Note: Descriptions are shown in the official language in which they were submitted.
CA 02207689 1997-06-13
WO 95/16518 PCT/US94I14316
io "IMPROVED SOLID FILTRATION MEDIA
INCORPORATING ELEVATED LEVELS OF
PERMANGANATE AND WATER"
Technical Field
is The present invention relates generally to an
improved composition and method for the removal of compounds
having toxic or corrosive properties or disagreeable odors,
especially sulfur containing compounds, from gaseous streams.
The invention more particularly relates to the use in filter beds of
- 2o an improved solid filtration media comprising a substrate
impregnated with elevated levels of potassium permanganate and
water. The media may also be impregnated with sodium
bicarbonate. The media is improved in that it has a substantially
higher capacity for the removal of the above compounds from gas
2s streams than the media of the prior art. Further, the media
contains significantly higher levels of potassium permanganate
and water than thought to be efficacious in the prior art.
Background of the Invention
3o Undesirable airborne compounds, including sulfur
compounds, ammonia, formaldehyde, urea, carbon monoxide,
oxides of nitrogen, mercaptans, amines, and ethylene, occur in a
number of environments, where most are primarily responsible
for the presence of disagreeable odors, or irritating or toxic
3s gases. Such environments include petroleum treatment and
CA 02207689 1997-06-13
WO 95/16518 PC'T/LTS94114316
r
2
storage areas, sewage treatment facilities, hospitals, morgues,
anatomy laboratories, animal rooms, and pulp and paper
production sites, among others. These undesirable compounds
may be bacterial breakdown products of higher organic -
compounds, or byproducts of industrial processes.
Hydrogen sulfide ("H2S"), a colorless, toxic gas with
a characteristic odor of rotten eggs, is produced in coal pits, gas
wells, sulfur springs, and from decaying organic matter
containing sulfur. Controlling emissions of this gas, particularly
io from municipal sewage treatment plants, has long been considered
desirable. More recently, protecting electronic apparatus from
the corrosive fumes of these compounds has become increasingly
important. Further, H2S is flammable.
Ammonia ("NH3"), also a colorless gas, possesses a
is distinctive, pungent odor and is a corrosive, alkaline gas. The gas
is produced in animal rooms and nurseries and its control also has
long been considered desirable.
Chlorine ("C12") is a greenish-yellow gas with a
suffocating odor. The compound is used for bleaching fabrics,
- 2o purifying water, treating iron, and other uses. Control of this
powerful irritant is most desirable for the well-being of those
who work with it or are otherwise exposed to it. At lower levels,
in combination v~ith moisture, chlorine has a corrosive effect on
electronic circuitry, stainless steel and the like.
2s Formaldehyde ("HCHO") is a colorless gas with a
pungent suffocating odor. It is present in morgues and anatomy
laboratories, and because it is intensely irritating to mucous
membranes, its control is desirable.
Urea ("CH4N20") is present in toilet exhaust and is
3o used extensively in the paper industry to soften cellulose. Its odor , .
makes control of this compound desirable.
Carbon monoxide ("CO"), an odorless, colorless, ,
toxic gas, is present in compressed breathing air. Oxygenation
requirements for certain atmospheres, including those inhabited
3s by humans, mandate its control.
CA 02207689 1997-06-13
WO 95/16518 PCTIUS94114316
3
Oxides of nitrogen, including nitrogen dioxide
("N02"), nitric oxide ("NO"), and nitrous oxide ("N20"), are
compounds with differing characteristics and levels of danger to
- humans, with nitrous oxide being the least irritating oxide.
s Nitrogen dioxide, however, is a deadly poison. Control of
pollution resulting from any of these oxides is desirable or
necessary, depending on the oxide.
Mercaptans and amines, including methyl mercaptan
("CH3SH"), butyl mercaptan ("C4H9SH") and methyl amine
io ("CHSN"), are undesirable gases present in sewerage odor. The
control of these gases is desired for odor control.
Ethylene ("C2H4") is a colorless, flammable gas that
is a simple asphyxiant which accelerates the maturation or
decomposition of fruits, vegetables, and flowers. Control of this
is compound prolongs the marketable life of such items.
Attempts have been made to provide solid filtration
media for removing the undesirable compounds listed above from
fluid streams. Desired features of such media are a high total
capacity for the removal of the targeted compound, a high
Zo efficiency in removing the compound from an air stream
contacting the media, and a high ignition temperature (non-
flammability).
The following approximate formulation is an
example of a solid filtration media produced by the current state
2s of the art: 69% activated alumina, 10% water, 4.5% potassium
permanganate, and 17% sodium bicarbonate. The above solid
filtration media is widely known in the art to have approximately
a 9% capacity for the uptake of hydrogen sulfide gas in a gas
stream.
3o One specific example of a solid filtration media for
the removal of undesirable compounds from gas streams is
described in U.S. Patent No. 4,235,750. The '750 patent discloses
an apparatus and method for adsorbing ethylene and other
gaseous contaminants, wherein the apparatus is a three-part
3s container comprising permanganate impregnated alumina in one
CA 02207689 1997-06-13
WO 95/16518 PCTlUS94114316
4
compartment, activated carbon in the second compartment, and a
mixture of molecular sieves and activated silica gel in the third
compartment. The '750 patent discloses that the concentration of
the aqueous potassium permanganate solution used to impregnate
s the alumina should be limited to one pound of permanganate
dissolved in one gallon of water since if more is dissolved, the
pores of the alumina will be clogged, therefore reducing its
oxidizing capacity. Ideally, the potassium permanganate and
water solution is applied to the substrate so that the dried, finished
io product contains about 4 to 5%, preferably 4.5% of potassium
permanganate by weight of the finished product. Also, the '750
patent teaches that water is simply a diluent which will eventually
be evaporated, and therefore, is not meaningful except as a
vehicle for application of the potassium permanganate to the basic
is alumina substrate. The product is deemed finished after a
substantial portion of the free water has been evaporated.
Preferably, the alumina is dried to remove 99% of the free water
after the aqueous potassium permanganate solution has been
applied to the alumina.
20 ~ Although the '750 patent discloses a potassium
permanganate impregnated alumina for the removal of
undesirable compounds from fluid streams, the capacity of the
impregnated alumina is limited. The efficiency of the
permanganate impregnated alumina of the '750 patent is limited
2.s as its optimal concentration of permanganate is 4.5%, and higher
concentrations of permanganate results in the clogging of the
pores of the substrate and therefore its oxidizing capacity being
reduced. Accordingly, this filtration media would be limited to
approximately a 9% capacity for the uptake of hydrogen sulfide
3o gas in a gas stream. Therefore, this filtration media could not be ,
efficiently used in small filter beds as larger quantities of the
impregnated alumina must be used to compensate for its limited ,
capacity. Further, the use of the impregnated alumina of the '750
patent would be more costly as the media would have to be
3s replaced more frequently, thereby incurring the cost of more
CA 02207689 1997-06-13
WO 95/16518 PC'TIUS94114316
J
frequently purchasing the media and also incurring the cost of the
_ ~ additional labor required for its more frequent replacement.
Finally, the permanganate impregnated alumina of the '750 patent
- is limited in that the failures in the adsorption of contaminants in
s fluid streams which occur at the end of the useful life of the
media would be more frequent due to the limited capacity of the
media. Therefore, the media of the '750 patent could not
practically be utilized in systems where the air quality is critical.
Another example of a solid oxidizing system in pellet
io form consisting of activated alumina ("A1203") impregnated with
potassium permanganate ("KMn04") is described in U.S. Patent
No. 3,049,399. The pellets disclosed in the '399 patent provide
air purification and odor control by both adsorbing and absorbing
odors, and then destroy the collected odors by the potassium
is permanganate's controlled oxidizing action. Apparently, the
permanganate destroys odors by the following oxidation
reactions:
Mn04-+8H++Se- ----> Mn+2+4H20 (acid)
Zo ~ 3Mn04+2H20 ----> Mn02+2Mn04+4~H (alkaline)
Mn04-+2H20+3e- ----> Ma102+2H20 (neutral)
Because the permanganate will not ionize to release the active
permanganate ion unless water is present, the substrate must be
2s hydrophilic and the reaction must take place in normal ambient
humidity. The '399 patent teaches that the amount of water
necessary to cause the oxidation reaction is supplied by a normal
ambient humidity. There is no teaching or suggestion in the '399
patent to elevate the amount of free water in the pellet. Further,
3o there is no teaching or suggestion in the '399 patent to elevate the
concentration of permanganate in the pellet above that obtained
with the 5% aqueous solution of permanganate.
The potassium permanganate impregnated alumina
pellets of the '399 patent are limited in that they have a limited
3s capacity for removing undesired contaminants from gas streams.
CA 02207689 1997-06-13
WO 95/16518 PCTIUS94/14316
6
In one specific example in the '399 patent, the adsorbent was
impregnated with a 5% aqueous solution of the permanganate and '
subsequently dried. ~No particular concentration range of
potassium permanganate or water is disclosed for the pellets of
s the '399 patent. As the '399 patent does not teach or suggest
elevated concentrations of permanganate or free water in its
pellets, the potassium permanganate impregnated alumina of the
'399 patent appears to have the same limitations as the potassium
permanganate impregnated alumina of the '750 patent as discussed
to above.
Yet another example of a solid filtration media for
removing undesirable compounds from a gas stream is disclosed
in U.S. Patent No. 3,226,332. The '332 patent teaches a method
of producing granular activated alumina uniformly impregnated
~s with a solid oxidizing agent, preferably potassium permanganate,
for use in treating fluid streams. This method includes the spray
addition of the impregnate, wherein the impregnate solution is
sprayed onto the dry combination being tumbled in a mixer
thereby forming pellets which are later dried to remove a
2o substantial portion of the remaining water. The preferred
impregnated concentration of potassium permanganate is 2.5 %,
by dry weight. The '332 patent discloses a range of 0.5 to 10%
of potassium permanganate, from about 2% to about 4% of
potassium permanganate being preferred. The '332 patent also
~.s discloses that the pellets are dried to remove at least a substantial
portion of the uncombined (free) water.
Although the permanganate impregnated alumina of
the '332 patent may contain approximately 0.5 to 10% potassium
permanganate, the preferred range of potassium permanganate
3o remains at from about 2% to about 4%. Accordingly, if the
concentration of the potassium permanganate in the solid substrate
is above 4%, it does not appear that the patentee expects any
significant improvement over its capacity at a concentration of
4%. Also, the '332 patent does not teach elevating the amount of
3s water remaining in the substrate. Further, there is no teaching in
CA 02207689 1997-06-13
WO 95116518 PCTIUS94/14316
7
the '332 patent of how to avoid clogging of the pores of the
'- ' substrate as expected from the high concentration of potassium
permanganate. Accordingly, the potassium impregnated alumina
as taught in the '332 patent would be expected by one skilled in
s the art to be limited in its capacity for the removal of undesirable
compounds from fluid streams, and therefor has the same
shortcomings as the potassium impregnated alumina of the '750
patent as discussed above.
As seen above, it is believed in the prior art that the
io adsorptivity of permanganate impregnated alumina is maximized
when the concentration of the potassium permanganate
impregnated in the alumina is approximately 4-5%. It is further
taught that at concentrations above 5%, the potassium
permanganate crystallizes and plugs the pores in the media, and
is therefore does not increase and may even decrease the
adsorptivity of the media.
For example, in a study entitled "Proposed Program
For Stage I of Research at Colorado School of Mines Research
Foundation" of Golden Co., for Marbon Chemical of Des Plaines,
2o IL., it was found that the acceptable range of Mn04- in alumina
pellets was between 2.5 to 3.0%, calculated as Mn04- ion weight
percent of the oven-dry alumina. The 3.0% cut-off was based on
prior experience 'where attempts to store greater amounts of
Mn04- in the pellets resulted in pore-plugging by small crystals
2s of KMnOq..
Also, in a conference report between Borg Warner
Corporation Research Center of Des Plaines, Illinois, and Kaiser
Aluminum and Chemical Corporation of Baton Rouge, Louisiana,
dated January 11, 1960, regarding a cooperative R&D program
0 on substrate improvement, it was concluded that a permanganate
("Mn~~-") concentration of 2.0% (dry basis) appeared to be
optimum. It was also concluded that for production purposes it
would be better to aim for 1.75 to 2.00% since it was thought that
the tendency toward pore-plugging increases very rapidly above
3s 2%.
CA 02207689 1997-06-13
WO 9s/~6518 PCT/US94/14316
Also, as seen above, it is believed that the
concentration of water should be minimized in solid filtration
media. In fact, the industry continues to minimize the water
content of such media. The resistance towards increasing the
s concentration of water in alumina filtration media results from
the belief that the activity of the media is directly related to its
surface area and pore size. Significantly increasing the water
content would therefore be expected to reduce the surface area
available for adsorption of contaminants, and therefore decrease
io the media' s efficacy.
Although there are a variety of permanganate
impregnated substrate known in the art for removing undesirable
contaminants from fluid streams, as demonstrated above, these
known impregnated substrates all have a limited capacity for the
is removal of undesirable compounds from gas streams, and
therefore have limitations and drawbacks in their use, and do not
meet the needs of various industries.
Therefore, what is needed is a high efficiency, high
capacity, low flammability permanganate impregnated substrate
zo for the removal of undesirable compounds from gas streams.
Further, this impregnated substrate needs to be long lasting,
requiring fewer replacements and thereby minimizing
replacement and 'maintenance costs. Also needed is a high
capacity impregnated substrate which may be used in small filter
2s beds, and therefore may allow the treatment of fluid streams
where there are significant space limitations.
Su~inary of the Invention
The present invention relates to an improved solid
3o filtration media, method of preparing the same, and method of
treating a fluid stream with the solid filtration media. The
improved solid filtration media comprises permanganate, water,
and a substrate, such as activated alumina. Sodium bicarbonate
may also be added. As shown above, in the filtration media of the
3s prior art the free water content is minimized, and the potassium
CA 02207689 1997-06-13
WO 95/16518 PCTlUS94/14316
9
permanganate concentration is maintained at approximately 4 to
S% to avoid pore-clogging problems. In direct contrast to the
prior art, the media of the present invention contains significantly
higher levels of permanganate and water. An improved
s efficiency of removal of compounds such as hydrogen sulfide is
achieved by this media. For example, the improved media of the
present invention has a hydrogen sulfide capacity of greater than
approximately 15%, whereas the media produced by the current
art has a maximum hydrogen sulfide capacity of approximately 9
to 10%.
The prior art does not teach or suggest the discovery
of the present invention, namely an improved permanganate
impregnated substrate having elevated levels of both
permanganate and water. No comparably effective combination
is of the materials of the present invention is taught or suggested in
the prior art.
The present invention addresses an existing need in
the prior art by providing a high efficiency, high capacity, low
flammability permanganate impregnated substrate for the
2o removal of undesirable contaminants from gas streams. The
present invention therefore provides a long lasting filtration
media which needs to be replaced less frequently and therefore
minimizes mainte~lance and replacement costs. Also provided by
the impregnated substrate of the present invention is a high
2s capacity filtration media which may be used in small filter beds,
and therefore may allow the treatment of fluid streams where
there are significant space limitations. The present invention
further provides methods of making and using the filtration
l edia.
3o The filtration media of the present invention has a
higher efficiency and capacity to remove certain undesired
.- compounds from gaseous streams than do the media in the prior
art. Further, the improved media of the present invention yields
an equivalent capacity as activated carbon adsorbents. However,
3s the filtration media of the present invention is much less
CA 02207689 1999-11-10
10
expensive, and is considerably less flammable than activated
carbon adsorbents.
Generally described, the present invention provides
a filtration media comprising at least approximately 5%
s permanganate salt, at least 10% water, and a porous substrate,
wherein these percentages are by weight of the composition.
Preferably, the permanganate salt is potassium permanganate.
The porous substrate is selected from the group consisting of
activated alumina, silica gel, zeolite, adsorbent clay, kaolin, and
io activated bauxite, the preferred porous substrate being alumina.
Preferably, the improved solid filtration media of the
present invention comprises between approximately 5 and 12%
permanganate salt, between approximately 10 and 35% water, and
a porous substrate, wherein the above percentages are by weight
is of the composition.
In another embodiment of the present invention, the
composition further comprises sodium bicarbonate, wherein the
sodium bicarbonate is between approximately 0 to 30%, and
preferably is between 15 to 20% by weight. The sodium
Zo bicarbonate enhances the retention of water in the solid filtration
media.
The present invention also provides a method of
preparing an improved filtration media composition. This
method comprises the steps of mixing water, a permanganate salt,
2s and a substrate, and then forming the mixture into at least one
cohesive porous unit. The unit is then cured at a temperature of
from about 37.8°C (100°F) to about 93.3°C (200°F),
until the
concentration of water is at least about 10% by weight of the
composition, and the concentration of the permanganate salt is at
30 least about 5% by weight of the composition.
Preferably, the method of the present invention
comprises forming an aqueous solution comprising the
permanganate salt and then mixing the aqueous solution with the
porous substrate. In an alternative aspect of the invention, the
3s method of the present invention comprises forming a dry mixture
CA 02207689 2001-12-03
11
comprising the permanganate salt and the substrate and then adding water to
the dry mixture.
In yet another aspect of the invention, the method of the present invention
comprises forming
a dry mixture comprising the permanganate salt and the substrate, forming an
aqueous solution
comprising the permanganate salt and then mixing the aqueous solution with the
dry mixture.
Optionally, sodium bicarbonate may be added either to the dry mixture, to the
water, or to both
in the method of preparing the filtration media.
Preferably, in the method of the present invention, the unit formed is cured
until
the concentration of water is from about 10 to about 30% by weight of the
composition and the
concentration of the permanganate salt is from about 5 to about 12% by weight
of the
composition. Preferably, where sodium bicarbonate has been added to the
composition, the unit
formed is cured until the concentration of sodium bicarbonate is between 15 to
20% by weight.
Yet another aspect of the present invention is a method of treating a
contaminated fluid stream with the improved solid filtration composition of
the present
invention. This method comprises contacting the contaminated fluid stream with
the improved
solid filtration composition.
The improved filtration media embodying the present invention, the method of
its preparation and the method of its use provide improved efficiency and
capacity in removing
contaminants from gas streams.
Accordingly, the present invention seeks to provide an improved solid
filtration
media for removing undesirable compounds from an air stream.
Further the present invention seeks to provide a solid filtration media having
a
high efficiency in removing unwanted compounds from an air stream flowing over
or through
the media.
CA 02207689 2001-12-03
12
In addition to other aspects noted previously, the invention in one broad
aspect
provides a method of treating a contaminated fluid stream containing a
contaminant, comprising
the step of contacting the contaminated fluid stream with a solid filtration
composition such that
the contaminant is removed from the contaminated fluid stream, wherein the
solid filtration
composition comprises at least 7% potassium permanganate by weight of the
composition,
between 20 and 25% water by weight of the composition, sodium bicarbonate and
activated
alumina.
Another broad aspect of the invention provides an improved solid filtration
composition comprising at least approximately 5% potassium permanganate salt,
at least
approximately 10% water, sodium bicarbonate and a porous substrate, wherein
the above
percentages are by weight of the composition.
These and other aspects, features and advantages of the present invention will
become apparent after a review of the following detailed description of the
disclosed
embodiments and the appended claims.
Brief Description of the Drawings
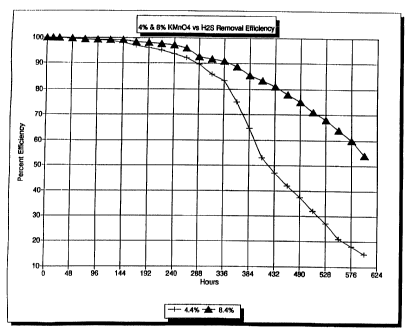
Fig. 1 is a graph representing the efficiency of removal of hydrogen sulfide
by
media comprising either 4.4% or 8.4% potassium permanganate, as described more
fully in
Example 7.
Detailed Description of the Invention
The present invention relates to an improved solid filtration media, method of
preparing the same and method of treating a fluid stream with the solid
filtration media. The
CA 02207689 1997-06-13
WO 95/16518
PCT/US94/14316
13
improved solid filtration media comprises permanganate, water,
and a substrate, such as activated alumina. Sodium bicarbonate
may also be added. The media c~nta_in~ ~~an;fi~antlcr h;.rha.. te.,m..
- ----- - ---- ...abaaurvuaW ~ Lll~ll~rl 1G V GL.7
of permanganate and water than thought possible in the prior art.
s An improved efficiency of removal of compounds is achieved by
this media. For example, a preferred embodiment of this
improved media has a hydrogen sulfide capacity of greater than
approximately 15%, whereas the media produced by the current
art has a maximum hydrogen sulfide capacity of approximately 9
io 10%.
Generally described, the present invention provides
an improved filtration media comprising a substrate impregnated
with high levels of both permanganate, and water. The filtration
media comprises at least approximately 5% permanganate salt,
is and at least 10% water, by weight of the composition. The
permanganate salt may be selected from the group consisting of,
but not limited to, potassium permanganate ("KMn04"), sodium
permanganate, magnesium permanganate, calcium permanganate,
_ barium permanganate, and lithium permanganate. Preferably, the
2o permanganate salt is potassium permanganate (Aldrich,
Milwaukee, WL). The concentration of the potassium
permanganate is between approximately 5 and 12%, and
preferably between 7 and 12%.
The porous substrate may be selected from the group
2s consisting of, but not limited to, activated alumina ("A1203")
(LaRoche Chemical, Baton Rouge, LA.), silica gels (J.M. Huber,
Chemical Division, Havre De Grace, MD.), zeolites (Steel Head
Specialty Minerals, Spokane, WA.), kaolin (Englehard Corp.,
Edison, N.J.), adsorbent clays (Englehard Corp., Edison, N.J.),
3o and activated bauxite. A preferred porous substrate is alumina.
Preferably, the concentration of substrate in the filtration media
_ is between 40 and 80%, and most preferably it is between 60 and
75% where the media does not contain sodium bicarbonate, and it
is preferably between.40 and 60% where the media contains
3s sodium bicarbonate.
CA 02207689 1997-06-13
> > .-
.. . ., , .
.. , ,.,~t T ,
. ~ ' , , ~. T 1 S
~ t t , T t -v ,
n A ~ . ~ 1
, -,
' , , , , , ~ ~ ,
v n .
I4
The concentration of water in the filtration media is
between approximately io and 35$,preferably between 20 and
25 %. One of ordinary skill in the ~ art will understand that the
concentration of free water in the filtration media may be altered
s by the conditions present, such as the humidity and the
temperature, during its storage and use.
- Preferably, the improved solid filtration media of the
present invention comprises between approximately s and 12%
potassium permanganate, between approximately io and 35$
. - 1o water, and between approximately 40 and 80% alumina, by
weight of the composition. Most preferably, the improved solid
filtration media comprises between approximately 7 and 12%
potassium permanganate, between approximately 20 and 25%
water, and between approximately 60 and 75% alumina, by
is weight.
In another embodiment of the present invention, the
composition further comprises sodium bicarbonate ("NaHC03")
(Rhone-Poulenc, Chicago Heights,1L.), wherein the concentration
. of sodium bicarbonate is between approximately 0 to 30%, . and
preferably is between 15 to 20%, by weight. The sodium
bicarbonate enhances the retention of water in the filtration
media. In the embodiment where the filtration media comprises
sodium bicarbonate,' the preferred concentration of alumina is
between approximately 40 and 60%.
2s It is to be understood that when referring to the
relative weight of components, the water referred to in the
present specification, examples, and tables is defined as the free
water, and does not include the bound water in the substrate.
Free water is driven off by an oven at approximately 93.3°C
30 (200°F), but if left in the substrate it is available for the
oxidation
reaction. In contrast, bound water is not driven out or
evaporated except by a kiln at 982.2° to 1093°C (I800 to
2000°F),
and the bound water functions by holding the substrate together.
Bound water is not available for reaction with the undesirable
3s contaminants.
. _.~._-_,_ _.. ~. __....__ ..... . . _._: . ,. ... CA 02207689 1997-06- 13 _
:,
=~ - ~ . ,
.~ 1 '~ , . .. S _ , ~ 1 , 1 ,
' , ~ v 1 7 1 1 1 1 7 1 1
n 1 , , . 7
' : 1 1 , 1 ] , 1 , , 1 . 7 1 S '
It is also to be understood that the term
permanganate in the present specification, examples, and tables
represents the permanganate salt, not the permanganate ion
(Mn~4-). Therefore, the percent ranges of permanganates in
s compositions in the present specification denote the percent of the
permanganate salt in the composition, not the percent of the
permanganate ion in the composition.
Terms such as filtration media, adsorbent
composition, and impregnated substrate are all interchangeable,
io and denote a substance which is capable of reducing or
eliminating the presence of unwanted contaminants in fluid
streams by the contact of such a substance with the fluid stream.
It is to be understood that the term fluid is defined as a liquid or
gas capable of flowing, and includes gaseous, aqueous, organic
is containing, and inorganic containing fluids.
The present invention also provides a method of
preparing an improved solid filtration composition. This method
comprises the steps of mixing water, a permanganate salt, and a
substrate, and then forming the mixture into at least one cohesive
porous unit. The unit is then cured at a temperature of from
about 37.8°C (100°F) to about 93.3°C (200°F),
until the
concentration of water is at least about 10% by weight of the
composition, and the concentration of the permanganate salt is at
least about 5% by weight of the composition. The size and shape
zs- of the cohesive porous unit is not critical to the present invention.
Any size and shape of a porous unit known in the art to reduce or
eliminate undesirable contaminants from fluid streams when in
contact with the unit may be used in the present invention.
Preferably, the porous unit is a nominal .3 cm. (1/8") diameter
so round pellet.
The method of the present invention preferably
comprises forming an aqueous solution comprising the
permanganate salt and then mixing the aqueous solution with the
porous substrate. To dissolve and maintain the permanganate salt
ss in solution, the aqueous solution should be heated to
::.:a....~_>:_~._..,.:.__._:.._.w____.--:- .__ .._ . CA 02207689 1997-06-13 --
~ - . _ .
-, , , n , . - 1> a, ,a,~
- . : , . ., , ., ~ ,
" :,
__. , . ", ., . , ~ , ~ - ~ ,
- , . ' ~ . , ,
., , - ., , . . . ,
16
approximately 71.1° to 93.3°C (160° to 200°F), and
preferably to
between approximately 82.2° to 87.8°C (I80° to
190°F~.
In another embodiment of the invention, the method
of the present invention comprises forming a dry mixture
s comprising the permanganate salt and the substrate, and then
adding water to the dry mixture. In yet another embodiment of
the invention, the method of the present invention comprises
forming a dry mixture comprising the permanganate salt and the
substrate, forming an aqueous solution comprising the
to permanganate salt, and then mixing the aqueous solution with the
dry mixture. Optionally, sodium bicarbonate may be added
either to the dry mixture, to the water, or to both in the above
methods of preparing the filtration media.
Preferably, in the method of the present invention,
is the unit formed is cured until the concentration of water is from
about 10 to about 30%, and most preferably between 20 and 25%,
by weight of the composition, and the concentration of the
permanganate salt is from about 5 to about 12% by weight of the
composition, and most preferably between 7 and 12%. Also,
2o where sodium bicarbonate has been added to the composition, the
unit formed is preferably cured until the concentration of sodium
bicarbonate is less than approximately 30%, and preferably
between IS to 20% by weight. .
In another embodiment of the present invention, the
2s media may be cured at approximately 54.4° to 60°C
(130° to
140°F). The presence of sodium bicarbonate allows for a lower
curing temperature such as 54.4° to 60°C (130° to
140°F), in
contrast to the prior art curing temperature of about 93.3°C
(200°F).
so The impregnation treatment of the activated starting
material in accordance with the present invention has not been
found to be critical with respect to the particular sequence in
- which the dry mix is impregnated with moisture and impregnates.
The above combinations may be mixed in any manner which
3s effectively produces the desired filtration media. Impregnation
CA 02207689 1999-11-10
17
may be carried out simply by immersing and soaking the solid combination in a
volume of
impregnate solution. Also, the impregnate solution may be passed through the
combination
rather than being used as a static immersion treatment. However, it has been
found that a
preferred method of impregnation is spray addition in which an impregnate
solution is sprayed
onto a dry combination being tumbled in a mixer. This method of impregnation
has been
described in U.S. Patent No. 3,226,332, which may be referred to for further
details. Other
methods of impregnating the combinations would suggest themselves as equally
appropriate
and these are included within the scope of the present invention.
In one embodiment utilizing the above spray addition method, the aqueous
impregnate solution of potassium permanganate is sprayed onto a dry
combination of sodium
bicarbonate and a substrate, such as activated alumina. For example, the dry
combination
would contain between approximately 80 to 85% activated alumina and between
approximately
to 20% of sodium bicarbonate.
The concentration of the potassium permanganate may vary in the solution to be
15 sprayed onto the dry combination. For example, to produce a solid
filtration medium
containing approximately 7% potassium permanganate, an aqueous solution
containing
approximately 20% of potassium permanganate, at between approximately
71.1° to 93.3°C
(160°F to 200°F) and preferably at between 82.2° to
87.8°C (180° to 190°F), should be
sprayed on the dry combination being tumbled in a mixer. Also,
to produce a solid filtration medium containing approximately 10% potassium
permanganate, a solution of approximately 25% potassium permanganate at
between
approximately 71.1° to 93.3°C (160°F to 200°F) and
preferably at about 82.2° to
87.8°C (180°F to 190°F) should be sprayed on the dry
combination being tumbled in
a mixer. Any concentration of permanganate in the aqueous solution which is
effective to yield
the composition of the present invention may be used. Further, where the
permanganate
.,._... .. ... _ . _ _. .. _ . _. CA 02207689 1997-06-13: _
_ _ _' ~ ,
~ . '1 . 1 > ~ 11' ' 1 9 ~ 1 1 ?
!~ ~ 1 1 1
1 '7 '. t
~ ' D 1 1 7
_ 1
1 1 ~ ~ 7 1 ~ ~_ . . 1
- . ~ .. v a ~ ~ ' _ 7 5 ~ ~ . . r
18
. salt is either in the dry feed mixture or in both the aqueous
solution and the dry feed mixture, any concentration of
permanganate in the dry mixture and/or the aqueous solution
which is effective to produce the composition of the present
s invention may be used. For example, the media may be used to
fill perforated modules to be inserted into air ducts in a manner
known in the art.
Yet another aspect of the present invention is a
method of treating a contaminated fluid stream with the improved
io solid filtration composition of the present invention. This method
comprises contacting the contaminated fluid stream with the
. above improved solid filtration compositions. Methods of
treating gaseous or other fluid streams are well known in the art.
As the method of treating fluid streams is not critical to the
is present invention, any method known in the art of treating fluid
streams with the media of the present invention may be used.
- The composition of the present invention is employed
to remove undesired compounds from gas streams. The
concentration of these undesirable compounds in the gas streams
2o is not considered critical -to the process of the present invention,
nor is the physical and chemical makeup of the gas stream from .
vt~hich it is desired to remove undesirable compositions considered
critical. Even concentrations of these undesirable compounds in
gas streams resulting in levels lower than one ppb of the
2s compounds passing through a solid filtration media bed per
minute can be removed.
However, it has been found that flow rates of the gas
stream being contacted wrath the bed of filtration media affect the
breakthrough capacities of the media. The preferred flow rate is
3o between 3 and 228.6 m./min. (10 and 750 ft./min.), and most
preferably is between 18.3 and 30.5 m./min. (60 and 100 ft/min.),
flowing perpendicularly to the face of the bed.
Also, it may be important that oxidizing conditions
prevail, but it is not known to what extent oxidation may affect
3s the purification achieved by the present invention. Typically, the
CA 02207689 1997-06-13
s~
~ ~ -_ ~ ~ 1 ] J 1 1
> > 7 7 J .) )
. ~ t ~ y ~ ~ 1 ~ ] 1 )
_.. ~t .) 1 1 7 1 n
i 1 , a
t i ~ ' ~ ) 7 Z .1
1~ .y s v . ~ ) 'f ) ~
19
. undesired compositions will be removed from air, especially
from air admixed with effluent gas streams resulting from
municipal waste treatment facilities, paper mills, petrochemical
refining plants, morgues, hospitals, anatomy laboratories, and
s hotel facilities, and so forth. The oxidizing conditions which may
be important are generally that oxygen preferably be present in
the gas stream being treated, at least in small amounts. This
oxygen content is readily found in the gas stream, if air comprises
a sufficient portion of the gas stream being treated. If oxygen is
to totally absent or present in insufficient amounts, oxygen may be
independently introduced into the gas stream being treated. A
number of factors affect the amount of oxygen which may be
required for maximum removal of the contaminants in a gas
stream in accordance with the present invention, including the
is concentration and absolute amount of compounds being removed
from the gas stream being treated.
With respect to the amount of compound removed, it
is believed that the following factors affect the process: the basic
degree of attraction of the activated substrate for the compound;
2o the pore structure and size of the substrate; the specific surface
area of the substrate; the surface characteristics of the substrate;
the amount of permanganate present; and the amount of water
. . present. '
The filtration media of the present invention is
2s appropriately used alone in filter beds for the removal of
undesirable compounds. It is also appropriate, however, to use
the composition of the present invention in conjunction with filter
beds containing other filtration media, and also in conjunction
with mechanical or electrostatic filters. Any such additional
3o filters may be placed either upstream (before the media of the
present invention with respect to the effluent gas being treated) or
downstream.
The above invention significantly increases the
efficiency and capacity of impregnated porous substrates
ss (filtration media) to remove certain undesired compounds from
CA 02207689 1997-06-13
. o , , , ,,
:, -., ;:~ , , , o ,
s . , , , w , _~ .~ r, ,
.o _ ~ n o " , ~ , .
,
a o o
_ ~ ~ . ~ , , , , . . , v a , , , . ,
gaseous streams over the capacity of impregnated porous
substrates of the prior art. Therefore, the lifetime of a specific
quantity of the improved filtration media will be much longer
than the same quantity of the currently available filtration media.
s The extension of the lifetime of the filtration products will
significantly reduce consumers' and businesses' purchasing,
servicing, and installation costs. Also, the enhanced efficiency of
the improved media allows for a new line of products which are
compact versions of currently available units, but have the same
to performance as the larger, currently available units. The
capability of creating significantly smaller filtration units is useful
for providing efficacious air filtration in space-limited quarters
which previously could not utilize the larger, currently available
units.
is Also, the improved permanganate media is less
expensive than other filtration media having a roughly equivalent
capacity. For example, the media of the present invention has an
equivalent capacity as activated carbon adsorbents. However, the
media of the present invention is considerably less expensive than
ao activated carbon adsorbents.
Further, the filtration media of the present invention
is safe as it is not flammable, in contrast to carbon containing
filtration products.- This aspect of the present invention is
significant to industries which manufacture or process flammable,
2s fume producing materials, such as the petroleum industry for
example. '
In media of the present invention, the increase in the
concentration of the potassium permanganate and water in the
solid filtration media has greatly increased the media's capacity
3o for removing contaminants from air streams. As described
earlier, the potassium permanganate impregnated alumina media
of the prior art has a capacity for the removal of hydrogen
sulfide of approximately 9-10%. In contrast, a composition of
the present invention containing approximately 53% activated
3s alumina, 23% water, 7% potassium permanganate, and 17%
_.
RCV.VON:EF'A-bIUE.NCHEN 05 :23- 2-~A 02207689 1997-06-13~~5 & ASKE1Y-. +.1-9
80 ~?35944f5:# 6
21
sodium bicarbonate, has a hydrogen sulfide capacity of
approximately ~ 5 to 170.
The rest~Its of accelerated efficacy tests comparing
the capacity of solid filtration media of the prior art and the solid
s filtration media described in this invention are fully discussed in
Examples S and ~. For example, a prior art media which
contained approximately 3.5-4,096 potassium. permanganate, arid
IO-I~9~b water, had a hydrogen sulfide capacity of approximately
Via- In contxast, the solid filtration media of the present
invention, which contained approximately 7~ potassium
permanganate, and about 22.5?9~ water, had a hydrogen sulfide
capacity of 15.9%. The effficacy tests were performed by
challenging a known quantity of the selected solid filtration rrledia
with I.0% hydrogen sulfndc gas at a constant flaw rate and
is monitoring the concentration of hydrogen sulfide in the gas
stream exciting the solid filtration media. The accelerated efficacy
test is fully described in Example 4.
The results of a non-accelerated test for the
determination of performance characteristics such as removal
~o efficiency and capacity for removal of various g~s_phase air
filtration media are discussed in; Example 8, and are graphically
illustrated in Figure I . The efficiency tests were performed on a
solid filtration media of the prior art, and on a solid filtration
media of the present invention. The media of the prior art
comprised 4.49'o potassium permanganate, 19.996 water, 17~v
sodium bicarbonate, and 58.7 °~o alununa. The media of the
present invention comprised 8.4% potassium permanganate,
24.4°w water, l79la sodium bicarbonate, and 50.2gb aluniina. pig.
1 illustrates the eff 'ciency of hydrogen sulfide removal of each of
so the media over an cxtGnded period of rime. As Can be seen from
Fig. 1, the media of the present invention has a percent efficiency
of hydrogen sulfide removal of 96°k at 260 hours, ?g°~ at 45G
hours, and 549ro at 6t)0 houxs. Ln catttrast, the media of the prior
art has a hydrogen sulfide removal efficiency of approximately
_ < . . -s .. _'_' . . ._
~~,~~t~DED SHEET
92% at 260 hours, 42% at 456 hours, and 15% at 600 hours. The
method of the efficiency test is fully described in Example 7.
Although the applicant does not know the precise
mechanisms by which the improved media operates, and is not
s bound by the following theory, it is believed that the oxidation
reactions between the permanganate and the undesirable
contaminants occur primarily near the surface of the filtration
media, rather than deep within its pores. Therefore it is critical
that the surface's oxidative capabilities be continually regenerated.
io It is believed that the oxidative capability of the surface of the
media is regenerated by the flow or migration of permanganate
from the center of the media to the surface of the media while the
products of the oxidation reactions flow or migrate from the
surface of the media to the center of the media. It is also believed
is that the higher the concentration of permanganate at the surface
of the media, the higher the capacity and efficiency of the media.
Further, the fluidity of the permanganate solution directly affects
the flow and thus the quantity of the permanganate reaching the
surface of the media. Therefore, it is critical to maintain an
2o elevated concentration of free water in the media so that the
permangante solution maintains a high level of fluidity and
readily flows to the surface of the media thereby maximizing the
efficiency and capacity of the media. A liquid path thus should be
established between the interior of the pores and the surface of
2s the media. This is contrary to prior art theory which teaches a
need for penetration of the gaseous contaminants into the pores of
the substrate.
The above theory explains why the capacity and
efficiency of the filtration media of the prior art could not
so surpass the capacity and efficiency obtained at the potassium
permanganate concentrations of 4-5%. As stated above, in the
prior art various attempts were made to impregnate the media
with higher quantities of potassium permanganate. However, the
majority of the free water has always been removed from the
3s prior art media. As stated above, the efficiency and capacity of
CA 02207689 1997-06-13 ,; ' _ " ~~w-'
_ . ,
' , 5 -,
, ,~ ~ "; ,., _.
\ 5 . .5 . \ . , , n . . ~ . .
23
the highly impregnated media of the prior art remains constant or
decreases relative to the capacity achieved by media impregnated
with 4-5% permanganate. There are three reasons for the failure
of the highly impregnated media of the prior art to obtain higher
s results. First, the high concentration of permanganate and the
low concentration of water causes the permanganate to crystallize
and clog the pores of the substrate thereby blocking the flow of
permanganate to the surface of the media. Second, the
crystallized permanganate remains in the center of the media and
to therefore cannot move to the surface of the media to oxidize
contaminants. Third, it is difficult for any permanganate that
may be in solution to move to the surface of the media as the
permanganate solution is very concentrated and has a low level of
fluidity. It is for these reasons that maintaining an elevated level
is of water in the media is believed to be critical for the improved
filtration media of the present invention.
The following examples will serve better to illustrate
the composition, the treatment methods of the present invention,
and the capacity for the removal of contaminants in gas streams
2o produced thereby. ' It should be noted that the continuous flow
systems described in several of the following examples all were
operated at a relative humidity of 40-50%.
~XAMpI,E 1
as A 7% potassium permanganate impregnated alumina
composition is prepared as follows:
A dried feed mix is prepared by combining, by
weight, 80 to 85% alumina, and IS to 20% sodium bicarbonate.
The dry feed mixture is sprayed with a heated aqueous potassium
so permanganate solution at 82.2° to 87.8°C (180 to
190°F) while the
dried feed mix is being tumbled in a tumble mill. The resulting
pellets are then dried at 54.4° to 60°C (130 to 140°F),
until the
pellets contain about 20 to 25% free water.
To prepare solid filtration media containing
3s approximately 7% potassium permanganate, by dry weight, the
CA 02207689 1999-11-10
24
aqueous potassium permanganate solution should preferably contain
approximately 20%
potassium permanganate by weight. It is to be understood that the aqueous
potassium
permanganate solution is sprayed on to the dry feed mix while the dry mix is
rolled in the
pelletizing disk as described in U.S. Patent No. 3,226,332, which may be
referred to for further
details.
EXAMPLE 2
A 10% potassium permanganate impregnated alumina composition is prepared
as follows:
A dried feed mix is prepared by combining, by weight, 80 to 85% alumina and
15 to 20% sodium bicarbonate. The dry feed mixture is sprayed with a heated
aqueous
potassium permanganate solution at 82.2° to 87.8°C (180 to
190°F) while the dried feed mix
is being tumbled in a tumble mill. The resulting pellets are then dried at
54.4° to 60°C (130
to 140°F) in air, until the pellets contain about 20 to 25% free water.
To prepare a solid filtration media containing approximately 10% potassium
permanganate, by dry weight, the aqueous solution should preferably contain
approximately
25% potassium permanganate, by weight. It is to be understood that the aqueous
potassium
permanganate solution is sprayed on to the dry feed mix while the dry mix is
rolled in the
pelletizing disk as described in U.S. Patent No. 3,226,332, which may be
referred to for further
details.
EXAMPLE 3
Compositions of Permanganate Impregnated Substrates
Using the method described in Example 1 or Example 2, the following
compositions, by dry weight, may also be prepared:
CA 02207689 1997-06-13
WO 95/16518 PCT/US94/14316
a
TAELE I: Composition of Solid Filtration Media
s (Substrate % Substrate %NaHCO~ %~~0 %KMn04
Alumina 5 0 18 2 0 12
Alumina 5 0 2 0 2 0 10
Alumina 5 0 2 0 2 2 8
1o Alumina 6 5 8 15 12
Alumina 7 5 0 15 10
Silica gel 5 0 18 2 2 10
Zeolite 5 5 10 2 3 12
Adsorbent Clay5 5 13 2 0 12
15
The dry deed mix is prepared by mixing appropriate
amounts of the substrate and the sodium bicarbonate together.
The dry feed mix is mixed in a tumbling mill with an appropriate
- 2o amount of an aqueous potassium permanganate solution sprayed
onto the dry feed mix while tumbling, in the manner described in
U.S. Patent No. 3,226,332. Curing is as in Example 1 or 2. The
cured pellets are a strong, non-dusting filter media suitable for
placement in filter beds. They provide an efficient media for the
zs reduction or elimination of many undesirable compounds.
EXAMPLE 4
Standard Accelerated Test Method for Capacity
Determination of Gas-Phase Air Filtration Media.
3o The following is an accelerated test method to be
used for determining the capacity of removal of various gas-phase
air filtration media when subjected to a flowing gas stream
containing high levels of contaminant(s). Low-level challenge
testing of gas-phase air filtration media, whether full-scale or
ss small-scale, usually takes long periods of time to obtain the
desired results. The following method provides an accelerated
-CA 02207689 1997-06-13
_. , . ,
' ' , , a
' ~ , W s s ., , > > a s .
... .', ' ~.- .
. -" " . _ ..
26
test for determining the removal capacities of various media by
exposing them to high levels of contaminants.
The method is briefly summarized as follows: a
known volume of media is placed in an adsorption tube and
s exposed to a known concentration (usually 1 % # vol./vol.) of
contaminant gases) in a tempered, humidified, clean air system.
The gas stream is calibrated to deliver a total flow rate of 1450 ~
20 ml/min. The removal capacity is calculated as the amount (in
grams) of contaminant removed from the air stream per volume
to (cubic centimeters) of media at a SO parts per million ("ppm")
breakthrough.
More specifically; the air utilized must be tempered,
humidified, clean, oil-free, and compressed. Accordingly, the air
must be passed through a bed of activated carbon followed by a
~s filter bed containing potassium permanganate impregnated
alumina pellets. Each filter bed should contain at least 300 ml.
(18.3 cu. in.) of media for each Iiter per minute or 991.1 ccm.
(0.035 cfm.) of air flow. The media in each filter bed should be
changed before each test.
2o Media samples are preferably obtained from
unopened original manufacturer's shipping or storage containers
chosen at randorrl whenever possible. The entire container,
whenever possible or practical, should be sampled by taking small
amounts of media from throughout the container and combining
' 2s them into one larger sample. The sample should be thoroughly
rriixed before being analyzed. Guidance on sampling may be
obtained from ASTM Standard E300, entitled Recommended
Practice for S_amnlina Industrial Chemicals. If a test is to be run
comparing media of the same size or different sizes, the sample
so collected may be screened through the appropriate sieves to sort
the media by size.
Using an appropriate sampling method, obtain a .
representative sample of media (approximately 400 grams should
be sufficient) and determine its apparent density as per ASTM
3s 2854, or an equivalent method. Obtain an adsorption tube which
CA 02207689 1997-06-13
WO 95/16518 PCTIUS94114316
27
is a cylindrical tube where glass wool and/or beads are optionally
placed below the media, and the media and optional glass wool or
beads are supported by stainless steel mesh, a perforated slotted
glass disc, or a perforated slotted ceramic disc positioned below
s the media and glass wool or beads. After the adsorption tube
having the glass wool or glass beads has been calibrated for the
volume of a known depth of media, weigh the adsorption tube to
the nearest I.0 mg. Fill the adsorption tube to the desired depth
via alternately filling and gently tamping the tube to eliminate any
io dead space until the desired depth is reached. Weigh the filled
adsorption tube to the nearest 1.0 mg.
The filled media tube is arranged such that a mixture
of air and contaminated gas enters the bottom of the tube, flows
through the glass wool or beads, flows through the filtration
is media, and is then analyzed by a gas analyzer. Leaks in the gas
system should be checked for and eliminated before beginning the
analysis of the sample. Rotameters, analyzers, recorders, etc.
should be calibrated over appropriate ranges according to the
. manufacturer's instructions or other standard methods such as
2o ASTM Standard D3195, before any media is introduced into the
system. Also, air and gas flow requirements should be
determined and checked against supply capabilities to assure
proper air and gas flows to the system.
Once the adsorption tube is. in position, start the flow
2s of the mixture of contaminated gas and air and record the time,
or time the test using a stop watch. Continue the flow of the
mixture of gas and air until a breakthrough of 50 ppm is
observed or indicated by the gas analyzer. Record the time at
breakthrough. It is preferable to use a gas analyzer capable of
3o variable scale readouts to 0 ppm (~5 ppm), having specific or
multiple gas capabilities.
The data obtained from the above analysis will yield
the gas capacity of the media tested using the following equation:
CA 02207689 1997-06-13
WO 95/16s18 PCT/US94114316
a ,
28
GAS CAPACITY (GM/CC) = K x 1 =~ F t ~
V
where:
s K = 1.52 for H2S, 2.86 for S02, 3.17 for C12, 2.15 for CH3SH,
0.76 for NH3, 2.05 for N02, and 1.39 for NO.
C = Concentration of challenge gas in airstream, Volume %.
F = otal stream flow rate, cc/min.
tb = Time to 50 ppm breakthrough, minutes.
to V = Volume of the adsorption tube media column, cc.
EXAMPLE 5
Capacity of Potassium Permanganate Impregnated
Alumina Pellets.
Ls- The results of efficacy tests comparing the capacity
of solid filtration media of the present invention are summarized
in Table II below. The efficacy tests were performed by
challenging a known quantity of the selected solid filtration media
with 1.0% hydrogen sulfide gas at a constant flow rate and
2o monitoring the concentration of hydrogen sulfide in the gas
stream exiting the solid filtration media as described in Example
4.
Table II summarizes the content and the capacity of
various permanganate containing, solid filtration media. Lot
2s 01083-8 is currently available solid filtration media. Lots 01183-
8 and 01223-8 are the improved solid filtration media of the
present invention.
TABLE II: %KMn04 vs. H .S CAPACITY
ILOT # Crush Abrasion 9~N~ ~~Ca~racitvl
01083-8 4.40 50.7 2.8 Is-20 19.s8 9.68
01183-8 s.43 s1.9 1.9 1s-20 24.48 11.06
01223-8 7.0 51.8 1.4 IS-20 22.s7 1s.90
CA 02207689 1997-06-13
WO 95/16518 PCTILTS94/14316
29
EXAMPLE 6
Capacity of Potassium Permanganate Impregnated
Alumina Pellets.
s Table III summarizes the content and the capacity of
solid filtration media produced by several different
manufacturers. Carus Chemical manufactured Lot 252 which
contains no sodium bicarbonate, approximately 3.5 to 4%
KMn04, and about 10 to 15% H20. Unisorb manufactured Mark
io II which contains no sodium bicarbonate, approximately 3.5 to
4% KMn04, and about 10 to 15% H20. The two media
manufactured by Purafil are the improved solid filtration media
of the present invention. The two Purafil media contain
approximately 5.4% KMn04 and 7.0% KMnOq., respectively.
TABLP III: COMPETIT~ MErna aNALY~I~ O~F
4_~2S CAPACITY
' % Mn04 % NaHCOY %0Q ~S CAPACITY
Carus
Chemical
Lot 252 3.5 - 4 0 10-15 7. 5
'
Unisorb
Mark 3.S _ d ~ - iv-i~ a.0~
Tf
5.4% 5.4 15-20 20-25 10.93
Purafil
7.0% 7.0 IS-20 20-25 13.44
CA 02207689 1997-06-13 ,
a
~, >,:.:
r . , , .. ,
a ~ i , n . ~ ~
~ . ~ ~
~ ~~
. , , , ~ , ~ ' '
EXAMP E 7
Standard Test Method For Determination Of Performance
Characteristics Of Gas-Phase Air Filtration Media.
The following is a method of determining the
s . Pe~onnance characteristics (removal efficiency and capacity for
removal) of various gas-phase air filtration media when subjected
to flowing gas streams of known concentrations and velocities.
This test provides a basis for comparing the characteristics of
different media.
to The method is briefly summarized as follows: a
weighed media sample is placed in a glass tube and exposed to a
known concentration of contaminant gases) in a tempered,
humidified, clean air stream. The inlet and outlet gas
concentrations are measured and recorded for use in determining
is media efficiency and capacnty. The tests can be run until a media
is exhausted or to a predetermined removal efficiency or percent
capacity. All parameters are held constant, and all samples are
run simultaneously (in parallel) until the test is completed.
Operating parameters may be altered between tests to create
2o simulated field conditions.
- More specifically, the air utilized must be tempered,
humid~ed, clean, oiI-free, and compressed. Accordingly, the air
must be -passed through a bed of activated carbon followed by a
filter bed containing , potassium permanganate impregnated
2s alumina pellets. Each filter bed should contain at least 300 ml.
(18.3 cu. in.) of media for each liter per minute or 991.1 ccm
(0.035 cfm.) of air flow. The media in each filter bed should be
changed before each test.
Media samples are preferably obtained from
3o unopened original manufacturer's shipping or storage containers
chosen at random if possible. The entire container, whenever
possible or practical, should be sampled by taking small amounts
of media from throughout the container and combining them into
one larger sample. Also, the sample should be thoroughly mixed
ss before being analyzed. Guidance on sampling may be obtained
CA 02207689 1997-06-13
WO 95!16518
PCT/LTS94l14316
31
from ASTM Standard E300- entitled Recommended Practice For
Samnlin Industrial C'hPr";~als. If a test is to be run comparing
media of the same size or different sizes, the sample collected
may be screened through the appropriate sieves to sort the media
s by size.
i
Using an appropriate sampling method, obtain a
representative sample of media (approximately 400 grams should
be sufficient) and determine its apparent density as per ASTM
Standard 2854, or an equivalent method. Obtain an adsorption
io tube which is a cylindrical tube having glass wool and/or beads
optionally placed below the media, and the media and optional
glass wool or beads being supported by stainless steel mesh, a
perforated slotted glass disc, or a perforated slotted ceramic disc
positioned below the media and glass wool or beads. After the
is adsorption tube having the glass wool or glass beads has been
calibrated for the volume of a known depth of media, weight the
adsorption tube to the nearest I.0 mg. Fill the adsorption tube to
the desired depth via alternately filling and gently tamping the
_ tube. to eliminate any dead space until the desired depth is
Zo reached. Weigh the filled adsorption tube to the nearest 1.0 mg.
In the alternative, the amount of media placed in the
tubes may be determined by filling the tube to the desired level of
media while taming gently to eliminate any dead space,
removing the media from the tubes, weighing the media, and
2s finally returning the media to the tube. This alternative method
eliminates the need to determine the density of the media as
required when filling the tube volumetrically.
The filled media tubes are then arranged in parallel
such that the gas-air mixture will simultaneously flow through all
so of the media tubes. Also placed in parallel with the filled media
- tubes is one empty tube for challenging gas concentration, relative
humidity, and temperature monitoring.
Leaks in the gas system should be checked for and
eliminated before beginning the analysis of the media samples.
3s Also, rotameters, analyzers, recorders, etc. should be calibrated
CA 02207689 1997-06-13
WO 95/16518 PCTIUS94/i4316
32
over appropriate ranges according to the manufacturer's
instructions or other standard methods such as ASTM Standard
D3195, before any media is introduced into the system. Air and
gas flow requirements should be determined and checked against
s supply capabilities to assure proper air and gas flows to the
system.
With the air/gas lines disconnected from the media
tubes, turn on the air and adjust the rotameters and the flow
meters to give the desired flow rates. Next, turn on the challenge
to gas and set the delivery pressure to approximately five pounds
above the system air pressure. Adjust to the desired
concentration of the contaminant gas in the air stream by
increasing or decreasing the flow through the rotameter/flow
meter and verify the concentration with the appropriate analyzer.
is If a multiple gas challenge is being used, each gas challenge
concentration should be set separately before combining the flows
and the challenge tube. Interferences, both in the gas stream and
on the analyzers, caused by the combining of multiple gases
- should be determined beforehand. The sample selector of the gas
2o analyzer may optionally be set for sampling at predetermined
intervals and durations.
The air/gas lines are then connected to the media
tubes. Purified and humidified air is then mixed with the
predetermined quantity of contaminant gas, and then the mixture
2s passes through the tubes and is then analyzed by a gas analyzer.
The filled media tubes are arranged such that the mixture of air
and contaminated gas enters the bottom of the tube, flows through
the glass wool or beads, flows through the filtration media, and is
then analyzed by a gas analyzer.
3o At predetermined sampling intervals, record the , .
outlet gas concentrations starting with the first media tube and
ending with the challenge tube for each set of readings.- Allow
the analyzers to equilibrate between each tube and to zero after
the challenge tube. After the initial set of readings (or when
3s outlet gas concentrations are detected), adjust the media tube
CA 02207689 1997-06-13
WO 95/16518
PCT/US94114316
33
sampling order to start with the tube showing the 'lowest outlet
concentration and move upward to the challenge tube. Each time
allow for equilibration between media tubes and zeroing after the
challenge tube.
If gas cylinders need to be changed before the test is
complete, first disconnect the air/gas lines to the media tubes.
Change the cylinders and adjust the challenge concentration as
outlined above. Note the time for placement and continue the test
to completion. Check and record the temperature and relative
to humidity in the challenge tube daily. Adjust as necessary.
Obtain the time and outlet gas concentration data, and
calculate the removal efficiency and capacity for removal
according to the following equations:
is REMOVAL EFFICIENCY f%) _ ((Clt - C2t) x 100)/Clt
where C 1 t = challenge gas concentration (in ppm) at time "t."
C2t = outlet gas concentration (in ppm) at time "t."
- CAPACITY FOR REMOVAL f WEIGHT %) _
2o n
x~F~ x MW x V x f60 min/hr)
106 x (24,450 mUmole) x W
t=1
where: n = hours since test start (for cumulative weight %)
Zs or hours to test completion (for total weight %).
Clt = challenge gas concentration (in ppm) at time "t."
Et = removal efficiency at time "t."
MW = molecular weight of challenge gas.
V = airflow rate (in ml/min).
3o W = media sample weight (in gm).
EXAMPLE 8
Efficiency of Potassium Permanganate Impregnated
Alumina Pellets.
3s The results of efficacy tests comparing the efficiency of
removal of hydrogen sulfide by solid filtration media of the prior
CA 02207689 1999-11-10
34
art and of the present invention are illustrated in Fig. 1. The efficiency
tests were performed
as described in Example 7, wherein solid filtration media comprising 4.4%
potassium
permanganate and solid filtration media containing 8.4% potassium permanganate
were
analyzed. The compositions of the analyzed media, by weight, are as follows:
% KMnO~ % H~O % NaHC03 % Alumina
8.4 24.4 17 50.2
4.4 19.9 17 58.7
Fig. 1 illustrates the efficiency of hydrogen sulfide removal of each of the
media
over an extended period of time. As can be seen from Fig. 1, the media
comprising 8.4%
potassium permanganate has a percent efficiency of hydrogen sulfide removal of
96% at 260
hours, 78% at 456 hours and 54% at 600 hours. In contrast, the solid
filtration media
comprising 4.4% potassium permanganate has a hydrogen sulfide removal
efficiency of
approximately 92% at 260 hours, 42% at 456 hours and 15% at 600 hours.
EXAMPLE 9
An alternative method of preparing potassium permanganate impregnated
alumina compositions prepared as follows:
A dry feed mix is prepared by combining, by weight, 75 to 85% alumina, 7 to
12% finely divided potassium permanganate and 10 to 20% sodium bicarbonate.
The dry feed
mixture is sprayed with water while the dried feed mix is being tumbled in a
tumble
mill. The resulting pellets are then dried at 54.4 to 60°C (130°
to 140°F), until the pellets
contain about 20 to 25% free water. It is to be understood that the dry feed
mix is rolled in
the pelletizing disc as described in U.S. Patent No. 3,226,332, while it is
being sprayed with
water. It is also to be understood that the dry feed mix may contain less
potassium
r.', -~::~:~:5;;~.~:~i::'.;'f_,._...'.,..~.y;~:_-~:,._;___._.~.-:.- _.-a_.. .
:...
CA 02207689 1997-06-13
~, ~s oo:_
. . . , . , ~ - ~ -
. , .. , , ' . - ., , " -.
"" ~ , ,
., .. _ ._: , , ~ _.,.'.::
' . ' . . 1 , 7 ;, ,
permanganate, and the water which is sprayed on the dry feed
mix may contain the corresponding amount of potassium
permanganate to yield the same final concentration of potassium
permanganate as when all of the potassium permanganate is in the
s dry feed mix. If such a method is utilized, the aqueous potassium
permanganate solution preferably is to be heated to approximately
82.2 to 87.8°C (180° to 190°F).
The invention having been fully described by the
above-detailed description and accompanying examples, other .
1o variations of, and uses for the present invention will become
apparent to one skilled in the art. All such variations and uses are
intended to be encompassed by the appended claims.