Note: Descriptions are shown in the official language in which they were submitted.
CA 02208541 1997-06-17
O.Z. 0050/45873
THE PRODUCTION OF COVERED TABLETS
The present invention relates to a process for
the production of covered tablets by molding a melt which
contains an active ingredient in a calender with counter-
rotating molding rolls which have on their surface
depressions for receiving and molding the tablet com-
position (melt calendering).
The production of tablets by calendering a melt
containing an active ingredient is disclosed in
DE-A 1 766 546 and US-A 4,880,585. The basis of this
process is the embedding of an active ingredient in a
melt of a carrier, eg. fatty substances or water-soluble
thermoplastic polymers. The melt is produced by melting
the mixture of active ingredient, polymer and, where
appropriate, other ancillary substances, for example in
an extruder, and molding the melt in a downstream molding
calender to give tablets, which harden on cooling. The
molding calender comprises a pair of counter-rotating
molding rolls which have on their surface engravings
(depressions) which correspond to the shape of one half
of the required tablet. The tablet molding takes place in
the region of contact of the two rolls by combination of
the tablet composition in one depression on one roll with
that in the opposite depression on the other roll.
Most of the tablets on the market are produced as
film-coated tablets, ie. a thin layer of water-soluble
polymers is applied to the tablets in the last production
step. This film-coating is often indispensable for
various reasons, because, for example,
a) a taste which is conferred by the 'active
ingredients and/or ancillary substances used must be
masked until the tablet reaches the stomach,
b) the active ingredient used is unstable to, for
example, light, moisture etc.,
c) the tablets require a colored coating for easier
identification.
CA 02208541 1997-06-17
- 2 - O.Z. 0050/45873
The protective layers (the coating) have to date
been applied almost exclusively by spraying on solutions
of water-soluble polymers (organic solvents and/or water)
with simultaneous drying. Besides the film coating (layer
thickness in the micrometer range) which is conventional
nowadays, there is the sugar-coating process in which
thick layers, which are sometimes in the millimeter
range, of sugar-containing mixtures are applied. These
widely used techniques are described in various textbooks
(see H. Sucker, P. Fuchs, P. Speiser: Pharmazeutische
Technologies 2nd edition, G. Thieme Verlag Stuttgart
(1991), pages 347-368).
If a coating was required over the tablets pro-
duced by melt calendering it was necessary to apply this
coating in a separate step after the tablets had cooled.
This took place in a conventional way, for example by
spraying on in rotating drums, by the dip pipe process
or
in a fluidized bed etc.
The conventional processes for applying coating
layers or for sugar coating all require comparatively
very high energy input, because the solvents used in the
spray solutions must be removed again rapidly after
spraying onto the tablets. In addition, a coating process
usually takes several hours because the spraying rate
cannot be set as high as may be required.
The application of the coating in a separate step
thus requires considerable expenditure of time,
additional machinery and additional staff, which has
marked effects on the production costs.
It is an object of the present invention to
provide a process for the production of covered tablets
by melt calendering in which covering of the tablets is
possible in a simple and cost-saving manner.
We have found that this object is achieved by
producing tablets by melt calendering with the melt
containing active ingredient being introduced between two
sheets of the covering material into the calender molding
i i
CA 02208541 2004-11-17
3
rolls.
The present invention therefore relates to a
process for the production of covered tablets by molding
a melt containing active ingredient in a calender with
two counter-rotating molding rolls which have on their
surface mutually opposite depressions for receiving and
molding the tablet composition, wherein the melt contain-
ing active ingredient is introduced between two sheets of
the covering material into the molding rolls.
More specifically the invention as claimed
relates to a process for the production of covered tablets
by the following steps:
(i) preparing a pharmaceutically mixture which
contains one or more pharmaceutical active ingredients and
one or more conventional ancillary substances and which
becomes pasty to viscous by melting or softening of at
least one component,
(ii) melting the pharmaceutical mixture, and
(iii) molding the melt containing the active
ingredients in a calender with counter-rotating molding
rolls which have on their surface depressions for receiving
and molding the pharmaceutical mixture, with the melt con-
taining the active ingredients) being introduced between
two sheets of a covering material into the molding rolls.
The tablets are produced starting from a mixture
which contains one or more pharmaceutical active ingre-
dients and one or more conventional ancillary substances
and which becomes a paste or viscous liquid (thermo-
plastic) , and can therefore be extruded, by melting or
softening of at least one component.
The pharmaceutical mixture is then melted in a
conventional way, preferably in an extruder, and fed to
the molding calender as described, for example, in
US-A 4,880,585.
CA 02208541 2004-05-06
3a
At the same time as the melt, two sheets which
form the covering material are fed to the molding rolls,
which are in contact along a surface line or are located
at only a very small distance from one another, in such
a way that the sheets are each located between molding
roll and melt. Subsequently during the calendering the
tablet composition is molded to the required tablet shape
with, simultaneously, the part forming the covering of
the tablets being cut out of the sheet and applied to the
tablets. The temperatures prevailing on the molding
to rolls, which are generally from 50 to 150°C, result in
softening of the sheet material and thus covering.of the
tablets. The sheet material melts at the edges of the
tablets and thereby envelopes the tablets singly so that
the tablet is completely and uniformly coated with the
covering material.
If necessary, the covered tablets are
subsequently subjected to a cooling process, for example
CA 02208541 1997-06-17
' - 4 - O.Z. 0050/45873
in an air or cooling bath.
The process according to the invention has the
advantage that the individual processes of granulation,
tableting and coating, which take place discontinuously
in conventional tablet production, are combined in a
single process step which, moreover, takes place con-
tinuously. Furthermore, the application of the coating
requires no additional energy input because it takes
place at the same time as the tableting (in this case:
calendering), which is anyway carried out at elevated
temperatures.
In a preferred embodiment, sheets suitable for
y forming a film coating on the tablets are used so that
film-coated tablets are obtained. The layer thickness of
the film can be varied over a wide range. This is pos-
sible in the conventional spraying-on only by changing
the process times (longer/shorter spraying time). The
superiority of the novel process (saving of time) is
particularly evident with thicker layers because these
thick layers can be applied extremely rapidly and very
uniformly. In general, sheets with a thickness of about
10 ~m to 500 ~m are used. It is possible to use sheets
which differ in thickness so that there is a different
thickness of film coating on the upper and lower halves
of the tablet, which makes it possible specifically to
influence, for example, the dissolution characteristics
of the tablet in the gastrointestinal tract.
The sheet material can be selected from a wide
range of materials. The only requirement is that the
material is pharmaceutically acceptable. The sheet
material can be chosen so that the resulting. tablet
dissolves in the gastric fluid or the resulting tablet
has modified release of active ingredient, for example a
tablet with enteric coating or a tablet with a prolonged
action, for example a tablet of the sustained release
type, prolonged release type, repeat release type or
delayed release type.
CA 02208541 2004-05-06
Sheet materials which are suitable for producing
such film-coated tablets and which rapidly dissolve in
the acidic gastric fluid are, in particular, gelatin,
polyvinyl alcohol, alkylcelluloses such as methyl-
celluloses, hydroxyalkylcelluloses such as hydroxyethyl-,
hydroxypropyl- or hydroxypropylmethylcelluose, polyvinyl-
pyrrolidone, certain acrylic resins such as copolymers
based on dimethylaminoethyl methacrylate and
methacrylates (Eudragit E~ etc., alone or mixed with one
another.
Examples of film formers which can be used
according to the invention for coatings with modified
release of active ingredient are alkylcelluloses such as
ethylcellulose, polyvinyl esters such as polyvinyl
acetate, certain acrylic resins such as copolymers based
on methacrylic acid and methacrylate (Eudragit L and S),
cellulose phthalates such as cellulose acetate phthalate
or hydroxypropylmethylcellulose phthalate etc. The
release characteristics can additionally be influenced by
using sheets of different materials, it also being
possible to use a plurality of sheets for covering one or
both tablet halves.
When water-soluble sheets are used, thermoplastic
polymers such as hydroxyalkylcelluloses, gelatin or
acrylic resins have proven particularly suitable as sheet
material. They can be used in a thickness of about 50 to
150 ~m and, in this case, form a thin, very uniform
water-soluble coating on the tablets.
The process according to the invention makes
substantially aseptic production of tablets possible. The
melting of the tablet composition and, if this takes
place in an extruder, the intense input of shear energy
into the product kills the microbes in the composition so
that it can be fed as sterile product to the molding
rolls. If sterilized polymer sheets are then used, and
the melt calendering is carried out under aseptic con-
ditions, for example with sterile air (laminar flow), the
* trademark
CA 02208541 1997-06-17
- 6 - O.Z. 0050/45873
tablets are obtained in sterile form. The tablets can
then be packed sterile in another process or, which is
particularly preferred, blister-packed simultaneously
with the molding of the tablets (see the statements
hereinafter). In the latter case, the risk of contami-
nation of the product with pathogenic microbes is con-
siderably reduced by comparison with a conventional
process with separate packaging.
The sheets can also according to the invention
contain another active ingredient. This can be an active
ingredient which is not compatible with one of the
components in the tablet composition. The incompatible
constituents are kept separate from one another in this
way. The inclusion of an active ingredient in the sheet
also makes it possible, however, to release an initial
dose owing to the active ingredient contained in the
sheet and then, with the actual tablet, to provide
another single dose or maintenance of the drug
concentration.
In another embodiment, the sheets used are those
suitable for packaging the tablets. These are, in par-
ticular, water-insoluble thermoforming sheets, the
preferred material being polyethylene, polypropylene,
polyvinyl chloride, polyethylene terephthalate, poly-
styrene, aluminum or coated aluminum. The tablets are in
this way immediately sealed in a blister pack. The
separate packaging step which is otherwise customary is
thus unnecessary and, moreover, it is possible in this
way to pack the tablets aseptically in an extremely
simple manner, especially when care is taken that the
outer edges of the tablet strip are sealed airtight.
It has surprisingly emerged that there is not, as
expected, vigorous adhesion of the hot tablet composition
to the water-insoluble thermoforming sheet so that later
removal of the tablets from the pack would be impeded or
even impossible.
It has proven particularly advantageous for
CA 02208541 1997-06-17
- 7 - O.Z. 0050/45873
packaging the tablets to combine a molding roll with the
depressions for receiving and molding the tablet com-
position with a smooth roll. This results in "half"
tablets which are sealed in a blister pack which has on
one side depressions for receiving the tablets and is
closed on the other side with a smooth sheet which can
be
pulled off. In this case, an aluminum sheet or a sheet
of
coated aluminum has proven particularly expedient for
closing the pack.
It may also prove to be expedient not to allow
the packaged tablets to cool in air, as otherwise usual,
but to provide a separate cooling step.. Suitable for this
purpose is a water bath, stream of cold air etc. This
prevents the tablets in the pack cooling too slowly,
which may lead to subsequent deformation of the tablets.
It is also possible to use the sheets for the
film coating of the tablets and the sheets for the
blister-packaging of the tablets simultaneously. In this
case, the melt in the molding rolls is covered by the
sheet for the film coating and simultaneously sealed in
the packaging sheet. This makes it possible to carry out
all the basic operations needed to produce the tablets,
namely tableting, coating and packaging, in a single step
which, moreover, takes place in a continuous manner. This
is associated with enormous savings in cost.
In some cases it has proven expedient to coat the
molding rolls or the sheets which are used, in particular
the outer sides thereof, with a mold release agent in
order to facilitate detachment of the tablets or the
packaging from the molding rolls. Examples of suitable
mold release agents are silicone resins, stearic acid,
calcium or magnesium stearate, paraffin, cetyl alcohol
or
lecithins.
It is also possible using the process according
to the invention to add further additives to the sheets
in a simple manner. Examples of such additives are
colored pigments, it being possible for the upper and
CA 02208541 1997-06-17
- 8 - O.Z. 0050/45873
lower sides of the tablets or the packaging to differ in
color, masking flavors, plasticizers etc. It is also
possible for one or both sheets to be printed, eg. with
numbers, names etc., in order to ensure unambiguous
identification of the tablets by the patients. This has
been possible to date only by subsequent printing with
ink jet printers.
The shape of the depressions and thus of the
tablets can be chosen substantially as desired. Depres-
sions which are elongate and in the shape of a segment
of
an ellipsoid, so that oblong tablets and lenticular
tablets are obtained, are particularly expedient.
It is also possible, if required, to produce
divisible tablets. For this purpose it is possible to
provide a small rib, which is often in the micrometer
range, on the bottom of the depressions, which leads to
formation of the score in the finished tablets. However,
it is preferable to use at least one molding roll in
which the depressions are divided by at least one bar
which extends essentially to the surface of the molding
roll and forms the score.
The abovementioned mixtures for producing the
tablets are, in particular, mixtures which contain
pharmacologically acceptable polymers (with the glass
transition temperature of the mixture being below the
decomposition temperature of all the components of the
mixture), for example polyvinylpyrrolidone (PVP), copoly-
mers of N-vinylpyrrolidone (NVP) and vinyl esters, in
particular vinyl acetate, copolymers of vinyl acetate and
crotonic acid, partially hydrolyzed polyvinyl acetate,
polyvinyl alcohol, ethylene/vinyl acetate copolymers,
poly(hydroxyethyl methacrylate), copolymers of methyl
methacrylate and acrylic acid, cellulose esters,
cellulose ethers, in particular hydroxypropylcellulose,
polyethylene glycol or polyethylene, preferably NVP
copolymers with vinyl acetate, hydroxypropylcellulose and
polyethylene glycols/polyethylene oxides. The K values
CA 02208541 1997-06-17
- 9 - O.Z. 0050/45873
(H. Fikentscher, Cellulose-Chemie 13 (1932) 58-64 and
71-74) of the polymers are in the range from 10 to 100,
preferably 12 to 70, in particular 12 to 35, for PVP
preferably 12-35, in particular 12-17.
The polymeric binder must soften or melt in the
complete mixture of all the components in the range from
50 to 180, preferably 60 to 130°C, so that the composi-
tion can be extruded. The glass transition temperature of
the mixture must therefore always be below 180, pre-
ferably below 130°C. It is if necessary reduced by
conventional pharmacologically acceptable plasticizing
ancillary substances such as long-chain alcohols, ethy-
l lene glycol, propylene glycol, trimethylolpropane,
triethylene glycol, butanediols, pentanols, hexanols,
polyethylene glycols, silicones, aromatic carboxylic
esters (eg. dialkyl phthalates, trimellitic esters,
benzoic esters, terephthalic esters) or aliphatic dicar
boxylic esters (eg. dialkyl adipates, sebacic esters,
azelaic esters, citric and tartaric esters) or fatty acid
esters.
Examples of conventional pharmaceutical ancillary
substances, whose total amount can be up to 100% by
weight based on the polymer, are extenders such as
silicates or diatomaceous earth, stearic acid or salts
thereof, eg. the magnesium or calcium salt, methylcellu-
lose, sodium carboxymethylcellulose, talc, sucrose,
lactose, cereal or corn starch, potato flour, polyvinyl
alcohol, also wetting agents, preservatives, disinte-
grants, absorbents, colorants, flavorings (cf., for
example, H. Sucker et al. Pharmazeutische Technologie,
Thieme-Verlag, Stuttgart 1978). The only condition for
suitability thereof is adequate thermal stability.
Pharmaceutical active ingredients mean for the
purpose of the invention all substances with a pharmaceu
tical effect and minimal side effects as long as they do
not decompose under the processing conditions. The amount
of active ingredient per dose unit and the concentration
CA 02208541 1997-06-17
- 10 - O.Z. 0050/45873
may vary within wide limits depending on the activity and
rate of release. The only condition is that they suffice
to achieve the desired effect . Thus, the concentration
of
active ingredient can be in the range from 0.1 to 95,
preferably from 20 to 80, in particular 30 to 70, % by
weight. It is also possible to use combinations of active
ingredients. Active ingredients for the purpose of the
invention are also vitamins and minerals, as well as crop
treatment agents and insecticides.
The process according to the invention is suit-
able, for example, for processing the following active
ingredients:
acebutolol, acetylcysteine, acetylsalicylic acid,
acyclovir, alprazolam, alfacalcidol, allantoin, allo-
purinol, ambroxol, amikacin, amiloride, aminoacetic acid,
amiodarone, amitriptyline, amlodipine, amoxicillin,
ampicillin, ascorbic acid, aspartame, astemizole,
atenolol, beclomethasone, benserazide, benzalkonium
hydroxide, benzocaine, benzoic acid, betamethasone,
bezafibrate, biotin, biperiden, bisoprolol, prazosin,
bromazepam, bromhexine, bromocriptine, budesonide,
bufexamac, buflomedil, buspirone, caffeine, camphor,
captopril, carbamazepine, carbidopa, carboplatin,
carotenoids such as ~-carotene or canthaxanthin,
cefachlor, cefalexin, cefatroxil, cefazolin, cefixime,
cefotaxime, ceftazidime, ceftriaxone, cefuroxime,
celedilin, chloramphenicol, chlorhexidine, chlorpheni-
ramine, chlortalidone, choline, cyclosporin, cilastatin,
cimetidine, ciprofloxacin, cisapride, cisplatin,
clarithromycin, clavulanic acid, clomipramine, clonaze-
pam, clonidine, clotrimazole, codeine, cholestyramine,
cromoglycic acid, cyanocobalamin, cyproterone, deso-
gestrel, dexamethasone, dexpanthenol, dextromethorphan,
dextropropoxiphene, diazepam, diclofenac, digoxin,
dihydrocodeine, dihydroergotamine, diltiazem, diphen-
hydramine, dipyridamole, dipyrone, disopyramide, domperi-
done, dopamine, enalapril, ephedrine, epinephrine,
CA 02208541 1997-06-17
- 11 - O.Z. 0050/45873
ergocalciferol, ergotamine, erythromycin, estradiol,
ethinylestradiol, etoposide, Eucalyptus globulus, famoti-
dine, felodipine, fenofibrate, fenoterol, fentanyl,
flavin mononucleotide, fluconazole, flunarizine, fluoro-
uracil, fluoxetine, flurbiprofen, furosemide, gemfibro-
zil, gentamicin, Ginkgo biloba, glibenclamide, glipizide,
clozapine, Glycyrrhiza glabra, guaifenesin, haloperidol,
heparin, hyaluronic acid, hydrochlorothiazide, hydro-
codone, hydrocortisone, hydromorphone, ipratropium
hydroxide, ibuprofen, imipenem, indomethacin, iohexol,
iopamidol, isosorbide dinitrate, isosorbide mononitrate,
isotretinoin, ketotifen, ketoconazole, ketoprofen,
(~ ketorolac, labetalol, lactulose, lecithin, levocarnitine,
levodopa, levoglutamide, levonorgestrel, levothyroxine,
lidocaine, lipase, lipoic acid, lisinopril, loperamide,
lorazepam, lovastatin, medroxyprogesterone, menthol,
methotrexate, methyldopa, methylprednisolone, metoclo-
pramide, metoprolol, miconazole, midazolam, minocycline,
minoxidil, misoprostol, morphine, multivitamin mixtures
or combinations and mineral salts, N-methylephedrine,
naftidrofuryl, naproxen, neomycin, nicardipine, nicer-
goline, nicotinamide, nicotine, nicotinic acid, nifedi-
pine, nimodipine, nitrendipine, nizatidine, norethis-
terone, norfloxacin, norgestrel, nortriptyline, nystatin,
ofloxacin, omeprazole, ondansetron, pancreatin, pan-
thenol, pantothenic acid, paracetamol, penicillin G,
penicillin V, phenobarbital, pentoxifylline, phenyl-
ephrine, phenylpropanolamine, phenytoin, piroxicam,
polymyxin B, povidone-iodine, pravastatin, prednisolone,
bromocriptine, propafenone, propranolol, pseudoephedrine,
pyridoxine, quinidine, ramipril, ranitidine, reserpine,
retinol, riboflavin, rifampicin, rutoside, saccharin,
salbutamol, salcatonin, salicylic acid, simvastatin,
somatropin, sotalol, spironolactone, sucralfate, sulbac-
tam, sulfamethoxazole, sulpiride, tamoxifen, tegafur,
teprenone, terazosin, terbutaline, terfenadine, theophyl-
line, thiamine, ticlopidine, timolol, tranexamic acid,
CA 02208541 2004-05-06
12
tretinoin, triamcinolone acetonide, triamterene, tri-
methoprim, troxerutin, uracil, valproic acid, vancomycin,
verapamil, vitamins B1, BZ, B" B6, Blz, D" E, K, folinic
acid, zidovudine.
In a few cases, solid solutions may form. The
term "solid solutions" is familiar to the skilled person,
for example from the literature cited at the outset. In
solid solutions of pharmaceutically active ingredients in
polymers, the active ingredient is present in a molecular
dispersion in the polymer.
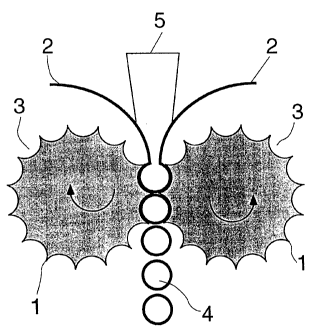
Figure 1 illustrates the claimed process. As is
shown in this figure, the molten pharmaceutical mixture 5
containing the active ingredients is introduced between two
sheets 2 of the covering material into counter-rotating
molding rolls 1 of a calendar, the counter-rotating molding
rolls 1 having on their surfaces depressions 3 for
receiving and molding the pharmaceutical composition.
The following examples illustrate the invention
without restricting it.
EXAMPLE 1
2o A mixture of 60.0 % by weight of Rollidon VA-64*
(BASF) (polyvinylpyrrolidone copolymer with vinyl acetate
(60:40)) and 40.0 % by weight of lactose monohydrate was
extruded in a twin screw extruder (ZSR-40* Werner +
Pfleiderer) under the following conditions:
- Temperatures:
Shot 1: 80°C
Shot 2: 100°C
Shot 3: 130°C
Shot 4: 130°C .
Dies: 135°C
30 _ Material throughput: 25 kg/h
- Screw speed: 160 rpm
* trademarks
CA 02208541 2004-05-06
12a
The melt 5 was introduced together with two
polypropylene sheets 2 about 300 micrometers thick
(thermoforming blister sheet) into the molding calender
with two molding rolls 1 which rotate in the direction of
the arrows (useful widths of the molding rolls about 14
cm). The depressions 3 in the molding rolls 1 were
designed so that oblong tablets (20 x 8.5 mm) 4 weighing
about 1000 mg were molded from the melt. The material
left the calender as strip of tablets packed in PP sheet.
The tablets were not sealed singly in the sheet because
CA 02208541 1997-06-17
- 13 - O.Z. 0050/45873
the molding rolls of the calender were adjusted so that
they were not in direct contact at any point (spacing
about 0.1 mmj. After cooling it was easy to remove the PP
sheet as a strip about 14 cm wide (7 parallel rows of
tablets on the molding roll) from the tablets. In no case
was there adhesion of the melt to the PP sheet. It was
possible to seal individual rows of tablets or the tablet
strip across the whole width of the molding roll by
subsequent welding of the outer edges (exclusion of airj .
The calender and molding rolls useful for the present
invention can be cooled or heated in a manner known per se and
the optimum surface temperature of the rolls for the relevant
processing step can be adjusted in this way.