Note: Descriptions are shown in the official language in which they were submitted.
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96101848
BANDED PROLONGED RELEASE ACTIVE AGENT DOSAGE FORM
2
FIELD OF THE INVENTION
4
s The present invention is related to the prolonged delivery of an active
s agent. More particularly, it is a banded active agent dosage form useful for
delivering a beneficial agent to a fluid environment of use. The invention is
s also directed to the method of making the banded active agent dosage form.
s
o BACKGROUND OF THE INVENTION
~2 Tablets, capsules, caplets and many other types of devices have been
s used for dispensing a beneficial agent to a fluid environment of use. Easy
a manufacture of a device that provides for prolonged delivery of an active
s agent in a controlled and predictable manner continues to be a goal,
s especially in the area of drug delivery.
~s US Patent No. 4,290,426 to Luschen et al describes a cylindrical
19 dispenser for releasing a beneficial agent into a fluid environment at a
rate
2o that is governed by the fluid induced relaxation of a polymeric agent
2~ contained within the dispenser. The cylindrical dispenser includes an
22 impermeable container that has within it a reservoir and a passageway from
2s the reservoir to the exterior of the container. The reservoir contains a
2a polymer and a beneficial agent. The polymer imbibes fluid from the
2s environment and thereby undergoes relaxation, releasing the beneficial
agent
zs from the device. The amount of agent released is dependent on the rate of
27 relaxation of the polymer over time.
28
2s Coated dosage forms have also been suggested for delivery of a
so controlled amount of a beneficial agent over a prolonged period of time. US
Patent No. 5,256,440 describes a process for producing a film coated dosage
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
form. A continuous groove is inscribed in a dosage form core. A latex film is
2 coated onto the core, the groove defining a fixed zone and a detachable zone
s for the film. The detachable portion of the latex film detaches when it is
4 exposed to the environment of use, thereby exposing a discrete portion of
the
s dosage form core surface. The remainder of the film remains attached to the
s dosage form core. The exposed portion of the dosage form surface erodes
~ and releases active agent to the environment of use.
s
s Coated tablets for constant and prolonged drug release are described
~o by Conte et al in J. Controlled Release, Vol. 26, (1993) pages 39-47. These
o GEOMATRIXTM Systems are swellable matrices that are coated or tableted
~2 with polymeric barrier layers. Release performances of the systems are
~s modulated as a result of the restriction of the releasing surface by the
~4 polymeric barrier layer coatings. As the extent of coating of the system's
~s surface is increased, the release kinetics of the system shift toward
constant
~s release. These systems are further described in US Patent No. 4,839,177 to
Colombo et al.
~s
~s As can be observed in the above-referenced patents and publications,
2o devices have been described that provide for prolonged delivery of an
active
2, agent. However, there remains a continuing need for improved systems for
22 delivering an active agent in a reliable and reproducible manner that are
easy
zs and inexpensive to manufacture.
24
2s SUMMARY OF THE INVENTION
zs
27 We have observed that devices such as those described above will
2s provide for prolonged delivery of an active agent formulation to a fluid
29 environment of use but that these devices may not necessarily provide for
so controlled and reliable release. Accordingly, the present invention is
directed
s, to a dispensing device that will release an active agent formulation in a
CA 02207098 2005-05-18
67696-344
3
reliably controllable manner, and further that is easy and
inexpensive to manufacture.
The invention is directed to an active agent
dosage form for the prolonged delivery of an active agent to
a fluid environment of use. The surface of an active agent
formulation matrix contains two or more insoluble bands.
In one aspect, the invention is directed to an
active agent dosage form for the prolonged delivery of an
active agent to a fluid environment of use. Two or more
insoluble bands surround an active agent formulation matrix.
The bands drop off the matrix as it erodes, providing a
decreasing path length for delivery of the drug.
In another aspect, the invention is to an active
agent dosage form for the prolonged delivery of an active
agent to a fluid environment of use where two or more
insoluble bands are printed or otherwise securely affixed to
the surface of the dosage form. The surface area of the
matrix between the bands erodes delivering active agent to
the fluid environment of use. The surface area not covered
by the rings increases with time.
The invention is also directed to a method for
preparing the active agent dosage form for prolonged
delivery of an active agent. An active agent formulation
matrix is prepared and insoluble material is placed or
printed onto the formulation to form two or more insoluble
bands on the surface of the matrix.
According to another aspect of the present
invention, there is provided an active agent dosage form for
the prolonged delivery of an active agent formulation to a
fluid environment of use, the dosage form comprising an
CA 02207098 2005-05-18
67696-344
3a
erodible active agent formulation matrix with at least two
insoluble bands positioned in spaced relationship on the
surface of the active agent formulation matrix with the
surface of the matrix on both sides of each band exposed to
the fluid environment of use.
According to still another aspect of the present
invention, there is provided an active agent dosage form for
the prolonged delivery of an active agent formulation to a
fluid environment of use, the dosage form comprising: a
first erodible active agent formulation matrix and at least
two insoluble bands positioned in spaced relationship on the
surface of the first active agent formulation matrix with
the surface of the first active agent formulation matrix on
both sides of each band exposed to the fluid environment of
use, and a second active agent formulation matrix, the first
active agent formulation matrix providing for the prolonged
delivery of a first active agent and a second active agent
formulation matrix providing an initial pulse of a second
active agent, the second active agent being the same as or
different than the first active agent.
According to yet another aspect of the present
invention, there is provided a method for making an active
agent dosage form for the prolonged release of an active
agent to a fluid environment of use, the method comprising:
(a) preparing an erodible active agent formulation matrix;
and (b) applying an insoluble material onto the active agent
formulation matrix to form at least two insoluble bands
positioned in spaced relationship on the surface of the
active agent formulation matrix with the surface of the
matrix on both sides of each band exposed to the fluid
environment of use.
CA 02207098 2005-05-18
67696-344
3b
According to a further aspect of the present
invention, there is provided a pharmaceutical composition
adapted to provide an active agent to a fluid environment of
use over a prolonged period of time, said composition
comprising: an active agent formulation matrix having at
least two insoluble bands positioned in spaced relationship
on its surface with the surface of the active agent
formulation matrix on both sides of each band exposed to the
fluid environment of use, wherein the matrix is adapted to
erode in the fluid environment of use, and wherein the bonds
are adapted to drop off the eroded matrix thereby exposing
the surface of the matrix to the fluid environment of use.
According to yet a further aspect of the present
invention, there is provided a pharmaceutical composition
adapted to provide an active agent to a fluid environment of
use over a prolonged period of time, said composition
comprising: an active agent formulation matrix having at
least two insoluble bands positioned in spaced relationship
on its surface with the surface of the active agent
formulation matrix on both sides of each band exposed to the
fluid environment of use, wherein the matrix is adapted to
erode in the fluid environment of use while the bands remain
positioned on the surface of the active agent formulation
matrix; and wherein the surface area of the matrix not
covered by the bands increases with time.
According to still a further aspect of the present
invention, there is provided a pharmaceutical composition
adapted to provide a first and second active agent to a
fluid environment of use, said composition comprising: a
first active agent formulation matrix comprising a first
active agent and having at least two insoluble bands
CA 02207098 2005-05-18
67696-344
3c
positioned in spaced relationship on its surface with the
surface of the first active agent formulation matrix on both
sides of each band exposed to the fluid environment of use
and a second active agent formulation matrix comprising a
second active agent, wherein the first active agent
formulation matrix is adapted to erode in the fluid
environment of use to provide prolonged delivery of said
first active agent; and wherein the second active agent
matrix is adapted to erode in the fluid environment of use
to provide an initial pulse of second active agent, the
second active agent being the same or different than the
first active agent.
According to another aspect of the present
invention, there is provided the pharmaceutical composition
as described herein wherein the bands are adapted to drop
off the surface of the first active agent formulation matrix
as the matrix erodes in the fluid environment of use.
According to yet another aspect of the present
invention, there is provided the pharmaceutical composition
as described herein wherein the bands remain positioned on
the surface of the first active agent formulation matrix as
the matrix erodes in the fluid environment of use.
According to another aspect of the present
invention, there is provided use of the pharmaceutical
composition as described herein for delivering an active
agent to a fluid environment of use for a prolonged period
of time .
According to still another aspect of the present
invention, there is provided use of the pharmaceutical
composition as described herein for delivering a first and
second active agent to a fluid environment of use.
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
1 DESCRIPTION OF THE DRAWINGS
2
s The figures are not drawn to scale, but are set forth to illustrate various
4 embodiments of the invention. Like numbers refer to like structures.
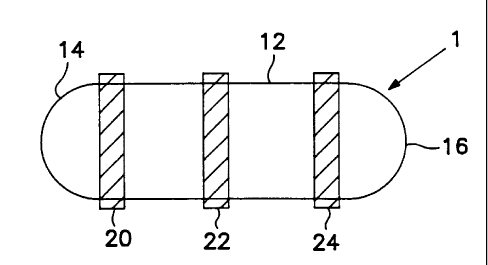
s FIG. 1 is a side elevational view of one embodiment of the delivery
~ device of the present invention, the device being in prepared form prior to
a placement in the environment of use.
s
1o FIG. 2 shows the device of FIG. 1 in operation after placement in the
11 environment of use, showing erosion of the active agent formulation matrix.
12
1s FIG. 3 shows the device of FIG. 1 in operation after sufficient erosion
1a of the matrix has caused separation of the banded sections of the device.
1s FIG. 4 is a side efevational view of a second embodiment of the
17 delivery device of the present invention, the device being in prepared form
1s prior to placement in the environment of use.
1s
2o FIG. 5 shows the device of FIG. 4 in operation after sufficient erosion of
21 the matrix has caused separation of the sections of the device.
22
2s FIG. 6 is a side elevational view of a third embodiment of the delivery
2a device of the present invention, the device being in prepared form prior to
is placement in the environment of use.
2s
z~ FIGs. 7, 8, 9, 10 and 11 (A and B) are graphs showing the performance
2s of ibuprofen matrices with bands of varying number and placed in different
2s positions on the matrices. FIGs. 7A, 8A, 9A, 10A and 11A show the release
so rates of the drugs from the devices while 7B, 8B, 9B, 1 OB and 11 B show
the
s1 cumulative doses of the drugs over a delivery period of up to 20 hours.
CA 02207098 1997-06-05
PCT/US96I01848
WO 96/25922
FIGs. 12, 13, 14 and 15 are graphs showing the release rates of ibuprofen
3 matrices with bands of varying number for a period of up to 4 hours.
4
5
s DETAILED DESCRIPTION OF THE INVENTION
s The present invention provides a device that is useful for the prolonged
s delivery of an active agent formulation to a fluid environment of use.
Definitions
12
~3 The phrase "prolonged delivery" intends a period of delivery that lasts for
,a several hours to about 24 hours, usually up to about 20 hours, and often
, s between about 3 and 16 hours.
1s
By "insoluble" is intended a material that will not dissolve, degrade or
,s erode in the environment of use during the delivery period.
19
zo By "apply" or "applied" or "application" intends the substantially uniform
z, deposition of insoluble material, in liquid or in molten form, onto the
active
2z agent formulation matrix. A variety of techniques may be used to apply the
z3 insoluble material, including but not limited to Gravure-type printing,
extrusion
Za coating, screen coating, spraying, painting, and the Capsealer process
2s developed by TAIT Design & Machine Co., Manheim, PA.
zs
27 The term "active agent formulation" intends the active agent or drug
za optionally in combination with pharmaceutically acceptable carriers and
i9 additional inert ingredients.
CA 02207098 1997-06-OS
WO 96125922 PCT/US96/01848
1 The term "active agent formulation matrix", as used herein, comprises the
z active agent formulation in combination with a hydrophilic polymeric
material.
3
4
s The term "active agent dosage form" intends the active agent formulation
s matrix as defined above with two or more bands of an insoluble material
applied onto its surface.
a
s As used herein, the terms "therapeutically effective" amount or rate refer
1o to the amount or rate of the active agent needed to effect the desired
11 pharmacologic, often beneficial, result.
12
13 The dispensing devices of the invention find use, for example, in humans
1a or other animals. The environment of use is a fluid environment and can
1s comprise the stomach, the intestinal tract, or a body cavity such as the
1s peritoneum or vagina. A single dispensing device or several dispensing.
17 devices can be administered to a subject during a therapeutic program.
1a
FIG. 1 depicts, in side elevational view, one embodiment of the delivery
2o device according to the present invention. The device is shown in prepared
21 form prior to placement in the environment of use. Dispensing device 1 is
22 shown in FIG. 1 to comprise a cylindrically shaped active agent formulation
23 matrix 12. The ends 14 and 16 of the matrix are preferably rounded and
2a convex in shape in order to ensure ease of insertion into the environment
of
2s use. Bands 20, 22 and 24 concentrically surround the cylindrical matrix 12.
26
27 FIG. 2 shows dispensing device 1 in operation after having been placed in
2s the fluid environment of use. The active agent formulation matrix 12
between
zs bands 20, 22 and 24 has begun to erode, thereby releasing active agent to
so the fluid environment of use.
31
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
1 FIG. 3 shows dispensing device 1 in operation after a length of time in the
2 fluid environment of use. The active agent formulation matrix 12 has eroded
3 between bands 20, 22 and 24 to such an extent that the matrix 12 is now in
4 three pieces, 30, 32 and 34. Erosion will continue until the matrix portions
of
s each of the pieces have completely eroded. Bands 20, 22 and 24 will
s thereafter be expelled from the fluid environment of use.
a FIG. 4 shows, in side elevational view, a second embodiment of the
s delivery device according to the present invention. The device is shown in
1o prepared form prior to placement in the environment of use. Dispensing
11 device 50 comprises a cylindrically shaped active agent formulation matrix
52
12 with convex ends 54 and 56. Bands 60, 62, 64 and 66 concentrically
13 surround the cylindrical matrix 52.
14
1s FIG. 5 shows dispensing device 50 in operation after a length of time in
1s the fluid environment of use. The active agent formulation matrix 52 has
17 eroded from the exposed ends of bands 60, and 66 to such an extent that the
1a device 50 is now in three pieces, 70, 72, and 74. The arrows show the
1s erosion of the matrix and therefore the extent of active agent delivery.
2o Erosion will continue from the exposed ends of bands 62 and 64 until the
21 matrix has completely eroded. Bands 60, 62, 64 and 66 will thereafter be
22 expelled from the fluid environment of use.
23
24 The active agent itself may be in liquid, solid or semisolid form. The
2s active agent formulation may contain additional materials and may be
2s designed in a multitude of ways to provide a specific drug delivery
profile.
27 One embodiment comprises a formulation that contains a biologically
28 acceptable hydrophilic polymer which is capable of slow dispersion in the
2s environmental fluid. In another embodiment, the formulation may contain a
3o hydrophilic polymer and a surfactant so that the formulation is susceptible
to
31 erosion in the environment. In still another embodiment, the formulation
may
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96I01848
include a solid surfactant and provide drug delivery in a finely dispersed
form.
z In yet a further embodiment, the formulation may include coated
s microspheres of an active agent and an adjuvant. The active agent and
a adjuvant can be delivered simultaneously from the microspheres either by
s diffusion or by osmosis. Suitable materials useful as active agent carriers
s and excipients are known in the art and are disclosed in US Patent Nos.
~ 4,595,583 and 4,874,388, for example.
8
s The terms "active agent" and "drug" are used interchangeably herein and
~o refer to an agent, drug, compound, composition of matter or mixture thereof
~~ which provides some pharmacologic, often beneficial, effect. This includes
~2 pesticides, herbicides, germicides, biocides, algicides, rodenticides,
~s fungicides, insecticides, antioxidants, plant growth promoters, plant
growth
~a inhibitors, preservatives, antipreservatives, disinfectants, sterilization
agents,
~s catalysts, chemical reactants, fermentation agents, foods, food
supplements,
~s nutrients, cosmetics, drugs, vitamins, sex sterilants, fertility
inhibitors, fertility
promoters, microorganism attenuators and other agents that benefit the
~s environment of use. As used herein, the terms further include any
physiologically or pharmacologically active substance that produces a
Zo localized or systemic effect or effects in animals, including warm blooded
mammals, humans and primates; avians; domestic household or farm animals
z2 such as cats, dogs, sheep, goats, cattle, horses and pigs; laboratory
animals
2s such as mice, rats and guinea pigs; fish; reptiles; zoo and wild animals;
and
Za the like. The active drug that can be delivered includes inorganic and
25 organic compounds, including, without limitation, drugs which act on the
2s peripheral nerves, adrenergic receptors, cholinergic receptors, the
skeletal
27 muscles, the cardiovascular system, smooth muscles, the blood circulatory
is system, synoptic sites, neuroeffector functional sites, endocrine and
hormone
2s systems, the immunological system, the reproductive system, the skeletal
3o system, autacoid systems, the alimentary and excretory systems, the
s, histamine system and the central nervous system. Suitable agents may be
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
1 selected from, for example, proteins, enzymes, hormones, polynucleotides,
z nucleoproteins, polysaccharides, glycoproteins, lipoproteins, polypeptides,
s steroids, hypnotics and sedatives, psychic energizers, tranquilizers,
a anticonvulsants, muscle relaxants, antiparkinson agents, analgesics, anti-
s inflammatories, local anesthetics, muscle contractants, antimicrobials,
s antimalarials, hormonal agents including contraceptives, sympathomimetics,
polypeptides and proteins capable of eliciting physiological effects,
diuretics,
a lipid regulating agents, antiandrogenic agents, antiparasitics, neoplastics,
s antineoplastics, hypoglycemics, nutritional agents and supplements, growth
1o supplements, fats, ophthalmics, antienteritis agents, electrolytes and
11 diagnostic agents.
12
1s Examples of beneficial agents useful in this invention include
1a prochlorperazine edisylate, ferrous sulfate, aminocaproic acid,
1s mecamylamine hydrochloride, procainamide hydrochloride, amphetamine
1s sulfate, methamphetamine hydrochloride, benzphetamine hydrochloride,
17 isoproterenol sulfate, phenmetrazine hydrochloride, bethanechol chloride,
1a methacholine chloride, pilocarpine hydrochloride, atropine sulfate,
1s scopolamine bromide, isopropamide iodide, tridihexethyl chloride,
phenformin
2o hydrochloride, methylphenidate hydrochloride, theophylline cholinate,
21 cephalexin hydrochloride, diphenidol, meclizine hydrochloride,
22 prochlorperazine maleate, phenoxybenzamine, thiethylperazine maleate,
Zs anisindione, diphenadione erythrityl tetranitrate, digoxin, isoflurophate,
Za acetazolamide, methazolamide, bendroflumethiazide, chlorpropamide,
zs tolazamide, chlormadinone acetate, phenaglycodol, allopurinol, aluminum
Zs aspirin, methotrexate, acetyl sulfisoxazole, hydrocortisone,
27 hydrocorticosterone acetate, cortisone acetate, dexamethasone and its
za derivatives such as betamethasone, triamcinolone, methyltestosterone, 17-
zs ~-estradiol, ethinyl estradiol, ethinyl estradiol 3-methyl ether,
prednisolone,
0 17-~i-hydroxyprogesterone acetate, 19-nor-progesterone, norgestrel,
s1 norethindrone, norethisterone, norethiederone, progesterone, norgesterone,
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96l01848
norethynodrel, aspirin, acetaminophen, indomethacin, naproxen, fenoprofen,
z sulindac, indoprofen, nitroglycerin, isosorbide dinitrate, propranolol,
timolol,
s atenolol, alprenolol, cimetidine, clonidine, imipramine, levodopa,
a chlorpromazine, methyldopa, dihydroxyphenylalanine, calcium gluconate,
s ketoprofen, ibuprofen, cephalexin, erythromycin, haloperidol, zomepirac,
s ferrous lactate, vincamine, phenoxybenzamine, diltiazem, milrinone,
captropril, mandol, quanbenz, hydrochlorothiazide, ranitidine, flurbiprofen,
a fenbufen, fluprofen, tolmetin, alclofenac, mefenamic, flufenamic, difuninal,
s nimodipine, nitrendipine, nisoldipine, nicardipine, felodipine, lidoflazine,
tiapamil, gallopamil, amlodipine, mioflazine, lisinopril, enalapril,
captopril,
ramipril, enalaprilat, famotidine, nizatidine, sucralfate, etintidine,
tetratolol,
~z minoxidil, chlordiazepoxide, diazepam, amitriptyline, and imipramine.
Further
~3 examples are proteins and peptides which include, but are not limited to,
a insulin, colchicine, glucagon, thyroid stimulating hormone, parathyroid and
~s pituitary hormones, calcitonin, renin, prolactin, corticotrophin,
thyrotropic
s hormone, follicle stimulating hormone, chorionic gonadotropin, gonadotropin
~ releasing hormone, bovine somatotropin, porcine somatropin, oxytocin,
a vasopressin, prolactin, somatostatin, lypressin, pancreozymin, luteinizing
s hormone, LHRH, interferons, interleukins, growth hormones such as human
zo growth hormone, bovine growth hormone and porcine growth hormone,
~ fertility inhibitors such as the prostaglandins, fertility promoters, growth
z2 factors, and human pancreas hormone releasing factor.
23
Za It is to be understood that more than one active agent may be
2s incorporated into the active agent formulation in a device of this
invention,
zs and that the use of the term "agent" or "drug" in no way excludes the use
of
z7 two or more such agents or drugs.
2a
2s The agents can be in various forms, such as uncharged molecules,
3o components of molecular complexes or nonirritating, pharmacologically
3, acceptable salts. Also, simple derivatives of the agents (such as ethers,
CA 02207098 1997-06-OS
WO 96/25922 PCTIUS96/01848
esters, amides, etc) which are easily hydrolyzed by body pH, enzymes, etc,
z can be employed.
3
a The amount of active agent employed in the delivery device will be that
s amount necessary to deliver a therapeutically effective amount of the agent
to
s achieve the desired result at the site of delivery. In practice, this will
vary
widely depending upon the particular agent, the site of delivery, the severity
8 of the condition, and the desired therapeutic effect. Thus, it is not
practical to
s define a particular range for the therapeutically effective amount of active
agent incorporated into the device.
~2 The hydrophilic polymeric material useful herein may comprise,
,s polysaccharides, methyl cellulose, sodium or calcium carboxymethyl
,a cellulose, hydroxypropylmethyl cellulose, hydroxypropyl cellulose,
,s hydroxyethyl cellulose, nitrocellulose, carboxymethyl cellulose and other
~s cellulose ethers, and polyethylene oxides (eg, Polyox~, Union Carbide).
Other materials useful as the hydrophilic polymeric material include but are
~8 not limited to methyl ethyl cellulose, ethylhydroxy ethylcellulose,
cellulose
s acetate, cellulose butyrate, cellulose propionate, gelatin, collagen,
starch,
2o maltodextrin, pullulan, polyvinyl pyrrolidone, polyvinyl alcohol, polyvinyl
2~ acetate, glycerol fatty acid esters, polyacrylamide, polyacrylic acid,
22 copolymers of ethacrylic acid or methacrylic acid (EudragitTM) or other
acrylic
2s acid derivatives, sorbitan esters, natural gums, lecithins, pectin,
alginates,
2a ammonia alginate, sodium or potassium alginate, calcium alginate, propylene
25 glycol alginate, potassium alginate, agar, gum arabic, gum karaya, locust
zs bean gum, gum tragacanth, carrageenans, gum ghatti, guar gum, xanthan
z7 gum, scleroglucan, and blends of the above.
28
2s The pharmaceutically acceptable carrier useful herein may comprise more
so than one ingredient, such as, for example, a buffer, a viscosity regulating
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
vehicle, a surtactant, a dye, a permeation enhancer, a proteinase inhibitor,
or
z other formulation ingredients and additives, as are known in the art.
3
a In addition to design of the active agent formulation to provide a specific
s drug delivery profile, the number, size, and placement of the insoluble
bands
s that are applied onto the active agent formulation matrix may be varied to
provide the desired drug delivery profile. For example, bands of from about
a 0.1 mm to about 12 mm in width, preferably between about 0.5 and 8 mm,
s may be applied onto the active agent formulation matrix surface. Further,
between about 2 and 10 bands may be used, but generally between about 2
» and 6 are affixed to the matrix. The bands may be placed close together (ie,
~z within about 0.5 mm of each other) or may be placed at opposite ends of the
s matrix (ie, spaced about 8 to 12 mm apart). The insoluble material may be
a any material that is nontoxic, biologically inert, nonallergenic and
nonirritating
~s to body tissue, and that maintains its physical and chemical integrity;
that is,
s the bands do not erode or degrade in the environment of use during the
dispensing period. Insoluble materials from which the bands may be prepared
,s include, for example, polyethylene, polystyrene, ethylene-vinyl acetate
s copolymers, polycaprolactone and Hytrel~ polyester elastomers (Du Pont).
zo Additional banding materials include but are not limited to
polysaccharides,
z~ cellulosics, powdered cellulose, microcrystalline cellulose, cellulose
acetate,
zz cellulose actetate pseudolatex (such as described in U.S. Patent
5,024,842),
2s cellulose acetate propionate, cellulose acetate butyrate, ethyl cellulose,
ethyl
za cellulose pseudolatex (such as Surelease~ as supplied by Colorcon, West
zs Point, PA or AquacoatTM as supplied by FMC Corporation, Philadelphia, PA),
2s nitrocellulose, polylactic acid, poly- glycolic acid, polylactide glycolide
z7 copolymers, collagen, polycaprolactone, polyvinyl alcohol, polyvinyl
acetate,
za polyethylene vinylacetate, polyethylene teraphthalate, polybutadiene
styrene,
zs polyisobutylene, polyisobutylene isoprene copolymer, polyvinyl chloride,
so polyvinylidene chloride-vinyl chloride copolymer, copolymers of acrylic
acid
~ and methacrylic acid esters, copolymers of methylmethacrylate and
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
13
ethylacrylate, latex of acrylate esters (such as Eudragit~ supplied by
z RohmPharma, Weiterstadt, Germany), polypropylene, copolymers of
s propylene oxide and ethylene oxide, propylene oxide ethylene oxide block
a copolymers, ethylenevinyl alcohol copolymer, poly sulfone, ethylene
s vinylalcohol copolymer, polyxylylenes, polyamides, natural and synthetic
s waxes, paraffin, carnauba wax, petroleum wax, white or yellow bees wax,
castor wax, candelilla wax, rice bran wax, microcrystalline wax, stearyl
s alcohol, cetyl alcohol, bleached shellac, esterified shellac, chitin,
chitosan,
s silicas, polyalkoxysilanes, polydimethyl siloxane, polyethylene glycol-
silicone
o elastomers, crosslinked gelatin, zein, electromagnetic irradiation
crosslinked
~ acrylics, silicones, or polyesters, thermally crosslinked acrylics,
silicones, or
~z polyesters, butadiene-styrene rubber, glycerol ester of partially dimerized
~s rosin, glycerol ester of partially hydrogenated wood rosin, glycerol ester
of tall
~a oil rosin, glycerol ester of wood rosin, pentaerythritol ester of partially
~s hydrogenated wood rosin, pentaerythritol ester of wood rosin, natural or
s synthetic terpene resin and blends of the above.
~s The banding materials often are also formulated with plasticizers, and
19 optionally with wetting agents, surfactants, opacifiers, colorants,
flavorants,
zo taste-masking agents, and the like. Examples of typical plasticizers are as
z~ follows: polyhydric alcohols, polyethylene glycol, glycerol, propylene
glycol,
zz acetate esters, glycerol triacetate, triethyl citrate, acetyl triethyl
citrate,
zs glycerides, acetylated monoglycerides, oils, mineral oil, castor oil and
the
za like.
zs
is The rate of release of the active agent from the active agent dosage form
z7 is predominantly controlled by erosion of the aqueous gel formed by
zs contacting the matrix with the fluid environment of use. The drug released,
m
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
1 at time t, is proportional to the surface area of the system and can be
written
2 as
3
dm/dt = KA. (Equation 1 )
s K is the erosion constant in mglcm2hr and varies according to the mol
A is the erosion area.
s
s The release profile dmldt is constant if K and A remain constant.
11 Substituting Equation 1 with the area of a cylindrical matrix with
negligible
12 end effects gives
13
dm/dt = K2~RL (Equation 2)
1s
1s L is the length of the cylinder and
17 R is the radius of the cylinder at time t.
1s
1s The mass release at time t is
21 m = ~ [Ro -R2]LCo (Equation 3)
22
23 Ro is the initial radius of the cylinder and
24 Co is the initial concentration of the drug in the cylinder.
2s Substituting Equation 3 into Equation 2 leads to
27
2s dR/dt = -K/Co (Equation 4)
2s
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
1 Integrating Equation 4 gives
2
3 R = Ro - (KICo)t (Equation 5)
4
s The fraction amount of drug release, F, can now be defined by
s substituting Equation 5 into Equation 3 as follows
s F = m/mo = n [Ro -R2]LCo I( ~ Ro2LCo) = 1 - [1 - Kt/(CoRo)]2 (Equation 6)
s
1o dF/dt = 2 KICoRo - (2 K2/Co Ro )t (Equation 7)
11
12 Equation 7 indicates that the plot of the rate of active agent released
from
13 a cylindrical dosage form without bands versus time will be linear and will
14 decrease with time, as is shown in Figure 7A. As drug is released from an
1s unbanded capsule, the diameter of the cylinder as well as the area of
erosion
1s decreases. In contrast, as the polymeric core of the banded cylinder of
this
17 invention shrinks, new surface area is created and exposed to the
1a environment of use (see Figure 2). As a result, the amount of active agent
1s released over time may remain constant or may increase with time depending
20 on the rate of the new surface area being generated. By arrangement of the
21 number, size and location of bands on the dosage form, the total new
surface
22 area created by erosion can be predicted and the desired release profile
can
23 be achieved.
24
25 The bands may be placed onto the surface of the matrix such that, as the
2s matrix erodes, the bands become loose and drop off the matrix. These bands
27 are easily excreted from the gastrointestinal tract. As the number of bands
2s remaining on the surface of the matrix decreases, more matrix surface area
2s will be exposed. The matrix will therefore erode in a fashion that
approaches
3o zero order.
31
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
1 The bands may also be printed onto the surtace of the matrix. The matrix
2 will erode where not covered by the bands as described above with reference
s to FIGs. 1-5.
4
s In order to prepare a device of the present invention, the active agent
s formulation is first prepared and formed into a matrix of the desired size
and
shape. The matrix in its initial prepared form is about the size and
s dimensions of a size "5" to size "000" hard gelatin capsule. The cross-
s sectional shape of the matrix may be circular or may be oval, triangular,
1o square, hexagonal or other shapes that are easily handled, especially by
11 patients with limited dexterity. The rings or bands are then placed onto
the
12 surface of active agent formulation matrix or printed onto the surface
using
1s conventional banding or printing techniques.
14
1s In addition to the devices described above, embodiments are
1s contemplated that include non-uniform matrices. These devices may include
17 matrices with two or more active agents or two or more pharmaceutically
1e acceptable carriers. An example of a device 80 with a non-uniform matrix is
1s shown in FIG. 6. A lower portion 82 of the matrix material is formed from
the
2o hydrophilic polymeric materials described above that erode over time upon
z1 exposure to a fluid environment of use. An upper portion 84 of the matrix
2z material disintegrates upon placement in the fluid environment of use. The
zs latter portion of the matrix will provide for an initial pulse of active
agent as
24 the material swells and separates from the rest of the matrix. The
remaining
Zs portion of the device, with bands 86 and 88 as described above, will
provide
zs for the prolonged delivery of the same or a different active agent.
Materials
27 that swell and disintegrate upon placement in the fluid environment of use
28 include, but are not limited to hydroxypropyl cellulose having a
is hydroxypropoxyl content of 7 to 16 weight percent, crosslinked polyvinyl
so pyrrolidone, crosslinked starch, microcrystalline cellulose, chitin,
cellulose
s1 fiber and the like .
CA 02207098 1997-06-OS
WO 96125922 PCTIUS96/01848
1
2 The following examples are illustrative of the present invention. They are
3 not to be construed as limiting the scope of the invention. Variations and
a equivalents of these examples will be apparent to those skilled in the art
in
s light of the present disclosure, the drawings and the claims herein.
s
EXAMPLE 1
s
s A delivery device according to the present invention was prepared as
follows. 58 grams of the analgesic drug, ibuprofen, 25 grams of
» hydroxypropyl methylcellulose having a number average molecular weight of
~2 9,200 grams per mote, and 15 grams of hydroxypropyl methylcellulose having
~3 a molecular weight of 242,000 grams per mole, were passed through a
a screen having a mesh size of 40 wires per inch. The celluloses each had an
average hydroxyl content of 8 weight percent and an average methoxyl
s content of 22 weight percent. The resulting sized powders were tumble
,~ mixed. Anhydrous ethyl alcohol was added slowly to the mixed powders with
s stirring until a dough consistency was produced. The damp mass was then
,s extruded through a 20 mesh screen and air dried overnight. The resulting
2o dried material was re-screened through a 20 mesh screen to form the final
2~ granules. 2 grams of the tabletting lubricant, magnesium stearate, which
had
22 been sized through an 80 mesh screen, was then tumbled into the granules.
23
2a 690 mg of the resulting granulation was placed in a die cavity having an
2s inside diameter of 9/32 inch and compressed with deep concave punch
Zs tooling using a pressure head of 2 tons. This formed a longitudinal capsule
27 core having an overall length, including the rounded ends, of 0.691 inch.
The
2a cylindrical body of the capsule, from tablet land to tablet land, spanned a
2s distance of 12 mm. Each core contained a unit dose of drug of 400 mg.
3o Rings of polyethylene having an inside diameter of 9/32 inch, a wall
thickness
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
of .013 inch, and a width of 2 mm were then fabricated. These rings, or
z bands, were press fitted onto the capsule to complete the dosage form.
3
EXAMPLE 2
s
s Drug release studies were performed by placing the dosage forms in a
slotted basket. The inside diameter of the basket was 14 mm and the length
s was 50 mm. The basket was attached to a reciprocating motor. The basket
s was then immersed in 50 ml of simulated intestinal fluid at 37°C, and
shaken
o vertically in the media with a amplitude of 3.8 cm and a frequency of 99-101
cycles per minute. After 1 hour of shaking, the basket was transferred to a
2 fresh 50 ml volume of the test media. This procedure was continued, hour by
~s hour, for nine hours. The systems then were allowed to release continuously
14 for another 13 hours to complete a 24 hour test duration. The release
~s receptor solutions were then analyzed for drug content by ultraviolet
s spectroscopy. The release rate as a function of time and cumulative release
7 as a function of time were computed.
~s
s Figure 7-10A show the release rates of the ibuprofen cylindrical matrix
2o formulation described in Example 1 and Figures 7-10B show the cumulative
2~ amount of drug released, FIGs. 7A and B show the 0.281 inch by 0.691 inch
z2 longitudinal capsule with no bands, FIGs. 8A and B show the capsule with
2s one 2 mm band positioned in the middle of the capsule, FIGs. 9A and B show
2a the capsule with two 2 mm bands one positioned at each end of the capsule
2s with an 8 mm distance between the bands, FIGs. 10A and B show the
is capsule with three 2 mm bands, one positioned in the middle of the capsule
27 and one positioned at each end with a distance of 3 mm between the bands.
2s The delivery rate approaches zero order as greater numbers of bands are
2s included. Further, as more of the surface area is covered by the insoluble
so material, the release rate is prolonged. The total delivery times to
release
31 90% of the initial dose are as follows: for no bands, 6.6 hours; for one 2
mm
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96/01848
band, 10.7 hours; for two 2 mm bands, 17.6 hours; and for three 2 mm bands,
2 19.2 hours.
3
EXAMPLE 3
s Figure 11 A shows the release rate as a function of time of the ibuprofen
~ cylindrical matrix formulation described in Example 1 except that the system
a banded with two rings was tested with a different positioning of the rings.
s Figure 11 B shows the corresponding cumulative release versus time. The
,o two rings were positioned such that they were equispaced along the 12 mm
cylindrical body, with 2.7 mm between the land and the ring, and 2.7 mm
~z between the rings. A comparison of these patterns with the patterns
~s illustrated in Figures 9A and 9B, demonstrates that different patterns can
be
~a achieved with different ring configurations. The total delivery time to
deliver
~s 90% of the dose is 10.2 hours rather than 17.6 hours.
CA 02207098 1997-06-OS
WO 96/25922 PCT/US96101848
zo
EXAMPLE 4
2
s A fast-release drug granulation was prepared as follows; 87 grams of
4 ibuprofen, 10 grams of hydroxypropyl cellulose having a hydroxypropoxyl
s content of 11 weight percent, and 1 gram of hydroxypropyl methyl cellulose
s having a hydroxypropoxyl content of 8 weight percent and a methoxy content
of 22 weight percent and having a number average molecular weight of 9,200
s grams per mole, were screened through a 40 mesh sieve. The sized
s powders were mixed and anhydrous ethanol was added with stirring until a
,o uniform, damp mass was produced. The mixture was extruded through a 20
11 mesh sieve. The elongated granules produced were air dried. The dried
~2 granules were re-screened through a 20 mesh sieve. 2 grams of stearic acid
~s which had been passed through an 80 mesh sieve were tumble mixed into
~4 the granules for 3 minutes.
~s
690 mg of the granulation of Example 1 were filled into a die cavity having
an inside diameter of 9/32 inch and lightly compressed with deep concave
~8 punch tooling. The upper punch was removed and 230 mg of the fast-release
,s granulation was placed on the lightly compressed core. The upper punch
2o was returned to the die cavity and a 2 ton compression force was applied,
2~ thereby forming a two-layered tablet. Two rings were press fitted onto the
22 690 mg portion of the dosage form according to the procedures specified in
2a Example 3.
24
zs An abrasion resistant, protective coating was applied to the banded, two-
2s layered tablet as follows. A coating solution was prepared by dissolving 63
27 grams of hydroxypropyl methylcellulose having a hydroxypropoxyl content of
2a 10 weight percent and a methoxy content of 29 weight percent with a number
2s average molecular weight of 11,900 grams per mole, and 7 grams of
3o polyethylene glycol having a molecular weight of 3,350 grams per mole, in
930 grams of water. The ringed tablets were then placed in a pan coating
CA 02207098 1997-06-OS
WO 96125922
21
PCT/US96/01848
1 machine. The coating solution was sprayed onto the ringed tablet in a
z current of warmed air until 40 mg of film were deposited on each tablet.
3
4 The resulting two-layer, film coated systems were tested for release of
s drug according to the procedures describe in Example 2, except that the
s release test media was simulated gastric fluid having a pH of approximately
1.2. In this test, the fast-release layer dispensed 200 mg of ibuprofen within
a 10 minutes, and the remaining 400 mg dose was dispensed slowly according
s to the release characteristics specified in Example 3.
11
12 EXAMPLE 5
13
14 78 grams of ibuprofen and 20 grams of hydroxypropylmethylcellulose
having a number average molecular weight of 9,200 grams per mole and a
1s hydroxyl content of 8 wt% and a methoxyl content of 22 wt% were passed
17 through a 40 mesh screen. The resulting sized powders were tumble mixed.
1s Anhydrous ethyl alcohol was added slowly to the powders with stirring until
a
1s dough consistency was produced. The damp mass was then extruded
Zo through a 20 mesh screen and air dried overnight. The resulting dried
z1 material was rescreened through a 20 mesh screen to form the final
granules.
2z 2 grams of tabletting lubricant, stearic acid, which had been sized through
an
z3 80 mesh screen, were then tumbled into the granules.
24
is 513 mg of the resulting granulation was placed in a die cavity having an
is inside diameter of 9132 inch and compressed with a deep concave punch
27 tooling using a pressure head of 2 tons, forming a longitudinal capsule
having
2a an overall length, including the rounded ends, of 0.543 inch. The
cylindrical
2s body of the capsule, from tablet land to tablet land, spanned a distance of
9
3o mm. Each capsule contained a unit dose of drug of 400 mg. Rings of
31 polyethylene having an inside diameter of 9/32, a wall thickness of 0.013
CA 02207098 1997-06-OS
WO 96125922 PCT/US96/01848
22
inch, and a width of 2 mm were then fabricated. These rings or bands were
z press fitted onto the capsule to complete the dosage form.
3
a Drug release studies were performed as described in example 2. Figures
s 12-15 show the release rates of the ibuprofen cylindrical matrix formulation
s described above. FIG. 12 shows the release rate of the matrix with no bands.
FIG. 13 shows the matrix with two 2 mm bands positioned in the middle of the
s matrix with a distance of 2 mm between the bands. FIG. 14 shows the matrix
s with three 2 mm bands, one positioned in the middle of the capsule and one
1o positioned at each end with a distance of 2 mm between the bands. FIG. 15
11 shows the matrix with four 2 mm bands, one positioned at each end and two
12 positioned in the middle, each separated by a distance of 0.33 mm.
13
1a As noted in the previous example, the delivery rates shown in FIGs. 12-15
1s approach zero order as greater numbers of bands are included. Further, as
1s more of the surface area is covered by the insoluble material, the release
rate
17 is prolonged. The total delivery times to dispense 90% of the initial dose
are
1s as follows: for no bands, 2.3 hours; for two bands, 2.8 hours; for three
bands
1s 2.8 hours; and for four bands, 3.7 hours.
21 EXAMPLE 6
22
23 A 550 mg unit dose for prolonged release of the analgesic drug
2a acetaminophen is prepared as follows. 78 grams of acetaminophen are
2s passed through a sizing screen having 40 wires per inch. 20 grams of a
2s hydroxypropyl methylcellulose having a hydroxypropyl content of 8 wt %, a
27 methoxyl content of 22 wt %, and a number average molecular weight of
2s 27,800 grams per mole are passed through a sizing screen with 100 wires
2s per inch. The sized powders are tumble mixed for 5 minutes. Anhydrous
so ethanol is added to the mixture with stirring until a damp mass is formed.
The
s1 damp mass is passed through a sizing screen with 20 wires per inch. The
CA 02207098 1997-06-OS
pCTIUS96/01848
WO 96125922
23
1 resulting damp granules are air dried overnight, and then passed again
2 through the 20 mesh sieve. 2 grams of the tabletting lubricant, magnesium
s stearate are passed through a sizing screen with 80 wires per inch. The
4 sized magnesium stearate is blended into the dried granules to form the
final
granulation.
s
705 mg portions of the final granulation are placed in die cavities having
s inside diameters of 0.281 inch. The portions are compressed with deep
s concave punches under a pressure head of 1 ton, forming longitudinal
1o capsule-shaped tablets.
11
12 The capsules are fed into a Tait Capsealer Machine (Tait Design and
1s Machine Co., Manheim, PA) where three bands are printed onto each
14 capsule. The material forming the bands is a mixture of 50 wt
ethylcellulose dispersion (Surelease~, Colorcon, West Point, PA) and 50 wt
1s % ethyl acrylate methylmethacrylate (Eudragit~ NE 30D, RohmPharma,
17 Weiterstadt, Germany). The bands are applied as an aqueous dispersion
1a and the excess water is driven off in a current of warm air. The diameter
of
1s the bands is 2 millimeters.
21 The above description has been given for ease of understanding only. No
22 unnecessary limitations should be understood therefrom, as modifications
will
zs be obvious to those skilled in the art.
24
26