Note: Descriptions are shown in the official language in which they were submitted.
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
1
s
RECIRCULATING A PORTION OF
HIGH INTERNAL PHASE EMULSIONS
PREPARED IN A CONTINUOUS PROCESS
to
FIELD OF THE INVENTION
This application relates to an improvement in a continuous process for
making high internal phase emulsions that are typically polymerized to provide
microporous, open-celled polymeric foam materials capable of absorbing aqueous
is fluids, especially aqueous body fluids such as urine. This application
particularly
relates to a continuous process for making high internal phase emulsions where
a
portion of the prepared emulsion is recirculated to improve the uniformity of
formation of such emulsions.
BACKGROUND OF THE INVENTION
Zo Water-in-oil emulsions having a relatively high ratio of water phase to oil
phase are known in the art as High Internal Phase Emulsions (hereafter
referred to as
"HIDE" or HIPEs). HIPEs possess radically different properties from emulsions
of
the low or medium internal phase ratio types. Because of these radically
different
properties, HIPEs have been used in such various applications such as fuels,
oil
is exploration, agricultural sprays, textile printing, foods, household and
industrial
cleaning, transport of solids, fire extinguishers, and crowd control to name
just a
few. HIPEs of the water-in-oil emulsion type have found use in several areas
such as
cosmetics and drugs and in foods such as in dietary products, dressings, and
sauces.
Water-in-oil HIPEs have also been used in emulsion polymerization to provide
3o porous, polymeric foam-type materials. See, for example, U.S. Patent
3,988,508
(Lissant), issued October 26, 1976; U.S. Patent 5,149,720 (DesMarais et al),
issued
September 22, 1992, U.S. patent 5,260,345 (DesMarais et al), issued November
9,
1993; and U.S. patent 5,189,070 (Brownscombe et al), issued February 23, 1993.
The dispersed droplets present in HIPEs are deformed from the usual
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
2
spherical shape into polyhedral shapes and are locked in place. For this
reason,
HIPEs are sometimes referred to as "structured" systems and display unusual
rheological properties that are generally attributed to the existence of the
polyhedral
droplets. For example, when HIPEs are subjected to sufficiently low levels of
shear
s stress, they behave like elastic solids. As the level of shear stress is
increased, a point
is reached where the polyhedral droplets begin to slide past one another such
that the
HIDE begins to flow. This point is referred to as the yield value. When such
emulsions are subjected to increasingly higher shear stress, they exhibit non-
Newtonian behavior, and the effective viscosity decreases rapidly.
io The difficulty in preparing HIPEs is in part due to these unusual
rheological
properties. The internal and external phases of the RIPE are themselves of
relatively
low viscosity, but as the emulsion is formed, its viscosity becomes very high.
Wheel
a small amount of low viscosity liquid is added to this high viscosity liquid,
it is
difficult to incorporate homogeneously with conventional mixing systems.
Without
is appropriate mixing, and as more of the low viscosity liquid is added, the
highly
viscous phase tends to break up and form a coarse dispersion in the thinner
liquid. It
is for this reason that HIPEs have been very difficult to prepare.
With the correct type and degree of mixing, however, the low viscosity liquid
can be adequately dispersed within the high viscosity liquid as it is added to
form a
Zo stable emulsion. The original processes for manufacturing HIPEs were
discontinuous
processes that have economic disadvantages in a commercial production
situation.
These discontinuous processes typically involve the preparation of a
dispersion
having a low portion of internal phase and subsequently adding more internal
phase
until the HIPE contains over 75% internal phase. Such processes are
cumbersome,
Zs but can be successfully employed using conventional mixing equipment.
Most continuous emulsification equipment used in preparing low- and
medium-internal-phase-ratio emulsions is unsuitable for preparing HIPEs. This
is
because this equipment: (1) does not provide a sufficient deforming force to
the
structured systems to move the polyhedral droplets past one another and
therefore
3o does not accomplish the required mixing; or (2) produces shear rates in
excess of the
inherent shear stability point. Most importantly, such equipment does not
provide
adequate mixing, particularly where there is a large disparity in the
viscosities of the
two phases.
One attempt at developing a continuous process for the production of HII'Es
3s is disclosed in U.S. Patent 3,565,817 (Lissant), issued February 23, 1971
and is
directed at achieving sufficient mixing by providing shear rates high enough
to
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
3
reduce the effective viscosity of the emulsified mass to near the viscosities
of the less
viscous external and internal phases. However, for certain types of emulsions,
it is
not possible to apply enough shear to effect an apparent viscosity near those
of the
external and internal phases without going above the shear stability point of
the
s emulsion. Low-fat spread emulsions (margarine) are examples of such
emulsions.
Although a variety of structurizing elements can achieve shear rates
sufficient to
reduce the effective viscosity of the emulsion phase to near the external and
internal
phase viscosities (thereby allowing the phases to be mixed to a certain
degree), such
elements do not always provide complete mixing, as evidenced by the presence
of
io some non-emulsified liquid in the HIDE.
U.S. Patent 4,844,620 (Lissant et al), issued July 4, 1989, also discloses a
continuous system for preparing HIPEs from internal and external phases having
highly disparate viscosities. The internal and external phase ingredients are
forced
through shearing a device 20 by a recirculating means 18. A recirculation loop
16 is
is adapted to provide for partial recirculation of the processed phase
materials as they
exit the shearing device such that the recirculating means draws a major
portion of
the processed materials through the recirculation loop for additional passes
through
the system. (The remaining portion of the processed phase materials are
continuously propelled from loop 16 as usable HIPE). The reason for.
recirculation
Zo appears to be to provide a preformed emulsion having the desired ratio of
internal to
external phase materials continuously circulating throughout loop 16. See Col.
3,
lines 39, 41. See also U.S. Patent 4,472,215 (Binet et al), issued September
18,
1984, which discloses a continuous HIDE making process for the manufacture of
a
water-in-oil explosive emulsion precursor where at least 80%, and up to 95%,
by
is volume of the coarse HIPS is drawn though a recirculation loop by a pump
and then
returned to be passed again through static mixer.
A continuous process for preparing HIDE useful in emulsion polymerization is
disclosed in U.S. Patent 5,149,720 (DesMarais et al), issued September 22,
1992. In
this continuous HIDE process, separate water and oil phase feed streams are
so introduced into a dynamic mixing zone (typically a pin impeller) and then
subjected
to sufficient shear agitation in the dynamic mixing zone to at least partially
form an
emulsified mixture while maintaining steady, non-pulsating flow rates for the
oil and
water phase streams. The water to oil weight ratio of the feed streams fed to
the
dynamic mixing zone is steadily increased at a rate that does not break the
emulsion
ss in the dynamic mixing zone. The emulsified contents of the dynamic mixing
zone are
continuously withdrawn and continuously feed into a static mixing zone to be
CA 02208419 2000-07-20
4
subjected to additional shear agitation suitable for forming a stable HIPE.
This HIPE
which contains the monomer components in the oil phase is particularly
suitable for
emulsion polymerization to provide absorbent polymeric foams.
As the oil and water phase streams are combined in this dynamic mixing zone
according to U.S. Patent 5,149,720, there is a transition point at the front
of this zone
where the oil and water streams go from two separate phases to an emulsified
phase. As
the rate of throughput of the oil and water phase streams through this dynamic
mixing
zone increases, it has been found that the extent of this transition point
also increases. As
a result, the water phase is less homogeneously dispersed in the oil phase and
the
resulting HIPE comprises water droplets that are less uniform in size. This
makes the
HIPE less stable during subsequent emulsion polymerization, especially if the
pour or
cure temperatures used are relatively high, e.g., at least about 65°C.
The cells formed in
the resulting polymeric foam are also less uniform in size.
Accordingly, it would be desirable to be able to make HIPE, and especially
HIDE
suitable for emulsion polymerization: (1) continuously; (2) with greater
uniformity of
dispersion of the water phase in the oil phase; (3) at higher throughputs; and
(4) with
greater ability to pour or cure the HIPE at higher temperatures during
emulsion
polymerization.
DISCLOSURE OF THE INVENTION
The present invention relates to an improved continuous process for obtaining
high internal phase emulsions (HIPEs), and particularly HIPEs useful in making
polymeric foams. This process comprises the steps of
A) providing a liquid oil phase feed stream comprising an effective amount of
a
water-in-oil emulsifier;
B) providing a liquid water phase feed stream comprising an aqueous solution
of a water-soluble electrolyte;
C) simultaneously introducing the liquid feed streams into a dynamic mixing
zone at flow rates such that the initial weight ratio of water phase to oil
phase
is in the range from about 2:1 to about 10:1;
D) subj ecting the combined feed streams in the dynamic mixing zone to
sufficient shear agitation to at least partially form an emulsified mixture in
the dynamic mixing zone;
E) continuously withdrawing the emulsified mixture from the dynamic mixing
zone;
CA 02208419 1999-10-18
F) recirculating from about 10 to about 50% of the withdrawn
emulsified mixture to the dynamic mixing zone;
G) continuously introducing the remaining withdrawn emulsified
s mixture into a static mixing zone where the remaining emulsified
mixture is further subjected to sufficient shear mixing to
completely form a stable high internal phase emulsion having a
water to oil phase weight ratio of at least about 4:1; and
H) continuously withdrawing the stable high internal phase emulsion
i o from the static mixing zone.
In accordance with an embodiment of the invention, continuous process
for the preparation of a high internal phase emulsion capable of forming a
polymeric foam material, which process comprises:
A) providing a liquid oil phase feed stream comprising:
is 1) from about 80 to about 97% by weight of a monomer
component capable of forming a polymer having a Tg of about
35°C or lower and comprising:
a) from about 50 to about 70% by weight of a
monomer selected from the group consisting of isodecyl
2o acrylate, n-dodecyl acrylate and 2-ethylhexyl acrylate, and
mixtures thereof:
b) from about 15 to about 30% by weight of the
comonomer selected from the group consisting of styrene,
ethyl styrene and mixtures thereof, and
2s c) from about 15 to about 25% by weight of a
crosslinking agent selected from the group consisting of
divinyl benzene, ethylene glycol dimethacrylate,
diethylene glycol dimethacrylate, 1,6-hexanediol
diacrylate, 2-butenediol dimethacrylate, ethylene glycol
CA 02208419 1999-10-18
Sa
diacrylate, trimethylolpropane triacrylate and
trimethacrylate, and mixtures thereof, and
2) from about 3 to about 20% by weight of an emulsifier
s component comprising an emulsifier selected from the group
consisting of sorbitan monoesters of branched C16-C24 fatty acids,
linear unsaturated C,6-C22 fatty acids, and linear saturated C~2-C,4
fatty acids; diglycerol monoesters of branched C~6-C24 fatty acids,
linear unsaturated C16-C22 fatty acids, and linear saturated C~2-C~4
io fatty acids; diglycerol monoaliphatic ethers of branched C,6-C24
alcohols, linear unsaturated C16-C22 alcohols, and linear saturated
C 12-C ~4 alcohols; and mixtures thereof
B) providing a liquid water phase feed stream comprising an
aqueous solution containing from about 0.2% to 20% by weight
i s of water-soluble electrolyte and an effective amount of a
polymerization initiator;
C) simultaneously introducing the liquid feed streams into a
dynamic mixing zone at flow rates such the initial weight ratio of
water phase to oil phase is in the range from about 2.5:1 to about
20 5:1;
D) subjecting the combined feed streams in the dynamic mixing
zone to sufficient shear agitation to at least partially form an
emulsified mixture in the dynamic mixing zone;
E) continuously withdrawing the emulsified mixture from the
2s dynamic mixing zone;
F) recirculating from about 15 to about 40% of the withdrawn
emulsified mixture to the dynamic mixing zone prior to step (D);
G) continuously introducing the remaining withdrawn emulsified
mixture into a static mixing zone wherein the remaining
CA 02208419 1999-10-18
Sb
emulsified mixture is further subjected to sufficient shear mixing
to completely form a stable high internal phase emulsion having a
water to oil phase weight ratio of from about 12:1 to about 250:1;
s and
H) continuously withdrawing the stable high internal phase emulsion
from the static mixing zone.
When the oil phase stream comprises one or more monomers capable of
forming a polymeric foam, when the water phase stream comprises an aqueous
io solution containing from about 0.2% to 20% by weight of water-soluble
electrolyte and when the oil or water phase stream comprises an effective
amount of a polymerization initiator, the resulting stable high internal phase
emulsion can be polymerized to form a polymeric foam.
The key improvement in the continuous process of the present invention
t s is the recirculation of a portion of the HIPE formed in the dynamic mixing
zone. It is believed that such recirculation modifies the extent of the
transition
point from separate water and oil phases to HIPE in the dynamic mixing zone.
This also improves the uniformity of the emulsion ultimately exiting the
static
mixer in terms of having the water droplets homogeneously dispersed in the
2o continuous oil phase. This improves the stability of the HIPE and expands
the
temperature range for pouring and curing this HIPE during subsequent
emulsion polymerization. Recirculation can provide other benefits, including:
(a) higher throughput of the RIPE throughout the entire process; and (b) the
ability to formulate HIPEs having much higher water to oil phase ratios, e.g.,
as
2s high as about 250:1. Indeed, HIPEs made by the process of the present can
readily achieve very high water to oil phase ratios of from about 150:1 to
about
250:1.
While the process of the present invention is particularly desirable for
making HIPEs useful in preparing polymeric foams, it is also useful for making
CA 02208419 1999-10-18
Sc
other water-in-oil type HEPEs. These include agricultural products such as
agricultural sprays, textile processing additives such as textile printing
pastes,
food products such as salad dressings, creams and margarines, household and
s industrial cleaning products such as hand cleaners, wax polishes, and
silicone
polishes, cosmetics such as insect repellent creams, antiperspirant creams,
suntan creams, hair creams, cosmetic creams, and acne creams, transportation
of solids through pipes, crowd control
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
6
products, fire extinguishing products, and the like.
BRIEF DESCRIPTION OF THE DRAWING
The Figure is side sectional view of the apparatus and equipment for carrying
out the process of the present invention.
s DETAILED DESCRIPTION OF THE INVENTION
I. Oil Phase and Water Phase Components of HIDE
A. In General
The process of the present invention is useful in preparing certain water-in-
oil
emulsions having a relatively high ratio of water phase to oil phase and are
io commonly known in the art as "HIPEs. These HIPEs can be formulated to have
a
relatively wide range of water-to-oil phase ratios. The particular water-to-
oil phase
ratio selected will depend on a number of factors, including the particular
oil and
water phase components present, the particular use to be made of the HIDE, and
the
particular properties desired for the HIDE. Generally, the ratio of water-to-
oil phase
is in the HIDE is at least about 4:1, and is typically in the range of from
about 4:1 to
about 250:1, more typically from about 12:1 to about 200:1, and most typically
from
about 20:1 to about 150:1.
For preferred HIPEs according to the present invention that are subsequently
polymerized to provide polymeric foams (hereafter referred to as "HIDE
foams"), the
Zo relative amounts of the water and oil phases used to form the HIDE are,
among many
other parameters, important in determining the structural, mechanical and
performance properties of the resulting HIDE foams. In particular, the ratio
of water
to oil phase in the HIDE can influence the density, cell size, and capillarity
of the
foam, as well as the dimensions of the struts that form the foam. HIPEs
according to
is the present invention used to prepare these foams will generally have water-
to-oil
phase ratios in the range of from about 12:1 to about 250:1, preferably from
about
20:1 to about 200:1, most preferably from about 25:1 to about 150:1.
CA 02208419 2000-07-20
B. Oil Phase Components
1. Th i
The oil phase of the HIPE can comprise a variety of oily materials. The
particular oily materials selected will frequently depend upon the particular
use to be
made of the HIPE. By "oily" is meant a material, solid or liquid, but
preferably liquid
at room temperature that broadly meets the following requirements: (1) is
sparingly
soluble in water; (2) has a low surface tension; and (3) possesses a
characteristic
greasy feel to the touch. Additionally, for those situations where the HIPE is
to be
used in the food, drug, or cosmetic area, the oily material should be
cosmetically and
pharmaceutically acceptable. Materials contemplated as oily materials for use
in
making HIPEs according to the present invention can include, for example,
various
oily compositions comprising straight, branched and/or cyclic paraffins such
as
mineral oils, petrolatums, isoparaffins, squalanes; vegetable oils, animal
oils and
marine oils such as tung oil, oiticica oil, castor oil, linseed oil, poppyseed
oil, soybean
oil, cottonseed oil, corn oil, fish oils, walnut oils, pineseed oils, olive
oil, coconut oil,
palm oil, canola oil, rapeseed oil, sunflower seed oil, safflower oil, sesame
seed oil,
peanut oil and the like; esters of fatty acids or alcohols such as ethyl
hexylpalmitate,
C 16 to C 1 g fatty alcohol di-isooctanoates, dibutyl phthalate, diethyl
maleate, tricresyl
phosphate, acrylate or methacrylate esters, and the like; resin oils and wood
distillates
including the distillates of turpentine, rosin spirits, pine oil, and acetone
oil; various
petroleum based products such as gasolines, naphthas, gas fuel, lubricating
and
heavier oils; ccal distillates including benzene, toluene, xylene, solvent
naphtha,
creosote oil and anthracene oil and ethereal oils: and silicone oils.
Preferably, the oily
material is non-polar.
For preferred HIPEs that are polymerized to form the polymeric foams , this
oil phase comprises a monomer component. In the case of HIPE foams suitable
for
use as absorbents, this monomer component is typically formulated to form a
copolymer having a glass transition temperature (Tg) of about 35°C or
lower, and
typically from about 15° to about 30 °C. (The method for
determining Tg by
Dynamic Mechanical Analysis (DMA) is described in CA 2209928. This monomer
component includes: (a) at least one monofunctional monomer whose atactic
amorphous polymer has a Tg of about 25°C or lower; (b) optionally a
CA 02208419 1997-06-20
WO 96121505 PCT/US96I00082
8
monofunctional comonomer; and (c) at least one polyfunctional crosslinking
agent.
Selection of particular types and amounts of monofunctional monomers) and '
comonomer(s) and polyfunctional cross-linking agents) can be important to the
realization of absorbent RIPE foams having the desired combination of
structure,
s mechanical, and fluid handling properties that render such materials
suitable for use
as absorbents for aqueous fluids.
For HIDE foams useful as absorbents, the monomer component comprises
one or more monomers that tend to impart rubber-like properties to the
resulting
polymeric foam structure. Such monomers can produce high molecular weight
io (greater than 10,000) atactic amorphous polymers having Tg's of about
25°C. or
lower. Monomers of this type include, for example, monoenes such as the (C4-C
14)
alkyl acrylates such as butyl acrylate, hexyl acrylate, octyl acrylate, 2-
ethylhexy~
acrylate, nonyl acrylate, decyl acrylate, dodecyl (lauryl) acrylate, isodecyl
acrylate
tetradecyl acrylate, aryl acrylates and alkaryl acrylates such as benzyl
acrylate,
is nonylphenyl acrylate, the (C6-C 16) alkyl methacrylates such as hexyl
acrylate, octyl
methacrylate, nonyl methacrylate, decyl methacrylate, isodecyl methacrylate,
dodecyl
(lauryl) methacrylate, tetradecyl methacrylate, (C4-C 12) alkyl styrenes such
as p-n-
octylstyrene, acrylamides such as N-octadecyl acrylamide, and polyenes such as
2-
methyl-1,3-butadiene (isoprene), butadiene, 1,3-pentadiene (piperylene), 1,3-
2o hexadiene, 1,3-heptadiene, 1,3-octadiene, 1,3-nonadiene, 1,3-decadiene, 1,3-
undecadiene, 1,3-dodecadiene, 2-methyl-1,3-hexadiene, 6-methyl-1,3-heptadiene,
7-
methyl-1,3-octadiene, 1,3,7-octatriene, 1,3,9-decatriene, 1,3,6-octatriene,
2,3-
dimethyl-1,3-butadiene, 2-methyl-3-ethyl-1,3-butadiene, 2-methyl-3-propyl-1,3-
butadiene, 2-amyl-1,3-butadiene, 2-methyl-1,3-pentadiene, 2,3-dimethyl-1,3-
zs pentadiene, 2-methyl-3-ethyl-1,3-pentadiene, 2-methyl-3-propyl-1,3-
pentadiene, 2,6-
dimethyl-1,3,7-octatriene, 2,7-dimethyl-1,3,7-octatriene, 2,6-dimethyl-1,3,6-
octatriene, 2,7-dimethyl-1,3,6-octatriene, 7-methyl-3-methylene-1,6-octadiene
(myrcene), 2,6-dimethyl-1,5,7-octatriene (ocimene), 1-methyl-2-vinyl-4,6-hepta-
dieny-3,8-nonadienoate, 5-methyl-1,3,6-heptatriene, 2-ethylbutadiene, and
mixtures
so of these monomers. Of these monomers, isodecyl acrylate, n-dodecyl acrylate
and 2-
ethylhexyl acrylate are the most preferred. The monomer will generally
comprise 30
to about 85%, more preferably from about 50 to about 70%, by weight of the
monomer component.
For HIDE foams useful as absorbents, the monomer component also typically
ss comprises one or more comonomers that are typically included to modify the
Tg
properties of the resulting polymeric foam structure, its modulus (strength),
and its
CA 02208419 1997-06-20
WO 96121505 PCT/US96/00082
9
toughness. These monofunctional comonomer types can include styrene-based
comonomers (e.g., styrene and ethyl styrene) or other monomer types such as
methyl
methacrylate where the related homopolymer is well known as exemplifying
toughness. Of these comonomers, styrene, ethyl styrene, and mixtures thereof
are
s particularly preferred for imparting toughness to the resulting polymeric
foam
structure. These comonomers can comprise up to about 40 % of the monomer
component and will normally comprise from about 5 to about 40%, preferably
from
about 10 to about 35%, most preferably from about 15 about 30%, by weight of
the
monomer component.
io For HIDE foams useful as absorbents, this monomer component also includes
one or more polyfunctional crosslinking agents. The inclusion of these
crosslinking
agents tends to increase the Tg of the resultant polymeric foam as well as its
strength
with a resultant loss of flexibility and resilience. Suitable crosslinking
agents include
any of those that can be employed in crosslinking rubbery diene monomers, such
as
is divinylbenzenes, divinyltoluenes, divinylxylenes, divinylnaphthalenes
divinylalkylbenzenes, divinylphenanthrenes, trivinylbenzenes,
divinylbiphenyls,
divinyldiphenylmethanes, divinylbenzyls, divinylphenylethers,
divinyldiphenylsulfides,
divinylfurans, divinylsulfone, divinylsulfide, divinyldimethylsilane, 1,1'-
divinylferrocene, 2-vinylbutadiene, maleate, di-, tri-, tetra-, penta- or
higher
Zo (meth)acrylates and di-, tri-, tetra-, penta- or higher (meth)acrylamides,
including
ethylene glycol dimethacrylate, neopentyl glycol dimethacrylate, 1,3-
butanediol
dimethacrylate, 1,4-butanediol dimethacrylate, 1,6-hexanediol dimethacrylate,
2-
butenediol dimethacrylate, diethylene glycol dimethacrylate, hydroquinone
dimethacrylate, catechol dimethacrylate, resorcinol dimethacrylate,
triethylene glycol
is dimethacrylate, polyethylene glycol dimethacrylate; trimethylolpropane
trimethacrylate, pentaerythritol tetramethacrylate, 1,3-butanediol diacrylate,
1,4-
butanediol diacrylate, 1,6-hexanediol diacrylate, diethylene glycol
diacrylate,
hydroquinone diacrylate, catechol diacrylate, resorcinol diacrylate,
triethylene glycol
diacrylate, polyethylene glycol diacrylate; pentaerythritol tetraacrylate, 2-
butenediol
3o diacrylate, tetramethylene diacrylate, trimethyol propane triacrylate,
pentaerythritol
tetraacrylate, N-methylolacrylamide, 1,2-ethylene bisacrylamide, 1,4-butane
bisacrylamide, and mixtures thereof.
The preferred polyfunctional crosslinking agents include divinylbenzene,
ethylene glycol dimethacrylate, diethylene glycol dimethacrylate, 1,6-
hexanediol
ss dimethacrylate, 2-butenediol dimethacrylate, ethylene glycol diacrylate,
diethylene
glycol diacrylate, 1,6-hexanediol diacrylate, 2-butenediol diacrylate,
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
trimethylolpropane triacrylate and trimethacrylate, and mixtures thereof.
Divinyl
benzene is typically available as a mixture with ethyl styrene in proportions
of about '
55:45. These proportions can be modified so as to enrich the oil phase with
one or
the other component. Generally, it is advantageous to enrich the mixture with
the
s ethyl styrene component while simultaneously omitting inclusion of styrene
from the
monomer blend. The preferred ratio of divinyl benzene to ethyl styrene is from
about
30:70 to 55:45, most preferably from about 35:65 to about 45:55. The inclusion
of
higher levels of ethyl styrene imparts the required toughness without
increasing the
Tg of the resulting copolymer to the degree that styrene does. The cross-
linking
to agent can generally be included in the oil phase of the HIDE in an amount
of from
about 5 to about 40%, more preferably from about 10 to about 35%, most
preferably
from about 15 to about 30%, by weight of the monomer component (100% basis).
The major portion of the oil phase of these preferred HIPEs will comprise
these monomers, comonomers and crosslinking agents. It is essential that these
is monomers, comonomers and crosslinking agents be substantially water-
insoluble so
that they are primarily soluble in the oil phase and not the water phase. Use
of such
substantially water-insoluble monomers ensures that HIDE of appropriate
characteristics and stability will be realized.
It is, of course, highly preferred that the monomers, comonomers and
zo crosslinking agents used herein be of the type such that the resulting
poiymeric foam
is suitably non-toxic and appropriately chemically stable. These monomers,
comonomers and cross-linking agents should preferably have little or no
toxicity if
present at very low residual concentrations during post-polymerization foam
processing and/or use.
zs 2. Emulsifier Component
Another essential component of the oil phase is an emulsifier (or emulsifiers)
that permits the formation of stable HIDE emulsions. Suitable emulsifiers for
use
herein can include any of a number of conventional emulsifiers applicable for
use in
low and mid-internal-phase emulsions. The particular emulsifiers used will
depend
3o upon an number of factors, including the particular oily materials present
in the oil
phase and the particular use to be made of the HIDE. Usually, these
emulsifiers are
nonionic materials and can have a wide range of HLB values. Examples of some
typical emulsifiers include sorbitan esters such as sorbitan laurates (e.g.,
SPAN~ 20),
sorbitan palmitates (e.g., SPAN~ 40), sorbitan stearates (e.g., SPAN~ 60 and
CA 02208419 2000-07-20
11
SPAN~ 65), sorbitan monooleates (e.g., SPAN~ 80), sorbitan trioleates (e.g.,
SPAN~
85), sorbitan sesquioleates (e.g., EMSORB~ 2502), and sorbitan isostearates;
polyglycerol
esters and ethers (e.g., TRIODAN~ 20); polyoxyethylene fatty acids, esters and
ethers
such as polyoxyethylene (2) oleyl ethers, polyethoxylated oleyl alcohols (e.g.
BRIJ~ 92
and SIMUSOL~ 92), etc.; mono-, di-, and triphosphoric esters such as mono-, di-
, and
triphosphoric esters of oleic acid (e.g., HOSTAPHAT K0300N), polyoxyethylene
sorbitol esters such as polyoxyethylene sorbitol hexastearates (e.g., ATLAS~ G-
1050),
ethylene glycol fatty acid esters, Iglycerol mono-180 stearates (e.g., IMWITOR
780K),
ethers of glycerol and fatty alcohols (e.g., CREMOPHOR WO/A), esters of
polyalcohols,
synthetic primary alcohol ethylene oxide condensates (e.g., SYNPERONIC A2),
mono
and diglycerides of fatty acids (e.g., ATMOS~ 300), and the like.
For preferred HIPEs that are polymerized to make polymeric foams, the
emulsifier
can serve other functions besides stabilizing the HIPE. These include the
ability to
hydrophilize the resulting polymeric foam. The resulting polymeric foam is
typically
washed and dewatered to remove most of the water and other residual
components. This
residual emulsifier can, if sufficiently hydrophilic, render the otherwise
hydrophobic foam
sufficiently wettable so as to be able to absorb aqueous fluids.
For preferred HIPEs that are polymerized to make polymeric foams, suitable
emulsifiers can include sorbitan monoesters of branched C16-C24 fatty acids,
linear
unsaturated C 16-C22 fatty acids, and linear saturated C 12-C 14 fatty acids,
such as
sorbitan monooleate, sorbitan monomyristate, and sorbitan monoesters derived
from
coconut fatty acids; diglycerol monoesters of branched C 16-C24 fatty acids,
linear
unsaturated C 16-C22 fatty acids, or linear saturated C 12-C 14 fatty acids,
such as
diglycerol monooleate (i.e., diglycerol monoesters of C18:1 fatty acids),
diglycerol
monomyristate, diglycerol monoisostearate, and diglycerol monoesters of
coconut fatty
acids; diglycerol monoaliphatic ethers of branched C16-C24 alcohols (e.g.
Guerbet
alcohols), linear unsaturated C16-C22 alcohols, and linear saturated C12-C14
alcohols
(e.g., coconut fatty alcohols), and mixtures of these emulsifiers. Preferred
emulsifiers
include
CA 02208419 1997-06-20
WO 96/21505 PGTIUS96/00082
12
sorbitan monolaurate (e.g., SPAN~ 20, preferably greater than about 40%, more
preferably greater than about 50%, most preferably greater than about 70%
sorbitan
monolaurate), sorbitan monooleate (e.g., SPAN~ 80, preferably greater than
about
40%, more preferably greater than about 50%, most preferably greater than
about '
s 70% sorbitan monooleate), diglycerol monooleate (e.g., preferably Beater
than
about 40%, more preferably greater than about 50%, most preferably greater
than
about 70% diglycerol monooleate), diglycerol monoisostearate (e.g., preferably
greater than about 40%, more preferably greater than about 50%, most
preferably
greater than about 70% diglycerol monoisostearate), diglycerol monomyristate
(e.g.,
io preferably greater than about 40%, more preferably greater than about SO%,
most
preferably greater than about 70% sorbitan monomyristate), the cocoyl (e.g.,
lauryl
and myristoyl) ethers of diglycerol, and mixtures thereof.
In addition to these primary emulsifiers, co-emulsifiers can be optionally
included in the oil phase. These co-emulsifiers are at least cosoluble with
the primary
is emulsifier in the oil phase. Suitable co-emulsifiers can be zwitterionic
types,
including the phosphatidyl cholines and phosphatidyl choline-containing
compositions
such as the lecithins and aliphatic betaines such as lauryl betaine; cationic
types,
including long chain 012-022 dialiphatic, short chain C1-C4 dialiphatic
quaternary
ammonium salts such as ditallow dimethyl ammonium chloride, bistridecyl
dimethyl
zo ammonium chloride, and ditallow dimethyl ammonium methylsulfate, the long
chain
C 12-022 dialkoyl(alkenoyl)-2-hydroxyethyl, short chain C 1-C4 dialiphatic
quaternary ammonium salts such as ditallowoyl-2-hydroxyethyl dimethyl ammonium
chloride, the ~ long chain C 12-022 diaIiphatic imidazolinium quaternary
ammonium
salts such as methyl-1-tallow amido ethyl-2-tallow imidazolinium methylsulfate
and
is methyl-1-oleyl amido ethyl-2-oleyl imidazolinium methylsulfate, the short
chain Cl-
C4 dialiphatic, long chain C 12-022 monoaliphatic benryl quaternary ammonium
salts
such as dimethyl stearyl benzyl ammonium chloride and dimethyl tallow benzyl
ammonium chloride, the long chain C 12-022 dialkoyl(alkenoyl)-2-aminoethyl,
short
chain C1-C4 rrionoaliphatic, short chain Cl-C4 monohydroxyaliphatic quaternary
.
so ammonium salts such as ditallowoyl-2-aminoethyl methyl 2-hydroxypropyl '
ammonium methyl sulfate and dioleoyl-2-aminoethyl methyl .2-hydroxyethyl
ammonium methyl sulfate; anionic types including the dialiphatic esters of
sodium
sulfosuccinic acid such as the dioctyl ester of sodium sulfosuccinic acid and
the
bistridecyl ester of sodium sulfosuccinic acid, the amine salts of
dodecylbenzene
3s sulfonic acid; and mixtures of these secondary emulsifiers. The preferred
secondary
emulsifiers are ditallow dimethyl ammonium methyl sulfate and ditallow
dimethyl
CA 02208419 1999-10-18
w0 96JZ1305 PC?/US96/00082
13
ammonium methyl chloride. When these optional secondary emulsifiers are
included
in the emulsifier component, it is typically at a weight ratio of primary to
secondary
emulsifier of from about 50:1 to about 1:4, preferably from about 30:1 to
about 2:1.
3. Oil Phase Comoo ition
s The oil phase used to form the Fi~E according to the process of the present
invention can comptix varying ratios of oily materials and emulsifier. The
particular
ratios xlected wiU depend on a number of factors including the oily materials
involved, the emulsifier used, and the ux to be made of the HIDE. Generally,
the oil
phax can comprise from about 50 to about 98% by weight oily materials and from
io about 2 to about 50% by weight emulsifier. Typically, the oil phase wiD
comprise
from shout 70 to about 97% by weight of the oily materials and from about 3 to
about 30 % by weight emulsifier, and more typically from about 85 to about 97%
by
weight of the oily materists and from about 3 to about 15% by weight emuisi5er
For preferred HIPEs used to make polymeric foams, the oil phase will
~s generslly comprix from about 65 to about 98% by weight monomer component
and
from about 2 to about 35% by weight emulsifier component. Preferably, the oil
phax will comprise from about 80 to about 97% by weight monomer component
and from about 3 to about 20% by weight emulsifier component. More preferably,
the oil phase will comprix from about 90 to about 97% by weight monomer
so component and from about 3 to about 10% by weight emulsifier component.
In addition to the monomer and emulsifier components, the oil phsx of thex
preferred HIPEs can contain other optional components. One such optional
component is an oil soluble polymerization initiator of the general type well
known
to those skilled in the art, such as described in U.S. vatent 5,290,820 (Bass
et al),
a issued March 1, 1994. Another possible optional
component is a substantially water insoluble solvent for the monomer and
emulsifier
components. Ux of such a solvent is not preferred, but if employed will
generally
comprix no more than about 10% by weight of the oil phax.
A preferred optional component is an antioxidant such as a Ii'tndaed Amine
ao Light Stabilizer (HAi.S), such as his-( 1.2.2,5,5-pentamethylpiperidinyl)
sebacate
(Ttnuvirt 765) or a Hindered Phenolic Stabilizer (IBS) such as Irganox 1076
and t
butylhydroxyquinone. Another preferred optional component is a plasticizer
such as
dioctyl azelate, dioctyl sebacate or dioctyl adipate. Other optional
components
CA 02208419 1997-06-20
WO 96/21505 PGT/US96/00082
14
include fillers, colorants, fluorescent agents, opacifying agents, chain
transfer agents,
and the like.
C. Water Phase Components
The internal water phase of the HIDE is generally an aqueous solution
s containing one or more dissolved components. One essential dissolved
component
of the water phase is a water-soluble electrolyte. The dissolved electrolyte
minimizes
the tendency of the components in the oil phase to also dissolve in the water
phase.
For preferred HIPEs used to make polymeric foams, this is believed to minimize
the
extent to which polymeric material fills the cell windows at the oiUwater
interfaces
to formed by the water phase droplets during polymerization. Thus, the
presence of
electrolyte and the resulting ionic strength of the water phase is believed to
determine
whether and to what degree the resulting preferred HIDE foams can be open-
celled.
Any electrolyte capable of imparting ionic strength to the water phase can be
used. Preferred electrolytes are mono-, di-, or trivalent inorganic salts such
as the
is water-soluble halides, e.g., chlorides, nitrates and sulfates of alkali
metals and
alkaline earth metals. Examples include sodium chloride, calcium chloride,
sodium
sulfate and magnesium sulfate. For HIPEs that are used to make polymeric
foams,
calcium chloride is the most preferred for use in the process according to the
present
invention. Generally the electrolyte will be utilized in the water phase of
the RIPE in
zo a concentration in the range of from about 0.2 to about 20% by weight of
the water
phase. More preferably, the electrolyte will comprise from about 1 to about
10% by
weight of the water phase.
For HIPEs used to make polymeric foams, a polymerization initiator is
typically included in the HIDE. Such an initiator component can be added to
the
zs water phase of the HIDE and can be any conventional water-soluble free
radical
initiator. These include peroxygen compounds such as sodium, potassium and
ammonium persulfates, hydrogen peroxide, sodium peracetate, sodium
percarbonate
and the like. Conventional redox initiator systems can also be used. Such
systems
are formed by combining the foregoing peroxygen compounds with reducing agents
so such as sodium bisulfite, L-ascorbic acid or ferrous salts. The initiator
can be present
at up to about 20 mole percent based on the total moles of polymerizable
monomers
in the oil phase. Preferably, the initiator is present in an amount of from
about 0.001
to 10 mole percent based on the total moles of polymerizable monomers in the
oil
phase.
CA 02208419 2000-07-20
II. Continuous Process for Making RIPE
The continuous process of the present invention for making HIPE includes the
following steps: A) introducing the oil phase and water phase feed streams
into the
dynamic mixing zone (and initially the recirculation zone); B) initially
forming the
emulsion in the dynamic mixing zone (and the recirculation zone); C) forming
HIPE
in the dynamic mixing zone; and D) transfernng the effluent from the dynamic
mixing zone to the static mixing zone. See U.S. Patent 5,149,720 (DesMarais et
al),
issued September 22, 1992. While this description of the continuous process of
the
present invention will be with reference to making preferred HIPEs useful for
obtaining polymeric foams, it should be understood that this process can be
used to
prepare other water-in-oil type HIPEs by using different oil and water phase
components and amounts, by appropriate modification of the process, and the
like.
A. Initial Introduction of Oil and Water Phase Feed Streams Into the
Dynamic Mixing and Recirculation Zones
The oil phase can be prepared in any suitable manner by combining the
essential and optional components using conventional techniques. Such a
combination of components can be carned out in either continuous or batch-wise
fashion using any appropriate order of component addition. The oil phase so
prepared
will generally be formed and stored in a feed tank, then provided as a liquid
feed
stream at any desired flow rate. The water phase stream can be prepared and
stored in
a similar manner.
The liquid streams of both oil and water phases are initially combined by
simultaneously introducing these feed streams together into a dynamic mixing
zone.
During this stage of initial combination of these oil and water phases, the
flow rates of
the feed streams are set so that the initial weight ratio of water phase to
oil phase
being introduced into the dynamic mixing zone is well below that of the final
weight
ratio of the HIPE produced by the process. In particular, flow rates of the
oil and
water phase liquid streams are set such that the water to oil weight ratio
during this
initial introduction stage is in the range of from about 2:1 to about 10:1,
more
preferably from about 2.5:1 to about 5:1. The purpose of combining the oil and
water
phase streams at these lower water to oil ratios is to permit formation in the
dynamic
mixing zone of at least some amount of water-in-oil emulsion which is
CA 02208419 1997-06-20
WO 96/21505 PCTIUS96/00082
16
relatively stable and does not readily "break" under the conditions
encountered in this
zone.
The actual flow rates of the oil and water phase liquid feed streams during
this stage of initial introduction into the dynamic mixing zone will vary
depending
s upon the scale of the operation involved. For pilot plant scale operations,
the oil
phase flow rate during this initial introduction stage can be in the range of
from about
0.02 to about 0.35 liter/minute, and the water phase flow rate can be in the
range of
from about 0.04 to about 2.0 liters/minute. For commercial scale operations,
the oil
phase flow rate during this initial introduction stage can be in the range of
from about
l0 10 to about 25 liters/minute, and the water phase flow rate can be in the
range of
from about 20 to about 250 liters/minute.
During the initial startup of this process, the dynamic mixing and
recirculatiolt
zones are filled with oil and water phase liquid before agitation begins.
During this
filling stage, the displaced headspace gas is vented from the dynamic mixing
zone.
is Before agitation begins, the liquid in these zones is typically in two
separate phases,
i.e., an oil phase and a water phase. (At lower water to oil ratios,
spontaneous
emulsification could occur such that there is essentially only one phase.)
Once the
dynamic mixing zone is filled with liquid, agitation is begun, and the
emulsion begins
to form in the dynamic mixing zone. At this point, oil and water phase. flow
rates
zo into the dynamic mixing zone should be set so as to provide a relatively
low initial
water to oil weight ratio within the range previously described. The
recirculation
zone should also be set at a rate approximating the sum of the introductory
oil and
water phase rates as described previously.
B. Initial Emulsion Formation in the Dynamic Mixing Zone
Zs As noted above, the oil and water phase feed streams are initially combined
by simultaneous introduction into a dynamic mixing zone (and in the
recirculation
zone during initial fill up). For the purposes of the present invention, the
dynamic
mixing zone comprises a containment vessel for iiquid components. This vessel
is
equipped with means for imparting shear agitation to the liquid contents of
the
so vessel. The means for imparting shear agitation should cause agitation or
mixing
beyond that which arises by virtue of simple flow of liquid material through
the
vessel.
The means for imparting shear agitation can comprise any apparatus or
device that imparts the requisite amount of shear agitation to the liquid
contents in
CA 02208419 1997-06-20
WO 96!21505 PCT/US96100082
17
the dynamic mixing zone. One suitable type of apparatus for imparting shear
agitation is a pin impeller that comprises a cylindrical shaft from which a
number of
rows (flights) of cylindrical pins extend radially. The number, dimensions,
and
configuration of the pins on the impeller shaft can vary widely, depending
upon the
s amount of shear agitation that is desired to be imparted to the liquid
contents in the
dynamic mixing zone. A pin impeller of this type can be mounted within a
generally
cylindrical mixing vessel which serves as the dynamic mixing zone. The
impeller
shaft is positioned generally parallel to the direction of liquid flow through
the
cylindrical vessel. Shear agitation is provided by rotating the impeller shaft
at a
io speed which imparts the requisite degree of shear agitation to the liquid
material
passing through the vessel. See Figure 2 of U. S. Patent 5,149,720
The shear agitation imparted in the dynamic mixing zone is sufficient to forts
at least some of the liquid contents into a water-in-oil emulsion having water
to oil
phase ratios within the ranges previously set forth. Frequently such shear
agitation at
. is this point will typically be in the range from about 1000 to about 10,000
sec.-1, more
typically, from about 1500 to 7000 sec.-1. The amount of shear agitation need
not
be constant but can be varied over the time needed to effect such emulsion
formation. As indicated, not all of the water and oil phase material that has
been
introduced into the dynamic mixing zone at this point need be incorporated
into the
zo water-in-oil emulsion so long as at least some emulsion of this type (e.g.,
the
emulsion comprises at least about 90% by weight of the liquid effluent from
the
dynamic mixing zone) is formed in and flows through the dynamic mixing zone.
In the continuous process described in U.S. Patent 5,149,720, it is taught
that
it is important that both the oil and water phase flow rates be steady and non
zs pulsating once agitation begins to avoid sudden or precipitous changes that
can cause
the emulsion formed in the dynamic mixing zone to break. See Col. 9, lines 31-
35.
An important advantage of the improved process according to the present
invention
is that the criticality of steady, non-pulsating flow rates is substantially
reduced by
using a recirculation zone as described hereafter. Indeed, it has been found
that the
so oil phase flow can be stopped for a period of time, as long as the
recirculation rate is
sufficient to return enough emulsified oil phase such that the ratio of total
oil phase
(unemulsified/emulsified) in this recirculating flow to the introduced water
phase
does not exceed the stabilizing capacity of the emulsifier.
CA 02208419 1997-06-20
WO 96/21505 PGT/US96/00082
18
C) HIDE Formation in Dynamic Mixing Zone
After a water-in-oil emulsion having a relatively low water-to-oil ratio is
formed in the dynamic mixing zone, the emulsion is converted, along with the
additional non-emulsified contents, into HIDE. This is accomplished by
altering the
s relative flow rates of the water and oil phase streams being fed into the
dynamic
mixing zone. Such an increase in the water-to-oil ratio of the phases can be
accomplished by increasing the water phase flow rate, by decreasing the oil
phase
flow rate or by a combination of these techniques. The water-to-oil ratios to
be
eventually realized by such an adjustment of the water phase and/or oil phase
flow
ro rates will generally be in the range of from about 12:1 to about 250:1,
more typically
from about 20:1 to 200:1, most typically from about 25:1 to 150:1.
Adjustment of the oil and/or water phase flow rates to increase the water to
oil phase ratio being fed to the dynamic mixing zone can begin immediately
after
initial formation of the emulsion. This will generally occur soon after
agitation is
is begun in the dynamic mixing zone. The length of time taken to increase the
water to
oil phase ratio to the ultimately desired higher ratio will depend on the
scale of the
process involved and the magnitude of the eventual water to oil phase ratio to
be
reached. Frequently the duration of the flow rate adjustment period needed to
increase water to oil phase ratios will be in the range of from about 1 to
about 5
zo mrnutes.
The actual rate of increase of the water-to-oil phase ratio of the streams
being
fed to the dynamic mixing zone wilt be dependent upon the particular
components of
the emulsion being prepared, as well as the scale of the process involved. For
any
given HIDE formula and process setup, emulsion stability can be controlled by
simply
zs monitoring the nature of the effluent from the process to ensure that it
comprises at
least some material (e.g., at least 90% of the total et~luent) in
substantially HIPS
form.
Conditions within the dynamic mixing zone during emulsion formation can
also affect the nature of the HIDE prepared by this process. One aspect that
can
so impact on the character of the HIDE produced is the temperature of the
emulsion
components within the dynamic mixing zone. Generally the emulsified contents
of
the dynamic mixing zone should be maintained at a temperature of from about
5° to
about 95°C., more preferably from about 35° to about
90°C., during HIDE
formation. An important advantage of the improved process according to the
ss present invention (relative to that described in is that U.S. Patent
5,149,720) is the
CA 02208419 2000-07-20
19
ability to increase the temperature at which uniform HIPE can be made by a
continuous
process. This is due to the addition of the recirculation zone (as described
below) where a
portion of the HIPE from the dynamic mixing zone is recirculated and combined
with the
oil and water phase streams introduced into the dynamic mixing zone.
Another aspect involves the amount of shear agitation imparted to the contents
of
the dynamic mixing zone both during and after adjustment of the water and oil
phase flow
rates. The amount of shear agitation imparted to the emulsified material in
the dynamic
mixing zone will directly impact on the size of the dispersed water droplets
(and
ultimately on the size of the cells that make up the polymeric foam). For a
given set of
emulsion component types and ratios, and for a given combination of flow
rates,
subjecting the dynamic mixing zone liquid contents to greater amounts of shear
agitation
will tend to reduce the size of the dispersed water droplets.
Foam cells, and especially cells which are formed by polymerizing a monomer-
containing oil phase that surrounds relatively monomer-free water-phase
droplets, will
frequently be substantially spherical in shape. The size or "diameter" of such
substantially spherical cells is thus a commonly utilized parameter for
characterizing
foams in general as well as for characterizing polymeric foams of the type
prepared from
the HIPE made by the process of the present invention. Since cells in a given
sample of
polymeric foam will not necessarily be of approximately the same size, an
average cell
size (diameter) will often be specified.
A number of techniques are available for determining average cell size in
foams.
These techniques include mercury porosimetry methods which are well known in
the art.
The most useful technique, however, for determining cell size in foams
involves simple
photographic measurement of a foam sample. Such a technique is described in
greater
detail in U.S. Pa:ent 4,788,225 (Edwards et al), issued November 29, 1988.
For purposes of the present invention, the average cell size of foams made by
polymerizing this HIPE can be used to quantify the amount of shear agitation
imparted to
the emulsified contents in the dynamic mixing zone. In particular, after the
oil and water
phase flow rates have been adjusted to provide the requisite water/oil ratio,
the emulsified
contents of the dynamic mixing zone should be subjected to shear agitation
which is
sufficient to eventually form a HIPE that, upon subsequent polymerization,
provides a
foam having an average cell size of from about 5 to about 100 pm. More
preferably, such
agitation will be that suitable to
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
realize an average cell size in the subsequently formed foam of from about 10
to
about 90 Vim. This will typically amount to shear agitation of from about 1000
to
about 10,000 sec.-1, more preferably from about 1500 to about 7000 sec.-1
As with the shear agitation utilized upon initial introduction of the oil and
s water phases into the dynamic mixing zone, shear agitation to provide HIDE
need
not be constant during the process. For example, impeller speeds can be
increased or
decreased during HIDE preparation as desired or required to provide emulsions
that
can form foams having the particular desired average cell size characteristics
described above.
to During the adjustment period, recirculation is adjusted to approximate the
current rate of total flow of the introductory oil and water phases. Thus,
when the
targeted oil and water phase flow rates are achieved, about half of the
effluent exiting
the dynamic mixing zone is withdrawn and passed through the recirculation
zone.
The flow rate through the recirculation zone can then conveniently be reduced.
is D) Transfer of Effluent from Dynamic Mixing_ Zone to Static Mixing
Zone
In the process of the present invention, the emulsion-containing liquid
contents of the dynamic mixing zone are continuously withdrawn and a portion
introduced into a static mixing zone where they are subjected to further
mixing and
2o agitation. The nature and composition of this effluent will, of course,
change over
time as the process proceeds from initial startup, to initial emulsion
formation, to
HIPS formation in the dynamic mixing zone, as the water-to-oil phase ratio is
increased. During the initial startup procedure, the dynamic mixing zone
effluent can
contain little or no emulsified material at all. After emulsion formation
begins to
zs occur, the effluent from the dynamic mixing zone will comprise a water-in-
oil
emulsion having a relatively low water-to-oil phase ratio, along with excess
oil and
water phase material that has not been incorporated into the emulsion.
Finally, after
the water-to-oil phase ratio of the two feed streams has been increased, the
dynamic
mixing zone effluent will primarily comprise HIDE, along with relatively small
3o amounts of oil and water phase materials that have not been incorporated
into this
HIDE.
Once steady state operation is achieved, the flow rate of effluent from the
dynamic mixing zone to the static mixing zone will equal the sum of the flow
rates of
the water and oil phases being introduced into the dynamic mixing zone. After
water
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
21
and oil phase flow rates have been properly adjusted to provide formation of
the
desired HIDE, the effluent flow rate from the dynamic mixing zone will
typically be in
the range of from about 35 to about 800 liters per minute for commercial scale
operations. For pilot plant scale operations, dynamic mixing zone effluent
flow rates
s will typically be in the range of from about 0.8 to about 9.0 liters per
minute.
The static mixing zone also provides resistance to the flow of liquid material
through the process and thus provides back pressure to the liquid contents of
the
dynamic mixing zone. However, the primary purpose of the static mixing zone is
to
subject the emulsified material from the dynamic mixing zone to additional
agitation
io and mixing in order to complete the formation of stable HIDE.
For purposes of the present invention, the static mixing zone can comprise
any suitable containment vessel for liquid materials. This vessel is internall
j~
configured to impart agitation or mixing to such liquid materials as these
materials
flow through the vessel. A typical static mixer is a spiral mixer that can
comprise a
is tubular device having an internal configuration in the form of a series of
helices that
reverse direction every 180° of helical twist. Each 180° twist
of the internal helical
configuration is called a flight. Typically, a static mixer having from 12 to
32 helical
flights that intersect at 90° angles will be useful in the present
process.
In the static mixing zone, shear forces are imparted to the liquid material
2o simply by the effect of the internal configuration of the static mixing
device on the
liquid as it flows therethrough. Typically such shear is imparted to' the
liquid
contents of the static mixing zone to the extent of from about 1000 to about
10,000
sec.-1, more preferably from about 1000 to about 7000 sec.-1.
In the static mixing zone, essentially all of the water and oil phase material
Zs that has not been previously incorporated into the emulsion will, after
HIPS water/oil
phase ratios are achieved, be formed into a stable HIDE. Typically, such HIPEs
will
have a water-to-oil phase ratio which is in the range of from about 12:1 to
about
250:1, more typically from about 20:1 to about 200:1, most typically from
about
25:1 to about 150:1. Such emulsions are stable in the sense that they will not
. . 3o significantly separate into their water and oil phases, at least for a
period of time
sufllcient to permit polymerization of the monomers present in the oil phase.
III. Recirculation of Portion of HIDE from Dynamic Mixing Zone
As noted above, a key aspect of the improved continuous process according to
the present invention is the addition of a recirculation zone. In this
recirculation
CA 02208419 1997-06-20
WO 96/21505 PCT/US96100082
22
zone, a portion of the emulsified mixture withdrawn from the dynamic mixing
zone is
recirculated and then combined with the oil and water phase streams being '
introduced to the dynamic mixing zone, as described previously. By
recirculating a
portion of the withdrawn emulsified mixture, the uniformity of the HIDE
ultimately
s exiting the static mixer is improved, especially in terms of having the
water droplets
homogeneously dispersed in the continuous oil phase. Recirculation may also
allow
higher throughput of HIPE through both the dynamic and static mixing zones, as
well as allow the formulation of HIPEs having higher water to oil phase
ratios.
The particular amount of HIDE that is recirculated will depend upon a variety
io of factors, including the particular components present in the oil and
water phases,
the rate at which the oil and water phase streams are introduced to the
dynamic
mixing zone, the rate at which the emulsified mixture is withdrawn from the
dynamic
mixing zone, the particular throughput desired through both the dynamic and
static
mixing zones, and like factors. For the purposes of the present invention,
from about
is 10 to about 50% of the emulsified mixture withdrawn from the dynamic mixing
zone
is recirculated. In other words, the ratio of the recirculated stream to the
combined
oil phase and water phase streams introduced to the dynamic mixing zone is
from
about 0. I 1:1 to about 1:1. Preferably, from about 15 to about 40% of the
emulsified
mixture withdrawn from the dynamic mixing zone is recirculated (ratio of
zo recirculated stream to combined oil phase and water phase streams of from
about
0.17:1 to about 0.65:1). Most preferably, from about 20 to about 33% of this
withdrawn emulsified mixture is recirculated (ratio of recirculated stream to
combined oil phase and water phase streams of from about 0.25: I to about
0.5:1 ).
The recirculated portion of the withdrawn emulsified mixture is returned to
the
is dynamic mixing zone at a point such that it can ~be combined with the oil
and water
phase streams that are being introduced to the dynamic mixing zone. Typically,
this
recirculated portion of the emulsified mixture (the recirculated stream) is
pumped
back to a point that is proximate the point where the oil and water phase
streams are
entering the dynamic mixing zone. The means used to pump this recirculated
stream
so should not induce shear higher than that previously described for the
dynamic mixing
zone. Indeed, it is typically preferred that this pumping means induce
relatively low
shear to this recirculated stream.
The volume of emulsified components present in the recirculated stream,
relative to the total volume of oil and water phase components present in the
ss dynamic mixing zone, can be important. For example, the recirculated stream
volume can affect the degree of stabilization of the emulsion present in the
dynamic
CA 02208419 1997-06-20
WO 96/21505 PG"f/US96/00082
23
mixing zone, especially if the rate of introduction of the oil phase stream to
the
dynamic mixing zone is reduced or stopped as described above. Conversely, the
higher the recirculation stream volume, the less responsive will be the
continuous
' process to changes in the flow rates or HIDE composition. For production
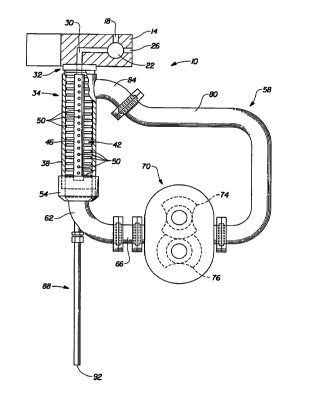
systems
s that are intended to operate for substantial periods of time to make only
one
particular type of HIDE, a relatively large recirculated stream volume is
recommended, i.e., the recirculated stream volume is on the order of from
about 2 to
about 10 times the total volume of oil and water phase components present in
the
dynamic mixing zone. For systems that require substantially faster response to
to changes in the flow rate or HIDE composition, a relatively smaller
recirculated
stream volume is preferred, i.e., the recirculated stream volume is on the
order of
from about 0.3 to about 3 times the total volume of oil and water phase
component
present in the dynamic mixing zone. In addition, if the length of the
recirculation
zone through which this recirculated stream passes is substantially greater
than the
is length of the dynamic mixing zone, e.g., about twice the length, the
inclusion of
static mixing elements in the recirculation zone can be desirable. This is
particularly
important to prevent the build up of the emulsified components on the interior
surfaces of conduits, pipes, etc. that are used to convey this recirculated
stream
through the recirculation zone.
Zo A suitable apparatus for carrying out the improved continuous process of
the
present invention is shown in the Figure and is indicated generally as 10.
Apparatus
has a shot block indicated generally as 14. The oil phase and water phase
streams
are fed from tanks (not shown) to block 14. These oil and water phase streams
enter
through a conduit 18 formed in block 14. A valve indicated generally as 22
controls
is the flow of these oil and water phase ingredients into either conduit 26 or
conduit 30
formed in block 14. Indeed, the relative position of valve 22 determines
whether the
oil and water phase streams flow out through conduit 26, as is shown in the
Figure,
or else flow into conduit 30. Conduit 30 feeds the oil and liquid phase
streams to the
head 32 of the dynamic mixing vessel generally indicated as 34. This vessel 34
is
- so fitted with a vent line (not shown) to vent air during the filling of
vessel 34 to
maintain and all-liquid environment in this vessel.
This dynamic mixing vessel has a hollow cylindrical housing indicated as 38
within which rotates a pin impeller 42. This pin impeller 42 consists of a
cylindrical
shaft 46 and a number of flights of cylindrical impeller pins SO protruding
radially
ss outwardly from this shaft. These flights of pins 50 are positioned in four
rows that
run along a portion of the length of shaft 46, the rows being positioned at
90° angles
CA 02208419 1997-06-20
WO 96/21505 PGT/US96/00082
24
around the circumference of this shaft. The rows of pins 50 are offset along
the
length of shaft 46 such that flights that are perpendicular to each other are
not in the
same radial plane extending from the central axis of shaft 46.
A representative impeller 42 can consist of a shaft 46 having a length of
about
s 18 cm and a diameter of about 1.9 cm. This shaft holds four rows of
cylindrical pins
50 each having a diameter of 0.5 cm and extending radially outwardly from the
central axis of shaft 42 to a length of 1 cm. This impeller 42 is mounted
within
cylindrical housing 38 such that the pins 50 have a clearance of 0.8 mm from
the
inner surface thereof. This impeller can be operated at a speed of from about
300 to
io about 3000 rpm.
Impeller 50 is used to impart shear agitation to the liquid contents present
in
dynamic mixing vessel 34 to form the emulsified mixture. This emulsified
mixture i~
withdrawn from the dynamic mixing vessel through housing cone 54 in which one
end of housing 38 fits. A portion of this withdrawn emulsified mixture is then
is recirculated through the recirculation zone indicated generally as 58. This
recirculation zone has an elbow shaped coupling 62, one end of which fits
within
housing cone 54 to receive that portion of the emulsified mixture to be
recirculated.
The other end of coupling 62 is connected to one end of a hose or conduit 66.
The
other end of hose or conduit 66 is connected to a pumping device generally
zo indicated as 70. A particularly suitable pumping device that imparts low
shear to this
recirculated stream is a Waukesha Lobe Pump. As shown in Figure 3, this
Waukesha pump has elements 74 and 76 that pump the recirculated stream through
the recirculation zone while at the same time imparting only low shear. The
other
end of pump 70 is connected to one end of a hose or conduit 80. The other end
of.
is hose or conduit 80 is connected to one end of coupling 84. The other end of
coupling 84 is connected to housing 38 of the dynamic mixing vessel 34 such
that the
recirculated stream from zone 58 is introduced near the head 30 of this
vessel.
The remaining portion of the withdrawn emulsified mixture that is not
recirculated is subjected to further agitation or mixing in a static mixing
vessel
so indicated as 88. One end of static mixing vessel 88 receives the remaining
portion of
the emulsified mixture exiting dynamic mixing vessel 34. One suitable static
mixer
(14 inches long by 1/2 inch outside diameter by 0.43 inch inside diameter) is
fitted
with a helical internal configuration of mixing elements so as to provide back
pressure to the dynamic mixing vessel 34. This helps keep vessel 34 full of
liquid
3s contents. This static mixer 88 insures appropriate and complete formation
of the
CA 02208419 2000-O1-10
HIPE from the oil and water phases. The RIPE from this static mixer 88 is then
withdrawn through end 92 for further processing such as emulsion
polymerization.
5
IV. Polymerizing HIPS to Obtain Polymeric Foams
HIDE can be continuously withdrawn from the static mixing zone at a rate
which approaches or equals the sum of the flow rates of the water and oil
phase
1o streams fed to the dynamic mixing zone. After the water-to-oil phase ratio
of the feed
materials has been increased to within the desired HIPE range and steady state
conditions have been achieved, the effluent from the static mixing zone will
essentially comprise a stable HIDE emulsion suitable for further processing
into
absorbent foam material. In particular, preferred HIPEs containing a
polymerizable
15 monomer component can be converted to polymeric foams. Polymeric foams of
this
type and especially their use as absorbents in absorbent articles is disclosed
in, for
example, U.S. Patent 5,268,224 (DesMarais et al), issued December 7, 1993.
This HIPS can be converted to a polymeric foam by the following
additional steps: A) polymerizing/curing the HIPS under conditions suitable
for
2o forming a solid polymeric foam structure; B) optionally washing the
polymeric foam
to remove the original residual water phase therefrom and, if necessary,
treating the
foam with a hydrophilizing surfactant and/or hydratable salt to deposit any
needed
hydrophilizing surfactant/hydratable salt, and C) thereafter dewatering this
polymeric
foam.
A. Polymerization/Curin~ of the HIDE
The formed HIPE will generally be collected or poured in a suitable
reaction vessel, container or region to be polymerized or cured. In one
embodiment,
3o the reaction vessel comprises a tub constructed of polyethylene from which
the
eventually polymerized/cured solid foam material can be easily removed for
further
processing after polymerization/curing has been carried out to the extent
desired. It is
usually preferred that the temperature at which the HIPE is poured into the
vessel be
approximately the same as the polymerization/curing temperature.
CA 02208419 2000-O1-10
25a
Suitable polymerization/curing conditions will vary depending upon the monomer
and
other makeup of the oil and water phases of the emulsion (especially the
emulsifier
systems used), and the type and amounts of polymerization initiators
CA 02208419 2000-07-20
26
used. Frequently, however, suitable polymerization/curing conditions will
involve
maintaining the HIPE at elevated temperatures above about 30°C, more
preferably
above about 35°C, for a time period ranging from about 2 to about 64
hours, more
preferably from about 4 to about 48 hours. The HIPE can also be cured in
stages such
as described in U.S. patent 5,189,070 (Brownscombe et al), issued February 23,
1993.
When more robust emulsifier systems such as diglycerol monooleate,
diglycerol isostearate or sorbitan monooleate are used in these HIPEs, the
polymerization/curing conditions can be carried out at more elevated
temperatures of
about 50°C or higher, more preferably about 60°C or higher.
Typically, the RIPE can
be polymerized~cured at a temperature of from about 60° to about
99°C, more
typically from about 65° to about 95°C.
A porous water-filled open-celled HIDE foam is typically obtained after
polymerization/curing in a reaction vessel, such as a tub. This polymerized
HIPE
foam is typically cut or sliced into a sheet-like form. Sheets of polymerized
RIPE
foam are easier to process during subsequent treating/washing and dewatering
steps,
as well as to prepare the HIPS foam for use in absorbent articles. The
polymerized
HIPE foam is typically cut/sliced to provide a cut thickness in the range of
from about
0.08 to about 2.5 cm. During subsequent dewatering, this can lead to collapsed
HIPE
foams having a thickness in the range of from about 0.008 to about 1.25 cm.
B. Treating Washing HIPE Foam
The solid polymerized HIPE foam formed will generally be filled with
residual water phase material used to prepare the HIPE. This residual water
phase
material (generally an aqueous solution of electrolyte and other residual
components
such as emulsifier) should be at least partially removed prior to further
processing and
use of the foam. Removal of this original water phase material will usually be
earned
out by compressing the foam structure to squeeze out residual liquid and/or by
washing the foam structure with water or other aqueous washing solutions.
Frequently several compressing and washing steps, e.g., from 2 to 4 cycles,
will be
used.
After the original water phase material has been removed to the extent
required, the HIPE foam, if needed, can be treated, e.g., by continued
washing, with
an aqueous solution of a suitable hydrophilizing surfactant and/or hydratable
salt.
When these foams are to be used as absorbents for aqueous fluids such as juice
spills,
milk, and the like for clean up and/or bodily fluids such as urine and/or
menses, they
CA 02208419 2000-07-20
27
generally require further treatment to render the foam relatively more
hydrophilic.
Hydrophilization of the foam, if necessary, can generally be accomplished by
treating
the HIPE foam with a hydrophilizing surfactant.
These hydrophilizing surfactants can be any material that enhances the water
wettability of the polymeric foam surface. They are well known in the art, and
can
include a variety of surfactants, preferably of the nonionic type. They will
generally
be liquid form, and can be dissolved or dispersed in a hydrophilizing solution
that is
applied to the HIPE foam surface. In this manner, hydrophilizing surfactants
can be
adsorbed by the preferred HIPE foams in amounts suitable for rendering the
surfaces
thereof substantially hydrophilic, but without substantially impairing the
desired
flexibility and compression deflection characteristics of the foam. Such
surfactants
can include all of those previously described for use as the oil phase
emulsifier for the
HIPE, such as diglycerol monooleate, sorbitan monooleate and diglycerol
monoisostearate. In preferred foams, the hydrophilizing surfactant is
incorporated
such that residual amounts of the agent that remain in the foam structure are
in the
range from about 0.5% to about 15%, preferably from about 0.5 to about 6%, by
weight of the foam.
Another material that needs to be incorporated into the HIDE foam structure is
a hydratable, and preferably hygroscopic or deliquescent, water soluble
inorganic salt.
Such salts include, for example, toxicologically acceptable alkaline earth
metal salts.
Salts of this type and their use with oil-soluble surfactants as the foam
hydrophilizing
surfactant is described in greater detail in U.S. Patent 5,352,711
(DesMarais), issued
October 4, 1994. Preferred salts of this type include the calcium halides such
as
calcium chloride. (As previously noted, these salts can also be employed as
the water
phase electrolyte in forming the HIPE).
Hydratable inorganic salts can easily be incorporated by treating the foams
with aqueous solutions of such salts. These salt solutions can generally be
used to
treat the foams after completion of, or as part of, the process of removing
the residual
water phase from the just-polymerized foams. Treatment of foams with such
solutions preferably deposits hydratable inorganic salts such as calcium
chloride in
residual amounts of at least about 0.1% by weight of the foam, and typically
in the
range of from about 0.1 to about 12%.
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
28
Treatment of these relatively hydrophobic foams with hydrophilizing
surfactants (with or without hydratable salts) will typically be carried out
to the '
extent necessary to impart suitable hydrophilicity to the foam. Some foams of
the
preferred HIDE type, however, are suitably hydrophilic as prepared, and can
have -
s incorporated therein sufficient amounts of hydratable salts, thus requiring
no
additional treatment with hydrophilizing surfactants or hydratable salts. In
particular,
such preferred HIPE foams include those where certain oil phase emulsifiers
previously described and calcium chloride are used in the HIPE. In those
instances,
the internal polymerized foam surfaces will be suitably hydrophilic, and will
include
to residual water-phase liquid containing or depositing sufficient amounts of
calcium
chloride, even after the polymeric foams have been dewatered.
C. Foam DewaterinQ
After the HIPE foam has been treated/washed, it will generally be dewatered.
Dewatering can be achieved by compressing the foam to squeeze out residual
water,
is by subjecting the foam, or the water therein, to temperatures of from about
60° to
about 200°C, or to microwave treatment, by vacuum dewatering or by a
combination
of compression and thermal drying/microwave/vacuum dewatering techniques. The
dewatering step will generally be carried out until the HIPE foam is ready for
use and
is as dry as practicable. Frequently such compression dewatered foams will
have a
Zo water (moisture) content of from about 50 to about 500%, more preferably
from
about 50 to about 200%, by weight on a dry weight basis. Subsequently, the
compressed foams can be thermally dried to a moisture content of from about 5
to
about 40%, more preferably from about 5 to about 15%, on a dry weight basis.
V. Uses of Polymeric Foams Made by Improved Continuous Process
Zs A. In General
Polymeric foams made according to the improved continuous process of the
present invention are broadly useful in a variety of products. For example,
these
foams can be employed as environmental waste oil sorbents; as absorbent
components in bandages or dressings; to apply paint to various surfaces; in
dust mop
so heads; in wet mop heads; in dispensers of fluids; in packaging; in
odor/moisture
sorbents; in cushions; and for many other uses.
CA 02208419 2000-O1-10
29
B. Absorbent Articles
Polymeric foams made according to the improved continuous process of the
present invention are particularly useful as absorbent members for various
absorbent
articles. See copending Canadian application number 2,208,506 which discloses
the
use of these absorbent foams as absorbent members and absorbent articles. By
"absorbent article" is meant a consumer product that is capable of absorbing
1o significant quantities of urine or other fluids (i.e. liquids), like
aqueous fecal matter
(runny bowel movements), discharged by an incontinent wearer or user of the
article.
Examples of such absorbent articles include disposable diapers, incontinence
garments, catamenials such as tampons and sanitary napkins, disposable
training
pants, bed pads, and the like. The absorbent foam structures herein are
particularly
suitable for use in articles such as diapers, incontinence pads or garments,
clothing
shields, and the like.
In its simplest form, such absorbent articles need only include a backing
sheet, typically relatively liquid-impervious, and one or more absorbent foam
structures associated with this backing sheet. The absorbent foam structure
and the
2o backing sheet will be associated in such a manner that the absorbent foam
structure is
situated between the backing sheet and the fluid discharge region of the
wearer of the
absorbent article. Liquid impervious backing sheets can comprise any material,
for
example polyethylene or polypropylene, having a thickness of about 1.5 mils
(0.038
mm), which will help retain fluid within the absorbent article.
More conventionally, these absorbent articles will also include a liquid-
pervious topsheet element that covers the side of the absorbent article that
touches the
skin of the wearer. In this configuration, the article includes an absorbent
core
comprising one or more absorbent foam structures positioned between the
backing
sheet and the topsheet. Liquid-pervious topsheets can comprise any material
such as
3o polyester, polyolefin, rayon and the like that is substantially porous and
permits body
fluid to readily pass there through and into the underlying absorbent core.
The
topsheet material will preferably have no propensity for holding aqueous
fluids in the
area of contact between the topsheet and the wearer's skin.
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
VI. Specific Examples
Example 1: Preparation of RIPE and Foams from a HIDE.
A) HIDE Preparation
Anhydrous calcium chloride (36.32 kg) and potassium persulfate (189 g) are
s dissolved in 378 liters of water. This provides the water phase stream to be
used in a
continuous process for forming a HIDE emulsion.
To a monomer combination comprising distilled divinylbenzene (40%
divinylbenzene and 60% ethyl styrene) (2100 g), 2-ethylhexyl acrylate (3300
g), and
hexanediol diacrylate (600 g) is added a diglycerol monooleate emulsifier (360
g~,
to and Tinuvin 765 (30 g). The diglycerol monooleate emulsifier (Grindsted
Products;
Brabrand, Denmark) comprises approximately 81% diglycerol monooleate, 1% other
diglycerol monoesters, 3% polyglycerols, and 15% other polyglycerol esters.
After
mixing, this combination of materials is allowed to settle ovennight. No
visible
residue is formed and all of the mixture is withdrawn and used as the oil
phase in a
is continuous process for forming a HIPS emulsion.
Separate streams of the oil phase (25°C) and water phase (53°-
55°C) are fed
to a dynamic mixing apparatus. Thorough mixing of the combined streams in the
dynamic mixing apparatus is achieved by means of a pin impeller. The pin
impeller
comprises a cylindrical shaft of about 21.6 cm in length with a diameter of
about 1.9
zo cm. The shaft holds 4 rows of pins, 2 rows having 17 pins and 2 rows having
16
pins, each having a diameter of 0.5 cm extending outwardly from the central
axis of
the shaft to a length of 1.6 cm. The pin impeller is mounted in a cylindrical
sleeve
which forms the dynamic mixing apparatus, and the pins have a clearance of 0.8
mm
from the walls of the cylindrical sleeve.
zs A minor portion of the effluent exiting the dynamic mixing apparatus is
withdrawn and enters a recirculation zone, as shown in the Figure. The
Waukesha
pump in the recirculation zone returns the minor portion to the entry point of
the oil
and water phase flow streams to the dynamic mixing zone.
A spiral static mixer is mounted downstream from the dynamic mixing
so apparatus to provide back pressure in the dynamic mixing apparatus and to
provide
improved incorporation of components into the HIDE that is eventually formed.
The
static mixer (TAH Industries Model 070-821, modified by cutting off 2.4 inches
(6.1
CA 02208419 1997-06-20
WO 96/21505 PGT/US96/00082
31
cm) of its original length) is 14 inches (35.6 cm) long with a 0.5 inch (1.3
cm)
outside diameter.
The combined mixing and recirculation apparatus set-up is filled with oil
phase and water phase at a ratio of 3 parts water to 1 part oil. The dynamic
mixing
s apparatus is vented to allow air to escape while filling the apparatus
completely. The
flow rates during filling are 3.78 g/sec oil phase and 11.35 cc/sec water
phase with
about 1 S cc/sec recirculation.
Once the apparatus set-up is filled, the water phase flow rate is cut in half
to
reduce the pressure build up while the vent is closed. Agitation is then begun
in the
io dynamic mixer, with the impeller turning at 1800 RPM. The flow rate of the
water
phase is then steadily increased to a rate of 45.4 cc/sec over a time period
of about 1
min., and the oil phase flow rate is reduced to 0.757 g/sec over a time period
of
about 2 min. The recirculation rate is steadily increased to about 45 cc/sec
during
the latter time period. The back pressure created by the dmamic and static
mixers at
~s this point is about 10 PSI (69 kPa). The Waukesha pump speed is then
steadily
decreased to a yield a recirculation rate of about 11 cc/sec.
B) Polymerization of HIDE
The HIDE flowing from the static mixer at this point is collected in a round
polypropylene tub, 17 in. (43 cm) in diameter and 7.5 in ( 10 cm) high, with a
Zo concentric insert made of Celcon plastic. The insert is 5 in ( 12.7 cm) in
diameter at
its base and 4.75 in (12 cm) in diameter at its top and is 6.75 in (17.1 cm)
high. The
HIDE-containing tubs are kept in a room maintained at 65 °C. for 18
hours to bring
about polymerization and form the foam.
C) Foam Washing and Dewatering
2s The cured HIDE foam is removed from the curing tubs. The foam at this
point has residual water phase (containing dissolved emulsifiers, electrolyte,
initiator
residues, and initiator) about 50-60 times (50-60X) the weight of polymerized
monomers. The foam 'is sliced with a sharp reciprocating saw blade into sheets
which are 0.160 inches (0.406 cm) in thickness. These sheets are then
subjected to
so compression in a series of 2 porous nip rolls equipped with vacuum which
gradually
reduce the residual water phase content of the foam to about 6 times (6X) the
weight
of the polymerized material. At this point, the sheets are then resaturated
with a
1.5% CaCl2 solution at 60°C., are squeezed in a series of 3 porous nip
rolls equipped
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
32
with vacuum to a water phase content of about 4X. The CaCl2 content of the
foam
is between 8 and 10 %.
The foam remains compressed after the final nip at a thickness of about 0.021
in. (0.053 cm). The foam is then dried in air for about 16 hours. Such drying
reduces the moisture content to about 9-17% by weight of polymerized material.
At
this point, the foam sheets are very drapeable. In this collapsed state, the
density of
the foam is about 0.14 glcc.
Example 2: Preparation of HIPEs Under Various Operatin~~Conditions
HIPEs are continuously prepared from oil phase stream consisting of 'a
monomer component having 40% divinylbenzene (50% purity) and 60% 2-ethylhexyl
acrylate to which is added diglycerol monooleate (6% by weight of the
monomers)
and Tinuvin 765 (0.5% by weight of the monomers). These HIPEs are prepared
with the apparatus shown in the Figure using the operating conditions shown in
Table 1 below:
Table 1
Run W/O Ratio Impeller TemperatureBack Recirculation
(RP1V~ (F) Pressure Rate
( si)
A 75 1800 130 9.8 3
B 90 1800 130 9.4 3
C 90 1200 130 6.7 3
D 100 1000 130 5.1 3
E 100 800 150 3.6 3
F 120 700 150 3.6 3
G 120 700 166 3.5 3
- -
H 140 700 166 3 .7 3
I
Example 3. Preparation of HIPEs Under Various Operating Conditions
HIPEs are continuously prepared from oil phase stream consisting of a
monomer component having 35% divinylbenzene (40% purity), 55% 2-ethylhexyl
acrylate and 10% hexanediol diacrylate to which is added diglycerol monooleate
(5%
CA 02208419 1997-06-20
WO 96/21505 PCT/US96/00082
33
by weight of the monomers), ditallow dimethyl ammonium methyl sulfate (1% by
weight of the monomers) and Tinuvin 765 (0.5% by weight of the monomers).
These FiIPEs are prepared with the apparatus shown in Figure 3 using the
operating
conditions shown in Table 2 below:
Table 2
Run W/O Ratio Impeller TemperatureBack Recirculation
(F) Pressure Rate
( si)
A 60 1800 130 10 6
B 60 1800 130 9.6 3
C 60 1800 130 9.6 1.5
D 60 1800 130 5 0
E 85 1500 130 5.8 3