.); lH NMR (300 MHz, D20) o 7.80 (d, 2 H), 7.45-7.30 (brs, 5 H), 6.95 (d, 2 H),
4.32 (s, 2 H), 3.80-3.66 (m, 4 H), 3.60-3.40 (m, 12 H), 3.30-3.20 (m, 4 H).
~Y~mrl~ 73
l-~-Cyanophenyl)-3-(4'-benzyl-1'-~ipt~ l)-1-propanonedihydrochlcri-ie: mpl90C
20 (dec.); lH NMR (300 MHz, DMSO-d6) o 8.29-7.90 (m, 4 H), 7.70-7.15 (m, 5 H), 4.45-4.05
(brs, 2 H), 4.00-3.00 (m, 12 H).
FY~mr1t 74
-56-
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
Wo 96/16052 PCr/US95/14987
MeSO2~ ~~N~ ~
l-(m-lvl~ ..P~..lfon~m; lophpnyl)-3-(4~-benzy~ -pi~ ;..yl)-l-plu~ e dihydrochl~nr~
mp 180-182-C; lH NMR (300 MHz, D2O) o 7.95-7.85 (m, 2 H), 7.65-7.,50 (m, 7 H), 4.49
(s, 2 H), 3.85-3.60 (brs, 12 H), 3.14 (s, 3 H).
Example 75
l-(m-Fl~lolù~henyl)-3-(4'-benzyl-l'-~ yl)-l-plul)anonedih~ lucllloride: mp251-253-C;
1H NMR (300 MHz, DMSO-d6) o 12.2 (brm, 2 H), 7.9-7.3 (m, 9 H), 4.4 (s, 2 H), 4.0-3.18
(m, 12 H).
Example 76
~ N~3
1-(2-Thiazolyl)-3-(4'-benzyl-1'-~ yl)-l-plù~ olledihydrochlon~e: Thesolidwasbasified with NH40H, e~ r~ with dichlulu...~Ll~ne and purified via flash clllulllaLo~ y
(Silica, EtOAc): IH NMR (300 MHz, CDC13) o 8.0 (d, 1 H), 7.66 (d, 1 H), 7.28 (m, 5 H),
3.5(s,2H),3.37(t,2H),2.88(t,2H),2.5(brm,8H).
1~ FY~n~rl~ 77
1-Lp-(Trifluljlulll~,lhyl)-phenyl]-3-(4'-benzyl-1'-pi~.~hlyl)-1-~lu~ one: Thesolidwas
basified with lN KOH, extracted with dichlc"u,l.~ane, dried and filtered: mp 59-60 C; lH
NMR (300 MHz, CDC13) ~ 8.06 (d, 2 H), 7.74 (d, 2 H), 7.3 (m, 5 H), 3.52 (s, 2 H), 3.2 (t, 2
H), 2.87 (t, 2 H), 2.53 (brm, 8 H).
Example 78
SUBSTITUTE SHEET (RULE 26)
-
CA 0220~x6 1997-0~-16
Wo 96/16052 PCT/US95/14987
1 -(5"-Bromo-2"-thienyl)-3-r4'-( 1 "'-p-chlorophenyl- 1 "'-phenylmethyl)- 1 '-pi~ inyl3- 1-
~lu~ olle dihydrochlon~ie mp 141-144 C; lH NMR (300 MHz, CDC13) ~ 7.44 (d, 1 H),
7.39-7.12 (m, 7 H), 7.07 (d, 1 H), 4.19 (s, 1 H), 3.10 (t, 2 H), 2.8 (t, 2 H), 2.6S-2.25 (brm, 8
H)-
S F-~mrl~ 79
i~Ji N~
1-(4" MLLIIOAY-1 "-n~ph-h~l~nyl)-3-(4'-benzyl-l'-pipcl~il~yl)-1~ ol-edihydro~hlon~e mp
230 C (dec.); lH NMR (300 MHz, D20/DMSO-d6) ~ 8.88 (d, I H), 8.27 (t, 2 H), 7.73-7.35
(m, 7 H), 7.1 (d, 1 H), 4.52-4.02 (m, 5 H), 3.65 (brm, 12 H).
- FY~mrlP 80
1-(5"-Nitro-3"-thienyl)-3-(4'-benzyl-1'-~ zinyl)-1-propanone dihydroch1orit1e mp 220'C
(dec.); lH NMR (300 MHz, D20/DMSO-d6) ~ 88. (d, 1 H), 8.47 (d, 1 H), 7.66-7.42 (m, 5
H), 4.3 (s, 2 H), 3.85-3.2 (m, 12 H).
Example 81
15 1-o-Iodophenyl-3-(4'-benzyl-1'-pip~ yl)-1-propanone dihydrochlonr1e: mp 240-242 C; lH
NMR of the free base (300 MHz, CDC13) ~ 7.9 (d, 1 H), 7.5 (m, 8 H), 3.5 (s, 2 H), 3.08 (t, 2
H), 2.78 (t, 2 H), 2.49 (brs, 8 H).
Example 82
Ç ~N~
-58-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US9~i;/14987
1 -(S"-Chloro-3 "-methylbenzo[b] -2"-thienyl)-3-(4'-benzyl- 1 '-pi~, ~inyl)- 1 -~luyanolle
dihydroehl~n~- mp 247 C (dec.); lH NMR of the free base(300 MHz, CDCl3) o 7.84 (d, I
H), 7.76 (d, 1 H), 7.45 (dd, 1 H), 73 (m, 5 H), 3.53 (s, 2 H), 3.15 (t, 2 H), 2.9 (t, 2 H), 2.71
(s,3H),2.55(brm,8H).
FY~mrl~ 83
l-(S"-r2"'-thienyl]-2"-thienyl)-3-(4'-benzyl-1'-~ h~yl)-1-1,lol)dnone dillydr~llloride: mp
249-252 C; lH NMR (300 MHz, D20/DMSO-d6) o 7.96 (d, 1 H), 7.67 (d, 1 H), 7.63-7.34
(m, 7 H), 7.15 (t, 1 H), 4.25 (brs, 2 H), 3.4 (brm, 12 H).
Example 84
10 1-(S"-Bromo-2"-thienyl)-3-(4'-o-fluorobcl"yl-1'-~ipeld~inyl)-1-propanonedihydrochlonr;~o: mp
224-226 C; IH NMR of the free base (300 MHz, CDC13) ~ 7.45 (d, 1 H), 7.36 (m, 1 H),7.23
(m, 1 H), 7.15-6.92 (m, 3 H), 3.6 (s, 2 H), 3.03 (t, 2 H), 2.82 (t, 2 H), 2.54 (brs, 8 H).
FY~mrle 85
l-(S''-Cyano-2''-thienyl)-3-(4'-benzyl-1'-pi~ yl)-l-~lu~anone dihydrochlonrl~ mp 256-
15 258 C; lH NMR of the free base (300 MHz, CDC13) ~ 7.63 (dd, 2 H), 7.27 (m, 5 H), 3.5 (s, 2
H),3.1(t,2H~,2.84(t,2H),2.5(brm,8H).
Example 86
~ ~N~
Br o
1 -(5"-Bromo-2"-thienyl)-3-[4'-(3"'-chlorobenzo~b]-2"'-thienoyl)- 1 '-~ yl]- 1-propanone
20 dihydrochloride: mp 188-189 C; lH NMR of the free base (300 MHz, CDC13) ~ 7.84 (m,2
H),7.48(m,3H),7.12(d, lH),3.83(brm,2H),3.5(brm,2H),3.04(t,2H),2.88(t,2H),
2.58 (brs, 4 H).
59
SUBSTITUTE SHEET (RULE 26~
CA 02205586 1997-05-16
WO 96/16052 PCI~/US95/14987
FY~rl~ 87
1-(6"-M~ y-2"-n~phth~l~nyl)-3-(4'-benzyl~ ip~,~dzillyl)-l-ll,updllo,-edihydrochloride: mp
243-246 C; lH NMR (300 MHz, D20/DMSO-d6) o 8.62 (s, 1 H), 8.12-7.86 (m, 3 H), 7.65-
7.34 (m, 6 H), 7.27 (m, 1 H), 7.42-7.32 (m, 17 H).
Fr~mpl~ 88
l-(p-Methylthiophenyl)-3-(4'-(2'',6''-difl,lolubGI,,.yl)-l'-~ ,dzinyl)-l-p,upallone
dihydroçhlnTi~ mp 187-188 C; lH NMR (300 MHz, CDCl3/MeOD) o 7.90 (d, 2 H),7.65-
7.50 (m, 1 H), 7.30 (d, 2 H), 7.23-7.10 (m, 2 H), 4.00-3.40 (m, 14 H~, 255 (s, 3 H).
Fr~mrl~ 89
o
~~~ ~N"~
1 -(2"-Thienyl)-3-r4'-(1 "'-p-chlorophenyl- 1 "'-phenylmethyl)- 1 '-~ ~nyl]- 1-~,upanu--c
dihydrochloride: mp 189-201 C; IH NMR (300 MHz, DMSO-d6) â 8.05 9d, 1 H), 8.00 (d, 1
H), 7.55-7.20 (m, 10 H),4.60 (brs, 1 H), 3.80-3.20 (m, 12 H).
~r~mpl~ 90
15 1-~D-N-Piperidinophenyl)-3-(4'-benzyl-1'-pi~ yl)-1-plu~anoné dihydrochloride: mp 135-C
(dec.); lH NMR (300 MHz, CDC13tDMSO-d6) o 8.00 (d, 2 H), 7.90-7.40 (m, 7 H), 4.35 (s, 2
H), 4.15-5.00 (m, 2 H), 4.00-3.80 (m, 2 H), 3.80-3.40 (m, 12 H), 2.15-1.95 (m, 4 H), 1.80-
I.65(m,2H).
Example 91
-60-
SUBSTITIJTE SHEET (RULE 26)
- CA 02205586 1997-05-16
Wo 96tl6052 PCT/US95/14987
N~
SO2
l-(N-Phenylsulfonylpyrro1-2-yl)-3-(4'-benzyl-1'-P;l ~ yl)-1-p,u,.)anul-cdihydrochlonde: mp
215-C (dec.); lH NMR (300 MHz, DMSO-d6) â 8.0807.85 (m, 2 H), 7.75-7.35 (m, 10 H),
6.60-6.50 (m, 1 H), 4.30 (brs, 2 H), 3.80-3.00 (m, 12 H).
Fy~mrle 92
1-[3',5'-Bis(llinuul~lLoxy)phenyl]-3-(4'-benzyl- 1 '-P;l~ yl)- I -propanone dihydrochlnnde
mp 170-172 C; lH NMR (300 MHz, DMSO-d6) ~ 7.70-7.20 (m, 8 H), 4.70-4.95 (m, 4 H),
7.25 (brs, 2 H), 3.80-3.20 (m, 12 H).
FY~mpl.o 93
10 1-(2''-Bromo-5''-l~iLlù~he,lyl)-3-(4'-benzyl-1'-piy~ lyl)-1-propanone dihydrochlon~ mp
230 C (dec.); lH NMR (300 MHz, DMSO-d6) ~ 8.60 (s, 1 H), 8.25 (d, 1 H), 8.05 (d, 1 H),
7.65-7.40 (m, 5 H), 4.30 (brs, 2 H), 3.80-3.20 (m, 12 H).
Example 94
3-(4'-Benzyl-l'-p;l.e..~ -yl)-thioch~ -4-one dihydrochloride: mp >250 C (dec.); lH
15 NMR (300 MHz, D2O) o 7.90 (d, 1 H), 7.37 (m, 6 H), 7.25 (m, 1 H), 7.14 (m, 1 H), 4.32 (s,
2 H), 3.70-3.33 (m, 10 H), 3.24 (m, 2 H), 3.17 (m, 1 H).
Example 95
1-(5"-Chloro-4"-nitro-2"-thienyl)-3-(4'-benzyl- 1 '-pi~l~lyl)- 1-~1upallullc dihydrochloride: To
a mixture of isoplv~ ol (25 mL) and concellt,~Led hydrochloric acid (1.45 mL,0.017 mol) was
20 added l-~,l~ylpi~ e (1.00 g, 5.68 mmol) to form the hydrochloride salt in situ.
-61-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCI~/USgS/14987
P~ d~hyde (0.34 g, 11.36 mmol) was then added followed by 5-acetyl-2-chloro-3-niL~ ic~hene (1.28 g, 6.25 mmol). The resulting mixture was refluxed for 72 h. A white
solid ~ formed, was filtered and the filter cake was ~ ,. ..lf d with ~ llr)l to give
0.99 g (44%) of the desired product: mp 220-223-C; lH NMR (300 MHz, D20/DMSO-d6) o
5 8.57 (s, l H), 7.65-7.45 (m, 5 H), 4.37 (s, 2 H), 7.65-7.45 (m, 5 H), 4.37 (s, 2 H), 3.65-3.30
(m, 12 H).
FY~PIO 96
I-(2"-Thienyl)-3-(4'-benzyl-1 '-piperidinyl)-1-propanone hydrochloride: mp 189-l90 C; 1H
NMR (300 MHz, CDC13) o 12.55-12.35 (brs, 1 H), 7.95 (d, 1 H), 7.73 (d, 1 H), 7.35-7.05
10 (m, 6 H), 3.73 (t, 2 H), 3.60-3.35 (m, 4 H), 2.75-2.55 (m, 4 H), 2.35-1.95 (m, 2 H), 1.90-
1.65 (m, 3 H).
FY~m~l~ 97
1 -(5~-Bromo-2~-thienyl)-3-(4~-p-fluorobenzoyl- 1 '-piperidinyl)- I -propanone hydrochloride: mp
201-203 C; IH NMR (300 MHz, DMSO-d6) o 10.55-10.33 (brs, 1 H), 8.30-8.20 (m, 2 H),
15 8.07 (d, 1 H), 7.70-7.50 (m, 3 H), 3.90-3.15 (m, 6 H), 2.25-1.95 (m, 5 H).
Example 98
NO2~ F
1 -t2 "-Mtro-4 "-thienyl)-3-(4'-p-fluul ~Ibcnzc yl- 1 '-piperidinyl)- 1 -propanone hydrochloride: mp
205-206 C; lH NMR (300 MHz, DMSO-d6) ~ 10.55-10.35 (brs, 1 H), 8.80 (s, 1 H), 8.08 (s,
20 lH),8.10(dd,2H),7.37(dd,2H),3.75-3.50(m,5H),3.50-3.35(m,2H),3.20-3.00(m,2
H), 2.10-1.80 (m, 4 H). '`
Example 99
-62-
SUBSTITUTE SHEET (RULE 26
,
CA 0220~86 1997-0~-16
WO 96/16052 PCr/US95/14987
-
1-(5"-Bromo-2"-thienyl)-3-(4'-benzyl-1'-pil,~,.i lillyl)-1-propanone hydrochlori(le. mp 205-
208 C; lH NMR (300 MHz, CDC13) o 7.44 (d, 1 H), 7.35-6.9~ (m, 6 H), 3.2 (t, 2 H), 2.88
(m, 2 H), 2.77 (t, 2 H), 2.53 (m, 2 H), 1.96 (m, 2 H), 1.74-1.43 (m, 3 H), 1.3 (m, 2 H).
FY~n~rl~ 100
1 -(5"-Bromo-2"-thienyl)-3-(4'-~2"'-chloro-6"'-methylpyrido-4"'-yl]- 1 '-piperazinyl)- 1 -
~lul)anone dihydrochlnri-le A mixture of 2-acetyl-~-blulllotl~iophene (0.50 g, 2.44 mmol), the
cG.ll...oul~d from ex~mp!e 49s (0.45 g, 1.88 m!I~.o!), p~ fc~ dehyde (73.0 mg, 2.44 m.m..o!),
and co,-rcf.~ .,d hydrochloric acid (0.19 mL, 2.26 mmol) in 20 mL iso~ a-~ol were refluxed
10 72 h. The reactiûn was cooled and filtered. The mother liquor was ~ol-ce ,I . ~.t. rl, basified
(NaHCO3) and c~ rt. ~ with CH2CI2. The c~,al)ulalGd residue was purified via flash
cL~,lllaLu~la~l.y (Sili~ ~-FtOAc). The resulting oil was dissolved in ether and forrned into its
coll~,;,yul~ding hydro~hlo~i~e salt by the ~rl~litio~ of dry ether/HCI to give 25 mg (2.7%) of the
desired pl~lu-;L. mp 214-215C; lH NMR of the free base (300 MHz, CDC13) ~ 7.46 (d, 1 H),
15 7.18-6.99 (m, 3 H), 3.75 (brm, 2 H), 3.35 (brm, 2 H), 3.04 (t, 2 H), 2.87 (t. 2 H), 2.70-2.32
(m, 7 H).
Example 101
1-(5"-Bromo-2"-thienyl)-3-(4'-b~,.-7~.~r~..lfonyl-l'-l)ipe,a~illyl)-1-~,upanolle dihydrochloride: A
mixture of 2-acetyl-5-b.~"l,oLl,iophene (0.33 g, 1.60 mmol), the co,ll~u"d of ex~mple 49r
20 (0.30 g, 1.33 mmol), paraformaldehyde (48.0 mg, 1.60 mmol), and concentrated
hydrochloric acid (0.17 mL, 1.99 mmol) in 15 mL isopropanol were refluxed for 16 h. The
reaction was cooled, filtered and the filter cake was recryst~lli7~d from meth~nol to give 40 mg
(7.4%) of the desired product: mp 129-130C; IH NMR free base (300 MHz, CDC13/NaOD)
-63-
SUBSTITUTE SHEET (RULE 26
=
CA 02205586 1997-05-16
WO 96/16052 PCr/US95114987
7.74 (m, 2 H), 7.65-7.45 (m, 3 H), 7.40 (m, 1 H), 7.07 (m, 1 H), 2.98 (m, 6 H), 2.8 (m, 2
H), 2.57 (m, 4 H).
FY~mI~l.o 102
Nillu~h~"~yl)-3-(4'-ben7. .~e;".ifonyl-l' p;l.~,.a,;.,yl)-l-prop~no~e dihydrochloride: mp 193-
5 195C; IH NMR free base (300 MHz, CDC13) ~ 8.30 (d, 2H), 8.10 (d, 2H), 7.75 (m, 2H),
7.57 (m, 3H), 3.18 (t, 2H), 3.03 (m, 4H), 2.86 (t, 2H), 2.60 (m, 4H).
FY~mrl~ 103
1-~D-Nitrophenyl)-3-[4'-(4"-methyl- 1 ",2",3"-thi~ 7-5"-oyl)]- 1 '-piperazinyl)- I -propanone
dihydrochlonde mp 207-209C; IH NMR (300 MHz, D20) o 8.38 (d, 2H), 8.21 (d, 2H~,10 4.40-3.25 (m, 12H~, 2.72 (s,3H).
FY~mrl~ 104
1-(4''-Morpholino-3''-nillupllenyl)-3-(4'-benzyl-l'-pip,,,~inyl)-1-propanone trihydroch1- n-ie:
IH NMR (300 MHz, DMSO-d6) ~ 12.25-11.55 (brs, 2H), 9.65-9.45 (brs, lH), 8.75 (s, lH),
8.75 (d, lH), 7.75-7.30 (m, 6H), 4.60-4.20 (brs, 2H3, 4.00-3.00(m, 20H).
FY~mrle 105
1-(3"-Nitro-4"-N-piperidinophenyl)-3-(4'-benzyl-1'-piperazinyl)-1-propanone trihydrochloride:
IH NMR (300 MHz, DMSO-d6) ~ 11.95-11.70 (brs, 3H), 8.35 (s, lH), 8.04 (d, IH), 7.75-
7.55 (brs, 2H), 7.55-7.40 (brs, 3H), 7.35 (d, lH), 4.60-3.00 (m, 18H), 1.80-1.30 (brs, 6H).
Example 106
20 1-(5"-Methyl-2"-thienyl)-3-(4'-benzyl-1'-pip~illyl)-1-propanone dihydrochloride: mp 220C
(dec.); IH NMR (300 MHz, CDCl3/NaOD) ~ 7.53 (m, I H), 7.40-7.08 (m, 5 H), 6.78 (m, 1
H), 3.50 (s, 2 H), 3.04 (t, 2 H), 2.83 (l, 2 H), 2 67-2.30 (m, 11 H).
FY~mrl~ 107
-64-
SUBSTlTlJTE SHEET (RULE 2~r,~
CA 0220~86 1997-0~-16
Wo 96/16052 PCT/US95/14987
1-[4"-p-(5"',l"'-Dichloroimi~7O1-l"'-yl)phenyl]-3-(4'-benzyl-l'-piperazinyl)-1-propanone
dihydrochloride: mp 200-202C; IH NMR (300 MHz, DMSO-d6) ~ 12.25-11.30 (brs, 2 H),
8.30-8.10 (m, 3 H), 7.77 (d, 2 H), 7.70-7.30 (m, 5 H), 4.35 (brs, 2 H), 3.90-3.20 (m, 12 H).
FY~n~ e 108
5 1-r5~-(phenylethynyl)-2~-thienyl]-3-(4-benzy~ -pi~e~ lyl)-l-plup~one dihydr~!chlnrirl~:
mp 205C (dec.); IH NMR (300 MHz, DMSO-d6) o 12.30-11.20 (brs, 2 H), 8.00 (d, 1 H),
7.80-7.30 (m, 11 H), 4.30 (brs, 2 H), 3.90-3.00 (m, 12 H).
Exarnple 109
1-(3"-Nillu~hc"yl)-3-(4'-~ lonyl-1'-~i~cla.i~yl)-1-1,l~onedil~y~llucl~loride: mp215C
lO (dec.); IH NMR (300 MHz, DMSO-d6) o 12.35-11.20 (brs, 2 H), 8.70 (s, 1 H), 8.52 (d, 1 H),
8.40 (d, 1 H), 7.70 (dd, 1 H), 7.25 (s, 1 H), 7.15-6.90 (m, 2 H), 6.05 (s, 1 H), 4.25 (brs, 2
H), 3.90-3.10 (m, 12 H).
FY~rnri~llO
1 -(5"-Methyl-3"-phenylisoxazol-4"-yl)-3-(4'-benzyl- 1 '-~ip~l~inyl)- 1 -l~lupallone
15 dihydrochloride: mp 220C (dec.); IH NMR (300 MHz, DMSO-d6) ~ 12.35-11.40 (brs, 2 H),
7.70-7.30 (m, 10 H), 4.35 (brs, 2 H), 3.70-3.10 (m, 12 H), 2.80 (s, 3 H).
FY~n rl-o. 1 1 1
1-(2"-Phenylthiazol-4"-yl)-3-(4'-benzyl-1'-pi~,la2ih~yl)-1 propanonedihydrochlonrlç: mp
220C (dec.); IH NMR (300 MHz, DMSO-d6) ~ 12.20-11.50 (brs, 2 H), 8.65 (s, 1 H), 8.10-
20 7.90 (brs, 2.H), 7.75-7.30 (m, 8 H), 4.30 (brs, 2 H), 3.80-3.20 (m, 12 H).
Fy~mrl~ 112
1 -(3"-Phenylisoxazol-4"-yl)-3-(4'-benzyl- 1 '-piperazinyl)- 1-propanone dihydrochloride: mp
212C (dec.); IH NMR (300 MHz, DMSO-d6) ~ 12.25-11.40 (brs, 2 H), 8.10-7.90 (m, 3.H),
7.70-7.30 (m, 8 H), 4.35 (brs, 2 H), 3.80-3.10 (m, 12 H).
Example 113
-65-
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO g6/16052 PCr/US95114987
1 -(4"-t2"',4"'-di~ ùphenylamino]phenyl)-3-(4'-benzyl- 1 '-piperazinyl)- 1 -propanone
dihydroehl~rid.o.: mp 248-250C; IH NMR (300 MHz, CDC13/NaOD) o 9.20 (m, 1 H), 8.26
(m, 1 H), 8.08 (m, 2 H), 7.50-7.10 (m, 8 Hj, 3.53 (s, 2 H), 3.21 (m, 2 H), 2.87 (m, 2 H),
2.55 (brrn, 8 H).
Exarnple 114
1 -(2",4"-dilll~;Ll,ylLl,iazol-5"-yl)-3-(4'-benzyl- I '-piperazinyl)- 1 -~ulupanol1e di~lyd~ uchloride:
mp: 228-C (dec); IH NMR (300 MHz, D20) ~ 7.55 (brs, 5 H~, 4.50 (s, 2 H), 3.87-3.42 (m,
12 H), 2.75 (s, 3 H), 2.67 (s, 3 H).
FYarnple 115
10 1-(m-NiL,u~,hc,lyl3-3-(4'-benzyl-l'-piperidinyl)-l-prûpanonehydrochloride: mpl89-191C;IH
NMR (300 MHz, CDC13) ~ 12.60-12.40 (brs, 1 H), 8.35 (d, 2 H), 8.18 (d, 2 H), 7.35-7.05
(m, 5 H), 3.95 (t, 2 H), 3.65-3.3S (m, 4 H), 2.80-2.55 (m, 4 H), 2.~0-1.65 (m, 5 H).
Exarnple 116
The following cullllJuull~L. may be ,u~ cd by the general method:
(a) 1-(6"-Blul,locoull,~ill-3"-yl)-3-(4'-benzyl-l'-pirer~7inyl)-l-propanone
dihy~lluchlnrit~ .
O
Br ~ N~ ~3
(b) 1-(6"-Bromocoumdlill-3"-yl)-3-(4'-benzoyl-1'-pip~ yl)-1-propanone
dihydrochln7 i~
(c) 1 -(I ",1 "-Dimethyl-6"-tert-butylindan-4"-yl)-3-(4'-benzyl- 1 '-piperazinyl)- 1 -
propanone dihydrochlorirl~
(d) 1 -(1 ",1 "-Dimethyl-6"-tert-butylindan-4"-yl)-3-(4'-benzoyl- 1 '-piperazinyl)- 1-
~,u~ano,le dihydrochlr~ri-i~
-66-
SUBSTITUTE SHEET (RUEE 26)
CA 02205586 1997-05-16
WO g6/16052 PCI`/US95tl4987
(e) l-(cou~ -3~r-yl)-3-(4~-benzy~ yl)- l-ylùl)anolle dihydro~hlt Ti~
(f) l-(Cou~ -3"-yl)-3-(4'-benzoyl-1'-~ip.~ yl)-1-,~1u~allûlle dihydrochlnnrle
(g) 1 -(5"-Cyano-4"-methyl-2"-(methylthio)thien-3"-yl)-3-(4'-benzyl- 1'-
-pl~:)pd~lollc clil~ydluc1~1nril1.o
(h) 1 -(5"-Cyano-4"-methyl-2"-(methylthio)thien-3"-yl)-3-(4'-benzoyl- 1'-
pip. .~illyl)- l-~l~anone dihydr~hlnnde
(i) 1 -~-(2",4"-Dichlorophenoxy)-phenyl)-3-(4'-benzyl- 1 '-pi~ zinyl)- 1-
pl~ ullC dihydroc hlnri~
(i) 1-~-(2",4"-Dichlorophenoxy)-phenyl)-3-(4'-benzoyl-1'-piperazinyl)-1-
10 plupanone dihydr~hlonde
(k) 1 -(5"'-(2",4"-Difluorophenoxy)-fur-2"'-yl)-3-(4'-benzyl- 1 '-piperazinyl)- 1-
propanone dihydroehlr)ri~
~ ~ N~
(1) 1-(5"'- (2",4"-Difluorophenoxy) -fur-2"'-yl)-3-(4'-benzoyl- 1 '-piperazinyl)- 1-
propanone dihydrochlnnrle
(m) 1 -(2",3"-Dihydrobenzo[b]fur-5"-yl)-3-(4'-benzyl- 1 '-piperazinyl)- 1 -propanone
dihydrochloride
-67-
SUBSTITUTE SHEET (RULE 2~)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
NJ~3
(n) 1 -(2",3"-Dihydrobenzorb~fur-5"-yl)-3-(4'-benzoyl- 1 '-pip~ lyl)- I -propanone
dihydrochloride
5(o) 1 -(3",5"-Dillletilylbenzorb]thien-2"-yl)-3-(4'-benzyl- 1'-~ d~inyl)- 1-
plo~anolle dihydroçhloritl~
(p) I -(3",5"-Dimethylbenzorb]thien-2"-yl)-3-(4'-benzoyl- 1 '-piper~7invl)- 1-
propanone dihydroçhlonrle
Me O
Me ~~N~N~
10(q) 1-(2",4"-Dimethylthien-5"-yl)-3-(4'-benzyl-1'-pi~ a~inyl)-1-~.op~,olle
dihydrochloride
Me o
Me N~N~ ~
(r) 1 -(2",4"-Dimethylthien-5"-yl)-3-(4'-benzoyl- 1 '-piperazinyl)- 1 -propanonedihydrochloride
15(s) 1-GD-[Phenylthio~phenyl)-3-(4'-benzyl-1'-~ilJel~hlyl)-l-propanone
dihydrochloride
-68-
SUBSTlTUrE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCrlUS95/14987
(t) l-(p-~Pl,~nyl~,io~iphenyl)-3-(4'-benzoyl-1'-pip~ yl)-1-plvpanone
dihydrorhlnntit.
(u) 1 -(3"-Metho,~ye~ bonyl-5"-methylisoxazol-4"-yl)-3-(4'-benzyl- 1 '-piperazinyl)-
u~lol~e dihydrochlnrid~
(v) 1-(3"-Metho,~yc~L.onyl-5"-methylisoxazol-4"-yl)-3-(4'-benzoyl-1'-
pi~e. ~ yl)- l-~lu~ one dihydrof hlnncle
(w) 1-(5''-(2'''-C~l~ulll~llloxythien-3~-yl)-furan-2~-yl)-3-(4~-b
~i~e.d~illyl)-l-~lop~-t,.~ dihydrothlnnrit~.
(x) 1 -(5"-(2"'-C~ bonl~,~hoxythien-3"'-yl)-furan-2"-yl)-3-(4'-benzoyl- 1'-
10 pi~.~inyl)-l-~lu~anone dihydroçhinn~
(y) l-(p-(4"-Methox~heno~y)-phenyl)-3-(4'-benzyl- 1 '-pi~,.~inyl)- l-~ro~ olle
dihy~lluchloride
(z) l -(p-(4"-Methoxyphenoxy)-phenyl)-3-(4'-benzoyl- 1 '-pi~,.azinyl)- 1 -1,-upanolle
dihydrochloride
1~ (aa) 1-(p-(4"-Nh~ul)hEnoxy)-phenyl)-3-(4'-benzyl-1'-1,ip~,.~illyl)-1-p~upanone
dihydrochloride
(bb) l-(p-(4''-Ni~uphelloxy)-phenyl)-3-(4'-benzoyl-l'-~ ,.a~illyl)-l-propanone
dihydrochloride
(cc) 1 -(m-Nitro-p-(phenylthio!-phenyl)-3-(4'-benzyl- 1 '-~i~.~inyl)- l-propanone
20 dihydror hlr)riti
~Oz~ N~
(dd) 1-(m-Nitro-p-(phenylthio)-phenyl)-3-(4'-benzoyl-1'-~ lyl)-1-~u~upallonc
dihydroçhk)rifi.-.
-69-
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
(ee) l-(m-Mtro-p-(1"-piperidinyl)-phenyl)-3-(4'-benzoyl-1'-pi~ inyl)-1-
plup&no~e dihydrochlnT-A~
(ff) I-(m-Nitro-p-(4"-morpholino)-phenyl)-3-(4'-benzoyl~ Jipc.~illyl)- 1-
p~u~ .,.e dihydro~hlnri-lP.
(gg) 1-(p-Nitrophenyl)-3-(4'-(5"-(1"'-methyl-3"'-trifluo.olllcLllylpyrazol-5"'-yl)-
thien-2"yl)- 1 '-p;l.~f ,~ yl)- l-l,-u~ano..e dihyd~ocll1nri~le
NO~ ~N~CF3
(hh) 1-(5"Bromo-2"-thienyl)-3-(4'-p-bromobc.,,~ne~lllfonyl-1'-pip~ illyl)-1-
~u~no~e hydrochlnride
lû (ii) l-(S"Bromo-2"-thienyl)-3-(4'-p-toluenesulfonyl-1'-pi~,e.~illyl)-1-plupanolle
hydroçhlnriflp
(ii) 1 -~D-Nillupl~e~lyl)-3-(4 -p-l~l u~ot~n ~f l~r i,ulfonyl- 1 '-pil,ei~zi--yl)- 1 -~-ùpanonc
hydrochlnnde
(ii) 1 -(5"-Chloro-4"-nitro-2"- thienyl)-3-(4'-bf n 7enes~ 1 If onyl- I '-piperazinyl)- 1-
15 ~ru~,anollc hydrochlnri~
(kk) 1-(2"-Chloro-3"-nitrothien-5"-yl)-3-(4'-o-trifluolo~llcLhylbenzoyl-l'-
P;l~ yl)-l-p.op~one dihydrochlnntie
(Il) 1 -~n-Nitrophenyl)-3-(4'-{4"-methyl- 1 ",2",3"-thiadiaz-5"-oyl]- 1 '-piperazinyl)-
u~allolle dihydrochloride.
(mm) 1 -(5"-Chloro-4"-nitro-2"-thienyl)-3-~4'-(4"'-methyl- 1 "',2"',3"'-thia~
5"'-ûyl)- 1'-pi~e.d~ yl]-1-propanone dihydrochloride.
-70-
S~B~ J I t s~E~ (RlJ~E 26)
CA 02205586 1997-05-16
WO 96/16052 PC'r/US95/14987
(nn) I -r5"-Cyano-4"-methyl-2"-(methylthio)-3"-thienyl~-3-(4'-benzyl- 1'-
P;l~ a~ yl)-1-y~ o,le dihydroçhlnri~ie
(oo) 1-(2",4"-Dimethyl-5"-thiazolyl)-3-(4'-benzyl-1'-~ ,c.azillyl)-1-propanone
dihydroçhlori~
S (pp) 1-(5"-Nitro-3"-thienyl)-3-(4'-o-fluoro~.~zoyl-1'-yil,e.~zinyl)-1-propanone
dihy~L~uçl .olr~ride
(qq) 1-(5"-Bromo-2"-thienyl)-3-(4'-o-fluolubenzc.yl-1'-~ip~ inyl)-l-plupanone
dihydrochnlori-le
F~m,rle 117
Effect of Chlc,-~lu.. ~ .~;.. e on Paired Helical Filament Levels
in Human Brain Tissue
The in vitro analyses desçrihed herein to investigate the activity of the co~ oullds of this
invention can also be used to ~letP-rmine the nature of abnorrnally phosphorylated paired helical
fil~m~nt epitopes involved in ~l7hpimer~s Disease, the regulatory mech~nicm~ involved with
15 ~17hrimrr~s Disease and mo l~;r;rAI;Ol-c of the protein tau and other polypeptides ~c$ociAtç~ with
PHFs and involved in ~1,1.~.;...~.. 's Disease.
Certain phenorhi~7in.-s, for e~ . .plc chlu~ ;nr. can mArk~Aly inhibit the expression
of PHF epitopes (as determined by loss of immllnoreactivity with ~l7hrim-or~s Disease specific
antibodies) upon ~ llllfnt of MSNla cells with okadaic acid (cf. Figure 33. These co,.l~lou~lds
20 are effective at micromolar conce-lL-ations, with increased ~OL..;~ y being evident when cells are
treated prior to the ad-iition of okadaic acid. The effective concelltlations appear to be in the
range at which these drugs are present in the brain following chronic treatment of psychiatric
patients (Svendsen, CN, Psychoph~rm~rolngy, 90, 316-321, 1986). These results indicate that
patients who were chronically treated with phenothi~7ines would be ~lotecled from the
25 development of PHF, and thus would have a low probability of development of Alzheimer's
Disease.
SUBSTITUTE SHEET (RULE 26
CA 0220~86 l997-0~-l6
WO 96/16052 PCrlUS95/14987
Frontal and cortical brain tissue ~I,ec-;...~,n~ obtained at autopsy from patients chronically
treated with chlo~lu~ 7;nr- were ana}yzed using the ALZ-EIA method previously described
(Gh~nh~ri, HA, et al, JAMA, 263, 2907, 1990). The results are shown in Figures 4A and 4B
frontal (Broadman area 10) and ltj11lPI)~ (Broadman area 38) regions, res~cc~ively. The brain
S tissue (post-mortem) from a~ d.. .~ y the sarne number of (age .. .~ -d) normal controls and
Al7h/ .... 's Disease patients were also analyzed and are ~ ,sented in these figures as nc~ativc
and positive controls (for normal and AD, rcsp.,~ively). As the data inrli~ ~tç, the samples from
chlol~lu~ treated patients (Rx) had low PHF epitope levels similar to normal controls,
wh~l~as the PHF epitope levels in the AD group were clearly much higher. EU1~ 1IU1C~ these
10 results were conci-ctent with histopathological diagnosis of the brain ~ec;.. -c. St~tictir~lly~ in
the general population, about 20-30% of the individuals in the Rx age group would have
developed AD and hence much higher PHF in the brain regions inriir~t~d (~t7m~n, R., Kawas.
C. H., pages 105-122, in ,~l,l.. ;.... r's Disease, Eds., Terry, RD; K~t7m~n, R; Bick, KL. Raven
Press, New York, 1994). This evidence shows that chronic Ll~O~ ent of patients with
15 chlol~,.,...~,;..e pl~ PHF formation and AD.
In a subsequent study, 51 additional patients were idtqntifiçd and the analysis was
Icl,ealcd as above using the TG3 antibody in an ELISA format. The results, ~u~ ali~ in
Table 1, clearly reproduce and confirm the first l~,~u~l,e~ e study. TmmllnohictQpathological
studies ca~Tied out on several brain regions from these patients were concict~nt with these ELISA
~0 results. Taken together, these two studies provide evidence of the therapeutic benefit of
chlo~ u...~,;ne in AD and validate the use of the MSNla model for the identification of
p~;r~lly usefulcompoundsforthe~lca~ ltofAD.
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
TABLE 1
N MEAN AGE + SEM SIGNAL$ + SEM
Frontal Temporal
Normal (12) 79.50 i 3.33 0.12 i 0.12 0.00 i 0.00
AD (28) 82.54 i 1.94 16.54 i 2.59 17.55 i 2.82
Rx (51) 76.88i 1.55 0.12iO.11 0.16iO.ll
* Signal is c~lr~ t~A by ~ the means of r~llrlir~t~ al~solbdllce readings (direct
ELISA using TG3 antibody) for 8 dilutions of all lysates ~ ~ by one to two serial
lillltion.$
s
Efforts to dissect the characteristic lesions of Alzheimer's Disease, namely the
neurofihnll~ry tangles and the neuritic plaques have inrlirterl that aberrant protein y~ yllu~ on
is a h~llm~rk of the ~;ylOS~ l abnorrn~lities in AD. Phosphorylated sites on the PHFs found in
10 neurofibrill~ry tangles and abnormal neurites are detected by AD-specific antibodies, such as
TG3 and PHF-1, in the brains of AD pati~ntc and not in normal or other neuro~eg,~ e
disease controls (cf. Figure 1, 2A). In the MSNla cell culture model (cf. Figure 2B, 3), these
ly phosphorylated PHF sites are also rlet~cted by these ~ntihodies
Th~.~r...~, the Ih~a~ lic activitv of the co~ c of the invention was ~csecsed by the
15 ability of these agents to affect the ~-u.lu~,Lion of abr-orm~lly phosphorvlated c~ u~ues p~Li~ to
the production of PHFs of AD, utili7ing a human neuroblastoma cell line (MSNla) in the
presence of the protein phosphatase inhibitor, okadaic acid (OKA). As described above,
chlo~ olllazine, a clinically used antipyschotic, prevents production of the aberrantly
phosphorylated PHF c~ ûl)es and, hence, the production of PHFs, thereby preventing AD. As
20 shown in Figures 2B and 3, and in Table 2, chlc,ll,lu.,,~7ine is effective in inhibiting the
production of these aberrant phosphorylated epitopes in the MSNla cell culture model as
det~ h~cd by the prevention of the imm~lnoreactivity ~ccoci~no~l with PHF antibodies such as
TG3 which was utilized in the l~o~ ;Live clinical analysis.
SUBSTITUTE SHEcT ~RULE 26)
CA 0220~86 1997-0~-16
Wo 96tl60S2 PCI/US95/14987
To detelllline the effect of chlorprom?7ine on the paired helical filament epitopes
~c~ 1 with AD, MSNla cells e,.~le~ing paired helical fil~ment c~,itopcs were i~ lt A with
100 mM chlc~ ,",~,;..e (CPZ) or a 0.2% DMSO vehicle control for 2 hours at 37 C. The cells
were isolated by centrifi-g~tirJn, boiled for 10 ...;..~ ,5 and 25 mg protein from the resulting heat
S stable s~ t~ were loaded per lane on an SDS-PAGE gel. The gel was clccLI~ph.lGlically
transferred to nitrc!ce~ lose m.omhr~nP and then immllnost~int~cl with PHF-l. As shown in
Figure 3, CPZ greatly dccl~ascd the pnxillrtion of paired helical fil~m-o.nt ~ opes by the MSNla
cells. In comr~ct, the control shows that without the ari iition of CPZ, there was prc.~-iurtion of
paired helical fil~mrnt ~ilupes by the MSNla cells. The use of the MSNla cell culture model
10 for scl~ g of novel cO~ ,ou,-ds for Lllc~ Lic activity in AD is, Ll,~ ,îulc, quite w,- .t;~nel1
This assay can thus be used to determine whether a drug is capable of pl~ r,tillg or
intervening in AD by ~ .n~ g the production of abnorrnally phosphorvlated paired helical
fil~m~ont ~iLc,~es :~c.coci~t~ with AD. To ~i~,~ ...;ne wl~cihcr a drug is capable of ~ ,cLing or
intervening in AD activity, the MSNla cells con~ g tau and ~csoci~t~ci ~lOkillS are treated
15 with OKA capable of greatly in~,~asi,lg ;, ....~ ea.;LiviLy of the MSNla cells with AD specific
mt~n~l~ n~i antibodies. Then, either prior to or concGIl,iLt;ulLly with OKA Ll~ 1, a coll,~oulld
of the present invention is added to the culture. If the c.,lll~ul-d ~ ,n~ the induction of the
AD e~ .pes in the MSNla cells treated with OKA, then it is said to have anti-AD activity. If the
AD-specific antibodies are highly reactive with the neurobl~ctom~ cells following OKA L~ealllle.,L
20 in the presence of compound, then the coll,~ound is said to be in~offectn~l against AD.
The MSNla human neurobl~ctom~ line was subclonrd from a population of MSN cells
obtained from the labold~ of Dr. Peter Davies (Albert Finctein College of Medicine, New
York, NY). Cells were grown and m~inr~inerl in flasks in RPMI 1640 Il-el~ l, suppl~m~nted
with lS% fetal calf serum, 100 U/ml penicillin, 100 U/ml S~ L.,-,ly~;in, 0.5 ug/ml fungizone
25 under7.5% CO2 at37 C.
Stock solutions of drugs were p.ep~d by dissolving the anhydrous powders to final
conre..l.,.l;ons of either 10 or 20 mM in 50% ac~ueous or 100% dill~lhyl sulfoxide (DMSO),
l~pccli~ely. Working solutions of drugs were ~ d by dilution of the stock solutions with
Kreb's Ringer in Hepes buffer (KRH) (128 mM NaC12 5 mM KCl, 2.5 mM CaCl2 1.2 mM
-74-
SUBSTITUTE SHEET (RULE 26)
CA 0220~86 1997-0~-16
WO 96/160S2 PCT/US9S/14987
MgS04, 1 mM Na2HPO4, 10 mM dextrose, 20 mM Hepes, pH 7.4). OKA is ",~h.~h~d as a 1
mM stock so~ nn in DMSO prior to dilution to 4 ~uM in KRH.
MSN cells grown in 225 cm2 flasks are removed from the inrubator, their .~fd;.~
der~ ed and ~ )laced with 50 ml pre-wa.lll~i KRH. The cells are harvested by 5~ping prior to
5 s~1;,n~...1i.1;1~n at 1000 rpm for 5 min. The s.~ la~nts are discarded and the cell pellets are
1, the cells counted and diluted to a density of 2 x 104 cells/50 ml. The cells are then
plated in a 96 well plate at 50 ~Vwell and 25 ~1 of 4 ~LM OKA and 25 ~ul of drug are then added to
the a~lOI,lialt; wells; the entire 96 well plate is then int~lb~ted for 2 hrs at 37 C. Depf ,~1;ng on
the dilution of drug added, this yields individual wells co~ h~;ng 1 ~M OKA, 0-64 ~lM drug and
10 2 x 104 cells in 0.26% DMSO in KRH. The il~;uh~t;oll is t~ d by the ~ 1i*o~ of 20 111 of
6X extraction buffer and immedi~7ts freezing at -80 C for at least one hr. The 6X extraction
buffer contains: 25 mM Tris-HCI, pH 7.6. 150 mM NaCI, 5 mM EDTA, 24 tnM ~-glycerol
l hosl.hal~, 10 ~g/ml APMSF (4-~min~inophe"ylll~ethane-sulfonyl flouride), and 2.5 ~g/ml each
of le.l~e~li", a~l.,Li"i." and p~ The plates are thawed in a 37 C water bath and 20 ~11 of
15 cell lysate is added to a replica well in an ELISA plate that already cont~in.c 80 ~,11 of coating
buffer (15 mM Na2CO3 and 35 mM NaHCO3, pH 9.6). The plates are allowed to coat
overnight at 4 C. The plates are washed four times with deionized water and then blocked for 30
min at 37 C with 200 ~Vwell pre-warmed 1% bovine serum ,7lbumin in 10 mM Tris-HCl, pH
7.4, 150 mM NaCI, 3 mM NaN3 (BSA-TBS). After the blocking solution is removed, 100
20 ~Vwell of an ~.~liate dilution of pre-warmed primary antibody (eg., TG3 or PHF-1) in BSA-
TBS is added and incubated at 37 C for 30 min. Tnrub~tion is tt~ tf:(l' by washing four times
in deionized water at which time 100 ~11 of an a~pl~.iate dilution of pre-warmed secondary
antibody (conjugated to alkaline pho,l,hatase) in BSA-TBS is added to each well in the ELISA
plate. Tnrnh~tion is ~lÇwmed for 30 min at 37 C followed by washing 4 times with deionized
25 water. 100 ~l/ml of prewarmed chromogenic substrate (1 mg/ml p-nitrophenyl phosphate in
10% t1isth~nt)lamine7 3 mM NaN3, 0.5 mM MgC12, pH 9.8) is added and the plate incubated for
30 min at 37 C. Absul bance at 405 nm is then determined by reading the plate on a BioRad UV
Microplate Reader.
SUBSTITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCr/US95/14987
Results oblailled from the MSNla cell culture model studies utilizing the TG3 antibody
are shown in Table 2.
TABLE 2
S IC50forinhihitionofTG3 h,,..,...,ulcacLi~ yin
OKA treated MSNla cells
FY~mrl~ ~ IC50 (~M)
yl. .~ 70.0
5-0
6.0
11 4.2
13 11.0
14 5.8
16 5.2
19 15.6
21 6.6
26 14.7
29 8.7
32 6.2
36 25.0
39 10.0
42 5.7
44 7.5
6.3
57 6.8
63 7.3
67 20.0
69 I5.0
76 10.5
5.7
81 45
91 9.5
2.8
` 78 34.0
-76-
SUBSTITUTE SHEET (RULE 26
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
As seen in Table 2, the co.l,l,o~ ds of the present invention are potent inhihitors of the
;nl- of the ~I~..,...~Iy pho~ ./ylated c~iLo~es as d~,t~,cl~d in tne MSNla cell culture model
system nti1i7ing the anti'oody TG3. As shown, the col.l~unds of the present invention are more
potent than chlu~ 7ine~ a co.ll~o~ d shown to inhibit the pl~luc~ion of the AD ~ou~
5 e~ilopcs and to prevent AD. The cc,~ .ouilds of the present invention are thus useful in the
~,d~ of AD.
FY~mrl~ 118
1~h..~ ll of ~ rv~ Activity
The collll~o.~l,ds of the invention according to formula I are effective in dest ~'t ili~ing
10 ~ ,lù~ .ul.oc, thus are useful in treating certain neoplastic lic~cçs Indirect imm~ln~ s~ nce
llliClosco~,y using the mnnoclon~l antibodies to tubulin is used herein to ~l~.h'.. .n;.~e the activity of
the co.ll~uu,lds of this invention in ~,ollluLh,g depol~ lir.n of the llli~,lolucjule cytos~ ton
in cultured cells. The following assay was employed.
Cultured human CG neurobl~ctom~ cells were seeded 1:3 from conflu~nt cultures onto
15 sterile llx22 mtn coverslips in 6 well tissue culture plates 16 hr prior to L.e~t~ nt The
fol~owing day, the tissue culture ..~ef~ " is removed and the cells are exposed to various
cni-~el.~ ;nnc of co-llpuu,lds of this invention in Krebs Ringer Hepes (KRH) buffer cou~ a
final concentration of 0.5% DMSO as carrier. Tncubations were pc,rulllled at 37 C in a
hnmi-lifie~l 5% C02 ~tmnsphPre for 2 hr. After incubation, the cells were fixed in 3% TEM
20 grade fnrm~ ohyde in PBS for 45 min at room ~elllp~;ld~
To visualize the effects of the compounds of the invention on the microtubule
cytoCL~I~tnn, the cells were processed for tubulin i.. ,.. l~oflnorescence Briefly, the cells were
permeabili7~ in 0.5% Triton-X 100 in PBS for 5 min. The cells were washed 2x with PBS and
aldehydes reduced using 0.5 mglml NaBH4 in PBS for 5 min. Subsequently, the cells were
25 blocked for 10 min in 1.5% BSA and 1.5 % nonfat dry milk in PBS (blocking solution, BS) for
10 min. The cells were then incllha~ecl for 30 min at 37 C with the mnnnelon~l antibody Tu27B
which recognizes b-tubulin (dilution 1:20 in BS). Subseu~u~,~ltly, the cells were washed 2x in
PBS and once in BS. The cells were then incub~te~ for 30 rnin at 37 C with sheep an~ luuse
IgG cûnjug~l~d to FITC. After in~ubation, the cells were washed 2x in PBS and once in BS and
Sl~STITUTE SHEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
inCllb~t~A for 30 min at 37 C with rabbit antisheep IgG conjugated to ~llC. The cells were
rinsed in PBS, cuu~ a~illcd with Hoechst 33285 in PBS (1 ~g/ml). and rinsed in PBS. The
co~ ,s were . . ,.,u..l~ in g: 1 glycerol:PBS cr)~ g 1 mg/ml p-phenylel~e~ " ,; "e and sealed
with nail polish.
S The cells were viewed using a Jen~ium~r epifluo~esc.,.. ec mi~_lu~co~e c~lui~ed with the
proper filters for Flllol~,scc;ll Tcothiocyanate (FlTC) eycit~tinn and c . "i~ Lack of effect of a
cu~ vulld is evidenced by the ~ sc~nce of an intact microtubule network in ir~ hase cells, or a
mitotic spindle in dividing cells. Antineoplastic col.~ .ric of the invention at effective doses
result in the di~a~ e of llli~;lu~ r c in both inte~ ase and mitotic cells.
The results of s.;l.,.,.li"g of cclll~uullds of the invention are shown in Table 3. Shown for
co...l~.vtive ~ul~oSes iS vinbl~cttne ("Velban", Lilly Research La~lalolics), a clinir~lly useful
amill~lastic agent which ~lulll~t~ microtubule rl~st~hili7~tir~n, hence llli~;lu~ubllle iic~ccr.mhly.
E 3
Al~ f.clulubllleActivity
C JQ. ~ 1 "~l1 r
F~mrle # ~M)
7 20
9 20
21 20
22 20
27 20
38 20
41 20
2 20
-78 -
SUBSTITUTE Sl-IEET (RULE 26)
CA 02205586 1997-05-16
WO 96/16052 PCT/US95/14987
113 20
vinhl~ctin~. 0 05
- * a CQ~e .~ 1;orl where ,l.icluLui,,lle ~st~hili7~tion occurs
As shown in Table 3, the compounds of the present invention ~ ol~; microtubule
iest~i1i7~tion and thus are useful in the ~ L~ nl of neoplastic lic~c~s
5FY~rnrl~o 119
,~ntih~rt~ri~l Activity Assay
The co~ .oullds of the present invention are also effective antibacterial and antifungal
agents.
The activity of the colllyounds is de~ n;ne~ using the following assay. The colllyound
10 is dissolved in DMSO at a concentration of 6 mg/ml and then diluted with water to a final
corlcentr~tion of 3 mglml in 50% DMSO (% DMSO was shown not to be inhihitnry to the test
org~nicmc). An aliquot (0.08 ml) of each drug solution was transferred to filter paper discs
(12.7 mm diameter, Scmeicherand Schuell) for use in the agar diffusion assay. The
CQnt~ 1 ;nn of each drug on the filter paper disc was 240 ug/disc. The test ol~liSll~
15 the following: Gram-positive aerobes (Staphylococcus aureus SRI 1323, Streptococcus
pneu~noniae SRI 1167); Gram-negative ærobe (Proteus vulgaris SR~ 180); and the yeast Candida
albicans S~ 523. An agar disc diffusion assay was used to evaluate the ~ntimicrobial activity of
the test compounds. Briefly, cultures were prepared from 16-20 hr agar slant cultures by
in~nl~ting in phyc~ ogir~l saline to a turbidity equivalent to a #0.5 McF~land turbidity ~ d~d
20 for the bacteria and a #3 McFarland st~n~rd for C. albicans. The surface of Mueller-Hinton agar
(lSxlOO mrn plates, mm) were evenly inoculated with each stand~di~d culture using a cotton
swab. Filter paper discs cont~ining the drug concentration listed above were then placed on the
inocul~ted agar plates along with a disc co..~ g the solvent used to dissolve each drug. The
plates were incubat~osi at 37 C for about 24 hr and the fli~meters of the zones of inhibition
25 lllea~ul~d with a vernier caliper. Positive control drugs consisted of ~ ycin for use with
the bacterial strains and 5-fluor~yL~sille for use with C. albicans. The positive control drugs
were assayed as described above for the cu.l~you~lds of the present invention using the same
-79-
SUBSTITUTE SHEET (RULE 2~)
CA 0220~86 1997-0~-16
WO 96/16052 PCT/US95/14987
concen-ration. Results of screeing a lc~ ,e,.Lative sample of the co~ ounds of the present
invention are shown in Table 4.
-8~
SUBSTITUTE SHEET (RULE 2c)
CA 0220~86 1997-0~-16
WO 96/16052 PCrrUS95/14987
Table 4
AIlLuluulubial and filngi~l activity
Di~ll~.,. of the zone of inhibirit-n (mm)
ExamDle # P. vulRaris S. pneu~noniae S. aureus C. albicans
17.3 29.9 22.817.6 =-
21 o1 40.7 33.826.6
16.0 36.3 27.526.0
43 15.9 25.3 17.722.5
76 20.5 35.5 19.320.9
78 14.5 19.7 16.215.2
13.8 +2 17.214.5
86 13.9 19.3 16.217.3
sLIc~ nlJycill 37.6 39.8 28.4ND3
5-fluorocvtosine ND ND ND 33.5
no IllF~ Ahle zone of inhihi*on a~.~und the disc.
2 zone of inhihition too large to 111~7U~C.
3 not ~1f ~ .rrl
As is shown in Table 4, the colll~ounds of the present invention inhibit growth and
proliferation of Gram positive and Grarn-negative or~nicmc to a degree similar to that
~e~ c(lal~d by the known thr~ c"~ ly useful agent sL~ L~ Iy-;ill. These co~ ou-lds also
inhibit growth and proliferation of C. albicans to a degree similar to that dem~nctrated by the
therapeutically useful 5-fluorocytosine. Therefore, the compounds are therapeutically useful
antibacterial andlor ~ntifilng~1 agents.
The foregoing is merely illustrative of the invention and is not intencled to limit the
15 invention to the ~liccloserl co,llpoullds. From the Ço.~going, it will be appreciated that although
specific embo l;...~...t~ of the invention have been described herein for pu-~uOses of illustration,
vatious i..~;r;-.~lir)nC may be made without deviating from the spirit or scope of the invention.
~ , .
-81-
SUBSTITUTE SHEET (RULE 26)