Note: Descriptions are shown in the official language in which they were submitted.
CA 022088~9 1997-06-26
W O96/21443 PCTADK~6/00014
5 Use of 3,4-diDhenvl chromans for the manufacture of a Dharmaceutical
comoosition for the treatment or DroDhvlaxis of hvDerliDoDroteinaemia,
hvoertriqlvceridaemia, hvDerliDidaemia or hvDercholesterolaemia or
arteriosclerosis or for anticoagulative treatment
FIELD OF THIS INVENTION
The present invention relates to the use of compounds of the general
formula I for the treatment of patients suffering from hyperlipoproteinae-
mia, hypertriglyceridaemia, hyperlipidaemia or hypercholesterolaemia and
15 prophylaxis hereof. The present invention furthermore relates to the use
of compounds of the general formula I for the treatment of patients
suffering from arteriosclerosis including atherosclerosis and prophylaxis
hereof, and furthermore to the use of compounds of the general formula
I for the treatment of patients in a need of an anticoagulative treatment
20 or prophylaxis, e.g. following a coronary thrombosis or postoperativelY
i.e. after surgery. The present invention also embraces pharmaceutical
compositions comprising these compounds and methods of using the
compounds and their pharmaceutical compositions.
BACKGROUND OF THIS INVENTION
Ischemic heart disease (IHD) the relevant cardiovascular disease in
relation to postmenopausal women, primarily is caused by
atherosclerosis (Havel and Rapaport, N Eng J Med 1995; 332:1491-
30 1498). Other frequent manifestations of atherosclerosis iscerebrovascular disease, and intermittent claudication. An important risk
factor for the development of atherosclerosis is an atherogenic lipid
CA 022088~9 1997-06-26
WO 96/21443 PCT/DK96/00014
profile, i.e. hyperlipidaemia with increased LDL-cholesterol and relatively
decreased HDL-cholesterol. In epidemiological studies (Samsioe G. Int J
Fertil, 1993;38,suppl. 1 :19-23) it has been indicated that estrogen
therapy in postmenopausal women reduces the stenosis of the coronary
5 arteries, thereby increasing survival rate compared to a non-treated
population. An important factor in this effect on the coronary system is a
reduction in serum lipids and a normalisation of the relation between
LDL-cholesterol and HDL-cholesterol (Samsioe G. Int J Fertil 1994; 39
suppl. 1:43-49).
One object of the present invention is to provide compounds which can
effectively be used in the treatment or prophylaxis of hyperlipoproteinae-
mia, hypertriglyceridaemia, hyperlipidaemia or hypercholesterolaemia.
15 Data on men suggest that a 35% reduction in LDL-cholesterol is required
to achieve a 50% reduction in cardiovascular disease, i.e. coronary
atherosclerosis provided that LDL-cholesterol reduction are the sole
cause of cardioprotection. However, estrogens reduce LDL-cholesterols
by only 5-10%. It is therefore now believed that the lipid effects account
20 for only 25-40% of the reduction in the incidence of coronary heart
disease after estrogen replacement therapy. A possible mechanism could
be a direct effect on the vessel wall improving blood flow and inhibiting
the atherogenic mechanisms independent on an effect on plasma lipids.
25 Another object of the present invention is to provide compounds which
can effectively be used in the treatment or prophylaxis of arteriosclerosis
including atherosclerosis.
Coagulation and thrombosis are important mechanisms involved in the
30 pathogenesis of atherosclerosis and its complications such as vascular
occlusion and embolism. Furthermore, blood clotting is important for the
development of vascular restenosis following surgical intervention of
CA 022088~9 1997-06-26
W O96/21443 PCTADK96/00014
blocked arteries. Restenosis occurs in about 35% of the patients after 6
months. Current therapy such as heparin, low molecular heparin and
aspirin or stents have failed to reduce the incidence of restenosis. New
therapeutic possibilities which can inhibit a tendency for thrombosis after
endothelial damage are therefore needed.
Another further object of the present invention is to provide compounds
which can effectively be used in the treatment or prophylaxis of patients
in a need of an anticoagulative treatment or prophylaxis, e.g. following a
coronary thrombosis or after surgery.
Recent studies (Writing group for the PEPI trial, JAMA 273:199, 1995)
confirm that oral estrogen taken alone or in combination with medroxy-
progesterone acetate or micronized progesterone is associated with a
beneficial effect on the risk of developing cardiovascular disease through
an improved effect on lipoprotein and the fibrinogen profile. However,
estrogen is also known to have adverse effects on endometrium and
perhaps breast tissue by increasing the frequency of malignancies in
these areas after prolonged treatment.
Thus, there is a need for new compounds which have beneficial effects
on hyperlipoproteinaemia, hypertriglyceridaemia, hyperlipidaemia or
hypercholesterolaemia or arteriosclerosis including atherosclerosis or as
an anticoagulant, but without introducing significant effects in the
reproductive tissues.
Centchroman is a non-steroidal compound known to have antiestrogenic
activity. It is in use in India as an oral contraceptive (see, for example,
Salman et al., U.S. Patent Specification No. 4,447,622; Singh et al.,
Acta Endocrinal (Copenh) 126 (1992), 444 - 450; Grubb, Curr Opin
Obstet Gvnecol 3 (1991), 491 - 495; Sankaran et al., Contraception _
(1974), 279 - 289; Indian Patent Specification No. 129187). Centchro-
CA 022088~9 1997-06-26
W O96/21443 PCT~DK96/00014
man has also been investigated as an anti-cancer agent for treatment of
advanced breast cancer (Misra et al., Int J Cancer 43 (1989), 781 -
783). Recently, centchroman as a racemate has been found potent as a
cholesterol lowering pharmaceutical expressed by a significant decrease
5 of the serum concentrations (S.D. Bain et al., J Min Bon Res 9 (1994), S
394).
U.S. patent 5,280,040 describes methods and pharmaceutical composi-
tions for reducing bone loss using 3,4-diarylchromans and their pharma-
10 ceutically acceptable salts.
BRIEF DESCRIPTION OF THIS INVENTION
It has, surprisingly, been found that compounds of the general formula I
15 as stated in claim 1 can be used in the treatment or prophylaxis ofhyperlipoproteinaemia, hypertriglyceridaemia, hyperlipidaemia or hyper-
cholesterolaemia and furthermore it has also, surprisingly, been found
that compounds of the general formula I as stated in Claim 2 and 3 can
be used in the treatment or prophylaxis of arteriosclerosis including
20 atherosclerosis and that compounds of the general formula I as stated in
claim 4 can be used in the treatment or prophylaxis of patients in a need
of an anticoagulative treatment or prophylaxis, e.g. following a coronary
thrombosis or after surgery.
DETAILED DESCRIPTION OF THIS INVENTION
The present invention is based in part on the discovery that a representa-
tive 3,4-diarylchroman, centchroman (3,4-trans-2,2-dimethyl-3-phenyl-4-
[p-(beta-pyrrolidinoethoxy)phenyl]-7-methoxychroman) is effective as a
30 hypolipoproteinaemic, hypotriglyceridaemic, hypolipidaemic or hypocho-
lesterolaemic compound, inter alia in rabbits fed with cholesterol contain-
ing diet. These animal models are generally recognized models of hyperli-
CA 022088~9 1997-06-26
W O96/21443 PCT~DK~6/00014
poproteinaemia, hypertriglyceridaemia, hyperlipidaemia or hypercholeste-
rolaemia. These data thus indicate that the 3,4-diarylchromans are useful
as therapeutive and preventive hypolipoproteinaemic, hypotriglyceri-
daemic, hypolipidaemic or hypocholesterolaemic agents in mammals,
including primates such as humans.
Furthermore, the present invention is based in part on the discovery that
a representative 3,4-diarylchroman, centchroman (3,4-trans-2,2-
dimethyl-3-phenyl-4-[p-(beta-pyrrolidinoethoxy) phenyl]-7-methoxychro-
man) is also effective against the direct vascular effects of arteriosclero-
sis including atherosclerosis, inter alia in rabbits fed with cholesterol
containing diet. These animal models are generally recognized models of
arteriosclerosis including atherosclerosis. These data thus indicate that
the 3,4-diarylchromans are useful as therapeutic agents against arterio-
sclerosis including atherosclerosis in mammals, including primates such
as humans.
The present invention is yet further based in part on the discovery that a
representative 3,4-diarylchroman, centchroman (3,4-trans-2,2-dimethyl-
3-phenyl-4-[4-(2-(pyrrolidin-1-yl)ethoxy)phenyl]-7-methoxychroman) has
preventive or therapeutic anticoagulative activities when administered
inter alia to rats. These animal models are generally recognized models of
patients in a need of an anticoagulative treatment or prophylaxis, e.g.
following a coronary thrombosis or after surgery. These data thus
indicate that the 3,4-diarylchromans of formula I are useful as thera-
peutic and preventive agents to mammals, including primates such as
humans, in a need of an anticoagulative treatment or prophylaxis, e.g.
following a coronary thrombosis or after surgery.
This invention is related to the treatment or prophylaxis of disorders as
defined in the LiDid Research Clinics Program. J.A.M.A. 251 (1984),
351-364 and J.A.M.A. 251 (1984), 365 - 374 or what a person skilled
CA 022088~9 1997-06-26
W O 96/21443 PCT~DK96/00014
in the art may consider as subject for treatment or prophylaxis.
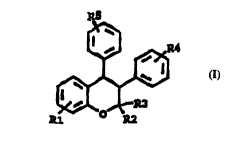
Within the present invention, compounds of formula I are used for the
indications as stated in claims 1-4 in a patient. Within formula 1, R1, R4
5 and R5 are individually hydrogen, halogen, trifluoromethyl, lower alkyl,
lower alkoxy or (tertiary amino)(lower alkoxy); and R2 and R3 are indivi-
dually hydrogen or a lower alkyl. As used herein, the term "lower alkyl"
includes straight and branched chain alkyl radicals containing from 1 to 6
carbon atoms, such as methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-
10 butyl, n-amyl, sec-amyl, n-hexyl, 2-ethylbutyl, 2,3-dimethylbutyl and the
like. The term "lower alkoxy" includes straight and branched chain
alkoxy radicals containing from 1 to 6 carbon atoms, such as methoxy,
ethoxy, n-propoxy, isopropoxy, n-butoxy, tert-butoxy, n-amyloxy, sec-
amyloxy, n-hexyloxy, 2-ethylbutoxy, 2,3-dimethylbutoxy and the like.
15 "Halogen" includes chloro, fluoro, bromo and iodo. The tertiary amino
radical may be a N,N-dialkylamine such as a N,N-dimethylamino, N,N-
diethylamino, N,N-dipropylamino and N,N-dibutylamino or a polymethy-
leneimine, e.g., piperidine, pyrrolidine, N-methylpiperazine or morpholine.
Herein, the term "(tertiary amino)(lower alkoxy)" is a lower alkoxy group
20 which is substituted by a tertiary amino group. Preferred compounds
include those in which R1 is lower alkoxy; R2 and R3 are lower alkyl,
especially methyl; R4 is hydrogen; and R5 is (tertiary amino)(lower
alkoxy) of the polymethyleneimine type. Within particularly preferred
embodiments, R1 is in the 7-position and is lower alkoxy, particularly
25 methoxy; each of R2 and R3 is methyl, R4 is hydrogen, and R5 is in the
4-position and is a (tertiary amino)(lower alkoxy) radical such as 2-
(pyrrolidin-1-yl)ethoxy. To be included by this invention are all pharma-
ceutically acceptable salts of the mentioned compounds of formula 1.
30 It is preferred to use the compounds of formula I in the transconfigura-
tion. These compounds may be used as racemic mixtures, or the isolated
stereoisomers e.g. d- or 1- enantiomers, may be used. The trans-l-en-
CA 022088~9 1997-06-26
WO96121443 PCT~DK96/00014
antiomers are more preferred.
- A particularly preferred compound for use within the present invention is
centchroman having the formula IV as stated in claim 14.
Although only one enantiomer is shown, it will be understood that the
formula IV is used herein to designate the transconfiguration of the 3-
and 4-phenyl groups and that both the d- and l-enantiomers, as well as
the racemic mixture are included.
3,4-diarylchromans are prepared according to known methods, such as
those disclosed in U.S. Patent Specification No. 3,340,276 to Carney et
al., U.S. Patent Specification No. 3,822,287 to Bolger, and Ray et al., J
Med Chem 19 (1976), 276 - 279, the contents of which are incorpo-
15 rated herein by reference. Conversion of the cis isomer to the transconfiguration by means of an organometallic base-catalyzed rearrange-
ment is disclosed in U.S. Patent Specification No. 3,822,287. The
optically active d- and l-enantiomers may be prepared as disclosed by
Salman et al. in U.S. Patent Specification No. 4,447,622 (incorporated
20 herein by reference) by forming an optically active acid salt which is
subjected to alkaline hydrolysis to produce the desired enantiomer. lf R2
is different from R3 and R4 is different from R5, the general formula I
covers 8 optical isomers.
25 Within the present invention, 3,4-diarylchromans may be prepared in the
form of pharmaceutically acceptable salts, especially acid-addition salts,
including salts of organic acids and mineral acids. Examples of such salts
include salts of organic acids such as formic acid, acetic acid, propionic
acid, fumaric acid, glycolic acid, lactic acid, pyruvic acid, oxalic acid,
30 succinic acid, malic acid, tartaric acid, citric acid, benzoic acid, salicylic
acid and the like. Suitable inorganic acid-addition salts include salts of
hydrochloric, hydrobromic, sulphuric and phosphoric acids and the like.
CA 022088~9 1997-06-26
WO 96/21443 PCI/DK96/00014
The acid addition salts may be obtained as the direct products of com-
pound synthesis. In the alternative, the free base may be dissolved in a
suitable solvent containing the appropriate acid, and the salt isolated by
evaporating the solvent or otherwise separating the salt and solvent.
3,4-diarylchromans and their salts are useful within human and veteri-
nary medicine, for example, in the treatment or prevention of patients
suffering from hyperlipoproteinaemia, hypertriglyceridaemia,
hyperlipidaemia or hypercholesterolaemia or arteriosclerosis including
10 atherosclerosis or in need of anticoagulative treatment or prophylaxis
e.g. following a coronary thrombosis or after surgery. For use within the
present invention, 3,4-diarylchromans and their pharmaceutically accept-
able salts are formulated with a pharmaceutically acceptable carrier to
provide a medicament for parenteral, oral, nasal, rectal, subdermal or
15 intradermal or transdermal administration according to conventional
methods. Formulations may further include one or more diluents, fillers,
emulsifiers, preservatives, buffers, excipients, etc. and may be provided
in such forms as liquids, powders, emulsions, suppositories, liposomes,
transdermal patches, controlled release, dermal implants, tablets, etc.
20 One skilled in this art may formulate the compounds in an appropriate
manner, and in accordance with accepted practices, such as those
disclosed in Remington's Pharmaceutical Sciences, Gennaro, ed., Mack
Publishing Co., Easton, PA, 1990.
25 Oral administration is preferred. Thus, the active compound is prepared
in a form suitable for oral administration, such as a tablet or capsule.
Typically, a pharmaceutically acceptable salt of the compound is com-
bined with a carrier and moulded into a tablet. Suitable carriers in this
regard include starch, sugars, dicalcium phosphate, calcium stearate,
30 magnesium stearate and the like. Such compositions may further include
one or more auxiliary substances, such as wetting agents, emulsifiers,
preservatives, stabilizers, colouring additives, etc.
CA 022088~9 1997-06-26
W O 96/21443 PC~rADK96/00014
Pharmaceutical compositions containing a compound of formula I may be
administered one or more times per day or week. An effective amount of
such a pharmaceutical composition is the amount that provides a clinical-
ly significant effect as hypolipoproteinaemic, hypotriglyceridaemic,
hypolipidaemic or hypocholesterolaemic agents or a clinically significant
effect against arteriosclerosis including atherosclerosis or provides a
clinically significant effect to patients in a need of an anticoagulative
treatment or prophylaxis e.g. following a coronary thrombosis or after
surgery. Such amounts will depend, in part, on the particular condition to
be treated, age, weight, and general health of the patient, and other
factors evident to those skilled in the art.
The pharmaceutical compositions containing a compound of formula I
may be administered in unit dosage form one or more times per day or
week. In the alternative, they may be provided as controlled release
formulations suitable for dermal implantation. Implants are formulated to
provide release of active compound over the desired period of time,
which can be up to several years. Controlled-release formulations are
disclosed by, for example, Sanders et al., J Pharm Sci 73 (1964), 1294 -
1297, 1984; U.S. Patent Specification No. 4,489,056; and U.S. Patent
Specification No. 4,210,644, which are incorporated herein by refe-
rence.
The following examples are offered by way of illustration, not limitation.
Examples of preferred compounds of formula I are centchroman as a
racemic mixture and as l-centchroman and d-centchroman. Furthermore,
3,4-trans-2,2-dimethyl-3-phenyl-4-[4-(2-(pyrrolidin-1 -yl)ethoxy)phenyl]-7-
hydroxychroman is a preferred compound. The more preferred compound
is trans-l-centchroman (1-3,4-trans-2,2-dimethyl-3-phenyl-4-[4-(2-(pyrro-
lidin-1 -yl)ethoxy)phenyl)-7-methoxychroman) .
CA 022088~9 1997-06-26
WO96/21443 PCTADK96/00014
- 10-
Examples of pharmaceutically acceptable acid addition salts are salts
with non-toxic acids, either inorganic acids such as hydrochloric acid,
sulphuric acid and phosphoric acid, or organic acids such as formic acid,
acetic acid, propionic acid, succinic acid, fumaric acid, glyconic acid,
5 lactic acid, citric acid, ascorbic acid, benzoic acid, embonic acid, metha-
nesulphonic acid and malonic acid.
The present invention is further illustrated by the following examples
which, however, are not to be construed as limiting the scope of protec-
10 tion. The features disclosed in the foregoing description and in thefollowing examples may, both separately and in any combination thereof,
be material for realising the invention in diverse forms thereof.
EXAMPLE 1
The effects of l-centchroman on plasma cholesterol have been investi-
gated in both rats and rabbits.
In the first study sham surgery or ovariectomy (OVX) was performed on
20 56 female Sprague-Dawley rats and the animals were assigned to the
following treatments (8 rats per group): 1 ) sham/vehicle; 2) OVX/vehicle,
3) OVX/ethynyl estradiol 0.2 mg/kg; 4) OVX/I-centchroman 1 mg/kg; 5)
OVX/I-centchroman 5 mg/kg; 6) OVX/I-centchroman 10 mg/kg;7)
OVX/I-centchroman 25 mg/kg. The doses were administered three times
25 per week for 5 weeks by oral gavage. At the conclusion of the experi-
ment serum was collected for determination of cholesterol level (US
patent 5,407,955).
I-centchroman had a marked effect on serum cholesterol in all doses and
30 reduced the cholesterol level even below the level of the sham operated
animals ~Table 1 ) .
CA 022088~9 1997-06-26
W O96/21443 PCT~DK96/00014
Table 1. Effect of l-centchroman on serum cholesterol in rats
Treatment Serum cholesterol (mmol/l)
Sham/vehicle 2.2 :i 0.3
OVX/vehicle 2.9 + 0.3
OVX/ethynyl estradiol 0.2 mg/kg1.9 + 0.4
OVX/I-centchroman 1 mg/kg 1.4 + 0.3*
OVX/I-centchroman 5 mg/kg 1.5 + 0.4
OVX/I-centchroman 10 mg/kg 1.5 + 0.2
OVX/I-centchroman 25 mg/kg 1.2 + 0.2*
Values are mean + SD. * indicate significant reduction of serum
cholesterol compared to OVX rats treated with ethynyl estradiol;
p < 0.002.
In order to show a clear dose-response effect on serum cholesterol a
further study was performed with l-centchroman.
Again sham surgery or ovariectomy was performed on 56 female rats
and the rats were assigned to the following treatments: 1 ) sham/vehicle;
2) OVX/vehicle, 3) OVX/ethynyl estradiol 0.2 mg/kg; 4) OVX/I-centchro-
man 0.05 mg/kg; 5) OVX/I-centchroman 0.1 mg/kg; 6) OVX/I-centchro-
man 0.5mg/kg;7) OVX/I-centchroman 1 mg/kg. The doses were admini-
stered three times per week for 5 weeks by oral gavage. At the con-
clusion of the experiment serum was collected for determination of
cholesterol level (US patent 5,407,955). In this experiment serum
cholesterol was reduced in a dose-dependent manner (table 2).
CA 022088~9 1997-06-26
W O96/21443 PCT~DK~6/00014
Table 2. Effect of l-centchroman on serum cholesterol in rats
Treatment Serum cholesterol (mmol/l)
Sham/vehicle 2.1 i 0.3
OVX/vehicle 2.7 i 0.3
OVX/ethynyl estradiol 0.2 mg/kg 1.6 i 0.4
OVX/I-centchroman 0 .05 mg/kg 2 .4 i 0.4
OVX/I-centchroman 0.1 mg/kg 2.3 i 0.3
OVX/I-centchroman 0.5 mg/kg 1.7 i 0.3*
OVX/I-centchroman 1.0 mg/kg 1.5 + 0.4*
Values are mean i SD. * indicate significant reduction of serum
cholesterol compared to sham/vehicle treated animals; p<O.003.
15 The effects of l-centchroman on plasma lipids were also investigated in
cholesterol fed rabbits. One week before the experiment was started 30
sexually mature New Zealand White rabbits were ovariectomized during
pentobarbital anaesthesia. Following recovery subcutaneous treatment
was started in three groups: Vehicle, 17-~-estradiol or l-centchroman 7.5
20 mg/kg. The doses were administered three times per week for 4 weeks.
At the end of the experiment plasma samples for analysis of serum
cholesterol were taken. As shown in table 3 I-centchroman reduced
serum cholesterol significantly compared to the level in vehicle treated
animals and estrogen treated animals.
Table 3. Effect of l-centchroman on serum cholesterol in ovariectomized
rabbits
Treatment Serum cholesterol (mmol/l~
OVX/vehicle 1 1.0 i 1.1
OVX/17-13-estradiol 50/Jg/kg 3x/week7.5 i 0.5*
OVX/I-centchroman 7.5 mg/kg 8.2 i 0.7*
3x/week
CA 022088~9 1997-06-26
W O96/21443 PCTADK~6/00014
Values are mean + SEM. * indicate significant reduction of serum
cholesterol compared to OVX/vehicle treated animals; p<O.05.
EXAMPLE 2
The effects of l-centchroman on the acummulation of lipid into the aortic
wall i.e. a central mechanism in the atherogenic process was investi-
gated in New Zealand White rabbits. One week before the experiment
was started 45 sexually mature New Zealand White female rabbits were
10 ovariectomized during pentobarbital anaesthesia. Additionally a group of
45 age and weight matched male rabbits were included in the experi-
ment. Following recovery oral treatment was commenced. Males and
females were treated in 3 groups tl5 animals per group): 1) Placebo
treated animals; 2) 17~ estradiol 2.5 mg/kg /day; 2) I-centchroman, 2.5
15 mg/kglday. After 14 days of treatment cholesterol was added to the
food. Every second day a blood sample was collected from each rabbit
and the total serum cholesterol was determined. (Holm P et al.
Atherosclerosis 1 1 5:1 91, 1 995). On basis of this measurement the
cholesterol concentration in the feeding was regulated on the following
20 days until next measurement. In that way serum cholesterol was kept
constant throughout the treatment period even though some of the
treatments are known to have effects on plasma cholesterol level. The
direct effect of treatment on the vessel wall could then be isolated. After
3 months of treatment the animals were killed, the aorta from each
25 animal was isolated and the cholesterol accumulated within the intima of
the aorta was determined. (Holm P et al Atherosclerosis, 115:191,
1 995).
The results are shown in table 4. It is clearly seen that both estrogen
30 and l-centchroman in both male and female rabbits had a significant
reducing effect on the intimal cholesterol content, which is independent
of the serum cholesterol since this was kept constant throughout the
CA 022088~9 1997-06-26
WO 96/21443 PCI~/DK96/00014
- 14-
experiment. The anti-atherosclerotic effect of l-centchroman may there-
fore partly be due to a direct vascular wall effect.
Table 4. Effect of l-centchroman and estrogen on intimal cholesterol
content
Treatment Intimal cholesterol Intimal cholesterol
(mmol/l) Female (mmol/l) Male
Placebo 45 i 9 27 i 5
17-13-estradiol 8 i 3* 8 i 2*
I-centchroman 2.518 i 5* 13 i 4*
1 0 mg/kg
Values are mean i SEM. * indicate significant reduction of intimal
cholesterol compared to placebo treated animals; p~0.05.
EXAMPLE 3
In vitro it has been shown that l-centchroman in rabbit platelet-rich
plasma inhibited platelet aggregation induced by platelet activating
factor (PAF). EC50 for this effect was between 3 and 10 ~g/ml. ADP-
induced aggregation was not affected.