Note: Descriptions are shown in the official language in which they were submitted.
CA 0220691~ 1997-06-04
W O 96/19240 PCTAEP95104933
Oiigonucleotide-dendrimer conjugates
The present invention relates to oligonucleotide-dendrimer conjugates, to a process for
preparing these conjugates, to the use of these conjugates and to pharmaceuticalpreparations which comprise these conjugates.
Oligonucleotides have attracted wides~,t~ad interest as antiviral active ingredients or as a
result of their ability to interact with nucleic acids (antisense oligonucleotides) and the
biological activity associated therewith. A substantial problem in this context is that they are
only taken up in small quan~ities by cells. Hitherto, efforts have been made to increase the
cellular uptake of antisense oligonucleotides by covalently linking the oligonucleotides to, or
substituting them by, various chemical groups. Examples are conjugates with cationic
compounds such as poly-(L~-lysine, poly-(L)-ornithine or aminoalkanes, conjugates with
lipophilic compounds such as cllole~ ;r~l, alkanes, phospholipids or aromatic substances,
or conjugates with other groups such as polyethylene glycol. These groups are attached at
any position in the oligonucleotide, for example at the 3'- or 5' end, at any base, at a sugar
or at another site in the backbone. In addition, liposome and nanoparticle formulations have
been used to increase the cellular uptake of oligonucleotides. A further possibility is to
admix cationic lipids with the oligonucleotides.
It has now been found that oligonucleotide-dendrimer conjugates have an improved cellular
uptake, a high resistance to nucle~-ses and advantageous pharmacokinetics. This is of
value for antisense oligonucleotide or antigen oligonucleotide applications, in the
transfection of cells with foreign hereditary material and in medical diagnosis. By means of
linking the oligonucleotides to dendrimers, groups having an extremely high degree of
lipophilia or an ionic character can be introduced in a simple manner. The influence of the
dendrimer moiety on the conjugate as a whole can readily be controlled by the size or
number of the end groups.
The invention relates to oligonucleotide-dendrimer conjugates, where dendrimer is the
monovalent residue of a dendrimer of the first to tenth generation and oligonucleotide is a
natural, modified or synthetic sequence which is composed of natural, modified or synthetic
deoxynucleosides or peptide nucleic acid building blocks which are linked via
CA 0220691~ 1997-06-04
W 096/19240 P~ o4933
internucleotide bridges and which encompasses a region which is complementary,
preferably completely complementary, to a target nucleic acid (target RNA or target DNA),
with the dendrimer being directly bonded, or bonded via a bridging group B, to an
internucleotide bridge, a nucleic acid base or a sugar of the oligonucleotide, and the
physiologically tolerated saits thereof.
Preferably, the target nucleic acid is a target ribonucleic acid (target RNA). Accordingly,
polyribonucleic acids (RNA) can be present. These are preferably mRNA (messenger RNA),
pre-mRNA (precursor mRNA) and viral RNA. The RNA has sufficient building blocks to
ensure that a complex (double strand) can be formed with the oligonucleotide.
The oligonucleotide can be partially or completely constructed of natural DNA building
blocks which are complementary to the target RNA or completely constructed of unnatural,
synthetic nucleotides which are likewise complementary to the target RNA, with partially
denoting that natural DNA building blocks which are complementary to the target RNA are
replaced in the oligonucleotide sequence by unnatural, synthetic nucleotides which are
likewise complementary. Synthetic building blocks comprise the modifications of natural
building blocks in the nucleic acid base, the furanose ring and/or the bridging groups of the
oligonucleotides. In general, synthetic building blocks are employed in order to strengthen
complex binding in duplex structures and/or to increase the stability of the oligonucleotides
towards degradation which is caused, for example, by nucleases. A wide variety of modified
nucleosides have become known which can be used, within the sphere of "antisensetechnology", for synthesizing or modifying complementary oligonucleotides and such
nucleotides will not, therefore, be dealt with in more detail here (cf., for example,
E. Uhlmann et al., Chemical rlcvicws, Volume 90, Number 4, pages 543 to ~84 (1990)).
Possible modiric~Lions are modifications in the nucleic acid base moiety (for example
sl~hstitut!ons or omission of substituents), in the nucleotide-bridging group (for example
modification of the phosphoric ester group or its replacement by other bridging groups) and
in the furanose ring (for example substitutions on the 2'-hydroxyl group, replacement of the
furanose O atom, replacement of the furanose ring by monocarbocyclic or bicarbocyclic
rings, or replacement of the furanose ring by open-chain structures).
CA 0220691~ 1997-06-04
Wo 96/19240 PCTIEP95/04933
The choice and the order of the building blocks in the sequence of the oligonucleotide is
determined by the necessity of forming a duplex with a target RNA. The nature and the site
of linkage to the dendrimer can also affect the choice and the order of the building blocks.
The number of building blocks in the oligonucleotide is designed so that hybridization is
achieved with the target RNA. The oligonucleotides can, for example, contain from 5 to 100,
preferably from 5 to 50, particularly preferably from 8 to 30 and, very partirJularly, from 10 to
25, building blocks. The nucleotide building blocks which pair with the target RNA are
preferably arranged in the central sequences of the oligonucleotide, for example between
the fourth building blocks from each end of the sequence, or between the third from each
end, or between the seconc from each end or between the last building blocks at each end
of the sequence. For example, in an oligonucleotide having 20 building blocks, building
blocks which pair are preferably located in the region From the fourth to the seventeenth
building block.
The oligonucleotides are preferably constructed from nucleosides of the purine series and
the pyrimidine series. They are particularly preferably constructed from 2'-deoxy-2-amino-
adenosine, 2'-deoxy-5-methylcytidine, 2'-deoxyadenosine, 2'-deoxycytidine, 2'-deoxyguano-
sine and thymidine. Very particular prerere"ce is given to 2'-deoxyadenosine (A), 2'-deoxy-
cytidine (C), 2'-deoxyguanosine (G) and thymidine (T). Modified building blocks are
preferably derived from natural nucleosides of the purine series and the pyrimidine series,
particularly preferably from adenosine, cytidine, guanosine, 2-aminoadenosine, 5-methyl-
cytosine, uridine and the previously mentioned deoxy derivatives. The nucleosides can also
be 2'-modified ribonucleosides.
In a very particularly p~r~r,ed embodiment of the invention, the oligonucleotide which is
co"lplementary to a target RNA is constructed from natural deoxynucleosides, particularly
preferably from the group 2'-deoxyadenosine (A), 2'-deoxycytidine (C), 2'-deoxyguanosine
(G), and 2'-thymidine (T), Ol from complementary, unnatural synthetic building blocks.
Within the scope of ~he invention, those modified nucleosides are particularly preferred
which increase the stability of the oligonucleotide towards nucleases.
The oligonucleotide can also consist of sequences of peptide nucleic acids (PNA), with the
dendrimer preferably being bonded to the amino end or the carboxyl end. The same
CA 0220691~ 1997-06-04
W O96/19240 PCT~EP9~ 1933
pr~ rences apply to the structure of the PNA sequence as to that of the oligonucleotides.
Examples of PNA's can be found in Science 254:1497-1500 (1991).
Within the scope of the present invention, the dendrimer contains an initiator core having at
least three valencies, with one valency being used for the bond to the oligonucleotide, and
at least two monovalent branches which are bonded to the initiator core, with each branch
consi~li"g of at least one branching point having at least three valencies. The dendrimer
itself, and alsio its building blocks, are physiologically tolerated or harmless.
The initiator core and the branching point can, independently of each other, be a single
atom, a cyclic or heterocyclic, saturated or unsaturated aliphatic radical having from three to
twelve, preferably from five to eight, ring members, a bicyclic or heterobicyclic aliphatic
radical having from five to twelve ring members or a mononuclear or polynuclear aromatic
or heleroar~,nlalic radical having from six to eighteen, preferably from six to fourteen, in
particular from six to twelve, ring members, where the ring members are carbon atoms
which are, where appropriate, interrupted by from one to three heteroatoms which are
selected from the group consisting of nitrogen, oxygen and su!fur. A pr~rer,ed embodiment
of the present invention is represented by those compounds in which the initiator core and
the branching point are, independently of each other, a single atom, a cyclic or heterocyclic,
saturated or unsaturated aliphatic radical or a mononuclear or polynuclear aromatic or
heteroaromal:ic radical. Compounds are particularly preferred in which the initiator core and
the branching point are, independently of each other, a cyclic or heterocyclic, saturated or
unsaturated aliphatic radical or a mononuclear or polynuclear aromatic or heteroaromatic
radical.
All atoms having at least three valencies are possible single atoms for the initiator core or
the branching point; those which are plt:r~r,ed are carbon, nitrogen, silicon or phosphorus,
in particular carbon.
In one embodiment of the present invention, the cyclic or heterocyclic aliphatic radical as
the meaning of the initiator core and of the branching point is derived from compounds
which are selected from the group consisting of cycloalkanes and cycloalkenes which
preferably have from 5 to 7 ring carbon atoms.
CA 0220691~ 1997-06-04
WO 9611g240 PCTIEP9~/0 1933
In another embodiment of the present invention, the bicyclic or heterobicyclic aliphatic
radical as the meaning of the initiator core and of the branching point is derived from
compounds which are selected from the group consisting of bicycloalkanes and
bicycloalkenes which preferably have from 5 to 7 ring carbon atoms.
In a further embodiment of the present invention, the aromatic or heteroaromatic radical as
the meaning of the initiator core and the branching point is derived from compounds which
are selected from the group consisting of benzene, naphthalene, anthracene,
phenanthrene, naphthacene, indene, fluorene, indacene, biphenylene, triphenylene,
pyrrole, indole, carb~ole, h3ran, benzofuran, dibenzofuran, thiophene, ben~ull,iophene,
dibenzothiophene, pyridine, quinoline, isoquinoline, acridine, phena~llllri~ e, pyridazine,
cinnoline, phthalazine, pyrirrlidine, quinazoline, pyrazine, quinoxaline, phenazine, pteridine,
purine, pyrazole, indazole, imidazole, benzimidazole, isoxazole, oxazole, furazan,
thianthrene, xanthene, triazine, phenar,li,n~line, benzoxazole and ben~uLl,i~ole.
rl~r~rence is given to compounds which are selected from the group consisting ofbenzene, naphthalene, fluorene, biphenylene, pyrrole, carbazole, furan, dibenzofuran,
thiophene, dibenzothiophene, pyridine, acridine, pyrazine, phenazine, furazan, thianthrene
and xanthene, with particular prer~r~nce being given to compounds which are selected from
the group consisting of benzene, pyrrole, furan, thiophene, pyridine, pyrazine and furazan,
with benzene being in particular pr~r~:"ed.
The valencies of the initiator core are occupied by the bond to the oligonucleotide and those
to the branches. The valencies of the first generation branching point are occupied by the
bond to the initiator core and those to the second generation branching point. The valencies
of the branching points of later generations are occupied by the bond to the branching polnt
of the preceding generation and those to the branching points of the subsequent
, t generation. If, in all these cases, valencies are still free, these free valencies are then
occupied, independently of each other, by hydrogen or a substituent selected from the
group consisting of halogen, C1-C6alkyl, C1-C6hydroxyalkyl, C1-C6alkoxy~ C1-C6alkylthio, C6-
C,2aryl, C6-C12Ar-C,-C6alkyl, -CN and -NO2 -
One of the valencies of the branching points at the periphery of the branch is occupied by
the bond to the branching point of the preceding generation. The free valencies are,
CA 0220691~ 1997-06-04
W O96/19240 PCTAEP95/04933
independently of each other, occupied by monovalent end groups. In one embodiment of
the present invention, end groups are understood to mean groups having a high degree of
lipophilia or an ionic character.
It has been found to be advantageous to select an end group from the group consisting of
hydrogen, C1-C6alkyl, C,-C6alkoxy, C,-C6alkylthio, C6-C,0aryl, C7-C,7aralkyl, hydroxyl, amino,
nitro and an organic radical which is derived from a carboxylic acid derivative. Preference is
given to an end group which is selected from the group consisting of hydrogen and C,-
C6alkoxy. End groups which are in particular preferred are hydrogen and -OCH3.
The invention also relates to oligonucleotide-dendrimer conjugates in which the initiator core
in the dendrirner residue is linked to the first generation branching point via a bivalent
bridging group Z.
The present invention also relates to oligonucleotide-dendrimer conjugates in which, in the
dendrimer residue, the branching points of consecutive generations are linked via a bivalent
bridging group Z.
Within the scope of rhe present invention, the bivalent bridging group Z is advantageously
selected from the group consisting of C1-C4alkylene; C,-C4alkylene which is interrupted
once or more than once by a representative selected from the group consisLing of oxygen
atom, sulfur atom, nitrogen atom, carbonyl radical, thio radical, sulfoxide radical and a
radical of the formula -Si(OR')(OR")-O-, in which R' and R" are, independently of each
other, hydrogen or C,-C6alkyl; C2-C4alkenylene; C2-C4alkenylene which is interrupted orice
or more than once by a representative selected from the group consisting of oxygen atom,
sulfur atom, nitrogen atom, carbonyl radical, thio radical, sulfoxide radical, and a radical of
the formula -Si(OR')(OR")-O-, in which R' and Ri' are, independently of each other,
hydrogen or C, C6alkyl; C2-C4alkynylene; C2-C4alkynylene which is interrupted once or more
than once by a representative selected from the group consisting of oxygen atom, sulfur
atom, nitrogen atom, carbonyl radical, thio radical, sulfoxide radical, and a radical of the
formula -Si(OR')(OR")-O-, in which R' and R" are, independently of each other, hydrogen or
C1 C6alkyl; aryl; aralkyl and alkyloxy. Particular preference is given to the bivalent bridging
group Z which is selected from the group consisting of C,-C4alkylene and C,-C4alkylene
which is interrupted once or more than once by a representative selected from the group
CA 0220691~ 1997-06-04
W O 96/19240 PCTAEP95/04933
consi~Li"g of oxygen atom, sulfur atom, nitrogen atom, carbonyl radical, thio radical,
sulfoxide radical, and a radical of the formula -Si(OR')(OR")-O-, in which R' and R" are,
independently of each other, hydrogen or C1-C6alkyl. r, ~rence is in particular given to the
bivalent bridging group Z being C,-C4alkylene or C,-C4alkylene which is interrupted once by
an oxygen atom. The most pi~r~r,ad bivalent bridging group Z is -OCH2-.
Within the scope of the present inven~ion, the number of the generations indicates the
number of consecutive brant:hing points. Those compounds are pre~"ed in which
dendrimer denotes the monovalent residue of a dendrimer of the first to seventh,particularly preferably of the first to fifth, in particular of the first to fourth, very particularly
preferably of the first to third, generation.
The novel residue of a dendrimer can be constructed in accordance with the formula 1,
(12' X3
/ - E
~ X2 E
(113 (~2) x3
Xl E (I)
(11) ~(12' X3
X2 E
'12' x3
- E
in which x, is the initiator core, (11) and (12) are each a bridging group Z, x2 and X3 are each a
branching point and E is an end group. As already indicated above, the initiator core and
the branching points may have more than three valencies, the initiator core may or may not
be linked to the first generation branching point via a bivalent bridging group Z, the
branching points of consecutive generations may or may not be linked via a bivalent
bridging group Z, and the generation number determines how often branching points
succeed each other and, correspondingly, how frequently bridging groups Z may be
CA 022069l~ l997-06-04
W 096/19240 PCT~EP9StO4933
present. According to the invention, the residue of the dendrimer of the formula (I) may be
branched to a greater extent than indicated in formula (I), i.e. the radical E in formula (I)
represents addilional bifurcations which are constructed from additional initiator cores X4, X5
etc., which, where a~Jpropri~le, are bridged via additional radicals (13), (14) etc., and which
end in an end group, for example H or -OCH3 .
Those dendrimer residues of the formula I are prer~"ed in which x1, x2 and X3 is benzene,
(11) and (12) are-O-CH2-, and E is H or-OCH3 .
The dendrimer is, for example, bonded to N, S or O atoms in the 3' or 5' end groups of the
oligonucleotide sequence. However, it can also be bonded to C, N or O atoms of nucleic
acid bases in or at the end of the sequence, to 2' positions in the furanose ring, to O, S or N
atoms in or at the end of the sequence, or to O, S or N atoms of the nucleotide-bridging
group in the sequence. The nature of the bond depends on the dendrimer and on the
nature of its functional groups. The bond to the oligonucleotide can be ionic or, preferably,
covalent. The dendrimer can also be bonded to the 6' carbon atom of a carbocyclic
nucleotide analogue.
It has been found to be particularly advantageous for the dendrimer to be bonded via a
bridging group B. Within the scope of the p,~se"l invention, the bridging group B is a group
of the formula ll
-Xp-[A-X~n~A'm~ (Il)
in which
X and X' are, independently of each other, a radical which is unsubstituted or is substituted
by Cl-C,Oalkoxy, preferably C1-C6alkoxy, F, Cl, Br, -CN, C,-~:lOalkyl, preferably C,-C6alkyl,
aryl, hydroxy-~,-C,Oalkyl, preferably hydroxy-C,-C6alkyl, amino-C,-C,Oalkyl, preferably
amino-C,-C6alkyl, OH, NR,2 or -NO2 and which is selected from the group consisting of
C, C20alkylene, C2-C,2alkenylene, C2-C12alkynylene, C3-C8cycloalkylene, C6-C,2arylene and
C7-C,2aralkylene,
A and A' are, independently of each other, -O-, -S-, -S-S-, -NR,2-CO-NR,2-, -NR,2-CS-NR,2-,
-NR,2-, -NR,2-C(O)-O-, -C(O)O-, -C(O)S-, -C(O)NR,2-, -C(S)S-, -C(S)O-, -C(S)NR,2-,
-SO2NR12-, -SO2-, -P(O)(OH)O-, -OP(O)(OH)O-, -P(S)(SH)O-, -OP(S)(SH)O-, -P(S)(OH)O-,
_
CA 0220691~ 1997-06-04
W O 96/19240 PCT/~5~ 33
-OP(S)(OH)O-, -P(O)(OH)-NR12-, -OP(O)(OH)-NR12-, -P(S)(SH)-NR12-, -OP(S)(SH)-NR12-,
-P(S) (OH)-NR12- or -OP(S) (OH)-NR12-,
R12 is H or C1-C10alkyl, prt7r~rably H or Cl-C6alkyl;
n is a number from 1 to 50, preferably from 1 to 20, particularly preferably from 1 to 5, in
particular from 1 to 3, where, when more than one tA-X') unit is present, the meanings of A
and X' in the individual units are identical or different, and
m and p are, independently of each other, 0 or 1.
Some examples of possible rneanings of X and X' are methylene, ethylene, 1,2- or 1,3-
propylene, 1,2-, 1,3- or 1,4-kutylene, 1,2-, 1,3-, 1,4- or 1,5-pentylene, 1,2-, t,3-, 1,4-, 1,5- or
1 ,6-hexylene, 1,2-, 1,3-, 1,4-, 1,5-, 1,6- or 1 ,7-heptylene, 1,2-, 1,3-, 1,4-, 1,5-, 1,6-, 1,7- or
1,8-octylene, and the isomers of nonylene, decylene, undecylene, dodecylene, tridecylene,
tetradecylene, pentadecylene, hexadecylene, heptadecylene, octadecylene, nonadecylene
and eicosylene; cyclopentylene, cyclohexylene; naphthylene, and particularly, phenylene;
benzylene and phenylethylene.
In one embodiment of the present invention, X is a radical which is unsubstituted or
substituted by C,-C10alkoxy, preferably C1-C6alkoxy, F, Cl, Br, -CN, C1-C10alkyl, preferably
C,-C6alkyl, aryl, hydroxy-C1-C,0alkyl, preferably hydroxy-C1-C6alkyl, amino-C1-C1Oalkyl,
preferably amino-C,-C6alkyl, OH, NR12 or -NO2 and which is selected from the group
consisting of C,-C20alkylene, C3-C8cycloalkylene, C6-C12arylene and C7-C12aralkylene.
In a pr~er,ed embodiment, X is C,-C20alkylene, particularly preferably C,-C,Oalkylene, in
particular C,-C5alkylene. The radical -CH2- has been found to be particularly advantageous.
Advantageously, the novel compounds contain X, that is p is preferably 1.
In a further embodiment of the present invention, X' is a radical which is unsubstituted or
sllh.stitllted by C,-C10alkoxy, preferably C,-C6alkoxy, F, Cl, Br, -CN, C,-C,Oalkyl, preferably
C~-C6alkyl, aryl, hydroxy-Cl-C10alkyl, preferably hydroxy-C,-C6alkyl, amino-C,-C,Oalkyl,
preferably amino-C,-C6alkyl, OH, NR,2 or -NO2 and which is selected from the group
consisting of C,-C20alkylene, C3-C8cycloalkylene, C6-C,2arylene and C~C,2aralkylene.
In a preferled embodiment, X' is a radical which is unsubstituted or substituted by hydroxy -
CA 0220691~ 1997-06-04
WO 9~/19240 PCT/EP95/04933
- 10-
C1-C10alkyl, preferably hydroxy-C1-C6alkyl, amino-C1-C,0alkyl, preferably amino-C1-C6alkyl or
OH and which is selected from the group consisting of C,-C20alkylene, C3-Cacycloalkylene,
C6-C,2arylene and CrC12aralkylene. Particularly preferably, X' is a radical which is
unsubstituted or sl l~stitl Ited by hydroxy-C1-C2alkyl or OH and which is selected from the
group consisl:ing of Cl-ClOalkylene and CrClOaralkylene. It is preferred, in particular, that X'
is selected from the group consisting of -(CH2)2-, -(CH2)6-, -(CH2)10-, -CH2CH(OH)CH2-,
-CH(CH20H)CH2-, --CH
and ---CH2~3CH2-- -
In one embodiment of the present invention, A is -O-, -NR12-CO-NR12-, -NR12-, -NR12-C(O)-
O-, -C(O)O-, -C(O)NR12-, -P(O)(OH)O-, -OP(O)(OH)O-, -P(O)(OH)-NR12- or-OP(O)(OH)-
NR,2-, particularly preferably, A is -O-, -NR12-CO-NR12-, -NR12-, -NR,2-C(O)-O-, -C(O)O- or -
C(O)NR12-, and, in particular prer~,~bly, -O-, -C(O)O- oder-C(O)NH-
Within the scope of the present invention, those compounds are preferred in which A' is notpresent, that is m is 0, or is -P(O)(OH)O- or -P(S)(OH)-.
The bridging groups of the formula Illa, Illb or Illc
-CH2-O-CH2~ ~ ~
W~NI~ p~O (Illa)
~NH'--~O~ ~0 (Illb)
O O O--
CA 02206915 1997-06-04
W 096/19240 PcT~ 5lolg33
-CH2-O-(CH2)10~ ~ NH~O~ ~'~ (IIIC)-
have been found to be particularly suitable as bridging groups B.
The bridging groups of the formula Illd or Ille
-CH2-O-CH2\~ OH
~"NH~O~ (Illd)
-CH2-O-CH2\,~
{o_ (Ille).
are likewise particularly suitable.
The present invention furthermore relates to intermediates in the preparation of the novel
compounds. These are the compounds of the formula IV -
dendrimer-Xp-[A-X~n-~
where dendrimer, X, p, A and X' have the abovementioned meanings, n' is a number from O
to 49, and R, is a monovalent functional group.
Within the scope of the present invention, the monovalent functional group is preferably
selected from the group consisting of -ORlo, -SRlo, -NCO, -NCS, -NHR", -C(O)OR",-C(O)SH, -C(O)CI, -C(S)SR", -C(S)OR", -S03R", -SO2CI, -OP(O)(OR)(OH),
CA 0220691~ 1997-06-04
W O96/19240 PCTnEPg5/04933
-OP(S)(OR)(C~H), -OP(O)(SR)(SH), -OP(O)(OH), -OP(O)(SH), -OP(OCH3)N[CH(CH3)2J2,
-OP(OCH2CH2CN)N[CH(CH3)232and P(OCH2CH2CN)N[CH(CH3)2~2, where R is a phosphdLe
proLecli"g group, for example ~-cyanoethyl, 2,6-dichlorobenzyl, 2-chlorophenyl, 4-chloro-
phenyl or S-phenyl, R~o is H, -C(O)NH2~ -C(S)NH2~ -C,-C6alkyl, -C~H2X-NH2~ -CxH2x-SH or
-(CxH~O)y~H and R.1 is H, -C,-C6alkyl, -CxH2x-NH2~ -CxHa~-SH or -(CxHa~O)y~H~ and x is a
number from 2 to 6, and y is a number from 1 to 20. The functional group is particularly
preferably selected from the group consisting of -OR10, -SR,0, -NCO, -NCS, -NHR11,
-C(O)OR" and -P(O)(OH)2, in particular selected from the group consisting of -NCS,
-C(O)OR~ and-P(O)(OH)2
The present invention furthermore relates to a process for preparing the novel compounds,
which comprises reacting a compound of the formula IV with a compound of the formula Va
R,--[A-X']n-A'm-oligonucleotide (Va)
in which R, and n" each have one of the meanings mentioned above for R1 and n', and A,
X', A', m and oligonucleotide have the abovementioned meanings.
The process can, for example, be carried out such that the compounds of the formulae IV
and Va are dissolved in a solvent, preferably in equivalent quantities, and then reacted with
each other at elevated temperatures. Expediently, condensation catalysts, for example
concentrated mineral acids, in particular hydrochloric acid, or acidic ion exchangers, are
used concomitantly. It can be expedient to add water-binding agents or to remove the water
of reaction from the reaction mixture.
The reaction temperature can, for example, be from 40 to 220~C, preferably from 50 to
1 50~C.
Examples of suitable solvents are water and polar protic solvents which advantageously are
miscible with water, and also polar aprotic and non-polar solvents. Examples of such
solvents are alcohols (methanol, ethanol, n- or i-propanol, butanol, ethylene glycol,
propylene glycol, ethylene glycol monomethyl ether, diethylene glycol, and diethylene glycol
monomethyl ether), ethers (diethyl ether, dibutyl ether, tetrahydrofuran, dioxane, ethylene
glycol dimethyl ether, ethylene glycol diethyl ether, diethylene glycol diethyl ether and
CA 0220691~ 1997-06-04
W 096/19240 PCT~EP95/04933
triethylene glycol dimethyl ether), halogenated hydrocarbons (methylene chloride,
chlor~,~c,r"" 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane and
chlorobenzene), carboxylic esters and lactones (ethyl acetate, methyl propionate, ethyl
benzoate, 2-methoxyethylac~tate, y-butyrolactone~ â-valerolactone and piv~lol~~tone),
N-alkylated carboxamides and lactams (N,~\l-dimethyl~ur,lla",ide, N,N-diethylformamide,
N,N-dimethylacetamide, tetramethylurea, hexamethyl~hospl~oric triamide, N-methyl-y-
butyrolactam, N-methyl-~-caprolactam and N-methylpyrrolidone), sulfoxides (dimethyl
sulfoxide and tetramethylene sulfoxide), sulfones (dimethyl sulfone, diethyl sulfone,
trimethylene sulfone and tetramethylene sulfone), tertiary amines (trimethylamine,
triethylamine, N-methylpiperidine, N-methylmorpholine and pyridine), substituted benzenes
(chlorobenzene, o-dichlorobenzene, 1,2,4-trichlorobenzene, nitrobenzene, toluene and
xylene) and nitriles (acetonitrile, propionitrile, benzonitrile and phenylacetonitrile).
The novel process for preparing the oligonucleotide conjugates can, for example, be carried
out such that an oligonucleo~ide which is or is not functionalized is dissolved in a solvent or
solvent mixture and the dendrimer carrying a suitable functional group is then added, and
the reaction mixture is subsequently allowed to react, if desired while stirring. The conjugate
which is formed can then be purified in a manner known per se and isolated, if desired.
The reaction temperature can, for example, be from 0 to 1 20~C, preferably from ~0 to 80~C.
Particularly preferably, the reaction is carried out at room temperature.
If the linking is an esterification, transesterification or amidalion reaction, corresponding
carboxylic acid groups can be activated in advance in a known manner, for example by
reaction with carbodiimides and N-hydroxysuccinimide.
The reactants are expediently employed in molar ratios. However, an excess of the catalyst
or the oligonucleotide can be used.
The customary methods, advantageously, for example, dialysis, electrophoresis and
chromatographic methods such as high pressure liquid chromatography (HPLC), reverse-
phase HPLC, affinity chromatography, ion exchange chromatography and gel
chromatography, can be used for the purification.
CA 0220691~ 1997-06-04
W O 96/19240 PCTAEP95/04933
The oligonucleotides which are to be used and which are or are not functionalized can be
prepared in a manner known per se using automated synthesizers which are commercially
available. Nucleosides for their synthesis are known and can either be obtained
commercially or prepared using analogous methods. The phosphorotriester method, the
phosphite triester method or the H-phosphonate method, with which the person skilled in
the art is familiar, can, for example, be used in the case of the bridging group -P(O)O -. In
the phosphite triester method, the approach can, for example, be to react the nucleosides
with a protecting group reagent, for example 4,4'-dimethoxytriphenylmethyl chloride, and to
use a linker, for example succinic anhydride, to bind the resulting compound to a solid
support material, for example to control pore glass (CPG) which contains long-chain
alkylamino groups. In a separate procedure, a hydroxyl group of such compounds is
derivatized, for example to form a phosphoramidite using R'OP[N(i-propyl)2)]2.
After the protecting group, for example the DMT group, of the material bound to the support
has been eliminated, coupling is carried out while eli,l,in~li"g -N(i-C3H7)2, any free hydroxyl
groups which are present are blocked (capped), and the phosphite which has been formed
is then oxidized to the phosphate. Following deprotection of the dimer, the reaction cycle is
repeated using another protected compound until an oligomer having the desired number of
monomer units has been synthesized, after which the product is released from the support
material. In this manner, oligonucleotides can be prepared having any monomer units in any
sequence, depending on the use of synthetic, natural and novel nucleoside building blocks
in the individual reaction cycles.
The novel compounds have anti-viral and anti-proliferative properties and can consequently
be used as pharmaceuticals. In addition, the novel oligonucleotides exhibit a high degree of
stability towards degradation by nucleases. Their unexpectedly high cellular uptake is
~ . particularly surprising. In addition, they pair in an outstanding manner with complementary
nucleic acid strands, especially of the RNA type. The novel oligonucleotides are therefore
particularly suitable for antisense technology, that is for inhibiting the expression of
undesirable protein products by means of binding to suitable, complementary mRNAnucleotide sequences (EP 266, 099, W087/07300 and WO89/08146). They may be
employed for treating infections and diseases, for example by means of blocking the'
~ . . _ _ _ _ _ _ _ _ _ _ _ . _ _ . . . . . .. . . . .
CA 0220691~ 1997-06-04
W O 96/19240 PCT~EP9S/04933
-15-
expression of bioactive proteins at the stage of the nucleic acids (for example oncogenes).
More than 30 families of such oncogenes are known which are thou~ht to be involved in the
formation of tumours in humans. An example of such a family is the raf gene family which
comprises three highly conserved genes which are designated A-raf, B-raf and c-raf (also
termed raf-1). The raf genes encode protein kinases which are assurned to play an
important role in cellular signal transduction which regulates cell proliferation. There are
indications that abnor"lal expression of the c-raf protein, in particular, is associated with
abnormal cell profileration. (Review: U. Rapp et al., "The Oncogene Handbook", E.P. Reddy
et al., eds., Elsevier Science Publishers, New York, 1988, pp. 213-253.)
Surprisingly, it has been observed, within the scope of the present invention, that
oligonucleotide-dendrimer conjugates whose olignucleotide sequence is complementary to
a segment of the 3'-non-translated region of human c-raf mRNA and has, in particular, the
sequence 5'-TCCCGCCTGTGACATGCATT-3' (nucleosides linked via -P(S)O-, SEQ. ID.
NO.5) possess outstanding properties as regards decreasing the expression of c-raf,
determined, for example, in cell cultures, and decreasing tumour growth in vivo.
Consequently, the invention 1urthermore relates to novel oligonucleotide-dendrimer
conjugates in which the oligonucleotide moiety has the sequence
5'-TCCCGC~I~IGACATGCATT-3' (nucleosides linked via -P(O)S~- ("PS"), SEQ. ID. NO.
5) and the dendrimer moiety is as defined above. Preference is given to oligonucleotide-
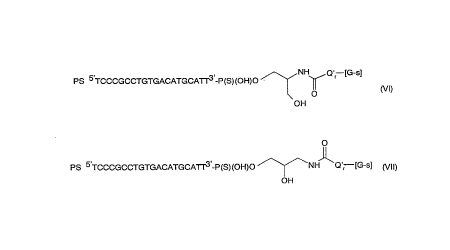
dendrimer conjugates of the formulae (Vl) and (~/II)
PS 5TCCCGCCTGTGACATGCAT~ -P(S)(OH)O ~NH~Q'r [G-s]
O (\/1)
OH
. ' . O
11
PS 5 TCCCGCCTGTGACATGCATT3 -P(S)(OH)O /\~--NH Q'r [G-s] (Vll)
OH
CA 0220691~ 1997-06-04
W O96/19240 PCTAEP95/04933
in which Q' is ~CH2-O-CH2- , r is 0 or 1, and s is 1, 2 or 3. Pl~r~:t~bly, r is 1 and
s is 2.
The protein kinase C (PKC) family, which comprises several isoforms (isozymes) such as
PKC ~, ~, y, ~, E, 1~ and r~, forms anotner class of proteins which play an important role in
signal transduction and abnormal cell proliferation (cf., for example, Gescher et al., Anti
Cancer Drug Design 4 (1989), pp. 93-105; Nishizuka, Nature 334 (1988), pp. 661-665).
Within the scope of the present invention, it has been observed, surprisingly, that
oligonucleoti~e-dendrimer conjugates which have an oligonucleotide sequence
5'4i l l C l C~iCTGGTGAGTTTCA-3' (nucleosides linked via -P(S)O-, SEQ. ID. NO. 6),
which is complementary to human PKC a mRNA, are outstandingly suitable for decreasing
the ex,uression of PKC a, for example in cell cultures, and for reducing tumour growth in
vivo.
The invention furthermore relates to novel oligonucleotide-dendrimer conjugates in which
the oligonucleotide moiety has the sequence 5'-GTTCTCGCTGGTGAG I I I CA-3'
(nucleosides linked via -P(O)S~- ("PS"), SEQ. ID. NO. 6) and the dendrimer moiety Is
defined as above. Preference is given to oligonucleotide-dendrimer conjugates of the
formulae (\/III) and (IX)
PS 5 G I I C I CGCTGGTGAG I I I CA3 -P(S)(OH)O /~NH~Q'r [G-s]
o (Vlll)
OH
O
PS 5(à 1 ~ CGCTGGTGAG I I I CA3-P(S)(OH)O/~--NH Q'r [G-s] (IX)
OH
CA 0220691~ 1997-06-04
W O 96/19240 ~CT/~r9~ g33
in which Q' is ~CH2-0-CH2- , r is 0 or 1, and s is 1, 2 or 3. Preferably, r is 1 and
s is 2.
The novel oligonucleotide-dendrimer conjugates are also suitable for use as diagnostic
agents and can be used as c~lene probes for detecting viral infections or genetically
determined diseases by means of selective interaction at the stage of single-stranded or
double-stranded nucleic acids (gene probes). In particular - as a result of the increased
stability towards nucle~es - it is possible to use the conjugates for diagnostic applic~tions
in vivo (for example tissue samples, blood plasma and blood serum) as well as in vitro.
Such possible uses are described, for example, in W0 91/06556.
The invention furthermore relates to the use of the novel compounds as diagnostic agents
for detecting viral infections or genetically determined diseases.
The invention also relates to the novel compounds for use in a therapeutic process for
treating diseases in homoiothermic animals, including man, by means of inactivating
nucleotide sequences in the body. When a~JI,,i,,i~Lering to homoiothermic animals of about
70 kg body weight, the dose can, for example, be from û.01 to 1000 mg per day.
Administration is pre~rably effected parenterally, for example intraveneously orintraperitoneally, in the form of pharmaceutical preparations.
The invention also relates to a pharmaceutical preparation which cornprises an effective
quantity of a novel compouncl, either alone or together with other active compounds, a
pharmaceutical excipient, preferably in a significant quantity, and auxiliary substances, if
desired.
The pharmacologically active novel compounds can be used in the form of preparations
which can be ac~",i"i:jL~red parenterally or of infusion solutions. Such solutions are,
preferably, isotonic aqueous solutions or suspensions, with it being possible for the latter,
for example in the case of Iyophilized preparations which comprise the active substance
. CA 0220691~ 1997-06-04
W O 96/19240 PCT/~195~01933
-18-
alone or together with an excipient, for example rllannilol, to be prepared prior to use. The
pharmaceutical preparations can be ~lerili~ed and/or comprise auxiliary substances, for
example preservatives, st~hili~ers, wetting agents and/or emulsifiers, soluhili,ers, salts for
regulating the osmotic pressure and/or buffers. The pharmaceutical preparalions, which, if
desired, can comprise additional pharmacologically active substances, for example
antibiotics, are produced in a manner which is known per se, for example by means of
conventional dissolution or Iyophilization methods, and comprise from about 0.1% to 90%,
in particular from about 0.5% to about 30%, for example from 1% to 5%, of activecompound(s) .
The following examples illustrate the invention in more detail.
In the examples, the dendrimers which conform to the foliowing scheme are described, with
the clarifications given above in association with the compounds of the formula tl) applying
in an analogous manner:
(12) X3
/ - E
2 E
~ ) (12~ x3E
Xl
\
(Il) ~ (l~ E
X2
\ E
(12) - X3
- E
CA 02206915 1997-06-04
Wo 96/19240 PCT/~;l 9~/0~1933
- 19-
[G-1]: x1 = X2 = \~ ,~ ; l, = -O-CH2-; E = H
~ ,[3
0~,0
[G-2~: x~ = X2= X3= ~ 3~'' ; 11 =l2=-O-CH2-; E = H
O O
' OJ~' ~0
~~~ ~13
~G-3]: x, = x~ = x = X4 = ~ 2 = ~ O-CH~-; E = H
CA 02206915 1997-06-04
W O96/19240 PCT~EP~/04933
-20-
[G-4]: x, = x2 = x~ = X4 = xs = ~/ ; I, = lz = 13 = l~ = -O-CH~-; E = H
[M-1]: xl = x2 = ~~ = -O-CH2-; E = -OCH3
[M-y: x~ = x2 = x3 = ~J ; 11 = 12 = -O-CH2-; E = -OCH3
The abbreviation Q denotes --CH2--O--CH2~
Q' denotes ~CH2-O-CH2-, and W is -CH2-O-(CH2)10-O-C(O)-(CH2)2-.
P~ rdlion of the st~, lin~ co."~ounds
e A1: Pl e~ar~lion of lG-2]-CH2-OH (4)
a) 15.1 9 of methyl 3,5-dihydroxybenzoate are dissolved in 400 ml of acetone, 4.75 9 of
1 8-crown-6, 26.6~ ml of benzyl bromide and 31.0 9 K2CO3 (anhydrous) are added and the
whole is heated under reflux for 40 h. The precipitate is filtered oK and washed with
acetone and the filtrate is concentrated in vacuo. H20 and CH2C12 are added to the residue
and the organic phase is separated off and dried and the solvent is removed; the residue is
recrystallized from diethyl ether/hexane (1:1). The compound (1)
CA 0220691~ 1997-06-04
W 096/19240 PCT/~195/O~
-21 -
[G-1 ]-C02-CH3 (1 )
is obtained.
b) 19.0 9 of compound (1 ) are dissolved in 300 ml of diethyl ether, the solution is cooled
and 3.8 9 of LiAlH4 are added in several portions. The mixture is subsequently heated under
reflux for a total of 18 h. The reaction mixture is hydrolysed dropwise with H20 and filtered,
the solvent is removed and the residue is then dried overnight under high vacuum. The
compound (2)
[G-1]-CH2-OH (2)
is obtained.
c) 14.0 9 of compound (2) are dissolved in 200 ml of tetrahydrofuran (argon), and 13.7 g of
triphenylphosphine and 17.4 9 of CBr4 are added at room temperature. The mixture is
stirred at room temperature for 1.5 h, the precipitate is filtered off and the filtrate is
concenlr~l~d in vacuo. CH2CI2 and H2O are added to ~he residue, the organic phase is
removed and dried (Na2SOd and the solvent is removed. Purification is effected by means
of flash chromatography. Eluent: CH2CI2:hexane (1:1). The compound (3) .
[G-1 ]-CH2Br (3)
is obtained.
d) 14.5 9 of compound (3) and 2.4 9 of 3,5-dihydroxybenzyl alcohol are dissolved in
acetone (argon atmosphere), 5.5 g of (K2C03) (anhydrous) and 0.9 g of 18-crown-6 are
~ . added and the whole is heated under reflux for 18 hours. The mixture is filtered and the
filtrate is concer,ll~led in vacuo. CH2CI2and H20 are added to the residue, and the organic
phase is separated off and dried and the solvent is removed; the residue is recrystallized
from toluene:hexane (3:1). The title compound, compound (4), is obtained.
Example A2: Pleparalics., of [G-31-CH2-OH (6)
_
CA 0220691~ 1997-06-04
WO 96/19240 PCTIEP95/04933
a) 6.0 9 of compound (4) are dissolved in 100 ml of tetrahydrofuran (argon), and 2.6 9 of
triphenylphosphine and 3.3 9 of CBr4 are added at room temperature. The mixture is stirred
at room temperature for 1.5 h, the precipitate is filtered off and the filtrate is concentrated in
vacuo. CH2GI2 and H2O are added to the residue, and the organic phase is removed and
dried (Na2SC)4) and the solvent is removed. Purification is effected by means of flash
chromatography. Eluent: CH2CI2:hexane (3:1). The compound (5)
[G-2]-CH2-Br (5)
is obtained.
b) 5.0 9 of compound (5) and 5.0 g of 3,5-dihydroxybenzyl alcohol are dissolved in 50 ml of
acetone (argon atmosphere), 1.03 g of K2CO3 (anhydrous) and 158 mg of 1 8-crown-6 are
added and the whole is heated under reflux for 18 hours. The mixture is filtered and the
filtrate is concentrated in vacuo. CH2C12 and H2O are added to the residue, and the organic
phase is separated off and dried and the solvent is removed. Purification is effected by
means of flash chromatography. Eluent: CH2C12. The title compound, compound (6) is
obtained.
Example A3: Pl e,~ardlion of [G-2]-CH2-O-(CH2ho~OH (7)
0.5 g of 1,10-decanediol are dissolved in 15 ml of tetrahydrofuran, 672 mg of NaH are
added and the whole is heated under reflux for 15 min. The mixture is brought to room
temperature and a solution of 0.3 g of compound (5) in tetrahydrofuran is added dropwise.
After this addition, the mixture is refluxed overnight and then cooled; the excess NaH is
hydrolysed by adding a few drops of H2O, and H2O/CH2C12 is added to the mixture. The
organic phase is separated off and dried (Na2SO4) and the product is purified by flash
chromatography. Eluent: CH2C12. The title compound, compound (7), is obtained.
E)c~,.~,:le A4: Preparation of lG-3]-CH2-O-(CH2)~o~0H (9)
250 mg of 1,10-decanediol are dissolved in 10 ml of tetrahydrofuran, 50 mg of NaH are
added and the whole is heated under reflux for 15 min. The mixture is brought to room
CA 0220691~ 1997-06-04
W O96/19240 PCT/~r5S/01933
-23-
temperature and a solution of 0.3 9 of compound (8)
[G-3]-CH2-Br (8)
in tetrahydrofuran is added dropwise. After this addition, the mixture is refluxed overnight
and then cooled; the excess NaH is hydrolysed by adding a few drops of H20, and
H2O/CH2CI2 is added to the Mixture. The organic phase is separated off and dried (Na2SO4)
and the product is purified by flash chromatography. Eluent: CH2CI2:acetone (19:1). The title
compound, compound (9) is obtained.
Example A5: r-~,ar~lion ~f lG~ CH20H (10)
1.4 9 of compound (8) and 5~ mg of 3~s-dihydroxybenzyl alcohol are dissolved in 40 ml of
acetone (argon atmosphere), 140 mg of K2C03 (anhydrous) and 53 mg of 18-crown-6 are
added and the whole is heated under reflux for 18 hours. The mixture is filtered and the
filtrate is concentrated in vacuo. CH2C12 and H2O are added to the residue, and the organic
phase is separated off and dried and the solvent is removed. Purification is effected by
means of flash chromatography. Eluent: CH2CI2/acetone (50:1). The title compound,
compound (10), is obtained.
Example A6: Preparation of [M-1l-CH20H (11)
4.0 9 of 3,5-dimethoxybenzyl bromide and 1.12 9 of 3,5-dihydroxybenzyl alcohol are
dissolved in 100 ml of acetone (argon atmosphere), 2.75 9 of K2C03 (anhydrous) and 0.45
g of 1 8-crown-6 are added and the whole is heated under reflux (argon) for 24 hours. The
mixture is filtered and the filtrate is concentrated in vacuo. CH2CI2 and H2O are added to the
residue, and the organic phase is separated off, dried (Na2SO4) and concentrated, and the
residue is purified by flash chromatography. Eluent: CH2CI2/acetone (9:1). The title
compound, compound (11), is obtained.
Example A7: r-~epa,alion of lM-2]-CHrOH (13)
10 9 of 3,5-dimethoxybenzyl bromide and 3.53 9 of methyl 3,5-dihydroxybenzoate are
added together to 200 ml of acetone, after which 1 .t 1 g of 1 8-crown-6 and 6.2 9 of K2CO3
CA 0220691~ 1997-06-04
W O 96/19240 PCT~EP95/n4933
-24-
are added, with the mixture subsequently being heated under reflux (argon) for about 20 h.
The residue is filtered off and the fiitrate is concer,L~ ed and CH2C12/H20 is added. The
organic phase is separated off, dried (Na2SO4) and concenl,~led, and the solid is
recryst~ 7ed from toluene/hexane. The compound (12)
[M-1]-COOCH3 (12)
is obtained.
b) 1.2 g of LAH are added to a solution of 8 9 of compound (12) in 150 ml of
tetrahydrofuran. The suspension is stirred at room temperature under argon for 0.5 h and
then at 40~C for 16 hours. While being cooled, the reaction mixture is carefully hydrolysed
with H20, acidified with dilute HCI and filtered. CH2C12/H20 is added to the filtrate, and the
organic phase is separated off and dried (Na2SO4). The solvent is removed and the residue
is dried under high vacuum, treated with hexane, filtered off and dried. The compound (11)
is obtained.
c) 1.5 9 of compound (11),1.05 9 of triphenylphosphine and 1.32 9 of CBr4 are stirred, at
room temperature for 1 h, in 50 ml of THF. The precipitate is filtered off and the fil~rate is
concentrated to dryness. CH2C12/H20 is added to the residue, and the organic phase is
separated off and dried (Na2SO4) and the solvent is removed. Purification is effected by
means of flash chromatography. Eluent: CH2C12. The compound (46)
[M-1]-CH2Br (46)
is obtained.
d) 0.8 9 of compound (46) and 105 mg of 3,5-dihydroxybenzyl alcohol are dissolved in 50
ml of acetone (argon atmosphere), and 276 mg of K2C03 (anhydrous) and 42.3 mg of 18-
crown-6 are added and the whole is heated under reflux (argon) for 24 hours. The mixture
is filtered and the filtrate is concentrated in vacuo. CH2CI2 and H20 are added to the
residue, and the organic phase is separated off, dried (Na2SO4) and concentrated; the
residue is purified by flash chromatography. Eluent: CH2CI2/acetone (19:1). The title
compound, compound (13), is obtained.
CA 0220691~ 1997-06-04
W O 96119240 PCTAEP95/04933
-25-
a,~ar~liu" of illte~ le~JF~ r~
o
,le B1: r~ aldlion of [G-2]-O--C--o--N~ (15)
a) 0.2 9 of compound (4) is added to a suspension of 18 mg of NaH in 10 ml of absolute
tetrahydrofuran and the mixture is heated under reflux for 15 min. It is then cooled down to
room temperature, 65 mg of c~-bromo-p-toluic acid are added and the whole is heated under
reflux overnight. The reaction mixture is allowed to cool down to roorn temperature, after
which it is carefully treated with a few drops of H2O and acidified with dilute HCI.
CH2CI2/H20 is added to the solution, and the organic phase is separated off and dried
(Na2SO4) and the solvent is removed. The residue is subsequently dried under high
vacuum. The compound (14~
[G-Y-Q-cooH (1 4)
is obtained.
b) 100 mg of compound (14) and 13.8 mg of N-hydroxysuccinimide are dissolved in 5 ml of
absolute tetrahydrofuran, the solution is cooled down to 0~C, and a solution of 25.8 ml of
DCC in 5 ml of tetrahydrofur,an is added. The mixture is stirred at room temperature
overnight, after which the solvent is removed in vacuo and the residue is purified by flash
chromatography. The title compound, compound (15), is obtained.
O 11
Example B2: Preparation of [G-3]-Q--C--O--N~l~ (17)
,, . o
.
a) 1.0 g of compound (6) is added to a suspension of 48 mg of NaH in 20 ml of absolute
tetrahydrofuran, and the mixture is heated under reflux for 15 min. It is then cooled down to
room temperature, 0.15 g of ~-bromo-p-toluic acid is added and the whole is heated under
reflux overnight. The reaction mixture is allowed to cool down to room temperature, after
which it is carefully treated with a few drops of H20 and acidified with dilute HCI.
CA 022069l~ l997-06-04
W O 96/19240 PCT/~5~01933
-26-
CH2CI2/H2O is added to the solution, and the organic phase is separated off and dried
(Na2SO4) and the solvent is removed. Purification is effected by means of flash
chromatography. Eluent: CH2CI2:acetone (9:1). The compound (16)
[G-3]~Q-COOH (1 6)
is obtained.
b) 200 mg of compound (16) and 14.0 mg of N-hydroxysuccinil~,ide are dissolved in 5 ml of
absolute tetrahydrofuran, the solution is cooled down to 0~C and a solution of 27 mg of
DCC in 5 ml of tetrahydrofuran is added. The mixture is stirred at room temperature
overnight, after which the solvent is removed in vacuo and the residue is purified by flash
chromatography. Eluent: CH2C12. The title compound, compound (17) is obtained.
O 11
E~a~ le B3 r,e;~ arc.lio.~ of [G-3]-~C--o--N~ (19)
o
a) 0.32 9 of compound (6) and 16 mg of 4-(dimethylamino)pyridine are dissolved in 2 ml of
pyridine, after which 25 mg of succinic anhydride are added. The mixture is stirred for 16
hours and then treated with CH2C12 and extracted twice with an ice-cold 10% solution of
citric acid on each occasion. The organic phase is separated off and dried (Na2SO4) and the
solvent removed; the residue is dried under high vacuum. The compound (18).
[G-3]-W-COOH (1 8)
.
is obtained.
b) 125 mg of compound (18) and 1 1.5 mg of N-hydroxysuccinimide are dissolved in 10 ml of
absolute tetrahydrofuran, the solution is cooled down to 0~C and 25 ml of DCC are added.
The mixture is allowed to stand overnight, after which the solvent is removed in vacuo and
the residue is purified by flash chromatography. Eluent: CH2CI2:acetone (19:1). The title
CA 0220691~ 1997-06-04
W O96/19240 PCT/~1~51~1g33
-27-
comopund, compound (19), is obtained.
O
0 11
Example B4: P,~.ar~lion of [G-2]-~e o N? (21)
a) 8.5 mg of 4-(dimethylamino)pyridine and 50 ml of triethylamine are added to 120 mg of
compound (7) and the reaction mixture is then stirred at room temperature overnight. It is
then treated with 3.0 ml of C1-12C12 and extracted twice with ice-cold 10% citric acid on each
occasion. The organic phase is subsequently washed with H2O and dried (Na2SO4) and the
solvent removed the residue is dried under high vacuum. The compound (20)
[G-Y-w-cooH (20)
which is used without further purification, is obtained.
b) 130 mg of compound (20) are dissolved in 5 ml of absolute tetrahydrofuran, the solutlon
is cooled down to ~C and 35 mg of N-hydroxysuccinimide and 17.5 mg of DCC are then
added. In order to ensure complete reaction, the reaction mixture is stirred at room
temperature overnight. The precirit~te is filtered off, the filtrate is concentrated and the
residue is purified by flash chromatography. Eluent: CH2CI2:acetone (19:1). The title
compound, compound (21), is obtained.
O 11
Exa~ .le B5: Plt~,aldlio-~ of [G-3]-~e ~ N~--l (23)
" . ~
O
a) 60 mg of 4-N,N-dimethylaminopyridine and ~.0 ~11 of triethylamine are added to 140 ml of
compound (9) and the reaction mixture is then stirred at room temperature overnight. It is
treated with 3.0 ml of CH2C12 and extracted twice with ice-cold 10% citric acid on each
occasion. The organic phase is subsequently washed with H20 and dried (Na2SO4) and the
CA 0220691~ 1997-06-04
W O 96/lg240 PCT/~l9~ 933
- 28 -
solvent removed; the residue is dried under high vacuum. The compound (22)
[G-3]-W-COOH (22)
which is used without further pu, i~ic~ion, is obtained.
b) 140 mg of compound (22) are dissolved in 5 ml of absolute tetrahydrofuran, the solution
is cooled down to 0~C and 11 mg of N-hydroxysuccinimide and 20.5 mg of DCC are then
added. In order to ensure complete reaction, the reaction mixture is stirred at room
temperature overnight. The ,l~r~ci,~ le is filtered off, the filtrate is concenl~led and the
residue is purified by flash chr~""~lography. Eluent: CH2CI2:acetone (19:1). The title
compound, compound (23), is obtained.
o
ExampleB6: Preparationof lG-4]-Q--C--O--N~ (25)
o
a) 1.0 9 of compound (10) is added to a suspension of 12 mg of NaH in 10 ml of absolute
tetrahydrofuran and the mixture is heated under reflux for 15 min. It is then cooled down to
room temperature, 36.5 mg of a-bromo-p-toluic acid are added and the whole is heated
under reflux overnight. The reaction mixture is allowed to cool down to room temperature
after which it is carefully treated with a few drops of H2O and acidified with dilute HCI.
CH2CI2/H2O is added to the solution, and the organic phase is separated off and dried
(Na2SO4) and the solvent removed. Purification is effected by means of flash
chro,,,~lu9raphy. Eluent: CH2CI2:acetone (19:1). The compound (24)
[G-4]-Q-COOH (24)
is obtained.
b) 100 mg of compound (24) and 4.5 mg N-hydroxysuccinimide are dissolved in 5 ml of
absolute tetrahydrofuran, the solution is cooled down to 0~C and a solution of 10 mg of
CA 022069l5 l997-06-04
W 096/19240 PCT/~l J~/O 1933
-29-
DCC in 5 ml of tetrahydrofuran is added. The mixture is stirred at room temperature
overnight, after which the solvent is removed in vacuo and the residue is purified by flash
chromatography. Eluent: CH2Ci2/acetone (60:1). The title compound, compound (25), is
obtained.
o
Il ~
Example B7: r.epar~tliG.. of [G-1]--Q--C--O--Nh l (B7.2)
a) 5.0 9 of compound (2) are reacted with 1.2 9 of sodium hydride and 3.44 g of a-bromo-p-
toluic acid in 200 ml of absolute tetrahydrofuran in analogy with method B1 a). The
compound (B7.1)
[G 1 ]-Q-COOH (B7. 1 )
is obtained.
b) 3.0 g of compound (B7.1) are reacted with 0.83 g of N-hydroxysuccinimide and 1.55 g of
N,N-dicyclohexylcarbodiimidle in 50 ml of absolute tetrahydrofuran in analogy with method
B1 b). The title compound, ~ompound (B7.2) is obtained.
~.eparalion of de--d~ er-f~ cliGI~ali~ed cG,.l-olled pore glass (CPG)
Ex~...~.~le C1: rl~.aralion of Compound No. 30
"
- [G-2]-~C--NH-CH2 CH----CH2 ODMTr
o--C--CH2 CH2 ICI--NH--CPG
a) 545 mg of 3-amino-1,2-propanediol are added, while stirring vigorously, to 3.19 of
CA 0220691~ 1997-06-04
W 096/19240 PCT/~19S/~933
-30-
compound (15) in 40 ml of tetrahydrofuran. The reaction mixture is stirred at room
temperature for a total of 3 h and at 50~C for 15 min. It is then concentrated and the residue
purified by column chromatography fflash chromatography). Eluent: MeOH:CH2CI2 (1:9).
The product is subsequently dried under high vacuum. The compound (26)
[G-2]-Q-C(O)-NH-CH2-CH(OH)-CH2-OH (26)
is obtained.
b) 2.6 9 of compound (26) are dissolved in 20 ml of absolute pyridine, the solution is cooled
down to 0~C and 1.01 9 of dimethoxytrityl chloride (DMTrCI) are added in several portions at
this temperature. After that, the mixture is stirred at 0~C for 1 h, slowly brought to room
temperature and stirred overnight. 2 ml of methanol are added to the reaction solution and
the solvent (pyridine + methanoi) is removed. 100 ml of CH2C12 are added and the mixture is
washed with a 5% solution of NaHCO3 and with H20. The organic phase is dried, the
solvent is removed and the residue is purified by flash chromatography. Eluent:
CH2CI2:acetone (9:1) + 0.5 % triethylamine. The compound (27)
[G-2]-Q-C(O)-NH-CH2-CH(OH)-CH2-O-DMTr (27)
is obtained.
c) 1.25 9 of compound (27) are dissolved in 5.0 ml of CH2CI2, and 0.15 9 of succinic
anhydride are added. 61 mg of 4-(dimethylamino)pyridine and 140 ~l of triethylamine are
then added to the reaction mixture and the whole is stirred at room temperature overnight.
The solvent is removed in vacuo and 20 ml of CH2C12 are added to the residue, and this
solution is washed with an ice-cold 10% solution of citric acid and with H2O. The organic
phase is separated off and dried (Na2SO4) and the solvent is removed in vacuo. The
residue is dissolved by adding 5 ml of CH2C12, and the product is precipitated out by adding
approximately 100 ml of hexane while at the same time stirring vigorously. The solvent is
decanted off and the residue is dried under high vacuum. The compound (28)
CA 02206915 1997-06-04
W O 9~/19240 PCT~EP9~1~;933
[G-2]-Q--C--NH-CH2 CH CH2 ODMTr (2~)
O--C--CH2 CH2 COOH
O
is obtained.
d) 258 mg of DCC are added to a reaction mixture consisting of 0.68 9 of compound (28),
70 mg of p-nitrophenol, 100 ~11 of pyridine and 2 ml of dioxane and the whole is stirred at
room temperature. After a total reaction time of 3 h, the mixture is filtered. The compound
(29)
[G-y-Q_e--NH--CH2 CH CH2 ODMTr
(29)
( )--C CH2 CH2 C--O~NO2
is obtained.
e) The resulting compound (:~9) is added to a suspension of 3 g of dried (H\/ pump over
P205) long chain alkylamine controlled pore glass (LCAA-CPG) in 7 rnl of
dimethylformamide and the whole is stirred overnight. The mixture is filtered, a further 3 9 of
LCAA-CPG is added to the filtrate, with the whole then being stirred overnight and
subsequently filtered. 1 ml of acetic anhydride, 50 mg of 4-(dimethylamino)pyridine and 10
ml of pyridine are in each case added to the solid support (2x 3 9 in each case) and the
mixture is stirred for 1 h. The support is filtered off, washed with dimethylformamide,
methanol and diethyl ether t100 ml in each case) and dried in a desicc~t--r. The title
compound, compound (30) is obtained.
Ex~ .le C2: rr~,ar~liG" of Compound No. 35
,0,
[G-3]-Q--C--NH-CH-CH2 ODMTr
(35)
CH2 O--C~CH2 CH2 C--NH--CPG
O O
CA 0220691~ 1997-06-04
W O96/1924~ PCT~EPg5/04933
a) 1.4 9 of compound (17) and 136 mg of serinol are added together to 20 ml of
tetrahydrofuran and the reaction mixture is stirred at room te",pe,dlure. CH2CI2 and H20 are
added to it, and the organic phase is separated off and dried and the solvent is removed.
The product is purified by flash cl~ror"~Lugraphy. Eluent: CH2CI2:MeOH (19:1). The
compound (~1)
[G-3]-Q-C(0)-NH-CH(CH20H)2 (31 )
is obtained.
b) 0.7 9 of compound (31) is dissolved in 15 ml of absolute pyridine, the solution is cooled
down to 0~C and 140 mg of dimethoxytrityl chloride (DMTrCI) are added. The mixture is
stirred at 0~C for 1 h, brought to room temperature and stirred overnight. Working-up is
effected in analogy with Example C1 b). The compound (32)
[G-3]-Q-C(0)-NH-CH(CH20H)-CH2-0-DMTr (32)
is obtained.
c) 0.4 g of compound (32) are dissolved in 5.0 ml of CH2C12, and 30 mg of succinic
anhydride are added. 12.5 mg of 4-N,N-dimethylaminopyridine and 30 ~Ll of triethylamine
are subsequently added to the reaction mixture, which is then stirred at room temperature
overnight. Working-up is effected in analogy with Example C1 c). The compound (33)
~G-3]-Q--C--NH CH--CH2--ODMTr (33)
CH20--,C, -CH2CH2--COOH
O
is obtained.
d) 82.5 mg of DCC are added to a reaction mixture consisting of 0.35 9 of compound (33),
28 mg of p-nitrophenol, 50 ~l of pyridine and 4 ml of dioxane and the whole is stirred at
room temperature. After a total reaction time of 3 h, the mixture is filtered. The compound
CA 02206915 1997-06-04
Wo 96/19240 PCT/EP95/04933
(34)
O
[G-3]-~C--NH--CH CH2 ODMTr
CH2--O---C CH2--CH2 8 O~NO ~
O O
is obtained.
e) The title compound, compound (35), is obtained after reacting the resulting compound
(34) with 3 9 of LCAAICPG (dried over P205 under high vacuum).
Example C3: rl~.aldlion of Compound No. C3.5
[G-l] Q--C--NH CH CH2 ODMTr
(C3.5)
CH2O IC~--CH2--CH2~ NH--CPG
O O
a) 1.5 g of compound (B7.2)) are reacted with 0.32 9 of serinol in 30 ml of absolute
tetrahydrofuran at room temperature for 3 hrs and then at 50~C for 1 hr, in analogy with
method C2a). The compound (C3.1)
[G1]-Q-c(o)-NH-cH(cH2oH)2 (C3-1)
is obtained.
b) 1.4 g of the compound (C3.1) are reacted with 0.95 9 of 4,4'-dimethoxytrityl chloride
(DMTrCI) in 30 ml of absolute pyridine in analogy with method C2b). The compound (C3.2)
[G1]-Q-C(O)-NH-CH(CH2OH)-CH2-O-DMTr (C3.2)
is obtained.
CA 02206915 1997-06-04
Wo 96/19240 PC r/~l ;,S/0 19~3
- 34 -
c) 0.60 g of the compound (C3.2) is reacted with 0.10 g of succinic anhydride, 49 mg of
4-N,N-dimethylaminopyridine and 110 ~l of triethylamine in 5 ml of methylene chloride, in
analogy with method C2c). The compound (C3.3)
[G~ Q---C--NH-- I H CH2 ODMTr
CH2~--8--CH2 CH2 COOH (C3.3)
is obtained.
d) 0.60 g of the compound (C3.3) is reacted with 91 mg of p-nitrophenol, 310 mg of
N,N-dicyclohexylcarbodiimide and 150 lli of pyridine in 5 ml of dioxane, in analogy with
method C2d). The compound (C3.4)
[G-1] Q--C--NH CH CH2 ODNlTr
CH O--C--CH CH C--O~ (C3.4)
is obtained.
e) The title compound, compound (C3.5), is obtained by reacting the resulting compound
(C3.4) with 6 g of LCAA/CPG, in analogy wiih method C1.
r~ dlion of de".l,i."er-pl,ospl,ord"~idile building blocks
Example D1: r, epa.dlion of Compound No. 36
[G-2~ CH2--O--P--O--CH2 CH2 CN (36)
N--(CH(CH3)2)2
CA 0220691~ 1997-06-04
W 096/19240 P~ 5~0l3~3
0.75 9 of compound (4) is dissolved in 30 ml of hot acetonil,ile, the solution is then cooled
down to room temperature and 85.5 mg of diisopropylammonium tetr~olide are added.
0.33 ml of [bis(diisopropylamino)-2-cyanoethoxy]phosphine is addecl dropwise, under an
argon atmosphere, and the mixture is stirred at room temperature for 1 h and at 30~C for 2
h. The title compound, compound (36), is obtained.
Sy.lt~.esis and pU.;tiCdliG., of oligon~lcl~a1;-1?s
The oligonucleotides are synthesized on an Applied Biosystems 392 DNA-RNA synthesizer
(synthesis scale, 0.1 - 10 ~mol) or on a Millipore 8800 large scale DNA synthesizer
(synthesis scale, 10 - 100 ~lmol) using the common cyanoethyl phosphoramidite method
and employing 4-tert.-butylphenoxyacetyl-protected building blocks on a solid phase
support.
If the dendrimer moiety is to be attached to the 3' end of the conjugate (cf. also Examples
E6 to E20), the oligonucleotide synthesis is then carried out using a dendrimer-modified
solid phase support, e.g. co~pounds 30, 35 or C3.5. After the synthesis, the crude
polynucleotides are detached from the solid phase support, and deprotected, by being
treated, at room temperature for 16 hrs, with a conc. aqueous solution of ammonia. The
solution is concentrated and the crude oligonucleotide is purified by reverse phase high
pressure chromatography (\Naters HPLC system) using a Nucleosil C 18 column (gradient:
from 85% 0.05 M triethylammonium acetate and 15% acetonitrile to 100% acetonitrile over
65 min). The oligonucleotide-containing fractions are collected and Iyophilized. Molecular
weights are determined on an LD1 1700 (Linear Scientific Inc., Reno, USA).
Introduction of the dendrimer at the 5~ end (cf. also Examples E1 to E5) is carried out, as
described, by using a suitable dendrimer derivative and an appropriately amino-substituted
oligonucleotide. Alternatively, the dendrimer can be introduced directly during the
oligonucleotide synthesis by using a dendrimer-phosphoramidite.
rr~t.ardlion of oligonucleotide conjugates
CA 02206915 1997-06-04
W O 96/19240 PCT~EP95/04933
The preparation of oligonucleotide-den~,i",er conjugates is described below.
Oligonucleotides having the f~l c~;,lg sequences are used:
PO: Linkage of the nucleo~ides via -P(O)O -
PS: Linkage of the nucleosides via -P(O)S -
SEQ. ID. NO.1:5'-111llC;Ic;l~ill;l(;l-3~ (PO)
SEQ. ID. NO.2:5'-111ll(;lC;I~;lOlCT-3' (PS)
SEQ. ID. NO.3:!~'-GCCCCCAGCATCGACATCTA-3' (PO)
SEQ. ID. NO. 4: 5'-GCCCCCAGCATCGACATCTA-3' (PS)
SEQ. ID. NO:5:5'-TCCCGCCTGTGACATGCATT-3' (PS)
SEQ.ID.NO.6:5'-GTTCTCGCTGGTGAG m CA-3' (PS)
Example E1: rrt ~,ardion of Compound No. 38 (cf. SEQ. ID. NO. 1)
[G-2]-Q-CoNH(cH2)6op(o)(oH)-os(lllll~l~l~lclol)3 (38)
2.0 mg of cornpound (15) are reacted with 2 OD of compound (37)
H2N(CH2)6op(o)(oH)-o~(lllll~lcl~lcl~l)3(37)
at room temperature for 20 h in the presence of 160 ul of solvent
(dimethylformamide:dioxane:H2O = 1:1:4) and 5~11 of N N-diisopropylethylamine. The title
compound compound (38),is obtained.
Example E2: PreparaliG~ of Compound No. 39 (cf. SEQ. ID. NO. 1)
[G-3]-Q-CONH(CH2)6OP(O)(OH)-O5~11111CTCTCTCTCT)3 (39)
The title compound compound (39),is obtained by reacting compound (17) in analogy with
Example (E1).
CA 02206915 1997-06-04
W O96/19240 PCTI~r~510~933
Example E3~ ai~lion oF Compound No. 41 (cf. SEQ. ID. NO. 11)
[G-3]-CH2OC(o)(CH2)2CoNH(cH2)6op(o)(oH)-o5(lllllcl~lcl~l)3 (41)
120 1ll of a mixture of dimethylformamide and H20 (5:1) and 2 !11 of diisopropylethylamine
are added to 2.0 mg of compound (40)
(40)
[G-3]--CH2--O--C--CH2 CH2 C--O--N~
o
together with compound (37), and the mixture is stirred at 40~C for 16 h. The title
compound, compound (41), is obtained.
Example E4: rre~ aralion olF Compound No. 42 (cf. SEQ. ID. NO. 1)
~G-2]-w-coNH(cH2)6op(o)(oH)-o5 ( I ~ ;lCI~ ;1)3 (42)
The title cornpound, compound (42), is obtained by reacting 2.0 mg of compound (21 ) and
4 OD of compound (37) in analogy with Example (E3).
Ex~l"~,le E5: r~ io" of Compound No. 43 (cf. SEQ. ID. NO. 1)
[G-3]-W-CoNH(CH2)6oP(o)(oH)-o5 ( I 1 111CICI(;ICI(;1)3 (43)
The title compound, compound (43), is obtained by reacting 2 mg of compound (23) and 4
~ . OD of compound 37 in analogy with Example (E4)
Examples E6 to E20:
The following 3'-oligonucleotide-dendrimer conjugates are synthesized by using dendrimers
which are bound to solid phase supports::
CA 02206915 1997-06-04
W O 96/19240 PCTAEP9S/04933
-38-
E6: PO (cf. SEQ. ID. NO. 1)
1~l
51 1 1 1 I CTt~ ; l --P(O)(OH)O/~/\NH Q--[G-2]
OH
E7: PO (cf. SEQ. ID. NO. 1)
1~l
F-51 I I I I~l~lC;lC;lc13-P(O)(OH)O/~/--NH Q'--[G-2]
OH
E8: PS (cf. SEQ. ID. NO. 2)
1~l
F- I I I I I ~i l (; l C I CTCP- P(S)(OH)O /\I/--NH Q'--[G-2]
OH
E9: PO (cf. SEQ. ID. NO. 1)
F- 5 1 1 ! I I C I CTCTCTCP- P(O)OH)O /~NH Q'--[G-3]
OH
E10: PS (cf. SEQ. ID. NO. 2)
1~l
F-51 1 1 1 IC;l~;lCTCTCT3-P(S)OH)O /~NH Q'--[G-3]
OH
Ell: PO (cf. SEQ. ID. NO. 3)
CA 02206915 1997-06-04
PCT~EP~/04933
W 096/19240
-39-
5GCCCCCAGCATCGACATCTA3-P(o)(oH)o /~NH Q'--[G-2]
OH
E12: PS (cf. SEQ. ID. NO. 4)
5 GCCCCCAGCATCGACATCTA3 -P (S) (OH) ~ /~--H~ NH o~--[G-2]
E13: PO (cf. SEQ. ID. NO. 3)
~ NH Q' [G-3]
5GCCCCCAGCATCGACATCTA3-P(o)(oH)o '~
OH o
E14: PS (cf. SEQ. ID. NO. 4~
NH Q' [G-3]
5 GCCCCCAGCATCGACATCTA3 -P(S) (OH)O /\~ l~
OH
E15: PS (cf. SEQ. ID. NO. 5~
J~ ' .
5TCCCGCCTGTGACATGCATT3-P(S)(oH)o/~NH Q'--[G-2]
OH
E16: PS (cf. SEQ. ID. NO. 5~
NH Q' [G-1]
5TCCCGCCTGTGACATGCATT3-P(S)(oH)o /~~
OH
CA 02206915 1997-06-04
W O96/19240 PCT/~l9_J~333
-40-
E17: PS (cf. SEQ. ID. NO. 5)
~ NH Q' [G-3
5TCCCGCCTGTGACATGCATT3-P(S)(oH)
OH
E18: PS (cf. SEQ. ID. NO. 6)
o
5GTTCTCGCTGGTGAGlllCA3 P(S)(OH)O ~ NH Q'-[G-2]
OH
E19: PS (cf. SEQ. ID. N0. 6)
5GTTCTCGCTGGTGAGIIICA3 P(S)(OH)O ~ NH Q'-[G-1]
OH
E20: PS (cf. SEQ. ID. NO. 6)
5 GTTCTCGCTGGTGAGmCA3 -P(S)(OH)O'--~ N~ Q'--[G-3]
F: Fluorescein
PO: Linking of the nucleosides via -P(O)O -
PS: Linking of the nucleosides via -P(O)S -
CA 0220691~ 1997-06-04
W O 96/19240 PCTAEr9~a:~33
-41-
Rj~ ~'activi~
In vivo anti tumor activity a~a;.~t human lung calci.,G.,-a A549 o~ oligon~le~ti~e
~~c.~ i",~r conj~ t~s
D~ lion of anti tumor activities are carried out in male Balb/c nude mice bearing
serially passaged (minimum of three consecutive transplantations) human lung carcinoma
A549 (CCL185, American Type culture collection ATCC; Rockville, Maryland, USA).The
cells are cultured as recommended by ATCC. Tumor fragments of approx;,nalely 25 mg are
transplanted into the left flank of each animal (n = 6 per group). Treatments with
oligonucleotide dendrimer conjugates according to the presen~ inven~ion are started when
the tumors reach a mean tumor volume of 150 - 200 mm3. Tumor growth is monitoredtwice weekly by measuring perpendicular diameters. Tumor volumes are determined as
described in T. Meyer et al. Int. J. Cancer 43 (1989) pp. 851-856. Treatment schedule
used in these experiments is once daily i.v (tail vein) starting day 10 after tumor
transplantation .
Example F1:
The novel oligonucleotide-dendrimer conjugate from Example E15 is employed for the
treatment in accordance with the above protocol.
Example F2:
The novel oligonucleotide-dend,i",er conjugate from Example E16 is employed in analogy
with Example F1.
Example F3:
.
The novel oligonucleotide-dendrimer conjugate from Example E17 is employed in analogy
with Example F1.
CA 02206915 1997-06-04
W O 96/19240 PCT~EP95/04933
-42-
Example F4:
The novel oligonucleotide-dendrimer conjugate from Example E18 is employed in analogy
with Example F1.
Example F5:
The novel oligonucleotide-dendrimer conjugate from Example E19 is employed in analogy
with Example F1.
Example F6:
The novel oligonucleotide-dendrimer conjugate from Example E20 is employed in analogy
with Example F1.
CA 02206915 1997-06-04
W O96119240 PCT1~9~1933
-43-
~U~N~ LISTING
(1) ~FN~RAT. INFORMATIONo
(i) APPLICANT:
(A) NAME: CI~- OE IGY AG
(B) STREET: Klybeckstr. 14l
(C) CITY: Basel
(E) Cuu~l~nY: .Switzerland
(F) POSTAL CODE (ZIP): 4002
(G) '~ :~HUN~- +41 61 69 ll ll
(H) ~ F~FAX: -~ 41 61 696 79 76
(I) ~TT~X: 262 99l
(ii) TITLE OF lNV~N'l'lU~: 91 ;~ml~leotide dendrimer conjugates
(iii) NUMBER OF S~YU~N~S: 6
(iv) COMPu~l~K R~AnART~T~ FORM:
(A) MEDIUM TYL'E: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #l.0, Version #1.30 (EPO)
(2) INFORMATION FOR SEQ ID NO: l:
( i ) ~UU~N~: cHARAr-TFRT~sTIcs:
. (A) LENGTH: 15 base pairs
~ (B) TYPE: ml~l~;~ acid
(C) S~ANl)~ Nl~:SS: single
(D) TOPOLOGY: linear
( ii ) M~T~T~'CTlT~T~ TYPE: other nucleic acid
CA 022069l5 l997-06-04
W O 96/19240 PCTnEPg5/04933
-44-
(A) DESCRIPTION: /desc = "ol;gnnllcleQtide"
(iv) ANTI-SENSE: YES
t x~ :UU~N~ DESCRIPTION: SEQ ID NO: 1:
'll-l'll~'l~'l~ TCTCT 15
(2) INFORMATION FOR SEQ ID NO: 2:
U~;N~ R z~t'T~R T !::TIcs:
(A) T-~N~,T~ 15 base pairs
(B) TYPE: nllrl~;r acid
(C) STF~NI~ l)N~ s single
( n ) TOPOLOGY: linear
( ii ) ~T.~.~ TYPE: other nurl e; r acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(iv) ANTI-SENSE: YES
(ix) FEATURE:
(A) NAME/ ~ Y: misc_feature
(B) LOCATION:1..15
(D) OTHER INFORMATION:/note= "~;f;~ hArkhnn~"
. ( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 2:
'll'll'l~'l~'l~ TCTCT 15
(2) INFORMATION FOR SEQ ID NO: 3:
CA 02206915 1997-06-04
W 096tl9240 PCT~EP9~/04933
-45-
;L2UI~;N(~; f~A~z~ rrFRIsTIcs:
(A) T~f,T~: 20 base pairs
(B) TYPE: ml~1~;~ acid
(C) STRAN~ S: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oli~omlcleotide"
(iv) ANTI-SENSE: YES
( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 3:
fX CCCfAf~r-A TCGACATCTA 20
(2) INFORMATION FOR SEf~ ID NO: 4:
( i ) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 20 base pairs
(B) TYPE: ml~l~;r acid
(C) S~RAN~ N~:~S: single
(D) TOPOLOGY: linear
(ii) Mf)T.F.f~.F. TYPE: other nll~le;c acid
(A) DESCRIPTION: /desc = "o1; goml~l eotide"
(iv) ANTI-SENSE: YES
.
(ix) EEATuRE:
(A) NAME/REY: misc_feature
(B) LOCATION:1..20
(D) OTHER INF~RMA~Ir)N /note= "mn~;f;~ h~Ckho~"
CA 02206915 1997-06-04
W O 96/19240 PCT/~5_/01533
-46-
(Xi) ~:yu~N~ ~scRTpTIoN SEQ ID NO: 4:
GCCC~(A~A TCGACATCTA 20
(2) INFORMA~ION FOR SEQ ID NO: 5:
(i) ~u~ CHAR~CTERISTICS:
(A) LENGTH: 20 base pairs
(B) TYPE: ml~l ~; C acid
( C ) S~ NI ~ N ~ S S; n~1 e
(D) TOPOLOGY: linear
( ii ) M~T-~lT-~ TYPE: other nn~l ~; ~ acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(iv) ANTI-SENSE: YES
(ix) FEATURE:
(A) NAME/KEY: misc_feature
(B) LOCATION:1..20
(D) CTELK INFORMATION:/notec "m~;f;~ backbone"
(xi) ~ U~N~: D~RTPTION: SEQ ID NO: 5:
~CCCGCCl~l GACATGCATT 20
(2) INFORMATION FOR SEQ ID NO: 6:
U N~ CHARAC~l~ISTICS:
(A) T.~N~ . 20 base pairs
(B) TYPE: nll~l ~; ~ acid
(C) STT~Nl)t~ N~ s s;n~e
CA 02206915 1997-06-04
WO 96/19240 PCT/E~P95/04933
- 47 -
(D) TOPOLOGY: linear
(ii) ~nT~ T-~ TYPE: other nllc~e;c acid
(A) ~ RTPTION: /desc = "ol i~o~ leotide~
(iv) ANTI-SENSE: YE5
(ix) FEATURE:
(A) ~/~Y: rnisc_ feature
(B) T~TON:1..20
(D) OrHER INEORM~ION:/note= "m~i f; ~ backbone"
(xi) .~ u~ D~R~ O~: SEQ ID NO: 6:
W~ GL~l~ GTGAGTTTCA 20