Note: Descriptions are shown in the official language in which they were submitted.
CA 02207462 1997-06-11
CARBONACEOUS ELECTRODE MATERIAL FOR SECONDARY BATTERY
AND PROCESS FOR PRODUCTION THEREOF
FIELD OF THE INVENTION AND RELATED ART
The present invention relates to a
carbonaceous material suitable as an electrode
material for a non-aqueous solvent-type secondary
battery, and a process for production thereof. The
present invention also relates to an electrode
structure comprising such a carbonaceous electrode
material, and a non-aqueous solvent-type secondary
battery having such an electrode structure.
Non-aqueous solvent-type lithium secondary
batteries having a negative electrode comprising a
carbonaceous material have been proposed as a type of
high energy-density secondary batteries (Japanese
Laid-Open Patent Application (JP-A) 57-208079, JP-A
62-90863, JP-A 62-122066, JP-A 2-66856, etc.). When
such a secondary battery is charged, lithium in a
positive electrode comprising a chalcogenide, such as
LiCoO2, is introduced into negative electrode carbon
(i.e., dopes the carbon) electrochemically. The
carbon thus doped with lithium functions as a lithium
electrode, from which the lithium is released (i.e.,
de-doped) during discharge to return to the positive
electrode. Thus, a secondary battery capable of
repetitive charge-discharge is formed.
CA 02207462 1997-06-11
As carbonaceous materials capable of
providing non-aqueous solvent-type lithium secondary
batteries, there have been known so-called "non-
graphitizable carbon" obtained by calcining phenolic
resin or furan resin, so-called "graphitizable carbon"
obtained by carbonizing pitch or tar; and activated
carbon having a large specific surface area on the
order of 900 - 2000 m2/g.
There has been also proposed a process for
producing a carbonaceous material for providing high-
performance secondary batteries comprising treating
plant fiber of, e.g., coconut shell with hydrochloric
acid, etc., for removal of an inorganic substance
contained therein and then carbonizing the treated
plant fiber at 900 - 1500 ~C under a reduced pressure
of at most 10 kPa (European Laid-Open Patent
Application (EP-A) 0700105.
However, the above-mentioned known
carbonaceous materials are accompanied with a problem
that a large amount of active substance, such as
lithium, remains in the carbon (i.e., the carbon shows
a large non-dedoping capacity) during the dedoping
step, so that the active substance is wasted
uselessly, and also a problem that the dedoping
capacity per se determining the battery performance
(discharge capacity) is relatively small.
SUMMARY OF THE INVENTION
CA 02207462 1997-06-11
As a result of study by our research and
development group, it has been already found that a
carbonaceous material obtained by carbonizing an
organic material of plant origin characterized by the
presence of vessel, sieve tube, plant fiber, etc., can
be doped with a large quantity of active substance,
and is therefore promising as a carbonaceous electrode
material, and a patent application (European Patent
Appln. No. 95305897.1, later laid open as EP-A
0767505) was filed based on the finding. However the
carbonaceous material is liable to exhibit a
relatively large non-dedoping capacity which is the
amount of active substance, such as lithium, that is
not completely de-doped from but remains within the
carbonaceous material during a discharge step, and a
reduction of the non-dedoing capacity has been
earnestly desired.
Accordingly, a principal object of the
present invention is to provide a carbonaceous
material useful as a carbonaceous electrode material
for providing high-performance secondary batteries
exhibiting large capacities for doping and de-doping
of an active substance, such as lithium, and also
exhibiting a reduced non-dedoping capacity, i.e., a
reduced amount of active substance remaining within
the carbonaceous material without de-doping.
Further objects of the present invention are
CA 02207462 1997-06-11
-4-
to provide a process for producing such a carbonaceous
material, an electrode structure composed from such a
carbonaceous material, and a high-performance
secondary battery including such an electrode
structure.
In the course of our study for obtaining
high-performance carbonaceous electrode materials more
suitably used for non-aqueous solvent-type secondary
batteries from organic materials of plant origin, it
has been found that not all the ash content (inorganic
substance) contained in such an organic material
adversely affect the resultant carbonaceous material
obtained therefrom but the potassium element contained
in the organic material adversely affects the
resultant carbonaceous material. It has been further
found that a carbonaceous material obtained by
removing the potassium from an organic material of
plant origin and then carbonizing the organic material
under appropriate conditions provides an excellent
carbonaceous electrode material having well-balanced
performances including a small non-dedoping capacity
and a large dedoping capacity in combination, and the
resultant carbonaceous material has a large pore
volume suitable for doping with a cell active
substance not found in conventional carbonaceous
electrode materials.
Incidentally, the above-mentioned EP-A
CA 02207462 1997-06-11
0700105 discloses an example of production of a
carbonaceous electrode material through de-ashing of a
carbon precursor of plant origin and carbonization of
the de-ashed carbon precursor (Example 5). It has
been however found that such an ordinary de-ashing
treatment is not effective enough to reduce the
potassium content, and the resultant carbonaceous
material cannot exhibit a sufficiently reduced non-
dedoping capacity (see Comparative Example 7 described
hereinafter).
According to the present invention, there is
provided a carbonaceous electrode material (first
carbonaceous material) for a non-aqueous solvent-type
secondary battery, having a pore volume of at least
0.55 ml/g of pores having a pore diameter of at most 5
~m as measured by mercury injection method, a
potassium content of at most 0.5 wt. % as measured by
fluorescent X-ray analysis, and a specific surface
area of at most 100 m2/g as measured by nitrogen
adsorption BET method.
According to another aspect of the present
invention, there is provided a carbonaceous electrode
material (second carbonaceous material) for a non-
aqueous solvent-type secondary battery, obtained by
carbonizing an organic material of plant origin, and
having a potassium content of at most 0.5 wt. % as
measured by fluorescent X-ray analysis, and a specific
CA 02207462 1997-06-11
surface area of at most 100 m2/g as measured by
nitrogen adsorption BET method.
It is preferred that both the first
carbonaceous material and the second carbonaceous
material of the present invention have a hydrogen-to-
carbon atomic ratio H/C of below 0.1 as measured by
elementary analysis, and an average (002)-plane
spacing of at least 0.365 nm as measured by X-ray
diffraction method.
According to the present invention, there is
further provided a process for producing a
carbonaceous electrode material for a non-aqueous
solvent-type secondary battery, comprising:
carbonizing a carbon precursor of plant origin having
a potassium content of at most 0.5 wt. % as measured
by fluorescent X-ray analysis, in contact with a
stream of an inert gas optionally containing a halogen
gas at a temperature of 700 - 1500 ~C.
According to the present invention, there is
also provided an electrode structure for a non-aqueous
solvent-type secondary battery, comprising: an
electroconductive substrate and a composite electrode
layer disposed on at least one surface of the
electroconductive substrate; the composite electrode
layer comprising the above-mentioned first or second
carbonaceous material in a particulate form, and a
binder.
CA 02207462 1997-06-11
According to the present invention, there is
further provided a non-aqueous solvent-type secondary
battery, comprising a positive electrode, a negative
electrode, and a separator and a non-aqueous
electrolytic solution disposed between the positive
and negative electrodes; wherein at least one of the
positive and negative electrodes comprises an
electrode structure as described above.
The carbonaceous material according to the
present invention is practically so-called non-
graphitizable carbon capable of storing a large amount
of active substance and accordingly has an essentially
large capacity for doping with an active substance.
In addition, the carbonaceous material according to
the present invention has many pores of a relatively
large diameter represented by a pore volume of at
least 0.55 ml/g of pores having a pore diameter of at
most 5 ~m as measured by mercury injection method
according to a first aspect, or is characterized by
relatively large penetrating or open pores originated
from structures, such as vessel, sieve tube and plant
fiber, attributable to the starting material according
to a second aspect.
Accordingly, the electrolytic solution is
allowed to easily penetrate into the interior of the
carbon through pores, and the active substance is
allowed to easily move between the inside and outside
CA 02207462 1997-06-11
of the carbon. As a result, it is possible to provide
a carbonaceous electrode material having a small non-
dedoping capacity and capable of effectively utilizing
an active substance.
Further, the carbonaceous material of the
present invention has a reduced content of potassium
element which adversely affects the doping and de-
doping characteristic of a carbonaceous material, so
that a high-performance secondary battery can be
prepared therefrom.
These and other objects, features and
advantages of the present invention will become more
apparent upon a consideration of the following
description of the preferred embodiments of the
present invention taken in conjunction with the
accompanying drawing.
BRIEF DESCRIPTION OF THE DRAWING
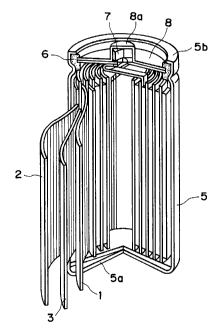
Figure 1 is a partially exploded perspective
view of a non-aqueous solvent-type se~o~ry battery
which can be constituted according to the invention.
Figure 2 is a partial sectional view of an
electrode structure adopted in the secondary battery.
DETAILED DESCRIPTION OF THE INVENTION
The carbonaceous materials according to the
present invention is characterized by a specific
CA 02207462 1997-06-11
surface area of at most lOO m2/g as measured by the
BET method using nitrogen as adsorbate gas, and a
potassium content of at most 0.5 wt. % as measured by
fluorescent X-ray analysis. The first carbonaceous
material is further characterized by a pore volume of
at least 0.55 ml/g of pores having a diameter of at
most 5 ~m as measured by mercury injection method. A
carbonaceous material having a specific surface area
in excess of lOO m2/g as represented by activated
carbon provides a large non-dedoping capacity, i.e., a
large amount of active substance left within the
carbonaceous material without dedoping. The specific
surface area may preferably be 0.5 - 50 m2/g, more
preferably 0.5 - lO m2/g.
A larger potassium content is not preferred
because it provides a smaller de-doping capacity and a
larger non-dedoping capacity. The potassium content
is preferably at most 0.4 wt. %, further preferably at
most 0.3 wt. %, particularly preferably at most 0.2
wt. %.
The large pore volume is a characteristic of
the carbonaceous material. A smaller pore volume
makes it difficult for the electrolytic solution to
penetrate into the inside of the carbon and hinders
free movement of the active substance within the
carbonaceous material, thereby resulting in a
remarkable increase in non-dedoping capacity defined
CA 02207462 1997-06-11
- 1 0 -
as a difference (A-B) between a doping capacity (A~
and a dedoping capacity (B) and a lowering in rate of
effective utilization of active substance. On the
other hand, an extremely large pore volume results in
a lowering in packing density of the carbonaceous
material for preparing a secondary battery.
Accordingly, the pore volume may preferably be 0.55 -
1.00 ml/g, further preferably 0.55 - 0.70 ml/g.
In the present invention, the carbonaceous
material should be construed as a term covering a
graphitic material having a developed graphite
structure as obtained through heat treatment at a
temperature of 2000 ~C or higher. However, a high-
temperature heat treatment causes a shrinkage of
carbon structure and is liable to deprive the pore
structures, such as vessel, sieve tube and plant
fiber, originated from the starting material.
Accordingly, the carbonaceous material according to
the present invention may be practically
advantageously realized as a carbonaceous material
having an average (002)-plane spacing as measured by
X-ray diffraction method (hereinafter sometimes
denoted by ''doo2ll) of at least 0.365 nm. If a
carbonaceous material having doo2 of below 0.365 nm is
used to constitute a negative electrode of a non-
aqueous solvent-type secondary battery, the negative
electrode is liable to exhibit a smaller doping
CA 02207462 1997-06-11
capacity for a cell active substance. On the other
hand, if a carbonaceous material having doo2 exceeding
0.390 nm is used to constitute a negative electrode of
a non-aqueous solvent-type secondary battery, the
negative electrode is liable to exhibit an increased
non-dedoping capacity. doo2 is preferably 0.365 -
0.390 nm, further preferably 0.370 - 0.390 nm.
The carbonaceous material may preferably have
a hydrogen-to-carbon atomic ratio H/C of below 0.1,
more preferably at most 0.07. A carbonaceous material
having an H/C of 0.1 or larger formed at a low
carbonization temperature is not suitable as a
carbonaceous electrode material for a non-aqueous
solvent-type secondary battery because of an increase
in non-dedoping capacity.
Now, the process for producing a carbonaceous
material according to the present invention will be
described.
The production process according to the
present invention comprises: carbonizing a carbon
precursor of plant origin having a potassium content
of at most 0.5 wt. % as measured by fluorescent X-ray
analysis, in contact with a stream of an inert gas
optionally containing a halogen gas at a temperature
of 700 - 1500 ~C. Herein, the term "carbon precursor"
includes a starting organic material of plant origin,
a product obtained by subjecting such an organic
CA 02207462 1997-06-11
material to a potassium removal treatment as described
hereinafter, a preliminarily calcined product of such
an organic material of plant origin, and a
preliminarily calcined organic material further
subjected to a potassium removal treatment.
Examples of the inert gas used in the process
of the present invention may include nitrogen gas,
argon gas and helium gas. Example of the halogen gas
optionally contained in the inert gas may include
chlorine gas, bromine gas, iodine gas, and fluorine
gas, but chlorine gas is particularly preferred.
The halogen-containing inert gas may preferably have
a halogen gas concentration on the order of 4 - 40
mol. %.
Preferred examples of the organic material of
plant origin used in the present invention as carbon
sources of the carbonaceous material may generally
include: coconut shell, coffee bean, chaffs, broad-
leaf tree wood, conifer wood, and bamboo. It is
preferred that such an organic material of plant
origin is preliminarily calcined at 300 - 800 ~C in an
inert gas atmosphere or under a reduced pressure so as
to preliminarily remove its tar and other volatile
contents therefrom prior to carbonization thereof.
The potassium content in such a plant material varies
depending on the plant species, but a carbonaceous
material obtained by carbonizing a starting material
CA 02207462 1997-06-11
having a large potassium content provides a
carbonaceous electrode material exhibiting inferior
performances, as represented by a small de-doping
capacity and a large non-dedoping capacity for an
active substance, such as lithium.
The potassium content in a carbon precursor,
such as an organic material of plant origin or
preliminary calcination product thereof may be reduced
by subjecting the carbon precursor to a deashing or
potassium removal treatment. The potassium content is
at most 0.5 wt. %, preferably at most 0.4 wt. %,
further preferably at most 0.3 wt. %, particularly
preferably at most 0.2 wt. %. This condition should
be satisfied by the carbonaceous material after
carbonization but may preferably be also satisfied by
the carbon precursor before the carbonization.
The removal of potassium from (i.e., deashing
of) an organic material of plant origin may preferably
be performed by pulverizing such an organic material
of plant origin as it is or after preliminary
calcination thereof at a temperature of ca. 300 - 800
~C into a carbon precursor, into fine particles, and
then dipping the fine particles within an acid, such
as hydrochloric acid, or water. If the particles to
be treated for potassium removal have a large particle
size, the potassium removal efficiency is liable to be
remarkably lowered. The particles to be treated may
CA 02207462 1997-06-11
-14-
preferably have a weight-average particle size
(diameter) of at most 100 ~um, further preferabIy at
most 50 ~m. The potassium removal treatment may
preferably be applied to a carbonaceous precursor
obtained by preliminarily calcining a starting organic
material at ca. 300 - 800 ~C so as to improve the
potassium removal efficiency. A high preliminary
calcination temperature in excess of 800 ~C is not
preferred because the potassium removal efficiency is
rather lowered thereby.
The potassium removal treatment may be
performed by dipping carbon precursor particles within
an aqueous liquid inclusive of acids (aqueous
solutions), such as hydrochloric acid, sulfuric
lS acid,nitric acidand hydrofluoric acid, and water per
se. In case where water is used as the treating
liquid, the water may preferably be used at an
elevated temperature of at least 50 ~C, more
preferably at least 80 ~C since a lower water
temperature results in a remarkably lower potassium
removal efficiency. The dipping treatment for
potassium removal may preferably be performed by
repeating plural times of dipping for a relatively
short period rather than a single dipping for a longer
period in order to effectively increase the potassium
removal efficiency. The potassium removal treatment
may preferably be performed as at least one cycle,
CA 02207462 1997-06-11
-15-
preferably at least two cycles, of dipping in an acid
and then dipping in water.
The carbonization may preferably be performed
while taking care so that tar or decomposition
products, such as hydrogen and methane, will not
hinder the pore formation in the carbon precursor.
In case where the carbon precursor is carbonized in an
environment rich or dense in decomposition product,
the formation of minute pores is liable to be
iO insufficient, thus resulting in a carbonaceous
material having a lower capacity for doping with
active substance.
As the carbon precursor inclusive of an
organic material of plant origin per se is inherently
porous because of the presence of vessel, sieve tube,
etc., the dissipation or removal of decomposition
products during the carbonization is facilitated to
result in a large volume of pores having a relatively
large diameter.
According to the production process of the
present invention, the carbon precursor of plant
origin is carbonized while flowing an inert gas or a
halogen-containing inert gas (hereinafter sometimes
inclusively referred to as a "treatment gas") in
contact with the carbon precursor. In this instance,
the material to be carbonized (i.e., carbon precursor)
may be disposed in a piled layer within a reactor and
CA 02207462 1997-06-11
-16-
is carbonized while flowing the inert gas in a space
outside but in contact with the layer (outside-layer
flow scheme), or the material to be carbonized (carbon
precursor) is disposed in a layer or bed and is
carbonized while flowing the inert gas through within
the layer or bed (intra-layer flow scheme).
In a batch-wise outside-layer flow scheme, it
is preferred to suppress the piled layer thickness of
the material to be carbonized as thin as possible so
as to increase the area of contact of the material
layer with the inert gas and allow quick removal of
the decomposition product from the material out of the
system. The piled layer thickness of the material to
be carbonized may preferably be at most 50 mm, more
preferably at most 30 mm. The inert gas may be
supplied or flowed at a vacant reactor-basis speed of
at least 1 mm/sec, more preferably at least 5 mm/sec.
It is preferred to adopt an intra-layer flow
scheme of a continuous-type or a batch-type using a
fluidized bed, a fixed bed, etc. In this case, the
inert gas may preferably be supplied or flowed at a
rate of at least lO ml, more preferably at least 50
ml, further preferably at least 100 ml, per gram of
the material to be carbonized, while it can depend on
the amount of the material to be carbonized per unit
time. A higher inert gas supply rate may be preferred
in view of the properties of the product carbonaceous
CA 02207462 1997-06-11
material, but practically the supply rate may be at
most 500 ml per gram of the material to be carbonized.
The gas supply rate referred to herein is calculated
based on the volume of the treatment gas under the
standard state (0 ~C and 1 atm).
The carbonization may be performed at a
temperature of 700 - 1500 ~C. Carbonization at a
temperature below 700 ~C results in an increased non-
dedoping active substance capacity of the product
carbonaceous material. Carbonization at a temperature
higher than 1500 ~C results in a decrease in capacity
for doping with active substance. The carbonization
temperature is 700 - 1500 ~C, preferably 900 - 1400
~C ~
Next, the non-aqueous solvent-type secondary
battery of the present invention will be described.
The carbonaceous material according to the
present invention has a micro-texture suitable for
doping with lithium and can be suitably used as an
electrode material for lithium batteries for
constituting a positive electrode or a negative
electrode to be doped with lithium as an active
substance. It is particularly preferred that the
carbonaceous material is used for constituting a
negative electrode for doping with lithium as a
negative electrode active substance of a non-aqueous
solvent-type lithium secondary battery.
CA 02207462 l997-06-ll
-18-
Figure 1 is a partially exploded perspective
view of a non-aqueous solvent-type lithium secondary
battery as a preferred embodiment of the battery
according to the present invention.
More specifically, the secondary battery
basically includes a laminate structure including a
positive electrode 1, a negative electrode 2 and a
separator 3 disposed between the positive and negative
electrodes 1 and 2 and comprising a fine porous film
of a polymeric material, such as polyethylene or
polypropylene, impregnated with an electrolytic
solution. The laminate structure is wound in a vortex
shape to form an electricity-generating element which
is housed within a metal casing 5 having a bottom
constituting a negative electrode terminal 5a. In the
secondary battery, the negative electrode 2 is
electrically connected to the negative electrode
terminal 5a, and the uppermost portion of the battery
is constituted by disposing a gasket 6 and a safety
valve 7 covered with a top plate 8 having a projection
constituting a positive electrode terminal 8a
electrically connected to the positive electrode.
Further, the uppermost rim 5b of the casing 5 is
crimped toward the inner side to form an entirely
sealed cell structure enclosing the electricity-
generating element.
Herein, the positive electrode l or negative
CA 02207462 1997-06-11
--19--
electrode 2 may be constituted by an electrode
structure 10 having a sectional structure as partially
shown in Figure 2. More specifically, the electrode
structure 10 includes an electroconductive substrate
11 comprising a foil or wire net of a metal, such as
iron, stainless steel, copper, aluminum, nickel or
titanium and having a thickness of, e.g., 5 - lO0 ,um,
or 5 - 20 ~m for a small-sized battery, and a composite
electrode layer (12a, 12b) of, e.g., 10 - 1000 ~m,
preferably lO - 200 ~m, in thickness for a small-sized
battery, on at least one surface, preferabl~ on both
surfaces as shown in Figure 2, of the
electroconductive substrate ll.
The composite electrode layers 12a and 12b
are respectively a layer comprising a particulate
carbonaceous material according to the present
invention, an electroconductive material such as
electroconductive carbon, optionally included, and a
binder such as a vinylidene fluoride resin, formed on
the electroconductive substrate 11.
More specifically, in case of using the
carbonaceous material according to the present
invention for producing an electrode structure 10 (in
Figure 2, corresponding to an electrode 1 or 2 in
Figure l) of a non-aqueous solvent-type secondary
battery as described above, the carbonaceous material
may be optionally formed into fine particles having an
CA 02207462 1997-06-11
-20-
average particle size of 5 - 100 ~m and then mixed
with a binder stable against a non-aqueous solvent,
such as polyvinylidene fluoride, polytetrafluoro-
ethylene or polyethylene, to be applied onto an
electroconductive substrate 11, such as a circular or
rectangular metal plate, to form, e.g., a 10 - 200 ,um-
thick layer. The binder may preferably be added in a
proportion of 1 - 20 wt. % of the carbonaceous
material. If the amount of the binder is excessive,
the resultant electrode is liable to have too large an
electric resistance and provide the battery with a
large internal resistance. On the other hand, if the
amount of the binder is too small, the adhesion of the
carbonaceous material particles with each other and
with the electroconductive substrate 11 is liable to
be insufficient. The above described formulation and
values have been set forth with respect to production
of a secondary battery of a relatively small size,
whereas, for production of a secondary battery of a
larger size, it is also possible to form the above-
mentioned mixture of the carbonaceous material fine
particles and the binder into a thicker shaped
product, e.g., by press-forming, and electrically
connect the shaped product to the electroconductive
substrate.
The carbonaceous material of the present
invention can also be used as a positive electrode
CA 02207462 l997-06-ll
-21-
material for a non-aqueous solvent-type secondary
battery by utilizing its good doping characteristic
but may preferably be used as a negative electrode
material of a non-aqueous solvent-type secondary
battery, particularly for constituting a negative
electrode to be doped with lithium as an active
substance of a lithium secondary battery, as mentioned
above.
In the latter case, the positive electrode
material may comprise a complex metal chalcogenide,
particularly a complex metal oxide, such as LiCoO2,
LiNiO2 or LiMn204. Such a positive electrode material
may be formed alone or in combination with an appro-
priate binder into a layer on an electroconductive
substrate.
The non-aqueous solvent-type electrolytic
solution used in combination with the positive
electrode and the negative electrode described above
may generally be formed by dissolving an electrolyte
in a non-aqueous solvent. The non-aqueous solvent may
comprise one or two or more species of organic
solvents, such as propylene carbonate, ethylene
carbonate, dimethyl carbonate, diethyl carbonate,
dimethoxyethane, diethoxyethane, ~-butyrolactone,
tetrahydrofuran, 2-methyl-tetrahydrofuran, sulfolane,
and 1,3-dioxolane. Examples of the electrolyte may
include LiC104, LiPF6, LiBF4, LiCF3S03, LiAsF6, LiCl,
CA 02207462 1997-06-11
-22-
LiBr, LiB(C6H5)4, and LiN(SO2CF3) 2'
As described above with reference to Figure
1, a secondary battery of the present invention may
generally be formed by disposing the above-formed
positive electrode 1 and negative electrode 2 opposite
to each other, optionally with a liquid-permeable
separator 3 composed of, e.g., unwoven cloth or other
porous materials, disposed therebetween, and dipping
the positive and negative electrodes together with an
intermediate permeable separator in an electrolytic
solution as described above.
In the above, a cylindrical battery has been
described as an embodiment of the non-aqueous solvent-
type secondary battery according to the present inven-
lS tion. However, the non-aqueous solvent-type secondary
battery according to the present invention can basi-
cally have any other shapes, such as those of a coin,
a rectangular parallelepiped, or a paper or sheet.
Incidentally, the measurement of various
parameters described herein, i.e., the pore volume by
mercury injection method, the specific surface area by
nitrogen adsorption, the hydrogen/carbon atomic ratio
(H/C), the potassium content by fluorescent X-ray
analysis and doo2 of carbonaceous materials, was
performed in the following manner.
[Pore volume by mercury injection method]
The measurement was performed by using
CA 02207462 1997-06-11
"AUTOPORE 9200~ (available from Micromeritics
Instrument Corp.) in the following manner.
A sample carbonaceous material in the form of
particles having an average diameter of 10 - 20 ~m was
placed in a sample vessel, which was then evacuated
for 30 min. at a pressure of at most 2.67 Pa. Then,
mercury was introduced into the sample vessel and
gradually injected into pores under a gradually
increasing pressure (up to a maximum pressure of 414
MPa). From a relationship between pressure P and
injected volume of mercury during the measurement, a
pore volume distribution of the carbonaceous material
sample was derived versus pore diameter D as a variant
by using formulae described below. The volume of
mercury injected from a pressure (0.25 MPa)
corresponding to a pore diameter of 5 ~m to the
maximum pressure (414 MPa; corresponding to a pore
diameter of 3 nm) was measured as a pore volume of
pores having a diameter of at most 5 ~m.
Formulae for pore diameter calculation were
as follows. In case where mercury is injected
(pressurized) into a cylindrical pore having a
diameter D under a pressure P, the following equation
is given based on a balance between a surface tension
and a pressure acting on a sectional area of the pore:
-~Dr-cos0 = ~(D/2)2P,
wherein ~ represents a surface tension of the mercury,
CA 02207462 1997-06-11
-24-
and ~ denotes a contact angle between the mercury and
the pore well. Accordingly,
D = (-4~-cosO)/P.
Herein, the surface tension (~) of mercury
was assumed to be 484 dyn/cm, the contact angle (0)
between mercury and carbon was assumed to be 130 deg.;
and the pressure P and the diameter D were expressed
in the units of MPa and ~m, respectively, whereby the
above equation was reduced to
D = 1.27/P.
[Specific surface area by nitrogen a~sorption]
An approximate equation
Vm = l/(V- ( l-x) )
derived from the BET equation was used to obtain vm
(amount (cm3/g-sample)) of adsorbed nitrogen re~uired
to form a mono-molecular layer of nitrogen on the
sample surface) from a measured nitrogen volume v at a
relative pressure x (= 0.3) according to the BET
single-point method using nitrogen adsorption. From
the thus-obtained vm-value, a specific surface area
SBET was calculated based on the following equation:
SBET = 4.35 x vm (m /g).
More specifically, the nitrogen adsorption
onto a carbonaceous material was performed at liquid
nitrogen temperature by using "Flow Sorb II 2300"
(available from Micromeritics Instrument Corp.) in
the following manner.
CA 02207462 1997-06-11
-25-
A sample carbonaceous material pulverized
into an average diameter of ca. 20 ~m was packed in a
sample tube, and the sample tube was cooled to -196 ~C
while flowing helium gas containing nitrogen at a
concentration of 30 mol. %, thereby to cause the
carbonaceous material to adsorb nitrogen. Then, the
sample tube was restored to room temperature to
measure the amount of nitrogen desorbed from the
sample by a thermal conductivity-type detector,
thereby to obtain the adsorbed nitrogen amount v
(cm3/g-sample).
[Hydrogen/carbon (H/C) atomic ratio]
A sample of carbonaceous material was
subjected to elementary analysis by using a CNH
analyzer, and a hydrogen/carbon(H/C) atomic ratio was
calculated as a ratio of numbers of atoms of
hydrogen/carbon based on the weight proportions of
hydrogen and carbon in the sample.
[Potassium content by fluorescent X-ray analysis]
For potassium content measurement, carbon
samples having prescribed potassium contents were
prepared and subjected to measurement by a fluorescent
X-ray analyzer to prepare a calibration curve for a
relationship between potassium Ka-ray intensity and
potassium content in advance. Then, sample
carbonaceous materials were subjected to measurement
of potassium Ka-ray intensities by the fluorescent X-
CA 02207462 1997-06-11
-26-
ray analysis to obtain the potassium contents based on
the calibration curve. The calibration curve was
approximated into a straight line passing through the
origin in a potassium content range of 0 - 2. 5 wt. %.
The carbon samples used for making the
calibration curve were prepared in the following
manner. Petroleum coke free from potassium content
prepared by calcination at 1200 ~C was pulverized to
an average particle size of 20 ~m to obtain powdery
carbonaceous materials. A prescribed amount of
potassium hydrogen carbonate was added to each
carbonaceous material and stirred after addition of
some deionized water, and the resultant mixture was
dried. In this way, several carbon samples having
prescribed potassium contents were prepared.
The fluorescent X-ray analysis was performed
by using "RIGAKU SYSTEM 3082E2" (available from Rigaku
Denki K.K.) in the following manner. An upper part
irradiation-type holder was used, and a sample
measurement area was set within a circle having a
diameter of 20 mm. More specifically, a ring having a
diameter of 20 mm and a height of 5 mm was placed on a
filter paper, and 0.935 g of a sample carbonaceous
material was placed within the ring and surface-
covered with a polyethylene terephthalate film to besubjected to the measurement. The measurement was
performed by using germanium as an analyzing crystal
CA 02207462 1997-06-11
-27-
and a proportional counter as a detector in a 20-range
of 60 - 73 deg. at a scanning speed of 1 deg./min.
[doo2 of carbonaceous material]
A powdery sample of a carbonaceous material
was packed in an aluminum-made sample cell and
irradiated with monochromatic CuKa rays (wavelength
= 0.15418 nm) through a graphite monochromator to
obtain an X-ray diffraction pattern. The peak
position of the diffraction pattern is determined by
the center of gravity method (i.e., a method wherein
the position of a gravity center of diffraction lines
is obtained to determine a peak position as a 20 value
corresponding to the gravity center) and calibrated by
the diffraction peak of (111) plane of high-purity
silicon powder as the standard substance. The doo2
value is calculated from the Bragg's formula shown
below.
doo2 = ~/(2 sinO) (Bragg's formula)
[Examples]
Hereinbelow, the present invention will be
described more specifically based on Examples and
Comparative Examples. All the volumes or flow rates
of treatment gases described hereinafter are values
calculated under the standard state (0 ~C, 1 atm).
Example 1
Coconut shell char (available from M.C.
Carbon K.K.) was pre-calcined for 1 hour at 600 ~C in
CA 02207462 1997-06-11
-28-
a nitrogen gas atmosphere (normal pressure) and then
pulverized to form a powdery carbon precursor having
an average particle size of 25 ~m Then, the powdery
carbon precursor was subjected to two cycles of
5 potassium removal treatment, each including dipping
within 35 %-hydrochloric acid for 1 hour and then
washing by dipping within boiling water for 1 hour, to
obtain a treated carbon precursor. The thus-treated
carbon precursor exhibited a potassium content of 100
ppm (by weight) or below. Then, 10 g of the treated
carbon precursor was piled in a ca. 1 - 2 mm-thick
layer in an alumina-made boat and then placed in a
horizontal tubular furnace of 100 mm in diameter to be
heated for 1 hour at 1100 ~C for carbonization while
flowing nitrogen gas at a rate of 10 liter/min.
The properties (including pore volume,
potassium content, specific surface area (SBET), H/C
and doo2) of the resultant carbonaceous material are
shown in Table 1 appearing hereinafter together with
those of other Examples and Comparative Examples.
Example 2
A carbonaceous material was prepared in the
same manner as in Example 1 except that the
carbonization temperature was changed to 1200 ~C.
Example 3
A carbonaceous material was prepared in the
same manner as in Example 1 except that the
CA 02207462 l997-06-ll
-29-
carbonization temperature was changed to 1300 ~C.
Example 4
The powdery carbon precursor prepared in
Example 1 was treated for potassium removal by dipping
in boiling water for 1 hour. The thus-treated carbon
precursor exhibited a potassium content of 0.4 wt. %.
The treated powdery carbon precursor was carbonized in
the same manner as in Example 1 except that the
carbonization temperature was changed to 1200 ~C to
prepare a carbonaceous material.
Example 5
Milled and extracted coffee bean was dried at
120 ~C for 1 hour and then subjected to pre-
calcination, pulverization and potassium removal
treatment under similar conditions as in Example 1 to
prepare a treated powdery carbon precursor. The
treated carbon precursor exhibited a potassium content
of 100 ppm or below. The treated carbon precursor was
carbonized in the same manner as in Example 1 except
that the carbonization temperature was changed to 1200
~C, to prepare a carbonaceous material.
Example 6
Mohsoh bamboo trunk (produce of Fukushima-
ken, Japan; age: 3, diameter: ca. 70 mm) was pre-
calcined and pulverized under the same conditions asin Example 1, to obtain a powdery carbon precursor,
which exhibited a potassium content of 3.1 wt. %. The
CA 02207462 1997-06-11
-30-
powdery carbon precursor was subjected to potassium
removal treatment in the same manner as in Example to
obtain a treated carbon precursor, which exhibited a
potassium content of 100 ppm or below. The treated
carbon precursor was carbonized in the same manner as
in Example 1 except that the carbonization temperature
was changed to 1200 ~C, thereby to obtain a
carbonaceous material.
Example 7
Oak wood (produce of Fukushima-ken, Japan,
age: 10, diameter: ca. 50 mm) was subjected to pre-
calcination, pulverization and potassium removal
treatment under similar conditions as in Example 1 to
prepare a treated powdery carbon precursor. The
treated carbon precursor exhibited a potassium content
of 100 ppm or below. The treated carbon precursor was
carbonized in the same manner as in Example 1 except
that the carbonization temperature was changed to 1200
~C, to prepare a carbonaceous material.
Example 8
A carbonaceous material was prepared in the
same manner as in Example 1 except that the
carbonization in the furnace was performed under the
following conditions.
The furnace temperature was raised at a rate
of 10 ~C/min. while flowing nitrogen gas at a rate of
10 liter/min. When the furnace temperature reached
CA 02207462 l997-06-ll
-31-
900 ~C, the nitrogen was was changed to a mixture of
nitrogen gas at a rate of 7 liter/min. and chlorine
gas at a rate of 3 liter/min., and the furnace
temperature was continually raised. After the furnace
temperature reached 1100 ~C, the temperature was
retained for 1 hour, and the chlorine gas supply was
terminated, followed by cooling while flowing nitrogen
gas at a rate of 10 liter/min., thereby to obtain a
carbonaceous material.
Comparative Example l
A carbonaceous material was prepared in the
same manner as in Example 1 except that the potassium
removal treatment of the powdery carbon precursor was
omitted, and the carbonization temperature was changed
to 1200 ~C.
Comparative Example 2
A carbonaceous material was prepared in the
same manner as in Example 1 except that the potassium
removal treatment of the powdery carbon precursor was
omitted, and the carbonization temperature was changed
to 1300 ~C.
Comparative Example 3
The powdery carbon precursor prepared in
Example 6 and having a potassium content of 3.1 wt. %
was, without being subjected to the potassium removal
treatment, carbonized in the same manner as in Example
1 except that the carbonization temperature was
CA 02207462 1997-06-11
changed to 1200 ~C, thereby to obtain a carbonaceous
material.
Comparative Example 4
Coconut shell-based activated carbon
(available from Kuraray Chemical K.K.) was pulverized
to an average particle size of 25 ~m to form a powdery
activated carbon, which was then carbonized in the
same manner as in Example 1 except that the
carbonization temperature was changed to 1200 ~C,
thereby to obtain a carbonaceous material.
Comparative Example 5
A petroleum pitch (softening point = 210 ~C,
quinoline-insoluble content = 1 wt. ~, H/C atomic
ratio = 0.63) was heated to 600 ~C and held at 600 ~C
for 1 hour for pre-calcination, followed by
pulverization to form a powdery carbon precursor
having an average particle size of 20 ,um, which was
then carbonized in the same manner as in Example 1
except that the carbonization temperature was changed
to 1200 ~C, thereby to obtain a carbonaceous material.
Comparative Example 6
A phenolic resin ("BELLPEARL S-870",
available from Kanebo K.K.) was preliminarily cured at
170 ~C for 3 min. and cured at 130 ~C for 8 hours to
prepare a powdery carbon precursor, which was then
carbonized in the same manner as in Example 1 except
that the carbonization temperature was changed to 1200
CA 02207462 1997-06-11
~C, thereby to obtain a carbonaceous material.
Comparative Example 7
In a 300 ml-Erlenmeyer flask, 30 g of coconut
shell char ("Yashibon No. l", available from Kuraray
Chemical K.K.) pulverized to an average particle size
of 1 mm or below and lO0 g of 35 %-hydrochloric acid
were placed and shaked at 50 ~C for l hour, followed
by filtration. The filration residue was sufficiently
washed with de-ionized water and dried at 120 ~C for 2
hours to obtain de-ashed char. The resultant de-ashed
char was pulverized into a powdery carbon precursor
having an average particle size of 20 ~m, which
exhibited a potassium content of 0.6 wt. %. The
powdery carbon precursor was then carbonized in the
same manner as in Example 1 except that the
carbonization temperature was changed to 1200 ~C,
thereby to obtain a carbonaceous material.
Comparative Example 8
The treated powdery carbon precursor obtained
in Example 1 was dipped in an aqueous solution of
potassium hydrogen carbonate (KHCO3) and then dried to
obtain a potassium-carrying powder carbon precursor,
which exhibited a potassium content of 2.8 wt. %. The
carbon precursor was then carbonized in the same
manner as in Example 1 except that the carbonization
temperature was changed to 1200 ~C, thereby to obtain
a carbonaceous material.
CA 02207462 1997-06-11
-34-
The basic properties of the carbonaceous
materials prepared in the above Examples and
Comparative Examples are inclusively shown in Table 1
appearing hereinafter.
[Doping/de-doping capacity for active substance]
The carbonaceous materials obtained in
Examples and Comparative Examples were respectively
used to prepare a non-aqueous solvent-type secondary
battery (cell) and the performances thereof were
evaluated in the following manner.
The carbonaceous material is generally suited
for constituting a negative electrode of a non-aqueous
solvent secondary battery. However, in order to
accurately evaluate the performances of a carbonaceous
material inclusive of a doping capacity (A) and a de-
doping capacity (B) and also a non-dedoping capacity
(A-B) for a cell active substance without being
affected by a fluctuation in performance of a counter
electrode material, a large excess amount of lithium
metal showing a stable performance was used as a
negative electrode, and each carbonaceous material
prepared above was used to constitute a positive
electrode, thereby forming a lithium secondary
battery, of which the performances were evaluated.
More specifically, the positive electrode
(carbon electrode) was prepared as follows. That is,
9O wt. parts of the carbonaceous material thus
CA 02207462 1997-06-11
formulated in the form of fine particles and 10 wt.
parts of polyvinylidene fluoride were mixed together
with N-methyl-2-pyrrolidone to form a paste composite,
which was then applied uniformly onto an aluminum
foil. The composite, after being dried, was peeled
off the aluminum foil and stamped into a 15 mm-dia.
disk-shaped carbonaceous film. Separately, a 17 mm-
dia. disk-shaped stainless steel net was spot-welded
to an inner lid of a coin-shaped cell can of 2016 size
(i.e., diameter of 20 mm x thickness of 1.6 mm), and
the above-prepared disk-shaped carbonaceous film was
press-bonded to the disk-shaped stainless steel net to
form a positive electrode containing ca. 20 mg of the
carbonaceous material.
On the other hand, a negative electrode
(lithium electrode) was prepared in a glove box of an
argon atmosphere in the following manner. A 17 mm-
dia. disk-shaped stainless steel net was spot-welded
to an outer lid of the coin-shaped cell can of 2016
size, and a 15 mm-dia. lithium disk formed by stamping
a 0.5 mm-thick metallic lithium plate was press bonded
onto the disk-shaped stainless steel net to form a
negative electrode.
The thus-prepared positive and negative
electrodes, a porous polypropylene film as a separator
disposed therebetween, and an electrolytic solution
comprising a 1:1 (by volume)-mixture solvent of
CA 02207462 1997-06-11
-36-
propylene carbonate and dimethoxyethane and LiC104
dissolved therein at a rate of 1 mol/liter, were used
to form a coin-shaped non-aqueous solvent-type lithium
secondary battery of 2016 size together with a
polyethylene-made gasket in an argon glove box.
In the lithium secondary battery thus
constituted, the carbonaceous material in the positive
electrode was subjected to doping and dedoping of
lithium to evaluate capacities therefor.
More specifically, the doping was effected by
repeating a cycle including 1 hour of current
conduction at a current density of 0.5 mA/cm2 and 2
hours of pause until the equilibrium potential between
the positive and negative electrodes reached 5 mV.
The electricity thus flowed was divided by the weight
of the carbonaceous material to provide a doping
capacity (A) in terms of Ah/kg. Then, in a similar
manner, a current was flowed in a reverse direction to
dedope the lithium from the doped carbonaceous
2~ material. The de-doping was effected by repeating a
cycle including 1 hour of current conduction at a
current density of 0.5 mA/cm2 and 2 hours of pause,
down to a cut-off voltage of 1.5 volts. The
electricity thus flowed was divided by the weight of
the carbonaceous material to provide a dedoping
capacity (B) in terms of Ah/kg. Then, a non-dedoping
capacity (A-B) was calculated as a difference between
CA 02207462 1997-06-11
-37-
the doping capacity (A) and the dedoping capacity (B),
and a discharge efficiency (%) was obtained by
dividing the dedoping capacity (B) with the doping
capacity (A) and multiplying the quotient (B/A) with
lO0. The discharge efficiency is a measure of
effective utilization of the active substance.
The performances of the lithium secondary
batteries using positive electrodes of the respective
carbonaceous materials measured in the above-described
manner are summarized in the following Table 2.
CA 02207462 1997-06-11
-38-
Table 1: Basic properties of carbonaceous material
Starting Carbon- Pore K- SBET H/C doo2
material ization volume content
temp.
(~C) (ml/g) (%) (m2/g) (nm)
Ex. 1 coconut 1100 0.63 0.01 0.9 0.01 0.377
shell
Ex. 2 coconut 1200 0.63 0.01 1.0 0.01 0.379
shell
Ex. 3 coconut 1300 0.60 0.01 1.2 0.00 0.380
shell
Ex. 4 coconut 1200 0.62 0.45 1.1 0.02 0.378
shell
Ex. 5 coffee 1200 0.61 0.02 1.2 0.00 0.371
bean
Ex. 6 bamboo 1200 0.82 0.01 3.0 0.02 0.375
Ex. 7 oak wood 1200 0.93 0.01 1.2 0.02 0.379
Ex. 8 coconut 1100 0.63 0.00 0.9 0.00 0.378
shell
____________________________________________________________________
Comp. coconut 1200 0.62 0.92 1.1 0.01 0.376
Ex. 1 shell
Comp. coconut 1300 0.59 0.83 1.2 0.00 0.378
Ex. 2 shell
Comp. bamboo 1200 0.82 2.2 3.5 0.03 0.374
Ex. 3
Comp. coconut 1200 1.05 0.60 1270 0.03 0.349
Ex. 4 shell
active
carbon
Comp. petroleum 1200 0.38 0.00 1.7 0.01 0.355
Ex. 5 pitch
Comp. phenolic 1200 0.42 0.00 1.0 0.02 0.377
Ex. 6 resin
Comp. coconut 1200 0.62 0.58 1.3 0.06 0.379
Ex. 7 shell
Comp. coconut 1200 0.63 1.52 1.4 0.01 0.378
Ex. 8 shell
CA 02207462 1997-06-11
-39-
Table 2: Battery performances
Starting Carbon- Doping Dedoping Non- Discharge
material ization capacity capacity dedoping efficiency
temp. (A) (B3 capacity
(A-B)
(~C) (Ah/kg) (Ah/kg) (Ah/kg) (~)
Ex. 1 coconut 1100 606 496 111 81.8
shell
Ex. 2 coconut 1200 560 473 87 84.5
shell
Ex. 3 coconut 1300 464 412 52 88.8
shell
Ex. 4 coconut 1200 544 445 99 81.8
shell
Ex. 5 coffee 1200 557 469 88 84.2
bean
Ex. 6 bamboo 1200 550 460 90 83.6
Ex. 7 oak wood 1200 544 470 74 86.4
Ex. 8 coconut 1100 600 495 105 82.5
shell
____________________________________________________________________
Comp. coconut 1200 538 437 101 81.2
Ex. 1 shell
Comp. coconut 1300 422 362 60 85.8
Ex. 2 shell
Comp. bamboo 1200 532 411 121 77.3
Ex. 3
Comp. coconut 1200 581 345 236 59.4
Ex. 4 shell
active
carbon
Comp. petroleum 1200 332 279 53 84.0
Ex. 5 pitch
Comp. phenolic 1200 504 294 210 58.3
Ex. 6 resin
Comp. coconut 1200 605 475 130 78.5
Ex. 7 shell
Comp. coconut 1200 530 420 110 79.2
Ex. 8 shell
CA 02207462 1997-06-11
-40-
In view of the battery performances shown in
Table 2 in comparison with the starting material-
carbonization temperatures and resultant basic
properties of the carbonaceous materials, it is
understood that the secondary batteries using
carbonaceous materials prepared through potassium
removal treatment and carbonization of carbon
precursors of plant origin according to Examples
exhibited a doping capacity (A) and a dedoping
capacity (B) which were both high and provided an
extremely small non-dedoping.capacity (A-B) as a
difference therebetween, thus allowing an effective
utilization of cell active substance.
Especially, the results of Examples 2, 3 and
6 show it possible to realize a remarkable improvement
in discharge efficiency, particularly through a
remarkably increased de-doping capacity, by using a
carbon precursor of a reduced potassium content in
comparison with those of Comparative Examples 1, 2 and
3, respectively, corresponding thereto.
Comparative Example 8 was performed in order
to confirm an ill effect of an increased potassium
content. Thus, the carbonaceous material of
Comparative Example 8 was prepared by causing the
treated carbon precursor having a potassium content of
lOO ppm or below prepared in Example 1 to carry
potassium hydrogen carbonate up to a potassium content
CA 02207462 l997-06-ll
-41-
of 2.8 wt. %, followed by carbonization under the same
condition as in Example 2. The resultant carbonaceous
material contained 1.52 wt. % of potassium as shown in
Table 1. As is understood from comparison between
performances of secondary batteries prepared by using
carbonaceous materials of Comparative Example 8 and
Example 2, the secondary battery prepared by using the
potassium-containing carbonaceous material of
Comparative Example 8 exhibited clearly inferior
performances. From these results, it is understood
that potassium adversely affects the performances of a
carbonaceous electrode material for secondary battery.
The carbonaceous material of the present
invention exhibits a larger de-doping capacity and a
smaller non-dedoping capacity for active substance and
therefore excellent performances as a battery
electrode material.
The process of the present invention can also
exhibit a remarkable effect of allowing easy
production of a carbonaceous electrode material for
secondary battery showing excellent performances as
described above by calcining and carbonizing a
carbon precursor of plant origin having a specifically
low potassium content under appropriate conditions.