Note: Descriptions are shown in the official language in which they were submitted.
CA 02207113 1997-06-0~
-
Th. G o 1 d s c h m i d t AG, Essen
a,~-Polyether polysilox~nec as W/O emulsifiers
The invention relates to the use of a,~-polyether polysiloxanes
as W/O emulsifiers, in particular in antiperspirant
formulations.
As a rule, the antiperspirant gels which are known and
available on the market consist of
- active ingredients having antiperspirant activity, such as,
for example, al-im;nllm chloride, alnm;nllm hydroxychloride,
active alllm;nllm hydroxychlorides and zirconium salts,
- water,
- oils, in particular silicone oils, and
- emulsifiers.
Customarily, these formulations are prepared by dissolving the
active compound salts in water and intro~llc; ng this solution
with stirring into the oil phase, which contains appropriate
W/O emulsifiers, as a batchwise component.
Normally, these W/O formulations are cloudy. However, if the
refractive index of the water phase is matched with the
refractive index of the oil phase, clear W/O gels result. For
1 --
CA 02207113 1997-06-OS
this purpose, polyalcohols, such as sugar alcohols, or poly-
ethylene glycols are customarily added to the water phase in
amounts from 25 to 50~ by weight. The amount nep~e~ is
~pen~ent on the nature and amount of the constituents of the
emulsion and can easily be determined by simple experiments.
- Further constituents of the emulsion, such as preservatives,
perfume oils, colorants and stabilizers, as a rule are added to
the phase in which they are soluble or easily dispersible. If
required, the mixture can then be hollloy~llized~ e.g. with the
aid of a colloid mill.
The use of emulsifier systems based on polys;lo~n~ is known
and described in detail, for- example, in US-A-4 988 504.
Co~red with the polysiloxane ~1l1 s; fiers known until then,
US-A-4 988 504 proposes those which consist of
a) units of the formula
R2SiO2/2
R = hydrogen or a substituted or unsubstituted hydrocarbon
radical having 1 to 12 carbon atoms,
b) units of the formula
RR1SiO22
R1 = polyalkylene ether of the formula
-- 2 --
CA 02207113 1997-06-0
~Ra3 ~ (oR2 ) n~OR4
R2 = -CH2CH2-,
R3 = substituted or unsubstituted alkylene group having 1
to 20 carbon atoms,
R4 = R,
n has a value from 5 to 20 and
a is 0 or 1, and
c) final member~ of the siloxane chain,
where the molecular weight of c~l-~onent a) should be
a~lo~imately 25,000 to 35,000.
These emulsifiers having lateral polyoxyalkylene groups should
achieve improved properties for antiperspirant sticks with
respect to stability and low wax content.
EP-B-0 407 089 relates to a transparent water-in-silicone oil
emulsion, suitable for external application to m~mm~ n skin
or hair, c~ ising, in addition to water:
i. 1 to 50~ by weight of a volatile polydimethylsiloxane;
ii. 0.1 to 20~ by weight of a silicone surfactant con-
stituent, colu~lising a polymer of
dimethylpolysiloxane having poly-
oxyethylene and/or polyoxypropylene
side chA; n~ having a molecular
-- 3
CA 02207113 1997-06-0~
weight of 10,000 to 50,000 and the
structural fonmula:
CH3 CH3 CH3 CH3
CH3~ O ~ i-O - i-O ~ i-CH3
CH3 CH3 - x - CH3
in which R is -H or
[cH2CH20]a[cH2cHO ]bH
~H3
a has a value from 9 to 115,
b has a value from 0 to 50,
x has a value from 133 to 673,
y has a value from 25 to 0.25 and
iii. 1 to 50~ by weight of a transparency-imparting agent,
which is at least a polyhydric
alcohol.
The siloxane emulsifiers proposed here accordingly also have
polyoxyethylene and polyoxypropylene groups in the side chain
and are characterized by a high molecular weight. The emulsions
described should have a good storage and temperature stability
as well as skin-compatible properties. A disadvantage of these
siloxane emulsifiers, however, is their extremely high visco-
sity, so that they can only be handled in solvents. They are
- 4
CA 02207113 1997-06-0~
therefore preferably employed as a dispersion in short-chain
(D4, D5) silicone oils, wh-ich must have a viscosity of below
5 mm2.s~1. Preferably, a 10~ strength emulsifier formulation
is employed here. The use of these emulsifiers is thus
inevitably associated with the introduction of short-chain
silicone oils and thus means a restriction in the selection of
the components of the desired antiperspirant gels.
The object of the present invention is the provision of
emulsifiers for antiperspirant formulations based on W/O emul-
sions which have a low viscosity and thus can be- handled
without the use of solvents or dispersions and display the
desired emulsifying action at the lowest po~sible concen-
tration. The emulsifiers should have high stability and also
retain this under thermal stress. On account of the cosmetic
application area, the emulsifiers must in this case be physio-
logically acceptable.
According to the invention, this object is achieved by the use
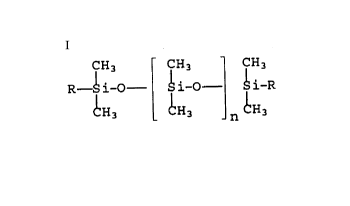
of copolymers of the general formula
CH3 CH3 CH3
R-- i-O i-O ~ i-R
CH3 CH3 n CH3
R = - (CH2-)mO- (c2H4o-)x(c3H6o-)yR
m = 2 to 4,
CA 02207113 1997-06-05
x = 3 to 100,
y = 0 to 50,
R1 = H, CH3, CH2CH3,
n = 50 to 200,
as emulsifiers in W/O emulsions in amounts from 0.1 to 20~ by
weight, based on the total weight of the emulsion.
The weight ratio of oxyethylene to oxypropylene groups is
preferably selected here such that the weight ratio of poly-
ethylene oxide to polypropylene oxide is 100 : 10 to 20 : 80.
Copolymers in which R1 = H and n = 100 to 150 and the weight
ratio of polyethylene oxide to polypropylene oxide is 80 : 20
to 40 : 60 are particularly preferred.
The oxyethylene and oxypropylene units can be r~n~o~ly
distributed or arranged blockwise.
In contrast to the known emulsifiers, the emulsifier according
to the invention is modified - at the termlnal siloxane groups
- by polyether groups. In contrast to the customary types which
are modified in a comb-like m~nner, this type of graft
copolymer has a lower degree of modification of two polyether
groups per polymer molecule. By modification of the hydrophobic
radicals (index y and index n) and the hydrophilic radicals
(index x) in the whole molecule, the polyethersilox~nes used
6 --
CA 02207113 1997-06-0~
according to the invention can be adjusted to the desired HLB
values and necess~ry interfacial activities. Emul-sifiers having
a relatively low molecular weight result here, using which it
is surprisingly possible to prepare emulsions having
outst~n~;ng stability with respect to elevated temperature,
freeze/thaw cycles and long-term storage. Com~red with the
prior art, the emulsifier employed according to the invention
here has a mlmh~r of advantages. As a 100~ strength product, it
is of low viscosity and readily handleable and can be prepared
more easily than defined compounds. In comp~rison with solvent-
cont~;n;ng products, lower use conce~trations are necessary,
which leads to considerable savings in the logistics area. In
the products of the prior art, silicone oil is used. In
contrast, it turns out to be an advantage of the compounds
according to the invention that, if required, silicone-oil-free
formulations are also possible.
The emulsifiers according to the invention are suitable for the
preparation of W/O emulsions using silicone oils which can be
linear or cyclic. Silicone oils such as octamethylcyclotetra-
siloxane are preferred. Further oils or waxes can be emulsified
together with the silicone oils.
Examples of such oily substances are synthetic skin oils, such
as isopropyl myristate, isopropyl stearate, hexyl laurate,
oleyl oleate. Liquid waxes, such as jojoba oil, are furthermore
suitable.
CA 02207113 1997-06-0~
Further constituents of the oily phase can be natural vegetable
or ~n;m~l oils, such as, for example, mink oil, fish oil,
neat's foot oil, bone oil, castor oil, sunflower oil, avocado
oil, olive oil, groundnut oil, palm oil. Microwaxes or other
thickeners by means of which the consistency of the emulsions
obt~;ne~ can be adjusted from liquid to solid can furthermore
be added to the ester and silicone oils to be emulsified.
To increase the stability to cold, polyalcohols, e.g. glycerol,
1,2-propylene glycol or sorbitol, can be added to the emulsion
in amounts from 0.5 to 15% by weight. The heat stability can be
~ uv~d by addition of electrolytes, e.g. sodium chloride,
calcium chioride and/or m~nesium sulfate. These additives are
employed at 0.5 to 10.0% by weight. Moreover, the stability of
the formulation can be inl~luved by addition of zinc stearate or
al-lm;m~m stearate.
The use conc~ntration of the emulsifier should be at least 1%
by weight; the oil phase can make up 15 to 50% by weight of the
total formulation.
At a water content of 70 to 85% by weight, the emulsion has a
pasty consistency. Less water lowers the viscosity of the
system to a lotion-like consistency, and even less water can be
used in order to prepare an emulsion of very low viscosity.
The antiperspirant W/O emulsions are prepared in a customary
mann~r by dissolving and dispersing silicone oils, preferably
the polysiloxanes D4 and D5, on their own or in combination
8 --
CA 02207113 1997-06-0~
with skin oils, in the emul~ifying agent, the polyether-
siloxane. If the latter co~ta; ns relatively high-melting
cu,.,~llents, e.g. silicone wax or beeswax, the oily phase is
warmed above the melting range of these constituents.
The water phase is then slowly added to the oily phase with
stirring. In the case of transparent W/O emulsions, the
refractive indices of the water phase are matched with those of
the oil phase. This can be carried out by ap~Lo~riate
adjustment of the ratio of polyalcohols (glycerol, propylene
glycol, sorbitol and the like) to water in the water phase.
Examples
1. Emulsifiers
The table shows the chemical structure of some emulsifiers
which are suitable for the use according to the invention:
CA 02207113 1997-06-05
Table 1: emulsifiers according to the invention
(C2H4o-)x(c3H6o-)yR~ Weight ratio
R' n average desired C2H4O : C3H6O
molecular weight
[g/mol]
Emulsifier 1 H 50 1 600 58 : 42
Emulsifier 2 H 150 400 100 : 0
Emulsifier 3 H 100 1 400 42 : 58
Emulsifier 4 H 200 500 60 : 40
Emulsifier 5CH3 100 2 550 42 : 58
Emulsifier 6 H 100 1 400 12 : 88
- 10 -
CA 02207113 1997-06-0~
2. For~lAtion examples
2.1. Preparation
Table 2 shows the composition of emulsions and emulsion gels
according to the invention.
The preparation of the emulsion is carried out in a cont~;n~r
which is provided with a stirrer. The constituents A are added
to the cont~;n~r and optionally fused. The water, in which, if
appropriate, sodium chloride, propylene glycol and, depending
on the recipe, all]m;nl~m salts (in solution) are dissolved, is
added to the mixture of emulsifier and oil with stirring.
In the case of clear, gelatinous formulations, the refractive
index of the aqueous phase is matched with the refractive index
of the oil phase by addition of polyalcohols (e.g. propylene
glycol, glycerol or sorbitol).
The W/O emulsions or emulsion gels obtained can, if required,
be homogenized, for example using a colloid mill or a
homogenizer which operates on the pressure principle.
The emulsions prepared have particle sizes of c 1 ~ particle
diameter.
2.2. Emll~ nn Examples 1 to 7
The emulsions 1, 3, 4, 5, 6 and 7 have a creamy or gelatinous
consistency; the emulsion 2 is liquid. The emulsions and the
emulsion gels are stable for 3 months, stored at 20~C and 45~C.
-- 11 --
CA 02207113 1997-06-0~
The emulsions show no ;mr~irment of the stability after cooling
to -15~C and subsequently warming to 20~C five times. Even
after storage of the emulsions at 20~C for six months, no
reduction in viscosity is to be found.
The exemplary formulations 2 and 5 are skin-care emulsions. The
emulsions have a smooth and brilliant appearance. They can be
distributed very easily on the skin, are absorbed rapidly and
impart a ple~nt, velvety, slightly cooling sensation to the
skin.
The exemplary formulations 1, 3, 4, 6 and 7 are transparent
emulsions for use as antiperspirant. The transparency of these
formulations is achieved by adjustment of the refractive index
of the aqueous phase to that of the oily phase. These
formulations can be distributed easily on the skin and leave a
velvety sleek sensation on the skin surface.
- 12 -
CA 02207113 1997-06-0~
Table 2: r ll~si~n fon~ll~t;~nc
r- l~ion example No.1 2 3 4 5 6 7
Constituents~ by ~ by ~ by ~ by ~ by ~ by ~ by
wt. wt. wt. wt. wt. wt. wt.
r llcifier 1 3.0 - - ~
;fier 2 ~ 4-0
r~llc;fier 3 - 1.5
Emulsifier 4 - - - 3.04.0
r ~1 Ri fi ~r 5 _ _ _ _ _ 1.0
~llc;fi~ 6 1.5
Phase A)
Octylm~ethylcyclo- 13.0 - 7.2 6.5 - 7.5 7.0
tetrAC; 1 C~Y~n~
De~: -hylcyclopenta- - - 7.3 6.5 - 7.5 7.5
s; 1 oX 3n~
Isopropyl myristate - - - - 8.0
Liquid paraffin GP 8 - 12.5 - - 13.0
Avocado oil - - - - 1.0
Cetyl ~; ~th;cone *1) - - - - 0.5
Caprylic/capric - 12.5
triglyceride
Zink ri~;nnl~te + - - - 2.5
tetrahydroxypropyl-
ethyl ~n~;: ' n~ +
laureth-3 + 1,2-pro-
pylene glycol *2)
Phase B)
Water 19.0 70.3 5.0 16.5 67.0 20.0 20.0
NaCl - 0.7 - - 2.0
1,2-Propylene glycol25.0 - 22.0 25.04.5 24.0 24.0
hydroxy- 40.0 ~ ~ 40-0
chloride solution *3)
Al~ 'm zirkonium- - - 57.0
tetrahydro-hydroxy-
chloride-glycine
complex *4)
Total 100.0100.0 100.0 100.0 100.0 100.0 100.0
CA 02207113 1997-06-0~
,
*1) A~3IL Wax 9801 (polysiloxane/polyalkylene copolymer),
Th. Goldschmidt AG
*2) TEGO Deo CW 90 (deodorant active ingredient), Th.
Goldschmidt AG
*3) Reach 501 (antiperspirant active ingredient, 50~ in
water), Reheis
*4) Reach 36GLY (antiperspirant active ingredient, 36~ in
water), Reheis
3. Formulation examples
3.1. E~ f~ers
For csmp~rison, the product "Formulation aid DC 3225 C", Dow
Corning, available on the market is employed. This is a cloudy
dispersion of a polydimethylsiloxane which is modified in a
comb-like m~nner with polyethylene glycol/polypropylene glycol
groups as the modification (graft copolymer) in
octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane.
A pale deposit precipitates from this dispersion after just a
short time.
The emulsifiers according to the invention, on the other hand,
are clear and homogeneous products. Separation phenomena as in
the case of the market comparison product have not been
observed with the emulsifiers according to the invention.
CA 02207113 1997-06-0
3.2. Formulations
The recipes in Table 3 are prepared as described previously.
Table 3: Formulation examples
Antiperspirant Skin-care emulsions
gels
Formulation example 3 89 10 11
Constituents % by % by% by % by % by
wt. wt. wt. wt. wt.
Emulsifier 3 1.5 - - - -
Emulsifier 4 - - 3.0 - 3.0
DC 3225 C - 15.0 - 30.0
Phase A)
Octamethylcyclotetra- 7.2 1.0 - - 13.5
siloxane
Decamethylcyclopenta- 7.3 - - - 13.5
siloxane
Isopropyl myristate - - 8.0 8.0 8.0
Liquid paraffin GP 8 - - 13.0
Avocado oil - - 1.0 1.0 1.0
Phase B)
Water 5.0 5.071.0 59.059.0
NaCl - - - 2.0 2.0 2.0
1,2-Propylene glycol 22.0 22.02.0
Alllm;nl~m-zirconium 57.0 57.0
tetrahydroglycine
complex *4)
Total 100.0 100.0100.0 100.0100.0
*4) Reach 36GLY (antiperspirant active ingredient, 36% in
water), Reheis
- 15 -
CA 02207113 1997-06-0~
3.3. Comparison: viscosity of gel formulations
Formulation example 3 8
Viscosity 45,000 mPas 49,000 mPas
The viscosities of the recipe examplés 3 and 8 are similar.
3.4. Comparison: silicone oil content in skin-care
formulations
Formulation example 9 10 11
Silicone oil content 0~ 27% 27
in ~
The silicone oil content in the recipe examples is fixed by the
emulsifer. The market comparison product, DC 3225 C, contains
89~ silicone oils. As example 10 shows, it is not possible-to
prepare DC 3225 C emulsions which do not contain any silicone
oil. With the emulsifiers according to the invention, on the
other hand, it is possible to prepare both silicone-free
formulations (example 9) and those formulations which contain
the same amount of silicone oils as the formulations which can
be prepared with DC 3225 C (example 11).
- 16 -
CA 02207113 1997-06-0~
4. InvestigationQ on the efficacy of antiperQpirant
formulations
4.1. Test recipeQ
The formulations listed in Table 4 are employed in all
investigations for determination of the efficacy. The content
of all~m;nl~m in all these formulations is 6~.
Table 4: Test recipes for efficacy determinations
Formulation examples 12 13
Emulsifier 3 1.1~ -
DC 3225 C - 10.0
Octamethylcyclotetrasiloxane 15.9~ 7.0~
Alnm;nl]m hydroxychloride 50.0~ 50.0%
solution *3)
Propylene glycol 16.0~ 16.0
Water, distilled 17.0~ 17.0
Total 100.0~ 100.0~
*3) Reach 501 (antiperspirant active compound, 50~ in water),
Reheis
4.2. Release of aluminum
In the formulations investigated, the active constituent
(all~m;nl~m hydroxychloride solution) is present in the internal,
aqueous phase as a solution. In order to be efficacious, the
alnm; n~m hydroxychloride solution must pass through the
-- 17 --
CA 02207113 1997-06-0~
external, oily phase. The rate of release of the active
constituent (alllm;nl~m hydroxychloride solution) is investigated
in Formulation Examples 12 and 13.
For this, the formulations are diluted with 95~ of water. After
shaking for 5 minutes, the alllm;nllm content in an aliquot
amount of the aqueous solution is determined. This 5-minute
value marks the starting point. The dilutions are shaken
further and, after 1 and 24 hours in each case 1 aliquot
amounts are again taken for analysis. The content of all~m;nllm
in the aqueous solution is determined by atomic absorption
spectroscopy.
The results are compiled in Table 5. It is clear that the
formulations 12 and 13 have an equally high rate of release of
al-lm; m~m .
Table 5: Release of all~m; n~m
-Formulation Example 12 ¦ 13
~ all~m;nllm recovered
5 minutes 15 16
1 hour ~ 90 > 90
24 hours ~ 90 ~ 90
- 18 -
CA 02207113 1997-06-0~
4.3. In-vivo test for antiperspirant effectiveness
4.3.1.Prel;~;nary investigations (selection of the subjects)
Subjects with a similar perspiration intensity (sweat
production) on the volar surface of the forearm are selected.
To do this, the appropriate skin areas are first swabbed with
alcohol. This is carried out to ~e-,love residual moisture. A
mixture of 3~ iodine in 95~ strength alcohol is then applied to
the entire area. After drying of the surface, a suspension of
starch and castor oil (50 : 50 w/w) is applied to the skin. The
subjects are exposed to a temperature of 37~C and a relative
humidity of 35~ in a climatically controlled room. The duration
of the test in this case depends on the production of sweat in
the individual subjects. An alternative procedure can consist
in stimulating the subjects to produce perspiration by rapid
walking.
As a result of perspiration, water is released. In the presence
of water, iodine and starch form an intensively blue-colored
inclusion compound (iodine-starch complex). The treated areas
are ~X~m; ned for their similarity by means of visual
lnspection.
-- 19 --
CA 02207113 1997-06-0~
4.3.2.Treatment with the antiperspirant formulation
For the treatment of the skin surface with the test
formulations 12 and 13, the occlusion by immersion technique is
used (OBIT).
The skin areas to be treated are first rinsed with water. Small
plastic beakers (2.5 cm diameter) having a hole (1 cm diameter)
in the bottom are fixed to the skin surface using an adhesive
tape. This is carried out on 12 subjects using 2 to 3 beakers
in each case on each forearm. Through the opening- in the
beakers, aliquot amounts of the sample solution from 1.0 to
1.4 ml are applied. After a contact time of 30 minutes, this
treatment is terminated. The beakers are then removed and
Pxce~s antiperspirant agent is rinsed off with water. For later
identification, the treated areas are marked.
4.3.3.Determ;n~tion of the antiper~pirant efficacy
30 minutes after the treatment and 4 hours after the treatment,
a determination of the efficacy of the antiperspirant
formulation is carried out. To do this, the appropriate skin
areas are first swabbed with alcohol. This is carried out to
remove residual moisture. A mixture of 3~ iodine in 95~
strength alcohol is then applied to the entire area. After
drying of the surface, a suspension of starch and castor oiI
(50 : 50 w/w) is applied to the skin. The subjects are made to
sweat in a climatically controlled room at a temperature of
- 20 -
CA 02207113 1997-06-0~
37~C and a relative humidity of 35~. The duration of this
treatment in this case depends on the production of sweat in
each individual subject. The treated areas are then e~m;neA
visually and the antiperspirant activity is assessed using a
scale from 0 to 100~. The numbers stand for the proportion of
sweat glands which do not secrete any sweat as a result of the
treatment with the antiperspirant agent.
Based on the abovementioned test design, in each case on
average a reduction of perspiration by 20 to 30~ is achieved by
each of the formulations 12 and 13 after 30 minutes or 4 hours.
The example shows that highly efficacious antiperspirant
formulations can be prepared using the emulsifiers according to
the invention.
The W/O emulsions prepared with the emulsifiers to be used
according to the invention are also suitable for industrial
purposes, e.g. for use as care compositions and polishes for
furniture, painted sheet metal and floors.
- 21 -