Note: Descriptions are shown in the official language in which they were submitted.
CA 02209658 1997-07-04
WO 96/24568 PCT/US96/01818
-1-
HEAVY AROMATICS PROCESSING
This invention relates to a process for converting a
feedstock containing heavy aromatics, specifically, C9+
aromatics, to lighter aromatic products, specifically
xylenes.
Para-xylene is an important by-product of petroleum
refining because it is used in significant quantities for
the manufacture of terephthalic acid which is reacted with
polyols such as ethylene glycol in the manufacture of
polyesters.
The major source of para-xylene is catalytic reformate
which is prepared by mixing petroleum naphtha with hydrogen
and contacting the mixture with a strong
hydrogenation/dehydrogenation catalyst such as platinum on
a moderately acidic support such as a halogen treated
alumina.
Usually, a C6 to Ce fraction is separated from the
reformate, extracted with a solvent selective for aromatics
or aliphatics to separate these two kinds of compounds and
produce a mixture of aromatic compounds which is relatively
free of aliphatics. This mixture of aromatic compounds
usually contains benzene, toluene and xylenes (BTX) along
with ethyl benzene. '
Liquids from extremely severe thermal cracking, e.g.
high temperature steam cracking of naphtha are also rich in
aromatics and may be used to prepare BTX in a similar
manner. '
Concentrated aromatic fractions are also provided by
severe cracking over such catalysts as ZSM-5 and by
conversion of methanol over ZSM-5.
Refineries have focused on the production of xylenes
by transalkylation of C9+ aromatics, which would normally
only be of value as fuel, and toluene, over zeolite
containing catalysts. The stability and transalkylation
selectivity of zeolite beta for this reaction is the
subject of several recent publications. See Das et al.
CA 02209658 1997-07-04
WO 96/24568 PCT/US96/01818
-2-
"Transalkylation and Disproportionation of Toluene and C9
Aromatics over Zeolite Beta" 23 Catalyst Letters pp. 161-
168 (1994); Das et al. "Zeolite Beta Catalyzed C~ and C9
Aromatics Transformation" 116 Applied Catalysis A:
General, pp. 71-79 (1994) and Wang et al.
"Disproportionation of Toluene and of Trimethylbenzene and
Their Transalkylation over Zeolite Beta", 29 Ind. Eng.
Chem. Res. pages 2005-2012 (1990).
Additionally, processes for producing xylenes from
hydrocarbon fractions containing substituted aromatics have
been disclosed in the patent literature. U.S. Patent No.
4,380,685 discloses the para-selective alkylation,
transalkylation or disproportionation of a substituted
aromatic compound to provide a mixture of dialkylbenzene
compounds employing as a catalyst a zeolite characterized
by a Constraint Index of 1 to 12 and a silica/alumina mole
ratio of at least 12/1, the catalyst having incorporated
thereon various metals and phosphorus.
During the dealkylation reactions that, typically,
accompany the conversion of heavy aromatics to xylenes,
olefins are formed which tend to undergo secondary
reactions resulting in the formation of coke which rapidly
deactivates the catalyst and of undesirable aromatic by-
products which can also contribute to catalyst
deactivation. One approach for solving the problem posed by
olefins formation has been to encourage olefin saturation.
Hydrogenation metals, such as platinum, are known to
saturate olefins and prevent coke formation and have been
incorporated into the catalysts. Similarly, employing high
hydrogen partial pressures or high hydrogen to hydrocarbon
mole ratios have been proposed to minimize olefin formation
and catalyst aging.
U.S. Patent No. 5,030,787 discloses a process for the
vapor-phase conversion of a feedstock containing at least
one C9+ aromatic compound to a product containing
CA 02209658 2005-09-14
-3-
substantial quantities of C6 to C8 compounds, e.g. benzene and
xylenes. The conversion occurs over a catalyst which contains
a zeolite having a Constraint Index of 1 to 3, e.g. zeolites
MCM-22, ZSM-12 and zeolite beta. Steam treatment of the
zeolite is proposed, see Col. 9, lines 66-67. A Group VIII
metal can be included with the catalyst. In the specific
examples of the disclosure the zeolite is subjected to the
steam treatment prior to incorporation of the hydrogenation
metal, see Examples 20-22.
However, the use of hydrogenation components and high
hydrogen partial pressures not only reduces olefin formation
and catalyst aging but also promotes saturation of the aromatic
compounds resulting in low yields of the desirable lighter
aromatics products such as benzene, toluene and xylenes. Also,
maintaining a high hydrogen to hydrocarbon mole ratio requires
large reactors which are costly to manufacture and maintain.
An object of the present invention is to obviate or alleviate
these disadvantages.
The invention is directed to a process for converting C9+
aromatic hydrocarbons to lighter aromatic products comprising
the step of contacting a feed comprising the C9+ aromatic
hydrocarbons, benzene and/or toluene with a catalyst
composition comprising a zeolite having a Constraint Index of
0.5 to 3 and a hydrogenation component to produce a product
comprising xylenes, wherein the catalyst composition having the
hydrogenation component is treated to reduce its aromatic
hydrogenation activity.
CA 02209658 2005-09-14
-3a-
In one particular embodiment there is provided a process
for converting C9+ aromatic hydrocarbons to a product which
includes xylenes comprising the step of contacting a feed
comprising the C9+ aromatic hydrocarbons, together with benzene
or toluene, with a catalyst composition comprising a zeolite
selected from beta, ZSM-12 and MCM-22 and a hydrogenation
component to produce a product comprising xylenes, wherein the
catalyst composition having the hydrogenation component is
treated with a source of sulfur or nitrogen or with steam to
reduce its aromatic hydrogenation activity.
The catalyst employed in the process of the invention
includes a zeolite having a Constraint Index of 0.5 to 3. The
method by which Constraint Index is determined is described
fully in U.S. Patent No. 4,016,218.
Suitable zeolites for use in the process of the invention
include MCM-22, ZSM-12 and Beta. ZSM-12 is more particularly
described in U.S. Patent No. 3,832,449 and has a Constraint
Index of 2.3 (316°C). Zeolite Beta is more
CA 02209658 1997-07-04
WO 96/Z4568 PCT/US96/01818
particularly described in U.S. Patent No. Re. 28,341 (of
original U.S. patent No. 3,308,069) and has a Constraint ,
Index of 0.6-2.0 (316-399°C). Zeolite MCM-22 is described
in U.S. Patent No. 4,954,325 and has a Constraint Index of
1.5 (454°C).
It may be desirable to incorporate the selected
zeolite catalyst with another material which is resistant
to the temperatures and other conditions employed in the
process of this invention. Such materials include active
and inactive materials and synthetic or naturally occurring
zeolites as well as inorganic materials such as clays,
silica and/or metal oxides such as alumina. The latter may
be either naturally occurring or in the form of gelatinous
precipitates or gels including mixtures of silica and metal
oxides. Use of a material in conjunction with the zeolite
catalyst, i.e. combined therewith or present during its
synthesis, which itself is catalytically active, may change
the conversion and/or selectivity of the catalyst.
Inactive materials suitably serve as diluents to control
the amount of conversion so that transalkylated products
can be obtained economically and orderly without employing
other means for controlling the rate of reaction. These
materials may be incorporated into naturally occurring
clays, e.g. bentonite and kaolin to improve the crush
~-strength of the catalyst under commercial alkylation
operating conditions. The materials, i.e. clays, oxides,
etc. function as binders for the catalyst. It is desirable
to provide a catalyst having good crush strength because in
commercial use, it is desirable to prevent the catalyst
from breaking down into powder-like materials. These clay
binders have been employed normally only for the purpose of '
improving the crush strength of the catalyst.
Naturally occurring clays which can be composited with '
the zeolite catalyst herein include the montmorillonite and
kaolin family, which families include the subbentonites,
and the kaolins commonly known as Dixie, McNamee, Georgia
CA 02209658 1997-07-04
WO 96124568 PC"T/US96/01818
-5-
and Florida clays or others in which the main mineral
constituent is halloysite, kaolinite, dickite, nacrite.or
anauxite. Such clays can be used in the raw state as
originally mined or initially subjected to calcination,
acid treatment or chemical modification. Binders useful
for compositing with zeolite also include inorganic oxides,
notably alumina.
In addition to the foregoing materials, the zeolite
catalyst can be composited with a porous matrix material
such as an inorganic oxide selected from the group
consisting of silica, alumina, zirconia, titania, thoria,
beryllia, magnesia, and combinations thereof such as
silica-alumina, silica-magnesia, silica-zirconia, silica-
thoria, silica-beryllia, silica-titania as well as ternary
compositions such as silica-alumina-thoria, silica-alumina-
zirconia, silica-alumina-magnesia and silica-magnesia-
zirconia. It may also be advantageous to provide at least
a part of the foregoing matrix materials in colloidal form
so as to facilitate extrusion of the bound catalyst
component(s).
The relative proportions of finely divided crystalline
material and inorganic oxide matrix vary widely, with the
,. crystal content ranging from 1 to 95 percent by weight and
more usually, particularly when the composite is prepared
in the form of beads, in the range of 2 to 80 weight
percent of the composite. The zeolite is employed in
combination with a hydrogenation component such.as a metal
selected from Group VIII of the Periodic Table of the
Elements (CAS version, 1979). Specific examples of useful
hydrogenation materials are iron, ruthenium, osmium,
nickel, cobalt, rhodium, iridium, or a noble metal such as
platinum.
The amount of the hydrogenation component is selected
according to a balance between hydrogenation activity and
catalytic functionality. Less of the hydrogenation
component is required when the most active metals such as
platinum are used as compared to molybdenum which does not
CA 02209658 1997-07-04
WO 96124568 PCT/LTS96/01818
-6-
possess such strong hydrogenation activity. Generally, the
catalyst contains 0.01 to 10 wt.%, preferably 0.05 to 5
wt%, of the hydogenation component.
The hydrogenation component can be incorporated into
the catalyst composition by co-crystallization, exchanged
into the composition to the extent a Group IIIA element,
e.g., aluminum, is in the structure, impregnated therein or
mixed with the zeolite and the inorganic oxide matrix.
Such component can be impregnated in, or on, the zeolite
such as for example, in the case of platinum, by treating
the zeolite with a solution containing a platinum metal-
containing ion. Suitable platinum compounds for
impregnating the catalyst with platinum include
chloroplatinic acid, platinous chloride and various
compounds containing platinum amine complex, such as
Pt (NH3 ) QC12 . H20.
After treatment with the hydrogenation function, the
catalyst composite is usually dried by heating the catalyst
at a temperature of 150 to 320°F (65 to 160°C), preferably
from 230°F to 290°F (110°C to 143°C) for at least
about 1
minute and generally not longer than about 24 hours.
Thereafter, the catalyst composite is calcined in a stream
of dry gas, such as air or nitrogen at temperatures of 500
°F to 1200°F (260°C to 649°C) for 1 to 20 hours.
Calcination is preferably conducted at pressures ranging
from 15 to 30 psia (100-200 kPa).
The catalyst composition is treated to reduce its
aromatics hydrogenation activity, without substantially
inhibiting its olefin saturation activity which prevents
formation of the desirable products.
Aromatics loss over the treated catalyst composition '
of this invention is substantially lower than the aromatics
loss sustained over the untreated catalyst. '
The activity of the catalyst composition for aromatics
ring loss relative to the entire amount of aromatics in the
feed, is an effective way to evaluate the aromatics
CA 02209658 1997-07-04
WO 96/24568 PCTlUS96/01818
hydrogenation activity of the catalyst. Ideally, aromatics
ring loss is less than 1 mole %. However, ring losses of
less than l0 mole %, specifically, less than 5 mole %, even
more specifically, less than 2 mole %, are acceptable,
based on the entire amount of aromatics in the feed. Ring
loss is determined using gas chromatography by comparing
the amount of aromatics in the feed with the amount of
aromatics in the product.
The benzene hydrogenation activity test (BHA test) can
also be used to determine catalytic activity for
hydrogenation of benzene to cyclohexane and it is a good
indicator of aromatics hydrogenation capability which, for
purposes of the instant invention is minimized. The test
is, typically, used to determine the activity of noble
metal catalysts. The conditions of the BHA test are
described below in the examples. The BHA test is also
described in U.S. Patent Nos. 5,188,996; 4,952,543;
4,837,397 and 4,849,385. This test provides a rate of
benzene hydrogenation in terms of moles benzene/moles
hydrogenation functionality/hour at 100'C. After treatment
the catalyst of the invention has a BHA less than 500, and
preferably less than 400, most preferably less than 20.
The extent and methods of treatment of the catalyst
including the hydrogenation functionality for minimizing
loss of aromatics may vary depending upon the catalyst
composition and its method of manufacture, e.g. the method
of incorporating the hydrogenation functionality.
Typically, steam treatment of the catalyst composition
is employed as an effective method for minimizing the
aromatics hydrogenation activity of the catalyst
composition. In the steaming process the catalyst is,
usually, contacted with 5 to 100% steam at a temperature of
500°F to 1200°F (260 to 910°C) for 1 to 20 hours at a
pressure of 100 to 2500 kPa.
Another method for minimizing the aromatics
hydrogenation activity of the catalyst composition is by
CA 02209658 2005-09-14
.g.
exposing it to a compound containing an element selected
from Group VA or VIA of the Periodic Table of the Elements
(CAS Version, 1979). The VIA element specifically
contemplated is sulfur, whereas a specifically contemplated
group VA element is nitrogen.
Effective treatment is accomplished by contacting the
catalyst with a source of sulfur at a temperature of 600 to
900'F (316 to 480'C). The source of sulfur can be
contacted with the catalyst via a carrier gas, typically,
l0 an inert gas such as hydrogen or nitrogen. In this
embodiment, the source of sulfur is typically hydrogen
sulfide .
Catalyst treatment can be effected ex-situ prior to
the process of the invention or can be effected in situ in
the process reactor either before or during at least part
of the process.
For example, a source of sulfur can be co-fed with the
hydrocarbon feedstream in a concentration ranging from 50
ppmw sulfur to 10,000 ppmw sulfur. Any sulfur compound
that will decompose to form H2S and a light hydrocarbon at
about 900'F (480'C) or less will suffice. Typical examples
of appropriate sources of sulfur include carbon disulfide
and alkylsulfides such as methylsulfide, dimethylsulfide,
dimethyldisulfide, diethylsulfide and dibutyl sulfide.
Sulfur treatment can be considered sufficient when sulfur
breakthrough occurs: that is, when sulfur appears in the
liquid product.
Typically, sulfur treatment is initiated by
incorporating a source of sulfur into the feed and
continuing sulfur treatment for a few days, typically, up
to 10 days, more specifically, from one to five days. The
sulfur treatment can be monitored by measuring the
concentration of sulfur in the product off gas. During
this treatment " the sulfur concentration in the off gas
should range from 20 to 500 ppmw sulfur, preferably 30-to
250 ppmw.
CA 02209658 1997-07-04
WO 96/24568 PCT/US96/01818
A further method for minimizing the aromatics
hydrogenation activity of the catalyst composition is by
the addition of an inactive or less active element, such as
an element selected from Group IB of the Periodic Table of
the Elements (CAS Version, 1979). The IB element
specifically contemplated is copper.
Any one or a combination of these in situ and/or ex
situ methods can be employed for minimizing the aromatics
hydrogenation activity of the catalyst. It has been found
that these methods minimize aromatics hydrogenation
activity while sustaining sufficient hydrogenation of
olefins which avoids rapid catalyst aging.
Aromatics hydrogenation activity is also controlled by
operating the process under conditions of low hydrogen
partial pressure. Typically, an appropriately low hydrogen
partial pressure is 100 to 3000 kPa, preferably 700 to 2100
kPa and hydrogen to hydrocarbon mole ratio of less than
3.0, preferably 1.0 to 2Ø
The heavy aromatics feed used in this process
comprises one or more aromatic compounds containing at
least 9 carbon atoms such as, e.g. trimethylbenzenes,
dimethylbenzenes, and diethylbenzenes, etc. Specific C9+
aromatic compounds include mesitylene (1,3,5-
trimethylbenzene), durene (1,2,4,5-tetramethylbenzene),
hemimellitene (1,2,4-trimethylbenzene), pseudocumene
(1,2,4-trimethylbenzene), 1,2-methylethylbenzene, 1,3-
methylethylbenzene, 1,4-methylethylbenzene, propyl-
substituted benzenes, butyl-substituted benzenes, isomers
of dimethyl-ethylbenzenes, etc.
Suitable feeds include a C9+ refinery fractions which
are rich in aromatics, that is contain at least 80 wt.% C9+
aromatics. Typical refinery fractions which may be useful
include catalytic reformate, FCC naphtha or TCC naphtha.
The feedstock employed contains benzene and/or toluene
in addition to the C9+ compounds. The feed can also
contain xylenes. This charge will normally constitute
CA 02209658 1997-07-04
WO 96/24568 PCTIUS96/01818
-10-
40% to 90 %, more specifically 50 to 70 %, by volume of the
entire feed, the balance of the feed is made up by C~+
aromatics.
The process can be conducted in any appropriate
reactor including a radial flow, fixed bed, continuous down
flow or fluid bed reactor.
The process is performed at a temperature of 600°F to
1100°F (315°C to 590°C), preferably 700°F to
950°F (370°C
to 510°C) a catalyst inventory of 0.5 to 4.0 WHSV and a
total system pressure'of 50 to 1000 psig (450 to 7000 kPa).
The invention will now be more particularly described
with reference to the accompanying drawings, in which:
Figure 1 is a simplified schematic flow diagram of one
embodiment of the process of this invention.
Figure 2 is a plot of hydrocarbon conversion at
constant pressure and hydrogen to hydrocarbon mole ratio at
a temperature of about 725°F (385°C) and a temperature of
about 850°F (454°C) as a function of days on stream vs.
average reactor temperature.
Figure 3 is a plot of hydrocarbon conversions at
constant temperature and pressure at hydrogen to
hydrocarbon mole ratios of 2:1 and 3:1 as a function of
days on stream vs. average reactor temperature.
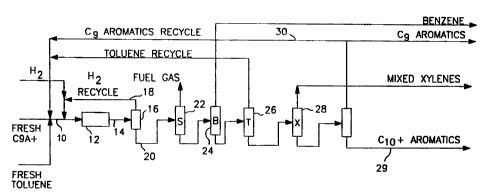
Referring to Figure 1, a C9+ aromatics stream along
with toluene and hydrogen are introduced via line 10 to
reactor 12 which contains the transalkylation catalyst of
this invention. The reactor is maintained under conditions
sufficient so that benzene and methyl aromatics (toluene,
xylenes, trimethylbenzenes and tetramethylbenzenes)
approach thermodynamic equilibrium through transalkylation
reactions. The C8 to C11 aromatics having CZ+ alkyl groups
undergo dealkylation to form light gas and benzene,
ethylbenzene and methylbenzenes which can undergo
transalkylation reactions. The conditions are maintained
to promote the secondary reactions which involve
hydrogenation of light olefins which reduce coke. The
CA 02209658 1997-07-04
WO 96/24568 PCT/US96/01818
-li-
conditions are also maintained to prevent olefins from
taking part in formation of heavy aromatics compounds that
can deactivate the catalyst or produce undesirable by-
products. These results are achieved without producing a
high yield of saturated aromatics. The product of reactor
12 is withdrawn via line 14 and introduced to hydrogen
separator 16 which separates hydrogen for recycle to
reactor 12 via line 18. The feed then passes via line 20
to a stabilizer section 22 which removes Cs- fuel gas by
known techniques. Thereafter, the product is fractionated
into benzene, toluene and xylenes streams in fractionators
24, 26 and 28, respectively, for separation of these
streams. The remaining product which comprises unreacted
C9+ feed and any heavy aromatics is separated into a C9
aromatics stream 30 and a Clo+ aromatics stream 29. Stream
30 is recycled back to the reactor feed, removed from the
process, or a combination of both (partial recycle). The
Clo+ aromatics stream 29 is suitable for gasoline blending
or other product such as solvents.
Example 1
This example demonstrates formation of a platinum
exchanged alumina bound ZSM-12 catalyst.
65 parts of ZSM-12 synthesized according to U.S.
Patent No. 3,832,449 was mixed with 35 parts of LaRoche
~lersal 250 alumina on a dry basis. The mixture was dry
mulled and formed into 1/16" (l.6mm) cylindrical
extrudates. The extrudates were dried, activated and
calcined. Platinum (0.1 wt.%) was exchanged into the
extrudates using [(NH3)~Pt)C12. The extrudates were washed,
dried and calcined at 660°F (350°C). The platinum
containing calcined extrudates were steamed at 900°F
(480°C) for 4 hours. The resulting catalyst was designated
as catalyst A and had an alpha activity of 53 and a surface
area of 281 m2/g.
The catalyst was tested for its activity for
hydrogenation of benzene to cyclohexane in the BHA test.
CA 02209658 1997-07-04
WO 96/24568 PCT/US96/01818
-12-
In this test, a gaseous mixture containing 100:1 molar
ratio of hydrogen and benzene was flowed through a vertical
quartz ("Vycor" trademark) tubular reactor, 1/4 inch (6 mm)
~in diameter and 5 inches (12 cm) in length, containing ,
about 250 mg of the catalyst, at a hydrogen flow rate of
200 cc/min, a total pressure of 1 atm. and temperatures
between 75'F (24'C) and 300'F (150'C), depending on the
activity of the catalyst. The benzene hydrogenation
activity was determined by measuring the ability of the
catalyst to hydrogenate benzene in terms of the moles of
benzene hydrogenated per moles of platinum per hour at
100'C. The catalyst had a BHA of 444 moles benzene/moles
Pt/hour.
Figure 2 is a plot of average reactor temperature vs.
days on stream which compared the performance of sulfided
catalyst A at a temperature of about 725°F (385°C) and a
temperature of about 850°F (454°C) at constant pressure of
400 psig (2860 kPa) and hydrogen to hydrocarbon mole ratio
of 4:1. The plot shows that the catalyst remained stable
at both temperatures within a period from about 10 to 100
days. Elevating the temperature to 850°F (454°C) after 40
days achieved a greater C9+ conversion which remained
relatively constant for up to 55 days longer.
Example 2
This example demonstrates formation of a platinum
impregnated alumina bound ZSM-12 catalyst.
65 parts of ZSM-12 (dry basis) synthesized according
to U.S. Patent No. 3,832,449 were mixed in a muller with 35
parts of LaRoche Versal 250 alumina (dry basis) and with a
platinum-containing solution using [(NH3)4Pt]C12. The
amount of platinum used gave a nominal loading of 0.1 wt.~
(dry basis). The mixture was mulled and formed into 1/16"
(l.6mm) cyclindrical extrudates. The extrudates were '
dried, activated and calcined. The platinum containing
calcined extrudates were steamed at 900°F (480°C) for 4
hours. The platinum loading of the finished catalyst was
CA 02209658 1997-07-04
WO 96/24568 PGT/US96l01818
-13-
0.09 wt.%. The resulting catalyst was designated as
catalyst B. The finished catalyst had an alpha activity of
77, a BHA of 118 moles benzene/moles of Pt/hour, and a
surface area of 280 m2/g.
Figure 3 is a plot of average reactor temperature vs.
days on stream which compares the performance of catalyst B
at a low hydrogen to hydrocarbon mole ratio (2:1) with the
performance of the same catalyst at a higher hydrogen to
hydrocarbon mole ratio (3:1). Catalyst B was steamed and
sulfided by exposing the catalyst to H2S in a concentration
ranging from between 0.05 and 4.0 wt.% in flowing hydrogen,
as carrier, at temperatures ranging from about 650°F
(340°C) and 800°F (430°C) until HZS was detected in the
product gas at a level of approximately 250 ppmw. The
plot shows that catalyst performance is relatively stable
over a period of over 100 days on stream at a relatively
constant temperature of 750 to 775°F (400 to 413°C). This
plot demonstrates how a low hydrogen to hydrocarbon mole
ratio during the start-up phase of the conversion enhances
catalyst stability.
Examples 3-4
These examples compare the performance of catalyst A
which was steamed after addition of the metal (Example 3)
with a catalyst made in accordance with Example 22 of U.S.
Patent No. 5,030,787 which was steamed prior to
incorporation of the metal (Example 4) in transalkylating
C9+ aromatics and toluene. The results are reported in
Table 1.
CA 02209658 1997-07-04
WO 96/24568 PCT/ITS96/01818
-14-
Table 1
Conversion of C9+ Aromatics
and Toluene
Over Steamed Catalysts
Example 3 Example
4
Reaction Conditions
Hydrogenation metal
Steamed Unsteamed
Pressure (psig) 300
250
(kPa) (2170) (1825)
Temperature (F) 800
800
(C) (430) (430)
WHSV (hr 1) 2.5 2.6
H2:Hydrocarbon mole
1/1 1/1
ratio
Composition (wt.%) Feed Product Feed Product
C6 - C8 Aromatics 48.5 70.1 62.7 77.4
C9 aromatics 43.0 20.5 30.4 11.0
Clo aromatics 7.8 4.3 4.8 3.1
Aromatic Ring -- 98.9 -- 96.7
Retention, mol. % (1.1) (3.3)
(loss) I
The data reported in Table 1 show that ring retention is
significantly better when the hydrogenation metal is
steamed as in Example 3. Additionally, the steamed
hydrogenation functionality of Example 3 enabled a lower C6
to Ce aromatics feed to be converted to a product
containing an amount of C6 to C8 aromatics which was
comparable to the amount of C6 to Ce aromatics produced over
the higher aromatics feed of Example 4. Moreover, although
not reported in Table 1, of the C6 to Ce aromatics produced
in Example 3, 36.0 wt.% were xylenes. In contrast, of the
C6 to C$ aromatics produced in Example 4, only 28.6 wt.%
were xylenes.
CA 02209658 1997-07-04
WO 96/24568 PG"T/US96l01818
-15-
examples 5-6
These examples demonstrate the advantage of sulfiding
catalyst A prior to introduction of the feed and also
compare the performance of the presulfided catalyst with a
presulfided catalyst which is further treated by adding
sulfur to the feed. In both examples catalyst A was
employed and the conditions of reaction included a
temperature of 800°F (430°C), pressure of 400 psig (2860
kPa), WHSV of 2.5 and hydrogen to hydrocarbon mole ratio of
4. In Example 5, sulfiding was accomplished by contacting
the steamed platinum exchanged ZSM-12 catalyst with about
50 cc/minute of 2% IiZS in hydrogen gas for about 40 minutes
at 660°F to 750°F (350°C to 400°C). In Example 6,
the
catalyst was further sulfided in situ by cofeeding 600 ppm
sulfur (in the form of dibutyl sulfide) with the
hydrocarbon feed for two hours. The results of conversion
over these sulfided catalysts are shown below in Table 2.
Table 2
Conversion of C9+
Aromatics and Toluene
Over Steamed and Sulfur
Treated Catalyst
A
Broduct, wt.% of Feed Example 5 Example 6
Feed
CS- 0 36.7 26.2
C6+, non-aromatics 0.4 1.3 1.0
Benzene 0 4.4 6.4
Toluene 56.0 19.1 24.0
Ethylbenzenes 0.1 0.8 1.0
Xylenes 1.8 25.2 28.0
C9+ Aromatics 41.7 16.2 16.0
Aromatic Ring 66.0 75.0
Retention, wt% (44.0) (25.0)
(loss)
CA 02209658 1997-07-04
WO 96/24568 PGTIUS96/01818
-16-
As the data in Table 2 show there are significant'
advantages, particularly in the aromatic ring retention and
xylenes production, to cofeeding sulfur.
Examples 7 and 8
These examples demonstrate that a lower aromatics
saturation activity of the hydrogenation functionality can
be established by operating the process at a low hydrogen
to hydrocarbon mole ratio. In these examples, Catalyst A
was employed in the transalkylation of a C9+ aromatic
hydrocarbon feedstream and toluene. The catalyst was not
exposed to sulfur treatment.
CA 02209658 1997-07-04
WO 96/24568 PCT/US96/01818
-17-
Table 3
Conversion
of C9+ Aromatics
and Toluene
Under
Different
Hydrogen:
Hydrocarbon
Mole Ratios
Example 7 Example 8
Reaction Conditions
Pressure, 400 (2860) 400 (2860)
psig (kPa)
Ave. Temperature, 780 (415) 811 (433)
F
( C)
Time On Stream, 3.1 6
days'
Space Velocity, 2.4 2.4
WHSV
HZ to hydrocarbon 2:1 l:l
mole
ratio
Product, Feed Product Product
~t.% of
Feed
CS- -- 13.9 4.0
C6+ non- 0.1 1.0 0.1
aromatic
Benzene -- 7.7 10.8
Toluene 57.0 29.0 34.8
Ethylbenze 0.1 1.8 1.5
ne
Xylenes 1.7 26.0 28.6
C9+ 41.1 2 2 . 0 2 0 . 4
Aromatics
Aromatic 87.1 98.7.
Ring (12.9) (1.3)
Retention,
mol %
(loss)
Examples 9-11
These examples demonstrate continuously cofeeding
sulfur to treat the catalyst in situ.
In these examples a Pt/ZSM-12 catalyst was presulfided
with about 50 cc/min 2% HzS/HZ for about 40 minutes at 660-
750°F (350-400°C). In each example the reaction was
CA 02209658 1997-07-04
WO 96/24568 PCTIUS96/01818
-18-
operated at a temperature of 800°F (430°C), pressure of 300
psig _(2170 kPa), WHSV of 2.5 and hydrogen to hydrocarbon
mole ratio of 2. After about 125 hours on stream 100 ppmw
sulfur Was added to the feed and the sulfur was
continuously cofed through about 170 hours on stream, at
which time the sulfur feed was discontinued to evaluate the
effect that continuous addition of sulfur had on the
product. Product samples were analyzed at 134 hours on
stream (Example 9) at 158 hours on stream (Example 10) and
at 206 hours on stream (Example 11). The following Table 4
reports the results of product analysis.
Table 4
Continuous Sulfur
Addition
Example . 9 10 11
Sulfur Cofeed Yes Yes No
Time on Stream, hours 134 158 206
Product, wt.% of
Feed
Feed Product Product Product
0 6.1 6.0 7.3
C6+ Non aromatics 0 0 0 0.1
C6 - CS Aromatics 58.0 78.1 78.0 76.6
C9+ Aromatics 42.1 16.1 16.6 16.4
Ring Retention, -- 97.6 97.9 96.1
mol.% (2.4) (2.1) (3.9)
(loss)
The data reported in Table 4 demonstrate the
advantages of continuously cofeeding sulfur. Comparing the
C6 to C8 aromatics of Example 11 with Examples 9 and 10, it
is apparent that continuously cofeeding sulfur maintained a
product of higher C6 to C8 aromatics content. Additionally,
in Examples 9 and 10 fewer CS- and C6+ nonaromatics (e. g.
methylcyclopentane) formed and fewer aromatics were lost.
Furthermore, although not reported in Table 4, the hydrogen
consumption was significantly reduced with sulfur cofeed.
At 134 hours on stream, with sulfur cofeed, the hydrogen
CA 02209658 1997-07-04
WO 96/24568 PCT/US96/01818
-19-
consumption was 222:f ~CF/B (Exau~le 9), at 158 hours on
stream, with sulfur cofeed, the hydrogen consumption was
202.1 SCF/B (Example 10). In Example 11, after the sulfur
cofeed was discontinued, the hydrogen consumption was 312.2
SCF/B.
Example 12
In this Example the Pt/ZSM-12 catalyst A (Example 1)
was compared with a Mo/zeolite beta catalyst in the
conversion of a feed comprising 55 wt% toluene and 45 wt%
l0 C9-Clo aromatics.
The beta catalyst was prepared by muller impregnation
of a 65 wt% zeolite beta/35 wt% alumina extrudate~with
phosphoric acid and ammonium heptamolybdate to give a
catalyst containing 4.2 wt% Mo and 1.7 wt% P. The activity
of the catalyst was reduced by steaming for l0hrs in 100%
steam at 1025°F (550°C) to give an alpha value of 17.
Prior to testing, each catalyst was pre-sulfided with
a flow of 50-60cc/min of 2%HZS/HZ at 100 kPa at 300-400°C
for 40-60 minutes.
Results obtained in processing the above feed, after
about 1 week on stream, at 800°F (430°C), 300 psig (2170
kPa), a hydrogen to hydrocarbon mole ratio of 2:1, and 2.5
WHSV are shown in Table 5 below:
Table 5
Pt/ZSM-12 Mo/beta
C9 aromatic conversion 64.8 wt% 62.4 wt%
Clo aromatic conversion 44.2 wt% 26.4 wt%
Xylene yield 31.6 wt% 29.7 wt%
Benzene yield 10.6 wt% 6.5 wt%
C6-Clo ring loss 3 . 9 mol % 1. 3 mol%
CA 02209658 1997-07-04
WO 96!24568 PCT/US96/01818
-20-
The Mo/beta catalyst exhibited a lowr ring loss, but
converted less C9-Clo aromatics, than the Pt/ZSM-12
catalyst.
Examt~le 13
In this Example the Pt/ZSM-12 catalyst B (Example 2)
was compared with a Cu-modified Pt/zeolite beta catalyst in
the conversion of a feed comprising 47 wt% toluene and 53
wt% C9-Cla aromatic blend.
The beta catalyst was prepared by impregnation using
the incipient wetness technique of a 65 wt% zeolite beta/35
wt% alumina extrudate with a platinum and copper salt
solution to give a nominal loading of 0.1 wt% Pt and 0.033
wt% Cu on the catalyst. Comparison of the benzene
hydrogenation activity of the copper-containing catalyst
with an identical catalyst without copper indicated that
the benzene hydrogenation activity had been reduced by the
copper modification. The BHA of the copper-containing
catalyst was 109 moles benzene/moles Pt/hour.
Both catalysts were presulfided for 30 minutes at
430°C with a feed doped with dibutylsulfide. The results
obtained in processing the above feed at 800°F (430°C), 300
psig (2170 kPa), a hydrogen to hydrocarbon mole ratio of
2:1, and 2.5 WHSV are shown in Table 6 below:
Table 6
Pt/ZSM-12 Cu/Pt/beta
C9 aromatic conversion 54.7 wt% 52.9 wt%
Clo aromatic conversion 53.3 wt% 43.7 wt%
Xylene yield 36.1 wt% 35.3 wt%
Benzene yield 6.3 wt% 4.1 wt%
C6-Clo ring loss 2.7 mol% 5.6 mol%